Abstract
Introduction:
A recent study reported an association between inactivated influenza vaccine (IIV) and spontaneous abortion (SAB), but only among women who had also been vaccinated in the previous influenza season. We sought to estimate the association between IIV administered in three recent influenza seasons and SAB among women who were and were not vaccinated in the previous influenza season.
Methods:
We conducted a case-control study over three influenza seasons (2012–13, 2013–14, 2014–15) in the Vaccine Safety Datalink (VSD). Cases (women with SAB) and controls (women with live births) were matched on VSD site, date of last menstrual period, age group, and influenza vaccination status in the previous influenza season. Of 1908 presumptive cases identified from the electronic record, 1236 were included in the main analysis. Administration of IIV was documented in several risk windows, including 1–28, 29–56, and >56 days before the SAB date.
Results:
Among 627 matched pairs vaccinated in the previous season, no association was found between vaccination in the 1–28 day risk window and SAB (adjusted odds ratio (aOR) 0.9; 95% confidence interval (CI) 0.6–1.5). The season-specific aOR ranged from 0.5 to 1.7 with all CIs including the null value of 1.0. Similarly, no association was found among women who were not vaccinated in the previous season; the season-specific aOR in the 1–28 day risk window ranged from 0.6 to 0.7 and the 95% CI included 1.0 in each season. There was no association found between SAB and influenza vaccination in the other risk windows, or when vaccine receipt was analyzed relative to date of conception.
Conclusion:
During these seasons we found no association between IIV and SAB, including among women vaccinated in the previous season. These findings lend support to current recommendations for influenza vaccination at any time during pregnancy, including the first trimester.
Keywords: Influenza vaccine, Spontaneous abortion, Influenza, Pregnancy
1. Introduction
Influenza infection can cause serious complications, especially in high-risk persons, which include pregnant women [1,2]. Studies show that pregnant women experience higher rates of morbidity, hospitalization, and mortality during seasonal influenza epidemics and pandemics, such as the 2009 pandemic caused by A/California/7/2009 (H1N1)pdm09 (pH1N1) [3–6]. Since 2004, the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) and other organizations have recommended vaccination of women who are or will be in any stage of pregnancy during the influenza season [7,8]. Numerous studies have established the safety of seasonal influenza vaccines administered to pregnant women [9–11]; particularly relevant for this study are the safety studies of vaccines containing pH1N1 antigens and the risk of spontaneous abortion [12–18].
In 2017, Vaccine Safety Datalink (VSD) investigators published a case-control study that reported an adjusted odds ratio (aOR) of 2.0 and a 95% confidence interval (CI) of 1.1 to 3.6 for spontaneous abortion (SAB) among women vaccinated with inactivated influenza vaccination (IIV) [19]. Post hoc analyses revealed that in each of the two influenza seasons studied (2010–11 and 2011–12), the association between IIV and SAB was found only in women vaccinated in the 1–28 days before the SAB who had also received a pH1N1-containing influenza vaccine the previous season (i.e., effect modification). Although the results were based on relatively small sample sizes, additional investigation did not identify any error or bias that might explain these findings. This study was preceded by a similarly designed VSD study of women pregnant in the influenza seasons of 2005–06 and 2006–07 that observed an aOR of 1.2 (95% CI 0.5–2.9) for the IIV-SAB association in the 128 day risk window [20]. However, the earlier study did not evaluate vaccination in the previous influenza season. Here we report the findings of a third VSD study specifically designed to determine if receipt of IIV was associated with SAB among women who had and had not been administered influenza vaccine the previous season. For the purposes of this report, we refer to the VSD study of women pregnant in the 2005–06 or 2006–07 influenza seasons as IIV-SAB-1 [20], the VSD study of women pregnant in the 2010–11 and 2011–12 influenza seasons as IIV-SAB-2 [19], and the current VSD study of women pregnant in the 2012–13, 2013–14, and 2014–15 influenza seasons as IIV-SAB-3.
2. Methods
2.1. Study population
In IIV-SAB-3, we included women who were pregnant during the 2012–13, 2013–14, or 2014–15 influenza seasons and were members of the VSD population at one of six integrated healthcare delivery organizations: Kaiser Permanente (Colorado, Northern California, Southern California, Northwest, Washington) and Marshfield Clinic in Wisconsin. Sites contributed subjects approximately proportionate to their size in the overall VSD population. The VSD was established in 1990 as a collaborative project between several integrated healthcare organizations and CDC; it is one of the primary post-licensure vaccine safety monitoring and research systems in the United States [21].
The study was approved by the Institutional Review Boards of each VSD organization.
2.2. Cases
We identified possible cases of SAB by searching VSD databases for spontaneous and unspecified abortion diagnosis codes (International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) 634.* and 637.* (Supplemental Table 1) assigned during encounters in ambulatory, urgent care, emergency department, and inpatient settings. To enhance our ability to identify effect modification by previous season influenza vaccination, we conducted stratified sampling to ensure 50% of the cases in each of the three seasons were vaccinated in the previous influenza season.
The primary analysis focused on cases with gestational ages between 6 and <20 weeks at time of estimated pregnancy loss; however, we also examined SABs between 5 and <20 weeks in a secondary analysis to be consistent with IIV-SAB-2 [19]. We only included SAB diagnoses assigned during the influenza season (September 1 through April 28) to avoid including women with no opportunity for influenza vaccination. We confirmed pregnancy using information abstracted from the medical record: obstetric ultrasound, clinic or hospital-based assay (e.g., human chorionic gonadotropin level), patient-reported test, and/or physician diagnosis.
Study eligibility criteria included: (1) documentation of last menstrual period (LMP) in the medical record, (2) continuous enrollment in the healthcare organization for 20 months prior to the LMP, (3) age 18–44 years on the date of LMP, and (4) SAB confirmed by ultrasound or by clinical diagnosis in the absence of ultrasound results. More than 90% of cases had ultrasound information available, but ultrasound was not required for eligibility. The continuous enrollment requirement was necessary to ensure capture of prior influenza vaccinations and chronic medical conditions from the medical record. Exclusion criteria included ectopic pregnancy, therapeutic abortion, multi-fetal pregnancy, or SAB occurring at <5 weeks’ gestation.
Documentation of all cases began with a search of electronic diagnosis codes and was supplemented with available information about care received for their SAB, which was obtained from the medical record. We abstracted information related to pregnancy, including ultrasound and medical history, from medical records of cases using trained abstractors. All potential cases with an ultrasound were adjudicated by the investigative team (blinded to influenza vaccination status) to determine the SAB date and gestational age at the time of pregnancy loss as accurately as possible following an algorithm that was developed and refined by the study obstetrician (M.A.M.).[22] The SAB date was based on the earliest clinical diagnosis for women without ultrasound results. The study obstetrician (M.A.M.), who was also blinded to vaccination status, adjudicated cases with equivocal ultrasound results. We defined the SAB date as the LMP date plus the estimated gestational age at the time of the SAB.
