Abstract
The hybrid variety of Cucurbita maxima is commercially available and frequently used as food in Bangladesh compared to the native variety. Although the seeds of Cucurbita maxima have nutritional and therapeutic values, people in Bangladesh typically throw them away as waste. If we can explore their physicochemical and biochemical properties, this will add new knowledge to nutrition and food science. Thus, in this study, we compare the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil. Solvent extraction methods were used to obtain oil. The physicochemical properties of the extracted seed oils were examined by titration methods and proximate compositions of pumpkin seeds were determined using the standard method. Tocopherols were analyzed by HPLC and fatty acids were identified by GC/MS as N-acylpyrolidines. The specific gravity, refractive index, viscosity, acid value, saponification value, iodine value and ester values were determined and among them iodine values and acid values were significant (p < 0.05) at native but saponification and ester values were very highly significantly (p < 0.001) at hybrid. The comparative lipid content in the seed of native variety was higher than hybrid (p < 0.01) and protein contents were higher but not significant. However, crude fiber content was higher in the hybrid (p < 0.01). There was no significant differences of moisture, ash, sugar and starch contents in between the two varieties. The total tocopherol, contents were very high (p < 0.001) in native. In hybrid, saturated fatty acids -capric acid, myristic acids were more (p < 0.001) but in native unsaturated fatty acid linoleic and linolenic acids were predominant (p < 0.05). Owing to the considerable differences in the physicochemical composition, the fatty acids and other properties, this study may suggest that the both varieties of pumpkin seed oil may be an alternative good source of edible oil.
Keywords: Food science, Chemistry, Agriculture, Pumpkin seed oil, Physicochemical properties, Unsaturated fatty acid, Native, Hybrid
Food Science; Chemistry; Agriculture; Pumpkin seed oil; Physicochemical properties; Unsaturated fatty acid; Native; Hybrid.
1. Introduction
Pumpkin (Curcurbita maxima L.) belong to the family cucurbitaceae is a popular vegetable in Bangladesh like all over the world. Two varieties (native and hybrid) of pumpkin (Curcurbita maxima) are widely cultivated and used as food sources in Bangladesh. Farmers are more interested in cultivating the hybrid variety due to its low cultivation cost and high production rate. As a result, hybrids are available on the market when compared to the native one. Plants and animals are the major sources of oils but plant sources of oils are mostly used throughout the world as cooking oils. The demand for cooking oil is increasing daily in both developed and developing countries. Fats and oils provide concentrated reserves of energy for maintaining optimum body temperature. Human consume approximately 40 million tons of fats and oils every year, which indicates their nutritional importance and widespread daily use (Dhiman et al., 2009). Pumpkin seeds account for approximately 30–50% of oil (El-Adaway and Taha, 2001) and they are rich in various bioactive components including unsaturated fatty acids, sterols, tocopherols, squalene and carotenoid (Younis et al., 2000; Rabrenović et al., 2014). Pumpkin seeds and seed oils are generally not used as food in Bangladesh. Usually people ingest pumpkin flesh as part of their diet and seeds are thrown away as waste. They have different useful bioactivities such as anti-diabetic, antihypertensive, antioxidant, antimicrobial and antitumor (Fu et al., 2006; Nawirska-Olszańska et al., 2013; Zuhair et al., 2000; Jian et al., 2005; Boaduo et al., 2014; Burt and Reinders, 2003). The antimicrobial activity of pumpkin has many applications, for example as a preservative, alternative medicine and natural therapies (Rajakaruna et al., 2002). Although the pumpkin seed oils’ ability to protect against disease is well recognized, what has not yet been done in a variety-based composition, physical properties and comparative protective action analysis. For this reason a comparative analysis of two varieties of Cucurbita maxima has captured our interest.
