Summary
We utilize polymer micelle to precisely control indocyanine green (ICG) J-aggregation in a fast and highly efficient way. In addition to simple encapsulation, the polymer micelle plays a role as a host template to drive ICG J-aggregation by the synergy of electrostatic and hydrophobic attractions. We further demonstrate that, due to the robust host-guest interaction, the intact of ICG J-aggregate will be secured by the polymer encapsulation during the intracellular and in vivo incubation. These features make this hierarchical assembly between ICG J-aggregate and the micelle polymer a promising biomedicine for cancer phototheranostics. Therefore the complex micelles are further modified by introduction of doxorubicin for chemotherapy and DNA aptamer for tumor targeting, and the final multi-functional micellar medicine shows high therapeutic efficacy for tumor, i.e., the tumor can be completely eliminated with no local reoccurrence and without long-term toxicity or side effects during a 24-day period after the treatment.
Subject Areas: Medical Imaging, Organic Chemistry, Materials Science
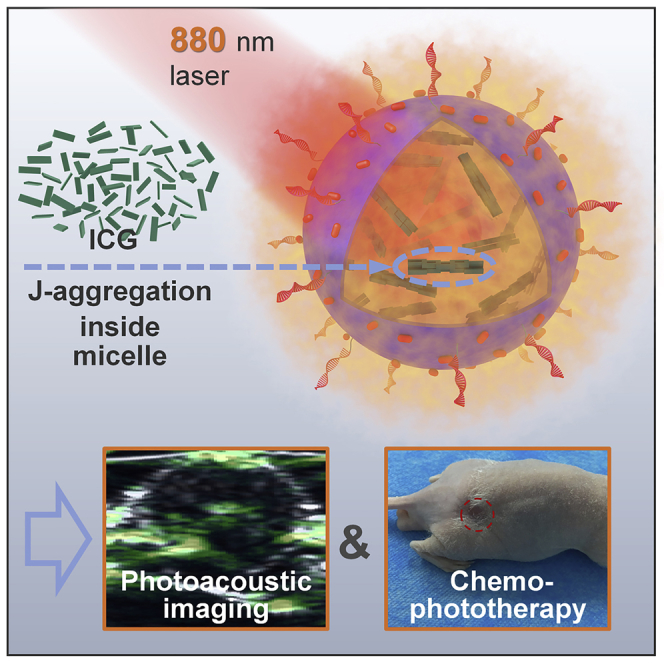
Graphical Abstract
Highlights
-
•
J-aggregation of ICG is facilitated by polymer micelle
-
•
Proper host-guest interactions are very critical
-
•
The aggregation significantly improves the capability of ICG in phototheranostics
-
•
The hierarchical assembly exhibits excellent photo/bio-stability
Medical Imaging; Organic Chemistry; Materials Science
Introduction
Nature has created remarkable examples of dye assembly that are vital for sustaining life. For instance, the formation of chlorophyll J-aggregates can afford intense near-infrared (NIR) absorption up to ∼900 nm, which enables the more efficient light harvesting of sunlight (Sengupta and Würthner, 2013). Organic biomedical materials have been quickly developed in recent years because of their biodegradability, robust biocompatibility, and long-term biological safety (Song et al., 2015a, Song et al., 2015b, Pu et al., 2014, Qi et al., 2017, Zheng et al., 2013). During the process, the supramolecular strategy plays an increasingly important role as weak noncovalent interactions will influence the properties of organic biomedicines by controlling the molecular packing structure and self-assembly morphology (Gorl et al., 2012, Yin et al., 2019, Varughese, 2014, Su et al., 2019, Cai et al., 2018a, Cai et al., 2018b, Cai et al., 2018c). For instance, Zheng and co-workers developed porphyrin-lipid conjugates, which self-assembled into porphysome. Although porphyrin has been extensively investigated in biomedical applications, the porphysome shows distinguished optical properties with improved performance in photothermal therapy (PTT) and photoacoustic imaging (PAI) (Lovell et al., 2011). In another example, supramolecular complex between cucurbit[7]uril and organic conjugated radical was developed by Zhang and co-workers; the organic radical itself suffers from radical annihilation caused by π-π stacking and its NIR absorption would be impaired. Accordingly, in addition to functioning as a drug delivery vehicle, the macrocycle acted as a shield to preserve the organic radical and its absorption, finally making the complex applicable in PTT (Jiao et al., 2015).
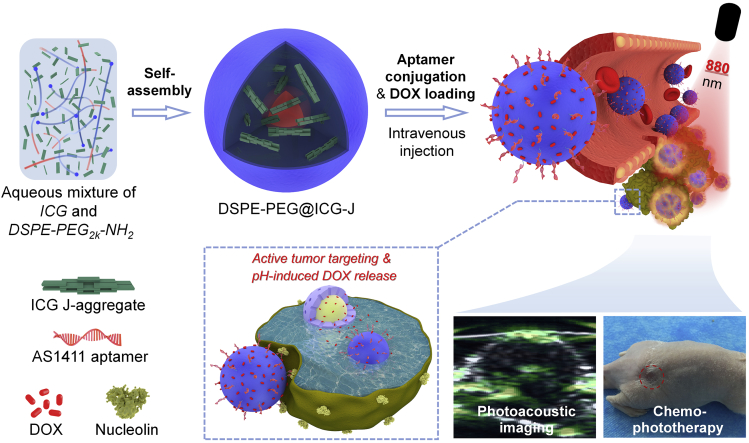
The NIR cyanine dyes with long-wavelength absorption and high extinction coefficient show great potential in phototheranostics nowadays (Luo et al., 2011, Porcu et al., 2016, Guo et al., 2014). However, as they are vulnerable to photo-irradiation and the complex biological environment (Cai et al., 2018a, Cai et al., 2018b, Cai et al., 2018c, Sheng et al., 2013), cyanine dyes are generally complexed with other materials for practical applications, and polymer encapsulation is one of the approaches (Zheng et al., 2013, Song et al., 2014, Song et al., 2015a, Song et al., 2015b). Herein, we report an example of advanced supramolecular medicine between amphiphilic polymer DSPE-PEG2k-NH2 and indocyanine green (ICG), a representative cyanine dye, in which the beneficial interactions between the polymer carrier and dye bring superior properties to the complex system (Scheme 1). Rather than a simple encapsulation, these polymer micelles can induce the guest ICG molecules to a complete J-aggregation and enhance their optical properties for phototheranostic applications, i.e., the micelle-encapsulated ICG J-aggregate showed a deeper NIR absorption (a redshift from 780 to 895 nm) with a higher absorption coefficient (78.0 L·g−1·cm−1 versus 45.0 L·g−1·cm−1 of free ICG), higher penetration capability, and enhanced photothermal effect and photostability. Recently, the construction of aggregates by noncovalent interaction has been proved to be an effective strategy to improve the function and stability of organic dyes (Song et al., 2016, Gaufres et al., 2014, Eisele et al., 