2.3. Controls
Controls met the same age, LMP, and enrollment inclusion criteria as cases but had a live delivery instead of an SAB diagnosis; live delivery was identified using a VSD pregnancy database [23] or by searching for specific ICD-9 or ICD-10 codes in VSD data files (Supplemental Table 1). Trained abstractors reviewed medical records to confirm the live delivery and collect additional clinical and pregnancy information.
2.4. Matching
We individually matched cases and controls (1:1 ratio) by VSD site, maternal age (18–24, 25–34, and 35–44 years), influenza vaccination status in the previous season, and LMP. While matching multiple controls to each case would have provided methodological advantages, a 1:1 ratio was considered optimal given the available resources. Matching on vaccination in the previous season increased statistical power to identify effect modification by previous season vaccination on the potential association between current-season IIV and SAB. Matching on LMP ensured that cases and controls would have similar opportunity for influenza vaccination in early pregnancy and nearly identical gestational ages relative to calendar time. Matching on LMP began after we identified and adjudicated cases; 10 potential controls with LMP dates within seven days of the case LMP were randomly selected. We selected the first eligible control with an LMP most closely matching the case LMP. The estimated SAB date for the case was used as the reference date for each case-control pair.
2.5. Influenza vaccine exposure
The composition of trivalent IIV for the 2012–13 season was: A/California/7/2009 (H1N1)-like, A/Victoria/361/2011 (H3N2)-like, and B/Wisconsin/1/2010-like (Yamagata lineage).[24] The composition of trivalent IIV for the 2013–14 and 2014–15 seasons was: A/California/7/2009 (H1N1)-like virus, A/Texas/50/2012 (H3N2)-like virus, and B/Massachusetts/2/2012-like (Yamagata lineage) virus. The quadrivalent IIVs for the 2013–14 and 2014–15 seasons contained the above antigens and B/Brisbane/60/2008-like (Victoria lineage) virus [25,26]. However, less than 3% of IIV administered to our study population was quadrivalent; therefore, we did not differentiate trivalent and quadrivalent IIV exposures in the analysis.
We abstracted vaccination dates for influenza and other vaccines administered during pregnancy from medical records. Vaccines administered between August 1 and June 30 in the influenza season where the SAB for the matched pair occurred were considered current-season vaccinations. Women were considered exposed only if the vaccine was administered before the reference date; we classified women receiving influenza vaccines on or after the reference date as unexposed in all analyses. For analyses examining the association of SAB and vaccinations relative to the reference date, we categorized current-season vaccinations into three mutually exclusive risk windows that preceded the reference date: 128, 29–56, and >56 days. For analyses examining the association of SAB and vaccinations relative to conception, current-season vaccinations were categorized into four risk windows relative to conception date: >42 days before, 0–42 days before, 1–28 days after (and before the reference date), and >28 days after (and before the reference date). Conception was defined as the date of the last menstrual period plus 14 days. We also documented influenza vaccines administered to women during the influenza season before the season in which the SAB for the matched pair occurred. We excluded matched pairs containing 22 women who were administered LAIV or high-dose IIV before the reference date in the current season. Valid previous season vaccinations included all vaccine types, except high-dose IIV.
2.6. Statistical analysis
We compared characteristics of matched cases and controls using Wilcoxon signed-rank tests for continuous variables, [27] McNemar tests for dichotomous variables, [28] and Bowkef’s test of symmetry for categorical variables with more than two levels [29]. P-values were based on two-sided tests. We used SAS 9.4 (SAS Institute, Cary, NC) for the analysis.
We performed conditional logistic regression to estimate the association between receipt of IIV and SAB. We decided a priori to build separate models (including separate confounding assessments) for women vaccinated in the previous influenza season and those that were not to facilitate assessment of effect modification. We built a third model using the pooled data from the above two strata after finding no statistical evidence of effect modification by previous season vaccination status.
We included three covariates in all multivariable models because they were suspected a priori to be associated with both SAB and vaccination: maternal age, pre-pregnancy body mass index (BMI), and previous healthcare utilization (defined as the number of days with at least one healthcare encounter (inpatient or outpatient) in the year before the LMP) [30]. We assessed other covariates based on associations observed between the covariate and the outcome (i.e., SAB) and included those with P-values ≤0.20.[31] Variables screened as potential confounders appear in Table 1 although some were not viable candidates due to sparse data or many missing values. As in the previous two studies, [19,20] we did not adjust for history of prior SAB to avoid potential bias [32], although we conducted a secondary analysis excluding matched pairs where either woman had a history of ≥2 SABs.
Table 1.
Demographic and clinical characteristics of SAB cases and controlsa.
