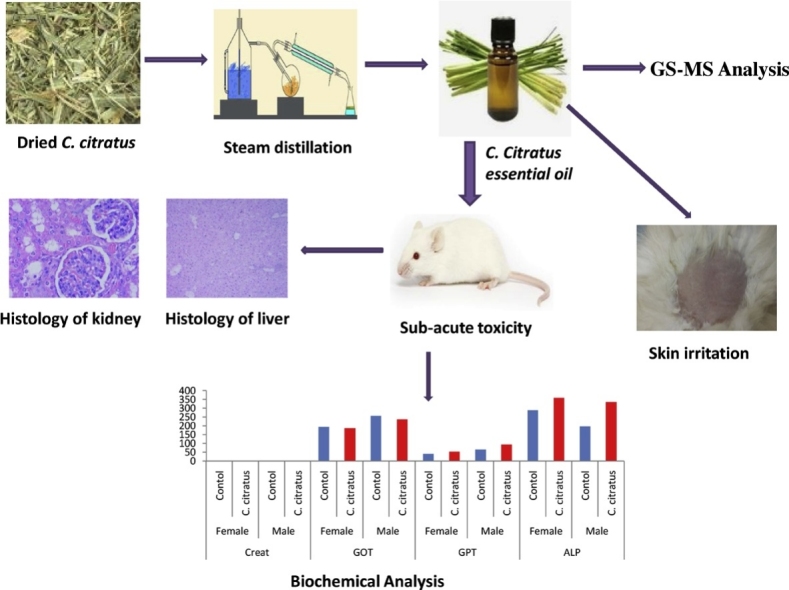
Graphical abstract
Keywords: Acute toxicity, Biochemical analysis, Cymbopogon citratus, Histopathology, Skin irritation, Sub-acute toxicity
Highlights
-
•
C. citrates essential oil contains about 71.297 % citral.
-
•
10 % ointment formulation of C. citratus oils did not cause skin irritation.
-
•
Oral LD50 of C. citratus essential oil was greater than 2000 mg/kg.
-
•
C. citratus essential oil didn’t produce any toxicity sign.
-
•
No histopathological changes were detected in the organs tested.
Abstract
Cymbopogon citratus has been used by the local people in Ankober district, northern Ethiopia, as traditional medicine to treat various ailments. Its essential oil has been shown to have antibacterial, antifungal, antiprotozoal, anti-carcinogenic, anti-inflammatory and antioxidant activities amongst others. This study was conducted to determine skin irritation, acute and subacute toxicity of C. citratus essential oil in mice and rabbits. The essential oil was analyzed using GC–MS. The essential oil at dose of 2000 mg/kg body weight was administered to mice for 21 consecutive days. The mice mortality, behavioral change, injury and other signs of illness were recorded once daily. Biochemical parameters were evaluated. Liver and kidney were taken after sacrifice for gross findings and histological analyses. 10 % ointment formulation of C. citratus oils was applied on the rabbit skin to determine skin irritation effects. The result revealed, the presence of citral (71.297%), myrcene (19.034%), 4, 5-epoxycarene (2.780%), linalool (1.713%), ((S)-cis-verbenol (1.110 %), linalool (1.713 %), ((S)-cis-verbenol (1.110 %) and undecan-2-one (1.001 %) in the C. citratus essential oil. There was no significant difference (p > 0.05) in the body weights, gross abnormalities of the organs and biochemical parameters compared to the control. No histopathological changes were detected in the organs tested. 10 % ointment formulation of C. citratus oils did not cause skin irritation. Analysis of results leads to the conclusion that Ethiopian C. citratus essential oil may be considered as relatively safe and non-toxic.
1. Introduction
Cymbopogon citratus is an economically important aromatic perennial plant of the Poaceae family. It is grown around the world and has a century-long record of extensive therapeutic applications in traditional and Ayurvedic medicine in a number of countries [1,2]. It is used in herbal medicine for a wide range of applications based on its therapeutic potentials including antibacterial [3], antifungal [4], antiprotozoal [5], anti-carcinogenic [6], anti-inflammatory [7], antioxidant [8], and cardio protective activities [9]. Furthermore, this plant has been used as an aromatherapy, to inhibit platelet aggregation [10], and to treat diabetes [11], dyslipidemia [12], anxiety [13] and pneumonia [14]. Aside its medicinal uses, C. citratus is used as a fragrance in manufacturing of perfumes, soaps, detergents, and body creams. It is also added to non-alcoholic beverages and baked foods as a flavoring and preservative agent [15].
