Abstract
FUN14 domain-containing protein 1 (FUNDC1) is a mitochondrial outer membrane protein which is responsible for hypoxia-induced mitophagy in mammalian cells. Knockdown of fundc1 is known to cause severe defects in the body axis of a rare minnow. To understand the role of Fundc1 in embryogenesis, we used zebrafish in this study. We used bioimaging to locate zebrafish Fundc1 (DrFundc1) with MitoTracker, a marker of mitochondria, and/or CellLight Lysosomes-GFP, a label of lysosomes, in the transfected ovary cells of grass carp. The use of Western blotting detected DrFundc1 as a component of mitochondrial proteins with endogenous COX IV, LC3B, and FUNDC1 in transgenic human embryonic kidney 293 T cells. DrFundc1 induced LC3B activation. The ectopic expression of Drfundc1 caused cell death and apoptosis as well as impairing cell proliferation in the 293 T cell line, as detected by Trypan blue, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and incorporation of BrdU. DrFundc1 up-regulated expression of both autophagy- and apoptosis-related genes, including ATG5, ATG7, LC3B, BECLIN1, and BAX in transgenic 293 T cells. A knockdown of Drfundc1 using short hairpin RNA (shRNA) led to midline bifurcation with two notochords and two spinal cords in zebrafish embryos. Co-injection of Drfundc1 mRNA repaired defects resulting from shRNA. Knockdown of Drfundc1 resulted in up- or down-regulation of genes related to autophagy and apoptosis, as well as decreased expression of neural genes such as cyclinD1, pax2a, opl, and neuroD1. In summary, DrFundc1 is a mitochondrial protein which is involved in mitophagy and is critical for typical body axis development in zebrafish.
Subject terms: Zebrafish, Body patterning, RNAi
Introduction
Mitochondria are essential for cellular energy metabolism and for controlling a series of key metabolic and biosynthetic pathways1–3. Fission and fusion of mitochondria regulate metabolism and embryonic development as well as autophagy and apoptosis of cells4–6. Mitochondrial dysfunction is the reason for many age-related diseases such as cancer7, deafness8, diabetes9, and neurodegenerative diseases (including Alzheimer’s and Parkinson’s diseases)10. Therefore, removal of dysfunctional or superfluous mitochondria by autophagy, known as mitophagy11,12, is crucial for survival.
During mitophagy, dysfunctional or superfluous mitochondria are incorporated into double-membrane structures known as autophagosomes, which are in turn delivered to lysosomes for degradation and reuse13,14. Interference with the expression of genes critical to mitophagy leads to severe defects. To give several examples, inactivation of 3-hydroxybutyrate dehydrogenase type 2 (bdh2) leads to mitochondrial dysfunction and mitophagy, which delays erythroid maturation in zebrafish15, cytosolic p53-mediated inhibition of mitophagy leads to heart failure in mice16, while in Drosophila, knockdown of optic atrophy 1 (opa1) results in smaller mitochondria, developmental defects, and muscle degeneration17.
FUN14 domain-containing protein 1 (FUNDC1), a mitochondrial outer membrane protein in mammalian cells, plays a key role in mitophagy by binding to light chain 3 (LC3), an autophagy marker, via its LC3-interacting region (LIR) motif18. FUNDC1 overexpression induces mitophagy in several cell lines, including HeLa (cervical cancer cells), MCF-7 (breast cancer cells), and MEF (mouse embryonic fibroblasts)19. During mitochondrial fission, FUNDC1 is also an adaptor for dynamin-related protein 1 (DRP1). FUNDC1 overexpression induces mitochondrial fission, while knockdown of FUNDC1 results in mitochondrial fusion18. Under hypoxia, FUNDC1 accumulates at endoplasmic reticulum (ER)-mitochondria contact sites through interactions with Calnexin20,21. Up-regulation of FUNDC1 mediates proliferation and invasion of human ovarian cancer cells22. FUNDC1 also protects against cardiac ischemia-reperfusion injury (IRI) through mitophagy and interacts with receptor-interacting serine-threonine kinase 3 (Ripk3)23,24.
Authors have previously found that knockdown of fundc1 causes severe defects in the body axis of a rare minnow (Gobiocypris rarus)25. However, the role of FUNDC1 in embryogenesis remains unclear. Therefore, zebrafish (Danio rerio) were used in this study due to their genomic sequences and well-documented embryonic development. We found that in zebrafish, Fundc1 (DrFundc1) is crucial for body axis formation.
Results
Location and function of DrFundc1 in vitro
Use of a multiple-sequence alignment of selected proteins as well as a molecular phylogenetic tree show that FUNDC1 is highly conserved in vertebrates (Fig. S1A,B). The open reading frame (ORF) of zebrafish fundc1 (Drfundc1) is 459 base pairs (bp) in length. This encodes DrFundc1, a protein of 152 amino acids (AAs). DrFundc1 contains a putative LIR motif (YEVV) and a FUN domain (AA 51–132; Fig. S1A). DrFundc1 is a putative transmembrane protein with three α-helical stretches and the LIR motif in the N-terminal out of the mitochondrial outer membrane (Fig. S1C).
DrFundc1’s location and function in vitro were studied in two available cell lines: the grass carp (a relative of zebrafish and rare minnow in Cyprinidae family) ovary (GCO) cell line and the human embryonic kidney (HEK) 293 T cell line. This was due to a lack of both a proper zebrafish cell line for gene transfer and antibodies for detection of zebrafish proteins. We ask whether DrFundc1 is located in mitochondria and if it can work as its homolog FUNDC1 in mammalian cells to induce mitophagy.
Through the use of bioimaging, we found that the red fluorescence of DrFundc1-Cherry overlapped with the green fluorescence from MitoTracker Green (Thermo Fisher Scientific, Carlsbad, CA, USA; M7514), a reagent labeling mitochondrion, in transgenic GCO cells (Fig. S2A). Use of Western blotting showed a clear band (~44 kD) of DrFundc1-Cherry-His in the mitochondrial extract of transgenic GCO cells (Fig. S2B). It was also observed that DrFundc1-Cherry co-located with CellLight Lysosomes-GFP (Thermo Fisher Scientific; C10596), a reagent labeling lysosome, in transgenic GCO cells (Fig. S2C).
