Abstract
Mucous membrane plasmacytosis (MMP) is an uncommon variant of mucositis represented by a polyclonal plasma cell infiltration of mucosal tissue. Various clinical presentations in the upper airway have been reported ranging from erythematous mucosa to fungating masses. Histologic features include mucosal epithelial hyperplasia or psoriasiform changes with a dense submucosal infiltrate of polytypic plasma cells. Molecular studies for immunoglobulin gene rearrangement should be performed in all cases of MMP to rule out clonal neoplastic expansion of plasma cells. We present a case of MMP with over 15 years of clinical follow-up, emphasizing the relatively benign clinical course of this disorder.
Keywords: Mucous membrane plasmacytosis, Long-term follow-up
1. Introduction
Mucous membrane plasmacytosis (MMP) is a rare variant of mucositis represented by a polyclonal plasma cell infiltration of the tissue. The diagnosis can be challenging considering significant histological similarities with neoplastic lesions. About 50 cases have been reported in the English literature including less than 10 published cases with long (more than 5 years) follow-up [1], [2], [3], [4], [5], [6]. The first evidence of unusual plasma cell infiltrate was described in glans penis by Zoon in 1952 which he termed a “chronic benign balanoposthitis with plasmacytes” [7]. Clinicopathologically similar process was later identified in orofacial mucosa by Schuermann [8] and Luders [9], and was originally named plasmacytosis circumficialis. Since then, multiple terms were introduced including plasmacytosis mucosae, plasma cell orificial mucositis, plasma cell mucositis, and MMP, however no consensus in terminology still exists. Exceedingly rare cases of cutaneous and systemic variants of plasmacytosis have been reported in patients of Asian descent but appear to be unrelated to MMP [10].
The etiology of MMP is not clear, but associations with some exogenous factors or immunologically mediated diseases were suggested [2]. Recognition of the entity is important as no progression to plasma cell or other hematological neoplasm was noted. Only one reported case showed concomitant squamous cell carcinoma with unclear etiopathogenetic association [11]. The median age of the patients at presentation is 60 years old and ranging from 17 to 84 years old. Various clinical presentations have been reported ranging from erythematous surface of mucosa to fungating mass. The major histological features include epithelial hyperplasia or psoriasiform changes with a dense infiltrate of polytypic plasma cells. The plasma cells can present with occasional Russel bodies but show no atypia. Molecular studies for immunoglobulin (Ig) gene rearrangement should be performed in all cases of MMP to rule out clonal expansion of plasma cells. Here we present a rare case of MMP with over 15 years follow-up.
2. Case report
We report a case of a 68-year-old female with a history of chronic obstructive pulmonary disease and smoking, who presented with a long history of dysphagia due to a persistent laryngeal lesion, first identified in 2002. A biopsy of the laryngeal lesion in 2002 showed a submucosal plasmacytosis. Definitive evidence of clonality was not seen by immunohistochemical (IHC) or molecular testing. The patient had normal immunoglobulin levels and serum protein immunofixation was negative for monoclonal proteins. Complete blood count and chemistry tests were within normal limits. Serum protein studies showed free kappa and lambda light a chain within normal limits, no monotypic immunoglobulin band on immunofixation electrophoresis was seen. No additional masses were identified. The patient had localized cervical lymphadenopathy in 2002 that was biopsied to reveal a benign reactive lymph node. The patient was treated with low dose prednisone which relieved the dysphagia in 2002. The patient experienced a waxing and waning course over the next 15 years that was fairly responsive to steroid therapy. She recently re-presented in 2017 with mild enlargement of a papillomatous laryngeal lesion, no monotypic immunoglobulin band on immunofixation electrophoresis was seen, and a repeat biopsy of the lesion was performed.
The histological findings were similar between the 2002 and 2017 biopsy. The sections from the laryngeal biopsies showed squamous mucosa with nodular psoriasiform epithelial hyperplasia (Fig. 1). No dysplasia was seen. There was neutrophilic inflammation of the epithelium with occasional microabscesses. A prominent, dense, submucosal infiltrate composed primarily of plasma cells was present. Plasma cells appeared mature with round nuclei and without prominent nucleoli. IHC studies showed that the plasma cells were positive for CD138 (variable expression) and CD38; and negative for CD56, CD20, cyclin D1 and HHV8 (Fig. 2). Kappa and lambda IHC and in-situ hybridization (ISH) studies showed elevated kappa:lambda ratio, but definitive evidence of monotypic light chain expression was not seen. EBER ISH was negative. Grocott's methenamine silver (GMS) and spirochete stains were negative for microorganisms. Molecular PCR studies performed in 2002 were negative for clonal immunoglobulin heavy chain (IGH) gene rearrangements. In the biopsy from 2017, PCR studies showed an equivocal gene rearrangement of the IGH gene in a polyclonal background, and no clonal rearrangements of IGK, IGL, and IGK deleting element.
