Abstract
BACKGROUND
Gallbladder squamous cell carcinoma (GBSCC) is a rare subtype of malignancy and accounts for only 2%-3% of gallbladder malignancies. Due to its rapid development, most patients with GBSCC initially present with an advanced stage of the disease and hence a poor prognosis. The clinicopathological and biological features of SCC remain to be fully elucidated, owing to its uncommon occurrence. The majority of currently available data only described individual case reports or series analyses of trivial cases.
CASE SUMMARY
A 64-year-old man was admitted for progressively poor abdominal distension and pain. Liver computed tomography (CT) showed infiltration of gallbladder carcinoma into the adjacent liver, and enlarged retroperitoneal lymph nodes. The patient underwent radical cholecystectomy. Part of the mass was grey and soft, and the neoplastic section showed a purulent-necrotic lesion. Hematoxylin and eosin staining revealed a moderately differentiated SCC. Immunohistochemical studies showed strong staining of the tumor for AE1/3 and CK5/6. Staining for CK19, CK7, and CAM5.2 was positive in the cytoplasm. Systemic chemotherapy was not administered because of the patient’s poor physical condition. After five months, CT and magnetic resonance cholangiopancreatography showed multiple metastases in the liver and abdominal cavity.
CONCLUSION
Squamous components of GBSCC may explain the complex biological behavior, and CD109 may be involved in the pathogenesis.
Keywords: Gallbladder, Squamous cell carcinoma, Squamous metaplasia, Cholecystectomy, Case report
Core tip: Squamous cell carcinoma of gallbladder (GBSCC) is a rare subtype, and it has a poor prognosis. The squamous components that may explain the complex biological behavior and its poor prognosis show greater capacities of proliferation and local invasive in gallbladder carcinoma. Surgical resection has been considered as the basis of treatment for years, and complete resection is associated with increased survival. CD109 may be involved in the pathogenesis of GBSCC and serves as a novel target for intervention.
INTRODUCTION
Gallbladder carcinoma (GBC) is a common type of cancer in the gastrointestinal tract, and adenocarcinoma (AC) is the most common type of gallbladder cancer[1]. Squamous cell carcinoma (SCC) is a rare subtype and is responsible for only 2%-3% of gallbladder malignancies[2,3]. Due to its rapid development, the majority of patients with gallbladder SCC (GBSCC) initially present with an advanced stage of the disease and hence a poor prognosis[1]. Although patients underwent surgical interventions, the 5-year survival rate remained at approximately 1%. The large majority of SCCs are invasive carcinoma entity, often invading the entire gallbladder wall. It had been speculated that adenosquamous carcinomas (ASCs)/SCCs are more aggressive than ordinary gallbladder ACs. It has been testified that the squamous component of GBCs proliferates at a higher rate than the glandular component. However, despite their high proliferation rate, these tumors appeared to less frequently present with lymph node metastasis than gallbladder ACs. Their aggressive biological behavior has been attributed to their potential for direct extension and early invasion into the liver and neighboring organs, such as the stomach, duodenum, and transverse colon. Generally, SCC is aggressive, with infiltration into the liver, and rarely metastasizes to lymph nodes[4]. The clinicopathological and biological features of SCC remain to be fully elucidated[5,6], owing to its uncommon occurrence. The majority of currently available data only described individual case reports or series analyses of trivial cases. Therefore, it is imperative to describe the clinicopathological and biological features of SCC[5,6]. To improve our understanding of SCC of the gallbladder, we conducted a retrospective case study and review of the literature[6].
CASE PRESENTATION
Chief complaints
A 64-year-old man presented with symptoms of constantly worsening right upper quadrant distension and pain for 1 d.
History of present illness
The patient’s symptoms started a month ago with symptoms of right upper quadrant distension and pain, which had worsened the last 48 h.
History of past illness
The patient did not have a previous medical history.
Personal and family history
The patient denied any personal or family history.