GCO cells grew poorly following Drfundc1 transfection. The more Drfundc1 was transfected, the poorer the cell growth (Fig. S2D). Cell numbers decreased significantly in the dosage of 400–500 ng of pCS2 + -Drfundc1-Cherry-His compared to control cells transfected with pCS2 + -Cherry plasmid. Cells transfected with Drfundc1 displayed low density, while some cells were round in shape, floating, and aggregating into clusters.
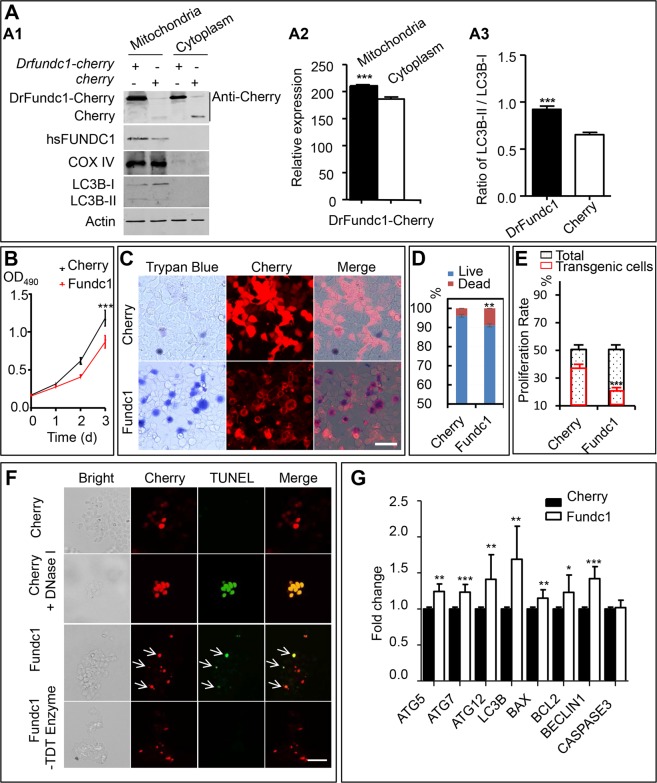
Use of Western blotting detected DrFundc1-Cherry fusion protein, mainly in the mitochondrial extract of transgenic 293 T cells which had been transfected with pCS2 + -Drfundc1-Cherry-His plasmid (Fig. 1A). DrFundc1-Cherry levels were significantly higher in mitochondria than in the cytoplasm of transgenic cells (P < 0.001), while endogenous cytochrome C oxidase subunit 4 (COX IV), LC3B, and endogenous FUNDC1 were primarily detected in mitochondria. Due to expression of DrFundc1 in the cells, more LC3B-I was converted to LC3B-II than in control cells (P < 0.001) (Fig. 1A). This suggests that DrFundc1 is a component of mitochondria and can lead to LC3 activation, which in turn causes mitophagy.
Figure 1.
DrFundc1 reduced cell viability while inducing autophagy and apoptosis in transgenic 293 T cells. (A) Western blotting of proteins extracted from mitochondria and cytoplasm from transgenic 293 T cells transfected with pCS2 + -Drfundc1-Cherry-His as well as pCS2 + -Cherry plasmids. Antibodies used were anti-Cherry (detecting Drfundc1-Cherry-His and Cherry), anti-FUNDC1, anti-COX IV, anti-LC3B, and anti-β-ACTIN. (A1) Results of Western blotting. (A2) Gray-scale analyses of DrFundc1 using ImageJ in mitochondria and cytoplasm of cells transfected with pCS2 + -Drfundc1-Cherry-His. (A3) LC3B-II:LC3B-I ratio in mitochondria of transgenic cells transfected with pCS2 + -Drfundc1-Cherry-His and pCS2 + -Cherry. (B) DrFundc1 decreased viability of 293 T cells, measured by MTT assay. (C-D) DrFundc1 increased 293 T cell mortality, measured by Trypan Blue staining. (E) DrFundc1 decreased cell proliferation in transgenic cells, detected using BrdU incorporation. (F) DrFundc1 led to apoptosis of 293 T cells, detected using TUNEL assay. Arrows show apoptotic cells. Red indicates DrFundc1-Cherry or Cherry, while green indicates TUNEL-positive cells. Positive control: Cherry + DNase I. Negative control: incubation without TdT enzyme. (G) Expressional fold change of autophagy- and apoptosis-related genes, detected using qRT-PCR. β-ACTIN was used as an internal control. Significant differences between cells transfected with different plasmids are shown as asterisks. *P < 0.05; **P < 0.01; ***P < 0.001.
Deliberate expression of DrFundc1 was harmful to 293 T cells, which decreased in density, changed in morphology, aggregated, and even died following Drfundc1 transfection (Fig. 1B,C, S3A,B). Use of a 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT) assay found a significant decrease in proliferation of transgenic 293 T cells following Drfundc1 transfection (P < 0.001) (Fig. 1B). Use of trypan blue staining and cell counting indicated a significant increase in mortality (P < 0.01) in cells by Drfundc1 expression (Fig. 1C,D). We used a 5-bromo-2′-deoxyuridine (BrdU) incorporation and found a decrease of cell proliferation in the transgenic cells with Drfundc1 expression (P < 0.001), although no apparent changes to cell proliferation in total cells were observed (Fig. 1E, S3C). This suggests that DrFundc1 caused cell death and impaired cell proliferation.
Transfection of Drfundc1 led to apoptosis of transgenic 293 T cells, as revealed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. TUNEL-positive cells were observed in cells transfected with Drfundc1-Cherry (Fig. 1F), while use of a quantitative reverse transcription polymerase chain reaction (qRT-PCR) demonstrated expressional change of autophagy- and apoptosis-related genes in cells. Autophagy-related genes ATG5, ATG7, ATG12, and LC3B, as well as apoptosis-related genes BCL2-associated X (BAX), B-cell CLL/lymphoma 2 (BCL2), and BECLIN1 were significantly up-regulated by use of DrFundc1 (Fig. 1G). Therefore, decreased cell viability was due to DrFundc1-induced autophagy and apoptosis. However, CASPASE3 expression did not change, suggesting that apoptosis may not depend on CASPASE3.