2. Materials and methods
2.1. Materials and chemicals
For the extraction and analysis of pumpkin seeds, Petroleum ether (Labscan, Thailand), n-Hexane (Duksan, Korea). Methanol (MeOH), Ethanol and Dichloromethane from Merck (Germany). Anhydrous Sodium sulfate, Potassium hydroxide and Sodium thiosulfate from Merck (India). To prepare the samples for tocopherol analysis, Whatman filter paper 44 and syringe filter (0.45 μm) were purchase from Whatman (England) and Restek (USA) respectively. The standards of alpha, gama and delta tocopherol and Fatty acid methyl ester (FAME) standards were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
2.2. Collection and processing
Cucurbita maxima is commonly known as “Mistikumra” in Bangladesh. Two varieties of mistikumra (native and hybrid) were collected from the same local pumpkin field at Ambottala which is located in the district of Jashore, Bangladesh. After collection, the seeds were washed and dried under shade for four consecutive days and crushed into a fine powder. The powdered material was dried at 50 °C for 3 h in an electric oven (PIMPF 50, Phoenix, Germany) and stored at 4 °C until used.
2.3. Extraction of oil
The Soxhlet method (AOAC, 2005) was employed to extract the crude oil from seeds. Petroleum ether (boiling point, 60–80 °C) served as the extraction solvent. The extraction process lasted 16 h and the petroleum ether was separated from the oil via distillation. Traces of petroleum ether were evaporated at 100 °C in an air-dried oven for 1 h. The oil was cooled in a desiccators to room temperature (25 °C), put into a dark glass bottle, and then stored at 4 °C until required for further analysis.
2.4. Refining crude oil
About 100 g of the extracted crude oil was put into a separating funnel followed by the addition of 100 mL of water, 200 mL of ether and 25 mL of saturated sodium chloride solution. The content of the separating funnel was shaken well and allowed to stand for some time until two distinct layers were separated. Discarding the aqueous layer, the organic layer was again shaken with 100 mL of distilled water and 25 mL of saturated solution of sodium chloride, and then subsequently allowed to stand. The ether layer was separated using the similar treatment once more. Finally, the resulting ether extract was put into a conical flask and dried over anhydrous sodium sulfate. The extract was then evaporated by a rotary vacuum evaporator (Witeg, 2600000, Germany) at 40 °C to obtain the refined oil.
2.5. Proximate analysis
Chemical composition of the pumpkin seeds including the contents of moisture, ash, total lipid, total protein, total sugar and crude fiber were served to determine by (AOAC, 2005). Moisture content was determined by drying the seeds in an oven at 105 ± 1 °C to a constant weight and the ash content by burning at 900 °C till constant mass (AOAC, 923.03). Total lipids were determined by continuous extraction in a Soxhlet apparatus for 12 h using hexane as solvent. The oil contents were determined by gravimetrically after evaporating the solvent. Ash was determined by incinerating the sample at 550 °C in a muffle furnace. Total protein was calculated from the nitrogen content measured with the Kjeldahl method (AOAC. 978.04) set to a factor of 6.25, and calculated as N x 6.25. The crude fiber content was determined according to the gravimetric procedure of AOAC (920.860). Total carbohydrate was obtained by subtracting (crude protein + crude fat + ash + crude fiber) from 100. The moisture content was expressed in g/100g (as is basis) and the other values were reported on dry basis. All analyses were conducted in triplicate.
2.6. Analysis of physicochemical properties
2.6.1. Determination of specific gravity
A dry density bottle (volume capacity of 25 mL) was weighed accurately, filled with distilled water and weighed again. Similarly, another pre-weighed dried density bottle was filled with oil and re-weighed. The experiment was repeated three times (Kukeera et al., 2015) and the specific gravity was calculated as follows:
where, Wb + 0 is the weight (g) of bottle filled with oil, Wb + w is the weight (g) of bottle filled with water and Wb is the weight (g) of the empty bottle.
2.6.2. Determination of refractive index
The refractive index of the oil samples was determined in triplicate at room temperature, employing an Abbe 60 refractometer as described by (AOAC, 2000).
2.6.3. Determination of viscosity
The viscosity of the oil sample was detected using an Ostwald U-Tube viscometer according to (AOAC, 2003).
2.6.4. Determination of saponification value
Two grams of oil and 25 mL of ethanolic potassium hydroxide solution were placed in a conical flask and heated under reflux for 30 min. After cooling, 2.0 mL of phenolphthalein was added to the saponified mixture and then titrated with 0.5 M HCl (Ogungbenle and Afolayan, 2015).
where: N is the normality of HCl; W is the weight of oil; and V1 and V2 are the titer volumes of the blank and sample, respectively.
