Abstract
The structures and functions of the cellular acidic compartments are strongly dependent on the pH gradients across vesicular membranes. Measurement and imaging of the vesicular pH require fluorophores with appropriate pKa values. In this report, we characterized the pH-dependent lifetime responses of a family of acidotropic probes, LysoSensors, to evaluate their usefulness to low-pH lifetime imaging. LysoSensors are cell-permeable weak bases that selectively accumulate in acidic vesicles after being protonated. They have higher quantum yields at lower pH ranges to allow visualization of the lysosomes. For LysoSensors DND-167, DND-189, and DND-153, raising the buffer pH increased the quenching effects of their basic side chains and substantially reduced their steady-state fluorescence and lifetimes. The apparent pKa values determined from their lifetime responses were shifted to near neutral values because of the dominant intensity contribution from their protonated species. One unique property of LysoSensor DND-189 is its non-monotonic lifetime responses of the maxima occurring between pH 4 and 5. LysoSensor DND-192 did not show significant lifetime changes over a wide pH range. LysoSensor DND-160, which was the only excitation and emission ratiometric probe, showed significant pH-dependent lifetime changes as well as its spectral shifts. Its apparent pKa values determined from the lifetime responses were comparable to the lysosomal pH because of its bright basic form. Because of the pH-dependent absorption spectra, the apparent pKa values could be manipulated between 3 and 5 by changing the excitation and/or emission wavelengths. These results indicate that LysoSensor DND-160 is a promising probe for lifetime imaging to determine lysosomal pH.
Keywords: lifetime-based sensing, lifetime imaging, pH probes, fluorescence
Endocytic vesicles are intracellular acidic compartments in the cytoplasm which perform transportation and biodegradation functions in cells. From their ultrastructural differences and subcellular distributions, endocytic vesicles can be classified as early endosomes, recycling endosomes, multivesicular endosomes, and late endosomes. The vesicles participate in processing and sorting of antigens, recycling and down-regulation of membrane receptors, and delivery and degradation of foreign materials internalized by the cells. Numerous biochemical assays and fluorescence imaging data have shown that the morphology and activity of acidic compartments are highly regulated by the pH gradients between their vesicular lumen and cytoplasm. For example, treating cells with different pH-perturbing reagents such as weak bases, ATP–H+ pump inhibitors(1), or proton-selective channels formed by membrane-spanning proteins (2) dissipated the pH gradient across the vesicle membranes and slowed down the transportation processes such as protein sorting or endocytosis. Biogenesis of endosomes from early endosomes to multivesicular body is also regulated by the pH-dependent ability of coatomer proteins to mediate the association of endosomal membranes (3).
The multidrug-resistant properties of certain cells are also correlated with their intracellular pH distributions. Drug-sensitive parental cells usually have greater extent of cytoplasm accumulation of chemo-therapeutic drugs than the drug-resistant cells derived from them, but the accumulation of drugs in their acidic vesicles is much less than that in drug-resistant cells (4–6). When treated with lysosomotropic agents such as chloroquine, the drug-resistant cells reversed their phenotype to be drug-sensitive, raised vesicular pH, and depleted drug accumulation in their acidic compartments (5–8). Imaging evidence from MCF-7 breast cancer cell lines also indicates that MCF-7 cells have a higher average endocytic pH than the multi-drug-resistant cells, but an average cytosolic pH lower than resistant cells (9–11). It was concluded that the decreased pH gradients across the endosomal membranes resulted in less active drug sequestration processes in these vesicles. Precise measurements of the vesicular pH are very important to understand the functions and regulations of acidic organelles in different living cells.
Conventional fluorescence microscopy uses very limited number of fluorophores such as fluorescein–dextran and acridine orange (12) to determine the pH in acidic organelles. However, fluorescein is easily photo-bleached and insensitive to pH values below 6, and the emission of acridine orange depends on its accumulation in acidic vesicles. Recently several alternative low-pH indicators have been developed for labeling and tracking the pH in acidic organelle with better sensitivity and selectivity. Molecular Probes (Eugene, OR) developed a family of acidotropic probes called LysoSensors, which can spontaneously partition into the acidic organelles and accumulate there after being protonated. They show great pH dependency of their fluorescence intensity and highlight the acidic compartments in the living cells with their blue or yellow fluorescence emissions (13–15). However, most of the published imaging results using LysoSensor probes allowed only semiquantitative estimation of the organelle pH and did not allow estimation of the intracellular pH.