2012). Moreover, compared with random aggregation, dye J-aggregate with regular head-to-tail molecular packing featuring a deeper and stronger absorption (Coles et al., 2010) has attracted extensive attentions (Wurthner et al., 2011, Cai et al., 2018a, Cai et al., 2018b, Cai et al., 2018c, Kim et al., 2018, Walker et al., 2011, He et al., 2017, Chen et al., 2017, Zhao et al., 2008, Horn and Rieger, 2001, Huang et al., 2015). However, the formation of dye J-aggregate and the subsequent biomedical application are challenging, which requires a long formation time at a high concentration and lacks structural stability for in vivo application (Liu et al., 2017, von Berlepsch et al., 2007, Egawa et al., 2007, von Berlepsch and Bottcher, 2010). In the system that we explored in this article, facilitated by the host-guest interactions of polymer micelles, the ICG molecules in dilute solution can convert to complete J-aggregate within 8 h. What's more, the ICG J-aggregate in the supramolecular assembly showed robust stability under intracellular and in vivo incubation, making this material more accessible to biomedical applications. To the best of our knowledge, there is rare report on the precise control of dye aggregation by adjusting the host-guest interactions between the polymer carrier and the encapsulated dye. As another benefit of micelle encapsulation, the as-fabricated complex micelle can be functionalized conveniently. In this work, to reach a better therapeutic effect, the negative charge and functional groups of the complex micelle with ICG J-aggregate in encapsulation were employed to introduce doxorubicin (DOX) through electrostatic interaction for chemotherapy and to covalently conjugate DNA aptamer for active tumor targeting (Scheme 1). The invivo anticancer experiment proved that the final multi-functional micellar medicine, which combined J-aggregation-enhanced PTT, aptamer targeting and enhanced permeability and retention (EPR)-promoted tumor enrichment, and chemotherapy based on DOX sustained release, accomplished complete tumor elimination with no local reoccurrence within 24 days. Last but not the least, in addition to the superior therapeutic performance, this micellar medicine showed reliable biocompatibility without long-term toxicity and side effects.
Scheme 1.
Illustration of the Formation of the Supramolecular Medicine Based on Polymer Micelle and ICG J-aggregate and Its Application in PAI-Guided Chemo-phototherapy
See also Table S1.
Results and Discussion
Fabrication and Characterization of DSPE-PEG@ICG-J
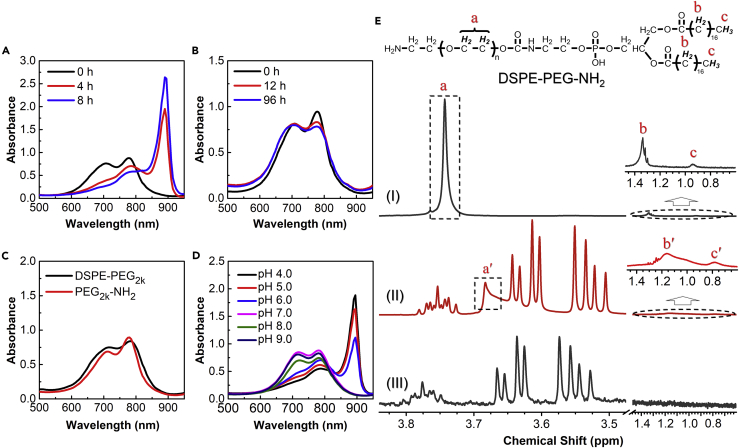
ICG aqueous solution showed two absorption peaks at 715 and 780 nm from its monomer and dimer, respectively. Occasionally, we observed an interesting phenomenon that, after being co-dissolved with phospholipid-poly(ethylene glycol), DSPE-PEG2k-NH2, in ultrapure water and stirred at room temperature for 8 h, ICG showed significantly changed absorption with a shaper and red-shifted peak at 895 nm (Figures 1A and S1A), which indicates the formation of ICG J-aggregate (Liu et al., 2017). However, without the presence of the micelle polymer, ICG under the same concentration (50 μg·mL−1) did not convert to J-aggregate at all, even after a long incubation of 96 h (Figure 1B), implying the important role of the micelle polymer. In the further study, we observed that ICG J-aggregate did not form with DSPE-PEG polymer without the amine functionalization (Figures 1C and S1B). It indicated that the positive charge of amine groups probably played an important role. Therefore, we modulated the charge of amine groups by adjusting the pH value. As shown in Figure 1D, under neutral or alkaline conditions, no ICG J-aggregate formed after an 8-h incubation at room temperature; on the contrary, the majority of free ICG converted to J-aggregate under acidic conditions, and the more acidic the buffer was, the higher the conversion yield achieved. All the results demonstrated that the electrostatic interaction between the positive charge of amine groups and the negatively charged ICG probably is critical for ICG J-aggregation.
Figure 1.
ICG J-aggregation under Different Conditions and 1H NMR Characterization
(A–E) Monitoring ICG J-aggregation (A) in the presence of DSPE-PEG2k-NH2 (0.2 mg·mL−1) with a 15-min 80°C heating before room temperature stirring in water, (B) in the absence of additive with room temperature stirring in water, (C) in the presence of DSPE-PEG2k or PEG2k-NH2 (0.2 mg·mL−1) with a 24-h room temperature stirring in water, and (D) in the presence of DSPE-PEG2k-NH2 (0.2 mg·mL−1) with room temperature stirring in buffers with various pH values. In these experiments, the concentration of ICG was 50.0 μg·mL−1. (E) 1H NMR spectra of (I) DSPE-PEG2k-NH2 micelle, (II) DSPE-PEG2k-NH2 micelle with ICG J-aggregate in encapsulation, and (III) naked ICG J-aggregate. See also Figures S1–S3.
