Highlights
-
•
SBRT for liver metastases is a safe alternative for surgical resection or ablation.
-
•
High dose (>100 Gy) SBRT provides better local control than low dose (≤100 Gy) SBRT.
-
•
Toxicity rates of SBRT are low and do not increase after dose escalation.
-
•
Dose escalation is positively associated with overall survival.
Keywords: Stereotactic Body Radiation Therapy, SBRT, Liver metastases, Local control, Dose-escalation
Abstract
Introduction
Stereotactic Body Radiation Therapy (SBRT) is a treatment option for patients with liver metastases. This study evaluated the impact of high versus low dose image-guided SBRT of hepatic metastases.
Methods and materials
This is a single-center retrospective study of patients with liver metastases treated with SBRT. For analyses, patients were divided into two groups: ≤100 Gy and >100 Gy near-minimum Biological Effective Doses (BED98%). The main outcomes were local control (LC), toxicity and overall survival (OS). Cox regression analyses were performed to determine prognostic variables on LC and OS.
Results
Ninety patients with 97 liver metastases (77% colorectal) were included. Median follow-up was 28.6 months. The two-year LC rates in the ≤100 Gy and >100 Gy BED98% group were 60% (CI: 41–80%) and 90% (CI: 80–100%), respectively (p = 0.004). Grade 3 toxicity occurred in 7% vs 2% in the ≤100 Gy and >100 Gy group (p = 0.23). Two-year OS rates in the ≤100 Gy and >100 Gy group were 48% (CI: 32–65%) and 85% (CI: 73–97%), respectively (p = 0.007). In multivariable Cox regression analyses, group dose and tumor volume were significantly correlated with LC (HR: 3.61; p = 0.017 and HR: 1.01; p = 0.005) and OS (HR: 2.38; p = 0.005 and HR: 1.01; p = <0.0001).
Conclusion
High dose SBRT provides significantly better local control and overall survival than low dose SBRT without increasing toxicity. When surgical resection is not feasible, high dose SBRT provides an effective and safe treatment for liver metastases.
1. Background
Liver metastases are the most common malignant tumors of the liver, with main primary sites in the breast, lung, colon, and rectum [1]. In patients with colorectal cancer, 25–50% will develop either synchronous or metachronous liver metastases [2]. Surgical resection is the standard of care for limited metastatic disease and the five-year survival rates after curative surgical resection are approximately 50–60% [3]. Unfortunately, many patients with liver metastases are not eligible for surgery due to e.g. extensive disease. In a proportion of patients with limited metastatic disease, surgery is an unfavorable option as a result of age, poor performance status, co-morbidity or involvement of adjacent anatomical structures (like large blood vessels). In these patients, studies show that alternative local therapies - including thermal ablation - can offer good rates of local control [4], [5], [6]. Thermal ablation may, however, fail when lesions are close to the biliary structures or large blood vessels because of damage to the bile duct and heat-sink respectively. Ablation is also limited in superficial lesions close to the liver capsule due to high incidence of complications [7]. Moreover, local control with this technique is significantly better when metastases are <3 cm in diameter [8], [9].
In the past, the role of radiotherapy in the treatment of liver metastases was limited due to toxicity in surrounding healthy tissue with increased risk of radiation-induced liver disease [10]. In the last decade, Stereotactic Body Radiation Therapy (SBRT) has been developed. Phase I and II studies showed that SBRT can deliver high doses of radiation with great precision and low damage to the surrounding healthy liver tissue [11], [12], [13], [14]. Compared to other local ablative treatments, SBRT is non-invasive and requires only brief outpatient clinic visits without hospital admission during treatment. Recently, a randomized controlled phase 2 trial compared palliative standard of care treatment with standard of care plus SBRT in patients with oligometastatic cancers [15]. They concluded that treatment with SBRT was associated with improved overall survival but showed more treatment-related deaths compared to the control group. Despite the increased amount of studies, the ultimate dose and fractionation schedule remain under debate.
At the Netherlands Cancer Institute, image-guided SBRT has been used for many years. Over time, our fractionation schedule for liver metastases changed after positive results published on high dose SBRT [16], [17]. The aim of this study was to evaluate the efficacy and safety of high versus low dose SBRT in patients with liver metastases in our institute and to determine prognostic variables on local control and overall survival.
