Highlights
-
•
Unilateral sound damage was used, survival time 25-30 days post damage
-
•
Significant hearing loss at 5/7 tested frequencies
-
•
Hearing loss related increases in DCX expression were observed in the cerebellum
-
•
Hearing loss related decreases in DCX expression were observed in the hippocampus
Keywords: Sensorineural Hearing Loss, Dorsal Cochlear Nucleus, Cerebellum, Hippocampus, Neuroplasticity, Tinnitus
Abstract
Sound damage induced hearing loss has been shown to elicit changes in auditory and non-auditory brain regions. A protein critical for neuronal migration and brain development, doublecortin (DCX), has been used as a marker of central nervous system (CNS) neuroplasticity. DCX is expressed in unipolar brush cells (UBCs) of the dorsal cochlear nucleus (DCN), cerebellar parafloccular lobe (PFL) and neuronal precursor cells in the sub-granular zone of the hippocampal dentate gyrus (DG). Sound damage induced hearing loss has been shown to differentially impact DCX expression months later. To identify earlier alterations in DCX expression, we utilized immunohistochemistry to detect DCX protein in three brain regions (DCN, PFL, DG) approximately one month following unilateral sound damage. Auditory brainstem response was used to measure hearing loss. Unilateral hearing loss was evident in all sound damaged animals. Hearing loss related decreases in DCX expression were evident bilaterally in the DG while hearing loss related increases in DCX expression were evident bilaterally in the PFL. No changes to DCX expression were evident in the auditory DCN. Gap detection was used to assess whether this sound damage paradigm induced tinnitus-like behavior. However, results obtained from this behavioral test as used here were inconclusive and are presented here only as a guide to others wishing to design similar studies.
INTRODUCTION
Hearing Loss and Tinnitus
Exposure to high intensity sound can induce not only cochlear hair cell damage and elevated thresholds (Kujawa and Charles Liberman, 2019) but may also result in other types of perceptual dysfunction. Tinnitus, the perception of sound in the absence of external auditory stimuli, affects approximately 1 in 10 adults in the United States, and a subpopulation of tinnitus sufferers are debilitated (Bhatt, Lin et al. 2016). The etiology of tinnitus is heterogeneous, with the most common cause being recreational, occupational, and firearm noise exposure capable of inducing damage (Agrawal, Platz et al. 2009, Shore and Wu 2019). Pathologic neural activity underlying sound damage induced hearing loss and tinnitus may result from plastic changes that are compensatory in nature. Animal studies have shown that noise and drug induced hearing loss and tinnitus are often accompanied by changes in spontaneous neuronal activity and protein expression in various auditory brain regions (Dong, Mulders et al. 2010, Baizer et al., 2012, Mazurek, Haupt et al. 2012, Brozoski, Wisner et al. 2013, Kennon-McGill 2014). Common sites of these central changes are the dorsal cochlear nucleus (Kaltenbach and Afman 2000, Kaltenbach, Rachel et al. 2002, Kaltenbach, Zhang et al. 2005), inferior colliculus (Bauer, Turner et al. 2008, Dong, Mulders et al. 2010), and primary auditory cortex (Seki and Eggermont 2003, Norena and Eggermont 2005). Single and multi-unit electrophysiological recordings used to measure spontaneous neural hyperactivity have yielded contradictory results that were dependent on brain region and presence or absence of anesthesia during recording sessions (Ma and Young 2006, Kennon-McGill 2014). Neurochemical studies have shown tinnitus may result from both down regulation of inhibitory glycinergic and GABAergic neurotransmission (Caspary, Pazara et al. 1987, Brozoski, Bauer et al. 2002, Caspary and Llano 2017) and upregulation of excitatory glutamatergic transmission (Bauer et al., 2013b, Brozoski, Wisner et al. 2013).
Hearing loss impacts non-auditory brain regions
Understanding CNS changes associated with hearing loss and tinnitus is complicated by the involvement of non-auditory brain regions including, but not limited to, the cerebellum and hippocampus (Brozoski, Ciobanu et al. 2007, De Ridder, Elgoyhen et al. 2011, Kraus and Canlon 2012, Bauer et al. 2013a, b). The cerebellar flocculus and parafloccular lobe, areas known to be involved in gaze-related motor control (vestibulo-ocular reflex, VOR) have been shown to be affected in tinnitus models of animals, though the exact role of the PFL in tinnitus is unknown. The cerebellum has been implicated in both generation and modulation of tinnitus (Brozoski, Ciobanu et al. 2007, Bauer et al., 2013a, Mennink, Van Dijk et al. 2018). The PFL has been shown to receive auditory input from the cochlea in chinchilla, cat, and monkey (Rasmussen 1990). The cerebellum also functions as an integrator of somatosensory information from multiple sites (Sawtell 2010, Voogd & Glickstein 1998). Moreover, PFL ablation eliminates behavioral evidence of tinnitus in rats with noise induced hearing loss (Bauer et al. 2013a) and the application of NMDA antagonists in the PFL has also been shown to modulate tinnitus behavior (Bauer et al., 2013b).
Brain regions with roles in memory and emotion, such as the hippocampus, have been shown to be impacted in both human and animal studies of tinnitus (Lockwood, Salvi et al. 1998, Kraus, Mitra et al. 2010, Kraus and Canlon 2012, Seydell-Greenwald, Raven et al. 2014, Gunbey et al., 2017). Neuroimaging studies in the clinical tinnitus population have found consistent pathophysiological changes in limbic brain regions, including the amygdala, hippocampus, and anterior cingulate cortex (Landgrebe, Langguth et al. 2009, De Ridder, Vanneste et al. 2013). More importantly, structural and functional changes in the auditory and limbic system are strongly correlated in tinnitus patients (Leaver, Renier et al. 2011), indicating the importance of further investigation into the role of auditory-limbic interactions in tinnitus. The hippocampus responds to auditory stimuli and likely plays a role in the formation and retrieval of auditory memories (Munoz-Lopez, Mohedano-Moriano et al. 2010). Noise induced hearing loss impairs spatial memory and hippocampal neurogenesis in mice (Liu, Shen et al. 2016). Further, decreased hippocampal neurogenesis is evident in sound damaged animals regardless of tinnitus presence (Kraus, Mitra et al. 2010). While changes to non-auditory brain regions have been observed in tinnitus patients and noise-induced hearing loss models of tinnitus, their contribution to the disorder is not well understood.
Doublecortin as a marker of neuroplasticity
Doublecortin (DCX) is a microtubule associated protein expressed exclusively in neuronal tissue. DCX has been used as a marker of migrating and immature neurons (Gleeson, Lin et al. 1999, Manohar, Paolone et al. 2012). Historically, DCX expression has been correlated with neurogenesis, as it is expressed in immature post-mitotic neurons of the developing brain. In the adult CNS, DCX labeling has been observed in the subventricular zone and the dentate gyrus of the hippocampus, a region known to exhibit neurogenesis beyond the developmental period (Francis, Koulakoff et al. 1999, Friocourt, Koulakoff et al. 2003, von Bohlen und Halbach 2011). Recently, doublecortin (DCX) protein expression has been evaluated in animal models of sound damage induced tinnitus. Unipolar brush cells in the auditory dorsal cochlear nucleus (DCN) and cerebellar parafloccular lobe have been shown to express DCX (Manohar, Paolone et al. 2012). While these two regions have been targeted in tinnitus research, the specific involvement and interaction of these two regions, in response to peripheral auditory damage, is not clear. They share similar cellular circuitry (Oertel and Young 2004, Singla, Dempsey et al. 2017) and both receive auditory input from the cochlea (Rasmussen 1990, Kaltenbach, Zhang et al. 2005, Baizer et al., 2012) as well as somatosensory and auditory input via the auditory cortex and inferior colliculus. Unipolar brush cells are classified as excitatory local circuit neurons as they receive glutamatergic input from mossy fibers and form glutamatergic synapses with their target cells (neuronal precursor cells and other UBCs). UBCs reside in the granular layer of discrete regions in the cerebellar cortex (vermis and flocculonodular node [PFL]) and the granule cell domain of the DCN (Mugnaini, Sekerkova et al. 2011, Manohar, Paolone et al. 2012). Due to their distinct morphology and corresponding cellular properties, UBCs have been described as having a role in automatic gain circuitry in which they aid in modulating sensory afferents (Brozoski et al., 2017).