In addition to the electrostatic interaction, the role of micellar structure formed by DSPE-PEG2k-NH2 was studied. As a control experiment, PEG2k-NH2 (both at 0.1–0.3 mg·mL−1), which are positively charged molecules but without micellar structure, were incubated with ICG under the same condition as for DSPE-PEG2k-NH2. However, no conversion trend was observed (Figures 1C and S1C). This result indicated that the micellar structure would also be necessary for the ICG J-aggregation. Subsequently, the location of ICG J-aggregates in DSPE-PEG2k-NH2 micelles was analyzed by 1H NMR. As shown in Figures 1E and S2, for the spectrum I of pure DSPE-PEG2k-NH2 micelles, the resonances of the hydrophobic DSPE core (b and c) and of the hydrophilic PEG shell (a) were well resolved; also, spectrum III of the naked ICG J-aggregates showed sharp resonances. In spectrum II, for the complex between DSPE-PEG2k-NH2 micelles and ICG J-aggregates, there were a set of resonance peaks showing the same pattern as that of the naked ICG J-aggregates but locating at relatively higher field; meanwhile, the resonances of both the DSPE core and the PEG shell of the DSPE-PEG2k-NH2 micelle became broader and shifted to a higher field region (a′, b′, and c′). All these resonance changes could be attributed to the shielding effect induced by guest encapsulation (Zhao et al., 2008, Hu et al., 2009), which fully demonstrated that the ICG molecules were encapsulated in the DSPE-PEG2k-NH2 micelles under the aggregated state.
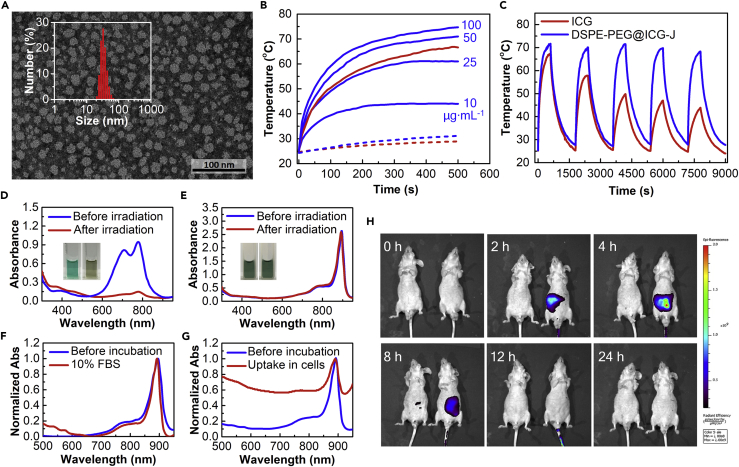
Above all, considering the amphiphilic nature of ICG, it can be arranged in the hydrophilic-hydrophobic interface of the micelle after mixing with the amphiphilic DSPE-PEG (Wan et al., 2014). In other words, the hydrophobic core of micelle will attract the head of ICG inside the micellar structure through hydrophobic interaction. At the same time, the positive charges on the micelle outer surface will attract the negatively charged tail of ICG. As a result, the two forces in opposite directions could preset the orientation of ICG in a head-to-tail fashion and cause the ICG J-aggregation. According to the previous studies, naked ICG J-aggregate requires a long formation time. A high concentration of ICG (∼5 mg·mL−1) in water could gradually convert to J-aggregate over 1 month (von Berlepsch and Bottcher, 2010), indicating it was a thermodynamically controlled process in the absence of any external forces. We also found that no J-aggregate formed when an aqueous solution of pure ICG at a high concentration (10.0 mg·mL−1) was stirred at room temperature for 24 h, whether at pH 4.0 or 7.0 (Figure S3). Here, a kinetically driven J-aggregation of ICG has been revealed by utilizing the host-guest interaction between polymer micelle and ICG molecules, which will significantly facilitate the process. The obtained micellar assembly between ICG J-aggregate and DSPE-PEG2k-NH2 is denoted as DSPE-PEG@ICG-J. The DSPE-PEG@ICG-J loaded with 33 wt. % ICG J-aggregates was chosen for the following experiments based on careful optimizations (Figure S4). Transmission electron microscopy and dynamic light scattering detections revealed that it was a spherical micelle with an average hydrodynamic diameter of 34.6 nm (Figure 2A), and its zeta potential was −19.3 mV owing to the presence of negatively charged ICG J-aggregates (Liu et al., 2017).
Figure 2.
Micelle Morphology, In Vitro Photothermal Properties, as well as Photo- and Bio-stability of DSPE-PEG@ICG-J
(A) Transmission electron microscopic image and dynamic light scattering analysis result (inset) of DSPE-PEG@ICG-J.
(B) Temperature profiles of PBS buffer (blue dashed line) and DSPE-PEG@ICG-J solutions at different concentrations (blue solid line) under the irradiation of 880-nm laser (0.8 W·cm−2), as well as of PBS buffer (red dash) and free ICG at 50 μg·mL−1 (red solid line) under the irradiation of 808-nm laser (0.8 W·cm−2).
(C–G) (C) The temperature profiles of DSPE-PEG@ICG-J and free ICG at 50 μg·mL−1 under five cycles of laser on-off (0.8 W·cm−2; 880-nm laser for DSPE-PEG@ICG-J; 808-nm laser for free ICG). The absorption spectra of (D) free ICG and (E) DSPE-PEG@ICG-J before and after 10-min laser irradiation, and the inserts show the before (left) and after (right) photographs. The stability of DSPE-PEG@ICG-J when incubated in (F) 10% fetal bovine serum or (G) with A549 cells.
(H) The fluorescence profiles of DSPE-PEG@ICG-J (left) and free ICG (right) after they were intravenously injected into mice (150.0 μL, at ICG equivalent concentration of 0.5 mg·mL−1).
See also Figures S4 and S5.
The aggregated ICG in DSPE-PEG@ICG-J showed a sharp absorption at 895 nm, therefore, in contrast to an 808-nm laser generally used for ICG irradiation, an 880-nm laser can be employed to irradiate DSPE-PEG@ICG-J in the related applications. The residual energy of the two beams, from an 808-nm and an 880-nm laser, were compared after they passed through pork slides with different thicknesses (Table S1), where the 880-nm laser exhibited a much better tissue penetration capability. The extinction coefficient by weight of ICG J-aggregates in DSPE-PEG@ICG-J was determined to be 78.0 L·g−1·cm−1 at 880 nm, which was much higher than that of free ICG at 808 nm (45.0 L·g−1·cm−1) and many other photothermal materials, such as reduced graphene (∼22.0 L·g−1·cm−1, 808 nm), single-walled carbon nanotubes (∼46.5 L·g−1·cm−1, 808 nm), and gold nanorods (∼13.9 L·g−1·cm−1, 808 nm) (Robinson et al., 2010, Robinson et al., 2011). Meanwhile, DSPE-PEG@ICG-J showed negligible fluorescence (Figure S5), which probably came from the super-quenching effect of J-aggregates (Lu et al., 2002). Both the enhanced absorption and fluorescence quenching can facilitate the energy conversion from light to heat, therefore at 50 μg·mL−1 equivalent concentration of ICG, the temperature of DSPE-PEG@ICG-J solution increased from 25.3°C to 71.7°C (ΔT ∼ 46.4°C) after a 10-min irradiation at 880 nm, whereas that of free ICG solution increased from 25.2°C to 67.1°C (ΔT ∼ 41.9°C) under 808 nm irradiation (Figure 2B). In addition to the superior optical properties, DSPE-PEG@ICG-J also exhibited excellent photostability. After five cycles of irradiation on-off process, DSPE-PEG@ICG-J held a consistent photothermal conversion capability, whereas free ICG (50 μg·mL−1) could only elevate 18°C in the end (Figure 2C). We noted that, after a 10-min laser irradiation, the absorption of free ICG decayed significantly, indicating the degradation of ICG molecules (Figure 2D); however, under the same irradiation time, the absorption spectrum of DSPE-PEG@ICG-J showed no visible change (Figure 2E).