| Vaccinated in previous seasonb | Not vaccinated in previous seasonc | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | Pd | Cases | Controls | Pd | |
| N | 627 | 627 | 609 | 609 | ||
| Age in years at LMP | <0.001 | <0.001 | ||||
| 18–19 | 23 (4) | 10 (2) | 9 (1) | 12 (2) | ||
| 20–23 | 30 (5) | 32 (5) | 43 (7) | 44 (7) | ||
| 24–27 | 58 (9) | 64 (10) | 64 (11) | 70 (11) | ||
| 28–31 | 88 (14) | 104 (17) | 139 (23) | 120 (20) | ||
| 32–35 | 162 (26) | 187 (30) | 105 (17) | 151 (25) | ||
| 36–39 | 156 (25) | 191 (30) | 147 (24) | 175 (29) | ||
| 40–44 | 110 (18) | 39 (6) | 102 (17) | 37 (6) | ||
| Median (IQR) | 35.0 (31, 39) | 34.9 (30, 37) | <0.001 | 34.4 (29, 39) | 34.2 (29, 37) | 0.001 |
| BMI (pre-pregnancy) | 0.01 | 0.16 | ||||
| <18.5 | 10 (2) | 19 (3) | 10 (2) | 14 (2) | ||
| 18.5-<25 | 286 (46) | 309 (49) | 232 (39) | 278 (46) | ||
| 25-<30 | 141 (23) | 169 (27) | 169 (28) | 164 (27) | ||
| ≥30 | 189 (30) | 129 (21) | 189 (32) | 152 (25) | ||
| Median (IQR) | 25.4 (22, 32) | 24.8 (22, 29) | 0.01 | 26.3 (23, 32) | 25.4 (22, 30) | 0.002 |
| Race | ||||||
| White | 346 (55) | 381 (61) | 0.04 | 353 (58) | 380 (62) | 0.11 |
| Black | 41 (7) | 27 (4) | 0.10 | 68 (11) | 42 (7) | 0.01 |
| Native American or Aleutian | 4 (1) | 5(1) | 1.00 | 5 (1) | 9 (1) | 0.42 |
| Native Hawaiian or other Pacific Islander | 8 (1) | 15 (2) | 0.21 | 6 (1) | 4 (1) | 0.75 |
| Asian | 124 (20) | 158 (25) | 0.02 | 65 (11) | 108 (18) | 0.001 |
| Other | 36 (6) | 18 (3) | 0.02 | 40 (7) | 23 (4) | 0.04 |
| Ethnicity | 0.002 | 0.41 | ||||
| Non-Hispanic | 428 (68) | 476 (76) | 427 (70) | 440 (72) | ||
| Hispanic | 199 (32) | 151 (24) | 182 (30) | 169 (28) | ||
| Occupation | 0.21 | 0.84 | ||||
| Healthcare | 122 (19) | 141 (22) | 64 (11) | 74 (12) | ||
| Education | 53 (8) | 74(12) | 62 (10) | 73 (12) | ||
| Other | 290 (46) | 296 (47) | 308 (51) | 323 (53) | ||
| Unknown | 162 (26) | 116 (18) | 175 (29) | 139 (23) | ||
| Parity (≥1) | 445 (71) | 452 (72) | 0.75 | 324 (54) | 355 (58) | 0.07 |
| Gravidity (≥1) | 513 (82) | 515 (82) | 1.00 | 424 (70) | 445 (73) | 0.33 |
| Previous SAB | ||||||
| ≥1 | 217 (35) | 207 (33) | 0.58 | 175 (29) | 175 (29) | 1.00 |
| ≥2 | 74 (12) | 63 (10) | 0.37 | 57 (9) | 58 (10) | 1.00 |
| Alcohol use during pregnancy | 49 (8) | 47 (8) | 0.92 | 70 (11) | 62 (10) | 0.52 |
| Smoked during pregnancy | 21 (3) | 29 (5) | 0.30 | 35 (6) | 35 (6) | 1.00 |
| Concomitant IIV and Tdap vaccination before reference date | 1 (0) | 3 (0) | 0.62 | 1 (0) | 3 (0) | 0.62 |
| Type I/II diabetes | 21 (3) | 8 (1) | 0.02 | 9 (1) | 11 (2) | 0.82 |
| Asthma | 83 (13) | 60 (10) | 0.06 | 72 (12) | 63 (10) | 0.46 |
| Pre-existing hypertension | 31 (5) | 22 (4) | 0.24 | 24 (4) | 19 (3) | 0.54 |
| Antiphospholipid syndrome | 0 (0) | 1 (0) | NA | 0 (0) | 1 (0) | NA |
| Celiac disease | 0 (0) | 1 (0) | NA | 1 (0) | 0 (0) | NA |
| Hashimoto’s thyroiditis | 5 (1) | 4 (1) | 1.00 | 4 (1) | 3 (0) | 1.00 |
| Systemic lupus erythematosus | 2 (0) | 0 (0) | NA | 1 (0) | 0 (0) | NA |
| Multiple sclerosis | 2 (0) | 1 (0) | 1.00 | 2 (0) | 0 (0) | NA |
| Febrile illness in the 1–14 days before reference/event date | 8 (1) | 2 (0) | 0.11 | 7 (1) | 3 (0) | 0.34 |
| Febrile illness in 1st trimester, but before the reference date | 10 (2) | 8 (1) | 0.81 | 13 (2) | 11 (2) | 0.84 |
| Number of days with outpatient diagnoses, vaccinations, or hospitalizations in the 365 days before LMP, median (IQR) | 6 (3, 11) | 6 (3, 10) | 0.35 | 4 (2, 8) | 4 (2, 8) | 0.35 |
Data are N (%) unless otherwise noted. IQR = Interquartile Range. Table includes data for cases (and matched controls) that are between 6 and less than 20 weeks’ gestation. Total N and (%) may not add up to the stratum-specific sample sizes since missing values are not shown in the table.
Missing data among those vaccinated in the previous season: One case and one control missing BMI, two cases missing parity, two cases missing gravidity, one case missing count of previous SAB.
Missing data among those NOT vaccinated in the previous season: Nine cases and one control missing BMI, four cases missing parity, six cases missing gravidity, two cases missing count of previous SABs.
For categorical variables, the statistical tests were McNemar’s test for 2x2 tables and Bowker’s test of symmetry for tables larger than 2 × 2. For continuous variables, the statistical test was the Wilcoxon signed-rank test.
All three models described above included the core covariates: maternal age, pre-pregnancy BMI, previous healthcare utilization, Hispanic ethnicity, and four non-mutually-exclusive race variables. We entered age, BMI, and resource utilization in the models as natural cubic splines [33]. Since age group was a matching factor, rather than a spline for age itself, we entered in the models a spline for the deviation of each woman’s age from the mean age in their matched pair [34]. In our study population 334 women (13.5%) did not have a specified race, and this was strongly linked to Hispanic ethnicity; 92.2% of those with no race specified were Hispanic, compared to 18.4% of those with a specified race. Therefore, we did not exclude persons from the analysis if race was not specified because we believed that such exclusion would introduce bias. However, because retaining these individuals in the analysis may also cause bias, we conducted a secondary analysis in which we excluded all matched pairs where at least one woman did not have a specified race (n = 315 pairs). The model for subjects vaccinated the previous season also included history of type 1 or 2 diabetes, asthma, and presence of febrile illness in the 14 days before the reference date. The model for subjects not vaccinated the previous season included the core covariates and parity. Finally, the overall model included all variables in the stratum-specific models plus pre-existing hypertension. The referent exposure group in all odds ratio (OR) calculations was comprised of women not vaccinated as of the reference date. We structured our power calculations on the power to detect an association between IIV and SAB in the 1–128 days before the reference date (the primary risk window) for a given influenza season and stratum (vaccinated in the previous season or not). We determined that 250 matched pairs per stratum per season were required to detect an OR of 3.5 with a power of 0.82 in the primary risk window. Pooling stratum-specific data for two seasons (e.g., seasons where the influenza vaccine composition was the same) resulted in power of 0.82 to detect an OR of 2.3. Pooling both strata and all seasons would result in power of 0.83 to detect an OR of 1.6. We based power calculations on a proportion of 0.106 for pairs that were discordant for current-season vaccination exposure, which was derived from IIV-SAB-2 [19]. Additional assumptions used in the power calculations were 1:1 matching of cases and controls and two-sided α = 0.05.