Progression from peripheral damage to CNS changes – the relationship between hearing loss and tinnitus
Although most individuals with subjective tinnitus have some degree of hearing loss, hearing loss does not guarantee tinnitus onset. Identifying differences between individuals with only sound damage-induced hearing loss and individuals with both hearing loss and tinnitus is a necessary step in the development of effective therapies. Additionally, past research has suggested a delay between peripheral damage and tinnitus onset, thereby highlighting the importance of establishing a timeline while documenting observed dynamics at relevant time points. At what time point does remodeling or neuroplasticity take place following a single auditory insult? DCX expression has been investigated 10 weeks post damage in the hippocampus (Kraus, Mitra et al. 2010) and 9-11 months post damage in the DCN and PFL (Bauer et al., 2013b, Brozoski et al., 2017), while changes at earlier time points have not been investigated using DCX expression as an index of change. Altered spontaneous firing rate (SFR) is evident in the auditory cortex just a few hours after acoustic trauma (Norena and Eggermont 2003) while the timeline of changes to SFR in the DCN is variable. Kaltenbach and Afman (2000) did not observe electrophysiological changes in the DCN until several days after noise exposure (Kaltenbach and Afman 2000) while Gao and colleagues (2016) observed changes in the DCN within minutes of exposure (Gao et al., 2016). Cochlear application of NMDA antagonists prior to sound damage prevents tinnitus development (Duan, Agerman et al. 2000). Guitton et al. (2007) observed that drug treatment targeted in the cochlea can prevent onset of noise induced tinnitus if intervention occurs within a brief time window, further suggesting long term tinnitus undergoes a consolidation period of several days (Guitton and Dudai 2007).
In the work presented here, we exposed young adult rats to high intensity sound, using a mild sound damage paradigm designed to induce hearing loss that may or may not result in tinnitus. To better understand specific neuroplastic changes underpinning hearing loss within the first month after sound damage, we utilized immunohistochemistry to detect doublecortin (DCX) protein in the dorsal cochlear nucleus, cerebellar paraflocculus and the dentate gyrus of the hippocampus of sound damaged animals. Animals were also evaluated for tinnitus-like behavior using gap detection, although these tests were not sensitive enough to provide conclusive evidence relating DCX labeling and tinnitus.
EXPERIMENTAL PROCEDURES
Subjects
Eighteen male Long-Evans rats (Charles River Laboratories, Wilmington, MA) that were 4-5 months old at the start of the experiment were used for immunohistochemical experiments (n = 12 sound damaged, n = 6 unexposed controls). Auditory brainstem response and gap detection data were collected for all sound-damaged animals at baseline and after sound damage. In a follow up experiment, gap detection data only was also collected in an additional group of control animals (n = 7). As will be described below, the results of gap detection tests were not robust enough for us to confidently assign animals to specific groups related to tinnitus-like behavior. Thus, we have drawn no conclusions about DCX labeling based on grouping animals based on these tests. However, we have included the behavioral data to assist others who might contemplate using the gap detection method in animals who also show hearing loss.
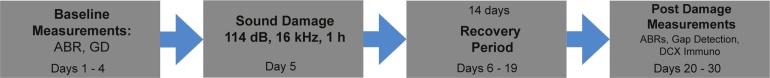
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center. All animals had ad libitum access to water and standard laboratory rodent chow. They were housed individually with environmental enrichment and maintained on a 12 -h light-dark cycle. A summary of our experimental timeline is shown in Fig. 1.
Fig. 1.
Experimental Timeline. Experimental timeline used to determine effect of sound damage on doublecortin labeling. ABR: auditory brainstem response, GD: gap detection, DCX: doublecortin. Figure includes the range of days for each type of testing. Sacrifice occurred 25-30 days after sound damage.
Sound Damage
Rats were placed in a sound attenuated booth (Industrial Acoustics Company, Bronx, NY) and anesthetized with Isoflurane (4% Isoflurane, 2 L/min induction, 2% isoflurane 1.5 L/min O2 maintenance) administered via the Matrx VP 3000 isoflurane vaporizer (Midmark, Kettering, OH). Respiration was monitored and body temperature was regulated by a feedback/automatic adjustment heating pad. A 16-kHz pure tone was continuously presented to the left ear at 114 dB for 1 hour from a loudspeaker (Radio Shack 40-1310-B) inside a plastic case. The loudspeaker was coupled to the left pinna via ½” flexible plastic tubing and sealed using Audalin ear mold compound (All American Mold Lab, Oklahoma City, OK). The intensity level of the stimulus measured outside the tubing was 45 dB less than the intensity of the stimulus within the tubing sealed to the head of the animal (Imig and Durham 2005), reducing the likelihood of any bilateral damage resulting from air conduction. A Macintosh computer with a MaLab synthesizer, event processor, and software (Kaiser Instruments, Irvine, CA) was used to control noise waveform synthesis.
Auditory Brainstem Response (ABR)
ABRs were recorded for each animal both at baseline and approximately 2-4 weeks post-damage using Intelligent Hearing Systems Smart EP hardware and software (IHS, Miami, Florida) in a sound attenuated booth (Industrial Acoustics Company, Bronx, NY). Rats were anesthetized with Isoflurane (2-2.5%) delivered via the Matrx VP 3000 isoflurane vaporizer (Midmark, Kettering, OH). Respiration was monitored, and body temperature was regulated by a feedback/automatic adjustment heating pad. A probe connected to a high frequency transducer was placed in the left ear and a series of tone bursts was presented at a range of frequencies (2, 4, 8, 11.3, 16, 22.6, and 32 kHz) and intensities. For each frequency, threshold was defined as the lowest intensity (dB SPL) for which a signal could be reliably observed in three or more repetitions. A high pass filter was used for the 22.6 and 32 kHz frequency sweeps to prevent artificially low thresholds at high frequencies. Stimulus presentation started at 70 dB and was decreased in 5-10 dB increments until no response was detected. Analysis of ABR thresholds gave us information about the degree of hearing loss induced by our mild sound damage paradigm.
Gap Detection
Animals were tested for evidence of tinnitus-like behavior using gap detection. This method exploits the startle reflex, a quick movement occurring in response to a loud sound. Gap detection refers to changes in the amplitude of the response when the startle stimulus is preceded by a continuous background tone. A gap in the background tone will reduce the amplitude of the startle response. Behavioral testing was conducted inside a sound attenuated booth with acoustic startle reflex software and hardware (Kinder Scientific, Poway, CA). Performance was tested using no gap (startle only) and gap trials. For the gap detection (GD) procedure each rat was presented with a constant, 60 dB SPL background sound consisting of 1 kHz bands centered at 12, 16, and 20 kHz. Background frequency presentation was intermixed such that 22 trials at each testing frequency (10 gap and 12 no gap trials) were presented in a random order for a total of 66 trials. A 120 dB SPL, 50 ms white noise burst was used to induce the acoustic startle reflex. During the background noise, the startle stimulus was presented alone (no gap trial) or 50 ms following a silent gap (100 ms) embedded in the background noise (gap trial). Animals were tested on 4 consecutive days at baseline and 4 consecutive days between 15 and 25 days post damage.
For each rat, all startle data (baseline and post damage) were combined into a single spreadsheet and sorted as a function of testing day, background frequency, and trial type. A single iteration of the Grubbs outlier detection test (Grubbs 1950, Longenecker, Chonko et al. 2014) was performed on each subset of data (e.g.12 kHz gap, 20 kHz no-gap, etc.), removing a maximum of 1 extreme outlier trial per subset. Outlier trials were excluded from all further analyses. We then compared the daily startle amplitudes in each individual animal during gap and no-gap trials at baseline. This comparison was repeated following sound damage.
We used this same method in a small group of control animals (n = 7) to determine if gap detection performance could change merely as a function of time between testing bouts. For this control group, gap detection performance was collected over four consecutive days at baseline and again after a post wait period of approximately 2-4 weeks.
Behavioral phenotype assignment
The gap detection test does not require animal training and the parameters for performing gap detection measurements are straightforward, making it a common behavioral test to assess tinnitus-like behavior. However, data analysis and interpretation is varied and gap detection assessment may include (1) statistically significant reduction of startle inhibition (Wang, Brozoski et al. 2009, Longenecker, Chonko et al. 2014); (2) fixed threshold cut-offs (Middleton, Kiritani et al. 2011); (3) inter-group differences (Fournier and Hebert 2013), and (4) development of custom tinnitus indices (Norman, Tomscha et al. 2012). We used baseline startle measures for individual animals to serve as a basis for post-exposure comparisons. In this experiment, we used paired t-tests in which each pair represented the mean gap vs. no-gap startle amplitude at one day to determine an individual animal’s gap detection performance during each trial type (background frequency) at baseline and following damage. Sound damaged animals were then sorted into three different groups based on how their gap detection performance changed post damage relative to baseline. Animals that displayed no change in performance following sound damage were assigned to an “unchanged” group (n = 3). This group consisted of animals showing a significant difference between startles at gap and no-gap at baseline as well as post damage, and, animals showing no significant differences between gap and no-gap startles, neither at baseline nor post-damage. Animals that were skilled at inhibiting their startle response during gap trials at baseline but lost this ability following sound damage were assigned to an “impaired” group. These were animals showing a significant difference between gap and no-gap startles at baseline, but no longer post-damage. Lastly, animals that were unable to detect the gap at baseline but displayed improved performance post damage were assigned to the “improved” group. This group was animals showing no significant difference between gap and no-gap startles at baseline, but a significant difference post damage.
