Abstract
BACKGROUND
Gastric cancer is the third most lethal malignant tumor worldwide. Metastasis has always been a major cause of poor prognosis. Epidemiological evidence shows that the most common sites for metastasis of gastric carcinoma are the liver (48%), peritoneum (32%), lung (15%), and bone (12%); however, subcutaneous metastasis is are and occurs in approximately 0.8% of cases. We report a rare case of armpit subcutaneous metastasis of gastric cancer. The best surgical window was missed, as a result of lacking attention of the mass.
CASE SUMMARY
A 69-year-old man who had previously undergone radical gastrectomy and received eight cycles of oral chemotherapy for gastric cancer showed a rapidly growing mass in his the left armpit; within just 3 mo, the mass grew to a size of 6.9 cm × 4.4 cm × 5.7 cm. Color Doppler ultrasonography and Positron emission tomography/computed tomography prompted the possibility of metastasis of the malignancy. Fine needle aspiration biopsy guided by color Doppler ultrasound showed the presence of cancer cells in the mass. Immunohistochemical examination showed CDX-2 (+), PCK (+), CK20 (+), CK7 (-), and TTF (-), which supported the metastasis of gastric cancer. Considering the risk of resection, the patient did not undergo surgical treatment.
CONCLUSION
The case indicates that unidentified subcutaneous masses in patients with a history of gastric cancer should be carefully evaluated.
Keywords: Stomach neoplasms, Neoplasm metastasis, Subcutaneous, Case report, Cancer therapy, Skin neoplasms
Core tip: Epidemiological evidence shows that the most common metastasis sites of gastric carcinoma are the liver (48%), peritoneum (32%), lung (15%), and bone (12%); however, subcutaneous metastasis is are and occurs in approximately 0.8% of cases. The recurrence and metastasis of malignant tumors still contribute to more than 90% of cancer mortalities. For the uncertainty of mechanism of metastasis and metastatic sites, and the limitations of monitoring methods, early detection of metastatic lesions of gastric cancer is difficult. This case demonstrates more sensitive and applicable monitoring methods and early attention may improve the early diagnosis rate.
INTRIDUCTION
Gastric cancer is prevalent worldwide, with an average of approximately 990000 new cases per year from 182 countries and 30 world regions[1]. The highest incidence is observed in Eastern Asia[2,3]. According to the Eindhoven Cancer Registry statistics, between 1995 and 2012, about 40% of gastric cancer patients had one metastasis at least[4]. The most common metastasis sites of gastric carcinoma are the liver (48%), peritoneum (32%), lung (15%), and bone (12%); however, relevant data indicated that the incidence of subcutaneous metastasis of gastric cancer is about 0.8%[5,6]. Today, there is no data referring to the left armpit metastasis of gastric carcinoma. Here we report the case of a patient with stage III gastric carcinoma who underwent curative intent resection (R0) and D2 lymph node dissection and received eight cycles of chemotherapy post-surgery. However, left armpit subcutaneous metastasis occurred in the fifth year after surgery. We report the case to promote the exploration and monitoring of unusual rare metastatic sites of advanced gastric cancer, and provide clinical evidence for the diagnosis and treatment of metastasis of gastric cancer.
CASE PRESENTATION
Chief complaints
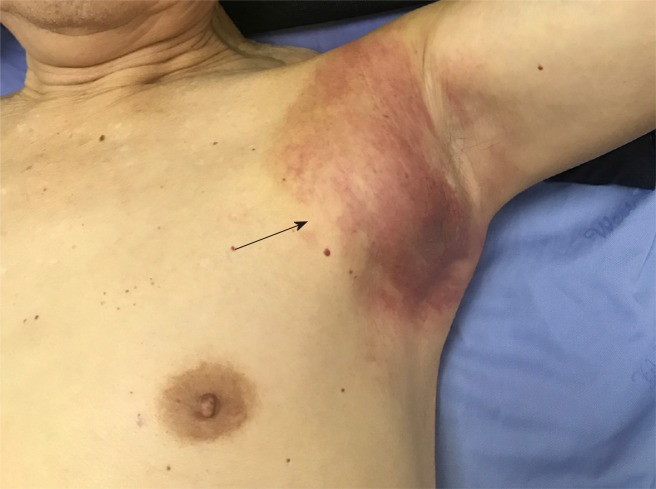
A 69-year-old man was re-admitted to West China Hospital of Sichuan University due to an asymptomatic lump in his left armpit for 3 mo (Figure 1).
Figure 1.
Left armpit subcutaneous metastasis arising from primary gastric cancer.
