Abstract
Grain filling is an important growth process in formation of yield and quality for barley final yield determination. To explore the grain development behavior during grain filling period in barley, a high-density genetic map with 1962 markers deriving from a doubled haploid (DH) population of 122 lines was used to identify dynamic quantitative trait locus (QTL) for grain filling rate (GFR) and five grain size traits: grain area (GA), grain perimeter (GP), grain length (GL), grain width (GW) and grain diameter (GD). Unconditional QTL mapping is to detect the cumulative effect of genetic factors on a phenotype from development to a certain stage. Conditional QTL mapping is to detect a net effect of genetic factors on the phenotype at adjacent time intervals. Using unconditional, conditional and covariate QTL mapping methods, we successfully detected 34 major consensus QTLs. Moreover, certain candidate genes related to grain size, plant height, yield, and starch synthesis were identified in six QTL clusters, and individual gene was specifically expressed in different grain filling stages. These findings provide useful information for understanding the genetic basis of the grain filling dynamic process and will be useful for molecular marker-assisted selection in barley breeding.
Subject terms: Agricultural genetics, Plant breeding
Introduction
Barley grain filling is an important growth process for yield and quality, which determines the thousand grain weight (TGW) and final yield1. Grain filling is mainly determined by filling rate and duration2. Genotypic variation between these two parameters have been reported in barley3–8. Metzger et al.9 found differences in the grain-filling duration between barley varieties, but no significant correlation between grain-filling duration and yield. Moreover, grain weight was correlated positively with grain filling rate, but not with grain filling duration in wheat and maize10,11.
Previous reports suggested that the grain filling rate was better than grain filling duration to reveal the difference in yield7,12,13. Grain filling duration is vulnerable to temperature, especially under stress conditions14–17. In addition, long-term grain filling affected the normal sowing of stubble crops and the regular maturation of barley, whereas the grain filling rate (GFR) seems to be dominated by the genetic factors6,7,12. Therefore, increasing grain filling rate was more important than increasing grain filling duration for crop yield.
The grain filling process can be divided into three periods: grain formation, linear growth and maturity period18. The grain formation period was an active phase of cell division with little accumulation of dry matter19. After entering the linear growth period, the accumulation of dry matter increases rapidly, and the GFR continues to rise and reaches its maximum value20. At the end of the linear growth period, the grain weight increases slowly, and the GFR decreases significantly, reaching the maturity period21. Although the environment such as temperature and humidity affects GFR during the filling process, genotype is still the main factor affecting GFR7,22.
Physiological mechanisms of grain filling have been extensively studied in barley23–25, but few reported the genetic basis for grain filling characteristics. To date, QTL for yield-related traits such as, grain per spike, grain weight per spike, grain size and thousand grain weight have been reported in barley26–30. The above mentioned traits were conventional QTL based on the final phenotype after maturation. However, many morphological traits are dynamic or progressive development31. According to the theory of developmental genetics, genes are selectively expressed at different growth stages, QTL mapping of these morphological traits should be analyzed to find out the authentic pattern of function of genes at different developmental stages32. Zhu33 proposed a method to map conditional QTL using the net genetic effect between two time points to reveal the gene interactions and regulatory mechanisms of quantitative traits in crop development. Since then, conditional QTL mapping methods have been used to study the agronomic traits, such as root growth and seed vigour in rice34,35, plant height and protein content in wheat36–38, and dry matter accumulation in soybean39.
The final yield and yield components are the result of a combination of physiological and biochemical processes, a genetic analysis of grain weight or size traits might not provide a reasonable explanation for yield determinants40, however, grain filling rate is crucial in determining the grain weight and yield during the physiological and biochemical processes of yield formation. In this study, a DH population containing 122 lines was used to study dynamic QTLs for grain-filling traits in barley. The objectives were to: (1) identify QTL for grain filling rate and grain size traits using conventional and conditional mapping methods, (2) explore the genetic basis of grain filling dynamic process in barley.
Materials and Methods
Plant materials and field experiment
The mapping population comprised 122 doubled haploid (DH) lines was obtained from anther culture by Huaai11 × Huadamai641. Huaai11 is a six-rowed dwarf barley variety selected from the barley landrace Daofu Baiqingke, Huadamai6 is an elite two-rowed feed barley developed by Huazhong Agricultural University. Besides, there were differences in GFR and grain size between two parents. In 2017 (Y1) and 2018 (Y2), the DH population and two parents were planted at the farm of Huazhong Agricultural University (Wuhan, 114°30′E, 30°60′N). The climatic conditions of the barley growing season during the two years are described in Supplementary Fig. S1. The field trial followed a completely randomized block design, with 3 replicates each year. All DH and parents were grown in two rows with a length of 1.5 m and 0.2 m spacing plots and 30 seeds in each row. The management of field experiments was in line with local standard practices.
Traits measurement
The traits evaluated in this study include: thousand grain weight (TGW), grain filling rate (GFR), mean grain filling rate (GFRmean), maximum grain filling rate (GFRmax), grain area (GA), grain perimeter (GP), grain length (GL), grain width (GW) and grain diameter (GD), the measurements were as follows:
At the flowering time, 40 spikes that were basically the same in the growth, size and flowering time were tagged for each line. Five tagged spikes were randomly sampled from each line at 7, 14, 21, 28, 35, 42 and 49 days after anthesis in 2017 and 2018, respectively. For convenience, the seven sampling stages were named as I, II, III, IV, V, VI and VII, respectively. The chaff of grain was peeled off and the GA, GP, GL, GW and GD traits were evaluated using WSeen SC-G automatic seed selection and thousand grain weight analysis system, then the grains were put at 105 °C for 15 minutes and dried at 65 °C until constant weight. The TGW was recorded from the first stage to the seventh stage, and the grain size traits were recorded from the second stage because the first sample was too small to be measured. The GFR (between two sampling stages) was calculated as: GFR = TGW of the difference between two sampling times/7. The grain filling process was adjusted by logistic equation (Y = K/(1 + ae−bt) using the days after flowering (t) as independent variable and grain weight (Y) as dependent variable, K was the maximum theoretical weight, a and b were modulus calculated by the regression equation. These parameters were determined according to the SAS NLIN procedure. The GFRmean and GFRmax parameters were calculated by the first and second order derivatives of the logistic equation, GFRmax = -Kb/4, and GFRmean = GWmax/GFD, GFD is the number of days from flowering to maturity of the plant, GWmax is the maximum grain weight in GFD42.
Phenotypic data statistics
The phenotypic data was analyzed using SAS v.9.2 (SAS Institute Inc, Cary, NC). Correlation analysis between the traits was performed using the “PROC CORR” program. ANOVA of each component was performed using PROC GLM procedure. The broad-sense heritability was calculated using the formula: hB2 = σg2/(σg2 + σge2/n + σe2/rn), where σg2 was the genetic variance, σge2 was the genetic-by environment interaction variance, σe2 was the error variance, and r and n were the number of repetitions for each genotype and environment, respectively.
Dynamic QTL analysis
The genetic map consisting of the 1962 markers cover a total of 1375.8 cM genomic regions was used to screen QTLs43. Unconditional phenotypic values were measured at different stages of I, II, III, IV, V, VI and VII after flowering time. Conditional phenotypic values were the incremental grain filling-related traits values in adjacent stages ΔT2 (II-I), ΔT3 (III-II), ΔT4 (IV-III), ΔT5 (V-IV), ΔT6 (VI-V) and ΔT7 (VII-VI), since the grain size traits were recorded from stage II, so the conditional phenotypic values were calculated from ΔT3, and the data collection method was used according to Zhu33. The conditional phenotypic value ΔT1 of GFR from the flowering time to the stage I was the phenotypic value of stage I, so the effects were considered to be the unconditional genetic effects of stage I. QTL IciMapping 4.1 with inclusive composite interval mapping (ICIM) model was used to analyze the locus and effects of unconditional QTLs44,45. Conditional variable analysis method33 combined with ICIM was used to perform conditional QTL analysis of GA, GP, GL, GW, GD and GFR at each stage. Furthermore, in order to eliminate the interference of row type (Rt) and caryopsis type (Ct), we used QTL.gCIMapping.GUI software46 for covariate QTL analysis using Rt and Ct as covariates. The scan step and probability in stepwise regression (PIN) were set to 1 cM and 0.001, respectively. The logarithm of the odds (LOD) threshold was set to 3.0 after 1000 permutations on a 0.05 Type 1 error, so the QTL was declared based on the LOD threshold of 3.0, and these QTLs were termed ‘identified QTLs’.
QTL integration
Goffinet and Gerber47 first used the QTL meta-analysis method to integrate QTLs from independent experiments and determine the corresponding confidence intervals for consensus QTLs, this method is currently the best way to solve QTL integration. In this study, BioMercator 4.2 was used to integrate QTLs identified in different years or stages and to determine the optimal number of consensus QTLs based on meta-analysis method48. If an identified QTL did not overlap CI with other QTLs, it was also considered a consensus QTL. According to Wang et al.49, the “two-round” QTL integration strategy was adopted here with minor modifications. In the first round, the unconditional and conditional identified QTLs for each trait in two environments were integrated into unconditional and conditional consensus QTLs using BioMercator 4.2. In the second round, these unconditional and conditional consensus QTLs for each trait of the first round of integration were integrated into a unique QTLs. The name of the consensus QTL follows the nomenclature rules reported by McCouch50. The designation was prefixed with ucq, cq, uq and qc followed by abbreviation of a trait, and a linkage group to represent the unconditional, conditional, unique QTL and covariate QTL, respectively. If two or more consensus QTLs were detected in the linkage group, a hyphen ‘-’ with a serial number was added to the linkage group. For example, QTL cqGFR1-2 indicates the second conditional consensus QTL for GFR on chromosome 1H. The phenotypic variation explained (PVE) by consensus QTL more than 20% or at least twice with PVE ≥ 10% was considered to be a major QTL, otherwise regarded as a minor QTL51. The unconditional and conditional consensus QTLs location on genetic map were drawn using MapChart ver. 2.2 software52.
Results
Phenotypic variation
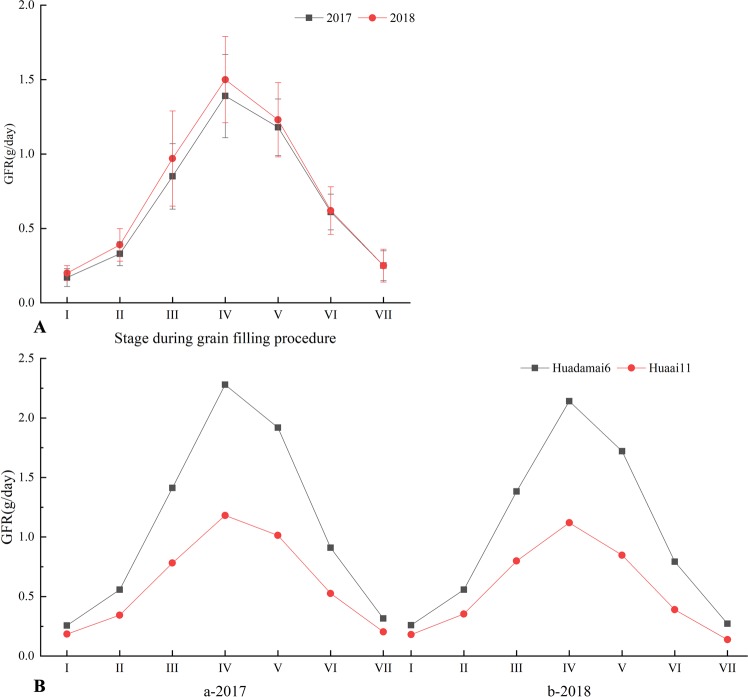
The phenotypic data of the parents and DH line were listed in Table 1. The value of each trait in Huadamai6 was significantly higher than Huai11 (P < 0.01) except for GW at stage II. For the two parents, GFR increased slowly during the initial two stages, after which GFR increased rapidly and reached its maximum at stage IV, and then decreased continuously in two years (Fig. 1B). In the DH population, the tendency of GFR in the seven sampling stages was basically the same as that of the parents (Fig. 1A). The frequency distribution of the five grain size traits was shown in Supplementary Fig. S2. Each trait was continuous variation in all sampling stages, and the absolute values of skewness and kurtosis of most traits were less than 1 except for GFR at stage VII, indicating that these traits followed a normal distribution and were suitable for QTL analysis (Table 1). ANOVA showed statistically significant effects for genotype, environment and G × E interaction with all traits (P < 0.01) (Supplementary Table S1). The broad-sense heritability (hB2) was estimated between 58.9% and 98.5% (Supplementary Table S1).
Table 1.
