Abstract
Background
Even low levels of substance misuse by people with a severe mental illness can have detrimental effects.
Objectives
To assess the effects of psychosocial interventions for reduction in substance use in people with a serious mental illness compared with standard care.
Search methods
The Information Specialist of the Cochrane Schizophrenia Group (CSG) searched the CSG Trials Register (2 May 2018), which is based on regular searches of major medical and scientific databases.
Selection criteria
We included all randomised controlled trials (RCTs) comparing psychosocial interventions for substance misuse with standard care in people with serious mental illness.
Data collection and analysis
Review authors independently selected studies, extracted data and appraised study quality. For binary outcomes, we calculated standard estimates of risk ratio (RR) and their 95% confidence intervals (CIs) on an intention‐to‐treat basis. For continuous outcomes, we calculated the mean difference (MD) between groups. Where meta‐analyses were possible, we pooled data using a random‐effects model. Using the GRADE approach, we identified seven patient‐centred outcomes and assessed the quality of evidence for these within each comparison.
Main results
Our review now includes 41 trials with a total of 4024 participants. We have identified nine comparisons within the included trials and present a summary of our main findings for seven of these below. We were unable to summarise many findings due to skewed data or because trials did not measure the outcome of interest. In general, evidence was rated as low‐ or very‐low quality due to high or unclear risks of bias because of poor trial methods, or inadequately reported methods, and imprecision due to small sample sizes, low event rates and wide confidence intervals.
1. Integrated models of care versus standard care (36 months)
No clear differences were found between treatment groups for loss to treatment (RR 1.09, 95% CI 0.82 to 1.45; participants = 603; studies = 3; low‐quality evidence), death (RR 1.18, 95% CI 0.39 to 3.57; participants = 421; studies = 2; low‐quality evidence), alcohol use (RR 1.15, 95% CI 0.84 to 1.56; participants = 143; studies = 1; low‐quality evidence), substance use (drug) (RR 0.89, 95% CI 0.63 to 1.25; participants = 85; studies = 1; low‐quality evidence), global assessment of functioning (GAF) scores (MD 0.40, 95% CI ‐2.47 to 3.27; participants = 170; studies = 1; low‐quality evidence), or general life satisfaction (QOLI) scores (MD 0.10, 95% CI ‐0.18 to 0.38; participants = 373; studies = 2; moderate‐quality evidence).
2. Non‐integrated models of care versus standard care
There was no clear difference between treatment groups for numbers lost to treatment at 12 months (RR 1.21, 95% CI 0.73 to 1.99; participants = 134; studies = 3; very low‐quality evidence).
3. Cognitive behavioural therapy (CBT) versus standard care
There was no clear difference between treatment groups for numbers lost to treatment at three months (RR 1.12, 95% CI 0.44 to 2.86; participants = 152; studies = 2; low‐quality evidence), cannabis use at six months (RR 1.30, 95% CI 0.79 to 2.15; participants = 47; studies = 1; very low‐quality evidence) or mental state insight (IS) scores by three months (MD 0.52, 95% CI ‐0.78 to 1.82; participants = 105; studies = 1; low‐quality evidence).
4. Contingency management versus standard care
We found no clear differences between treatment groups for numbers lost to treatment at three months (RR 1.55, 95% CI 1.13 to 2.11; participants = 255; studies = 2; moderate‐quality evidence), number of stimulant positive urine tests at six months (RR 0.83, 95% CI 0.65 to 1.06; participants = 176; studies = 1) or hospitalisations (RR 0.21, 95% CI 0.05 to 0.93; participants = 176; studies = 1); both low‐quality evidence.
5. Motivational interviewing (MI) versus standard care
We found no clear differences between treatment groups for numbers lost to treatment at six months (RR 1.71, 95% CI 0.63 to 4.64; participants = 62; studies = 1). A clear difference, favouring MI, was observed for abstaining from alcohol (RR 0.36, 95% CI 0.17 to 0.75; participants = 28; studies = 1) but not other substances (MD ‐0.07, 95% CI ‐0.56 to 0.42; participants = 89; studies = 1), and no differences were observed in mental state general severity (SCL‐90‐R) scores (MD ‐0.19, 95% CI ‐0.59 to 0.21; participants = 30; studies = 1). All very low‐quality evidence.
6. Skills training versus standard care
At 12 months, there were no clear differences between treatment groups for numbers lost to treatment (RR 1.42, 95% CI 0.20 to 10.10; participants = 122; studies = 3) or death (RR 0.15, 95% CI 0.02 to 1.42; participants = 121; studies = 1). Very low‐quality, and low‐quality evidence, respectively.
7. CBT + MI versus standard care
At 12 months, there was no clear difference between treatment groups for numbers lost to treatment (RR 0.99, 95% CI 0.62 to 1.59; participants = 327; studies = 1; low‐quality evidence), number of deaths (RR 0.60, 95% CI 0.20 to 1.76; participants = 603; studies = 4; low‐quality evidence), relapse (RR 0.50, 95% CI 0.24 to 1.04; participants = 36; studies = 1; very low‐quality evidence), or GAF scores (MD 1.24, 95% CI ‐1.86 to 4.34; participants = 445; studies = 4; very low‐quality evidence). There was also no clear difference in reduction of drug use by six months (MD 0.19, 95% CI ‐0.22 to 0.60; participants = 119; studies = 1; low‐quality evidence).
Authors' conclusions
We included 41 RCTs but were unable to use much data for analyses. There is currently no high‐quality evidence to support any one psychosocial treatment over standard care for important outcomes such as remaining in treatment, reduction in substance use or improving mental or global state in people with serious mental illnesses and substance misuse. Furthermore, methodological difficulties exist which hinder pooling and interpreting results. Further high‐quality trials are required which address these concerns and improve the evidence in this important area.
Plain language summary
Psychosocial interventions for people with both severe mental illness and substance misuse.
What is the aim of this review?
The aim of this Cochrane Review is to find out if psychosocial interventions aimed at reducing substance abuse in people with a serious mental illness improve patient outcomes compared to standard care. Researchers in the Cochrane collected and analysed all relevant studies that randomly allocated people with severe mental illness and substance misuse to a psychosocial treatment or standard care to answer this question and found 41 relevant studies.
Key message
From these 41 studies we did not find any high‐quality evidence to support any one psychosocial intervention over standard care. However, the differences in study designs made comparisons between studies problematic.
What was studied in the review?
“Dual” diagnosis is the term used to describe people who have a mental health problem and also have problems with drugs or alcohol. In some areas, over 50% of all those with a serious mental illness (these include schizophrenia, bipolar disorders and major depression) will have problems with drugs or alcohol that have negative and damaging effects on the illness symptoms and the way their medication works. People who have substance misuse problems can be treated via a variety of psychosocial interventions. These include motivational interviewing, or MI, that looks at people’s motivation for change; cognitive behavioural therapy, or CBT, which helps people adapt their behaviour by improving coping strategies; contingency management which rewards patients to abstain from taking substances, psycho‐education for patients and their carers or family, group and individual skills training. Other interventions include provider‐oriented long‐term interventions unifying services to provide integrated treatment so patients do not have to negotiate separate mental health and substance abuse treatment programmes. Integrated care is often linked to assertive community treatment (ACT) for patients with a dual diagnosis. There are a variety of psychosocial interventions that can be added to routine care and these can be provided individually or in various combinations. Currently, we do not know if any psychosocial treatment is better or worse than standard care or if they work better given in combination or individually.
What are the main results of the review?
The review found 41 relevant studies with a total of 4024 people. These studies looked at a variety of different psychosocial interventions (including CBT, MI, skills training, integrated models of care and contingency management) and compared them to standard care (the care a participant in the trial would normally receive). Main results showed there was:
1. no real difference in terms of numbers lost to treatment (low‐quality evidence);
2. no real difference in terms of death (low‐quality evidence);
3. no real difference in alcohol or substance used (low‐quality evidence);
4. no real difference in global functioning or general life satisfaction (low‐ to moderate‐quality evidence).
In addition, studies had high numbers of people leaving early, differences in outcomes measured, and differing ways in which psychosocial interventions were delivered. More large‐scale, high‐quality and better reported studies are required to address these shortcomings. This will better address whether psychosocial interventions are effective for people with serious mental illness and substance misuse problems.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to October 2018.
Summary of findings
Background
Description of the condition
Substance misuse among people with a severe mental illness is a major concern, with prevalence rates over 50%. This figure varies across studies, depending on location, methodologies and by the way substance misuse problems and severe mental illness are defined (Brunette 2018; Carra 2009; Green 2007; Gregg 2007; Hartz 2014; Lai 2012a; Lai 2012b; Lowe 2004; Morgan 2017; Regier 1990; Todd 2004). Recent meta‐analyses have shown high rates of co‐morbidity between substance use disorders (SUD) and schizophrenia (Hunt 2018), bipolar disorders (Hunt 2016) and major depression (Lai 2015). Improving services for people with a serious mental illness (often labelled as having a 'dual diagnosis') is a priority as using drugs or consuming alcohol, even at low levels, is associated with a range of adverse consequences, including higher rates of non‐adherence, relapse, suicide, HIV, hepatitis, homelessness, aggression, incarceration, and fewer social supports or financial resources (Donald 2005; Green 2007; Hunt 2002; Khokhar 2018; Pettersen 2014; Schmidt 2011; Tsuang 2006). Further, co‐morbidity places an additional burden on families, psychiatric and government resources and is particularly challenging to those providing services as these patients have lower rates of treatment completion and higher rates of relapse (Khokhar 2018; Mueser 2013; Siegfried 1998; Tyrer 2004; Warren 2007).
Description of the intervention
It is important that co‐occurring substance use is detected as early as possible and that appropriate and effective treatment is provided (Green 2007; Mueser 2013). Treatment has traditionally been complicated by different approaches and philosophies among mental health and drug services as they may differ in their theoretical underpinnings, policies and protocols. Separate treatment programmes have been offered in parallel or sequentially by different clinicians, which may result in less than optimum patient care with the patient having to negotiate two separate treatment systems (De Witte 2014; Drake 2008; Green 2007). Another approach to care is the integrated treatment model where mental health and substance use treatments are brought together simultaneously by the same service, clinician or team of clinicians who are competent in both service areas and place similar importance on both (Drake 2004). Basic elements include an assertive style of engagement, techniques of close monitoring, comprehensive services (including inpatient, day hospital, community team and outpatient care), supportive living environments, flexibility and specialisation of clinicians, step‐wise treatment, and a long‐term perspective and optimism (Bond 2015; Drake 1993; Drake 2008). Assertive Community Treatment (ACT) and residential programmes are generally long term and can form a basis for integrated programmes (Mueser 2013).
How the intervention might work
As many substance users in the general population have benefited from a range of psychosocial interventions, it would follow that these same interventions should also benefit people with psychosis when their mental health problems are taken into account (Barrowclough 2006a). Most, if not all, substances of abuse increase dopaminergic activity in the brain (Koob 2010). Given that schizophrenia and other forms of psychosis are characterised by heightened dopaminergic transmission and that neuroleptics decrease activity or block dopamine receptors (Kapur 2005), it stands to reason that most substances of abuse increase symptoms, the risk of relapse and compromise the beneficial effects of neuroleptics (Khokhar 2018; Large 2014; LeDuc 1995; Seibyl 1993). This is especially true for stimulant drugs like amphetamine, cocaine and concentrated forms such as crack cocaine and methamphetamine ('ice') that can exacerbate or mimic psychotic symptoms and elevate risk of readmission (Callaghan 2012; Lappin 2016; McKetin 2013; Pluddemann 2013; Romer Thomsen 2018; Sara 2015). Substance use is also related to poor adherence with treatment, further increasing the risk of relapse (Hunt 2002). Interventions that reduce substance use are likely to improve symptoms, relapse rates, recovery and other outcomes (Cleary 2009a; Drake 2008; Horsfall 2009). Common psychosocial interventions to reduce substance use and misuse include Twelve‐Step recovery, which adopts a supportive approach such as that used by Alcoholics Anonymous (AA); motivational interviewing, which aims to increase an individual's motivation for change; group and individual skills training; family psycho‐education regarding the signs and effects of substance use; and individual or group psychotherapy involving cognitive or behavioural principles, or both, which aim to increase coping strategies, awareness and self‐monitoring behaviour (Mueser 2013). All of these interventions can vary in intensity and duration, and can be offered in a variety of settings, either individually or as part of an integrated programme. Integrated treatment ensures mental health and substance misuse services are available in the same setting and delivered in a coherent fashion.
Why it is important to do this review
While encouraging, results of trials assessing the effectiveness of these psychosocial interventions for mental health consumers are equivocal (for reviews, see: Bennett 2017; Bogenschutz 2006; Cleary 2009a; De Witte 2014; Dixon 2010; Drake 1998b; Drake 2004; Drake 2008; Horsfall 2009; Ley 2000; Mueser 2005; Mueser 2013; NICE 2011). Many studies have been hampered by small heterogeneous samples, poor experimental design (for example non‐random assignment), high attrition rates, short follow‐up periods, lack of accuracy of measuring substance use, skewed data, use of non‐standardised outcome measures and unclear descriptions of treatment components (Barrowclough 2006a; Cleary 2008; Hunt 2013). When assessing integrated programmes, it can also be difficult to determine exactly which part of the programme is the most effective, and control groups (particularly in the USA) may involve a certain level of service integration, making interpretations difficult (Drake 1996). Moreover, study methodologies, interventions and outcome measures vary across studies, as do patterns of participants' readiness to change, severity and type of illness and substance use, all of which make combining results in a review problematic (De Witte 2014; Donald 2005; Drake 2008).
This current review updates the 2013 Cochrane Review on "Psychosocial treatment programmes for people with both severe mental illness and substance misuse". The previous review (Hunt 2013) included any programme of substance misuse treatment and located 32 randomised controlled trials (RCTs). The authors from three previous reviews found no evidence to support any one substance misuse programme as being superior to another (Cleary 2008; Hunt 2013; Ley 2000). We felt an update of this review was warranted as there are several new studies that have been conducted in the last five years.
Objectives
To assess the effects of psychosocial interventions for reduction in substance use by people with a serious mental illness compared with standard care.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant, randomised controlled trials (RCTs) with or without blinding if they utilised a psychosocial intervention to reduce substance use in people with severe mental illness and substance misuse compared with standard care. We excluded quasi‐randomised trials, such as those where allocation was alternate or sequential.
Types of participants
We included people diagnosed with a severe mental illness (for example, mixed patient populations with schizophrenia, bipolar disorder, major depression and other psychosis) and concurrent problem of substance misuse. We have defined people with 'severe' illness as those with a chronic mental illness like schizophrenia who present to adult services for long‐term care. Those with an organic disorder, non‐severe mental illness (for example, personality disorder, post‐traumatic stress disorder (PTSD), anxiety disorders, depressive symptoms based on scores from a scale) or those who solely abused tobacco were, if possible, excluded. Trials that included a mixture of patients with a severe mental illness diagnosis were included if a large proportion had a schizophrenia‐like illness or psychosis (see Characteristics of included studies). Studies were excluded if all of the participants had a diagnosis of bipolar disorder or major depressive disorder from 2013 on, so they do not overlap with affective disorder reviews.
