Abstract
BACKGROUND
Groove pancreatitis (GP) is a type of chronic pancreatitis occurring in an anatomic area between the duodenum, head of the pancreas, and common bile duct. Duodenal obstruction is always caused by malignant pancreatic diseases, such as pancreatic head carcinoma, while is rarely induced by benign pancreatic diseases, such as pancreatitis.
CASE SUMMARY
A 39-year-old man presented with a 1-mo history of upper abdominal discomfort. His concomitant symptoms were abdominal distension, postprandial nausea, and vomiting. Contrast-enhanced computed tomography of the abdomen showed thickening of the intestinal wall with enhancement of the descending segment of the duodenum, which could not be clearly differentiated from the head of the pancreas. Upper gastrointestinal radiographs and gastrointestinal endoscopy showed a complete obstruction of the descending duodenum. An operation found that a 3-cm mass was located in the “groove part” of the pancreas and oppressing the descending duodenum. Pancreaticoduodenectomy was performed to relieve the obstruction and thoroughly remove the pancreatic lesions. The pathologic diagnosis was pancreatitis. The patient had an uneventful recovery with no complications.
CONCLUSION
Because of the special location and the contracture induced by long-term chronic inflammation, our case reminds surgeons that some benign pancreatic diseases, such as GP, can also present with symptoms similar to those of pancreatic cancer. This knowledge can help to avoid an unnecessary radical operation.
Keywords: Groove pancreatitis, Duodenal obstruction, Pancreatic head carcinoma, Case report
Core tip: Duodenal obstruction is always caused by malignant pancreatic diseases, such as pancreatic head carcinoma. Here, we report a case of complete duodenal obstruction which was caused by groove pancreatitis, a benign pancreatic disease. This case reminds surgeons that some benign pancreatic diseases, such as groove pancreatitis, can also present with symptoms similar to those of pancreatic cancer, and this knowledge can help to avoid an unnecessary radical operation.
INTRODUCTION
Groove pancreatitis (GP) is a type of chronic pancreatitis occurring in an anatomic area between the duodenum, head of the pancreas, and common bile duct[1,2]. The causes may be associated with smoking, long-term alcohol abuse, and gastric resection. Its low incidence and very similar presentation to pancreatic head adenocarcinoma make its diagnosis extremely challenging. The symptoms of GP are always nontypical, including upper abdominal pain, postprandial nausea, vomiting, and weight loss. Unfortunately, patients with pancreatic cancer can also present with these same nontypical symptoms[3,4]. However, there are some characteristics that differ between GP and pancreatic carcinoma. GP primarily affects the pancreatic head in the groove area and rarely affects other organs, such as the duodenum[5]. In the present case, complete duodenal obstruction caused by GP was reported to be a rear but easy to be misdiagnosed symptom of extrapancreatic organs.
CASE PRESENTATION
Chief complaints
A 39-year-old man presented with a 1-month history of upper abdominal discomfort.
History of present illness
The patient’s symptoms were abdominal distension, postprandial nausea, and vomiting. The symptom aggravated gradually.
History of past illness
The patient had a history of drinking about 750g of wine every day for 10 years and denied a history of tobacco use, hypertension, diabetes, or prior surgery.
Personal and family history
The patient had no significant personal or family history.
Physical examination upon admission
Physical examination revealed no other remarkable findings with the exception of slightly elevated upper abdomen.
Laboratory examinations
Laboratory tests showed a white blood cell count of 7.54 × 109/L, and C-reactive protein level of 2.41 mg/L; the alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, amylase, and total bilirubin levels were normal. Blood tests also showed a cancer antigen 19-9 level < 2 U/mL, cancer antigen 125 level of 8.4 U/mL, carcinoembryonic antigen level of 4.11 ng/mL, and cancer antigen 50 level of 0.5 U/mL.