History of present illness
The patient had a history of gastric neoplasms. Five years ago, he had undergone curative gastrectomy, followed by eight cycles of oral chemotherapy.
History of past illness
The patient had a free previous medical history.
Physical examination
Physical examination after admission showed that the patient’s body temperature was 36 °C, heart rate was 106 bpm, respiratory rate was 20 breaths per minute, and blood pressure was 140/68 mmHg. A mass of approximately 7.0 cm × 4.5 cm × 5.7 cm mass was observed in the left armpit of the patient. The skin of the mass was reddish, and the temperature was high. There was no skin ulceration or itching. The patient experienced no pain when the mass was pressed. The mass was hard, fixed, and had an unclear boundary.
Laboratory examinations
Blood analysis did not reveal raised levels of tumor markers. Prothrombin and partial thromboplastin times were normal and serum C-reactive protein level had increased to 4.5 mg/dL (normal range: < 0.8 mg/dL).
Imaging examinations
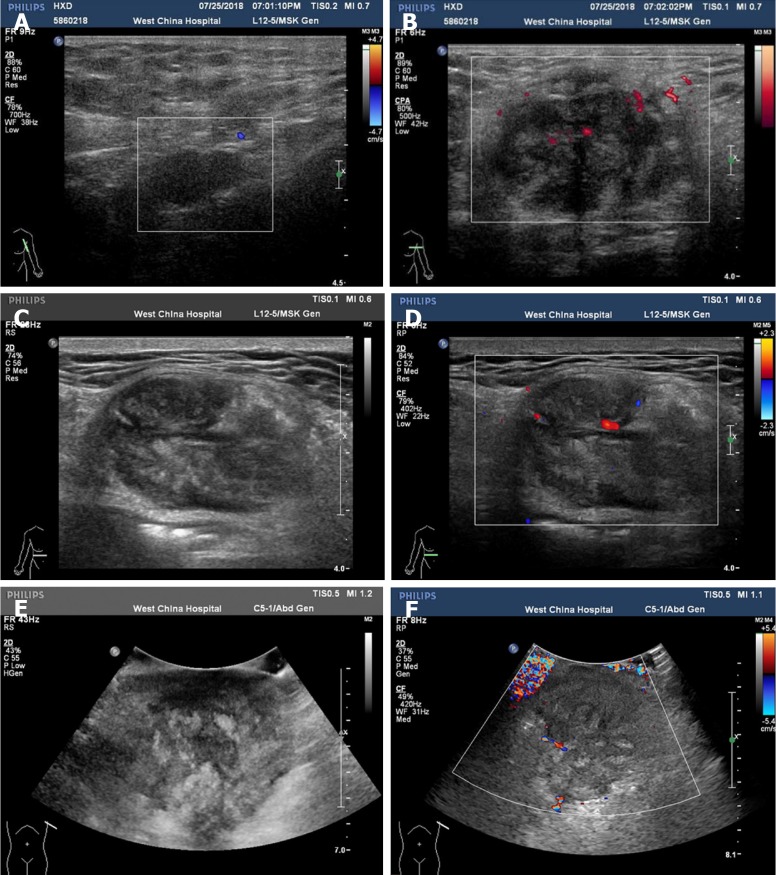
Initial color Doppler ultrasound imaging of the left axillary lump showed a heterogeneous echo pattern sized approximately 4.2 cm × 2.4 cm × 3.8 cm, with an unclear boundary and irregular shape. There was linear blood flow signal observed in the mass. Several abnormally enlarged lymph nodes were observed around the mass. After three months, color Doppler examination revealed that the mass grew to a size of 6.9 cm × 4.4 cm × 5.7 cm (Figure 2). A chest computed tomography (CT) scan revealed a 3.9 cm × 5.7 cm soft tissue lump in the left armpit.
Figure 2.
Color Doppler ultrasound images. A and B: Initial color Doppler ultrasound images of the left axillary lump; C-F: Color Doppler ultrasound images of the left axillary lump after three months.
