Abstract
Infection is the leading cause of non-relapse-related mortality after allogeneic haematopoietic stem cell transplantation (HSCT). Altered functions of immune cells in nasal secretions may influence post HSCT susceptibility to viral respiratory infections. In this prospective study, we determined T and NK cell numbers together with NK activation status in nasopharyngeal aspirates (NPA) in HSCT recipients and healthy controls using multiparametric flow cytometry. We also determined by polymerase chain reaction (PCR) the presence of 16 respiratory viruses. Samples were collected pre-HSCT, at day 0, +10, +20 and +30 after HSCT. Peripheral blood (PB) was also analyzed to determine T and NK cell numbers. A total of 27 pediatric HSCT recipients were enrolled and 16 of them had at least one viral detection (60%). Rhinovirus was the most frequent pathogen (84% of positive NPAs). NPAs of patients contained fewer T and NK cells compared to healthy controls (p = 0.0132 and p = 0.120, respectively). Viral PCR + patients showed higher NK cell number in their NPAs. The activating receptors repertoire expressed by NK cells was also higher in NPA samples, especially NKp44 and NKp46. Our study supports NK cells relevance for the immune defense against respiratory viruses in HSCT recipients.
Subject terms: Viral infection, Paediatric cancer
Introduction
Allogeneic haematopoietic stem cell transplant (HSCT) is a potentially curative procedure for a variety of pediatric hematologic disorders. Post-transplant infections contribute significantly to the morbidity associated with this procedure. The ablation of HSCT recipient’s immune system by the conditioning regimen increases the susceptibility to infections until they reach immune reconstitution from donor stem cells. The morbidity and mortality from respiratory viruses in immunocompromised patients, including those undergoing HSCT, is well established for respiratory syncytial virus (RSV)1–4, parainfluenza viruses (PIV)5,6, influenza viruses7,8, adenovirus9, and human metapneumovirus (HMPV)4,10. Recent data suggest that common human coronaviruses (HCoV) are also associated with significant mortality, similar to that seen with RSV, PIV and influenza11. There is less information regarding infections caused by human rhinoviruses (HRV) and human bocavirus (HBoV)12,13.
Natural Killer (NK) cells in the nasal secretions are a first line of defense against respiratory viruses both in healthy individuals and in those undergoing HSCT. They have a protective role against many viral infections commonly encountered after HSCT, including herpes simplex virus, varicella-zoster virus, cytomegalovirus and influenza virus as well as some bacterial infections14–16. NK cells play important roles in the host’s immune defense through the production of cytotoxic granules containing molecules such as perforins and granzymes that lyse infected cells. Other important features of NK cells are the production of cytokines including interferons, their enhancement of local immune responses by directly acting on target cells, and their role in the recruitment and activation of other immune cells, including T cells and macrophages17.
NK cells recognize virally infected cells through the integration of signaling from activating and inhibitory germ line encoded receptors on the NK cell surface17. Membrane bound Killer-cell Activation Receptors (KARs) work together with inhibitory Killer Immunoglobulin-like Receptors (KIRs), in order to regulate the NK cells functions on infected or transformed cells. The interaction between the KIR family on NK cells and HLA Class I molecules results in inhibitory signals. KARs, like the natural cytotoxicity receptors (NCRs), comprising NKp30, NKp46, and NKp44 are key receptors in the recognition and elimination of virally infected and tumor cells18,19.
Because immune cells in the nasal mucosa can control and regulate viral infections via killing infected respiratory epithelial cells, low numbers of NK and or T cells in the nasopharyngeal aspirates (NPAs) of HSCT pediatric recipients could be associated with an increased susceptibility to viral respiratory infections. Although blood immune reconstitution after an HSCT has been widely studied20–26, very little is known about the immune reconstitution in HSCT recipients’ mucosa. In the present study, our goals were: 1) to determine NK and T cell numbers in the nasal passages of HSCT recipients; 2) to determine the activation phenotype of NPAs’ NK cells using multiparametric flow cytometry; 3) to determine the presence and functional profile of these cells within the context of a viral infection; and 4) to assess the effects of viral infections on nasal immune reconstitution.
