Summary
A novel nickel/Brønsted acid-catalyzed asymmetric hydroamination of acyclic 1,3-dienes has been established. A wide array of primary and secondary amines can be transformed into allylic amines with high yields and high enantioselectivities under very mild conditions. Moreover, our method is compatible with various functional groups and heterocycles, allowing for late-stage functionalization of biologically active complex molecules. Remarkably, this protocol exhibits good chemoselectivity with respect to amines bearing two different nucleophilic sites. Mechanistic studies reveal that the enantioselective carbon-nitrogen bond-forming step is reversible.
Subject Areas: Catalysis, Organic Synthesis, Materials Chemistry
Graphical Abstract
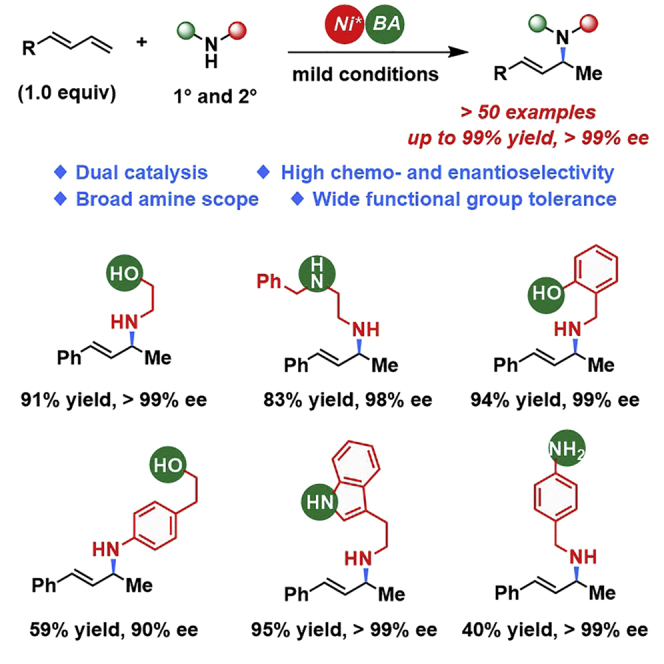
Highlights
-
•
Nickel/Brønsted acid-catalyzed asymmetric hydroamination of conjugated dienes
-
•
High regio-, chemo-, and enantioselectivity
-
•
Broad range of substrate scope
-
•
Wide functional group tolerance
Catalysis; Organic Synthesis; Materials Chemistry
Introduction
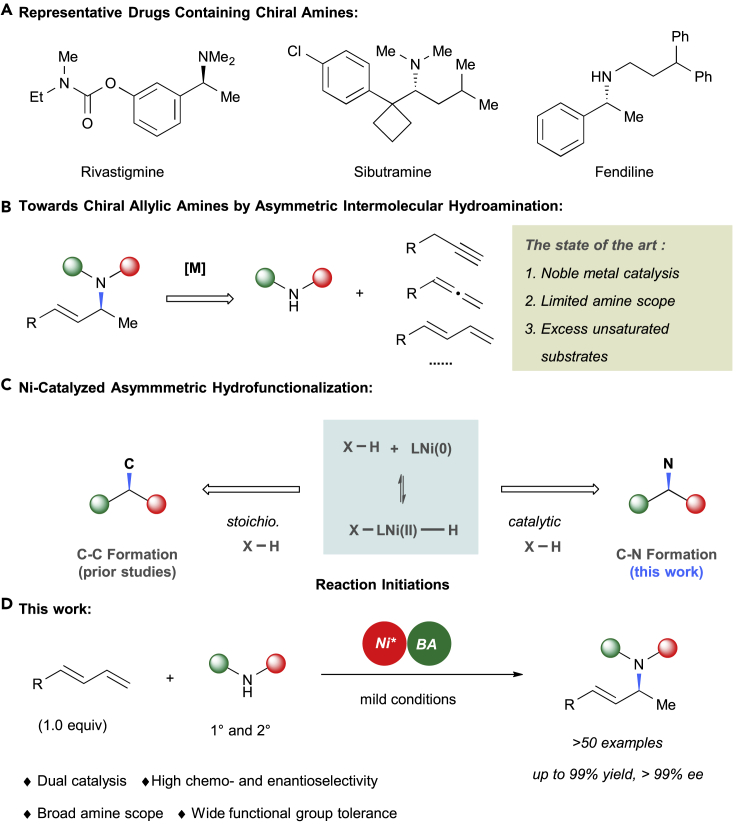
Chiral amines represent a privileged pharmacophore and are present in a myriad of natural products and drugs (Figure 1A) (Francotte and Lindner, 2006, Lough and Wainer, 2002, Nugent, 2010). Therefore, organic chemists have made considerable efforts toward their synthesis during the last decade (Grogan, 2018, Li and Zhang, 2014, Nugent and El-Shazly, 2010, Patil et al., 2018, Robak et al., 2010). Among them, asymmetric hydroamination of unsaturated C-C bonds serves as an efficient and powerful tool in organic synthesis, particularly hydroamination using free amines (Aillaud et al., 2007, Clement and Jerome, 2017, Dondoni, 2015, Hannedouche and Schulz, 2013, Hannedouche and Schulz, 2018, Hii, 2006, Huang et al., 2015, Huo et al., 2019, Jerome, 2018, Müller et al., 2008, Patel et al., 2017, Pirnot et al., 2016, Reznichenko and Hultzsch, 2016, Zi, 2009, Zi, 2011). In this context, transition-metal-catalyzed intermolecular asymmetric hydroamination of allenes (Berthold and Breit, 2018, Berthold et al., 2019, Cooke et al., 2012, Dion and Beauchemin, 2011, Lin et al., 2019, Parveen et al., 2017, Xu et al., 2016), alkynes (Athira et al., 2018, Liu et al., 2011, Lutete et al., 2004, Patil et al., 2006, Xu et al., 2019), and conjugated dienes (Adamson et al., 2017, Dion and Beauchemin, 2011, Lin et al., 2019, Löber et al., 2001, Park and Malcolmson, 2018, Xiong et al., 2018, Yang and Dong, 2017, Zhou and Hartwig, 2008) has been extensively studied (Figure 1B). Nevertheless, the use of noble transition metals such as rhodium and palladium are often mandatory (Adamson et al., 2017, Aillaud et al., 2007, Athira et al., 2018, Berthold et al., 2019, Berthold and Breit, 2018, Clement and Jerome, 2017, Cooke et al., 2012, Dion and Beauchemin, 2011, Dondoni, 2015, Hannedouche and Schulz, 2013, Hannedouche and Schulz, 2018, Hii, 2006, Huang et al., 2015, Huo et al., 2019, Jerome, 2018, Lin et al., 2019, Liu et al., 2011, Löber et al., 2001, Lutete et al., 2004, Müller et al., 2008, Park and Malcolmson, 2018, Parveen et al., 2017, Patel et al., 2017, Patil et al., 2006, Pirnot et al., 2016, Reznichenko and Hultzsch, 2016, Xiong et al., 2018, Xu et al., 2016, Xu et al., 2019, Yang and Dong, 2017, Zhou and Hartwig, 2008, Zi, 2009, Zi, 2011); in addition, these methods suffer from limited amine scope (Adamson et al., 2017, Dion and Beauchemin, 2011, Lin et al., 2019, Löber et al., 2001, Park and Malcolmson, 2018, Xiong et al., 2018, Yang and Dong, 2017, Zhou and Hartwig, 2008), as well as excessive quantities of the unsaturated substrate are always required to achieve a high level of efficiency (Adamson et al., 2017, Dion and Beauchemin, 2011, Lin et al., 2019, Löber et al., 2001, Park and Malcolmson, 2018, Yang and Dong, 2017, Zhou and Hartwig, 2008).