In this report, we characterized the lifetime responses of the five LysoSensor probes and evaluated their potential usage in lifetime-based pH sensing and imaging. In general, their lifetimes increased upon acidification because of the decreased quenching effects of their protonated weak base side chains. However, lifetime response of LysoSensor DND-1894 was not monotonic, showing lifetime maxima around pH4.5. The same profile was obtained with the pH-dependent emission intensity. Another unique case is the dual-excitation and dual-emission properties of LysoSensor yellow/blue DND-160. DND-160 is brightly fluorescent at both high and low pH values, and its pH-dependent spectral shift helped us to manipulate its pH sensitivity and apparent pKa values by changing the excitation and observation wavelengths (16). Whereas the apparent pKa values determined from the phase angle responses of other LysoSensor probes are above the pH value of the acidic vesicles, DND-160 has apparent pKa values falling in the same pH range of the acidic organelles. Besides, the free form of DND-160 showed significant lifetime changes in this pH range. The lifetime characterizations presented here implicate that DND-160 is a promising lifetime-based probe for endocytic pH imaging.
MATERIALS AND METHODS
The LysoSensor pH indicators, LysoSensor blue DND-167, LysoSensor blue DND-192, LysoSensor green DND-189, LysoSensor green DND-153, and LysoSensor yellow/blue DND-160 were purchased from Molecular Probes. Because their chemical structures show high hydrophobicity (Fig. 1), they can permeate through the membranes spontaneously. All other chemicals used for preparing pH buffers, such as citrate, phosphate, Tris, and bicarbonate, were obtained from Sigma (St. Louis, MO). The buffer concentrations were kept at 50 μM in the presence of 0.1 M KCl to maintain a constant ionic strength. Stock solutions of LysoSensor probes were first prepared in distilled water at a concentration of 0.1 μM and then diluted with pH buffers to a final concentration of 2 μM.
FIG. 1.
Chemical structures of the LysoSensor pH indicators, DND-167, DND-192, DND-153, DND-189, and DND-160.
The fluorescence excitation and emission spectra were measured on Aminco Bowman Series 2 and SLM8000 (Spectronic Instruments, Inc., Rochester, NY) spectrometers. Quantum yield measurements were performed according to the previously established method (17). The optical densities of both samples and standards were kept below 0.05 at the excitation wavelengths to avoid the inner filter effects, and the emission intensities were integrated through the whole spectra. The time-resolved fluorescence intensity decays of LysoSensors were measured by a frequency-domain phase/modulation instrumentation (ISS, Inc., Champaign, IL), which can be adapted to different lasers as the light sources. We used a continuous wave He-Cd laser with an output at 442 nm and a frequency-doubled synchronously pumped Pyridine 2 dye laser tunable between 350 and 380 nm. The 442-nm excitation light was modulated by an electrooptic modulator (ISS, Inc.). The lifetime standards were selected according to the broadband emission filters: aqueous solution of rhodamine B with lifetime of 1.68 ns for emission at 600 nm; cyclohexane solution of MSB with lifetime of 1.68 ns (18) was for emission at 450 nm; and methanol solution of DCS with lifetime of 0.47 ns (19) for emission at 550 nm.
The fluorescence intensity decays were recovered from the multifrequency data and analyzed with double or triple exponential decay models:
| [1] |
where Ik(t) is the fluorescence intensity decay at given pH value (k), and αik are the pH-dependent amplitudes for individual lifetimes τi. The summation of αik equals to 1 and N represents the number of exponentials. The fractional intensity of each decay component was also determined:
| [2] |
Detailed description of the theories and instruments has been published previously (20, 21). For global intensity decay analysis, the individual lifetime τi was set as global parameters, and the amplitudes αik were dependent on the pH environments. The use of this equation with global analysis assumes that the decay time τi of various protonated species are independent of pH. The mean lifetimes τk at difference pH values were calculated using:
| [3] |
RESULTS
Chemical structures of the five LysoSensor probes are shown in Fig. 1. LysoSensor probes can be divided into three families based on their emission wavelengths: LysoSensor blue, LysoSensor green, and LysoSensor yellow/blue. LysoSensor blue DND-167 and DND-192 have a same anthracene skeleton but with different side chains N-morpholinomethyl and dimethylaminomethyl, which bring their pKa values to 5.0 and 7.5, respectively. They emit bright blue fluorescence with quantum yields up to 0.9 in acidic solutions (Table 1). Similarly, different aliphatic amine substitutions on the 1,2-naphthoylenebenzimidazole structures of LysoSensor green DND-153 and DND-189 make them sensitive to neutral and low pH range. LysoSensor yellow/blue DND-160 is a pyridyl oxazole dye, emitting yellow or blue light according to the pH. Based on the spectral evidence from its analogous compounds (22), its protonation was predicted to occur on the pyridine ring instead of the oxazole moiety.
TABLE 1.
