CTE is often conceptualized as a delayed-onset and progressive neurodegenerative disease with unique pathology. However, based on a comprehensive review of the evidence, Iverson et al. argue that CTE pathology cannot be considered inexorably progressive or wholly specific to those who have experienced repetitive neurotrauma.
Keywords: hyperphosphorylated tau, sports, concussion, mild TBI, neurodegenerative disease
Abstract
In the 20th century, chronic traumatic encephalopathy (CTE) was conceptualized as a neurological disorder affecting some active and retired boxers who had tremendous exposure to neurotrauma. In recent years, the two research groups in the USA who have led the field have asserted definitively that CTE is a delayed-onset and progressive neurodegenerative disease, with symptoms appearing in midlife or decades after exposure. Between 2005 and 2012 autopsy cases of former boxers and American football players described neuropathology attributed to CTE that was broad and diverse. This pathology, resulting from multiple causes, was aggregated and referred to, in toto, as the pathology ‘characteristic’ of CTE. Preliminary consensus criteria for defining the neuropathology of CTE were forged in 2015 and published in 2016. Most of the macroscopic and microscopic neuropathological findings described as characteristic of CTE, in studies published before 2016, were not included in the new criteria for defining the pathology. In the past few years, there has been steadily emerging evidence that the neuropathology described as unique to CTE may not be unique. CTE pathology has been described in individuals with no known participation in collision or contact sports and no known exposure to repetitive neurotrauma. This pathology has been reported in individuals with substance abuse, temporal lobe epilepsy, amyotrophic lateral sclerosis, multiple system atrophy, and other neurodegenerative diseases. Moreover, throughout history, some clinical cases have been described as not being progressive, and there is now evidence that CTE neuropathology might not be progressive in some individuals. Considering the current state of knowledge, including the absence of a series of validated sensitive and specific biomarkers, CTE pathology might not be inexorably progressive or specific to those who have experienced repetitive neurotrauma.
Introduction
In the 20th century, chronic traumatic encephalopathy (CTE) was conceptualized as a neurological disorder affecting some active and retired boxers who had tremendous exposure to neurotrauma (Martland, 1928; Roberts, 1969). CTE was known by a number of terms, such as ‘punch drunk’ (Martland, 1928; Critchley, 1949), traumatic encephalopathy (Parker, 1934), traumatic encephalopathy of boxers (Grahmann and Ule, 1957), chronic progressive traumatic encephalopathy (Critchley, 1957), and dementia pugilistica (Millspaugh, 1937; Grahmann and Ule, 1957). An early clinical description by Martland (1928) of the neurological problems in some boxers included dysarthria, gait disturbance, tremor, and cognitive impairment. He reported that some cases remain mild and do not progress, but other cases progress to advanced parkinsonism and dementia. The neurological problems were often described as ‘pyramidal’ (e.g. abnormal reflexes) and ‘extrapyramidal’ (tremors and Parkinsonian gait disturbance), and this chronic brain damage was often first noticed in some active boxers who were in their twenties and thirties (Parker, 1934; Critchley, 1957; Sercl and Jaros, 1962; Mawdsley and Ferguson, 1963; Johnson, 1969; Roberts, 1969; Jedlinski et al., 1970; Corsellis et al., 1973; Harvey and Davis, 1974). Researchers reported that some boxers with chronic brain damage exhibited diverse neuropsychiatric problems that co-occurred with the more characteristic neurological features (Mawdsley and Ferguson, 1963; Payne, 1968; Johnson, 1969; Roberts, 1969), such as euphoria (Critchley, 1949, 1957), a child-like demeanour (Corsellis et al., 1973) or ‘fatuous cheerfulness’ (Critchley, 1957), and aggression, volatility, and anger dyscontrol (Critchley, 1957; Mawdsley and Ferguson, 1963; Payne, 1968; Johnson, 1969; Corsellis et al., 1973; Harvey and Davis, 1974).
It has never been clear to the medical and scientific community the extent to which CTE in boxers was static, progressive, or whether its course represented two or more different conditions (Martland, 1928; Parker, 1934; Carrol, 1936; Critchley, 1949; Grahmann and Ule, 1957; Courville, 1962; Johnson, 1969; Roberts, 1969; Mendez, 1995; Victoroff, 2013). When progressive, at least in a subgroup, it was not clear the extent to which the progression was driven by specific neuropathology associated with CTE, other co-occurring neuropsychiatric, medical, and/or neurodegenerative conditions.
Prior to 2016, there were no agreed upon clinical or neuropathological diagnostic criteria for CTE. Preliminary criteria for defining the post-mortem neuropathology of CTE were published in 2016 (McKee et al., 2016). CTE, as a clinical disorder or disease, has never been included in the Diagnostic and Statistical Manual of Mental Disorders or the International Classification of Diseases—and as of 2019, there are still no agreed upon criteria for diagnosing CTE in a living person, although five overlapping sets of criteria have been proposed (Jordan, 2013; Victoroff, 2013; Montenigro et al., 2014; Reams et al., 2016; Laffey et al., 2018). The prevalence of CTE is unknown, and resistance and resilience factors relating to the neuropathology and the presumed clinical disease have not been identified.
In recent years, the two research groups in the USA who have led the field have stated definitively that CTE is a delayed-onset and progressive neurodegenerative disease, with symptoms appearing ‘in midlife’ (Gavett et al., 2011b; Stern et al., 2011b) or decades after exposure (McKee et al., 2009; Omalu et al., 2010a, 2011; Gavett et al., 2011b; Stern et al., 2011b, 2013; Baugh et al., 2012; Montenigro et al., 2014; Omalu, 2014). The modern putative theory is that CTE is a distinct (McKee et al., 2009; Gavett et al., 2011b; Baugh et al., 2012, 2014; Mez et al., 2013) and unique (Baugh et al., 2014) neurodegenerative disease (Gavett et al., 2011a; Baugh et al., 2012, 2014; Mez et al., 2013; McKee et al., 2014). It has also been asserted that CTE pathology is only found in individuals (i.e. mostly boxers, football players, and contact sport athletes) who have been exposed to repetitive subclinical to clinical neurotrauma (Stern et al., 2011a, 2013; Baugh et al., 2012, 2014; Mez et al., 2013; McKee et al., 2014). However, in the past few years CTE pathology has been identified in individuals with (i) substance abuse (Noy et al., 2016); (ii) temporal lobe epilepsy (Puvenna et al., 2016); (iii) schizophrenia who have undergone leukotomy (Shively et al., 2017); (iv) amyotrophic lateral sclerosis (ALS) (Fournier et al., 2015; Gao et al., 2017); (v) multiple system atrophy (Koga et al., 2016); and (vi) other neurodegenerative diseases (Ling et al., 2015) who have no known participation in collision or contact sports and no known exposure to repetitive neurotrauma. It is one thesis of this review that the neuropathology of the modern descriptions of CTE may not be inexorably progressive. A second thesis is that CTE neuropathology might not be unique to those who have experienced repetitive neurotrauma.
Macroscopic and microscopic pathology of CTE
The seminal neuropathological criteria associated with dementia pugilistica were described in 1973. Corsellis and colleagues (1973) examined the brains of 15 boxers and described both the gross and microscopic neuropathology that was common, although not invariable, to these cases (e.g. neurofibrillary degeneration, neuronal loss, ‘scarring’ of the cerebellar tonsils, and fenestrated cavum septum pellucidum). They noted, like previous authors (Grahmann and Ule, 1957; Constantinidis and Tissot, 1967), that extensive neurofibrillary tangles were present in boxers but senile plaques (i.e. amyloid plaques characteristic of Alzheimer’s disease) were sparse to absent. Corsellis and colleagues used Bielschowsky silver impregnation to detect neurofibrillary degeneration, a methodology that does not detect many of the subtle hyperphosphorylated tau (p-tau) lesions, such as astrocytic p-tau or pretangles, described in the modern version of CTE in this century using immunohistochemistry (McKee et al., 2016). A recent study re-examined the original Corsellis case series using modern immunohistochemistry and applying the new consensus criteria for defining the tau pathology of CTE, described below, and found that only 50% of the cases met neuropathological criteria for CTE (Goldfinger et al., 2018).
In 1990, Roberts re-examined 14 of 15 cases from the Corsellis series, using a modern immunohistochemistry staining technique that was more sensitive and more consistent than the previously used silver staining, and reported that extensive amyloid-β plaques, of a comparable extent to that seen in Alzheimer’s disease, were common in these reported cases of dementia pugilistica (Roberts et al., 1990a). This was an important finding because prior to 1990, it was assumed that ‘senile plaques’ (i.e. ‘neuritic’ plaques) were not present in dementia pugilistica (Grahmann and Ule, 1957; Constantinidis and Tissot, 1967). This rekindled theorizing about a link between boxing, Alzheimer’s disease neuropathological change, and progressive dementia (Roberts et al., 1990a; Tokuda et al., 1991). What is now considered a defining feature of CTE, p-tau in depths of cortical sulci (McKee et al., 2016), was identified by Hof et al. (1991) in a patient with autism who had a long history of head banging behaviour. Geddes et al. (1999) suggested that the distribution of the tau pathology associated with repetitive head injuries might relate to damage to blood vessels or perivascular elements, and blood–brain barrier disruption has more recently been reported in a case study (Doherty et al., 2016). Recent preclinical studies have examined blood–brain barrier disruption association with experimental neurotrauma (Jullienne et al., 2016; Kenney et al., 2016) and identified associated p-tau immunolabelling (Goldstein et al., 2012; Meabon et al., 2016). More research is needed to determine the translational relevance of these experimental studies to human disease, and whether p-tau immunolabelling in experimental rodents is similar to structural p-tau accumulations in neurons or astrocytes in human brains.
In 2005, Omalu et al. published the first case description of the neuropathology of CTE in a retired National Football League (NFL) player. The pathology in this case report was depicted in a four-panel figure. Panel A depicted a collection of diffuse amyloid-β plaques, said to be frequent in the neocortex, although the extent of amyloid-β was not further characterized or depicted other than the single high magnification field. It is important to appreciate that the plaques depicted in this figure are not unique to CTE; they occur in adults in association with ageing (Braak and Braak, 1991; Morris et al., 1996) and in individuals with other neurodegenerative diseases (Nelson et al., 2012). Panels B and C show relatively small amounts of p-tau in neocortex (panel B) and in a neocortical neuron (panel C). Panel D shows no amyloid-β immunoreactivity in the hippocampal cornu ammonis (CA-1) region, an expected finding for a 50-year-old. It is difficult to discern neuronal loss in panel D, although the authors reported mild neuronal dropout in this region. The text of the paper also indicated, although did not depict, the impression of mild to moderate neuronal loss in the substantia nigra with extraneuronal pigment. This finding is sometimes present in association with ageing (Fearnley and Lees, 1991) and with other neurodegenerative diseases (Dickson, 2012). Overall, a notable feature of this index case is the relatively small amount of neuropathology depicted in the figures. This same research group published a second case in 2006 (Omalu et al., 2006) and two additional cases in 2010 (Omalu et al., 2010b, c), in which some degree of neocortical p-tau were depicted. They also visually depicted p-tau in the locus coeruleus, a non-specific finding common in ageing (Braak et al., 2011). In 2009, McKee and colleagues reviewed the world literature and identified 51 neuropathologically confirmed cases of CTE, of which 46 (90%) were athletes and 39 (76.5%) were boxers. Clinical and neuropathological characteristics were documented (McKee et al., 2009). The review included three cases from their research group—two boxers and one retired NFL player.
Four proposed ‘phenotypes’ of CTE neuropathology
Omalu and colleagues introduced four neuropathological ‘phenotypes’ of CTE in 2011 (Omalu et al., 2011). Phenotype 1 was described as ‘a combination of sparse to frequent neurofibrillary tangles (NFTs) and neuropil threads (NTs) in the cerebral cortex and brainstem, with or without NFTs and NTs in the subcortical nuclei/basal ganglia, no NFTs and NTs in the cerebellum, and no diffuse amyloid plaques in the cerebral cortex’ (Omalu et al., 2011, p.178). It is important to appreciate that sparse amounts of NFTs and NTs in brainstem and some parts of the neocortex are commonly associated with ageing (Braak and Braak, 1991), so phenotype 1 is likely common in the general population of aged individuals who have not had exposure to repetitive neurotrauma. Phenotype 2 was described as ‘a combination of sparse to frequent NFTs and NTs in the cerebral cortex and brainstem with or without NFTs and NTs in the subcortical nuclei/basal ganglia, no NFTs and NTs in the cerebellum, and sparse to frequent diffuse amyloid plaques in the cerebral cortex’ (Omalu et al., 2011, pp.178–79 of the article). Phenotype 2 thus adds the non-specific finding of ‘sparse to frequent diffuse plaques’ to the non-specific phenotype 1 pathology, expanding the scope and permissibility of the CTE pathological diagnosis. Again, the neuropathology described in phenotype 2 is likely common in the general aged population, because diffuse plaques are a feature of ageing and Alzheimer’s disease as noted above. Phenotype 3 was described as ‘a combination of moderate to frequent NFTs and NTs in brainstem nuclei (brainstem predominant), none to sparse NFTs and NTs in the cerebral cortex and subcortical nuclei/basal ganglia, no NFTs and NTs in the cerebellum, and no diffuse amyloid plaques in the cerebral cortex’ (Omalu et al., 2011, p.179). It is important to note that the brainstem is the earliest structure in which p-tau accumulates with normal ageing (Braak et al., 2011), and p-tau is present in larger amounts in the brainstem in a number of neurodegenerative diseases (Dickson et al., 2011).