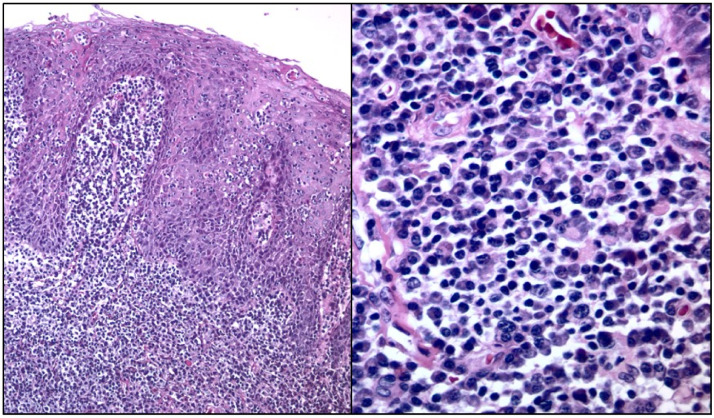
Fig. 1.
Hematoxylin-eosin stain demonstrating hyperplastic epithelium with plasma cells rich underlying lamina propria. A. (× 100 magnification). B. Prominent, dense, subepithelial infiltrate composed primarily of mature plasma cells without atypia and rare Russel bodies (× 400 magnification).
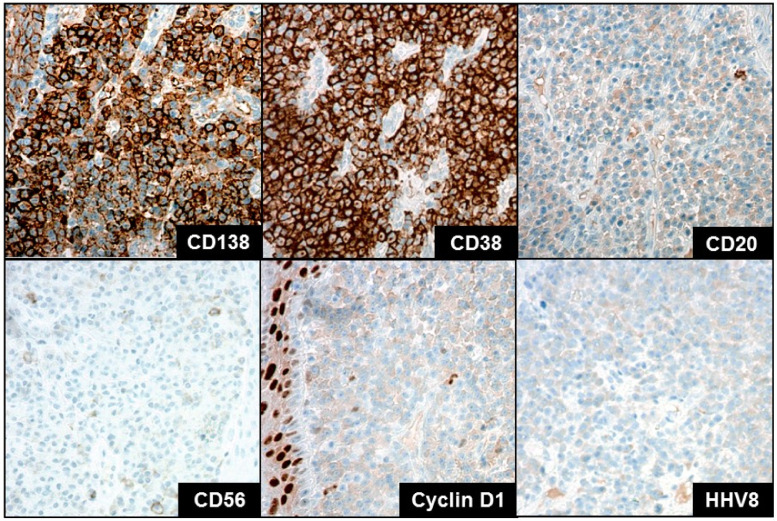
Fig. 2.
Selected immunohistochemical stains demonstrated in tumor cells. Positive staining for CD138 and CD38, and negative staining for CD20, CD56, Cyclin D1 and HHV8. (× 200 magnification).
3. Discussion
Few reports are available regarding long-term histopathological and clinical follow-up of mucous membrane plasmacytosis (MMP). In Table 1, we summarized all such cases published in English literature. The median age at presentation was 46 years old and ranging from 27 to 69 years old. We found that this age range is significantly less compared to short-term follow-up cases of MMP. It can be explained by quicker loss of follow-up in older population due to comorbidities. No deference was found in gender predilection in long-term vs short-term follow-up cases. The clinical course of MMP is typically waxing and waning with periods of long remissions. Progression to loss of organ functions in treatment-resistant cases is not uncommon. Histological representation of cases with long-term follow-up shows a few distinctive features that are discussed in greater details further. There is no consensus on the treatment for MMP; it includes steroids as first-line therapy and various topical or systemic immunosuppressive agents, i.e. tacrolimus, cyclosporin, methotrexate, chlorambucil, and dapsone [2,12,13]. Low-dose radiotherapy was also reported [1].
Table 1.
Summary of reported cases of mucous membrane plasmacytosis.