Expression of Drfudnc1 in adult tissues and embryos of zebrafish
Use of a qRT-PCR detected Drfundc1 in selected tissues (brain, eye, heart, intestine, liver, muscle, kidney, testis, and ovary; see Fig. S4A). Expression of Drfundc1 was highest in the brain, followed by a moderate expression in the liver, ovary, testis, and kidney, while the lowest expression was in the heart and muscle. Use of a qRT-PCR also detected Drfundc1 in zygotes throughout zebrafish embryogenesis (Fig. S4B). Expression of Drfundc1 increased from the 1-cell stage, peaked at the gastrula stage (6 h post fertilization [hpf]), decreased at 12 hpf, and was then maintained at a low level from 24 hpf until hatching. We further studied the expression pattern of Drfundc1 using a whole-mount in situ hybridization (WISH; see Fig. S4C). Use of WISH detected Drfundc1 in embryos from zygote until hatching. Drfundc1 was found in all blastomeres at early stages, from the 1-cell stage to the gastrula stage. Expression of Drfundc1 was enriched in embryos’ heads – including brains and eyes – from 24 hpf onwards.
Knockdown of Drfundc1 caused serious defects in the body axis
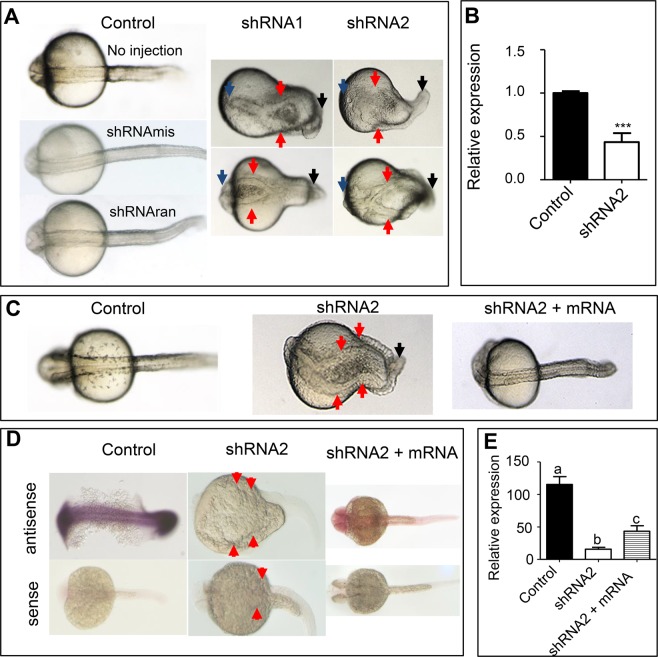
Knockdown of Drfundc1 was induced by microinjecting specific Drfundc1 short hairpin RNAs (shRNAs: shRNA1 and shRNA2) into zygotes. Both Drfundc1 shRNA1 and Drfundc1 shRNA2 caused similar defects in the body axis; that is, bifurcation in the midline, short body length, curved tail, and/or head dysplasia (Table 1 and Fig. 2A). Abnormalities were significantly high in embryos which had been microinjected with Drfundc1 shRNAs (10.15–17.67%) compared to embryos with/without microinjection of the pSuper-puro plasmid and control shRNAs (mismatched shRNA [shRNAmis] or random shRNA [shRNAran]) (1.75–3.77%) (P < 0.05). Drfundc1 shRNA2 caused higher abnormality rates in embryos than shRNA1. We therefore used Drfundc1 shRNA2 for subsequent experiments.
Table 1.
Rates of abnormal embryos.
| Experiment | Group | Total embryos | Abnormal embryos | Abnormal rate % (mean ± SE) |
|---|---|---|---|---|
| 1 | No injection | 3146 | 55 | 1.75 ± 0.08 a |
| pSuperpuro | 2742 | 103 | 3.75 ± 0.11 a | |
| shRNAmis | 1326 | 50 | 3.77 ± 0.35 a | |
| shRNAran | 1146 | 20 | 1.75 ± 0.02 a | |
| shRNA1 | 3566 | 362 | 10.15 ± 0.30 b | |
| shRNA2 | 7153 | 1264 | 17.67 ± 1.52 c | |
| 2 | Control | 2888 | 55 | 1.90 ± 0.09 a |
| shRNA2 | 3098 | 505 | 16.30 ± 0.58 b | |
| shRNA2 + mRNA | 2205 | 104 | 4.72 ± 0.31 c |
Note: Significant differences between groups are shown by different letters following the data (P < 0.05). The shRNAs – shRNA1, shRNA2 – target nucleotide 77–99 and 217–237 of Drfundc1 ORF respectively. shRNAmis, mismatched shRNA; shRNAran, random shRNA without any target gene.
Figure 2.
Knockdown of Drfundc1 caused abnormal body axis development in zebrafish embryos (A,B), which was repaired by Drfundc1 mRNA (C–E). (A) Midline bifurcation and headless embryos caused by microinjection of Drfudnc1 shRNA1 or shRNA2 into zygotes at 24–28 hpf. (B) shRNA2 significantly decreased endogenous Drfundc1 in the embryos (***P < 0.001), as measured by qRT-PCR with normalization by β-actin. (C) Co-injection of Drfundc1 mRNA repaired defects caused by shRNA2. Before injection, 200 pg/nL of shRNA2 was mixed with 400 pg/nL of mRNA. Control embryos were not injected. (D) WISH results of Drfundc1 in embryos with different treatments. (E) Gray-scale analysis of WISH results of Drfundc1 (D), measured using ImageJ. Letters a, b, and c indicate significance (P < 0.05). All embryos were oriented with a dorsal view. Arrows point to abnormal sites in the embryos.
Compared to control embryos, endogenous Drfundc1 decreased to 43.28% in embryos which had been microinjected with shRNA2 (Fig. 2B). To examine whether Drfundc1 messenger RNA (mRNA) could repair Drfundc1 shRNA-induced abnormalities, Drfundc1 mRNA was co-injected with Drfundc1 shRNA2 into zygotes, which developed typically (Fig. 2C). Drfundc1 expression was highest in control embryos, followed by a moderate expression in embryos co-injected with Drfundc1 mRNA and shRNA2, while only traces were detected in those microinjected with Drfundc1 shRNA2 (Fig. 2D,E). Co-injection of Drfundc1 mRNA and shRNA2 decreased abnormality from 16.30% (by Drfundc1 shRNA2 microinjection) to 4.72% (P < 0.05) (Table 1). This suggests that Drfundc1 mRNA repairs defects caused by Drfundc1 shRNA2.