Types of interventions
We anticipated that studies included in the review would use a wide variety of psychosocial interventions for substance misuse, making direct comparisons difficult. In order to enhance the utility of the review, we developed a priori categories within which we made planned comparisons. These categories were developed from theoretical models of the types of behavioural and psychosocial interventions offered to clients and the context in which they are delivered. The types of interventions were grouped in two strata, based on duration and intensity of treatment. The first stratum describes long‐term interventions for dual diagnosis patients that offered an array of services with different levels of integration and assertive outreach (taking place over years rather than weeks or months), and the second describes stand‐alone psychosocial interventions that clients received over shorter periods. We did not include interventions for informal carers (partner or family members) as separate categories, though we did sometimes include them as part of the treatments mentioned below.
1. Provider‐oriented long‐term interventions: integrated and non‐integrated care by community mental health teams for dual diagnosis populations
1.1 Integrated models of care with assertive community treatment (ACT)
Integrated treatment models for patients with a dual diagnosis unify services at the provider level rather than forcing clients to negotiate separate mental health and substance abuse treatment programmes (Drake 1993; Drake 2008; Mueser 2013). The range of services provided varies according to client needs and should be able to handle patients at differing stages of readiness to change (Tsuang 2006). Substance abuse treatments are integrated into an array of direct services, such as frequent home visits, crisis intervention, housing skills training, vocational rehabilitation, medication monitoring, and family psycho‐education. Integrated treatment means that the same clinicians or teams of clinicians in the one setting provide long‐term treatments in a co‐ordinated fashion (Barrowclough 2006a; Green 2007). Teams consist of three to six clinicians and attempt to remain faithful to a specified model of care. To the client, the services should appear seamless with a consistent approach, philosophy and set of recommendations. Usually the caseloads of dual diagnosis teams are lower (approximately 10 to 15 clients shared within a team) than for standard case managers (approximately 20 to 30). Integrated treatment is a process that takes place over years rather than weeks or months. Studies included in this category must have clearly demonstrated the following: 1) assertive community outreach to engage and retain clients and to offer services to reluctant or uncooperative clients, 2) staged interventions to reduce substance use, and 3) adherence to the integrated team philosophy. The intervention could be community‐based or provided for special populations, such as homeless people or forensic patients.
1.2 Non‐integrated models of care (ACTO) or intensive case management
Non‐integrated treatment entails similar interventions by community teams, as described above, except the same members do not deliver them in a co‐ordinated fashion and assertive community outreach is not included. Normally, case managers in this category are better trained and have higher clinical qualifications and better therapeutic skills than standard case managers. Intensive case management is defined as lower case load size (approximately 10 to 15 clients) than for standard case managers and tends to have a 'psychodynamic' flavour (see Marshall 1998; Mueser 2013). To be included in this category, part of the intervention had to address the client's drug and alcohol misuse.
2. Patient or client focused short‐term interventions for substance misuse
These interventions can be broadly grouped into individual and group modalities. They are offered in addition to routine care (treatment as usual, standard case management) and are based on different theoretical models. Although they could be part of the provider‐oriented packages described above, studies included here were easier to evaluate since they described a simplified intervention that can be easily reproduced. As some studies used more than one intervention (for example, cognitive behavioural therapy combined with motivational interviewing), these were included in a separate category.
2.1 Individual approaches
2.1.1 Cognitive behavioural therapies
Cognitive behavioural approaches include a variety of interventions (Rector 2012; Work Group 2007). The defining features are: 1) emphasis on functional analysis of drug use, understanding the reasons for use and consequences; and 2) skills training for recognising the situations where a person is most vulnerable to drug use and avoiding these situations. A cognitive behavioural intervention seeks to establish links between drug misuse, irrational beliefs, and misperceptions at a personal level and endeavours to correct the thoughts, feelings and actions of the recipient with respect to and the promotion of alternative ways of coping (Jones 2004; Jones 2012; Thoma 2015). The target symptom that is usually focused on is reducing problematic substance use or harm minimisation, such as reducing the risk of contracting HIV.
2.1.2 Motivational interviewing
Motivational interviewing takes a non‐confrontational approach to treating substance misuse and is intended to enhance the individual's intrinsic motivation for change, in patients who often find it difficult to commit to change (Tsuang 2006). It matches the patient's level of problem recognition to change with specific strategies and goals and can be delivered in brief sessions or over a number of weeks. It is based on four key principles: 1) expressing empathy, 2) developing discrepancy, 3) supporting self‐efficacy, and 4) rolling with resistance (Chanut 2005); and is directed at five stages: 1) pre‐contemplation, 2) contemplation, 3) preparation, 4) action, and 5) maintenance (Tsuang 2006). A key hypothesis is that the patient's perspective on the importance of change is fundamental to the patient's readiness to address the problem. Developing the patient's confidence in their ability to achieve the desired change is also a key issue of motivational interviewing. This treatment is delivered individually or in small group settings.
2.1.3 Contingency management
Based on principals of operant conditioning, contingency management (CM) offers incentives or rewards to reinforce specific goals (reduced substance use, risky behaviours etc). Typically, rewards are provided if a negative substance test is provided (urine test or breath test). Rewards can vary widely, ranging from encouraging statements ('keep up the good work') to large or small financial prize (vouchers for food, cash etc). This approach has shown consistent success with various drug use disorders: cannabis, opiate and cocaine dependence and polysubstance use disorders (Dutra 2008). Contingency management has also been added with other psychosocial interventions, for example, motivational interviewing plus cognitive behavioural therapies (Bellack 2006). Thus, contingency management was added to the previous review due to the number of current and ongoing trials using this intervention.
2.2 Group approaches
2.2.1 Social skills training
These groups are aimed at helping clients develop interpersonal skills for establishing and maintaining relationships with others, dealing with conflict, and handling social situations involving substance misuse (Mueser 2004; Mueser 2013). They are taught in a highly structured way by using role play, corrective feedback and homework. This usually occurs in a group format, although the methods can also be employed in individual work as a type of cognitive behavioural counselling.
3. Standard care
For this review, standard care or 'treatment as usual' or 'routine' care was defined as the care that a person would normally receive had they not been included in the research trial. This could include standard case management (e.g. see Dieterich 2017Mas‐Esposito 2015). Standard care varies between settings and can be supplemented by additional components, including psycho‐educational material, family therapy, or referral to self‐help groups (for example, Alcoholics Anonymous) or other agencies for substance abuse treatment.
Types of outcome measures
We intended to group data into short‐, medium‐ and long‐term outcomes. However, this would have resulted in much data loss as outcome periods varied and therefore, we reported for the following time periods: 3, 6, 9, 12, 18, 24 and 36 months (where applicable).
We endeavoured to report binary outcomes, recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale ‐ as defined within the trials) before any others. Thereafter we listed other binary outcomes and then those that are continuous*.
We only extracted data from valid scales (see Data collection and analysis).
For outcomes measured and scales used by included studies see (Included studies).
Primary outcomes
1. Leaving the study early
1.1 Lost to treatment: this is a measure of stability and engagement (number of participants who did not continue with the treatment following randomisation; however, some may have provided data for the study. This varies with study design as some treatments are ongoing for the study duration and some are short term. When studies reported exactly the same data for both lost to treatment and lost to evaluation (see below), and if there were no other studies with which to pool data, then we only reported the numbers lost to treatment (to reduce the number of comparison tables). We did not adjust numbers lost to treatment for death (see below).
2. Substance use: changes in substance use (alcohol or drugs, or both) as defined by each of the studies
2.1 Clinically important change or any change in substance use 2.2 Average endpoint or change score on substance use scale
3. Mental state (mental health symptoms)
3.1 Clinically important change or any change in mental state 3.2 Average endpoint or change score on mental state scale
Secondary outcomes
1. Leaving the study early
1.1 Lost to evaluation (number of people lost to the study who did not provide data at particular time points). 1.1.1. For any reason 1.1.2 For specific reason
2. Adverse events
2.1 Death (all causes)
Some studies may not have reported on the number of participants dying over the treatment or evaluation period. If reported, we recorded death in a separate table, but these cases were retained in the lost to treatment and lost to evaluation figures as it is often unclear when the death occurred or the cause of death was not stated as unlikely to be linked to the intervention.
3. Global state (a clinical measure of patient's psychological, social and occupational functioning in a single measure)
3.1 Clinically important change or any change in global state 3.2 Average endpoint or change score on global state scale
4. Social functioning (social assessment measures and basic skills necessary for community living)
4.1 Clinically important change or any change in social functioning 4.2 Average endpoint or change score on quality of life/life satisfaction scale
5. Quality of life/life satisfaction (objective and subjective quality of life satisfaction measures, measures of global patient satisfaction with daily living and services provided)
5.1 Clinically important change or any change in quality of life/life satisfaction 5.2 Average endpoint or change score on quality of life/life satisfaction scale
6. Service use (including relapse)
Relapse is often used as an outcome measure. Relapse can be measured as a dichotomous event (re‐admitted to hospital), or continuous measure such as number of admissions over a specified period or time to admission (number of weeks, days).
6.1 Re‐admission to hospital 6.2 Number of admissions 6.3 Number of days in community
7. Homelessness: homelessness is common in this patient population. It is often used to measure a favourable outcome if a person finds stable living accomodation over a specified period
7.1 Number of days in stable living accomodation 7.2 Number of days on street
8. Economic outcomes
8.1 Direct costs: e.g. costs to the patient for supplying a drug‐free urine sample 8.2 Indirect costs: e.g. savings based on number of days living in the community, versus costs of inpatient treatment or days in jail
9. Compliance with treatment and medication (as defined by individual studies)
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011); and used GRADEpro GDT to export data from our review to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rate as important to patient care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' tables.
Leaving the study early: numbers lost to treatment (medium term: 12 months; if these data were not available we used the short‐term data).
Adverse event: death.
Substance use: alcohol use (as measured in the trials). Described as a clinically important reduction in alcohol use.
Substance use: drug use (as measured in the trials). Described as a clinically important reduction in illicit drug use.
Mental state: (as measured in the trials, and if no specific scale assessment was done we reported on relapse or hospitalisation).
Global state: (as measured in the trials) described as a clinically important change in functioning.
Quality of life ‐ life satisfaction: (as measured in the trials) described as a clinically important change in satisfaction.
If data were not available for these pre‐specified outcomes but were available for ones that were similar, we presented the closest outcome to the pre‐specified one in the table but took this into account when grading the finding*.
* see Differences between protocol and review Note 5.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Study‐Based Register of Trials
On 2 May 2018, the Information Specialist searched the register using the following search strategy:
(*{PSY}* in Intervention) AND (*Substance Use* in Healthcare Condition) of STUDY
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017).
This register is compiled by systematic searches of major resources (AMED, BIOSIS, CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, Chinese databases (CBM, CNKI, and Wanfang) and their annual updates, handsearches, grey literature, and conference proceedings (see Group's website). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous searches, please see Appendix 1.
Searching other resources
1. Reference lists
We searched all references of articles selected for inclusion, major review articles (Baker 2012; Bennett 2017; De Witte 2014; Dixon 2010; Drake 2008; Dutra 2008; Galletly 2016; Horsfall 2009; Kelly 2012), as well as recent guidelines (NICE 2011; Hasan 2015) on this topic for further relevant trials.
2. Journal databases
Further searches were completed (September 2018) by the principal review author (GEH) using the Cochrane Database of Systematic Reviews, MEDLINE (daily update, PREMEDLINE), and PsycINFO. A separate search for randomised trials using contingency management was completed as this was an additional intervention category for the previous update. We also searched MEDLINE for recent articles (2013 to 2018) by the first authors of all included studies in order to get a more complete list of recent publications.
We also did 'forward' searches to identify trials that cited previously included RCTs using Web of Science and Scopus. Scopus was used to identify trials that cited the most recent versions of this review (Cleary 2008, Hunt 2013) up to October 2018.
3. Trials registries
In addition, web sites and journals that list ongoing trials in the USA, UK, Australia and various European countries were searched for RCTs through the Cochrane Schizophrenia Group Trials Register. The principal review author (GEH) searched www.clinicaltrials.gov for protocols of current and previously included studies for proposed outcome measures to assess selective reporting bias.
4. Personal contact
We contacted the first author (or corresponding author) of newly included studies for this update regarding their knowledge of ongoing or unpublished trials.
Data collection and analysis
Methods for data collection and analysis methods employed for the 2018 search are below. For data collection and analysis methods in earlier versions see Appendix 2 and Appendix 4.
Selection of studies
Review authors GEH and MC inspected all citations from the new electronic search and identified relevant abstracts, full‐text articles and trials against the inclusion criteria. To ensure reliability, KM inspected all full‐text articles for inclusion. Where there were uncertainties or disagreements, two additional review authors provided resolution (NS and CB‐S). Where disputes could not be resolved, these studies remained as awaiting assessment or ongoing studies and we contacted the authors for clarification.
Data extraction and management
1. Extraction
GEH and KM extracted data from the included studies. We resolved disputes by discussion and adjudication from the other review authors (NS and MC) when necessary. If it was not possible to extract data or if further information was needed, we attempted to contact the authors. We extracted data presented only in graphs and figures whenever possible, but the data were included only if two review authors independently had the same result. When further information was necessary, we contacted authors of studies in order to obtain missing data or for clarification of methods.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b) the measuring instrument has not been written or modified by one of the trialists for that particular trial; c) the instrument should be a global assessment of an area of functioning and not sub‐scores which are not, in themselves, validated or shown to be reliable. However there are exceptions, we included sub‐scores from mental state scales measuring positive and negative symptoms of schizophrenia.
Ideally the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, we noted in Description of studies if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we preferred to use mean differences (MD) rather than standardised mean differences (SMD) throughout (Deeks 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant data before inclusion.
Please note, we entered data from studies of at least 200 participants in the analysis, because skewed data pose less of a problem in large studies. We also entered all relevant change data as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not.
For endpoint data from studies < 200 participants:
(a) when a scale starts from zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than 1, it strongly suggests a skew and we excluded these data. If this ratio was higher than one but below 2, there is suggestion of skew. We entered these data and tested whether its inclusion or exclusion change the results substantially. Finally, if the ratio was larger than 2 we included these data, because skew is less likely (Altman 1996; Higgins 2011).
(b) if a scale starts from a positive value such as the Positive and Negative Syndrome Scale (PANSS, Kay 1986), which can have values from 30 to 210, we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
Where relevant, to facilitate comparison between trials, we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we converted continuous outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this can be considered as a clinically important response (Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the psychosocial intervention. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not un‐improved'), we presented data where the left of the line indicates an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review authors (GEH, NS, CB‐S) independently assessed risk of bias within the included studies by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011a). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, we made the final rating by consensus with all review authors. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies contacted in order to obtain further information. If non‐concurrence occurred, we reported this.