Tissue Preparation
On the day of sacrifice (25-30 days post damage), rats were deeply anesthetized with an intraperitoneal injection of 5 mg/kg of Beuthenasia and perfused through the heart with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS). Brains were removed, divided in the coronal plane into a rostral and a caudal block just rostral to the cerebellum, and postfixed in 4% PFA at 4 °C, for 24 hours to 1 week. The brain blocks were then cryoprotected in 30% sucrose in PBS for approximately 24 hours or until the brain sank to the bottom of the container. Then the brain blocks were placed in plastic cases, covered with OCT and flash frozen in heptane cooled with dry ice. Frozen brains were stored at −80 °C until further processing.
The brains were then cut into 40 μm coronal sections on a cryostat and every section was collected. Sections were stored in 12 well culture plates in a cryoprotectant solution (30% ethylene glycol and 30% glycerol in PBS) at −20 °C until further processing.
Immunohistochemistry
All tissue processing was done on free-floating sections in 12 well plates. On the first day of processing, sections were removed from cryoprotectant and rinsed with PBS. Sections were placed in 3% hydrogen peroxide at room temperature (RT) for 5-10 min to quench endogenous peroxidase activity and then rinsed in PBS for 10 min. Non-specific binding of primary antibodies was blocked by incubating the sections in a solution of 10% normal horse serum (NHS, Vector Laboratories), containing 0.1% Triton X-100 in PBS for 1 hour at RT. Primary antibody (1:500, Doublecortin- Santa Cruz sc-8066) was diluted in 1% NHS, and 0.1% Triton X-100 in PBS. Sections in the 12 well plates incubated in primary antibody overnight on a rocker at 4 °C.
On day 2, sections were removed from the primary antibody solution and rinsed 3x for 10 min each in PBS. Biotintylated secondary antibody (1:300, donkey anti-goat, Vector Laboratories) was diluted in 1% NHS, and 0.1% Triton X-100 in PBS, added to sections and incubated for 1 hour at RT. Sections were then rinsed 3x for 10 min each with PBS. Sections were prepared for antibody visualization using the ABC elite kit according to manufacturer instructions (Vector Laboratories PK-6100). Following a 45-60 minute incubation at RT, sections were rinsed 2x for 5 min each in PBS. Immunoreactivity was visualized using the DAB peroxidase substrate kit with nickel enhancement (Vector Laboratories SK-4100) per manufacturer instructions. Incubation time varied from 5-10 min according to manufacturer instructions. Sections were then washed in PBS several times until sections were free of reaction precipitates.
Labeled sections were floated onto slides in PBS and slides were left to dry overnight. Sections then were dehydrated using increasing concentrations of ethanol (70%, 95%, 95%, 100%, 100%), cleared in xylene and cover-slipped with DPX (Millipore Sigma).
The DCX antibody used for IHC in the present study is a polyclonal antibody with a high affinity meaning that it can bind to more than one epitope of a target protein. At the time this study was done there were few monoclonal DCX antibodies available, thus we chose an antibody whose specificity had been verified in previously published reports. Preadsorption tests demonstrated a lack of non-specific labeling in both the hippocampus (Hinduja, Kraus et al. 2015) and the DCN and PFL (Manohar et al. 2012).
Imaging and Immunolabeling Quantification
Immunostained sections were examined with a Nikon 80i bright-field microscope, and digital images of specific regions of interest (ROIs) were captured using a Nikon DS-Fi1 High- Definition color camera head and NIS-Elements imaging software. Digital images were collected bilaterally and labeling was measured on 20x (200x total magnification) images stitched together to provide a complete rendition of each ROI. Assembly of images for all ROIs was accomplished using a combination of Adobe Illustrator and Adobe Photoshop.
Dorsal cochlear nucleus (DCN)
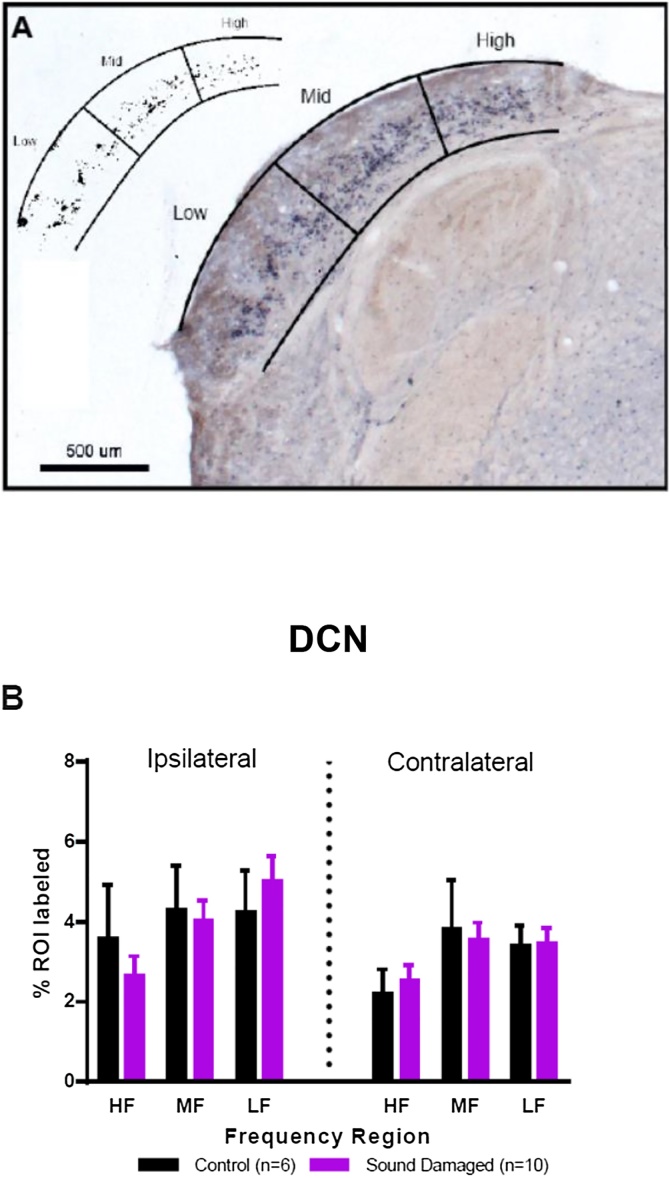
The entirety of the DCN was collected during sectioning and the midpoint region (50% along the rostral to caudal extent) of the DCN was used for immunohistochemical labeling. Within the DCN, our primary goal was to measure tonotopic frequency-specific changes. Therefore we chose a section that has been shown to represent all frequencies contained within the rat tonotopic map (Ryan, Furlow et al. 1988). As shown in Fig. 3B, the DCN was divided into frequency regions as defined by metabolic mapping (Ryan, Furlow et al. 1988), using Adobe Illustrator. First the dorsal and ventral boundaries of the DCN were drawn and then two additional lines were drawn to divide the DCN into three equal regions, which correspond to low, middle and high frequency region. Illustrator images with divisions then were opened in ImageJ, converted to 8-bit, and frequency region specific boundaries were drawn (Fig. 3B). Binary images were created using the Otsu local-area thresholding method (Otsu 1979) and percent area labeled was measured within the entire frequency region. Percent area was calculated in this ROI as distinct individual cells were unable to be objectively counted due to unipolar cell morphology and dense cell labeling. Measurements were made in DCN ipsilateral and contralateral to the damaged ear.
Fig. 3.