3. Results
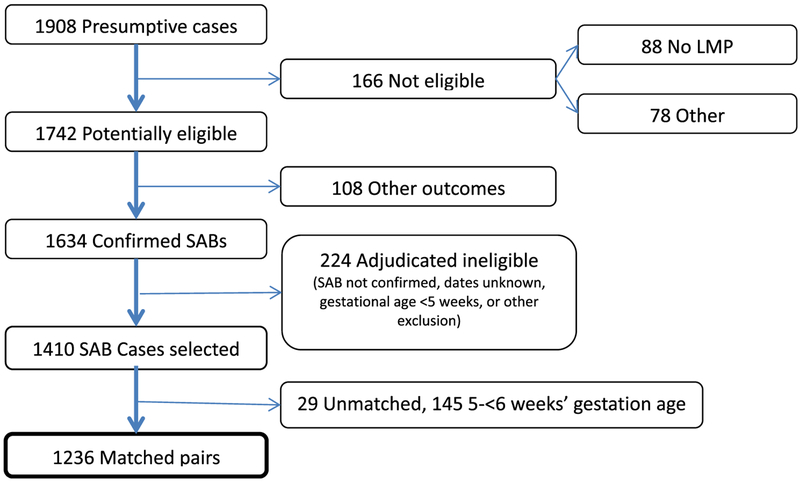
We identified 1908 presumptive SAB cases from automated data using diagnosis codes (Fig. 1). Of these, 166 (8.7%) were ineligible due to the absence of LMP or other reasons (e.g., unconfirmed pregnancy, multi-fetal pregnancies, LMP outside eligible period, age <18 years), and an additional 108 (5.7%) had non-SAB outcomes (e.g., therapeutic abortion, ectopic pregnancy, live birth). Of 1634 confirmed cases of SAB, we excluded 224 (13.7%) after adjudication due to gestational age being out of range, adjudicated SAB date prior to September 1, or other reasons (e.g., therapeutic abortion). Of 1410 eligible and confirmed cases of SAB, we were unable to match 29 (2.1%) to an eligible control. The remaining 1381 cases (72.4% of 1908) had a gestational age of 5-<20 weeks and were matched to 1381 controls resulting in a total study population of 2762. Of these, 1236 cases (89.5%) had a gestational age of 6-<20 weeks and were the focus of the primary analysis.
Fig. 1.
Identification and confirmation of spontaneous abortions.
We compared the demographic and clinical characteristics of cases and controls, stratified by previous season vaccination status (Table 1). Of the 1236 matched pairs in the main analysis, 627 were vaccinated in the previous season and 609 were not. Within each stratum, the median maternal age of cases was greater than controls; a larger proportion of cases were 40–44 years old compared to controls; pre-pregnancy BMI was greater among cases than controls; and cases were more likely to be Black/African American, less likely to be Asian, and more likely to be Hispanic than controls. Cases and controls were similar with respect to parity (≥1) and gravidity (≥1), but both were lower among subjects not vaccinated the previous season. The proportion of cases and controls with a history of ≥1 or ≥2 miscarriages was similar in both strata. Alcohol and smoking exposure during pregnancy did not vary by case-control status, but was greater among women not vaccinated the previous season. Among women vaccinated the previous season, cases were more likely than controls to have a history of diabetes. Asthma and febrile illness in the 1–14 days before the reference date were also more common among cases. Other factors were similar in cases and controls or were uncommon. Selected characteristics of study subjects showed minimal variation by season of enrollment (Supplemental Table 2).
Overall and season-specific proportions of women vaccinated for influenza in the various risk windows before the reference date were generally greater among women vaccinated the previous season compared to those not vaccinated previously; however, within each stratum cases and controls had similar vaccination proportions (Table 2). For example, among cases in all seasons who were vaccinated the previous season, 10% (n = 70) were vaccinated in the 1–28 day risk window compared to 9% (n = 64) of controls.
Table 2.
Influenza vaccinations before the reference date among cases and controls, by timing of vaccination and prior season vaccination statusa.
| Influenza season | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012–13 | 2013–14 | 2014–15 | All seasons combined | |||||||||||||
| Vaccinated in previous season? | Vaccinated in previous season? | Vaccinated in previous season? | Vaccinated in previous season? | |||||||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | |||||||||
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | |
| Days from vaccination to ref. date | ||||||||||||||||
| 1–28 | 21 (9) | 27 (11) | 12 (5) | 18 (8) | 30 (13) | 25 (11) | 10 (4) | 20 (9) | 19 (8) | 12 (5) | 13 (6) | 17 (7) | 70 (10) | 64 (9) | 35 (5) | 55 (8) |
| 29–56 | 23 (10) | 27 (11) | 2 (1) | 5 (2) | 16 (7) | 27 (12) | 1 (0) | 9 (4) | 18 (8) | 18 (8) | 3 (1) | 4(2) | 57 (8) | 72 (11) | 6 (1) | 18 (3) |
| >56 | 85 (35) | 97 (40) | 16 (7) | 20 (9) | 88 (39) | 80 (36) | 23 (10) | 22 (10) | 78 (34) | 89 (39) | 25 (11) | 31 (14) | 251 (36) | 266 (39) | 64 (9) | 73 (11) |
| Not vaccinatedb | 109 (45) | 85 (35) | 201 (87) | 188 (81) | 91 (40) | 90 (40) | 187 (84) | 172 (77) | 115 (50) | 108 (47) | 187 (82) | 177 (77) | 315 (45) | 283 (41) | 575 (85) | 537 (79) |
Data are N (%). Column percentages may not add to 100% due to rounding and missing values: 10 in 2012–13, 5 in 2013–14, and 6 in 2014–15.
Before the reference date.
Among women vaccinated the previous season, the aOR for influenza vaccine receipt in the 1–28 day risk window was 0.9 (95% CI, 0.6 to 1.5) (Table 3). Season-specific aORs for the 128 day window ranged from 0.5 to 1.7; the 95% CIs included 1.0 for each season; similar results were noted for other risk windows (29–56 and > 56 days). Among women not vaccinated the previous season, the overall aOR in the 1–28 day window and season-specific aORs were all less than 1.0 with 95% CI that included 1.0. The aORs for other risk windows ranged from 0.1 to 1.8 in the three seasons, with 95% CI that included 1.0 in each instance.
Table 3.