Imaging examinations
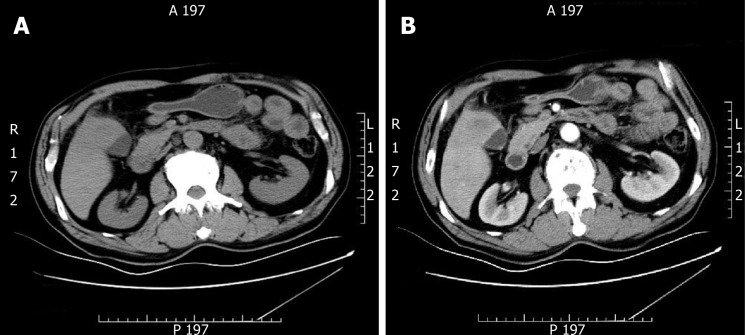
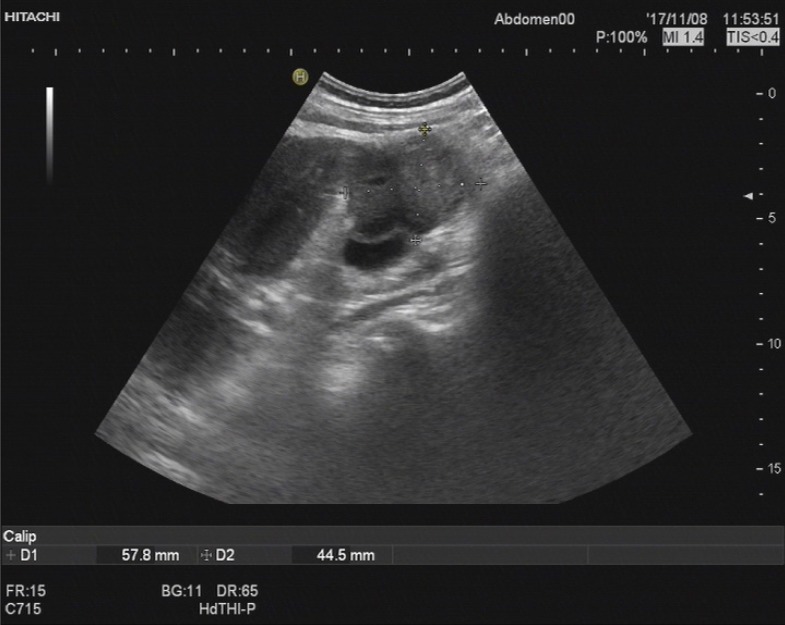
Ultrasonography (USG) of the abdomen showed a 5.8 cm × 4.5 cm heterogeneous mass localized in both the gallbladder and liver and a gallbladder with remarkably thickened wall, indicating a malignancy. Computed tomography (CT) and contrast-enhanced CT of the liver revealed infiltration of GBC into the adjacent liver, with enlarged retroperitoneal lymph nodes (Figure 1).
Figure 1.
Gallbladder carcinoma infiltrating the adjacent liver, with enlarged retroperitoneal lymph nodes. A: Computed tomography; B: Contrast enhanced computed tomography.
The patient underwent radical cholecystectomy for GBC. The gallbladder was intraoperatively observed to adhere tightly to the greater omentum. Moreover, miliary lesions were observed in a portion of the omentum. Neoplastic entities were present at the bottom of the gallbladder, which had invaded the V hepatic segments of the liver.
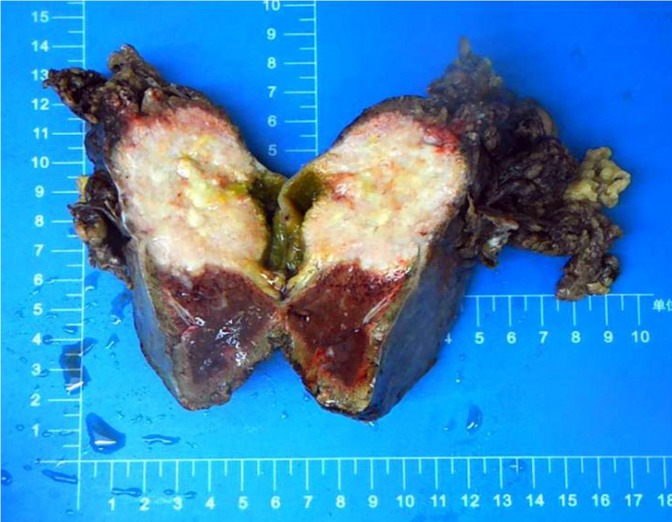
The gallbladder and a part of the liver tissue were surgically resected (dimension of 13 mm × 5 mm × 8 mm). The gallbladder contained dark green bile. The thickness of the gallbladder wall was approximately 0.2-0.3 cm, and the size of the tumor near the neck of the gallbladder was approximately 5 cm × 4 cm × 6 cm. Part of the mass was grey and soft, and the neoplastic section of the tumor revealed purulent necrosis. The boundaries of the mass and surrounding liver tissue were vague (Figure 2).
Figure 2.
