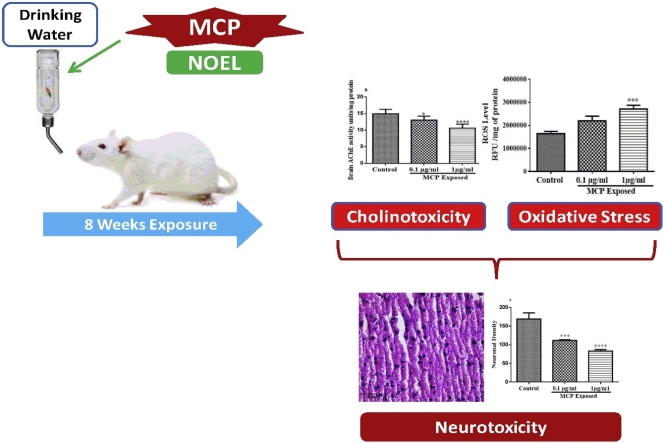
Graphical abstract
Abbreviations: OP, organophosphate; MCP, monocrotophos; ChE, cholinesterase; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde; ROS, reactive oxygen species; BSA, bovine serum albumin; DTNB, 5, 5-dithiobis (2-nitro-benzoic acid); ATCI, acetylthiocholineiodide; rGSH, reduced glutathion; Na2CO3, sodium carbonate; NaOH, sodium hydroxide; SDS, sodium dodecyl sulphate; TBA, thiobarbituricacid; DCFDA, 2, 7-dichlrofluorescein diacetate; DMS, dimethyl sulfoxide; NO, nitric oxide; H2O2, hydrogen peroxide; SOD, superoxide dismutase; PMSP, henazinemethosulphate; NBT, nitrobluetetrazolium; NADH, nicotinamide adenine dinucleotide reduced; MCP, monocrotophos; NOEL, no observed effect level
Keywords: Monocrotophos, Oxidative stress, Nitrosative stress, Cholinesterase inhibition, Neuronal loss, Rat
Abstract
Monocrotophos (MCP) is an organophosphate mainly used as insecticides in agriculture, and veterinary practice to control pests. Exposure to MCP is known to induce significant systemic toxicity in animals and humans. Short term exposure to a high dose of MCP has been reported to cause systemic toxicity, however limited information is available regarding low dose long term exposure in rats. We studied the effects of low dose long term exposure to MCP on oxidative/nitrosative stress, cholinesterase activity and neuronal loss in rat. Male rats were exposed to MCP (0.1 μg or 1 μg/ml) via drinking water for 8 weeks. The pro-oxidant markers such as reactive oxygen species (ROS), lipid peroxidation (MDA), nitrite level and antioxidant markers such as reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and inhibition of cholinesterase activities were measured to evaluate the effects of MCP on brain along with plasma cholinesterase activity. Neuronal loss was analyzed in cortical region using H&E stained slices. The results suggested that exposure to MC even at the low dose, increased reactive oxygen species, thiobarbituric acid reactive substance levels and decreased glutathione, superoxide dismutase, catalase and cholinesterase activities in brain. No significant effect however, was observed on nitrite levels. Histological analysis revealed that low dose MCP exposure lead to structural changes in the cortical neurons in rats. It can be concluded from the study that low dose long term exposure (lower than No Observed Effect Level) of MCP may lead to the generation of oxidative stress by elevation of pro-oxidants markers and depletion of antioxidant enzymes markers along with inhibition of cholinesterase activity. These changes might thus be considered as the possible mechanism of cortical neuronal loss in these animals.
1. Introduction
Organophosphates (OP) are the organic molecules containing one or more phosphate ester groups. It has been extensively used to control pests in agriculture, veterinary practice and home garden [1]. Deliberate self-poisoning with OP has been a major concern worldwide, especially in the developing countries [2]. Acute OP poisoning has been reported to cause more than 0.2 million death per year, worldwide. However, long term exposure to OP may lead to acute neuro-, delayed and developmental neurotoxicity, contributing thus to the pathogenesis of progressive neurodegenerative diseases viz. Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis [3,4].The potential toxicological mechanism of OP has not been fully explored, which undermines the development of specific approaches for the management of OP poisoning.
Acetylcholinesterase (AChE) inhibition may be considered as one of the primary modes of action for OP induced neurotoxicity [5,6]. AChE is an important enzyme which regulates the cholinergic innervations by facilitating degradation of acetylcholine into choline and acetate through hydrolysis [6]. OP binds to the estratic site of AChE irreversibly leading to the inhibition of its activity which further leads to cholinergic crisis in the central nervous system [7].
The redox imbalance has also been reported in OP induced neurotoxicity [8]. Inhibition of AChE with OP may cause hyperactivity of cholinergic innervations which in turn facilitate the production of reactive oxygen species [9]. Brain is known to be highly susceptible to oxidative stress mediated cellular damage due to its high oxygen consumption and high lipid content and low antioxidant defense enzymes [10].
Monocrotophos (MCP), a water soluble OP, has been reported to cause systemic and dermal toxicity. MCP has been reported to cause functional and developmental neurotoxicity, possibly via inhibiting AChE, and impairing cholinergic, dopaminergic, serotonergic innervations, oxidative stress and mitochondrial dysfunction in central nervous system [2].
The LD50 (lethal dose on 50 % population) of MCP has been reported to be 18 mg/kg (orally) in rats [11]. Most of the earlier reported toxicity studies have been carried out using a relatively higher dose of MCP, which simulates the acute OP poisoning. However, the presence of varied amount of these OPs has been noticed in ground water, which may expect its low dose chronic exposure induced neurotoxicity. The NOEL of MCP in rats has been reported to be 0.025 mg/kg daily through diet [12].This study thus was envisaged to investigate low dose prolonged exposure of MCP induced biochemical and structural changes in rat brain below the NOEL range of MCP dose.
2. Materials and Methods
2.1. Chemicals
Monocrotophos, Acetylthiocholine iodide, 5, 5 o-Dithiobis (2-nitrobenzoic acid) (DTNB), were procured from Sigma–Aldrich Chemicals Co., St. Louis (USA).