Odds ratios for the association of SAB with influenza vaccination before the reference date, by influenza season and vaccination status in the previous seasona
| Vaccination to reference date (days) | 2012–13 | Influenza season 2013–14 | 2014–15 | All seasons combined | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc. pairsb | Crude ORc | aORd | Disc. pairssb | Crude ORc | aORd | Disc. pairssb | Crude ORc | aORd | Disc. pairssb | Crude ORc | aORd | ||
| Vaccinated in previous season | 1–28 | 6/15 | 0.5 | 0.5 (0.2, 1.1) | 11/14 | 1.0 | 1.1 (0.6, 2.3) | 8/6 | 1.4 | 1.7 (0.7, 4.0) | 25/35 | 0.9 | 0.9 (0.6, 1.5) |
| 29–56 | 4/11 | 0.6 | 0.6 (0.3, 1.3) | 4/9 | 0.5 | 0.8 (0.3, 1.8) | 6/6 | 0.9 | 1.0 (0.4, 2.5) | 14/26 | 0.6 | 0.7 (0.5, 1.2) | |
| >56 | 22/32 | 0.5 | 0.5 (0.3, 0.9) | 25/21 | 1.0 | 0.9 (0.5, 1.7) | 19/31 | 0.6 | 0.5 (0.3, 1.0) | 66/84 | 0.7 | 0.6 (0.5, 0.9) | |
| Not vaccinated in previous season | 1–28 | 9/13 | 0.5 | 0.7 (0.3, 1.6) | 8/18 | 0.4 | 0.6 (0.2, 1.4) | 11/13 | 0.8 | 0.7 (0.3, 1.8) | 28/44 | 0.6 | 0.7 (0.4, 1.1) |
| 29–56 | 2/2 | 0.7 | 1.8 (0.2, 14.9) | 0/6 | 0.1 | 0.1 (0.0, 1.2) | 2/1 | 0.6 | 0.5 (0.1, 3.0) | 4/9 | 0.4 | 0.4 (0.1, 1.3) | |
| >56 | 12/19 | 0.7 | 0.8 (0.4, 1.8) | 15/14 | 0.9 | 1.3 (0.6, 2.8) | 14/18 | 0.9 | 0.8 (0.4, 1.7) | 41/51 | 0.8 | 0.9 (0.6, 1.4) | |
The referent exposure group in all odds ratio calculations was comprised of women unvaccinated as of the reference date. Gestational age of cases ranges from 6 to <20 weeks. Based on the sets of covariates included in stratum-specific models, there were 2 discordant pairs with missing values for >1 covariate (for either the case or control in the pair) among women vaccinated in the previous season; there were 4 among women not vaccinated in the previous season.
Discordant pairs: the first number is the number of matched pairs where the case was vaccinated in the relevant exposure window (1–28, 29–56, >56 days before the reference date) and the control was unvaccinated as of the reference date, the second number is the number of matched pairs where the control was vaccinated in the window and the case was unvaccinated as of the reference date.
Crude odds ratios are controlled for matching variables: VSD site, LMP, vaccination in the previous season, and age group (18–24, 25–34, and 35–44).
In addition to the matching variables, adjusted odds ratios (aOR) are adjusted in all models for maternal age, BMI, selected race variables, Hispanic ethnicity, and healthcare utilization. Models for the stratum, vaccinated in prior season = Yes, also adjusted for diabetes, asthma, and febrile illness in the 14 days before the reference date. Models for the stratum, vaccinated in prior season = No, also adjusted for parity. Numbers in parentheses represent 95% confidence intervals.
Because effect modification of the association of IIV and SAB by vaccination in the previous season was not observed, we pooled stratum-specific data to produce aORs for each risk window in each season and for all seasons combined. The aORs were less than or close to 1.0 in every risk window and season (Table 4).
Table 4.
Odds ratios for the association of SAB with influenza vaccination before the reference date for all women, by influenza season and all seasons combineda.
| Time from vaccination to reference date | Disc.pairsb | Influenza season | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012–13 | 2013–14 | 2014–15 | All seasons combined | |||||||||
| Crude ORc | aORd | Disc.pairsb | Crude ORc | aORd | Disc.pairsb | Crude ORc | aORd | Disc.pairsb | Crude ORc | aORd | ||
| 1–28 days | 15/28 | 0.5 | 0.6 (0.3, 1.0) | 19/32 | 0.7 | 0.8 (0.5, 1.4) | 19/19 | 1.1 | 1.1 (0.6, 2.0) | 53/79 | 0.7 | 0.8 (0.6, 1.1) |
| 29–56 days | 6/13 | 0.6 | 0.7 (0.3, 1.5) | 4/15 | 0.3 | 0.5 (0.2, 1.0) | 8/7 | 0.8 | 0.8 (0.4, 1.7) | 18/35 | 0.6 | 0.6 (0.4, 1.0) |
| >56 days | 34/51 | 0.6 | 0.6 (0.4, 1.0) | 40/35 | 0.9 | 1.0 (0.6, 1.6) | 33/48 | 0.7 | 0.6 (0.4, 1.0) | 107/134 | 0.7 | 0.7 (0.6, 0.9) |
The referent exposure group in all odds ratio calculations was comprised of women unvaccinated as of the reference date. Gestational age of cases: 6 to <20 weeks. Based on the set of covariates included in models that pooled data across prior season vaccination status strata, there were 4 discordant pairs with missing values for >1 covariate (for either the case or control in the pair) among women in 2012–13, 2 in 2013–14, and 1 in 2014–15.
Discordant pairs: the first number is the number of matched pairs where the case was vaccinated in the relevant exposure window (1–28, 29–56, >56 days before the reference date) and the control was unvaccinated as of the reference date, the second number is the number of matched pairs where the control was vaccinated in the window and the case was unvaccinated as of the reference date.
Crude odds ratios are controlled for matching variables: VSD site, LMP, vaccination in previous season, and age group (18–24, 25–34, and 35–44).
In addition to the matching variables, adjusted odds ratios are adjusted for maternal age, BMI, selected race variables, Hispanic ethnicity, healthcare utilization, diabetes, asthma, febrile illness in the 14 days before the reference date, parity, and pre-existing hypertension. Numbers in parentheses represent 95% confidence intervals.
In the analyses of vaccination relative to conception, aORs for IIV receipt in each risk window and for all seasons combined were generally less than or close to 1.0 among women who were and were not vaccinated the previous season (Table 5). However, several subgroups had aOR point estimates greater than 1.0, although in each instance the lower bound of the 95% CI was less than 1.0. In a combined analysis that included all women, the aOR for all seasons combined was less than 1.0 in each risk window (Supplemental Table 3).
Table 5.
