Highlights
-
•
Parkinson patients with hallucinations exhibit more perceptual errors.
-
•
Perceptual errors are due to decrease in perceptual sensitivity and not to response bias.
-
•
Parkinson patients with hallucinations exhibit increased performance fluctuations.
-
•
Deficits correlate with functional connectivity changes in fronto-parietal networks.
Keywords: Parkinson's disease, Visual hallucinations, Misperceptions, Trial-by-trial variability, Continuous flash suppression
Abstract
Patients with Parkinson's disease (PD) frequently suffer from visual misperceptions and hallucinations, which are difficult to objectify and quantify. We aimed to develop an image recognition task to objectify misperceptions and to assess performance fluctuations in PD patients with and without self-reported hallucinations. Thirty-two non-demented patients with Parkinson's disease (16 with and 16 without self-reported visual hallucinations) and 25 age-matched healthy controls (HC) were tested. Participants performed a dynamic image recognition task with real and scrambled images. We assessed misperception scores and intra-individual variability in recognition times. To gain insight into possible neural mechanisms related to misperceptions and performance fluctuations we correlated resting state network connectivity to the behavioral outcomes in a subsample of Parkinson's disease patients (N = 16). We found that PD patients with self-reported hallucinations (PD-VH) exhibited higher perceptual error rates, due to decreased perceptual sensitivity and not due to changed decision criteria. In addition, PD-VH patients exhibited higher intra-individual variability in recognition times than HC or PD-nonVH patients. Both, misperceptions and intra-individual variability were negatively correlated with resting state functional connectivity involving frontal and parietal brain regions, albeit in partly different subregions. Consistent with previous research suggesting that hallucinations arise from dysfunction in attentional networks, misperception scores correlated with reduced functional connectivity between the dorsal attention and salience network. Intra-individual variability correlated with decreased connectivity between somatomotor and right fronto-parietal networks. We conclude that our task can detect visual misperceptions that are more prevalent in PD-VH patients. In addition, fluctuating visual performance appear to be a signature of PD-VH patients, which might assist further studies of the underlying pathophysiological mechanisms and cognitive processes.
1. Introduction
Patients with Parkinson's disease (PD) frequently experience non-motor symptoms such as cognitive and perceptual deficits (Armstrong, 2011). Visual misperceptions and visual hallucinations (VH) involving complex images are highly prevalent, have a negative impact on quality of life and represent a key predictor for dementia with disease progression (Diederich et al., 2009; Weil et al., 2017). In clinical studies, the presence of hallucinations is typically assessed with questionnaires such as the University of Miami Parkinson's disease Hallucinations Questionnaire (UM-PDHQ) (Papapetropoulos et al., 2008) that do not discriminate between hallucinations and misperceptions and thus appear entangled in the majority of studies (for exceptions see: Shine et al. (2012)). Visual hallucinations are more likely to occur at advanced disease stages and are co-morbid with REM sleep disorder (Manni et al., 2011), cognitive and attentional dysfunction (Koerts et al., 2010; Meppelink et al., 2008; Shine et al., 2014) as well as with sensory impairments such as reduced visual acuity, color and contrast sensitivity (Matsui et al., 2006; Pieri et al., 2000; Ramirez-Ruiz et al., 2007). While object recognition deficits involving visual search and object invariance are observable in Parkinson patients without self-reported VH (Weil et al., 2018), they are even more pronounced in PD patients with self-reported hallucinations (Meppelink et al., 2008; Shine et al., 2012).
These empirical observations suggest that hallucinations in PD patients are modulated by both top-down (i.e. ‘attentional’) and bottom-up (‘perceptual’) deficits, which is also reflected in current theoretical models (Muller et al., 2014). Functional models implicate that VH's occur as a consequence of deficient reality monitoring due to the misattribution of self-generated information (Collerton et al., 2005) or dysfunctional attentional networks leading to the intrusion of illusory object representations (Shine et al., 2015). Specifically, Shine et al. (2015) showed that impaired resting state functional connectivity between the dorsal attention network (DAN) and the ventral attention network is related to higher rates of misperceptions in (self-reported) hallucinating PD patients.
Although there has been considerable progress in recent years, visual misperceptions and hallucinations in PD remain poorly understood and are difficult to track and to treat.
The first aim of the present study was to derive a quantifiable misperception measure that is sensitive to the occurrence of (self-reported) hallucinations in PD and that lends itself to trial-based designs to study neural activity during misperceptions. The second aim was to assess intra-individual performance variability across the task. The third aim was to relate the misperceptions and the intraindividual variability to putative changes in resting state functional connectivity in the PD patients. To our knowledge, intra-individual variability and its neuronal signature has not been investigated as a potential marker of visual misperceptions/hallucinations in PD, although intra-individual performance fluctuations have been described as sensitive markers to detect subtle cognitive deficits in a wide range of psychiatric and neurological diseases such as autism, head injury and dementia (MacDonald et al., 2009). Previous research in patients with schizophrenia and drug-induced psychosis also suggests a direct link between intraindividual variability and hallucinations (Fassbender et al., 2015,2014; Rentrop et al., 2010). Cognitive fluctuations, which might be reflected in task performance variability, are related to desynchronization of fronto-parietal networks in patients with Lewy Body Dementia, which is characterized by hallucinations (Peraza et al., 2014).
Based on previous research showing increased occurrence of visual misperceptions as measured in psychophysical tasks in PD patients with self-reported hallucinations (Koerts et al., 2010; Shine et al., 2012), we employed a dynamic image recognition paradigm where image contrast was stepped up and subjects reported the detection of a face or car image while rejecting a scrambled image. The scrambled images were used to assess whether PD-VH patients are prone to report real objects while none is physically present. Since recent studies suggested that the usage of visual illusions might contribute to the sensitivity detecting Parkinson patients with hallucinations (Shine et al., 2012; Straughan et al., 2016), we also implemented a Continuous Flash Suppression condition (CFS) that suppresses salient stimuli from perceptual awareness due to binocular rivalry (Stein et al., 2011; Tsuchiya and Koch, 2005).
We calculated signal detection parameters such as visual sensitivity and bias. As clinically applicable parameters we calculated perceptual error scores and intra-individual recognition time variability and correlated those outcome parameters to resting state functional connectivity as assessed with functional magnetic resonance imaging (fMRI). We tested the following hypotheses: (1) PD patients with self-reported visual hallucinations will make more perceptual errors such as reporting scrambled images as real images, possibly more so when the images are further suppressed from conscious perception. (2) PD patients with self-reported hallucinations will show increased intra-individual variability in their task performance and (3) Perceptual error scores (PES) and intra-individual variability (CVRT) are associated with impaired functional connectivity within or between attention-related networks that involve prefrontal cortices.
2. Materials and methods
2.1. Participants
Thirty-two patients with Parkinson's disease (16 PD patients without self-reported hallucinations (PD-nonVH) and 16 with self-reported hallucinations (PD-VH)) and 25 healthy age matched controls without a history of neurological or psychiatric disease (assessed via questionnaire) were recruited. Exclusion criteria were moderate to severe general cognitive impairment/dementia (Mini Mental State Examination (MMSE) score < 26), visual acuity below 80% (corrected with glasses as necessary) and psychiatric disorders other than minor depression. We matched the groups based on demographic variables such as age, gender and years of post-secondary education (Table 1). Diagnosis of idiopathic Parkinson's disease was made according to the UK Parkinson's Disease Society Brain Bank criteria by experienced neurologists.
Table 1.
Demographic and neuropsychological characteristics of volunteers that participated in the image recognition task.
| Healthy controls (n = 25) | PD-all (n = 32) | PD-nonVH (n = 16) | PD-VH (n = 16) | P- value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Healthy controls vs. PD-all | PD-VH vs. PD-nonVH | |
| Age (years)a | 68.24 (4.67) | 70.34 (6.36) | 70.19 (6.92) | 70.50 (5.92) | = 0.17 | = 0.89 |
| Gender (female/male)c, n(%) | 5 (20%) / 20 (80%) | 9 (28%) / 23 (72%) | 4 (25%) / 12 (75%) | 5(31%)/ 11 (69%) | = 0.48 | = 0.69 |
| Years of postsecondary educationb | 3.82 (2.35) | 3.56 (2.10) | 3.59 (2.09) | 3.53 (2.17) | = 0.76 | = 0.47 |
| MMSEb | 29.16 (1.07) | 28.59 (1.21) | 28.87 (1.03) | 28.31 (1.35) | = 0.06 | = 0.25 |
| Hoehn and Yahr stageb | – | 2.13 (0.81) | 1.88 (0.79) | 2.38 (0.79) | – | = 0.10 |
| Disease durationb | – | 7.20 (6.22) | 4.61 (3.68) | 9.78 (7.22) | – | = 0.007* |
| UPDRS IIIa | – | 23.78 (9.97) | 20.94 (10.64) | 26.63 (8.67) | – | = 0.11 |
| LEDDa, mg=day | – | 591.76 (353.84) | 385.53 (253.42) | 797.98 (322.19) | – | = 0.0004* |
| UM-PDHQ | – | – | – | 9.13 (2.53) | – | – |
| BDIb | 3.16 (2.70) | 8.44 (5.55) | 7.75 (5.50) | 9.13 (5.69) | = 0.00005† | = 0.45 |
| Visual acuity (%)b | 97.20 (6.78) | 93.75 (9.42) | 95.00 (8.94) | 92.50 (10.00) | = 0.16 | = 0.45 |
| Mars Letters Contrast Sensitivity Testb | 1.76 (0.05) | 1.71 (0.09) | 1.74 (0.08) | 1.68 (0.10) | = 0.009† | = 0.11 |
t-test; bMann-Whitney-U-Test; BDI, Beck's, Depression Inventory; cchi-square-test; HC, healthy controls; LEDD, levodopa-equivalent daily dose; MMSE, Mini Mental State Examination; PD, Parkinson patients; PD-nonVH, Parkinson patients without visual hallucinations; PD-VH, Parkinson patients with visual hallucinations; UM-PDHQ, University of Miami Parkinson's disease Hallucinations Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale; †HC vs. PD p < 0.05; *PD-nonVH vs. PD-VH p < 0.05.
Healthy controls and PD patients as a whole group did not differ in age (p = 0.17), gender (p = 0.48), education levels ranging from 0 (elementary school not finished) to 5 (PhD) (p = 0.59) and years of post-secondary education (p = 0.76). Compared to healthy controls PD patients tended to have lower MMSE scores (p = 0.06). Consistent with previous research, PD patients had increased Beck's Depression Inventory (BDI) scores (p = 0.00005) (Kritzinger et al., 2015) and lower contrast sensitivity (p = 0.009) (Meppelink et al., 2009) compared to healthy controls.
PD-VH and PD-nonVH did not differ in age (p = 0.89), gender (p = 0.69), education levels (p = 0.76), years of post-secondary education (p = 0.47), MMSE scores (p = 0.25), depression (BDI) scores (p = 0.45), contrast sensitivity (p = 0.11) and Hoehn & Yahr stage (p = 0.10). Consistent with co-morbidity studies, PD-VH as compared to PD-nonVH patients had significantly longer disease durations (p = 0.007) and higher levodopa-equivalent daily dose (LEDD, p = 0.0004) (Gupta et al., 2004) (Table 1).