Gross morphology of a 5 cm × 4 cm × 6 cm tumor seen near the gallbladder neck and liver tissue. Part of the mass was grey and soft, part of the mass section showing purulent necrosis was visible, and the boundary between the mass and surrounding liver tissue was not clear.
Pathological findings
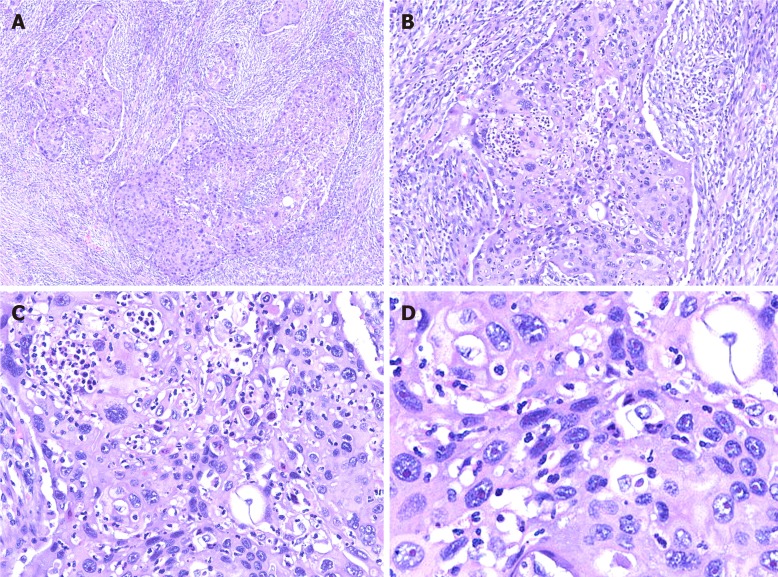
Hematoxylin and eosin staining: For paraffin-embedded tissue specimens, consecutive 3-5 µm thick sections were cut and used for histological analysis[7]. Hematoxylin and eosin staining revealed moderately differentiated SCC. The tumor cells invaded the full-thickness of the serosa and approached the surrounding adipose tissue. Part of the tumor also infiltrated the liver tissue. Lymph nodes in the hepatic duodenal ligament were examined, and resected lymph nodes were negative for carcinoma metastasis (Figure 3).
Figure 3.
Hematoxylin and eosin staining of tumor cells. A: Image showing diffuse growth replacing the mucosa and invading the muscularis propria; B: Image showing densely packed tumors formed by squamous cells; C: Image showing squamous cell carcinoma with keratin pearl formation; D: Image showing that tumor cell proliferation is markedly atypical, and karyokinesis is increased.
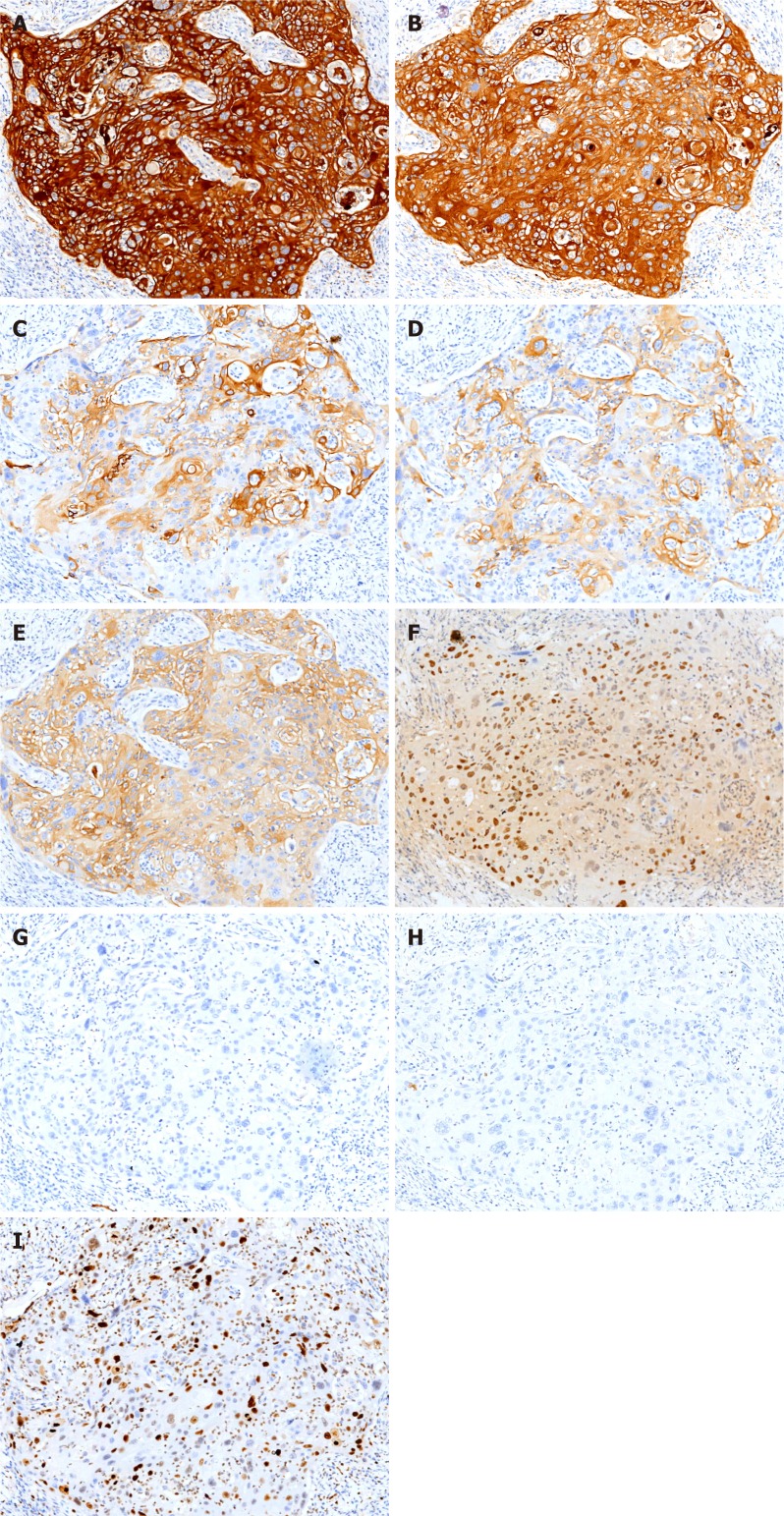
