Highlights
-
•
Scoring the tumor-stroma ratio is a simple and reproducible method.
-
•
Tumor-stroma ratio and response to neoadjuvant chemoradiotherapy are correlated.
-
•
Stroma-low tumors are likely to respond better to neoadjuvant chemoradiotherapy.
Keywords: Esophageal cancer, Neoadjuvant chemoradiotherapy, Pathologic response, Prediction, Tumor-stroma ratio
Abstract
Background and purpose
With currently available techniques, the prediction of pathologic complete response after neoadjuvant chemoradiotherapy is insufficient. The tumor-stroma ratio (TSR) has proven to be a predictor of survival for several types of cancer, including esophageal. The aim of this study was to investigate the value of TSR in predicting pathologic response after neoadjuvant chemoradiotherapy in esophageal cancer patients.
Materials and methods
Patients with esophageal adenocarcinoma or squamous cell carcinoma who received neoadjuvant chemoradiotherapy followed by a resection were selected. Haematoxylin and eosin (H&E) stained sections of diagnostic biopsies were collected and TSR was independently assessed by two investigators. Patients were categorized in stroma-low (≤50% stroma) and stroma-high (>50% stroma) groups for further analyses. The tumor regression grade (TRG) was assessed on H&E stained sections of the resected primary tumor to determine pathologic response.
Results
A total of 94 patients were included in this study, of which 76 patients were categorized as stroma-low and 18 as stroma-high. Forty-two (45%) patients had a major pathologic response (TRG 1–2), whereas 52 (55%) were considered non-responders. After adjustment for gender, tumor type, cT-status and differentiation grade, patients with a stroma-high tumor showed a higher chance of no response compared to patients with a stroma-low tumor (OR 3.57, 95%CI 1.03–12.31, P = 0.04).
Conclusion
TSR showed to have the potential to aid in the prediction of pathologic response in esophageal cancer patients receiving neoadjuvant chemoradiotherapy. Larger validation studies are necessary before implementing this method in daily practice.
1. Introduction
Esophageal cancer is the 9th most common cancer, affecting >570.000 people each year worldwide, and the 6th most common cause of cancer related deaths [1]. Currently, the standard treatment for patients with resectable disease is neoadjuvant chemoradiotherapy (nCRT) followed by surgery. Since the addition of nCRT as part of esophageal cancer treatment, survival improved compared to patients who only underwent surgery [2], [3]. Treatment with nCRT leads to a pathologic complete response (pCR) in approximately 30% of patients, were another 30% of patients reach a near complete response [4]. It is debatable whether these patients should receive an additional resection or whether they should be followed up by an active wait-and-see procedure [5], [6], [7], [8]. Achieving pCR proved to be associated with improved survival in patients with esophageal cancer [9]. In contrast, non-responders on nCRT have no survival benefit compared to primary surgery alone, but are still exposed to the potential side effects of nCRT [10], [11], [12]. Hence, it is important to define factors that predict whether or not a patient with esophageal cancer will benefit of nCRT.
Several imaging studies with endoscopic ultrasonography (EUS), computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) tried to assess the response to nCRT in patients with esophageal cancer. Unfortunately, for EUS and CT no predictive capacity could be found, whereas for FDG-PET the results were contradictory [7], [13], [14]. Moreover, a meta-analysis on the use of endoscopic biopsy and EUS for the detection of residual disease after nCRT, in order to use an organ-preserving approach, revealed both methods not suitable (yet) for withholding surgery [15]. Furthermore, there is an increase in the number of molecular and genetic studies aiming to identify markers that will predict the pathologic response after nCRT. These studies showed promising results, but need to be validated before implementation in clinical routine [16], [17], [18].
In the past decades, cancer research mainly focused on the malignant cell itself by understanding the role of tumor suppressor and oncogenic factors in the transformation to malignancy. Currently the stromal part of the tumor is subject of investigation. It is increasingly known that the malignant cell relies on the so-called tumor microenvironment (TME) and therefore does not act alone. Intratumoral stroma within the TME is variable and different cell-types like infiltrating immune cells, cancer-associated fibroblasts, endothelial cells and pericytes all play a role in supporting malignant transformation, invasion of the tumor and metastasis [19], [20]. Some studies have demonstrated that intratumoral stroma is associated with reduced chemotherapy delivery [21] and increased chemotherapy resistance [22] and consequently could play a role in patient treatment outcome.