Figure 1.
Reaction Design
(A) Representative drugs containing chiral amines.
(B) Toward chiral allylic amines by asymmetric intermolecular hydroamination.
(C) Ni-catalyzed asymmetric hydrofunctionalization.
(D) Nickel/Brønsted acid-catalyzed chemo- and enantioselective intermolecular hydroamination of conjugated dienes.
In recent years, research toward nickel-catalyzed oxidative addition with X-H (X = C, O …) bonds has become a hot theme owing to earth-abundance of nickel and its great potential in oxidative addition (Ananikov, 2015, Tasker et al., 2014, Wang, 2016; Figure 1C). Significant progress has been made in the asymmetric hydrofunctionalization of alkenes through nickel-catalyzed reactions (Bezzenine-Lafollee et al., 2017, Cai et al., 2019, Chen and Lu, 2018, Cheng et al., 2018, Cheng et al., 2019, Diesel et al., 2018, Diesel et al., 2019, Donets and Cramer, 2013, Li et al., 2018, Li et al., 2019a, Lv et al., 2018, Richmond and Moran, 2018, Woźniak and Cramer, 2019, Xiao et al., 2016, Xiao et al., 2018, Zhang et al., 2019). Chiral centers are generally induced via a carbon-carbon bond-forming process, involving the direct oxidative addition of C-H bonds (Cai et al., 2019, Cheng et al., 2018, Cheng et al., 2019, Diesel et al., 2018, Diesel et al., 2019, Donets and Cramer, 2013, Li et al., 2019a, Lv et al., 2018, Woźniak and Cramer, 2019, Zhang et al., 2019) or an external stoichiometric reductant, such as alcohol (Chen et al., 2019) or hydrosiloxane (Ahlin and Cramer, 2016). However, nickel-catalyzed asymmetric hydrofunctionalization of unsaturated compounds involving a carbon-heteroatom bond formation has not been studied much (Tran et al., 2019). As an extension of our studies with nickel-catalyzed carbon-carbon bond formations (Li et al., 2019b, Wang et al., 2019), we turned our attention to carbon-heteroatom bonds. Inspired by the recent reports on metal/Brønsted acid dual catalysis (Adamson et al., 2017, Dion and Beauchemin, 2011, Han et al., 2018, Kathe and Fleischer, 2019, Lin et al., 2019, Liu and Feng, 2018, Löber et al., 2001, Park and Malcolmson, 2018, Yang and Dong, 2017, Zhou and Hartwig, 2008), we have developed a novel, room temperature nickel/Brønsted acid-catalyzed asymmetric hydroamination using conjugated dienes as a limiting reagent (Figure 1D). This protocol can transform a wide array of primary and secondary amines into allylic amines in high yields with excellent enantioselectivities. Significantly, good regio-, chemo-, and enantioselectivity have been achieved using amines bearing potentially competitive nucleophilic sites. It is noteworthy that the nickel-catalyzed racemic hydroamination of cyclic dienes has only been reported by the Hartwig group before, wherein they also demonstrated the challenge for the development of an enantioselective variant (Pawlas et al., 2002).
Results
Optimization Reaction Conditions
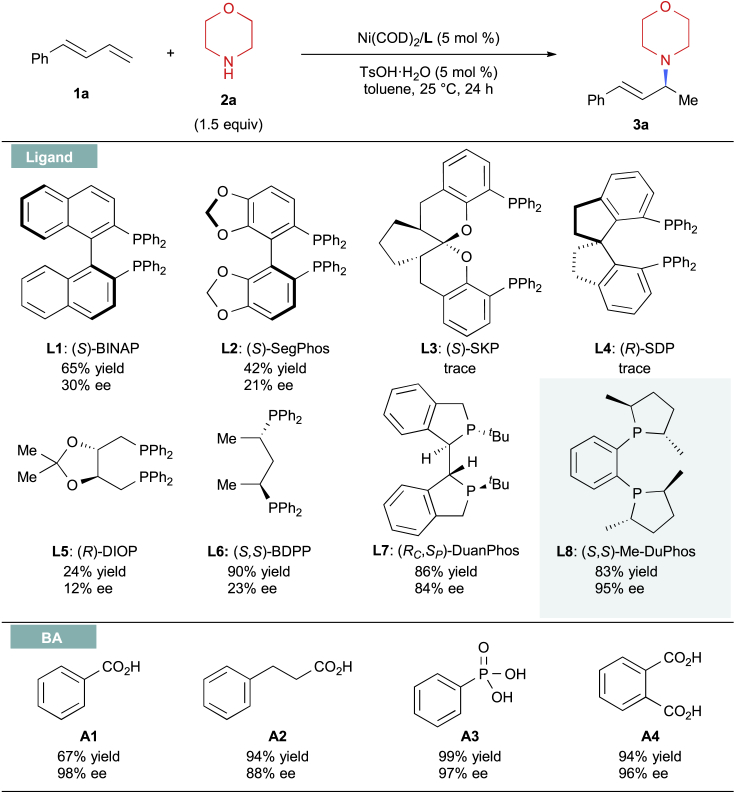
We initiated this study by choosing phenyl-1,3-diene (1a) and morpholine (2a) as model substrates. Ligand evaluations were conducted using Ni(COD)2 as the precatalyst and TsOH⋅H2O as a cocatalyst. As shown in Figure 2, a series of bisphosphine ligands were examined; the 1,2-hydroamination product 3a (Wang et al., 2014) was obtained in a moderate yield with a low enantiomeric excess (ee) when chiral BINAP (L1) or SEGPHOS (L2) was used, which demonstrated the feasibility of this hydroamination reaction. Unfortunately, (S)-SKP (L3), (R)-SDP (L4), and (R)-DIOP (L5) as ligand were not effective for this transformation, although (S,S)-BDPP (L6), a flexible bisphosphine ligand, yielded 3a in an excellent yield, but with low enantioselectivity (23% ee). However, both high yields and enantioselectivities were achieved by (RC,SP)-DuanPhos (L7). To our delight, excellent ee (95% ee) was obtained when (S,S)-Me-DuPhos (L8), as a more rigid ligand, was used. In addition, the Brønsted acid cocatalyst can also affect the efficiency and enantioselectivity of this hydroamination reaction. Further studies demonstrated that the desired product can also be obtained in high yields without a decrease in enantioselectivity when switching the acid cocatalyst to phenylphosphonic acid (A3) or phthalic acid (A4). To easily weighout, we selected A4 as cocatalyst. Moreover, control experiments indicated that both nickel catalysts and the Brønsted acids were crucial to the success of this reaction. Notably, no other regioisomers were detected in these reactions.