2. Methods
This was a retrospective study of patients with liver metastases who underwent SBRT at the Netherlands Cancer Institute, a tertiary referral center for patients with cancer in the Netherlands. Consecutive patients treated with SBRT between April 2009 and April 2017 were included. Hepatic metastases from any primary solid tumor were included. Patients with primary liver cancer were excluded. Before SBRT, all patients were individually discussed in a multidisciplinary meeting including radiologists, oncologists, radiation and surgical oncologists. Indications for SBRT included inoperable liver metastases due to age, anatomic location or poor performance status and inoperable recurrent disease. Extrahepatic disease was permitted when controlled with other local or systemic treatment modalities. The study was approved by the institutional review board of the Netherlands Cancer Institute (METC18.1493/N18SBR).
2.1. Treatment
Prior to SBRT, fiducial Twist markers were placed by an interventional radiologist using computer tomography (CT) or ultrasound guidance. When patients had hepatic surgery prior to SBRT, surgical clips could be used as markers. Patients were placed in a Thorax support (Macromedics BV, Waddinxveen, the Netherlands) for immobilization and a phased contrast-enhanced CT scan was made to define the gross tumor volume (GTV), which equals the clinical target volume (CTV). When available, the CT scan was registered to planning Magnetic Resonance Imaging (MRI) in radiotherapy-position. A 4D planning CT was made to assess motion and was used to generate a mid-position CT. The contrast-enhanced CT was deformed to match the mid-position geometry [18]. The anisotropic CTV to planning target volume (PTV) margins were based on the tumor motion (8 to 15 mm in anterior-posterior and left-right direction, 9 to 16 mm in caudal-cranial direction). Patients received a prescribed dose of 37.5 – 60 Gy in 3 fractions, in which tumor size was not taken into account. Doses up to 150% of the prescribed dose was allowed in parts of the PTV that did not overlap with organs at risk. Dose constraints for organs at risk were: duodenum (Dmax <30 Gy), heart (Dmax <30 Gy), great vessels (Dmax <45 Gy), kidneys (V 15 Gy at <35%), esophagus (Dmax <27 Gy), spinal cord (Dmax <18 Gy), and stomach (Dmax <30 Gy), depending on the location of the tumor. A Volumetric Modulated Arc Therapy (VMAT) technique planned in Pinnacle (Philips Medical Systems, Fitchburg, WI) was used. Patients were treated on an Elekta Agility or MLCi (Elektra AB, Stockholm, Sweden) accelerator with 4D – Cone Beam CT guidance.
2.2. Follow up and endpoints
Standard follow-up in the outpatient clinic was performed two months after completing SBRT treatment and included an abdominal CT-scan and in individual cases an MRI-scan or PET scan. Thereafter, patients were scheduled for abdominal CT-scans at four-month intervals.
The main outcomes were infield local control, toxicity, and overall survival. Infield local control was defined as the absence of new or re-growth of existing lesions within or at the margin of the PTV. The local control was calculated from the first day of radiation treatment until the first date of infield progression. Toxicity was evaluated using the National Cancer Institute CTCAE v4.0 criteria [19]. Acute and late toxicity were defined as toxicities within or after 3 months. Overall survival was calculated from the first day of treatment until the last date of follow up or death.
2.3. Evaluation
The follow-up scans of all patients were retrospectively reviewed by four individual observers, including two dedicated radiation oncologists, one hepatobiliary surgeon, and one liver radiologist. All relevant follow up scans were compared to the three-dimensional (3D) image-based radiation treatment plan to determine if recurrence had occurred in the PTV area. Infield recurrence, out-of-field liver recurrence, and extrahepatic recurrence were individually scored by all observers. In case of interobserver disagreement, a joint meeting was planned to re-evaluate until consensus was reached.
2.4. Statistical analysis
All analyses were performed using SPSS® version 24.0 (IBM Corporation; Armonk, NY, USA) and R version 3.5.1. Patients and treatment characteristics were presented as number (percentage) or median (range). For analyses, patients were divided into a BED98% low dose group of ≤100 Gy and a high dose group of >100 Gy. The median follow up time was calculated using the reverse Kaplan-Meier method [20]. Categorical variables were compared using Fisher’s test and continuous variables with the Mann-Whitney U test. Local control and overall survival curves were calculated with the Kaplan-Meier method. For local control, patients who died from other causes without infield progression were censored. Local control was analyzed per lesion. Overall survival was analyzed per patient. The difference between the survival curves were analyzed with the Log-rank test.