Knockdown of Drfundc1 formed two notochords and two spinal cords as well as affecting expression of autophagy-, apoptosis-, and neural development-related genes
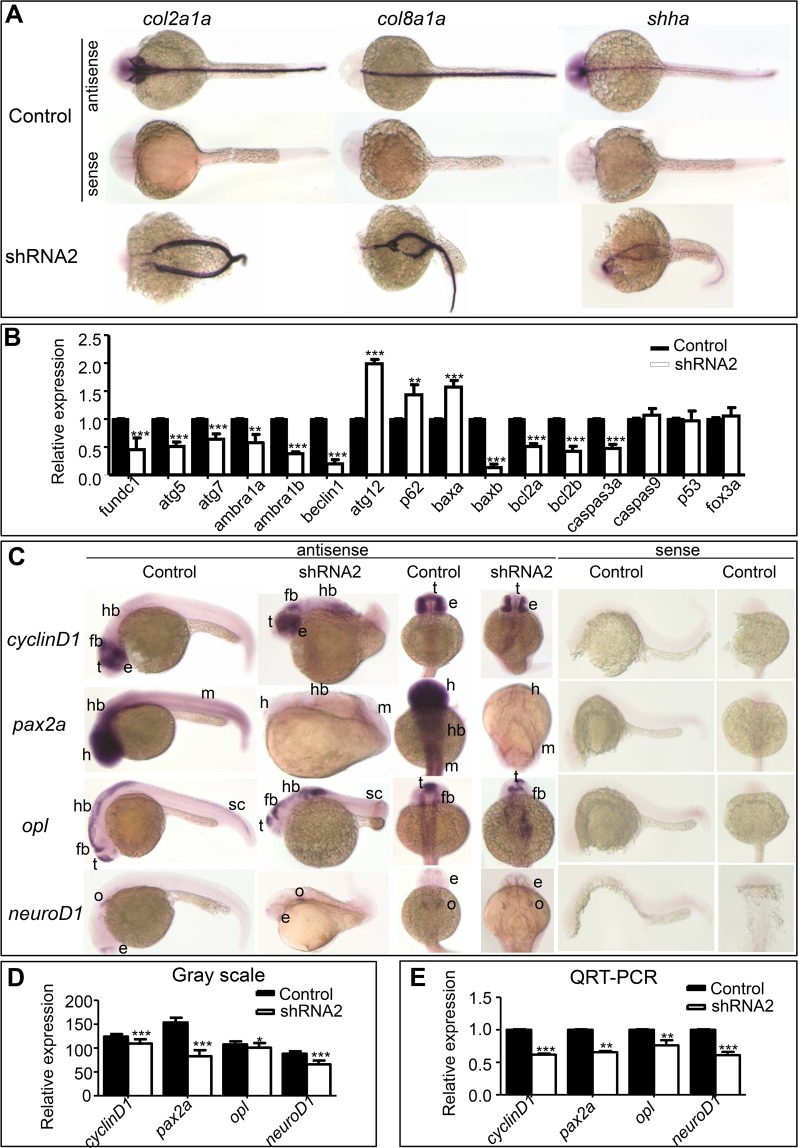
To understand midline bifurcation, we subjectively studied collagen 2a1a (col2a1a) and col8a1a as notochord markers and sonic hedgehog protein a (shha) as a spinal cord marker26–29. Use of WISH clearly demonstrated that interference with Drfundc1 induced formation of two notochords as well as two spinal cords in the embryo, while the control embryo was typical, with one notochord and one spinal cord (Fig. 3A), suggesting that midline bifurcation is a malformation of the body axis.
Figure 3.
Expression of genes related to body axis formation, autophagy, and apoptosis. (A) WISH of col2a1a, col8a1a, and shha in embryos at 24–28 hpf. (B) Expression of autophagy- and apoptosis-related genes in embryos at 24–28 hpf, detected using qRT-PCR. β-actin was used as an internal control. (C) WISH of cyclinD1, pax2a, opl, and neuroD1, which are involved in neural system development. (D) Gray-scale analysis of WISH results of genes in (C), whose signal strength was measured using ImageJ. (E) Expression of cyclinD1, pax2a, opl, and neuroD1 in embryos, detected using qRT-PCR, was normalized using β-actin.
We hypothesized that insufficient mitophagy may be the reason for midline bifurcation due to Drfundc1 knockdown. Authors of several studies have claimed that autophagy tends to be accompanied by a certain degree of apoptosis30,31. We therefore measured expressions of the following autophagy- and apoptosis-related genes: atg5, atg7, atg12, autophagy and beclin 1 regulator 1a (ambra1a), ambra1b, nucleoporin 62 (p62), beclin1, bcl2a, bcl2b, baxa, baxb, caspase3a, caspase9, p53, and forkhead transcription factor 3a (fox3a)32,33.
Use of a qRT-PCR found that atg5, atg7, ambra1a, and ambra1b were down-regulated, while p62 and atg12 were significantly up-regulated by interference of Drfundc1 (P < 0.01 or 0.001; Fig. 3B). In addition, beclin1, bcl2a, bcl2b, and caspase3a were significantly down-regulated (P < 0.001) by interference of fundc1. Interference of fundc1 down-regulated baxb but up-regulated baxa (P < 0.001) and did not affect expression of caspase9, p53, and fox3a. This suggests that knockdown of Drfundc1 interfered with autophagy and apoptosis, leading to body axis malformation in zebrafish embryos, and that this process depends on Caspase3 rather than Caspase9.
Neural system-related genes cyclinD1, paired box 2a (pax2a), odd-paired-like (opl), and neuroD1 were detected using WISH and qRT-PCR. CyclinD1 is associated with cell proliferation in the spinal cord34, pax2a is a mesencephalic marker participating in midbrain-hindbrain boundary development35,36, opl is a marker for forebrain cell fate which controls midline formation and forebrain patterning37,38, while neuroD1 is critical for neuronal cell fate39. Use of WISH indicated that Drfundc1 knockdown attenuated expression of cyclinD1 in the telencephalon, opl in the spinal cord, pax2a in the head and muscle, and neuroD1 in the eye (Fig. 3C). We used gray-scale analyses to find that gene expression significantly decreased in embryos which had been microinjected with Drfundc1 shRNA2 (Fig. 3D). Subsequently, qRT-PCR confirmed cyclinD1, pax2a, opl, and neuroD1 down-regulation due to Drfundc1 knockdown (Fig. 3E).