Frequency specific doublecortin (DCX) labeling in the dorsal cochlear nucleus (DCN) of control (n = 6) and sound damaged animals (n = 10). The DCN was divided tonotopically into high, mid, and low frequency regions (as shown in Fig. 3A). After thresholding using the Otsu method (upper left, panel A), % ROI labeled with DCX was quantified. Panel B compares ipsilateral and contralateral DCX labeling in control and noise exposed animals as a function of frequency region. HF: high frequency, MF: mid frequency, LF: low frequency, ROI: region of interest. There were no statistically significant differences between controls and any sound damaged group (Two-way ANOVA; F = 2.027, p = 0.12).
Cerebellar parafloccular lobe (PFL)
DCX labeled images were opened in ImageJ, converted to 8-bit, and the transition zone between the ventral PFL and flocculus, as defined by Manohar et al. (2012) was outlined. Binary images were created using the Otsu thresholding method and percent area labeled was measured bilaterally. Percent area was calculated in this ROI as distinct individual cells were unable to be objectively counted due to unipolar cell morphology and dense cell labeling. Labeling density was consistent among sections in a given brain and thus we performed measurements in one section.
Dentate gyrus of the hippocampus (DG)
DCX labeled images were opened with Adobe Illustrator and the length of the dentate gyrus was measured. Labeled cells in the subgranular zone of the dentate granule cell layer were counted. Cells immunopositive for DCX were counted when the cell body was recognizable and there was at least 1 labeled process extending from the cell body as described in previously published reports (Kraus, Mitra et al., 2010). This quantification method differed from the other two ROIs due to distinct cell morphology in this region. Specifically, labeled cells were clearly darker than the surroundings with cytoplasm homogenously labeled (see Fig. 6) enabling each individually labeled cell to be counted. Cell density (number of cells/length of DG) was measured bilaterally in three consecutive sections. Average cell density from all three sections was used in our analysis.
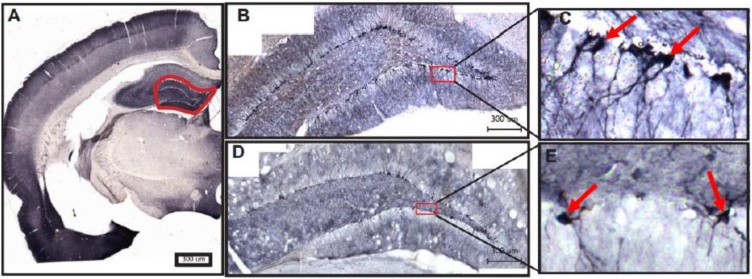
Fig. 6.
Doublecortin immunohistochemical staining in the dentate gyrus of the hippocampus in a representative control and sound damaged animal. 6A shows the rostral to caudal level of the hippocampus at which the dentate gyrus (red outline) was sampled. A magnified view of the left dentate gyrus from representative control (B & C) and sound damaged (D & E) animals demonstrates staining intensity. Individual cells labeled with DCX (red arrows) can be seen in C and E.
Data Analysis
All statistical tests were carried out using Prism v6.0 (GraphPad, La Jolla, CA) with the level of significance set at p = 0.05 for all analyses. Auditory brainstem response threshold changes (post-damage vs. baseline, as a function of frequency) were assessed using repeated-measures Two-way ANOVA, with Fisher’s LSD post-hoc test. Doublecortin immunolabeling was assessed using One-way ANOVA, with Sidak’s multiple comparisons test (DCN, PFL, DG) to compare pairs of means, and the Mann-Whitney test (PFL and DG) for remaining comparisons. Gap detection performance was evaluated using paired sample t-tests but yielded inconclusive results. Therefore, these data are included at the end of the results section under heading – ‘Inconclusive behavioral data’. Limitations of this method, as used here, are indicated in the discussion.
RESULTS
Hearing loss
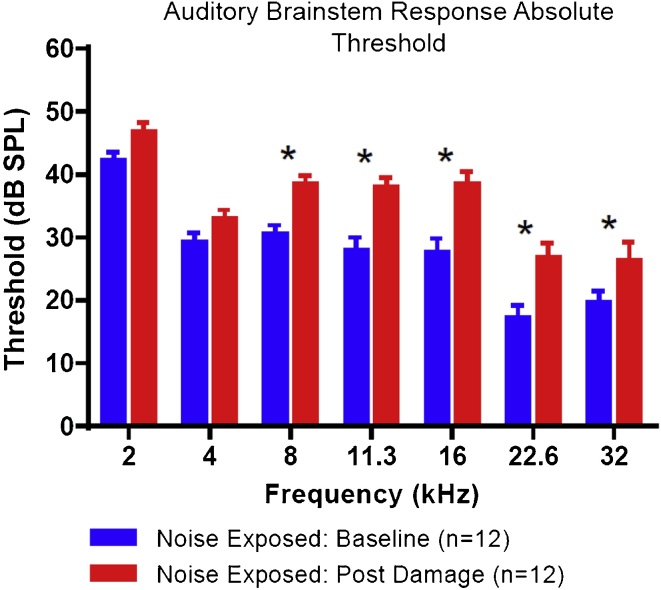
Fig. 2 shows the degree of hearing loss that occurred in the ear ipsilateral to the damaging stimulus. When examining all animals, our 114 dB, 1 -h exposure resulted in significantly increased hearing thresholds of (average 10 dB) at 5 of 7 tested frequencies (8 kHz, p ≤ 0.01; 11.3, 16, and 22.6 kHz, p ≤ 0.001; 32 kHz, p < 0.05) relative to baseline.
Fig. 2.
Hearing loss data as evaluated by auditory brainstem response (ABR). The average threshold of hearing at baseline and ∼2 weeks post damage (n = 12 animals) is plotted as a function of frequency. There were statistically significant threshold increases relative to baseline ∼2 weeks after damage, at 5 of 7 tested frequencies (repeated measures ANOVA, asterisk indicates p < 0.05).
Neuroplasticity
We evaluated doublecortin (DCX) staining in the dorsal cochlear nucleus (DCN) as well as two non-auditory regions (hippocampal dentate gyrus [DG] and cerebellar parafloccular lobe [PFL]). Representative images of DCX labeling in selected areas of interest appear in Figs. 3, 4 and 6. Regional labeling density was evaluated in DCN (Fig. 3) and the cerebellar PFL (Fig. 5), while individual cells were counted, and cell density was evaluated in the hippocampal dentate gyrus (Fig. 6). For each region we evaluated labeling for all sound damaged animals combined relative to controls. For statistical analysis, immunoreactivity (IR) was quantified both ipsilateral and contralateral to the damaged ear. This is especially important for the DCN, where input from the eighth nerve is relayed to higher CNS structures in a specific sequence that is dependent on the location of an auditory stimulus. The number of animals (control, sound damaged) included in the IR evaluation varied somewhat between brain regions due to occasional loss of adequately stained tissue.
Fig. 5.
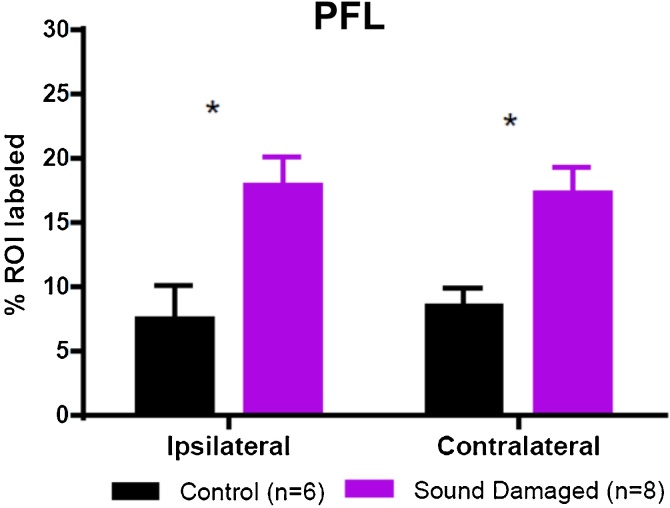
DCX labeling in the transition zone of the PFL of control (n = 6) and sound damaged animals (n = 8). Data are shown as percentage of ROI labeled in the ipsilateral and contralateral PFL. Asterisk indicates there was a significant bilateral increase of DCX labeling in the PFL of sound damaged animals relative to controls (One-Way ANOVA F = 6.551, p = 0.0022).
Dorsal cochlear nucleus (DCN)
DCX immunoreactivity (IR) was quantified as percent area labeled in high, mid and low frequency regions of the DCN both ipsilateral and contralateral to the damaged ear. In Fig. 3B control labeling was compared to labeling in all sound damaged animals. Again, there were no ipsilateral or contralateral differences in DCX IR of sound damaged animals relative to controls.