Research findings indicated that all herbal medicines are not safe [3]. For instance, studies have revealed that side effect such as diarrhea and skin necrosis are observed with the use of certain traditional herbs [16]. Moreover, the limited number of toxicological studies on medicinal plants also raises a legitimate concern regarding the potential toxic effects associated with their chronic use. In Ankober, northern Ethiopia, medicinal plants such as C. citratus have been used as traditional medicine to treat different livestock and human sickness and disorders from time immemorial. So far, only few studies have been conducted on the toxicity of the essential oil of C. citratus. Review and analysis of published scientific materials retrieved from online bibliographical databases such as PubMed, Science Direct and Google Scholar revealed only one study on a single dose toxicity of the Ethiopian C. citratus essential oil emulsion [17]. However, the study area addressed by Gebremickael [17] was ecologically different from the present study site. Moreover, it had a clear lack of repeated dose and dermatotoxicity data. Therefore, overall objective of this study is to evaluate skin irritation, acute and sub-acute toxicity of essential oil from C. citratus in mice and rabbits.
2. Methods
2.1. Collection and identification of plant material
C. citratus Stapf specimen comprising leaves and stalks were collected from Ankober Project Nursery Site which is Located in North Shoa Zone, Amhara Regional State, Ethiopia. The taxonomic identity was confirmed and specimen was deposited at the National Herbarium (ETH) in Addis Ababa University, Ethiopia with voucher number of EL896.
2.2. Extraction of essential oil
Collected samples were air dried under shade at the ambient temperature, protected from the direct sun light for a week. Both leaves and stalks of C. citratus were extracted for 3 h using steam distillation method. The oil obtained was pale yellow in color and has 0.79 % volume by mass. The oil was freed from water by adding anhydrous sodium sulfate and stored in sealed vials at 6 °C until use.
2.3. Gas chromatography-Mass spectrometry analyses
GC–MS analyses were carried out using Agilent 5977A GC–MS system equipped with a 30 m HP-5 capillary column with 0.25 mm internal diameter and 0.25 μm film thickness. Oven temperature was increased from 50 to 180 °C at a rate of 5 °C/min, transfer line temperature was held at 250 °C, carrier gas was helium with a flow rate of 1 ml/min, spilt ratio 1:20, ionization energy 70 eV, and mass range 40–400 a.m.u. The constituents were identified by comparison of their mass spectra with those of NIST 14 library data for the GC–MS system. GC–MS chromatogram obtained was compared with two libraries (NIST and Wily) which provide best information about the identification of constituent present in samples. Based on this information the percentage composition of each compound was calculated from peak area using normalization method.
2.4. Ointment formulation
The 10 % (w/w) ointment was prepared for skin irritation test, where 10 g of the oil was mixed in 90 g petroleum jelly base. Physical parameters including homogeneity and physical appearance, viscosity, extrudability and spreadability were assessed according to procedures described in Rahul et al. [18] and Bora et al. [19].
2.5. Experimental animals
Both gender of Swiss albino mice (25–35 g) and New Zealand female rabbits (1.4–2.3 kg) were obtained from the School of Pharmacy of Addis Ababa University and Ethiopian Public Health Institute respectively. The study animals were housed in an air-conditioned room at 25 ± 2 °C temperature and were adapted for 5 days before the experiment during which they were exposed to 12 h light/dark cycle. During acclimatization and study periods the animals were given water and food pellets ad libitum. Maintenance of animals and experimentation were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 85-23 revised in 1985) and the Animal Ethics Committee of Debre Berhan University.
2.6. Rabbit skin irritation test
The tests were carried out by employing OECD [20] guidelines with little modification. Approximately 24 h before the test, fur was shaved from dorsal area at different site of the trunk and the herbal ointment (500 mg) was applied to the three sites. A separate untreated site was used as a control. Observation of the sites was done at 24 h after application, and repeated at 48 h, 72 h, 7th day and 15th day thereafter. The reactions, defined as erythema and edema, were evaluated according to skin reactions scoring system [20,21].
2.7. Acute toxicity in mice
Food was deprived for 8 h prior to commencing the experiment. C. citratus essential oil was then administered orally at 2000 mg/kg body weight to one female mouse [22]. After two days, the same dose was administered orally to four female mice increasing the number of treatment animals to five. The second group of 5 female mice (negative control group) was administered with equal volume of saline. All the mice were observed critically for the first 4 h, periodically during the first 24 h and once a day for 14 days. During this period, the activities related to motor-muscle coordination as well as central and autonomic nervous system were analyzed.
2.8. Sub-acute toxicity in mice
The study was conducted following OECD guidelines 407 [23]. Twenty mice (10 male and 10 female) were randomly divided into two groups each having 5 female and 5 male mice. C. citratus essential oil was given once daily at 2000 mg/kg body weight for treatment group for 21 days while the control group orally received saline. The animals were observed for any signs of toxicity throughout the experimental period. The feed consumption of each cage and the body weight of all mice were measured weekly. Blood was withdrawn from a common carotid artery and all mice from both study groups were sacrificed using Sodium pentobarbital at the end of the experiment. The serum was separated and creatinine level, alkaline phosphatase (ALP), glutamic-oxaloacetic transaminase (GOT), and glutamic-pyruvic transaminase (GPT) activities were determined. Blood chemistry was analyzed using Mindray automated chemistry analyzer, China. Kidney and liver of mice were examined for gross pathological changes, weighed and preserved in formalin for histopathological examination. The tissues were embedded in paraffin wax and sectioned 5 μm thick [24]. They were then stained with haematoxylin and eosin, mounted on glass slides and observed under a standard light microscope [25].
