Summary
Mitochondrial sulfide quinone oxidoreductase (SQR) catalyzes the oxidation of H2S to glutathione persulfide with concomitant reduction of CoQ10. We report herein that the promiscuous activity of human SQR supported the conversion of CoA to CoA-SSH (CoA-persulfide), a potent inhibitor of butyryl-CoA dehydrogenase, and revealed a molecular link between sulfide and butyrate metabolism, which are known to interact. Three different CoQ1-bound crystal structures furnished insights into how diverse substrates access human SQR, and provided snapshots of the reaction coordinate. Unexpectedly, the active site cysteines in SQR are configured in a bridging trisulfide at the start and end of the catalytic cycle, and the presence of sulfane sulfur was confirmed biochemically. Importantly, our study leads to a mechanistic proposal for human SQR in which sulfide addition to the trisulfide cofactor eliminates 201Cys-SSH, forming an intense charge-transfer complex with FAD, and 379Cys-SSH, which transfers sulfur to an external acceptor.
Graphical Abstract
eTOC Blurb
The catalytic promiscuity of human sulfide quinone oxidoreductase supports formation of CoA-persulfide, a known inhibitor of short-chain fatty acid oxidation in colonocytes. Landry et al describe four crystal structures of human SQR unraveling a different mechanism for sulfide oxidation, which is catalyzed by an active site cysteine trisulfide.
Introduction
Since its discovery as a gaseous signaling molecule (Abe and Kimura, 1996), an array of physiological effects have been associated with hydrogen sulfide (H2S) in the cardiovascular, nervous and gastrointestinal systems (Filipovic et al., 2018; Kabil and Banerjee, 2010; Kimura, 2010). Cells maintain low steady-state levels of H2S, a well-known respiratory toxin (Nicholls and Kim, 1982), and efficiently oxidize it via the mitochondrial sulfide oxidation pathway to generate thiosulfate and sulfate (Hildebrandt and Grieshaber, 2008; Libiad et al., 2014). Sulfide quinone oxidoreductase (SQR), a member of the flavin disulfide reductase superfamily (Argyrou and Blanchard, 2004), is anchored in the inner mitochondrial membrane and catalyzes the first and committing step in the sulfide oxidation pathway (Figure 1A). While 10–80 nM intracellular H2S is typical in various tissues (Furne et al., 2008; Vitvitsky et al., 2012), ~0.2–2.4 mM H2S has been reported in the colonic lumen (Deplancke et al., 2003; Macfarlane et al., 1992), which hosts ~100 trillion microbes (Turnbaugh and Gordon, 2009), including those that produce H2S via sulfate reduction, or desulfuration of cysteine (Linden, 2014). Hence, in addition to producing the gas endogenously, colonic epithelial cells are routinely exposed to microbiota-derived H2S. The hydrophobicity of H2S promotes its unfacilitated membrane transport (Mathai et al., 2009), potentially increasing exposure of colonocytes to the gas. Accordingly, colonic epithelial cells are adapted to survive acute exposure to high H2S levels (Levitt et al., 1999).
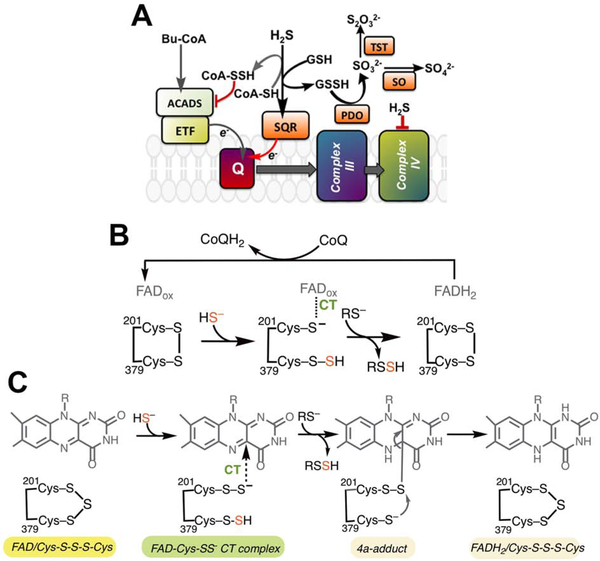
Figure 1. Intersection between sulfide and butyrate oxidation pathways and the proposed mechanisms for the SQR reaction.
(A) The canonical SQR reaction utilizes GSH as an acceptor to generate GSSH, with electrons driven into the CoQ10 (Q) pool. ACADS via the electron transferring flavoprotein (ETF), also drives electrons into the Q pool via oxidation of butyrate. We hypothesized that SQR utilizes CoA as an alternate acceptor generating CoA-SSH, which inhibits ACADS and blocks electron flow from butyrate oxidation. PDO (or ETHE1), TST and SO represent persulfide dioxygenase, rhodanese and sulfite oxidase, respectively in the mitochondrial sulfide oxidation pathway. (B) The previously proposed SQR reaction mechanism in which the active site cysteines in the resting enzyme form a disulfide. Cys201 participates in a CT complex with FAD while Cys379 is the sulfane sulfur carrier. (C) A proposal for the SQR-catalyzed oxidative half reaction, informed by our crystal structures, invokes a trisulfide configuration of the active site cysteines. Sulfide addition leads to persulfides on both cysteines. 201Cys-SS− forms a CT complex with FAD while 379Cys-SSH serves as the sulfur donor.
Butyrate is the preferred fuel source for normal colonocytes and is generated via microbial fermentation of undigested fiber remnants that reach the large intestine (Roediger, 1980). Butyryl-CoA is metabolized via butyryl-CoA dehydrogenase (or ACADS) to acetyl-CoA, which enters the Krebs cycle or is converted to ketone bodies. An unusual feature of ACADS is that it is purified as a “green” protein due to the presence of an inhibitory charge-transfer (CT) complex between a tightly bound CoA persulfide (CoA-SSH) and the FAD cofactor (Williamson et al., 1982). The remarkable stability of this complex has raised the obvious but as yet unanswered question as to the biological source of CoA-SSH. Sulfide inhibition of ACADS-dependent butyrate oxidation in colonocytes is associated with ulcerative colitis (Babidge et al., 1998). Given the promiscuity of SQR with respect to its sulfane sulfur acceptor (Jackson et al., 2012; Libiad et al., 2014) and its key role in detoxifying colonic H2S (Roediger et al., 1993), we hypothesized that SQR is a source of CoA-SSH whose formation allows prioritization of sulfide over butyrate oxidation.
SQR catalyzes two half reactions: (i) sulfur transfer from H2S to an acceptor via an active site cysteine persulfide (Cys-SSH) intermediate, and (ii) electron transfer from H2S to coenzyme Q10 (CoQ10) via an FADH2 intermediate (Figure 1B) (Jackson et al., 2012; Landry et al., 2017; Mishanina et al., 2015). The first step in the proposed mechanism is addition of the sulfide anion to an active site disulfide between Cys201 and Cys379, generating a persulfide intermediate on Cys379 (379Cys-SSH) with concomitant release of the Cys201 thiolate (Figure 1B) (Jackson et al., 2012). SQR forms an intensely absorbing CT complex centered at ~675 nm (Jackson et al., 2012; Libiad et al., 2014), suggesting formation of an electronic species that is distinct from those seen in other members of the flavin disulfide reductase superfamily (Mishanina et al., 2015). While the identity of the physiological acceptor has been controversial (Jackson et al., 2012; Libiad et al., 2014), kinetic simulations predict that GSH is the physiological sulfur acceptor for SQR (Landry et al., 2017, 2018; Libiad et al., 2014; Mishanina et al., 2015). The resulting glutathione persulfide (GSSH) product is then used by persulfide dioxygenase (PDO or ETHE1), the next enzyme in the pathway (Kabil and Banerjee, 2012). In the final step, electron transfer to CoQ10 regenerates FAD and connects SQR to the electron transfer chain at the level of complex III, making H2S the only known inorganic substrate for ATP synthesis in humans (Goubern et al., 2007).
Prior to a recent report of the crystal structure of human SQR (Jackson et al., 2019), structural perspective on the reaction was provided by bacterial homologs (Brito et al., 2009; Cherney et al., 2010; Marcia et al., 2009) which are, however, mechanistically distinct. In bacterial SQRs, sulfane sulfur does not exit the active site at the end of each catalytic cycle; instead, a hydropolysulfide bridge extends between the active site cysteines and is released as a linear polysulfide chain or after cyclization to elemental octasulfur.
Herein, the known substrate promiscuity of SQR is expanded to include CoA as an alternate sulfur acceptor, forming CoA-SSH, which is an ACADS inhibitor. We also report four structures of human SQR in the absence and presence of CoQ1, a soluble CoQ10 analog, and with/without sulfite or sulfide soaking, at resolutions ranging from 2.0–2.8 Å. Notably, soaking SQR-CoQ1 crystals with sodium sulfide provided evidence for in crystallo catalysis and captured CoQ1 exiting a long hydrophobic channel leading from FAD to the putative membrane-facing surface of SQR. Importantly, the structures together with biochemical data, led us to postulate a different mechanism for human SQR by assigning the 201Cys-SS− (versus the Cys201 thiolate) as the species involved in CT complex formation with FAD, and 379Cys-SSH as the sulfane sulfur donor to an external acceptor (Figure 1C).