Parafloccular lobe (PFL)
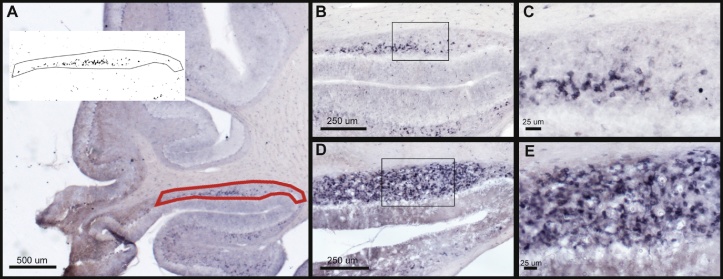
DCX IR was quantified as percent area labeled in the unipolar brush cell (UBC) rich transition zone between the flocculus and paraflocculus of the cerebellum. Representative images of a control animal (B & C) and a sound damaged animal (D & E) can be seen in Fig. 4. In the PFL, unipolar brush cells (UBCs) in the granule cell layer are densely labeled with DCX. Quantitative analysis (Fig. 5) revealed a significant bilateral increase of DCX labeling when comparing sound damaged animals to controls (One-Way ANOVA F = 6.551, p = 0.0022). These results suggest increased PFL labeling is a function of hearing loss.
Fig. 4.
Doublecortin immunohistochemical staining in the transition zone of the cerebellar parafloccular lobe (PFL): control vs. sound damaged animals. A low magnification image of the cerebellar parafloccular lobe control animal is shown in A. The area outlined in red (the UBC rich transition zone between the paraflocculus and flocculus of the cerebellum) was outlined and thresholded using the Otsu Method in ImageJ (inset in A). % ROI labeled (black puncta) in this region was measured bilaterally. A magnified view of DCX labeling in the transition zone of a control animal (B) and sound damaged animal (D) are shown to the right of A. C and E are magnified views of areas containing dense concentrations of labeled cells within the insets shown in B and D.
Dentate Gyrus of the Hippocampus (DG)
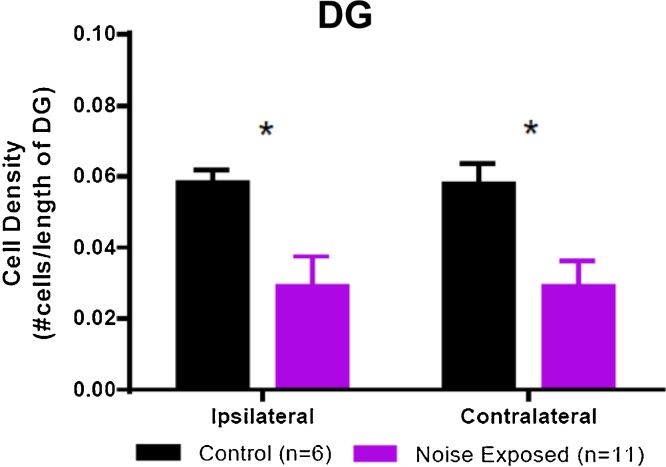
DCX cell density (number of labeled cells/length of the SGZ) was quantified in the dentate gyrus of the hippocampus. Fig. 6 shows a representative section from a control animal and a sound damaged animal, in which density appears to be decreased. When DCX cell density was quantified (Fig. 7), there was a significant bilateral decrease in DCX cell density when comparing all sound damaged animals to controls (F = 22.04, p = <0.0001). Unlike the results in the PFL, in the dentate gyrus hearing loss results in a decrease in DCX labeling.
Fig. 7.
Doublecortin labeling in the dentate gyrus (DG) of the hippocampus of control (n = 6) and sound damaged animals (n = 11). A bilateral decrease in density of labeled cells (number of labeled cells/length of the DG) was observed in sound damaged animals relative to controls (One-Way ANOVA F = 22.04, p < 0.0001).
Inconclusive behavior data: Gap Detection
Our goal at the start of the study was to employ gap-detection performance of individual animals as a means of exploring whether doublecortin staining is related to tinnitus-like behavior. We compared gap detection performance after sound damage to baseline performance to examine whether gap detection performance changed as a result of sound damage. We also included a group of control animals (no sound damage) evaluated at baseline and after a waiting period equivalent to that experienced by the sound damaged animals as a way of determining the stability of gap detection performance over time. The experimental timeline can be viewed in Fig. 1. The results described below demonstrate that the gap detection results are not robust enough to accomplish our initial goal, likely due to too few testing days. Thus, we have not drawn any conclusions that relate DCX staining to gap detection performance. However, we have chosen to report the data we obtained to make others aware of the pitfalls of using gap detection as commonly described in the literature, particularly for those animals who demonstrate hearing loss.
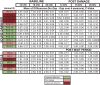
We evaluated individual animals across three testing frequencies to identify behavioral phenotypes related to how sound damage impacted gap-detection performance. Table 1 shows the mean of gap detection amplitude differences and corresponding statistical evaluations calculated by comparing acoustic startle responses during gap and no gap trials at both baseline and post damage (Sound Damaged), and for a group of control animals. If our test were adequately robust, we would expect that all animals at baseline would uniformly demonstrate significant differences in startle responses between gap and no-gap trials. We would expect this same result for control animals tested at a later date. For sound damaged animals we would expect a percentage of animals to no longer show differences in startle between gap and no-gap trials (tinnitus-like behavior) following sound damage. As Table one demonstrates, some animals in both sound damaged and control groups did not show significant gap detection at baseline (e.g. control GD-6 at 12 kHz and 16 kHz) and the response of control animals was not constant over time (e.g. GD-8). Because of these results, in particular the behavior of control animals, we are not confident that our test adequately identifies behavior that can be classified as “tinnitus-like”.
Table 1.
Experimental animals and control gap detection data. Performance data was collected over four consecutive testing days at baseline and four consecutive days post damage, or post wait period in the case of controls. The mean of differences at each testing frequency (12, 16, 20 kHz) was calculated as the average of daily responses during gap trials subtracted from the average of daily responses during no gap trials. P-values indicating paired t-tests comparing daily gap vs no-gap performance are included in the table.
 |
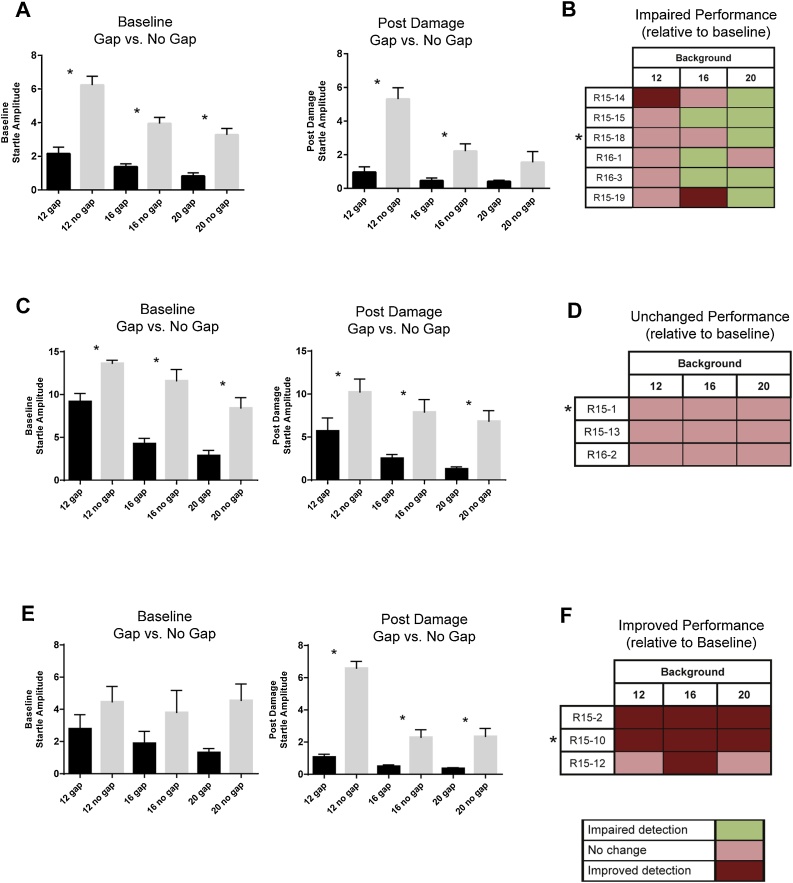
Despite this unexpected outcome, we decided to present our observations of gap detection behavior of sound damaged animals when comparing baseline to post sound damage performance. These patterns are displayed as a ‘heat map’ of each individual animal’s performance as shown in Fig. 8 B, D, and F. Using these heat maps, we identified three behavioral subgroups based on how their gap detection performance changed after sound damage relative to baseline performance. Figs. 8 A, C, and E show data from a representative animal in each behavioral phenotype group. The impaired group displayed mildly impaired performance (ability to detect the gap at baseline while not being able to detect the gap post damage) at one or more frequencies following sound damage (Fig. 8A, B). The unchanged group displayed no change in performance after sound damage (Fig. 8C, D). This included animals that did not demonstrate gap detection at either time point, and those animals who displayed gap detection at both time points. In the example shown in Fig. 8C, this animal was able to detect the gap both at baseline and following sound damage. Finally, we observed an improved group that displayed an improved ability to detect the gap at one or more frequencies post damage (Fig. 8E, F). Overall, fifty percent of animals were impaired, displaying tinnitus-like behavior at 16 or 20 kHz. Twenty-five percent of the animals showed unchanged performance at all frequencies, and twenty-five percent of animals showed improved performance at one or more frequencies.