2.2. Animals
Male Sprague Dawley rats (10–12 weeks old) were procured from the animal facility of CSIR-Central Drug Research Institute, Lucknow, India. The animals were housed (3 rats/cage) in controlled environment on a 12-h light/dark cycle, with free access to standard laboratory food and distilled water. The experimental protocol and animal handling were in accordance with the guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA, Government of India). The study was approved by the Institutional Review Board and Animal Ethics Committee of the National Institute of Pharmaceutical Education and Research (NIPER).
2.3. Experimental protocol
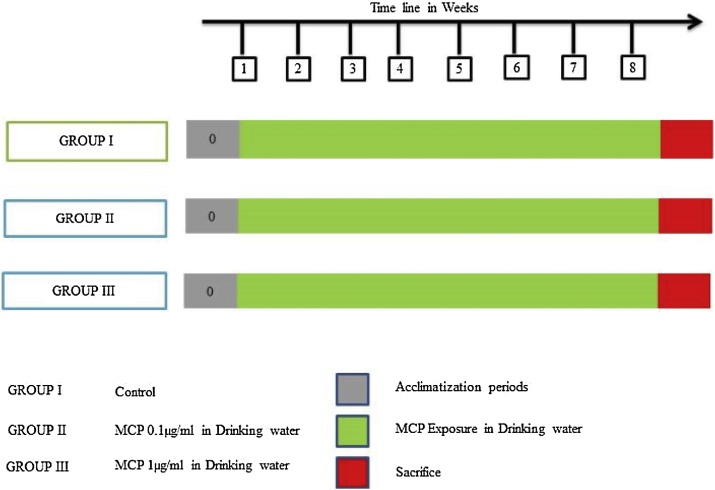
All the animals were randomly divided into 3 groups of 9 rats each. Group I served as vehicle control and received normal drinking water, while Group II and group III were administered two doses of MCP (0.1 and 1 μg/ml) through drinking water for 8 weeks (Fig. 1).
Fig. 1.
Experimental study design.
After 8 weeks of exposure, all the animals were sacrificed under anesthesia (urethane 1.5 g/kg). Blood was collected by cardiac puncture and cardiac perfusion was done with ice-cold normal saline. After the perfusion, brains were removed for following biochemical and histological analysis.
3. Biochemical Parameters
3.1. Cholinesterase activity in plasma and brain
Determination of cholinesterase (ChE) activity was carried out using the method described by Ellman et al. [14]. Brain homogenate (25 %, w/v) was prepared in 100 mM phosphate buffer (pH 7.4), and centrifuged at 10,000 rpm for 10 min at 4 °C. 10 μl of sample (plasma/brain homogenate of supernatant) was mixed with 272 μl of mixture containing (10 mM DTNB, 75 mM ATCI and 50 mM phosphate buffer pH 7.4) in micro plate. The absorbance of the reaction mixture was read at 412 nm on kinetic loop using Multimode plate Reader (SynergyH1M, Biotech). The results were expressed as units/mg protein [14]. Standard plot of AChE activity was recorded with different concentrations of AChE (2 fold of 35 nM). The straight line equation of AChE activity comes out to be Y = 1.4286x, Y is change in optical density/min; x is concentration of AChE (units/mg protein).
3.2. Reactive oxygen species levels
The level of reactive oxygen species (ROS) was determined following the protocol described by Socci et al. [15]. The reaction mixture was prepared with 5 μl supernatant of tissue homogenate which was mixed with 5 μl of 5 μM DCFDA (2, 7-dichlrofluorescein diacetate) and 990 μL of water. After 30 min, the fluorescence was measured at 485/525 nm. Results were expressed as fluorescence units per milligram of protein [15].
3.3. Thiobarbituric acid reactive substance (TBARS)
Malondialdehyde (MDA) is the end product of lipid per oxidation and could be measured in brain tissues by using thiobarbituric acid reactive substance (TBARS) method [16] with some modifications. In brief, the brain tissues were collected and rinsed with ice-cold PBS, minced and homogenates were prepared in phosphate buffer (pH 7.4) containing EDTA (1 mM). The samples were centrifuged and the supernatants were used for the determination of TBARS (MDA) levels. The absorbance was measured at 532 nm. MDA levels were calculated from the standard curve using the 1, 1, 3, 3-tetramethoxy propane (TMP) the straight line equation was Y = 0.0023x + 0.0004 where Y is change in optical density at 532 nm; x is concentration TMP per mg of protein. The results were expressed as μM MDA/mg protein [16].
3.4. Nitrite level
The Nitrite contents were determined in the tissue homogenate according to the method described by Giustarini et al., [17] with some modifications. Briefly, equal volumes of Griess reagent and supernatant were added in a 96- well plate and incubated for 10 min in dark condition with shaking, and final absorbance was measured at 540 nm. Nitrite levels were calculated using a standard curve of sodium nitrite as a standard at different concentrations and straight line equation was comes to be Y = 0.0086x + 0.1852, where Y is the change in optical density of nitrite at 540 nm; x is concentration of nitrite per mg of protein. The results were expressed as μM/mg protein [17].
3.5. Superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity was determined according to the method of Flora et al. [18]. The reaction mixture was prepared which contained 1.2 ml of sodium pyrophosphate, 0.3 ml of PMS, 0.3 ml of NBT, 0.2 ml of supernatant, 0.8 ml of distilled water and 0.2 ml of NADH. The control reaction mixture was prepared and contained 1.2 ml of sodium pyrophosphate, 0.3 ml of PMS, 0.3 ml of NBT, 1 ml of distilled water and 0.2 ml of NADH. Both mixtures were incubated at 37 °C for 90 s and then reaction was stopped by adding 1 ml of acetic acid and the mixture was allowed to stand for 10 min. The absorbance was measured at 560 nm.
Measurement of protein level: Supernatants (5 μL) of the brain tissue homogenate were incubated with solution D (2 % sod. carbonate, 0.4 % sod. hydroxide, 2 % sod. tartrate, and 1 % copper sulphate) for 10 min at 37 °C. Resulting solution was treated with Folin’s reagent in 1:1 ratio for 30 min at 37 °C. Blue colour was developed. The absorbance was measured at 660 nm along with standard prepared with known concentration of BSA.