We noted the level of risk of bias in the text of the review and in Figure 1; Figure 2 and 'Summary of findings' tables.
1.
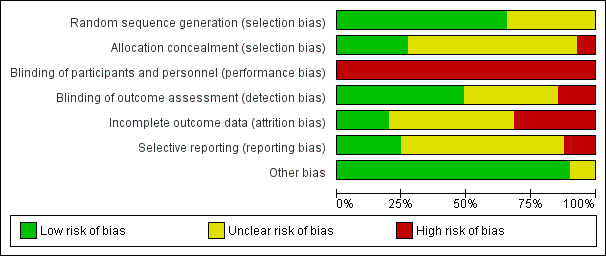
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
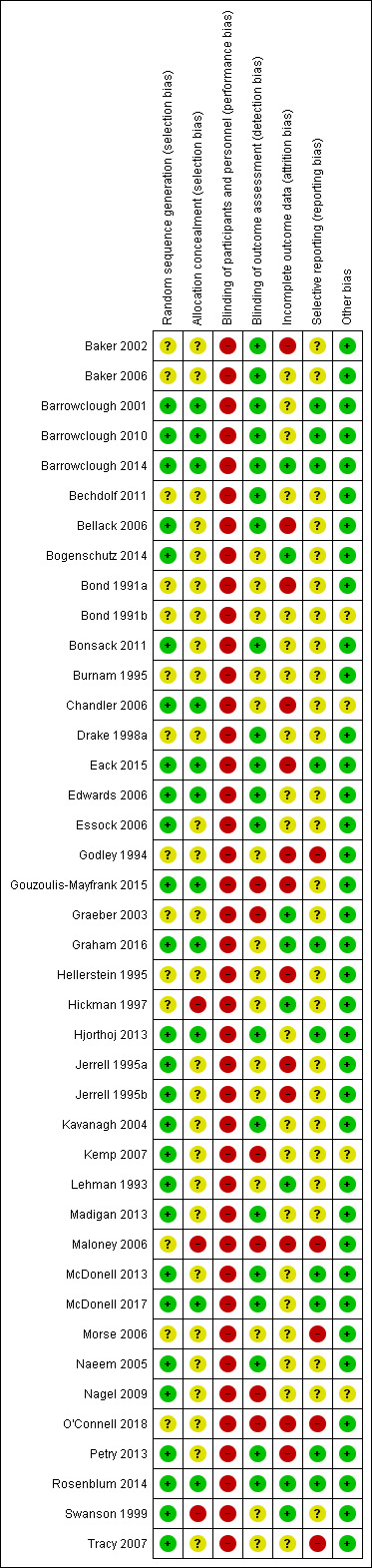
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios as odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference SMD). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
None of the presently included trials used cluster randomisation. For the purposes of future updates of this review, where clustering is not accounted for in primary studies we planned to present data in a table with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, should we include cluster‐RCTs, we will seek to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we plan to present these data as if from a non‐cluster randomised study but adjust for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC (design effect = 1 + (m ‐ 1)*ICC) (Donner 2002). If the ICC is not reported we will assume it to be 0.1 (Ukoumunne 1999).
If we had identified cluster trials, we would have analysed them taking into account ICCs and relevant data documented in the report. Synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
None of the presently included studies employed a cross‐over trial design. For the purposes of future updates of the review, a major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (for example, pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we proposed to only use the data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary we simply added and combined within the two‐by‐two table. If data were continuous we combined data following the formula in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not use data where the additional treatment arms were not relevant.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' tables by down‐rating quality. We also planned to downgrade quality within the 'Summary of findings' tables should loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome is between 0% and 50% and where these data are not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). We assumed all those leaving the study early to have the same rates of negative outcome as those who completed ‐ except for the outcomes of death and adverse effects ‐ for these outcomes we used the rate of those who stayed in the study (in that particular arm of the trial) for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change by comparing data only from people who completed the study to that point to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
We reported and used data where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either 'P' value or 't' value available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). The Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple imputation or mixed‐effects models for repeated measurements have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, we preferred to use the more sophisticated approaches. (e.g. MMRM or multiple‐imputation) and only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item "incomplete outcome data" of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on: i) magnitude and direction of effects, and ii) strength of evidence for heterogeneity (for example, P value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic was interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for the heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Chapter 10. of the Cochrane Handbook for Systemic reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We tried to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies are of similar size. In future versions of this review, if funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies, even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the random‐effects model for main analyses (see also Sensitivity analysis)
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses ‐ only primary outcomes
1.1 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of psychosocial interventions for people with schizophrenia and other psychoses, in general. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we have reported this. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review we decided that should this occur, with data contributing to the summary finding of no more than around 10% of the total weighting, we would present the data. If not, then we did not pool the data and discussed the issues. We know of no supporting research for this 10% cut‐off, but we use prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious we simply stated hypotheses regarding these for future reviews or versions of this review. We do not anticipate undertaking analyses relating to these.
Sensitivity analysis
We conducted sensitivity analyses on outcomes of comparisons with four or more trials where studies with different quality were combined to ascertain if there were substantial differences in the results when lesser quality trials or those comprising participants with schizophrenia (or other psychoses) were compared to trials of higher quality or using mixed diagnostic groups. We applied all sensitivity analyses to the primary outcomes based on randomised sequence, allocation concealment and blinding of outcome measurement. We only conducted sensitivity analyses on comparisons with four or more studies as analyses with less than four trials would provide unclear decisions on whether there have been any possible biases in the estimate of effects.
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way so as to imply randomisation. For the primary outcomes, we included these studies and if there was no substantive difference when the implied randomised studies were added to those with a better description of randomisation then we entered all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumptions and when we used data only from people who completed the study to that point. If there was a substantial difference, we reported the results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing sSD data (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumptions and when we used data only from people who completed the study to that point. A sensitivity analysis was undertaken to test how prone results were to change when completer‐only data were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (see Assessment of risk of bias in included studies) for the meta‐analysis of the primary outcomes. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
A sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials was not carried out as there are no cluster‐randomised trials included in the review.
5. Fixed‐ and random‐effects
We synthesised data using a random‐effects model; however, we also synthesised data for the primary outcomes using fixed‐effect method to evaluate whether this altered the significance of the results.
If we noted substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome but presented them separately.
Results
Description of studies
Results of the search
A total of 4866 citations were found using the search strategy devised for the original version of this review. The inclusion of the word 'drug' in the search strategy produced a vast number of irrelevant references. For the current update 113 additional relevant records were scrutinised which resulted in an additional nine studies considered for inclusion (Barrowclough 2014; Bogenschutz 2014; Eack 2015; Gouzoulis‐Mayfrank 2015; Graham 2016; McDonell 2017; O'Connell 2018; Petry 2013; Rosenblum 2014). Together with the 32 studies from previous reviews, the current review included 41 studies. See also Figure 3.
3.
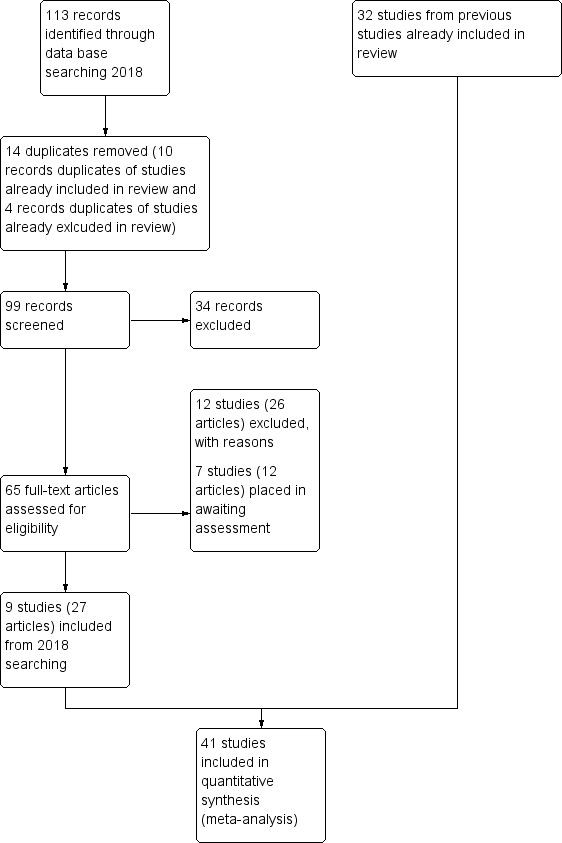
Study flow diagram for 2018 search
Included studies
In the previous review (Hunt 2013), 32 randomised controlled trials (RCTs) were selected for inclusion. Three studies (Godley 1994; Maloney 2006; Morse 2006) from the first update (Cleary 2008) contained only skewed data (shown as 'other data' within the Data and analyses). The remaining trials provided usable data (either dichotomous or continuous parametric data). For the current update, nine new trials were selected for inclusion thus in total, 41 RCTs were included in the current review.
1. Design
Three trials were set exclusively in hospital (Baker 2002; Bechdolf 2011; Swanson 1999) and 26 in the community. Ten trials recruited patients or were conducted in both the community (outpatients) and in hospital (Bellack 2006; Bonsack 2011; Gouzoulis‐Mayfrank 2015; Graeber 2003; Graham 2016; Hellerstein 1995; Hjorthoj 2013; Kavanagh 2004; Madigan 2013; Naeem 2005), and two were set in the community and in jail (Chandler 2006; Maloney 2006).
Most studies randomly allocated participants to one of two treatment conditions; the exceptions were Barrowclough 2014; Burnam 1995; Jerrell 1995a; Jerrell 1995b; Maloney 2006; Morse 2006 and O'Connell 2018). These trials randomly allocated participants to one of three or four (Maloney 2006) interventions. We have used only two of the intervention arms in Burnam 1995 as the other did not fit into any a priori category described for inclusion in this review. Data are shown in additional tables. Study durations ranged from three months to three years and the length of the interventions ranged from less than one hour to three years. There were 25 trials from the USA, six from Australia, five from the UK, two from Germany and one each from Denmark, Ireland and Switzerland.
2. Participants
A total of 4024 people participated in the trials after giving informed consent and were randomised into one of the treatment arms. All participants were adults (aged 18 to 65 years) who were 'severely mentally ill' with the majority having a diagnoses of schizophrenia, schizoaffective disorder or psychosis (affective and non‐affective). All had a current diagnosis of substance use disorder or had documented evidence of substance misuse. Some were homeless or had a history of unstable accommodation (Burnam 1995; Essock 2006; Morse 2006; Tracy 2007), and some were incarcerated at the time of the study (Chandler 2006; Maloney 2006).
3. Interventions
Integrated models of care (four RCTs).
Non‐integrated models of care (four RCTs).
Combined cognitive behavioural therapy and motivational interviewing (nine RCTs).
Cognitive behavioural therapy (two RCTs).
Motivational interviewing (nine RCTs).
Skills training (six RCTs).
Contingency management (four RCTs).
4. Outcomes
Where possible, we included dichotomous data relating to loss to treatment, loss to evaluation, death, abstinence or reduced substance use, relapse, attendance at aftercare, and arrests.
All of the outcome scales and their abbreviations are listed in Table 10, together with the reference of the source of the scale. See below for descriptions of the continuous data scales that reported data used in the analyses. For a full list of the scales mentioned in each of the studies see Characteristics of included studies.
1. List of scales and abbreviations used in included studies.
| Name of tool | Abbreviation | Source of scale ‐ reference |
| Diagnostic tools | ||
| Diagnostic and Statistical Manual of Mental Disorders, 4th edition | DSM‐IV | DSM‐IV |
| The classification of mental and behavioural disorders | ICD‐10 | ICD‐10 |
| Structured Clinical Interview for Diagnosis | SCID | Spitzer 1990 |
| Diagnostic Interview Schedule (DIS), computerised scoring for DSM‐III‐R criteria | C‐DIS‐R | DSM III‐R |
| Substance use scales | ||
| Addiction Severity Index | ASI | McLellan 1980; McLellan 1992 |
| Alcohol Use Inventory | AUI | Horn 1987 |
| Alcohol Use Scale | AUS | Mueser 1995 |
| Alcohol Use Disorders Identification Test | AUDIT | Saunders 1993 |
| Brief Drinker Profile | BDP | Miller 1987 |
| Drug and Alcohol Problem Scale | DAPS | adapted non‐peer reviewed version of this scale used; see Bond 1991a |
| Drug Use Scale | DUS | Mueser 1995 |
| Opiate Treatment Index | OTI | Darke 1991 |
| Change Questionnaire‐Cannabis | RTCQ‐C | Rollnick 1992 |
| Substance Abuse Treatment Scale | SATS | McHugo 1995 |
| Schedule for Clinical Assessment in Neuropsychiatry | SCAN | Wing 1990 |
| Substance Use Severity Scale | USS | Carey 1996 |
| Mental state scales | ||
| Addiction Severity Index (psychiatric subscale) | ASI | McLellan 1980 |
| Beck Depression Inventory ‐ Short Form | BDI‐SF, BDI‐11 | Beck 1972 |
| Brief Psychiatric Rating Scale | BPRS | Lukoff 1986 |
| Brief Scale for Anxiety | BSA | Tyrer 1984 |
| Brief Symptom Inventory | BSI | Derogatis 1983a |
| Calgary Depression Scale | CDS | Addington 1992 |
| Comprehensive Psychopathological Rating Scale | CPRS | Asberg 1978 |
| Hamilton Rating Scale for Depression | HAM ‐ D | Hamilton 1960 |
| Hospital Anxiety and Depresion Scale | HADS | Zigmond 1983 |
| Insight Scale | David 1992 | |
| Montgomery Asberg Depression Rating Scale | MADRS | Montgomery 1979 |
| Positive & Negative Syndrome Scale for schizophrenia | PANNS | Kay 1987 |
| Psychiatric Epidemiologic Research Interview | PERI | Dohrenwend 1980 |
| Scale for the Assessment of Negative Symptoms | SANS | Andreasen 1982 |
| Scale for the assessment of Positive Symptoms | SAPS | Norman 1996 |
| Symptom Checklist 90 | SCL‐90 | Derogatis 1973; Derogatis 1975 |
| Symptom Checklist 90‐revised | SCL‐90‐R | Derogatis 1983b |
| Schizophrenia Change Scale | SCR | Montgomery 1978 |
| Young Mania Rating Scale | YMRS | Young 1978 |
| General function scales | ||
| Global Assessment of Functioning | GAF | DSM‐IV |
| Health of the Nation Outcome Scale | HoNOS | Wing 1996 |
| Role Functioning Scale | RFS | Green 1987 |
| Social Adjustment Scale for the Severely Mentally Ill | SAS‐SMI | Wieduwilt 1999 |
| Social Functioning Scale | SFS | Birchwood 1990 |
| The Social and Occupational Functioning Scale | SOFAS | Goldman 1992 |
| Quality of life scales | ||
| Brief Quality of Life Scale | BQOL | Lehman 1995 |
| Life Satisfaction Checklist | LSC | Bond 1988; Bond 1990 |
| Manchester Short Assessment of Quality of Life | MANSA | Priebe 1999 |
| Quality of Life Interview | QOLI | Lehman 1988 |
| Satisfaction with Life Scale | SLS | Stein 1980 |
| World Health Organization's Quality of Life assessment scale, short version | WHOQOL‐BREF | Skevington 2004 |
| Other | ||
| Client Satisfaction Questionnaire | CSQ | Larsen 1979 |
| Medication Adherence Rating Scale | MARS | Thompson 2000 |
| The Service Utilization Rating Scale | SURS | Mihalopoulos 1999 |
4.1 Substance use scales
a. Drug and alcohol scales from Addiction Severity Index (ASI)
The ASI (McLellan 1980) provides two summary scores of problems of functioning in seven areas, including psychiatric problems, and those concerning drug and alcohol use. Severity ratings range from zero to nine and are assessments of lifetime and current problem severity derived by the interviewer. Composite scores are mathematically derived and are based on client responses to a set of items based on the last 30 days. Although difficulties have been reported concerning the use of the ASI with people who have severe mental illness (Corse 1995), the psychometric properties of the subscales with this population have been reported by a number of authors (Appleby 1997; Hodgins 1992; Zanis 1997). Given that the problems encountered by the scale are likely to be encountered by any other similar instrument based on self‐reports of those with severe and persistent mental illness, it was decided to include data obtained with the ASI (used in Barrowclough 2001; Bechdolf 2011; Bellack 2006; Drake 1998a; Eack 2015; Essock 2006; Hellerstein 1995 and Lehman 1993).