Phenotypic values of grain filling rate and grain size traits in parents and DH population at different grain filling stages over two years.
| Year | Traita | Stageb | Huaai11 | Huadamai6 | STc | DH lines | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Range | Mean | Skew | Kurt | ||||
| 2017 | GFR | I | 0.18 ± 0.02 | 0.26 ± 0.01 | 0.000** | 0.03–0.39 | 0.17 ± 0.01 | 0.44 | −0.20 |
| II | 0.34 ± 0.04 | 0.56 ± 0.01 | 0.000** | 0.08–0.76 | 0.33 ± 0.01 | 0.86 | 0.93 | ||
| III | 0.78 ± 0.03 | 1.41 ± 0.03 | 0.000** | 0.32–2.21 | 0.85 ± 0.03 | 0.97 | 0.73 | ||
| IV | 1.18 ± 0.04 | 2.28 ± 0.07 | 0.000** | 0.47–2.80 | 1.39 ± 0.05 | 0.72 | −0.64 | ||
| V | 1.01 ± 0.04 | 1.92 ± 0.5 | 0.000** | 0.55–2.55 | 1.18 ± 0.04 | 1.08 | 0.78 | ||
| VI | 0.53 ± 0.03 | 0.91 ± 0.04 | 0.000** | 0.13–1.33 | 0.61 ± 0.02 | 0.39 | 0.12 | ||
| VII | 0.20 ± 0.01 | 0.32 ± 0.04 | 0.000** | 0.02–0.73 | 0.25 ± 0.01 | 0.91 | 0.40 | ||
| GFRmean | 0.60 ± 0.04 | 1.09 ± 0.04 | 0.000** | 0.36–1.16 | 0.69 ± 0.02 | 0.68 | −0.43 | ||
| GFRmax | 1.21 ± 0.02 | 2.45 ± 0.05 | 0.000** | 0.57–3.14 | 1.54 ± 0.06 | 0.76 | −0.43 | ||
| GA | II | 6.39 ± 0.08 | 7.53 ± 0.15 | 0.002** | 3.69–10.00 | 6.43 ± 0.13 | 0.28 | −0.48 | |
| III | 8.72 ± 0.11 | 12.06 ± 0.18 | 0.000** | 5.33–14.86 | 9.45 ± 0.17 | 0.50 | −0.01 | ||
| IV | 11.39 ± 0.08 | 16.32 ± 0.25 | 0.000** | 8.48–18.12 | 12.80 ± 0.23 | 0.52 | −0.90 | ||
| V | 12.09 ± 0.12 | 20.12 ± 0.21 | 0.000** | 10.11–22.65 | 15.28 ± 0.26 | 0.72 | −0.24 | ||
| VI | 14.53 ± 0.15 | 22.26 ± 0.26 | 0.000** | 11.89–25.61 | 17.08 ± 0.30 | 0.98 | 0.29 | ||
| VII | 14.64 ± 0.14 | 22.55 ± 0.18 | 0.000** | 11.26–25.68 | 17.04 ± 0.30 | 0.90 | 0.17 | ||
| GP | II | 12.13 ± 0.08 | 14.51 ± 0.11 | 0.000** | 7.78–16.62 | 12.08 ± 0.15 | −0.11 | −0.42 | |
| III | 14.22 ± 0.13 | 16.26 ± 0.11 | 0.000** | 11.53–18.09 | 15.05 ± 0.13 | 0.05 | −0.29 | ||
| IV | 14.82 ± 0.12 | 17.80 ± 0.18 | 0.000** | 13.89–19.05 | 16.09 ± 0.12 | 0.42 | −0.69 | ||
| V | 15.58 ± 0.16 | 19.78 ± 0.22 | 0.000** | 14.22–20.25 | 16.93 ± 0.12 | 0.50 | −0.45 | ||
| VI | 15.98 ± 0.20 | 20.93 ± 0.14 | 0.000** | 15.41–21.64 | 17.72 ± 0.12 | 0.72 | 0.00 | ||
| VII | 16.14 ± 0.14 | 21.07 ± 0.15 | 0.000** | 15.65–22.84 | 17.75 ± 0.14 | 0.93 | 0.85 | ||
| GL | II | 4.92 ± 0.04 | 6.25 ± 0.06 | 0.000** | 2.88–7.13 | 4.94 ± 0.07 | −0.06 | −0.18 | |
| III | 6.00 ± 0.08 | 6.95 ± 0.05 | 0.000** | 4.74–7.77 | 6.40 ± 0.06 | −0.11 | −0.12 | ||
| IV | 6.15 ± 0.08 | 7.41 ± 0.11 | 0.000** | 5.72–8.18 | 6.82 ± 0.05 | 0.20 | −0.62 | ||
| V | 6.32 ± 0.06 | 7.87 ± 0.08 | 0.000** | 5.97–8.66 | 7.15 ± 0.05 | 0.39 | −0.23 | ||
| VI | 6.41 ± 0.10 | 8.60 ± 0.08 | 0.000** | 5.90–9.26 | 7.41 ± 0.06 | 0.30 | −0.28 | ||
| VII | 6.27 ± 0.08 | 8.84 ± 0.11 | 0.000** | 5.56–9.10 | 7.46 ± 0.07 | 0.00 | −0.42 | ||
| GW | II | 1.67 ± 0.02 | 1.49 ± 0.03 | 0.000** | 1.34–2.15 | 1.65 ± 0.01 | 0.47 | −0.29 | |
| III | 1.88 ± 0.03 | 2.32 ± 0.03 | 0.000** | 1.46–2.77 | 1.93 ± 0.02 | 1.07 | 1.09 | ||
| IV | 2.51 ± 0.03 | 2.94 ± 0.05 | 0.000** | 1.87–3.46 | 2.54 ± 0.04 | 0.48 | −0.90 | ||
| V | 2.73 ± 0.05 | 3.49 ± 0.05 | 0.000** | 2.19–3.70 | 2.92 ± 0.04 | 0.34 | −1.03 | ||
| VI | 3.13 ± 0.04 | 3.80 ± 0.04 | 0.000** | 2.32–3.87 | 3.17 ± 0.03 | 0.33 | −0.84 | ||
| VII | 3.13 ± 0.03 | 3.71 ± 0.03 | 0.000** | 2.34–3.94 | 3.22 ± 0.03 | 0.29 | −0.89 | ||
| GD | II | 2.81 ± 0.02 | 3.07 ± 0.03 | 0.000** | 2.13–3.55 | 2.80 ± 0.03 | 0.13 | −0.56 | |
| III | 3.30 ± 0.03 | 3.91 ± 0.05 | 0.000** | 2.51–4.34 | 3.42 ± 0.03 | 0.20 | −0.19 | ||
| IV | 3.79 ± 0.04 | 4.54 ± 0.04 | 0.000** | 3.26–4.80 | 3.99 ± 0.04 | 0.43 | −0.97 | ||
| V | 3.91 ± 0.04 | 4.92 ± 0.04 | 0.000** | 3.58–5.34 | 4.32 ± 0.04 | 0.60 | −0.59 | ||
| VI | 4.29 ± 0.03 | 5.24 ± 0.05 | 0.000** | 3.63–5.64 | 4.52 ± 0.04 | 0.78 | −0.10 | ||
| VII | 4.30 ± 0.04 | 5.37 ± 0.04 | 0.000** | 3.69–5.68 | 4.56 ± 0.04 | 0.66 | −0.33 | ||
| 2018 | GFR | I | 0.20 ± 0.01 | 0.34 ± 0.02 | 0.000** | 0.09–0.43 | 0.20 ± 0.01 | 0.67 | 0.12 |
| II | 0.39 ± 0.02 | 0.61 ± 0.02 | 0.000** | 0.18–0.73 | 0.39 ± 0.01 | 0.79 | 0.47 | ||
| III | 0.88 ± 0.04 | 1.52 ± 0.05 | 0.000** | 0.41–2.32 | 0.97 ± 0.03 | 1.06 | 1.03 | ||
| IV | 1.23 ± 0.04 | 2.36 ± 0.06 | 0.000** | 0.70–2.68 | 1.50 ± 0.05 | 0.70 | −0.62 | ||
| V | 0.93 ± 0.02 | 1.90 ± 0.03 | 0.000** | 0.63–2.27 | 1.23 ± 0.03 | 0.90 | 0.22 | ||
| VI | 0.43 ± 0.02 | 0.87 ± 0.04 | 0.000** | 0.14–1.17 | 0.62 ± 0.02 | 0.09 | −0.05 | ||
| VII | 0.15 ± 0.01 | 0.30 ± 0.02 | 0.000** | 0.02–0.62 | 0.25 ± 0.01 | 0.42 | −0.02 | ||
| GFRmean | 0.60 ± 0.03 | 1.12 ± 0.03 | 0.000** | 0.39–1.18 | 0.74 ± 0.02 | 0.57 | −0.51 | ||
| GFRmax | 1.27 ± 0.03 | 2.51 ± 0.04 | 0.000** | 0.72–3.22 | 1.61 ± 0.05 | 0.78 | −0.39 | ||
| GA | II | 7.23 ± 0.11 | 8.33 ± 0.11 | 0.000** | 4.01–10.99 | 7.08 ± 0.12 | 0.20 | −0.20 | |
| III | 9.55 ± 0.16 | 13.25 ± 0.15 | 0.000** | 5.93–15.99 | 10.25 ± 0.18 | 0.40 | −0.20 | ||
| IV | 13.64 ± 0.21 | 18.64 ± 0.20 | 0.000** | 8.94–20.35 | 13.70 ± 0.25 | 0.41 | −0.83 | ||
| V | 15.67 ± 0.18 | 22.69 ± 0.26 | 0.000** | 11.24–23.46 | 16.42 ± 0.29 | 0.53 | −0.39 | ||
| VI | 17.59 ± 0.26 | 25.61 ± 0.30 | 0.000** | 12.14–26.61 | 18.39 ± 0.32 | 0.61 | −0.11 | ||
| VII | 17.16 ± 0.21 | 25.07 ± 0.22 | 0.000** | 11.42–27.16 | 18.56 ± 0.35 | 0.54 | −0.29 | ||
| GP | II | 12.37 ± 0.15 | 14.94 ± 0.12 | 0.000** | 8.03–17.32 | 12.50 ± 0.16 | −0.14 | −0.32 | |
| III | 14.67 ± 0.14 | 16.21 ± 0.21 | 0.000** | 11.61–18.95 | 15.63 ± 0.14 | −0.09 | −0.50 | ||
| IV | 15.84 ± 0.16 | 18.66 ± 0.16 | 0.000** | 13.51–20.15 | 16.90 ± 0.15 | −0.19 | −0.98 | ||
| V | 16.33 ± 0.14 | 19.85 ± 0.22 | 0.000** | 14.51–22.02 | 17.76 ± 0.16 | −0.06 | −0.86 | ||
| VI | 16.54 ± 0.18 | 21.69 ± 0.31 | 0.000** | 15.07–23.54 | 18.66 ± 0.18 | 0.19 | −0.69 | ||
| VII | 16.92 ± 0.16 | 22.68 ± 0.25 | 0.000** | 15.06–23.45 | 18.68 ± 0.20 | 0.13 | −1.04 | ||
| GL | II | 5.26 ± 0.06 | 6.14 ± 0.05 | 0.000** | 2.71–7.11 | 4.97 ± 0.08 | −0.12 | 0.25 | |
| III | 6.31 ± 0.06 | 7.11 ± 0.06 | 0.000** | 4.53–8.14 | 6.42 ± 0.07 | 0.00 | −0.49 | ||
| IV | 6.51 ± 0.08 | 7.60 ± 0.06 | 0.000** | 5.81–8.61 | 6.93 ± 0.06 | 0.37 | −0.56 | ||
| V | 6.64 ± 0.07 | 8.26 ± 0.11 | 0.000** | 6.19–8.89 | 7.36 ± 0.07 | 0.11 | −0.99 | ||
| VI | 6.79 ± 0.11 | 8.61 ± 0.08 | 0.000** | 6.31–9.37 | 7.66 ± 0.08 | 0.03 | −1.21 | ||
| VII | 6.76 ± 0.10 | 8.81 ± 0.11 | 0.000** | 6.02–9.50 | 7.55 ± 0.09 | 0.05 | −1.21 | ||
| GW | II | 1.71 ± 0.02 | 1.53 ± 0.02 | 0.000** | 1.34–2.13 | 1.77 ± 0.01 | −0.23 | 0.73 | |
| III | 1.94 ± 0.03 | 2.39 ± 0.03 | 0.000** | 1.68–2.65 | 2.15 ± 0.02 | 0.41 | 0.06 | ||
| IV | 2.65 ± 0.03 | 3.15 ± 0.04 | 0.000** | 2.07–3.41 | 2.74 ± 0.03 | 0.42 | −0.69 | ||
| V | 3.15 ± 0.04 | 3.75 ± 0.04 | 0.000** | 2.28–3.86 | 3.13 ± 0.03 | 0.37 | −0.89 | ||
| VI | 3.21 ± 0.03 | 3.85 ± 0.04 | 0.000** | 2.32–3.96 | 3.30 ± 0.03 | 0.11 | −0.50 | ||
| VII | 3.31 ± 0.04 | 3.86 ± 0.04 | 0.000** | 2.51–4.19 | 3.33 ± 0.03 | 0.25 | −0.56 | ||
| GD | II | 2.87 ± 0.02 | 3.21 ± 0.04 | 0.002** | 2.20–3.91 | 2.97 ± 0.03 | 0.36 | −0.08 | |
| III | 3.42 ± 0.03 | 4.26 ± 0.04 | 0.000** | 2.81–4.52 | 3.66 ± 0.03 | −0.08 | −0.16 | ||
| IV | 3.97 ± 0.03 | 4.87 ± 0.03 | 0.000** | 3.36–5.05 | 4.23 ± 0.04 | 0.02 | −0.97 | ||
| V | 4.21 ± 0.04 | 5.29 ± 0.05 | 0.000** | 3.67–5.43 | 4.52 ± 0.04 | 0.34 | −0.59 | ||
| VI | 4.40 ± 0.04 | 5.61 ± 0.06 | 0.000** | 3.62–5.81 | 4.72 ± 0.04 | 0.31 | −0.36 | ||
| VII | 4.51 ± 0.05 | 5.60 ± 0.04 | 0.000** | 3.72–5.86 | 4.81 ± 0.05 | 0.27 | −0.57 | ||
aGFR, grain filling rate; GFRmean, mean grain filling rate; GFRmax, maximum grain filling rate; GA, grain area; GP, grain perimeter; GL, grain length; GW, grain width; GD, grain diameter. bI, II, III, IV, V, VI and VII represent 7, 14, 21, 28, 35, 42 and 49 days after flowering, respectively. cST: Significant; **Significant at 0.01 level.
Figure 1.
Grain filling rate for parents (B) and DH population (A) at different grain filling stages in 2017 and 2018 at Wuhan, China.