Results
CoA is an alternative sulfur acceptor for SQR
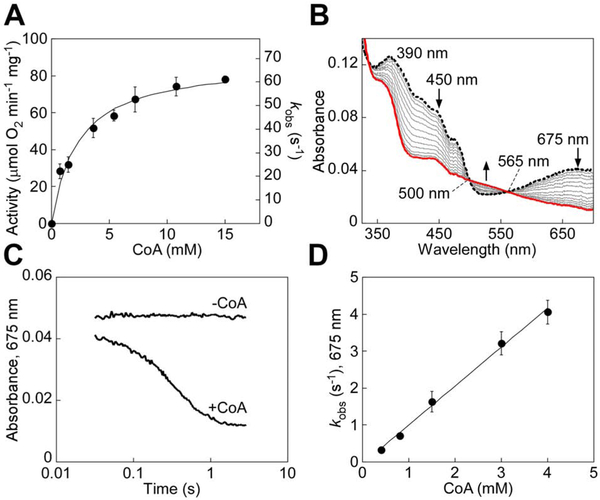
To test our hypothesis that SQR is a potential source of CoA-SSH, we characterized the kinetics of sulfur transfer from sulfide to CoA. Since the absorption spectrum of CoA interfered with our ability to monitor CoQ1 reduction at 278 nm in the steady-state SQR assay, we developed an alternative coupled assay using PDO, which oxidizes CoA-SSH in an O2-dependent reaction (Kabil and Banerjee, 2012). Michaelis-Menten analysis of SQR activity at varying CoA concentrations yielded a specific activity of 88 ± 4 μmol O2 min−1 mg−1, KM(CoA) of 2.3 ± 0.4 mM, and kcat of 69 ± 3 s−1 at 25 °C (Figure 2A). The kcat/KM for CoA (3 × 104 M−1s−1) was ~2-fold higher than for GSH (1.6 × 104 M−1s−1) (Landry et al., 2017).
Figure 2. Pre-steady state kinetic analysis of ndSQR-mediated sulfur transfer to CoA.
(A) Dependence of ndSQR activity on CoA concentration assessed in the coupled assay described under Star Methods. The data represent the mean ± S.D. of three independent experiments, each performed in duplicate. (B) ndSQR (40 μM) in 100 mM potassium phosphate buffer, pH 7.4, was rapidly mixed 1:1 (v/v) with Na2S (80 M) for ~35 msec to form the sulfide-induced CT complex at 675 nm (dashed black line), followed by a second rapid 1:1 (v/v) mixing with CoA (3 mM). Spectral changes associated with CT complex decay in ndSQR and concomitant FAD reduction (red line) were monitored over a period of 3 sec. (C) Comparison of the decay kinetics of the sulfide-induced CT complex in ndSQR with or without mixing with CoA (3 mM) monitored at 675 nm over 3 sec. (D) Dependence of the kobs for sulfide-induced CT complex decay on CoA concentration. The data represent the mean ± S.D. of two independent experiments.
The sulfide-induced CT intermediate on SQR (λmax = 675 nm) is stable for several seconds, but decayed upon rapid mixing with CoA, and led to the concomitant reduction of FAD (Figure 2B,C). A kon of 1.05 × 103 M−1s−1 at 4 °C was obtained from the dependence of the kobs for CT complex decay on the concentration of CoA (Figure 2D). CoA, like other thiols (Landry et al., 2018), was also capable of nucleophilic addition to the resting enzyme, forming a CT complex (Figure S1A). Compared to solubilized SQR, the intensity of the CT complex with nanodisc-embedded SQR (ndSQR), formed under the same conditions, was ~80% lower (Figure S1B), consistent with a role for the membrane environment in limiting this unwanted side reaction, as seen previously with GSH (Landry et al., 2018).
SQR-derived CoA-SSH forms a CT complex with ACADS
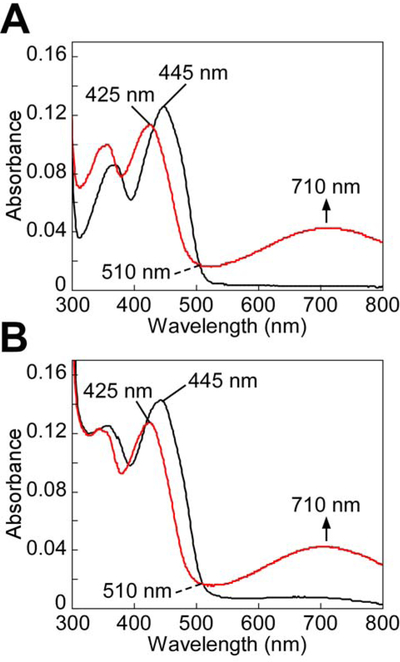
Incubation of oxidized ACADS with chemically prepared CoA-SSH led to the appearance of a CT band centered at 710 nm and a shift in the FAD absorption peak from 445 to 425 nm (Figure 3A), which were consistent with the formation of a CoA-SSH to FAD CT complex (Williamson et al., 1982). Next, the ability of SQR-derived CoA-SSH to form a CT complex on human ACADS was examined. Addition of a catalytic amount of ndSQR to the reaction mixture containing sulfide, CoA and CoQ1 reproduced the spectral changes in ACADS, consistent with the formation of a CoA-SSH to FAD CT complex (Figure 3B).
Figure 3. CT complex formation in ACADS by ndSQR-derived CoA-SSH.
(A) ACADS (10 μM) in 100 mM potassium phosphate buffer, pH 7.4 at 25 °C (black line) was mixed with CoA-SSH (20 μM) and incubated for 1 min. Formation of the CoA-SSH-induced CT complex in ACADS was indicated by the 710 nm absorbance band (red line). (B) ACADS (10 μM) in the same buffer as in (A), was mixed with Na2S (150 μM), CoA (3 mM), and CoQ1 (60 μM) and incubated for 1 min (black line), followed by addition of ndSQR (100 nM) and incubation for 1 min. Formation of the CoA-SSH-induced CT complex in ACADS (red line) was observed. The data are representative of three independent experiments.
Sulfide inhibits butyrate oxidation in colon epithelial cells
The effect of sulfide on butyrate oxidation was monitored by the release of 14CO2 from 14C-butyrate in the malignant human colon epithelial cell line HT-29. 14CO2 release was inhibited 20 or 37% upon bolus exposure to 0.3 or 0.5 mM Na2S, respectively (Figure S1C). While our data and those reported previously with human colonocytes exposed to 1.5 mM H2S (Babidge et al., 1998) could be consistent with sulfide inhibition of butyrate metabolism via CoA-SSH, it is important to note that both the sulfide and butyrate oxidation pathways are dependent on Complex IV, which is also inhibited by sulfide (Nicholls and Kim, 1982).
Crystal structures of human SQR
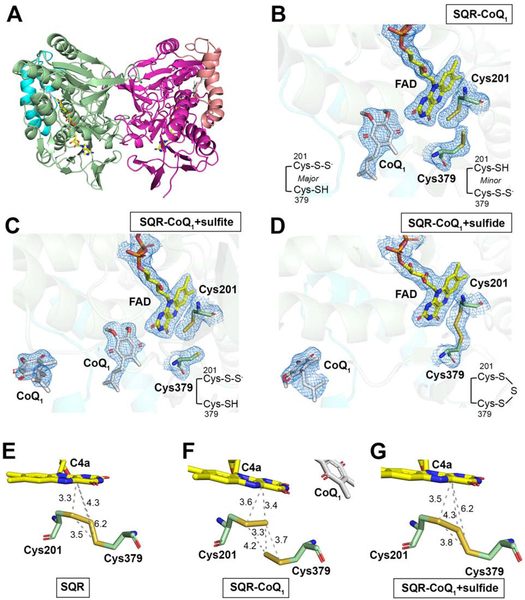
Table 1 summarizes the crystallographic data collection and refinement statistics for the following four structures: SQR (SQR, 2.80 Å), SQR co-crystallized with CoQ1 (SQR-CoQ1, 2.03 Å), SQR-CoQ1 soaked with sulfite (SQR-CoQ1 + sulfite, 2.21 Å), and SQR-CoQ1 soaked with sulfide (SQR-CoQ1 + sulfide, 2.55 Å). The initial phases were obtained from selenomethionine-substituted SQR at 3.27 Å resolution using the single-wavelength anomalous dispersion (SAD) method. The SQR model built from the SAD map was then employed for molecular replacement to calculate electron density maps for other high-resolution datasets. Two copies of monomeric SQR were present in the asymmetric unit forming a crystallographic dimer (Figure 4A). The overall structure is similar to the Acidithiobacillus ferrooxidans SQR (Cherney et al., 2010) and the flavoprotein subunit of the periplasmic Allochromatium vinosum flavocytochrome sulfide dehydrogenase (Chen et al., 1994) which uses cytochrome c instead of quinone as an electron acceptor (Figure S2). Each monomer of human SQR comprises the tandem Rossmann fold repeat signature of the flavin disulfide reductase superfamily, along with two amphipathic C terminal helices that anchor SQR to the membrane (Figure 4A), as also seen in A. ferrooxidans SQR (Cherney et al., 2010), and described in detail for human SQR (Jackson et al., 2019). The four SQR structures overlay well, with Cα rmsd ranging from 0.19–0.32 Å (Figure S3A), revealing the absence of major conformational changes. Our SQR structure overlays with a Cα rmsd of 0.29 Å on the recently described SQR structure (PDB ID: 6MP5) (Jackson et al., 2019). The membrane anchoring helices are at opposite ends in the crystallographic dimer, indicating that the observed dimer of detergent-solubilized SQR is not biologically relevant.
Table 1.