Discussion
We identified DrFundc1 as a mitochondrial protein associated with mitophagy. Additionally, DrFundc1 is critical for proper body axis formation in zebrafish. Knockdown of Drfundc1 causes down- or up-regulation of autophagy-, apoptosis-, as well as neural development-related genes.
DrFundc1 was co-localized with MitoTracker, LC3B, COX IV, and FUNDC1 in transgenic GCO and 293 T cells. Deliberate expression of DrFundc1 caused conversion of LC3B-I to LC3B-II, which induced autophagy40. Co-localization of DrFundc1 with CellLight Lysosomes-GFP indicated occurrence of mitophagy. This suggests that DrFundc1 is a mitochondrial protein which is responsible for mitophagy.
In this study, ectopic DrFundc1 induced cell death through both autophagy and apoptosis. Use of a TUNEL assay demonstrated that apoptosis occurred in cells transfected with Drfundc1. Expression of autophagy- and apoptosis-related genes, including ATG5, ATG7, ATG12, LC3B, BECLIN1, BAX, and BCL2 increased due to Drfundc1 expression. As mammalian FUNDC1, DrFundc1 may regulate mitophagy via interaction with LC318–20,40–43. Conversion of LC3-I to LC3-II by Fundc1 activates the autophagy pathway. Following this, ATG7 increases and activates ATG12, which binds to ATG5 and BECLIN1, forming an autophagy complex42,43. Finally, autophagy occurs, which in turn induces apoptosis via BAX, a pro-apoptotic factor, and BCL2, a survival factor44,45. Cell death can result from apoptosis and/or autophagy.
Mitophagy is pivotal for the survival of organisms while dysfunction of mitochondria causes severe deficiencies in zebrafish15, mice16, and fruit flies17. Zebrafish Fundc1 is involved in mitophagy, as described above. In this study, Drfundc1 knockdown caused severe defects in the body axis, such as midline bifurcation and headlessness, which had been previously observed in rare minnows25. Co-injection of Drfundc1 mRNA repaired these defects, although Drfundc1’s expression level did not go back to typical levels. This suggests that Fundc1 is necessary and critical for typical embryogenesis in fish.
The genes atg5, atg7, ambra1a, ambra1b, beclin1, p62, and atg12 code major proteins for autophagy30,31,40–46. In mammals, down-regulation of ATG5 family genes as well as up-regulation of P62 lead to insufficient autophagy42,43,46. ATG12 is crucial for autophagosome elongation, but requires activation by ATG7. ATG12 conjugates with ATG5, forming a complex42,43. In this study, p62 was increased, while atg5, atg7, ambra1a, ambra1b, and beclin1 expression was decreased in zebrafish embryos due to interference with Drfundc1. This indicates that knockdown of Drfundc1 resulted in insufficient autophagy in zebrafish embryos, although atg12 expression was increased.
Apoptosis is associated with mitophagy30,31. Knockdown of Drfundc1 decreased expression of the apoptosis-related genes baxb, bcl2a, bcl2b, and caspase3a32,33,45,47,48, contrasting to results of 293 T cells due to ectopic expression of Drfundc1. This means that Drfundc1 knockdown interferes with apoptosis in zebrafish embryos. Apoptosis is a major part of typical development in many organisms, including zebrafish49. Interference with the process of apoptosis is likely to result in defects.
Midline bifurcation had been reported in cases of forced expression of insulin-like growth factor 2a (IGF-2a)27 as well as loss of Squint28 in zebrafish. Overexpression of IGF-2a induced Akt phosphorylation and caused midline bifurcation27. Loss of Squint, a nodal-related protein, resulted in midline bifurcation due to the failing expression of wnt5b28. Nodal regulatory factors contribute to mitochondrial homeostasis50. Nodal factors can down-regulate phospho-Akt while activating Smad2 and mitophagy to induce granulosa cell apoptosis51. Activation of Wnt signaling and Akt maintains the mitochondrial membrane and regulates apoptosis repressor Bcl-xL52. Overexpression of IGF-2a and loss of Squint may impair mitophagy and apoptosis in zebrafish embryos. However, IGF-1 induces mitophagy through BNIP3 accumulation in mitochondria, stimulates mitochondrial biogenesis53, and protects mitochondria from apoptosis54. IGF-IIR, the receptor of IGFII, induces mitophagy through Rab9-dependent alternative autophagy55. In contrast, it has been reported that IGF-IIRα disrupts mitochondrial membrane potential, induces perturbation of mitophagy, and can lead to mitochondrial oxidative stress56. Further study is needed to clarify these controversial results. Drfundc1 knockdown impaired mitophagy and apoptosis and resulted in a similar phenotype and midline bifurcation in our experiment, suggesting that midline bifurcation results from impairment of mitophagy and apoptosis during embryogenesis.
In addition to midline bifurcation, Drfundc1 knockdown led to other severe defects in the anterior section of zebrafish embryos. Knockdown of Drfundc1 decreased expression of cyclinD1, pax2a, opl, and neuroD1, which are involved in development of the neural system34–39. However, the ways in which these genes are affected remains unclear.
Overall, we found that Fundc1 is critical for proper body axis formation in fish. Fundc1 knockdown induces insufficient mitophagy or autophagy, interferes with apoptosis, and leads to body axis malformation. Interference with Fundc1 expression impacts on genes which are important for typical embryogenesis.
Materials and Methods
Ethics
This study was conducted in strict accordance with recommendations for the Regulation for the Management of Laboratory Animals from the Ministry of Science and Technology in China. The animal protocol was approved by the Animal Care and Use Committee of Hubei Province in China (No. SYXK(E)2015-0012).
Experimental animals
Wild-type zebrafish were kept at 28 °C in circulating water on a 14 h:10 h light:dark cycle and fed twice daily. Fertilized eggs were incubated at 28.5 °C in an embryo medium and staged according to hours post fertilization (hpf) and days post fertilization (dpf), following standard criteria57.