Furthermore, different studies found that the tumor-stroma ratio (TSR) is an independent predictor of survival in different types of carcinomas, for instance colon [23] and esophageal cancer [24], [25]. A high proportion of stroma is associated with poor clinical outcome. A study for assessment of TSR in esophageal biopsy specimens has been performed by Courrech Staal et al. [26], which showed that scoring TSR in biopsy specimens is representative and reproducible. The relationship between the proportion of tumor in diagnostic biopsies and the pathologic response has been studied in esophageal cancer patients, who received neoadjuvant chemotherapy followed by resection [27]. However, the relationship between TSR and pathologic response after concurrent chemotherapy and radiotherapy has, to our knowledge, not yet been investigated.
The aim of this current study was to evaluate the association of TSR in pre-treatment biopsies and the pathologic response after nCRT in esophageal cancer patients.
2. Materials and methods
2.1. Patients and tissue material
The retrospective patient cohort consisted of consecutive patients with esophageal cancer clinical stage I–III with adenocarcinoma or squamous cell carcinoma, who underwent neoadjuvant chemoradiotherapy followed by resection at the Leiden University Medical Centre (LUMC) between 2010 and 2016. The cohort was part of an existing study cohort available in the LUMC, which ended including patients at the end of 2016. Patients were diagnosed with an esophagogastroduodenoscopy and a biopsy for histological confirmation. All patients underwent external beam radiotherapy using a 3D conformal planning with a four-field box technique. A total dose of 41.4 Gy was given in 23 fractions of 1.8 Gy, 5 fractions per week. Concurrent chemotherapy consisted of 5 weekly administrations of Carboplatin (AUC 2) and Paclitaxel (50 mg/m2) [2]. Clinical data were retrospectively collected from the electronic patient files. Haematoxylin and eosin (H&E) stained pre-operative biopsies taken from the primary tumor were collected together with the related resection specimens from the Department of Pathology of the LUMC. In case of referred patients, the original biopsy slides were collected from regional hospitals using the Dutch Pathology Registry (PALGA) [28]. All tissue samples were coded and handled according to ethical standards (‘Code for Proper Secondary Use of Human Tissue’, Dutch Federation of Medical Scientific Societies). This study was approved by the Medical Ethics Committee of the LUMC.
2.2. Histopathological procedure
For determining the TSR, 3 μm H&E-stained sections of biopsy specimens were microscopically analyzed using a 2.5× or 5× objective to select the part with the largest amount of stroma. Then, with the 10× objective the image fields were scored for the percentage of stroma by increments of 10%. Tumor cells had to be present at 4 borders of the field of vision. When multiple sections per patient were available, all biopsies were assessed for TSR. The highest score was decisive for final stroma classification. All biopsies were independently assessed by two investigators (GvP, JK). After six weeks, one investigator (JK) assessed all samples a second time to determine intra-observer variation. A cut-off value of 50% stroma was used to categorize patients as stroma-low (≤50%) or stroma-high (>50%) as determined in earlier research to be most discriminative [29]. In case consensus could not be reached, a third observer (expert pathologist, AFS) was decisive.
The response to nCRT was assessed on the primary tumor resection specimens by a gastrointestinal pathologist using the tumor regression grade (TRG) defined by Mandard [30]. This classification is defined by 5 categories. TRG 1 is defined as complete regression with no residual cancer but only fibrosis through all layers of the esophageal wall and is called pathologic complete response (pCR). TRG 2 is characterized by scattered residual cancer cells or groups of cells within the fibrosis. TRG 3 shows an increase of residual cancer cells but fibrosis predominates. TRG 4 is characterized by residual cancer outgrowing the fibrosis. TRG 5 is defined by absence of any regressive changes. The TRG scores were taken from the clinical reports, however, they were all determined by the same, experienced pathologist (AFS).