3.6. Catalase activity
The catalase activity was estimated by the method described by Goth et al., [19]. Ammonium molybdate forms a yellow complex with H2O2 and is suitable for measuring serum and tissue catalase activity. To analyze the catalase activity, 0.2 ml of tissue homogenate was incubated with 1 ml 65 μM H2O2 in 6.0 mM sodium potassium phosphate buffer, pH 7.4 for 60 s (sample 1). Control reactions were done with 1 ml H2O2 plus 0.2 ml buffer (no enzyme control; blank 2) and 1.2 ml buffer (no enzyme/no substrate, blank 3). The reaction was stopped by adding 1.0 ml of 32.4 mM ammonium molybdate to the sample and control reactions and the absorbance was determined at 405 nm [19].
3.7. Reduced glutathione level
Reduced glutathione GSH(r) was estimated by the method of Gupta and Flora [20] with some modification. The tissue homogenate was prepared as indicated above and equal volume of 5 % sulfosalicylic acid was added and vortexed. The mixture was kept for 30 min in ice bath. After centrifugation, the supernatant was collected. GSH content was measured using Ellman’s reagent 5, 5-dithiobis (2-nitrobenzoic acid) (DTNB) solution. GSH levels were calculated using a standard reference curve of reduced glutathione as a standard and straight line equation cpmes to be Y = 0.002x - 0.0015, where Y is change in optical density of r GSH at 412 nm; x is concentration of r GSH per mg of protein. The results were expressed in μM GSH/mg protein [20].
4. Histological analysis
The structural changes in brain were determined using normal hematoxylin and eosin (H&E) staining procedure. The brain parts were fixed in Bouin’s fluid, embedded in paraffin, sectioned at 5 μm thicknesses by using the microtome (York scientific, India) and stained with H&E. Brain sections were analyzed for neuronal loss in cortical region of rat brain. The brain sections were analyzed at 100 and 400 magnifications using optical microscope (DM11, Leica).The neuronal density was quantified using image J software.
5. Statistical analyses
All the results were expressed as the mean ± standard error (SEM). Graph Pad (Prism 6.1) software was used for the statistical analysis. Statistical differences between the groups were analyzed by one-way analysis of variance (ANOVA) followed by multiple comparisons with Tukey’s test and regression analysis was performed along with correlation. The level of statistical significance was set at *p < 0.05. **p < 0.01, ***p<0.001, and ****p < 0.0001.
6. Results
6.1. Cholinesterase activity
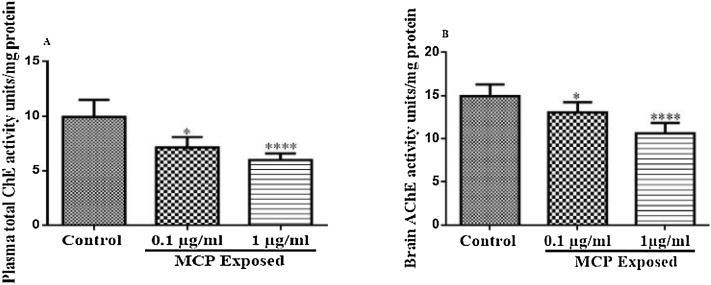
The changes in plasma ChE activity are depicted in Fig. 2(A). There was a significant change in plasma ChE activity after 8 weeks of exposure to monocrotophos (F (2, 15) = 19.99, p < 0.0001). Exposure to monocrotophos (0.1 and 1 μg/ml) resulted in a significant decrease in plasma ChE activity (p < 0.0001, 0.01 respectively) compared to control animals.
Fig. 2.
Effect of monocrotophos on ChE activity in plasma (A), and brain (B). All values were expressed as Mean ± SEM of six animals. Statistical analysis was done using one way ANOVA followed by Tukey’s multiple comparison test and statistical significance was considered at p < 0.05. *p < 0.05, and ****p < 0.0001 as compared to control animals.
The change in brain ChE activity is depicted in Fig. 2(B). A significant change in brain ChE activity was noted after 8 weeks exposure to monocrotophos (F (2, 15) = 17.76, P = 0.0001). Both the doses (1 μg/ml and 0.1 μg/ml) of MCP exposure resulted in a significant decrease in plasma ChE activity (p < 0.0001, 0.05 respectively) compared to control animals.
6.2. ROS and TBARS levels in rat brain
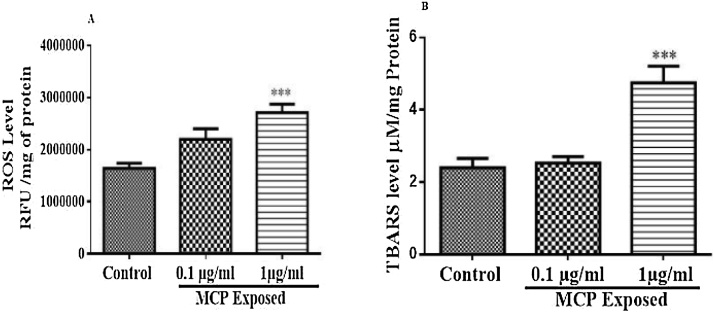
The changes in brain ROS level are shown in Fig. 3(A). Brain ROS level increased significantly after 8 week exposure to monocrotophos (F (2, 12) = 11.43, p = 0.0017). A 1 μg/ml dose of monocrotophos exposure resulted in a significant increase (p < 0.01) in brain ROS level compared to controls while, a dose of 0.1μ/ml dose of monocrotophos exposure had no appreciable change in ROS compared to control animals.
Fig. 3.
(A) Effect of monocrotophos on brain ROS level, (B) Effect of monocrotophos on brain TBARS level, All values were expressed as Mean ± SEM; n = 6. Statistical analysis was done using one way ANOVA followed by Tukey’s multiple comparison tests and statistical significance was considered at P < 0.05. *p < 0.05, and ****p < 0.0001 as compared to control animals.