Immunohistochemical staining: Immunohistochemical analysis showed strong staining for AE1/3 and CK5/6 in the tumor. Staining for CK19, CK7, and CAM5.2 was positive in the cytoplasm, and P40 and Ki-67 were positive in the nucleus. In contrast, the expression of hepatocyte marker and AFP was not detected (Figure 4).
Figure 4.
Immunohistochemical staining shows tumor cells in gallbladder squamous cell carcinoma (×100). A: AE1/AE3 (+); B: CK5/6 (+); C: CAM5.2 (+); D: CK7 (+); E: CK19 (+); F: P40 (+); G: Hepatocyte (-); H: AFP (-); I: Ki-67 (60%+).
FINAL DIAGNOSIS
The final pathological diagnosis was moderately differentiated SCC of the gallbladder.
TREATMENT
Due to the patient’s poor physical condition, systemic chemotherapy was not administered.
OUTCOME AND FOLLOW-UP
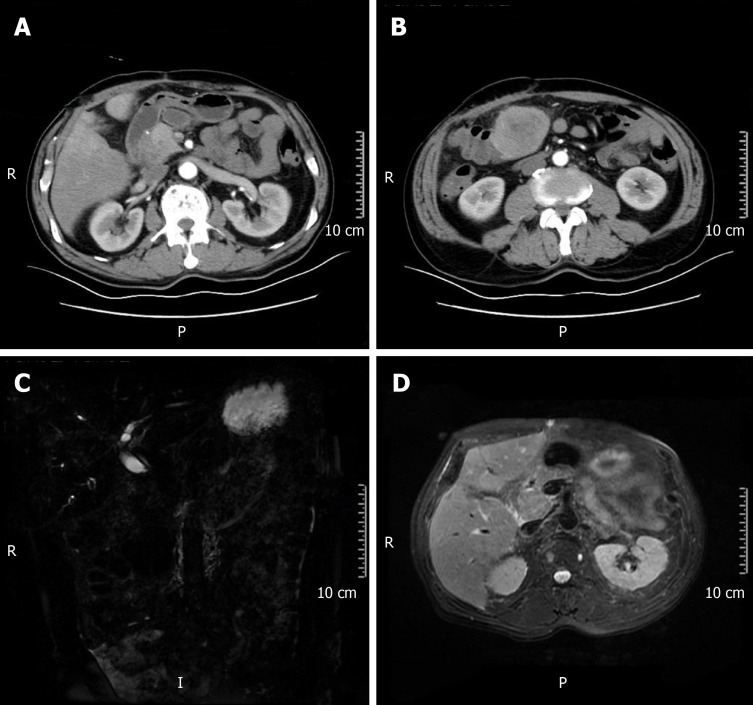
After five months, the patient was re-admitted to the hospital owing to fever (Table 1). A space-occupying lesion in the right posterior aspect of the first hepatic portal region was observed by abdominal USG, and considered metastatic cancer (Figure 5). Re-examination of CT and magnetic resonance cholangiopancreatography (MRCP) revealed postoperative changes in the GBC and multiple metastases in the liver and abdominal cavity (Figure 6).