Cell culture and transfection
An ovary cell line (GCO) of grass carp (Ctenopharyngodon idellus) was obtained from the National Key Laboratory of Fresh Water Ecology and Biotechnology at the Institute of Hydrobiology, Chinese Academy of Sciences. GCO cells were cultured in M199 medium (Thermo Fisher Scientific, Carlsbad, CA, USA; C11150500BT) and supplemented with 10% inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific)58. HEK 293 T cells were cultured in high-glucose Dulbecco’s modified Eagle medium (Thermo Fisher Scientific; C11995500BT) and supplemented with 10% FBS59. GCO and 239 T cells were incubated at 28 °C and 37 °C respectively in a humidified atmosphere of 95% air and 5% CO2.
A fusion gene of Drfundc1 with Cherry and His tags (Drfundc1-Cherry-His) was subcloned into pCS2 + vector as pCS2 + -Drfundc1-Cherry-His. pCS2 + -Cherry was constructed as a control. Plasmids were transfected into GCO or 239 T cells using PolyJet (SignaGen Laboratories, Rockville, MD, USA; SL100688) at a cell density of 70–80% in plates, or at a cell density of 50% on a coverslip, following manufacturer’s instructions. Transfection medium was replaced with a fresh complete medium at 12–18 h post-transfection.
Extraction of mitochondrial protein and Western blotting
We isolated mitochondrial and cytoplasmic proteins from transgenic GCO and 239 T cells transfected with pCS2 + -Drfundc1-Cherry-His and/or pCS2 + -Cherry, strictly following the mitochondria protein extraction kit manual (Jiancheng Bioengineering Institute, Nanjing, China; G006). DrFundc1, Cherry, FUNDC1, COX IV, LC3B, and β-ACTIN were detected by use of Western blotting. Antibodies used were anti-His (Beyotime Biotechnology, Shanghai, China; AH367), anti-Cherry (ABclonal, Wuhan, China; AE002), anti-FUNDC1 (Bioss Inc., Boston, MA, USA; bs-13227R), anti-COX IV (ABclonal; A10098), anti-LC3B (ABclonal; A7198), and anti-β-ACTIN (Bioss Inc.; bsm-33036M).
Cell staining and fixation
In order to label mitochondria, GCO and 239 T cells were incubated with pre-warmed (37 °C) MitoTracker Green FM probes (Thermo Fisher Scientific; M7514) at a concentration of 200 nmol/L for 40 min under growth conditions in the dark. Nuclei were stained with Hoechst 33258 (Thermo Fisher Scientific; H3569). After staining, the solution was replaced with a fresh pre-warmed culture medium. Images were taken using an EVOS FL auto fluorescence microscope (Thermo Fisher Scientific).
In order to label lysosomes, CellLight Lysosomes-GFP reagent (Thermo Fisher Scientific; C10596) was added to cells in a complete culture medium, following the manufacturer’s instructions, and was gently mixed. Cells were incubated with the reagent overnight (≥16 h) in the dark. Images were taken with a confocal microscope (Leica, Germany; SP5) following cell fixation.
TUNEL assay
A one-step TUNEL apoptosis assay kit (Beyotime Biotechnology; C1088) was utilized to detect apoptosis in 293 T cells. The TUNEL reaction was carried out according to the protocol60 and the manufacturer’s instructions. After fixation with freshly prepared 4% paraformaldehyde (PFA) for 30 min, cells were washed once with phosphate-buffered saline (PBS) and then incubated at room temperature with PBS containing 0.3% Triton X-100 for 5 min. Next, cells were incubated with 100 μL TUNEL detection solution in a 24-well plate at 37 °C for 60 min in the dark. Following Triton X-100 treatment and PBS immersion, positive controls were treated with DNase I reaction solution (Beyotime Biotechnology; C1082) at room temperature for 10 min, while negative controls were incubated without the terminal transferase (TdT) enzyme reaction solution during the labeling reaction. Coverslips were washed three times with PBS and mounted onto slides using antifade mounting medium (Beyotime Biotechnology; P0126). Images were taken with an EVOS FL auto fluorescence microscope (Thermo Fisher Scientific).
Cell death and cell proliferation assay
An MTT proliferation assay kit (Beyotime Biotechnology; C0009) was used to measure cell viability. Briefly, 293 T cells were seeded into 96-well plates and cultured. Cell viability was measured according to the manual of the kit post-transfection.
Trypan Blue (Thermo Fisher Scientific; T10282) was used to detect dead cells; 10 μl 0.4% Trypan Blue solution was added into 100 μl cell suspensions. Blue staining cells and total cells were counted using a hemocytometer. Cells were also stained with Trypan Blue in plates after fixation with 4% PFA followed by a wash with PBS.
Cell proliferation was also detected with BrdU incorporation61. The 293 T cells were seeded onto cover slips and transfected with plasmids, as described above. After transfection, cells were incubated with 10 μmol/L 5-bromo-2′-deoxyuridine (BrdU) (Thermo Fisher Scientific; B23151) in culture medium for 9 h in dark and then fixed with 4% PFA. Cells were washed with a Tris-buffered saline Tween-20 (TBST) containing 0.1% Triton X-100 (TBSTx) after fixing and were incubated in 1.5 mol/L HCl for 30 min to expose antigens. After washing, 5% BSA in TBSTx was applied onto cells for blocking. Cells were then incubated with BrdU monoclonal antibody (ABclonal; A1482) for 12 h at 4 °C. After washing three times with TBSTx, Alexa Flour 488-conjugated AffiniPure goat anti-mouse IgG (H + L) (ABclonal; AS076) was applied for 1 h in the dark. Cells were stained with Hoechst 33258 after washing and mounted onto slides with antifade mounting medium (Beyotime Biotechnology; P0126). Images were taken with an EVOS FL auto fluorescence microscope (Thermo Fisher Scientific).
Quantitative real-time PCR (qRT-PCR)
Total RNA of different cells, tissues and/or embryos at different stages was extracted with TRIzol reagent (Thermo Fisher Scientific; 15596026) and then reverse-transcribed into complementary DNA (cDNA) with a FastQuant RT Kit (with gDNase; TIANGEN Biotech, Beijing, China; KR106) after digestion of genomic DNA with RNase-free DNase I, following the manufacturer’s instructions.
QRT-PCR was carried out in a reaction volume of 20 μL containing template cDNA, primers, RNase-free H2O, and 10 μL of 2х SuperReal premix plus (SYBR Green) (TIANGEN Biotech; FP205) using the following cycle settings: 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s and 62 °C for 30 s. Samples were analyzed in triplicate, and gene expression values calculated on technical triplicates and biological replicates. Expression levels of target genes were measured and normalized with that of β-actin according to the 2-DCt or 2-DDCt calculation method62.