The changes in brain TBARS level are depicted in Fig. 3(B). A significant change in brain TBARS level (F (2, 15) = 16.90, P = 0.0001) was noted on monocrotophos administration on administration of 1 μg/ml dose (p < 0.001) while, 0.1 μg/ml dose of monocrotophos exposure had no effect on TBARS level compared to control animals.
6.3. Nitrite and anti-oxidant enzymes level
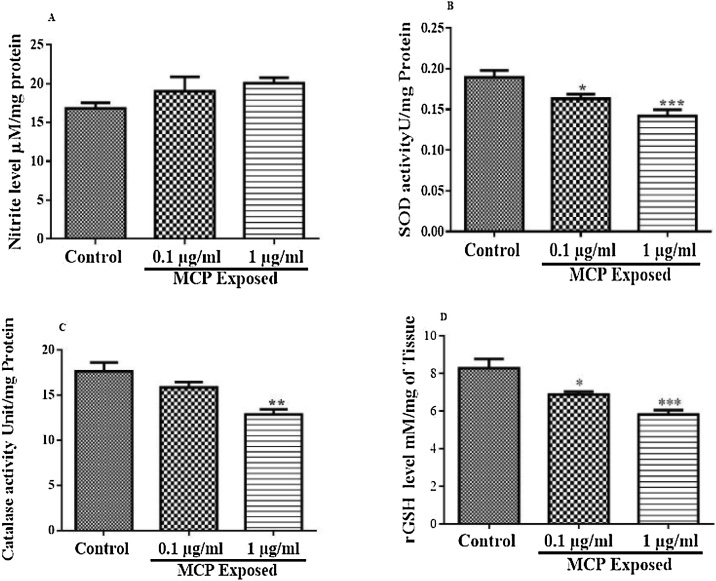
The change in brain Nitrite level has been depicted in Fig. 4(A). There was no change in brain nitrite level after 8 week exposure to monocrotophos (F (2, 15) = 1.903, P = 0.1834). Monocrotophos exposure at various doses did not alter nitrite level significantly (p < 0.05) compared to control animals.
Fig. 4.
(A) Effect of monocrotophos on brain Nitrite level, (B) Effect of monocrotophos on brain SOD level, (C) Effect of monocrotophos on brain Catalase level, (D) Effect of monocrotophos on brain rGSH level. All values were expressed as Mean ± SEM; n = 6, Statistical analysis was done using one way ANOVA followed by Tukey’s multiple comparison test and statistical significance was considered at P < 0.05. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to control animals.
The change in brain SOD activity has been depicted in Fig. 4(B). There was significant change in brain SOD activity after 8 week exposure to monocrotophos (F (2, 15) = 10.26, p = 0.0016). At 1 μg/ml and 0.1 μg/ml doses of monocrotophos exposure resulted in a significant (p < 0.001, 0.05 respectively) inhibition of brain SOD activity compared to control animals.
The changes in brain Catalase activity are shown in Fig. 4(C). There was significant change in brain catalase activity after 8 week exposure to monocrotophos (F (2, 15) = 10.58, p = 0.0014). At 1 μg/ml dose of monocrotophos exposure resulted in a significant (p < 0.01) decrease in brain catalase activity compared to control animals. However 0.1 μg/ml dose of monocrotophos exposure did not produce any significant (p < 0.05) change in catalase activity compared to control animals.
The change in brain rGSH level has been shown in Fig. 4(D). A significant change in brain rGSH level after 8 week exposure to monocrotophos (F (2, 15) = 14.06, P = 0.0004) was observed at both the dose level (1 μg/ml and 0.1 μg/ml) of monocrotophos exposure (p < 0.001, 0.05 respectively) compared to control animals.
6.4. Correlation of analysis
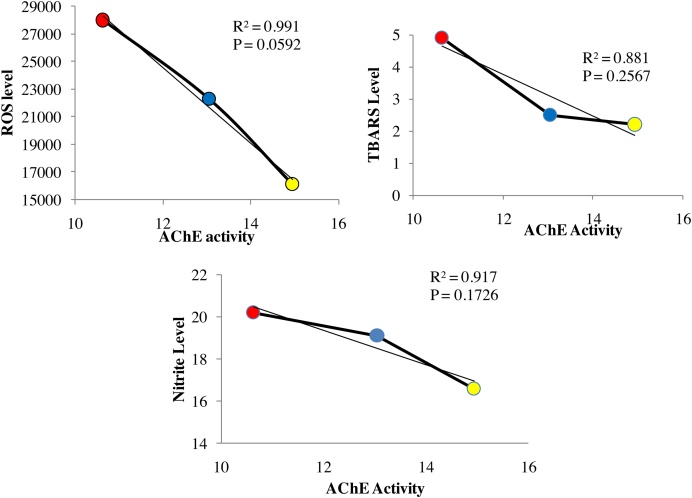
The effect of MCP on AChE activity was negatively correlated with the level of level of ROS (r2 = 0.9914 and p = 0.0592) and is depicted in Fig. 5A, TBARS level (r2 = 0.8460 and p = 0.2567) depicted in Fig. 5B, while Nitrite level (r2 = 0.9283 and p = 0.1726) is shown in Fig. 5C.
Fig. 5.
Correlation of analysis of linear regression showing the correlation between inhibition of Acetyl cholinesterase activity and pro-oxidant markers level rats were treated with monocrotophos at the doses of (0.1, 1 μg/ml) for a period 8 weeks. All the values are expressed as mean ± SD; n = 6.