Fig. 8.
Inconclusive gap detection performance data. Figs. 8A, C, E show data obtained from representative animals in each behavioral phenotype at baseline and post damage during gap and no gap trials at each testing frequency (12, 16, 20 kHz; Paired sample t-test, asterisk indicates p < 0.05). A heat map was generated to show each individual animal’s post damage performance relative to baseline (color code noted in bottom legend). Heat maps (B, D and F) were used to sort animals into three different groups based on tinnitus-like behavior following damage; impaired (n = 6; significant gap detection at baseline while no significant gap detection post damage), unchanged (n = 3, either significant gap detection at baseline as well as post damage, or no significant gap detection at baseline as well as post damage) and improved performance (n = 3, no significant gap detection at baseline but present post damage). Because of too few testing days, we present these results as observations only, as described more fully in the text.
Inconclusive immunolabeling data: DCX expression in groups sorted by gap detection performance
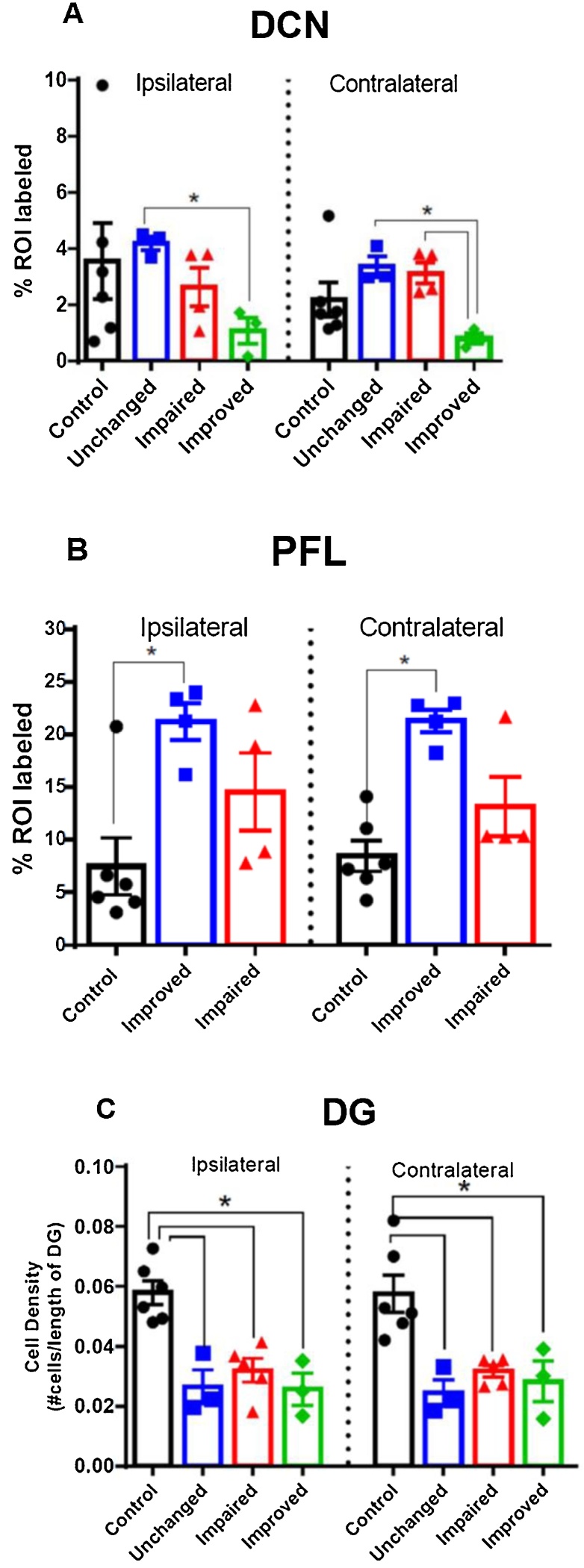
Despite our unwillingness to draw any conclusions relating DCX labeling to tinnitus behavior based on our gap detection results, we did compare labeling among the animals grouped into the empirical categories we observed, and we observed differences in DCX staining among them. For each region we compared DCX labeling among all sound damaged animal subgroups (unchanged, impaired, improved) to controls. In the DCN, there were no significant differences between control animals and any subgroup for low or mid frequency regions (data not shown). Statistically significant differences were observed only in the high frequency region of the DCN when compared between behavioral groups (One-Way ANOVA; F = 9.519, p = 0.0004). As shown in Fig. 9A, we observed a bilateral decrease in immunoreactivity in improved animals relative to unchanged animals and a decrease in improved animals relative to impaired in DCN contralateral to sound damage. In the PFL (Fig. 9B) the increase in DCX labeling seen for all sound damaged animals is more prominent in “improved” animals. Finally, for the dentate gyrus (Fig. 9C) a similar decrease in labeling is seen in all subgroups, consistent with the decrease we see comparing controls to all sound-damaged animals (Fig. 7).
Fig. 9.
Inconclusive doublecortin labeling of gap detection subgroups. In the DCN, scatterplots of high frequency region labeling (9A) show a significant, bilateral difference in DCX labeling between unchanged animals (n = 3) and animals with improved (n = 3) gap detection performance (ipsilateral, p ≤ 0.01; contralateral, p ≤ 0.05). In the contralateral DCN, labeling in impaired animals was greater than that in animals with improved gap detection performance (p ≤ 0.01). In the transition zone of the PFL (9B), there was a significant bilateral increase of DCX labeling of 3 improved and 1 unchanged animals (grouped together) relative to controls (Mann-Whitney ipsilateral, p = 0.0190; contralateral, p = 0.0095). Mean DCX labeling in the ipsilateral PFL for impaired animals (n = 4) was greater than that for controls but did not reach statistical significance (ipsilateral, p = 0.0667, contralateral, p = 0.23). There was no difference between the impaired relative to the improved group (ipsilateral, p = 0.20; contralateral, p = 0.0857). *n = 8 because we had PFL tissue for 8 animals. We only had PFL tissue from one of the animals in the unchanged group so we grouped that animal with the improved animals for PFL labeling analysis only. In the Dentate Gyrus (9C) there was a significant bilateral decrease in density of labeled cells in unchanged (n = 3, p = 0.0238), impaired (n = 5, p = 0.0043) and improved animals (n = 3, p = 0.0022) relative to controls (Mann-Whitney).
DISCUSSION
The goal of this study was to better understand the timeline of neuroplastic changes that occur as a result of sound damage induced hearing loss. To identify early alterations in DCX expression, we utilized immunohistochemistry to detect DCX protein in three brain regions (DCN, PFL, DG) within the first month post damage. A second goal was to obtain data which may help us to understand how hearing loss progresses to the onset of tinnitus-like behavior, and to identify DCX expression profiles in animals with and without evidence of tinnitus-like behavior. We were successful in identifying early cell specific plastic changes in hearing impaired animals. Changes in DCX expression in two brain regions within the first month post damage are an indicator of early onset unipolar brush cell (UBC) plasticity in the PFL as well as neuronal precursor cell plasticity in the hippocampal dentate gyrus. Prior to addressing the importance of our results related to hearing loss we will discuss limitations imposed by our gap detection methods.