Table S1 shows primers used.
In situ hybridization
Whole mount in situ hybridization (WISH) was carried out on zebrafish embryos following the protocol reported previously63. Sense and antisense digoxigenin (DIG)-labeled RNA riboprobes were produced, as reported previously25. Embryos older than 20 hpf were digested with proteinase K and then hybridized with appropriate riboprobes at 70 °C for 12–16 h. After thoroughly washing and blocking, embryos were incubated with anti-DIG antibodies which conjugated with alkaline phosphatase. Embryos were then stained using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP). Images were taken under an MZ16F stereomicroscope (Leica) which was equipped with a digital camera. Grey scales of the genes in WISH were quantified depending on embryos’ grey strengths, using the software ImageJ (https://imagej.nih.gov/ij/). Optical density of the areas stained (S) and neighboring background (B) were measured. Signal strengths were calculated as S-B64. The mean strength of each gene was obtained from 10 to 15 embryos in each group.
Knockdown of Drfundc1
In this study, shRNAs which interfered with Drfundc1 were designed online (http://rnaidesigner.thermofisher.com/rnaiexpress/design.do). Two shRNAs – shRNA1 and shRNA2 – targeted nucleotides of Drfundc1 ORF from 77 to 99 and 217 to 237 respectively. A mismatched shRNA (shRNAmis) as well as a random shRNA (shRNAran) without any target were designed as controls. Vector pSuper-puro (OligoEngine) was used to construct shRNA expression plasmids. shRNAs or pSuper-puro were microinjected into zygotes at a dosage of 200 pg. Drfundc1 mRNA was synthesized from pCS2 + -Drfundc1 in vitro using an mMESSAGE mMACHINE SP6 transcription kit (Thermo Fisher Scientific; AM1340). To repair defects caused by Drfundc1 shRNA, 400 pg of Drfundc1 mRNA was microinjected with 200 pg of the shRNA plasmid into zygotes. Embryos with/without injection of pSuper-puro, shRNAmis, and shRNAran were treated as controls.
Statistical analyses
Data analyses were carried out using SPSS 17.0 (IBM, Armonk, NY, USA). Differences between groups were analyzed using a one-way analysis of variance (ANOVA). A post-hoc Duncan’s multiple range test was utilized to determine significant differences. All data were obtained from at least three independent experiments (n ≥ 3) and were described as mean ± standard error (SEM). P < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 31672284, 31272645 to HZ.)
Author contributions
G.X., H.S., E.N., K.W., X.K., M.A.A.H. and X.L. performed the experiments. X.Z., Q.Z., C.Q. and H.Z. designed the experiment. G.X. and H.Z. wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55415-0.
References
- 1.Honscher C, et al. Cellular metabolism regulates contact sites between vacuoles and mitochondria. Dev. Cell. 2014;30:86–94. doi: 10.1016/j.devcel.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur. J. Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 4.Qu X, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan MX. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion. 2011;11:237–245. doi: 10.1016/j.mito.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006;212:167–78. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 11.Bhujabal Z, et al. FKBP8 recruits LC3A to mediate Parkin-independent mitophagy. EMBO Rep. 2017;18:947–961. doi: 10.15252/embr.201643147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, et al. Mitochondrial E3 ligase MARCH5 regulates FUNDC1 to fine-tune hypoxic mitophagy. EMBO Rep. 2017;18:495–509. doi: 10.15252/embr.201643309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng D, Liu L, Zhu Y, Chen Q. Molecular signaling toward mitophagy and its physiological significance. Exp. Cell. Res. 2013;319:1697–1705. doi: 10.1016/j.yexcr.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat. Rev. Mol. Cell. Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davuluri G, et al. Inactivation of 3-hydroxybutyrate dehydrogenase 2 delays zebrafish erythroid maturation by conferring premature mitophagy. Proc. Natl. Acad. Sci. USA. 2016;113:E1460–1469. doi: 10.1073/pnas.1600077113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino A, et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 2013;4:2308. doi: 10.1038/ncomms3308. [DOI] [PubMed] [Google Scholar]
- 17.Rai M, Katti P, Nongthomba U. Drosophila Erect wing (Ewg) controls mitochondrial fusion during muscle growth and maintenance by regulation of the Opa1-like gene. J. Cell Sci. 2014;127:191–203. doi: 10.1242/jcs.135525. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 19.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12:689–702. doi: 10.1080/15548627.2016.1151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 2016;17:69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Y, Tang J. Effects of silencing FUNDC1 gene on proliferation and invasion of ovarian cancer cell SKOV3. J. Shanghai Jiaotong. Med. Sci. 2016;36:218–223. [Google Scholar]
- 23.Zhang W, et al. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife. 2016;5:e21407. doi: 10.7554/eLife.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, et al. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu G, et al. Expression pattern and functional analysis of fundc1 in rare minnow (Gobiocypris rarus) Gene. 2017;626:149–157. doi: 10.1016/j.gene.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Tellez A, Zampedri C, Ramos-Balderas JL, Garcia-Hernandez F, Maldonado E. Zebrafish scarb2a insertional mutant reveals a novel function for the Scarb2/Limp2 receptor in notochord development. Dev. Dyn. 2016;245:508–519. doi: 10.1002/dvdy.24383. [DOI] [PubMed] [Google Scholar]
- 27.Zou S, Kamei H, Modi Z, Duan C, Zebrafish IGF. genes: gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS One. 2009;4:e7026. doi: 10.1371/journal.pone.0007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei W, Feldman B. Identification of common and unique modifiers of zebrafish midline bifurcation and cyclopia. Dev Biol. 2009;326:201–211. doi: 10.1016/j.ydbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gansner JM, Gitlin JD. Essential role for the alpha 1 chain of type VIII collagen in zebrafish notochord formation. Dev. Dyn. 2008;237:3715–3726. doi: 10.1002/dvdy.21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J., Chen, Z., Guo, J., Wang, L. & Liu, X. Ambra1 induces autophagy and desensitizes human prostate cancer cells to cisplatin. Biosci. Rep. 3, 10.1042/BSR20170770 (2017). [DOI] [PMC free article] [PubMed]