Odds ratios for the association of SAB with influenza vaccination relative to conceptiona, by influenza season and vaccination status in the previous seasonb
| Vaccination to reference date (days) | 2012–13 | Influenza season 2013–14 | 2014–15 | All seasons combined | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc. Pairsc | Crude ORd | aORe | Disc. Pairsc | Crude ORd | aORe | Disc. Pairsc | Crude ORd | aORe | Disc. Pairsc | Crude ORd | aORe | ||
| Vaccinated in previous season | >42 days before | 18/25 | 0.6 | 0.6 (0.3, 1.0) | 17/14 | 1.0 | 0.8 (0.4, 1.7) | 12/20 | 0.6 | 0.5 (0.2, 1.0) | 47/59 | 0.7 | 0.6 (0.4, 0.9) |
| 0–42 day before | 8/9 | 0.7 | 0.8 (0.4, 1.7) | 9/11 | 1.0 | 1.1 (0.5, 2.3) | 8/16 | 0.7 | 0.6 (0.3, 1.2) | 25/36 | 0.8 | 0.8 (0.5, 1.2) | |
| 1–28 days after | 3/14 | 0.3 | 0.3 (0.1, 0.7) | 9/7 | 0.9 | 1.3 (0.6, 3.0) | 7/4 | 1.6 | 2.1 (0.8, 5.2) | 19/25 | 0.7 | 0.9 (0.5, 1.4) | |
| >28 days after | 3/10 | 0.5 | 0.5 (0.2, 1.3) | 5/12 | 0.7 | 0.8 (0.3, 1.8) | 6/3 | 1.2 | 1.6 (0.5, 4.6) | 14/25 | 0.7 | 0.8 (0.5, 1.4) | |
| Not vaccinated in previous season | >42 days before | 8/11 | 0.9 | 1.0 (0.4, 2.7) | 10/10 | 0.8 | 0.9 (0.4, 2.3) | 9/13 | 0.7 | 0.6 (0.3, 1.4) | 27/34 | 0.8 | 0.8 (0.5, 1.3) |
| 0–42 day before | 3/8 | 0.3 | 0.4 (0.1, 1.6) | 4/6 | 1.0 | 1.6 (0.5, 5.2) | 6/5 | 1.7 | 1.6 (0.5, 5.0) | 13/19 | 0.9 | 1.1 (0.6, 2.2) | |
| 1–28 days after | 7/3 | 1.8 | 3.2 (0.9, 11.9) | 3/4 | 0.4 | 1.0 (0.2, 4.5) | 6/6 | 0.8 | 0.4 (0.1, 1.4) | 16/13 | 0.9 | 1.1 (0.5, 2.2) | |
| >28 days after | 5/12 | 0.3 | 0.4 (0.1, 1.2) | 6/18 | 0.4 | 0.4 (0.2, 1.0) | 6/8 | 0.8 | 0.9 (0.3, 3.0) | 17/38 | 0.4 | 0.5 (0.3, 0.9) | |
Conception was defined as the date of the last menstrual period plus 14 days.
The referent exposure group in all odds ratio calculations was comprised of women unvaccinated as of the reference date. Numbers in parentheses represent 95% confidence intervals. Gestational age of cases ranges from 6 to <20 weeks. Based on the sets of covariates included in stratum-specific models, there were 2 discordant pairs with missing values for >1 covariate (for either the case or control in the pair) among women vaccinated in the previous season; there were 4 among women not vaccinated in the previous season.
Discordant pairs: the first number is the number of matched pairs where the case was vaccinated in the relevant exposure window and the control was unvaccinated as of the reference date, the second number is the number of matched pairs where the control was vaccinated in the window and the case was unvaccinated as of the reference date.
Crude odds ratios are controlled for matching variables: VSD site, LMP, vaccination in previous season, and age group (18–24, 25–34, and 35–44).
In addition to the matching variables, adjusted odds ratios (aOR) are adjusted in all models for maternal age, BMI, selected race variables, Hispanic ethnicity, and health care utilization. Models for the stratum, vaccinated in prior season = Yes, also adjusted for diabetes, asthma, and febrile illness in the 14 days before the reference date. Models for the stratum, vaccinated in prior season = No, also adjusted parity.
We also performed secondary analyses that included all SAB cases with gestational age 5-<20 weeks (1381 matched pairs) to be consistent with IIV-SAB-2 [19]; the findings were similar to the primary analyses (Supplemental Table 4). Analysis of risk windows relative to conception also yielded similar results (Supplemental Table 5). In a separate secondary analysis that excluded women with a history of >2 prior SABs, the results were largely unchanged (Supplemental Tables 6 and 7). Exclusion of the 315 matched pairs where at least one woman did not have a specified race showed no meaningful differences compared to the primary results (Supplemental Tables 8–10). Nearly all adjusted ORs are less than or close to 1.0 and all 95% confidence intervals for aORs above 1.0 include the null.
4. Discussion
We conducted a third matched case-control study of pregnant women in the VSD and found no association between IIV and SAB, providing reassurance regarding the safety of IIV in pregnancy. CDC and VSD investigators conducted this study to follow up on a safety signal for SAB following IIV detected in IIV-SAB-2, which included women who were pregnant in the two seasons immediately following the 2009 influenza pandemic (2010–11, 2011–12) [19]. The most notable finding from IIV-SAB-2 was an association of SAB in women who received IIV in the 1–28 days before the reference date. Subgroup analyses revealed that this association was found in both seasons, but only in women who were also vaccinated in the previous influenza season. Additional analyses failed to identify sources of confounding or bias that could explain the findings. The results of IIV-SAB-2 were unexpected because aORs were greater than those observed in IIV-SAB-1, which evaluated IIV and SAB in women who were pregnant in 2005–06 and 2006–07 [19,20]. While biologic processes for a relationship between IIV and SAB are theoretically possible, no plausible causal pathway has been established [35–38].
We designed IIV-SAB-3, which included 1236 matched pairs in the main analysis, to have sufficient power to detect an association between IIV and SAB, both for women vaccinated the previous season and those not vaccinated the previous season. Among women vaccinated the previous season, the aORs across all seasons were <1.0 for all three risk windows preceding the reference date, including the 1–28 day window. Similar results were observed for women not vaccinated the previous season. When we combined data for both strata and all seasons, aORs in each risk window were <1.0 with relatively narrow 95% confidence intervals. Similarly, when we analyzed the data based on number of days between conception and vaccination, aORs with all seasons combined were less than or close to 1.0 in the four risk windows (range 0.5 to 1.1) regardless of whether women were vaccinated the previous season or not. A number of our aOR estimates were less than 1.0, although in nearly all cases the 95% CI included 1.0. Other influenza vaccine studies have also reported risk estimates less than 1.0 for various pregnancy outcomes [17,39,40]. However, methodological limitations and residual bias are considered more likely explanations than an actual protective effect [41,42].
