Abstract
Objective:
Our study described the occurrence, assessment, prevention, and management practices of pain, agitation, and delirium (PAD) in four intensive care units (ICUs) from the Puerto Rico Medical Center and compared findings with the 2013 PAD guidelines.
Methods:
A descriptive study, with repeated bedside measures (two times a day/two times a week) of PAD and review of patient clinical records.
Results:
Eighty ICU patients (20 per ICU) were evaluated, (median 3 times [IQR, 2–7]). At least once during the assessment period, 57% percent of patients had significant pain and 34% had delirium. Moreover, 46% were deeply sedated, 17.5% had agitation, and 52.5% of patients were within the recommended Richmond Agitation-Sedation Scale (RASS) scores. The Numeric Rating Scale and RASS were the most common tools used by clinicians to evaluate pain and agitation/sedation levels, respectively. Clinicians did not assess pain in patients unable to self-report with any guideline-recommended tools, as was the case for delirium. Fentanyl and morphine were the most commonly used analgesics, while benzodiazepines were used for sedation.
Conclusion:
Although pain, agitation, and delirium occurrence were similar to other studies, patients continue to suffer. A gap exists between clinical practices in these ICUs and current guidelines. Strategies that contribute to integrating guidelines into these ICUs should be developed, studied, and implemented.
Keywords: Pain, Analgesia, Agitation, Sedation, Delirium, Intensive care unit
Resumen
Objetivos:
Se describió la ocurrencia, estimado, prevención y prácticas de manejo de dolor, agitación y delirium (DAD) en cuatro unidades de cuidado intensivo (UCI) del Centro Médico de Puerto Rico y comparó los hallazgos con las guías de DAD del 2013.
Metodología:
Estudio descriptivo con medidas repetidas (dos veces al día/dos veces en semana) de DAD y la revisión de expedientes clínicos.
Resultados:
Ochenta pacientes de UCI (20/unidad) fueron evaluados (mediana 3 veces, [IQR, 2–7]). Al menos una vez durante el periodo de estimado, el 57% de los pacientes tenía dolor significativo y el 34% tenía delirium. Además, el 46% estaba profundamente sedado, el 17.5% tenía agitación y el 52.5% de los pacientes se encontraban dentro de los niveles recomendados en la Escala de Agitación-Sedación de Richmond (RASS). La Escala de Valoración Numérica y el RASS fueron las herramientas más comúnmente utilizadas por los clínicos para evaluar los niveles de dolor y agitación/sedación, respectivamente. Los clínicos no evaluaron el dolor en pacientes incapaces de auto-reportarlo usando instrumentos recomendados por las guías, como fue el caso con el delirium. El fentanilo y la morfina fueron los medicamentos más utilizados para la analgesia, mientras que las benzodiacepinas se utilizaron para la sedación.
Conclusiones:
Aunque la ocurrencia de DAD es similar a otros estudios, los pacientes continúan sufriendo estos fenómenos. Existe una brecha entre las prácticas clínicas de las UCI y las guías actuales. Se deben desarrollar, estudiar e implementar estrategias que contribuyan a la integración de estas guías en las UCI.
Patients in intensive care units (ICU) suffer from pain, agitation, and delirium (PAD) which can have negative effects on their clinical outcomes. The incidence of pain at rest in ICU patients fluctuates from 33% to 51% (1,2), agitation from 55% to 59% (3,4), deep sedation from 41 to 57% (1), and delirium from 27 to 64% (5–8). While treating these conditions, ICU patients must also face added risks incurred from the treatments employed (e.g., sedative and analgesics). The use of benzodiazepines and opioids has been implicated in increases in both length of ICU stay and days on mechanical ventilation (MV), development of delirium, and mortality (9–12). As a response to the lack of structured protocols that consider these complexities, there has been an increase in studies during the past few decades regarding treating PAD. These studies served as the basis for the development of the clinical practice guidelines with bundled recommendations for the assessment, management, and prevention of PAD (13). The goal of these guidelines is to provide clinicians recommendations about the best practices to manage PAD in adults critically ill patients (13). Clinicians are expected to adapt these guidelines to their context and use them to improve their clinical outcomes (13).