Phenotype 4, as written, is perplexing. Phenotype 4 was described as ‘a combination of none to sparse (several) NFTs and NTs in the cerebral cortex, brainstem, and subcortical nuclei/basal ganglia (incipient); no NFTs and NTs in the cerebellum; and no diffuse amyloid plaques in the cerebral cortex’ (Omalu et al., 2011, p.179). As written, a brain with no pathology and no immunolabelling for p-tau or amyloid-β could qualify for phenotype 4—which of course cannot be true. What the authors appear to mean is that a brain with ‘several’ NFTs and NTs in the cerebral cortex, brainstem, and/or subcortical nuclei/basal ganglia could qualify as having CTE. We are not aware of any published studies to date that have examined the specificity of the four phenotypes. In short, the four phenotypes put forward by Omalu and colleagues are sensitive, broad, and encompassing; thus, many middle-aged and older adults in the general population, even with no history of neurotrauma, could meet the minimal criteria.
‘Characteristic’ neuropathology of CTE
In the past few years, the neuropathology described as ‘characteristic’ of CTE has included broad and diverse macroscopic and microscopic features (Table 1), such as (i) frontal and temporal atrophy, thinning of the hypothalamic floor, shrinkage of the mammillary bodies, pallor of the substantia nigra, hippocampal sclerosis, and reduction in brain mass; (ii) enlarged ventricles; (iii) cavum septum pellucidum with or without septal fenestrations; (iv) localized neuronal and glial accumulations of phosphorylated tau involving perivascular areas of the cerebral cortex and sulcal depths, and with a preference for neurons within superficial cortical laminae; (v) multifocal axonal varicosities involving deep cortex and subcortical white matter; (vi) variable and often absent amyloid-β deposits; and (vii) TAR DNA-binding protein 43 (TDP-43)-positive inclusions and neurites (McKee et al., 2009; Gavett et al., 2011a; Baugh et al., 2012).
Table 1.
Neuropathology described as ‘characteristic’ of CTE in the literature
| Gross pathological features |
| Cavum septum pelluciduma |
| Lateral or third ventricle enlargementa |
| Frontal atrophy |
| Temporal atrophy |
| Diencephalon atrophy |
| Basal ganglia atrophy |
| Brainstem atrophy |
| Cerebellar atrophy |
| Thinning of the hypothalamic floor |
| Shrinkage of the mammillary bodiesa |
| Pallor of the substantia nigra |
| Hippocampal sclerosis |
| Reduced brain weight |
| Microscopic neuropathology |
| Amyloid-β deposition (variable) |
| Multifocal axonal varicosities |
| Frontal and temporal cortex |
| Subcortical white matter |
| Deep white matter tracts |
| Diffuse axonal loss |
| Subcortical white matter |
| White matter tracts |
| Neuronal loss |
| Hippocampus |
| Entorhinal cortex |
| Amygdala |
| Locus coeruleus |
| Substantia nigra |
| Medial thalamus |
| TDP-43 |
| Frontal cortex |
| Medial temporal cortexa |
| Hippocampusa |
| Amygdalaa |
| Insular cortices |
| Basal ganglia |
| Thalamus |
| Hypothalamus |
| Brainstem |
| Hyperphosphorylated tau |
| Perivascular in the neocortex |
| Depths of sulcib |
| Superficial layers of cerebral cortexa |
Supportive criteriaa and pathognomonic criterionb for CTE based on the preliminary consensus criteria (McKee et al., 2016). Prior to 2015, diverse and widespread non-specific neuropathology was described as ‘characteristic’ of CTE in some articles. Most of the neuropathology described in past articles is also present in association with ageing and in other neurological, neuropsychiatric, and neurodegenerative disorders—and is not unique to CTE. As of 2015, a small number of these are now considered preliminary asupportive criteria for the neuropathology of CTE, and a single finding, bp-tau in depths of sulci (neuronal and astrocytic), particularly in a perivascular distribution, is considered the sole necessary and sufficient pathognomonic criterion of CTE (McKee et al., 2016).
Most of those features, however, are associated with ageing, other neurological diseases, or both, such as: enlarged ventricles (Jackson et al., 2011; Apostolova et al., 2012), frontal and temporal atrophy (Jack et al., 1997; Moran et al., 2001; Fjell et al., 2009; Nyberg et al., 2010), shrinkage of the mammillary bodies (Raz et al., 1992; Shear et al., 1996; Kumar et al., 2008), hippocampal sclerosis (Berkovic et al., 1991; Nelson et al., 2013; Nag et al., 2015), reduction in brain volume (Scahill et al., 2003; Fjell et al., 2009), axonal loss (Medana and Esiri, 2003), neuronal loss (Morrison and Hof, 1997), amyloid-β accumulation (Fukumoto et al., 1996), and TDP-43 deposition (Nag et al., 2015). Moreover, subcortical and cortical accumulation of p-tau is associated with normal ageing (Giannakopoulos et al., 1995; Hof et al., 1996; Price and Morris, 1999; Braak et al., 2011), opiate abuse (Kovacs et al., 2015), and other neurological and neurodegenerative diseases (Rademakers et al., 2004; Lewis and Dickson, 2016). The unique and defining microscopic feature for CTE is p-tau aggregates in neurons, astrocytes, and cell processes around small blood vessels in an irregular pattern at the depths of the cortical sulci (McKee et al., 2013, 2016).
Four proposed ‘stages’ of CTE neuropathology
In 2013, McKee and colleagues presented a large case series and hypothesized a diverse set of four neuropathological ‘stages’ of CTE (McKee et al., 2013). The stages are described in detail in the article, and summarized in the note below their figure 3, as follows:
‘In stage I CTE, p-tau pathology is restricted to discrete foci in the cerebral cortex, most commonly in the superior, dorsolateral or lateral frontal cortices, and typically around small vessels at the depths of sulci. In stage II CTE, there are multiple epicentres at the depths of the cerebral sulci and localized spread of neurofibrillary pathology from these epicentres to the superficial layers of adjacent cortex. The medial temporal lobe is spared of neurofibrillary p-tau pathology in stage II CTE. In stage III, p-tau pathology is widespread; the frontal, insular, temporal and parietal cortices show neurofibrillary changes with greatest severity in the frontal and temporal lobe, concentrated at the depths of the sulci. In addition, the amygdala, hippocampus and entorhinal cortex show neurofibrillary pathology. In stage IV CTE, there is severe p-tau pathology affecting most regions of the cerebral cortex and the medial temporal lobe, sparing calcarine cortex in all but the most severe cases’ (McKee et al., 2013, figure 3, p.52).
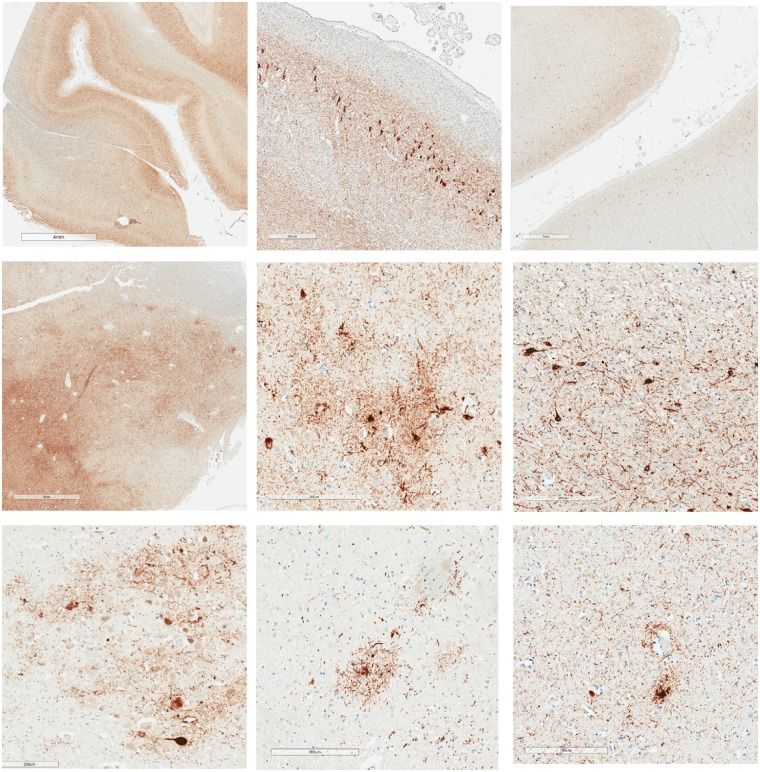
The stages of CTE pathology are said to advance as a function of age, with rough correlations to clinical signs attempted (although not statistically verified). McKee and colleagues have suggested that stage I CTE might be asymptomatic or preclinical in some individuals (McKee et al., 2013, 2015). As a practical matter, the precise boundaries between different stages cannot be easily gleaned from the description of the stages in the original paper (McKee et al., 2013), making it difficult to apply them in research and clinical practice. The diagnostic error rate for stage I and stage II CTE within and between neuropathologists has not been explored. Moreover, stage III and IV cases are often diagnosed with comorbid canonical neurodegenerative diseases, such as Alzheimer’s disease or frontotemporal dementia (McKee et al., 2013), making it difficult to separate some of the pathology of ageing and sporadic neurodegenerative diseases from the pathology believed to underlie CTE specifically. This is illustrated in Fig. 1. The accumulation of p-tau in normal ageing and in association with neurodegenerative diseases is distributed throughout many brain regions. The images depict p-tau in a variety of areas, such as near blood vessels, in hippocampus, and in amygdala, in men with no known history of TBI, repetitive mild neurotrauma, or participation in contact sports.
Figure 1.
P-tau accumulates with ageing and in neurodegenerative diseases in diverse brain regions including depths of cortical sulci, in hippocampus, amygdala, mammillary bodies, thalamus, locus coeruleus, raphe nucleus, and around small blood vessels. Images of p-tau accumulations from three males with no known history of TBI, repetitive neurotrauma, or participation in contact or collision sports (Iverson et al., 2019) (Cases 2, 3, and 5). Case 2 was an 82-year-old male who was not reported to be cognitively impaired at the time of death. He might have had mental health problems at some point during later adulthood, although this was not clearly documented. His cause of death was aortic valve stenosis. His APOE genotype was ε4-3. This case was rated as Braak NFT stage V, Thal amyloid-β phase 3-4, with moderate CERAD neuritic plaques, and NIA-AA designation A3B3C2. Case 3 was an 80-year-old male who was not reported to be cognitively impaired at the time of death. His cause of death was cardiomyopathy. His APOE genotype was ε3-3. This case was rated as Braak NFT stage IV, Thal amyloid-β phase 0, with no CERAD neuritic plaques, and NIA-AA Designation A0B2C0. PART and ARTAG pathology were present. Case 5 was a 73-year-old male who was not reported to be cognitively impaired at the time of death. His cause of death was cardiomyopathy. His APOE genotype was ε3-3. This case was rated as Braak NFT stage IV, Thal amyloid-β phase 1, with sparse CERAD neuritic plaques, and NIA-AA designation A1B2C1. PART and ARTAG pathology were present. Top row: Left = extensive, diffusely distributed p-tau with NFT at low magnification (illustrating uniform involvement of neocortex including sulcal depths that occurs with ageing and with Alzheimer’s disease; scale bar = 4 mm; Case 5, age = 73); middle = CA-2 region of Ammon’s horn with extensive p-tau including NFTs (scale bar = 400 µm; Case 2, age = 82); right = low magnification showing extensive p-tau including NFTs with preferential involvement of neocortical layers 2 and 3 (scale bar = 1 mm; Case 5, age = 73). Middle row: Left = abundant p-tau in amygdala at low magnification (scale bar = 3 mm; Case 5, age = 73); middle = irregularly distributed p-tau involving neurons and astrocytes in amygdala (scale bar = 200 µm; Case 3, age = 80); right = extensive p-tau with NFT involving the mamillary body (scale bar = 200 µm; Case 5, age = 73). Bottom row: Left = p-tau involving the locus coeruleus (scale bar = 200 µm; Case 3, age = 80); middle = p-tau involving the pontine raphe nucleus (scale bar = 200 µm; Case 3, age = 80); right = p-tau within cell processes near a small blood vessel (scale bar = 200 µm; Case 2, age = 82).
Consensus meeting to define the neuropathological criteria for CTE
Before 2015, there were no well-validated or consensus-based neuropathological criteria for CTE, and the criteria put forward by the two research groups in the USA had problematic specificity and differed substantially in their definitions (Omalu et al., 2011; McKee et al., 2013). To develop agreed upon pathology criteria for CTE, a consensus panel of seven neuropathologists, convened by the National Institutes of Health (NIH), were provided 10 cases of advanced CTE pathology [two with stage III and eight with stage IV pathology (McKee et al., 2013)] and 15 cases with primary tau-related neurodegenerative diseases to examine blindly (McKee et al., 2016). The pathologists were provided pre-prepared virtual slides from researchers at Boston University. The pathologists were given the a priori presumptive definition and criteria for the neuropathology of CTE set out in Box 1 (found in the online supplementary material 1 of the original article) and were blinded to demographic data, clinical history (including type and degree of neurotrauma exposure), and all gross neuropathological data. The inter-rater reliability (kappa) for a diagnosis of CTE at the consensus conference was 78%.