3. Statistical analysis
Results were expressed as mean ± standard errors of the mean (SEM). Independent samples t test was used for comparison between two groups. Differences were considered significant at P < 0.05. Statistics were performed using SPSS Software version 20.
4. Results
4.1. Cymbopogon citratus chemical composition
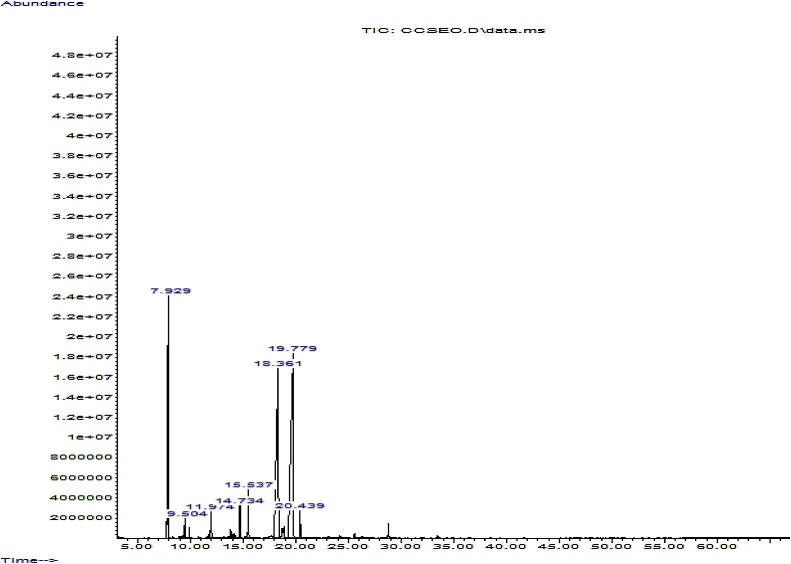
The gas chromatogram and constituents of C. citrates essential oil following GC–MS analysis are present in Fig. 1 and Table 1 respectively. Eight major components, representing 97.477 % were identified. Among the identified components, citral which is the marker comment of C. citratus essential oil represents 71.297 % of which geranial and neral represented 40.708 % and 30.589 % respectively. Myrcene (19.034 %), 4,5-epoxycarene (2.780 %), linalool (1.713 %), (S)-cis-verbenol (1.110 %) and undecan-2-one (1.001 %) are other major constituents of the identified components.
Fig. 1.
Gas Chromatogram of Cymbopogon citratus essential oil.
Table 1.
Constituents of Cymbopogon citratus essential oil.
| RT | Constituent | % Composition | MF | Mass Fragments |
|---|---|---|---|---|
| 7.929 | Beta-myrcene | 19.034 | C10H16 | 41,43,53,67,74,77,89,90,105,121,136 |
| 9.504 | (Z)- beta -Ocimene | 0.542 | C10H16 | 41,43,51,53,67,74,79,89,91,93,105,107,121,136 |
| 11.974 | Linalool | 1.713 | C10H18O | 43, 55,71,80,83,93,107,136,154 |
| 14.734 | (S)-Cis-verbenol | 1.110 | C10H16O | 41,55,59,69,79,91,94,109,119,137,150 |
| 15.537 | 4,5-Epoxycarene | 2.780 | C10H16O | 41,43,53,55,67,59,69,77,79,81,91,95,109,119,123,137,152 |
| 18.361 | Neral | 30.589 | C10H16O | 41,43,51,53,59,65,67,69,79,84,91,94,109,119,137,151 |
| 19.779 | Geranial | 40.708 | C10H16O | 41,43,51,53,65,67,69,84,91,94,105,109,123,137,152 |
| 20.439 | undecan-2-one | 1.001 | C11H22O | 41,43,45,55,58,69,71,69,85,95,100,112,127,141,155,170 |
| Total | 97.477 |
MF = Molecular Formula, RT = Retention Time.
4.2. Physicochemical characteristics C. citratus oil ointment
Physicochemical characteristics of C. citratus oil ointment were presented in Table 2. The ointment was found to be smooth, free from grittiness and whitish in color. The physicochemical properties investigated show satisfactory results for spreadability, extrudability and viscosity.
Table 2.