Figure 2.
Reaction Optimization
Reactions were conducted at 0.2 mmol scale, see Supplemental Information for reaction details. See also Tables S1–S3.
Substrate Scope Study
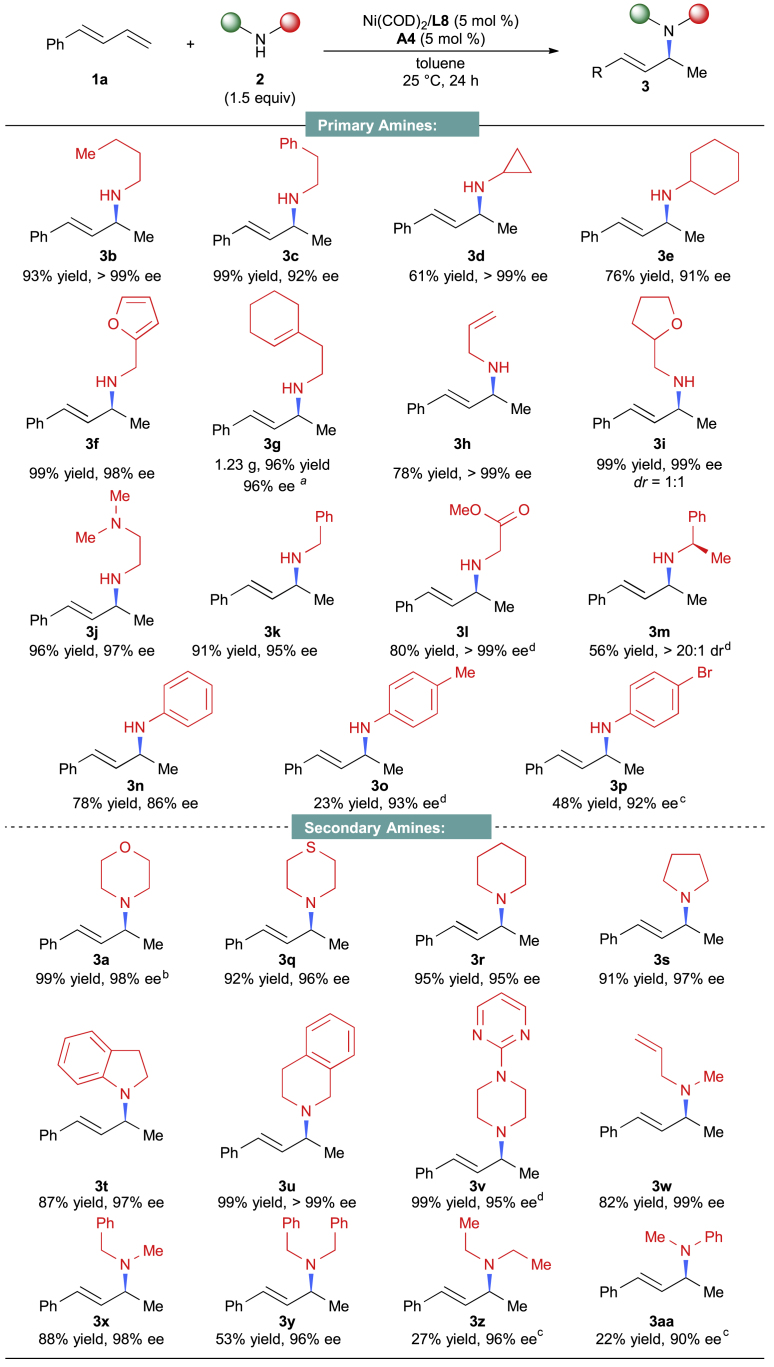
With the optimal conditions in hand, we shifted our attention to investigate the generality of this Ni-catalyzed asymmetric hydroamination reaction. Utilizing 1a, we examined the scope of the amines. As illustrated in Figure 3, a series of primary amines bearing various functional groups produced the corresponding hydroamination products 3b-3l with good to excellent yields with excellent enantioselectivities. Notably, (R)-(+)-1-Phenylethylamine, a chiral amine, also gave the hydroamination product in a moderate yield with an excellent diastereomeric ratio (dr > 20:1, 3m). In addition to the aliphatic amines, primary arylamines were also suitable for the reaction to generate the chiral amine products with excellent enantioselectivities, albeit in lower yields under the current reaction conditions. It is noteworthy that the aryl bromide is compatible with this nickel-catalyzed reaction (3p). To assess the practicality of this asymmetric hydroamination reaction, a gram-scale experiment was conducted. When the reaction of 1a with 2g was performed on a 5 mmol scale, it still was able to furnish 3g without loss of reaction efficiency and optical enantioselectivities, even in the presence of 1 mol % catalysts.
Figure 3.
Scope of Primary and Secondary Amines
Reactions were conducted at 0.2 mmol scale, see Supplemental Information for reaction condition details. aReactions were conducted at 5 mmol scale. b12 h; c36 h; d48 h. See also Scheme S3.