2.6.5. Determination of iodine value
An amount of oil (0.20 g) was dissolved in 10 mL of carbon tetrachloride and then 25 mL of Wijs solution was added. The mixture was stored in the dark for 30 min at 25 °C. Thereafter, 15 mL of potassium iodine solution and 100 mL of distilled water were added and mixed thoroughly. The mixture was titrated with 0.1 M sodium thiosulphate using 1% starch as an indicator (Ogungbenle and Omodara, 2014).
where, N is the normality of sodium thiosulphate, and TB and TS are the titer volumes of the blank and sample, respectively.
2.6.6. Determination of acid value
Five grams of oil were poured into a 250 mL conical flask containing 50 mL of neutralized alcohol and then boiled. Following this, 5 drops of phenolphthalein were added. The oil solution was titrated with 0.1 M NaOH using phenolphthalein as an indicator (Ogungbenle and Omodara, 2014)
where, N is the normality of NaOH, and TB and TS are the titer volumes of the blank and sample, respectively.
2.6.7. Determination of ester value
The ester value was calculated according to Akinola et al. (2010) by subtracting the acid value of oil from the saponification value of the corresponding oils.
2.7. Analysis of tocopherols
The tocopherols contents in the seed of two pumpkin varieties (native and hybrid) were determined by slightly modification of the method as described by Nada et al. (2010).
2.7.1. Preparation of standards
Stock solutions of alpha, gamma, delta tocopherols were prepared by dissolving nearest 50 mg in 50 mL amber volumetric flask and make it 50 mL by adding methanol (HPLC grade, Merck, Germany).
2.7.2. Sample preparation
Experimental pumpkin seed samples, 1g were weighed and taken into a 50 mL amber volumetric flask and mixed with 10 mL of dichloromethane and finally make the volume 50 mL by methanol. The mixture was then sonicated for 10 min and filtered through Whatman filter paper 44, and finally a small portion of the supernatant was filtered through 0.45 μm syringe filter and kept in 1.5 mL vial for HPLC injection.
2.7.3. High performance liquid chromatography (HPLC) analysis
HPLC Shimadzu 10A VP (Shimadzu, Japan) equipped with UV-Vis detector was used to analyze tocopherols. Degassed HPLC grade methanol was used as mobile phase and the flow rate was 1 mL/min. A portion of 20 μL sample was injected and the oven temperature was maintained 40 °C. Rec® C18 column (4.6 × 250 mm, 5 μm) (Restek, USA) was used to separate the compounds. Alpha, gamma and delta tocopherols were identified by comparing with the retention time of the authentic standard and quantified by comparing the peak area of the standard. Class VP software was use for the HPLC analysis.
2.8. Analysis of fatty acid composition
2.8.1. Sample preparation for fatty acid composition by gas chromatography
2.8.1.1. Preparation of fatty acid methyl ester (FAME)
Relative concentrations of fatty acid (FA) derived from the oil samples were measured as their corresponding methyl esters according to the method described in IUPAC with only minor modifications. 5 to 7 drops of oil were added into a 15 mL test tube and 3 mL of 0.5 M sodium methoxide (prepared by mixing metallic sodium in methanol) was added and digested by stirring in a boiled water bath for approximately 15 min. It was allowed to cool to room temperature and then 1 mL of petroleum ether (b.p 40–60 °C) was added followed by 10 mL deionized water, mixed gently and allowed to settle for some time. The distinct upper layer of methyl ester in the petroleum ether was separated carefully in a capped vial and used for analysis. 200 mg of different fatty acid standards (FAME mix; Sigma-Aldrich, St. Louis, Missouri, USA) in their respective methyl ester form were dissolved separately in 10 mL petroleum ether (b.p 40–60 °C) in a series of screw-capped test tubes. Aliquots of 1μL FAME (Fatty Acid Methyl Ester) were injected and the peaks of fatty acids were recorded for their respective retention times and presented as relative percentages. This was done utilizing the automated GC software (V6.14 SP1).