b. Alcohol Use Inventory (AUI)
This inventory assesses alcohol use (Horn 1987) (used by Hickman 1997).
c. Alcohol Use Scale (AUS)
A five‐point scale based on clinicians' ratings of severity of disorder, ranging from one (abstinence) to five (severe dependence) (Mueser 1995). This was used in Drake 1998a and Essock 2006.
d. Alcohol Use Disorders Identification Test (AUDIT)
The AUDIT is a 10‐item questionnaire covering the domains of alcohol consumption, drinking behaviour and alcohol‐related problems (Saunders 1993). Responses to each question are scored from zero to 4. A sum score of 8 or more indicates hazardous or harmful alcohol use. This was used in Graham 2016.
e. Cannabis and Substance Use Assessment Schedule (CASUAS) (modified from the SCAN)
This measures cannabis use and includes similar information to the ASI, such as percentage of days using cannabis in the past four weeks, frequency of cannabis use, and an index of severity (range zero to 4) with higher scores indicating greater severity (Wing 1990) (used by Edwards 2006).
f. Drug Use Scale (DUS)
A five‐point scale based on clinicians' ratings of severity of disorder, ranging from one (abstinence) to five (severe dependence) (Mueser 1995) (used in Drake 1998a and Essock 2006).
g. Opiate Treatment Index (OTI)
The OTI has six domains reflecting treatment outcomes of: drug use, HIV risk‐taking behaviour, social functioning, criminality, health status and psychological adjustment (Darke 1991; Darke 1992). The drug use domain consists of 11 items measuring drug use over the last three days (recent drug use) or previous month (28 days) for alcohol, cannabis, amphetamines, cocaine, opiates and other drugs. Clients are asked to estimate the number of drinks or usage of drugs on the two most recent use days in the previous month. The quantity over the two days (q1 + q2) is divided by day interval (t1 + t2). Thus, an OTI score of 1.0 indicates one drink, injection or joint per day; 0.14 to 0.99 more than once a week; 0.01 to 0.13 once a week or less, and 2.0 or more indicates use more than once a day. Higher scores indicate a greater degree of dysfunction or substance use. Baker 2002 and Baker 2006 used the OTI to measure substance use over the previous month.
h. Substance Abuse Treatment Scale (SATS)
An eight‐point scale indicating progression toward recovery ranging from one (early stages of engagement) to eight (relapse prevention). Higher scores indicate greater progression (McHugo 1995). This was used by Drake 1998a, Essock 2006 and Graham 2016.
i. Alcohol and drug use disorders section of the Structured Clinical Interview for DSM‐III‐R (Patient Edition) (SCID)
Items relate to substance use in the past month (Spitzer 1990). Higher scores indicate a greater degree of dysfunction (used by Baker 2002).
j. Substance Use Severity Scale (USS)
This is a five‐point scale, ranging from one (not using) to five (meets criteria for severe use) (Carey 1996), used by Morse 2006.
4.2 Mental state assessment
a. Beck Depression Inventory (BDI)
This contains 21 self‐report items which measure the severity of depression (Beck 1972). Each item comprises four statements (rated from zer to 4) describing increasing severity on how they felt over the preceding week. Scores range from zero to 84, with higher scores indicating more severe symptoms (used in Baker 2006; Edwards 2006 used the short form of this scale (BDI‐SF)).
b. Brief Psychiatric Rating Scale (BPRS)
Used to assess the severity of a range of psychiatric symptoms, including psychotic symptoms (Lukoff 1986), the scale has 24 items, of which 14 are based on the person's self‐report in the last two weeks and 10 on the person's behaviour during the interview. Each item can be defined on a seven‐point scale from one (not present) to seven (extremely severe). Total scoring ranges from 24 to 168 and there are five subscales with minimum scores ranging from three to four depending on the subscale (used in Baker 2006; Drake 1998a; Eack 2015; Edwards 2006; and Essock 2006).
c. Brief Symptom Inventory (BSI)
This measures psychiatric symptomatology (Derogatis 1983a). A brief rating scale is used by an independent rater to assess severity of psychiatric symptoms. Scores range from zero to 4 with higher scores indicating more symptoms (used by Baker 2002, Bogenschutz 2014, McDonell 2013, McDonell 2017 and Petry 2013).
d. Comprehensive Psychopathological Rating Scale (CPRS)
This is an interview rating scale covering a wide range of psychiatric symptoms, and can be used in total or as subscales. The Montgomery Asperg Depression Rating Scale (MADRS), Brief Scale for Anxiety (BSA) and the Schizophrenia Change Scale (SCR) are all subscales of the CPRS. It comprises 65 items that cover the range of psychopathology over the preceding week (40 symptom items are rated by the participant) (Asberg 1978). Each item is rated on a zero to 3 scale, varying from 'not present' to 'extremely severe', with high scores indicating more severe symptoms and a worse outcome (used by Naeem 2005).
Global state: (a clinical measure of patient's psychological, social and occupational functioning in a single measure)
e. Global Assessment of Functioning (GAF)
The Global Assessment of Functioning is a revised version of the Global Assessment Scale (GAS) (Endicott 1976). The (GAF) scale allows the clinical progress of the patient to be expressed in global terms using a single measure. The GAF allows the clinician to express the patient's psychological, social and occupational functioning on a continuum extending from superior mental health, with optimal social and occupational performance, to profound mental impairment when social and occupational functioning is precluded. Developed by DSM‐IV to report global assessment of functioning on the Axis V (DSM‐IV), it ranges from 1 to 100 (zero is used to acknowledge inadequate information). Higher scores indicate a better outcome; scores ranging from 1 to 20 indicate a person unable to function independently; 21 to 40 indicate major impairment, severely impaired by delusions; 41 to 60 moderately impaired, having serious symptoms and these patients usually need continuous treatment in a partial hospitalisation or outpatient setting; 61 to 80 indicate slight or mild impairment with transient symptoms; and 81 to 100, good or superior functioning. Baker 2006, Barrowclough 2001, Barrowclough 2010, Barrowclough 2014, Bechdolf 2011, Bonsack 2011, Essock 2006, Gouzoulis‐Mayfrank 2015 and Madigan 2013 used this scale.
f. Hospital Anxiety and Depression Scale (HADS)
The HADS is a 14‐item self‐assessment scale developed to detect states of depression and anxiety (Zigmond 1983). Each of the items is rated on a four‐point Likert scale (zero to 3) with half of the items rated on a reverse scale (I feel cheerful, I can sit at ease and feel relaxed). Scale scores can be categorised into non‐cases (scores of zero to 7), doubtful cases (8 to 10) and definite cases (>11) for depression (HADS‐D) and anxiety (HADS‐A) (used by Graham 2016).
g. Health of the Outcome Nation Outcomes Scale (HoNOS)
HoNOS is a 12‐item instrument on a scale of zero to 4 used to rate patients' symptoms and progress towards health (Wing 1996). Item 3 can be used to rate drug and alcohol use (zero = no problem, 1 = some over‐indulgence but within social norm, 2 = loss of control, 3 = marked craving, 4 = incapacitated by alcohol or drug problem) and other items can be used to assess social functioning. Thus, ratings range from zero to 48 and higher scores indicate a poorer outcome (used by Naeem 2005).
h. Insight Scale
This is used to assess the patient's level of insight about their illness (David 1992). Seven self‐report items are scored from zero = no insight to 2 = full insight. One additional self‐report item is scored zero to 4 (used by Graham 2016 and Naeem 2005).
i. The Positive and Negative Syndrome Scale (PANSS)
The PANSS was developed from the BPRS and the Psychopathology Rating Scale (Kay 1987). It is used as a method for evaluating positive, negative and other symptom dimensions in schizophrenia. The scale has 30 items and each item can be defined on a seven‐point scoring system, varying from one (absent) to seven (extreme), so total scores range from 30 to 210. This scale can be divided into three subscales for measuring the severity of general psychopathology (range 16 to 112), positive symptoms (PANSS‐P, range 7 to 49) and negative symptoms (PANSS‐N, range 7 to 49). A low score indicates low levels of symptoms. This was used by Barrowclough 2001, Barrowclough 2010, Barrowclough 2014, Bechdolf 2011, Bonsack 2011, Gouzoulis‐Mayfrank 2015, Kemp 2007, McDonell 2017 and O'Connell 2018.
j. Psychiatric scale from Addiction Severity Index (ASI‐psychiatric)
Psychiatric subscores (McLellan 1980) were reported in Lehman 1993 and Hellerstein 1995. See the ASI scoring above.
k. Scale for the Assessment of Negative Symptoms (SANS)
The scale assesses negative symptoms for schizophrenia (Andreasen 1982). This assesses five symptoms complexes to obtain the clinical rating of negative symptoms over the preceding week. They are affective blunting, alogia, apathy, anhedonia and disturbance of attention. Each item uses a six‐point scale ranging from zero (not at all) to 5 (indicating severe). High scores indicate a worse outcome (used by Edwards 2006).
l. Symptom Checklist 90 (revised) (SCL‐90‐R)
Used to measure psychiatric symptoms (Derogatis 1983b), the scale has 90 self‐report items designed to measure nine symptom dimensions. Each item has a five‐point Likert scale ranging from zero (mild or not at all) to 4 (severe or extremely distressing), with higher scores indicating greater symptomatology (used by Hickman 1997).
4.3 Quality of life and client satisfaction; quality of life scales measure objective and subjective quality of life satisfaction and global patient satisfaction with daily living and services provided (see Results, section 4.3).
a. The Quality of Life Interview (QOLI) and the Brief Quality of Life Scale (BQOL)
The QOLI contains 153 items that measure global life satisfaction as well as objective and subjective quality of life (Lehman 1988; Lehman 1995). It has eight domains (for example, living situations, daily activities and functioning, family relations, social relations). Rated on a seven‐point scale (1 = terrible, 2 = unhappy, 3 = mostly dissatisfied, 4 = equally satisfied and dissatisfied, 5 = mostly satisfied, 6 = pleased, and 7 = delighted), with higher scores indicating better quality of life. It was used by Baker 2006, Bellack 2006, Drake 1998a, Essock 2006 and Lehman 1993.
b. World Health Organization's Quality of Life scale (WHOQOL‐BREF)
The WHOQOL‐BREF is a 26‐item scale (Skevington 2004) assessing physical health, psychological well being, social relationships, and environmental factors (for example, home environment, recreation, access to health care, physical safety and financial resources). It also contains two general items and each item is rated on a five‐point scale (1 to 5, with higher scores = better quality) (used by Madigan 2013).
c. Client Satisfaction Questionaire (CSQ)
The CSF questionnaire (CSQ) (Larsen 1979) is a self‐report instrument that consists of eight items designed to measure global patient satisfaction of services provided and if they met their needs or approval. The items are rated on a four‐point scale (minimum of 1 = no definitely not to maximum 4 = very satisfied), with a minimum score of 8 and maximum of 32 and higher scores indicating greater satisfaction (used by Hjorthoj 2013).
4.4 Social functioning: social function scales measure degree of social assessment and basic skills necessary for community living (see Results, section 4.4, Social functioning).
a. Role Functioning Scale (RFS)
This is a self‐report scale whereby the total of four subscales measures global role functioning (Green 1987). Scores reported are summary scores derived from four independent raters. Higher scores indicate better functioning (used by Jerrell 1995a and Jerrell 1995b).
b. Social Adjustment Scale for the Severely Mentally Ill (SAS‐SMI)
An abbreviated version of the Social Adjustment Scale II is used to assess social adjustment (Wieduwilt 1999), with a self‐reported scale composed of 24 items covering seven areas including social, family and work functioning designed specifically for use with schizophrenic populations. Scores range from 1 to 7, with a high score indicating poor outcome (used by Jerrell 1995a and Jerrell 1995b).
c. Social Functioning Scale (SFS)
A self‐report scale developed for people with schizophrenia which enumerates basic skills necessary for community living and performance (Birchwood 1990), the SFS is a 79‐item questionnaire that uses a four‐point rating scale (zero to 3) of frequency or ability. Items are grouped into seven domains. Raw scores for each subscale are converted to a standard score; overall functioning is based on the mean standard score (Burns 2007). Higher standardised scores indicate better functioning (range 55 to 135) (Birchwood 1990). This was used by Barrowclough 2001 and O'Connell 2018.
d. The Social and Occupational Functioning Scale (SOFAS)
SOFAS was derived from the GAF scale and is used to assess levels of physical and mental functioning in social and work settings (Burns 2007; Goldman 1992). Scored similarly to the GAF (see above) by an observer it ranges from zero to 100 with zero representing inadequate information. Higher scores indicate better outcomes (used by Bonsack 2011 and Edwards 2006).
e. Service Utilisation Rating Scale (SURS)
This measures inpatient and outpatient attendance and medication usage (Mihalopoulos 1999) (used by Edwards 2006).
Excluded studies
In the current update (May 2018 search), we excluded (with reasons) 28 studies/reports or trials: six were not randomised, 15 did not include participants with a concurrent diagnosis of severe mental illness and substance misuse and five studies did not use a psychosocial intervention. One study identified from an on‐going trial list Castle 2002 (was abandoned due to lack of funds according to email from the primary author and excluded as there was no usable data) and another from the 'to be assessed' list (Odom 2005) was excluded as feedback from the author indicated there was no usable or published data.