To maintain a balance between the occurrence of PAD and their treatment, the guidelines have recommended targeting both pain intensity and sedation to the lowest possible level. They also stress the importance of routinely assessing PAD with validated scales such as the Numeric Rating Scale (NRS) (14) for patients able to self-report, the Critical-Care Pain Observation Tool (CPOT) (15) or the Behavioral Pain Scale (16) for patients unable to self-report, the Richmond Agitation Sedation Scale (RASS) (17) or the Sedation Agitation Scale for sedation assessment, and the Confusion Assessment Method for ICU (CAM-ICU) (18) or the Intensive Care Delirium Screening Checklist (19) to measure delirium. Yet, regarding the use of tools to assess PAD, a survey from multiple European hospitals showed that only 43% of patients were evaluated for both pain and sedation using validated tools, and 27% of patients evaluated with validated delirium tools (20).
While midazolam has been the most commonly used sedative in the ICU (1,21), the PAD bundle also recommends that non-benzodiazepines be used for sedation (13). In addition to assessment and management strategies, the guidelines addressed prevention practices such as a spontaneous awakening trial (SAT), a spontaneous breathing trial (SBT), and early mobilization. Although there is some evidence of how these 2013 guidelines are being applied in critical care settings (22–25), the occurrence, assessment, prevention, and management practices of PAD in ICUs in Puerto Rico (PR) has not been documented.
Our preliminary study was aimed at describing the occurrence of PAD as well as PAD assessment, prevention, and management practices in four ICUs at the PR Medical Center. We also aimed to compare the findings according to the PAD guidelines to determine if gaps exist between the clinical practices and the current guidelines.
Materials and Methods
Design and Setting
A prospective, descriptive preliminary study with repeated bedside measures (two times a day/two times a week) of PAD and review of patient clinical records was conducted in four ICUs from four different hospitals at the PR Medical Center where medical-surgical, cardiac-surgical, or trauma patients are admitted.
Sample
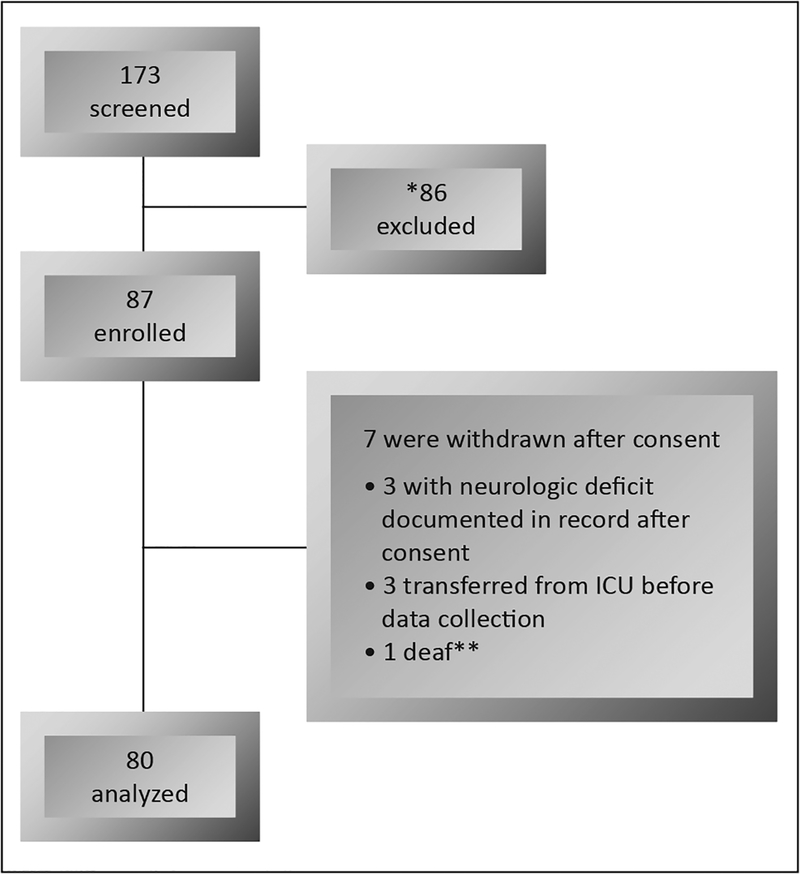
A convenience sample of 20 patients (80 total) from each ICU was recruited (see Figure 1). Those who were 21 years of age or more were eligible if they did not have the following exclusion criteria: head trauma or acute neurological injury, Glasgow Coma Scale score less than 8, pregnancy, psychiatric disease, or prisoners.