2.3. Statistics
IBM SPSS version 25.0 (Statistical Package for the Social Sciences, Chicago, IL) was used for statistical analysis. Differences in categorical variables between patient, tumor and treatment characteristics for the TRG groups were analyzed using the Fisher’s exact test or the Chi-square test. For continuous variables the Mann-Whitney test was used. Inter- and intra-observer variability was performed using the Cohen’s Kappa coefficient (Κ). TRG was dichotomized in TRG 1–2 (major responders) and TRG 3–5 (non-responders), as found to be of prognostic significance as well [31]. Uni- and multivariable logistic regression analyses were performed to investigate the relationship between TSR and other baseline factors for a major response. Factors known to be predictive for pathologic response (gender, tumor type, cT-stage and differentiation grade) were added to a multivariable model [32], [33]. For multivariable analysis, missing cases for cT-stage (cTx, N = 6) were imputed using the mode as default. A two-tailed P value ≤0.05 was considered significant in all analyses.
3. Results
3.1. Patient and tumor characteristics
The cohort consisted of 115 patients. Thirteen cases (11%) were excluded as invasive carcinoma within the biopsy could not be established with certainty. In 8 cases (7%) TSR could not be assessed due to insufficient quality of the tissue, leaving a total of 94 patients available for analysis. Median age was 64 years (range 25–82) at the start of nCRT, 76% (N = 71) were men and 80% (N = 75) of the tumors were adenocarcinoma. All patients completed radiotherapy as intended. However, 13 patients (14%) received <5 cycles of chemotherapy (Table 1).
Table 1.
Patients, tumor and treatment characteristics, stratified by tumor regression grade (TRG).
| Total N = 94 (%) |
TRG 1–2 N = 42 (%) |
TRG 3–5 N = 52 (%) |
P-value |
|
|---|---|---|---|---|
| Gender | ||||
| Male | 71 (76) | 28 (67) | 43 (83) | 0.07 |
| Female | 23 (25) | 14 (33) | 9 (17) | |
| Median age (years)[range] | 64 [25–82] | 64 [39–74] | 65 [25–82] | 0.69 |
| Weight loss at presentation | ||||
| None | 29 (31) | 18 (43) | 11 (21) | 0.06 |
| ≤10% | 42 (45) | 14 (33) | 28 (54) | |
| ˃10% | 23 (25) | 10 (24) | 13 (25) | |
| Alcohol consumption | ||||
| None or stopped | 28 (30) | 14 (33) | 14 (27) | 0.78 |
| Yes | 64 (68) | 27 (64) | 37 (71) | |
| Unknown | 2 (2) | 1 (2) | 1 (2) | |
| Smoking | ||||
| Never or stopped | 57 (61) | 25 (60) | 32 (62) | 0.63 |
| Yes | 36 (38) | 17 (41) | 19 (37) | |
| Unknown | 1 (1) | 0 (0) | 1 (2) | |
| Tumor location | ||||
| GEJ | 11 (12) | 3 (7) | 8 (15) | 0.46 |
| Middle | 9 (10) | 4 (10) | 5 (10) | |
| Low | 74 (79) | 35 (83) | 39 (75) | |
| Median length tumora(cm) [range] | 5 [1–11] | 5 [2–11] | 6 [1–10] | 0.53 |
| Histology | ||||
| Adenocarcinoma | 75 (80) | 32 (76) | 43 (83) | 0.44 |
| Squamous cell carcinoma | 19 (20) | 10 (24) | 9 (17) | |
| Cycles of chemotherapy | ||||
| <5 cycles | 13 (14) | 4 (10) | 9 (17) | 0.28 |
| 5 cycles | 81 (86) | 38 (91) | 43 (83) | |
| Median time interval between nCRT and surgery (days)[range] | 44 [25–85] | 43 [25–58] | 47 [31–85] | 0.12 |
| cT status | ||||
| cT2 | 16 (17) | 7 (20) | 9 (6) | 0.84 |
| cT3 | 72 (77) | 33 (74) | 39 (89) | |
| cTx | 6 (6) | 2 (7) | 4 (6) | |
| cN status | ||||
| cN0 | 23 (25) | 8 (19) | 15 (29) | 0.48 |
| cN1 | 42 (45) | 19 (45) | 23 (44) | |
| cN2 | 28 (30) | 14 (33) | 14 (27) | |
| cN3 | 1 (1) | 1 (2) | 0 (0) | |
| Differentiation grade | ||||
| Well/Moderate | 44 (47) | 20 (48) | 24 (47) | 0.98 |
| Poor | 50 (53) | 22 (52) | 28 (53) |
Abbreviations: GEJ: Gastro-esophageal junction.