C. citratus oil 10 % ointment formulation.
| Formulation | Color | Viscosity (cP) at 100 rpm (Mean ± SD) | Spreadability (g.Cm/min) (Mean ± SD) | Extrudability (g) (Mean ± SD) |
|---|---|---|---|---|
| C. citratus oil ointment | White | 8167.3 ± 31.7 | 2295.9 ± 94.2 | 0.104±0.048 |
4.3. Rabbit skin irritation test
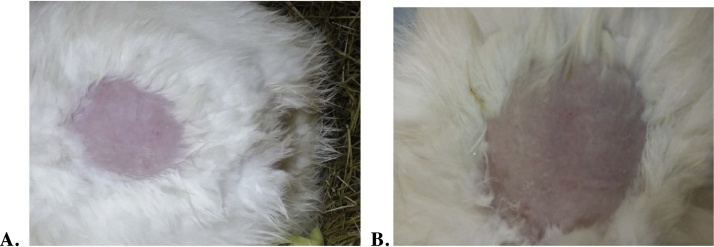
Dermal irritation result is presented in Table 3. There was no evidence of any visible skin irritation (no edema and erythema) and inflammation during the study period as compared with control (Fig. 2). At 1 h after removal of test substance and thereafter, it was found that in all rabbits, edema and erythema score of “0″.
Table 3.
Score of erythema and edema after application of test materials 10 % C. citratus essential oil ointment.
| Reaction | 1 hr |
24hrs |
48hrs |
72hrs |
7th day |
15th day |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl | Trt | Cl | Trt | Cl | Trt | Cl | Trt | Cl | Trt | Cl | Trt | |
| Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Primary Irritation Index (PII) = 0/3, PII = 0, Category of irritation based on PII is Negligible. Cl = Control, Trt = Treatment.
Fig. 2.
Photograph of skin of rabbit before (A) and 1 h after removal of test formulation (B).
4.4. Result of acute toxicity test
No mortality and sign of toxicity was observed in female mice treated with 2000 mg/kg of the of C. citratus essential oil. Hence, the lethal dose (LD50) of C. citratus essential oil oral administration was found to be greater than 2000 mg/kg.
4.5. Result of sub-acute toxicity test
4.5.1. General observation (food consumption and body weight)
Oral administration of C. citratus essential oil at the dose of 2000 mg/kg body weight for 21 days produced no toxicity signs or death in all of the experimental animals. Mice were active and responsive to stimuli, with no clinical signs or death that could be associated with local or systemic toxicity. The body weight gain was higher (70 ± 7.9 %) in male treatment group as compared to female treatment group (48.8 ± 8.5 %) and both control groups (Table 4). Similarly, food consumption in male treatment group was higher than female treatment group (48.8 ± 8.5 %) and both control groups. However, the differences were not statistically significant.
Table 4.
Body weight gain and food consumption of mice treated orally with C. citratus oil for 21 days.
| Control | CC 2000 mg/kg | ||
|---|---|---|---|
| Female | |||
| Initial Body Weight (g) | 20 | 20 | |
| Final Body Weight (g) | 33.5 ± 2.4 | 29.8 ± 1.7 | |
| Body Weight Gain (%) | 67.5 ± 11.9 | 48.8 ± 8.5 | |
| Food Intake (g/week) | 171.3 ± 37.6 | 110.5 ± 41.9 | |
| Male | |||
| Initial Body Weight (g) | 20 | 20 | |
| Final Body Weight (g) | 28.5 ± 0.7 | 34 ± 1.6 | |
| Body Weight Gain (%) | 42.5 ± 3.5 | 70 ± 7.9 | |
| Food Intake (g/week) | 122 ± 48.1 | 175.5 ± 44.5 | |
Values expressed as mean ± SEM, n = 5 animals/group, p > 0.05 (Independent-samples T test). CC=C. citratus.
4.5.2. The effect of C. citratus oil on organs relative weight and serum biochemical parameters
Statistically, there was no significant difference in relative organ weight gain between treatment and control groups (Table 5). Similarly, sub-acute administration of the oil did not cause any significant (P > 0.05) changes on biochemical parameters such as creatinine, GOT, GPT and ALP when compared to control groups (Table 6). Nonetheless, as compared to the control group serum activity of GOT was lower, while the level of GPT and ALP was higher in both male and female treatment groups.
Table 5.
Relative organ weight (g/100 g of body weight) of mice treated orally with C. citratus oil for 21 days.
| Control | CC 2000 mg/kg | ||
|---|---|---|---|
| Female | |||
| Liver (g) | 5.6 ± 0.9 | 5.7 ± 0.8 | |
| Kidney (g) | 1.5 ± 0.2 | 1.5 ± 0.23 | |
| Male | |||
| Liver (g) | 8.1 ± 0.6 | 6 ± 0.7 | |
| Kidney (g) | 2.1 ± 0.1 | 1.7 ± 0.4 | |
Values expressed as mean ± SEM, n = 5 animals/group, p > 0.05 (Independent-samples T test), CC=C. citratus.