Materials and Methods
Study participants
Prospective study including patients under 18 years of age who received allogenic HSCT at La Paz University Hospital (Madrid, Spain), from January 2017 to June 2018. Exclusion criteria included chronic lung disease prior to HSCT. HSCT was performed according to current centre protocols, using bone marrow or peripheral blood stem cells as haematopoietic stem cell source. Acute and chronic graft versus host disease (GVHD) were defined and graded as previously reported27,28.
Age- and sex-matched controls were recruited among healthy patients undergoing elective surgery. Cases and controls were also matched according to procedure date (HSCT and elective surgery, respectively) ± 1 week.
All research was performed in accordance to local regulations. Informed consent in accordance with the Declaration of Helsinki was obtained from the parents of all children. The study was approved by the Clinical Research Ethics Committee at La Paz University Hospital.
Study samples
Study samples were peripheral blood (PB) and NPAs that were collected prior to the conditioning regimen and after HSCT at days 15 and 30 for PB mononuclear cells and before the conditioning regimen and at days 0, 10, 20 and 30 after HSCT for NPAs. The following tests were performed in each sample type:
PB: total, T and NK cell counts.
NPAs: total T and NK cell counts, natural cytotoxicity receptors, viral detection.
For NPA collection, 1 mL of 0.9% saline solution was instilled into each nostril and the nasopharynx was suctioned using a sterile catheter with a mucus trap. NPAs were not performed if the patient had active bleeding or his platelet count was below 20.000/mm3. NPA samples containing blood were considered not valid and discarded. Each specimen was sent to the Respiratory Virus and Influenza Unit at the National Microbiology Center (ISCIII, Madrid, Spain) for viral analysis and to the Translational Research in Paediatric Oncology, Hematopoietic Transplantation & Cell Therapy Unit at La Paz Hospital Research Institute (Madrid, Spain) for flow cytometry assays. NPAs were processed within 24 hours after collection. In the control group, NPAs were collected on the day of surgery.
Viral detection by RT-PCR
Upon reception of the samples, three aliquots were prepared and stored at −80 °C. Both the reception and the NPA sample processing areas are separated from those defined as working areas. Three independent RT-PCR assays were performed to detect sixteen respiratory viruses as previously published by our group29–31. Influenza A, B and C viruses were detected by using previously described primer sets only to amplify influenza viruses in a multiplex PCR assay29. A second multiplex PCR was used to detect parainfluenza viruses 1 to 4, human coronaviruses 229E and OC43, enteroviruses and HRV30. Presence of RSV A and B types, HMPV, HBoV and adenoviruses was established using a third multiplex RT-nested PCR−BRQ method31.
Cell characterization by flow cytometry
For flow cytometry assays, NPA samples were filtered with a 70 µm cell strainer (Falcon), centrifuged at 1400 rpm (5 min) and suspended in 100 µl of PBS. Total sample volume was acquired using a Navios flow cytometer (Beckman Coulter).
The total cell count per sample was determined. T cell (CD45+, CD3+ CD56−) and Natural killer (NK) cell (CD45+, CD56+, CD3−) populations were identified using the following labelled antibodies: CD45-FITC/APCCy7 (clone 2D1, BD Pharmingen), CD56-APC (clone NCAM16.2, BD Pharmingen) and CD3-PECy7 (clone UCHT1, Biolegend). Fluorescent cell viability dye 7-AAD (BD Pharmingen) was used to exclude dead cells. FlowJo v10.0.7 software (TreeStar) was used for data analysis.
The expression of natural cytotoxicity receptors (NCRs), NKG2A and NKG2D receptors on NK cells subpopulation in NPA was evaluated with the following fluorochrome-conjugated antibodies: NKG2D CD314-PE (clone BAT221, MiltenyiBiotec); NKp46 CD335-FITC (clone 9E2, AbDSerotec); NKp44 CD-336-PE (clone 44, BD Pharmingen); NKp30 CD337-PE (clone REA823, MiltenyiBiotec); NKG2A-PE (clone 131411, R&D systems; T1, Biolegend).
Statistical analysis
Values are expressed as percentages for discrete variables and as median and interquartile range (IQR) for continuous variables. Comparisons between groups (controls and HSCT patients before conditioning, and patients with and without viral respiratory infections) were made using Mann-Whitney U test, and Fisher’s exact test) as appropriate. A value of P < 0.05 was considered statistically significant. All analyses were performed using the SPSS statistical software, version 21.0 (IBM Corp.).