Tumor length was determined by endoscopy. If tumor length by endoscopy was not reported, tumor length on CT scan was used instead.
3.2. Histopathology
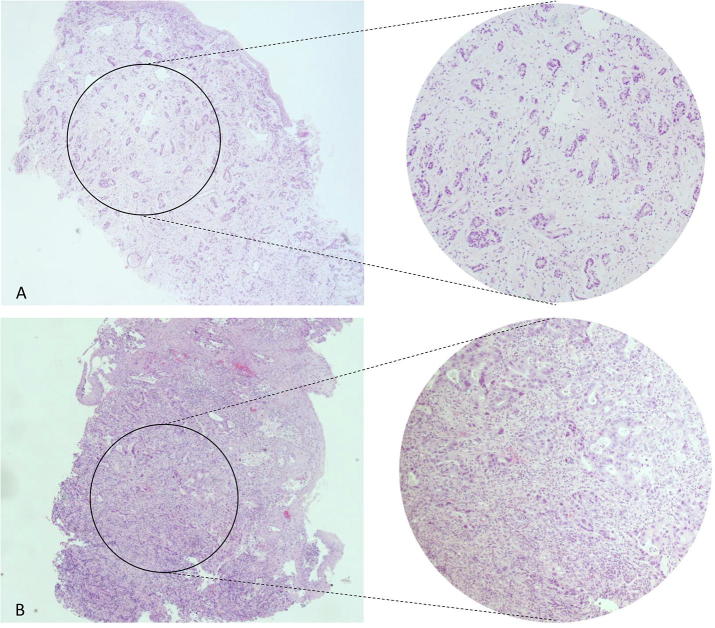
A total of 142 H&E biopsy sections of 94 patients were available and evaluated. Seventy-six patients (81%) were categorized as stroma-low and 18 patients (19%) as stroma-high. Fig. 1 shows examples of stroma-low and -high tumor biopsies. Intra-observer agreement was good (Κ = 0.81, 93% agreement), whereas a substantial inter-observer agreement was found for the assessment of TSR (Κ = 0.73, 91% agreement). In 5 out of 9 discrepant cases, consensus could not be reached and the pathologists’ assessment was decisive.
Fig. 1.
H&E stained biopsy sections of esophageal carcinoma. (A) Represents a tumor with high stromal proliferation (stroma-high). As shown by the magnification on the right there is evident stromal proliferation between the tumor cells. (B) Shows a tumor with few spots of stromal tissue (stroma-low). The magnification shows almost no stromal proliferation between tumor cells.
The assessment of the pathological response revealed 28 cases (29.8%) to have a complete pathologic response (TRG 1) whereas 2 cases did not show any regressive changes at all (TRG 5). The other cases were categorized as TRG 2 (N = 14), TRG 3 (N = 31) and TRG 4 (N = 19), respectively. After dichotomization, 42 cases were classified as major pathologic responders (TRG 1–2), whereas 52 cases were considered non-responders (TRG 3–5). The distribution of TRG categories versus TSR classification is shown in Table 2 and Fig. 2.
Table 2.
Distribution of TRG categories versus TSR categories.
| Major pathologic responders |
Non-responders |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| TRG 1 | TRG 2 | TRG 1–2 | TRG 3 | TRG 4 | TRG 5 | TRG 3–5 | ||
| Stroma-low | 25 | 13 | 38 (50%) | 23 | 13 | 2 | 38 (50%) | 76 |
| Stroma-high | 3 | 1 | 4 (22%) | 8 | 6 | 0 | 14 (78%) | 18 |
| Total | 28 | 14 | 42 (45%) | 31 | 19 | 2 | 52 (55%) | 94 |
Fig. 2.