The pKa Values and Quantum Yields (Q) of LysoSensor Probes
| Quantum yield | ||||
|---|---|---|---|---|
| LysoSensor probe | pH | Q | pKaa | b |
| DND-167 | 3.0 | 0.80c | 5.0 | 6.5 |
| DND-192 | 4.0 | 0.88c | 7.5 | N/A |
| DND-153 | 4.0 | 0.34d | 7.5 | 8.9 |
| DND-189 | 3.0 | 0.41d | 5.2 | 6.6 |
| 4.4 | 0.48d | |||
| DND-160 | 3.0 | 0.31d | 4.2 | 3.5e |
| 7.7 | 0.34c | 4.0f | ||
| 4.5g | ||||
pKa values are from the “Handbook of Fluorescent Probes and Research Chemicals,” Molecular Probes, Inc.
values were derived from the pH-dependent phase-angle-responsive curves of each LysoSensor probes fitted to the Henderson–Hasselbalch equation.
Quinine sulfate in 1.0 N H2SO4 (Q = 0.546) served as a quantum yield standard.
Fluorescein in 0.1 N NaOH (Q = 0.9) was employed as a quantum yield standard.
λex = 350 nm.
λex = 365 nm.
λex = 380 nm.
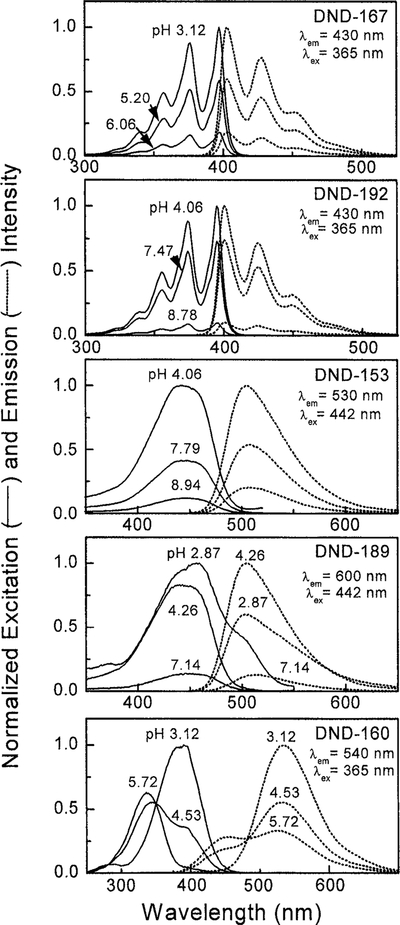
Quenching effects from the basic side chains of LysoSensor probes were observed from the excitation and emission spectra of DND-167, DND-192, and DND-153 (Fig. 2). An increase in the extent of protonation of the amino side chains relieved their quenching effects and increased the steady-state fluorescence intensity of the probes. Because of its pKa value at 5.0, DND-167 had very weak fluorescence at neutral pH but became much brighter at pH 3.0. DND-192 and DND-153 with their pKa values close to neutral were still strongly fluorescent at pH 8.
FIG. 2.
Excitation and emission spectra of LysoSensor probes in different pH buffers. DND-160 can be used as a dual-excitation and dual-emission pH indicator since both of its excitation and emission maxima show blue shift as pH increases.
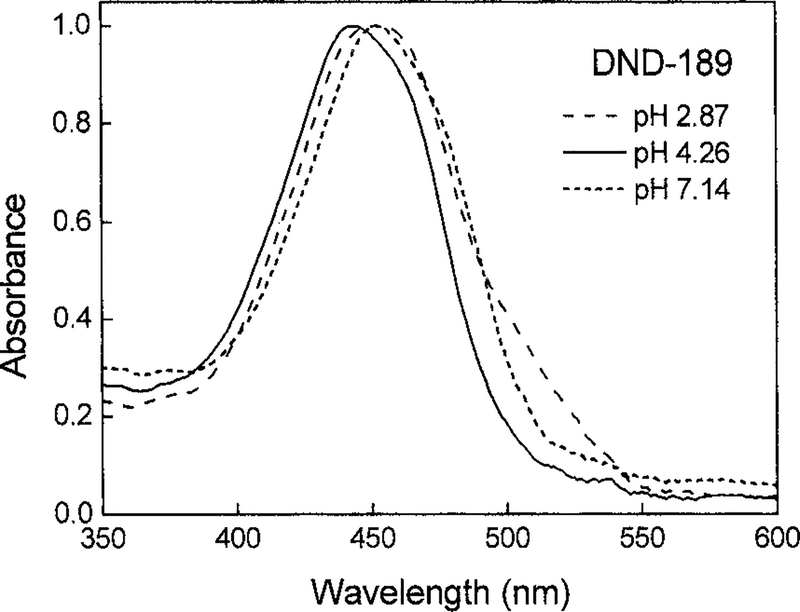
DND-189 and DND-160 showed different pH dependency in their excitation and emission spectra. The steady-state fluorescence intensity of DND-189 at 500 nm reached the maxima between pH 4 and 5, and its excitation spectra obtained at 600 nm emission were broadened and red shifted at higher (pH 7.1) and lower (pH 2.9) values (Fig. 2). The same red-shifts phenomena were observed in its absorption spectra, indicating the multiple protonation steps of this probe (Fig. 3). DND-160 is a unique ratiometric LysoSensor probe since its excitation and emission spectra are significantly blue shifted as the buffer pH increased, with isosbestic points at 365 and 470 nm (Fig. 2). Both its protonated and its basic forms had comparable quantum yields of ≈0.3 (Table 1). No significant quenching effects were observed.