Figure 1.
Patients recruitment process. Legend: *neurologic deficit, no family available for consent, patient or family refused to participate, and other exclusion criteria; **difficult to assess.
Ethical considerations
This study was approved by the University of PR, Medical Sciences Campus Institutional Review Board (A5570113). Consent was signed by patients who agreed to participate. For patients unable to consent, signed consent was obtained from a family member (next-of-kin). During the study, if patients became capable of providing his or her own consent, they were asked to sign the consent form if they desired to continue or their participation was terminated.
Measures
Pain intensity at rest was measured by self-report using the 0 to 10 NRS. For patients unable to self-report their pain at rest, the CPOT (15) was used except for patients with a sedation level of −4 or −5 according to the RASS. Significant pain was defined as a NRS greater than 3 or a CPOT greater than 2, according to PAD guidelines (13).
Agitation and sedation levels were measured with the RASS (17). Agitation and deep sedation were described as a RASS from +1 to +4 and from −3 to −5, respectively as defined by the PAD guidelines (13). In addition, patients were classified as being in the recommended RASS in PAD guidelines if they were between −2 and 0.
Delirium was measured with the CAM-ICU, Spanish version (26,27). Motoric subtypes of delirium was classified according to the following criteria: (a) hypoactive, positive CAM-ICU with a RASS 0 to −3; (b) hyperactive, positive CAM-ICU with a RASS +1 to +4; and (c) mixed, positive CAM-ICU assessments that alternate between hyperactive and hypoactive (28,29).
Procedures
The first day of evaluation was the day on which the patient or family member was consented. Pain, agitation/sedation, and delirium were prospectively measured at the patient’s bedside by researchers two times a day (AM/PM, with at least 6 hours between assessments) on two days per week (Tuesday and Thursday) for a maximum of three weeks while the patient was in the ICU (i.e., maximum of 12 assessments) at different length of ICU stay. Assessment practices (i.e., scales for PAD assessment); prevention practices (i.e, SAT, SBT, and early mobilization); and management practices (i.e., drug, type and route of sedative, analgesic, and antipsychotic) used by the nurses and physicians were recorded. Baseline measures were also obtained from the clinical record during each 24-hour period that corresponded to the day of patient evaluation: (1) demographics; (2) type of patient (i.e., surgical, medical, or trauma); and (3) use of MV.
Descriptive statistics were used. Results are expressed in medians (interquartile range [IQR]) or frequency (percent [%]). Data were analyzed using SPSS version 21.
Results
A total of 80 patients, 20 patients in each one of the study ICUs, were individually assessed a median of 3.0 [IQR, 2.0–7.0] times at different intervals during their stay at ICU. Patients on MV were individually assessed more times than those not on MV (4.0 [IQR, 2.0–8.3] vs. 2.0 [IQR, 1.0–2.0] due to their increased length of ICU stay. Median age of participants was 59 [IQR, 41–68] years and 46 were male (57.5%). Twenty (25%) were trauma patients, 30 (37.5%) surgical, and 30 (37.5%) medical patients. Among the 80 patients, 62 (77.5%) were on MV for 6.5 days [IQR, 2.0–13.0]. Median length of stay in ICU was 7.0 (IQR, 3.0–12.0) days for all patients, decreasing for those without MV to 3.0 [IQR, 1.0–5.0], and increasing in those who were on MV to 9.0 [IQR, 4.0–14.0] days.
Study results are described as follows: (1) percent of patients who had pain, agitation/ sedation and/or delirium at least once during their assessment period; and, (2) percent of patients who experienced pain, agitation/ sedation and/or delirium at half or more of their assessments, and (3) mean percentage of PAD total scores. These results are described for the total sample (i.e., both non-mechanically and mechanically ventilated patients) in text and described in Table 1 according to MV status using PAD guidelines definitions and classifications (13).
Table 1.