Biological effective doses (BED) were calculated according to the formula:
where n is the number of fractions, d the prescribed dose per fraction and α/β the fractionation sensitivity for tumor tissue of 10 Gy. Near-minimum BED (BED98%) was defined as the dose received by 98% of the PTV volume [21], [22]. Near-maximum BED (BED2%) was defined as the dose received by 2% of the PTV volume.
Relative near-minimum PTV dose was defined as the dose received by 98% of the PTV volume relative to the prescribed dose and was calculated using the following formula:
where PTV D98% is the near-minimum dose, n is the number of fractions and d the dose per fraction.
Regression analyses were performed using Cox proportional hazard models. A frailty model was used to allow for clustering of lesions within a patient for the local control endpoint. Multivariable models were used to evaluate whether the association of dose group with local control and overall survival remained significant after adjusting for factors that were significant at the 0.10 level in univariable analysis. The association of local recurrence with overall survival was analyzed using a cox proportional hazard model with local recurrence as a time-dependent variable. Statistical significance was accepted with a p-value of <0.05.
3. Results
3.1. Patient and treatment characteristics
Ninety patients with 97 liver metastases were included. Primary tumor origin was colorectal in 76.7% of the patients. Extrahepatic disease was present in 29 patients (32.2%). The median follow up of all patients was 28.6 months (range: 1.2–62.4 months), and was significantly longer in the ≤100 Gy group (54.2 months) than in the >100 Gy group (25.0 months) (p = <0.001). The median GTV of all tumors was 14.4 cm3 (range: 1.0 – 194.9 cm3). No significant baseline differences were found in patient characteristics between the groups. Patient and treatment characteristics are described in Table 1.
Table 1.
Patient and treatment characteristics.
| ≤100 Gy group | >100 Gy group | P-value | |
|---|---|---|---|
| No. of patients/treated lesions | 40/41 | 50/56 | |
| Sex | 1.00 | ||
| Male | 23 (57.5%) | 29 (58.0%) | |
| Female | 17 (42.5%) | 21 (42.0%) | |
| Age at treatment (years) | 64.3 (40.6–84.9) | 64.2 (38.5–85.8) | 0.54 |
| Primary tumor location | 0.81 | ||
| Colorectal | 30 (75.0%) | 39 (78.0%) | |
| Other gastrointestinal tumor | 3 (7.5%) | 2 (4.0%) | |
| Lung | 3 (7.5%) | 1 (2.0%) | |
| Breast | 1 (2.5%) | 3 (6.0%) | |
| Melanoma | 0 (0%) | 1 (2.0%) | |
| Other | 3 (7.5%) | 4 (8.0%) | |
| Extrahepatic disease | 0.50 | ||
| None | 26 (65.0%) | 35 (70.0%) | |
| Lung | 7 (17.5%) | 9 (18.0%) | |
| Peritoneum | 3 (7.5%) | 1 (2.0%) | |
| Lymph nodes | 2 (5.0%) | 1 (2.0%) | |
| Other | 2 (5.0%) | 4 (8.0%) | |
| Previous treatment | 0.71 | ||
| None | 5 (12.5%) | 5 (10.0%) | |
| Local therapy (surgery, radiotherapy, ablation) | 1 (2.5%) | 3 (6.0%) | |
| Systemic therapy | 31 (77.5%) | 31 (62.0%) | |
| Combination of local and systemic treatment | 3 (7.5%) | 11 (22.0%) | |
| Volumes | |||
| GTV (cm3) | 18.4 (1.3 – 195.0) | 14.5 (1.0 – 157.3) | 0.31 |
| PTV (cm3) | 86.5 (19.0 – 481.6) | 66.0 (18.4 – 400.4) | 0.094 |
| Prescribed dose | |||
| Dose per fraction (Gy) | 12.5 (12.0 – 15.0) | 20.0 (12.0 – 20.0) | <0.001 |
| Total dose (Gy) | 37.50 (36.0 – 45.0) | 60.0 (51.0 – 60.0) | <0.001 |
| Biologically Effective Dose | |||
| BED98% (Gy) | 77.6 (8.1 – 93.1) | 175.2 (103.7 – 355.8) | <0.001 |
| BED2% (Gy) | 143.3 (71.1 – 336.8) | 300.8 (143.6 – 347.5) | <0.001 |
| Relative near-minimum PTV dose (%) | 90.9 (16.0 – 98.84) | 94.6 (40.0 – 98.8) | 0.22 |
Data are reported as number (percent) or median (range) Abbreviations: GTV: gross tumor volume. PTV: planned target volume. BED98% = near minimum BED. BED2% = near-maximum BED.