Table 6.
Biochemical parameters of mice treated orally with C. citratus oil for 21 days.
| Control | CC 2000 mg/kg | ||
|---|---|---|---|
| Female | |||
| CREAT | 0.8 ± 0.1 | 0.8 ± 0.1 | |
| GOT | 194 ± 36.8 | 185.8 ± 43.2 | |
| GPT | 39.7 ± 12.7 | 52 ± 30.8 | |
| ALP | 289.3 ± 77.2 | 359 ± 112.6 | |
| Male | |||
| CREAT | 0.8 ± 0.1 | 0.7 ± 0.4 | |
| GOT | 256.5 ± 68.6 | 236.2 ± 128.4 | |
| GPT | 65 ± 24.04 | 92.8 ± 23.5 | |
| ALP | 197.5 ± 92.6 | 334.6 ± 73.9 | |
Values expressed as mean ± SEM, n = 5 animals/group, p > 0.05 (Independent-samples T test), CC=C. citratus.
4.5.3. Histopathology
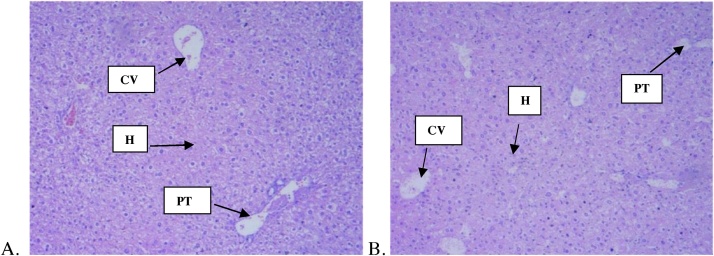
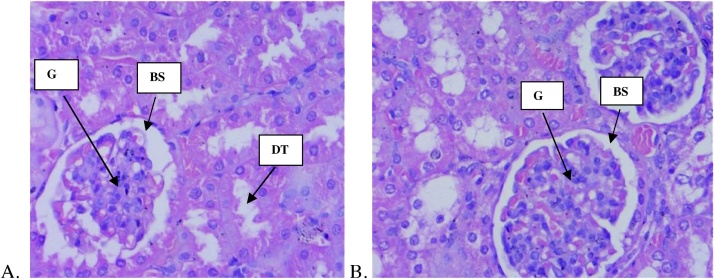
The histopathological examinations of liver and kidney of both treatment groups did not reveal any negative pathological changes (Fig. 3, Fig. 4). The result shows normal hepatocellular morphology, normal periportal area with no evidence of necrosis and inflammation. Correspondingly, all sections from the kidney showed normal glomerular architecture and typical tubule interstitial parenchyma, with no vascular necrosis or hyaline changes.
Fig. 3.
Histology of liver (H&E, magnification x 4) of control and C. citratus oil treated animals. (A) Section of liver from control animals revealed normal architecture and hepatic cells; (B), liver from C. citratus oil (2000 mg/kg)-treated animals exhibited normal architecture and hepatic cells. CV = Central Vein, PT = Portal Tract, H= Hepatocytes.
Fig. 4.
Histology of kidney (magnification x 40) of control and C. citratus oil treated animals. (A) Section of kidney from control animal showed normal size of glomeruli with normal tubules; (B), kidney from C. citratus oil (2000 mg/kg) treated animals exhibit normal size of glomeruli with normal tubules. G = Glomerulus, BS = Bowman’s Space, DT = Distal Convoluted Tube.
5. Discussion
Considering the numerous reported therapeutic and cosmetic potentials of C. citratus, it is only pertinent to establish safety profile as a guide for its use. In this study, single dose of 2000 mg/kg C. citratus essential oil administration did not cause any toxicity signs or mortality in the first 24 h of the experiment and all the way to the 14th day. This finding is in agreement with the study conducted by Costa et al [26] and Nakavuma et al. [27]. Further, in line with our finding, [28] reported LD50 value of 3250 mg/kg body weight in rats. On the other hand, unlike our study, they reported signs of toxicity at 2000 mg/kg. This might be due to difference in the composition of secondary metabolites among various C. citratus varieties collected from different agro-ecological zones. Several investigations have shown that the chemical composition of C. citratus extracts varies according to the geographical origin, genetic differences, part of the plant used, method of extraction, age/stage of maturity, and season of harvest [29].
According to Nair [30] the C. citratus essential oil is usually made up of citral at an average of 65–80%. The percentage of citral determines the quality of C. citratus essential oil [31]. In this study citral represents 71.297 % of C. citratus essential oil of which geranial and neral represented 40.708 % and 30.589 % respectively. The result of this study is in good agreement with study done by Kassahun et al. [32]. However, there is a contradiction with the study conducted by Abegaz and Yohannes [33] which reported that Ethiopian C. citratus essential oil contains exceptionally lower amount of citral (13 %). The difference may be due to difference in the environmental factors such as light, temperature, water, soil fertility and salinity at the present time and 36 years back.