Pain, agitation/sedation, and delirium by mechanical ventilation status
| Variables | Mechanically ventilated | Not mechanically ventilated | ||
|---|---|---|---|---|
| n | % | n | % | |
| Total patients | 62 | 77.5 | 18 | 22.5 |
| Pain Occurrence | ||||
| Pain (NRS or CPOT > 0) in one or more assessments | 36* | 62 | 11 | 61 |
| Significant pain (NRS > 3 or CPOT > 2) in one or more assessments | 26* | 45 | 10 | 56 |
| Significant pain in half or more assessments | 16* | 28 | 9 | 50 |
| Mean percentage of total pain score within acceptable pain score (NRS < 4 or CPOT < 3) | 46* | 79 | 9 | 50 |
| Agitation Occurrence (RASS +1 to +4) | ||||
| Agitation in one or more assessments | 13 | 21 | 1 | 6 |
| Agitation in half or more assessments | 5 | 8 | 1 | 6± |
| Mean percentage of RASS score within agitation score | 4 | 6 | 1 | 6 |
| Deep Sedation (RASS −3 to −5) | ||||
| Deep sedation in one or more assessments | 37 | 60 | 0 | 0 |
| Deep sedation in half or more assessments | 21 | 34 | 0 | 0 |
| Mean percentage of RASS score within deep sedation score | 13 | 21 | 0 | 0 |
| Recommended RASS (RASS 0 to −2) | ||||
| Recommended RASS in one or more assessment | 24 | 39 | 18 | 100 |
| Recommended RASS in half or more times of the assessments | 41 | 66 | 18 | 100± |
| Mean percentage of RASS score within recommended RASS score | 45 | 73 | 17 | 94 |
| Delirium | ||||
| Developed delirium at least one time | 24^ | 45 | 0 | 0 |
| Had delirium in half of more times of assessments | 17^ | 35 | 0 | 0 |
Legend: RASS, Richmond Agitation Sedation Scale;
from a sample of 58;
from a sample of 53;
same patient had only two assessments with RASS 0 and RASS +1
Occurrence of pain, agitation, and delirium
Pain at rest
Pain at rest was present (i.e., NRS or CPOT > 0) in 47 (62%) of 76 patients that were evaluated for pain at rest. Thirty-six patients (47% of the 76 patients) presented with significant pain at rest (i.e., NRS >3 or CPOT > 2) during at least one assessment and 25 patients (33%) during half or more of the assessments. Mean percentage of total pain scores was within an acceptable pain range in 72% of patients. Patients not mechanically ventilated had more instances of significant pain than those on MV (Table 1).
Agitation and sedation levels
Fourteen patients (17.5%) had agitation (RASS +1 to +4) at least one time, and only six patients (7.5%) were agitated in half or more assessment times. The mean percent of total RASS scores within agitation levels was 6%. Only one patient in the unventilated cohort had a RASS of +1 one time.
Thirty-seven patients (46%) presented with deep sedation (RASS −3 to −5) at least one time during the assessment period and 21 patients (26%) in half or more times during assessments. The mean percent of total RASS scores within deep sedation was 16%.
For the total sample (n=80), 42 patients (52.5%) were in the recommended RASS level (−2 to 0) at least one time, and 59 patients (74%) were in the recommended RASS level in half or more times of the assessments. The mean percent of total RASS scores was within recommended RASS level in 78% of patients.
Delirium
Of the total sample, 71 patients were evaluated for delirium and 24 of them (34%) were delirious at least one time. Seventeen (24%) of these had delirium in half or more of the assessments. The most common type of delirium was hypoactive (67%), followed by hyperactive (21%), and mixed (12%). Only patients who were mechanically ventilated developed delirium (see Table 1). Nine mechanically ventilated patients were unable to be assessed for delirium at all time measures, and 41% of the time delirium was unable to be assessed in all patients because RASS scores were −4 or −5 or the patient was clinically unstable.
Practices of assessment, prevention, and management of PAD
The most common tool used to measure pain was the verbal 0–10 NRS. The CPOT was used by the study’s research team but it was not used by clinicians (i.e., nurses and physicians) in any of the study ICUs. For those patients who were not able to self-report their pain, two ICUs used observation of pain behaviors and the assumption that pain is present according to pain risk factors, respectively. Nurses in all four sites assessed and recorded pain; however, physicians only recorded pain in one of the four ICUs. For agitation and sedation levels, the RASS was used in three of the ICUs. The other ICU measured agitation by recording “yes or no” if the patient was observed to be agitated. Sedation and agitation levels were also primarily measured by nurses. In two of the ICUs, physicians measured sedation/agitation levels sporadically; in one ICU, consistently; and, for the fourth ICU, physicians did not record sedation/agitation levels. Assessments of pain and agitation/sedation were not performed in a consistent manner (i.e., not during every shift). Delirium was not assessed by clinicians in any of the ICUs with use of the CAM-ICU or any other validated method.