Crystallographic Data collection and refinement statistics
| SeMet-apo-SQR | SQR | SQR-CoQ1 | SQR-CoQ1 + sulfite | SQR-CoQ1 + sulfide | |
|---|---|---|---|---|---|
| Space group | P212121 | P212121 | P212121 | P212121 | P212121 |
| Unit cell parameters (Å) | a=77.58 | a=76.729 | a=78.516 | a=76.11 | a=76.295 |
| b=111.42 | b=110.424 | b= 111.512 | b=108.75 | b=111.768 | |
| c=133.92 | c=132.124 | c=133.648 | c=131.06 | c=137.032 | |
| α=β=γ=90° | α=β=γ=90° | α=β=γ=90° | α=β=γ=90° | α=β=γ=90° | |
| Wavelength (Å) | 0.9786 | 1.12713 | 1.12713 | 1.12713 | 1.12713 |
| Data collection statistics | |||||
| Resolution range (Å) | 66.92–3.27 (3.33–3.27)* | 50.00–2.80 (2.85–2.80) | 51.46–2.03 (2.08–2.03) | 43.69–2.11 (2.16–2.11) | 50.00–2.55 (2.60–2.55) |
| Number of unique reflections | 18411 (831) | 26934 (1198) | 73848 (4449) | 61813 (4480) | 38478 (1642) |
| Completeness (%) | 99.72 (92.03) | 96.5 (89.1) | 96.9 (78.9) | 98.0 (97.3) | 99.2 (85.7) |
| Rmerge | 0.508 (0.864) | 0.134 (0.747) | 0.184 (1.664) | 0.140 (1.300) | 0.202 (1.139) |
| Rpim | 0.127 (0.306) | 0.077 (0.485) | 0.079 (0.798) | 0.065 (0.588) | 0.095 (0.522) |
| CC1/2 | 0.315 (0.537) | 1.002 (0.568) | 0.875 (0.302) | 0.982 (0.637) | 0.984 (0.673) |
| CC1/2(anom) | 0.091 (0.323) | ||||
| Redundancy | 24.31 (9.87) | 3.6 (3.1) | 6.6 (5.8) | 5.6 (5.8) | 5.3 (5.4) |
| Mean I/σ | 9.09 (2.24) | 11.7 (1.6) | 6.0 (1.3) | 6.4 (1.1) | 4.5 (1.3) |
| Refinement statistics | |||||
| Resolution range (Å) | 33.18–2.81 | 41.38–2.03 | 41.92–2.21 | 50.93–2.56 | |
| Rwork/Rfree (%) | 21.74/27.32 | 22.01/25.53 | 19.02/22.60 | 22.41/27.19 | |
| RMSD bonds (Å) | 0.005 | 0.007 | 0.012 | 0.005 | |
| RMSD angles (deg) | 0.882 | 0.951 | 1.243 | 0.825 | |
| Average B factor (Å2) | 48.8 | 24.8 | 30.6 | 34.1 | |
| Number of water molecules | 30 | 191 | 101 | 41 | |
| Ramachandran | 95 | 94 | 97 | 97 | |
| favored (%) | |||||
| allowed (%) | 5 | 6 | 3 | 3 | |
| not allowed (%) | 0 | 0 | 0 | 0 | |
| PDB ID | 6O15 | 6O1B | 6O1C | 6O16 |
Values in parentheses are for highest-resolution shell
Figure 4. Overall structure and active site architecture of SQR.
(A) Overall structure of SQR-CoQ1 in which the monomers in the crystallographic dimer are shown in pale green and pink, respectively. The C terminal membrane anchoring helices are highlighted in cyan and salmon pink. FAD and CoQ1 are shown in stick display. The electron density maps (2Fo-Fc) contoured at 1.0 σ of Cys379, Cys201, FAD and CoQ1 are shown as mesh in SQR-CoQ1 (B), SQR-CoQ1 + sulfite (C) and SQR-CoQ1 + sulfide (D). Note that the cysteine backbone carbons are shown in green in B-D and that CoQ1 has moved away from FAD in the sulfide-soaked crystal structure in (D). (E-G) Close up of the active site redox centers in the indicated structures in which the distances between sulfur atoms and between sulfur atoms and the flavin C4a are labeled. The diffraction data precision indicator values are 0.37 Å (E), 0.16 Å (F), and 0.28 Å (G), respectively.
Configuration of redox active cofactors
The cache of redox cofactors in SQR comprises a noncovalent FAD and a pair of cysteine residues, Cys201 and Cys379 (Figure 4B–D, Figure S3B). FAD is bound in an extended conformation via multiple electrostatic and hydrogen-bonding interactions with main chain and side chain groups (Figure S3C). The FAD walls off the active site into a re-face and matrix-facing sulfur substrate channel, and a si-face and membrane-facing CoQ channel. An additional solvent-exposed channel connects FAD to the protein surface. Sulfur atoms in the active site were assigned using composite or polder omit maps calculated in the absence of FAD, CoQ1 and sulfur atoms, to limit biases introduced during model building. In the apo-SQR structure, the extra electron density between the cysteine thiols is assigned as a sulfur atom connecting Cys201 and Cys379 in a trisulfide bridge (Figure S3B). This configuration, also seen in the recent human SQR structure, was designated as the catalytically inactive “thiocystine” state of the enzyme (Jackson et al., 2019). The S-S distances in the trisulfide are 2.1 Å and 2.0 Å between the bridging sulfur and the sulfurs on Cys201 and Cys379, respectively (Figure 4E). The additional sulfur in human SQR as isolated was confirmed biochemically by cold cyanolysis, which detected 1.0 ± 0.2 mol sulfane sulfur per mol monomer.
In the SQR-CoQ1 structure, electron density assigned to CoQ1 is present in a hydrophobic pocket that opens to the putative membrane side (Figure 4B, Figure S4A,B), identified by the location of the membrane anchoring C-terminal helices (Jackson et al., 2019). Assumptions about equivalent thermal parameters or occupancy were not made, although the higher B-factors for CoQ1 likely reflect incomplete ligand occupancy and/or greater mobility. Unexpectedly, the sulfur bridge between Cys201 and Cys379 is broken in this structure. The strong additional density near Cys201 is assigned as the major 201Cys-SSH species; the weak electron density at Cys379 is assigned as the minor 379Cys-SSH species (Figure 4B, Figure S4C). The outer sulfur in 201Cys-S-S− is 3.7 Å away from 379Cys-SH, i.e. too far for an S-S bond (Figure 4F). Instead, it is rotated toward the FAD and is at a distance of 3.4 Å from C4a, forming the basis of our assignment that the major species in the SQR-CoQ1 structure is the 201Cys-S-S− persulfide forming a CT complex with FAD. In the minor 379Cys-S-S− species, the distance between the outer sulfur and 201Cys-SH is 4.2 Å, also too far for a bridging 201Cys-S-S-S-379Cys trisulfide.
In principle, soaking sulfite into SQR-CoQ1 crystals, which have a sulfane sulfur at either Cys201 or Cys379, could have led to a single sulfur transfer reaction to form thiosulfate (Mishanina et al., 2015). The active site cysteine configuration in the SQR-CoQ1 + sulfite structure is similar to that in the SQR-CoQ1 structure (Figure 4B,C), except that the additional electron density in the vicinity of Cys379 is almost completely absent in the polder omit map (Figure S4C). Density assigned to CoQ1 is found in the active site in chain B, and in the active site or at the putative membrane-entry site in chain A (Figure 4C).
Soaking SQR-CoQ1 crystals with sulfide could, in principle, support in crystallo catalysis (Jackson et al., 2012). Unexpectedly, sulfide addition to the SQR-CoQ1 crystals restored the resting trisulfide configuration (Figure 4D,G). The S-S distances in the trisulfide are 2.1 Å and 2.0 Å between bridging sulfur and the sulfurs on Cys201 and Cys379, respectively similar to those in the apo-SQR structure (Figure 4E). The distance between the flavin C4a and the Sγ of Cys201 versus C4a and the bridging sulfur is 3.5 and 4.3 Å, respectively. Furthermore, the CoQ binding site proximal to FAD is vacant (Figure 4D). In solution, when SQR was allowed to undergo 1.7 turnovers in the presence of sulfide and CoQ1, the sulfane sulfur atom was retained on the enzyme and detected by cold cyanolysis (1.5 ± 0.2 mol sulfane sulfur per mol monomer), consistent with the cysteine trisulfide being regenerated following turnover. We note that the slight increase in the sulfane sulfur detected at the end, compared to the beginning of turnover, might have resulted from spurious persulfidation of a surface-exposed Cys127 by HSSH, the reaction product.