2.8.1.2. Gas chromatography analysis
The fatty acid compositions were analyzed with a Shimadzu GC-14B series gas chromatograph equipped with a flame ionization detector and fused silica capillary column (FAMEWAX, Crossbond® polyethylene glycol, 15 m × 0.25 mm × 0.25μm film thickness, Restek; Pennsylvania, USA). Split-less injection technique with nitrogen as carrier gas at a constant flow rate of.
20 mL/min was employed. The injector temperature was 250 °C, initial oven temperature was 150 °C and maintained for 5 min. Temperature was increased at 8 °C/min. to 190 °C and then elevated to 200 °C at a rate of 2 °C/min. and held for 10 min. The fatty acids were identified by using respective fatty acid methyl ester standards (FAME mix; Sigma-Aldrich, St. Louis, Missouri, USA; CAS 71076-49.8).
2.9. Statistical analysis
All the analysis were carried out in triplicate. SPSS statistical package for WINDOWS (version 23.0; SPSS Inc, Chicago) was used for statistical analysis. Values were expressed as mean ± S.E.M and statistical significance between native and hybrid varieties were evaluated by Independent Sample t-Test. Levels of significance were denoted as p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
3. Results and discussion
In this study proximate analysis that is summarized in Table 1 and confirmed that moisture and ash contents were higher in the native but not significant. Crude fibers were significantly higher in the hybrid (p < 0.01) but the lipid contents were rich in the native variety (p < 0.01). Total protein and sugar contents were higher in the native but not significant. On the other hand starch content was higher in the hybrid but did not prove to be statistically significant.
Table 1.
Proximate composition of composition of two varieties (native and hybrid) of pumpkin seed.
| Parameter | Native Pumpkin seed (g/kg) | Hybrid Pumpkin seed (g/kg) |
|---|---|---|
| Moisture | 42.7 ± 0.5 | 42.6 ± 0.7 |
| Ash | 3.4 ± 0.6 | 3.2 ± 0.6 |
| Crude Fiber | 46.3 ± 0.6 | 52.6 ± 0.6** |
| Total lipid | 23.5 ± 0.4** | 17.6 ± 0.6 |
| Total protein | 21.2 ± 0.6 | 20.5 ± 0.6 |
| Sugar | 1.5 ± 0.5 | 1.5 ± 0.5 |
| Starch | 2.2 ± 0.3 | 2.4 ± 0.4 |
Values represent means ± standard deviations (n = 3). *p < 0.05; **p < 0.01 and ***p < 0.001 are considered as significant.
Most of the inorganic constituents or minerals are present in ash. The levels of ash present in the seeds of native and hybrid varieties of pumpkin were 3.4 g/kg and 3.2 g/kg, respectively. No significant variation in the ash content was observed between the two varieties of pumpkin seed, but these values were lower than that recorded (4.62 g/kg) by Viswanathan et al. (2001) for Teramnus labialis seed. Furthermore the fiber contents of the seeds of the native and hybrid varieties of pumpkin were 46.3 g/kg and 52.6 g/kg, respectively. Similar results were reported for the seeds of C. pepo which had significantly less fiber than other species of pumpkin (Achu et al., 2005).
Total sugar contents of the native and hybrid pumpkin seeds were found to be 1.5g. This value is lower than that for Brazil nuts, i.e. 1.9 g as reported by Chinedu et al. (2017). Starch is the most important source of carbohydrate in the human diet. In most of the plant, usually it is found that seed serves as the nutritional reserve of carbohydrate. Starch contents of the two varieties of pumpkin seed were reported as being 2.2g and 2.4g, respectively, which were lower than that of 5.25 g for loofah seeds (Stanisavljevic et al., 2009).
The physicochemical analysis (Table 2) reveals that the specific gravity and refractive index were higher in the hybrid but not statistically significant. The viscosity, iodine, acid and ester values were higher in the native variety although viscosity was not significant. Conversely, the iodine and acid values were significant (p < 0.05) but saponification and ester values were higher and highly significant (p < 0.001) in native.
Table 2.