Allergy is a state of hypersensitivity of the skin or an excessive immune response to an antigen, and is manifested as edema and erythema [34]. Erythema is redness of the skin or mucous membranes, caused by hyperemia of superficial capillaries while edema is a buildup of excess serous fluid between tissue cells [35]. Recent animal studies have shown that Citral, a major component of the C. citratus oil, can induce skin irritation. Additionally, a lemon-scented detergent was also implicated in an outbreak of eczema in human being [29]. On the contrary in this study there was no positive skin reactions associated with the application of C. citratus essential oil. This difference could be because of the use of oil extract in the current study and thus different constituents other than citral that could have opposite effect on allergic reaction. But the other studies have utilized pure citral. Moreover difference in dose and duration of treatment could be the reason for the observed difference.
Repeated exposure toxicological evaluations are vital to characterize the toxicological profile of xenobiotics [23]. Changes in body and organ weights are clear indications of damage caused by the substance being tested [36]. In the present study, after sub-acute exposure, there were no significant differences in mean food consumption, percentage weight gain and relative organ weight gain between treatment and control groups. These findings are however in contradiction with the reports of Fandohan et al [28] in which all rats treated with C. citratus essential oils at 2000 and 3000 mg/kg died the 2nd day of treatment. These discrepancies may be due to agro-ecology of the plant which has a pronounced impact on composition and proportion of secondary metabolites produced by C. citratus [29].
Serum biochemicals (creatinine, ALP, GOT and serum SGPT) are used as biomarkers to measure hepatorenal injuries ([37,38]). In this study, there are no statistically significant differences in all biochemical parameters were observed between the treated and control groups as indicated in (Table 6). The absence of significant increase observed for GPT and GOT strongly suggests that sub-acute administration of C. citratus oil did not alter hepatocytes and consequently the metabolism in the mice. The excretion function of kidney can be assessed by measuring the levels of plasma creatinine, urea and uric acid concentrations. Increased blood levels of these parameters indicate some problems with ability of the kidneys to filter the blood [24,39]. The non-significant difference in the values of creatinine in the control and treated groups suggest that the essential oil did not create any sort of disturbance in the renal function. The histopathological examination of organs and tissues is best for evaluating treatment related pathological changes which further confirms alteration in their cellular structure. In the current study, liver architecture of both control and treatment animals are almost the same with normal morphology of hepatocytes, central vein and portal triads. There was no sign of necrosis. Further, no change was observed in kidney function in both the treatment and control animals as evidenced by no histological changes. Therefore, C. citratus essential oil falls in Class 5 (LD50 >2000 mg/kg) and can be considered as a low toxicity substance [22].
5.1. Conclusion
The present study indicates absence of acute and sub-acute toxicity attributable to C. citratus essential oil oral administration at the dose of 2000 mg/kg. Moreover, the data obtained from present study are relevant as they provide the safety of the oil for cosmetic use. Generally, Ethiopian C. citratus essential oil may be considered as relatively safe and non-toxic. However, studies on chronic toxicity of the oil should be conducted in the future to evaluate the safety of this oil for human use.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We are indebted to Debre Berhan University and the Ethiopian Ministry of Science and Technology for financing the field and laboratory works. W/O Aagere Yigezu is deeply acknowledged for her support in all aspects of laboratory activities pertinent to this work. Staff members of the National Herbarium, Addis Ababa University are highly appreciated for their services in taxonomic identification of plant specimen used in this research.
Contributor Information
Ermias Lulekal, Email: Zeaklog@gmail.com.
Solomon Tesfaye, Email: soltesfa2010@gmail.com.
Selam Gebrechristos, Email: selam3148@gmail.com.
Kassahun Dires, Email: kassh2009@gmail.com.
Tizazu Zenebe, Email: tizazuzenebe@yahoo.com.
Gezu Feleke, Email: gezufeleke@gmail.com.
Abayneh Kassahun, Email: abayneh2002@gmail.com.
Awol Mekonnen, Email: awolalim@gmail.com.