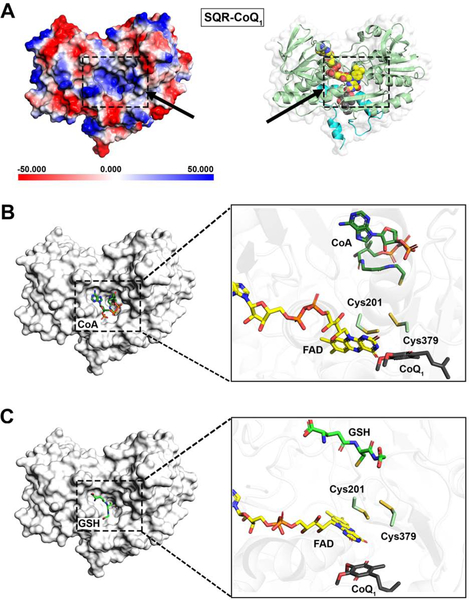
Structural perspective on GSH and CoA binding
The structures of detergent-solubilized SQR reported herein, correspond to the promiscuous form of the enzyme, which allows diverse nucleophiles to add into the oxidized cysteine cofactor and/or serve as sulfur acceptors. On the other hand, embedding SQR in nanodiscs restricts some of these adventitious reactions (Landry et al., 2018). The matrix-facing side of solubilized SQR provides some structural perspective on its catalytic promiscuity, as discussed for the apo-SQR structure (Jackson et al., 2019). A large channel leads from the surface to the relatively exposed 379Cys-SH or 379Cys-SSH in the active site (Figure S5). The electropositive potential energy surface could be important for guiding deprotonated substrates (sulfide, GSH, or CoA) during the sulfide oxidation and sulfur transfer reactions (Figure 5A). The top docking poses for GSH and CoA bound to SQR-CoQ1 reveal that they are lodged in the concavity at the surface of the channel (Figure 5B,C), with GSH being in a similar orientation as reported (Jackson et al., 2019). In these modeled structures, the thiols of GSH and CoA reach into the active site with their sulfur atom pointing toward, but distant from the Cys379 thiol (~6.3 Å for CoA and ~8.2 Å for GSH). These thiols would be closer to the outer sulfur in the putative 379Cys-SSH intermediate generated during the catalytic cycle.
Figure 5. The sulfur substrate entry site is on the matrix side.
(A) Electrostatic potential energy surface map showing the sulfur substrate entry/exit site in SQR-CoQ (dashed box and arrow, left panel). In the right panel, the structure is shown in the same orientation as in the left panel, with the FAD and CoQ1 displayed as yellow and dark gray spheres, respectively. (B) Docking model of SQR-CoQ1 with CoA (left) and close-up view of the active site (right). (C) Docking model of SQR-CoQ1 with GSH (left) and close-up view on the active site (right).
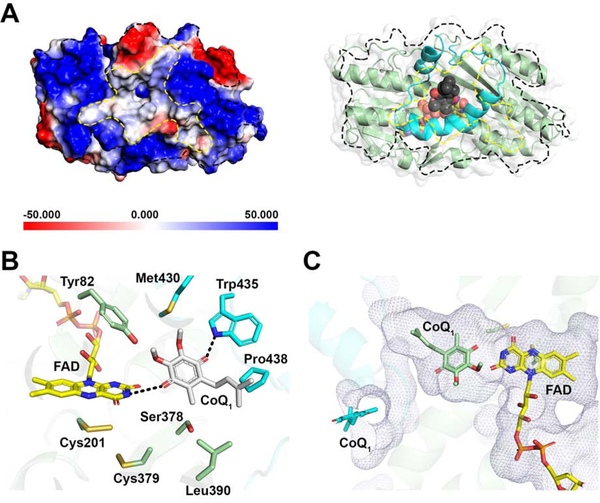
CoQ1 movement through a hydrophobic tunnel
The largely electropositive potential energy surface on the putative membrane-facing side of SQR frames a central hydrophobic patch that narrows into a deep tunnel (Figure 6A). CoQ1, with its C1 rather than C10 isoprenoid tail found in the natural CoQ10 substrate, resides at the end of the tunnel. CoQ1 is held at the edge of the isoalloxazine ring via interactions with active site residues on the si face of FAD (Figure 6B), similar to the interactions noted with a docked decylubiquinone moiety (Jackson et al., 2019). The C10-isoprenoid tail of the physiological substrate for SQR presumably occupies the length of the tunnel in the membrane environment. In the SQR-CoQ1 + sulfite structure, density assigned as CoQ1 is observed both at the entrance and the end of the hydrophobic tunnel, while it is only seen at the entrance in the SQR-CoQ1 + sulfide structure (Figure 4C–D, 6C).
Figure 6. CoQ entry from the mitochondrial membrane side.
(A) Electrostatic potential energy surface map showing the CoQ entry/exit site denoted by the dashed line in the SQR-CoQ1 structure (left panel). In the right panel, the structure is shown in the same orientation as the left panel, with FAD and CoQ1 displayed as yellow and grey spheres, respectively. (B) A close-up showing the interactions between CoQ1 and active site residues. (C) Location of CoQ1 near FAD at the end of the hydrophobic tunnel (in mesh representation) as seen in the SQR-CoQ1 structure (green) versus at the entrance to the tunnel (cyan) in the SQR-CoQ1 + sulfide structure.
The apparent movement of CoQ1 away from the active site and to the tunnel entrance in the sulfide-treated SQR-CoQ1 crystals suggests that in crystallo catalysis occurred, as discussed below. Additional density assigned as CoQ1 is seen at a third location in chain A in the SQR-CoQ1 and SQR-CoQ1 + sulfite structures (not shown). This site is likely to be nonspecific since it is on the surface of the matrix-facing side of SQR, and would not be contact with the CoQ10 pool in the mitochondrial membrane.
Discussion
SQR exists at the membrane-water interface, and its development as a potential therapeutic target for regulating H2S has been impeded by the lack of structural perspective on its catalytic mechanism, and complicated by its substantial substrate promiscuity. We report for the first time that CoA can serve as a sulfur acceptor for human SQR, generating CoA-SSH. The specificity constant (kcat/KM) for sulfide oxidation is ~2-fold higher with CoA than with GSH. Intracellular CoA levels are estimated to be ~15–175 μM in various tissues (Shurubor et al., 2017), while GSH is present at 1–10 mM (Kosower and Kosower, 1978), predicting that CoA-SSH production will be minor relative to GSSH. However, it is not known whether colonocytes, which preferentially rely on β-oxidation for their energy metabolism, have higher CoA concentrations. Our study establishes the feasibility of SQR-derived CoA-SSH to bind to and inhibit ACADS, thus identifying a biological source of CoA-SSH associated with “green” ACADS preparations that were first reported 65 years ago (Green et al., 1954; Shaw and Engel, 1984; Steyn-Parve and Beinert, 1958).
Both butyrate and sulfide oxidation rely on the electron transfer chain for provision of electron acceptors (Figure 1A). A possible rationale for sulfide modulation of ACADS in colonocytes via SQR-derived CoA-SSH is prioritization of sulfide oxidation under conditions of acute H2S exposure from gut microbial metabolism. Under these conditions, the high sulfide oxidation activity of SQR would rapidly decrease ambient H2S levels if competition for the oxidized CoQ pool is reduced by inhibition of ACADS (Figure 1A). We posit that CoA-SSH-induced ACADS inhibition helps shield the electron transfer chain from the effects of H2S poisoning, and allow cells to return to butyrate-oxidation fueled metabolism when H2S levels decrease. Impaired butyrate utilization is correlated with ulcerative colitis (Roediger, 1980a), and sulfide exposure reportedly leads to increased butyryl-CoA and decreased crotonyl-CoA in human colonocytes (Babidge et al., 1998). While our observation that HT-29 cells exhibit sulfide-sensitive impairment of butyrate oxidation (Figure S1C) is consistent with the postulated inhibition of ACADS by CoA-SSH (Shaw and Engel, 1987), it is important to note that Complex IV poisoning by H2S also contributes to inhibition of β-oxidation. Exploring the role of SQR in this process in vivo is an intriguing next step that merits investigation.
While our manuscript was in preparation, the structure of human apo-SQR at 2.59 Å resolution was reported (Jackson et al., 2019). The observed “thiocystine,” which is analogous to the active site trisulfide described in this study, was interpreted as the noncatalytic state of SQR and decylubiquinone was modeled into the CoQ10 binding pocket. The structure was used as a framework to propose a chemically unusual mechanism for converting the “noncatalytic” trisulfide into the active disulfide form (Reaction 1). Jackson et al postulated that sulfide (or an alternative nucleophile) attacks the trisulfide, generating 201Cys-SS− and 379Cys-SSH; the outer sulfur atom of 201Cys-SS− then attacks Cβ of Cys379 eliminating hydrodisulfide and forming the 379Cys-S-S-Cys201 disulfide (Jackson et al., 2019). Notably, the sulfide sulfur atom replaces the original Cys379 Sγ in the proposed mechanism to build the active site disulfide.
| [Rxn 1] |
We propose an alternative mechanism (Figure 1C), which is supported by analysis of the sulfane sulfur associated with SQR at the start and end of catalytic turnover. Furthermore, the movement of CoQ1 out of the active site and reformation of the resting trisulfide in the SQR + CoQ1 + sulfide structure, provide evidence for in crystallo sulfide-dependent turnover and support the postulated mechanism.
We propose that SQR, as isolated with a cysteine trisulfide cofactor (Figure S6, [1]), represents the active form of the enzyme. Surprisingly, co-crystallization with CoQ1 leads to loss of continuous electron density between the cysteines and to S-S bond distances that indicates the absence of a trisulfide. Instead, the extra electron density was modeled as a mixture of a major 201Cys-SS− [2] and a minor 379Cys-SS− species [3]. The ruptured trisulfide suggests that the S-S bond was either reduced, or that nucleophilic addition led to elimination of 201Cys-SS− (major) or 379Cys-SS− (minor), followed by hydrolysis of the resulting Cys-S-Nuc species. The source of the nucleophile, if any, is unknown, and could be an impurity introduced with CoQ1 (2.5 mM) or the carrier, dimethylsulfoxide (2.5% v/v) used during crystallization. We tentatively attribute the 201Cys-SS− species as the product of a nucleophilic addition/hydrolysis, as a nucleophile would more readily access the exposed Cys379 to cleave the trisulfide. Alternatively, intermittent light exposure during crystallization conditions, which included carboxylates that can participate as electron donors, could have led to CoQ1 photoreduction (Gorner, 2004) and electron transfer to FAD. This could account for the formation of the 379Cys-SS− species by reduction of the proximal 201Cys-S-SR bond in the trisulfide leading to [3]. Notably, electron density assigned as CoQ1 proximal to FAD was observed in the SQR-CoQ1 crystals, as also seen in the homologous A. ferrooxidans SQR structure (Zhang et al., 2016).