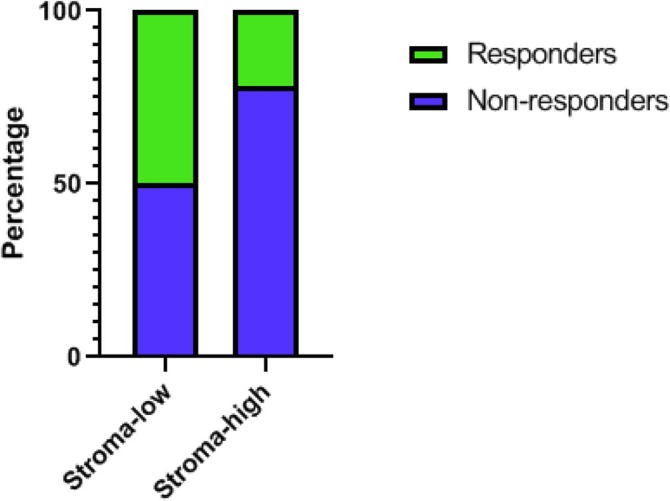
The distribution of pathologic major responders within the stroma categories. The percentage of responders (in green) versus non-responders (in blue) within stroma-low and stroma-high categories, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. TSR and other predictive factors for pathologic response
No significant differences in baseline characteristics and possible predictors of pathologic response were seen between both TRG groups (Table 1). However, there was a significant difference for TSR between the group TRG 1–2 and the group TRG 3–5 (P = 0.033).
As shown in Table 2, 78% (14/18) of the patients with a stroma-high tumor did not have a response to nCRT, whereas patients with a stroma-low tumor have only a 50% chance on a pathologic major response. In univariable analyses TRG 3–5 was used as reference category for all factors that potentially could influence pathologic response. Univariable analyses showed a significant higher chance for patients with a stroma-high tumor to have no response to nCRT (OR 3.50, 95%CI 1.06–11.61, P = 0.04). In multivariable analysis, after adjusting for gender, histology, differentiation grade and clinical T-stage, a stroma-high tumor remained an independent predictive factor for a higher chance of no response to nCRT (OR 3.57, 95%CI 1.03–12.31, P = 0.04) (Table 3).
Table 3.
Uni- and multivariable logistic regression analyses for TRG group 3–5.
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | AOR | 95% CI | P-value | |
| Gender | ||||||
| Male | Ref | |||||
| Female | 2.39 | 0.91–6.26 | 0.08 | |||
| Age (years) | 0.99 | 0.95–1.04 | 0.85 | |||
| Weight loss at presentation | ||||||
| None | Ref | |||||
| ≤10% | 0.31 | 0.11–0.82 | 0.02 | |||
| ˃10% | 0.47 | 0.15–1.43 | 0.18 | |||
| Alcohol consumption | ||||||
| None or stopped | Ref | |||||
| Yes | 0.73 | 0.30–1.78 | 0.49 | |||
| Smoking | ||||||
| Never or stopped | Ref | |||||
| Yes | 1.15 | 0.50–2.65 | 0.75 | |||
| Tumor location | ||||||
| GEJ | Ref | |||||
| Middle | 2.13 | 0.33–13.81 | 0.43 | |||
| Low | 2.39 | 0.59–9.74 | 0.22 | |||
| Length tumor (cm) | 0.97 | 0.81–1.17 | 0.78 | |||
| Histology | ||||||
| Adenocarcinoma | Ref | |||||
| Squamous cell carcinoma | 1.49 | 0.54–4.10 | 0.44 | |||
| Cycles of chemotherapy | ||||||
| <5 cycles | Ref | |||||
| 5 cycles | 1.99 | 0.57–6.98 | 0.28 | |||
| cT status | ||||||
| cT2 | Ref | |||||
| cT3 | 1.05 | 0.35–3.09 | 0.93 | |||
| cN status | ||||||
| cN0 | Ref | |||||
| cN+ | 1.72 | 0.65–4.57 | 0.28 | |||
| Differentiation grade | ||||||
| Well/Moderate | Ref | |||||
| Poor | 0.94 | 0.42–2.13 | 0.89 | |||
| Tumor-stroma ratio | ||||||
| Stroma-low | Ref | Ref | ||||
| Stroma-high | 3.50 | 1.06–11.61 | 0.04 | 3.57 | 1.03–12.31 | 0.04 |
Abbreviations: OR: Odds ratio; CI: Confidence interval; TRG: Tumor regression grade; GEJ: Gastro-esophageal junction; AOR: Adjusted odds ratio.