The naked ICG J-aggregate suffers from poor intracellular and in vivo bio-stability (Liu et al., 2017), which was completely overcome by DSPE- PEG@ICG-J. After a 24-h incubation in 10% fetal bovine serum (mimicking the biological condition) or a 12-h incubation with A549 cells, the characteristic absorption of ICG J-aggregate in DSPE-PEG@ICG-J showed no obvious change (Figures 2F and 2G), demonstrating that the molecular structure and the aggregation state of ICG were well reserved. The in vivo structural integration of DSPE-PEG@ICG-J was investigated through intravenous injection in mice, and the mice injected with free ICG were tested as control. Emitting NIR fluorescence at 845 nm, free ICG can be detected by in vivo fluorescence imaging, which is used to indicate the disassembly of ICG J-aggregates in animal body. For the mice injected with free ICG, obvious fluorescence was detected post injection and before the complete metabolism. On the other hand, for the mice injected with DSPE-PEG@ICG-J, no detectable fluorescence was observed during a 24-h period (Figure 2H). Thus the ICG J-aggregates in DSPE-PEG@ICG-J complex micelles show robust stabilities under intracellular and in vivo incubations.
Compared with the naked ICG J-aggregate, DSPE-PEG@ICG-J is convenient to be modified for multi-functionalization, which is another benefit of the hierarchical assembly. First, the amine groups on the PEG shell, which were necessary for the formation of ICG J-aggregate, were utilized to conjugate cancer-targeting ligand after the formation of the complex micelle. A DNA aptamer, AS1411, was selected, which could recognize nucleolin, a specific cellular protein commonly overexpressed on cancer cell membranes (Li et al., 2017). Through NHS/EDC amidation reaction, a high density of AS1411 aptamers (∼114 μmol·g−1) could be coupled on the surface of DSPE-PEG@ICG-J (the product was named as DSPE-PEG-1411@ICG-J). Second, due to the negatively charged surface of the complex micelle, a large quantity of positively charged DOX for chemotherapy could be loaded through electrostatic interactions (∼0.2 g DOX on 1.0 g of DSPE-PEG@ICG-J) (Scheme 1). We found that the AS1411 conjugation and DOX loading did not alter the aggregation state of ICG (Figure S6A). The fully functionalized micelle was named as DSPE-PEG-1411@ICG-J/DOX, which showed a hydrodynamic diameter enlarged to 48.4 nm (inset of Figure S6B) and an electrically neutral surface (−1.3 mV). The photothermal properties as well as the photo- and bio-stabilities of this multifunctional micelle were fully characterized according to the same procedures for DSPE-PEG@ICG-J, and its performances were consistent with those of DSPE-PEG@ICG-J in all the tests (Figures S7A–S7D), which demonstrated that the incorporation with DOX and DNA aptamer did not affect the photothermal and structural properties of the complex micelle. Subsequently, DOX release from DSPE-PEG-1411@ICG-J/DOX was monitored over a period of 72 h in PBS buffer (pH 7.4) and HAc-NaAc buffer (pH 5.5), which mimicked the pH values of blood and tumor endosome, respectively (Zheng et al., 2016). The DOX release at pH 5.5 was much quicker than that at pH 7.4; in the end, ∼ 90% DOX was released at pH 5.5, whereas only ∼49% of DOX could be released at pH 7.4 (Figure S7E). These results demonstrated that the DOX release from DSPE-PEG-1411@ICG-J/DOX exhibited a sustained and pH-responsive nature. Taking into account that the in vivo application of the micelles would span several weeks, we further evaluated the long-term stability of the micelles at 37°C for 5 weeks. The PBS dispersions of DSPE-PEG@ICG-J, DSPE-PEG-1411@ICG-J, and DSPE-PEG-1411@ICG-J/DOX all maintained excellent colloidal stability at the endpoint, and there is no significant change in their characteristic absorption and the average hydrodynamic diameter of the micelles (Figure S8).
Anticancer Therapy
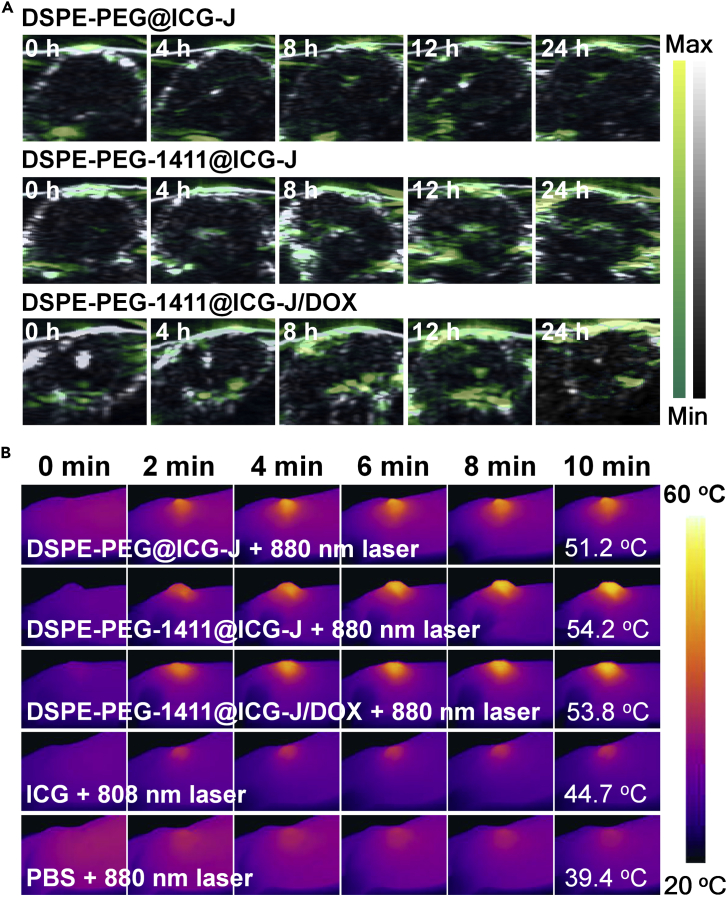
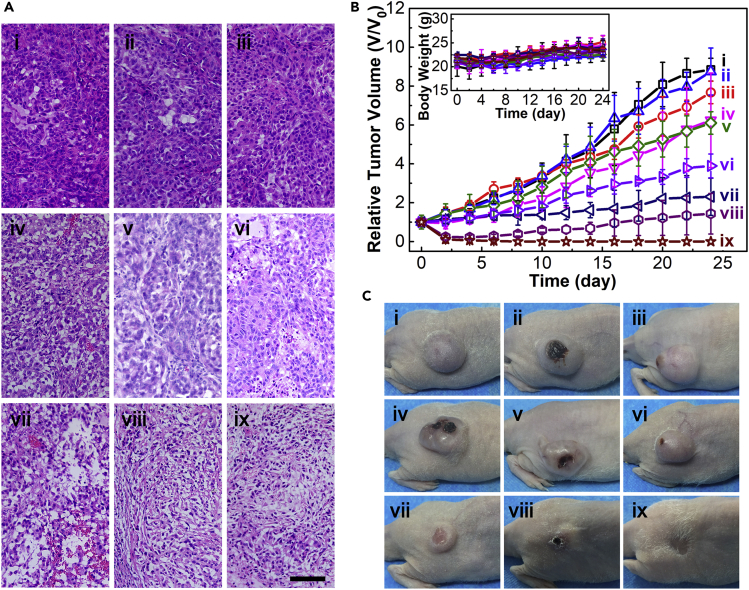