3.2. Local control
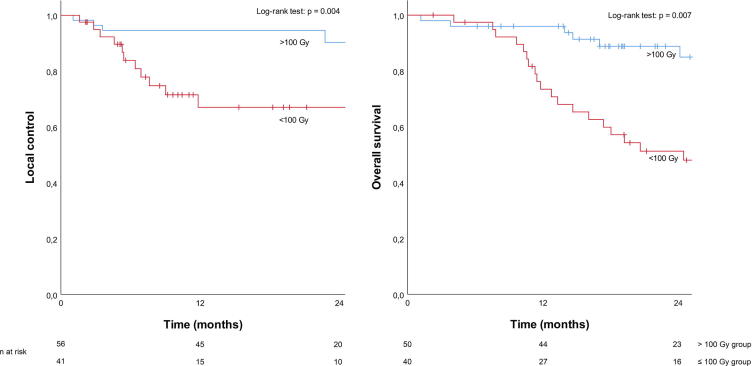
The one-year LC rate in the ≤100 Gy group was 67.0% (CI 95%: 50.5–83.5%) and in the >100 Gy group 94.6% (CI 95%: 88.9% − 100.0%). Two-year LC rates in these groups were 60.3% (CI 95%: 40.9–79.7%) and 90.3% (CI 95%: 80.3–100.0%), respectively. A significant difference was found between the two groups (p = 0.004) (Fig. 1).
Fig. 1.
Kaplan Meier curves for local control and overall survival.
In univariable Cox regression analyses, a larger GTV was significantly associated with a worse LC (HR: 1.02; p = 0.001) (Table 2). Group dose was a significant predictor of LC (HR: 4.20; p = 0.007). Multivariable Cox proportional hazard analysis was performed to evaluate whether the association of dose group with LC remained significant after adjusting for tumor volume. Since dose group (low vs high dose) is correlated to the BED2% and BED98 %, these were not included in the model. In multivariable analyses, both dose group and GTV variables were significant predictors for LC (p = 0.017 and p = 0.0046).
Table 2.
Univariable and multivariable analyses for local control and overall survival.
| Local control |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Univariate analysis | ||||||
| Group dose (≤100 Gy vs. >100 Gy) | 4.20 | 1.47 – 11.98 | 0.007 | 2.67 | 1.34 – 5.33 | 0.005 |
| Age at treatment (continues) | 1.00 | 0.95 – 1.05 | 0.99 | 1.00 | 0.97 – 1.04 | 0.80 |
| Primary tumor (CRC vs. other) | 2.09 | 0.48 – 9.17 | 0.33 | 0.76 | 0.37 – 1.55 | 0.45 |
| Extrahepatic disease (present vs. absent) | 0.91 | 0.32 – 2.57 | 0.85 | 1.16 | 0.58 – 2.30 | 0.67 |
| Prior chemotherapy (Yes vs. no) | 1.49 | 0.55 – 4.02 | 0.43 | 1.04 | 0.54 – 1.99 | 0.91 |
| GTV (cm3) | 1.02 | 1.01 – 1.03 | 0.001 | 1.02 | 1.01 – 1.02 | <0.001 |
| BED98% (Gy) per 10 Gy | 0.90 | 0.78 – 0.95 | 0.013 | 0.90 | 0.84 – 0.99 | <0.001 |
| BED2% (Gy) per 10 Gy | 0.94 | 0.85 – 0.97 | 0.029 | 0.95 | 0.91 – 0.99 | 0.012 |
| Relative near-min. PTV dose | 0.99 | 0.97 – 1.01 | 0.20 | 0.99 | 0.90 – 1.01 | 0.27 |
| Infield recurrencea | 3.55 | 1.77 – 7.13 | <0.001 | |||
| Multivariate analysis | ||||||
| Group dose (≤100 Gy vs. >100 Gy) | 3.61 | 1.25 – 10.40 | 0.017 | 2.38 | 1.16 – 4.90 | 0.005 |
| Age at treatment (continues) | ||||||
| Primary tumor (CRC vs. other) | ||||||
| Extra hepatic disease (present vs. absent) | ||||||
| Prior chemotherapy (Yes vs. no) | ||||||
| GTV (cm3) | 1.01 | 1.00 – 1.02 | 0.005 | 1.01 | 1.01 – 1.02 | <0.0001 |
| BED98% (Gy) per 10 Gy | ||||||
| BED2% (Gy) per 10 Gy | ||||||
| Relative near-min. PTV dose | ||||||
Abbreviations: HR = hazard ratio. CI = Confidence Interval. CRC = colorectal cancer. GTV = Gross Tumor Volume. BED = Biologically Effective Dose. BED98% = near-minimum BED. BED2% = near-maximum BED. PTV = Planning Target Volume.
Infield recurrence as a time dependent variable.
3.3. Toxicity