In the 2013 update, we excluded 46 studies or trials identified through the initial search (July 2012 search): five were not randomised, 30 did not include participants with a concurrent diagnosis of severe mental illness and substance misuse, 10 studies used a non‐psychosocial intervention or did not include a specific substance misuse treatment programme, and one trial had no usable data. Four further full text articles that were identified through subsequent searches (Bagoien 2013; Sigmon 2000; Smeerdijk 2012; Weiss 2009) were excluded.
In the 2008 review, we excluded 68 studies (this did not include related studies, please see Characteristics of excluded studies). Twenty‐six were not randomised or used a quasi‐randomisation method, 18 did not have participants with a concurrent diagnosis of severe mental illness and substance misuse, and 14 used a non‐psychosocial intervention or did not include a specific substance misuse treatment programme. A further 10 RCTs were excluded either due to high attrition rates or unclear reporting (attempts were made to contact all authors for further information). One study previously listed in the 2008 review as ongoing (Sitharthan 1999) was excluded in the current review. Two studies previously included in the 2008 review (Schmitz 2002; Weiss 2007) were excluded as all the participants had bipolar disorder so this criteria was applied retrospectively as well as prospectively.
Two studies were listed as awaiting assessment (Meister 2010; Odom 2005). Both are dissertations: one requires translation from German to English. Further correspondence indicated no publication arose from latter dissertation so this was moved to the excluded category as there were no data available.
We found 12 studies where we require more information from authors or text translated. WE have placed these in awaiting assessment until we have a response.There are currently five ongoing studies.
Risk of bias in included studies
For a summary of the overall risk of bias in the included trials please see Figure 1 and Figure 2.
Allocation
1. Random generation
All 41 studies were stated to be randomised. Some used blocking or stratification methods in the sequence to obtain evenly balanced groups or different proportions (2:1) for each intervention, site, or to control for various demographic variables (type or degree of substance use, gender or psychiatric diagnosis). Fourteen studies stated the sequence was computer‐generated (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Bonsack 2011; Chandler 2006; Eack 2015; Edwards 2006; Essock 2006; Graham 2016; Hjorthoj 2013; Madigan 2013; Maloney 2006; Naeem 2005; Petry 2013), but often it was unclear how the sequence was generated (for example, random number table). Nine studies used urn randomisation or placed cards in envelopes that were shuffled to produce a random sequence (Bellack 2006; Bogenschutz 2014; Gouzoulis‐Mayfrank 2015; Jerrell 1995a; Jerrell 1995b; Kemp 2007; Lehman 1993; McDonell 2013; McDonell 2017). Five other studies mentioned that a numbered table or random sequence was used to generate the sequence or that the sequence was stratified (Kavanagh 2004; Nagel 2009; Rosenblum 2014; Swanson 1999; Tracy 2007), but it was not clear if a computer was involved as no further details were provided. The 27 studies listed above (with the exception of Maloney 2006) were classified as low risk of selection bias as they provided some details of the allocation process or further particulars were provided by the researchers. Some participants in the Maloney 2006 study were not randomised due to procedural difficulties. The remaining studies did not provide enough details of how the allocation sequence was generated to make a judgement so were classified as of unclear quality with a moderate risk of selection bias and an overestimate of positive effect.
2. Allocation concealment
Two studies provided a full description of the methods used to generate the random sequence and allocation concealment (Barrowclough 2010; Barrowclough 2014). Ten studies provided some details or made explicit their method used for allocation concealment. Two studies used urn method randomisation (Gouzoulis‐Mayfrank 2015; McDonell 2013), which has a low risk of bias if used properly, and some confirmed this via personal emails. Eight trials stated that allocation concealment was achieved by a third party or researcher who was independent of the treating team (Barrowclough 2001; Chandler 2006; Eack 2015; Edwards 2006; Graham 2016; Hjorthoj 2013; Madigan 2013; Rosenblum 2014), but often no further details were provided. The studies listed above were judged as low risk as it was implied that the allocation concealment was adequate. Three trials (Maloney 2006; Hickman 1997; Swanson 1999 ) were judged high risk of bias as the researcher or therapist was involved with allocating patients or affected randomisation schedule. Six studies used sealed envelopes or patients selected a card (Baker 2006; Bogenschutz 2014; Bonsack 2011; Kemp 2007; McDonell 2017; Naeem 2005). However, it was not clear if the envelopes were opaque or if other measures were taken to ensure concealment, so these were judged as unclear risk. The remaining studies were classified as of unclear quality with a moderate risk of selection bias and overestimate of positive effect as no details were provided regarding allocation concealment, but this may be due to incomplete reporting and not how the study was conducted.
Blinding
1. Performance bias
We classified blinding in respect to primary outcomes for performance and detection bias. Due to intervention characteristics, that is being a therapy or model of service, we assumed the participants and clinicians as being implicitly not blind to treatment assignment when considering performance bias. Therefore, we judged performance bias of all trials to be of high risk of bias since it is not possible to blind participants or personnel for a psychosocial intervention.
2. Detection bias
Overall, 20 studies stated that independent raters were blinded to allocation when assessing clinical ratings of mental state or substance use, or the primary outcome involved urine or saliva samples. For 15 other studies it was unclear if the raters were blind to treatment as this was not stated. Six studies (Gouzoulis‐Mayfrank 2015; Graeber 2003; Kemp 2007; Maloney 2006; Nagel 2009; O'Connell 2018) were judged at high risk of bias because it was not clearly stated that the outcome assessors were blind to treatment allocation and it was therefore possible to assess the risk of bias in these studies with higher confidence for clinical‐based ratings. In three of these studies blinding status would not influence the primary outcome data as these were administrative measures (hospital readmissions, convictions, time to first outpatient appointment etc), however they were still judged high risk as less effort may have been made to follow‐up those in the control arm.
Incomplete outcome data
We only rated risk of incomplete outcome data in respect to the primary outcome. The number of participants lost to treatment or evaluation across studies ranged from 0% to 65%. The following trials were judged as adequately addressing incomplete outcome data and were rated as low risk of attrition bias because there were no missing outcome data (Hickman 1997; Lehman 1993; Swanson 1999), or less than 25% attrition with no differences between treatment groups (Barrowclough 2014; Bogenschutz 2014; Graham 2016; Rosenblum 2014) and for one study (Graeber 2003) there were no missing values for the primary outcome.
The following trials were rated as high risk. Bellack 2006 excluded 46 of 175 participants after they were randomised, a further 19 participants because they did not become engaged in treatment, and a further 27 were lost to follow‐up. Therefore, a high proportion of participants (92/175, 53%) were excluded from the analysis, which may have a clinically relevant bias in intervention effect estimates. The attrition rate was greater than 50% for Hellerstein 1995 (at eight months), Gouzoulis‐Mayfrank 2015 (at 12 months) and Bond 1991a, Godley 1994 (at 18 months) and so data from these trials were excluded from the analysis as per protocol. In the Chandler 2006 trial the attrition rate for the primary outcome measure was 37% (68/182); and for the controls no interviews were conducted to ascertain their whereabouts (moved from area, re incarcerated or died) and this may have led to severe bias. More than half the urine samples for the primary outcome were missing in Petry 2013. Six further trials were rated as high risk as more than 40% of participants were lost to follow‐up; no reasons were given for them being missing or a full intention‐to‐treat (ITT) analysis was not reported (Baker 2002; Eack 2015; Maloney 2006; Jerrell 1995a; Jerrell 1995b; O'Connell 2018).
Many, but not all, included studies provided reasons for attrition. Reasons given included: some of the participants died during the trial, some could not be contacted or moved elsewhere, and some withdrew. Eight studies reported their results based on a full ITT analysis with all missing data imputed for primary outcomes using appropriate methods (Barrowclough 2010; Bechdolf 2011; Bonsack 2011; Edwards 2006; Hjorthoj 2013; McDonell 2017; Naeem 2005; Nagel 2009). These were rated as unclear as all imputation strategies can bias study results. The remaining trials were rated as 'unclear'. They either did not address this issue, presented insufficient information of attrition or exclusions to permit judgement (that is, no reasons for missing data provided or numbers lost to evaluation not stated for each group) or did not report a full ITT analysis with imputed missing values (Baker 2006; Barrowclough 2001; Bond 1991b; Burnam 1995; Drake 1998a; Essock 2006; Kavanagh 2004; Kemp 2007; Madigan 2013; Maloney 2006; McDonell 2013; Morse 2006; Tracy 2007).
Selective reporting
The following studies were rated as high quality in reporting outcomes with a low risk of reporting bias (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014;Eack 2015; Graham 2016; Hjorthoj 2013; McDonell 2013; McDonell 2017; Petry 2013; Rosenblum 2014) as the pre‐specified outcomes listed in the trial protocol were fully reported (cases or means, SD and number (n) for each outcome at specific time points) in the study report. Conversely, five studies were rated as low quality with a high risk of reporting bias (Godley 1994; Maloney 2006; Morse 2006; O'Connell 2018; Tracy 2007) as they presented data in a way we could not consider as free of suggestion of selective outcome reporting. For these studies, there were no usable data or data were reported incompletely for each treatment arm or in a way (for example, as correction matrix, graphically or in a mixed‐methods model) that they could not be entered in a meta‐analysis. For the rest of the studies the risk of bias was assessed as unclear with a moderate risk of reporting bias due to insufficient information to permit judgement of yes or no; there was no protocol to assess the presence of selective reporting.
Other potential sources of bias
Overall risk of other potential sources of bias was rated as low or unclear. Most were publicly funded trials. No declaration of interest was made by authors, and we are assuming there was none to be made. However, many study authors were active pioneers in developing and the implementation of the experimental intervention model across the scientific community and clinical world. This raises the issue of how researcher beliefs could affect the entire process of evaluating an intervention in an RCT. Although conscious of this issue, we decided not to make any attempt to rate it as it is very difficult to judge, and erroneous quantification could drive bias into our conclusions.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings for the main comparison. Integrated models of care compared to standard care for both severe mental illness and substance misuse.
| INTEGRATED MODELS OF CARE compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: outpatient Intervention: INTEGRATED MODELS OF CARE Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | INTEGRATED MODELS OF CARE | |||||
| Leaving the study early: Lost to treatment Follow‐up: mean 36 months | 212 per 1000 | 231 per 1000 (174 to 308) | RR 1.09 (0.82 to 1.45) | 603 (3 studies) | ⊕⊕⊝⊝ low1,2 | Data were available for 36 months only |
| Adverse event: Death Follow‐up: mean 36 months | 28 per 1000 | 33 per 1000 (11 to 101) | RR 1.18 (0.39 to 3.57) | 421 (2 studies) | ⊕⊕⊝⊝ low3,4 | Data were available for 36 months only |
| Substance use: any change ‐ Alcohol use (not in remission) Follow‐up: mean 36 months | 500 per 1000 | 575 per 1000 (420 to 780) | RR 1.15 (0.84 to 1.56) | 143 (1 study) | ⊕⊕⊝⊝ low4,5 | Data were available for 36 months only |
| Substance use: any change ‐ Drug use (not in remission) Follow‐up: mean 36 months | 650 per 1000 | 578 per 1000 (409 to 812) | RR 0.89 (0.63 to 1.25) | 85 (1 study) | ⊕⊕⊝⊝ low4,5 | Data were available for 36 months only |
| Mental state | See comment | See comment | Not estimable | 0 (3 studies) | See comment | No data were available for this important outcome. |
| Global state: average score (GAF scale of 1 ‐ 100)Follow‐up: mean 12 months | The mean global assessment of functioning in the intervention groups was 0.70 higher (‐2.07 lower to 3.47 higher) |
171 (1 study) | ⊕⊕⊝⊝ low5,6 | NOTE: The GAF measures functioning on a scale of 1 to 100 and the difference detected in this single trial is not of clinical importance. | ||
| Quality of life/life satisfaction: avearge score (General life satisfaction) Quality of Life Interview section, scale of 1 to 7 Follow‐up: mean 12 months | The mean general life satisfaction in the intervention groups was 0.02 higher (‐0.28 lower to 0.32 higher) | 372 (2 studies) | ⊕⊕⊕⊝ moderate3 | The scale is from 1 to 7 and the very small difference was not statistically significant and is not of clinical importance. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as SERIOUS: Blinding of participants and personnel in all three trials was not possible and performance bias was rated as unclear risk of bias. Similarly, all trials were at an unclear risk of detection bias. 2 Imprecision: Rated as SERIOUS: The number of events is less than 300 and the overall sample size is small. 3 Risk of bias: Rated as SERIOUS: Blinding of participants and personnel in both trials was not possible and performance bias was rated as unclear risk of bias. Similarly all trials were at an unclear risk of detection bias as outcomes ratings were not blinded. 4 Imprecision: Rated as SERIOUS: The event rate is very low and the 95% confidence interval is wide. 5 Risk of bias: Rated as SERIOUS: Blinding of participants and personnel was not possible and performance bias was rated as unclear risk of bias. Similarly there was an unclear risk of detection bias as outcomes ratings were not blinded. 6 Imprecision: Rated as SERIOUS: The confidence interval is very wide and the sample size small.
Summary of findings 2. Non‐integrated models of care ‐ Assertive community treatment only/Intensive case management/Specialised case management services versus standard care for both severe mental illness and substance misuse.
| ASSERTIVE COMMUNITY TREATMENT INTENSIVE CASE MANAGEMENT compared to STANDARD CARE for people with both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: outpatient Intervention: ASSERTIVE COMMUNITY TREATMENT/INTENSIVE CASE MANAGEMENT Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | NON‐INTEGRATED MODELS OF CARE OR INTENSIVE CASE MANAGEMENT | |||||
| Leaving the study early: Lost to treatment Follow‐up: mean 12 months | 239 per 1000 | 289 per 1000 (174 to 475) | RR 1.21 (0.73 to 1.99) | 134 (3 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Adverse event: Death ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Death was not measured in any of the trials. |
| Substance use: Alcohol ‐ average score (C‐DIS‐R computer program for Diagnostic Interview Schedule: average score) Follow‐up: mean 12 months | See comment | See comment | See comment | Data were skewed from one trial and there was no analysis of the difference between randomised arms. | ||
| Substance use: Drug ‐ average score C‐DIS‐R computer program for Diagnostic Interview Schedule: average score Follow‐up: mean 12 months | See comment | See comment | See comment | Data were skewed from one trial and there was no analysis of the difference between randomised arms. | ||
| Mental state: Average score Schizophrenia symptoms on C‐DIS‐R computer program for Diagnostic Interview Schedule: average score Follow‐up: mean 12 months | See comment | See comment | See comment | Data were skewed from one trial and there was no analysis of the difference between randomised arms. | ||
| Global State: Average score (Role Functioning Scale, high = better functioning) Follow‐up: mean 12 months | The mean global assessment of functioning in the intervention groups was 0.70 higher (1.56 lower to 2.96 higher) | 50 (1 study) | ⊕⊝⊝⊝ very low3,4 | NOTE: the scale is 1 to 7 and the difference observed is not of clinical importance and is not statistically significant. | ||
| Quality of life/life satisfaction: (Quality of Life Interview section, scale of 1 to 7) Follow‐up: mean 12 months | See comment | Data were skewed from one trial and there was no analysis of the difference between randomised arms. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as VERY SERIOUS: Random generation and allocation concealment was not adequately reported and the risk of bias is unclear. Both performance and detection bias was unclear as blinding was not performed or was unclearly reported. Attrition was unclear or very high (57% in Bond‐Anderson 91) so overall the risk of bias was rated as very serious. 2 Imprecision: Rated as SERIOUS: The confidence interval is wide and the sample size is very small. 3 Risk of bias: Rated as SERIOUS: Blinding of participants and personnel was not possible and performance bias was rated as unclear risk of bias. Similarly there was an unclear risk of detection bias as outcomes ratings were participant/clinician mediated. 4Imprecision: Rated as VERY SERIOUS: The sample size is small and the confidence interval is very wide.