Results
HSCT recipients and control group characteristics
During the study period, 27 HSCT recipients were recruited. Their main characteristics are summarized in Table 1. The median age of patients was 7.7 years (IQR 9.2).
Table 1.
Characteristics of haematopoietic stem cell transplantation recipients (n = 27).
| Age (years) | 6 (9.8) |
| Female gender | 14 (52) |
| Indication for HSCT | |
| PID | 9 (33) |
| SAA | 5 (19) |
| ALL | 5 (19) |
| AML | 2 (7) |
| MDS | 3 (11) |
| Other | 3 (11) |
| Donor type | |
| MRD | 4 (15) |
| MMRD | 8 (30) |
| MUD | 12 (44) |
| MMUD | 3 (11) |
| Graft source | |
| Bone marrow | 14 (52) |
| Mobilized peripheral blood | 13 (48) |
| Conditioning regimen | |
| Reduced intensity | 17 (63) |
| Myeloablative | 10 (37) |
| GVHD prophylaxis* | 25 (93) |
| In vivo: ATG/alemtuzumab | 14 (52) |
| Ex vivo: T-celldepletion (CD45RA/ TCRαβ) | 11 (41) |
Data are n (%) or median (interquartile range) where appropriate.
Abbreviations: HSCT, haematopoietic stem cell transplantation; PID, primary immunodeficiency; SAA, severe aplastic anemia; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; MDS, myelodysplastic syndrome; MRD, matched related donor; MMRD, mismatched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; GVHD, graft versus host disease; ATG, anti-thymocyte globulin.
*Two patients transplanted from HLA-identical siblings received neither ATG nor T-cell depletion.
All HSCT were allogenic. Twenty-five patients underwent HSCT for the first time (93%) and two received their second HSCT (7%).
Eighteen healthy children were included in the control group, 10 male (56%) and 8 female (44%), with a median age of 8.7 years (IQR 9). There were no significant differences regarding age and sex between HSCT recipients and healthy controls.
Viral infections
A total of 83 samples were collected from the 27 HSCT recipients, and 77 were valid for viral studies (median number of valid samples per patient: 3; IQR 2). Twenty-five samples (32%) were positive, and 16 of 27 HSCT recipients (60%) had at least one viral detection. Among HSCT recipients with viral infection, the median number of positive samples per patient was 1 (IQR 1). HRV was isolated in 21 samples (84% of positive NPA) from 12 patients, followed by adenovirus and parainfluenza type 1 (two positive samples from two different patients each, 8%). There were no viral coinfections among HSCT recipients. Detailed information regarding positive samples is given in Table 2.
Table 2.
Samples with positive viral detection.
| Sample type | Valid samples* | Positive samples (%) | Respiratory viruses (n°) | |
|---|---|---|---|---|
| HSCT recipients | Day 7 | 20 | 9 (45) | HRV (6), ADV (2), PIV (1) |
| Day 0 | 21 | 6 (29) | HRV (6) | |
| Day 10 | 15 | 3 (20) | HRV (3) | |
| Day 20 | 12 | 4 (33) | HRV (4) | |
| Day 30 | 6 | 3 (50) | HRV (2), PIV (1) | |
| After day 30 | 3 | 0 | ||
| Healthy controls | 17 | 4 (24) | HRV (1), ADV (1), HRV + AV (1), HRV + HBoV (1) | |
Abbreviations: ADV, adenovirus; HBoV, human bocavirus; HRV, human rhinovirus; PIV, parainfluenza virus.
*A total of five samples were not valid because they contained blood or because polymerase chain reaction was inhibited.
Infections caused by HRV were symptomatic in 2 of 12 patients (17%): one had low-grade fever and the other persistent rhinorrhea. Both patients with adenovirus infections had fever, mucositis and elevated levels of C-reactive protein (above 100 mg/L). Infections by parainfluenza type 1 virus were also symptomatic (one patient with fever and another with laryngitis and pneumonia). None of the patients required admission to the intensive care unit (ICU) nor died as a result of a viral infection. There were no differences regarding age between HSCT recipients with and without viral infections (median [IQR] 7.5 [8.8] and 6 [10.2] years of age, respectively, p = 0.94), but patients below two years of age tested positive more frequently (11/21 samples, 52% vs. 14/56, 25%, p = 0.03).