References
- 1.Aftab K., Ali M., Aijaz P., Beena N., Gulzar H., Sheikh K., Sofia Q., Tahir Abbas S. Determination of different trace and essential element in lemon grass samples by x-ray fluorescence spectroscopy technique. Int. Food Res. J. 2011:18. [Google Scholar]
- 2.Tarkang P., Agbor G., Tsabang N., Tchokouaha R., Tchamgoue D., Kemeta D., Mengue Y., Mba J., Weyepe F. Effect of long-term oral administration of the aqueous and ethanol leaf Extracts of Cymbopogon citratus (DC. ex Nees) stapf. Ann. Biol. Res. 2012;3:5561–55570. [Google Scholar]
- 3.Wannissorn B., Jarikasem S., Siriwangchai T., Thubthimthed S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia. 2005;76:233–236. doi: 10.1016/j.fitote.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T., Mazzali M., Kang D.-H., Kanellis J., Watanabe S., Sanchez-Lozada L.G., Rodriguez-Iturbe B., Herrera-Acosta J., Johnson R.J. Hyperuricemia causes glomerular hypertrophy in the rat. Am. J. Nephrol. 2003;23:2–7. doi: 10.1159/000066303. [DOI] [PubMed] [Google Scholar]
- 5.Holetz F.B., Ueda-Nakamura T., Filho B.P.D., Cortez D.A.G., Morgado-Diaz J.A., Nakamura C.V. Effect of essential oil of Ocimum gratissimum on the trypanosomatid herpetomonas samuelpessoai. Acta Protozoologica. 2003;42:269–276. [Google Scholar]
- 6.Puatanachokchai R., Kishida H., Denda A., Murata N., Konishi Y., Vinitketkumnuen U., Nakae D. Inhibitory effects of lemon grass (Cymbopogon citratus, Stapf) extract on the early phase of hepatocarcinogenesis after initiation with diethylnitrosamine in male Fischer 344 rats. Cancer Lett. 2002;183:9–15. doi: 10.1016/s0304-3835(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 7.Abe S., Maruyama N., Hayama K., Inouye S., Oshima H., Yamaguchi H. Suppression of neutrophil recruitment in mice by geranium essential oil. Mediators Inflammation. 2004;13:21–24. doi: 10.1080/09629350410001664798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda T., Odaka Y., Ogawa N., Nakamoto K., Kuninaga H. Identification of geranic acid, a tyrosinase inhibitor in lemongrass (Cymbopogon citratus) J. Agric. Food. Chem. 2007;56:597–601. doi: 10.1021/jf072893l. [DOI] [PubMed] [Google Scholar]
- 9.Gazola R., Machado D., Ruggiero C., Singi G., Alexandre M.M. Lippia alba, Melissa officinalis and Cymbopogon citratus: effects of the aqueous extracts on the isolated hearts of rats. Pharmacol. Res. 2004;50:477–480. doi: 10.1016/j.phrs.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Tognolini M., Barocelli E., Ballabeni V., Bruni R., Bianchi A., Chiavarini M., Impicciatore M. Comparative screening of plant essential oils: phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006;78:1419–1432. doi: 10.1016/j.lfs.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Mansour H.A., Newairy A.-S.A., Yousef M., Sheweita S. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology. 2002;170:221–228. doi: 10.1016/s0300-483x(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 12.Carlini E.A., Contar J.D.D., Silva-Filho A.R., Da Silveira-Filho N.G., Frochtengarten M.L., Bueno O.F. Pharmacology of lemongrass (Cymbopogon citratus Stapf). I. Effects of teas prepared from the leaves on laboratory animals. J. Ethnopharmacol. 1986;17:37–64. doi: 10.1016/0378-8741(86)90072-3. [DOI] [PubMed] [Google Scholar]
- 13.Xaio P. Recent developments on medicinal plants in China. J. Ethnopharmacol. 1983;7:95–109. doi: 10.1016/0378-8741(83)90083-1. [DOI] [PubMed] [Google Scholar]
- 14.Negrelle R., Gomes E. Cymbopogon citratus (DC.) Stapf: chemical composition and biological activities. Rev. Bras. Pl. Med. 2007;9:80–92. [Google Scholar]
- 15.Lorenzetti B.B., Souza G.E., Sarti S.J., Santos Filho D., Ferreira S.H. Myrcene mimics the peripheral analgesic activity of lemongrass tea. J. Ethnopharmacol. 1991;34:43–48. doi: 10.1016/0378-8741(91)90187-i. [DOI] [PubMed] [Google Scholar]
- 16.Limenih Y., Umer S., Wolde-Mariam M. Ethnobotanical study on traditional medicinal plants in Dega Damot woreda, Amhara Region, North Ethiopia. Int. J. Res. Pharm. Chem. 2015;5:258–273. [Google Scholar]
- 17.Gebremickael A. Single dose acute toxicity testing and preliminary safety evaluation of lemongrass oil in mice. Int. J. Current Res. 2017;9:56297–56299. [Google Scholar]
- 18.Rahul N., Sevukarajan M., Badivaddin M., Jayraj K. Formulation of Microemulsion based vaginal gel-in vitro and in vivo evaluation. Der. Pharmacia. Lettre. 2010;2:99–105. [Google Scholar]