Summary of findings 3. Cognitive behavioural therapy versus standard care for both severe mental illness and substance misuse.
| COGNITIVE BEHAVIOUR THERAPY compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: outpatient Intervention: COGNITIVE BEHAVIOUR THERAPY Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | COGNITIVE BEHAVIOUR THERAPY | |||||
| Leaving the study early: Lost to treatment Follow‐up: mean 3 months | 97 per 1000 | 108 per 1000 (43 to 277) | RR 1.12 (0.44 to 2.86) | 152 (2 studies) | ⊕⊕⊝⊝ low1,2 | |
| Adverse event: Death | See comment | See comment | Not estimable | See comment | Death was not measured in any of the trials. | |
| Substance use: Alcohol | See comment | See comment | Not estimable | See comment | See comment | Naeem 2005 measured alcohol together with drug use in the Health of the Nation Outcome (HoNOS) scale. Edwards did not report on alcohol. |
| Substance use: Drug (Cannabis) Percentage of participants who used cannabis in last 4 weeks Follow‐up: mean 6 months | 500 per 1000 | 650 per 1000 (395 to 1000) | RR 1.30 (0.79 to 2.15) | 47 (1 study) | ⊕⊝⊝⊝ very low1,3 | Data for other drugs were skewed and were not compared between intervention and control. |
| Mental state: average score (Insight scale) Follow‐up: by 3 months | The mean mental state in the intervention groups was 0.52 higher (0.78 lower to 1.82 higher) | 105 (1 study) | ⊕⊕⊝⊝ low1,3 | The difference noted is unlikely to be clinically important | ||
| Social functioning: Average score* The Social and Occupational Functioning Scale (SOFAS): scale of 1 to 100 Follow‐up: mean 6 months | The mean global assessment of functioning in the intervention groups was 4.70 lower (14.52 lower to 5.12 higher) | 47 (1 study) | ⊕⊝⊝⊝ very low1,4 | * Global state data were not reported by the trials The other trial in this comparison (Naeem 2005) measured Functioning with the HoNOS scale. Data were skewed and meta‐analysis was not possible |
||
| Quality of life/ life satisfaction | See comment | Not estimable | See comment | No study measured Quality of life | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as SERIOUS: The participants and personnel were not blinded and performance bias may be present. Missing data were addressed by Last Observation Carried Forward in Edward 2006 but attrition bias may be present as loss to follow‐up was 30% at 9 months. 2 Imprecision: Rated as SERIOUS: The event rate is low and the confidence interval is wide. 3 Imprecision: Rated as VERY SERIOUS: The event rate is low, the sample size small and the confidence interval is wide. 4 Imprecision: Rated as VERY SERIOUS: The confidence interval is very wide and the sample size small.
Summary of findings 4. Contingency management versus standard care for both severe mental illness and substance misuse.
| CONTINGENCY MANAGEMENT compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: community Intervention: Contingency Management Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | CONTINGENCY MANAGEMENT | |||||
| Leaving the study early: Lost to treatment Follow‐up: mean 3 months | 315 per 1000 | 487 per 1000 (355 to 664) | RR 1.55 (1.13 to 2.11) | 255 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Adverse event: Death ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | None of the trials measured death as an outcome. |
| Substance use: Alcohol | See comment | See comment | Data were skewed and no between‐arm comparison were reported. | |||
| Substance use: Drug (non‐alcohol) Number with stimulant‐positive urine test Follow‐up: mean 6 months | 647 per 1000 | 537 per 1000 (421 to 686) | RR 0.83 (0.65 to 1.06) | 176 (1 study) | ⊕⊕⊝⊝ low1,3 | |
| Service use*: Number hospitalised Follow‐up: mean 6 months | 106 per 1000 | 22 per 1000 (5 to 98) | RR 0.21 (0.05 to 0.93) | 176 (1 study) | ⊕⊕⊝⊝ low1,4 | Predefinded outcome of Mental state data were skewed and unuseable. |
| Global state | See comment | See comment | Not estimable | ‐ | See comment | Neither trial measured Global state |
| Quality of life/life satisfaction | See comment | See comment | Not estimable | ‐ | See comment | Neither trial measured Quality of life |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as SERIOUS: Blinding was not possible so performance bias was rated as an unclear risk of bias. Primary outcome in both trials was urinalysis so detection bias was unlikely, Attrition bias was an unclear risk and greater than 20% across groups in both trials. 2 Imprecision: We did not downgrade. The confidence interval is wide although it does not cross 1 indicating appreciable harm.
3 Impresion: Rated as SERIOUS: The event rate is low (less than 300 according to GRADE) and the confidence interval includes 1 and appreciable benefit. 4 Imprecision: Rated as SERIOUS: The event rate is very low and the confidence interval is wide.
Summary of findings 5. Motivational interviewing versus standard care for both severe mental illness and substance misuse.
| MOTIVATIONAL INTERVIEWING compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: hospital and community Intervention: MOTIVATIONAL INTERVIEWING Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | MOTIVATIONAL INTERVIEWING | |||||
| Lost to treatment Follow‐up: mean 6 months | 156 per 1000 | 266 per 1000 (94 to 560) | RR 1.71 (0.63 to 4.64) | 62 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Death Follow‐up: mean 18 months | 40 per 1000 | 42 per 1000 (3 to 629) | RR 1.04 (0.07 to 15.73) | 49 (1 study) | ⊕⊝⊝⊝ very low3,4 | |
| Alcohol use Not abstaining from alcohol Follow‐up: mean 6 months | 923 per 1000 | 332 per 1000 (157 to 692) | RR 0.36 (0.17 to 0.75) | 28 (1 study) | ⊕⊝⊝⊝ very low5,6 | |
| Drug (non‐alcohol) use Polydrug consumption levels measured by Opiate Treatment Index (OT) Follow‐up: mean 12 months | The mean drug (non‐alcohol) use in the intervention groups was 0.07 lower (0.56 lower to 0.42 higher) | 89 (1 study) | ⊕⊝⊝⊝ very low7,8 | |||
| Mental state Symptom Checklist 90‐revised ‐ General Severity Index: Scale 0 to 4: Average score Follow‐up: mean 3 months | The mean mental state in the intervention groups was 0.19 lower (0.59 lower to 0.21 higher) | 30 (1 study) | ⊕⊝⊝⊝ very low9,10 | This is unlikely to be of clinical significance. | ||
| Global Assessment of Functioning GAF scale of 1‐ 100 Follow‐up: mean 12 months | The mean global assessment of functioning in the intervention groups was 2.30 higher (1.30 lower to 5.90 higher) | 54 (1 study) | ⊕⊝⊝⊝ very low1,9 | The difference is unlikely to be of clinical significance given the scale is from 1 to 100. | ||
| Quality of life/life satisfaction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | None of the eight trials contributing data to this comparison measured general life satisfaction. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as SERIOUS: This single trial has unclear risk of allocation concealment and an unclear risk for performance bias. 2 Imprecision: Rated as VERY SERIOUS: The sample size is very small (N = 62), the event rate very low and the confidence interval very wide. 3 Risk of bias: Rated as SERIOUS: Allocation concealment was unclear and blinding was not possible so performance bias is unclear. Assessors were not blinded so there is a high risk of detection bias. Attrition was 29% at 18 months. 4 Imprecision: Rated as VERY SERIOUS: The event rate is very small and the confidence interval is very wide. 5 Risk of bias: Rated as SERIOUS: Random generation, allocation concealment and performance bias (lack of blinding) posed an unclear risk of bias. Detection bias was likely as assessors were not blinded. 6 Imprecision: Rated as VERY SERIOUS: The sample size was extremely small (N = 30), and the event rate very low. 7 Risk of bias: Rated as SERIOUS: Selection bias was unclear and performance bias may be present as personnel and participants were not blinded. Assessors were blinded. Attrition bias is a high risk as 44% were lost to follow‐up by 12 months. 8 Imprecision: Rated as VERY SERIOUS: The single trial has a very small sample size (N = 30) and imprecision is very likely. 9 Imprecision: Rated as VERY SERIOUS: The single trial sample size is very small (N = 54) and the confidence interval is very wide. 10 Risk of bias: Rated as VERY SERIOUS: Selection bias was a high risk as allocation concealment was modified to allow for participant refusal and to minimise disruption to the treatment programme. Performance and detection bias were unclear as blinding was not possible for personnel and participants and assessor blinding was not reported.
Summary of findings 6. Skills training versus standard care for both severe mental illness and substance misuse.
| SKILLS TRAINING compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: community and outpatient Intervention: SKILLS TRAINING Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | SKILLS TRAINING | |||||
| Leaving the study early: Lost to treatment Follow‐up: mean 12 months | 138 per 1000 | 196 per 1000 (28 to 1000) | RR 1.42 (0.20 to 10.1) | 122 (3 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Adverse event: Death | 79 per 1000 | 12 per 1000 (2 to 112) |
RR 0.15 (0.02 to 1.42 |
121 (1 study) |
⊕⊕⊝⊝ low3,4,5 | |
| Substance use: Alcohol (C‐DIS‐R average score) Follow‐up: mean 12 months | See comment | See comment | Not estimable | See comment | See comment | Data were skewed and no estimate of effect was calculated between randomised arms. |
| Substance use: Drug (non‐alcohol) (C‐DIS‐R average score) Follow‐up: mean 12 months | See comment | See comment | Not estimable | See comment | See comment | Data were skewed and no estimate of effect was calculated between randomised arms. |
| Mental state | See comment | See comment | Not estimable | No data were available for analyses. | ||
| Social functioning*: Role Functioning Scale: scale 1 to 7 Follow‐up: mean 12 months | The mean assessment of functioning in the intervention groups was 1.07 higher (1.15 lower to 3.29 higher) | 47 (1 study) | ⊕⊝⊝⊝ very low6,7 | * Global state data were not reported. NOTE: the scale is 1 to 7 and the difference observed is not of clinical importance and is not statistically significant. |
||
| Quality of life/life satisfaction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Neither trial measured general life satisfaction. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of Bias: Rated as VERY SERIOUS: Blinding was not possible and performance bias may be present. It was unclear whether assessors were blinded and detection bias may be present. Attrition bias was a high risk in Hellerstein 1995 with 47% loss to follow‐up at 4 months and 64% at 8 months with no reasons for drop‐outs provided and not addressed in analysis. 2 Imprecision: Rated as SERIOUS: The event rate was low (zero events in one trial) with a wide confidence interval. 3 Risk of Bias: Rated as SERIOUS: The trial outcomes were self‐reported abstinence and there is no report of whether raters were blind to treatment allocation when assessing the participant's self‐report.
4 Inconsistency: this is not possible to evaluate as there is only a single study.
5 Imprecision: The single trial has a small sample size of 121 with very low event rates. The confidence interval crosses the line of no effect and appreciable harm. 6 Risk of Bias: Rated as SERIOUS: Blinding was not possible and performance bias may be present. It was unclear whether 7 Imprecision: Rated as VERY SERIOUS: The single trial has a very small sample size (N =47) and the confidence interval is wide.
Summary of findings 7. Cognitive behavioural therapy + motivational interviewing versus standard care for both severe mental illness and substance misuse.
| COGNITIVE BEHAVIOURAL THERAPY + MOTIVATIONAL INTERVIEWING compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: outpatient Intervention: COGNITIVE BEHAVIOUR THERAPY + MOTIVATIONAL INTERVIEWING Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | COGNITIVE BEHAVIOUR THERAPY + MOTIVATIONAL INTERVIEWING | |||||
| Leaving the study early: Lost to treatment Follow‐up: mean 12 months | 178 per 1000 |
176 per 1000 (110 to 283) |
RR 0.99 (0.62 to 1.59) | 327 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Adverse event: Death Follow‐up: mean 12 months | 32 per 1000 |
19 per 1000 (6 to 56) |
RR 0.60 (0.20 to 1.76) | 603 (4 studies) | ⊕⊕⊝⊝ low2,3 | |
| Substance use: Alcohol ( Estimated daily consumption in previous month) Follow‐up: mean 12 months | See comment | Moderate | 46 (1 study) | See comment | Data were skewed from one trial and there was no analysis of the difference between randomised arms. | |
| Substance use: Drug (non‐alcohol) use (Average number of different drugs used during the past month measured by the Opiate Treatment Index) Follow‐up: mean 6 months |
32 per 1000 The average number of different drugs used in past month in the intervention group was 0.19 higher (0.22 lower to 0.60 higher) |
19 per 1000 (6 to 56) | 119 (1 study) | ⊕⊕⊝⊝ low4,5 | ||
| Relapse:* Relapse at 3 months after 9 months of treatment Follow‐up: mean 12 months | 667 per 1000 | 333 per 1000 (160 to 693) | RR 0.50 (0.24 to 1.04) | 36 (1 study) | ⊕⊝⊝⊝ very low6,7 | * Clinically important change in mental state not reported. |
| Global State: GAF scale of 1‐ 100 Follow‐up: mean 12 months | The mean Global Assessment of Functioning in the intervention groups was 1.24 higher (1.86 lower to 4.34 higher) | 445 (4 studies) | ⊕⊝⊝⊝ very low8,9,10 | NOTE: The GAF measures functioning on a scale of 1 to 100 and the difference detected in this single trial is not of clinical importance. | ||
| Quality of life / life satisfaction: Brief Quality of Life Scale Follow‐up: mean 6 months | The mean general life satisfaction in the intervention groups was 0.58 higher (0.00 to 1.16 higher) | 110 (1 study) | ⊕⊕⊝⊝ low11,12 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as SERIOUS: The single trial which reported on 12 month loss to treatment had adequate random generation and allocation concealment. However, we down‐graded it for possible performance bias as participants and clinicians were not blinded. Detection bias was a low risk as outcome assessors were blinded. 2 Imprecision: Rated as SERIOUS: The event rate is low and the confidence interval is wide and includes the line of no effect and appreciable harm. 3 Risk of bias: Rated as SERIOUS: The four trials included in this meta‐analysis were well‐conducted. A lack of blinding is unlikely to affect measurement of death. However, attrition was > 20% in all four trials and although missing data were balanced across groups, there is an unclear risk of bias due to attrition bias. 4 Risk of bias: Rated as SERIOUS: Random generation and allocation concealment were unclear and blinding was not possible for participants or clinicians. Attrition was high at 20% at 12 months, but missing outcome data were balanced between groups. 5 Imprecision: Rated as SERIOUS: The sample size is small and the confidence interval is wide. 6 Risk of bias: Rated as SERIOUS: The risk of attrition bias is unclear (22% across both groups at 18 months) despite missing outcome balanced between groups. A lack of blinding of participants and clinicians may result in performance bias. 7 Imprecision: Rated as VERY SERIOUS: The event rate is extremely low in this very small single trial (N = 36) and the confidence interval is wide. 8 Risk of bias: Rated as SERIOUS: Attrition was > 20% in all four trials and although missing data were balanced across groups, there is an unclear risk of bias due to attrition bias. 9 Inconsistency: Rated as SERIOUS: Heterogeneity was present (Chi² = 5.20, df = 3 (P = 0.16); I² = 42%). One trial (Barrowclough) showed significant improvement in the treatment group compared with the others, but we were unable to explain the reason for this. 10 Imprecision: Rated as SERIOUS: Four trials provided data for this meta‐analysis. The confidence interval is wide. 11 Risk of bias: Rated as SERIOUS: Blinding of participants and clinicians was not possible and performance bias may be a risk. Attrition was 25% at 6 months and missing data were not balanced across interventions. Missing outcomes are enough to induce clinically relevant bias in observed effect size. 12 Imprecision: Rated as SERIOUS: The sample size of the single trial is small and the confidence interval is wide.