FIG. 3.
Absorption spectra of DND-189 at pH 2.87, 4.26, and 7.14. The red-shift phenomena were observed at pH 2.87 and 7.14, which were the same as those observed in the excitation spectra of DND-189.
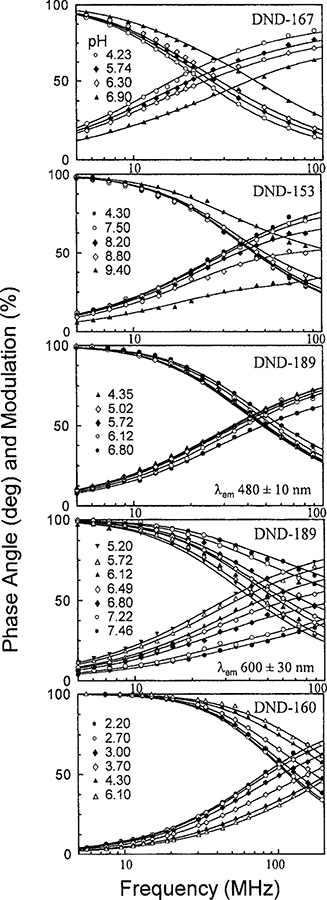
In order to determine the optimal conditions for low pH measurements using LysoSensor probes, we measured their frequency-domain lifetime responses with different pairs of excitation and emission wavelengths. Their phase angles and modulation responses at different pH values are illustrated in Fig. 4, and the global data analysis results are summarized in Table 2. LysoSensor blue DND-167 and DND-192 were excited at 365 nm and the emissions were collected at the blue region with a broadband interference filter (450 ± 20 nm). Their mean lifetimes in acidic solutions were relatively long at 11 ns, but the mean lifetime of DND-167 dropped to 7 ns in neutral pH. DND-192 did not show significant pH-dependent lifetime changes between pH 4 and 8. LysoSensor green DND-153 and DND-189 were excited at 442 nm and the emissions from their blue-green (centered at 480 nm) and red (centered at 600 nm) regions were measured. Very little pH-dependent lifetime changes for DND-153 were observed from its blue-green emission region, at most 8° in phase angle and 5% in modulation (data not shown). In contrast, large difference (40° in phase angle) was observed for DND-153 between pH 4 and 9 at its red emission region, corresponding to the mean lifetime difference, between 6.4 and 3.6 ns.
FIG. 4.
Phase and modulation responses of LysoSensor probes at different pH values with frequency domain measurements. The observed emission depends on the emission filters.
TABLE 2.