Imaging examinations
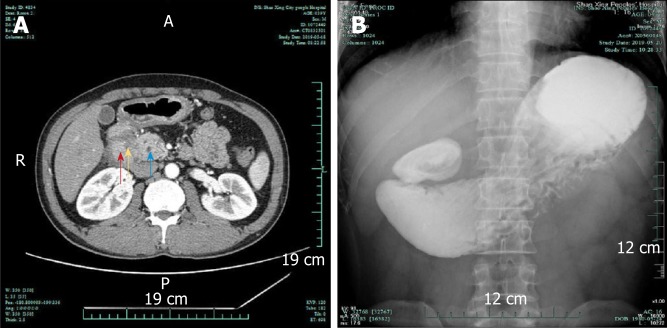
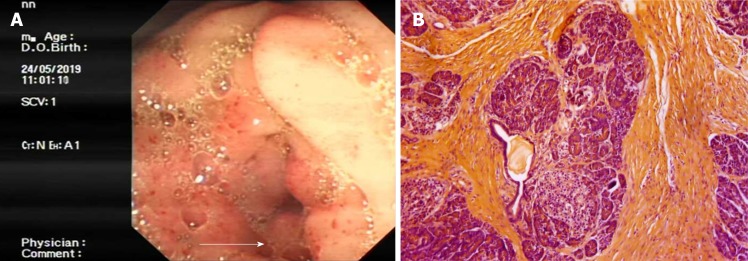
Contrast-enhanced computed tomography of the abdomen showed thickening of the intestinal wall with enhancement of the descending segment of the duodenum, which could not be clearly differentiated from the head of the pancreas (Figure 1A). It looks like an increased fat tissue concentration around the duodenum (Figure 1A). Both the common bile duct and pancreatic duct were dilated (Figure 1A). The radiologists provided an imaging diagnosis of a mass in the head of the pancreas. Upper gastrointestinal radiographs showed no contrast medium entering the descending duodenum (Figure 1B). Furthermore, gastrointestinal endoscopy showed a complete obstruction of the descending duodenum, and contrast medium accumulating at site of the obstruction (Figure 2A). Because no mucosal lesion was found by gastrointestinal endoscopy, no biopsy specimen was taken. The patient was presumptively diagnosed with a solid neoplasm located in the pancreatic head. Because the patient urged to have an operation as soon as possible to determine the property of lesion, no more image examination was performed.
Figure 1.
Contrast-enhanced computed tomography image and upper gastrointestinal radiograph. A: Contrast-enhanced computed tomography image of the lesion. Red arrow: Thickened and obstructed duodenum (the lesion looks like an increased fat tissue concentration around the duodenum); yellow arrow: Pancreaticoduodenal groove; blue arrow: Dilated pancreatic duct; B: Upper gastrointestinal radiograph showing that there was no contrast agent entering the descending duodenum.
Figure 2.
Duodenoscopy and histopathological examination. A: Duodenoscopic image showing complete obstruction of the descending duodenum (white arrow); B: Histopathological examination showing fibrous proliferation and chronic inflammation in the groove area. (Hematoxylin and eosin staining; magnification, 20×).
FINAL DIAGNOSIS
The patient was finally diagnosed with GP.
TREATMENT
An operation was performed followed by gastrointestinal decompression and intravenous nutrition because of the complete obstruction of the duodenum and poor general condition. Surgical exploration revealed obvious contracture and edema of the second part of the duodenal wall. After incising the duodenum, the bowel lumen was found to be exceedingly narrow, but the inner wall of the duodenum was smooth. Further exploration found a 3-cm mass located in the “groove part” of pancreas, specifically the mass was at the pancreatic head and oppressing the descending duodenum. No enlarged lymph nodes were found. Pancreaticoduodenectomy (PD) was performed to relieve the obstruction and thoroughly remove the pancreatic lesions. Pathologic examination of the specimens showed fibrous tissue hyperplasia in the head of the pancreas with acute and chronic inflammatory changes (Figure 2B).
OUTCOME AND FOLLOW-UP
The patient had an uneventful recovery with no complications.
DISCUSSION
There are many causes of duodenal obstruction, including both intra- and extra-duodenal factors. As a malignant disease with a poor prognosis, pancreatic head carcinoma is the most common cause of complete obstruction of the duodenum. However, benign pancreatic diseases, including GP, rarely induce complete duodenal obstruction. GP is a type of chronic pancreatitis occurring in an anatomic area between the duodenum, head of the pancreas, and common bile duct. Because of its special location and the contracture induced by long-term chronic inflammation, the small mass lesions in patients with GP can lead to thickening of the wall of the duodenum and stenosis of the intestinal cavity. Notably, some cases of chronic pancreatitis can convert to pancreatic cancer in a short term, and small pancreatic cancer lesions can sometimes be found during the pathological examination. We herein report a case of complete duodenal obstruction caused by GP. In fact, a mass lesion in the pancreas with complete duodenal obstruction can be easily misdiagnosed as pancreatic cancer, as in our case. Some image examinations, such as multidetector computed tomography, magnetic resonance imaging, and ultrasound-guided fine needle aspiration, may provide more evidence for differential diagnosis between GP and pancreatic head carcinoma[6-10]. PD is a candidate surgical operation for GP while it is the only and standard surgical option for carcinomas of the pancreatic head[11-14]. Different from malignant diseases, groove resection of the pancreatic head is also considered a candidate surgical operation for patients with symptomatic GP that require a surgical intervention[15]. Complete duodenal obstruction was an important and specific symptom in our case. It also led to our performance of PD to thoroughly relieve the obstruction.
CONCLUSION
Because of the special location and the contracture induced by long-term chronic inflammation, our case reminds surgeons that some benign pancreatic diseases, such as GP, can also present with symptoms similar to those of pancreatic cancer. This knowledge can help to avoid an unnecessary radical operation.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: There are some conflicts in funding. Authors obtained the grant support from National Natural Science Foundation of China; Zhejiang Provincial Natural Science Foundation of China; Zhejiang Provincial Public Welfare Technology Application Research Projects; and Research Foundation of Health Bureau of Zhejiang Province.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: September 27, 2019
First decision: October 24, 2019
Article in press: November 15, 2019
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maetani I S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Liu MY
Contributor Information
Ya-Li Wang, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Chen-Hao Tong, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Jian-Hua Yu, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Zhi-Liang Chen, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Hong Fu, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Jian-Hui Yang, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Xin Zhu, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China.