Summary of findings 8. Cognitive behavioural therapy + psychosocial rehabilitation versus standard care for both severe mental illness and substance misuse.
| COGNITIVE BEHAVIOUR THERAPY and PSYCHOSOCIAL REHABILITATION compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: jail and community Intervention: COGNITIVE BEHAVIOUR THERAPY and PSYCHOSOCIAL REHABILITATION Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | COGNITIVE BEHAVIOUR THERAPY and PSYCHOSOCIAL REHABILITATION | |||||
| Leavint the study early: Loss to Treatment Follow‐up: mean 12 months | See comment | See comment | Not estimable | 61 (1 study) | ⊕⊝⊝⊝ very low1,2 | Data were only reported per trial and not per randomised arm. |
| Adverse event: Death ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | The trial did not measure death as an outcome. |
| Substance use: Alcohol ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. Data were skewed and not compared between arms. |
| Substance use: Drug (non‐alcohol) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. The data were skewed and was not compared between arms. |
| Mental state ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. The data were skewed and was not compared between arms. |
| Global State‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. The data were skewed and was not compared between arms. |
| Quality of life/life satisfaction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. The data were skewed and was not compared between arms. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as VERY SERIOUS: Although the random generation is reported as computer‐generated the numbers in the arms vary and no report is made as to whether randomisation was done in a ratio fashion. Blinding was not done and performance bias may be unclear and detection bias is high risk as the assessors were not blinded. There is a high risk of selective reporting bias as few outcomes are reported per arm and mainly by site. 2 Imprecision: Rated as VERY SERIOUS: The sample size is small and any estimate of effect (had it been reported per arm) is highly likely to be imprecise.
Summary of findings 9. Cognitive behavioural therapy + intensive case management versus standard care for both severe mental illness and substance misuse.
| COMBINED COGNITIVE BEHAVIOUR THERAPY and INTENSIVE CASE MANAGEMENT compared to STANDARD CARE for both severe mental illness and substance misuse | ||||||
| Patient or population: people with both severe mental illness and substance misuse Settings: jail and community Intervention: COMBINED COGNITIVE BEHAVIOUR THERAPY and INTENSIVE CASE MANAGEMENT Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | COMBINED COGNITIVE BEHAVIOUR THERAPY and INTENSIVE CASE MANAGEMENT | |||||
| Leaving the study early: Loss to Treatment Follow‐up: mean 12 months | See comment | See comment | Not estimable | 59 (1 study) | ⊕⊝⊝⊝ very low1,2 | Unable to use data ‐ (no breakdown by treatment arms) |
| Adverse event: Death | See comment | See comment | Not estimable | ‐ | See comment | The trial did not measure death. |
| Substance use: alcohol ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. Data were skewed and not compared between arms. |
| Substance use: Drug (non‐alcohol) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. Data were skewed and not compared between arms. |
| Mental state ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. Data were skewed and not compared between arms. |
| Global State ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. Data were skewed and not compared between arms. |
| Quality of life/life satisfaction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This trial focused on criminal outcomes of jail and offences. Data were skewed and not compared between arms. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Risk of bias: Rated as VERY SERIOUS: Although the random generation is reported as computer‐generated the numbers in the arms vary and no report is made as to whether randomisation was done in a ratio fashion. Blinding was not done and performance bias may be unclear and detection bias is high risk as the assessors were not blinded. There is a high risk of selective reporting bias as few outcomes are reported per arm and mainly by site. 2 Risk of bias: Rated as VERY SERIOUS: Although the random generation is reported as computer‐generated the numbers in the arms vary and no report is made as to whether randomisation was done in a ratio fashion. Blinding was not done and performance bias may be unclear and detection bias is high risk as the assessors were not blinded. There is a high risk of selective reporting bias as few outcomes are reported per arm and mainly by site.
Comparison 1: Integrated models of care versus standard care
See Table 1. Data for this comparison came from four trials (Burnam 1995; Chandler 2006; Drake 1998a; Essock 2006).
1.1 Leaving the study early: 1. Lost to treatment ‐ by 36 months
By the end of treatment (36 months) we found no clear difference in the likelihood of participants being lost to treatment from the pooled results of Chandler 2006; Drake 1998a and Essock 2006 (treatment group 24% lost, control group 21% lost) (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.82 to 1.45; participants = 603; studies = 3). Statistical heterogeneity was not present (Chi² = 1.95, df = 2 (P = 0.38); I² = 0%). (Analysis 1.1).
1.1. Analysis.
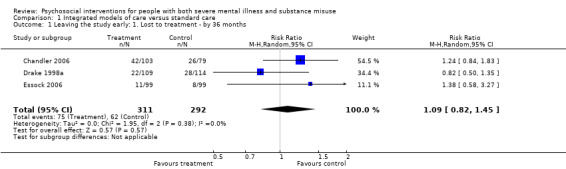
Comparison 1 Integrated models of care versus standard care, Outcome 1 Leaving the study early: 1. Lost to treatment ‐ by 36 months.
1.2 Leaving the study early: 2. Lost to evaluation
The control group for Burnam 1995 were 46% more likely to be lost to evaluation by three months (treatment group 15% lost, control group 28% lost), although this is not a clear difference between groups (RR 0.54, 95% CI 0.27 to 1.08; participants = 132; studies = 1). Six months data (Burnam 1995; Essock 2006) also did not reveal any clear difference between groups (RR 0.69, 95% CI 0.27 to 1.73; participants = 330; studies = 2). Numbers lost to evaluation at nine months (RR 0.76, 95% CI 0.49 to 1.19; participants = 132; studies = 1), 12 months (RR 0.54, 95% CI 0.22 to 1.29; participants = 198; studies = 1), 24 months (RR 1.00, 95% CI 0.47 to 2.12; participants = 198; studies = 1), and 36 (RR 0.76, 95% CI 0.35 to 1.66; participants = 603; studies = 3) were also equivocal. For the 36‐month data, we combined the results from three studies (Chandler 2006; Drake 1998a; Essock 2006) in a meta‐analysis . There was considerable statistical heterogeneity (Chi² = 7.70, df = 2 (P = 0.02); I² = 74%). Closer inspection of the forest plot indicated a higher retention rate in the treatment group in Drake 1998a, likely to account for this heterogeneity. (Analysis 1.2).
1.2. Analysis.
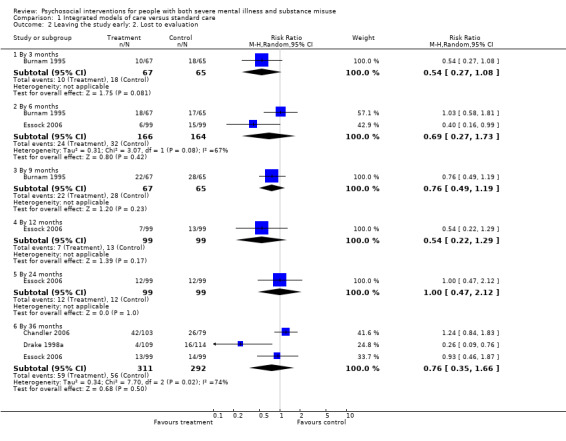
Comparison 1 Integrated models of care versus standard care, Outcome 2 Leaving the study early: 2. Lost to evaluation.
1.3 Adverse event: 1. Death ‐ by 36 months
We found no clear differences in the pooled results of Drake 1998a and Essock 2006 with regards to the likelihood of participants dying by the end of 36 months of treatment (treatment 3% died, control 3% died; (RR 1.18, 95% CI 0.39 to 3.57; participants = 421; studies = 2). Statistical heterogeneity was not present (Chi² = 0.68, df = 1, P = 0.41; I² = 0%). (Analysis 1.3).
1.3. Analysis.
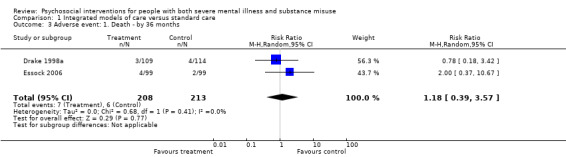
Comparison 1 Integrated models of care versus standard care, Outcome 3 Adverse event: 1. Death ‐ by 36 months.
1.4 Substance use 1. Clinically important change (not in remission) ‐ by 36 months
Drake 1998a reported data for remission from substance use (Analysis 1.4) The likelihood of participants not being in remission for alcohol use in the group was treatment 57% and 50% in the control group, which is not a clear difference between treatment groups (RR 1.15, 95% CI 0.84 to 1.56; participants = 143; studies = 1). The likelihood of participants not being in remission for drug use in the treatment group was 58% and 65% in the control group, which is also not a clear difference between treatment groups (RR 0.89, 95% CI 0.63 to 1.25; participants = 85; studies = 1).
1.4. Analysis.
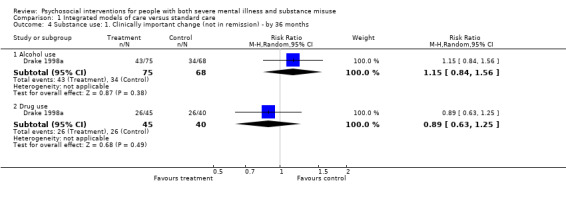
Comparison 1 Integrated models of care versus standard care, Outcome 4 Substance use: 1. Clinically important change (not in remission) ‐ by 36 months.
1.5 Substance use: 2. Average score for progress towards recovery (SATS, low = poor)
Drake 1998a reported average SATS scores (Analysis 1.5). No clear difference in participants average SATS scores were found by six months (mean difference (MD) 0.07, 95% CI ‐0.28 to 0.42; participants = 203; studies = 1) or 36 months (MD 0.11, 95% CI ‐0.41 to 0.63; participants = 203; studies = 1).
1.5. Analysis.
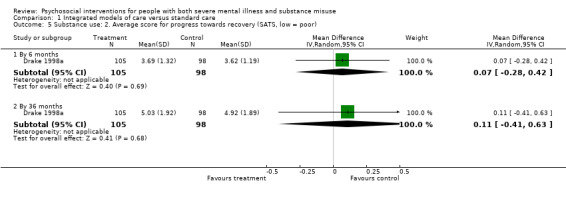
Comparison 1 Integrated models of care versus standard care, Outcome 5 Substance use: 2. Average score for progress towards recovery (SATS, low = poor).
1.6 ‐ 1.11 Substance use: skewed data
Further outcome data related to substance use (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10) contained skewed data and are presented in 'Other data' tables.
1.6. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 6 Substance use: 3a. Alcohol use: average score (AUS) (skewed data).
| Substance use: 3a. Alcohol use: average score (AUS) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Drake 1998a | Treatment | 3.12 | 1.03 | 70 |
| Drake 1998a | Control | 2.97 | 1.09 | 65 |
| Essock 2006 | Treatment | 2.90 | 1.30 | 68 |
| Essock 2006 | Control | 3.00 | 1.10 | 61 |
| 12 months | ||||
| Drake 1998a | Treatment | 3.17 | 1.05 | 75 |
| Drake 1998a | Control | 2.84 | 1.23 | 66 |
| Essock 2006 | Treatment | 2.80 | 1.30 | 64 |
| Essock 2006 | Control | 3.10 | 1.0 | 62 |
| 18 months | ||||
| Drake 1998a | Treatment | 3.07 | 1.15 | 75 |
| Drake 1998a | Control | 2.79 | 1.10 | 65 |
| Essock 2006 | Treatment | 2.80 | 1.20 | 65 |
| Essock 2006 | Control | 2.90 | 1.20 | 65 |
| 24 months | ||||
| Drake 1998a | Treatment | 2.98 | 1.07 | 73 |
| Drake 1998a | Control | 2.79 | 1.16 | 67 |
| Essock 2006 | Treatment | 2.60 | 1.20 | 60 |
| Essock 2006 | Control | 3.0 | 1.20 | 63 |
| 30 months | ||||
| Drake 1998a | Treatment | 2.86 | 1.09 | 72 |
| Drake 1998a | Control | 2.96 | 1.18 | 65 |
| Essock 2006 | Treatment | 2.80 | 1.20 | 58 |
| Essock 2006 | Control | 2.80 | 1.20 | 59 |
| 36 months | ||||
| Drake 1998a | Treatment | 2.70 | 1.12 | 74 |
| Drake 1998a | Control | 2.82 | 1.16 | 68 |
| Essock 2006 | Treatment | 2.70 | 1.0 | 58 |
| Essock 2006 | Control | 2.80 | 1.30 | 60 |
1.7. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 7 Substance use: 3b. Alcohol use: average number of days using in last 6 months (skewed data).