Sulfite can act as a sulfur acceptor, forming thiosulfate. The structure of SQR-CoQ1 + sulfite revealed loss of the extra electron density on Cys379 seen in the SQR-CoQ1 structure, but retention of a strong electron density peak in the polder omit map on the Sγ of Cys201 (Figure S4C). Implications of the observed sulfite-induced changes are that: (i) the CT complex with FAD involves 201Cys-SS− rather than the postulated 201Cys-S−, and (ii) sulfur transfer to the acceptor occurs from 379Cys-SSH. The distance between either sulfur in 201Cys-SS− and C4a on flavin is 3.5 Å in the SQR-CoQ1 + sulfite structure. Electropositive stabilization of the persulfide-CT intermediate could be provided by the helix dipole of α-helix 11 with its N-terminus oriented towards the FAD ring. The α-amino group of Lys207, oriented towards the outer sulfur in 201Cys-SS− at a distance of ~3.65 Å, could also help stabilize the persulfide intermediate. Strikingly, the CT complex in “green” human ACADS also arises from the interaction between a persulfide and FAD, in which the outer sulfur of CoA-SS− is 3.8 Å away from the flavin C4a (Figure S3D).
In the presence of sulfite, we presume that sulfane sulfur transfer occurred from the accessible 379Cys-SSH position leading to the presence of [2] in the crystal structure (Figure S6). Sulfide can act as both a sulfur donor and an acceptor in the SQR reaction (kcat =74 s−1 with sulfide, versus 113 s−1 with sulfide + GSH) (Libiad et al., 2014). Addition of sulfide to the SQR-CoQ1 crystals resulted in regeneration of the resting trisulfide and movement of CoQ1 out of the active site.
Based on our model, neither species observed in the SQR-CoQ1 crystals is catalytically active. The trisulfide could be rebuilt from [2] and [3] in crystallo in the presence of HSSH, a common contaminant in sulfide (Greiner et al., 2013), during sulfide soaking of SQR-CoQ1 crystals. Once reconstituted, the active enzyme would support sulfide oxidation and reduction of CoQ1, which was captured exiting the active site in the SQR-CoQ1 + sulfide structure (Figure 4D). Sulfane sulfur, detected by cold cyanolysis, remained associated with SQR following catalytic turnover in solution, consistent with the proposed regeneration of the resting trisulfide seen in crystallo. A CT complex and a C4a adduct between a persulfide rather than a thiolate on the proximal cysteine has been proposed for A. ferrooxidans SQR (Cherney et al., 2010). Similarly, a catalytic mechanism featuring a CT complex between a cysteine persulfide and FAD and a trisulfide resting form, has been postulated for the Aquifex aeolicus SQR (Marcia et al., 2009), and now visualized in our structures. The mechanism by which a redox active trisulfide cofactor is initially built in human SQR is currently under investigation in our laboratory.
As is clear from the above discussion, the redox activity of sulfur compounds introduces a layer of complexity in working with them, but fortuitously, led to the in crystallo formation and trapping of reactive species in human SQR. Structures of bacterial SQRs with linear and branched polysulfides extending between the analogous active site cysteine residues have been reported (Cherney et al., 2010; Marcia et al., 2009). The distance between the cysteine thiols in bacterial SQRs is significantly greater (~8.8 Å in A. ferrooxidans and 8.3 Å in A. aeolicus SQR) than in human SQR (~4.6 Å), consistent with the difference in length of the bridging sulfane sulfur intermediates that are formed in their respective catalytic cycles.
The large cavity extending from the matrix face to the active site exposes Cys379 and explains the vulnerability of solubilized SQR to errant attack by nucleophiles (sulfite, GSH, homocysteine, cysteine and methanethiol). Attack on the Sγ of the exposed Cys379 in the resting trisulfide would release 201Cys-SS− and form the CT complex (Figure 1C). However, these adventitious reactions lead to a dead end, and the active enzyme was only slowly recovered by reversal of the reaction and release of the nucleophile (Landry et al., 2018). Promiscuity is equally a problem in the subsequent sulfur transfer step, i.e. from 379Cys-SSH to an acceptor. Small molecule acceptors like sulfite and methanethiol exhibit higher catalytic efficiencies than GSH or CoA, which could reflect their more facile access to the active site (Jackson et al., 2012; Landry et al., 2018; Libiad et al., 2014). Docking models reveal that either GSH or CoA can be accommodated at the mouth of the cavity with their thiol moieties oriented to interact with the 379Cys-SSH intermediate (Figure 5B,C). The significantly greater intracellular concentration of GSH than sulfite or methanethiol, except perhaps under pathological conditions (Shih et al., 1977; Yaegaki and Sanada, 1992), is predicted to favor GSSH formation. In contrast to human SQR, the A. ferrooxidans SQR has a sheltered active site in which sulfide is polymerized rather than being transferred to an exogenous acceptor (Cherney et al., 2010). Since the propensity for adventitious addition of GSH (Landry et al., 2018) or CoA (Figure S1A, B) to the resting form of ndSQR is ~100-fold lower than the solubilized enzyme, we speculate that the active site in ndSQR might be less exposed than observed in the crystal structures of the solubilized enzyme. Membrane anchoring and/or dimerization might reduce the size of the substrate entry channel, thereby restricting access of larger substrates in the resting enzyme.
In summary, we have identified CoA as an additional sulfur acceptor for human SQR, and demonstrated that its generation in situ leads to formation of the inhibitory CoA-SS− to FAD CT complex in human ACADS. Our results implicate SQR in the coordinate regulation of sulfide and butyrate oxidation, potentially enabling the prioritization of sulfide clearance in colonic epithelial cells exposed to acute H2S stress. In addition, our structures have provided unexpected insights into the catalytic mechanism of SQR and implicated a cysteine trisulfide as the active form of the redox cofactor. Another feature of the postulated mechanism, also supported by the crystal structure, is that the CT complex forms between FAD and 201Cys-SS−, rather than with the thiolate 201Cys-S−. A potential catalytic advantage of this redox motif is that the nucleophilic addition of sulfide to a trisulfide is chemically more facile than addition to a disulfide, explaining in part the ~2×107 rate enhancement for sulfide addition to SQR versus sulfide addition to a disulfide in solution (0.6 M−1 s−1 at pH 7.4, 25 °C) (Cuevasanta et al., 2015).
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ruma Banerjee (rbanerje@umich.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
HT-29 colorectal adenocarcinoma cells (ATCC® HTB-38™, adult Caucasian female origin) were passaged at 37 °C in 5% CO2 and 95% air in RPMI 1640 medium lacking glucose, supplemented with fetal bovine serum (10%) and penicillin/streptomycin (0.5 mg mL−1) to induce differentiation (Zweibaum et al., 1985) and promote butyrate utilization (Leschelle et al., 2000). The cells were initially grown from a frozen stock that was authenticated by short-tandem repeat profiling performed by ATCC before this study.
METHOD DETAILS
Preparation of solubilized human SQR, ndSQR, and ACADS
Polyhistidine-tagged human SQR lacking the N-terminal mitochondrial leader sequence (amino acids 1–40) (Jackson et al., 2012) was expressed and purified as the selenomethionine-labeled enzyme as described previously (Libiad et al., 2014) with the following modifications. Briefly, a 1 L overnight culture of E. coli in Luria Bertani medium co-expressing plasmids for human SQR and cold-adapted chaperonins was pelleted and resuspended in 12 L of complete minimal medium supplemented with amino acids (80 μg mL−1 each, lacking methionine), glycerol (0.4% v/v), thiamine (5 μg mL−1), and the appropriate antibiotics. The initial A600 of the culture was ~0.4. The culture was incubated at 37 °C, 230 rpm for 2 h, followed by addition of selenomethionine (40 μg mL−1) and incubation for 1 h until A600 was ~0.9. The cells were then incubated at 17 °C, 170 rpm for 1 h before induction with isopropyl β-D-1-thioglactopyranoside (300 μM) and incubation was continued overnight under the same conditions. The purification procedure for SeMet-SQR was identical to that described previously (Libiad et al., 2014), except that 1,2-diheptanoyl-sn-glycero-3-phosphocholine was substituted with n-dodecyl-β-D-maltoside (DDM, 0.05% w/v) as the solubilizing detergent.