Next, the scope of secondary amines was tested. Various secondary cyclic amines afforded the chiral allylic amines in both remarkable yields and enantioselectivities (3a-3v). Moreover, acyclic secondary amines were also able to produce the desired hydroamination products with excellent enantioselectivities under the same reaction conditions (3w-3aa). Interestingly, although catalytic amount of Brønsted acid was used as a cocatalyst, amines containing other nitrogen atoms did not affect this asymmetric transformation (3j and 3v). Additionally, a series of functional groups, including ethers (3i and 3a), esters (3l), thioethers (3q), terminal alkenes (3h and 3w), and heterocycles such as furan (3f) and pyrimidines (3v), all were well tolerated in this reaction.
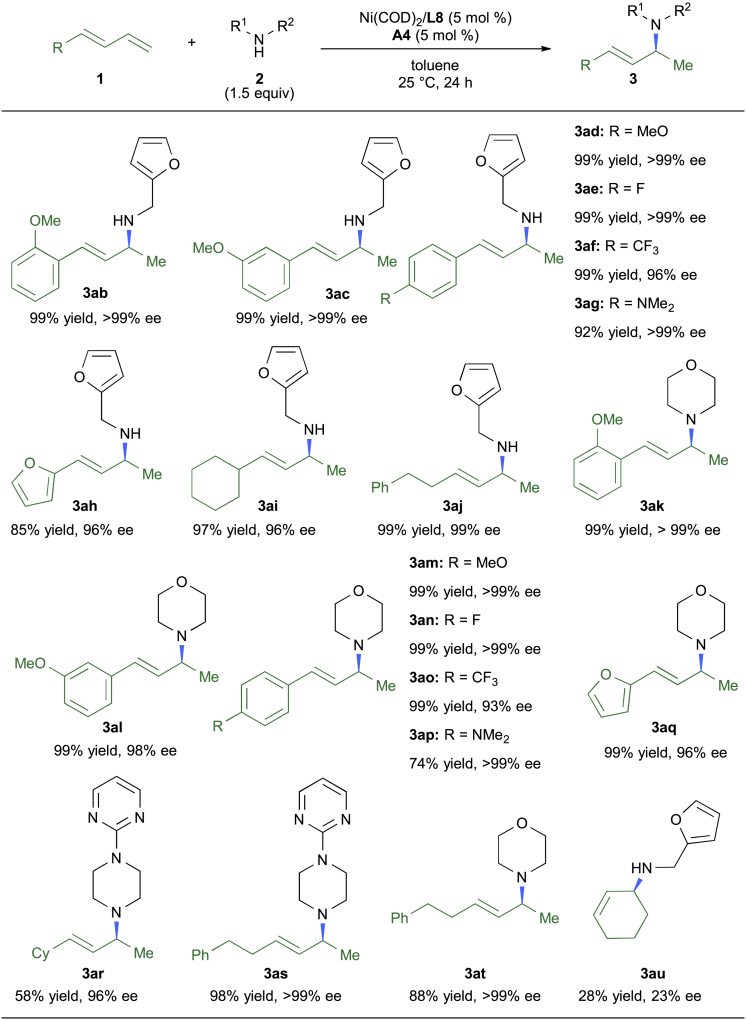
Subsequently, the scope of 1,3-dienes was studied. A set of aryl-substituted linear 1,3-butadienes were examined with both primary and secondary amines under the optimal conditions. As shown in Figure 4, both electron-rich and deficient substituents did not affect the efficiency or enantioselectivity. Alkyl-substituted butadienes were also capable of producing the Markovnikov hydroamination products (3ai, 3aj, 3ar, 3as, and 3at) in excellent yields with an excellent ee value. Notably, no other regioisomers were detected in these reactions. Furthermore, the hydroamination product (3au) could also be synthesized from 1,3-cyclohexadiene, albeit in low yields and enantioselectivity under the current conditions.
Figure 4.
Scope of Conjugated Dienes
Reactions were conducted at 0.2 mmol scale, see Supplemental Information for reaction condition details. See also Scheme S3.
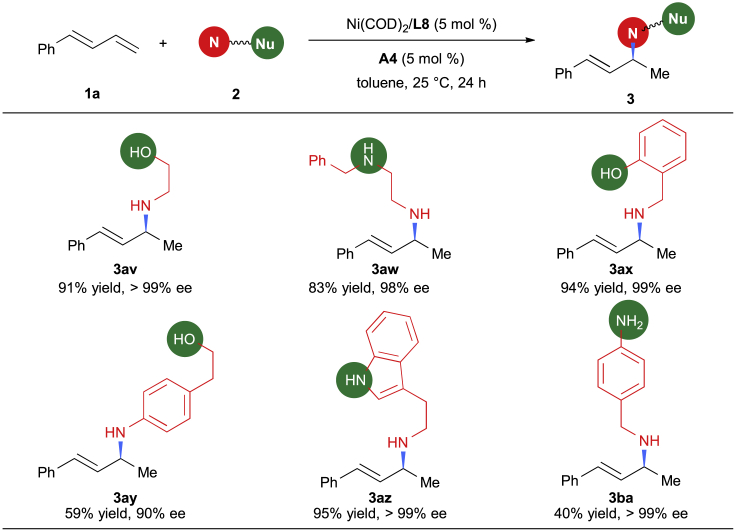
As we have highlighted earlier, both primary and secondary alkyl and aryl amines can produce satisfactory results in this nickel/Brønsted acid-catalyzed reaction. We were curious about the chemoselectivity when using one substrate containing two different nucleophilic sites. Guided by this idea, a set of more complex amines were tested under the optimal conditions and the results have been displayed in Figure 5. With aminoethanol, only the 1,2-hydroamination product (3av) was isolated with an excellent yield and ee value. Notably, the less sterically encumbered primary amine was found to be more reactive than the secondary amine when N-benzylethylediamine was used (3aw). Interestingly, the acidic phenol did not affect the amination (3ax), and the hydroamination reaction of the aryl amine (3ay) was not affected by the presence of an alcohol. Moreover, a single isomer with both excellent ee and yield could be obtained from tryptamine (3az). Finally, high chemoselectivity was shown at the aliphatic amine part when 4-aminobenzylamine was used (3ba). Collectively, these results suggest that this nickel-catalyzed reaction exhibits good chemoselectivity toward hydroamination and also demonstrates the potential of this method in the late-stage diversification of biomolecules.
Figure 5.
Substrates Containing Two Nucleophilic Sites
Reactions were conducted at 0.2 mmol scale, see Supplemental Information for reaction condition details. See also Scheme S3.