Box 1.
Definition of CTE neuropathology provided to the independent neuropathologists prior to the consensus conference [Supplementary material in McKee et al. (2016)]
‘CTE is a tauopathy and is characterized by the deposition of hyperphosphorylated tau (p-tau) protein as neurofibrillary tangles (NFTs), astrocytic tangles (ATs) and neurites in the neocortex and medial temporal lobe. The NFTs in CTE often show a perivascular distribution and an irregular clustering at the depths of the sulci. NFTs also preferentially involve of the superficial cortical layers, a feature that is most prominent in temporal isocortex. The frontal, temporal, septal, insular and parietal cortices are primarily affected, while primary visual and occipital cortices are generally spared. In advanced disease, the medial temporal lobe structures show pronounced neuronal loss and gliosis, with a high density of NFTs, including extracellular ghost tangles. In approximately 80% of cases, there are also TDP-43 immunoreactive neurites and intraneuronal inclusions. The following criteria for the neuropathological diagnosis of CTE are proposed (McKee et al., 2013):
Perivascular foci of p-tau immunoreactive neurofibrillary tangles (NFTs) and astrocytic tangles (ATs) in the neocortex
Irregular distribution of p-tau immunoreactive NFTs and ATs at the depths of cerebral sulci
NFTs in the cerebral cortex located preferentially in the superficial layers (often most pronounced in temporal cortex)
Supportive features: Clusters of subpial ATs in the cerebral cortex, most pronounced at the sulcal depths.’
Note that this definition was not adopted in whole by the consensus group. The consensus panel defined only one pathognomonic neuropathological criterion for CTE as an accumulation of abnormal p-tau in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci. They also identified some supportive criteria for the diagnosis.
Ultimately, after reviewing the cases and deliberating, the consensus panel did not adopt the complete criteria set out in Box 1. The panel defined the single pathognomonic criterion for the neuropathology of CTE as an accumulation of abnormal p-tau in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci (McKee et al., 2016). Additional supportive criteria relating to p-tau, macroscopic features (e.g. dilation of the third ventricle and septal abnormalities), and TDP-43 inclusions and dot-like structures in the hippocampus, anteromedial temporal cortex, and amygdala were identified. These consensus criteria are set out in Box 2. The preliminary consensus criteria substantially narrowed the pathology previously reported to be characteristic of CTE. The large majority of the pathology described in Table 1 was not included in the definition.
Box 2.
Preliminary consensus criteria for the neuropathological diagnosis of CTE
Pathognomonic criterion: an accumulation of abnormal p-tau in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci (McKee et al., 2016).
Supportive criteria were defined as follows:
‘(1) abnormal p-tau immunoreactive pretangles and NFTs preferentially affecting superficial layers (layers II–III), in contrast to layers III and V as in AD; (2) in the hippocampus, pretangles, NFTs or extracellular tangles preferentially affecting CA2 and pretangles and prominent proximal dendritic swellings in CA4. These regional p-tau pathologies differ from the preferential involvement of CA1 and subiculum found in AD; (3) abnormal p-tau immunoreactive neuronal and astrocytic aggregates in subcortical nuclei, including the mammillary bodies and other hypothalamic nuclei, amygdala, nucleus accumbens, thalamus, midbrain tegmentum, and isodendritic core (nucleus basalis of Meynert, raphe nuclei, substantia nigra and locus coeruleus); (4) p-Tau immunoreactive thorny astrocytes at the glial limitans most commonly found in the subpial and periventricular regions; and (5) p-tau immunoreactive large grain-like and dot-like structures (in addition to some threadlike neurites)’ (McKee et al., 2016).
Additional pathologies that were considered supportive of the diagnosis were as follows:
‘(1) macroscopic features: disproportionate dilatation of the third ventricle, septal abnormalities, mammillary body atrophy, and contusions or other signs of previous traumatic injury; and (2) TDP-43 immunoreactive neuronal cytoplasmic inclusions and dot-like structures in the hippocampus, anteromedial temporal cortex and amygdala’ (McKee et al., 2016).
AD = Alzheimer’s disease; CA = cornu ammonis; TDP-43 = TAR DNA-binding protein 43.
Importantly, the consensus panel recognized the proposed criteria as preliminary. They noted several limitations to their study including the small sample size, the cases of suspected CTE were selected from a single source with moderate to late-stage pathological severity, and the presence of age-related co-morbidities in the samples (McKee et al., 2016). For example, of the 10 presumptive CTE cases, seven had co-morbid amyloid-β deposits and one had co-morbid Lewy body disease, according to the research centre that characterized the cases (online supplementary material, table 1 in McKee et al., 2016). The consensus panel did not evaluate the four neuropathological phenotypes (Omalu et al., 2011) or stages of CTE (McKee et al., 2013), and they did not address whether CTE was a progressive neurodegenerative disease, or whether there were known or predictable clinicopathological correlations. The authors indicated that the preliminary criteria were a ‘first step along the path to standardizing the neuropathology of CTE’ (McKee et al., 2016).
Large scale CTE study in former high school contact sport athletes
Bienieck and colleagues recently published the largest neuropathology study of CTE to date (Bieniek et al., 2019). They examined historical obituary and yearbook records for 2566 cases within the Mayo Clinic Tissue Registry. From those, they identified 300 former athletes and 450 individuals who were not athletes (i.e. non-athlete controls). Restricted sampling of neocortical tissue was screened for CTE pathology (single samples of frontal and temporal tissue in all cases and parietal tissue in 75.3%). Clinical information was derived from a medical records-linkage system (Bieniek et al., 2019).
Tau pathology, in general, was present in 87.0% of athletes and 76.4% of controls. Alzheimer’s disease pathology was present in 24.0% of athletes and 23.3% of controls. Some degree of CTE pathology was found in 27 of the athletes (9%) and 15 controls (3.3%)—and half of those subjects met consensus criteria for CTE neuropathology [5.0% of athletes (15/300) and 1.3% of controls (6/450)]. Stratified by sports, the percentages with CTE pathology were as follows: baseball = 14.1% (9/64), basketball = 12.0% (11/92), boxing = 28.6% (2/7), football = 15.0% (21/140), hockey = 10.0% (2/20), soccer = 0% (0/5), and wrestling = 7.7% (3/39). There were 15 subjects who played football beyond high school, and seven had some form of CTE pathology (46.7%). Only one female was found to have CTE pathology (0.4%), and she was a control subject. Males with CTE pathology, compared to males who did not have CTE pathology, were significantly older at death (average age = 73 versus 65), had a lower likelihood of problems with anxiety (4.9% versus 18.8%), and had a higher likelihood of dementia (43.9% versus 25.7%), movement disorders (29.3% versus 14.7%), psychosis (63.4% versus 39.9%), and Alzheimer’s disease neuropathology (36.6% versus 20.2%). Males with CTE pathology, compared to males with no CTE pathology, had similar rates of depression (34.1% versus 33.3%), suicide (2.4% versus 7.8%), and alcoholism (24.4% versus 23.6%) (Bieniek et al., 2019). The tissue sampling in this study was substantially less than other CTE studies, which might have artificially lowered the rates at which CTE pathology was identified.
The mechanisms by which p-tau accumulates in depths of sulci are unknown
At present, the mechanisms by which p-tau accumulates in the depths of sulci, and the clinical significance or correlates of this region-specific accumulation (insofar as neuropathologists agree on its presence), are unknown. Moreover, the focus of the pathology at the depths of sulci does not allow effective modelling in rodents given their lissencephalic brains. In 1999, Geddes et al. reported that the distribution of the tau pathology associated with repetitive head injuries suggests that the pathogenesis might relate to damage to blood vessels or perivascular elements. McKee et al. (2009) speculated that ischaemia might contribute to the development of p-tau in depths of sulci, or that p-tau in those regions might be due to mechanical strain forces (McKee et al., 2009). In 2017, a computational modelling study of single TBIs, from different mechanisms of injury, illustrated high strain and strain rate in sulci (Ghajari et al., 2017). The possibility of blood–brain barrier disruption has been raised by a number of researchers (Goldstein et al., 2012; Doherty et al., 2016; Jullienne et al., 2016; Kenney et al., 2016; Meabon et al., 2016). Schwab et al. (2019) identified DNA damage throughout the frontal cortex, hippocampus, and brainstem in tissue from two males with CTE pathology. Gene expression profiling revealed greater ataxia telangiectasia mutated and checkpoint kinase 2, two serine/threonine kinases recruited in response to double-strand breaks in the DNA damage response. Vascular events and oxidative stress might precede tau deposition in depths of sulci. Recently, several research groups have speculated that the glymphatic system might play a role in the accumulation of p-tau in depths of sulci in a perivascular distribution (Daneshvar et al., 2015; Dadas et al., 2016; Puvenna et al., 2016; Kulbe and Hall, 2017; Shively et al., 2017; Thomsen et al., 2017; Kriegel et al., 2018; Sullan et al., 2018). The glymphatic system (Iliff et al., 2012, 2013) is believed to be a brain-wide perivascular pathway that supports the clearance and removal of interstitial solutes from the brain, including amyloid-β and tau, especially during sleep (Boespflug and Iliff, 2018).
The morphology of tau filaments in CTE and the way they are formed is unknown
A recent study by Falcon et al. (2019) suggests that tau filaments in CTE have a distinct structure that forms a hydrophobic cavity suitable for cofactors such as fatty acids to occupy and assist in tau aggregation. This study supports the idea that different conformations of filamentous tau might distinguish different tauopathies (such as Alzheimer’s disease and Pick’s disease), and therefore specific tracer compounds might be helpful in distinguishing diagnoses in the future. They used three cases with CTE neuropathology (one former football player and two former boxers). Using electron microscopy in the temporal cortex, they found a predominant helical filament type in all three cases. In contrast, the filaments in Alzheimer’s disease were paired helical and straight. Using cryo-electron microscopy they found that in all three cases, the predominant tau filament had additional density not seen in Alzheimer’s disease. The secondary structure of tau in CTE had a unique beta-helix motif that creates a hydrophobic ‘cavity’, likely due to the extra density seen in CTE tau filaments. The extra density in the cavity is very strong, and it is surrounded by hydrophobic chains. The authors suggest that non-polar sterols and fatty acids could occupy the cavity, making CTE tau the only known amyloid-like structure to incorporate non-protein molecules. The authors suggest that these molecules function as cofactors in order to stabilize tau folding and aggregation. Indeed, fatty acids and cholesterol accumulation lead to the formation of filamentous tau inclusions in the brain. The distribution of tau around blood vessels may suggest that cofactors initially enter the brain through the periphery from head trauma. It is important to appreciate that the cases upon which these findings are based are complex. Two cases had ALS, both with a positive family history and one with 145 C9orf72 repeats, and a third case had Parkinson’s disease and dementia, with Lewy bodies in the substantia nigra. It is not clear how these structural findings in CTE tau compare with tau in other diseases, such as frontotemporal lobar degeneration (FTLD)-tau, or with ageing-related tau astrogliopathy (ARTAG). The implications and functional importance of the ultrastructural tau findings reported by Falcon and colleagues are unknown.
It is unknown whether CTE pathology is unique, specific, or inexorably progressive
Accumulation of p-tau in ageing and neurological diseases
With ageing and a number of disease states, tau can become excessively and abnormally phosphorylated and then misfolded such that it resists degradation and normal turnover—leading to accumulation within the cell (von Bergen et al., 2005; Braak et al., 2011; Alonso et al., 2018). The transition from physiological p-tau to pathological p-tau in disease states is poorly understood (Arendt et al., 2016). The kinetics (i.e. linear increase, abrupt increase with stabilization, irregular increases, and/or decreases) of p-tau accumulation over time have been studied for years and are not well understood (Hyman and Gomez-Isla, 1997; Iacono et al., 2014). The presence of disease per se is implied in the term ‘tauopathy,’ but it is important to keep in mind that p-tau accumulation as identifiable lesions of varying light microscopic appearances accompanies ageing (Morsch et al., 1999; Crary et al., 2014; Kovacs et al., 2016), and it can accumulate for many years without obvious clinical manifestations. Tauopathy in the context of neurodegenerative diseases more appropriately refers to clinicopathological entities in which p-tau accumulates in substantial amounts and, importantly, is usually associated with a clinical phenotype with neurological deterioration and eventual death from disease (Arendt et al., 2016). Such tauopathies include progressive supranuclear palsy and corticobasal degeneration. Alzheimer’s disease is characterized by abundant accumulation of p-tau, which is a component of consensus recommendations for autopsy interpretation of Alzheimer’s disease neuropathological change (Montine et al., 2012). Considerable evidence to date, however, supports that p-tau in Alzheimer’s disease is a secondary phenomenon (Selkoe and Hardy, 2016), downstream of amyloid-β deposits, which are also required for a pathological diagnosis of Alzheimer’s disease (Montine et al., 2012). There is clear evidence that extensive neocortical p-tau is associated with dementia in Alzheimer’s disease (Braak and Braak, 1991; Nelson et al., 2012).
Age-related p-tau (PART and ARTAG)
In recent years, two broad groups of age-related p-tau have been described: primary age-related tauopathy (PART) published in 2014 (Crary et al., 2014) and ageing-related tau astrogliopathy (ARTAG) published in 2016 (Kovacs et al., 2016). Both are associated with advanced ageing, neither have a consistent association with neurological signs or problems, and they may occur separately or in combination.