Bao-Chun Lu, Department of Hepatobiliary Surgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing 312000, Zhejiang Province, China. sygd_lbc@126.com.
References
- 1.Jani B, Rzouq F, Saligram S, Nawabi A, Nicola M, Dennis K, Ernst C, Abbaszadeh A, Bonino J, Olyaee M. Groove Pancreatitis: A Rare form of Chronic Pancreatitis. N Am J Med Sci. 2015;7:529–532. doi: 10.4103/1947-2714.170624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pallisera-Lloveras A, Ramia-Ángel JM, Vicens-Arbona C, Cifuentes-Rodenas A. Groove pancreatitis. Rev Esp Enferm Dig. 2015;107:280–288. [PubMed] [Google Scholar]
- 3.Tezuka K, Makino T, Hirai I, Kimura W. Groove pancreatitis. Dig Surg. 2010;27:149–152. doi: 10.1159/000289099. [DOI] [PubMed] [Google Scholar]
- 4.Frutos-Pérez JM, Perea-Ribis M, Martínez-Pascual MA, Llopis-Sanchis M, Tornero-Estébanez C. Constitutional Syndrome, Ascites and Duodenal Thickening Presenting as Groove Pancreatitis. Eur J Case Rep Intern Med. 2018;5:000789. doi: 10.12890/2017_000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jun JH, Lee SK, Kim SY, Cho DH, Song TJ, Park DH, Lee SS, Seo DW, Kim MH. Comparison between groove carcinoma and groove pancreatitis. Pancreatology. 2018;18:805–811. doi: 10.1016/j.pan.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Patel BN, Brooke Jeffrey R, Olcott EW, Zaheer A. Groove pancreatitis: a clinical and imaging overview. Abdom Radiol (NY) 2019 doi: 10.1007/s00261-019-02239-1. [DOI] [PubMed] [Google Scholar]
- 7.Addeo G, Beccani D, Cozzi D, Ferrari R, Lanzetta MM, Paolantonio P, Pradella S, Miele V. Groove pancreatitis: a challenging imaging diagnosis. Gland Surg. 2019;8:S178–S187. doi: 10.21037/gs.2019.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oría IC, Pizzala JE, Villaverde AM, Spina JC, Pasqua AV, Lazarte JC, Mazza OM, Marcolongo MM. Endoscopic Ultrasound in the Diagnosis of Pancreatoduodenal Groove Pathology: Report of Three Cases and Brief Review of the Literature. Clin Endosc. 2019;52:196–200. doi: 10.5946/ce.2018.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal PK, Harri P, Nandwana S, Moreno CC, Muraki T, Adsay V, Cox K, Pehlivanoglu B, Alexander LF, Chatterjee A, Miller FH. Paraduodenal pancreatitis: benign and malignant mimics at MRI. Abdom Radiol (NY) 2017;42:2652–2674. doi: 10.1007/s00261-017-1238-9. [DOI] [PubMed] [Google Scholar]
- 10.Triantopoulou C, Dervenis C, Giannakou N, Papailiou J, Prassopoulos P. Groove pancreatitis: a diagnostic challenge. Eur Radiol. 2009;19:1736–1743. doi: 10.1007/s00330-009-1332-7. [DOI] [PubMed] [Google Scholar]
- 11.Aguilera F, Tsamalaidze L, Raimondo M, Puri R, Asbun HJ, Stauffer JA. Pancreaticoduodenectomy and Outcomes for Groove Pancreatitis. Dig Surg. 2018;35:475–481. doi: 10.1159/000485849. [DOI] [PubMed] [Google Scholar]
- 12.Dua MM, Visser BC. Surgical Approaches to Chronic Pancreatitis: Indications and Techniques. Dig Dis Sci. 2017;62:1738–1744. doi: 10.1007/s10620-017-4526-x. [DOI] [PubMed] [Google Scholar]
- 13.Levenick JM, Sutton JE, Smith KD, Gordon SR, Suriawinata A, Gardner TB. Pancreaticoduodenectomy for the treatment of groove pancreatitis. Dig Dis Sci. 2012;57:1954–1958. doi: 10.1007/s10620-012-2214-4. [DOI] [PubMed] [Google Scholar]
- 14.Rahman SH, Verbeke CS, Gomez D, McMahon MJ, Menon KV. Pancreatico-duodenectomy for complicated groove pancreatitis. HPB (Oxford) 2007;9:229–234. doi: 10.1080/13651820701216430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu C, Huang Q, Zhu J, Zhang X, Qin X. Groove resection of pancreatic head in groove pancreatitis: A case report. Exp Ther Med. 2017;14:1983–1988. doi: 10.3892/etm.2017.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]