Discussion
Mechanism Study
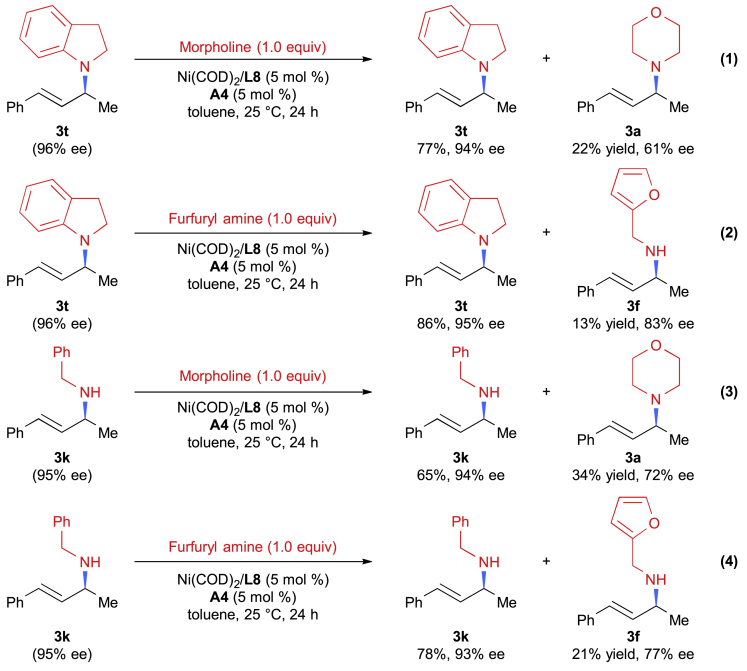
To get more details of this transformation, a preliminary mechanistic investigation was conducted. In Hartwig's reaction, a reversible carbon-nitrogen bond formation was observed. To determine if this phenomenon also exists in our reaction, amine exchange experiments were performed first. When the enantioenriched 3t and stoichiometric morpholine were subjected to the optimal conditions, both 3t and 3a were detected (Scheme 1-1). A similar phenomenon was also observed in the reaction of 3t with a primary amine (Scheme 1-2). This reversible effect was also found when a primary amine-based product was used (Schemes 1-3 and 1-4). These findings strongly suggested that a reversibility of carbon-nitrogen bond formation was involved in this reaction. These results are in consistence with Hartwig's results (Pawlas et al., 2002) but inconsistent with the results of Mazet's conditions (Tran et al., 2019).
Scheme 1.
Amine Exchange Experiment
(1) Exchange experiment of secondary amine-based product (3t) with secondary amine (morpholine).
(2) Exchange experiment of secondary amine-based product (3t) with primary amine (furfuryl amine).
(3) Exchange experiment of primary amine-based product (3k) with secondary amine (morpholine).
(4) Exchange experiment of primary amine-based product (3k) with primary amine (furfuryl amine).
Data are represented as mean value of three times; see also Scheme S5.
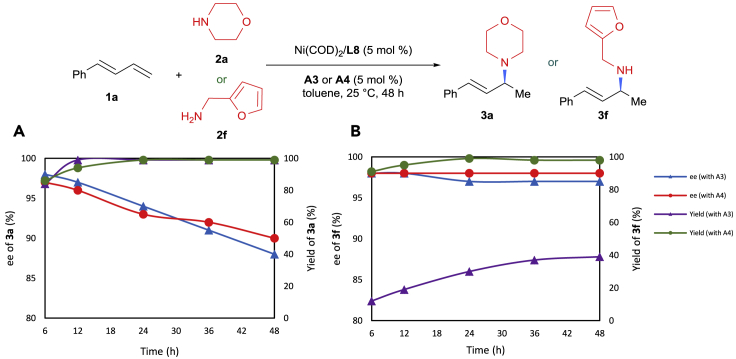
Furthermore, a decrease in enantioselectivity over time has been observed in the palladium-catalyzed hydroamination reactions (Löber et al., 2001, Pawlas et al., 2002). To determine if this phenomenon also exists in our reaction, time course experiments were conducted for both primary and secondary amines (Figure 6). To our surprise, significant racemization was observed for the reaction with a secondary amine (Figure 6A), whereas there was nearly no alteration of enantioselectivity in a reaction with a primary amine (Figure 6B). Moreover, similar results were also obtained switching A4 to A3.
Figure 6.
Reaction Profiles
(A) Time course experiments of secondary amine.
(B) Time course experiments of primary amine.
Data are represented as mean value of three times; see also Scheme S6 and Figure S246.
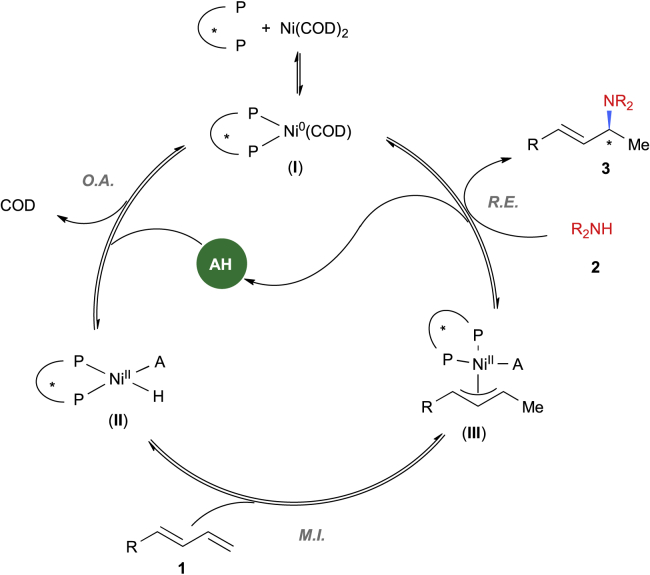
Finally, based on precedent studies (Adamson et al., 2017, Dion and Beauchemin, 2011, Lin et al., 2019, Löber et al., 2001, Park and Malcolmson, 2018, Xiong et al., 2018, Yang and Dong, 2017, Zhou and Hartwig, 2008) and the above-mentioned findings (see Supplemental Information for more results), a mechanistic profile is proposed for this transformation. As illustrated in Scheme 2, the reaction is initiated by a Ni(0) species (I), which undergoes oxidative addition to form a Ni(II)-H species (II). Subsequently, a 1,3-diene migratory insertion leads to the formation of a π-allylNi(II) intermediate (III). The hydroamination product 3 is ultimately generated from the π-allylNi(II) complex by an amine nucleophilic attack (McDonald et al., 2011), accompanied by releasing of a Ni(0) species and regeneration of the acid cocatalyst.
Scheme 2.