PART refers to neuronal p-tau, with neurofibrillary degeneration (p-tau) in the medial temporal lobe, basal forebrain, deep grey matter, brainstem, and olfactory areas (bulb and cortex) in significant amounts, but with sparse or absent amyloid-β, which distinguishes PART from Alzheimer’s disease (Crary et al., 2014). Prior to the publication of the PART article in 2014, and the CTE consensus criteria definition published in 2016 (McKee et al., 2016), PART pathology in the medial temporal lobe, basal forebrain, and brainstem was described as characteristic of CTE (McKee et al., 2013; Baugh et al., 2014). Interestingly, whole mount depictions of CTE often highlight substantial temporal lobe p-tau or brainstem p-tau. The extent to which similar p-tau staining would appear in whole mount preparations from control subjects or individuals with canonical neurodegenerative diseases has not been explored. It should also be pointed out that p-tau pathology in PART reacts with Bielschowsky silver impregnation, and that historical descriptions of dementia pugilistica pathology highlight temporal lobe NFT, although McKee et al.’s (2013) proposed staging criteria differentiate low stages based on medial temporal lobe involvement in stage II, but not stage I. Thus, there may be some pathological characteristics unique to PART versus CTE, but overall the NFT component in classical dementia pugilistica might be, in some cases, very difficult to distinguish from PART (particularly in cases with limited pathological evaluation). Indeed, the authors of the PART publication noted that the pathology overlapped with what was previously described as CTE (Crary et al., 2014).
ARTAG refers to ageing-related accumulation of p-tau in astrocytes, often in certain distributions (e.g. subpial, subependymal, and perivascular), in individuals with or without cognitive problems (Kovacs et al., 2016). Prior to the CTE consensus criteria definition published in 2016 (McKee et al., 2016), ARTAG pathology, such as astrocytic p-tau in subpial, subependymal, and perivascular locations, was described as characteristic of CTE (McKee et al., 2009, 2010, 2013; Omalu et al., 2011; Stern et al., 2011b, 2013; Mez et al., 2013; McKee and Robinson, 2014; Omalu, 2014; Montenigro et al., 2015; Riley et al., 2015). Kovacs and colleagues (2016) noted that ‘ARTAG has features that overlap those of CTE, including the accentuation of tau pathology around small cerebral vessels and in subpial and periventricular areas. On the other hand, tau pathology, including neuronal and astroglial, in CTE is more abundant in the depths of the convexity cerebral sulci, especially in early stages, an aspect that has not been reported in tau astrogliopathy in the ageing brain’ (p.94). This important distinction was not made prior to 2015.
Forrest and colleagues (2019) examined the prevalence of CTE pathology and age-related tauopathy by studying brain tissue from 310 individuals who came to autopsy between 2000 and 2016. All subjects were enrolled in the Vienna Trans-Danube Ageing (VITA) study and ranged in age from 76 to 91 years. ARTAG pathology was identified in 38% of the sample. Of the 117 cases with ARTAG, approximately half (47.9%) had the pathology in two or more brain regions. Given that ARTAG pathology and CTE pathology involve substantial overlap in morphology and similar anatomical locations, and may co-exist, the authors established rather strict criteria for defining CTE pathology. They introduced the terminology ‘higher density’ and ‘greater density’ when comparing NFTs in cerebral sulci versus cerebral gyri. They noted that NFTs related to Alzheimer’s disease and CTE can be present in the same person, and they stated ‘CTE-associated NFTs were defined as a higher density of NFTs in the cerebral sulci than observed in the cerebral gyri’. They noted that 25 of the cases had CTE-like pathology in the depths of sulci, such as astrocytic or neuronal tau, but when applying strict criteria for CTE pathology they concluded that no cases met criteria. They concluded that CTE pathology should be interpreted conservatively in cases that do not show the full constellation of tau pathologies occurring at the depths of sulci. They also noted that the earliest stages of CTE pathology, which are likely to be preclinical with individuals being asymptomatic and perhaps not progressing to develop neurological dysfunction, have not been determined. They proposed a new approach to classifying CTE pathology. They suggested the term ‘components of CTE’ to define tau pathologies at the depths of sulci that are seen in higher density than what is observed in cortical gyri, but that are not sufficient to meet full criteria for the pathognomonic lesion of CTE. This study illustrates that CTE pathology can be judged strictly, conservatively, or permissively in clinical practice and research.
In a study published at the end of 2018, Goldfinger and colleagues established that ARTAG pathology and CTE pathology co-occur. They re-examined 14 of 15 boxing cases described in the seminal 1973 paper by Corsellis and colleagues, using modern immunohistochemical techniques and the NINDS/NIBIB neuropathological consensus criteria for CTE (McKee et al., 2016), and discovered that only seven (50%) of these seminal cases of chronic brain damage in boxers met the consensus criteria for having the neuropathology of CTE. Ten of the 14 cases (71%) had ARTAG, including all seven of the boxers who had CTE pathology. The authors suggested that the co-occurrence raises the question whether ARTAG and CTE pathology are distinct entities or on the spectrum of the same disorder (Goldfinger et al., 2018).
Similarly, in a recent study, Lee and colleagues carefully documented a diverse range of post-mortem neuropathology in 11 former soccer (n = 7) and rugby (n = 4) players with a clinical history of dementia [age of dementia onset 60 ± 8.6 years (range 48–75)]. They found CTE neuropathology in 8 of 11 cases. They documented ARTAG or PART in five of eight cases and Alzheimer’s disease neuropathologic change in seven of eight cases that had CTE pathology (Lee et al., 2019).
Most cases of elderly males described as having CTE may also have PART and/or ARTAG, because the pathology as described spatially overlaps with neuronal p-tau of PART (primarily localized to medial temporal lobe structures) and glial p-tau of ARTAG [arranged in a wide array of possible distributions including focal, patchy, and diffuse patterns localized to subpial, subependymal, perivascular, and other parenchymal (including depth of sulcus) regions]. PART and ARTAG are characterized by the accumulation of large amounts of cortical and limbic p-tau, and the accumulation in PART may be more uniform and linear, and less ‘patchy’, ‘irregular’, ‘accentuated’, or ‘geographic’, while the accumulation in ARTAG may be patchy and irregular but it is only within astrocytes (the required CTE criterion requires neuronal and astrocytic p-tau). Stated differently, an individual who has ‘patchy’ p-tau in depths of sulci and uniform p-tau in depths of sulci, may be said to have both CTE pathology and age-related pathology. If the p-tau in depths of sulci is abundant and uniform, and the person has extensive amyloid-β accumulation with neuritic plaques, then the person could be said to have age-related p-tau, Alzheimer’s disease-related p-tau, and CTE pathology. In summary, there is currently no definitive way to attribute with certainty overlapping p-tau pathologies of CTE and age-related tau pathologies in the elderly, and it is not known if similar processes that result in PART and ARTAG in the elderly may operate in younger individuals (although there is currently no evidence to support this possibility). Thus, more studies on CTE neuropathology should be focused in younger populations, unbiased by selection for neurotrauma and other exposures, and cognizant of possible confounding factors that could drive p-tau pathology.
CTE pathology has been found in the brains of individuals with no known repetitive neurotrauma
Researchers have asserted that CTE pathology has only been found in individuals with exposure to repetitive neurotrauma (Stern et al., 2011a, 2013; Baugh et al., 2012, 2014; Mez et al., 2013; McKee et al., 2014). However, that is not the case as CTE pathology has been discovered in individuals who had no known history of participation in collision or contact sports and had no known exposure to repetitive neurotrauma. Specifically, CTE pathology has been identified in individuals with (i) schizophrenia who have undergone leukotomy (Shively et al., 2017); (ii) temporal lobe epilepsy (Puvenna et al., 2016); (iii) ALS (Fournier et al., 2015; Gao et al., 2017); (iv) multiple system atrophy (Koga et al., 2016); and (v) other neurodegenerative diseases (Ling et al., 2015) who have no known participation in collision or contact sports and no known exposure to repetitive neurotrauma. It should be noted, however, that many of these older adult patients with neurological diseases are at increased risk for falls as a result of their disease, and the threshold for neurotrauma exposure necessary to initiate CTE pathology is unknown.
The first female to have ever been identified as having CTE pathology was reported in 1990; she was repeatedly battered and she also had cerebrovascular disease (Roberts et al., 1990b). Specifically, her neuropathological examination showed limited neurofibrillary degeneration, abundant diffuse plaques, and a cavum septum pellucidum. No other females have been identified with CTE neuropathology until Ling and colleagues conducted a large-scale post-mortem study of CTE in the UK. Their total sample included 268 individuals, most of whom had neurodegenerative diseases identified at autopsy such as progressive supranuclear palsy, Parkinson’s disease, corticobasal degeneration, frontotemporal dementia, Alzheimer’s disease, and multiple system atrophy, and they reported that 94% had some form of history of TBI with or without loss of consciousness. They identified 32 (11.9%) cases in their sample as having mild forms of neuropathology consistent with CTE (Ling et al., 2015). Remarkably, of those 32 cases, 13 were female (40.6%). The extent to which this pathology was associated with clinical symptoms was unknown, and the authors assumed that they were likely asymptomatic from this pathology. However, Ling and colleagues used the pathological criteria for CTE proposed by McKee and colleagues (2013), not the new NIH consensus criteria (McKee et al., 2016), so it is not known how many females would have met consensus-based criteria.
In addition, Noy and colleagues prospectively examined 111 brains in a routine neuropathology service in Canada for the presence of CTE pathology (Noy et al., 2016). The subjects were between the ages of 18 and 60, and they were collected from a non-selected community-based neuropathology referral base. The researchers set their cut-off age at 60 to reduce the confounding effects of ageing and preclinical neurodegenerative diseases. They identified CTE pathology, based on the staging system of McKee et al. (2013), in 4.5% of their cases (three cases of stage I and two cases of stage II). However, they made the important observation that there is no lower bound for classifying stage I CTE pathology, so if sparse pathology is characteristic of stage I, an additional 34 cases were identified (30.6% of the sample). Therefore, of the total sample, 35.1% had some degree of mild CTE-like pathology. Only one subject had a known history of contact or collision sports participation. The CTE-like pathology was more common in males, and those with a history of traumatic brain injury, substance abuse, or both. However, some of the cases with CTE pathology had no known history of even a single traumatic brain injury.
Iverson and colleagues examined a post-mortem case series of eight males with no known participation in contact sports and no known history of repetitive neurotrauma. Two of the eight males had a known history of a single TBI. Six of the eight cases (i.e. 75%), including one of the cases with a history of TBI, showed characteristic CTE p-tau in neurons, astrocytes, and cell processes around small blood vessels in an irregular pattern at the depths of the cortical sulci. Of the six males with no known history of neurotrauma, five met consensus criteria for having CTE pathology. The changes were focal and limited in terms of overall extent, and some of the cases had a clearer pattern of pathology and some could be considered equivocal. In addition, PART was present in four cases and ARTAG was present in five (Iverson et al., 2019).
CTE pathology associated with a single traumatic brain injury
Different types of neuropathology, such as neuroinflammation (Frugier et al., 2010), amyloid-β deposition (Johnson et al., 2012), accumulation of TDP-43 (Johnson et al., 2011), and p-tau (Johnson et al., 2012; Kondo et al., 2015; Shultz et al., 2015) can and do arise following a single moderate-severe TBI (Smith et al., 2003, 2013); Blennow et al., 2012, and there is emerging evidence that multiple types of neuropathology can be present following repetitive mild TBI (Smith et al., 2013; Hay et al., 2016). Regarding axonal pathology, theoretically, there might be two phases of degeneration, an immediate process that arises from mechanical disruption and other cellular and extracellular processes, and a delayed process by which initially intact axons undergo degeneration months or years after injury (Chen et al., 2009). The formation of amyloid plaques following TBI has been reported to occur quickly, over the course of hours and days (Roberts et al., 1991, 1994; Ikonomovic et al., 2004), perhaps in association with axonal injury. However, the natural history of these plaques is not well understood, and there is some evidence that they regress over weeks and months (Chen et al., 2009), and much more research is needed in this area. In a post-mortem study of long-term survivors of TBI, amyloid plaques were observed in greater density than was present in control subjects, and they were more often fibrillary, as opposed to diffuse, compared to controls (Johnson et al., 2012). However, in the largest pathology study to date (Crane et al., 2016), in a sample of 1537 autopsy cases, a TBI with >1 h of loss of consciousness was not associated with future mild cognitive impairment, dementia, Alzheimer’s disease, neuritic plaques, or neurofibrillary tangles, but was associated with risk of Lewy body accumulation, progression of parkinsonism, and Parkinson’s disease.