The prevention strategies for agitation/sedation and delirium (i.e., SAT, SBT, and early mobilization) were evaluated only in our mechanically ventilated cohort. Daily SAT and daily SBT were performed in 13% and 28% of patients, respectively, in those who qualified for the intervention. Only one type of early mobilization activity, sitting on a chair at bedside, was performed in 13% of all mechanically ventilated patients in only one of the ICUs.
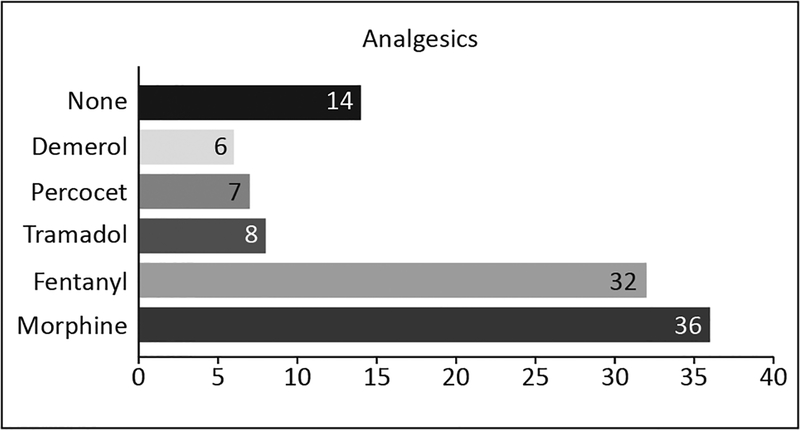
Pain was treated mainly with the intravenous opioids, morphine and fentanyl (see Figure 2). Analgesics were administered intermittently (62%), by continuous infusion (17%), or with a combination of both intermittent and continuous infusion (21%). Twelve (16%) patients who reported significant pain using the NRS during 19 assessments did not receive any analgesic during an evaluation day.
Figure 2.
Frequency of analgesics administered. Note: Some patients received more than one type of analgesic.
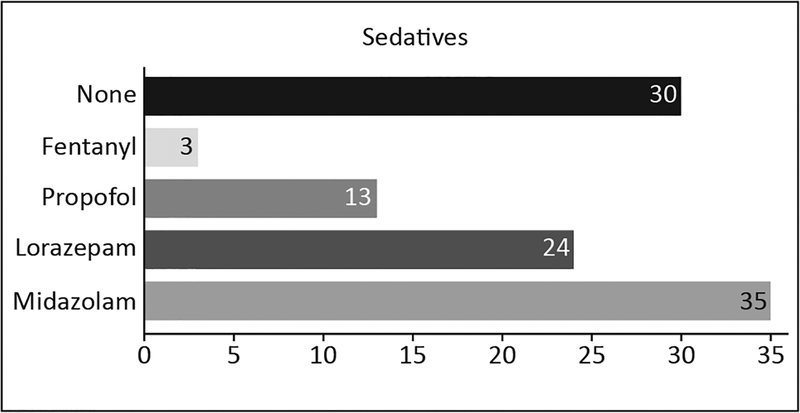
Only mechanically ventilated patients were treated with sedatives. The most frequently used sedative was midazolam (see Figure 3). In the 50 patients who received sedatives, the sedatives were primarily administered by continuous infusion (52%), intermittently (18%), or with a combination of both intermittent and continuous infusion (30%). Twenty-nine (36%) patients, all mechanically ventilated, received concurrent sedatives and analgesics. Haloperidol alone or haloperidol and olanzapine were used to treat delirium in six patients. However, six patients presenting with hyperactive or mixed delirium during our assessments did not receive any antipsychotic treatment.
Figure 3.
Frequency of sedatives administered. Note: Some patients received more than one type of sedative. In one of the sites fentanyl was given for sedation purpose.
Discussion
The occurrence, assessment, and management practices for PAD in PR have not been documented previously. We found that there is a gap between the clinical practices used in these ICUs and current practices recommended in the PAD guidelines.