Further diagnostic work-up
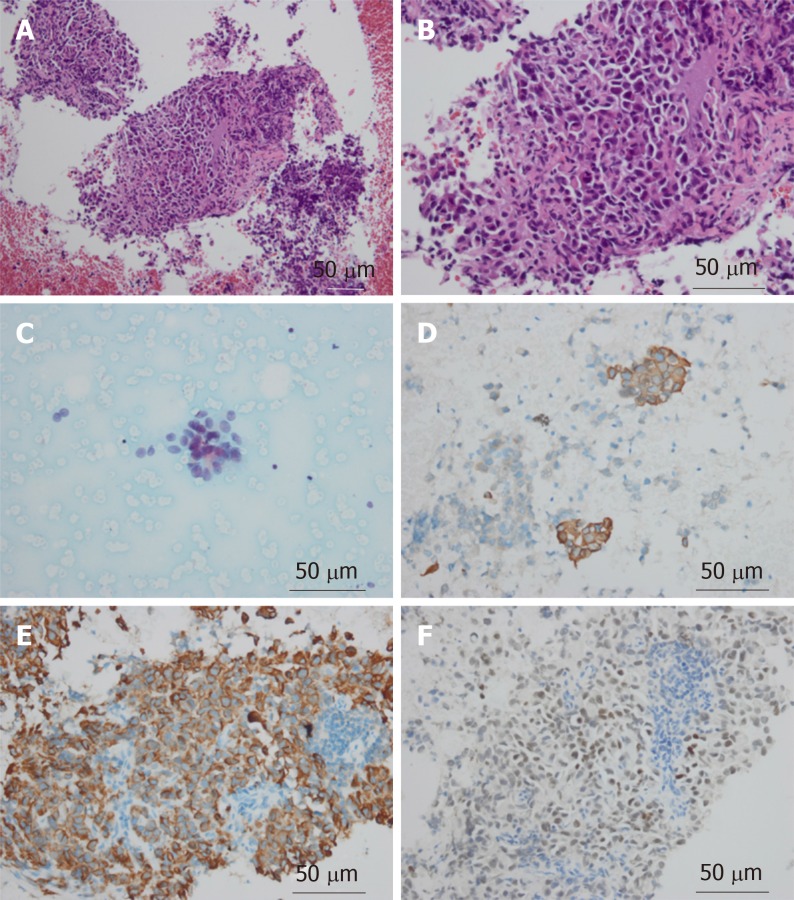
Fine needle aspiration biopsy of the mass guided by color Doppler ultrasound found cancer cells in the mass. Immunohistochemical examination showed that the mass was CDX2-positive, PCK-positive, CK20-positive, CK7-negative, and TTF-negative; this confirmed gastric cancer metastasis (Figure 3).
Figure 3.
Pathological images of the axillary mass. A-C: Stomach tumor cells were detected by cell smear (A: HE staining, ×200; B: HE staining, ×400; C: HE staining, ×400); D-F: The tumor cells were positive for CK20 (D), PCK (E), and CDX-2 (F) (immunohistochemical staining, ×400).
Positron emission tomography/CT (PET/CT) identification of the distant metastasis
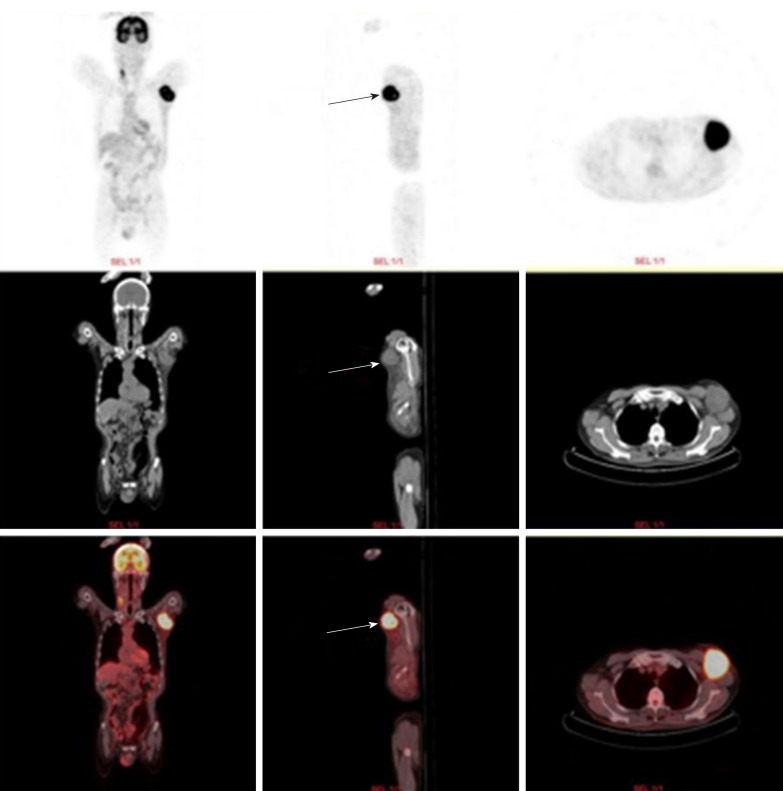
PET/CT examination showed a soft tissue mass sized approximately 6.2 cm × 5.5 cm in the left axilla. The internal density was uneven, and 18F-fludeoxyglucose uptake was abnormally high. The maximum SUV was 11.13 (Figure 4).