Global Intensity Decay Analysis of LysoSensor Probesa
| LysoSensor | pH | α1b | α2b | α3b | f1b | f2b | f3b | 〈τ〉 |
|---|---|---|---|---|---|---|---|---|
| DND-167 | 4.2 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 11.4 |
| 5.7 | 0.651 | 0.215 | 0.134 | 0.894 | 0.100 | 0.006 | 10.6 | |
| 6.3 | 0.436 | 0.304 | 0.260 | 0.797 | 0.189 | 0.014 | 9.8 | |
| 6.9 | 0.144 | 0.471 | 0.385 | 0.457 | 0.508 | 0.035 | 7.2 | |
| τ1 = 11.4 ns, τ2 = 3.9 ns, τ3 = 0.3 ns, = 7.2c | ||||||||
| DND-153 | 4.3 | 1.000 | 0.000 | 1.000 | 0.000 | 6.4 | ||
| 7.5 | 0.838 | 0.162 | 0.979 | 0.021 | 6.2 | |||
| 8.2 | 0.588 | 0.412 | 0.927 | 0.073 | 5.9 | |||
| 8.8 | 0.329 | 0.671 | 0.814 | 0.186 | 5.3 | |||
| 9.4 | 0.106 | 0.894 | 0.515 | 0.485 | 3.6 | |||
| τ1 = 6.4 ns, τ2 = 0.7 ns, = 6.4c | ||||||||
| DND-189 | 3.1 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 4.1 |
| 3.7 | 0.577 | 0.422 | 0.001 | 0.651 | 0.349 | 0.000 | 5.1 | |
| 4.1 | 0.857 | 0.126 | 0.017 | 0.898 | 0.096 | 0.006 | 5.4 | |
| 4.4 | 0.990 | 0.009 | 0.001 | 0.993 | 0.007 | 0.000 | 5.6 | |
| 5.2 | 0.999 | 0.000 | 0.001 | 1.000 | 0.000 | 0.000 | 5.6 | |
| 5.7 | 0.908 | 0.000 | 0.092 | 0.968 | 0.000 | 0.032 | 5.5 | |
| 6.8 | 0.509 | 0.000 | 0.491 | 0.758 | 0.000 | 0.242 | 4.7 | |
| τ1 = 5.6 ns, τ2 = 4.1 ns, τ3 = 1.8 ns, = 7.0c | ||||||||
| DND-189 | 5.2 | 0.970 | 0.030 | 0.000 | 0.996 | 0.004 | 0.000 | 6.4 |
| 5.7 | 0.537 | 0.142 | 0.321 | 0.679 | 0.024 | 0.297 | 5.8 | |
| 6.1 | 0.141 | 0.365 | 0.494 | 0.256 | 0.089 | 0.655 | 4.8 | |
| 6.5 | 0.076 | 0.546 | 0.378 | 0.179 | 0.173 | 0.648 | 4.4 | |
| 6.8 | 0.048 | 0.706 | 0.246 | 0.149 | 0.295 | 0.556 | 3.8 | |
| 7.2 | 0.013 | 0.864 | 0.123 | 0.059 | 0.532 | 0.409 | 2.8 | |
| 7.5 | 0.000 | 0.903 | 0.097 | 0.000 | 0.632 | 0.368 | 2.3 | |
| τ1 = 6.4 ns, τ2 = 0.9 ns, τ3 = 4.7 ns, c | ||||||||
| DND-160d | 2.2 | 1.000 | 0.000 | 1.000 | 0.000 | 2.4 | ||
| 2.7 | 0.871 | 0.129 | 0.956 | 0.044 | 2.3 | |||
| 3.0 | 0.627 | 0.373 | 0.846 | 0.154 | 2.1 | |||
| 3.7 | 0.356 | 0.644 | 0.643 | 0.357 | 1.8 | |||
| 4.3 | 0.167 | 0.833 | 0.395 | 0.605 | 1.4 | |||
| 6.1 | 0.112 | 0.888 | 0.291 | 0.709 | 1.2 | |||
| τ1 = 2.4 ns, τ2 = 0.7 ns, = 2.3c | ||||||||
The average lifetime of LysoSensor DND-192 (λex = 365 nm, λem = 450 ± 20 nm) is 12 ns at either acidic (pH 4.3) or basic (pH 8.8) buffers.
The amplitudes and fractional intensities are normalized to unity.
The accuracies of phase angle and modulation for the instrumentation were 0.3° and 0.008, respectively.
The average lifetimes of LysoSensor DND-160 (λex = 350 nm, λem = 535 ± 25 nm) is 2.37 ns at either acidic (pH 3.0) or basic (pH 8.1) buffers.