| Case | Age (years), gender | Location | Presumable etiological factor | Histologic findings | Ancillary studies | Follow up |
|---|---|---|---|---|---|---|
| White et al., 1986 | 47, F | lips, mouth, tongue, supraglottic larynx | Smoker for 32 years | Epidermal acanthosis with intercellular edema, mild dyskeratosis and exocytosis; dense bandlike infiltrate of plasma cells in the upper dermis | Stained polytypic for κ and λ light chains as well as ß and γ heavy chains | Persistent disease, 9 yrs |
| Timms MS et al., 1991 | 32, F | supraglottis, gingiva, mucobuccal | Not reported | Marked pseudoepitheliomatous hyperplasia with dense, predominantly plasmacytic infiltration of the lamina propria; the plasma cells are mature and lacked pleomorphism | Stained polytypic for κ and λ light chains and various heavy chains | Persistent disease, 7 y |
| Grattan et al., 1992 | 68, M | papillary hyperplasia extending from the hard palate to the uvula | Long-term dentures | Hyperplastic epithelium with a diffuse infiltrate of neutrophils in the deeper layers forming spongiform micro-abscesses in the subcorneal region; dense inflammatory infiltrate in the lamina propria consisting almost entirely of mature plasma cells. | Stained polytypic for IgG; PAS and GMS stains were negative for fungi. | Persistent disease, 15 y |
| Ferreiro et al., 1994 | 41, F | Supraglottic, glottic larynx, nose, pharynx | Not reported | Psoriasiform changes with parakeratosis, elongation of rete ridges, suprapapillalry epithelial thinning. Marked dyskeratosis. Dispersed exocytosis and aggregation of PMNs with the epidermis. Munro-microabscess like lesions in 2 cases. Diffuse expanse of inflammatory cells in submucosal tissue composed largely of mature plasma cells with scattered PMNs and lymphocytes. Foamy macrophages are not present. The plasma cells did not show anaplasia, prominent nucleoli or Dutcher body. Occasional Russel bodies are present. No associated endothelial hyperplasia. | Stained polytypic for κ and λ light chains. Only one case was studied and found to be negative for gene rearrangement in Ig-encoding region. Warthin-Starry, GMS, Gramm stains were negative for microorganisms. | Persistent disease, 7y, tracheostomy |
| 62, F | Supraglottic, glottic larynx, trachea | Not reported | Persistent disease, 16 y, tracheostomy | |||
| 45, F | Supraglottic larynx, lips, mouth, tongue | Not reported | Persistent disease, 9 y | |||
| 40, M | Supraglottic, glottic larynx, lips, mouth, tongue, palate, pharynx | Not reported | Persistent disease, 15 years, apnea | |||
| Fogarty et al. 2001 | 27, F | Gingiva progressed to supraglottic and glottic larynx | Chewing gum | Pseudo-epitheliomatous hyperplasia of the surface epithelium, with a densely cellular infiltrate, almost entirely plasmacytic. | The IHC revealed small amounts of IgA, abundant IgG. Stained polytypic for κ and λ light chains. | Persistent disease, 21 y |
| Galvin et al., 2016 | 68 F | Gingival | Long-term dentures | Partly ulcerated squamous epithelium with an underlying dense polyclonal plasma cell infiltrate. No interface mucositis was seen. | Stained polytypic for κ and λ light chains. | Remission for 7 y |
| 69, M | Oropharynx | Not reported | Polyclonal plasmacytic inflammatory infiltrate throughout the connective tissue. | Persistent disease, 10 y, recent flare | ||
| Our case | 53, F | Supraglottic, glottic larynx | Smoker for over 20 years | Stained polytypic for κ and λ light chains; negative for gene rearrangement in Ig-encoding region. Warthin-Starry, GMS, Gramm stains were negative for microorganisms. | Persistent disease, 15 y |
The etiological factors of MMP are still not clear, but role of exogenous factors was suggested based on possible association with smoking and long-term use of dentures [5]. On the other hand, a number of MMP patient with autoimmune or immune-mediated disease were observed suggesting a possible implication by the systemic processes [2,14]. Gross presentation of MMP shows significant variation and includes papillomatous, cobblestone, nodular, or granulomatous mucosa and sometimes hyperplastic proliferation of mucosa resulting in fungating ulcerated mass. The later can grossly mimic squamous cell carcinoma (SSC). Considering commonly found florid pseudo-epitheliomatous hyperplasia in overlying epithelium of MMP, serious caution should be taken when evaluating such cases. Only one case of MMP with associated invasive SSC was reported in an ex-smoker patient with a history of chemotherapy [11]. Other clinical differential diagnosis commonly includes lichen planus, lymphoproliferative disorders, infection, sarcoidosis, and granulomatosis with polyangiitis. Close histopathological examination is important to rule out most of these conditions. Rhinoscleroma can be considered in differential diagnosis since it can present with hyperplastic mucosa with inflammatory infiltrate with abundant plasma cells. However, vasculitis is commonly seen in rhinoscleroma, and foamy macrophages with gram negative bacteria, confirmed by special stains are diagnostic. Plasma cell gingivitis shows similar histological features, but it is a localized hypersensitivity reaction to offending agents, which typically resolves after their removal, thus confirming the diagnosis clinically [15].