Apart from 1 non-medicated PD-nonVH, all PD patients were tested under stable dopaminergic medication, 7 patients (4 PD-VH, 3 PD-nonVH) were taking antidepressants, and 4 were using opioids (2 PD-VH, 2 PD-nonVH). The levodopa-equivalent daily dose (LEDD) was calculated for all patients, according to the formula described in the paper by Tomlinson et al. (2010). In the PD-VH group, 44% (N = 7) reported visual hallucinations multiple times per day, 25% (N = 4) multiple times per week, and 31% (N = 5) less than weekly. PD-VH reported complex visual hallucinations such as people (63%), animals (50%), insects (25%), objects (38%) and simple visual hallucinations (6%). Five (31%) PD-VH also reported auditory hallucinations.
A sample of 16 (10 PD-nonVH, 6 PD-VH) Parkinson patients and 19 age-matched healthy control subjects participated in a resting state scan (∼6 min), performed either on the same or one to eight days after the behavioral experiment. Demographic and clinical variables of the fMRI sample are shown in Table 2. In short, HC and PD patients did not differ with respect to age (p = 0.66), education level (p = 0.69), years of post-secondary education (p = 0.88), contrast sensitivity (p = 0.78). PD patients had higher depression (BDI) scores (p = 0.02) and tended to have lower cognitive (MMSE) scores (p = 0.06).
Table 2.
Demographic and neuropsychological characteristics of subjects that participated in the Resting State fMRI and image recognition task.
| Healthy controls (N = 19) | PD (N = 16) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 68.32 (6.30) | 69.44 (8.52) |
| Gender (female/male), n (%) | 0 (0%) / 19 (100%) | 3 (19%) / 13 (81%) |
| Years of postsecondary education | 3.74 (2.06) | 3.44 (1.62) |
| MMSE | 29.16 (0.90) | 28.38 (1.31) |
| Hoehn and Yahr stage | – | 2.06 (0.70) |
| Disease duration | – | 7.67 (6.99) |
| UPDRS III | – | 20.44 (11.91) |
| LEDD, mg/day | – | 614.78 (397.31) |
| UM-PDHQ | – | 3.63 (4.89) |
| BDI | 3.32 (2.83) | 6.81 (4.64) |
| Visual acuity (%) | 97.89 (6.31) | 92.50 (10.00) |
| Mars Letters Contrast Sensitivity Test | 1.73 (0.05) | 1.72 (0.08) |
The subgroup of the volunteers who participated in the Resting State experiment was comparable to the complete behavioral sample in terms of Levodopa dose, disease duration and cognition as measured by MMSE. For the severity of motor deficits measured by the UDPRS, the subsample that participated in the resting state had a lower score on motor severity (mean: 20.44) compared to the full behavioral sample (mean: 23.78).
BDI, Beck's, Depression Inventory; HC, healthy controls; LEDD, levodopa-equivalent daily dose; MMSE, Mini Mental State Examination; PD, Parkinson patients UM-PDHQ, University of Miami Parkinson's disease Hallucinations Questionnaire UPDRS, Unified Parkinson's Disease Rating Scale.
2.2. Clinical test batteries
Motor symptoms were assessed with the Movement Disorder Society (MDS)‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS), part III (Goetz et al., 2008). General cognitive performance was tested with the Mini Mental State Examination (MMSE) (Folstein et al., 1975). Participants filled in a self-report depression scale (Beck's Depression Inventory, BDI) (Beck et al., 1961). Severity of hallucinations was assessed by a structured interview based on the hallucinations questionnaire (University of Miami Parkinson's disease Hallucinations Questionnaire (UM-PDHQ)) (Papapetropoulos et al., 2008). Close vision test was performed to assess visual acuity, which testified 80% visual acuity if the volunteers could read 1 mm large font size from a distance of 40 cm Contrast sensitivity was evaluated with the Mars Letters Test (Mars Perceptrix Corporation, Chappaqua, NY, USA).
2.3. Experimental design and procedures
2.3.1. General procedure
The session started with the neuropsychological testing including MMSE, BDI, Visual acuity test, Contrast sensitivity test and in addition for the patients UM-PDHQ and MD-UPDRS. The session continued with an instruction and a training task, where participants completed 10 CFS and 10 non-CFS trials where the image contrast was continuously stepped up. Volunteers were instructed to press the pre-assigned button as soon as they recognized a face or a car and not to press a button during stimulus presentation if a scrambled image was shown. In this training period, subjects could ask questions anytime, and in the rare cases that the task was still not entirely clear, the training was repeated until the participants had no further questions.
2.3.2. Control task with unmasked images at 100% luminance contrast
Before the start of the main experiment, participants were presented with 20 car or face images at 100% contrast. Subjects were instructed to press a pre-assigned button according to the image category. Maximal response time was 12 s. The purpose of the control task was twofold: 1) to test whether the instruction was understood and 2) to estimate response times under optimal (i.e. 100%) contrast conditions.
2.3.3. Main perceptual task
Participants were sitting in a dimly lit room in front of an LCD monitor. Target stimuli consisted of photographs of faces (Ekman and Friesen, 1976), cars (http://vision.caltech.edu/archive.html) or their scrambled versions, presented in a pseudorandom order (Fig. 1). Stimuli were presented on a 27 inch LCD monitor. The screen resolution was 1920 × 1080 pixels. The monitor had a vertical refresh rate of 60 Hz. The eye-to-screen distance was 57 cm. Visual stimuli were presented with custom-written software using Matlab (Version R2011b, 32bit) and the Psychtoolbox for Microsoft Windows (Brainard, 1997). Responses were recorded with a USB button-box and behavioral responses were recorded in Matlab and stored together with the stimulus information on an x64-based PC. Target stimuli consisted of photographs of cars (http://vision.caltech.edu/archive.html), faces (Ekman and Friesen, 1976) or their scrambled versions. Each image subtended 480 pixels by 480 pixels and was presented in the center of the screen with 12° of visual angle. Face stimuli were randomly drawn from different facial expressions. Scrambled images were generated using Matlab by dividing the images in 10 × 10 pixels blocks and randomizing their position (Fig. 1A). Example stimuli are illustrated in Supplementary Fig. S1. Target images were presented to the right eye by using red-blue goggles, displaying the image solely by the blue gun (stereomode of the psychophysics toolbox (Carmel et al., 2010)). In CFS trials, which were designed to suppress the images from visual awareness, high contrast and rapidly changing red-colored Mondrian patterns were flashed to the left eye at a frequency of 10 Hz (Tsuchiya and Koch, 2005). Thus, the right eye perceived the target image and the left eye perceived the Mondrian mask (Carmel et al., 2010) (Fig. 1C). Mondrian patterns were drawn from a pool of 100 randomly generated patterns (http://martin-hebart.de/webpages/code/stimuli.html) (Stein et al., 2011). In the non-CFS condition, only the target image was presented to the right eye without the mask (Fig. 1B).
Fig. 1.
Task design and trial structure. (A) Example images of face, car and scrambled images used in the experiment. Trial structure: (B) non-CFS and (C) CFS condition. Image contrast was continuously stepped up to reach 100% after 10 s. Maximum response time was 12 s. Subjects pressed the button as soon as they recognized the image category. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
Each trial started with the presentation of a central fixation cross, followed by the onset of the target image 600 ms later. Subjects were asked to maintain central fixation and to avoid eye blinks throughout the trial. Image luminance contrast was continuously stepped up over 10 s. Subjects were instructed to press a pre-assigned button as quickly as possible when they detected a target image and to not press any button when the image category was not recognized or when a scrambled image was shown. A trial ended either after the response was given, or at 12 s after image onset (Fig. 1B and C).
We created a ‘visible’ (Fig. 1B) and an ‘invisible’ condition (Fig. 1C). For the ‘invisible’ condition we used continuous flash suppression (CFS), designed to suppress the images from consciousness for several seconds via the presentation of high contrast and rapidly changing Mondrian patterns to the eye opposite of the target image (Tsuchiya and Koch, 2005) (Fig. 1C). CFS has been widely used in healthy and psychiatric populations to investigate the properties of unconscious vision (Seymour et al., 2016; Sterzer et al., 2011; Yang et al., 2011).
Non-CFS and CFS trials were presented in a pseudorandomized order. Since the CFS condition did merely provide confirmatory information about differences between hallucinating and non-hallucinating PD patients compared to the CFS condition, we primarily focus the presentation of the results on the non-CFS condition as depicted in Fig. 1B. Each, the non-CFS and the CFS condition consisted of 100–120 trials per subject (1/3 of each image category). Including breaks, the whole experiment with its 200–240 trials took about 1 ½ to complete.
2.3.4. Resting state image acquisition
Subjects were instructed to stay awake with their eyes closed and not to think of anything in particular during the resting state scan (∼ 6 min.) fMRI data were acquired using a 3 Tesla MR system (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany) with a 32-channel phased-array head coil. fMRI resting state experiment was performed using the 2D multiband gradient-echo planar imaging sequence from the Center for Magnetic Resonance Research, University of Minnesota (Moeller et al., 2010; Setsompop et al., 2012) with T2*-weighting at 3 × 3 mm² in-plane resolution, oriented parallel to the AC-PC plane (TR: 1800 ms, TE: 30 ms, flip angle: 70°, 34 slices of 3 mm thickness with 20% inter-slice gap, field-of-view: 192 mm x 192 mm, matrix size: 64 × 64, multiband acceleration factor 3, 194 whole-brain volumes per functional run).
2.4. Analyses
2.4.1. Demographic, clinical and behavioral statistical data analysis
Data analysis was performed using custom written scripts in MATLAB R2012b and SPSS (version 24; SPSS, Inc., Chicago, IL). Univariate ANOVA and t-tests were used to compare age, MDS-UPDRS-III and LEDD. Non-parametric Mann-Whitney-U-Tests were used to compare years of post-secondary education, education level, years of disease duration, Hoehn and Yahr stage, BDI and MMSE scores, visual acuity and contrast perception between groups. Chi-Square-Test was used to compare the groups with respect to gender.
Unless otherwise noted, behavioral analysis was performed by a mixed ANOVA with the within-subject factor “Category” (faces vs. cars) and the between-subject factor “Group”. Two types of ANOVAs were calculated: 1) ANOVA with the “Group” factor HC vs. all PD patients (PD-VH + PD-nonVH) together (in the following “ANOVA_1″). 2) To investigate behavioral markers that are specific for VH in PD we used a separate ANOVA with the “Group” factor PD-nonVH vs. PD-VH (in the following “ANOVA_2″).
The description of the results will focus on main and interaction effects of "Group", additional ANOVA results are provided in the referred tables. We were primarily interested to derive a potentially clinically applicable perceptual error score and in the intraindividual variability that might allow the discrimination between PD-VH from PD-nonVH patients. The behavioral measures were: Perceptual Error Score (PES), depending on the proportion correct categorization, proportion misses and proportion erroneous object recognition in scrambled images. In addition, we applied Signal Detection Theory (SDT) to calculate detection sensitivity (d’) and response criterion (c) for faces vs. cars (Subset 1), faces or cars vs. scrambled (Subset 2), faces vs. scrambled (Subset 3) and cars vs. scrambled (Subset 4). In order to investigate intraindividual variability we analyzed mean recognition times and the coefficient of recognition time variability (CVRT). Since we used two different task conditions (non-CFS and CFS) and performed two independent group comparisons, the significance level was adjusted to p < 0.0125.