SBRT was well tolerated. There was no significant difference in grade ≥ 3 treatment related-toxicity between both groups (p = 0.23). Acute toxicity grade 3 was observed in 2 patients of the ≤100 Gy group and in 1 patient of the >100 Gy group, all consisting of biliary obstruction. One patient (BED98%: 56 Gy; BED2%: 141 Gy) developed fibrosis of the biliary ducts requiring a biliary stent. The other 2 patients (BED98%: 32 Gy and 107 Gy; BED2%: 245 Gy and 275 Gy) suffered from local edema near the biliary ducts and received pharmacological treatment with dexamethasone. Late toxicity grade 3 was observed in 1 patient (BED98%: 79 Gy and BED2%: 123 Gy) in the ≤100 Gy group who developed a cholecystitis caused by biliary fibrosis after which a cholecystectomy was performed. In both groups, grade 4 or 5 treatment-related toxicity was not observed.
3.4. Overall survival
Median OS was 32.3 months for all patients. The one and two-year OS rates in the ≤100 Gy group were 70.8% and 48.1% (CI: 56.2–85.3%; 31.6–64.6%), respectively. In the >100 Gy group the one and two-year OS rates were 96.0% and 85.0% (CI: 90.5–100%; 73.4–96.6%). A significant difference was observed between the groups (p = 0.007) (Fig. 1).
Univariable analyses showed that group dose, BED2% and BED98% had a significant impact on OS (Table 2) (p = 0.005, p = <0.001 and p = 0.012). A larger tumor volume (GTV) was associated with a worse OS (p = <0.0001). Infield recurrence significantly increased the chance of death (HR: 3.55; 95% CI: 1.77 – 7.13; p = <0.001). In multivariable analyses, dose group and GTV were significant predictors for OS (p = 0.019 and p = <0.0001).
4. Discussion
This study shows that high dose image-guided SBRT provides significantly better local control when treating liver metastases than low dose SBRT. Overall survival was also significantly better in the >100 Gy dose group compared to the ≤100 Gy dose group. Treatment was furthermore well tolerated with similar toxicity rates in both groups.
Previous studies investigated SBRT in the treatment of liver metastases and showed promising results [17], [23], [24]. A small phase I study of Rule et al. was one of the first that investigated dose escalation for liver metastases [14]. The authors included 27 patients who were divided into 3 cohorts who were treated with 30 Gy in 3 fractions, 50 Gy in 5 fractions and 60 Gy in 5 fractions. The two-year local control rates were 56%, 89%, and 100% respectively. Grade 3–5 toxicity was not observed. A significant difference in local control was only seen comparing the 30 Gy and 60 Gy group. More recently, a systematic review of liver SBRT has been published in which local control rates and the effect of dose regimen on local control was evaluated [25]. Thirteen articles - including 290 patients with liver metastases - were included. They showed that local control rates were significantly better for metastatic lesions treated with BED’s >100 Gy compared to lesions treaded with BED’s <100 Gy. In the current study, local control rates were concordant, and the difference in local control between the >100 Gy group and the ≤100 Gy group was statistically significant. Consistent with these previous studies, toxicity rates remained similar regardless of the dose escalation.