In our study, more than half of the patients had pain at rest; almost half had significant pain at least one time; and one-third had significant pain in half or more of the assessments. Furthermore, after 19 NRS significant pain assessments, patients did not receive any analgesics. These findings highlight the importance of guideline recommendations because patients are most vulnerable in ICU settings and continue experiencing pain. In our mechanically ventilated cohort, the occurrence of significant pain was within the range of previously reported frequencies (33 to 51%) (1,2).
A recent published study from Jablonski and colleagues (25), conducted during the same time period as our study, evaluated PAD occurrence in mechanically ventilated patients before and after implementation of PAD guidelines. Although the PAD guidelines recommend classifying significant pain as a NRS > 3, they considered a score < 3 to be an acceptable pain score (i.e., excluding a rating of 3 as a non-significant pain). If we compare our results with their pre-intervention group (using their same pain classification), 74% of our mechanically ventilated patients had an average pain score that was within an acceptable pain level. Conversely, 86% of patients in Jablonski and colleagues’ study were within the acceptable pain level (25). This 12% difference could be clinically meaningful for an individual patient and deserves future attention.
Patients in our mechanically ventilated cohort developed agitation less than half of the time than what has been reported (3,4), while time in deep sedation was found to be 6% more than what has been reported in the literature (1). Although Jablonski established a stricter range for recommended RASS score (i.e., −1 to 0 instead of −2 to 0), 48% of our mechanically ventilated patients spent more time in the desired RASS range-using Jablonski’s criteria- versus 38% of mechanically ventilated patients in the Jablonski study (25). Differences could be due to a variation of data collection times: Jablonski measured patients during their entire ICU stay for up to 28 days, while we only measured twice a day, two times a week for a maximum of three weeks. It was possible we missed some measurements on days when patients had an inadequate RASS score.
Delirium occurred only in our mechanically ventilated cohort and within the percent frequency reported in the literature; i.e., 27 to 64%. This is expected because risk factors for delirium include being on MV and being on benzodiazepines (30). Patients presented more with hypoactive delirium, as has been reported in other trauma and surgical patients (28). The next most frequent type of delirium was hyperactive, a different finding from other studies in which mixed-type delirium was more prevalent (28,29). It is important to note that our measurement times could have decreased the possibility of detecting delirium and determining subtype because we did not measure on consecutive days.
Assessment is the basis for any intervention. For example, pain assessment with validated tools is associated with improvement in clinical outcomes (31). In our study, results show that patients who could self-report their pain were assessed by using the recommended NRS. However, for patients unable to self-report, none of the ICUs used the validated and recommended tools. Instead, two of the ICUs used observation of pain behaviors, which is the main feature of CPOT. In these two ICUs, clinicians made assumptions that pain was present based on risk factors recommended by the American Society for Pain Management Nursing for patients unable to self-report their pain (32). In all but one of the ICUs, the RASS was used to assess sedation/agitation, as recommended by the guidelines. Yet, neither pain nor agitation/sedation scores were consistently assessed, this finding being similar to other studies (1,20,33). Delirium was not assessed using any validated tools. This could contribute to underestimating the presence of delirium, specifically the hypoactive type which is the most difficult to detect. Lack of assessment lessens the possibility of providing adequate treatment.
Prevention strategies such as SAT and SBT were not performed daily, as recommended by the guidelines. Both strategies have been found to contribute to a decrease in the amount of sedatives administered, decreased levels of sedation and time on MV, among others positive outcomes (9,34). In addition, early mobilization, which is a delirium prevention strategy (35,36), was not performed in three of the ICUs. Early mobilization includes different levels of activities (37). However, the only activity in one of the ICUs studied was sitting on a chair at bedside which is a more passive activity.
The PAD guidelines establish that opioids should be the primary medications to treat pain in ICU patients (38). Consistent with this, we found that fentanyl and morphine were the most commonly used opioids. Surprisingly, clinicians in one of our studied ICUs treated pain with Demerol. This opioid is not recommended due to its neurologic toxicity effects and was not recommended in even earlier published 2002 guidelines (39,40). Benzodiazepines were the most commonly used sedatives, contrary to 2013 guidelines’ recommendations that use of non-benzodiazepine sedatives such as propofol or dexmedetomedine be considered. Propofol was used as the only sedative in two of our patients, and no dexmedetomedine was used in any patient. A change toward the use of non-benzodiazepine sedatives in ICU is strongly suggested.