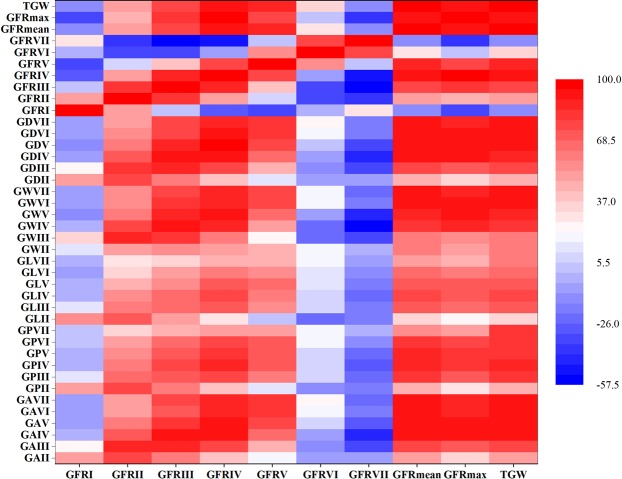
The Pearson correlation coefficients (r) between GA, GP, GL, GW, GD, TGW, GFRmean, GFRmax and GFR traits at seven sampling stages over two years were given in Fig. 2. Grain size traits were significantly correlated with each other at each stage (not listed), and grain size traits at all stages were significantly positively correlated with GFR at stage II, III, IV and V, GFRmean, GFRmax and TGW, whereas GFR at stages I, VI and VII were mostly unrelated or negatively correlated with grain size traits at each stage. TGW showed high positive correlation coefficients with GFRmean and GFRmax (r > 0.9), while GFR at stage III, IV and V were also positive correlation coefficients with TGW (r > 0.7). The initial and final stages of GFR was not correlated with GFRmean and TGW, but negatively with GFRmax (Fig. 2).
Figure 2.
Correlation coefficients among grain filling rate, grain size and thousand grain weight averaged across two years.
Unconditional QTL analysis
A total of 204 unconditionally identified QTLs for GFRmean, GFRmax, GA, GP, GL, GW, GD and GFR were detected on 7 chromosomes, individually accounted for 1.12–78.03% phenotypic variation (Supplementary Table S2). Up to 152 identified QTLs with overlapping CIs were detected and integrated into 38 consensus QTLs. The other 52 non-overlapping identified QTLs were also considered to be consensus QTLs, of which 8 QTLs for GFRmean and GFRmax were regarded as the unconditional consensus QTLs (Table 2, Supplementary Table S2). In total, 90 unconditional consensus QTLs were detected (Fig. 3, Table 2).
Table 2.
Unconditional consensus QTLs underlying the grain filling rate and grain size traits at different grain filling stages.
| Trait | Consensus QTLs | Chr.a | Peak | The closest marker | CIb | LOD | R2c | Addd | Stagee |
|---|---|---|---|---|---|---|---|---|---|
| GFR | ucqGFR1–1 | 1H | 37.79 | 1H_82851228 | 37.35–38.24 | 33.24–34.12 | 16.33–19.47 | 0.10–0.11 | Y1.II/Y2.II |
| ucqGFR1–2 | 1H | 42 | 1H_10863328 | 41.71–42.28 | 7.03–47.17 | 7.12–36.22 | −0.18 to −0.12 | Y2.II,III | |
| ucqGFR1–3 | 1H | 54 | 1H_58188215 | 52.4–54.5 | 6.81 | 4.40 | −0.11 | Y2.IV | |
| ucqGFR2–1 | 2H | 68.5 | M_1999039_479 | 68.28–68.87 | 3.10–4.12 | 2.69–2.83 | −0.11 to 0.09 | Y1.IV/Y2.III | |
| ucqGFR2–2 | 2H | 88 | Bmag829 | 87.5–89.5 | 4.85 | 12.20 | −0.05 | Y2.VII | |
| ucqGFR2–3 | 2H | 126.25 | 2HL_22930005 | 125.75–127.5 | 15.57–46.56 | 33.71–71.54 | 0.08–0.51 | Y1.II,III,IV,V/Y2.III,IV,V | |
| ucqGFR3–1 | 3H | 28 | 3_525094736 | 27.64–28.35 | 6.04–8.81 | 9.66–14.26 | 0.13–0.14 | Y1.V/Y2.V | |
| ucqGFR3–2 | 3H | 32 | 3HL_15958290 | 31.71–32.28 | 4.39–7.32 | 4.76–24.12 | −0.10 to 0.11 | Y1.II,III,VI/Y2.III,VI,VII | |
| ucqGFR5 | 5H | 0 | 5HS_7374618 | 0–0.5 | 4.12–4.39 | 5.76–6.24 | −0.09 to −0.08 | Y1.V/Y2.V | |
| ucqGFR7–1 | 7H | 66.5 | 7_501748124 | 66.25–66.75 | 5.35–12.69 | 7.66–9.96 | 0.10–0.17 | Y1.IV,V/Y2.V | |
| ucqGFR7–2 | 7H | 180 | 7HS_16458224 | 179.5–180.5 | 4.74–8.18 | 7.63–8.07 | 0.03–0.10 | Y1.II,III | |
| GA | ucqGA1–1 | 1H | 22 | 1_306394013 | 21.64–22.35 | 3.23–3.57 | 7.65–8.64 | −0.41 to −0.38 | Y1.II/Y2.II |
| ucqGA1–2 | 1H | 64 | M_2579923_225 | 62.5–64.5 | 16.69 | 7.44 | 0.81 | Y1.IV | |
| ucqGA1–3 | 1H | 87 | 1H_2112459 | 86.5–87.5 | 26.95 | 15.06 | −1.15 | Y1.IV | |
| ucqGA1–4 | 1H | 117 | 1_41186142 | 116.5–117.5 | 5.49 | 3.32 | −0.48 | Y2.IV | |
| ucqGA1–5 | 1H | 133 | 1_19825452 | 130.5–133.5 | 3.92–4.60 | 1.29–4.31 | −0.50 to −0.39 | Y2.VI,VII | |
| ucqGA2 | 2H | 126.13 | 2_527241334 | 125.55–126.64 | 9.51–55.19 | 25.91–75.68 | 0.76–2.85 | Y1.II,III,IV,V,VI,VII | |
| Y2.II,III,IV,V,VII | |||||||||
| ucqGA3–1 | 3H | 30 | 3_510997641 | 29.5–30.5 | 4.39 | 1.54 | 0.46 | Y2.VII | |
| ucqGA3–2 | 3H | 57 | 3HL_34537138 | 56.5–57.5 | 5.13 | 1.70 | 0.45 | Y2.VII | |
| ucqGA3–3 | 3H | 91 | 3_267212934 | 90.5–91.5 | 5.03 | 4.85 | −0.53 | Y2.VI | |
| ucqGA4 | 4H | 10 | 4HL_29463683 | 9.5–11.5 | 3.931 | 1.74 | −0.37 | Y1.V | |
| ucqGA5 | 5H | 141 | M_1634918_588 | 140.5–141.5 | 3.19 | 1.43 | −0.35 | Y1.V | |
| ucqGA7–1 | 7H | 65 | 7HL_8312277 | 64.71–65.28 | 12.66–35.07 | 9.43–30.11 | 0.8–1.6 | Y2.IV,VI,VII | |
| ucqGA7–2 | 7H | 94 | Bmag746 | 93.64–94.35 | 13.42–13.47 | 10.99–11.34 | 1.07–1.10 | Y1.VI,VII | |
| ucqGA7–3 | 7H | 151 | Bmac31 | 150.5–151.5 | 15.61 | 6.44 | 0.87 | Y2.VII | |
| GP | ucqGP1–1 | 1H | 22 | 1_306394013 | 21.5–22.5 | 4.04 | 10.26 | −0.54 | Y1.II |
| ucqGP1–2 | 1H | 42 | 1H_10863328 | 41.5–42.5 | 3.62 | 7.82 | −0.51 | Y2.II | |
| ucqGP2–1 | 2H | 126.25 | 2_527241334 | 125.93–126.54 | 8.21–37.66 | 15.96–62.17 | 0.52–1.21 | Y1.II,III,IV,V,VI,VII | |
| Y2.II,III,IV,VI,VII | |||||||||
| ucqGP2–2 | 2H | 132 | 2HL_43143355 | 131.5–132.5 | 25.32 | 24.71 | 0.90 | Y2.V | |
| ucqGP3–1 | 3H | 55 | 3HL_48064911 | 54.13–55.86 | 3.39–9.21 | 3.25–10.44 | 0.24–0.39 | Y1.V,VI/Y2.IV | |
| ucqGP3–2 | 3H | 91 | 3_267212934 | 90.64–91.35 | 6.36–11.91 | 5.14–13.83 | −0.45 to −0.30 | Y1.V,VI | |
| ucqGP5 | 5H | 203 | 5_226253827 | 202.5–203.5 | 4.76 | 4.78 | −0.26 | Y1.VI | |
| ucqGP6 | 6H | 102 | M_1661027_233 | 101.5–102.5 | 30.3724 | 31.46 | 0.94 | Y2.V | |
| ucqGP7–1 | 7H | 49 | GBM1102 | 47.5–50.5 | 5.26 | 5.45 | 0.31 | Y2.III | |
| ucqGP7–2 | 7H | 65.5 | 7HL_37199773 | 65.25–65.75 | 11.84–31.98 | 13.73–44.71 | 0.49–1.23 | Y2.IV,V,VI,VII | |
| ucqGP7–3 | 7H | 94 | Bmag746 | 93.64–94.35 | 9.36–14.66 | 9.99–13.89 | 0.42–0.69 | Y2.III,VII | |
| ucqGP7–4 | 7H | 116 | 5_496886371 | 115.5–116.5 | 17.12 | 21.04 | 0.61 | Y2.III | |
| ucqGP7–5 | 7H | 136 | 7HS_29196961 | 135.5–136.5 | 27.36 | 26.39 | −1.04 | Y2.VI | |
| ucqGP7–6 | 7H | 140 | 7_319506952 | 138.5–140.5 | 21.31 | 30.24 | 0.77 | Y1.III | |
| ucqGP7–7 | 7H | 151.77 | 2_287569753 | 151.44–152.11 | 7.18–22.28 | 16.00–30.90 | 0.52–0.75 | Y1.VI/Y2.II,IV | |
| GL | ucqGL1 | 1H | 22 | 1_306394013 | 21.5–22.5 | 5.33 | 12.70 | −0.29 | Y1.II |
| ucqGL2–1 | 2H | 125.94 | 2_527241334 | 125.76–126.11 | 12.44–29.66 | 15.52–51.03 | 0.35–0.45 | Y1.III,IV,V,VI,VII | |
| Y2. V,VI,VII | |||||||||
| ucqGL2–2 | 2H | 133.1 | 2_534686550 | 132.62–133.57 | 5.39–5.79 | 12.84–14.43 | 0.31–0.34 | Y1.II/Y2.II | |
| ucqGL3–1 | 3H | 48 | 3HL_33828484 | 47.18–47.87 | 4.35–5.53 | 3.96–7.23 | 0.12–0.13 | Y1.V/Y2.IV | |
| ucqGL3–2 | 3H | 91 | 3_267212934 | 90.5–91.5 | 6.59 | 6.36 | −0.15 | Y1.V | |
| ucqGL5 | 5H | 157 | 5_306133226 | 156.5–157.5 | 3.25 | 2.94 | −0.11 | Y1.V | |
| ucqGL7–1 | 7H | 65.25 | 7HL_13143105 | 65.0–65.5 | 15.66–50.97 | 25.01–45.19 | 0.25–0.61 | Y2.IV,V,VI,VII | |
| ucqGL7–2 | 7H | 116 | 5_496886371 | 115.5–116.5 | 10.26 | 21.79 | 0.27 | Y2.III | |
| ucqGL7–3 | 7H | 136 | 7HS_29196961 | 135.64–136.35 | 28.14–38.74 | 23.52–27.20 | −0.48 to −0.37 | Y2.VI,VII | |
| ucqGL7–4 | 7H | 140 | 7_319506952 | 138.5–140.5 | 22.26 | 37.83 | 0.40 | Y1.III | |
| ucqGL7–5 | 7H | 150.92 | Bmac31 | 150.64–151.2 | 6.67–24.79 | 17.10–43.21 | 0.35–0.50 | Y1.V,VI,VII/Y2.II | |
| ucqGL7–6 | 7H | 175 | 7_144173681 | 174.5–175.5 | 3.20 | 7.47 | 0.22 | Y1.II | |
| GW | ucqGW1–1 | 1H | 19.76 | 1H_45582581 | 19.42–20.09 | 5.08–7.31 | 2.71–5.45 | −0.09 to −0.06 | Y1.IV,V,/Y2.V,VII |
| ucqGW1–2 | 1H | 48 | 1_167351152 | 47.5–48.5 | 6.47 | 3.25 | −0.06 | Y2.VI | |
| ucqGW2 | 2H | 124.69 | 2HL_18970523 | 124.44–125.25 | 8.21–53.91 | 32.27–75.51 | 0.09–0.39 | Y1.II,III,IV,V,VI,VII | |
| Y2.II,III,IV,V,VI,VII | |||||||||
| ucqGW3–1 | 3H | 26 | 3_528470541 | 25.5–26.5 | 7.01 | 3.06 | 0.07 | Y1.VI | |
| ucqGW3–2 | 3H | 31.33 | 2HL_33741786 | 31.04–31.62 | 3.62–4.75 | 2.16–6.75 | −0.07 to 0.06 | Y1.III/Y2.VI,VII | |
| ucqGW3–3 | 3H | 52 | 3HL_45910009 | 51.5–52.5 | 3.53 | 1.43 | −0.05 | Y1.VI | |
| ucqGW4 | 4H | 52 | 4_474989327 | 51.64–52.35 | 4.09–4.48 | 1.18–2.32 | −0.05 | Y2.IV,VII | |
| ucqGW6 | 6H | 25 | 6_518846666 | 23.5–25.5 | 3.79 | 1.63 | −0.05 | Y1.VII | |
| ucqGW7–1 | 7H | 57 | 1_4088556 | 56.5–57.5 | 5.82 | 4.28 | 0.08 | Y1.IV | |
| ucqGW7–2 | 7H | 65.13 | 7HL_13143105 | 64.78–65.66 | 7.63–46.68 | 5.61–34.11 | −0.18 to 0.26 | Y1.IV,V,VII/Y2.IV,V,VI | |
| GD | ucqGD1–1 | 1H | 22 | 1_306394013 | 21.64–22.35 | 3.94–4.05 | 8.34–9.90 | −0.11 to −0.10 | Y1.II/Y2,II |
| ucqGD1–2 | 1H | 42 | 1H_10863328 | 41.5–42.5 | 4.14 | 5.92 | −0.09 | Y2.III | |
| ucqGD1–3 | 1H | 64 | M_2579923_225 | 62.5–64.5 | 16.54 | 7.09 | 0.12 | Y1,IV | |
| ucqGD1–4 | 1H | 89.17 | Bmag770 | 88.89–89.46 | 14.26–27.03 | 6.30–14.59 | −0.18 to 0.12 | Y1.IV,V | |
| ucqGD2 | 2H | 125.93 | 2_527241334 | 125.69–126.44 | 8.35–56.41 | 18.90–78.30 | 0.18–0.42 | Y1.II,III,IV,V,VI,VII | |
| Y2. II,III,IV,V,VI,VII | |||||||||