Control task analysis
In the control task in which either face or car images were presented at 100% contrast, the proportion of correctly categorized face and car images did not significantly differ between healthy controls and PD patients (ANOVA_1: F(1,55) = 0.02, p = 0.88) nor between the two PD patient groups (ANOVA_2: PD-nonVH vs. PD-VH: (F(1,30) = 0.06, p = 0.81). In all three groups the proportion correct image recognition in the control task was above 95% (HC: 97.2%, PD-nonVH: 97.19%, PD-VH: 97.19%) verifying that all groups were able to perform the task. In the 100% contrast condition, PD patients reporting visual hallucinations showed longer recognition times compared to PD patients without visual hallucinations (F(1,30) = 5.29, p = 0.028, PD-nonVH: 1.01 s., PD-VH: 1.62 s).
Additional control analyses
When there was a significant difference between PD-VH and PD-nonVH we performed an additional control analysis by an ANCOVA with levodopa daily dose and disease duration as covariates. The covariate analyses were performed since disease duration and LEDD significantly differed between PD-nonVH and PD-VH.
To exclude possible influences of individual values on the group differences, reliability of the results for the main outcome measures, perceptual error score and coefficient of recognition time variability, was tested applying the jackknife procedure by repeating the patient comparison by systematically removing one sample and re-calculating the mean (Wilke, 2012).
2.4.2. Key performance measures
Performance parameters were calculated separately for each image category (faces or cars).
Image Recognition Performance
(I) Proportion Correct Categorization (face reported as a face and car reported as a car); (II) Proportion Misses: Trials in which a face or a car was presented but the subject did not provide a button response, divided by the overall number of image presentations; (III) Proportion erroneous face or car reports in scrambled images; (IV) Perceptual error score (PES): Proportion of errors consisting of category confusions (face reported as car and vice versa), misses and false real image detection in scrambled images.
Recognition times: means and intra-individual variability
Mean recognition times (RT) denote the time between image onset and button response. Only correct responses were used to compute mean RT. Coefficient of recognition time variability (CVRT) was calculated by dividing the standard deviation of individual recognition times for a given condition and image category (car, face) by the individual mean of recognition times (standard deviation/mean) (Flehmig et al., 2007).
2.4.3. Signal detection analysis
Using standard signal detection theory approach we calculated detection sensitivity (d') and criterion (c) as:
where Z is the inverse of the cumulative normal distribution, H is the hit rate, and FA is the false alarm rate (Stanislaw and Todorov, 1999). Since each trial in our task included one of three possible stimuli (faces, cars, scrambled) and one of three corresponding responses (left button: face, right button: car, no button: scrambled), for these analyses we separated the trials into two subsets: (1) trials where faces or cars were presented and the response was a face or a car and (2) trials where an image (a face or a car) or scrambled image was presented and the response was a (correct) image or a scrambled image. For each subset, trials were sorted into hits (H), misses (M), false alarms (FA), and correct rejections (CR) as follows:
Subset 1. Faces vs. cars (faces represent “signal”, cars – “noise”)
H: face presented, face reported; M: face presented, car reported; FA: car presented, face reported; CR: car presented, car reported
Subset 2. Faces or cars vs. scrambled (faces or cars represent “signal”, scrambled – “noise”)
H: face presented and face reported or car presented and car reported; M: face or car presented, scrambled reported; FA: scrambled presented, face or car reported, CR: scrambled presented, scrambled reported.
The subset 1 was used to check the discrimination between face and car images, the subset 2 – the discrimination between either a face or a car and scrambled images. Furthermore, each of these subsets has been split into trials with continuous flash suppression (CFS) masking and without CFS. Additionally, faces vs. scrambled and cars vs. scrambled were analyzed separately (subsets 3 and 4, Supplementary Methods and Tables S6-7). This resulted in 8 pairs of d' and c values for each group. In the main results, we focus on the analysis of subsets 1 and 2.
Note that the criterion is negative when there is a bias to select “yes” (“signal”), and positive when there is a bias to select “no” (“noise”).
Statistical analysis of sensitivity (d') and criterion/response bias (c).
Since some of the d' and c distributions violated parametric assumptions, we used the Mann–Whitney U test for comparing the groups. Equivalent to the ANOVAs described for the other behavioral variables, we compared the healthy subjects (HC) with all Parkinson patients (PD) and then the PD-VH with the PD-nonVH: 1) U test with the “Group” factor HC vs. all PD patients (PD-VH + PD-nonVH) and 2) To investigate behavioral markers that are specific for VH in PD we used a separate U test with the “Group” factor PD-nonVH vs. PD-VH.
More methodological details are given in the Supplementary Methods.
2.4.4. Resting state functional connectivity
Preprocessing: All fMRI data processing was performed using the CONN Toolbox (www.nitrc.org/projects/conn, RRID: SCR_009550) (Whitfield-Gabrieli and Nieto-Castanon, 2012). For each subject's data set, preprocessing steps included: functional motion estimation and correction (i.e. realignment using a 6 parameter rigid-body model of head motion), functional and structural data translations to center (0,0,0 coordinates), functional data slice-timing correction, functional ART-based outlier detection (identifying single volumes with high instantaneous motion or sudden changes in global signal intensity), direct, simultaneous gray matter/white matter/cerebral spinal fluid (GM/WM/CSF, respectively) segmentation and MNI normalization applied to functional and structural data separately, and 6 mm full-width-at-half-maximum-kernel Gaussian smoothing. The following denoising steps were implemented: linear regression of confounding effects, including WM and CSF signals (CompCor method) (Behzadi et al., 2007), realignment parameters and their first derivatives (12 motion confounds), ART-detected outlier volumes, and the effect of the resting task (temporal filter reducing weight of initial 10 scans in the run). Linear detrending was applied prior to a 0.008 - 0.09 Hz bandpass filter.
Statistical analyses: voxelwise volume analysis: Group-level independent component analysis (ICA) was applied to the whole sample of combined patients and healthy controls in order to spatially define resting state networks (RSNs). Data dimensions were reduced through retaining 40 components of an initial principle component analysis of the temporally-concatenated runs for the whole group, followed by 20-component ICA. Of the 20 resulting components, eight were visually identified as RSNs of interest as previous imaging studies reported impairments in frontal, parietal and visual brain regions (Baggio et al., 2015; Prell, 2018): default mode (DMN), dorsal attention (DAN), left and right fronto-parietal (lFP, rFP, respectively), medial and lateral visual (medVIS and latVIS, respectively), somatomotor (SMN), and salience (SAL) (for more detailed network description see Table 3). Dual regression was applied to the group ICA results, using all components generated from the initial analysis, resulting in subject-level beta maps of all components, including the RSNs of interest (Beckmann et al., 2009). For second-level statistical tests, comparisons were made with network-level beta averages against behavioral statistics as well as equivalent full-volume voxelwise tests that included behavioral covariates. To generate masks for subject-level RSN averages, for each of the selected components, a one-sample t-test was performed using all subjects, and an uncorrected voxel-level p = 0.05 and FWE-corrected cluster-level p = 0.05 were applied. Fig. 2 shows the resting state network maps from the group-level ANOVA results of the ICA analysis. Resulting thresholded maps were binarized to be used as RSN masks to summarize within- and between-network functional connectivity for group-level statistics. For within- and between-network functional connectivity measures, for every subject, each group-level RSN mask was applied to each network volume map to extract average beta values. Subject-level within- and between-network average beta values were used in further analysis with behavioral data in SPSS.
Table 3.
Overview of resting state networks of interest.
| Network | Brain areas |
|---|---|
| Dorsal Attention | Precuneus, Inferior and Superior Parietal Lobule, Pre- and Postcentral Gyrus Cuneus, Paracentral Lobule, Middle and Superior Occipital Gyrus, Middle and Superior Frontal Gyrus, Fusiform Gyrus,Inferior Temporal Gyrus |
| Default Mode | Precuneus, Posterior Cingulate, Inferior, Middle and Superior Temporal Gyrus, Cuneus, Parahippocampal Gyrus, Angular Gyrus, Superior Occipital Gyrus, Inferior Parietal Lobule, Anterior Cingulate, Fusiform Gyrus, Right Insula, Middle and Superior Frontal Gyrus |
| Salience | Medial and Superior Frontal Gyrus, Cingulate Gyrus, Anterior Cingulate, Precuneus, Superior Temporal, Insula, Pre- and Postcentral Gyrus, Lingual Gyrus, Cuneus, Middle Occipital Gyrus, Lentiform Nucleus, Inferior Parietal Lobule |
| Left fronto-parietal | Left Inferior, Middle and Superior Frontal Gyrus, Left Precentral Gyrus, Left Middle and Superior Temporal Gyrus, Left Inferior and Superior Parietal Lobule, Left Precuneus, Left Supramarginal Gyrus |
| Right fronto-parietal | Right Inferior, Middle and Superior Frontal Gyrus, Right Precentral Gyrus, Right Inferior and Superior Parietal Lobule, Right Precuneus, Right Angular Gyrus, Right Superior Temporal Gyrus |
| Somatomotor | Medial and Superior Frontal Gyrus, Post- and Precentral Gyrus, Paracentral Lobule |
| Visual lateral | Inferior, Middle and Superior Occipital Gyrus, Cuneus, Fusiform Gyrus |
| Visual medial | Cuneus, Lingual Gyrus, Precuneus |
Fig. 2.