Physicochemical properties of native and hybrid varieties of pumpkin seed oil.
| Parameter | Native pumpkin seed oil | Hybrid pumpkin seed oil |
|---|---|---|
| Specific gravity (25 °C) | 0.9 ± 0.01 | 0.9 ± 0.01 |
| Refractive index (40 °C) | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Viscosity (21 °C), mPas | 4.7 ± 0.6 | 4.6 ± 0.4 |
| Saponification value | 236.0 ± 0.6*** | 115.7 ± 0.6 |
| Iodine value | 113.2 ± 1.9* | 106.6 ± 2.1 |
| Acid Value | 13.5 ± 0.6* | 11.5 ± 0.6 |
| Ester Value | 222.6 ± 0.4*** | 104.4 ± 0.4 |
Values represent means ± standard deviations (n = 3). *p < 0.05; **p < 0.01 and ***p < 0.001 are considered as significant.
The oil extracted from the seeds of the two varieties was a brown color liquid at room temperature (25 °C). The specific gravities of the two varieties of seeds oil were 0.91 and 0.92, respectively, which is comparable to the specific gravities of oils from watermelon (Egbuonu et al., 2015) and African wild mango (Etong et al., 2014). Similar results have been documented by Kukeera et al. (2015) for the seed of pumpkin. The refractive index analysis shows that the native and hybrid varieties of seeds oil with values between 1.4 and 1.5, met the ASTM values which range from 1.476 to 1.479 ASTM International (2002). The refractive index values were similar to those reported by Izuagie et al. (2008), for the following species: Cucumeropsis edulis, Colocynthis citrillus and Prunusa mygdalus. The values of the viscosity of the two varieties (native and hybrid) were 4.7 and 4.6, respectively.
The higher saponification value indicates a higher molecular weight fatty acids in triglyceride (Esan and Fasasi, 2013). Iodine value refers to the extent of unsaturated fatty acids present in the fat. Having an iodine value greater than 100 means that it can be regarded as a drying oil (Adu et al., 2015). Since the observed iodine value above 100, it is evident that both the native and hybrid varieties oil are in fact drying oils. The higher iodine value of the native variety confirmed it contained more unsaturated fatty acids compared to the hybrid variety. The ester value is an indication of the saponifiable fatty acids excluding the free acids of the fat (Aremu et al., 2015).
The tocopherol contents in the seeds of the two pumpkin varieties are summarized in Table 3 and the results obtained from these investigations indicated that in the native variety, the gama–tocopherol, delta-tocopherol and total Vit.E contents were very rich and found significant (p < 0.001). However, it is interesting to note that no significant differences of alpha-tocopherol contents were observed between the two pumpkin varieties. In this study, it is observed that gama-tocopherol and delta tocopherol contents in the native variety were 2.5–3 times higher than that of the hybrid. Similar results have been observed in C. pepo and C. moschata as reported by Kim et al. (2012). It has also been documented that alpha tocopherol has the greatest bioavailability, yet gama may have more anti-oxidant properties (Wagner et al., 2004; Salden and Salden, 2005). This could be important for controlling or preventing prediabetes, diabetes in patients or those with a vascular injury or condition (Yadav et al., 2010).
Table 3.
Tocopherol contents of two varieties (native and hybrid) of pumpkin seed.
| Parameter | Native Pumpkin seed (mg/kg) | Hybrid Pumpkin seed (mg/kg) |
|---|---|---|
| Tocopherols | ||
| Alpha-tocopherol | 54.0 ± 5.0 | 51.0 ± 2.0 |
| Gama-tocopherol | 112.0 ± 5.0*** | 24.0 ± 4.0 |
| Delta-tocopherol | 544 ± 8.0*** | 218.0 ± 6.0 |
| Total Vit. E (ppm) | 640 ± 2.9*** | 260 ± 2.9 |
Values represent means ± standard deviations (n = 3). *p < 0.05; **p < 0.01 and ***p < 0.001 are considered as significant.