| ucqGD3–1 | 3H | 27 | 3_529115904 | 26.5–27.5 | 4.19 | 1.96 | 0.06 | Y1.VI | |
| ucqGD3–2 | 3H | 32 | 3HL_15958290 | 31.5–32.5 | 9.02 | 2.93 | 0.09 | Y2.VII | |
| ucqGD3–3 | 3H | 90 | 3HL_14205585 | 89.64–90.35 | 4.49–4.78 | 2.29–2.46 | −0.07 to −0.06 | Y1.VI,VII | |
| ucqGD4–1 | 4H | 12 | 4_528451537 | 11.5–12.5 | 4.22 | 1.22 | −0.05 | Y2.V | |
| ucqGD4–2 | 4H | 52 | 4_474989327 | 51.5–52.5 | 4.59 | 1.39 | −0.05 | Y2.VII | |
| ucqGD5–1 | 5H | 102 | 5HS_16446198 | 100.5–102.5 | 11.71 | 3.87 | 0.10 | Y2.V | |
| ucqGD5–2 | 5H | 123.5 | 5HS_4157152 | 122.79–124.2 | 4.05–21.31 | 1.12–8.72 | −0.14 to −0.05 | Y2.V,VII | |
| ucqGD5–3 | 5H | 155 | M_81421_1318 | 154.5–156.5 | 5.41 | 2.71 | −0.07 | Y1.VI | |
| ucqGD6 | 6H | 78 | GBM1256 | 77.5–78.5 | 5.54 | 1.66 | 0.06 | Y2.V | |
| ucqGD7–1 | 7H | 65.88 | 7HL_37199773 | 65.63–66.12 | 13.71–44.92 | 10.08–31.65 | 0.13–0.26 | Y1.VI/Y2.IV,V,VI,VII | |
| ucqGD7–2 | 7H | 76 | 7_500661278 | 75.5–76.5 | 17.85 | 31.06 | 0.21 | Y2.III | |
| ucqGD7–3 | 7H | 94 | Bmag746 | 93.5–94.5 | 10.79 | 3.43 | 0.09 | Y2.VII | |
| ucqGD7–4 | 7H | 151 | Bmac31 | 150.5–151.5 | 9.59 | 6.33 | 0.11 | Y2.VI | |
| ucqGD7–5 | 7H | 167 | Bmag900 | 166.5–167.5 | 5.46 | 3.04 | 0.08 | Y1.VII | |
| GFRmax | ucqGFRmax1 | 1H | 54 | 1H_58188215 | 52.5–54.5 | 5.85 | 4.11 | −0.11 | Y2 |
| ucqGFRmax2–1 | 2H | 96 | 2HL_43859802 | 95.5–96.5 | 3.58 | 2.44 | 0.09 | Y1 | |
| ucqGFRmax2–2 | 2H | 127 | 2HL_22930005 | 126.5–127.5 | 43.38–43.39 | 68.26–70.11 | 0.5–0.54 | Y1/Y2 | |
| ucqGFRmax7–1 | 7H | 65 | 7HL_8312277 | 64.5–65.5 | 13.51 | 10.99 | 0.19 | Y1 | |
| ucqGFRmax7–2 | 7H | 142 | 7HL_38122468 | 141.5–142.5 | 13.53 | 11.38 | 0.19 | Y2 | |
| GFRmean | ucqGFRmean2–1 | 2H | 125 | 2HL_22930294 | 124.5–125.5 | 49.39–56.94 | 69.98–74.83 | 0.17–0.18 | Y1/Y2 |
| ucqGFRmean7–1 | 7H | 97 | 7_440111505 | 96.5–97.5 | 13.31 | 9.23 | 0.06 | Y1 | |
| ucqGFRmean7–2 | 7H | 107 | 7_266425095 | 106.5–107.5 | 21.68 | 11.69 | 0.06 | Y2 | |
aChromosome. bThe 1.5-LOD confidence interval of QTLs. cThe phenotypic variance explained by each QTL. dAdditive effect. eY1 and Y2 represent 2017 and 2018, respectively. Abbreviations are shown in the footnote of Table 1.
Figure 3.
Chromosomes location of the consensus QTLs associated with GFR, GFRmean, GFRmax and five grain size traits. The marker names are listed on the right of the linkage groups, and the positions on the left, given in centimorgan (cM). Solid and hollow bars represent the QTLs that were mapped using unconditional and conditional methods, respectively, the brown area on the linkage groups represents the QTL cluster. Abbreviations are shown in the footnote of Table 1.
For the GFRmax and GFRmean, eight QTLs were detected on chromosome 1H, 2H, and 7H over two years, individually explaining 2.44–74.83% of PVE (Table 2). Among these QTLs, two main-effect QTLs were detected on 2H, ucqGFRmean2 was located at 125 cM close to the marker 2HL_22930294, and ucqGFRmax2–2 was located at 127 cM near the marker 2HL_22930005. These alleles from Huadamai6 contributed PVE 68.26–74.83%.
In total, 30 identified QTLs located on 7 chromosomes except 4H and 6H were detected for GFR. Of those, 28 overlapping QTLs were integrated into 9 unconditional consensus QTLs and 2 non-overlapping QTLs also regarded as consensus QTLs (Table 2, Supplementary Table S2). Among these consensus QTLs, 4 QTLs were detected repeatedly at the same sampling stage over two years. ucqGFR1-2 located on 1H, linked to the SNP marker 1H_10863328 of 42 cM, was expressed at stage II and III in Y2, explaining 7.12–36.22% of PVE. ucqGFR3-2 linked to the marker 3HL_15958290 of 32 cM was detected at stage II, III and VI in Y1 and at stage III, VI and VII in Y2 with PVE ranging from 4.76 to 24.12%. The QTL ucqGFR3-3 showed negative additive effects and reduced the GFR from the Huadamai6 alleles. ucqGFR2-3 closely linked to the marker 2HL_22930005 of 127 cM was expressed simultaneously at stage II, III, IV and V in Y1 and at stage III, VI and V in Y2, accounting for 33.71–71.54% PVE, with the allele increasing GFR contributed by Huadamai6.
A total of 29 identified QTLs on 7 chromosomes were detected for GA. Among these QTLs, 20 overlapping QTLs were integrated into 5 consensus QTLs (Table 2, Supplementary Table S2). Only one QTL (ucqGA2) tightly linked to 2_527241334 of 126.13 cM was consistently expressed at all stages in Y1 and at stage II, III, IV, V and VII in Y2, explaining 25.91–75.68% of PVE, with additive effects varying from 0.76 to 2.68; another QTL ucqGA7-1 was detected simultaneously at three different stages in Y2, explaining 12.66–35.07% of PVE. Additionally, two QTLs were detected at two different stages in particular year, and ucqGA1-1 was detected at stage II in both years. The remaining 9 consensus QTLs were only identified at one stage in a particular year.
For the GP, 35 identified QTLs located across 7 chromosomes except 4H were detected over two years. Of these, 26 overlapping QTLs and 9 non-overlapping QTLs were integrated into 15 consensus QTLs (Table 2, Supplementary Table S2). Among these consensus QTLs, ucqGP2-1 was 126.25 cM from 2_527241334 and consistently detected at all stages in Y1 and at stage II, III, IV, VI and VII in Y2, which individually explained 15.96–62.17% of PVE. ucqGP7-2 tightly linked to the 7HL_37199773 of 65.5 cM, was expressed at four different stages in Y2, and contributed 13.73–44.71% PVE. In addition, ucqGP7-7 closely linked to the 2_287569753 at a genetic distance of 151.77 cM was detected at stage VI in Y1 and at stage II and IV in Y2, individually explaining up to 16–30.9% of PVE. These alleles increasing GP were from Huadamai6. ucqGP3-1 was detected consistently at stage V and VI in Y1 and at stage IV in Y2. Another two QTLs (ucqGP3-2 and ucqGP7-3) were detected at two different stages in particular year.
A total of 30 identified QTLs on 5 chromosomes (excluding 4H and 6H) were detected for GL. Of these QTLs, integrating 24 QTLs with overlapping CIs into 6 consensus QTLs and 6 non-overlapping QTLs were also considered to be consensus QTLs (Table 2, Supplementary Table S2). At stages III-VII, the major QTL ucqGL2-1 was 125.94 cM from 2_527241334 and explained up to 15.52–51.03% of PVE. At stages IV-VII in Y2, the main-effect ucqGL7-1 was closely linked to the marker 7HL_13143105 of 65.25 cM, accounting for 25.01–45.19% PVE. ucqGL7-3 tightly linked to the 7HS_29196961 was detected at stage VI and VII in Y2, explaining 23.52–27.20% of PVE. ucqGL7-5 closely linked to the marker Bmac31 of 150.92 cM was expressed at stages V-VII in Y1 and at stage II in Y2, explaining 17.10–43.21% of PVE. In addition, two QTLs ucqGL2-2 and ucqGL3-1 were detected at one or two stages in both years.
For the GW, 33 identified QTLs distributed on 7 chromosomes except 5H were detected over two years. Of which, 28 QTLs with overlapping CIs and 5 non-overlapping QTLs were integrated into 10 consensus QTLs (Table 2, Supplementary Table S2). ucqGW1-1, and ucqGW3-2 were expressed at one or two stages across two years. ucqGW4 was detected at stage IV and VII in Y2 and exhibited negative additive effects. In addition, ucqGW7-2 was 65.13 cM from 7HL_13143105 and expressed at stage IV, V and VII in Y1 and at stages IV-VI in Y2, explaining 7.63-46.68 of PVE. ucqGW3-2 and ucqGW7-2 expressed additive effects in opposite directions at different stages. Only ucqGW2 closely linked to the 2HL_22930294 of 124.69 cM was identified steadily at all stages in both years, explaining up to 32.27–75.51% of PVE.
For the GD, 39 identified QTLs located on 7 chromosomes were detected across two years. Of which, 26 overlapping were integrated into 6 consensus QTLs, the other 13 non-overlapping QTLs were also considered as consensus QTLs (Table 2, Supplementary Table S2). ucqGD1-1, ucqGD3-3 and ucqGD5-2 were detected at one or two stages over two years with −0.14 to −0.05 additive effects. The major QTL ucqGD2 closely linked to the 2_527241334 of 125.93 cM was stably expressed at all stages in both years, accounting for 18.90-78.03% PVE. In addition, ucqGD7-1 tightly linked to the 7HL_37199773 of 65.88 cM was expressed at stage VI in Y1 and at stages IV-VII in Y2, accounting for 10.08–31.65% PVE.
Conditional QTL analysis
In total, 95 conditionally identified QTLs were detected for GA, GP, GL, GW, GD and GFR across two years, and individually explained 1.26–65.72% of PVE (Supplementary Table S3). Among these QTLs, 55 conditional consensus QTLs consisted of 22 consensus QTLs integrated from 62 overlapping identified QTLs and 33 non-overlapping identified QTLs (Fig. 3, Table 3).
Table 3.