Most diagnostic challenges of MMP arise from its histologic similarities with plasma cell neoplasm and lymphomas that can also manifest as dense infiltrate of plasmocytoid cells in the lamina propria. Thus, Ig clonality studies are warranted for appropriate diagnosis. Virtually all cases with long-standing history of MMP and treatment resistance develop significant changes in overlying epithelium including pseudo-epitheliomatous and/or psoriasiform changes. Interestingly, dyskeratosis in the absence of other Bowenoid features have been frequently reported. Secondary reactive changes in epithelium could be seen and include mixed inflammatory infiltrate, polymorphonuclear leukocyte exocytosis and even intraepithelial microabscess formation. The plasma cells in MMP usually do not show atypical features such as prominent nucleoli or Dutcher bodies, but Russel bodies are not uncommon. Plasma cells stain positive for CD138 and CD38 without expression of aberrant markers such as CD56. It is reasonable to assess tissues for HHV8 since mixed inflammatory infiltrate enriched by polytypic plasma cells can be seen in Kaposi sarcoma. As noted before, it is important to assess polyclonal nature of plasma cell infiltrate by surface Ig immunochemical stains or in-situ hybridization. The most reliable study to evaluate clonality is Ig gene rearrangements by PCR. Only very few reported cases of MMP including ours were assessed by clonality studies. Interpretation of plasma cell clonality by Ig gene rearrangement can be challenging considering presence of non-specific pick(-s) in reactive polyclonal background especially in long-standing MMP. These cases should be reassessed by comparing sub-clones from previous biopsies.
To conclude, nearly all reported cases of CP have required multiple biopsies and numerous additional studies before a definitive diagnosis could be achieved. Clinicopathological correlation is crucial in confirming the diagnosis.
References
- 1.Fogarty G., Turner H., Corry J. Plasma cell infiltration of the upper aerodigestive tract treated with radiation therapy. J. Laryngol. Otol. 2001;115(11):928–930. doi: 10.1258/0022215011909396. [DOI] [PubMed] [Google Scholar]
- 2.Galvin S. Circumorificial plasmacytosis/plasma cell orificial mucositis: a case series and a review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016;122(3):e77–e81. doi: 10.1016/j.oooo.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 3.White J.W., Jr., Olsen K.D., Banks P.M. Plasma cell orificial mucositis. Report of a case and review of the literature. Arch Dermatol. 1986;122(11):1321–1324. doi: 10.1001/archderm.122.11.1321. [DOI] [PubMed] [Google Scholar]
- 4.Timms M.S., Sloan P. Association of supraglottic and gingival idiopathic plasmacytosis. Oral Surg. Oral Med. Oral Pathol. 1991;71(4):451–453. doi: 10.1016/0030-4220(91)90428-f. [DOI] [PubMed] [Google Scholar]
- 5.Grattan C.E., Gentle T.A., Basu M.K. Oral papillary plasmacytosis resembling candidosis without demonstrable fungus in lesional tissue. Clin. Exp. Dermatol. 1992;17(2):112–116. doi: 10.1111/j.1365-2230.1992.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferreiro J.A. Mucous membrane plasmacytosis of the upper aerodigestive tract. A clinicopathologic study. Am. J. Surg. Pathol. 1994;18(10):1048–1053. [PubMed] [Google Scholar]
- [7.Zoon J.J. [Chronic benign circumscript plasmocytic balanoposthitis] Dermatologica. 1952;105(1):1–7. [PubMed] [Google Scholar]
- 8.Schuermann H. Plasmacytosis circumorificialis. Dtsch. Zahnarztl. 1960;(15):601–610. [Google Scholar]
- [9.Luders G. Plasmocytosis mucosae: ein oft verkanntes neues Krankheitsbild. Munch Ed Wochenschr. 1972;(114):8–12. [PubMed] [Google Scholar]
- 10.Georgesen C., Kheterpal M., Pulitzer M. A rare case of cutaneous plasmacytosis in a Korean male. Case Rep. Pathol. 2017;2017 doi: 10.1155/2017/3032941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper T. Squamous cell carcinoma arising in mucosal plasmacytosis. Br. J. Oral Maxillofac. Surg. 2010;48(3):208–210. doi: 10.1016/j.bjoms.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Khan N.A., McKerrow W.S., Palmer T.J. Mucous membrane plasmacytosis of the upper aerodigestive tract. A case report with effective treatment. J. Laryngol. Otol. 1997;111(3):293–295. doi: 10.1017/s0022215100137132. [DOI] [PubMed] [Google Scholar]
- 13.Bharti R., Smith D.R. Mucous membrane plasmacytosis: a case report and review of the literature. Dermatol. Online J. 2003;9(5):15. [PubMed] [Google Scholar]
- 14.Solomon L.W. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;106(6):853–860. doi: 10.1016/j.tripleo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Silverman S., Jr., Lozada F. An epilogue to plasma-cell gingivostomatitis (allergic gingivostamtitis) Oral Surg. Oral Med. Oral Pathol. 1977;43(2):211–217. doi: 10.1016/0030-4220(77)90158-x. [DOI] [PubMed] [Google Scholar]