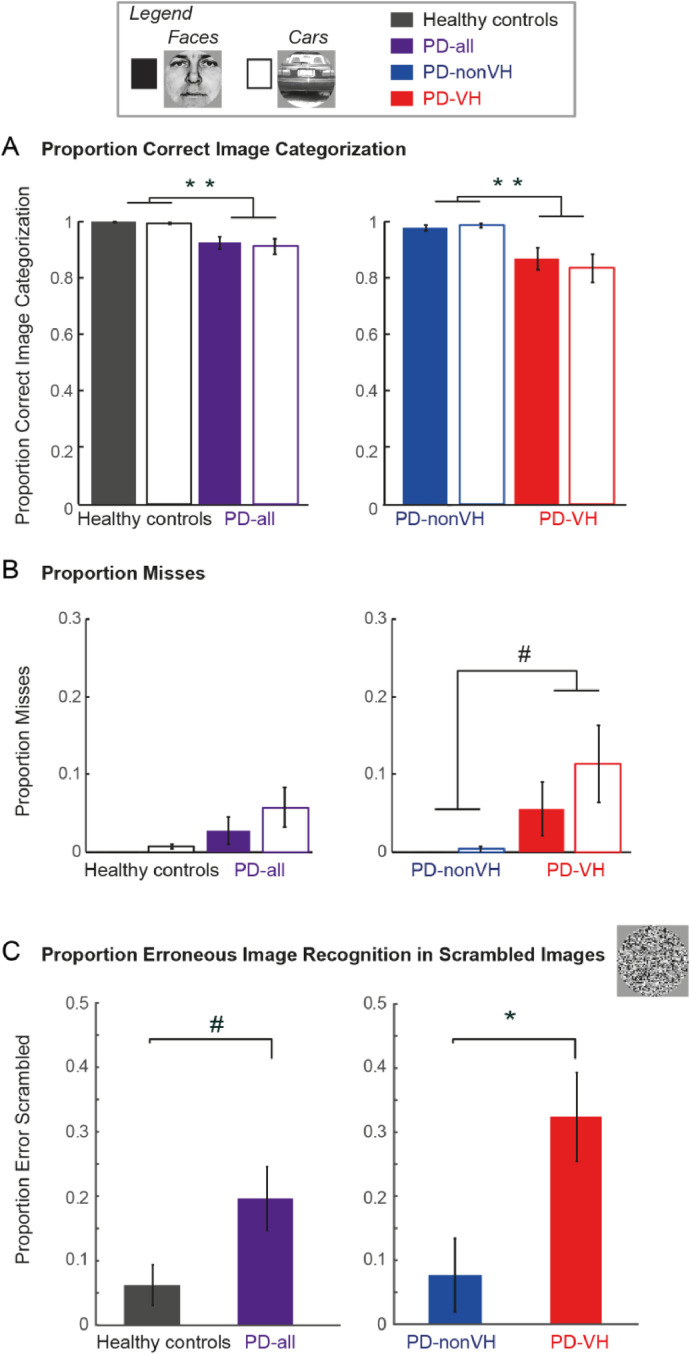
ICA Resting state networks. Spatial maps representing the eight resting state networks of interest, generated through ANOVAs on each chosen ICA result, displaying the simple main effect of each RSN component for all subjects (left), healthy controls (center), and all PD patients (right; for all maps: uncorrected voxel-level p < 0.05, FWE-corrected cluster p < 0.05).
2.4.5. Group differences in resting state networks
To investigate differences between HC and PD patients we performed full-volume voxelwise comparisons for the eight networks of interest (default mode, dorsal attention, salience, right and left fronto-parietal, somatomotor, visual medial and lateral). To test differences of within network connectivity, averages of the eight networks were submitted to a univariate ANOVA, with “Group” (HC and PD) as a between subject factor. Given the limited sample size (PD-nonVH = 10, PD-VH = 6) we did not compare the PD patient subgroups but focus on correlations between individual networks and behavioral values.
2.4.6. Correlation between resting state functional connectivity and perceptual
Error Score (PES) and Coefficient of intra-individual variability (CVRT)
In order to investigate the relationship between functional connectivity averages and the behavioral markers PES and CVRT, we performed for the PD group Spearman's rho correlations. Partial correlations of PD patients including disease duration and daily levodopa dose (LEDD) were used as control variables (Baggio et al., 2015; Shine et al., 2015). Given the limited fMRI sample size, we did not separate the PD patient groups when correlating the functional connectivity estimates with the main outcomes measures. PES scores violated the assumptions of linear correlation and were thus transformed using logarithmic transformation (log (PES + 1)) (Bartlett, 1947; Maria et al., 2003; Morrison et al., 2000).
3. Results
3.1. Correct image categorization in the main task (non-CFS)
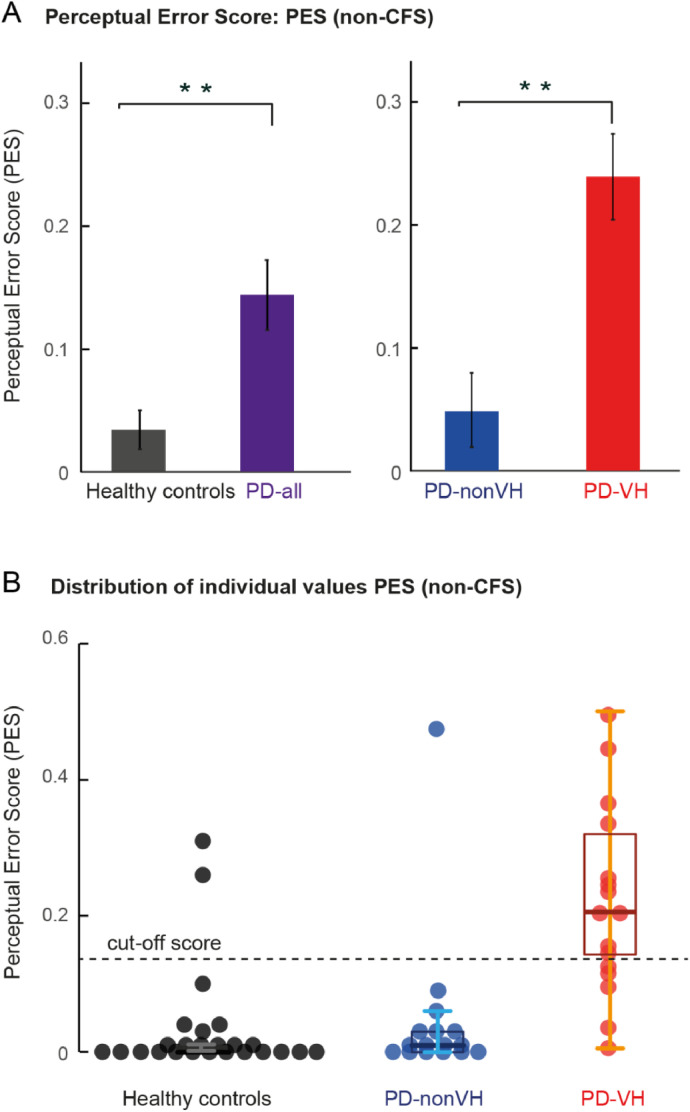
Fig. 3A shows that HC correctly categorized the images in 99%, while PD patients reached on average only 96.5%. Accordingly, the ANOVA_1 showed a main group effect between HC and PD patients (F(1,55) = 11.77, p = 0.001) (Supplementary Table S1).
Fig. 3.
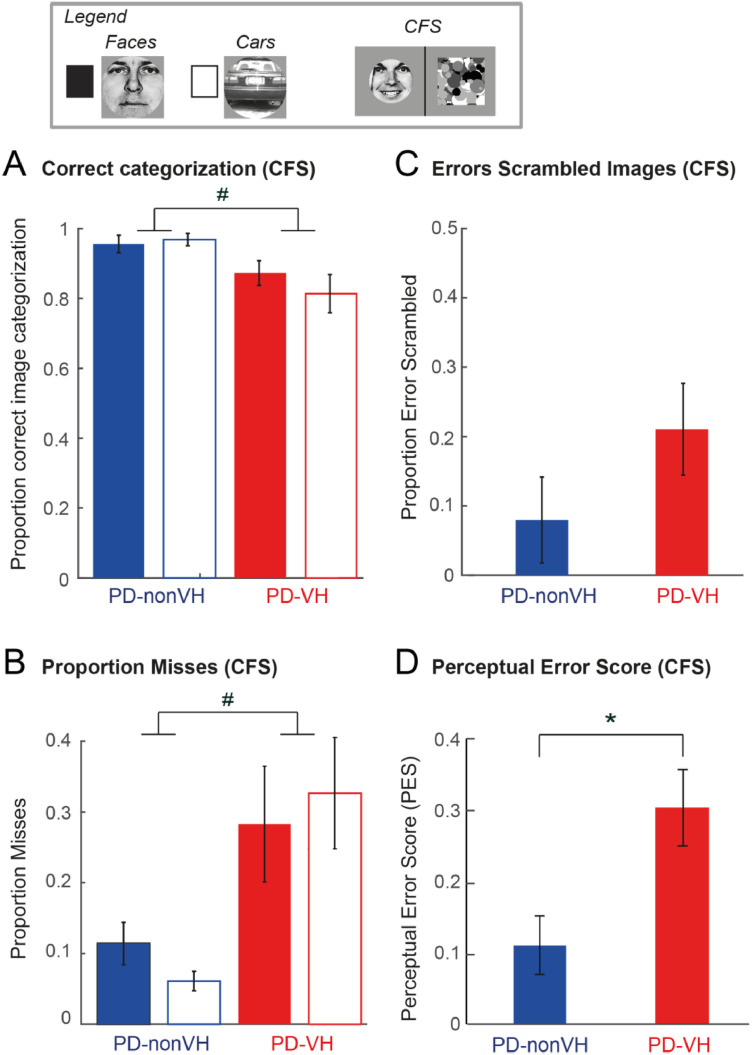
Recognition performance in the non-CFS condition. (A) Mean proportion of correct image categorization of faces and cars. (B) Mean proportion of missed faces and cars. (C) Mean proportion of erroneous image recognition in scrambled images. In (A–C): (left) Healthy controls (N = 25) vs. PD-all (N = 32), (right) PD-nonVH (N = 16) vs. PD-VH (N = 16). ** denotes a significant group difference, p < 0.005; * significant after Bonferroni correction p < 0.0125 and # denotes p < 0.05 without Bonferroni correction. Error bars denote S.E.M. across subjects.
The separate comparison between the two PD patient groups (ANOVA_2) showed that PD-VH patients categorized fewer images correctly than the PD-nonVH, expressed as a significant group effect (F(1,30) = 9.53, p = 0.004) (Fig. 3A, right column, Table 4). Given the larger levodopa daily dose (LEDD) and longer disease durations of PD-VH patients as compared to PD-nonVH, we conducted an additional analysis including LEDD and disease duration as covariates in the ANCOVA. This control analysis did not yield a significant difference in respect to correct image categorization between the two PD groups (F(1,30) = 1.09, p = 0.31) (Supplementary Table S2), indicating that dopaminergic medication and/or disease duration might be confounds explaining the lower image categorization performance in PD-VH patients.
Table 4.
ANOVA_2: repeated measures mixed ANOVA with the factors category, group and interaction effects for Parkinson patients without (PD-nonVH) and Parkinson patients with self-reported visual hallucinations (PD-VH).
| Factor | Proportion Correct Categorization | Proportion Misses | Proportion Erroneous Object Recognition Scrambled Images | Perceptual Error Score | CVRT | RT |
|---|---|---|---|---|---|---|
| F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | |
| Category | 0.07 (= 0.79) | 9.63 (= 0.004)** | 0.01 (= 0.92) | 164.96 (< 0.0001)** | ||
| Group | 9.53 (= 0.004)** | 5.12 (= 0.03)# | 7.56 (= 0.010)* | 17.16 (= 0.0003)** | 8.43 (= 0.007)* | 13.58 (= 0.001)** |
| Category x Group | 0.004 (= 0.95) | 7.98 (= 0.008)* | 0.60 (= 0.45) | 2.95(= 0.09) |
PD-nonVH, Parkinson patients without visual hallucinations (N = 16); PD-VH, Parkinson patients with visual hallucinations (N = 16) RT, Recognition times; CVRT, Mean individual variability coefficients (individual SD/individual mean) of recognition times. Between-subject factor: Group: PD-nonVH vs. PD-VH; Within-subject factor: Category: faces vs. car (except for PES and Proportion Erroneous Recognition in Scrambled Images). ** p < 0.005 significant between and within subject main and interaction effects, * p < 0.0125 significant with Bonferroni correction, # p < 0.05 significant only without Bonferroni correction.