Conditional consensus QTLs underlying the grain filling rate and grain size traits at different grain filling periods.
| Trait | Consensus QTLs | Chra | Peak | The closest marker | CIb | LOD | R2c | Addd | Stagee |
|---|---|---|---|---|---|---|---|---|---|
| GFR | cqGFR1–1 | 1H | 22 | M_1601731_2584 | 21.5–22.5 | 3.22 | 3.99 | −0.02 | Y1.ΔT2 |
| cqGFR1–2 | 1H | 54 | 1H_58188215 | 52.5–54.5 | 3.27–4.97 | 1.54–5.54 | −0.02 to 0.08 | Y2.ΔT2,ΔT5 | |
| cqGFR2–1 | 2H | 88 | Bmag829 | 87.35–88.24 | 3.74–4.69 | 1.26–12.78 | −0.12 to 0.05 | Y1.ΔT3,ΔT5, Y2.ΔT3,ΔT5 | |
| cqGFR2–2 | 2H | 126.83 | 2HL_22930005 | 126.45–127.25 | 6.51–39.08 | 14.92–65.72 | −0.28 to 0.23 | Y1.ΔT2,ΔT3,ΔT4,ΔT6,ΔT7 | |
| Y2.ΔT2,ΔT3, ΔT4,ΔT5,ΔT6,ΔT7 | |||||||||
| cqGFR2–3 | 2H | 132 | 2HL_43143355 | 131.5–132.5 | 8.69 | 26.63 | −0.19 | Y1.ΔT5 | |
| cqGFR3–1 | 3H | 28 | 3_525094736 | 27.64–28.35 | 4.88–6.94 | 12.25–14.46 | −0.06 to 0.10 | Y1.ΔT7/Y2.ΔT4 | |
| cqGFR3–2 | 3H | 33 | 3_511749149 | 32.66–33.33 | 4.06–9.63 | 1.40–22.86 | −0.07 to 0.16 | Y1.ΔT3,ΔT5/Y2. ΔT2,ΔT3,ΔT5,ΔT7 | |
| cqGFR5 | 5H | 0.03 | 5HS_7374618 | 0.21–0.28 | 3.19–3.68 | 5.17–5.35 | −0.07 to −0.06 | Y1.ΔT4/Y2.ΔT4 | |
| cqGFR7–1 | 7H | 58 | 1_4089724 | 57.5–58.5 | 5.06–5.52 | 7.49–9.44 | 0.08–0.09 | Y1.ΔT4/Y2.ΔT4 | |
| cqGFR7–2 | 7H | 65.5 | 7HL_37199773 | 65.25–65.75 | 3.77–5.75 | 8.46–11.91 | −0.09 to −0.04 | Y1.ΔT6,ΔT7/Y2.ΔT7 | |
| cqGFR7–3 | 7H | 122 | 7HS_21726812 | 120.5–122.5 | 6.25 | 8.52 | −0.08 | Y1.ΔT6 | |
| cqGFR7–4 | 7H | 133 | 7HS_17906516 | 132.5–134.5 | 8.66 | 16.27 | −0.11 | Y1.ΔT7 | |
| GA | cqGA2–1 | 2H | 121 | 2_506545106 | 120.5–122.5 | 4.00 | 8.35 | 0.30 | Y1.ΔT5 |
| cqGA2–2 | 2H | 127.54 | 2HL_17075593 | 127.13–127.84 | 9.25–15.63 | 27.71–40.97 | 0.77–0.88 | Y1.ΔT4/Y2.ΔT3,ΔT4 | |
| cqGA3–1 | 3H | 33.3 | 3_511749149 | 32.47–34.13 | 3.12–4.22 | 6.79–9.36 | 0.23–0.35 | Y1.ΔT6/Y2.ΔT6 | |
| cqGA3–2 | 3H | 41 | 3_499436820 | 39.93–42.06 | 5.06–5.14 | 10.48–12.13 | 0.32–0.38 | Y1.ΔT5/Y2.ΔT5 | |
| cqGA4 | 4H | 90 | 4HL_42790942 | 89.5–90.5 | 3.48 | 7.42 | −0.23 | Y2.ΔT6 | |
| cqGA5–1 | 5H | 11 | 5HS_10560611 | 9.5–11.5 | 4.79 | 9.98 | −0.30 | Y1.ΔT5 | |
| cqGA5–2 | 5H | 203 | 5_226253827 | 202.5–203.5 | 3.74 | 8.59 | −0.31 | Y2.ΔT5 | |
| cqGA7–1 | 7H | 58 | 1_4089724 | 57.5–58.5 | 4.78 | 11.32 | 0.46 | Y2.ΔT4 | |
| cqGA7–2 | 7H | 65 | 7HL_8312277 | 64.5–65.5 | 7.85 | 27.58 | 0.22 | Y2.ΔT7 | |
| cqGA7–3 | 7H | 97 | 7_440111505 | 96.5–97.5 | 7.54 | 18.97 | 0.46 | Y2.ΔT5 | |
| cqGA7–4 | 7H | 165 | 7_194302352 | 164.29–165.7 | 3.22–4.48 | 11.45–12.31 | 0.42–0.52 | Y1.ΔT3/Y2.ΔT3 | |
| GP | cqGP7–1 | 7H | 57 | 1_4088556 | 56.5–57.5 | 3.50 | 14.30 | 0.52 | Y2.ΔT3 |
| cqGP7–2 | 7H | 68.99 | 7HL_3360534 | 68.64–69.35 | 5.01–13.78 | 17.05–41.39 | 0.20–0.52 | Y2.ΔT6,ΔT7 | |
| cqGP7–3 | 7H | 124 | 7HS_32890650 | 123.5–124.5 | 25.21 | 16.88 | 1.57 | Y1.ΔT3 | |
| cqGP7–4 | 7H | 153 | M_90598_706 | 152.5–153.5 | 18.28 | 10.41 | −1.22 | Y1.ΔT3 | |
| GL | cqGL1–1 | 1H | 19 | 1H_17392124 | 18.64–19.35 | 3.02–8.28 | 11.34–14.00 | 0.21–0.32 | Y1.ΔT3/Y2.ΔT3 |
| cqGL1–2 | 1H | 114 | 1H_34522869 | 113.5–115.5 | 5.68 | 9.06 | −0.26 | Y2.ΔT3 | |
| cqGL3 | 3H | 19 | 3HL_42780152 | 17.5–19.5 | 4.61 | 7.29 | 0.24 | Y2.ΔT3 | |
| cqGL7–1 | 7H | 66 | 7HL_4313756 | 65.71–66.28 | 4.22–8.07 | 9.08–27.07 | 0.09–0.26 | Y1.ΔT6/Y2.ΔT3,ΔT5 | |
| cqGL7–2 | 7H | 92.5 | 7HL_27996637 | 92.14–92.85 | 4.37–12.36 | 12.70–38.03 | 0.06–0.11 | Y1.ΔT7/Y2.ΔT7 | |
| cqGL7–3 | 7H | 109 | 7HS_35580690 | 108.5–110.5 | 3.22 | 12.19 | −0.11 | Y2.ΔT4 | |
| cqGL7–4 | 7H | 124 | 7HS_32890650 | 123.5–124.5 | 3.20 | 12.31 | 0.22 | Y1.ΔT3 | |
| GW | cqGW2–1 | 2H | 53 | 2HL_17013042 | 52.5–53.5 | 4.08 | 15.00 | −0.06 | Y1.ΔT6 |
| cqGW2–2 | 2H | 86 | M_207663_1931 | 85.5–86.5 | 3.82 | 12.43 | −0.05 | Y2.ΔT6 | |
| cqGW2–3 | 2H | 116 | 2HL_13832944 | 115.5–116.5 | 5.00 | 18.33 | 0.06 | Y2.ΔT5 | |
| cqGW2–4 | 2H | 126 | 2_527241334 | 125.75–126.25 | 4.29–23.87 | 13.33–55.68 | 0.05–0.16 | Y1.ΔT3,ΔT4/Y2.ΔT3,ΔT4 | |
| cqGW3–1 | 3H | 27.5 | 3HL_38740410 | 27.14–27.85 | 3.09–4.08 | 9.85–13.72 | −0.04 to 0.06 | Y1.ΔT5/Y2.ΔT3 | |
| cqGW3–2 | 3H | 32.19 | 3HL_32354397 | 31.75–32.64 | 4.74–6.31 | 16.11–20.59 | −0.09 to 0.06 | Y1.ΔT3/Y2.ΔT6 | |
| cqGW3–3 | 3H | 83 | 3HL_3720522 | 82.5–83.5 | 3.07 | 10.93 | −0.05 | Y1.ΔT6 | |
| cqGW3–4 | 3H | 98 | 3_219109045 | 96.5–98.5 | 4.17 | 15.39 | −0.05 | Y2.ΔT5 | |
| cqGW5 | 5H | 4 | 5_3871196 | 1.5–5.5 | 3.71 | 11.83 | −0.05 | Y1.ΔT5 | |
| cqGW6 | 6H | 32 | 6_518728726 | 31.5–33.5 | 3.22 | 11.98 | −0.04 | Y2.ΔT7 | |
| cqGW7 | 7H | 130 | 7HS_25905506 | 129.5–130.5 | 3.93 | 7.76 | 0.07 | Y1.ΔT4 | |
| GD | cqGD2–1 | 2H | 96 | 2HL_43859802 | 95.5–96.5 | 4.24 | 13.74 | 0.11 | Y1.ΔT3 |
| cqGD2–2 | 2H | 129 | 2HL_34260490 | 128.64–129.35 | 6.05–6.11 | 18.55–20.41 | 0.10–0.11 | Y1.ΔT4/Y2.ΔT4 | |
| cqGD3 | 3H | 27 | 3_529115904 | 26.5–27.5 | 3.71 | 10.91 | 0.05 | Y1.ΔT5 | |
| cqGD4 | 4H | 152 | 4_16819133 | 151.5–154.5 | 3.31 | 9.05 | −0.04 | Y1.ΔT7 | |
| cqGD5–1 | 5H | 9 | 5_51657943 | 8.5–10.5 | 3.04 | 9.30 | 0.04 | Y1.ΔT7 | |
| cqGD5–2 | 5H | 17 | GBM1176 | 11.5–24.5 | 3.08 | 9.62 | −0.04 | Y1.ΔT5 | |
| cqGD7–1 | 7H | 57.6 | 7_523855164 | 57.15–58.04 | 3.14–3.36 | 10.74–12.89 | 0.09 | Y1.ΔT3/Y2.ΔT3 | |
| cqGD7–2 | 7H | 70 | M_363857_407 | 69.5–70.5 | 10.99 | 34.69 | 0.09 | Y2.ΔT7 | |
| cqGD7–3 | 7H | 80 | GMS46 | 78.5–80.5 | 6.18 | 17.99 | 0.06 | Y1.ΔT7 | |
aChromosome. bThe 1.5-LOD confidence interval of QTLs. cThe phenotypic variance explained by each QTL. dAdditive effect. eY1 and Y2 represent 2017 and 2018, respectively, ΔT2, ΔT3, ΔT4, ΔT5, ΔT6 and ΔT7 represent the time intervals I-II, II-III, III-IV, IV-V, V-VI and VI-VII, respectively. Abbreviations are shown in the footnote of Table 1.
For the GFR, 36 identified QTLs distributed on 7 chromosomes except 6H and 4H were detected in both years. The 36 identified QTLs were integrated into 12 consensus QTLs, including 4 non-overlapping identified QTLs (Table 3, Supplementary Table S3). The main-effect QTL cqGFR2-2 was stably expressed at ΔT2, ΔT3, ΔT4, ΔT6 and ΔT7 in Y1 and at all intervals in Y2, explaining 14.92–65.72% of PVE and the additive effects had opposite directions in different intervals. Another major QTL cqGFR3-2 was detected at ΔT3 and ΔT5 in Y1, and ΔT2, ΔT3, ΔT5 and ΔT7 in Y2, accounting for 1.40–22.86% PVE with additive effects ranging from −0.07 to 0.16. Similarly, cqGFR2-1 and cqGFR3-1 had opposite additive effects at different time intervals.
Sixteen identified QTLs, including three QTLs each at ΔT3, ΔT4 and ΔT6, six at ΔT5 and one at ΔT7 were detected for the GA. Nine overlapping and 7 non-overlapping QTLs were integrated into 11 conditional consensus QTLs (Table 3, Supplementary Table S3). cqGA3-1, cqGA3-2 and cqGA7-4 were repeatedly detected at a specific time interval in both years, and the alleles increasing GA were contributed by Huadamai6. Another QTL cqGA2-2 closely linked to the 2HL_17075593 of 127.54 cM was consistently expressed at ΔT4 in Y1 and at ΔT3 and ΔT4 in Y2, explaining 27.71–40.97% of PVE. The remaining 7 conditional consensus QTLs were only detected at a single interval in a particular year.
For the GP, five identified QTLs consisting of three QTLs at ΔT3 and one each at ΔT6 and ΔT7 were detected on 7H. The five identified QTLs were integrated into 4 conditional consensus QTLs (Table 3, Supplementary Table S3). Only cqGP7-2 tightly linked to the 7HL_3360534 of 68.99 cM was expressed simultaneously at two intervals (ΔT6 and ΔT7) in Y2, with explaining 17.05–41.39% of PVE. Among these consensus QTLs, three of them increased GP contributing from the Huadamai6 allele, and one from Huaai11.
In total, 11 identified QTLs, including six QTLs at ΔT3, one each at ΔT4, ΔT5 and ΔT6 and two at ΔT7 detected for the GL were integrated into 7 conditional consensus QTLs, including 4 non-overlapping identified QTLs (Table 3, Supplementary Table S3). cqGL1-1 and cqGL7-2 were repeatedly detected at specific intervals over two years, with PVE of 11.34-14.00% and 12.70–38.03%, respectively. In addition, cqGL7-1 was consistently expressed at ΔT6 inY1 and at ΔT3 and ΔT5 in Y2, explaining 9.08–27.07% of PVE.
For the GW, 16 identified QTLs were detected, including four each at ΔT3, ΔT5 and ΔT6, three at ΔT4 and one at ΔT7. Eight identified QTLs with overlapping CIs and 8 non-overlapping identified QTLs were integrated into 11 conditional consensus QTLs (Table 3, Supplementary Table S3). cqGW2-4 was expressed at ΔT3 and ΔT4 in both years, explaining 13.33–55.68% of PVE. Another major QTL cqGW3-2 closely linked to the 3HL_32354397 of 32.19 cM was repeatedly detected at ΔT3 in Y1 and at ΔT6 in Y2, and explained 16.11–20.59% of PVE. However, the additive effects of cqGW3-2 at different intervals were opposite. In addition, cqGW3-1 was detected at ΔT5 in Y1 and at ΔT3 in Y2, with opposite additive effects. The remaining 8 consensus QTLs were detected at a single interval in particular year, 6 of them increased the GW were from Huaai11 alleles.