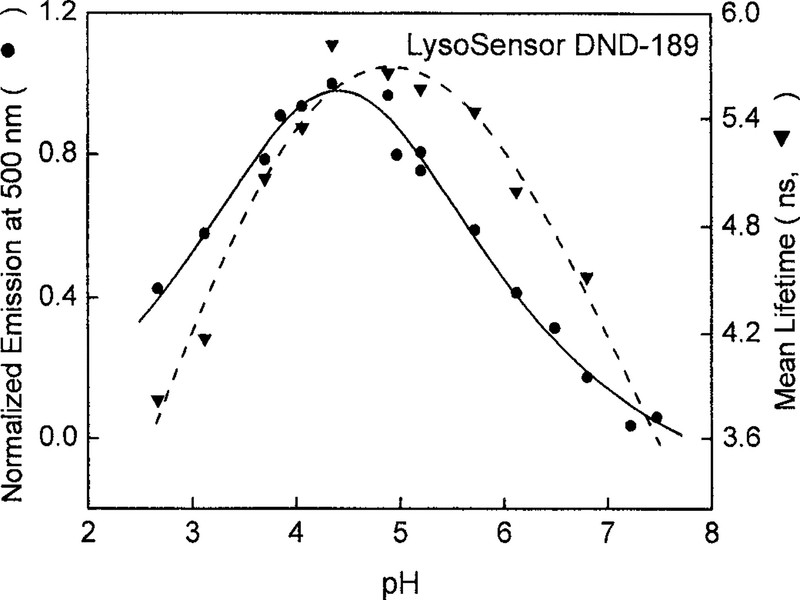
Though with similar structure to DND-153, DND-189 did not show monotonic lifetime changes upon pH but had the mean lifetime maximum occurring around pH 5 from its blue-green emission (Fig. 5). This unique observation is probably because of the three lifetime components derived from the global intensity decays analysis of DND-189 (Table 2). The two longer components, 5.6 and 4.1 ns, were dominant at pH values below 4.4, and the amplitude of lifetime component of4.1 ns diminished at pH 5 where contribution of the shortest component 1.85 ns started to become important. These results from the multiexponential decays analysis explained why the mean lifetime maximum occurs between pH 4 and 5. The steady-state fluorescence intensity of DND-189 at 500 nm showed the same pH dependency (Fig. 5). These observations implicated a multistep protonation on DND-189 and the existence of at least three emitting species in its aqueous solutions. Similar to DND-153, the pH-dependent lifetime changes obtained from the red emission of DND-189 were larger than those from its blue-green emission. Its mean lifetime reached 6.4 ns in acidic solution and then decreased at pH values above 5 with the shortest lifetime component (0.87 ns) dominating at near-neutral pH (Table 2).
FIG. 5.
The pH dependence of the steady-state fluorescence and mean lifetimes of DND-189. The excitation wavelength was 442 nm for both measurements, and the emission observation wavelengths were 500 and 470 ± 10 nm, respectively. Both profiles showed their maxima between pH 4 and 5.
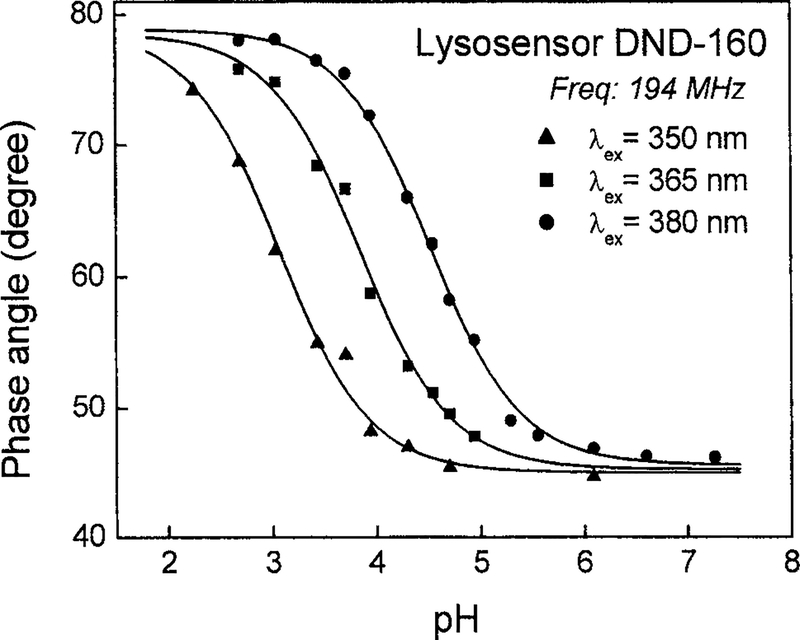
DND-160 is a good ratiometric probe for dual-excitation and dual-emission measurements because of its pH-dependent spectral shifts. Using single excitation and emission wavelengths, our lifetime measurements revealed substantial pH-dependent lifetime changes of DND-160 and its other advantages as a low-pH probe (Fig. 6). When we excited DND-160 at three different wavelengths, 350, 365, and 380 nm, and collected its blue emission (450 ± 20 nm), we determined two fixed lifetime components of 2.4 and 0.7 ns from the global intensity decay analysis. These two lifetime components came from the protonated and basic form ofDND-160 and were independent of the excitation wavelength (data not shown). However, the amplitudes of the two lifetime components could be adjusted by the excitation wavelengths, resulting in the shift of the apparent pKa values. The apparent pKa values were determined from the pH-dependent phase angle responsive curves fitted to the Henderson–Hasselbalch equation (Fig. 6). The overall phase angle changes were close to 30°. On the other hand, the mean lifetime obtained from the green emission region (535 ± 20 nm) of DND-160 remained at 2.4 ns and was not affected by the pH. We suggested that it is because of the dominant green emission from its protonated forms. The expandable pH working range and the significant lifetime changes of DND-160 make it a promising low-pH lifetime probes.