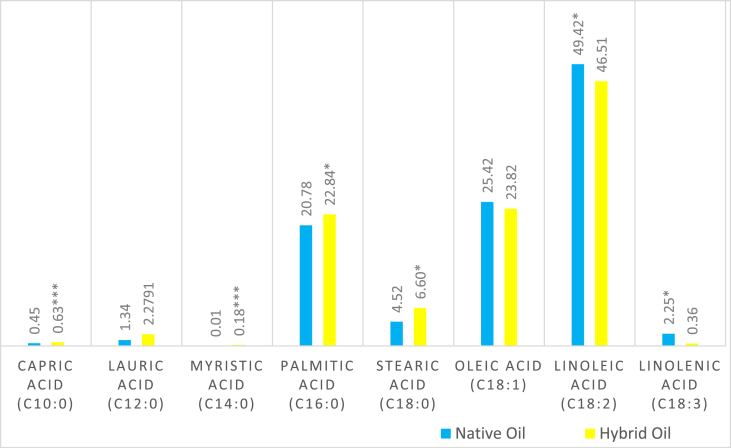
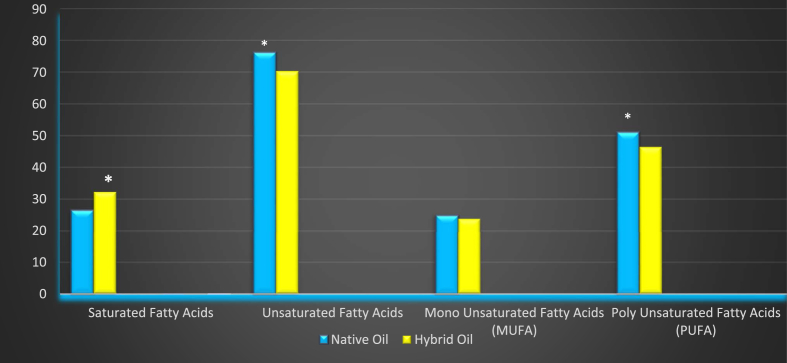
Results obtained from the analysis of fatty acid composition (Figures 1 and 2) indicated that saturated fatty acids, i.e. stearic acids, palmitic acids, myristic acids and capric acid contents were higher in the hybrid variety and found to be significant (p < 0.05 and 0.001,respectively). Lauric acid was predominant in the hybrid but not statistically significant. Conversely, the unsaturated fatty acids - linoleic acids and linolenic acids - were prominent in native pumpkin seed oils and found to be significant (p < 0.05). The oil profile confirms that in the native pumpkin seed oil the content of monounsaturated acids was 25.1 mg/kg but not significant, whereas there was 51.2 mg/kg of polyunsaturated acids and this was significant (p < 0.05). It is worth mentioning here that saturated fatty acids content in the hybrid pumpkin seed oil amounted to 32.3 mg/kg and was found significant (p < 0.05). The overall unsaturated fatty acid contents and polyunsaturated fatty acid contents in the native pumpkin seed oil were higher than the hybrid one and found significant (p < 0.05). Applequist et al., (2006) made the same observations in the case of native pumpkin seeds.
Figure 1.
Fatty acid composition of native and hybrid varieties of pumpkin seed oil (mg/kg). Levels of Significance p < 0.05, p < 0.01 and p < 0.001 are considered to be (*), (**) and (***), respectively.
Figure 2.
Different types of fatty acids present in native and hybrid varieties of pumpkin seed oil (mg/kg). Levels of Significance p < 0.05, p < 0.01 and p < 0.001 are considered to be (*), (**) and (***), respectively.
4. Conclusion
In this study, the results of fatty acid composition and biochemical analysis revealed that linoleic acid, linolenic acid, gama-tocopherol, lipid and protein are present in both varieties of pumpkin seed but predominant in native. Therefore, this study may suggest that the both varieties of pumpkin seed oil may be an alternative good source of edible oil and people may prefer for its nutritional advantages.
Declarations
Author contribution statement
Ziaul Amin: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tehera Islam, Farhana Mostofa: Performed the experiments.
M. Jashim Uddin: Analyzed and interpreted the data; Wrote the paper.
M.M. Rahman, Mohammed A. Satter: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Centre for Sophisticated Instrumentation and Research Laboratory, Jashore University of Science and Technology, Jashore-7408, Bangladesh. It provided the facilities for investigating the various physicochemical properties, proximal and biochemical analysis. The authors would also like to thank Absa Jabin and Nusrat Abadin, SSO, IFST laboratory, BCSIR, Dhanmondi Dhaka, Bangladesh for their technical assistance in the HPLC analysis for the determination of tocopherol contents, GC-MS analysis of fatty acid composition. The authors acknowledge Mr. Philip Thomas, Hamstead Gardens S.A-5086 for his generous help in critically reading the manuscript.