For the GD, 3, 2, 2 and 4 identified QTLs were detected at ΔT3, ΔT4, ΔT5 and ΔT7, respectively. The 11 identified QTLs were integrated into 9 conditional consensus QTLs, the 7 non-overlapping identified QTLs were also regarded as consensus QTLs (Table 3, Supplementary Table S3). cqGD2-2 and cqGD7-1 were stably detected at the same interval over two years. The major QTL cqGD2-2 was 129 cM from the 2HL_34260490, accounting for 18.55–20.41% PVE. The other 7 consensus QTLs were only detected at one interval in a particular year. None of the consensus QTLs were expressed at all intervals, and some QTLs showed opposite effects in different intervals.
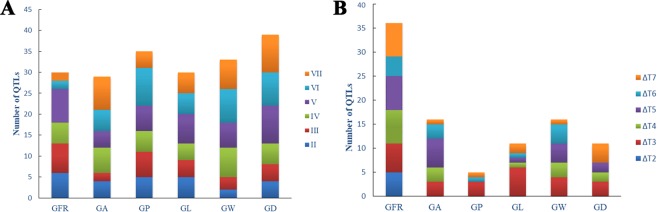
In conclusion, most of the QTLs detected by the unconditional QTL mapping method were detected in the V, VI and VII stages, while the QTLs identified by the conditional QTL mapping method were expressed in different periods (Fig. 4).
Figure 4.
The number of QTLs for grain filling rate and five grain size traits at different stages. A Number of QTLs in each stage identified by unconditional mapping method. B Number of QTLs in each period identified by conditional mapping method.
In addition, to reduce the interference of Rt and Ct on the grain shape and grain filling rate, we used the genome-wide composite interval mapping (GCIM) to perform covariate QTL analysis with Rt, Ct, Rt + Ct as covariates, and detected 118, 109 and 84 QTLs on all seven chromosomes, respectively (Supplementary Table S4). Among these 311 covariate QTLs, 140 covariate QTLs were consistent with unconditional or conditional consensus QTLs, and the remaining 171 were new QTLs. Comparing the consensus QTLs for grain filling rate and grain size traits detected by the five QTL mapping methods, we identified 34 major consensus QTLs (repeatedly detected in at least two QTL mapping methods), including 2, 4, 7, 9, 5, 5, 1 and 1 consensus QTLs for GFR, GA, GP, GL, GW, GD, GFRmax and GFRmean, respectively (Table 4). Of these major consensus QTLs, most of them were identified in two QTL mapping methods, and only six were simultaneously identified in at least four QTL mapping methods.
Table 4.
The major consensus QTLs identified for grain filling rate and five grain size traits using multiple mapping method.
| Trait | Consensus QTLs | Chr.a | Position (cM) | LOD | R2b | Addc | Mapping methodd |
|---|---|---|---|---|---|---|---|
| GFR | qcGFR2–3 | 2H | 125.73 | 4.19–46.56 | 16.30–71.54 | −0.28 to 0.51 | Ct,UC,C |
| qcGFR3–1 | 3H | 32.63–32.72 | 3.06–10.61 | 1.40–28.61 | −0.10 to 0.18 | Rt,Ct,Rt + Ct,UC,C | |
| GA | qcGA2–1 | 2H | 124.44–126.63 | 3.17–49.18 | 12.39–75.68 | 0.30–2.89 | Rt,Ct,Rt + Ct,UC,C |
| qcGA3–2 | 3H | 41.41 | 3.61–5.50 | 6.86–12.13 | 0.29–0.38 | Rt,Ct,Rt + Ct,C | |
| qcGA7–2 | 7H | 66.14 | 75.85–35.07 | 9.43–35.07 | 0.22–1.60 | Rt,UC,C | |
| qcGA7–4 | 7H | 92.90 | 12.61–13.47 | 10.99–36.31 | 0.51–1.10 | Rt,UC | |
| GP | qcGP2–1 | 2H | 125.53 | 3.46–37.66 | 15.96–68.01 | 0.52–1.21 | Ct,UC |
| qcGP2–2 | 2H | 133.61 | 5.93–25.32 | 20.26–24.71 | 0.79–0.90 | Ct,UC | |
| qcGP7–2 | 7H | 66.67 | 4.85–31.98 | 9.30–44.71 | 0.20–1.27 | Rt,UC,C | |
| qcGP7–5 | 7H | 115.22-118.25 | 4.78-17.12 | 11.32-21.04 | 0.56-0.62 | Rt,Rt + Ct,UC | |
| qcGP7-1 | 7H | 123.44 | 5.74-25.21 | 6.51-28.38 | 0.54-0.57 | Ct,Rt + Ct,C | |
| qcGP7-3 | 7H | 139.84-142.42 | 10.79-21.31 | 21.72-30.24 | 0.74-0.77 | Ct,Rt + Ct,UC | |
| qcGP7-8 | 7H | 151.09 | 5.48-22.28 | 8.48-30.90 | −1.22 to 0.79 | Rt,UC,C | |
| GL | qcGL1 | 1H | 18.81 | 3.02-8.28 | 10.36-14.00 | 0.21-0.32 | Rt,Ct,Rt + Ct,C |
| qcGL2-1 | 2 H | 125.94-126.13 | 6.59-29.66 | 15.47-51.03 | 0.35-0.45 | Ct,UC | |
| qcGL2-3 | 2H | 133.1-133.61 | 4.52-5.79 | 12.84-17.26 | 0.31-0.36 | Ct,UC | |
| qcGL7-2 | 7H | 66.67 | 4.22-50.97 | 9.08-45.19 | 0.09-0.61 | Rt,UC,C | |
| qcGL7-3 | 7H | 91.38-93.97 | 4.19-13.23 | 3.74-38.03 | 0.06-0.33 | Rt,Rt + Ct,C | |
| qcGL7-5 | 7H | 116.44 | 10.26-22.32 | 21.79-37.53 | 0.27-0.40 | Rt,UC | |
| qcGL7-6 | 7H | 123.33-123.44 | 3.20-10.51 | 6.51-31.13 | 0.37-0.57 | Rt,Ct,C | |
| qcGL7-7 | 7H | 135.24-136.75 | 9.15-38.74 | 23.52-47.29 | −0.48 to 0.55 | Rt,Rt + Ct,UC | |
| qcGL7-8 | 7H | 149.42-151.09 | 6.67-24.79 | 17.10-43.21 | 0.35-0.52 | Rt,Rt + Ct,UC | |
| GW | qcGW2-1 | 2H | 52.61 | 4.08-4.26 | 15-15.51 | −0.06 | Ct,C |
| qcGW2-4 | 2H | 125.19 | 4.10-53.91 | 11.06-75.51 | 0.05-0.39 | Ct,UC,C | |
| qcGW3-4 | 3H | 30.33-31.47 | 3.26-6.31 | 2.16-23.36 | −0.09 to 0.08 | Rt,Ct,Rt + Ct,UC,C | |
| qcGW5 | 5H | 3.58-4.49 | 3.43-11.83 | 10.66-11.83 | −0.05 | Rt,Ct,Rt + Ct,C | |
| qcGW7-2 | 7H | 66.53 | 5.62-46.68 | 5.20-34.11 | −0.18 to 0.26 | Rt,UC | |
| GD | qcGD2-2 | 2H | 125.73 | 5.48-56.41 | 18.90-78.03 | 0.18-0.42 | Ct,UC |
| qcGD7-1 | 7H | 65.75 | 4.12-44.92 | 9.80-31.65 | 0.12-0.26 | Rt,UC | |
| qcGD7-2 | 7H | 71.08 | 10.99-19.79 | 14.11-34.69 | 0.09-0.18 | Rt,C | |
| qcGD7-3 | 7H | 76.14 | 3.18-17.85 | 11.11-31.06 | 0.09-0.21 | Rt,UC | |
| qcGD7-7 | 7H | 151.09 | 9.59-31.02 | 6.33-34.85 | 0.11-0.30 | Rt,UC | |
| GFRmax | qcGFRmax2-3 | 2H | 126.97 | 33.18-43.39 | 68.04-70.11 | 0.49-0.54 | Ct,UC |
| GFRmean | qcGFRmean2 | 2H | 125.19 | 35.14-56.94 | 66.66-74.83 | 0.16-0.18 | Ct,UC |
aChromosome. bThe phenotypic variance explained by each QTL. cAdditive effect. dRt, covariate QTL analysis using row type as a covariate; Ct, covariate QTL analysis using caryopsis type as a covariate; Rt + Ct, covariate QTL analysis using row type and caryopsis type as a covariate; UC, unconditional QTL analysis; C, conditional QTL analysis. Abbreviations are shown in the footnote of Table 1.
Integration of unconditional and conditional QTLs
Unconditional and conditional consensus QTLs were further integrated into unique QTLs (Details of unique QTLs were shown in Supplementary Table S5), and listed in Table 5. For the GFR, unique QTL (uqGFR2-3) was repeatedly detected at three different stages and multiple time intervals over two years. For the GA, unique QTL uqGA2-2 was stably detected at five different periods and multiple intervals across two years. For the GL, uqGL7-1 was steadily expressed in several different periods and time intervals. For the GW, unique QTL uqGW2-4 was repeatedly expressed at all sampling stages. For unconditional and conditional QTL integration, some unique QTLs were detected at multiple stages, but not detected at the final stage, these unique QTLs might play an important role in the grain filling stage.
Table 5.
Number of unique QTLs that integrated from the unconditional and conditional consensus QTLs for GFR and five grain size traits.
| Trait | No. of uniques QTL | No. of QTL detected by unconditional | No. of QTL detected by conditional | No. of QTL detected by both conditional and unconditional |
|---|---|---|---|---|
| GFR | 16 | 4 | 5 | 7 |
| GA | 23 | 12 | 9 | 2 |
| GP | 18 | 14 | 3 | 1 |
| GL | 18 | 11 | 6 | 1 |
| GW | 18 | 7 | 8 | 3 |
| GD | 25 | 16 | 6 | 3 |
QTL clusters in genome
QTL clusters were defined as a region containing multiple QTLs of various traits within approximately 20 cM53. Total of 11 QTL clusters were detected on 1H (two clusters), 2H (one cluster), 3H (three clusters), 5H (one cluster), and 7H (four clusters) (Table 6). Among these QTL clusters, three QTL clusters (C1, C6 and C8) affected all grain size traits, and the cluster C3 affected all traits. The remaining seven QTL clusters affected at least four traits and three of them were associated with three different grain size traits. The consensus QTLs associated with all traits in C1 and C6 showed negative additive effects, and the alleles that increased GFR and grain size were from Huaai11. Conversely, the alleles of consensus QTLs that increased all grain size traits in C3 were from Huadamai6. In addition, QTLs in these three QTL clusters (C2, C7 and C10) increased GFR and grain size traits alleles were from different parents.
Table 6.
The QTL clusters simultaneously affecting several traits in this study.
| Clusters | Chra | Marker interval | position (cM) | No. of QTLs | Physical position(Mb) | Traits |
|---|---|---|---|---|---|---|
| C1 | 1H | 1H_37679977 – 1_306380656 | 18.42–22.5 | 7 | 415.6–429.8 | GFR, GA, GP, GL, GW, GD |
| C2 | 1H | 1H_58042355 – 1H_22104337 | 37.35–54.5 | 8 | 301.5–373.1 | GFR, GP, GW, GD, GFRmax |
| C3 | 2H | 2HL_16222169 – M_149956_1482 | 115.5–133.57 | 18 | 622.6–668.0 | GFR, GA, GP, GL, GW, GD, GFRmax, GFRmean |
| C4 | 3H | 3HL_32425970 – 3_504106156 | 17.5–34.13 | 12 | 626.2–672.6 | GFR, GA, GL, GW, GD |
| C5 | 3H | 6HL_29119759 – 3HL_24669457 | 39.93–57.5 | 5 | 585.4–622.4 | GA, GP, GL, GW |
| C6 | 3H | 3_411835196 – 3_428733082 | 82.5–98.5 | 6 | 438.1–512.9 | GA, GP, GL, GW, GD |
| C7 | 5H | 5HS_7374618 – 5_6313908 | 0–11.5 | 5 | 0.4–8.1 | GFR, GA, GW, GD |
| C8 | 7H | 7_552887354 – M_363857_407 | 56.5–70 | 15 | 543.7–585.3 | GFR, GA, GP, GL, GW, GD, GFRmax |
| C9 | 7H | 7_428184911 – 7_270366894 | 92.14–110.5 | 8 | 262.1–482.4 | GA, GP, GL, GD, GFRmean |
| C10 | 7H | 7HL_22161891 – 7HL_28498244 | 120.5–140.5 | 9 | 248.8–423.1 | GFR, GP, GL, GW |
| C11 | 7H | 7HL_6335336 – M_1645585_244 | 150.5–153.5 | 5 | 227.8–345.7 | GA, GP, GL, GD |
aChromosome. Abbreviations are shown in the footnote of Table 1.
Discussion
Many studies have reported that genetic difference in grain yield was related to difference in GFR in barley7,9,10. In addition, GFRmean and GFRmax have been reported in maize and wheat as important factors regulating grain weight42,54. The GFRmean and GFRmax were significantly associated with TGW (r > 0.9) in our experiments (Fig. 2), indicating that GFRmean and GFRmax also promoted grain weight in barley.
Grain development is a dynamic process that is regulated by three physiological stages: (1) grain formation period, mainly the division of endosperm cells and the formation of basic structure of seeds, during which there is almost no accumulation of dry matter; (2) the linear growth period of dry matter, the accumulation of dry matter in the most vigorous period of the grain, the grain weight during the period almost increased linearly; (3) maturity, the grain weight increased slowly during this period18–21,55. The substance accumulation mainly occurs in the linear growth period of dry matter. In this study, seven sampling from grain formation period to maturity were used to evaluate GFR. The GFR at stage III, IV and V were significantly positively correlated with TGW (r > 0.7), while, there was no correlation between the initial and final stages of GFR and TGW (Fig. 2). This results were basically consistent with the previous studies on the grain weight of linear dry matter accumulation period20.