In the current study, local control is correlated with overall survival. Conflicting results on the relation between local control and overall survival have been published. In some studies, local control of the liver metastases did not affect overall survival [23], [26], [27]. While other studies reported improved overall survival after adequate local control. The study of McPartlin et al. reported the long-term results of 60 patients treated with SBRT in previous phase 1 and 2 studies [28]. This study showed that infield progression after SBRT was significantly associated with overall survival (HR 2.46; P = <0.001). Similar results were published by Joo et al. who reported that local recurrence after SBRT is associated with death with an HR of 4.95 (p = <0.01) [29].
In the Netherlands Cancer Institute, liver metastases are treated with SBRT when other treatment options are not feasible. Patients can be medically not fit enough for surgery due to age or bad performance state or have inoperable metastases due to anatomic location. Thermal ablation is another common alternative to treat lesions in these patients but this technique is also limited in frail patients since it is an invasive treatment, requiring hospital admission and complete anesthesia. Despite the population treated with SBRT is expected to have worse baseline characteristics, the median overall survival of all patients was 32.3 months. This is remarkable as patients selected for SBRT were often old, frail or had recurrent disease. This study shows that SBRT is a good modality to treat liver metastases in a selected population, with the benefit of treating patients in an outpatient setting.
Whether thermal ablation or SBRT is superior for local treatment of liver metastases remains unclear. The ultimate way to compare these two treatments would be with prospective randomized trials. Unfortunately, some randomized controlled trials tried and were closed prematurely due to insufficient recruitment (NCT01233544). It is unlikely that they will occur in the near future. A recent study retrospectively compared the treatment of liver metastases with SBRT (n = 170) and radiofrequency ablation (RFA) (n = 112) [30], [31]. Both treatments provided excellent and similar local control rates for lesions <20 mm. For lesions >20 mm, SBRT was correlated with better local control. Therefore it was concluded that treatment with SBRT might be favorable over RFA in lesions larger than 20 mm. A drawback of this study, besides the retrospective nature, was that <25% of the patients treated with SBRT had lesions >40 mm. Therefore, SBRT local control rates of lesions larger than 40 mm remain unknown.
The present study had several limitations. These include selection bias, inclusion of multiple tumor types and development of treatments over time. Selection bias is inherently present in a retrospective study. The authors chose to include consecutive patients with metastases from various tumors. Tumor biology will influence local control and survival, and tumor biology certainly correlates to tumor origin [32]. Comparing two sequential historical cohorts does not correct for all other changes over time in oncology including indications for local treatment (i.e. surgical, thermal or radiation) and indication for systemic treatment and systemic treatment options. Over the past decade, some biologicals have become available for several tumors. Furthermore, imaging and radiotherapy technology has improved in the last decade but it remains unknown what the impact of these changes on local control and survival are.
This study shows that high dose SBRT provides significantly better local control rates in patients with liver metastases compared to low dose SBRT. Survival is associated with tumor volume and dose escalation. Toxicity rates are low and did not increase after dose escalation. When surgical resection is not feasible, high dose SBRT in patients with liver metastases is safe and effective.