Table 1.
Timeline
| Time | Measure | Results |
| November 8, 2017 | Abdominal ultrasonography | A 5.8 cm × 4.5 cm heterogeneous mass localized in both the gallbladder and liver and a gallbladder with remarkably thickened wall, indicating a malignancy |
| November 15, 2017 | CT and contrast-enhanced CT | Gallbladder carcinoma infiltrating the adjacent liver, with enlarged retroperitoneal lymph nodes |
| November 22, 2017 | Radical cholecystectomy | The gallbladder adhered tightly to the greater omentum. Neoplastic entities were present at the bottom of the gallbladder, which had invaded the V hepatic segments of the liver |
| November 23, 2017 | Pathological findings | A moderately differentiated SCC |
| November 24, 2017 | Immunohistochemistry | A moderately differentiated SCC |
| April 15, 2018 | Abdominal ultrasonography | A space-occupying lesion in the right posterior aspect of the first hepatic portal region, which was considered as cancer metastasis |
| April 16, 2018 | CT and magnetic resonance cholangiopancreatography | Postoperative changes in gallbladder carcinoma and multiple metastases in the liver and abdominal cavity |
CT: Computed tomography; SCC: Squamous cell carcinoma.
Figure 5.
Abdominal ultrasonography revealed a space-occupying lesion in the right posterior aspect of the first hepatic portal region, which was considered as cancer metastasis.
Figure 6.
Postoperative changes in gallbladder carcinoma and multiple metastases in the liver and abdominal cavity. A and B: Computed tomography; C and D: Magnetic resonance cholangiopancreatography.
DISCUSSION
GBC is one of the most aggressive malignancies of the gastrointestinal tract[1]. GBC includes several subtypes, including AC, SCC, and ASC. Particularly, SCC has a very low incidence rate and represents only 2%-3% of all GBC cases. The progression and overall prognosis of SCC appear to be worse than those of AC and ASC[4,8,9]. ASC of the gallbladder is also rare, and its incidence rate is only 7%-10.6%. ASC includes squamous cells and glandular components. The squamous components that may explain the complex biological behavior and poor prognosis show greater capacities of proliferation and local invasion than the glandular components. Lymphatic metastasis and hematogenous metastasis are the principal types of tumor metastasis.
In clinical practice, pain occurs in 66% of patients, and is the most significant symptom. In patients with GBC, the detection of a palpable mass in the right upper quadrant is not specific, but could be a crucial hint that indicates the presence of a malignancy. Some patients may present with a tumor in the right hypochondrial region. However, it could be clinically misdiagnosed as abscess of the gallbladder. USG is the preferred method for diagnosis, and suspected cases can be diagnosed by CT, percutaneous transhepatic cholangiography, MRCP, and fine needle aspiration[4]. SCC of the gallbladder has a very poor prognosis, and the involvement of the serosa and lymphatic metastasis are the most important prognostic factors. The size of tumors at diagnosis is the most significant determinant of survival. Therefore, it is especially important to improve the survival index, thus providing useful strategies for early diagnosis and prevention of SCC of the gallbladder[1].
Studies have shown that surgical resection has been considered as the cornerstone of treatment for many years, and complete resection is associated with increased survival[10,11]. However, most patients died approximately 6 mo after diagnosis without radical surgery[4]. Currently, cholecystectomy, hepatectomy, and lymphadenectomy contribute to patient’s recovery. Some studies demonstrated that the 5-year survival rate may still be low owing to systemic failure. Weatherall et al[11] have confirmed that although patients with SCC of the gallbladder have a larger tumor size and higher possibility of involvement of adjacent organs, complete resection is possible[12]. However, there is limited information on the optimal treatment for SCC of the gallbladder. Therefore, physicians have been striving to optimize assisted therapy and improve survival. After surgical resection, the majority of physicians offer adjuvant chemotherapy using 5-fluorouracil. The benefits of the best adjuvant therapy or accompanying chemoradiotherapy remain controversial.