Grain size can be divided into components such as GA, GP, GL, GW and GD56,57. In barley, previous studies on grain shape were mainly at maturity, and only major QTLs controlling GL traits were found57–60. However, QTLs detected at maturity may not observe their genetic effects during specific periods of crop development, and dynamic QTL analysis can better understand the developmental behavior of quantitative traits. We found that some of the QTLs for GFR and grain size traits identified on the 2H and 7H chromosomes co-localized with QTLs for yield-related traits, seedling traits and dwarf gene btwd1 detected in previous studies. For example, certain major consensus QTLs tightly linked to 2_527241334 at 126 cM for grain filling rate and grain size detected here, are likely the same to seedling traits QTL qSH2-191 and qSFW2-191 identified by Wang et al.61 and also likely same to the qSms2-7 and qTgw2-1 for yield-related traits reported by Wang et al.29. In addition, a major QTL ucqGL7-5 for grain length closely linked to the Bmac31 of 151 cM identified on 7H chromosome, which may be in the same locus as the dwarfing gene btwd1 reported by Ren et al.43. The findings indicated that GFR and grain size traits are closely related to yield and yield-related traits.
In previous studies, QTLs for grain area were detected on chromosomes 1H, 2H, 3H, 4H, 5H and 6H57,62,63, and QTLs associated with grain perimeter were identified on chromosomes 1H and 3H57. QTLs associated with grain length and width were previously detected on all seven chromosomes57–60,62–67, and QTLs for grain diameter were detected on chromosomes 2H, 3H, 4H, 6H, and 7H67. Ayoub et al.57 and Sharma et al.62 reported a major QTL on chromosome 2H, nearby the locus vrs1 and affected all grain size traits. Most of QTLs for grain sizes identified on 2H in this study were also distributed near the morphological marker vrs1, which are likely same to the QTL reported by Ayoub et al.57. Walker et al.66 detected two QTLs for grain length on 3H, located near markers 2_0662 and 2_1272, respectively. The physical location of the two genetic markers was queried using the Barleymap website (http://floresta.eead.csic.es/barleymap/), we found that the marker 3HL_42780152 in this experiment was close to the physical position of 2_1272, indicating that the QTL cqGL3 adjacent to 3HL_42780152 is likely the same locus reported by Walker et al.66. Many new QTLs controlling grain size were detected in our experiment, in which some major QTLs (ucqGA7-1, ucqGP7-2, cqGP7-2, ucqGL7-1 and ucqGD7-1) located near 65 cM of the 7H chromosome were repeatedly detected at different stages or at the same stage of different environments, indicating that these regions might be an important novel locus affecting grain size traits.
Dynamic QTL mapping identified 196 unconditional QTLs and 95 conditional QTLs (Supplementary Table S2, Table S3). Most QTLs were detected at V, VI and VII stages (Fig. 4). By integrating the unconditional QTL for GFR and grain size traits, some unconditional consensus QTLs were detected simultaneously at several stages, and the majority of the QTLs were identified at stages IV-VII. These results indicated that QTLs associated with grain size traits are selectively expressed during grain filling period. Additionally, we found that some unconditional and conditional consensus QTLs, such as ucqGW3-2, cqGFR2-2, and cqGW3-1, had a combination of identified QTLs with opposite additive effects, indicating that certain QTLs had different expression patterns at different developmental stages or environments. This expression pattern has also been reported for plant height in rapeseed68 and for grain filling rate in corn69.
In this study, 11 clustered QTL regions controlling grain filling rate and grain size traits were found on chromosomes 1H, 2H, 3H, 5H and 7H (Fig. 3, Table 6), and these co-localized QTLs were mainly concentrated on the chromosome 2H, 3H and 7H. Anchoring the SNP markers located in important QTL regions to the Morex genome via the barleymap website (http://floresta.eead.csic.es/barleymap/) identified eight related candidate genes70, of which, five associated with plant height and grain size, and three genes were involved in the biosynthesis and metabolism of starch and (1,3;1,4)-β-glucan. Notably, the most important QTL cluster region at the 2HL_16222169 - M_149956_1482 was located on chromosome 2H, containing 18 QTLs controlling all grain size and grain filling rate traits (Fig. 3, Table 6). The vrs1 gene was detected in this region, which was reported in previous studies to control row-number phenotype, affecting grain size and thousand grain weight traits29,57,61,62. The Nud gene controlling the hulled/naked caryopsis was detected within QTL cluster affecting GFR, GFRmax, GA, GP, GL, GW and GD in interval 56.5–70 cM on chromosome 7H, and QTLs affected TGW were located at this locus71. Since these two genes have large effects on the population used here, some of the minor-effect QTLs may be affected and difficult to detect. Therefore, to eliminate the interference of these two genes, we performed a covariate QTL analysis to find more new QTLs. Through covariate QTL analysis, we found that 171 (55%) of the new QTLs were undetected by either unconditional or conditional QTL mapping methods (Supplementary Table S4). The effects of these QTLs on grain filling rate and grain size traits are not as obvious as the vrs1 and Nud genes, but they had an important role underlying these traits. Gibberellin 20-oxidase gene (Hv20ox2) was found in a QTL cluster for GFR, GA, GL, GW and GD between 3HL_32425970 and 3_504106156, located in the interval of 17.5–34.13 cM on chromosome 3H, which was a functional gene regulating barley sdw1/denso, and certain QTLs for yield, grain size and plumpness were co-localized with this gene72,73. Within another QTL cluster for GA, GP, GL and GW between 6HL_29119759 and 3 HL_24669457 on chromosome 3H, the flowering time gene HvFT1 was detected, which was the dominant plant transition from vegetative state to reproductive state, affecting the flowering and maturity of barley74,75. Furthermore, within the QTL cluster associated with GA, GP, GL and GD in the interval of 150.5–153.5 cM on chromosome 7 H, the novel dwarf gene btwd1 was identified, which not only affect plant height, but affect grain yield at the btwd1 locus29,43.
The content of starch in barley is 62% to 77% of the grain dry weight, and the grain filling process is mainly the accumulation process of starch. The biosynthesis of starch mainly involves ADP glucose pyrophosphorylase, starch synthase, starch branching enzyme and starch debranching enzyme76–78. Interestingly, two genes related to starch biosynthesis and metabolism, as well as a gene involved in (1,3;1,4)-β-glucan synthesis, were found in three QTL clusters in this study. The GBSS1b gene, the key gene involved in amylose synthesis, was detected in the C3 region of chromosome 2H, which was close to the 2HL_22930294 marker. The ucqGFR2-3 closely linked to the marker 2HL_22930294 was detected simultaneously at stage II over two years. The GBSS1b transcripts were abundant in the pericarp of flowering and initial grouting79, so we considered this to be a candidate gene for the QTL locus. The amy2 gene, which was the most important gene for starch degradation during malting and saccharification80, was located in the C8 region of chromosome 7H, close to the 7HL_37199773 marker at 65 cM. Previous studies have reported QTLs underlying malt activity and amylopectin content at the amy2 locus81. The HvCslF6, a key gene regulating (1,3;1,4)-β-D-glucans biosynthesis, was found in the C9 region of chromosome 7H82. This gene was expressed low in the early stage of grain development and then rapidly up-regulated as the activity of synthetase increased83.
In summary, we identified 90, 55 and 311 consensus QTLs using unconditional, conditional and covariate QTL mapping methods, respectively, and detected 34 main-effect QTLs that were simultaneously expressed at multiple stages. The results indicated that these major QTLs were not only expressed at maturity but were also in the early stages of grain development. In addition, eight predicted candidate genes involved in grain yield and starch synthesis pathways were identified in the six clustered QTL regions, which might play an important role in controlling GFR and grain size traits. These findings enhance our understanding of the genetic mechanism of barley grain filling process.
Supplementary information
Mapping dynamic QTL dissects the genetic architecture of grain size and grain filling rate at different grain-filling stages in barley
Acknowledgements
This project was supported by the earmarked fund for China Agriculture Research System (CARS-5).
Author contributions
Conceived and designed the experiments: D.S., G.S. Performed the experiments: B.D., Q.W., Y.C., Y.W., S.G. Analyzed the data: B.D., X.R. Contributed reagents/materials/analysis tools: X.R., D.S., G.S. Wrote the paper: B.D., D.S., G.S., C.L. produced the Huaai 11 and Huadamai 6 D.H. population. All authors have read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53620-5.
References
- 1.Paynter, B. H. & Young, K. J. Environment influences rate of grain filling in barley. ‘Proceedings of the 8th International Barley Genetics Symposium’. Adelaide, S Aust. Vol. 3. (Ed. S Logue) pp. 279–281. (Department of Plant Sciences, Adelaide University: Adelaide, S. Aust.) (2000).
- 2.Darroch BA, Baker RJ. Grain filling in three spring wheat genotypes: statistical analysis. Crop Sci. 1990;30:525–529. doi: 10.2135/cropsci1990.0011183X003000030009x. [DOI] [Google Scholar]
- 3.Riggs TJ, Gothard PG. The development of barley grain: comparisons between cultivars for growth rate and α-amylase activity. J Agric Sci. 1976;86:603–608. doi: 10.1017/S0021859600061165. [DOI] [Google Scholar]
- 4.Rasmusson DC, Mclean I, Tew TL. Vegetative and Grain-Filling Periods of Growth in Barley. Crop Sci. 1979;19:5–9. doi: 10.2135/cropsci1979.0011183X001900010002x. [DOI] [Google Scholar]
- 5.Scott WR, Appleyard M, Fellowes G, Ejm K. Effect of genotype and position in the ear on carpel and grain growth and mature grain weight of spring barley. J Agric Sci. 1983;100:383–391. doi: 10.1017/S0021859600033530. [DOI] [Google Scholar]
- 6.Ho KM, Jui PY. Duration and Rate of Kernel Filling in Barley (Hordeum vulgare L.) Cereal Res Commmu. 1989;17:69–76. [Google Scholar]
- 7.Le G, Lille PCD. Grain filling and shoot growth of 2-row and 6-row winter barley varieties. Agronomie. 1993;13:545–552. doi: 10.1051/agro:19930612. [DOI] [Google Scholar]
- 8.Aksel R, Johnson LPV. Genetic studies in sowing-to-heading and heading-to-ripening periods in barley and their relation to yield and yield components. Can J Genet Cytol. 2011;3:242–259. doi: 10.1139/g61-028. [DOI] [Google Scholar]
- 9.Metzger DD, Czaplewski SJ, Rasmusson DC. Grain-Filling Duration and Yield in Spring Barley1. Crop Sci. 1984;24:1101–1105. doi: 10.2135/cropsci1984.0011183X002400060022x. [DOI] [Google Scholar]
- 10.Mou B, Kronstad WE, Saulescu NN. Grain Filling Parameters and Protein Content in Selected Winter Wheat Populations: II. Associations. Crop Sci. 1994;34:838–841. doi: 10.2135/cropsci1994.0011183X003400040004x. [DOI] [Google Scholar]
- 11.Wang G, Kang MS, Moreno O. Genetic analyses of grain-filling rate and duration in maize. Field Crops Res. 1999;61:211–222. doi: 10.1016/S0378-4290(98)00163-4. [DOI] [Google Scholar]
- 12.Van Sanford DA. Variation in Kernel Growth Characters Among Soft Red Winter Wheats. Crop Sci. 1985;25:626–630. doi: 10.2135/cropsci1985.0011183X002500040012x. [DOI] [Google Scholar]
- 13.Bruckner PL, Frohberg RC. Rate and Duration of Grain Fill in Spring Wheat. Crop Sci. 1987;27:451–455. doi: 10.2135/cropsci1987.0011183X002700030005x. [DOI] [Google Scholar]
- 14.Sofield I, Evans LT, Cook MG, Wardlaw IF. Factors Influencing the Rate and Duration of Grain Filling in Wheat. Funct Plant Biol. 1977;4:785–797. doi: 10.1071/PP9770785. [DOI] [Google Scholar]
- 15.Wiegand CL, Cuellar JA. Duration of grain filling and kernel weight of wheat as affected by temperature. Crop Sci. 1980;21:95–101. doi: 10.2135/cropsci1981.0011183X001100010027x. [DOI] [Google Scholar]
- 16.Knott DR, Gebeyehou G. Relationship between the lengths of the vegetative and grain filling periods and the agronomic characters in three durum wheat crosses. Crop Sci. 1987;27:857–860. doi: 10.2135/cropsci1987.0011183X002700050003x. [DOI] [Google Scholar]
- 17.Hunt LA, Poorten G, Pararajasingham S. Postanthesis temperature effects on duration and rate of grain filling in some winter and spring wheats. Can. J. Plant Sci. 1991;71:609–617. doi: 10.4141/cjps91-092. [DOI] [Google Scholar]
- 18.Bewley, J. D. & Black, M. Seeds: Physiology of development and germination, Plenum Press, New York, London (1994).