Proposed Mechanism
Conclusion
In summary, we have developed a novel nickel and Brønsted acid-cocatalyzed asymmetric hydroamination reaction. The choice of chiral bisphosphine ligand and the use of a suitable Brønsted acid in catalytic amount are crucial to the success of this transformation. This protocol allows access to a series of enantiopure secondary and tertiary allylic amines from linear conjugated dienes and free amines. This method provides high enantioselectivity and a broad substrate scope for the synthesis of various chiral amines. Importantly, a set of complex amines have been accomplished with excellent chemo- and enantioselectivity in this system. The good functional group tolerance and the scalability demonstrates the potential of this method in the synthesis of enantiopure amines. Mechanistic studies indicate that the C-N bond formation is a reversible step. Moreover, racemization over time exists in the reaction with secondary amines but not for primary amines. We believe this chemistry will greatly benefit medicinal chemistry and further reaction development.
Limitations of the Study
The disubstituted diene was not suitable in this methodology.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Profs. Xumu Zhang, Hui Lv, and Xiuqin Dong at Wuhan University for lending laboratory space and sharing the basic instruments. We are grateful for the financial support from National Natural Science Foundation of China (21702151, 21871211) and the Fundamental Research Funds for Central Universities (2042019kf0208).
Author Contributions
G.Y. conceived the project and designed the experiments. J.L. discovered the reported process and designed and carried out almost all the experiments. P.W. participated in synthesizing partial substrates. W.W. helped in executing isotopic labeling studies, and Y.L. helped in analyzing the data. G.Y. wrote the manuscript. J.L. wrote Supplemental Information. All the authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.008.
Data and Code Availability
All data and methods can be found in the Supplemental Information.
Supplemental Information
References
- Adamson N.J., Hull E., Malcolmson S.J. Enantioselective intermolecular addition of aliphatic amines to acyclic dienes with a Pd–PHOX catalyst. J. Am. Chem. Soc. 2017;139:7180–7183. doi: 10.1021/jacs.7b03480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlin J.S.E., Cramer N. Chiral N-heterocyclic carbene ligand enabled nickel(0)-catalyzed enantioselective three-component couplings as direct access to silylated indanols. Org. Lett. 2016;18:3242–3245. doi: 10.1021/acs.orglett.6b01492. [DOI] [PubMed] [Google Scholar]
- Aillaud I., Collin J., Hannedouche J., Schulz E. Asymmetric hydroamination of non-activated carbon-carbon multiple bonds. Dalton Trans. 2007:5105–5118. doi: 10.1039/b711126f. [DOI] [PubMed] [Google Scholar]
- Ananikov V.P. Nickel: the “spirited horse” of transition metal catalysis. ACS Catal. 2015;5:1964–1971. [Google Scholar]
- Athira C., Changotra A., Sunoj R.B. Rhodium catalyzed asymmetric hydroamination of internal alkynes with indoline: mechanism, origin of enantioselectivity, and role of additives. J. Org. Chem. 2018;83:2627–2639. doi: 10.1021/acs.joc.7b03047. [DOI] [PubMed] [Google Scholar]
- Berthold D., Breit B. Chemo-, regio-, and enantioselective rhodium-catalyzed allylation of triazoles with internal alkynes and terminal allenes. Org. Lett. 2018;20:598–601. doi: 10.1021/acs.orglett.7b03708. [DOI] [PubMed] [Google Scholar]
- Berthold D., Geissler A.G.A., Giofre S., Breit B. Rhodium-catalyzed asymmetric intramolecular hydroamination of allenes. Angew. Chem. Int. Ed. 2019;58:9994–9997. doi: 10.1002/anie.201904833. [DOI] [PubMed] [Google Scholar]
- Bezzenine-Lafollee S., Gil R., Prim D., Hannedouche J. First-Row late transition metals for catalytic alkene hydrofunctionalisation: recent advances in C-N, C-O and C-P bond formation. Molecules. 2017;22:1901–1930. doi: 10.3390/molecules22111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Ye X., Liu S., Shi S.-L. Nickel/NHC-Catalyzed asymmetric C-H alkylation of fluoroarenes with alkenes: synthesis of enantioenriched fluorotetralins. Angew. Chem. Int. Ed. 2019 doi: 10.1002/anie.201907387. [DOI] [PubMed] [Google Scholar]
- Chen J., Lu Z. Asymmetric hydrofunctionalization of minimally functionalized alkenes via earth abundant transition metal catalysis. Org. Chem. Front. 2018;5:260–272. [Google Scholar]
- Chen Y.-G., Shuai B., Xu X.-T., Li Y.-Q., Yang Q.-L., Qiu H., Zhang K., Fang P., Mei T.-S. Nickel-catalyzed enantioselective hydroarylation and hydroalkenylation of styrenes. J. Am. Chem. Soc. 2019;141:3395–3399. doi: 10.1021/jacs.8b13524. [DOI] [PubMed] [Google Scholar]
- Cheng L., Li M.-M., Xiao L.-J., Xie J.-H., Zhou Q.-L. Nickel(0)-Catalyzed hydroalkylation of 1,3-dienes with simple ketones. J. Am. Chem. Soc. 2018;140:11627–11630. doi: 10.1021/jacs.8b09346. [DOI] [PubMed] [Google Scholar]
- Cheng X., Lu H., Lu Z. Enantioselective benzylic C-H arylation via photoredox and nickel dual catalysis. Nat. Commun. 2019;10:3549. doi: 10.1038/s41467-019-11392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L., Jerome H. First-Row late transition metals for catalytic (formal) hydroamination of unactivated alkenes. Synthesis. 2017;49:1158–1167. [Google Scholar]
- Cooke M.L., Xu K., Breit B. Enantioselective rhodium-catalyzed synthesis of branched allylic amines by intermolecular hydroamination of terminal allenes. Angew. Chem. Int. Ed. 2012;51:10876–10879. doi: 10.1002/anie.201206594. [DOI] [PubMed] [Google Scholar]
- Diesel J., Finogenova A.M., Cramer N. Nickel-catalyzed enantioselective pyridone C-H functionalizations enabled by a bulky N-heterocyclic carbene ligand. J. Am. Chem. Soc. 2018;140:4489–4493. doi: 10.1021/jacs.8b01181. [DOI] [PubMed] [Google Scholar]