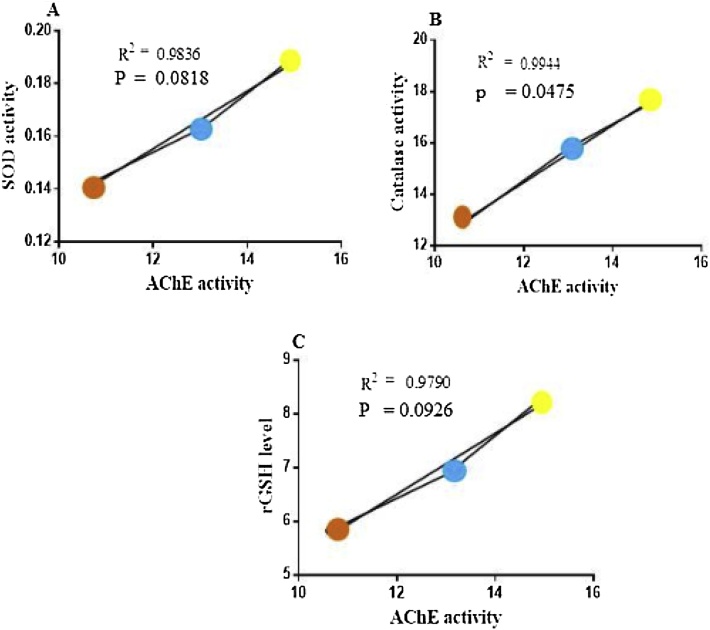
On the other hand, the effect of MCP on AChE activity was significantly and positively correlated with SOD activity (r2 = 0.9836 and p = 0.0818) (Fig. 6A), catalase activity (r2 = 0.9944 and p = 0.0475) (Fig. 6B) and reduced glutathione level (r2 = 0.9790 and p = 0.0926) (Fig. 6C).
Fig. 6.
Correlation of Analysis of linear regression showing the correlation between inhibition of Acetyl cholinesterase activity and anti-oxidant markers level rats were treated with monocrotophos at the doses of (0.1, 1 μg/ml) for a period 8 weeks. All the values are expressed as mean ± SD, n = 6.
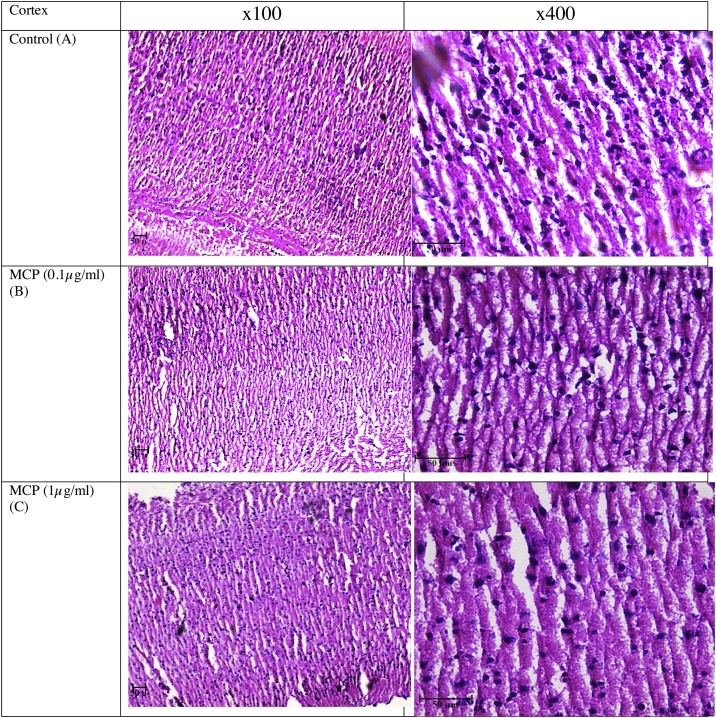
7. Effect of MCP on structural changes in cortical neurons of rat brain
H&E stained brain cortical sections are shown in Fig. 7. While Fig. 7 (A) reflects control group cortical sections of rat brain, Fig (B) shows MCP low dose (0.1 μg/ml) treated group and Fig. 7 (C) depicts MCP high dose(1 μg/ml) treated groups.
Fig. 7.
(A) The cortical regions of control group animals; Figure (B) Figure and (C) represent the (0.1, 1 μg/ml) of monocrotophos exposed cortical regions of treated group animals. Magnification x100 and x400.
Control section has the healthy and high density neurons with shape of spherical or slightly oval nucleus. Neurodegeneration are considered as the form of sequential specialized changes in morphology of neuronal cells. In the present study, row (B) shows that the low dose of MCP (0.1 μg/ml) treated brain cortical neurons led to a decreased neuronal density along with pyknotic neuronal cells. These stained were dark with fragmented or no nucleus, few of the cells were shrunken and sickle shaped while some cells were clumped together compared to control. Similar observations were found in the high dose (1 μg/ml) of MCP treated groups in (C) as compared to control group.
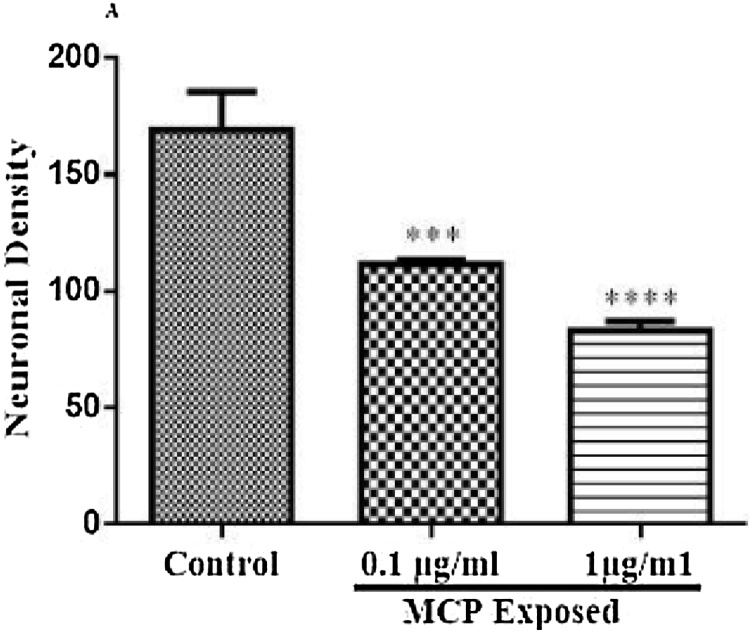
7.1. Effect of MCP on neuronal density of rat cortex
The loss of neuronal density in cortical regions of rat brain is depicted in Fig. 8. There was a significant decreased neuronal density in cortical regions after 8 week exposure to monocrotophos (F (2, 6) = 58.93, P = 0.0001). At 0.1 μg/ml and 1 μg/ml doses of MCP exposure a decreased neuronal density in rat cortex was noted (p < 0.001, 0.0001respectively) as compared to control animals.