Limitations of study conclusions - gap detection methods
Our ABR measurements were consistent enough among animals to allow us to draw conclusions about DCX changes due to hearing loss. However, we were not successful in reliably identifying tinnitus-like behavior using our gap detection methods. We provide those data as a guide to others who may wish to design similar experiments. The difficulty with our behavioral data is likely due to false negatives resulting from a small sample size caused by too few repeated testing days. Normal hearing (control) animals, with few exceptions, should be able to detect a gap at both baseline and 15-25 days later. The lack of a significant difference between startle amplitude in gap and in no-gap trials in all control animals is a strong indication of false negatives. Second, we found that both control and sound damaged animals show the same types of differences (unchanged, impairment, as well as improvement) when comparing baseline and subsequent testing (Table 1). We would not expect control animals to change their gap detection behavior within such a short time after their baseline testing. Thus, they would be expected to fall into the “unchanged” post-damage group instead. Instead, controls and sound damaged animals fell into the same three types of post-damage categories. Based on these results we concluded it is not possible to properly or reliably sort sound damaged animals into subgroups based on their behavioral performance in this study. Thus, to answer a question regarding tinnitus-like behavior and plasticity, this experimental design should be repeated with in a larger group of animals while also increasing the number of repeated behavioral testing days to provide conclusive results.
Sound damage induced hearing loss does not impact DCX expression in the DCN
While increased DCX (Bauer et al., 2013b) and unchanged DCX expression (Brozoski et al., 2017) have been reported in the DCN of animals with behavioral evidence of tinnitus at a chronic time point (3 months), we did not observe any significant difference in DCX labeling of any frequency region of the DCN following sound damage. This difference may be due to the shorter survival time after sound damage that we examined.
Sound damage positively impacts cerebellar plasticity
The PFL is responsive to acoustic stimuli and receives input from the auditory inferior colliculus and auditory cortex (Chen, Li et al. 2015). Additionally, connectivity studies have shown that there is direct interaction between the auditory brain regions (i.e. DCN) and PFL (Du, Liu et al. 2015). Bilaterally increased DCX expression was observed in the PFL of all sound damaged animals relative to controls. These results obtained at an early time point (within one month after damage) are in line with previously reported DCX measures obtained at a chronic (∼9-11 months) time point post damage (Bauer et al., 2013b). That the increase was seen for all sound damaged animals compared to controls suggests that DCX upregulation in the UBC is a result of sound damage. Our gap detection methods do not allow us to determine whether differences in DCX immunoreactivity might be related to tinnitus.
Sound damage negatively impacts hippocampal plasticity
Sound damage has been shown to produce long-term decreased DCX expression in the hippocampus (Kraus, Mitra et al. 2010), while early changes in expression have not been explored. We found that DCX is downregulated in the hippocampal dentate gyrus within the first month post damage. These changes occur as a result of sound damage induced hearing loss. The hippocampus undergoes constitutive neurogenesis long into adulthood and the valence of life experiences can influence this process. Stress and depression have been shown to decrease hippocampal neurogenesis (Duric and McCarson 2006) while learning and exercise increase the turnover of new cells (Zhao, Deng et al. 2008, Ming and Song 2011). Forty to seventy percent of individuals with tinnitus report concurrent emotional distress (e.g. depression and anxiety) (Joos, Vanneste et al. 2012, Gomaa, Elmagd et al. 2013). The hippocampus is affected in individuals with tinnitus and likely plays a role in assigning meaning to and recalling memories associated with a sound (Chen, Li et al. 2015). The hippocampus receives auditory information via auditory association areas and directly interacts with the primary auditory cortex. The findings presented here, in combination with previously reported results, suggest that noise induced hearing loss has an early and lasting impact of the turnover of new cells in the hippocampal dentate gyrus. Damage induced DCX changes in the hippocampus with concurrent behavioral measures (for depression, anxiety, memory) could determine if decreased hippocampal neurogenesis is associated with evidence of emotionally-driven behavior. Additionally, similar studies could identify specific ways in which hearing loss negatively impacts quality of life.
Neurogenesis and neuroplasticity
Since DCX has been associated with both developmental and adult neurogenesis (Francis, Koulakoff et al. 1999, Gleeson, Lin et al. 1999, Friocourt, Koulakoff et al. 2003, Walker, Yasuda et al. 2007, Klempin, Kronenberg et al. 2011, Ernst, Alkass et al. 2014), and the hippocampus undergoes neurogenesis into adulthood, decreased DCX expression in the dentate gyrus may reflect decreased neurogenesis. However, the interpretation of DCX expression in the DCN and PFL is not as clear, as these brain regions are not thought to undergo neurogenesis beyond embryonic development. Paolone et al. (2014) investigated whether DCX in these non-neurogenic brain regions signifies neurogenesis by co-labeling cells with DCX and BrdU. While they identified BrdU labeled cells in the brainstem and cerebellum the numbers and distribution of labeled nuclei did not support the hypothesis that DCX was labeling newly generated cells (Paolone, Manohar et al. 2014). Additionally, DCX has a restricted expression pattern and is limited to post-mitotic cells, showing no expression in proliferating cells. DCX has been shown to be localized to the tip of growing neuronal processes where it potentially plays a role in axonal guidance (Friocourt, Koulakoff et al. 2003). Taken together, these findings suggest that altered DCX expression may not be associated with the generation of new neurons in the DCN and PFL following sound damage, but rather play a unique role in CNS plasticity in these regions. Here, we observed differential changes to DCX expression in the PFL and DG that were related to sound damage induced hearing loss while hearing loss had no impact on DCX expression in the DCN relative to controls.
CONCLUDING REMARKS
The study presented here utilized immunohistochemistry to detect DCX protein in three brain regions (DCN, PFL, DG) within the first month after sound damage. Auditory brainstem response was used to measure hearing loss, and a measurable hearing loss was evident in all sound damaged animals. No changes in DCX expression were observed in any frequency region of the DCN. Decreased DCX expression related to hearing loss was evident in the DG. Increased DCX expression related to hearing loss was evident in the PFL.
While altered DCX expression in relation to noise damage and tinnitus-like behavior has been investigated by several groups, cross study comparisons reveal inconsistent results. These seemingly contradictory findings could largely be due to differences in experimental timelines and specifics in the methodology. Tinnitus onset does not immediately follow noise induced hearing loss and hearing loss alone is not enough to induce tinnitus. While the findings presented here indicates neuroplastic changes specific to hearing loss alone are evident in non-auditory brain regions, additional studies with reliable behavioral data are needed to provide information about the dynamics and subsequent role of DCX expression in the presence and absence of tinnitus. A comprehensive timeline will benefit auditory researchers by identifying when and where to target specific treatments in hopes of either preventing or reversing the impact that damage to the auditory periphery has on the CNS.
Author contributions
All authors have approved the final article. Study concept and design: A.F. and D.D. Data acquisition: A.F. and C.N. Data analysis and interpretation: A.F. and C.N. Drafting of the manuscript: A.F. Critical revision of the manuscript for important intellectual content: C.N., D.D., and H.S. Statistical analysis: A.F. Study supervision: J.B. and D.D.
Conflicts of interest
None.
Funding sources
This work is supported by the Department of Defense Grant # CDMRP PR08124, the Department of Otolaryngology- Head and Neck Surgery & the Madison and Lila Self Graduate Fellowship at the University of Kansas.
Acknowledgements
We would like to thank the University of Kansas Medical Center and the Kansas Intellectual and Development Disabilities Research Center (NICHD HD02528), the Confocal and Light Microscopy Core and Michelle Winter for her assistance with rodent behavior data analysis.