A total of 17 samples from healthy controls were analyzed, and viruses were identified in 4 (24%): two single infections (HRV and adenovirus) and two coinfections (HRV and HBoV, HRV and adenovirus) (Table 2). Controls with viral infections were younger, but this difference did not reach statistical significance (median [IQR] 4.1 [6.6] vs. 8.9 [8.5] years, p = 0.07). All infections were asymptomatic.
No significant differences were found regarding viral isolation rate between patients and healthy controls (32% and 24% of samples, respectively, p = 0.57).
NPA cellular composition of HSCT recipients
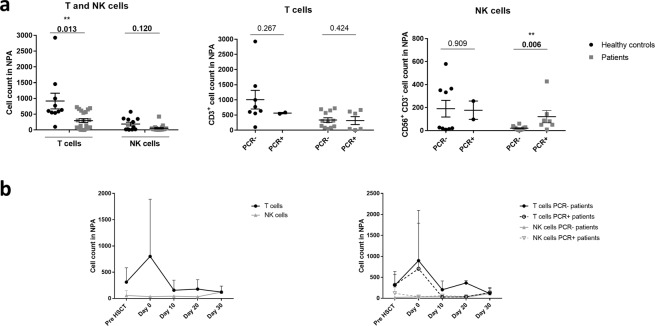
The NPAs of patients prior to HSCT conditioning contained fewer T and NK cells as compared with healthy controls p = 0.0132 and p = 0.120, respectively (Fig. 1A). Additionally, PCR + patients prior to HSCT conditioning showed statistically significant higher numbers of NK cells in NPAs than PCR− patients (p = 0.006). Those differences were not observed in healthy controls or in the T cell populations isolated from NPAs.
Figure 1.
T and NK cells in nasopharyngeal aspirates (NPAs) of patients prior to the HSCT conditioning are reduced as compared to healthy controls and have similar post HSCT kinetics in patients with and without viral respiratory infection. (a) Total number of T (CD45+, CD3+ CD56−) and NK cells (CD45+, CD3-, CD56+) in NPA of healthy controls and patients prior to the HSCT conditioning was determined by multiparametric flow cytometry. (b) Number of T and NK cells in NPAs was also monitored for one month post HSCT.
We further analyzed T and NK cell kinetics after the HSCT. We observed an increase in T cells in the NPA samples at infusion day. This increase is reverted at day 10 post HSCT, when NPA cell counts reach levels similar to those observed prior to the HSCT. This effect was observed both in PCR+ and in PCR− patients (Fig. 1B and Suppl. Table 1). However, we did not observe that peak with NK cells. There were no differences regarding NPA T and NK cell counts over time in patients with and without respiratory infection.
PB cellular composition of HSCT recipients
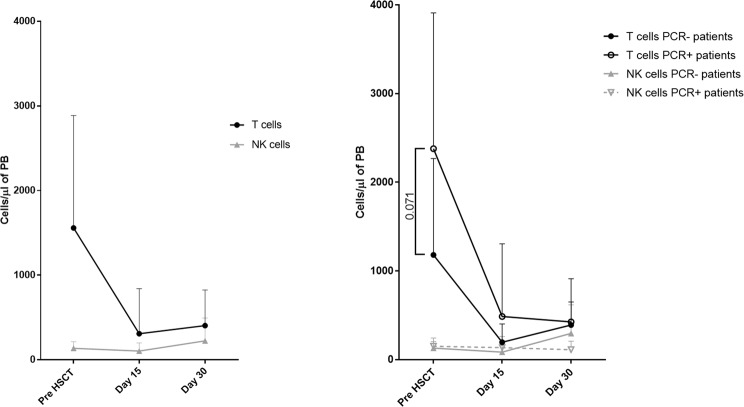
Prior to the HSCT, the initial T cell counts were higher in PCR+ patients than in PCR− patients (median, IQR 2014, 3677-1090 vs. 945, 1751-249 cells/µl, p = 0.07)) (Fig. 2 and Suppl. Table 2). However, PCR + and PCR− patients had similar initial NK cells counts prior to the HCT (median, IQR 154, 225-61 vs. 111, 185-62 cells/µl). Median T cell counts decreased notably after the HSCT, both in PCR+ and PCR− patients and on day 30 post HSCT the numbers were still far from the initial values. This effect was not observed in PB NK cell counts either.
Figure 2.