Fig. 8.
Effect of monocrotophos exposure on neuronal density in cortical region of rat brain. ***p < 0.001, ****p < 0.0001compared to control animals.
8. Discussion
Extensive use of pesticides, especially monocrotophos (MCP) has been reported especially from the developing countries, which might be resulting in ground water contamination. MCP is known to cause neurotoxicity in humans and animals. Most of the studies pertaining to MCP neurotoxicity have been carried out at relatively higher doses. It is however expected that people gets exposed to a low dose and for a long time. As varied amounts of MCP may be observed in the ground water, thus long term low dose exposure to MCP may be expected. Limited information is available for the neurotoxic potential of MCP at the lower doses. This study was thus envisaged to determine the neurotoxic effect of low dose MCP exposure in rats.
We noted a significant inhibition of acetylcholineesterase activity following exposure to MCP accompanied by oxidative stress and neuronal loss. AChE is an important enzyme involved in the maintenance of cholinergic neurotransmission in the brain, and serves as an important target for organophosphates. Most of the organophosphates binds to the esteratic site of AChE leading to the irreversible loss of AChE activity [21]. Irreversible binding of organophosphates with AChE may lead to the inhibition of AChE activity and converse to augment ACh level at synaptic junctions [22,23]. The over-activity of cholinergic at synapses may lead to the desensitization of cholinergic receptors and may culminate to neuronal death [24]. MCP has also been reported to cause neurotoxicity by inducing cholinergic over activity [21]. Twenty five and 40 % reduction in plasma cholinesterase activity has been reported in animals exposed to MCP at 0.1 and 1.0 μg/ml, respectively. Decrease in plasma cholinesterase activity is reported to be the early marker of OP poisoning in humans as well as in animals [24]. Similar reduction in AChE activity has been recorded with high dose of MCP exposure in humans [25] and other species [26]. More than 20 % reduction in AChE activity has been reported to be linked with developmental neurotoxicity, while more than 70 % inhibition of AChE activity has been conversed to cause cholinergic over activation [27]. Thus, in the present study, low dose of MCP appears to be capable of inhibiting plasma ChE activity and might be responsible for reported neurotoxic effects.
Long term exposure to MCP, may lead to the inhibition of brain AChE activity. In the present study, inhibition of brain AChE activity was noted with low doses of MCP exposure. However, the degree of reduction in brain AChE was found to be 10 and 27 % with 0.1 and 1.0 μg/ml of MCP exposure, respectively. It may be concluded that even at the low dose, MCP exposure may lead to cholinergic over activation and enhances risk of neurotoxicity in exposed individuals. In the present study, change in brain AChE activity has been found to be negatively correlated with pro-oxidant markers and positively correlated with antioxidant defense system, in MCP exposed animals. Monocrotophos is an AChE inhibitor and produced major inhibitory effect in plasma. Monocrotophos and its metabolites being lipophilic in nature were expected to result in more toxicity in brain compared to plasma. However, comparative less inhibitory effect were noted in brain. This might be due to the fact that in our study animals were exposed to monocrotophos through oral route. After oral exposure, Monocrotophos and its metabolites gets readily absorbed through intestine and distributed evenly in different organs and tissues [28,29]. Higher concentration of monocrotophos in liver and kidney is reported though no organ specific retention of monocrotophos is known [30].Thus the effects of Monocrotophos and its metabolites on brain/ central nervous are generally delayed. Log P value of monocrotophos is -0.2, however the Log P value of their metabolites is lower than the parent molecule.
The change in redox balance has been linked to various neurological diseases and neurodegenerative diseases. A balance in pro and anti- oxidant status generally maintains normal cellular homeostasis. Oxidative stress has been considered as one of the toxicological mechanism of MCP induced neurotoxicity. The central nervous system is known to be more susceptible to oxidative stress, thus in the present study, MCP even at the low dose is eliciting oxidative stress in the brain.
Oxidative stress is the imbalance between biochemical processes leading to the production of reactive oxygen species (ROS) and antioxidant defense. ROS are known to damage all cellular bio-macromolecules (lipids, sugars, proteins, and polynucleotides), and these damage may lead to the secondary products that can be just as damaging as the initial ROS. Mitochondrial production of ROS initially arises as superoxide anion radical from the side reaction of oxygen intercept single electrons from the electron transport chain. In mitochondria, these ROS are neutralized with superoxide dismutase and get further converted to hydrogen peroxide (which is detoxified by catalase into water and oxygen). Mitochondria play a vital role in apoptotic pathways, and possess decisive apoptotic factors, including cytochrome C in their inter-membranous space [31]. Once cytochrome C is released into cytosol, it initiates caspase mediated apoptosis [32,33]. If the level of ROS supersedes, it may cause mitochondrial dysfunction and may lead to apoptosis mediated neuronal death [34].
The generation of most reactive substances exerts their effect on the endogenous antioxidants activity system, antioxidants of biological system have the capacity to fend off this unlimited generation of singlet oxygen molecules as well as avoid the harmful reaction with biological molecules, both enzymatic and non-enzymatic antioxidant pathways work together to secure the oxidative damage of the cells [35]. Organophosphate pesticides affect the redox condition of the tissues by induction of the oxidative stress [36]. Following increased ROS production; TBARS may cause an alteration of antioxidant enzyme activities. Superoxide dismutase is an antioxidant enzyme which protects the tissue oxidative injury caused by the formation of superoxide radicals by conversion of superoxide radicals into hydrogen peroxides. The SOD converts the superoxide radicals to hydrogen peroxide to provide the protective role against the oxidative damage. Hydrogen peroxide molecules also have the highly reactive nature so these molecules can convert into water and oxygen molecules. Catalase is an enzyme which helps the conversion of hydrogen peroxide molecules into water and oxygen molecules.