Various hypotheses have been proposed for the etiology of gallbladder SCC, including: (1) Ectopic squamous epithelium with malignant transformation[1]; (2) Metaplastic squamous epithelium with malignant transformation which describes the evolution of metaplasia-dysplasia-carcinoma in progressive development[1,13]. The gallbladder may trigger differentiation of glandular cells into squamous cells due to chronic irritation from gallstones. Thereafter, the squamous metaplastic cells may undergo malignant transformation into tumor cells[4,8,13-16]; and (3) Adenocarcinoma with squamous metaplasia. Here, squamous cell elements of mixed ASC of the gallbladder undergo excessive growth and eventually replace all the adenocarcinoma components, resulting in SCC development[4,8,13-16].
The molecular mechanisms for the occurrence and progression of SCC remain unclear. Further studies are needed to determine the etiopathogenetic and molecular differences between this histologic type and conventional adenocarcinomas. Therefore, it is essential to investigate the malignant biological behavior and clinicopathological characteristics of GBSCC. According to studies, CD109 may be associated with the development and clinicopathological characteristics of SCC. CD109 is a glycosylphosphatidylinositol-anchored cell-surface glycoprotein that negatively regulates transforming growth factor-β (TGF-β) signaling as a novel TGF-β co-receptor[17-22]. CD109 is a member of the α2-macroglobulin/C3, C4, C5 family of thioester-containing proteins[21,22]. CD109 could be a potential biological marker for SCC of the gallbladder[23,24]. Furthermore, a higher level expression of the CD109 protein was also reported in SCCs of other organs[24-27]. TGF-β is a multipotent cytokine implicated in many physiological processes and regulates a wide variety of cellular processes including cell proliferation, differentiation, adhesion, extracellular matrix deposition, wound healing, and inflammation[18,28-30]. TGF-β signal transduction maintains the homeostasis of epithelial cells[24,31], and defective TGF-β signaling may lead to cellular hyper-proliferation, reduced apoptosis, and increased genomic instability[24,32]. The transducing factor of the TGF-β signaling pathway is commonly downregulated in human SCCs[23,24]. CD109 is a co-receptor of TGF-β1 and increases the binding of TGF-β1 to its receptor[24,33]. CD109 is associated with caveolin-1, which can promote the localization of TGF-β receptors in degraded cavities[24,34]. Therefore, CD109 regulates TGF-β signaling and enhances TGF-β receptor endocytosis actively. CD109 upregulation in SCC of the gallbladder potentially inhibits TGF-β signal transduction and subsequently promotes SCC progression. In summary, CD109 may be involved in the pathogenesis of gallbladder SCC and serves as a novel target for intervention[24].
Recent findings demonstrate that the clinicopathological features of SC remain to be well recognized. Consequently, the therapeutic interventions remain to be fully elucidated. Although biomarkers for predicting the prognosis of GBSCC have been recognized, they still lack clinical applicability currently. Therefore, it is vital to record the biological and clinicopathological characteristics of GBSCC.
CONCLUSION
Only scarce reports of SCC of the gallbladder have been reported; GBSCC is a rare subtype, and it has a poor prognosis. The squamous components that may explain the complex biological behavior and poor prognosis show greater capacities of proliferation and local invasion in GBC. Surgical resection has been considered as the basis of treatment for years, and complete resection is associated with increased survival. CD109 may be involved in the pathogenesis of gallbladder SCC and serves as a novel target for intervention.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: June 26, 2019
First decision: September 9, 2019
Article in press: October 15, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: González-González R S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu MY
Contributor Information
Shan Jin, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Lu Zhang, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Yuan-Feng Wei, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Hai-Jun Zhang, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Cheng-Yan Wang, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Hong Zou, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Jian-Ming Hu, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Jin-Fang Jiang, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China.
Li-Juan Pang, Department of Pathology, the First Affiliated Hospital to Shihezi University School of Medicine and Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China. ocean123456@163.com.