After coupling with AS1411 aptamer, the cell uptake amount of the complex micelles was about 2.6 times higher than that of their unfunctionalized counterpart on human lung carcinoma A549 cell line overexpressing nucleolin on the cell membrane (Figure S9) (Li et al., 2014). Coupling with the active targeting, ICG J-aggregate-dominated PTT, and DOX-dominated chemotherapy, DSPE-PEG-1411@ICG-J/DOX could kill 99.2% A549 cells at an ICG equivalent concentration of 100 μg·mL−1 under a 10-min 880-nm laser irradiation (0.8 W·cm−2) (Figures S10 and S11). Therefore, the in vivo anti-tumor efficacies of the micelles based on ICG J-aggregate were systematically evaluated on A549 tumor-bearing BALB/c nude mice. The tumor accumulations of the micelles following intravenous injection were visualized by PAI. Before the in vivo PAI, the concentration-dependent photoacoustic signal of the micelles had been confirmed by the in vitro studies (Figure S12). All the micelles, DSPE-PEG@ICG-J, DSPE-PEG-1411@ICG-J, and DSPE-PEG-1411@ICG-J/DOX, showed time-dependent accumulation at tumor tissues and reached peak levels at 12 h post the injection (Figure 3A). Therefore, 12 h post administration was an ideal time point for micelles, whereas the PTT time point for ICG was determined to be 1 h after intravenous administration according to the in vivo fluorescence imaging (Figure S13). Subsequently, we examined the tumoral temperature profiles during the in vivo PTT. As shown in Figure 3B, infrared thermography disclosed that the surface temperatures of the tumors in mice treated with DSPE-PEG@ICG-J, DSPE-PEG-1411@ICG-J, and DSPE-PEG-1411@ICG-J/DOX rapidly increased to over 50°C in 4 min and reached peak temperatures of 51.2°C, 54.2°C, and 53.8°C, respectively. In contrast, the highest temperature of the tumor in free ICG-treated mouse reached 44.7°C. The tumor site of PBS-treated mouse showed slight temperature elevation under irradiation, proving no background interference here. It has been reported that strong photothermal heating, which raises the tumor local temperature over 48°C, will result in irreversible tissue damage, whereas the tumor temperature between 40°C and 45°C can only bring sublethal damage to tumor cells (Yoo et al., 2013). This declaration was proved by the histopathological tissue analysis following H&E staining of tumor sections from mice after the PTT treatments. As shown in Figure 4A, compared with free ICG, DSPE-PEG@ICG-J and DSPE-PEG-1411@ICG-J resulted in more necrotic areas at the tumor. Moreover, compared with its counterpart without AS1411 conjugation, DSPE-PEG-1411@ICG-J led to severe tumor damage. Therefore, compared with free ICG, the strengthened light absorption and superior stability of the ICG J-aggregate, as well as the increased tumor accumulation through the EPR effect (Blanco et al., 2015, Stylianopoulos, 2013) and AS1411 active targeting, could indeed improve the PTT performance of the micelles.
Figure 3.
Detecting Tumor Accumulation of the Micelles through PAI and Monitoring Temperature Changes of the Tumors in the In Vivo PTT Process
(A) Time-dependent tumor site photoacoustic images (yellow-green scale) of the A549 tumor-bearing mice intravenously injected with various samples; the tumor boundaries were delineated by ultrasound images (gray scale). A pulsed 880-nm laser was used as excitation source, and the induced photoacoustic waves were collected by a point-focused 7.5-MHz ultrasound transducer.
(B) The infrared (IR) thermography of the tumors from A549 tumor-bearing mice intravenously injected with various samples at different time points after laser irradiation (the power density of both 808- and 880-nm lasers is 0.8 W cm−2). In both the photoacoustic imaging and the IR thermography experiments, the intravenous injection volume of the samples is 150.0 μL; for ICG and the micelles, ICG concentration equivalent to 0.5 mg·mL−1.
See also Table S1, Figures S6–S8, S12, S13, and S18.
Figure 4.
In Vivo Anticancer Therapy Record
(A) H&E-stained slices of tumors collected from A549 tumor-bearing mice 12 h after receiving the treatments. Scale bar, 100 μm.
(B) Tumor growth curves and the body weight (inset) of the mice in different groups; data are presented as the average ± SD (n = 3).
(C) Photographs of mice in different groups at the end of treatments. The samples in (A–C): (i) control (without any treatment); (ii) [-] DOX; (iii) [+] PBS; (iv) [+] ICG; (v) [-] Doxil; (vi) [-] DSPE-PEG-1411@ICG-J/DOX; (vii) [+] DSPE-PEG@ICG-J; (viii) [+] DSPE-PEG-1411@ICG-J; and (ix) [+] DSPE-PEG-1411@ICG-J/DOX; [+] and [-] stand for laser-irradiated and non-laser-irradiated groups, respectively; 808-nm laser was used for group (iv) and 880-nm laser was used for the other groups. The power density of both 808- and 880-nm lasers is 0.8 W cm−2. In the experiment, intravenous injection volume of the samples is 150.0 μL; for ICG and the micelles, ICG concentration equivalent to 0.5 mg·mL−1; for DOX and Doxil, DOX concentration equivalent to 0.3 mg·mL−1.