- 19.Brocklehurst PA. Factors controlling grain weight in wheat. Nature. 1977;24:348–349. doi: 10.1038/266348a0. [DOI] [Google Scholar]
- 20.Gupta PK, Rustgi S, Kumar N. Genetic and molecular basis of grain size and grain number and its relevance to grain productivity in higher plants. Genome. 2006;49:565–571. doi: 10.1139/g06-063. [DOI] [PubMed] [Google Scholar]
- 21.Borrás L, Westgate ME. Predicting maize kernel sink capacity early in development. Field Crops Res. 2006;95:223–233. doi: 10.1016/j.fcr.2005.03.001. [DOI] [Google Scholar]
- 22.Seka D, Cross HZ. Xenia and Maternal Effects on Maize Kernel Development. Crop Sci. 1995;35:80–85. doi: 10.2135/cropsci1995.0011183X003500010014x. [DOI] [Google Scholar]
- 23.Walpole PR, Morgan DG. A Quantitative Study of Grain Filling in Three Cultivars of Hordeum Vulgare L. Ann Bot. 1971;35:301–310. doi: 10.1093/oxfordjournals.aob.a084479. [DOI] [Google Scholar]
- 24.Walpole PR, Morgan DG. Physiology of Grain Filling in Barley. Nature. 1972;240:416–417. doi: 10.1038/240416a0. [DOI] [Google Scholar]
- 25.Holmes DP. Physiology of Grain Filling in Barley. Nature. 1974;247:297–298. doi: 10.1038/247297a0. [DOI] [Google Scholar]
- 26.Hori K, Kobayashi T, Sato K, Takeda K. QTL analysis of Fusarium head blight resistance using a high-density linkage map in barley. Theor. Appl. Genet. 2005;111:1661–1672. doi: 10.1007/s00122-005-0102-4. [DOI] [PubMed] [Google Scholar]
- 27.Li JZ, Huang XQ, Heinrichs F, Ganal MW, Röder MS. Analysis of QTLs for yield components, agronomic traits, and disease resistance in an advanced backcross population of spring barley. Genome. 2006;49:454–466. doi: 10.1139/g05-128. [DOI] [PubMed] [Google Scholar]
- 28.Baghizadeh A, Taleei AR, Naghavi MR. QTL Analysis for Some Agronomic Traits in Barley (Hordeum vulgare L.) Int. J. Agric. Biol. 2007;9:372–374. [Google Scholar]
- 29.Wang JB, et al. QTL underlying some agronomic traits in barley detected by SNP markers. BMC Genet. 2016;17:103. doi: 10.1186/s12863-016-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vafadar SF, Jamali SH, Sadeghzadeh B, Abdollahi MB. Genetic mapping of quantitative trait loci for yield-affecting traits in a barley doubled haploid population derived from clipper × sahara 3771. Front Plant Sci. 2017;8:1–9. doi: 10.3389/fpls.2017.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu YB, Shen ZT. Diallel analysis of tiller number at different growth stages in rice (Oryza sativa L.) Theor. Appl. Genet. 1991;83:243–249. doi: 10.1007/BF00226258. [DOI] [PubMed] [Google Scholar]
- 32.Xu YB. Quantitative trait loci: separating, pyramiding, and cloning. Plant Breed Rev. 1997;15:85–139. [Google Scholar]
- 33.Zhu J. Analysis of conditional genetic effects and variance components in developmental genetics. Genetics. 1995;141:1633–1639. doi: 10.1093/genetics/141.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu YY, et al. Mapping QTLs of root morphological traits at different growth stages in rice. Genetica. 2008;133:187–200. doi: 10.1007/s10709-007-9199-5. [DOI] [PubMed] [Google Scholar]
- 35.Zuo HL, et al. Molecular Detection of Quantitative Trait Loci for Leaf Chlorophyll Content at Different Growth-Stages of Rice (Oryza sativa L.) Asian J Plant Sci. 2007;6:518–522. doi: 10.3923/ajps.2007.518.522. [DOI] [Google Scholar]
- 36.Wang ZH, et al. QTL mapping for developmental behavior of plant height in wheat (Triticum aestivum L.) Euphytica. 2010;174:447–458. doi: 10.1007/s10681-010-0166-3. [DOI] [Google Scholar]
- 37.Wu XS, Wang ZH, Chang XP, Jing RL. Genetic dissection of the developmental behaviours of plant height in wheat under diverse water regimes. J Exp Bot. 2010;61:2923–2927. doi: 10.1093/jxb/erq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu ZL, et al. Dynamic QTL mapping of wheat protein content in developing grains. Scientia Agricultura Sinica. 2011;44:3078–3085. [Google Scholar]
- 39.Han YP, et al. Dynamic QTL analysis of dry matter accumulation in soybean seed at different developmental stages. Scientia Agricultura Sinica. 2010;43:1328–1338. [Google Scholar]
- 40.Cui K, et al. Molecular dissection of the genetic relationships of source, sink and transport tissue with yield traits in rice. Theor. Appl. Genet. 2003;106:649–658. doi: 10.1007/s00122-002-1113-z. [DOI] [PubMed] [Google Scholar]
- 41.Ren XF, et al. Inheritance and identification of molecular markers associated with a novel dwarfing gene in barley. BMC Genet. 2010;11:89. doi: 10.1186/1471-2156-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang RX, et al. QTL mapping for grain filling rate and yield-related traits in RILs of the Chinese winter wheat population Heshangmai x Yu8679. Theor. Appl. Genet. 2009;118:313–325. doi: 10.1007/s00122-008-0901-5. [DOI] [PubMed] [Google Scholar]
- 43.Ren XF, et al. SNP-based high density genetic map and mapping of btwd1 dwarfing gene in barley. Sci. Rep. 2016;6:31741. doi: 10.1038/srep31741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li HH, Ye GY, Wang JK. A modified algorithm for the improvement of composite interval mapping. Genetics. 2007;175:361–374. doi: 10.1534/genetics.106.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Li HH, Zhang LY, Wang JK. QTL IciMapping:Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. The Crop J. 2015;3:269–283. doi: 10.1016/j.cj.2015.04.004. [DOI] [Google Scholar]
- 46.Wang SB, et al. Mapping small-effect and linked quantitative trait loci for complex traits in backcross or DH populations via a multi-locus GWAS methodology. Sci. Rep. 2016;6:29951. doi: 10.1038/srep29951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goffinet B, Gerber S. Quantitative trait loci: a meta-analysis. Genetics. 2000;155:463–473. doi: 10.1093/genetics/155.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arcade A, et al. BioMercator: integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics. 2004;20:2324–2326. doi: 10.1093/bioinformatics/bth230. [DOI] [PubMed] [Google Scholar]
- 49.Wang XD, et al. Identification of QTLs Associated with Oil Content in a High-Oil Brassica napus Cultivar and Construction of a High-Density Consensus Map for QTLs Comparison in B. napus. PloS one. 2013;8:e80569. doi: 10.1371/journal.pone.0080569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCouch SR. Gene nomenclature system for rice. Rice. 2008;1:72–84. doi: 10.1007/s12284-008-9004-9. [DOI] [Google Scholar]
- 51.Shi JQ, et al. Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics. 2009;182:851–861. doi: 10.1534/genetics.109.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voorrips R. MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 53.Said JI, et al. A comparative meta-analysis of QTL between intraspecific Gossypium hirsutum and interspecific G. hirsutum x G. barbadense populations. Mol. Genet. Genomics. 2015;290:1003–1025. doi: 10.1007/s00438-014-0963-9. [DOI] [PubMed] [Google Scholar]
- 54.Huang ZH, et al. Study on Characteristic of Grain-Filling of Super High-yield Maize. Acta Agric Boreali Occidentalis Sin. 2007;16:14–18. [Google Scholar]
- 55.Perez CM, Perdon AA, Resurreccion AP, Villareal RM, Juliano BO. Enzymes of carbohydrate metabolism in the developing rice grain. Plant Physiol. 1975;56:579–583. doi: 10.1104/pp.56.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell KG, et al. Quantitative Trait Loci Associated with Kernel Traits in a Soft × Hard Wheat Cross. Crop Sci. 1999;39:1275–1285. doi: 10.2135/cropsci1999.0011183X003900040039x. [DOI] [Google Scholar]
- 57.Ayoub M, Symons S, Edney M, Mather D. QTLs affecting kernel size and shape in a two-rowed by six-rowed barley cross. Theor. Appl. Genet. 2002;105:237–247. doi: 10.1007/s00122-002-0941-1. [DOI] [PubMed] [Google Scholar]
- 58.Backes G, et al. Localization of quantitative trait loci (QTL) for agronomic important characters by the use of a RFLP map in barley (Hordeum vulgare L.) Theor. Appl. Genet. 1995;90:294–302. doi: 10.1007/BF00222217. [DOI] [PubMed] [Google Scholar]
- 59.Zhou H, et al. Mapping and validation of major quantitative trait loci for kernel length in wild barley (Hordeum vulgare ssp.spontaneum) BMC Genet. 2016;17:130. doi: 10.1186/s12863-016-0438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watt, C., Zhou, G. F., McFawn, L., Chalmers, K. J. & Li, C. D. Fine mapping of qGL5H, a major grain length locus in barley (Hordeum vulgare L.). Theor. Appl. Genet, 1–11 (2018). [DOI] [PubMed]
- 61.Wang QF, et al. Detection of QTLs for seedling characteristics in barley (Hordeum vulgare L.) grown under hydroponic culture condition. BMC Genet. 2017;18:94. doi: 10.1186/s12863-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma R, et al. Genome-wide association of yield traits in a nested association mapping population of barley reveals new gene diversity for future breeding. J Exp Bot. 2018;69:3811–3822. doi: 10.1093/jxb/ery178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X, et al. Genome-Wide Association Analysis of Grain Yield-Associated Traits in a Pan-European Barley Cultivar Collection. Plant. Genome. 2018;11:170073. doi: 10.3835/plantgenome2017.08.0073. [DOI] [PubMed] [Google Scholar]
- 64.Coventry SJ, Barr AR, Eglinton JK, Mcdonald GK. The determinants and genome locations influencing grain weight and size in barley (Hordeum vulgare L.) Aust J Agric Res. 2003;54:1103–1115. doi: 10.1071/AR02194. [DOI] [Google Scholar]
- 65.Kalladan R, et al. Identification of quantitative trait loci contributing to yield and seed quality parameters under terminal drought in barley advanced backcross lines. Mol Breed. 2013;32:71–90. doi: 10.1007/s11032-013-9853-9. [DOI] [Google Scholar]
- 66.Walker CK, Ford R, Munoz-Amatriain M, Panozzo JF. The detection of QTLs in barley associated with endosperm hardness, grain density, grain size and malting quality using rapid phenotyping tools. Theor. Appl. Genet. 2013;126:2533–2551. doi: 10.1007/s00122-013-2153-2. [DOI] [PubMed] [Google Scholar]
- 67.Cu ST, et al. Genetic analysis of grain and malt quality in an elite barley population. Mol Breed. 2016;36:129. doi: 10.1007/s11032-016-0554-z. [DOI] [Google Scholar]
- 68.Wang XD, et al. Dynamic and comparative QTL analysis for plant height in different developmental stages of Brassica napus L. Theor. Appl. Genet. 2015;128:1175–1192. doi: 10.1007/s00122-015-2498-9. [DOI] [PubMed] [Google Scholar]
- 69.Zhang ZH, et al. Genetic analysis of grain filling rate using conditional QTL mapping in maize. PloS one. 2013;8:e56344. doi: 10.1371/journal.pone.0056344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mascher M, et al. A chromosome conformation capture ordered sequence of the barley genome. Nature. 2017;544:427–433. doi: 10.1038/nature22043. [DOI] [PubMed] [Google Scholar]
- 71.Gong X, Wheeler R, Bovill WD, McDonald GK. QTL mapping of grain yield and phosphorus efficiency in barley in a Mediterranean-like environment. Theor. Appl. Genet. 2016;129:1657–1672. doi: 10.1007/s00122-016-2729-8. [DOI] [PubMed] [Google Scholar]
- 72.Cuesta-Marcos A, et al. Yield QTL affected by heading date in Mediterranean grown barley. Plant Breed. 2009;128:46–53. doi: 10.1111/j.1439-0523.2008.01510.x. [DOI] [Google Scholar]
- 73.Jia QJ, et al. Expression level of a gibberellin 20-oxidase gene is associated with multiple agronomic and quality traits in barley. Theor. Appl. Genet. 2011;122:1451–1460. doi: 10.1007/s00122-011-1544-5. [DOI] [PubMed] [Google Scholar]
- 74.Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol. 2008;147:355–366. doi: 10.1104/pp.108.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nitcher R, et al. Increased copy number at the HvFT1 locus is associated with accelerated;flowering time in barley. Mol Genet Genomics. 2013;288:261–275. doi: 10.1007/s00438-013-0746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ball SG, Morell MK. From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol. 2003;54:207–233. doi: 10.1146/annurev.arplant.54.031902.134927. [DOI] [PubMed] [Google Scholar]
- 77.James MG, Denyer K, Myers AM. Starch synthesis in the cereal endosperm. Curr Opin Plant Biol. 2003;6:215–222. doi: 10.1016/S1369-5266(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 78.Zhao BH, Zhang WJ, Wang ZQ, Zhu QS, Yang JC. Changes in Activities of the Key Enzymes Related to Starch Synthesis in Rice Grains During Grain Filling and Their Relationships with the Filling Rate and Cooking Quality. Agri Sci China. 2005;4:26–33. [Google Scholar]
- 79.Radchuk VV, et al. Spatiotemporal Profiling of Starch Biosynthesis and Degradation in the Developing Barley Grain. Plant Physiol. 2009;150:190–204. doi: 10.1104/pp.108.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang QS, Li CD. Comparisons of Copy Number, Genomic Structure, and Conserved Motifs for α-Amylase Genes from Barley, Rice, and Wheat. Front Plant Sci. 2017;8:1727. doi: 10.3389/fpls.2017.01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shu XL, Rasmussen SK. Quantification of amylose, amylopectin, and beta-glucan in search for genes controlling the three major quality traits in barley by genome-wide association studies. Front Plant Sci. 2014;5:197. doi: 10.3389/fpls.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tonooka T, Aoki E, Yoshioka T, Taketa S. A novel mutant gene for (1-3, 1-4)-β-D-glucanless grain on barley (Hordeum vulgare L.) chromosome 7H. Breed Sci. 2009;59:47–54. doi: 10.1270/jsbbs.59.47. [DOI] [Google Scholar]
- 83.Taketa S, et al. Functional characterization of barley betaglucanless mutants demonstrates a unique role for CslF6 in (1,3;1,4)-β-D-glucan biosynthesis. J Exp Bot. 2012;63:381–392. doi: 10.1093/jxb/err285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapping dynamic QTL dissects the genetic architecture of grain size and grain filling rate at different grain-filling stages in barley