- 19.Bora A., Deshmukh S., Swain K. Recent advances in semisolid dosage form. Int. J. Pharm. Sci. Res. 2014;5:3596. [Google Scholar]
- 20.OECD . Organization for Economic Cooperation and Development; Paris, France: 2002. Guideline for Testing of Chemicals 404. Acute Dermal Irritation/Corrosion. [Google Scholar]
- 21.Kamkaen N., Phuntuwate W., Samee W., Boonrod A., Treesak C. The investigation of the rabbit and human skin irritation of herbal anti-wrinkle cream. Thai Pharm Health Sci J. 2007;2:20–25. [Google Scholar]
- 22.OECD . Organization for Economic Cooperation and Development; Paris, France: 2001. Guideline for Testing Chemicals 425. Acute Oral Toxicity-up and Down Procedure; pp. 12–16. [Google Scholar]
- 23.OECD . Organization for Economic Cooperation and Development; Paris, France: 2008. Guidelines for the Testing of Chemicals. Repeated Dose 28-Day Oral Toxicity Study in Rodents, Draft Updated Test Guideline 407. [Google Scholar]
- 24.Tabarraei H., Hassan J., Parvizia M., Golshahic H., Tarikhi H. Evaluation of the acute and sub-acute toxicity of the black caraway seed essential oil in Wistar rats. Toxicol. Rep. 2019;6:869–874. doi: 10.1016/j.toxrep.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olaniyan J., Muhammad H., Makun H., Busari M., Abdullah A. Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonia campestris in rats. J. Acute Dis. 2016;5:62–70. [Google Scholar]
- 26.Costa R.A., Bidinotto L.T., Takahira R.K., Salvadori D.F., Barbisan L.F., Mirtes Costa M. Cholesterol reduction and lack of genotoxic or toxic effects in mice after repeated 21-day oral intake of lemongrass (Cymbopogon citratus) essential oil. Food Chem. Toxicol. 2011;49:2268–2272. doi: 10.1016/j.fct.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Nakavuma J.L., Matasyoh J.C., Wagara I.N., Kalema J., Alinaitwe L. Toxicity studies on anti-fungal essential oils extracted from selected aromatic plants from Mabira and Kakamega forests, East Africa. Eur. J. Med. Plants. 2016;14:1–14. [Google Scholar]
- 28.Fandohan P., Gnonlonfin B., Laleye A., Gbenou J., Darboux R., Moudachirou M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem. Toxicol. 2008;46:2493–2497. doi: 10.1016/j.fct.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Ekpenyong C.E., Akpan E.E., Daniel N.E. Phytochemical constituents, therapeutic applications and toxicological profile of Cymbopogon citratus Stapf (DC) leaf extract. J. Pharmacogn. Phytochem. 2014:3. [Google Scholar]
- 30.Nair E.V. Essential oil of East Indian lemongrass: present position in India and scope of its development. Cultiv. Util. Med. Aromat. Plants. 1977:204–206. [Google Scholar]
- 31.Hanaa A.R., Sallam Y.I., El-Leithy A.S., Aly S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012;57:113–116. [Google Scholar]
- 32.Kassahun B.M., Mekonnen S.A., Abedena Z.T., Kidanemariam H.G., Yalemtesfa B., Melka B., Mengesha W.K., Teixeira da Silva J.A. Performance of Lemongrass (Cymbopogon citratus L. (DC) Stapf) agronomic and chemical traits in different agro-ecologies of Ethiopia. Med. Aromat. Plant Sc. Biotechnol. 2011;5:133–138. [Google Scholar]
- 33.Abegaz B., Yohannes P.G. Constituents of the essential oil of Ethiopian cymbopogon citratus Stapf. J. Nat. Prod. 1983;46:424–426. [Google Scholar]
- 34.James O., Sunday A.B. Evaluation of acute dermal irritation and wound contraction by Gymnema Sylvestre and Datura metel extracts in rats. Am. J. Biomed. Life Sci. 2014;2:83–88. [Google Scholar]
- 35.Gatne M., Tambe K., Ravikanth K. Acute dermal irritation study of polyherbal gel Mastilep in rabbits. Int. J. Pharm. Sci. Res. 2015;6:3473. [Google Scholar]
- 36.Berenguer R., Azalea C., Castillo A., SalasH P.E., Betancourt J. Acute oral toxicity of Azadirachtaindica (Neem Tree) Rev Cubana Plant Med. 2013;18:502–507. [Google Scholar]
- 37.Brandt A.P., Oliveira L.F.Sd., Fernandes F.B., Alba J. Evaluation of prospective hypocholesterolemic effect and preliminary toxicology of crude extract and decoction from Vitex megapotamica (Spreng) Moldenke (V. montevidensis Cham.) in vivo. Revista Brasileira de Farmacognosia. 2009;19:388–393. [Google Scholar]
- 38.Lameire N., Van Biesen W., Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 39.Abdulrahman F., Onyeyili P., Sanni S., Ogugbuaja V. Toxic effect of aqueous root-bark extract of Vitex doniana on liver and kidney functions. Int. J. Biol. Chem. 2007;1:184–195. [Google Scholar]