See also Table S1, Figures S6–S11 and S14–S18.
Finally, A549 tumor-bearing mice were randomly divided into nine groups to comprehensively evaluate the in vivo therapeutic efficacies of the micelles over a 24-day follow-up period. Groups with and without laser irradiation are marked with [+] and [-], respectively; therefore the 9 groups include: (i) control (without any treatment); (ii) [-] DOX; (iii) [+] PBS, 880-nm laser; (iv) [+] ICG, 808-nm laser; (v) [-] Doxil (a clinically approved liposomal doxorubicin formulation); (vi) [-] DSPE-PEG-1411@ICG-J/DOX; (vii) [+] DSPE-PEG@ICG-J, 880-nm laser; (viii) [+] DSPE-PEG-1411@ICG-J, 880-nm laser; (ix) [+] DSPE-PEG-1411@ICG-J/DOX, 880-nm laser. As shown in Figure 4B and 4C and Figures S14A and B, for mice in the two control groups (i) and (iii), the tumors eventually increased 7- to 9-fold in volume. Free ICG in group (iv) exhibited poor photothermal therapeutic efficiency, which was probably due to the instability and quick metabolism of ICG molecules. After the assembly, significantly improved PTT efficiency was observed in group (vii), where the tumor growth rate was greatly inhibited (only ∼2.3-fold increase). Notably, through the introduction of AS1411 aptamer, DSPE-PEG-1411@ICG-J in group (viii) realized complete tumor ablation at the beginning, although tumor reoccurred at the end, which demonstrated the important role of AS1411 aptamer in active targeting to enhance accumulation of micellar medicines inside tumor. As a direct evidence, the bio-distribution determination 12 h after intravenous injection of the micelles indicated that the intra-tumor accumulation efficiency of DSPE-PEG@ICG-J was 3.00% ID·g−1, whereas benefiting from the active targeting ability of AS1411 aptamer, the accumulation amounts of DSPE-PEG-1411@ICG-J and DSPE-PEG-1411@ICG-J/DOX increased to 4.23% and 4.11% ID·g−1, respectively (Figure S14C). Compared with free DOX in group (ii) and Doxil in group (v), the chemotherapeutic efficiency was greatly improved when DOX was loaded on the micelle coupled with AS1411 active targeting in group (vi), which was consistent with the histopathological tissue analysis. Free DOX and Doxil, respectively, caused negligible and slight injury to the tumor, whereas for DSPE-PEG-1411@ICG-J/DOX in group (vi), even without laser irradiation, more visible injury to the tumor was observed (Figure 4A). To the end, [+] DSPE-PEG-1411@ICG-J/DOX in group (ix), which synergistically combined active targeting, PTT, and chemotherapy, displayed the best therapeutic effect, with complete tumor elimination and no local reoccurrence during the 24-day period. The mice in all the treatment groups did not show weight loss (inset of Figure 4B) or other abnormality during the whole process, suggesting that both ICG and micelles showed negligible acute toxicity for in vivo applications. Subsequently, a blood circulation analysis of DSPE-PEG-1411@ICG-J/DOX was conducted on Sprague Dawley rats; the result showed that the half-life time of the micelle in blood was ∼6.5 h (Figure S15), whereas that of ICG is just 2–4 min (Mundra et al., 2015). This long blood circulation property can strengthen the tumor accumulation of the micelle through EPR effect (Robinson et al., 2012). Furthermore, we found that one week after the intravenous injection of DSPE-PEG-1411@ICG-J/DOX, there was almost no ICG in the main organs of mice (Figure S16), which means the micelles had been completely metabolized and would have no potential toxicity to the mice. The histopathological analysis and the blood routine test on the healed mice in group (ix) confirmed this inference. We found that, after the treatment, the mice showed no visible signs of damage in the main organs (Figure S17A) and had normal blood parameters with no statistical difference to those of the healthy mice (Figure S17B). These results convinced that, in addition to the superior therapeutic performance, DSPE-PEG-1411@ICG-J/DOX is also a safe and reliable organic medicine without long-term toxicity and side effects.
Conclusion
We revealed that through a hierarchical assembly with micelle polymer, ICG can efficiently convert to J-aggregate in several hours without a high-concentration requirement. The careful investigation demonstrated that the amphiphilic structure of the micelle and the positive charge on the micelle surface were crucial in this process, which synergistically afforded electrostatic and hydrophobic attractions to the negatively charged amphiphilic ICG molecules, so that the molecules could be concentrated and arranged in the head-to-tail fashion in the micelle. Compared with free ICG, the as-fabricated DSPE-PEG@ICG-J complex micelle exhibited some advanced properties for cancer theranostics, including (1) an 880-nm laser with higher tissue penetration depth could be used for phototheranostics, (2) enhanced photothermal conversion resulted in a higher PTT efficiency, (3) robust photo- and bio-stability, and (4) excellent functional scalability through covalent grafting as well as physisorption. Based on DSPE-PEG@ICG-J, a multifunctional micellar medicine, DSPE-PEG-1411@ICG-J/DOX, was finally fabricated, which combined with J-aggregation-enhanced PTT, aptamer and EPR-effect-promoted tumor enrichment, and chemotherapy based on DOX sustained release, could promote complete tumor elimination with no local reoccurrence and without long-term toxicity or side effects during a 24-day period. To the best of our knowledge, this is the first report of controlling the J-aggregation of a classical clinical dye in polymer micelles to achieve enhanced properties. Like “old wine in a new bottle,” many “old” materials, which have been developed and well studied at the molecular level, will probably show new opportunity at the supramolecular level.
Limitations of the Study
In this study, we first report the method of controlling the J-aggregation of a cyanine dye within amphiphilic polymer micelles; however, only ICG as the cyanine dye model has been investigated. In future work, the applicability of this method to other NIR dyes and amphiphilic polymer will be investigated.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors acknowledge financial support by grants from Shenzhen Fundamental Research Programs (No. JCYJ20160226193029593, No. JCYJ20170817105645935), Shenzhen Science and Technology Innovation Commission (Grant No. KQTD20170810111314625), Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06G587), and the National Natural Science Foundation of China (No. 51503096).
Author Contributions
L.T. and C.S. conceived the project and designed the experiments. C.S. and D.J. carried out the experiments of material fabrication and characterizations; C. S., J.Y., and F.X. carried out the cellular and in vivo experiments; H.G. and L.X. carried out photoacoustic imaging experiments; L.T. and C.S. analyzed the data and co-wrote the manuscript with input from the other co-authors. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.022.