4. Discussion
Our results show that patients with high stromal tumors have a significantly higher chance to not respond on nCRT (TRG 3–5) compared to patients with tumors with a low amount of stroma. Seventy-eight percent of the stroma-high patients did not have a response on nCRT. This suggests that assessment of TSR could fulfill a role in identifying patients that will or will not respond well to nCRT, next to currently used (imaging) methods, adding to the realization of personalized medicine. It could be possible that stroma-high tumors represent a group of tumors with an environment that is well armed against chemoradiation, or even become resistant to therapy [34]. This might indicate that, for obtaining a pathologic response in stroma-high tumors, it might be necessary to adjust the current therapy strategy. For instance, these tumors could be future candidates for therapies targeting the stromal compartment of the tumor, by targeting activated oncogenic pathways (e.g. the TGF-β or PDGFR pathway), angiogenesis (VEGF) or cancer associated fibroblasts [35]. Another option could be not to treat these patients with nCRT and continue with resection instead, thereby avoiding exposing the patients to the side effects of chemoradiation treatment.
There is evidence that the interaction between cancer cells and the TME can affect sensitivity of the cancer cells to chemotherapy [36] and radiotherapy [37]. However, the exact underlying mechanisms and interactions within the TME and their role in protection of cancer cells from eradicating therapy have to be further explored.
Several phase I and II studies are currently ongoing targeting different components of the TME of advanced esophageal carcinoma, e.g. angiogenesis, immune cells and stroma. However, as the TME has the paradoxical capacity to both promote and inhibit tumor growth and progression, effective intervention can be challenging [38].
Our results are in contrast with those of the study of Hale et al., who found a high proportion of tumor (PoT) (=stroma-low) in the diagnostic biopsy to be associated with no evidence of tumor regression (TRG 4 or 5) after neoadjuvant chemotherapy [27]. However, this relationship was only found when the PoT was analyzed as continuous variable. Furthermore, the assessment of PoT was performed with a different (semi-automated) method. In addition, the TRG was categorized into different categories compared to our study (TRG 1, 2, 3/TRG 4, 5 versus TRG 1, 2/TRG 3, 4, 5, respectively).
Previous studies identified female gender, squamous cell carcinoma and cT1-2 stage as favorable factors in the prediction of complete pathologic response [32], [33]. However, in our current study none of these factors were significantly associated with pathologic tumor response grading. This might be explained by the smaller number of cases in our study.
We showed that assessment of TSR is simple and reliable as demonstrated by the substantial to good inter- and intra-observer agreement, allowing it to be easily implemented in routine pathology diagnostics.
A limitation of this study is the retrospective nature and the small sample size (N = 94) which means that results should be interpreted with caution. Furthermore, not all biopsy material suitable for diagnosing cancer, is suitable for assessment of TSR. Stroma has to be surrounded by tumor cells at four sides of the microscopic field in order to score TSR, which is not always possible with biopsy specimens. This might be solved by visually diminishing the field of vision and determine whether more stroma is present in comparison to tumor or vice versa. Still, approximately 11–18% of the biopsies are not suitable for TSR scoring (this study and [26]). Nevertheless, it seems that TSR predicts pathologic response after nCRT independently of other well-known factors.
In conclusion, this study shows that TSR might be an additional parameter in the prediction of pathologic response in esophageal cancer patients treated with nCRT. This relationship needs further exploration and validation in a larger population, preferably prospective, before implementing TSR as a novel predictor of pathologic response in daily practice.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
G.W. van Pelt, Email: g.w.van_pelt@lumc.nl.
I.M. Lips, Email: i.m.lips@lumc.nl.