With respect to misses (i.e. no indicated image recognition within the 12 s of a trial), PD patients as a group had a non-significant tendency for more misses (Fig. 3B). The comparison between the two PD groups yielded a tendency for a higher proportion of misses in the PD-VH group (PD-VH: 9%, PD-nonVH: 0.16% (ANOVA_2: main effect of “Group”: F(1,30) = 5.12, p = 0.03)), albeit not passing the Bonferroni correction.
3.2. Erroneous detection of real life images in scrambled images (non-CFS)
Fig. 3C shows that PD patients tended to report real images in scrambled images more often than healthy controls (Healthy controls: 6%, PD: 20%, ANOVA_1: F(1,55 = 4.86, p = 0.03, n.s. after Bonferroni correction). The ANOVA_2 comparing the group of PD-nonVH patients with the PD-VH patients revealed that this effect was mainly driven by the PD-VH, who reported a real image in 32% of trials as compared to PD-nonVH with only 8% (F(1,30) = 7.56, p = 0.010) (Fig. 3C, right column). The higher proportion of false image reports in the PD-VH group is also supported by a significant effect of “Group” (F(1,28) = 12.75, p = 0.001) in the ANCOVA control analysis using LEDD and disease duration as covariates (Supplementary Table S2).
3.3. Perceptual error scores (non-CFS)
Assuming that a combined sum score of different image recognition errors might be more robust in clinical applications than the separate ones, we computed a sum perceptual error score (“PES”) of category confusion, misses and false real image detection in scrambled images. As illustrated in Fig. 4A, PD patients (14%) had higher PES scores compared to healthy controls (3%) which is also revealed by the main effect of “Group” (ANOVA_1: F(1,55) = 9.81, p = 0.003) (Supplementary Table S1).
Fig. 4.
Perceptual Error Scores (PES) in the non-CFS condition. (A) Mean Perceptual Error Scores, separated by subject group. (left) Healthy controls (N = 25) vs. PD (N = 32); (right) PD-nonVH (N = 16) vs. PD-VH (N = 16). ** denotes a significant group difference, p < 0.005, * Bonferroni correction p < 0.0125 as assessed by ANOVA_1 (Healthy controls vs. PD-all) and ANOVA_2 (PD-nonVH vs. PD-VH). Error bars denote S.E.M. across subjects. (B) Individual Perceptual Error (PES) scores. Individual PES values and boxplots illustrating the median in the center of the box, error bars the 95% confidence interval separately for Healthy controls (N = 25) in black, PD-nonVH (N = 16) in blue and PD-VH (N = 16) in red. Grey line indicates the cut-off score (mean Healthy controls + 1.5 SD Healthy controls). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4A (right column) shows that this effect is led by the higher PES scores in PD-VH (24%) compared to PD-nonVH (5%), expressed also by the main effect of “Group” in the ANOVA comparing PD-nonVH and PD-VH (ANOVA_2: F(1,30) = 17.16, p = 0.0003) (Table 4). The ANCOVA comparing PD-nonVH with PD-VH that included levodopa daily dose and disease duration as covariates showed the PES is a robust effect, with a significant effect of “Group” (F(1,28) = 13.88, p = 0.001) (Supplementary Table S2). This suggests that the differences in PES cannot be explained only by differences in LEDD and disease duration.
To further demonstrate the stability of the group difference in PES between PD-VH and PD-nonVH, a Jackknife procedure was applied. Systematically leaving out one subject from the analysis and recomputing the ANOVA_2 always resulted in significant main effects of “Group” (all p values were in the range [0.0001 – 0.0005]). Finally, the calculation of PES cut-off scores underlines its potential to discriminate between PD-nonVH and PD-VH. Specifically, 11 PD-VH patients (69%) scored above the PES cut off score of 0.15 (average PES score of HC + 1.5 SD of HC) as compared to only 1 PD-nonVH (6%) and 2 HC (8%), showing its potential discriminative value in detecting hallucinating PD patients (Fig. 4B).
3.4. CFS condition: all perceptual measures
To investigate how visual awareness might contribute to the sensitivity of behavioral markers of VH, we also assessed correct categorization, misses and erroneous image recognition in scrambled images along with the perceptual error sum score (PES) in the ‘invisible’ condition created by using CFS (Tsuchiya and Koch, 2005) (Fig. 5). In the CFS condition, where the images were perceptually suppressed for several seconds, healthy controls and PD patients did not significantly differ in respect to proportion misses (F(1,55) = 0.93, p = 0.34), proportion misperceived scrambled images (F(1,55) = 1.21, p = 0.28) nor in respect to PES (F(1,55) = 3.33, p = 0.07) (HC vs. PD, ANOVA_1, Supplementary Table S3). PD patients had less correct image categorization than HC (F(1,55) = 7.66, p = 0.0076).
Fig. 5.
Perceptual performance in the CFS condition. All plots show the comparison between PD-nonVH (N = 16) vs. PD-VH (N = 16). (A) Mean proportion of correct image categorization of faces and cars. (B) Mean proportion of missed faces and cars. (C) Mean proportion of erroneous image recognition in scrambled images. (D) Mean Perceptual Error Score, separated by subject group. In (A–D) * denotes a significant Bonferroni corrected group difference, p < 0.0125, # Significant effects without Bonferroni correction p < 0.05 as assessed by ANOVA_2 (PD-nonVH vs. PD-VH). Error bars denote S.E.M. across subjects.
Comparable to the non-CFS condition, PD-VH patients missed more images than the PD-nonVH (F(1,30) = 7.01, p = 0.013 (n.s. after applying Bonferroni correction) (ANOVA_2, Supplementary Table S4). These differences were not significant in the ANCOVA model with LEDD and disease duration as covariates (p > 0.07) (Supplementary Table S5). PD-VH made more image categorization errors (ANOVA_2, F(1,30) = 6.77, p = 0.014 (n.s. after applying Bonferroni correction); ANCOVA, F(1,28) = 6.50, p = 0.02). PD-VH patients had significantly higher perceptual error scores (ANOVA_2, F(1,30) = 8.27, p = 0.007; significant also in the co-variate analysis (ANCOVA, F(1,28) = 7.66, p = 0.01) (Supplementary Tables S4 and S5). Thus, the results in the CFS condition confirmed the non-CFS condition, while discriminating slightly worse between PD-nonVH and VH than the non-CFS condition.
3.5. Signal detection analysis
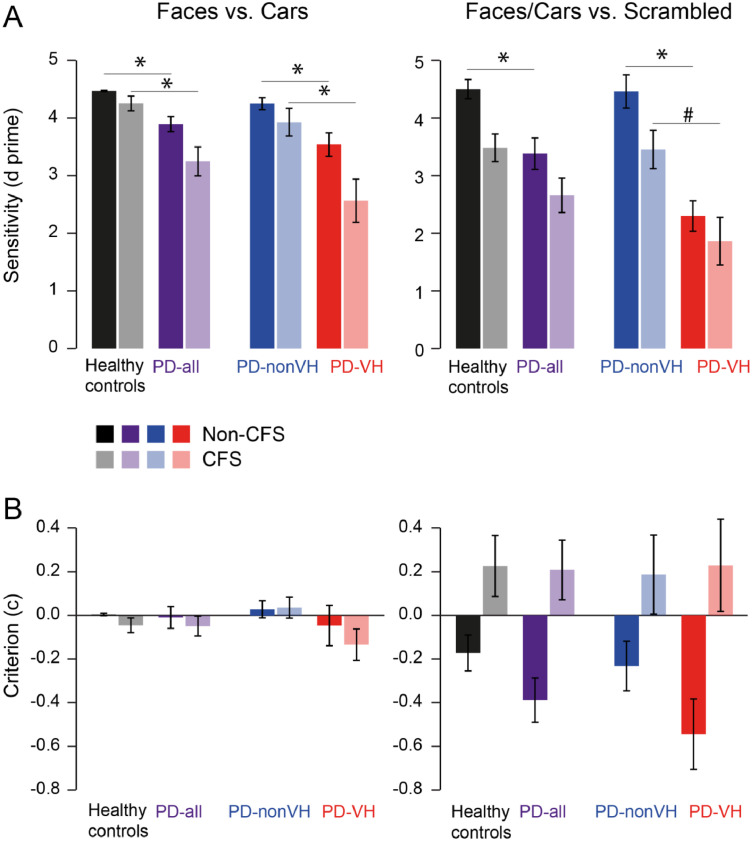
Finally, we assessed whether the increased recognition errors in the PD-VH patients are due to changes in visual sensitivity or are due to a response bias, e.g. towards reporting an image when there is none. To this end we analyzed our data in a signal detection framework, calculating visual sensitivity (d-prime) and criterion/response bias (c) from hits, misses, false alarms and correct rejections. Hit rate, false alarm rate, visual sensitivity and criterion are shown in Supplementary Tables S6 and S7. Sensitivity and criterion for the non-CFS and CFS condition are depicted in Fig. 6. Since our experiment had three instead of two potential response outcomes we resorted our data into a subset 1 that tested the detection of faces vs. cars (excluding the scrambled images) and for the complementary analysis - into subset 2 that tested detection of faces or cars vs. scrambled images (Supplementary Methods). For faces vs. cars (subset 1), PD patients as a group had lower sensitivity indices (d') than the healthy controls (p = 0.0001, non-CFS; p = 0.0006, CFS). In contrast, PD patients did not differ in respect to the criterion (c), i.e. no systematic response bias was observed. The decrease in perceptual sensitivity was mainly driven by the PD-VH group, as indicated by the significant differences between PD-nonVH and PD-VH (p = 0.0077, non-CFS; p = 0.0047, CFS). For faces/car images vs. scrambled images (subset 2), a similar decrease in the sensitivity was observed comparing HC with PD, reaching significance in the non-CFS condition (p = 0.0053, non-CFS; p = 0.0631, CFS). Importantly, the PD-VH group had significantly lower sensitivity indices than the PD-nonVH group in the non-CFS condition (p < 0.0001, non-CFS; p = 0.0167, CFS n.s. after Bonferroni correction).
Fig. 6.
Sensitivity and response bias. (A) sensitivity d’ and (B) response bias criterion c for Faces vs. Cars (left), and Faces/Cars (combined) vs. Scrambled images (right) plotted separately for healthy controls in black, PD-all in purple, PD-nonVH in blue and PD-VH red. Low transparency indicates the non-CFS and higher transparency the CFS condition (see color legend). * indicates significant Bonferroni corrected difference as tested by Mann-Whitney U Test with p < 0.0125, # significant effects without Bonferroni correction p < 0.05. Error bars denote S.E.M. across subjects. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
None of the response criterion comparisons reached significance, indicating that the PD patients did not differ from the controls in respect to response bias. In all groups there was a response bias to report faces or cars when the scrambled image was presented (the negative criterion). This effect was strongest in PD-VH, as expected from hallucinating patients, although the differences between the groups were not significant. Importantly though, this response bias was reversed by CFS to positive values in all groups, indicating that CFS prompted all subjects to miss face/car images, as expected from a perceptual suppression.