Researchers report that a single concussion does not lead to CTE (Stein et al., 2015). Whether a single moderate or severe TBI can cause CTE pathology is not well understood. Johnson and colleagues reported that NFTs were present following a single remote TBI in 34% of cases aged 60 or younger compared to only 9% of control subjects, and these NFTs were commonly observed in superficial cortical layers with clustering in the depths of sulci (Johnson et al., 2012). Although they reported pathology similar to CTE, they did not attempt to diagnose CTE in that study. In one large-scale CTE study, a subgroup of 33 patients were identified as having at least one TBI from motor vehicle accidents, assaults, domestic violence, or falls, and none of these individuals had the neuropathology characteristic of CTE (Bieniek et al., 2015). In a more recent study, 2 of 12 individuals with a history of severe TBI had neuropathology consistent with CTE (Zanier et al., 2018). In a sample of 155 veterans who died with ALS, 5.8% had CTE pathology (Walt et al., 2018). There were nine veterans who had a history of TBI (and no known history of participation in contact sports), and of those nine, three had CTE pathology (33.3%). More research is needed to determine the nature, extent, and natural history of CTE pathology following a single moderate or severe TBI.
Summary of current limitations of CTE neuropathology research
Currently CTE can only be diagnosed at autopsy using preliminary neuropathological consensus criteria. For any disease diagnosable only at autopsy, which by definition represents a static time point for each individual case, it is impossible to definitively ascribe a progressive neurodegenerative course. Only when validated biomarkers, such as CTE-specific blood or CSF molecules, or PET with radiotracers specific to CTE tau, or other CTE-specific targets, are available can one determine with reasonable certainty the natural course of a disease and the effects of exposures and interventions. Thus, it might be premature to describe CTE as inexorably progressive.
CTE is ascribed to chronic repetitive concussive or subconcussive neurotrauma exposure, and claims that no cases have been described in individuals without these exposures may not be correct. However, it is extremely difficult, if not impossible, to know for any individual all the neurotraumatic and other environmental, drug, medical health, nutritional, and other exposures that might contribute to neuropathological changes that develop over a lifetime. This issue is always a challenge in human clinical research but is especially troublesome when a disease, condition, or pathology can only be diagnosed at autopsy. A validated biomarker is essential to evaluate in-life changes with respect to exposures to trauma and other hazards that could potentially lead to CTE or CTE-like neuropathological change. There appears to be a correlation in cohorts enriched for subjects with high-level exposures to contact sports and variably prominent behavioural and cognitive impairments with the neuropathological changes of CTE. However, much more work is needed to test and validate current preliminary criteria for specificity and sensitivity and ultimately, without a biomarker, assertions regarding progression, exposures, and interventions on the neuropathological features of the disease are questionable.
The challenge of clinical diagnosis
During the 20th century, CTE was conceptualized as an uncommon neurological disorder afflicting a minority of long-career, high exposure boxers. Over the past 15 years, the two leading research groups in the USA have asserted that modern cases of CTE are characterized by an extraordinarily broad range of mild to severe clinical features, including: (i) psychological and psychiatric problems, such as depression and anxiety (Omalu et al., 2011; Baugh et al., 2012; McKee et al., 2013) or suicidality (Omalu et al., 2010a; Gavett et al., 2011b; Stern et al., 2011b, 2013; Baugh et al., 2012; McKee et al., 2013; Omalu, 2014); (ii) personality changes, anger control problems, and violence (Omalu et al., 2011; Baugh et al., 2012; McKee et al., 2013); (iii) poor financial decisions, financial problems, and bankruptcy (Omalu et al., 2011); (iv) gambling (Montenigro et al., 2014); (v) excessive shopping or unusual purchases (Montenigro et al., 2014); (vi) marital problems, separation, and divorce (Omalu, 2014); (vii) substance abuse (Montenigro et al., 2014); (viii) headaches (McKee et al., 2009, 2013; Omalu et al., 2011); (ix) generalized body aches and pain (Omalu et al., 2011); (x) insomnia (Omalu, 2014); (xi) parkinsonism (Omalu et al., 2005; Baugh et al., 2012; McKee et al., 2013); (xii) mild cognitive impairment (Omalu et al., 2011; Baugh et al., 2012; McKee et al., 2013); (xiii) dementia (Omalu et al., 2011; Baugh et al., 2012; McKee et al., 2013); and (xiv) motor neuron disease resembling ALS (McKee et al., 2010). Researchers from Boston University introduced preliminary proposed diagnostic criteria for ‘traumatic encephalopathy syndrome’ (TES) in 2014 (Montenigro et al., 2014), and the TES criteria are being used in several studies as part of a multicentre grant entitled ‘Diagnostics, Imaging, And Genetics Network for the Objective Study and Evaluation of Chronic Traumatic Encephalopathy’ (DIAGNOSE CTE; NIH/NINDS Grant No. U01NS093334). The proposed diagnostic criteria are presented in Box 3. All five of the criteria in Box 3 must be present to diagnose TES.
Box 3.
Proposed criteria for TES (Montenigro et al., 2014). All five criteria must be present
Types of injuries: History of multiple impacts to the head (e.g. four concussions, two moderate-severe TBIs, or multiple ‘subconcussive traumas’).
No other neurological disorder (including chronic residual symptoms from a single TBI or persistent post-concussion syndrome) that likely accounts for all clinical features, although concomitant diagnoses of substance abuse, PTSD, mood/anxiety disorders, or other neurodegenerative diseases (for example, Alzheimer’s disease and frontotemporal dementia) or a combination of these can be present.
Clinical features must be present for a minimum of 12 months. However, if treatment (for example, ‘antidepressant’ medication) results in an improvement in select symptoms, the clinician should use her or his best judgment to decide whether the symptoms would have persisted or progressed if treatment had not been initiated.
-
At least one of the three core clinical features must be present and should be considered a change from baseline functioning:
Cognitive: Cognitive problems reported by the person and/or an informant and low cognitive test scores on one or more tests of episodic memory, executive function, and/or attention (defined by scores at a level of at least 1.5 standard deviations below appropriate norms).
Behavioural: Being described as emotionally explosive (for example, having a ‘short fuse’ or being ‘out of control’), physically violent, and/or verbally violent, as reported by self or informant, by history of treatment, or by clinician’s report. A formal diagnosis of intermittent explosive disorder would meet this criterion but is not necessary.
Mood: Feeling overly sad, depressed, and/or hopeless, as reported by self or informant, by history of treatment, or by clinician’s report. A formal diagnosis of major depressive disorder or persistent depressive disorder would meet this criterion but is not necessary.
-
At least two supportive features must be present. The seven supportive criteria include:
Impulsivity (e.g. ‘new behaviors such as excessive gambling, increased or unusual sexual activity, substance abuse, or excessive shopping or unusual purchases’);
Anxiety (e.g. ‘anxious mood, agitation, excessive fears, obsessive or compulsive behavior (or both)’, and ‘a formal diagnosis of anxiety disorder would meet this criterion but is not necessary’);
Apathy (e.g. ‘loss of interest in usual activities, loss of motivation and emotions, and/or reduction of voluntary, goal-directed behaviors’);
Paranoia (e.g. ‘delusional beliefs of suspicion, persecution, and/or unwanted jealousy’);
Suicidality (i.e. ‘history of suicidal thoughts or attempts’);
Headache (‘significant and chronic headache with a least one episode per month for a minimum of 6 months’);
Motor signs (e.g. ‘dysarthria, dysgraphia, bradykinesia, tremor, rigidity, gait disturbance, falls, and/or other features of parkinsonism’);
Documented decline (i.e. ‘progressive decline in function and/or a progression in symptoms and/or signs’ that occurs ‘for a minimum of 1 year’);
Delayed onset (i.e. ‘delayed onset of clinical features after significant head impact exposure, usually at least 2 years and in many cases several years after the period of maximal exposure. It should be noted, however, that individual cases may begin to develop the clinical features of TES during their period of head impact exposure (for example, while still actively involved in a collision sport)’. PTSD = posttraumatic stress disorder.
Seven methodological issues and problems, with applying these proposed criteria in research and in clinical practice, are set out below. First, an individual is allowed to have a diverse range of primary or secondary psychiatric or neurological disorders assuming that the clinician or researcher can, somehow, differentiate the effects of TES from the other disorder(s) (see criterion ii). For example, an individual could have depression caused almost entirely by other factors if the clinician or researcher concludes that TES, and presumably underlying CTE neuropathology, is also contributing to that clinical presentation—although it is entirely unclear how a clinician would make such a determination.
Second, as written, a former contact sport athlete who presents for treatment for depression and headaches, for the first time, in his or her thirties, who improves with antidepressants and psychotherapy, could still be diagnosed with TES (at the clinician’s discretion; see criterion iii). Normally, under such circumstances, many clinicians would assume that the person simply had depression and not assume that the person was suffering from a putative neurodegenerative disease.
Third, the clinical features are extraordinarily broad and nonspecific (see criteria iv and v). Some examples of primary or secondary disorders or diseases that can mimic the symptoms and problems associated with TES are as follows: mood disorders [e.g. major depressive disorder; persistent depressive disorder (dysthymia), bipolar I disorder, bipolar II disorder, and unspecified depressive disorder]; impulse control disorders (e.g. intermittent explosive disorder); adjustment disorders (adjustment disorder with depressed mood, adjustment disorder with mixed anxiety and depressed mood, and adjustment disorder with anxiety); anxiety disorders (e.g. generalized anxiety disorder, obsessive-compulsive disorder, posttraumatic stress disorder, agoraphobia, panic disorder, and social anxiety disorder); substance-related and addictive disorders (e.g. alcohol use disorder, substance use disorder, and gambling disorder), and a variety of neurological diseases (e.g. Alzheimer’s disease, Parkinson’s disease, chronic cerebrovascular disease, and frontotemporal dementia). According to criterion ii in Box 3, an individual can have any of these diagnoses as a primary disorder, a secondary disorder, or a comorbidity and the clinician can still diagnose TES. This is challenging, of course, because those disorders in the general population are assumed to be independent of CTE neuropathology—and individuals with those disorders can easily present with a core feature (criterion iv) and other supportive features (criterion v), and thus mistakenly be diagnosed as having TES (and CTE).
Fourth, there is no requirement that the clinical condition have a delayed onset [see criterion v(i)]. As written, the onset can be while still an athlete, after a brief delay, after several years, or after decades. Therefore, by definition, if an individual is healthy during the decade of his or her twenties, and then develops mental health or neurological problems any time between the ages of 30 and 80, that person will have fulfilled one of the two required supportive features (i.e. delayed onset). As such, the criteria, in a practical sense, only require one supportive feature, not two, because the vast majority of former athletes will meet the delayed onset criterion because they do not show clinical evidence of TES while still participating in their sports. In general, criterion v(i) maximizes the likelihood that clinical problems, manifesting at any time in life, could be interpreted as being caused by CTE neuropathology and diagnosed as TES, when, in fact, they might be caused mostly or entirely by other factors.
Iverson and Gardner (2019) used data from the US National Comorbidity Survey Replication, an in-person survey that examined the prevalence and correlates of mental disorders in the USA, to identify a sample of 101 males from the general population who were diagnosed with a major depressive episode in the past 30 days. The clinical problems attributed to TES, such as depression, suicidality, anxiety, anger control problems, and headaches, were comorbid in this sample of males with depression from the general population—illustrating that they are not unique or specific to TES. Nearly half (45.5%) reported a lifetime history of an anger attack leading to hitting or attempting to hit another person, and 52.5% reported feeling mad or angry over the past month. Nearly one in five (17.8%) reported feeling ‘angry and out of control’ in the past month. It was common for these males to experience alcohol or drug abuse (20.8%), anxiety (73.3%), loss of interest (36.6%), suicidality (20.8%), and headaches (23.8%). Approximately half of the sample (52.5%) met the proposed research criteria for TES. Extrapolating to former athletes and military veterans, and accepting that the delayed-onset criterion would be met if they were presenting with depression years after retirement (from sports or the military), then 83.2% of this sample met the research criteria for TES. Thus, depression in males in the general population can easily mimic TES, as defined in Box 3.
Fifth, there is no requirement that the clinical condition be progressive—which runs counter to the assumption that TES is a neurodegenerative disease [see criterion v(h)]. Sixth, when examining the ‘core’ and ‘supportive’ features, it is not clear if there is a specific syndrome or syndromes that are being described because such a broad range of combinations of core and supportive features can meet criteria for TES. For example, if the person is healthy during the decade of his or her twenties, and he or she develops any of the following combinations of health problems between the age of 30 and 80 (thus meeting the supportive criterion of ‘delayed onset’), that person would screen positively for having the core and supportive criteria for TES: (i) depression and suicidality; (ii) depression and anxiety; (iii) depression and headaches; (iv) depression and substance abuse; (v) anger control problems and anxiety; (vi) anger control problems and substance abuse; (vii) anger control problems and headaches; (viii) mild cognitive impairment and anxiety; (ix) mild cognitive impairment and substance abuse; (x) Parkinson’s disease or parkinsonism and mild cognitive impairment; (xi) parkinsonism and apathy; (xii) Alzheimer’s disease and apathy; (xiii) Alzheimer’s disease and paranoia; (xiv) frontotemporal dementia and impulsivity; and (xv) frontotemporal dementia and paranoia.
Finally, as written, all individuals meeting the earlier-in-life exposure criterion for neurotrauma (criterion i), who develop mental health or neurological problems after the age of 40 that persist and/or worsen for at least 1 year, actually meet the two required supportive features—‘delayed onset’ [criterion v(i)] and ‘progressive’ [criterion v(h), ‘a progression in symptoms and/or signs’ that occurs ‘for a minimum of 1 year’]. Thus, as written, they do not need to have a single additional clinical supportive feature—the onset and course of their depression, anger control problems, or cognitive impairment are sufficient for meeting TES diagnostic criteria. This is problematic because this onset and course represents the natural history of many psychiatric and neurological conditions experienced by individuals in the general population who have no known exposure to repetitive neurotrauma.