References
- Achu B.M., Fokou E., Tchiegang E.C., Fotso M., Tchouanguep F.M. Nutritive value of some Cucurbitaceae oil seeds from different regions in Cameroon. Afr. J. Biotechnol. 2005;4(11):1329–1334. [Google Scholar]
- Adu O.B., Ogundeko T.O., Ogunrinola O.O., Saibu G.M., Elemo B.O. The effect of thermal processing on protein quality and free amino acid profile of Terminalia catappa(Indian Almond) seed. J. Food Sci. Technol. 2015;52(7):4637–4641. doi: 10.1007/s13197-014-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinola F.F., Ogutibeju O.O., Adisa A.W.O.S., Owojuyigbe O.S. Physico-chemical properties of palm oil from different palm oil local factories in Nigeria. J. Food Agric. Environ. 2010;8(3&4):264–269. [Google Scholar]
- AOAC . Association of Official Analytical Chemists; Washington, DC: 2005. Official Methods of Analysis. (2005) (AOAC 992.23) [Google Scholar]
- AOAC . seventeenth ed. Association of Official AnalyticalChemists; 2003. Official Methods of Analysis. [Google Scholar]
- AOAC . seventeenth ed. Association of Official Analytical Chemists, William Hurwitz; 2000. Official Methods of Analysis. [Google Scholar]
- Applequist W.L., Bharathi A., Schaneberg B.T., Yan-Hong W., Ikhlas A.K. Comparative fatty acid content of seeds of four Cucurbita species grown in a common (shared) garden. J. Food Compos. Anal. 2006;19(6):606–611. [Google Scholar]
- Aremu M.O., Ibrahim H., Bamidele T.O. Physicochemical Characteristics of the oil extracted from some Nigerian plant foods. Chem. Process Eng. Res. 2015;32:22–25. [Google Scholar]
- ASTM International . Annual Book of Standards, Section 12.10. ASTM International; West Conshohocken: 2002. Standard test method for oxidation onset temperature of hydrocarbons by differential scanning calorimetry (E-2009-02) pp. 734–738. [Google Scholar]
- Boaduo N.K., Katerere D., Eloff J.N., Naidoo V. Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in South Africa using in vitro methods. Pharm. Biol. 2014;52(6):756–761. doi: 10.3109/13880209.2013.869828. [DOI] [PubMed] [Google Scholar]
- Burt S.A., Reinders R.D. Antibacterial activity of selected plant essential oils against Escherichia coli 0157: H7. Lett. Appl. Microbiol. 2003;36(3):162–167. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Chinedu E.E., Ebere E.C., Emeka A.C. Quality assessment of palm oil from different palm oil local factories in Imo state, Nigeria. World Sci. News. 2017;88(2):152–167. [Google Scholar]
- Dhiman A.K., Sharma K.D., Attri S. Functional constituents and processing of pumpkin: a review. J. Food Sci. Technol. 2009;46(5):411–417. [Google Scholar]
- Egbuonu A.C.C., Aguguesi R.G., Samuel R., Ojunkwu O., Onyenmeri F., Uzuegbu U. Some physicochemical properties of the petroleum ether-extracted watermelon(Citrulluslanatus) seed oil. J. Asian Sci. Res. 2015;8(4):519–525. [Google Scholar]
- El-Adaway T.A., Taha K.M. Characteristics and composition of watermelon, pumpkin and paprika seed oils and flours. J. Agric. Food Chem. 2001;49(3):1253–1259. doi: 10.1021/jf001117+. [DOI] [PubMed] [Google Scholar]
- Esan Y.O., Fasasi O.S. Amino acid composition and antioxidant properties of African yam bean (Spenostylisstenocarpa) protein hydrolysates. Afr. J. Food Sci.Technol. 2013;4(5):100–105. http://www.interesjournals.org/AJFST [Google Scholar]
- Etong D.I., Mustapha A.O., Taleat A.A. Physicochemical properties and fatty acid composition of Dikanut (IrvingiaGabonensis) seed oil. Res. J. Chem. Sci. 2014;4(12):70–74. [Google Scholar]