T and NK cells in peripheral blood (PB) of HSCT recipients. Number of T (CD45+, CD3+ CD56-) and NK cells (CD45+, CD3−, CD56+) in PB of HSCT patients was determined by multiparametric flow cytometry. Differences in T and NK cells numbers in PB of patients with PCR− and PCR+ for respiratory virus are indicated (n = 18).
Natural cytotoxicity receptors (NCRs) expression in NPA’s NK cells
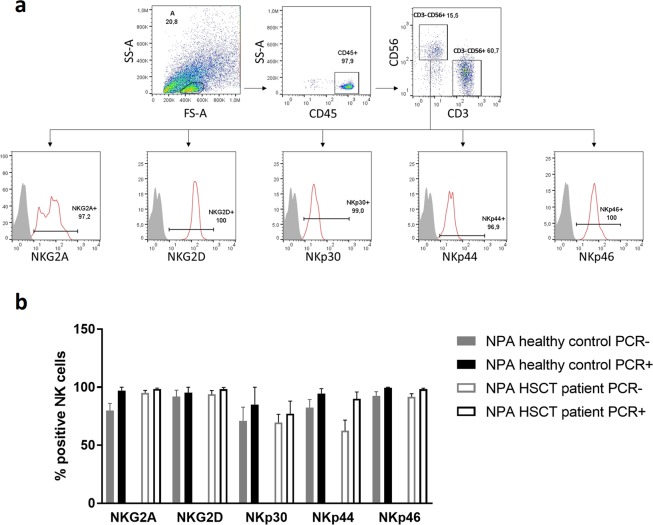
The NK cells found in the NPA samples had a highly activated status as determined by flow cytometry analysis (Fig. 3A). The percentage of NK cells expressing different activating receptors was higher in PCR + NPA samples than in PCR− samples for all the receptors analyzed, and especially significant for NKp44 and NKp46 receptors (Fig. 3B). The highly activated status was observed both in healthy donors and HSCT patients’ NPAs.
Figure 3.
NK cells in NPAs have a high activating status especially in PCR+ samples. (a) One representative flow cytometry analysis of an HSCT recipient NPA. CD45+, CD3−, CD56− cells were analyzed for the indicated NCRs (red lines) and compared with fluorescence minus one controls (grey). (b) Percentage of NK cells expressing activating receptors in PCR+ and PCR− NPAs of healthy controls versus patients prior to the HSCT.
HSCT recipient outcomes
The outcomes of HSCT recipients are summarized in Table 3. There were no significant differences according to the presence of viral infection. The number of T or NK cells in patients’ NPAs pre-, during- or after-HSCT was not correlated with a higher risk of GVHD, graft failure, ICU admission or death (Suppl. Tables 3 and 4).
Table 3.
Outcomes of haematopoietic stem cell transplantation recipients.
| All cases | Positive viral detection | Negative viral detection | p | |
|---|---|---|---|---|
| Number of cases | 27 | 16 | 11 | |
| Acute GVHD | 11 (41) | 7 (44) | 4 (36) | 1 |
| I-II | 4 (15) | |||
| III-IV | 7 (26) | |||
| Chronic GVHD | 3 (11) | 2 (13) | 1 (9) | 1 |
| Mild | 1 (4) | |||
| Moderate | 1 (4) | |||
| Severe | 1 (4) | |||
| Graft failure | 2 (7) | 1 (6) | 1 (9) | 1 |
| ICU admission | 7 (26) | 2 (12) | 5 (45) | 0.08 |
| Death | 7 (26) | 3 (19) | 4 (36) | 0.391 |
Data are n (%).
Abbreviations: GVHD, graft versus host disease; ICU, intensive care unit.
Discussion
The nasal mucosa is the first place within the respiratory system to be exposed to inhaled viral pathogens. Therefore, nasal immune cells are likely to play an important role in early innate immune responses to those environmental factors, both in healthy individuals and in immunocompromised patients like HSCT recipients. NK cells are thought to play a particularly important role in this context, since they are able to influence both innate and adaptive immune responses32,33 and are the first lymphocyte subset to appear in PB after HSCT20–26. While macrophages and dendritic cells have been identified in the nasal submucosa34, and neutrophils have been identified in the nasal cavity35, the immune cells present in NPAs have not been fully characterized. To phenotype NPA cells, previous researchers have relied on cell differential analysis of cytocentrifuge slides stained with haematoxylin and eosin. However, flow cytometry may be a superior method to fully characterize NPA immune cells since it allows for a precise quantification of cell populations as well as for phenotype studies, making it possible to determine the activation status of such specific cell populations.