FIG. 6.
Excitation wavelength dependence of the apparent pKa of LysoSensor yellow/blue DND-160. When excitation wavelength was adjusted from 380 and 365 to 350 nm, the apparent pKa dropped from4.5 and 3.8 to 3.1. The modulation frequency was fixed at 194.3 MHz.
Although most LysoSensor probes showed pH-dependent lifetime changes, not all of them had appro priate lifetime sensitivity at low pH range. Except for DND-160, the apparent pKa values determined from the phase angle changes of the other LysoSensor probes were about 1 to 1.5 units higher than their true pKa values (Table 1). This is because of the lower quantum yields and relatively smaller fractional intensity contribution of their basic forms. Since contributions of different lifetime components to the mean lifetime are intensity weighted, τ = ∑fiτi, the bright protonated form dominates the lifetime responses until the contribution from the basic form increases at higher pH. The high apparent pKa values obtained from LysoSensor blue and green probes made their best pH working ranges much higher than the lysosomal pH. The basic form of DND-160 brightly fluoresced in the blue region of the spectrum and counterbalanced the contribution from its protonated form, so its apparent pKa values were comparable to its true pKa values and lysosomal pH (Table 1). As a result, we believe that DND-160 is the only perspective candidate among the LysoSensor probes for lifetime imaging in acidic vesicles.
DISCUSSION
Precise determination of the endosomal/lysosomal pH is a critical issue for the effective chemotherapy on cancer cells. Since LysoSensor probes have several advantages of high quantum yields, good photostability, and cell permeability, they have been widely utilized to vitally stain the acidic compartments of cells. Some of their applications were visualizing lysosomes only (13,14), and some were predicting lysosomal/endosomal pH qualitatively based on their fluorescence intensity (2, 3,15). Fluorescence intensities of most of the LysoSensor probes are related to the extent of quenching from their basic side chains. We characterized the pH-dependent lifetime of these LysoSensor probes since the frequency responses were less sensitive to experimental artifacts than intensity-based measurements. These artifacts include variations in loading concentrations, unstable or nonuniform illumination, and photobleaching.
What characteristics are important for an appropriate lifetime-based pH indicator? First, both the protonated and the unprotonated forms of the pH probes should fluoresce at the observation wavelengths and have distinguishable lifetimes. If one of the forms is nonfluorescent or severely quenched, pH-induced lifetime changes will not be detectable. This is probably the reason why DND-197 does not show any lifetime changes but DND-167 shows significant lifetime changes, although they share the same anthracene structure. In the case of DND-153 and DND-160, the mean lifetimes derived from the green part of their emission spectra barely showed pH dependency. However, mean lifetimes associated with the red emission of DND-153 and with the blue emission of DND-160 had substantial pH sensitivity. These results indicate that careful selection of the observation wavelengths could balance the relative emission contributions of the two forms and provide detectable lifetime changes. The wavelength selection is also related to the second requirement for good pH sensors; the determined pKa should reside within the desired pH working range. Since the overall phase angle and modulation responses to pH changes are weighted by the fractional intensities from individual emitting species of the probes, we can manipulate those fractional intensities by proper adjustments of the excitation and emission wavelengths. Such adjustments would shift the titration curves of the indicators to the desired pH working range. Unfortunately, because the strong emission of the protonated forms of DND-167 and DND-189 completely dominated the mean lifetime, we could not shift the apparent pKa values close to the lysosomal pH by manipulating the excitation and emission wavelengths. Their applications might be restricted only for presenting the very acidic vesicles in cells. Finally, dual-excitation and/or dual-emission probes are usually good candidates for lifetime-based sensing. Previous results (16, 23) and our characterizations of DND-160 support this conclusion.
In conclusion, we characterized a series of novel acidotropic pH probes, LysoSensors, and provided some criteria for choosing appropriate lifetime-based pH probes because of the requirements of precise determination of the acidic-vesicular-pH and the low-pH gradients developed in the deep microbial biofilms (24). Besides, only a limited number of fluorescence pH indicators are available for the low-pH imaging in biological systems, and most of them are fluorescence excitation ratio probes. We found that DND-160 is the only LysoSensor probe that shows appropriate lifetime sensitivity and good working range at low pH values. With the advanced fluorescence lifetime imaging technology supported with the confocal laser scanning and two-photon excitation capability, we believe that LysoSensor DND-160 is a promising lifetime-based pH indicator to explore the pH gradients in endosomes/lysosomes and in the microbial biofilms.