3.6. Mean recognition times and intra-individual variability (CVRT)
3.6.1. Recognition times and CVRT: non-CFS condition
As expected from previous studies (e.g. (Meppelink et al., 2008), PD patients were slower in image recognition than healthy controls (F(1,55) = 8.87, p = 0.004) (Supplementary Table S1). Comparing the two PD groups, PD-VH had longer mean recognition times than PD-nonVH (F(1,30) = 13.58, p = 0.001) (Table 4). However, when LEDD and disease duration were included in an ANCOVA, the two patient groups did not significantly differ in respect to mean recognition times (F(1,28) = 2.78, p = 0.11), indicating that the LEDD and/or disease duration contributed to this recognition time effect (Supplementary Table S2).
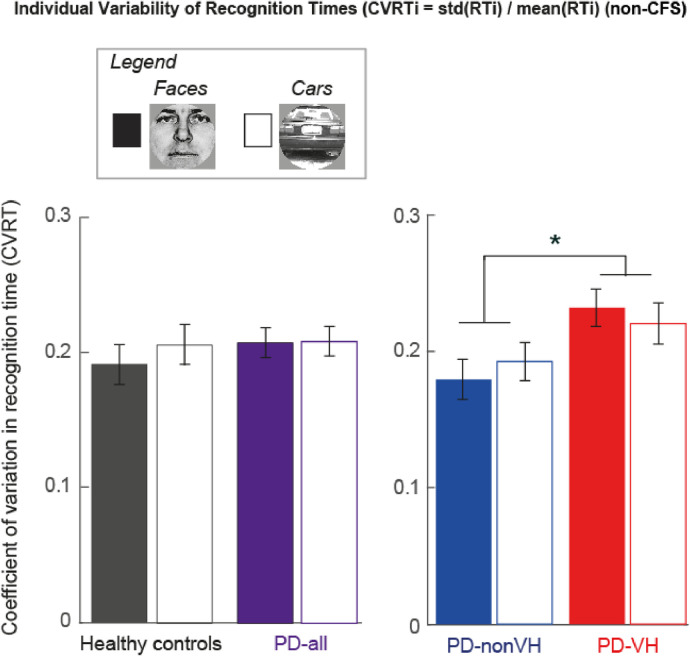
So far we considered only mean recognition times (RT). However, intra-individual variability in PD patients is discussed as a possible marker of ensuing cognitive dysfunction in PD (Camicioli et al., 2008; de Frias et al., 2007) and linked to information stability in other disease groups with hallucinations such as schizophrenia (Rentrop et al., 2010). In the next step we assessed the intra-individual variability of RTs expressed as the coefficient of individual recognition time variability across trials (CVRT) (MacDonald et al., 2009). As shown in Fig. 7, the CVRT of the PD patients as a whole group, did not differ from the HC (ANOVA_1, F(1,55) = 0.47, p = 0.5).
Fig. 7.
Coefficient of recognition time variability of the non-CFS condition. Average of intra-individual variability in RT (CVRT = individual standard deviation of RT divided by the individual RT mean). (left) No significant group differences between healthy controls and PD-all as analyzed by ANOVA are shown. (right) PD-nonVH vs. PD-VH, *denotes a significant group difference, Bonferroni corrected for multiple comparisons, p < 0.0125.
The direct comparison between the two PD groups showed a larger intra-individual RT variability for faces and cars in the PD-VH (0.23) as compared to PD-nonVH (0.19), which is also expressed in the main effect of “Group” (ANOVA_2, F(1,30) = 8.43, p = 0.007) (Fig. 7).
The CVRT difference between PD-VH and PD-nonVH patients remained significant when including disease duration and LEDD as covariates in the ANCOVA (F(1,28)= 4.34, p = 0.047) (Supplementary Table S2). Supplementary Fig. S3 illustrate that this effect cannot be explained by outliers or by an increase in RT during the experiment. To ensure the stability of this finding we performed the Jackknife procedure, which showed reliability of the CVRT group differences of p-values in the significant range [0.005; 0.01]. Supplementary Figs S4 and S5 demonstrate that this CVRT effect in the non-CFS condition cannot be explained by motor slowing or time restrictions during the experiment.
3.6.2. Recognition times and CVRT: CFS condition
In the CFS condition, PD patients did not need more time to respond and did not differ in respect to variability of RT in comparison to HC (p > 0.57) (ANOVA_1, Supplementary Table S4). In contrast to the non-CFS condition, there was also no difference in RT and CVRT between PD-nonVH and PD-VH (p > 0.19) (ANOVA_2, Supplementary Table S5). Most likely, the lack of group RT effects in the CFS condition is due to the fact that recognition times in the CFS condition vary strongly across trials in all groups due to the additional perceptual suppression component.
3.7. Resting state
3.7.1. Group resting state networks of HC and PD patients
We first tested group differences of network functional connectivity estimates of the eight networks of interest using univariate ANOVAs. This analysis showed no significant differences between HC and PD patients (default mode: F(1,33) = 1.94, p = 0.17, dorsal attention: F(1,33) = 2.15, p = 0.15, Salience: F(1,33) = 1.19, p = 0.28, left fronto-parietal: F(1,33) = 0.03, p = 0.86, right fronto-parietal: F(1,33) = 1.78, p = 0.19, Somatomotor: F(1,33) = 3.89, p = 0.06, Visual medial: F(1,33) = 0.16, p = 0.69, Visual lateral: F(1,33) = 0.02, p = 0.9). Given that we had a smaller subsample of PD patients (10 PD-nonVH and 6 PD-VH), we did not compare the different PD patient groups. Functional connectivity maps of HC and PD patients are presented in Fig. 2.
3.7.2. Correlation of resting state functional connectivity and perceptual error score (PES) and intra-individual variability of recognition times (CVRT)
Spearman's rho correlations were used to investigate the relationship between our main outcome variables of interest: PES, CVRT and resting state network functional connectivity in the PD group. To ensure reliability of our findings we also performed partial correlations including disease duration and LEDD as control variables. The partial correlations for all networks of interest are summarized in Supplementary Table S8.
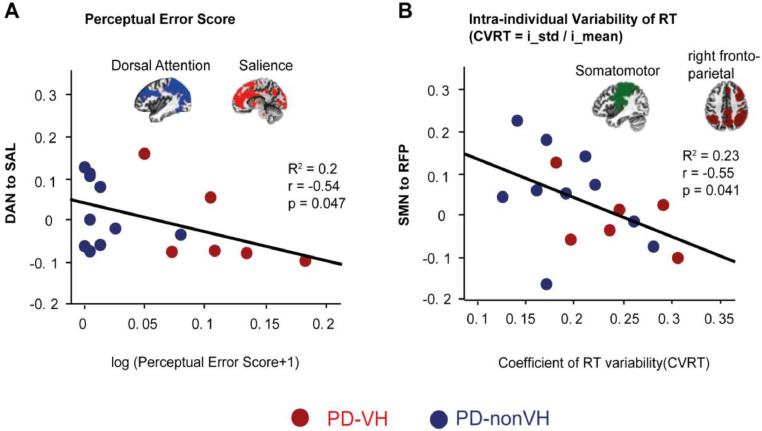
Among the PD patients, perceptual error scores (log (PES + 1)) were negatively correlated with functional connectivity between the left fronto-parietal network and the somatomotor network (rs = -0.56, p = 0.03), which did not remain stable after performing partial correlations including LEDD and disease duration (r = -0.12, p = 0.72). PES was also negatively correlated with functional connectivity between the dorsal attention network and salience network (rs = -0.50, p = 0.049), which remained stable after performing partial correlations including LEDD and disease duration (r = -0.54, p = 0.047). (Fig. 8A, see Supplementary Table S8, for values of all networks). Among the PD patients, CVRT was negatively correlated with functional connectivity between the right fronto-parietal network and default mode network (rs = -0.54, p = 0.03), which did not remain significant when LEDD and disease duration were included as control variables (r = -0.53, p = 0.053). CVRT was also negatively correlated with functional connectivity between somatomotor network and right fronto-parietal network (rs = -0.51, p = 0.044) which remained stable after performing partial correlations using LEDD and disease duration as control variables (r = -0.55, p = 0.041) (Fig. 8B, Supplementary Table S8).
Fig. 8.
Within- and between-network connectivity associated with perceptual error score (PES) and individual variability of RT (CVRT). (A) Scatter plot of logarithmic transformation of the PES on the x axis and connectivity of the dorsal attention network to salience network on the y axis of PD patients (N = 16). (B) Scatter plot of CVRT on the x axis and connectivity of somatomotor to right fronto-parietal networks on the y axis (N = 16). Blue dots show the individual values of PD-nonVH (N = 10) and red dots of PD-VH (N = 6). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We showed that perceptual errors and elevated intra-individual behavioral variability characterize PD patients with self-reported hallucinations compared to PD patients not reporting VH. We showed that these behavioral measures are associated with functional connectivity changes in overlapping, but slightly different neural networks: higher PES correlated with lower functional connectivity between dorsal attention network and salience network, while higher intra-subject variability in RT correlated with lower functional connectivity between somatomotor and right fronto-parietal networks.
4.1. Comparison with previous findings on misperceptions in hallucinating PD patients
In accordance with previous studies, PD-VH patients exhibited lower rates of correct image recognition and reported real images in scrambled images more often than PD-nonVH (Meppelink et al., 2008; Ramirez-Ruiz et al., 2007; Shine et al., 2012). It remains to be investigated which score is more sensitive for the identification of hallucinating patients and which can serve best to identify patients experiencing or being at risk to develop hallucinations. The signal detection analysis revealed that the recognition deficits of the PD-VH patients were mainly due to a reduction of perceptual sensitivity rather than to criterion shifts resulting in response bias. Thus, it appears that PD-VH patients have difficulties to discern signals from noise, leading to the observed recognition errors. The sensitivity reduction is reminiscent of previous studies that compared hallucinating and non-hallucinating patients with Lewy Body disease (Bowman et al., 2017) and two recent studies that compared Parkinson patients with healthy participants (Weil et al., 2018, 2017). Using several types of object recognition tasks, the latter study reported image recognition deficits in PD patients, while not finding a significant difference between PD-VH and PD-nonVH patients (Weil et al., 2018). Apart from the fact that the PD-VH patients in their study had slightly less disease progression, it appears that the inclusion of the scrambled image condition might have also led to better discrimination in our study. Specifically, while PD-VH differed from PD-nonVH also in respect to correct image categorization between faces and cars, error rates and perceptual sensitivity measures, the comparison between images (faces and cars) vs. the scrambled condition yielded larger differences between the two PD groups. The perceptual error score (PES) that combines the image categorization with the image detection in scrambled images might prove the most robust or practical measure to produce cut-off scores. The discriminatory and/or predictive value of the PES should be further tested in longitudinal studies involving larger patient cohorts.
In addition to the scrambled images we had also compared a non-masked (non-CFS) and a masked (CFS) condition, where perceptual image suppression is induced by means of binocular rivalry. Similar differences in perceptual error rates and perceptual sensitivity deficits in the PD-VH patients were observed in both the non-CFS and CFS condition. On the other hand, the intra-individual variability of RT between PD-VH and PD-nonVH patients were most pronounced in the non-CFS condition, while no differences in the perceptual breakthrough times (e.g. no differences in stimulus processing outside of awareness (Yang et al., 2014)) were revealed. Thus, while our data suggest that scrambled images add considerable value for objectifying misperceptions in PD patients, the CFS condition can likely be dismissed in future studies to streamline the experimental design.