Contributor Information
Lei Xi, Email: xilei@sustech.edu.cn.
Leilei Tian, Email: tianll@sustech.edu.cn.
Supplemental Information
References
- Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X.L., Mao D., Wang C., Kong D.L., Cheng X.M., Liu B. Multifunctional liposome: a bright AIEgen-lipid conjugate with strong photosensitization. Angew. Chem. Int. Ed. 2018;57:16396–16400. doi: 10.1002/anie.201809641. [DOI] [PubMed] [Google Scholar]
- Cai Y., Si W.L., Huang W., Chen P., Shao J.J., Dong X.C. Organic dye based nanoparticles for cancer phototheranostics. Small. 2018;14:1704247. doi: 10.1002/smll.201704247. [DOI] [PubMed] [Google Scholar]
- Cai K., Xie J.J., Zhang D., Shi W.J., Yan Q.F., Zhao D.H. Concurrent cooperative J-aggregates and anticooperative H-aggregates. J. Am. Chem. Soc. 2018;140:5764–5773. doi: 10.1021/jacs.8b01463. [DOI] [PubMed] [Google Scholar]
- Chen Z.J., Liu Y., Wagner W., Stepanenko V., Ren X.K., Ogi S., Wurthner F. Near-IR absorbing J-aggregate of an amphiphilic BF2-azadipyrromethene dye by kinetic cooperative self-assembly. Angew. Chem. Int. Ed. 2017;56:5729–5733. doi: 10.1002/anie.201701788. [DOI] [PubMed] [Google Scholar]
- Coles D.M., Meijer A.J.H.M., Tsoi W.C., Charlton M.D.B., Kim J.-S., Lidzey D.G. A characterization of the Raman modes in a J-aggregate-forming dye: a comparison between theory and experiment. J. Phys. Chem. A. 2010;114:11920–11927. doi: 10.1021/jp107646p. [DOI] [PubMed] [Google Scholar]
- Egawa Y., Hayashida R., Anzai J.I. pH-induced interconversion between J-aggregates and H-aggregates of 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin in polyelectrolyte multilayer films. Langmuir. 2007;23:13146–13150. doi: 10.1021/la701957b. [DOI] [PubMed] [Google Scholar]
- Eisele D.M., Cone C.W., Bloemsma E.A., Vlaming S.M., van der Kwaak C.G.F., Silbey R.J., Bawendi M.G., Knoester J., Rabe J.P., Vanden Bout D.A. Utilizing redox-chemistry to elucidate the nature of exciton transitions in supramolecular dye nanotubes. Nat. Chem. 2012;4:655–662. doi: 10.1038/nchem.1380. [DOI] [PubMed] [Google Scholar]
- Gaufres E., Tang N.Y.W., Lapointe F., Cabana J., Nadon M.A., Cottenye N., Raymond F., Szkopek T., Martel R. Giant Raman scattering from J-aggregated dyes inside carbon nanotubes for multispectral imaging. Nat. Photonics. 2014;8:73–79. [Google Scholar]
- Gorl D., Zhang X., Wurthner F. Molecular assemblies of perylene bisimide dyes in water. Angew. Chem. Int. Ed. 2012;51:6328–6348. doi: 10.1002/anie.201108690. [DOI] [PubMed] [Google Scholar]
- Guo Z.Q., Park S., Yoon J., Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014;43:16–29. doi: 10.1039/c3cs60271k. [DOI] [PubMed] [Google Scholar]
- He H., Ji S.S., He Y., Zhu A.J., Zou Y.L., Deng Y.B., Ke H.T., Yang H., Zhao Y.L., Guo Z.Q. Photoconversion-tunable fluorophore vesicles for wavelength-dependent photoinduced cancer therapy. Adv. Mater. 2017;29:1606690. doi: 10.1002/adma.201606690. [DOI] [PubMed] [Google Scholar]
- Horn D., Rieger J. Organic nanoparticles in the aqueous phase - theory, experiment, and use. Angew. Chem. Int. Ed. 2001;40:4331–4361. doi: 10.1002/1521-3773(20011203)40:23<4330::aid-anie4330>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hu J.J., Cheng Y.Y., Ma Y.R., Wu Q.L., Xu T.W. Host-guest chemistry and physicochemical properties of the dendrimer-mycophenolic acid complex. J. Phys. Chem. B. 2009;113:64–74. doi: 10.1021/jp8078919. [DOI] [PubMed] [Google Scholar]
- Huang P., Gao Y., Lin J., Hu H., Liao H.S., Yan X.F., Tang Y.X., Jin A., Song J.B., Niu G. Tumor-specific formation of enzyme-instructed supramolecular self-assemblies as cancer theranostics. ACS Nano. 2015;9:9517–9527. doi: 10.1021/acsnano.5b03874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Liu K., Wang G.T., Wang Y.P., Zhang X. Supramolecular free radicals: near-infrared organic materials with enhanced photothermal conversion. Chem. Sci. 2015;6:3975–3980. doi: 10.1039/c5sc01167a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.I., Hwang B., Lee B., Bae J., Kim Y. Selective monitoring and imaging of eosinophil peroxidase activity with a J-aggregating probe. J. Am. Chem. Soc. 2018;140:11771–11776. doi: 10.1021/jacs.8b07073. [DOI] [PubMed] [Google Scholar]
- Li L.Y., Hou J.J., Liu X.J., Guo Y.J., Wu Y., Zhang L.H., Yang Z.J. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials. 2014;35:3840–3850. doi: 10.1016/j.biomaterials.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Li F.F., Lu J., Liu J., Liang C., Wang M.L., Wang L.Y., Li D.F., Yao H.Z., Zhang Q.L., Wen J. A water-soluble nucleolin aptamer-paclitaxel conjugate for tumor-specific targeting in ovarian cancer. Nat. Commun. 2017;8:1390. doi: 10.1038/s41467-017-01565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Tang J., Xu Y., Zhou Y., Dai Z. Nano-sized indocyanine green J-aggregate as a one-component theranostic agent. Nanotheranostics. 2017;1:430–439. doi: 10.7150/ntno.19935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell J.F., Jin C.S., Huynh E., Jin H.L., Kim C., Rubinstein J.L., Chan W.C.W., Cao W.G., Wang L.V., Zheng G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011;10:324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- Lu L.D., Jones R.M., McBranch D., Whitten D. Surface-enhanced superquenching of cyanine dyes as J-aggregates on laponite clay nanoparticles. Langmuir. 2002;18:7706–7713. [Google Scholar]