For biochemical studies, SQR was purified as described (Libiad et al., 2014). Solubilized SQR was incorporated into nanodiscs formed with the MSP1E3D1 scaffold protein (Denisov et al., 2007) containing 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as described to generate ndSQR (Landry et al., 2017). The concentration of solubilized SQR and ndSQR was estimated from the absorbance of the bound FAD cofactor using an extinction coefficient of 11,300 M−1cm−1 at 450 nm. Human ACADS (lacking the mitochondrial leader sequence and extending from residues 25–412) was expressed using the ACADS-pNIC-CTHF vector (plasmid #38870, from Addgene, deposited by Nicola Burgess-Brown). Briefly, a 200 mL overnight culture of E. coli in Luria Bertani medium co-expressing plasmids for human ACADS and the GroEL/GroES chaperonins was diluted 1:50 (v/v) into 6 L of Terrific Broth supplemented with the appropriate antibiotics, and grown at 37 °C with shaking at 230 rpm. Upon reaching an A600 of ~0.8, the cells were incubated for 1.5 h at 18 °C before induction with isopropyl β-D-1-thioglactopyranoside (300 μM) and incubation was continued overnight under the same conditions. The cells were harvested at 4 °C, and all subsequent purification steps were performed at the same temperature. The cells were re-suspended in 50 mM Tris-acetate, pH 7.6, containing 150 mM NaCl and protease inhibitor cocktail (Roche), lysed by sonication, and centrifuged at 34,000 rpm for 45 min. The supernatant was loaded onto a 10 mL Ni-NTA column pre-equilibrated with 50 mM Tris-HCl buffer, pH 8.0, containing 300 mM NaCl. The column was then washed with 10 column volumes of the same buffer containing 20 mM imidazole, and ACADS was eluted with 20 mL of 250 mM imidazole in the same buffer. The eluted fractions were pooled, concentrated, and applied to a 25 mL Superdex 200 column (GE Healthcare) equilibrated with 50 mM Tris-HCl buffer, pH 8.0, containing 300 mM NaCl. The concentration of the eluted ACADS was estimated from the bound FAD cofactor using an extinction coefficient of 12,500 M−1cm−1 at 450 nm (Engel and Massey, 1971). CoA-SSH used for generating the CT complex in ACADS was prepared by reacting Na2S with CoA disulfide (CoAS-SCoA), as described previously (Kabil and Banerjee, 2012)
Stopped-flow spectroscopy
Stopped-flow spectroscopy experiments were conducted in 100 mM potassium phosphate buffer pH 7.4, at 4 °C on a SF-DX2 double mixing stopped flow system from Hi-Tech Scientific, equipped with a 300–700 nm range photodiode array detector. The spectra and kobs for CoA-dependent decay of the sulfide-induced CT complex in ndSQR were obtained in similar double mixing experiments as described previously (Landry et al., 2017, 2018). For these, ndSQR was first rapidly mixed 1:1 (v/v) with Na2S and aged for ~35 ms to generate the CT complex, followed by a second 1:1 (v/v) rapid mixing with different concentrations of CoA. The kinetic traces at 675 nm monitoring the CT complex decay were fitted to a single-exponential equation using the KinetAsyst software (Cousins, 1997). The spectra and kobs for CoA-induced CT complex formation in solubilized SQR and ndSQR were obtained from similar single mixing experiments as described (Landry et al., 2017, 2018). For these, solubilized SQR or ndSQR were rapidly mixed 1:1 (v/v) with different concentrations of CoA, and the kinetic traces at 675 nm monitoring the CT complex formation were fitted to a single-exponential equation using the KinetAsyst software. All enzyme and substrate concentrations for stopped-flow experiments reported in the respective figure legends are before any 1:1 (v/v) mixing steps.
Coupled enzyme activity assay for ndSQR with CoA as an acceptor
Recombinant human PDO (Tiranti et al., 2009) was purified as described previously (Kabil and Banerjee, 2012). Oxygen consumption rates in the coupled enzyme assay for ndSQR with PDO were determined as described (Landry et al., 2018). All reactions were conducted using a Clark oxygen electrode housed in a temperature-controlled, 1.5 mL Gilson-type chamber with constant magnetic stirring. PDO (2 μM) in 100 mM potassium phosphate, pH 7.4, at 25 °C was mixed with 0.06 mg mL−1 BSA, CoA (0–15 mM), and CoQ1 (150 μM). The reactions were initiated by injection of Na2S (150 μM), followed immediately by injection of ndSQR (1 nM). Oxygen consumption was monitored with a Kipp and Zonen BD single channel chart recorder, with oxygen consumption expressed as μmol O2 consumed min−1 mg−1 enzyme. To ensure complete coupling of the ndSQR and PDO reactions, the assay described above was first conducted using GSH (0–40 mM) as the acceptor, and the oxygen consumption rates were checked for consistency with CoQ1 reduction rates obtained previously from ndSQR steady-state kinetic analyses (Landry et al., 2017).
Butyrate oxidation assay
Butyrate oxidation in HT-29 cells was monitored by conversion of [1-14C] butyrate to 14CO2 as described previously (Roediger, 1982), with the following modifications. Briefly, cells were divided into 2 × 106 cells per well in 6-well plates. The plated cells were incubated for 24 h, after which the media was replaced with serum-free RPMI 1640 medium lacking glucose, supplemented with 1 mM butyrate (containing 10 nCi [1-14C] butyrate) and L-carnitine (100 μM). Cells were then incubated for 3 h at 37 °C at atmospheric CO2, with each well covered by a filter paper saturated with NaOH (1 M) to capture the released 14CO2 from butyrate as 14C-bicarbonate. The reactions were terminated by addition of 125 mM citric acid, pH 6.0, and incubated for an additional 3 h at 25 °C to capture the remaining 14CO2. The filter papers were then collected and suspended in EcoLite(+) liquid scintillation cocktail for counting. Counts were corrected for background levels of butyrate volatilization by using filter papers from plates with the same culture medium but lacking cells.
Crystallization of SQR
All SQR crystals were grown at 20 °C by the hanging drop vapor diffusion method using solubilized human SQR (17.4 mg mL−1) in 50 mM Tris-HCl pH 8.0, containing sodium chloride (300 mM) and n-dodecyl β-D-maltoside (0.05% w/v). SeMet SQR crystals were grown by mixing protein (14 mg mL−1) at a 1:1 (v/v) ratio with reservoir solution containing 100 mM N-(2-acetamido) iminodiacetic acid (ADA) pH 6.5, lithium sulfate (300 mM) and PEG 400 (30% w/v). Wedge-shaped crystals grew within a couple of weeks. The crystals were harvested in the aforementioned reservoir solution, which was supplemented with an additional 20% (w/v) PEG 400 as a cryoprotectant. Crystals were then flash-frozen in liquid N2 and used for data collection. Apo-SQR crystals were grown by mixing purified SQR with an equal volume of reservoir solution containing 100 mM ADA pH 7.0, PEG 400 (30% w/v) and lithium sulfate (400 mM). The crystals were harvested in the aforementioned reservoir solution supplemented with glycerol (25% v/v) as a cryoprotectant, and flash-frozen in liquid nitrogen. SQR-CoQ1 crystals were grown by combining SQR (17.4 mg mL−1) and CoQ1 (5 mM, in DMSO), followed by 1:1 (v/v) mixing with reservoir solution containing 200 mM ammonium tartrate dibasic, pH 6.6, and PEG 3350 (20% w/v) to yield a final CoQ1 concentration of 2.5 mM, with initial crystals used as seed stock. Crystals from SQR-CoQ1 co-crystallization wells were used for sulfite soaking, which was performed by incubation with Na2SO3 (2 mM). Similarly, sulfide-soaking of SQR-CoQ1 crystals was performed by addition of Na2S (2 mM). Crystals were then cryoprotected in their respective reservoir solutions supplemented with glycerol (35% v/v) and flash-frozen in liquid nitrogen.
X-ray data collection, structure determination, and data deposition
Diffraction data for SeMet SQR crystals was collected at the LS-CAT beamline 21-ID-G (Advanced Photon Source, Argonne National Laboratory) at 0.9786 Å wavelength. The space group and the unit cell size of the crystal were P212121 and a=77.58Å, b=111.42Å, c=133.92Å, α=90°, β=90°, γ=90°, respectively. Three single-wavelength anomalous dispersion (SAD) data sets (3.27 Å resolution) from SeMet SQR crystals were merged, indexed, integrated and scaled by DIALS (Winter et al., 2018) via Xia2 (Winter et al., 2013). A total of 22 selenium sites from two copies of SeMet SQR in the asymmetric unit were found using SHELXC/D/E/ (Sheldrick, 2010). The low-resolution initial model was built using the buccaneer program in the CCP4 package (Cowtan, 2006). The model was further built manually using Coot (Emsley and Cowtan, 2004), and refinement calculations were carried out using Phenix.Refine (Afonine et al., 2010). Ligand restraints and libraries were applied during the refinements. A single asymmetric unit contains two copies of SeMet SQR. The final refined SeMet SQR model was then used as an input model for molecular replacement toward higher resolution datasets obtained from SQR (2.80 Å resolution), SQR-CoQ1 (2.03 Å resolution), SQR-CoQ1 + sulfite (2.21 Å resolution), and SQR-CoQ1 + sulfide (2.55 Å resolution) crystals. Crystallographic data statistics are summarized in Table 1. Diffraction data for SQR crystals were collected at the LS-CAT beamline 21-ID-D (Advanced Photon Source, Argonne National Laboratory). The diffraction images were processed using HKL2000 (Otwinowski and Minor, 1997) for SQR, and DIALS for SQR-CoQ1, SQR-CoQ1 + sulfite, and SQR-CoQ1 + sulfide. The molecular replacement solutions for SQR, SQR-CoQ1, SQR-CoQ1 + sulfite, and SQR-CoQ1 + sulfide were determined using the SeMet SQR model as a search model. The final structures were completed using alternate cycles of manual fitting in Coot (Emsley and Cowtan, 2004) and refinement in REFMAC5 (Murshudov et al., 1997). The stereochemical quality of the final models was assessed using MolProbity (Chen et al., 2010). The omit maps (2Fo-Fc) were calculated by iteratively omitting 10% of the total region in the absence of sulfur, CoQ1 and FAD using the composite omit map program in the PHENIX package (Afonine et al., 2012). Polder omit maps were calculated for the FAD cofactor, active site cysteines, and CoQ1 ligand were calculated by omitting the surrounding electron density derived from bulk solvent, using the polder maps program in the PHENIX package. The diffraction data precision indicator values were estimated using SFCHECK in CCP4 (Vaguine et al., 1999). Structures presented in figures were generated using PyMOL 2.0.6.