Our study showed that HSCT patients, as expected -due to conditioning regimens and underlying disease- had fewer T and NK cells in their NPAs as compared to healthy controls. Interestingly, those patients exposed to viral pathogens showed statistically significant higher NK cell counts in their NPAs, which is in accordance with their role in the “front line” defense against viral infections. The phenotypes and functions of NK cells differ depending on the source organ or tissue36–38. Our current research further explores the particular phenotype of nasal NK cells, which show a higher activation status than those found in PB, comparing the results obtained in this study with previous publications from our group24. This was especially true for those individuals with positive viral detection in their nasal secretions. Okada et al. recently reported that NK cells found in the nasal passages of mice belong to the conventional NK cell linage and characteristically demonstrate an immature and activated phenotype39. Our results constitute the first evidence of such observations in humans.
In our study, we observed an unexpected T cells increase in NPAs on HSCT day 0 samples, despite conditioning regimens. We attribute it to the fact that all NPA samples were collected in the morning, and almost all patients receiving non-manipulated grafts (60% of our cohort) had finished their infusions prior to NPA collection. Pre-transplant conditioning, such as high-dose chemotherapy is toxic to many cells and render the endothelium vulnerable40 which could lead to increased T cells in NPA of many patients.
Natural killer cell activity is regulated through the balance of signals from inhibitory and activating receptors. The natural cytotoxicity receptors (NCRs) NKp46 (NCR1), NKp44 (NCR2), and NKp30 (NCR3), as well as NKG2D, are the main activating receptors involved in mediating NK cell function in health and disease18,19. The most overexpressed activating receptors we found in NK cells of NPAs were NKp44 (NCR2) and NKp46 (NCR1). This is consistent with Shemer-Avni et al.’s findings41. In nasal lavage samples of patients infected with respiratory viruses, they found NKp46 as the most abundantly expressed NCR. However, in their RTqPCR analysis they detected no NKp44 expression and very little of NKp30. Altogether, our findings point to NKp44 as a key player in nasal mucosa elimination of virally infected cells by NK cells.
Conditioning regimens used for allogeneic HSCT, high dose chemotherapy and/or radiotherapy, induce serious damage in mucosal, humoral and cellular immune function. Moreover, those regimens can harm the host’s thymopoiesis resulting in further delayed immune recovery25,26. Collectively, these factors predispose the host to a variety of infections. In fact, despite the routine use of prophylactic antimicrobials in the peri-transplant period42,43, infections occur is about 80–85% of HSCT recipients and are one of the leading causes of non-relapse-related mortality after allogeneic HSCT44,45.
More than half of the HSCT recipients included in our study presented a viral respiratory infection at some point. To date, most studies of these infections in pediatric HSCT recipients have focused on symptomatic individuals, reporting an incidence of 5–11%46–48. Asymptomatic respiratory infections in children undergoing HSCT have been barely studied. A prospective study of 33 patients detected respiratory viruses in 24% of them before HSCT, mainly HRV and adenovirus49. In our series, infections caused by HRV were the most common, and were not associated with increased morbidity. These findings are in concordance with those reported by Srinivasan et al. in children with cancer or post-HSCT and upper or lower respiratory infection13 and asymptomatic children before HSCT49. Other authors have reported implementing routine extensive pre-HSCT lung screening, including bronchoalveolar lavage for the detection of viruses, bacteria and fungi. In that study, almost one third of asymptomatic patients had viral respiratory infections, mainly caused by rhinovirus, and none of these worsened during HSCT50. On the other hand, infections caused by adenovirus and parainfluenza virus were less frequent but more often symptomatic in our patients. Adenovirus has been described as a cause of lower respiratory tract infection with high mortality after HSCT in studies involving symptomatic children46,51. We did not find a correlation between viral infection and HSCT outcomes, which could be caused by small sample size and low incidence of symptomatic infection.