Consistent with previous research we found a negative correlation between misperceptions and functional connectivity between dorsal attention network and the salience network (Shine et al., 2015). The salience network is related to the degree of personal salience in the cognitive, emotional or homeostatic domain and interoceptive-autonomic processing (Seeley et al., 2007). Distortion in attentional processes might be a common mechanism for the development of visual hallucinations given that attentional deficits have also been shown in hallucinating patients suffering from Charles Bonnet syndrome and dementia (Graham et al., 2011; Makin et al., 2013).
4.2. Intra-individual variability in recognition times
PD-VH patients showed higher intra-individual variability in recognition times than PD-nonVH, which to our knowledge has not been reported before. Performance fluctuations are problematic for the development of objective measures for VH based on mean performance (MacDonald et al., 2009). At the same time, intra-individual variability appears to be a promising variable as previous research indicates that trial-by-trial fluctuations in RT correlate negatively with cognitive performance and can even serve as a sensitive predictor for age-related cognitive decline (Bielak et al., 2010; MacDonald et al., 2006), incipient dementia in PD (de Frias et al., 2012) and identification of individuals at risk to develop hallucinations such as persons at risk for schizophrenia (Shin et al., 2013). In healthy aging populations, larger intra-individual variability has been linked to attentional lapses (Bunce, Warr, & Cochrane, 1993) and deficits in executive control (West, Murphy, Armilio, Craik, & Stuss, 2002). Since previous studies reported a strong link between visual hallucinations in PD and deficits in attentional and executive processes (Barnes, Boubert, Harris, Lee, & David, 2003; Gallagher et al., 2011, Hall et al., 2016; Meppelink et al., 2008), this functional interpretation is plausible (Muller et al., 2014; Shine et al., 2014). Concerning neural correlates of performance variability, the most consistent correlation with increased intra-individual variability in healthy elderly and other patient groups has been found with structural and functional alterations of prefrontal cortices (MacDonald et al., 2006; Murtha, Cismaru, Waechter, & Chertkow, 2002) and alterations in DMN and attentional networks (Kelly, Uddin, Biswal, Castellanos, & Milham, 2008), which both include prefrontal regions. We found that PD patients with higher intra-individual variability show reduced functional connectivity between somatomotor and right fronto-parietal networks. Previous research reported hyperconnectivity of fronto-parietal regions in hallucinating PD patients compared to non-hallucinating PD patients (Franciotti et al., 2015; Yao et al., 2014). The sensory-motor network is important for voluntary movements and shows deviant functional connectivity in PD (Biswal et al., 1995, Hohenfeld et al., 2014). However, exploratory analyses of our data show that the severity of the motor symptoms measured with the MDS-UPDRS score III was not related to functional connectivity between SMN and right fronto-parietal network using LEDD and disease duration as covariates, indicating that the correlation between CVRT and functional connectivity between SMN and right fronto-parietal network is not driven by movement disabilities in PD (r = 0.17, p = 0.62). Hypoconnectivity within the somatosensory and between fronto-parietal and ventral attentional network has also been reported in schizophrenia which indicates their possible involvement in cognitive changes (Dong, Wang, Chang, Luo, & Yao, 2018). Furthermore, during working memory maintenance, patients with mild cognitive impairment show hypoactivation in right fronto-parietal regions (Melrose et al., 2018) indicating its relation to cognitive decline. Increased intra-individual behavioral fluctuations were also reported in multiple other diseases such as schizophrenia, autism, attention deficit hyperactivity disorder (ADHD) as well as in aging populations exhibiting cognitive decline (Dinstein, Heeger, & Behrmann, 2015; MacDonald et al., 2009). Thus, the intra-individual variability observed in our task together with the hypoconnectivity of the somatomotor and fronto-parietal networks might be useful to predict cognitive changes in PD patients rather than the occurrence of visual hallucinations itself.
4.3. Limitations
One limitation is that we cannot be sure about the perceptual experience of the patients when they reported a real image in a scrambled image. As such there is a tradeoff between verbally reporting patients potentially providing more insight into their phenomenology, and trial-based measures offering the opportunity to collect many trials of the same type to assess neural activity or to assess inter-individual trial-by-trial variability.
Another potential limitation of our task design was the requirement of a time-limited response. Theoretically, the time limit could affect error rates and misses, becoming an issue in PD patients with motor slowing. However, the response limit of 12 s was relatively long, and our cumulative response data indicate that button presses in the non-CFS condition (including the slow contrast increase) were asymptotic at about 4 s in the HC group and at 6–8 s in the PD group. Moreover, in our control task with 100% contrast, the individual response times did not exceed 2 s in either group, suggesting that the 12 s time limit did not majorly contribute to the recognition errors or the misses in our study.
Another limitation of the current study is the relatively small sample size of the demographically matched PD patients and the subsample participating in the resting state. For this reason the correlational analyses of resting state functional connectivity need to be interpreted with caution as the subsample did not provide sufficient power to apply Bonferroni correction with confidence about possible negative results. We also had a bias towards less severe motor symptoms in the PD sample that participated in the fMRI study, although it is not entirely clear how this variable could be practically fixed in future studies. Clearly, a study with a larger PD sample where it is not only possible to control, but to match the patients for clinical variables such as LEDD and disease duration, is necessary. Albeit we tested for visual impairments and general cognitive state, we did not include assessments of attentional or executive functions in our study. Future studies would profit from the identification of different phenotypes in cognition and attention such as memory, sustained and divided attention. Moreover, differences in phenotype profiles of different clinical groups such as patients with mild cognitive impairments and different types of dementia would also be helpful to distill the best predictors for hallucinations versus cognitive decline.
Funding
This work was supported by the Herman and Lilly Schilling Foundation (to MW), the DFG Center for Nanoscale Microscopy & Molecular Physiology of the Brain (CNMPB) and the Leibniz Science Campus Primate Cognition.
Ethics
The study was approved by the medical ethics committees of the University Medical Center Goettingen, Germany. All participants signed an informed consent form prior to the examination.
CRediT authorship contribution statement
Kristina Miloserdov: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Carsten Schmidt-Samoa: Conceptualization, Writing - review & editing. Kathleen Williams: Formal analysis, Writing - review & editing. Christiane Anne Weinrich: Data curation, Writing - review & editing. Igor Kagan: Formal analysis, Writing - review & editing. Katrin Bürk: Writing - review & editing. Claudia Trenkwalder: Writing - review & editing. Mathias Bähr: Writing - review & editing. Melanie Wilke: Conceptualization, Formal analysis, Writing - review & editing, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank Severin Heumüller for excellent computer and programming support and Dr. Holger Sennhenn-Reulen for helpful statistical discussions. Dr. Dechent we thank for setting up the MRI scanning protocols and Britta Perl and Ilona Pfahlert for assistance with the acquisition of the MRI data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102076.
Appendix. Supplementary materials
References
- Armstrong R.A. Visual symptoms in Parkinson's disease. Parkinsons Dis. 2011;2011 doi: 10.4061/2011/908306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio H.C., Segura B., Sala-Llonch R., Marti M.J., Valldeoriola F., Compta Y., Tolosa E., Junqué C. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum. Brain Mapp. 2015;36:199–212. doi: 10.1002/hbm.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J., Boubert L., Harris J., Lee A., David A.S. Reality monitoring and visual hallucinations in Parkinson's disease. Neuropsychologia. 2003;41:565–574. doi: 10.1016/s0028-3932(02)00182-3. [DOI] [PubMed] [Google Scholar]
- Bartlett M.S. The use of transformations. Biometrics. 1947;3:39–52. [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. Group comparison of resting-state fMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148. [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for bold and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak A.A., Hultsch D.F., Strauss E., MacDonald S.W., Hunter M.A. Intraindividual variability in reaction time predicts cognitive outcomes 5 years later. Neuropsychology. 2010;24:731. doi: 10.1037/a0019802. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bowman A.R., Bruce V., Colbourn C.J., Collerton D. Compensatory shifts in visual perception are associated with hallucinations in lewy body disorders. Cogn. Res. Princ. Implic. 2017;2:26. doi: 10.1186/s41235-017-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D.H. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Bunce D.J., Warr P.B., Cochrane T. Blocks in choice responding as a function of age and physical fitness. Psychol Aging. 1993;8:26–33. doi: 10.1037//0882-7974.8.1.26. [DOI] [PubMed] [Google Scholar]
- Camicioli R.M., Wieler M., de Frias C.M., Martin W.R.W. Early, untreated parkinson's disease patients show reaction time variability. Neurosci. Lett. 2008;441:77–80. doi: 10.1016/j.neulet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Carmel D., Arcaro M., Kastner S., Hasson U. How to create and use binocular rivalry. J. Vis. Exp. 2010 doi: 10.3791/2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton D., Perry E., McKeith I. Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 2005;28:737–757. doi: 10.1017/S0140525X05000130. discussion 757-794. [DOI] [PubMed] [Google Scholar]
- de Frias C.M., Dixon R.A., Camicioli R. Neurocognitive speed and inconsistency in Parkinson's disease with and without incipient dementia: an 18-month prospective cohort study. J. Int. Neuropsychol. Soc. 2012;18:764–772. doi: 10.1017/S1355617712000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias C.M., Dixon R.A., Fisher N., Camicioli R. Intraindividual variability in neurocognitive speed: a comparison of Parkinson's disease and normal older adults. Neuropsychologia. 2007;45:2499–2507. doi: 10.1016/j.neuropsychologia.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Diederich N.J., Fénelon G., Stebbins G., Goetz C.G. Hallucinations in Parkinson disease. Nat. Rev. Neurol. 2009;5:331–342. doi: 10.1038/nrneurol.2009.62. [DOI] [PubMed] [Google Scholar]
- Dinstein I., Heeger D.J., Behrmann M. Neural variability: friend or foe? Trends Cogn. Sci. (Regul. Ed.) 2015;19:322–328. doi: 10.1016/j.tics.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Dong D., Wang Y., Chang X., Luo C., Yao D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P., Friesen W. Consulting Psychologists Press; Palo Alto, CA: 1976. Pictures of Facial Affect. [Google Scholar]