Figure 4.
Positron emission tomography computed tomography examination showed that a 6.2 cm × 5.5 cm soft tissue mass was visible in the left axilla (arrow).
MULTIDISCIPLINARY EXPERT CONSULTATION
Wen Zhuang, MD, PhD, Professor and Chief Physician, West China Hospital of Sichuan University
The patient should undergo surgical resection of the left axillary tumor and adjuvant radiotherapy, chemotherapy, or targeted therapy.
Jie Chen, MD, PhD, Professor, Department of Breast Surgery, West China Hospital of Sichuan University
The patient should undergo surgical treatment of the left mass in his left armpit and total excision of the lesion. If necessary, we will assist in the operation.
Zhi-Xing Chen MD, PhD, Associate Professor, Department of Plastic Surgery, West China Hospital of Sichuan University
Surgical resection of the left axillary subcutaneous tumor may require skin grafting.
FINAL DIAGNOSIS
Left axillary subcutaneous metastasis of gastric cancer.
TREATMENT
Due to the adhesion between the large subcutaneous mass of the left axilla and surrounding tissues and severe local inflammation, skin grafting might be required after operation. We asked plastic surgery experts to assist in the operation of tumor resection. However, considering the risk of resection, the patient did not agree to undergo surgical treatment.
OUTCOME AND FOLLOW-UP
After discharge, the patient was lost to follow-up. However, through telephonic communication, we know that he is on long-term medication and is alive.
DISCUSSION
Epidemiological studies on metastasis of gastric cancer are rare. Currently, the TNM system is used to stage malignant tumors, and cancer registries often only use “M0” and “M1” to indicate the absence or presence of distant metastasis. Therefore, there is a lack of information regarding specific distant metastasis sites[5].
The five-year cumulative risk of relapse (restricted to patients who undergo R0 resections and excluding in-hospital deaths) for patients with pathological stage T3 tumors is 83% for D1 dissection and 72% for D2 dissection[7]. Although considered a “localized tumor”, gastric cancer may show locoregional metastasis and this can be the most important signal of negative prognosis[8-10].
Metastasis is mostly driven by the acquisition of genetic and/or epigenetic alterations within tumor cells and the formation of the tumor microenvironment[11]. Metastasis of malignant tumors can occur at an early stage of primary tumorigenesis[12]. If metastasis of cancer cells occurs before clinical detection, surgical resection may not prevent recurrence, invasion, and further metastasis. In our case, the patient was followed regularly and monitored through dynamic imaging, and no sign of recurrence was observed. However, in the fifth year, he was diagnosed with distant subcutaneous metastasis. Because of mild clinical manifestations and metastasis into a rare site, the lump was not considered severe. Consequently, we missed the best surgical window.
Currently, ultrasound and color Doppler are the preferred non-invasive imaging modalities of choice allowing to diagnose superficial masses, which can not only differentiate the nature of masses, but also provide detailed information about vascular anatomy[13-15]. Color Doppler, in particular, is highly specific in the identification of benign and malignant nodules of the skin and subcutaneous tissue[16,17]. In addition, high-resolution ultrasound may contribute to the differential diagnosis of skin and subcutaneous lesions[17].
Treatment of even well-confined tumors can become difficult due to repeated changes in molecular phenotypes[18], immune evasion[19], and drug resistance[20]. Through a long-term observation, it has been shown that some types of malignant tumors metastasize only to specific target organs[20]. Cutaneous or subcutaneous tissues may not provide a better growth microenvironment than the liver or peritoneum. However, several cases of subcutaneous metastasis of gastric cancer, including scalp metastasis and mandibular metastasis and so like, have been reported in succession[21,22]. Here we report a rare case that provides clinical evidence for studying the specific metastatic sites of gastric cancer.
CONCLUSION
We should pay enough attention to any local mass developing in patients with a history of gastric cancer. We believe that in the future more sensitive, specific, and inexpensive techniques, such as nanoparticles[23] and liquid biopsy, would contribute to the detection of metastasis[24].
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: September 26, 2019
First decision: October 24, 2019
Article in press: November 14, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aydin M, Corvino A, Fiori E S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Li X
Contributor Information
Feng-Jun He, Department of Gastrointestinal Surgery, West China Hospital Sichuan University, Chengdu 610041, Sichuan Province, China.