- Fu C.L., Shi H., Li Q.H. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006;61(2):73–80. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- Izuagie A., Akpambang V.O., Amoo E.A. Comparative compositional analysis on two varieties of melon (ColocynthiscitrullusandCucumeropsisedulis) and a variety of almond (Prunusamygdalus) Res. J. Agric. Biol. Sci. 2008;4(6):639–642. [Google Scholar]
- Jian L., Du C.L., Lee A.H., Binns C.W. Do dietary lycopene and other carotenoids protect against prostate cancer? Int. J. Cancer. 2005;113(6):1010–1014. doi: 10.1002/ijc.20667. [DOI] [PubMed] [Google Scholar]
- Kim M.Y., Kim E.J., Kim Y.N., Choi C., Lee B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012;6(1):21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukeera T., Banadda N., Tumutegyereize P., Kiggundu N., Asuman R. Extraction, quantification and characterization of oil from pumpkin seeds. Int. J. Agric. Biol. Eng. 2015;8(1):98–102. [Google Scholar]
- Nada A., Krishnaiah Y.S.R., Zaghloul A.A., Khattab I. Analysis of vitamin E in commercial cosmetic preparations by HPLC. J. Cosmet. Sci. 2010;61:353–365. 2010. [PubMed] [Google Scholar]
- Nawirska-Olszańska A., Kita A., Biesiada A., Sokół-Łętowska A., Kucharska A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013;139(1-4):155–161. doi: 10.1016/j.foodchem.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Ogungbenle H.N., Afolayan M.F. Physical and chemical characterization of roasted cashew nut (Anacardiumoccidentale) flour and oil. Int. J. Food Sci. Nutr. Eng. 2015;5(1):1–7. [Google Scholar]
- Ogungbenle H.N., Omodara O.P. Physico chemical and fatty acid composition of nicker bean (Entadagigas) seed oil. Adv. Anal. Chem. 2014;4(2):35–39. [Google Scholar]
- Rabrenović B.B., Dimic E.B., Novakovic M.M., Tesevic V.V., Basic Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbitapepo L.) seeds. LWT - Food Sci. Technol. 2014;55(2):521–527. [Google Scholar]
- Rajakaruna N., Cory S.H., Towers G.H.N. Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka. Pharm. Biol. 2002;40(3):235–244. [Google Scholar]
- Salden K., Salden T. Importance of tocopherols beyond alpha-tocopherol: evidence from animal and human studies. Nutr. Res. 2005;25:877–889. [Google Scholar]
- Stanisavljevic I.T., Velickovic D.T., Todorovic Z.B., Lazic M.L., Veljkovic V.B. Comparison of techniques for the extraction of tobacco seed oil. Eur. J. Lipid Sci. Technol. 2009;111(5):513–518. [Google Scholar]
- Viswanathan M.B., Thangadurai D., Ramesh N. Biochemical and nutritional evaluation of Neonotonia wightii(Wight &Arn.) Lackey (Fabaceae) Food Chem. 2001;75(3):275–279. [Google Scholar]
- Wagner K.H., Kamal-Eldin A., Elmadfa I. Gama-tocopherolan underestimated vitamin? Ann. Nutr. Metab. 2004;48(3):169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- Yadav M., Jain S., Tomar R., Prasad G.B., Yadav H. Medicinal and biological potential of pumpkin: an updated review. Nutr. Res. Rev. 2010;23(2):184–190. doi: 10.1017/S0954422410000107. 2010. [DOI] [PubMed] [Google Scholar]
- Younis Y.M., Ghirmay S., Al-Shihry S.S. African Curcurbita pepo L.: properties of seed and variability in fatty acid composition of seed oil. Phytochemistry. 2000;54(1):71–75. doi: 10.1016/s0031-9422(99)00610-x. [DOI] [PubMed] [Google Scholar]
- Zuhair H.A., AbdEl-Fattah A.A., El-Sayed M.I. Pumpkin-seed oil modulates the effect of felodipine and captopril in spontaneously hypertensive rats. Pharmacol. Res. 2000;41(5):555–563. doi: 10.1006/phrs.1999.0622. [DOI] [PubMed] [Google Scholar]