The design and implementation of IIV-SAB-3 was similar to the previous two VSD studies, with several key differences: (1) we matched subjects on influenza vaccination status in the previous season and sampled to ensure approximately equal distribution of subjects by previous season vaccination status; (2) we used three more recent influenza seasons; (3) we matched cases and controls on three age groups (18–24, 25–34, 35–44 years) rather than two (<30 and ≥ 30 years); and (4) the study population was approximately three times larger than IIV-SAB-2, permitting more precise estimates for individual seasons and for women stratified by previous season vaccination.
Characteristics of the IIV-SAB-3 study population are similar to those of the previous two studies (Supplemental Table 11). Women in IIV-SAB-3 were somewhat older than those in the previous two studies, and the proportion of women with a history of one or more births (parity) was greater than that observed in IIV-SAB-2, but similar to IIV-SAB-1. Median gestational age at the time of SAB in IIV-SAB-3 was 7 weeks, which was nearly identical to the previous studies [19,20]. The distribution of gestational age at SAB for women in IIV-SAB-2 and IIV-SAB-3 was similar (Supplemental Fig. 1), although a greater proportion of women in IIV-SAB-2 had a SAB at six weeks.
Early studies of influenza vaccine safety in pregnant women did not report any unexpected or concerning findings. However, between 1997 and 2004 only women in the second and third trimesters were advised to be vaccinated for influenza [9,43,44] resulting in limited information on exposures and outcomes like SAB in the first trimester [7,8,10,11,20,45,46]. More recently, studies and systematic reviews investigating pH1N1-containing vaccines have not identified excess risks of adverse events [3,12,13,15,18,40,47–49]. Overall, evidence to support the safety of influenza vaccine in pregnant women is substantial.
A strength of our investigation is the large study population; the total number of case-control pairs in the primary analysis (n = 1236) makes it one of the largest case-control studies of the association between IIV and SAB reported. Also, as in the previous two VSD studies, we abstracted medical records for all cases and controls to collect information on the pregnancy and to estimate the date of SAB (more than 2700 records were reviewed). We closely matched cases and controls on LMP to ensure that they were in a similar stage of pregnancy; the mean pair-wise difference in LMP was zero. Finally, the study population is demographically and geographically diverse; the combined membership of the healthcare organizations in VSD represents ~3% of the U.S. population [21].
This study has a number of limitations. A difficulty common to all studies of spontaneous abortion is estimation of the SAB date. We attempted to estimate the date of pregnancy loss when possible and relied on an algorithm developed and refined in the previous two studies[19,20], as well as guidance from an obstetrician to integrate various types of information from the medical record, such as ultrasound results, clinical and laboratory findings, and provider notes. We estimated SAB dates without knowledge of vaccination status, so any misclassification should be unrelated to exposure status. Misclassification of vaccination status is possible, particularly for women who appeared to be unvaccinated, since influenza vaccination is commonly available outside of healthcare systems. However, given the strong recommendations for vaccination of pregnant women, we expect that out-of-system vaccinations would be documented by the provider in the medical record. Finally, women in our study were members of their respective healthcare organization for at least 20 months prior to their LMP and may not be representative of all pregnant women.
In the context of safety, the results of this study lend support to the ACIP recommendation for IIV at any time during pregnancy. We found no clear evidence of an association between IIV and SAB; reasons for the apparent discrepancy between IIV-SAB-2 and IIV-SAB-3 are unknown. We cannot rule out residual confounding or random error to explain the results of IIV-SAB-2; however, differences in the time periods studied might also be relevant. The current investigation included three seasons that were further removed from the 2009 influenza pandemic, while IIV-SAB-2 evaluated the two seasons immediately after the pandemic. While the challenges and risks associated with influenza pandemics may be very different compared to those observed in seasonal epidemics, assessment of influenza-related morbidity and vaccine safety in both settings should include maternal and pregnancy related outcomes.
Supplementary Material
Acknowledgements
We are grateful to the following individuals for the help with this research: KP, Northern California: Kristin Goddard, Pat Ross, Margarita Magallon, Virginia Robinson, and Sandy Bauska. KP, Colorado: Jo Ann Shoup, Katherine Burniece, Courtney Anderson. KP, Northwest: Mara Kalter, Stacy Harsh, Kim Olson, and Brad Crane. KP, Washington: Erika Kiniry and Jennifer Covey. KP, Southern California: Bernadine Dizon, Bianca Cheung, Cheryl Carlson, Claire Jang, Denison Ryan, Gina Lee, Joy Gelfond, Karen Schenk, Kerresa Morrissette, Lee Tillman, Lindsay Lyons, Nancy Cannizzaro, Nancy Canul-Jauriga, Radha Bathala, and Sunhea Kim. Marshfield Clinic: Deanna Cole, Tara Johnson, Sarah Kopitzke, Karen McGreevey, Suellyn Murray, Rebecca Pilsner, Carla Rottscheit, Erica Scotty, Sandy Strey, Elizabeth Vickers, and Jane Wesely. Results from this study were presented at the February 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).
Funding
This work was supported by a contract (200-2012-53587-0007) from the Centers for Disease Control and Prevention. CDC scientists participated in the design and conduct of the study, analysis and interpretation of the data, and preparation, review, and approval of the manuscript for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations:
- aOR
adjusted odds ratio
- ACIP
advisory committee on immunization practices
- BMI
body mass index
- CDC
centers for disease control and prevention
- CI
confidence interval
- IIV
inactivated influenza vaccine
- ICD-9-CM
international classification of diseases, ninth revision, clinical modification
- ICD-10-CM
international classification of diseases, tenth revision, clinical modification
- LMP
last menstrual period
- SAB
spontaneous abortion
- pH1N1
influenza virus A/H1N1pdm09
- VAERS
vaccine adverse event report system
- VSD
vaccine safety datalink
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Dr. Klein reports research support from Sanofi Pasteur, GlaxoSmithKline, Protein Science (now Sanofi Pasteur), Pfizer, and Merck. Dr. Naleway has received research support from Pfizer and Merck for unrelated studies. Ms. Hanson reports research support from Seqirus for unrelated studies. All other co-authors report no conflicts].
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.09.035.