Directions for future research
There are numerous gaps in our knowledge relating to CTE, and these gaps could be the focus for future research. Nine suggestions for research are summarized below. First, research is needed to determine whether, and the extent to which, the neuropathology of CTE is progressive—and if so, the mechanisms by which it is progressive. Second, the lower limit of pathology, described as ‘stage I’ (McKee et al., 2013) or ‘less than stage I’ (Noy et al., 2016), needs to be carefully defined. In its mildest form, the identification of CTE might be based entirely on immunohistochemistry, with no discernible changes by macroscopic or routine microscopic pathological evaluation, i.e. no detectable alterations in cellular structure. The preliminary consensus criteria for defining the neuropathology were based entirely on 10 cases selected with stage III and stage IV pathology (McKee et al., 2016). Researchers have identified small amounts of CTE pathology in community autopsy subjects with no known exposure to repetitive neurotrauma (Noy et al., 2016; Iverson et al., 2019). A preliminary consensus definition of the minimum criteria for identifying CTE pathology is needed. Third, more studies are needed to determine how often CTE pathology is present in community control subjects, of different ages, who have no known history of repetitive neurotrauma. Permissive versus strict criteria for identifying CTE pathology should be compared and contrasted.
Fourth, given that sleep apnoea is associated with the accumulation of both amyloid-β and tau (Baril et al., 2018; Bubu et al., 2019), and the glymphatic system (during sleep) has been hypothesized to play a role in CTE pathology (Daneshvar et al., 2015; Dadas et al., 2016; Puvenna et al., 2016; Kulbe and Hall, 2017; Shively et al., 2017; Thomsen et al., 2017; Kriegel et al., 2018; Sullan et al., 2018), it is important to do post-mortem studies looking for CTE pathology in individuals who had sleep apnoea (especially in combination with other comorbidities) but no known history of repetitive neurotrauma. Fifth, given that prescription opioid use (Flanagan et al., 2018) and opioid drug abuse (Anthony et al., 2010) are both associated with greater than expected tau in frontal cortex, it will be important to examine the brains of opiate users with no known history of repetitive neurotrauma for CTE pathology.
Sixth, from a methodological perspective, research is needed to develop a consensus definition of the depth of a sulcus. There is no accepted definition of the depth of a sulcus in the CTE literature, and in the published literature to date it is rarely clearly defined. A notable exception relating to the definition of a sulcal depth in CTE research is a recent study by Hsu et al. (2018). For this study, it was necessary to define the depth of sulci so that quantitative analyses of pathology could be conducted. The authors wrote:
‘A sulcal depth was defined as the bottom two-thirds of connecting gyri, extending into the gray matter from the gray/white matter boundary. White matter adjacent to sulcal depths was defined as a sub-region of superficial white matter, extending from boundary of the sulcal depth ROI to 1 mm into white matter’.
In both clinical practice and research, there is likely variability in how neuropathologists define the depths and how they interpret the ‘patchiness’ or ‘irregularity’ of the pattern of tau staining within those depths. There is also likely variability in how neuropathologists quantify the amount of irregular tau and differentiate it from the more evenly-distributed tau in sulcal depths that is apparent in association with ageing and Alzheimer’s disease. More research is needed to define and quantify p-tau staining patterns better within the depths of sulci.
Seventh, studies using sledge microtome, free-floating immunohistochemical preparations for examining community control subjects and subjects with other neurological diseases are needed to comprehensively interrogate tissue for p-tau in a manner that has been done in past cases examined for CTE. A sledge microtome relies on a moveable long blade to slice large hemispheric tissue slabs, as opposed to a conventional microtome that has a moveable paraffin block sliced by a fixed blade. Sledge microtomes are not used in mainstream diagnostic neuropathology or neuropathology research. This methodology allows for large slabs of brain to be sliced into 50–100-μm thick sections, floated into reagent, and hand-immunostained, allowing the researcher to stain a slice that is 10 or more times the thickness of standard immunohistochemistry, many times the surface area, and many times the volume. It has been reported that this technique may detect CTE pathology in ∼20% of cases that are not otherwise detectable by routine histopathological and immunohistochemical methods that are commonly used in neuropathology research and clinical practice (McKee et al., 2016).
Eighth, a large amount of research is needed on the presumed clinical characteristics of CTE. Research is needed to examine the extent to which certain clinical features correlate with pathology that is believed to be associated with CTE. To date, there is very little CTE research using rigorous clinicopathological correlational methods. Prior studies imply that virtually any neurological or psychiatric symptoms and problems present prior to death, in someone with post-mortem CTE neuropathology, are manifestations of CTE (i.e. TES). Lee and colleagues (2019) have made a first step in the direction of more refined research in this area. They proposed an ‘integrated’ diagnostic approach to CTE. They examined post-mortem pathology from 11 former soccer and rugby athletes with known dementia prior to death. CTE pathology was common, as was Alzheimer’s disease pathology, cerebrovascular disease, and ARTAG. The authors suggest three diagnostic categories for CTE: (i) patients with dementia but no evidence of CTE neuropathological change; (ii) patients with an integrated diagnosis of CTE dementia; and (iii) patients with CTE neuropathological change as a co-morbidity in the context of an alternate integrated diagnosis (such as Alzheimer’s disease or vascular dementia). Their findings further support the notion that CTE neuropathological change can represent incidental pathology without clinical effect (as has been observed in other dementias such as Alzheimer’s disease) (Lee et al., 2019).
Finally, research is needed to determine how to differentiate CTE and TES from other common psychiatric disorders. The proposed criteria for TES, set out in Box 3, are known to occur in combination in individuals from the general population who have no known history of repetitive neurotrauma. That is, the criteria for TES, especially for the ‘mood’ and ‘behavioural’ subtypes, are commonly comorbid in individuals from the general population. For example, depression commonly co-occurs with anxiety (American Psychiatric Association, 2000, 2013) and headaches (Chai et al., 2012). Males with depression frequently have anger control problems and anger attacks (Painuly et al., 2005, 2011; Winkler et al., 2005; Busch, 2009; Martin et al., 2013). Individuals from the USA general population with diagnoses of anxiety disorders, dysthymia, and major depression are three to four times more likely to admit to violent behaviours than those with no disorders, and individuals with drug and alcohol abuse are eight times more likely to report violent behaviours (Corrigan and Watson, 2005). People with serious and persistent anger control problems, such as intermittent explosive disorder, are at risk for developing substance abuse disorders (Coccaro et al., 2017). People with substance abuse disorders have higher rates of depression (Lai et al., 2015) and anxiety (Lai et al., 2015), and the relationship between substance abuse and mental health problems appears to be bidirectional (Bulloch et al., 2012). Some individuals with substance abuse disorders had difficulty with comorbid headache conditions (El-Mallakh et al., 1991; Beckmann et al., 2012; Rossi et al., 2012; McDermott et al., 2014). Anhedonia, i.e. a markedly diminished interest or pleasure in activities, is associated with both mood and substance abuse disorders [see criterion v(c) in Box 3] (Destoop et al., 2019). It is common for individuals with problematic gambling [see criterion v(a) in Box 3] to have comorbid psychiatric disorders, such as current mood disorders, alcohol use disorders, and anxiety disorders (DowLing et al., 2015; Sundqvist and Rosendahl, 2019). Males with gambling problems are at increased risk for depression and suicidality (Ronzitti et al., 2017; Sundqvist and Rosendahl, 2019), and suicide rates are significantly elevated in individuals with gambling disorders. In one population study, individuals with gambling disorders had a 15-fold increase in suicide mortality compared to the general population (Karlsson and Hakansson, 2018). Therefore, the proposed nosology set out for TES requires careful study and refinement because it combines well-established psychiatric comorbidities in the general population, such as depression, anxiety, suicidality, anger, apathy/anhedonia, headaches, and substance abuse, and classifies them as TES. There is a need to identify rates of false positive diagnoses of TES in the general population and in individuals with psychiatric and neurological disorders with no known exposure to repetitive neurotrauma.
Conclusions
In the modern descriptions of autopsy cases of former boxers, football players, and other athletes between 2005 and 2012, the neuropathology attributed to CTE was broad and diverse. This ‘CTE pathology’ included (i) TBI pathology in situ (e.g. remote contusion); (ii) microscopic pathology that can be associated with advanced age, such as PART and ARTAG; (iii) the accumulation of amyloid-β; (iv) macroscopic pathology associated with ageing, such as cortical thinning and heterospatial atrophy; and (v) macroscopic and microscopic pathology associated with canonical sporadic neurodegenerative diseases. This diverse pathology, arising from multiple causes, was aggregated and referred to, in toto, as the pathology ‘characteristic’ of CTE (McKee et al., 2009; Gavett et al., 2011a; Baugh et al., 2012). In 2015 (published in 2016), preliminary consensus criteria were developed for defining the neuropathology of CTE. Most of the neuropathology described as ‘characteristic’ of CTE in studies published before 2015 (Table 1) is not included in the new research criteria for defining the pathology of CTE (Box 2).
There is steadily emerging evidence that the neuropathology described as unique to CTE may not be completely unique. CTE pathology has been identified in individuals with no known participation in collision or contact sports and no known exposure to repetitive neurotrauma (Fournier et al., 2015; Ling et al., 2015; Koga et al., 2016; Noy et al., 2016; Puvenna et al., 2016; Iverson et al., 2019). Moreover, throughout history, some clinical cases have been described as not being progressive—and there is now evidence that CTE neuropathology might not be progressive in some individuals. The CTE pathology found in a large study in the UK (Ling et al., 2015) was mostly conceptualized as stage I, and in a large study in Canada it was sparse, almost always less than what has been described as stage I (Noy et al., 2016), and not supportive of progressive pathology or a substrate for a progressive clinical disease. Similarly, some retired professional football players or boxers have sparse p-tau (‘stage I’ or ‘stage II’ CTE) (McKee et al., 2013), and some of these individuals died in their 80s—which seems to be inconsistent with the pathology being inexorably progressive.
Over the past 5 years, there have been numerous reviews that have concluded that there are tremendous gaps in our knowledge relating to both the neuropathology and the clinical features of CTE (Gardner et al., 2014; Solomon and Sills, 2014; Iverson et al., 2015, 2018; Asken et al., 2016, 2017; Agoston et al., 2017; Aldag et al., 2017; Asken and Bauer, 2018; Solomon, 2018; Zuckerman et al., 2018). In fact, in March of 2019, Stewart and colleagues published a statement from 61 authors from diverse disciplines and multiple countries expressing concern that the media attention, and how CTE has been portrayed in the medical and scientific literature, risks causing harm. The statement emphasized that the clinical syndrome of CTE has not been fully defined, its prevalence is not known, the neuropathological criteria are preliminary, a single focus of pathology is insufficient to diagnose a disease, and we have an incomplete understanding of the extent and/or distribution of neuropathology required to produce clinical symptoms or neurological deficits (Stewart et al., 2019).
In conclusion, it is not known the extent to which the emergence, course, or severity of clinical symptoms are caused by or correlated with CTE pathology. It is also unclear whether symptoms can be predicted by specific combinations of CTE pathology and other co-morbid neuropathologies, thresholds for accumulation of pathology, or regional distributions of pathologies. More research is needed to determine the extent to which the perivascular deposition of p-tau in neurons and astrocytes is present in individuals with no known history of repetitive neurotrauma, and to determine whether or not, or the extent to which, the neuropathology of CTE is unique, distinct, progressive, and associated with specific clinical phenotypes.
Glossary
Abbreviations
- ALS =
amyotrophic lateral sclerosis
- ARTAG =
ageing-related tau astrogliopathy
- CTE =
chronic traumatic encephalopathy
- NFT =
neurofibrillary tangle
- NT =
neuropil thread
- PART =
primary age-related tauopathy
- TBI =
traumatic brain injury
- TES =
traumatic encephalopathy syndrome
Funding
No funding was received towards this work.