Detection of sulfane sulfur in SQR
Solubilized SQR (60 M) in 50 mM Tris-HCl, pH 8.0, containing NaCl (300 mM) and DHPC (0.03%) was assayed for sulfane sulfur according to the cold cyanolysis method as previously described (Wood, 1987). SQR was combined with KCN (50 mM) and ammonium hydroxide (80 mM) in a volume of 1 mL and incubated for 45 min at 25 °C, followed by addition of formaldehyde (0.76%) and 200 L of Goldstein’s reagent (50 mM ferric nitrate in 25% nitric acid). Precipitated protein was removed by centrifugation at 15,000 × g, and samples were measured at 460 nm for formation of the ferric thiocyanate complex. Sulfane sulfur concentration was calculated using a standard curve generated with thiocyanate (0–500 M). To correct for absorbance from FAD released from acidification of SQR, a sample containing an equimolar concentration of FAD in buffer was used as a control. To detect sulfane sulfur in SQR after catalytic turnover, the same enzyme was mixed with Na2S (200 μM) and CoQ1 (300 μM) and incubated for 5 min at 25 °C, followed by buffer exchange at 4 °C by passage through a PD-10 desalting column before cyanolysis. The data for mol sulfane sulfur per mol monomer are presented as the mean ± SD for three independent preparations of SQR.
Docked models for GSH and CoA binding to human SQR
The AutoDock Vina program (The Scripps Research Institute, CA, USA) (Trott and Olson, 2010) was used for the docking simulations. GSH and CoA structures were obtained from the PDB. The grid maps for docking studies were centered on the putative GSH and CoA binding site on the matrix facing side of SQR and comprised 22 × 22 × 22 and 20 × 20 × 20 points with 1.0 Å spacing, respectively. The docked models were selected among the nine top candidates based on the orientation of the thiol group in GSH and CoA.
QUANTIFICATION AND STATISTICAL ANALYSIS
Kinetic data are presented as mean ± SD, with the number of technical repeats indicated in the corresponding figure legends. A Student’s paired t-test was used to estimate the significance of sulfide treatment on butyrate oxidation in HT-29 cells, where p<0.05 was considered statistically significant, with the number of technical repeats indicated in the corresponding figure legend.
DATA AND CODE AVAILABILITY STATEMENT
The atomic coordinate and structure factors for SQR (6OI5), SQR-CoQ1 (6OIB), SQR-CoQ1 + sulfite (6OIC), and SQR-CoQ1 + sulfide (6OI6), were deposited in the RCSB Protein Data Bank.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| E. coli BL21 (DE3) | Invitrogen | Cat#C600003 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Selenomethionine | Molecular Dimensions | MD12–503B |
| Human SQR | Libiad et al 2015 | N/A |
| 1,2-diheptanoyl-sn-glycero-3-phosphocholine | Avanti Polar Lipids | 850306P |
| n-dodecyl-β-D-maltoside | Sigma-Aldrich | D4641 |
| MSP1E3D1 nanodisc scaffold protein | Landry et al 2017 | N/A |
| Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine | Anatrace Lipids | P516 |
| Human ACADS | This study | N/A |
| Coenzyme Q1 | Sigma-Aldrich | C7956 |
| Sodium sulfite | Sigma-Aldrich | S0505 |
| Sodium sulfide nonahydrate | Sigma-Aldrich | 431648 |
| Human PDO | Kabil et al 2012 | N/A |
| Coenzyme A persulfide | Kabil et al 2012 | N/A |
| Butyric acid, sodium salt | Alfa-Aesar | A11079 |
| [1-14C] butyric acid, sodium salt | ViT rax | VC 171 |
| Deposited Data | ||
| Crystal structure of selenomethionine-labeled SQR | This study | PDB ID: 6OI5 |
| Crystal structure of SQR-CoQ1 | This study | PDB ID: 6OIB |
| Crystal structure of SQR CoQ1 + sulfite | This study | PDB ID: 6OIC |
| Crystal structure of SQR CoQ1 + sulfide | This study | PDB ID: 6OI6 |
| Crystal structure of native SQR | Jackson et al 2019 | PDB ID: 6MP5 |
| Crystal structure of A. ferrooxidans SQR | Zhang et al 2016 | PDB ID: 3T31 |
| Crystal structure of A. vinosum FCSD | Chen et al 1994 | PDB ID: 1FCD |
| Experimental Models: Cell Lines | ||
| HT-29 human colorectal adenocarcinoma | ATCC | HTB-38 |
| Recombinant DNA | ||
| Expression vector pET23a+-MATopzSQOR | Jackson et al 2012 | N/A |
| Expression vector pCPN10/60 | Aligent Technologies | 230192 |
| Expression vector pNIC-CTHF-ACADS | Burgess-Brown, unpublished | Addgene 38870 |
| Expression vector pET28b+-MSP1E3D1 | Denisov et al 2007 | Addgene 20066 |
| Expression vector pMW172-ETHE1 (PDO) | Tiranti et al 2009 | N/A |
| Software and Algorithms | ||
| KinetAsyst | Cousins 1997 | https://www.hi-techsci.com/instruments/kinetasyst-stopped-flow-systems/ |
| DIALS | Winter et al 2018 | https://dials.github.io/ |
| Xia2 | Winter et al 2013 | https://xia2.github.io/ |
| SHELXC/D/E | Sheldrick 2010 | http://shelx.uni-goettingen.de/ |
| CCP4 | Cowtan 2006 | https://www.ccp4.ac.uk/ |
| Coot | Emsley and Cowtan 2004 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Phenix.Refine | Afonine et al 2010 | https://www.phenix-online.org/ |
| HKL2000 | Otwinowski and Minor 1997 | http://www.hkl-xray.com/ |
| REFMAC5 | Murshudiov et al 1997 | http://www.ccp4.ac.uk/html/refmac5.html |
| MolProbity | Chen et al 2010 | http://molprobity.biochem.duke.edu/ |
| PHENIX | Afonine et al 2012 | https://www.phenix-online.org/ |
| SFCHECK | Vaguine et al 1999 | http://www.ccp4.ac.uk/html/sfcheck.html |
| PyMOL | Schrodinger | https://pymol.org |
Highlights.
Human SQR utilizes CoA as an alternate sulfur acceptor generating CoA-persulfide
SQR-derived CoA-persulfide inhibits ACADS and butyrate oxidation
Four crystal structures of human SQR provide snapshots of its reaction coordinate
Cysteine trisulfide is the active cofactor and reforms at the end of turnover
Significance.
Mitochondrial membrane-anchored SQR catalyzes the committing step in H2S clearance in a reaction comprising sulfur transfer from H2S to a thiophilic acceptor and electron transfer from H2S via FAD to CoQ10, which links to the electron transfer chain. We have identified CoA as an alternate sulfur acceptor for SQR, and demonstrated that the CoA-SSH product forms a stable CT complex with FAD in human ACADS, which is known to be inhibitory. The ability of SQR to generate CoA-SSH has important implications for regulating colon bioenergetics, since colonocytes preferentially utilize microbiota-derived butyrate as a biofuel. Our study suggests the hypothesis that CoA-SSH could help prioritize sulfide over butyrate oxidation to shield cells from respiratory poisoning during acute gut exposure to H2S. Our crystallographic analyses have provided rich structural insights and lead us to postulate a different reaction mechanism. We have demonstrated that the active form of the enzyme has a redox-active cysteine trisulfide motif before and after in crystallo catalysis. The spectrally intense CT complex on SQR forms between 279Cys-SS− and FAD, rather than between 279Cys-S− and FAD, and was captured in sulfite-soaked SQR-CoQ1 crystals. By implication, the subsequent flavin C4a adduct involves a covalent interaction with 279Cys-SS−. The structural basis for the substrate promiscuity exhibited by solubilized SQR is amply explained by the structures, which reveal a large entrance exposing the active site trisulfide to solvent and providing access to the thiolate sulfur acceptor moieties of GSH and CoA alike.
Acknowledgements
We thank Dr. Sang Ho Park (University of Michigan) for his assistance with generating docking models of SQR with CoA and GSH. This work was supported in part by the National Institutes of Health (GM130183 to R.B., DK111465 to U.S.C. and F32GM122357 to A.P.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors declare that no competing financial interests exist.