- 31.Liu L, et al. Effect of inhibiting Beclin-1 expression on autophagy, proliferation and apoptosis in colorectal cancer. Oncol. Lett. 2017;14:4319–4324. doi: 10.3892/ol.2017.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miccoli A, et al. Beneficial bacteria affect Danio rerio development by the modulation of maternal factors involved in autophagic, apoptotic and dorsalizing processes. Cell Physiol. Biochem. 2015;35:1706–1718. doi: 10.1159/000373983. [DOI] [PubMed] [Google Scholar]
- 33.Miccoli A, Dalla Valle L, Carnevali O. The maternal control in the embryonic development of zebrafish. Gen. Comp. Endocrinol. 2017;245:55–68. doi: 10.1016/j.ygcen.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 35.Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–3062. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- 36.Krauss S, et al. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. EMBO J. 1991;10:3609–3619. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurus D, Harris WA. Zic-associated holoprosencephaly: zebrafish Zic1 controls midline formation and forebrain patterning by regulating Nodal, Hedgehog, and retinoic acid signaling. Genes Dev. 2009;23:1461–1473. doi: 10.1101/gad.517009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grinblat Y, Gamse J, Patel M, Sive H. Determination of the zebrafish forebrain: induction and patterning. Development. 1998;125:4403–4416. doi: 10.1242/dev.125.22.4403. [DOI] [PubMed] [Google Scholar]
- 39.Ochocinska MJ, Hitchcock PF. Dynamic expression of the basic helix-loop-helix transcription factor neuroD in the rod and cone photoreceptor lineages in the retina of the embryonic and larval zebrafish. J. Comp. Neurol. 2007;501:1–12. doi: 10.1002/cne.21150. [DOI] [PubMed] [Google Scholar]
- 40.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv M, et al. Structural insights into the recognition of phosphorylated FUNDC1 by LC3B in mitophagy. Protein Cell. 2017;8:25–38. doi: 10.1007/s13238-016-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushita M, et al. Structure of Atg5.Atg16, a complex essential for autophagy. J. Biol. Chem. 2007;282:6763–6772. doi: 10.1074/jbc.M609876200. [DOI] [PubMed] [Google Scholar]
- 43.Mai S, Muster B, Bereiter-Hahn J, Jendrach M. Autophagy proteins LC3B, ATG5 and ATG12 participate in quality control after mitochondrial damage and influence lifespan. Autophagy. 2012;8:47–62. doi: 10.4161/auto.8.1.18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fimia GM, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 45.Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Huang C, et al. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975–e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng EH, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/S1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 48.Kratz E, et al. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13:1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- 49.Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev. Biol. 2001;240:123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- 50.Palikaras K, Daskalaki I, Markaki M, Tavernarakis N. Mitophagy and age-related pathologies: Development of new therapeutics by targeting mitochondrial turnover. Pharmacol. Ther. 2017;178:157–174. doi: 10.1016/j.pharmthera.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Jiang JY, Zhu C, Peng C, Tsang BK. Role and regulation of nodal/activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol. Endocrinol. 2006;20:2469–2482. doi: 10.1210/me.2005-0446. [DOI] [PubMed] [Google Scholar]
- 52.Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol. Histopathol. 2004;19:495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyons A, et al. Insulin-like growth factor 1 signaling is essential for mitochondrial biogenesis and mitophagy in cancer cells. J. Biol. Chem. 2017;292:16983–16998. doi: 10.1074/jbc.M117.792838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen D, et al. The role of insulin-like growth factor 1 in ALS cell and mouse models: A mitochondrial protector. Brain Res. Bull. 2019;144:1–13. doi: 10.1016/j.brainresbull.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Huang CY, et al. Rab9-dependent autophagy is required for the IGF-IIR triggering mitophagy to eliminate damaged mitochondria. J. Cell Physiol. 2018;233:7080–7091. doi: 10.1002/jcp.26346. [DOI] [PubMed] [Google Scholar]
- 56.Pandey Sudhir, Kuo Wei-Wen, Shen Chia-Yao, Yeh Yu-Lan, Ho Tsung-Jung, Chen Ray-Jade, Chang Ruey-Lin, Pai Pei-Ying, Viswanadha Vijaya Padma, Huang Chih-Yang, Huang Chih-Yang. Insulin-like growth factor II receptor-α is a novel stress-inducible contributor to cardiac damage underpinning doxorubicin-induced oxidative stress and perturbed mitochondrial autophagy. American Journal of Physiology-Cell Physiology. 2019;317(2):C235–C243. doi: 10.1152/ajpcell.00079.2019. [DOI] [PubMed] [Google Scholar]
- 57.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 58.Zhang QY, Li ZQ, Gui JF. Isolation of a lethal rhabdovirus from the cultured Chinese sucker Myxocyprinus asiaticus. Dis. Aquat. Organ. 2000;42:1–9. doi: 10.3354/dao042001. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, et al. Graphene oxide regulates cox2 in human embryonic kidney 293T cells via epigenetic mechanisms: dynamic chromosomal interactions. Nanotoxicology. 2018;12:117–137. doi: 10.1080/17435390.2018.1425498. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez JC, Vazquez FJ, Rodriguez LE, Farfan MJ, Vega MM. Determination of apoptosis in actinic prurigo by TUNEL technique. Photodermatol. Photoimmunol. Photomed. 2015;31:115–117. doi: 10.1111/phpp.12153. [DOI] [PubMed] [Google Scholar]
- 61.Da SM, Munoz P, Armelin HA, Elias MC. Differences in the detection of BrdU/EdU incorporation assays alter the calculation for G1, S, and G2 phases of the cell cycle in Trypanosomatids. J. Eukaryot. Miccrobiol. 2017;64:756–770. doi: 10.1111/jeu.12408. [DOI] [PubMed] [Google Scholar]
- 62.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 63.Jowett T. Double in situ hybridization techniques in zebrafish. Methods. 2001;23:345–358. doi: 10.1006/meth.2000.1147. [DOI] [PubMed] [Google Scholar]
- 64.Wang H, Zhou Q, Kesinger JW, Norris C, Valdez C. Heme regulates exocrine peptidase precursor genes in zebrafish. Exp. Biol. Med. (Maywood). 2007;232:1170–1180. doi: 10.3181/0703-RM-77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.