References
- 1.Khan N, Afroz N, Haider N, Khan MA. A case of pure endophytic squamous cell carcinoma of the gallbladder: a rare entity with aggressive behaviour. Turk Patoloji Derg. 2012;28:181–183. doi: 10.5146/tjpath.2012.01122. [DOI] [PubMed] [Google Scholar]
- 2.Yıldız İ, Koca YS, Barut İ. Overlap of Acute Cholecystitis with Gallstones and Squamous Cell Carcinoma of the Gallbladder in an Elderly Patient. Case Rep Surg. 2015;2015:767196. doi: 10.1155/2015/767196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo Y, Mun S. Synchronous double primary squamous cell carcinoma and adenocarcinoma of the extrahepatic bile duct: a case report. J Med Case Rep. 2015;9:116. doi: 10.1186/s13256-015-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti I, Giri A, Ghosh N. Cytohistopathological correlation of a case of squamous cell carcinoma of gallbladder with lymph node metastasis. Turk Patoloji Derg. 2014;30:81–84. doi: 10.5146/tjpath.2013.01180. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Yang ZL, Ren X, Zou Q, Yuan Y, Liang L, Chen M, Chen S. ACE2 and FZD1 are prognosis markers in squamous cell/adenosquamous carcinoma and adenocarcinoma of gallbladder. J Mol Histol. 2014;45:47–57. doi: 10.1007/s10735-013-9528-1. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Yang Z, Jiang S, Zou Q, Yuan Y, Li J, Li D, Liang L, Chen M, Chen S. MCM2 and TIP30 are prognostic markers in squamous cell/adenosquamous carcinoma and adenocarcinoma of the gallbladder. Mol Med Rep. 2016;14:4581–4592. doi: 10.3892/mmr.2016.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Tao L, Chen D, Gao R, Zhao L, Li G, Chen H, Ren Y, Li X, Wang Y, Pang L. Immunohistochemical findings in small cell neuroendocrine carcinoma of the cervix: a report of two cases. Transl Cancer Res. 2017;6:860–868. [Google Scholar]
- 8.Roppongi T, Takeyoshi I, Ohwada S, Sato Y, Fujii T, Honma M, Morishita Y. Minute squamous cell carcinoma of the gallbladder: a case report. Jpn J Clin Oncol. 2000;30:43–45. doi: 10.1093/jjco/hyd010. [DOI] [PubMed] [Google Scholar]
- 9.Kendre P, Kataria P, Patel A, Mittal LC, Mule T. Metastasis as initial presentation of squamous cell carcinoma of gallbladder: A rare clinical entity. Indian J Pathol Microbiol. 2017;60:440–441. doi: 10.4103/IJPM.IJPM_113_17. [DOI] [PubMed] [Google Scholar]
- 10.Oohashi Y, Shirai Y, Wakai T, Nagakura S, Watanabe H, Hatakeyama K. Adenosquamous carcinoma of the gallbladder warrants resection only if curative resection is feasible. Cancer. 2002;94:3000–3005. doi: 10.1002/cncr.10578. [DOI] [PubMed] [Google Scholar]
- 11.Weatherall TJ, Fenton M, Munene G, Dickson PV, Deneve JL. Locally Advanced, Unresectable Squamous Cell Carcinoma of the Gallbladder. Case Rep Surg. 2015;2015:424650. doi: 10.1155/2015/424650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalayarasan R, Javed A, Sakhuja P, Agarwal AK. Squamous variant of gallbladder cancer: is it different from adenocarcinoma? Am J Surg. 2013;206:380–385. doi: 10.1016/j.amjsurg.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Alpuerto AC, Mora ME, Robitsek RJ, Schubl SD. Primary Pure Squamous Cell Carcinoma of the Gallbladder Locally Invading the Liver, Duodenum, and Stomach: A Case Report and Literature Review. Case Rep Surg. 2017;2017:2534029. doi: 10.1155/2017/2534029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseinzadeh M, Shokripur M, Salahi H. Primary pure squamous cell carcinoma of gallbladder presenting as acute cholecystitis. Iran J Med Sci. 2012;37:271–273. [PMC free article] [PubMed] [Google Scholar]
- 15.Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, Saka B, Losada H, Bagci P, Adsay NV. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol. 2011;24:1069–1078. doi: 10.1038/modpathol.2011.68. [DOI] [PubMed] [Google Scholar]
- 16.Rao S, Arya A, Aggarwal S, Gupta K, Arora R, Dhawan I. Pure squamous cell carcinoma of the gall bladder. Indian J Pathol Microbiol. 2007;50:599–600. [PubMed] [Google Scholar]