F.P. Peters, Email: f.p.peters@lumc.nl.
D. van Klaveren, Email: d.van_klaveren@lumc.nl.
J.J. Boonstra, Email: j.j.boonstra@lumc.nl.
W.O. de Steur, Email: w.o.de_steur@lumc.nl.
R.A.E.M. Tollenaar, Email: r.a.e.m.tollenaar@lumc.nl.
A. Farina Sarasqueta, Email: a.farina@lumc.nl.
W.E. Mesker, Email: w.e.mesker@lumc.nl.
M. Slingerland, Email: m.slingerland@lumc.nl.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro J., van Lanschot J.J.B., Hulshof M., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Sjoquist K.M., Burmeister B.H., Smithers B.M., Zalcberg J.R., Simes R.J., Barbour A. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Castoro C., Scarpa M., Cagol M., Alfieri R., Ruol A., Cavallin F. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg. 2013;17:1375–1381. doi: 10.1007/s11605-013-2269-3. [DOI] [PubMed] [Google Scholar]
- 6.Furlong H., Bass G., Breathnach O., O'Neill B., Leen E., Walsh T.N. Targeting therapy for esophageal cancer in patients aged 70 and over. J Geriatr Oncol. 2013;4:107–113. doi: 10.1016/j.jgo.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Noordman B.J., Spaander M.C.W., Valkema R., Wijnhoven B.P.L., van Berge Henegouwen M.I., Shapiro J. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19:965–974. doi: 10.1016/S1470-2045(18)30201-8. [DOI] [PubMed] [Google Scholar]
- 8.Taketa T., Xiao L., Sudo K., Suzuki A., Wadhwa R., Blum M.A. Propensity-based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology. 2013;85:95–99. doi: 10.1159/000351999. [DOI] [PubMed] [Google Scholar]
- 9.Donahue J.M., Nichols F.C., Li Z., Schomas D.A., Allen M.S., Cassivi S.D. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–398. doi: 10.1016/j.athoracsur.2008.11.001. discussion 8-9 10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Bakker C.M., Smit J.K., Bruynzeel A.M.E., van Grieken N.C.T., Daams F., Derks S. Non responders to neoadjuvant chemoradiation for esophageal cancer: why better prediction is necessary. J Thorac Dis. 2017;9:S843–S850. doi: 10.21037/jtd.2017.06.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittrick G.W., Weber J.M., Shridhar R., Hoffe S., Melis M., Almhanna K. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrate no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol. 2012;19:1678–1684. doi: 10.1245/s10434-011-2078-4. [DOI] [PubMed] [Google Scholar]
- 12.Hsu P.K., Chien L.I., Huang C.S., Hsieh C.C., Wu Y.C., Hsu W.H. Comparison of survival among neoadjuvant chemoradiation responders, non-responders and patients receiving primary resection for locally advanced oesophageal squamous cell carcinoma: does neoadjuvant chemoradiation benefit all? Interact Cardiovasc Thorac Surg. 2013;17:460–466. doi: 10.1093/icvts/ivt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwee R.M. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology. 2010;254:707–717. doi: 10.1148/radiol.09091324. [DOI] [PubMed] [Google Scholar]
- 14.Westerterp M., van Westreenen H.L., Reitsma J.B., Hoekstra O.S., Stoker J., Fockens P. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology. 2005;236:841–851. doi: 10.1148/radiol.2363041042. [DOI] [PubMed] [Google Scholar]
- 15.van Rossum P.S., Goense L., Meziani J., Reitsma J.B., Siersema P.D., Vleggaar F.P. Endoscopic biopsy and EUS for the detection of pathologic complete response after neoadjuvant chemoradiotherapy in esophageal cancer: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:866–879. doi: 10.1016/j.gie.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Ajani J.A., Wang X., Song S., Suzuki A., Taketa T., Sudo K. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol. 2014;8:142–149. doi: 10.1016/j.molonc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu F.M., Cheng J.C., Chang Y.L., Lee J.M., Koong A.C., Chuang E.Y. Circulating mRNA profiling in esophageal squamous cell carcinoma identifies FAM84B as a biomarker in predicting pathological response to neoadjuvant chemoradiation. Sci Rep. 2015;5:10291. doi: 10.1038/srep10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner H.D., Lee J.H., Bhutani M.S., Weston B., Hofstetter W., Komaki R. A validated miRNA profile predicts response to therapy in esophageal adenocarcinoma. Cancer. 2014;120:3635–3641. doi: 10.1002/cncr.28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bremnes R.M., Donnem T., Al-Saad S., Al-Shibli K., Andersen S., Sirera R. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale M.D., Hayden J.D., Grabsch H.I. Tumour-microenvironment interactions: role of tumour stroma and proteins produced by cancer-associated fibroblasts in chemotherapy response. Cell Oncol (Dordr) 2013;36:95–112. doi: 10.1007/s13402-013-0127-7. [DOI] [PubMed] [Google Scholar]