References
- Abe K, and Kimura H (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of neuroscience : the official journal of the Society for Neuroscience 16, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, and Adams PD (2012). Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 68, 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonine PV, Mustyakimov M, Grosse-Kunstleve RW, Moriarty NW, Langan P, and Adams PD (2010). Joint X-ray and neutron refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr 66, 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyrou A, and Blanchard JS (2004). Flavoprotein disulfide reductases: advances in chemistry and function. Progress in nucleic acid research and molecular biology 78, 89–142. [DOI] [PubMed] [Google Scholar]
- Babidge W, Millard S, and Roediger W (1998). Sulfides impair short chain fatty acid beta-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis. Mol. Cell. Biochem 181, 117–124. [DOI] [PubMed] [Google Scholar]
- Brito JA, Sousa FL, Stelter M, Bandeiras TM, Vonrhein C, Teixeira M, Pereira MM, and Archer M (2009). Structural and functional insights into sulfide:quinone oxidoreductase. Biochemistry 48, 5613–5622. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, and Richardson DC (2010). MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Koh M, Van Driessche G, Van Beeumen JJ, Bartsch RG, Meyer TE, Cusanovich MA, and Mathews FS (1994). The structure of flavocytochrome c sulfide dehydrogenase from a purple phototrophic bacterium. Science 266, 430–432. [DOI] [PubMed] [Google Scholar]
- Cherney MM, Zhang Y, Solomonson M, Weiner JH, and James MN (2010). Crystal structure of sulfide:quinone oxidoreductase from Acidithiobacillus ferrooxidans: insights into sulfidotrophic respiration and detoxification. Journal of molecular biology 398, 292–305. [DOI] [PubMed] [Google Scholar]
- Cousins J (1997). KinetAsyst, Version 2.0. Hi-Tech Scientific Ltd, Salisbury, United Kingdom. [Google Scholar]
- Cowtan K (2006). The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr 62, 1002–1011. [DOI] [PubMed] [Google Scholar]
- Cuevasanta E, Lange M, Bonanata J, Coitino EL, Ferrer-Sueta G, Filipovic MR, and Alvarez B (2015). Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J Biol Chem 290, 26866–26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisov IG, Baas BJ, Grinkova YV, and Sligar SG (2007). Cooperativity in cytochrome P450 3A4: linkages in substrate binding, spin state, uncoupling, and product formation. J Biol Chem 282, 7066–7076. [DOI] [PubMed] [Google Scholar]
- Deplancke B, Finster K, Graham WV, Collier CT, Thurmond JE, and Gaskins HR (2003). Gastrointestinal and microbial responses to sulfate-supplemented drinking water in mice. Exp Biol Med (Maywood) 228, 424–433. [DOI] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Engel PC, and Massey V (1971). The purification and properties of butyryl-coenzyme A dehydrogenase from Peptostreptococcus elsdenii. Biochem J 125, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic MR, Zivanovic J, Alvarez B, and Banerjee R (2018). Chemical Biology of H2S Signaling through Persulfidation. Chem Rev 118, 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furne J, Saeed A, and Levitt MD (2008). Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295, R1479–1485. [DOI] [PubMed] [Google Scholar]
- Gorner H (2004). Photoprocesses of p-naphthoquinones and vitamin K(1): effects of alcohols and amines on the reactivity in solution. Photochem Photobiol Sci 3, 71–78. [DOI] [PubMed] [Google Scholar]
- Goubern M, Andriamihaja M, Nubel T, Blachier F, and Bouillaud F (2007). Sulfide, the first inorganic substrate for human cells. FASEB J 21, 1699–1706. [DOI] [PubMed] [Google Scholar]
- Green DE, Mii S, Mahler HR, and Bock RM (1954). Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem 206, 1–12. [PubMed] [Google Scholar]
- Greiner R, Palinkas Z, Basell K, Becher D, Antelmann H, Nagy P, and Dick TP (2013). Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19, 1749–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt TM, and Grieshaber MK (2008). Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275, 3352–3361. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Loll PJ, and Jorns MS (2019). X-Ray Structure of Human Sulfide:Quinone Oxidoreductase: Insights into the Mechanism of Mitochondrial Hydrogen Sulfide Oxidation. Structure 27, 794–805 e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MR, Melideo SL, and Jorns MS (2012). Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 51, 6804–6815. [DOI] [PubMed] [Google Scholar]
- Kabil O, and Banerjee R (2010). The redox biochemistry of hydrogen sulfide. J Biol Chem 285, 21903–21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O, and Banerjee R (2012). Characterization of Patient Mutations in Human Persulfide Dioxygenase (ETHE1) Involved in H2S Catabolism. J Biol Chem 287, 44561–44567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H (2010). Hydrogen sulfide: from brain to gut. Antioxid Redox Signal 12, 1111–1123. [DOI] [PubMed] [Google Scholar]
- Kosower NS, and Kosower EM (1978). The glutathione status of cells. Int Rev Cytol 54, 109–160. [DOI] [PubMed] [Google Scholar]
- Landry AP, Ballou DP, and Banerjee R (2017). H2S oxidation by nanodisc-embedded human sulfide quinone oxidoreductase. J Biol Chem 292, 11641–11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry AP, Ballou DP, and Banerjee R (2018). Modulation of Catalytic Promiscuity during Hydrogen Sulfide Oxidation. ACS Chem. Biol 13, 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschelle X, Delpal S, Goubern M, Blottiere HM, and Blachier F (2000). Butyrate metabolism upstream and downstream acetyl-CoA synthesis and growth control of human colon carcinoma cells. European journal of biochemistry / FEBS 267, 6435–6442. [DOI] [PubMed] [Google Scholar]
- Levitt MD, Furne J, Springfield J, Suarez F, and DeMaster E (1999). Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. The Journal of clinical investigation 104, 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libiad M, Yadav PK, Vitvitsky V, Martinov M, and Banerjee R (2014). Organization of the human mitochondrial H2S oxidation pathway. J Biol Chem 289, 30901–30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR (2014). Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid Redox Signal 20, 818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GT, Gibson GR, and Cummings JH (1992). Comparison of fermentation reactions in different regions of the human colon. The Journal of applied bacteriology 72, 57–64. [DOI] [PubMed] [Google Scholar]
- Marcia M, Ermler U, Peng G, and Michel H (2009). The structure of Aquifex aeolicus sulfide:quinone oxidoreductase, a basis to understand sulfide detoxification and respiration. Proceedings of the National Academy of Sciences of the United States of America 106, 9625–9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, and Pohl P (2009). No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci U S A 106, 16633–16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishanina TV, Yadav PK, Ballou DP, and Banerjee R (2015). Transient Kinetic Analysis of Hydrogen Sulfide Oxidation Catalyzed by Human Sulfide Quinone Oxidoreductase. J Biol Chem 290, 25072–25080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, and Dodson EJ (1997). Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr 53, 240–255. [DOI] [PubMed] [Google Scholar]
- Nicholls P, and Kim JK (1982). Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can J Biochem 60, 613–623. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, and Minor W (1997). Processing of X-ray diffraction data collected in oscillation mode. Methods in enzymology 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Roediger WE (1980). Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21, 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger WE (1982). Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83, 424–429. [PubMed] [Google Scholar]
- Roediger WE, Duncan A, Kapaniris O, and Millard S (1993). Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis? Clin Sci (Lond) 85, 623–627. [DOI] [PubMed] [Google Scholar]
- Shaw L, and Engel PC (1984). The purification and properties of ox liver short-chain acyl-CoA dehydrogenase. Biochem J 218, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, and Engel PC (1987). CoA-persulphide: a possible in vivo inhibitor of mammalian short-chain acyl-CoA dehydrogenase. Biochim Biophys Acta 919, 171–174. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM (2010). Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D Biol. Crystallogr 66, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih VE, Abroms IF, Johnson JL, Carney M, Mandell R, Robb RM, Cloherty JP, and Rajagopalan KV (1977). Sulfite oxidase deficiency. Biochemical and clinical investigations of a hereditary metabolic disorder in sulfur metabolism. N Engl J Med 297, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Shurubor YI, D’Aurelio M, Clark-Matott J, Isakova EP, Deryabina YI, Beal MF, Cooper AJL, and Krasnikov BF (2017). Determination of Coenzyme A and Acetyl-Coenzyme A in Biological Samples Using HPLC with UV Detection. Molecules 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn-Parve EP, and Beinert H (1958). On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. VII. The nature of the green color of butyryl dehydrogenase. J Biol Chem 233, 853–861. [PubMed] [Google Scholar]
- Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, et al. (2009). Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med 15, 200–205. [DOI] [PubMed] [Google Scholar]
- Trott O, and Olson AJ (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, and Gordon JI (2009). The core gut microbiome, energy balance and obesity. J Physiol 587, 4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaguine AA, Richelle J, and Wodak SJ (1999). SFCHECK: a unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr D Biol Crystallogr 55, 191–205. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V, Kabil O, and Banerjee R (2012). High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid Red Signal 17, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G, Engel PC, Mizzer JP, Thorpe C, and Massey V (1982). Evidence that the greening ligand in native butyryl-CoA dehydrogenase is a CoA persulfide. J Biol Chem 257, 4314–4320. [PubMed] [Google Scholar]
- Winter G, Lobley CM, and Prince SM (2013). Decision making in xia2. Acta Crystallogr D Biol Crystallogr 69, 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G, Waterman DG, Parkhurst JM, Brewster AS, Gildea RJ, Gerstel M, Fuentes-Montero L, Vollmar M, Michels-Clark T, Young ID, et al. (2018). DIALS: implementation and evaluation of a new integration package. Acta Crystallogr D Struct Biol 74, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JL (1987). Sulfane sulfur. Methods in enzymology 143, 25–29. [DOI] [PubMed] [Google Scholar]
- Yaegaki K, and Sanada K (1992). Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res 27, 233–238. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qadri A, and Weiner JH (2016). The quinone-binding site of Acidithiobacillus ferrooxidans sulfide: quinone oxidoreductase controls both sulfide oxidation and quinone reduction. Biochem. Cell Biol. 94, 159–166. [DOI] [PubMed] [Google Scholar]
- Zweibaum A, Pinto M, Chevalier G, Dussaulx E, Triadou N, Lacroix B, Haffen K, Brun JL, and Rousset M (1985). Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J. Cell. Physiol 122, 21–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinate and structure factors for SQR (6OI5), SQR-CoQ1 (6OIB), SQR-CoQ1 + sulfite (6OIC), and SQR-CoQ1 + sulfide (6OI6), were deposited in the RCSB Protein Data Bank.