- 17.Hagiwara S, Murakumo Y, Mii S, Shigetomi T, Yamamoto N, Furue H, Ueda M, Takahashi M. Processing of CD109 by furin and its role in the regulation of TGF-beta signaling. Oncogene. 2010;29:2181–2191. doi: 10.1038/onc.2009.506. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Hancock MA, Sehgal P, Zhou S, Reinhardt DP, Philip A. Soluble CD109 binds TGF-β and antagonizes TGF-β signalling and responses. Biochem J. 2016;473:537–547. doi: 10.1042/BJ20141488. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama M, Ichinoe M, Okina S, Sakurai Y, Nakada N, Yanagisawa N, Jiang SX, Numata Y, Umezawa A, Miyazaki K, Higashihara M, Murakumo Y. CD109, a negative regulator of TGF-β signaling, is a putative risk marker in diffuse large B-cell lymphoma. Int J Hematol. 2017;105:614–622. doi: 10.1007/s12185-016-2173-1. [DOI] [PubMed] [Google Scholar]
- 20.Shiraki Y, Mii S, Enomoto A, Momota H, Han YP, Kato T, Ushida K, Kato A, Asai N, Murakumo Y, Aoki K, Suzuki H, Ohka F, Wakabayashi T, Todo T, Ogawa S, Natsume A, Takahashi M. Significance of perivascular tumour cells defined by CD109 expression in progression of glioma. J Pathol. 2017;243:468–480. doi: 10.1002/path.4981. [DOI] [PubMed] [Google Scholar]
- 21.Mii S, Hoshino A, Enomoto A, Murakumo Y, Ito M, Yamaguchi A, Takahashi M. CD109 deficiency induces osteopenia with an osteoporosis-like phenotype in vivo. Genes Cells. 2018;23:590–598. doi: 10.1111/gtc.12593. [DOI] [PubMed] [Google Scholar]
- 22.Qi R, Dong F, Liu Q, Murakumo Y, Liu J. CD109 and squamous cell carcinoma. J Transl Med. 2018;16:88. doi: 10.1186/s12967-018-1461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517–523. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- 24.Dong F, Lu C, Chen X, Guo Y, Liu J. CD109 is a novel marker for squamous cell/adenosquamous carcinomas of the gallbladder. Diagn Pathol. 2015;10:137. doi: 10.1186/s13000-015-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong F, Wang Y, Li L, Wang Y, Liu X, Liu J. CD109 expression is increased in cutaneous squamous cell carcinoma. J Dermatol. 2014;41:947–949. doi: 10.1111/1346-8138.12620. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JM, Hashimoto M, Kawai K, Murakumo Y, Sato T, Ichihara M, Nakamura S, Takahashi M. CD109 expression in squamous cell carcinoma of the uterine cervix. Pathol Int. 2005;55:165–169. doi: 10.1111/j.1440-1827.2005.01807.x. [DOI] [PubMed] [Google Scholar]
- 27.Dong F, Liu F, Yan S, Liu X, Jiang Z, Liu J. Elevated Expression of CD109 in Esophageal Squamous Cell Carcinoma. Pathol Oncol Res. 2015;21:1273–1275. doi: 10.1007/s12253-014-9894-3. [DOI] [PubMed] [Google Scholar]
- 28.Vorstenbosch J, Nguyen CM, Zhou S, Seo YJ, Siblini A, Finnson KW, Bizet AA, Tran SD, Philip A. Overexpression of CD109 in the Epidermis Differentially Regulates ALK1 Versus ALK5 Signaling and Modulates Extracellular Matrix Synthesis in the Skin. J Invest Dermatol. 2017;137:641–649. doi: 10.1016/j.jid.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Bizet AA, Tran-Khanh N, Saksena A, Liu K, Buschmann MD, Philip A. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J Cell Biochem. 2012;113:238–246. doi: 10.1002/jcb.23349. [DOI] [PubMed] [Google Scholar]
- 30.Man XY, Finnson KW, Baron M, Philip A. CD109, a TGF-β co-receptor, attenuates extracellular matrix production in scleroderma skin fibroblasts. Arthritis Res Ther. 2012;14:R144. doi: 10.1186/ar3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor MA, Lee YH, Schiemann WP. Role of TGF-β and the tumor microenvironment during mammary tumorigenesis. Gene Expr. 2011;15:117–132. doi: 10.3727/105221611x13176664479322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White RA, Malkoski SP, Wang XJ. TGFβ signaling in head and neck squamous cell carcinoma. Oncogene. 2010;29:5437–5446. doi: 10.1038/onc.2010.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, Bizet AA, Philip A. Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J. 2006;20:1525–1527. doi: 10.1096/fj.05-5229fje. [DOI] [PubMed] [Google Scholar]
- 34.Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, Buschmann MD, Philip A. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim Biophys Acta. 2011;1813:742–753. doi: 10.1016/j.bbamcr.2011.01.028. [DOI] [PubMed] [Google Scholar]