- Diesel J., Grosheva D., Kodama S., Cramer N. A bulky chiral N-heterocyclic carbene nickel catalyst enables enantioselective C-H functionalizations of indoles and pyrroles. Angew. Chem. Int. Ed. 2019;58:11044–11048. doi: 10.1002/anie.201904774. [DOI] [PubMed] [Google Scholar]
- Dion I., Beauchemin A.M. Asymmetric brønsted acid catalysis enabling hydroaminations of dienes and allenes. Angew. Chem. Int. Ed. 2011;50:8233–8235. doi: 10.1002/anie.201102408. [DOI] [PubMed] [Google Scholar]
- Dondoni A. New feats of alkene and alkyne asymmetric hydroamination catalyzed by copper and rhodium hydrides. Asymmetric Catal. 2015;2:51–54. [Google Scholar]
- Donets P.A., Cramer N. Diaminophosphine oxide ligand enabled asymmetric nickel-catalyzed hydrocarbamoylations of alkenes. J. Am. Chem. Soc. 2013;135:11772–11775. doi: 10.1021/ja406730t. [DOI] [PubMed] [Google Scholar]
- Francotte E., Lindner W. Wiley-VCH Verlag Gmbh; 2006. Chirality in Drug Research. [Google Scholar]
- Grogan G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018;43:15–22. doi: 10.1016/j.cbpa.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Han X.-W., Zhang T., Zheng Y.-L., Yao W.-W., Li J.-F., Pu Y.-G., Ye M., Zhou Q.-L. Bronsted acid enabled nickel-catalyzed hydroalkenylation of aldehydes with styrene and its derivatives. Angew. Chem. Int. Ed. 2018;57:5068–5071. doi: 10.1002/anie.201801817. [DOI] [PubMed] [Google Scholar]
- Hannedouche J., Schulz E. Asymmetric hydroamination: a survey of the most recent developments. Chem. 2013;19:4972–4985. doi: 10.1002/chem.201203956. [DOI] [PubMed] [Google Scholar]
- Hannedouche J., Schulz E. Hydroamination and hydroaminoalkylation of alkenes by group 3–5 elements: recent developments and comparison with late transition metals. Organometallics. 2018;37:4313–4326. [Google Scholar]
- Hii K.K. Development of palladium catalysts for asymmetric hydroamination reactions. Pure Appl. Chem. 2006;78:341–349. [Google Scholar]
- Huang L., Arndt M., Gooßen K., Heydt H., Gooßen L.J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 2015;115:2596–2697. doi: 10.1021/cr300389u. [DOI] [PubMed] [Google Scholar]
- Huo J., He G., Chen W., Hu X., Deng Q., Chen D. A minireview of hydroamination catalysis: alkene and alkyne substrate selective, metal complex design. BMC Chem. 2019;13:89–101. doi: 10.1186/s13065-019-0606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome H. Mechanistic insights into first-row late transition metal-catalysed (formal) hydroamination of unactivated alkenes. Chimia. 2018;72:635–641. doi: 10.2533/chimia.2018.635. [DOI] [PubMed] [Google Scholar]
- Kathe P., Fleischer I. Cooperative use of brønsted acids and metal catalysts in tandem isomerization reactions of olefins. ChemCatChem. 2019;11:3343–3354. [Google Scholar]
- Li R., Ju C.-W., Zhao D. Rhodium(III) vs. Cobalt(III): a mechanistically distinct three-component C–H bond addition cascade using a Cp*RhIII catalyst. Chem. Commun. (Camb.) 2019;55:695–698. doi: 10.1039/c8cc08792j. [DOI] [PubMed] [Google Scholar]
- Li K., Li M.-L., Zhang Q., Zhu S.-F., Zhou Q.-L. Highly enantioselective nickel-catalyzed intramolecular hydroalkenylation of N- and O-tethered 1,6-dienes to form six-membered heterocycles. J. Am. Chem. Soc. 2018;140:7458–7461. doi: 10.1021/jacs.8b04703. [DOI] [PubMed] [Google Scholar]
- Li Y., Pang H., Wu D., Li Z., Wang W., Wei H., Fu Y., Yin G. Nickel-catalyzed 1,1-alkylboration of electronically unbiased terminal alkenes. Angew. Chem. Int. Ed. 2019;58:8872–8876. doi: 10.1002/anie.201903890. [DOI] [PubMed] [Google Scholar]
- Li W., Zhang X. springer verlag; 2014. Stereoselective Formation of Amines. [Google Scholar]
- Lin J.-S., Li T.-T., Jiao G.-Y., Gu Q.-S., Cheng J.-T., Lv L., Liu X.-Y. Chiral brønsted acid catalyzed dynamic kinetic asymmetric hydroamination of racemic allenes and asymmetric hydroamination of dienes. Angew. Chem. Int. Ed. 2019;58:7092–7096. doi: 10.1002/anie.201900955. [DOI] [PubMed] [Google Scholar]
- Liu W., Chen C., Zhang Q. Highly stereoselective synthesis of tetrasubstituted alkenes via hydroamination of alkynes and C–H acetoxylation. Org. Biomol. Chem. 2011;9:6484–6486. doi: 10.1039/c1ob05958k. [DOI] [PubMed] [Google Scholar]
- Liu X., Feng X. Dual nickel and brønsted acid catalysis for hydroalkenylation. Angew. Chem. Int. Ed. 2018;57:16604–16605. doi: 10.1002/anie.201810708. [DOI] [PubMed] [Google Scholar]
- Löber O., Kawatsura M., Hartwig J.F. Palladium-catalyzed hydroamination of 1,3-dienes: a colorimetric assay and enantioselective additions. J. Am. Chem. Soc. 2001;123:4366–4367. doi: 10.1021/ja005881o. [DOI] [PubMed] [Google Scholar]
- Lough W.J., Wainer I.W. Oxford University Press; 2002. Chirality in Natural and Applied Science. [Google Scholar]
- Lutete L.M., Kadota I., Yamamoto Y. Palladium-catalyzed intramolecular asymmetric hydroamination of alkynes. J. Am. Chem. Soc. 2004;126:1622–1623. doi: 10.1021/ja039774g. [DOI] [PubMed] [Google Scholar]
- Lv H., Xiao L.-J., Zhao D., Zhou Q.-L. Nickel(0)-Catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids. Chem. Sci. 2018;9:6839–6843. doi: 10.1039/c8sc02101e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R.I., Liu G., Stahl S.S. Palladium(II)-Catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T.E., Hultzsch K.C., Yus M., Foubelo F., Tada M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]
- Nugent T.C. Wiley-VCH Verlag Gmbh; 2010. Chiral Amine Synthesis: Methods, Developments and Applications. [Google Scholar]
- Nugent T.C., El-Shazly M. Chiral amine synthesis - recent developments and trends for enamide reduction, reductive amination, and imine reduction. Adv. Synth. Catal. 2010;352:753–819. [Google Scholar]