Footnotes
Abbreviations used: DND-167, LysoSensor blue DND-167, 9,10-bis(N-morpholinomethyl)anthracene; DND-192, LysoSensor blue DND-192, 9,10-bis(N,N-dimethylaminomethyl)anthracene; DND-153, LysoSensor green DND-153, 3-(and-4)-(2-dimethylaminoethylamino)-7H-benzimidazole-(2,1a)-benz-(d,e)-isoquionlin-7-one; DND-189,LysoSensorgreenDND-189,3-(and-4)-(2-morpholinoethylamino)-7H-benz-imidazole-(2,1a)-benz-(d,e)-isoquionlin-7-one; DND-160, LysoSensor yellow/blue DND-160, 2-(4-pyridyl)-5-(4-(2-dimethylaminoethylaminocarbamoyl)methoxy)phenoxazole; MSB, p-bis(o-methylstyryl)benzene; DCS, 4-(dimethylamino)-4′-cyanostibene.
REFERENCES
- 1.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, and Tashiro Y (1991) J. Biol. Chem 266, 17707–17712. [PubMed] [Google Scholar]
- 2.Henkel JR, Popovich JL, Gibson GA, Watkins SC, and Weisz OA (1999) J. Biol. Chem 274, 9854–9860. [DOI] [PubMed] [Google Scholar]
- 3.Gu F, Aniento F, Parton RG, and Gruenberg J (1997)J. Cell Biol 139, 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altan N, Chen Y, Schindler M, and Simon SM (1998) J. Exp. Med 187, 1583–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crivellato E, Candussio L, Rosati AM, Decorti G, Klug-mann FB, and Mallardi F (1999) Histochem. J 31, 635–643. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz SJ, Terashima M, Mizunuma N, and Slapak CA (1997) Blood 89, 3745–3754. [PubMed] [Google Scholar]
- 7.Simon S, Roy D, and Schindler M (1994) Proc. Natl. Acad. Sci. USA 91, 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altan N, Chen Y, Schindler M, and Simon SM (1999) Proc. Natl. Acad. Sci. USA 96, 4432–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman MM, Wei LY, and Roepe PD (1996) J. Gen. Physiol 108, 295–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindler M, Grabski S, Hoff E, and Simon SM (1996) Biochemistry 35, 2811–2817. [DOI] [PubMed] [Google Scholar]
- 11.Thiebaut F, Currier SJ, Whitaker J, Haugland RP, Gottesman MM, Pastan I, and Willingham MC (1990)J. Histochem. Cytochem 38, 685–690. [DOI] [PubMed] [Google Scholar]
- 12.Millot C, Millot J-M, Morjani H, Desplaces A, and Manfait M (1997) J. Histochem. Cytochem 45, 1255–1264. [DOI] [PubMed] [Google Scholar]
- 13.King SM, Barbareses E, Dillman JFI, Benashski SE, Do KT, Patel-King RS, and Pfister KK (1998) Biochemistry 37, 15033–15041. [DOI] [PubMed] [Google Scholar]
- 14.Siemasko K, Eisfelder BJ, Williamson E, Kabak S, and Clark MR (1998) J. Immunol 160, 5203–5208. [PubMed] [Google Scholar]
- 15.Cousin MA, and Nicholls DG (1997) J. Neurochem 69, 1927–1935. [DOI] [PubMed] [Google Scholar]
- 16.Szmacinski H, and Lakowicz JR (1993) Anal. Chem 65, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demas JN, and Crosby GA (1971) J. Phys. Chem 75, 991–1024. [Google Scholar]
- 18.Lakowicz JR, and Gryczynski I (1993) Biophys. Chem 47, 1–7. [DOI] [PubMed] [Google Scholar]
- 19.Gryczynski I, Kusba J, and Lakowicz JR (1994) J. Phys. Chem 98, 8886–8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakowicz JR, and Maliwal BP (1985) Biophys. Chem 21, 61–78. [DOI] [PubMed] [Google Scholar]
- 21.Lakowicz JR (1991) in Topics in Fluorescence Spectroscopy, Vol. 1, Techniques; (Lakowicz JR, Ed.), pp. 293–355l, Plenum, New York. [Google Scholar]
- 22.Diwu Z, Chen CS, Zhang C, Klaubert DH, and Haugland RP (1999) Chem. Biol 6, 411–418. [DOI] [PubMed] [Google Scholar]
- 23.Lin H-J, Szmacinski H, and Lakowicz JR (1999) Anal. Biochem 269, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vroom JM, Grauw K. J. d., Gerritsen HC, Bradshaw DJ, Marsh PD, Watson GK, Birmingham JJ, and Allison C (1999) Appl. Environ. Microbiol 65, 3502–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]