Important strengths of our study include its prospective design and the determination of number, activation phenotype and functional profile of NK cells together with respiratory virus detection, assessing HSCT recipients over time and comparing them with healthy controls. Potential limitations of our study relate to sample size and losses to follow-up. NPA cell counts have great variability so larger cohorts would be desirable to draw stronger conclusions. Many patients did not tolerate well NPAs and withdrew during the course of the study. The small number of patients further precluded correlation of viral detection and transplantation outcomes, and limits our ability to examine the implications of asymptomatic viral respiratory infections in HSCT recipients. NPAs were collected up to 30 days after HSCT, and some infections occurring later may have been missed. However, most respiratory viral infections in HSCT recipients occur soon after transplantation, and adverse clinical outcomes are more likely to occur in the same period46,51.
In conclusion, we provide data suggesting NK cells as major effectors in respiratory viral control in the nasal mucosa. We also characterized this NK cell population finding a highly activated phenotype compared with that of their PB counterparts. Further study of this unique mucosal immune cell population will be beneficial in assessing the effects of viral infections on upper respiratory immune responses in HSCT recipients as well as in healthy individuals.
Supplementary information
Acknowledgements
This work was supported in part by the National Health Service of Spain, Instituto de Salud Carlos III (ISCIII), FONDOS FEDER grant (FIS) PI18/01301, CRIS Foundation to Beat Cancer, Patients’ Support Associations Fundación Mari Paz Jiménez Casado and La Sonrisa de Álex and a Small Grant Award from the European Society for Paediatric Infectious Diseases.
Author contributions
Research idea and study design: C.C. and A.P.-M. Data acquisition; C.C., J.V., I.C., F.P., F.R., D.B., D.C., A.M., Y.M. Data analysis and interpretation: M.V., T.R., J.V., C.C., A.P.-M. Original draft preparation: M.V., T.R. Funding acquisition: A.P.-M., T.R. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maria Vela and Teresa del Rosal.
Supplementary information
is available for this paper at 10.1038/s41598-019-55398-y.
References
- 1.Khanna N, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin. Infect. Dis. 2008;46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 2.Waghmare A, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin. Infect. Dis. 2013;57:1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo S, et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol. Blood Marrow Transplant. 2013;19:589–596. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renaud C, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol. Blood Marrow Transplant. 2013;19:1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan A, et al. Parainfluenza virus infections in children with hematologic malignancies. Pediatr. Infect. Dis. J. 2011;30:855–859. doi: 10.1097/INF.0b013e31821d190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan A, et al. Symptomatic Parainfluenza Virus Infections in Children Undergoing Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2011;17:1520–1527. doi: 10.1016/j.bbmt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kmeid J, et al. Outcomes of Influenza Infections in Hematopoietic Cell Transplant Recipients: Application of an Immunodeficiency Scoring Index. Biol. Blood Marrow Transplant. 2016;22:542–548. doi: 10.1016/j.bbmt.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah DP, et al. Characteristics and outcomes of pandemic 2009/H1N1 versus seasonal influenza in children with cancer. Pediatr. Infect. Dis. J. 2012;31:373–378. doi: 10.1097/INF.0b013e3182481ef8. [DOI] [PubMed] [Google Scholar]
- 9.Hale GA, et al. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant. 1999;23:277–282. doi: 10.1038/sj.bmt.1701563. [DOI] [PubMed] [Google Scholar]
- 10.Shah DP, Shah PK, Azzi JM, El Chaer F, Chemaly RF. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review. Cancer Lett. 2016;379:100–106. doi: 10.1016/j.canlet.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogimi C, et al. Clinical Significance of Human Coronavirus in Bronchoalveolar Lavage Samples From Hematopoietic Cell Transplant Recipients and Patients With Hematologic Malignancies. Clin. Infect. Dis. 2017;64:1532–1539. doi: 10.1093/cid/cix160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waghmare A, Englund JA, Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood. 2016;127:2682–2692. doi: 10.1182/blood-2016-01-634873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan A, et al. Prospective detection of respiratory pathogens in symptomatic children with cancer. Pediatr. Infect. Dis. J. 2013;32:e99–e104. doi: 10.1097/INF.0b013e31829862b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol. 2011;2:88. doi: 10.3389/fimmu.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adib-Conquy M, Scott-Algara D, Cavaillon J-M, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol. Cell Biol. 2014;92:256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. J HematolOncol. 2013;6:94. doi: 10.1186/1756-8722-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 18.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–191. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson CA, et al. Immune Reconstitution after Double Umbilical Cord Blood Stem Cell Transplantation: Comparison with Unrelated Peripheral Blood Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small TN, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. doi: 10.1182/blood.V93.2.467. [DOI] [PubMed] [Google Scholar]