Competing interests
G.L.I. has been reimbursed by the government, professional scientific bodies, and commercial organizations for discussing or presenting research relating to MTBI and sport-related concussion at meetings, scientific conferences, and symposiums. He has a clinical practice in forensic neuropsychology involving individuals who have sustained mild TBIs (including athletes). He has received honorariums for serving on research panels that provide scientific peer review of programs. He is a co-investigator, collaborator, or consultant on grants relating to mild TBI funded by the federal government and other organizations. He has received research support from test publishing companies in the past, including ImPACT® Applications Systems, Psychological Assessment Resources, and CNS Vital Signs. He has received grant funding from the National Football League and salary support from the Harvard Integrated Program to Protect and Improve the Health of NFLPA Members. He serves as a scientific advisor for BioDirection, Inc, SWAY Operations, LLC, and Highmark, Inc. He acknowledges unrestricted philanthropic support from the Mooney-Reed Charitable Foundation, Heinz Family Foundation, and ImPACT® Applications, Inc. A.J.G. has a clinical practice in neuropsychology involving individuals who have sustained sport-related concussion (including current and former athletes). He has been a contracted concussion consultant to the Rugby Australia since July 2016. He has received travel funding from the Australian Football League (AFL) to present at the Concussion in Football Conference in 2013 and 2017. Previous grant funding includes the NSW Sporting Injuries Committee, the Brain Foundation (Australia), and the Hunter Medical Research Institute (HMRI), supported by Jennie Thomas, and the HMR, supported by Anne Greaves. He is currently funded through an NHMRC Early Career Fellowship, and Hunter New England Local Health District, Research, Innovation and Partnerships Health Research & Translation Centre and Clinical Research Fellowship Scheme, and the University of Newcastle’s Priority Research Centre for Stroke and Brain Injury. S.R.S. is funded through an NHMRC Career Development Fellowship and an NHMRC Project Grant. He has also received research funding from the Australian Football League (AFL) to investigate the neurological effects of participating in amateur Australian rules football. He is an employee of Monash University and Alfred Health. G.S.S. has received honoraria and expense reimbursements from government, professional and scientific organizations for presenting research relating to sport-related concussion at meetings, scientific conferences, and symposiums. He is a full-time employee of the Vanderbilt University Medical Center, where he has a clinical practice in sports neuropsychology involving patients who have sustained concussions. He is the consulting neuropsychologist for the Nashville Predators, Tennessee Titans, and the athletic departments of the University of Tennessee, Tennessee Tech University, and Vanderbilt University, fees paid to institution. He is the Senior Medical Advisor for the National Football League Department of Health and Safety. P.M. is a co-investigator on competitive grants relating to mild TBI funded by several governmental and other organizations. He is funded under a Fellowship awarded by the National Health and Medical Research Council of Australia and is employed at the Florey Institute of Neuroscience and Mental Health. He has a clinical consulting practice in neurology, including medico-legal work. He has been reimbursed by the government, professional scientific bodies, and commercial organizations for discussing or presenting research relating to MTBI and sport-related concussion at meetings, scientific conferences, and symposiums. He has not directly received any research funding, or monies other than travel reimbursements from the AFL, FIFA or the NFL and does not hold any individual shares in or receive monies from any company related to concussion or brain injury assessment or technology. He acknowledges unrestricted philanthropic support from CogState Inc. (2001–16). He is the chair of the scientific committees of the International Concussion and Head Injury Research Foundation in London and the Sports Surgery Clinic in Dublin. P.M. did not receive any form of financial support directly related to this manuscript. R.Z. has been partially supported by NIDILRR, NIH, and USARMC: NIDILRR: 90DP07039-03-00, 90SI5007-02-04,90 D P0060; USAMRC-W81XWH-112-0210, NIH: 4 U01NS086090-04; 5R24HD082302-02;5U01NS091951-03. He also serves as co-principal investigator on a T-32 and receives funding from the Football Players Health Study at Harvard University, which is funded by the NFL Players Association. R.Z. received royalties from (i) Oakstone for an educational CD-Physical Medicine and Rehabilitation a Comprehensive Review; and (ii) Demos publishing for serving as co-editor of the text Brain Injury Medicine. R.Z. serves on the Scientific Advisory Board of Myomo, Oxeia Biopharma, ElMINDA, and BioDirection. He also evaluates patients in the MGH Brain and Body-TRUST Program which is funded by the NFL Players Association. G.P. acknowledges funding from the Alzheimer's Association, Semmes Foundation, NIH (G12-MD007591), and Lowe Foundation. He is on the board and owns equity in Neurotrope and Neurotez, he is an advisor and owns equity in Investicure, and he has research support and is an advisor for Phoenix Biotech. C.D.K. has received honoraria and expense reimbursements from government, professional, and scientific organizations for presenting research relating to sport-related concussion and traumatic brain injury at meetings, scientific conferences, and symposiums. He is supported in part through the Nancy and Buster Alvord Chair in Neuropathology, intramural support unrelated to this study, and NIH (U01 AG006781, P50 AG05136, P30 AG049638, R01 ES02618, R01 AG057915, R21 AG046883, U01 AG006781), DOD (W81XWH-17-1-0330), and foundation (Henry Jackson Foundation and Allen Institute for Brain Science) grants. R.J.C. has been reimbursed for travel for speaking at educational programs and has received fees for medicolegal consulting. He has received intramural research funding at Western Michigan University School of Medicine, unrelated to this study. He is subcontracted to the NIH Neurobiobank at the University of Maryland and the Lieber Institute for Brain Development. L-N.H. acknowledges research funding from the Canadian Institutes of Health Research and the National Institute of Neurological Disorders and Stroke. She has served as a consultant and expert witness in the area of neurotrauma and neuropathology.
References
- Agoston D, Arun P, Bellgowan P, Broglio S, Cantu R, Cook D, et al. Military blast injury and chronic neurodegeneration: research presentations from the 2015 International State-of-the-Science Meeting. J Neurotrauma 2017; 34: S6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldag M, Armstrong RC, Bandak F, Bellgowan PSF, Bentley T, Biggerstaff S, et al. The biological basis of chronic traumatic encephalopathy following blast injury: a literature review. J Neurotrauma 2017; 34: S26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, et al. Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci 2018; 12: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain 2010; 133: 3685–98. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, et al. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord 2012; 26: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull 2016; 126: 238–92. [DOI] [PubMed] [Google Scholar]
- Asken BM, Bauer RM. Chronic traumatic encephalopathy: the horse is still chasing the cart. J Orthop Sports Phys Ther 2018; 48: 672–5. [DOI] [PubMed] [Google Scholar]
- Asken BM, Sullan MJ, DeKosky ST, Jaffee MS, Bauer RM. Research gaps and controversies in chronic traumatic encephalopathy: a review. JAMA Neurol 2017; 74: 1255–62. [DOI] [PubMed] [Google Scholar]
- Asken BM, Sullan MJ, Snyder AR, Houck ZM, Bryant VE, Hizel LP, et al. Factors influencing clinical correlates of chronic traumatic encephalopathy (CTE): a review. Neuropsychol Rev 2016; 26: 340–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril AA, Carrier J, Lafreniere A, Warby S, Poirier J, Osorio RS, et al. Biomarkers of dementia in obstructive sleep apnea. Sleep Med Rev 2018; 42: 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh CM, Robbins CA, Stern RA, McKee AC. Current understanding of chronic traumatic encephalopathy. Curr Treat Options Neurol 2014; 16: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav 2012; 6: 244–54. [DOI] [PubMed] [Google Scholar]
- Beckmann YY, Seckin M, Manavgat AI, Zorlu N. Headaches related to psychoactive substance use. Clin Neurol Neurosurg 2012; 114: 990–9. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Andermann F, Olivier A, Ethier R, Melanson D, Robitaille Y, et al. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol 1991; 29: 175–82. [DOI] [PubMed] [Google Scholar]
- Bieniek KF, Blessing MM, Heckman MG, Diehl NN, Serie AM, Paolini MA II, et al. Association between contact sports participation and chronic traumatic encephalopathy: a retrospective cohort study. Brain Pathol 2019. doi: 10.1111/bpa.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015; 130: 877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012; 76: 886–99. [DOI] [PubMed] [Google Scholar]
- Boespflug EL, Iliff JJ. The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-beta, and sleep. Biol Psychiatry 2018; 83: 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70: 960–9. [DOI] [PubMed] [Google Scholar]
- Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, et al. Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep 2019; 42: zsz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch A, Lavorato D, Williams J, Patten S. Alcohol consumption and major depression in the general population: the critical importance of dependence. Depress Anxiety 2012; 29: 1058–64. [DOI] [PubMed] [Google Scholar]
- Busch FN. Anger and depression. Adv Psychiatr Treat 2009; 15: 271–78. [Google Scholar]
- Carrol EJ. Punch-drunk. Am J Med Sci 1936; 191: 706–12. [Google Scholar]
- Chai NC, Rosenberg JD, Peterlin BL. The epidemiology and comorbidities of migraine and tension-type headache. Tech Reg Anesth Pain Manag 2012; 16: 4–13. [Google Scholar]
- Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol 2009; 19: 214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Fanning JR, Lee R. Intermittent explosive disorder and substance use disorder: analysis of the national comorbidity survey replication sample. J Clin Psychiatry 2017; 78: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis J, Tissot R. Generalized Alzheimer's neurofibrillary lesions without senile plaques. (presentation of one anatomo-clinical case). Schweiz Arch Neurol Psychiatr 1967; 100: 117–30. [PubMed] [Google Scholar]
- Corrigan PW, Watson AC. Findings from the national comorbidity survey on the frequency of violent behavior in individuals with psychiatric disorders. Psychiatry Res 2005; 136: 153–62. [DOI] [PubMed] [Google Scholar]
- Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973; 3: 270–303. [DOI] [PubMed] [Google Scholar]
- Courville CB. Punch drunk. Its pathogenesis and pathology on the basis of a verified case. Bull Los Angeles Neurol Soc 1962; 27: 160–8. [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, Dams-O'Connor K, Trittschuh E, Leverenz JB, Keene CD, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016; 73: 1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014; 128: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley M. Punch-drunk syndromes: the chronic traumatic encephalopathy of boxers In: Maloine, editor. Hommage a Clovis Vincent. Strasbourg: Imprimerie Alascienne; 1949. p. 131–45. [Google Scholar]
- Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J 1957; 1: 357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadas A, Washington J, Janigro D. Cerebral waste accumulaton and glyphatic clearance mechanisms of human neurological diseases. J Neurol Neuromed 2016; 1: 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar DH, Goldstein LE, Kiernan PT, Stein TD, McKee AC. Post-traumatic neurodegeneration and chronic traumatic encephalopathy. Mol Cell Neurosci 2015; 66: 81–90. [DOI] [PubMed] [Google Scholar]
- Destoop M, Morrens M, Coppens V, Dom G. Addiction, anhedonia, and comorbid mood disorder. A narrative review. Front Psychiatry 2019; 10: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2012; 2: a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 2011; 45: 384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CP, O'Keefe E, Wallace E, Loftus T, Keaney J, Kealy J, et al. Blood-brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2016; 75: 656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling NA, Cowlishaw S, Jackson AC, Merkouris SS, Francis KL, Christensen DR. Prevalence of psychiatric co-morbidity in treatment-seeking problem gamblers: a systematic review and meta-analysis. Aust NZ J Psychiatry 2015; 49: 519–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mallakh RS, Kranzler HR, Kamanitz JR. Headaches and psychoactive substance use. Headache 1991; 31: 584–7. [DOI] [PubMed] [Google Scholar]
- Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 2019; 568: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991; 114: 2283–301. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. One-year brain atrophy evident in healthy aging. J Neurosci 2009; 29: 15223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan ME, Larson EB, Walker RL, Keene CD, Postupna N, Cholerton B, et al. Associations between use of specific analgesics and concentrations of amyloid-beta 42 or phospho-tau in regions of human cerebral cortex. J Alzheimers Dis 2018; 61: 653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest SL, Kril JJ, Wagner S, Honigschnabl S, Reiner A, Fischer P, et al. Chronic traumatic encephalopathy (CTE) is absent from a European community-based aging cohort while cortical aging-related tau astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 2019; 78: 398–405. [DOI] [PubMed] [Google Scholar]
- Fournier CN, Gearing M, Upadhyayula SR, Klein M, Glass JD. Head injury does not alter disease progression or neuropathologic outcomes in ALS. Neurology 2015; 84: 1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier T, Morganti-Kossmann MC, O'Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma 2010; 27: 497–507. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Asami-Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T. Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer's disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am J Pathol 1996; 148: 259–65. [PMC free article] [PubMed] [Google Scholar]
- Gao AF, Ramsay D, Twose R, Rogaeva E, Tator C, Hazrati LN. Chronic traumatic encephalopathy-like neuropathological findings without a history of trauma. Int J Pathol Clin Res 2017; 3: 50. [Google Scholar]
- Gardner A, Iverson GL, McCrory P. Chronic traumatic encephalopathy in sport: a systematic review. Br J Sports Med 2014; 48: 84–90. [DOI] [PubMed] [Google Scholar]
- Gavett BE, Cantu RC, Shenton M, Lin AP, Nowinski CJ, McKee AC, et al. Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Curr Opin Neurol 2011a; 24: 525–31. [DOI] [PubMed] [Google Scholar]
- Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011b; 30: 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol 1999; 98: 171–8. [DOI] [PubMed] [Google Scholar]
- Ghajari M, Hellyer PJ, Sharp DJ. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017; 140: 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Giannakopoulos AS, Herrmann FR, Michel JP, Bouras C. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol 1995; 52: 1150–9. [DOI] [PubMed] [Google Scholar]