The prospective use of recommended assessment tools by trained researchers to measure PAD was a major strength of this study. However, a limitation was that our assessments were not performed daily. This could have increased the probability of underestimating the occurrence of PAD since they could occur on days when data were not collected. Conversely, the limited assessments could have also increased the probability of overestimating the percent of patients who experienced PAD in half or more of the assessments. Most of those patients were measured only two times. Another limitation was that both PAD prevention strategies and pharmacologic management strategies were obtained from record review, which could be subject to missing data.
Conclusions
Although the occurrence of PAD is similar to that reported in other studies, patients in the PR ICUs continue to suffer from PAD. Such a study in a non-research intensive environment that may be similar to ICUs in smaller geographical countries may provide guidance to others. We believe that results could provide a database of PAD practices in select PR ICUs. Establishing baseline practices such as these could help to define further research and establish strategies to guide clinical practices in order to improve patients’ clinical outcomes.
Acknowledgments
Scientific writing support was provided by Post-doctoral Master of Science in Clinical and Translational Research Program/Hispanic Clinical and Translational Research Education and Career Development Program (HCTRECD) R25MD007607. Scientific editing support was provided by the PR Clinical and Translational Research Consortium, supported by the National Institute on Minority Health and Health Disparities (NIMHD) and the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under Award Number U54MD007587.
Footnotes
The author/s has/have no conflict/s of interest to disclose. This study was funded by Capacity Advancement in Research Infrastructure University of Puerto Rico UPR MFP-6251123.
References
- 1.Payen J-F, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou J-L, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 2007;106:687–695. [DOI] [PubMed] [Google Scholar]
- 2.Chanques G, Sebbane M, Barbotte E, Viel E, Eledjam J-J, Jaber S. A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology 2007;107:858–860. [DOI] [PubMed] [Google Scholar]
- 3.Burk RS, Grap MJ, Munro CL, Schubert CM, Sessler CN. Agitation onset, frequency, and associated temporal factors in critically ill adults. Am J Crit Care 2014;23:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaber S, Chanques G, Altairac C, Sebbane M, Vergne C, Perrigault P-F, et al. A prospective study of agitation in a medical-surgical ICU: Incidence, risk factors, and outcomes. Chest. 2005;128:2749–57. [DOI] [PubMed] [Google Scholar]
- 5.Bigatello LM, Amirfarzan H, Haghighi AK, Newhouse B, Del Rio JM, Allen K, et al. Effects of routine monitoring of delirium in a surgical/trauma intensive care unit. J Trauma Acute Care Surg 2013;74:876–883. [DOI] [PubMed] [Google Scholar]
- 6.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med 2010;38:2311–2318. [DOI] [PubMed] [Google Scholar]
- 7.Tomasi CD, Grandi C, Salluh J, Soares M, Giombelli VR, Cascaes S, et al. Comparison of CAM-ICU and ICDSC for the detection of delirium in critically ill patients focusing on relevant clinical outcomes. J Crit Care 2012;27:212–217. [DOI] [PubMed] [Google Scholar]
- 8.van den Boogaard M, Peters SA, van der Hoeven JG, Dagnelie PC, Leffers P, Pickkers P, et al. The impact of delirium on the prediction of in-hospital mortality in intensive care patients. Crit Care 2010;14:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–134. [DOI] [PubMed] [Google Scholar]
- 10.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471–1477. [DOI] [PubMed] [Google Scholar]
- 11.Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Tan MATI, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: A prospective longitudinal multicentre cohort study. Intensive Care Med 2013;39:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamdar BB, Niessen T, Colantuoni E, King LM, Neufeld KJ, Bienvenu OJ, et al. Delirium transitions in the medical ICU: Exploring the role of sleep quality and other factors. Crit Care Med 2015;43:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- 14.Chanques G, Viel E, Constantin JM, Jung B, de Lattre S, Carr J, et al. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain 2010;151:711–721. [DOI] [PubMed] [Google Scholar]