- 23.Mesker W.E., Liefers G.J., Junggeburt J.M., van Pelt G.W., Alberici P., Kuppen P.J. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol. 2009;31:169–178. doi: 10.3233/CLO-2009-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Courrech Staal E.F., Wouters M.W., van Sandick J.W., Takkenberg M.M., Smit V.T., Junggeburt J.M. The stromal part of adenocarcinomas of the oesophagus: does it conceal targets for therapy? Eur J Cancer. 2010;46:720–728. doi: 10.1016/j.ejca.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Wang K., Ma W., Wang J., Yu L., Zhang X., Wang Z. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol. 2012;7:1457–1461. doi: 10.1097/JTO.0b013e318260dfe8. [DOI] [PubMed] [Google Scholar]
- 26.Courrech Staal E.F., Smit V.T., van Velthuysen M.L., Spitzer-Naaykens J.M., Wouters M.W., Mesker W.E. Reproducibility and validation of tumour stroma ratio scoring on oesophageal adenocarcinoma biopsies. Eur J Cancer. 2011;47:375–382. doi: 10.1016/j.ejca.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 27.Hale M.D., Nankivell M., Hutchins G.G., Stenning S.P., Langley R.E., Mueller W. Biopsy proportion of tumour predicts pathological tumour response and benefit from chemotherapy in resectable oesophageal carcinoma: results from the UK MRC OE02 trial. Oncotarget. 2016;7:77565–77575. doi: 10.18632/oncotarget.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casparie M., Tiebosch A.T., Burger G., Blauwgeers H., van de Pol A., van Krieken J.H. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesker W.E., Junggeburt J.M., Szuhai K., de Heer P., Morreau H., Tanke H.J. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387–398. doi: 10.1155/2007/175276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandard A.M., Dalibard F., Mandard J.C., Marnay J., Henry-Amar M., Petiot J.F. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Barbour A.P., Jones M., Gonen M., Gotley D.C., Thomas J., Thomson D.B. Refining esophageal cancer staging after neoadjuvant therapy: importance of treatment response. Ann Surg Oncol. 2008;15:2894–2902. doi: 10.1245/s10434-008-0084-y. [DOI] [PubMed] [Google Scholar]
- 32.Ajani J.A., Correa A.M., Hofstetter W.L., Rice D.C., Blum M.A., Suzuki A. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–2642. doi: 10.1093/annonc/mds210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toxopeus E.L., Nieboer D., Shapiro J., Biermann K., van der Gaast A., van Rij C.M. Nomogram for predicting pathologically complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Radiother Oncol. 2015;115:392–398. doi: 10.1016/j.radonc.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Lotti F., Jarrar A.M., Pai R.K., Hitomi M., Lathia J., Mace A. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Zhang G., Wang J., Wang L., Huang X., Cheng Y. The role of cancer-associated fibroblasts in esophageal cancer. J Transl Med. 2016;14:30. doi: 10.1186/s12967-016-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castells M., Thibault B., Delord J.P., Couderc B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci. 2012;13:9545–9571. doi: 10.3390/ijms13089545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellevik T., Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front. Oncol. 2014;4:1. doi: 10.3389/fonc.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin E.W., Karakasheva T.A., Hicks P.D., Bass A.J., Rustgi A.K. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337–5349. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]