- Fassbender C., Lesh T.A., Ursu S., Salo R. Reaction time variability and related brain activity in methamphetamine psychosis. Biol. Psychiatry Neural Mech. Addict. 2015;77:465–474. doi: 10.1016/j.biopsych.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Scangos K., Lesh T.A., Carter C.S. RT distributional analysis of cognitive-control-related brain activity in first-episode schizophrenia. Cogn. Affect. Behav. Neurosci. 2014;14:175–188. doi: 10.3758/s13415-014-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flehmig H.C., Steinborn M., Langner R., Scholz A., Westhoff K. Assessing intraindividual variability in sustained attention: reliability, relation to speed and accuracy, and practice effects. Psychol. Sci. 2007;49:132–149. [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franciotti R., Delli Pizzi S., Perfetti B., Tartaro A., Bonanni L., Thomas A., Weis L., Biundo R., Antonini A., Onofrj M. Default mode network links to visual hallucinations: A comparison between Parkinson's disease and multiple system atrophy. Mov. Disord. 2015;30:1237–1247. doi: 10.1002/mds.26285. [DOI] [PubMed] [Google Scholar]
- Gallagher D.A., Parkkinen L., O'Sullivan S.S., Spratt A., Shah A., Davey C.C., Bremner F.D., Revesz T., Williams D.R., Lees A.J., Schrag A. Testing an aetiological model of visual hallucinations in Parkinson's disease. Brain. 2011;134:3299–3309. doi: 10.1093/brain/awr225. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez‐Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., Hilten J.J.van, LaPelle N. Movement disorder society-sponsored revision of the unified parkinson's disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Graham G., Dean J., Mosimann U.P., Colbourn C., Dudley R., Clarke M., Collerton D. Specific attentional impairments and complex visual hallucinations in eye disease. Int. J. Geriatr. Psychiatry. 2011;26:263–267. doi: 10.1002/gps.2522. [DOI] [PubMed] [Google Scholar]
- Hall J.M., O'Callaghan C., Shine J.M., Muller A.J., Phillips J.R., Walton C.C., Lewis S.J.G., Moustafa A.A. Dysfunction in attentional processing in patients with Parkinson's disease and visual hallucinations. J Neural Transm (Vienna) 2016;123:503–507. doi: 10.1007/s00702-016-1528-3. [DOI] [PubMed] [Google Scholar]
- Hohenfeld C., Werner C.J., Reetz K. Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? Neuroimage Clin. 2018;18:849–870. doi: 10.1016/j.nicl.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gupta M., Singh G., Khwaja G.A., Mehndiratta M.M. Hallucinations in Parkinson's disease–a study of forty three patients. J. Assoc. Physicians India. 2004;52:703–706. [PubMed] [Google Scholar]
- Koerts J., Borg M.A.J.P., Meppelink A.M., Leenders K.L., van Beilen M., van Laar T. Attentional and perceptual impairments in Parkinson's disease with visual hallucinations. Parkinsonism Relat. Disord. 2010;16:270–274. doi: 10.1016/j.parkreldis.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Kritzinger C., Vollstedt E.-J., Hückelheim K., Lorwin A., Graf J., Tunc S., Klein C., Kasten M. Qualitative characteristics of depression in Parkinson's patients and controls. Behav. Neurol. 2015;2015 doi: 10.1155/2015/961372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald S.W.S., Li S.-C., Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychol. Aging. 2009;24:792–808. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- MacDonald S.W.S., Nyberg L., Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Makin S.M., Redman J., Mosimann U.P., Dudley R., Clarke M.P., Colbourn C., Collerton D. Complex visual hallucinations and attentional performance in eye disease and dementia: a test of the perception and attention deficit model. Int. J. Geriatr. Psychiatry. 2013;28:1232–1238. doi: 10.1002/gps.3947. [DOI] [PubMed] [Google Scholar]
- Manni R., Terzaghi M., Ratti P.-L., Repetto A., Zangaglia R., Pacchetti C. Hallucinations and REM sleep behaviour disorder in Parkinson's disease: dream imagery intrusions and other hypotheses. Conscious. Cognit. 2011;20:1021–1026. doi: 10.1016/j.concog.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Maria B., Sophia S., Michalis M., Charalampos L., Andreas P., John M.E., Nikolaos S.M. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir. Med. 2003;97:1151–1157. doi: 10.1016/s0954-6111(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Matsui H., Udaka F., Tamura A., Oda M., Kubori T., Nishinaka K., Kameyama M. Impaired visual acuity as a risk factor for visual hallucinations in parkinson's disease. J. Geriatr. Psychiatry Neurol. 2006;19:36–40. doi: 10.1177/0891988705284739. [DOI] [PubMed] [Google Scholar]
- Melrose R.J., Jimenez A.M., Riskin-Jones H., Weissberger G., Veliz J., Hasratian A.S., Wilkins S., Sultzer D.L. Alterations to task positive and task negative networks during executive functioning in Mild Cognitive Impairment. Neuroimage Clin. 2018;19:970–981. doi: 10.1016/j.nicl.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meppelink A.M., de Jong B.M., Renken R., Leenders K.L., Cornelissen F.W., van Laar T. Impaired visual processing preceding image recognition in Parkinson's disease patients with visual hallucinations. Brain. 2009;132:2980–2993. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- Meppelink A.M., Koerts J., Borg M., Leenders K.L., van Laar T. Visual object recognition and attention in Parkinson's disease patients with visual hallucinations. Mov. Disord. 2008;23:1906–1912. doi: 10.1002/mds.22270. [DOI] [PubMed] [Google Scholar]
- Moeller S., Yacoub E., Olman C.A., Auerbach E., Strupp J., Harel N., Uğurbil K. Multiband multislice ge-epi at 7 T, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A.P., Wells A., Nothard S. Cognitive factors in predisposition to auditory and visual hallucinations. Br. J. Clin. Psychol. 2000;39(Pt 1):67–78. doi: 10.1348/014466500163112. https://onlinelibrary.wiley.com/doi/pdf/ [DOI] [PubMed] [Google Scholar]
- Muller A.J., Shine J.M., Halliday G.M., Lewis S.J.G. Visual hallucinations in Parkinson's disease: theoretical models. Mov. Disord. 2014;29:1591–1598. doi: 10.1002/mds.26004. [DOI] [PubMed] [Google Scholar]
- Murtha S., Cismaru R., Waechter R., Chertkow H. Increased variability accompanies frontal lobe damage in dementia. Journal of the International Neuropsychological Society. 2002;8:360–372. doi: 10.1017/s1355617702813170. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S., Katzen H., Schrag A., Singer C., Scanlon B.K., Nation D., Guevara A., Levin B. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol. 2008;8:21. doi: 10.1186/1471-2377-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza L.R., Kaiser M., Firbank M., Graziadio S., Bonanni L., Onofrj M., Colloby S.J., Blamire A., O'Brien J., Taylor J.-P. fMRI resting state networks and their association with cognitive fluctuations in dementia with lewy bodies. Neuroimage Clin. 2014;4:558–565. doi: 10.1016/j.nicl.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri V., Diederich N.J., Raman R., Goetz C.G. Decreased color discrimination and contrast sensitivity in Parkinson's disease. J. Neurol. Sci. 2000;172:7–11. doi: 10.1016/s0022-510x(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Prell T. Structural and functional brain patterns of non-motor syndromes in Parkinson's disease. Front. Neurol. 2018;9:138. doi: 10.3389/fneur.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ruiz B., Junque C., Marti M.-J., Valldeoriola F., Tolosa E. Cognitive changes in Parkinson's disease patients with visual hallucinations. Dement. Geriatr. Cognit. Disord. 2007;23:281–288. doi: 10.1159/000100850. [DOI] [PubMed] [Google Scholar]
- Rentrop M., Rodewald K., Roth A., Simon J., Walther S., Fiedler P., Weisbrod M., Kaiser S. Intra-individual variability in high-functioning patients with schizophrenia. Psychiatry Res. 2010;178:27–32. doi: 10.1016/j.psychres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K., Gagoski B.A., Polimeni J.R., Witzel T., Wedeen V.J., Wald L.L. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012;67:1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour K., Rhodes G., Stein T., Langdon R. Intact unconscious processing of eye contact in schizophrenia. Schizophr. Res. Cognit. 2016;3:15–19. doi: 10.1016/j.scog.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.S., Kim S.N., Shin N.Y., Jung W.H., Hur J.-W., Byun M.S., Jang J.H., An S.K., Kwon J.S. Increased intra-individual variability of cognitive processing in subjects at risk mental state and schizophrenia patients. PLoS One. 2013;8:e78354. doi: 10.1371/journal.pone.0078354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J.M., Halliday G.H., Carlos M., Naismith S.L., Lewis S.J.G. Investigating visual misperceptions in Parkinson's disease: a novel behavioral paradigm. Mov. Disord. 2012;27:500–505. doi: 10.1002/mds.24900. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Halliday G.M., Gilat M., Matar E., Bolitho S.J., Carlos M., Naismith S.L., Lewis S.J.G. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Hum. Brain Mapp. 2014;35:2206–2219. doi: 10.1002/hbm.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J.M., Keogh R., O'Callaghan C., Muller A.J., Lewis S.J.G., Pearson J. Imagine that: elevated sensory strength of mental imagery in individuals with parkinson's disease and visual hallucinations. Proc. Biol. Sci. 2015;282 doi: 10.1098/rspb.2014.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H., Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Stein T., Hebart M.N., Sterzer P. Breaking continuous flash suppression: A new measure of unconscious processing during interocular suppression? Front. Hum. Neurosci. 2011;5:167. doi: 10.3389/fnhum.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P., Hilgenfeldt T., Freudenberg P., Bermpohl F., Adli M. Access of emotional information to visual awareness in patients with major depressive disorder. Psychol. Med. 2011;41:1615–1624. doi: 10.1017/S0033291710002540. [DOI] [PubMed] [Google Scholar]
- Straughan S., Collerton D., Bruce V. Visual priming and visual hallucinations in Parkinson's disease. evidence for normal top-down processes. J. Geriatr. Psychiatry Neurol. 2016;29:25–30. doi: 10.1177/0891988715598237. [DOI] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N., Koch C. Continuous flash suppression reduces negative afterimages. Nat. Neurosci. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Weil R.S., Pappa K., Schade R.N., Schrag A.E., Bahrami B., Schwarzkopf D.S., Crutch S.J., O'Keeffe A.G., Morris H.R. The cats-and-dogs test: A tool to identify visuoperceptual deficits in Parkinson's disease. Mov. Disord. 2017;32:1789–1790. doi: 10.1002/mds.27176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R.S., Schwarzkopf D.S., Bahrami B., Fleming S.M., Jackson B.M., Goch T.J.C., Saygin A.P., Miller L.E., Pappa K., Pavisic I., Schade R.N., Noyce A.J., Crutch S.J., O'Keeffe A.G., Schrag A.E., Morris H.R. Assessing cognitive dysfunction in Parkinson's disease: An online tool to detect visuo-perceptual deficits. Mov. Disord. 2018;33:544–553. doi: 10.1002/mds.27311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R., Murphy K.J., Armilio M.L., Craik F.I., Stuss D.T. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilke M. An iterative jackknife approach for assessing reliability and power of fMRI group analyses. PLoS One. 2012;7:e35578. doi: 10.1371/journal.pone.0035578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Brascamp J., Kang M.-S., Blake R. On the use of continuous flash suppression for the study of visual processing outside of awareness. Front. Psychol. 2014;5:724. doi: 10.3389/fpsyg.2014.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Zhao J., Jiang Y., Li C., Wang J., Weng X., Northoff G. Altered negative unconscious processing in major depressive disorder: an exploratory neuropsychological study. PLoS One. 2011;6:e21881. doi: 10.1371/journal.pone.0021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N., Shek-Kwan Chang R., Cheung C., Pang S., Lau K.K., Suckling J., Rowe J.B., Yu K., Ka-Fung Mak H., Chua S.-E., Ho S.L., McAlonan G.M. The default mode network is disrupted in Parkinson's disease with visual hallucinations. Hum Brain Mapp. 2014;35:5658–5666. doi: 10.1002/hbm.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.