- Park S., Malcolmson S.J. Development and mechanistic investigations of enantioselective Pd-catalyzed intermolecular hydroaminations of internal dienes. ACS Catal. 2018;8:8468–8476. [Google Scholar]
- Parveen S., Li C., Hassan A., Breit B. Chemo-, regio-, and enantioselective rhodium-catalyzed allylation of pyridazinones with terminal allenes. Org. Lett. 2017;19:2326–2329. doi: 10.1021/acs.orglett.7b00718. [DOI] [PubMed] [Google Scholar]
- Patel M., Saunthwal R.K., Verma A.K. Base-mediated hydroamination of alkynes. Acc. Chem. Res. 2017;50:240–254. doi: 10.1021/acs.accounts.6b00449. [DOI] [PubMed] [Google Scholar]
- Patil M.D., Grogan G., Bommarius A., Yun H. Oxidoreductase-catalyzed synthesis of chiral amines. ACS Catal. 2018;8:10985–11015. [Google Scholar]
- Patil N.T., Lutete L.M., Wu H., Pahadi N.K., Gridnev I.D., Yamamoto Y. Palladium-catalyzed intramolecular asymmetric hydroamination, hydroalkoxylation, and hydrocarbonation of alkynes. J. Org. Chem. 2006;71:4270–4279. doi: 10.1021/jo0603835. [DOI] [PubMed] [Google Scholar]
- Pawlas J., Nakao Y., Kawatsura M., Hartwig J.F. A general nickel-catalyzed hydroamination of 1,3-dienes by alkylamines: catalyst selection, scope, and mechanism. J. Am. Chem. Soc. 2002;124:3669–3679. doi: 10.1021/ja017575w. [DOI] [PubMed] [Google Scholar]
- Pirnot M.T., Wang Y.M., Buchwald S.L. Copper hydride catalyzed hydroamination of alkenes and alkynes. Angew. Chem. Int. Ed. 2016;55:48–57. doi: 10.1002/anie.201507594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznichenko A.L., Hultzsch K.C. Hydroamination of alkenes. Organic Reactions. 2016;88:1–554. [Google Scholar]
- Richmond E., Moran J. Recent advances in nickel catalysis enabled by stoichiometric metallic reducing agents. Synthesis. 2018;50:499–513. [Google Scholar]
- Robak M.T., Herbage M.A., Ellman J.A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 2010;110:3600–3740. doi: 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- Tasker S.Z., Standley E.A., Jamison T.F. Recent advances in homogeneous nickel catalysis. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran G., Shao W., Mazet C. Ni-catalyzed enantioselective intermolecular hydroamination of branched 1,3-dienes using primary aliphatic amines. J. Am. Chem. Soc. 2019 doi: 10.1021/jacs.9b07253. During we are preparing this manuscript, a Ni-catalyzed enantioselective hydroamination of branched 1,3-dienes has been reported: [DOI] [PubMed] [Google Scholar]
- Wang T.-T., Wang F.-X., Yang F.-L., Tian S.-K. Palladium-catalyzed aerobic oxidative coupling of enantioenriched primary allylic amines with sulfonyl hydrazides leading to optically active allylic sulfones. Chem. Commun. (Camb.) 2014;50:3802–3805. doi: 10.1039/c4cc00275j. The absolute configuration of compound 3a was assigned by comparison of the optical rotation with that reported in the literature: [DOI] [PubMed] [Google Scholar]
- Wang W., Ding C., Li Y., Li Z., Li Y., Peng L., Yin G. Migratory arylboration of unactivated alkenes enabled by nickel catalysis. Angew. Chem. Int. Ed. 2019;58:4612–4616. doi: 10.1002/anie.201814572. [DOI] [PubMed] [Google Scholar]
- Wang Z. Nickel-based catalysts. RSC Green Chemistry Series. 2016;38:407–468. [Google Scholar]
- Woźniak Ł., Cramer N. Enantioselective C-H bond functionalizations by 3d transition-metal catalysts. Trends Chem. 2019;1:471–484. [Google Scholar]
- Xiao L.-J., Fu X.-N., Zhou M.-J., Xie J.-H., Wang L.-X., Xu X.-F., Zhou Q.-L. Nickel-catalyzed hydroacylation of styrenes with simple aldehydes: reaction development and mechanistic insights. J. Am. Chem. Soc. 2016;138:2957–2960. doi: 10.1021/jacs.6b00024. [DOI] [PubMed] [Google Scholar]
- Xiao L.-J., Ye M.-C., Zhou Q.-L. Nickel-catalyzed highly atom-economical C–C coupling reactions with π components. Synlett. 2018;30:361–369. [Google Scholar]
- Xiong Y., Sun Y., Zhang G. Recent advances on catalytic asymmetric difunctionalization of 1,3-dienes. Tetrahedron Lett. 2018;59:347–355. [Google Scholar]
- Xu C., Feng Y., Li F., Han J., He Y.-M., Fan Q.-H. A synthetic route to chiral benzo-fused N-heterocycles via sequential intramolecular hydroamination and asymmetric hydrogenation of anilino-alkynes. Organometallics. 2019 [Google Scholar]
- Xu K., Wang Y.H., Khakyzadeh V., Breit B. Asymmetric synthesis of allylic amines via hydroamination of allenes with benzophenone imine. Chem. Sci. 2016;7:3313–3316. doi: 10.1039/c5sc04984a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-H., Dong V.M. Rhodium-catalyzed hydrofunctionalization: enantioselective coupling of indolines and 1,3-dienes. J. Am. Chem. Soc. 2017;139:1774–1777. doi: 10.1021/jacs.6b12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.-B., Yang X.-T., Ma J.-B., Su Z.-M., Shi S.-L. Regio- and enantioselective C-H cyclization of pyridines with alkenes enabled by a nickel/N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 2019;141:5628–5634. doi: 10.1021/jacs.9b00931. [DOI] [PubMed] [Google Scholar]
- Zhou J., Hartwig J.F. Intermolecular, catalytic asymmetric hydroamination of bicyclic alkenes and dienes in high yield and enantioselectivity. J. Am. Chem. Soc. 2008;130:12220–12221. doi: 10.1021/ja803523z. [DOI] [PubMed] [Google Scholar]
- Zi G. Asymmetric hydroamination/cyclization catalyzed by organolanthanide complexes with chiral biaryl-based ligands. Dalton Trans. 2009;42:9101–9109. doi: 10.1039/b906588a. [DOI] [PubMed] [Google Scholar]
- Zi G.F. Asymmetric hydroamination/cyclization catalyzed by group 4 metal complexes with chiral biaryl-based ligands. J. Organomet. Chem. 2011;696:68–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and methods can be found in the Supplemental Information.