Funding
The author(s) received no specific funding for this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Doherty G.M. 14th ed. 2006. CURRENT Diagnosis & Treatment: Surgery. [Google Scholar]
- 2.van der Pool A.E., Damhuis R.A., Ijzermans J.N. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis. 2012;14(1):56–61. doi: 10.1111/j.1463-1318.2010.02539.x. [DOI] [PubMed] [Google Scholar]
- 3.Zaydfudim V.M., McMurry T.L., Harrigan A.M. Improving treatment and survival: a population-based study of current outcomes after a hepatic resection in patients with metastatic colorectal cancer. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2015;17(11):1019–1024. doi: 10.1111/hpb.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillams A.R., Lees W.R. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19(5):1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 5.Groeschl R.T., Pilgrim C.H., Hanna E.M. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259(6):1195–1200. doi: 10.1097/SLA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 6.Beermann M., Lindeberg J., Engstrand J. 1000 consecutive ablation sessions in the era of computer assisted image guidance - Lessons learned. Eur J Radiol Open. 2018;6:1–8. doi: 10.1016/j.ejro.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhim H., Goldberg S.N., Dodd G.D. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics. 2001;21(suppl_1):S17–S35. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 8.Shady W., Petre E.N., Gonen M. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillams A., Goldberg N., Ahmed M. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence T.S., Robertson J.M., Anscher M.S. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31(5):1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 11.Katz A.W., Carey-Sampson M., Muhs A.G. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67(3):793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 13.Lee M.T., Kim J.J., Dinniwell R. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27(10):1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 14.Rule W., Timmerman R., Tong L. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18(4):1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 15.Palma D.A., Olson R., Harrow S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 16.Scorsetti M., Arcangeli S., Tozzi A. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys. 2013;86(2):336–342. doi: 10.1016/j.ijrobp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Dewas S., Bibault J.E., Mirabel X. Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol. 2012;7:166. doi: 10.1186/1748-717X-7-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruis M.F., van de Kamer J.B., Sonke J.J. Registration accuracy and image quality of time averaged mid-position CT scans for liver SBRT. Radiother Oncol. 2013;109(3):404–408. doi: 10.1016/j.radonc.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 19.Common terminology criteria for adverse events (CTCAE) v.4.0. [cited 2018 July 2018]; Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 21.Wilke L., Andratschke N., Blanck O. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams : Statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. Strahlenther Onkol. 2019;195(3):193–198. doi: 10.1007/s00066-018-1416-x. [DOI] [PubMed] [Google Scholar]
- 22.Report 91. Journal of the International Commission on Radiation Units and Measurements, 2017. 14(2): p. 1-160. [DOI] [PubMed]
- 23.Andratschke N., Alheid H., Allgäuer M. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer. 2018;18 doi: 10.1186/s12885-018-4191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scorsetti M., Comito T., Tozzi A. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141(3):543–553. doi: 10.1007/s00432-014-1833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohri N., Tome W.A., Mendez Romero A. Local control after stereotactic body radiation therapy for liver tumors. Int J Radiat Oncol Biol Phys. 2018 doi: 10.1016/j.ijrobp.2017.12.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klement R.J., Abbasi-Senger N., Adebahr S., Alheid H., Allgaeuer M., Becker G., Blanck O., Boda-Heggemann J., Brunner T., Duma M., Eble M.J., Ernst I., Gerum S., Habermehl D., Hass P., Henkenberens C., Hildebrandt G., Imhoff D., Kahl H., Klass N.D., Krempien R., Lewitzki V., Lohaus F., Ostheimer C., Papachristofilou A., Petersen C., Rieber J., Schneider T., Schrade E., Semrau R., Wachter S., Wittig A., Guckenberger M., Andratschke N. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer. 2019;19(1) doi: 10.1186/s12885-019-5362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fode M.M., Hoyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114(2):155–160. doi: 10.1016/j.radonc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 28.McPartlin A., Swaminath A., Wang R. Long-Term Outcomes of Phase 1 and 2 Studies of SBRT for Hepatic Colorectal Metastases. Int J Radiat Oncol Biol Phys. 2017;99(2):388–395. doi: 10.1016/j.ijrobp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Joo J.H., Park J.H., Kim J.C. local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99(4):876–883. doi: 10.1016/j.ijrobp.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Jackson W.C., Tao Y., Mendiratta-Lala M. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiation Oncol Biol Phys. 2018;100(4):950–958. doi: 10.1016/j.ijrobp.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franzese C., Comito T., Clerici E. Liver metastases from colorectal cancer: propensity score-based comparison of stereotactic body radiation therapy vs. microwave ablation. J Cancer Res Clin Oncol. 2018 doi: 10.1007/s00432-018-2692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klement R.J., Guckenberger M., Alheid H. Stereotactic body radiotherapy for oligo-metastatic liver disease - Influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol. 2017;123(2):227–233. doi: 10.1016/j.radonc.2017.01.013. [DOI] [PubMed] [Google Scholar]