In the present study, MCP at a dose of 1 μg/ml, caused a significant elevation of ROS and TBARS levels in brain tissues, while at the lower dose of MCP (at 0.1 μg/ml) did not shown any appreciable change in brain ROS and TBARS levels. ROS are very reactive molecules which may easily interact with lipid membrane and cause membrane damage. The degree of lipid peroxidation in tissues is assessed by measuring TBARS. Production of brain ROS level with MCP exposure may thus be responsible for the elevated levels of TBARS. These findings indicate elevation in pro-oxidant levels in brain on low dose of MCP. On the other hand, reduction in antioxidant defense system has been recorded with low dose of MCP as evident from reduced activities of brain SOD and catalase and the decreased level of GSH. The reduction in antioxidant defense system suggests reduced free radicals scavenging. Overall, MCP exposure at low dose leads to redox imbalance in brain. Similar observations were reported with comparative high doses of MCP in different experimental models [37]. MCP is lipophilic in nature, thus it has the tendency to accumulate in brain which might be responsible for increased pro-oxidant markers in brain. Oxidative stress and mitochondrial dysfunction are considered to be the key mechanism for organophosphates induced apoptosis [38] which share a similar pathology with Alzheimer's disease.
This redox imbalance has been reported to be associated with mitochondrial dysfunction which may lead to oxidative damage to neurons. Similar redox imbalance has been observed in MCP induced neurotoxicity possibly via induction of oxidative stress and severe inhibition of AChE activity in brain [11].
As discussed earlier MCP has been reported to cause oxidative stress mediated mitochondrial dysfunction leading to apoptosis dependent neuronal death [39]. Similar observations were reported suggesting that exposure to MCP might cause the expression of apoptotic proteins of Bax, caspase-3 and leading to neuronal cell death [40].
Nitric Oxide (NO), in low concentrations, exerts beneficial effect on neuronal cells. However its augmented levels may elicit damaging effects on the neurons. In the presence of superoxides the nitric oxide gets converted into peroxynitriles, which may lead to more pronounced nitrosative damage to cells. In the present study, we did not observe any significant changes in brain NO level with low dose MCP exposure. In contrast, MCP at a comparatively higher dose showed an elevation in the brain NO level possibly via elevating inducible nitric oxide synthase expression [41]. The principle component analysis suggested the negative correlation between AChE activity and the oxidative stress level. Reduction in AChE activity and elevation in oxidative stress is linked with neuronal damage. Thus might be the cause of observed neuronal density in cortical neurons.
9. Conclusion
We conclude from the current study that exposure to MCP exposure, even at a lower dose than NOEL is capable of inhibiting AChE activity and induction of oxidative stress in the brain. The changes in brain AChE activity and oxidative stress may be the causative factor for the neuronal damage in cortical region of the brain. However, nitrosative stress was not found to be involved in the low dose MCP induced cortical damage in rats. These findings thus suggest the revision of minimum permissible limits of MCP in drinking water and food.
Declaration of Competing Interest
Authors declare no conflict of interest in this manuscript.
Acknowledgements
Authors acknowledge Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India, and National Institute of Pharmaceutical Education and Research (NIPER), Raebareli for their support and providing necessary infrastructure and facility. NIPER-R Communication # 81.
References
- 1.Migliore L., Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. 2009;674(1-2):73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Sankhwar M.L., Yadav R.S., Shukla R.K., Singh D., Ansari R.W., Pant A.B., Parmar D., Khanna V.K. Impaired cholinergic mechanisms following exposure to monocrotophos in young rats. Hum. Exp. Toxicol. 2012;31(6):606–616. doi: 10.1177/0960327111405860. [DOI] [PubMed] [Google Scholar]
- 3.Naughton S.X., Terry A.V., Jr Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101–112. doi: 10.1016/j.tox.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgiadis N., Tsarouhas K., Tsitsimpikou C., Vardavas A., Rezaee R., Germanakis I., Kouretas D. Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 2018;353:1–14. doi: 10.1016/j.taap.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Astiz M., Diz-Chaves Y., Garcia-Segura L.M. Sub-chronic exposure to the insecticide dimethoate induces a proinflammatory status and enhances the neuroinflammatory response to bacterial lypopolysaccharide in the hippocampus and striatum of male mice. Toxicol. Appl. Pharmacol. 2013;272(2):263–271. doi: 10.1016/j.taap.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Masoud A., Kiran R., Sandhir R. Modulation of dopaminergic system and neurobehavioral functions in delayed neuropathy induced by organophosphates. Toxicol. Mech. Methods. 2011;21(1):1–5. doi: 10.3109/15376516.2010.529182. [DOI] [PubMed] [Google Scholar]
- 7.Elersek T., Filipic M. Organophosphorous pesticides-mechanisms of their toxicity. In: Stoytcheva M., editor. Pesticides-the Impacts of Pesticides Exposure. InTech Press; 2011. pp. 243–262. [Google Scholar]
- 8.Thakur S., Dhiman M., Mantha A.K. APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells. Mol. Cell. Biochem. 2018;441(1-2):201–216. doi: 10.1007/s11010-017-3186-7. [DOI] [PubMed] [Google Scholar]
- 9.Júnior H.V., de França Fonteles M.M., Mendes de Freitas R. Acute seizure activity promotes lipid peroxidation, increased nitrite levels and adaptive pathways against oxidative stress in the frontal cortex and striatum. Oxid. Med. Cellular Long. 2009;2(3):130–137. doi: 10.4161/oxim.2.3.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla S., Jhamtani R.C., Dahiya M.S., Agarwal R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Reports. 2017;4:240–244. doi: 10.1016/j.toxrep.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazi A.I., Oommen A. Monocrotophos induced oxidative damage associates with severe acetylcholinesterase inhibition in rat brain. Neurotoxicol. 2012;33(2):156–161. doi: 10.1016/j.neuro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Tomlin C. 5.0. British Crop Protection Council; 2010. The Pesticide Manual (A World Compendium) [Google Scholar]