Peng Zhang, Department of Gastrointestinal Surgery, West China Hospital Sichuan University, Chengdu 610041, Sichuan Province, China.
Mo-Jin Wang, Department of Gastrointestinal Surgery, West China Hospital Sichuan University, Chengdu 610041, Sichuan Province, China.
Yi Chen, Department of Gastrointestinal Surgery, West China Hospital Sichuan University, Chengdu 610041, Sichuan Province, China.
Wen Zhuang, Department of Gastrointestinal Surgery, West China Hospital Sichuan University, Chengdu 610041, Sichuan Province, China. zhuangwen1966@163.com.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 5.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307–52316. doi: 10.18632/oncotarget.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379–387. doi: 10.1016/j.jaad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 8.Imano M, Yasuda A, Itoh T, Satou T, Peng YF, Kato H, Shinkai M, Tsubaki M, Chiba Y, Yasuda T, Imamoto H, Nishida S, Takeyama Y, Okuno K, Furukawa H, Shiozaki H. Phase II study of single intraperitoneal chemotherapy followed by systemic chemotherapy for gastric cancer with peritoneal metastasis. J Gastrointest Surg. 2012;16:2190–2196. doi: 10.1007/s11605-012-2059-3. [DOI] [PubMed] [Google Scholar]
- 9.Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H, Nagawa H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. doi: 10.1093/annonc/mdp260. [DOI] [PubMed] [Google Scholar]
- 10.Ishigami H, Kitayama J, Kaisaki S, Yamaguchi H, Yamashita H, Emoto S, Nagawa H. Phase I study of biweekly intravenous paclitaxel plus intraperitoneal cisplatin and paclitaxel for gastric cancer with peritoneal metastasis. Oncology. 2010;79:269–272. doi: 10.1159/000323272. [DOI] [PubMed] [Google Scholar]
- 11.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 13.Corvino A, Catalano O, Corvino F, Sandomenico F, Setola SV, Petrillo A. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound? J Ultrasound. 2016;19:197–201. doi: 10.1007/s40477-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corvino A, Corvino F, Catalano O, Sandomenico F, Petrillo A. The Tail and the String Sign: New Sonographic Features of Subcutaneous Melanoma Metastasis. Ultrasound Med Biol. 2017;43:370–374. doi: 10.1016/j.ultrasmedbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Corvino A, Sandomenico F, Setola SV, Corvino F, Pinto F, Catalano O. Added value of contrast-enhanced ultrasound (CEUS) with Sonovue® in the diagnosis of inferior epigastric artery pseudoaneurysm: report of a case and review of literature. J Ultrasound. 2019;22:485–489. doi: 10.1007/s40477-019-00398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovagnorio F, Andreoli C, De Cicco ML. Color Doppler sonography of focal lesions of the skin and subcutaneous tissue. J Ultrasound Med. 1999;18:89–93. doi: 10.7863/jum.1999.18.2.89. [DOI] [PubMed] [Google Scholar]
- 17.Catalano O, Roldán FA, Varelli C, Bard R, Corvino A, Wortsman X. Skin cancer: findings and role of high-resolution ultrasound. J Ultrasound. 2019;22:423–431. doi: 10.1007/s40477-019-00379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura J, Okuyama K, Sato H, Yoda Y, Kai K, Noshiro H. Repeated changes of the molecular subtype in gastric metastasis from breast cancer: A case report. Mol Clin Oncol. 2016;4:695–698. doi: 10.3892/mco.2016.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 21.Kawai S, Nishida T, Hayashi Y, Ezaki H, Yamada T, Shinzaki S, Miyazaki M, Nakai K, Yakushijin T, Watabe K, Iijima H, Tsujii M, Nishida K, Takehara T. Choroidal and cutaneous metastasis from gastric adenocarcinoma. World J Gastroenterol. 2013;19:1485–1488. doi: 10.3748/wjg.v19.i9.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchberger MC. Unusual presentation of a cutaneous metastasis in the face arising from gastric cancer: a case report. SAGE Open Med Case Rep. 2018;6:2050313X18795080. doi: 10.1177/2050313X18795080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Liu B, Gao J. The application of nanoparticles in diagnosis and theranostics of gastric cancer. Cancer Lett. 2017;386:123–130. doi: 10.1016/j.canlet.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409–424. doi: 10.1038/s41571-019-0187-3. [DOI] [PubMed] [Google Scholar]