- Goldfinger MH, Ling H, Tilley BS, Liu AKL, Davey K, Holton JL, et al. The aftermath of boxing revisited: identifying chronic traumatic encephalopathy pathology in the original Corsellis boxer series. Acta Neuropathol 2018; 136: 973–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012; 4: 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahmann H, Ule G. Diagnosis of chronic cerebral symptoms in boxers (dementia pugilistica & traumatic encephalopathy of boxers). Psychiatr Neurol (Basel) 1957; 134: 261–83. [PubMed] [Google Scholar]
- Harvey PK, Davis JN. Traumatic encephalopathy in a young boxer. Lancet 1974; 2: 928–9. [DOI] [PubMed] [Google Scholar]
- Hay J, Johnson VE, Smith DH, Stewart W. Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu Rev Pathol 2016; 11: 21–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Glannakopoulos P, Bouras C. The neuropathological changes associated with normal brain aging. Histol Histopathol 1996; 11: 1075–88. [PubMed] [Google Scholar]
- Hof PR, Knabe R, Bovier P, Bouras C. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathologica 1991; 82: 321–6. [DOI] [PubMed] [Google Scholar]
- Hsu ET, Gangolli M, Su S, Holleran L, Stein TD, Alvarez VE, et al. Astrocytic degeneration in chronic traumatic encephalopathy. Acta Neuropathol 2018; 136: 955–72. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T. The natural history of Alzheimer neurofibrillary tangles and amyloid deposits. Neurobiol Aging 1997; 18: 386–7; discussion 9–92. [DOI] [PubMed] [Google Scholar]
- Iacono D, Resnick SM, O'Brien R, Zonderman AB, An Y, Pletnikova O, et al. Mild cognitive impairment and asymptomatic alzheimer disease subjects: equivalent beta-amyloid and tau loads with divergent cognitive outcomes. J Neuropathol Exp Neurol 2014; 73: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, et al. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 2004; 190: 192–203. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013; 123: 1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Gardner AJ. Risk for misdiagnosing chronic traumatic encephalopathy in men with depression. J Neuropsychiatry Clin Neurosci. 2019. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Gardner AJ, McCrory P, Zafonte R, Castellani RJ. A critical review of chronic traumatic encephalopathy. Neurosci Biobehav Rev 2015; 56: 276–93. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Keene CD, Perry G, Castellani RJ. The need to separate chronic traumatic encephalopathy neuropathology from clinical features. J Alzheimers Dis 2018; 61: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Luoto TM, Karhunen PJ, Castellani RJ. Mild chronic traumatic encephalopathy neuropathology in people with no known participation in contact sports or history of repetitive neurotrauma. J Neuropathol Exp Neurol 2019; 78: 615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 1997; 49: 786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Irwin W, Dabbs K, Lin JJ, Jones JE, Hsu DA, et al. Ventricular enlargement in new-onset pediatric epilepsies. Epilepsia 2011; 52: 2225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlinski J, Gatarski J, Szymusik A. Chronic posttraumatic changes in the central nervous system in pugilists. Pol Med J 1970; 9: 743–52. [PubMed] [Google Scholar]
- Johnson J. Organic psychosyndromes due to boxing. Br J Psychiatry 1969; 115: 45–53. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol 2012; 22: 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Trojanowski JQ, Smith DH. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol 2011; 122: 715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 2013; 9: 222–30. [DOI] [PubMed] [Google Scholar]
- Jullienne A, Obenaus A, Ichkova A, Savona-Baron C, Pearce WJ, Badaut J. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res 2016; 94: 609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson A, Hakansson A. Gambling disorder, increased mortality, suicidality, and associated comorbidity: A longitudinal nationwide register study. J Behav Addict 2018; 7: 1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, et al. Cerebral vascular injury in traumatic brain injury. Exp Neurol 2016; 275: 353–66. [DOI] [PubMed] [Google Scholar]
- Koga S, Dickson DW, Bieniek KF. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 2016; 75: 963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 2015; 523: 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 2016; 131: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Horvath MC, Majtenyi K, Lutz MI, Hurd YL, Keller E. Heroin abuse exaggerates age-related deposition of hyperphosphorylated tau and p62-positive inclusions. Neurobiol Aging 2015; 36: 3100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel J, Papadopoulos Z, McKee AC. Chronic traumatic encephalopathy: is latency in symptom onset explained by tau propagation? Cold Spring Harb Perspect Med 2018; 8: a024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbe JR, Hall ED. Chronic traumatic encephalopathy-integration of canonical traumatic brain injury secondary injury mechanisms with tau pathology. Prog Neurobiol 2017; 158: 15–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, et al. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett 2008; 438: 330–4. [DOI] [PubMed] [Google Scholar]
- Laffey M, Darby AJ, Cline MG, Teng E, Mendez MF. The utility of clinical criteria in patients with chronic traumatic encephalopathy. NeuroRehabilitation 2018; 43: 431–41. [DOI] [PubMed] [Google Scholar]
- Lai HM, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990-2014: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 154: 1–13. [DOI] [PubMed] [Google Scholar]
- Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 2019; 138: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW. Propagation of tau pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 2016; 131: 27–48. [DOI] [PubMed] [Google Scholar]
- Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015; 130: 891–3. [DOI] [PubMed] [Google Scholar]
- Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry 2013; 70: 1100–6. [DOI] [PubMed] [Google Scholar]
- Martland HS. Punch drunk. J Am Med Assoc 1928; 19: 1103–7. [Google Scholar]
- Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet 1963; 2: 795–801. [DOI] [PubMed] [Google Scholar]
- McDermott MJ, Tull MT, Gratz KL, Houle TT, Smitherman TA. Comorbidity of migraine and psychiatric disorders among substance-dependent inpatients. Headache 2014; 54: 290–302. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016; 131: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009; 68: 709–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol 2014; 127: 29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2010; 69: 918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimer's Dement 2014; 10: S242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015; 25: 350–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF, et al. Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci Transl Med 2016; 8: 321ra6. [DOI] [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain 2003; 126: 515–30. [DOI] [PubMed] [Google Scholar]
- Mendez MF. The neuropsychiatric aspects of boxing. Int J Psychiatry Med 1995; 25: 249–62. [DOI] [PubMed] [Google Scholar]
- Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep 2013; 13: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millspaugh J. Dementia pugilistica. US Nav Med Bull 1937; 35: 297–361. [Google Scholar]
- Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 2014; 6: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenigro PH, Corp DT, Stein TD, Cantu RC, Stern RA. Chronic traumatic encephalopathy: historical origins and current perspective. Annu Rev Clin Psychol 2015; 11: 309–30. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NF, Lemieux L, Kitchen ND, Fish DR, Shorvon SD. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain 2001; 124: 167–75. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW Jr., Rubin EH, Price JL, Grant EA, et al. Cerebral amyloid deposition and diffuse plaques in "normal" aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology 1996; 46: 707–19. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science 1997; 278: 412–9. [DOI] [PubMed] [Google Scholar]
- Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 1999; 58: 188–97. [DOI] [PubMed] [Google Scholar]
- Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 2015; 77: 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, Neltner JH, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 2013; 126: 161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy S, Krawitz S, Del Bigio MR. Chronic traumatic encephalopathy-like abnormalities in a routine neuropathology service. J Neuropathol Exp Neurol 2016; 75: 1145–54. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, et al. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A 2010; 107: 22682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu B. Chronic traumatic encephalopathy. Prog Neurol Surg 2014; 28: 38–49. [DOI] [PubMed] [Google Scholar]
- Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery 2011; 69: 173–83; discussion 83. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist. Am J Forensic Med Pathol 2010a; 31: 130–2. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery 2006; 59: 1086–92; discussion 92–3. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005; 57: 128–34; discussion 34. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs 2010b; 6: 130–6. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: case report and emerging medicolegal practice questions. J Forensic Nurs 2010c; 6: 40–6. [DOI] [PubMed] [Google Scholar]
- Painuly N, Sharan P, Mattoo SK. Relationship of anger and anger attacks with depression: a brief review. Eur Arch Psychiatry Clin Neurosci 2005; 255: 215–22. [DOI] [PubMed] [Google Scholar]
- Painuly NP, Grover S, Gupta N, Mattoo SK. Prevalence of anger attacks in depressive and anxiety disorders: implications for their construct? Psychiatry Clin Neurosci 2011; 65: 165–74. [DOI] [PubMed] [Google Scholar]
- Parker HL. Traumatic encephalopathy (‘Punch Drunk’) of professional pugilists. J Neurol Psychopathol 1934; 15: 20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne EE. Brains of boxers. Neurochirurgia (Stuttg) 1968; 11: 173–88. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol 1999; 45: 358–68. [DOI] [PubMed] [Google Scholar]
- Puvenna V, Engeler M, Banjara M, Brennan C, Schreiber P, Dadas A, et al. Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res 2016; 1630: 225–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 2004; 24: 277–95. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Acker JD. Age-related shrinkage of the mamillary bodies: in vivo MRI evidence. Neuroreport 1992; 3: 713–6. [DOI] [PubMed] [Google Scholar]
- Reams N, Eckner JT, Almeida AA, Aagesen AL, Giordani B, Paulson H, et al. A clinical approach to the diagnosis of traumatic encephalopathy syndrome: a review. JAMA Neurol 2016; 73: 743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley DO, Robbins CA, Cantu RC, Stern RA. Chronic traumatic encephalopathy: contributions from the Boston University Center for the Study of Traumatic Encephalopathy. Brain Inj 2015; 29: 154–63. [DOI] [PubMed] [Google Scholar]
- Roberts A. Brain damage in boxers: a study of prevalence of traumatic encephalopathy among ex-professional boxers. London: Pitman Medical Scientific Publishing Co; 1969. [Google Scholar]
- Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry 1990a; 53: 373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Graham DI. beta A4 amyloid protein deposition in brain after head trauma. Lancet 1991; 338: 1422–3. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry 1994; 57: 419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Whitwell HL, Acland PR, Bruton CJ. Dementia in a punch-drunk wife. Lancet 1990b; 335: 918–9. [DOI] [PubMed] [Google Scholar]
- Ronzitti S, Soldini E, Smith N, Potenza MN, Clerici M, Bowden-Jones H. Current suicidal ideation in treatment-seeking individuals in the United Kingdom with gambling problems. Addict Behav 2017; 74: 33–40. [DOI] [PubMed] [Google Scholar]
- Rossi P, Allena M, Tassorelli C, Sances G, Di Lorenzo C, Faroni JV, et al. Illicit drug use in cluster headache patients and in the general population: a comparative cross-sectional survey. Cephalalgia 2012; 32: 1031–40. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 2003; 60: 989–94. [DOI] [PubMed] [Google Scholar]
- Schwab N, Tator C, Hazrati LN. DNA damage as a marker of brain damage in individuals with history of concussions. Lab Invest 2019; 99: 1008–18. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016; 8: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercl M, Jaros O. The mechanisms of cerebral concussion in boxing and their consequences. World Neurol 1962; 3: 351–8. [PubMed] [Google Scholar]
- Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res 1996; 20: 1489–95. [DOI] [PubMed] [Google Scholar]
- Shively SB, Edgerton SL, Iacono D, Purohit DP, Qu BX, Haroutunian V, et al. Localized cortical chronic traumatic encephalopathy pathology after single, severe axonal injury in human brain. Acta Neuropathol 2017; 133: 353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 2015; 138: 1297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013; 9: 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Uryu K, Saatman KE, Trojanowski JQ, McIntosh TK. Protein accumulation in traumatic brain injury. Neuromol Med 2003; 4: 59–72. [DOI] [PubMed] [Google Scholar]
- Solomon G. Chronic traumatic encephalopathy in sports: a historical and narrative review. Dev Neuropsychol 2018; 43: 279–311. [DOI] [PubMed] [Google Scholar]
- Solomon GS, Sills A. Chronic traumatic encephalopathy and the availability cascade. Phys Sportsmed 2014; 42: 26–31. [DOI] [PubMed] [Google Scholar]
- Stein TD, Alvarez VE, McKee AC. Concussion in chronic traumatic encephalopathy. Curr Pain Headache Rep 2015; 19: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013; 81: 1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 2011a; 3: S460–7. [DOI] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 2011b; 3: S460–7. [DOI] [PubMed] [Google Scholar]
- Stewart W, Allinson K, Al-Sarraj S, Bachmeier C, Barlow K, Belli A, et al. Primum non nocere: a call for balance when reporting on CTE. Lancet Neurol 2019; 18: 231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev 2018; 84: 316–24. [DOI] [PubMed] [Google Scholar]
- Sundqvist K, Rosendahl I. Problem gambling and psychiatric comorbidity-risk and temporal sequencing among women and men: results from the Swelogs case-control study. J Gambl Stud 2019; 35: 757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen GM, Ko A, Harada MY, Ma A, Wyss L, Haro P, et al. Clinical correlates to assist with chronic traumatic encephalopathy diagnosis: insights from a novel rodent repeat concussion model. J Trauma Acute Care Surg 2017; 82: 1039–48. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers' brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol 1991; 82: 280–5. [DOI] [PubMed] [Google Scholar]
- Victoroff J. Traumatic encephalopathy: review and provisional research diagnostic criteria. NeuroRehabilitation 2013; 32: 211–24. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Biernat J, Mandelkow EM, Mandelkow E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim Biophys Acta 2005; 1739: 158–66. [DOI] [PubMed] [Google Scholar]
- Walt GS, Burris HM, Brady CB, Spencer KR, Alvarez VE, Huber BR, et al. Chronic traumatic encephalopathy within an amyotrophic lateral sclerosis brain bank cohort. J Neuropathol Exp Neurol 2018; 77: 1091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler D, Pjrek E, Kasper S. Anger attacks in depression—evidence for a male depressive syndrome. Psychother Psychosom 2005; 74: 303–7. [DOI] [PubMed] [Google Scholar]
- Zanier ER, Bertani I, Sammali E, Pischiutta F, Chiaravalloti MA, Vegliante G, et al. Induction of a transmissible tau pathology by traumatic brain injury. Brain 2018; 141: 2685–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman SL, Brett BL, Jeckell A, Yengo-Kahn AM, Sills AK, Solomon GS. Chronic traumatic encephalopathy and neurodegeneration in contact sports and american football. J Alzheimers Dis 2018; 66: 37–55. [DOI] [PubMed] [Google Scholar]