References
- Agrawal Y., Platz E.A., Niparko J.K. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30(2):139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- Baizer J.S., Manohar S., Paolone N.A., Weinstock N., Salvi R.J. Understanding tinnitus: the dorsal cochlear nucleus, organization and plasticity. Brain Res. 2012;1485:40–53. doi: 10.1016/j.brainres.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.A., Kurt W., Sybert L.T., Brozoski T.J. The cerebellum as a novel tinnitus generator. Hear Res. 2013;295:130–139. doi: 10.1016/j.heares.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.A., Turner J.G., Caspary D.M., Myers K.S., Brozoski T.J. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86(11):2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.A., Wisner K.W., Baizer J.S., Brozoski T.J. Tinnitus, unipolar brush cells, and cerebellar glutamatergic function in an animal model. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0064726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt J.M., Lin H.W., Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the united states. JAMA Otolaryngology–Head & Neck Surgery. 2016;142(10):959–965. doi: 10.1001/jamaoto.2016.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T., Brozoski D., Wisner K., Bauer C. Chronic tinnitus and unipolar brush cell alterations in the cerebellum and dorsal cochlear nucleus. Hearing Research. 2017;350:139–151. doi: 10.1016/j.heares.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T.J., Bauer C.A., Caspary D.M. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22(6):2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T.J., Ciobanu L., Bauer C.A. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228(1-2):168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Brozoski T.J., Wisner K.W., Odintsov B., Bauer C.A. Local NMDA receptor blockade attenuates chronic tinnitus and associated brain activity in an animal model. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary D.M., Llano D.A. Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology. Hear Res. 2017;349:197–207. doi: 10.1016/j.heares.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary D.M., Pazara K.E., Kossl M., Faingold C.L. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987;417(2):273–282. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Li X., Liu L., Wang J., Lu C.Q., Yang M., Jiao Y., Zang F.C., Radziwon K., Chen G.D., Sun W., Krishnan Muthaiah V.P., Salvi R., Teng G.J. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife. 2015;4 doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Elgoyhen A.B., Romo R., Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011;108(20):8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Weisz N., Londero A., Schlee W., Elgoyhen A.B., Langguth B. An integrative model of auditory phantom perception: Tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Dong S., Mulders W.H., Rodger J., Woo S., Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci. 2010;31(9):1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Du Y., Liu J., Kang W. The role of the cerebellum in auditory process and tinnitus. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(13):1231–1234. [PubMed] [Google Scholar]
- Duan M., Agerman K., Ernfors P., Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000;97(13):7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V., McCarson K.E. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7(8):544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H., Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Fournier P., Hebert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res. 2013;295:16–23. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Francis F., Koulakoff A., Boucher D., Chafey P., Schaar B., Vinet M.C., Friocourt G., McDonnell N., Reiner O., Kahn A., McConnell S.K., Berwald-Netter Y., Denoulet P., Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Friocourt G., Koulakoff A., Chafey P., Boucher D., Fauchereau F., Chelly J., Francis F. Doublecortin functions at the extremities of growing neuronal processes. Cereb Cortex. 2003;13(6):620–626. doi: 10.1093/cercor/13.6.620. [DOI] [PubMed] [Google Scholar]
- Gao Y., Manzoor N., Kaltenbach J.A. Evidence of activity-dependent plasticity in the dorsal cochlear nucleus, in vivo, induced by brief sound exposure. Hearing research. 2016;341:31–42. doi: 10.1016/j.heares.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J.G., Lin P.T., Flanagan L.A., Walsh C.A. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron. 1999;23(2):257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gomaa M.A., Elmagd M.H., Elbadry M.M., Kader R.M. Depression, Anxiety and Stress Scale in patients with tinnitus and hearing loss. Eur Arch Otorhinolaryngol. 2013 doi: 10.1007/s00405-013-2715-6. [DOI] [PubMed] [Google Scholar]
- Grubbs F.E. Sample Criteria for Testing Outlying Observations. Ann. Math. Statist. 1950;21(1):27–58. [Google Scholar]
- Guitton M.J., Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast. 2007;2007:80904. doi: 10.1155/2007/80904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunbey H.P., Gunbey E., Aslan K., Bulut T., Unal A., Incesu L. Limbic-Auditory Interactions of Tinnitus: An Evaluation Using Diffusion Tensor Imaging. Clin Neuroradiol. 2017;27(2):221–230. doi: 10.1007/s00062-015-0473-0. [DOI] [PubMed] [Google Scholar]
- Hinduja S., Kraus K.S., Manohar S., Salvi R.J. D-methionine protects against cisplatin-induced neurotoxicity in the hippocampus of the adult rat. Neurotox Res. 2015;27:199–204. doi: 10.1007/s12640-014-9503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig T.J., Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490(4):391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Joos K., Vanneste S., De Ridder D. Disentangling depression and distress networks in the tinnitus brain. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach J.A., Afman C.E. "Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140(1-2):165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A., Rachel J.D., Mathog T.A., Zhang J., Falzarano P.R., Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88(2):699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A., Zhang J., Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206(1-2):200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Klempin F., Kronenberg G., Cheung G., Kettenmann H., Kempermann G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus K.S., Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res. 2012;288(1-2):34–46. doi: 10.1016/j.heares.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Kraus K.S., Mitra S., Jimenez Z., Hinduja S., Ding D., Jiang H., Gray L., Lobarinas E., Sun W., Salvi R.J. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167(4):1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S.G., Charles Liberman M. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear Res. 2019;377(1):44–52. doi: 10.1016/j.heares.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M., Langguth B., Rosengarth K., Braun S., Koch A., Kleinjung T., May A., de Ridder D., Hajak G. Structural brain changes in tinnitus: Grey matter decrease in auditory and non-auditory brain areas. NeuroImage. 2009;46(1):213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Leaver A.M., Renier L., Chevillet M.A., Morgan S., Kim H.J., Rauschecker J.P. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69(1):33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Shen P., He T., Chang Y., Shi L., Tao S., Li X., Xun Q., Guo X., Yu Z., Wang J. Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Sci Rep. 2016;6:20374. doi: 10.1038/srep20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood A.H., Salvi R.J., Coad M.L., Towsley M.L., Wack D.S., Murphy B.W. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50(1):114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Longenecker R.J., Chonko K.T., Maricich S.M., Galazyuk A.V. Age effects on tinnitus and hearing loss in CBA/CaJ mice following sound exposure. Springerplus. 2014;3:542. doi: 10.1186/2193-1801-3-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W.L., Young E.D. Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity. Hear Res. 2006;216-217:176–188. doi: 10.1016/j.heares.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S., Paolone N.A., Bleichfeld M., Hayes S.H., Salvi R.J., Baizer J.S. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience. 2012;202:169–183. doi: 10.1016/j.neuroscience.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek B., Haupt H., Olze H., Szczepek A.J. Stress and tinnitus-from bedside to bench and back. Front Syst Neurosci. 2012;6:47. doi: 10.3389/fnsys.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennink L.M., Van Dijk J.M.C., Van Der Laan B., Metzemaekers J.D.M., Van Laar P.J., Van Dijk P. The relation between flocculus volume and tinnitus after cerebellopontine angle tumor surgery. Hear Res. 2018 doi: 10.1016/j.heares.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Middleton J.W., Kiritani T., Pedersen C., Turner J.G., Shepherd G.M., Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108(18):7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E., Sekerkova G., Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev. 2011;66(1-2):220–245. doi: 10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Lopez M.M., Mohedano-Moriano A., Insausti R. Anatomical pathways for auditory memory in primates. Front Neuroanat. 2010;4:129. doi: 10.3389/fnana.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena A.J., Eggermont J.J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183(1-2):137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Norena A.J., Eggermont J.J. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci. 2005;25(3):699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M., Tomscha K., Wehr M. Isoflurane blocks temporary tinnitus. Hear Res. 2012;290(1-2):64–71. doi: 10.1016/j.heares.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Oertel D., Young E.D. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27(2):104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Otsu N. A Threshold Selection Method from Gray-Level Histograms. IEEE Transactions on Systems, Man, and Cybernetics. 1979;9(1):62–66. [Google Scholar]
- Paolone N., Manohar S., Hayes S.H., Wong K.M., Salvi R.J., Baizer J.S. Dissociation of doublecortin expression and neurogenesis in unipolar brush cells in the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience. 2014;265:323–331. doi: 10.1016/j.neuroscience.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen G. National Library of Medicine; Washington D.C: 1990. Remarks on the cochleo-cerebellar connections. Research Notebooks. History of Medicine Division: 1-42. [Google Scholar]
- Ryan A.F., Furlow Z., Woolf N.K., Keithley E.M. The spatial representation of frequency in the rat dorsal cochlear nucleus and inferior colliculus. Hear Res. 1988;36(2-3):181–189. doi: 10.1016/0378-5955(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Shore S.E., Wu C. Mechanisms of Noise-Induced Tinnitus: Insights from Cellular Studies. Neuron. 2019;103(1):8–20. doi: 10.1016/j.neuron.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Eggermont J.J. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180(1-2):28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Seydell-Greenwald A., Raven E.P., Leaver A.M., Turesky T.K., Rauschecker J.P. Diffusion Imaging of Auditory and Auditory-Limbic Connectivity in Tinnitus: Preliminary Evidence and Methodological Challenges. Neural Plasticity. 2014;2014 doi: 10.1155/2014/145943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla S., Dempsey C., Warren R., Enikolopov A.G., Sawtell N.B. A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat Neurosci. 2017;20(7):943–950. doi: 10.1038/nn.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345(1):1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Walker T.L., Yasuda T., Adams D.J., Bartlett P.F. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci. 2007;27(14):3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Brozoski T.J., Turner J.G., Ling L., Parrish J.L., Hughes L.F., Caspary D.M. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164(2):747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]