- 22.Saliba RM, et al. General and Virus-Specific Immune Cell Reconstitution after Double Cord Blood Transplantation. Biol. Blood Marrow Transplant. 2015;21:1284–1290. doi: 10.1016/j.bbmt.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen SL, et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant. 2003;32:65–72. doi: 10.1038/sj.bmt.1704084. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Martínez A, et al. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant. 2012;47:1419–1427. doi: 10.1038/bmt.2012.43. [DOI] [PubMed] [Google Scholar]
- 25.Ringhoffer S, Rojewski M, Döhner H, Bunjes D, Ringhoffer M. T-cell reconstitution after allogeneic stem cell transplantation: assessment by measurement of the sjTREC/βTREC ratio and thymic naive T cells. Haematologica. 2013;98:1600–1608. doi: 10.3324/haematol.2012.072264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komanduri KV, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris AC, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016;22:4–10. doi: 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagasia MH, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coiras MT, Pérez-Breña P, García ML, Casas I. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J. Med. Virol. 2003;69:132–144. doi: 10.1002/jmv.10255. [DOI] [PubMed] [Google Scholar]
- 30.Coiras MT, Aguilar JC, García ML, Casas I, Pérez-Breña P. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J. Med. Virol. 2004;72:484–495. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvo C, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. ActaPaediatr. 2010;99:883–887. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 33.Fehniger TA, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 34.Jahnsen FL, Gran E, Haye R, Brandtzaeg P. Human nasal mucosa contains antigen-presenting cells of strikingly different functional phenotypes. Am. J. Respir. Cell Mol. Biol. 2004;30:31–37. doi: 10.1165/rcmb.2002-0230OC. [DOI] [PubMed] [Google Scholar]
- 35.Noah TL, et al. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ. Health Perspect. 2011;119:78–83. doi: 10.1289/ehp.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 38.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada K, et al. Identification and Analysis of Natural Killer Cells in Murine Nasal Passages. PLoS ONE. 2015;10:e0142920. doi: 10.1371/journal.pone.0142920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaby EG, Gilbert RD. Thrombotic microangiopathy following haematopoietic stem cell transplant. Pediatr Nephrol. 2018;33:1489–1500. doi: 10.1007/s00467-017-3803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shemer-Avni Y, et al. Expression of NKp46 Splice Variants in Nasal Lavage Following Respiratory Viral Infection: Domain 1-Negative Isoforms Predominate and Manifest Higher Activity. Front Immunol. 2017;8:161. doi: 10.3389/fimmu.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majhail NS, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:337–341. doi: 10.1038/bmt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomblyn M, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol. Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young J-AH, et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol. Blood Marrow Transplant. 2016;22:359–370. doi: 10.1016/j.bbmt.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parody R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol. Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Hutspardol S, et al. Significant Transplantation-Related Mortality from Respiratory Virus Infections within the First One Hundred Days in Children after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2015;21:1802–1807. doi: 10.1016/j.bbmt.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luján-Zilbermann J, et al. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin. Infect. Dis. 2001;33:962–968. doi: 10.1086/322628. [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, et al. Respiratory viral infections during the first 28 days after transplantation in pediatric hematopoietic stem cell transplant recipients. Clin Transplant. 2012;26:736–740. doi: 10.1111/j.1399-0012.2012.01607.x. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan A, et al. Detection of respiratory viruses in asymptomatic children undergoing allogeneic hematopoietic cell transplantation. Pediatr Blood Cancer. 2013;60:149–151. doi: 10.1002/pbc.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Versluys AB, et al. High Diagnostic Yield of Dedicated Pulmonary Screening before Hematopoietic Cell Transplantation in Children. Biol. Blood Marrow Transplant. 2015;21:1622–1626. doi: 10.1016/j.bbmt.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo MS, et al. The impact of RSV, adenovirus, influenza, and parainfluenza infection in pediatric patients receiving stem cell transplant, solid organ transplant, or cancer chemotherapy. Pediatr Transplant. 2013;17:133–143. doi: 10.1111/petr.12022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.