- 15.Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care 2006;15:420–427. [PubMed] [Google Scholar]
- 16.Payen JF, Bru O, Bosson JL, Lagrasta a, Novel E, Deschaux I, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001;29:2258–2263. [DOI] [PubMed] [Google Scholar]
- 17.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001;29:1370–1379. [DOI] [PubMed] [Google Scholar]
- 19.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: Evaluation of a new screening tool. Intensive Care Med 2001;27:859–864. [DOI] [PubMed] [Google Scholar]
- 20.Luetz A, Balzer F, Radtke FM, Jones C, Citerio G, Walder B, et al. Delirium, sedation and analgesia in the intensive care unit: A multinational, two-part survey among intensivists. PLoS One 2014;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randen I, Bjørk IT. Sedation practice in three Norwegian ICUs: A survey of intensive care nurses’ perceptions of personal and unit practice. Intensive Crit Care Nurs 2010;26:270–277. [DOI] [PubMed] [Google Scholar]
- 22.Balas MC, Vasilevskis EE, Olsen KM, Schmid KK, Shostrom V, Cohen MZ, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014;42:1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balas MC, Burke WJ, Gannon D, Cohen MZ, Colburn L, Bevil C, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med 2013;41:S116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, et al. Worldwide Survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) Bundle. Crit Care Med 2017;45:e1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jablonski J, Gray J, Miano T, Redline G, Teufel H, Collins T, et al. Pain, Agitation, and Delirium Guidelines: Interprofessional Perspectives to Translate the Evidence. Dimens Crit Care Nurs 2017;36:164–173. [DOI] [PubMed] [Google Scholar]
- 26.Tobar E, Romero C, Galleguillos T, Fuentes P, Cornejo R, Lira MT, et al. [Confusion Assessment Method for diagnosing delirium in ICU patients (CAM-ICU): Cultural adaptation and validation of the Spanish version]. Med Intensiva 2010;34:4–13. [DOI] [PubMed] [Google Scholar]
- 27.Toro AC, Escobar LM, Franco JG, Díaz-Gómez JL, Muñoz JF, Molina F, et al. [Spanish version of the CAM-ICU (Confusion Assessment Method for the Intensive Care Unit). Pilot study of validation]. Med Intensiva 2009;34:14–21. [DOI] [PubMed] [Google Scholar]
- 28.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman Pun B, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33:1726–1731. [DOI] [PubMed] [Google Scholar]
- 29.Peterson JF, Pun BT, Dittus RS, Thomason JWW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: A study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. [DOI] [PubMed] [Google Scholar]
- 30.Mehta S, Cook D, Devlin JW, Skrobik Y, Meade M, Fergusson D, et al. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med 2015;43:557–566. [DOI] [PubMed] [Google Scholar]
- 31.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain Assessment Is Associated with Decreased Duration of Mechanical Ventilation in the Intensive Care Unit. Anesthesiology 2009;111:1308–1316. [DOI] [PubMed] [Google Scholar]
- 32.Herr K, Coyne PJ, McCaffery M, Manworren R, Merkel S. Pain Assessment in the Patient Unable to Self-Report: Position Statement with Clinical Practice Recommendations. Pain Manag Nurs 2011;12:230–250. [DOI] [PubMed] [Google Scholar]
- 33.Wøien H, Vaerøy H, Aamodt G, Bjørk IT. Improving the systematic approach to pain and sedation management in the ICU by using assessment tools. J Clin Nurs 2014;23:1552–1561. [DOI] [PubMed] [Google Scholar]
- 34.Hooper MH, Girard TD. Sedation and Weaning from Mechanical Ventilation: Linking Spontaneous Awakening Trials and Spontaneous Breathing Trials to Improve Patient Outcomes. Crit Care Clin 2009;25:515–525. [DOI] [PubMed] [Google Scholar]
- 35.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 2010;91:536–542. [DOI] [PubMed] [Google Scholar]
- 37.Engel HJ, Needham DM, Morris PE, Gropper M a. ICU Early Mobilization. Crit Care Med 2013;41:S69–80. [DOI] [PubMed] [Google Scholar]
- 38.Barr J, Kishman CP, Jaeschke R. The methodological approach used to develop the 2013 Pain, Agitation, and Delirium Clinical Practice Guidelines for adult ICU patients. Crit Care Med 2013;41:S1–15. [DOI] [PubMed] [Google Scholar]
- 39.Erstad BL, Puntillo K, Gilbert HC, Grap MJ, Li D, Medina J, et al. Pain Management Principles in the Critically Ill. Chest 2009;135:1075–2264. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119–141. [DOI] [PubMed] [Google Scholar]