References
- [1].Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 2011;205:10–8. 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- [2].Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol 2012;207:S3–8. 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- [3].Fell DB, Azziz-Baumgartner E, Baker MG, Batra M, Beaute J, Beutels P, et al. Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine 2017;35:5738–50. 10.1016/j.vaccine.2017.08.037, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rasmussen SA, Jamieson DJ. Influenza and pregnancy in the United States: before, during, and after 2009 H1N1. Clin Obstet Gynecol 2012;55:487–97. 10.1097/GRF.0b013e31824df23e. [DOI] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention. Maternal and infant outcomes among severely ill pregnant and postpartum women with 2009 pandemic influenza A (H1N1)-United States, April 2009-August 2010. MMWR Morb Mortal Wkly Rep 2011;60:1193–6. [PubMed] [Google Scholar]
- [7].Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004;53:1–40. [PubMed] [Google Scholar]
- [8].ACOG Committee Opinion No. 468: Influenza vaccination during pregnancy. Obstet Gynecol 2010;116:1006–7. 10.1097/AOG.0b013e3181fae845. [DOI] [PubMed] [Google Scholar]
- [9].Munoz FM. Safety of influenza vaccines in pregnant women. Am J Obstet Gynecol 2012;207:S33–7. 10.1016/j.ajog.2012.06.072. [DOI] [PubMed] [Google Scholar]
- [10].Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol 2011;204(146):e1–7. 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- [11].Bednarczyk RA, Adjaye-Gbewonyo D, Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am J Obstet Gynecol 2012;207:S38–46. 10.1016/j.ajog.2012.07.002. [DOI] [PubMed] [Google Scholar]
- [12].Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the vaccine adverse event reporting system. Am J Obstet Gynecol 2011;205(473):e1–9. ). ajog.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis 2014. 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- [14].Chambers CD,Johnson DL, Xu R, Luo YJ, Louik C, Mitchell AA, et al. Safety of the 2010–11, 2011–12, 2012–13, and 2013–14 seasonal influenza vaccines in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine 2016;34:4443–9. 10.1016/j.vaccine.2016.06.054. [DOI] [PubMed] [Google Scholar]
- [15].Zhang C, Wang X, Liu D, Zhang L, Sun X. A systematic review and meta-analysis of fetal outcomes following the administration of influenza A/H1N1 vaccination during pregnancy. Int J Gynaecol Obstet 2018;141:141–50. 10.1002/ijgo.12394. [DOI] [PubMed] [Google Scholar]
- [16].Haberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 2013;368:333–40. 10.1056/NEIMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pasternak B, Svanstrom H, Molgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ 2012;344: e2794 10.1136/bmj.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015;33:2108–17. 10.1016/j.vaccine.2015.02.068, [DOI] [PubMed] [Google Scholar]
- [19].Donahue JG, Kieke BA, King JP, DeStefano F, Mascola MA, Irving SA, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010–11 and 2011–12. Vaccine 2017;35:5314–22. 10.1016/j.vaccine.2017.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol 2013;121:159–65. , http://10.1097/AOG.0b013e318279f56f. [DOI] [PubMed] [Google Scholar]
- [21].Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127 (Suppl 1):S45–53. 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- [22].Doubilet PM. Should a first trimester dating scan be routine for all pregnancies? Semin Perinatol 2013;37:307–9. 10.1053/j.semperi.2013.06.006, [DOI] [PubMed] [Google Scholar]
- [23].Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31:2898–903. 10.1016/j.vaccine.2013.03.069. [DOI] [PubMed] [Google Scholar]
- [24].Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)-United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2012;61:613–618. [PubMed] [Google Scholar]
- [25].Advisory Committee on Immunization Practices. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices-United States, 2013–2014. MMWR Recomm Rep 2013;62:1–43. [PubMed] [Google Scholar]
- [26].Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- [27].Lehmann EL, D’Abrera HJM. Nonparametrics: statistical methods based on ranks. San Francisco: Holden-Day; 1975. [Google Scholar]
- [28].McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947;12:153–7. [DOI] [PubMed] [Google Scholar]
- [29].Bowker AH. A test for symmetry in contingency tables. J Am Stat Assoc 1948;43:572–4. [DOI] [PubMed] [Google Scholar]
- [30].Tulandi T. Al-Fozan HM. Spontaneous abortion: risk factors, etiology, clinical manifestations, and diagnostic evaluation UpToDate. Waltham, MA: Wolters Kluwer; 2013. [Google Scholar]
- [31].Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- [32].Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol 1993;137:1–8. [DOI] [PubMed] [Google Scholar]
- [33].Hastie TJ, Tibshirani RJ, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: Springer-Verlag; 2001. [Google Scholar]
- [34].Re Greenland S. Estimating relative risk functions in case-control studies using a nonparametric logistic regression. Am J Epidemiol 1997;146:883–4. [DOI] [PubMed] [Google Scholar]
- [35].Christian LM, Porter K, Karlsson E, Schultz-Cherry S, Iams JD. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am J Reprod Immunol 2013;70:45–53. 10.1111/aji.12117, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Christian LM, Iams JD, Porter K, Glaser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine 2011;29:8982–7. 10.1016/j.vaccine.2011.09.039, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kay AW, Fukuyama J, Aziz N, Dekker CL, Mackey S, Swan GE, et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci USA 2014;111:14506x11 10.1073/.pnas.1416569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11:302–8. ). siny.2006.03.001, [DOI] [PubMed] [Google Scholar]
- [39].Sammon CJ, Snowball J, McGrogan A, de Vries CS. Evaluating the hazard of foetal death following H1N1 influenza vaccination; a population based cohort study in the UK GPRD. PLoS ONE 2012;7:e51734 , journal.pone.0051734, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fell DB, Sprague AE, Liu N, Yasseen AS 3rd, Wen SW, Smith G, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health 2012;102:e33–40. 10.2105/AJPH.2011.300606, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fell DB, Platt RW, Lanes A, Wilson K, Kaufman JS, Basso O, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. Br J Obstet Gynecol. 2015;122:17–26. [DOI] [PubMed] [Google Scholar]
- [42].Hutcheon JA, Fell DB, Jackson ML, Kramer MS, Ortiz JR, Savitz DA, et al. Detectable Risks in Studies of the Fetal Benefits of Maternal Influenza Vaccination. Am J Epidemiol 2016;184:227–32. 10.1093/aje/kww048, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Prevention and control of influenza recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Crevention. MMWR Recomm Rep; 1995;44:1–22. [PubMed] [Google Scholar]
- [44].Arden NH, Cox NJ, Schonberger LB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(RR-9):1–25. [PubMed] [Google Scholar]
- [45].Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2005;192:1098–106. 10.1016/j.ajog.2004.12.019. [DOI] [PubMed] [Google Scholar]
- [46].Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2009;201:547–52. 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- [47].Chambers CD, Johnson D, Xu R, Luo Y, Louik C, Mitchell AA, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine 2013;31:5026–32. 10.1016/j.vaccine.2013.08.097, [DOI] [PubMed] [Google Scholar]
- [48].Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, et al. First Trimester Influenza Vaccination and Risks for Major Structural Birth Defects in Offspring. J Peds. 2017. 10.1016/j,jpeds.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Giles ML, Krishnaswamy S, Macartney K, Cheng A. The safety of inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccin Immunother. 2018:1–13. 10.1080/21645515.2018.1540807, [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.