- Luo S.L., Zhang E.L., Su Y.P., Cheng T.M., Shi C.M. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Mundra V., Peng Y., Rana S., Natarajan A., Mahato R.I. Micellar formulation of indocyanine green for phototherapy of melanoma. J. Control Release. 2015;220:130–140. doi: 10.1016/j.jconrel.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Porcu E.P., Salis A., Gavini E., Rassu G., Maestri M., Giunchedi P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016;34:768–789. doi: 10.1016/j.biotechadv.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Pu K.Y., Shuhendler A.J., Jokerst J.V., Mei J.G., Gambhir S.S., Bao Z.N., Rao J.H. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014;9:233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Fang Y., Kwok R.T.K., Zhang X.Y., Hu X.L., Lam J.W.Y., Ding D., Tang B.Z. Highly stable organic small molecular nanoparticles as an advanced and biocompatible phototheranostic agent of tumor in living mice. ACS Nano. 2017;11:7177–7188. doi: 10.1021/acsnano.7b03062. [DOI] [PubMed] [Google Scholar]
- Robinson J.T., Welsher K., Tabakman S.M., Sherlock S.P., Wang H.L., Luong R., Dai H.J. High performance in vivo near-IR (> 1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010;3:779–793. doi: 10.1007/s12274-010-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.T., Tabakman S.M., Liang Y.Y., Wang H.L., Casalongue H.S., Vinh D., Dai H.J. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133:6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- Robinson J.T., Hone G., Liang Y., Zhang B., Yaghi O.K., Dai H. In vivo fluorescence imaging in the second near-infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake. J. Am. Chem. Soc. 2012;134:10664–10669. doi: 10.1021/ja303737a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S., Würthner F. Chlorophyll J-aggregates: from bioinspired dye stacks to nanotubes, liquid crystals, and biosupramolecular electronics. Acc. Chem. Res. 2013;46:2498–2512. doi: 10.1021/ar400017u. [DOI] [PubMed] [Google Scholar]
- Sheng Z.H., Hu D.H., Xue M.M., He M., Gong P., Cai L.T. Indocyanine green nanoparticles for theranostic applications. Nano Micro Lett. 2013;5:145–150. [Google Scholar]
- Song X.J., Gong H., Liu T., Cheng L., Wang C., Sun X.Q., Liang C., Liu Z. J-aggregates of organic dye molecules complexed with iron oxide nanoparticles for imaging-guided photothermal therapy under 915-nm light. Small. 2014;10:4362–4370. doi: 10.1002/smll.201401025. [DOI] [PubMed] [Google Scholar]
- Song X.J., Chen Q., Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015;8:340–354. [Google Scholar]
- Song X.J., Zhang R., Liang C., Chen Q., Gong H., Liu Z. Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials. 2015;57:84–92. doi: 10.1016/j.biomaterials.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Song Q., Jiao Y., Wang Z.Q., Zhang X. Tuning the energy gap by supramolecular approaches: towards near-infrared organic assemblies and materials. Small. 2016;12:24–31. doi: 10.1002/smll.201501661. [DOI] [PubMed] [Google Scholar]
- Stylianopoulos T. EPR-effect: utilizing size-dependent nanoparticle delivery to solid tumors. Ther. Deliv. 2013;4:421–423. doi: 10.4155/tde.13.8. [DOI] [PubMed] [Google Scholar]
- Su M.H., Li S.X., Zhang H., Zhang J.Q., Chen H.L., Li C.H. Nano-assemblies from J-aggregated dyes: a stimuli-responsive tool applicable to living systems. J. Am. Chem. Soc. 2019;141:402–413. doi: 10.1021/jacs.8b10396. [DOI] [PubMed] [Google Scholar]
- Varughese S. Non-covalent routes to tune the optical properties of molecular materials. J. Mater. Chem. C. 2014;2:3499–3516. [Google Scholar]
- von Berlepsch H., Bottcher C. Cryo-transmission electron microscopy reveals mesoscopic H- and J-aggregates of near infrared cyanine dyes. J. Photochem. Photobiol. A. 2010;214:16–21. [Google Scholar]
- von Berlepsch H., Kirstein S., Hania R., Pugzlys A., Bottcher C. Modification of the nanoscale structure of the J-aggregate of a sulfonate-substituted amphiphilic carbocyanine dye through incorporation of surface-active additives. J. Phys. Chem. B. 2007;111:1701–1711. doi: 10.1021/jp065826n. [DOI] [PubMed] [Google Scholar]
- Walker B.J., Dorn A., Bulovic V., Bawendi M.G. Color-selective photocurrent enhancement in coupled J-aggregate/nanowires formed in solution. Nano Lett. 2011;11:2655–2659. doi: 10.1021/nl200679n. [DOI] [PubMed] [Google Scholar]
- Wan Z.H., Mao H.J., Guo M., Li Y.L., Zhu A.J., Yang H., He H., Shen J.K., Zhou L.J., Jiang Z. Highly efficient hierarchical micelles integrating photothermal therapy and singlet oxygen-synergized chemotherapy for cancer eradication. Theranostics. 2014;4:399–411. doi: 10.7150/thno.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurthner F., Kaiser T.E., Saha-Moller C.R. J-aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. 2011;50:3376–3410. doi: 10.1002/anie.201002307. [DOI] [PubMed] [Google Scholar]
- Yin L., Sun H., Zhang H., He L., Qiu L., Lin J.G., Xia H.W., Zhang Y.Q., Ji S.J., Shi H.B. Quantitatively visualizing tumor-related protease activity in vivo using a ratiometric photoacoustic probe. J. Am. Chem. Soc. 2019;141:3265–3273. doi: 10.1021/jacs.8b13628. [DOI] [PubMed] [Google Scholar]
- Yoo D., Jeong H., Noh S.-H., Lee J.-H., Cheon J. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew. Chem. Int. Ed. 2013;52:13047–13051. doi: 10.1002/anie.201306557. [DOI] [PubMed] [Google Scholar]
- Zhao L.Z., Ma R.J., Li J.B., Li Y., An Y.L., Shi L.Q. J- and H-aggregates of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin and interconversion in PEG-b-P4VP micelles. Biomacromolecules. 2008;9:2601–2608. doi: 10.1021/bm8004808. [DOI] [PubMed] [Google Scholar]
- Zheng M., Yue C., Ma Y., Gong P., Zhao P., Zheng C., Sheng Z., Zhang P., Wang Z., Cai L. Single-step assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7:2056–2067. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- Zheng T.T., Li G.G., Zhou F., Wu R., Zhu J.J., Wang H. Gold-nanosponge-based multistimuli-responsive drug vehicles for targeted chemo-photothermal therapy. Adv. Mater. 2016;28:8218–8226. doi: 10.1002/adma.201602486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.