- 14.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 15.Socci D.J., Bjugstad K.B., Jones H.C., Pattisapu J.V., Arendash G.W. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp. Neurol. 1999;155(1):109–117. doi: 10.1006/exnr.1998.6969. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Giustarini D., Rossi R., Milzani A., Dalle-Donne I. Nitrite and nitrate measurement by Griess reagent in human plasma: evaluation of interferences and standardization. Methods Enzymol. 2008;440:361–380. doi: 10.1016/S0076-6879(07)00823-3. [DOI] [PubMed] [Google Scholar]
- 18.Flora S.J.S., Bhadauria S., Pant S.C., Dhaked R.K. Arsenic induced blood and brain oxidative stress and its response to some thiol chelators in rats. Life Sci. 2005;77(18):2324–2337. doi: 10.1016/j.lfs.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta. 1991;196(2-3):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R., Flora S.J.S. Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J. Appl. Toxicol. 2006;26(3):213–222. doi: 10.1002/jat.1131. [DOI] [PubMed] [Google Scholar]
- 21.Dwivedi N., Bhutia Y.D., Kumar V., Yadav P., Kushwaha P., Swarnkar H., Flora S.J.S. Effects of combined exposure to dichlorvos and monocrotophos on blood and brain biochemical variables in rats. Hum. Exp. Toxicol. 2010;29(2):121–129. doi: 10.1177/0960327109357212. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H., Yuyama A., Chiba K. Cholinergic system of brain tissue in rats poisoned with the organophosphate, 0, 0-dimethyl 0-(2, 2-dichlorovinyl) phosphate. Toxicol. Appl. Pharmacol. 1986;82(1):32–39. doi: 10.1016/0041-008x(86)90434-5. [DOI] [PubMed] [Google Scholar]
- 23.Moser V.C. Age-related differences in acute neurotoxicity produced by mevinphos, monocrotophos, dicrotophos, and phosphamidon. Neurotoxicol. Teratol. 2011;33(4):451–457. doi: 10.1016/j.ntt.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Haughey N.J., Bandaru V.V., Bae M., Mattson M.P. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim. Biophy. Acta. 2010;1801(8):878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aroonvilairat S., Kespichayawattana W., Sornprachum T., Chaisuriya P., Siwadune T., Ratanabanangkoon K. Effect of pesticide exposure on immunological, hematological and biochemical parameters in Thai orchid farmers—a cross-sectional study. Int. J. Environ. Res. Public Health. 2015;12(6):5846–5861. doi: 10.3390/ijerph120605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das G.P., Jamil K., Rahman M.F. Effect of four organophosphorus compounds on human blood acetylcholinesterase: in vitro studies. Toxicol. Mech. Methods. 2006;16(8):455–459. doi: 10.1080/15376520600719281. [DOI] [PubMed] [Google Scholar]
- 27.Narra M.R., Rajender K., Reddy R.R., Murty U.S., Begum G. Insecticides induced stress response and recuperation in fish: biomarkers in blood and tissues related to oxidative damage. Chemosphere. 2017;168:350–357. doi: 10.1016/j.chemosphere.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 28.Kumar B.K., Reddy A.G., Krishna A.V., Quadri S.S., Kumar P.S. Developmental neurotoxicity of monocrotophos and lead is linked to thyroid disruption. Vet. World. 2016;9(2):133. doi: 10.14202/vetworld.2016.133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selmi S., Rtibi K., Grami D., Sebai H., Marzouki L. Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep. 2018;5:189–195. doi: 10.1016/j.toxrep.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mücke W. Metabolism of monocrotophos in animals. Rev. Environ. Contam. Toxicol. 1994;139:59–65. doi: 10.1007/978-1-4684-7071-0_5. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Lein P.J., Liu C., Bruun D.A., Tewolde T., Ford G., Ford B.D. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol. Appl. Pharmacol. 2011;253(3):261–269. doi: 10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y.P., Mou D.L., Song J.F., Rao Z.R., Li D., Ju G. Aberrant activation of CDK5 is involved in the pathogenesis of OPIDN. J. Neurochem. 2006;99(1):186–197. doi: 10.1111/j.1471-4159.2006.04027.x. [DOI] [PubMed] [Google Scholar]
- 33.Owumi S.E., Dim U.J. Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicol. Rep. 2019;6:202–209. doi: 10.1016/j.toxrep.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayre L.M., Perry G., Smith M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2007;21(1):172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 35.Irshad M., Chaudhuri P.S. Oxidant-antioxidant system: role and significance in human body. Ind. J. Exp. Biol. 2002;40(11):1233–1239. [PubMed] [Google Scholar]
- 36.Mishra V., Srivastava N. Organophosphate pesticides‐induced changes in the redox status of rat tissues and protective effects of antioxidant vitamins. Environ. Toxicol. 2015;30(4):472–482. doi: 10.1002/tox.21924. [DOI] [PubMed] [Google Scholar]
- 37.Yaduvanshi S.K., Ojha A., Pant S.C., Lomash V., Srivastava N. Monocrotophos induced lipid peroxidation and oxidative DNA damage in rat tissues. Pest. Biochem. Physiol. 2010;97(3):214–222. [Google Scholar]
- 38.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. Suppl. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 39.Masoud A., Kiran R., Sandhir R. Impaired mitochondrial functions in organophosphate induced delayed neuropathy in rats. Cell. Mol. Neurobiol. 2009;29(8):1245–1255. doi: 10.1007/s10571-009-9420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar V., Tripathi V.K., Singh A.K., Lohani M., Kuddus M. Trans-resveratrol restores the damages induced by organophosphate pesticide-monocrotophos in neuronal cells. Toxicol. Int. 2013;20(1):48–55. doi: 10.4103/0971-6580.111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar B., Dhiman M., Mittal S., Mantha A.K. Curcumin revitalizes Amyloid beta (25–35)-induced and organophosphate pesticides pestered neurotoxicity in SH-SY5Y and IMR-32 cells via activation of APE1 and Nrf2. Metabolic Brain Dis. 2017;32(6):2045–2061. doi: 10.1007/s11011-017-0093-2. [DOI] [PubMed] [Google Scholar]