| Substance use: 3b. Alcohol use: average number of days using in last 6 months (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 month | ||||
| Drake 1998a | Treatment | 56.80 | 56.40 | 75 |
| Drake 1998a | Control | 47.50 | 58.40 | 68 |
| Essock 2006 | Treatment | 36.60 | 50.1 | 65 |
| Essock 2006 | Control | 38.60 | 54.20 | 53 |
| 12 month | ||||
| Drake 1998a | Treatment | 59.10 | 53.30 | 75 |
| Drake 1998a | Control | 42.80 | 52.90 | 68 |
| Essock 2006 | Treatment | 35.80 | 50.00 | 64 |
| Essock 2006 | Control | 44.80 | 58.80 | 61 |
| 18 month | ||||
| Drake 1998a | Treatment | 53.80 | 57.80 | 75 |
| Drake 1998a | Control | 33.50 | 44.40 | 68 |
| Essock 2006 | Treatment | 30.00 | 46.90 | 64 |
| Essock 2006 | Control | 37.00 | 46.80 | 66 |
| 24 month | ||||
| Drake 1998a | Treatment | 52.40 | 55.90 | 75 |
| Drake 1998a | Control | 31.00 | 43.00 | 68 |
| Essock 2006 | Treatment | 31.30 | 46.70 | 57 |
| Essock 2006 | Control | 47.60 | 62.80 | 64 |
| 30 month | ||||
| Drake 1998a | Treatment | 54.80 | 60.90 | 75 |
| Drake 1998a | Control | 54.00 | 63.00 | 68 |
| Essock 2006 | Treatment | 39.40 | 52.40 | 60 |
| Essock 2006 | Control | 42.80 | 59.80 | 62 |
| 36 month | ||||
| Drake 1998a | Treatment | 46.40 | 53.60 | 75 |
| Drake 1998a | Control | 43.60 | 57.30 | 68 |
| Essock 2006 | Treatment | 33.50 | 47.30 | 60 |
| Essock 2006 | Control | 31.10 | 49.90 | 59 |
1.8. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 8 Substance use: 4a. Drug use: average score (DUS) (skewed data).
| Substance use: 4a. Drug use: average score (DUS) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Drake 1998a | Treatment | 3.07 | 1.10 | 45 |
| Drake 1998a | Control | 3.26 | 1.03 | 39 |
| Essock 2006 | Treatment | 3.50 | 1.10 | 81 |
| Essock 2006 | Control | 3.30 | 1.20 | 61 |
| 12 months | ||||
| Drake 1998a | Treatment | 2.93 | 1.10 | 45 |
| Drake 1998a | Control | 3.17 | 1.15 | 39 |
| Essock 2006 | Treatment | 3.10 | 1.20 | 75 |
| Essock 2006 | Control | 3.20 | 1.20 | 61 |
| 18 months | ||||
| Drake 1998a | Treatment | 2.80 | 1.23 | 45 |
| Drake 1998a | Control | 2.90 | 1.16 | 39 |
| Essock 2006 | Treatment | 3.10 | 1.30 | 76 |
| Essock 2006 | Control | 3.10 | 1.50 | 62 |
| 24 months | ||||
| Drake 1998a | Treatment | 2.73 | 1.21 | 44 |
| Drake 1998a | Control | 2.94 | 1.15 | 39 |
| Essock 2006 | Treatment | 2.80 | 1.30 | 67 |
| Essock 2006 | Control | 3.10 | 1.40 | 63 |
| 30 months | ||||
| Drake 1998a | Treatment | 2.71 | 1.28 | 43 |
| Drake 1998a | Control | 3.03 | 1.19 | 40 |
| Essock 2006 | Treatment | 3.10 | 1.40 | 71 |
| Essock 2006 | Control | 2.90 | 1.50 | 55 |
| 36 months | ||||
| Drake 1998a | Treatment | 2.61 | 1.23 | 44 |
| Drake 1998a | Control | 2.89 | 1.12 | 40 |
| Essock 2006 | Treatment | 2.80 | 1.30 | 67 |
| Essock 2006 | Control | 2.90 | 1.40 | 59 |
1.9. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 9 Substance use: 4b. Drug use: average number of days using in last 6 months (skewed data).
| Substance use: 4b. Drug use: average number of days using in last 6 months (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Drake 1998a | Treatment | 42.60 | 53.50 | 45 |
| Drake 1998a | Control | 45.30 | 44.30 | 40 |
| Essock 2006 | Treatment | 40.80 | 50.90 | 75 |
| Essock 2006 | Control | 44.10 | 57.70 | 55 |
| 12 months | ||||
| Drake 1998a | Treatment | 26.90 | 33.40 | 45 |
| Drake 1998a | Control | 47.30 | 54.50 | 40 |
| Essock 2006 | Treatment | 36.80 | 55.30 | 76 |
| Essock 2006 | Control | 36.30 | 51.70 | 60 |
| 18 months | ||||
| Drake 1998a | Treatment | 41.60 | 54.90 | 45 |
| Drake 1998a | Control | 34.40 | 53.30 | 40 |
| Essock 2006 | Treatment | 37.70 | 54.20 | 78 |
| Essock 2006 | Control | 39.90 | 51.70 | 64 |
| 24 months | ||||
| Drake 1998a | Treatment | 35.50 | 48.00 | 45 |
| Drake 1998a | Control | 37.00 | 48.80 | 40 |
| Essock 2006 | Treatment | 28.20 | 47.20 | 69 |
| Essock 2006 | Control | 39.10 | 56.20 | 66 |
| 30 months | ||||
| Drake 1998a | Treatment | 33.90 | 50.00 | 45 |
| Drake 1998a | Control | 46.40 | 53.10 | 40 |
| Essock 2006 | Treatment | 35.00 | 51.30 | 73 |
| Essock 2006 | Control | 35.90 | 54.50 | 60 |
| 36 months | ||||
| Drake 1998a | Treatment | 38.20 | 54.70 | 45 |
| Drake 1998a | Control | 51.50 | 67.20 | 40 |
| Essock 2006 | Treatment | 31.70 | 47.80 | 71 |
| Essock 2006 | Control | 32.10 | 49.00 | 60 |
1.10. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 10 Substance use: 5. Avearage score (SATS, low = poor) (skewed data).
| Substance use: 5. Avearage score (SATS, low = poor) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Essock 2006 | Treatment | 3.3 | 1.60 | 93 |
| Essock 2006 | Control | 3.3 | 1.50 | 83 |
| 12 months | ||||
| Drake 1998a | Treatment | 3.99 | 1.60 | 105 |
| Drake 1998a | Control | 4.10 | 1.59 | 98 |
| Essock 2006 | Treatment | 3.80 | 1.70 | 87 |
| Essock 2006 | Control | 3.40 | 1.50 | 84 |
| 18 months | ||||
| Drake 1998a | Treatment | 4.19 | 1.77 | 105 |
| Drake 1998a | Control | 4.32 | 1.75 | 98 |
| Essock 2006 | Treatment | 4.00 | 1.90 | 88 |
| Essock 2006 | Control | 3.90 | 1.80 | 84 |
| 24 months | ||||
| Drake 1998a | Treatment | 4.50 | 1.76 | 105 |
| Drake 1998a | Control | 4.51 | 1.73 | 98 |
| Essock 2006 | Treatment | 4.20 | 2.0 | 79 |
| Essock 2006 | Control | 3.7 | 1.90 | 83 |
| 30 months | ||||
| Drake 1998a | Treatment | 4.76 | 1.92 | 105 |
| Drake 1998a | Control | 4.48 | 1.88 | 98 |
| Essock 2006 | Treatment | 4.10 | 2.0 | 80 |
| Essock 2006 | Control | 4.0 | 1.9 | 75 |
| 36 months | ||||
| Essock 2006 | Treatment | 4.40 | 1.80 | 78 |
| Essock 2006 | Control | 4.30 | 2.10 | 79 |
1.12 Mental state: 1. Average score (BPRS, high = poor) (skewed data)
We found that the BPRS scores reported by Drake 1998a and Essock 2006 contained wide confidence intervals (skewed data) and have presented these in 'Other data' tables (Analysis 1.12).
1.12. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 12 Mental state: 1. Average score (BPRS, high = poor) (skewed data).
| Mental state: 1. Average score (BPRS, high = poor) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Drake 1998a | Treatment | 42.20 | 12.18 | 105 |
| Drake 1998a | Control | 42.85 | 12.09 | 98 |
| Essock 2006 | Treatment | 46.10 | 13.70 | 78 |
| Essock 2006 | Control | 43.40 | 11.20 | 75 |
| 12 months | ||||
| Drake 1998a | Treatment | 41.57 | 10.80 | 105 |
| Drake 1998a | Control | 43.19 | 11.96 | 98 |
| Essock 2006 | Treatment | 46.20 | 13.80 | 86 |
| Essock 2006 | Control | 43.60 | 11.50 | 82 |
| 18 months | ||||
| Drake 1998a | Treatment | 43.89 | 11.58 | 105 |
| Drake 1998a | Control | 44.37 | 13.39 | 98 |
| Essock 2006 | Treatment | 45.60 | 12.50 | 89 |
| Essock 2006 | Control | 45.00 | 12.10 | 84 |
| 24 months | ||||
| Drake 1998a | Treatment | 42.45 | 11.03 | 105 |
| Drake 1998a | Control | 43.32 | 12.36 | 98 |
| Essock 2006 | Treatment | 43.70 | 12.40 | 79 |
| Essock 2006 | Control | 45.90 | 12.80 | 86 |
| 30 months | ||||
| Drake 1998a | Treatment | 39.63 | 9.60 | 105 |
| Drake 1998a | Control | 42.27 | 12.13 | 98 |
| Essock 2006 | Treatment | 46.60 | 16.10 | 83 |
| Essock 2006 | Control | 44.60 | 12.80 | 77 |
| 36 months | ||||
| Drake 1998a | Treatment | 40.89 | 10.82 | 105 |
| Drake 1998a | Control | 41.11 | 11.69 | 98 |
| Essock 2006 | Treatment | 43.00 | 11.20 | 85 |
| Essock 2006 | Control | 43.20 | 12.50 | 81 |
1.13 Global state: 1. Average score (GAF, low = poor)
Only Essock 2006 reported data for global state (Analysis 1.13). We found no clear differences for average global functioning scores (GAF) at six months (MD 1.10, 95% CI ‐1.58 to 3.78; participants = 162; studies = 1), 12 months (MD 0.70, 95% CI ‐2.07 to 3.47; participants = 171; studies = 1), 18 months (MD 1.00, 95% CI ‐1.58 to 3.58; participants = 176; studies = 1), 24 months (MD 1.70, 95% CI ‐1.18 to 4.58; participants = 166; studies = 1), 30 months (MD ‐0.60, 95% CI ‐3.56 to 2.36; participants = 164; studies = 1) or 36 months (MD 0.40, 95% CI ‐2.47 to 3.27; participants = 170; studies = 1).
1.13. Analysis.
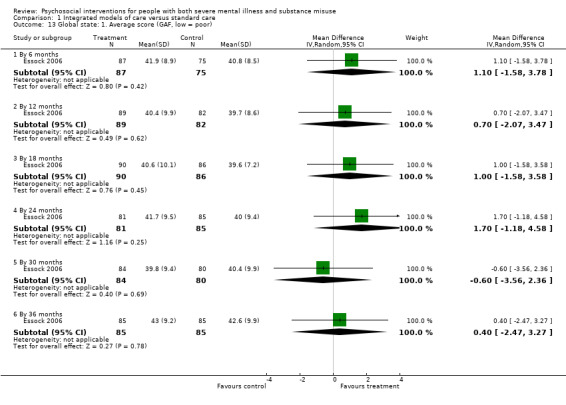
Comparison 1 Integrated models of care versus standard care, Outcome 13 Global state: 1. Average score (GAF, low = poor).
1.14 Global state: 2. Forensic measures (skewed data)
Data reported by Chandler 2006 on arrests, convictions, felony, hospitalisation and jail were skewed and are presented in 'Other data' tables. (Analysis 1.14).
1.14. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 14 Global state: 2. Forensic measures (skewed data).
| Global state: 2. Forensic measures (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Arrests ‐ 36 months | ||||
| Chandler 2006 | Treatment | 2.21 | 4.06 | 103 |
| Chandler 2006 | Control | 2.61 | 3.31 | 79 |
| Convictions ‐ 36 months | ||||
| Chandler 2006 | Treatment | 0.59 | 0.90 | 103 |
| Chandler 2006 | Control | 0.73 | 0.98 | 79 |
| Felony ‐ 36 months | ||||
| Chandler 2006 | Treatment | 0.31 | 0.46 | 103 |
| Chandler 2006 | Control | 0.28 | 0.50 | 79 |
| Hospital or jail ‐ 3 months | ||||
| Essock 2006 | Treatment | 118 | 221 | 99 |
| Essock 2006 | Control | 112 | 210 | 99 |
| Jail days ‐ 36 months | ||||
| Chandler 2006 | Treatment | 60.71 | 73.03 | 103 |
| Chandler 2006 | Control | 59.39 | 66.86 | 79 |
1.15 Quality of life/ life satisfaction: Average general score (QOLI, range 1‐7, low = poor)
The pooled results of Drake 1998a and Essock 2006 revealed no clear difference between treatment groups in average general life satisfaction (QOLI) scores by 6 months (MD ‐0.11, 95% CI ‐0.41 to 0.20; participants = 361; studies = 2), 12 months (MD 0.02, 95% CI ‐0.28 to 0.32; participants = 372; studies = 2), 18 months (MD 0.09, 95% CI ‐0.27 to 0.44; participants = 377; studies = 2), 24 months (MD 0.02, 95% CI ‐0.29 to 0.33; participants = 370; studies = 2), 30 months (MD 0.02, 95% CI ‐0.27 to 0.32; participants = 366; studies = 2), and 36 months (MD 0.10, 95% CI ‐0.18 to 0.38; participants = 373; studies = 2). Statistical heterogeneity was not present at any of the six time points (for example, 24 months: Chi² = 1.09, df = 1, P = 0.30; I² = 8%). (Analysis 1.15).
1.15. Analysis.
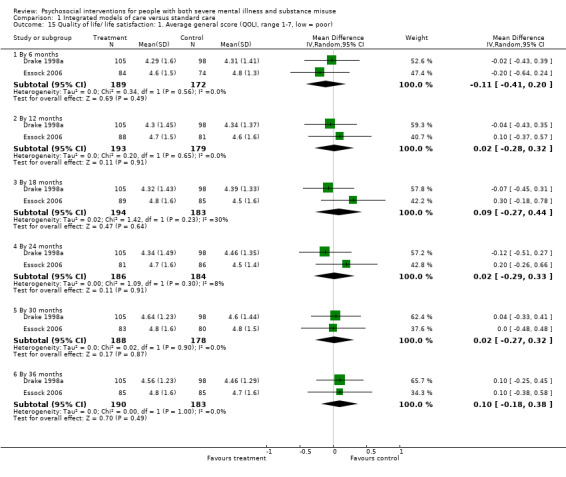
Comparison 1 Integrated models of care versus standard care, Outcome 15 Quality of life/ life satisfaction: 1. Average general score (QOLI, range 1‐7, low = poor).
1.16 Service use: 1. Days in stable community residences (not in hospital)
We found that the pooled results of two studies (Drake 1998a; Essock 2006) for average number of days spent in stable community residences (not in hospital) by 12 months were equivocal (MD ‐10.00, 95% CI ‐38.61 to 18.60; participants = 378; studies = 2), and also at 24 months (MD 7.40, 95% CI ‐6.32 to 21.12; participants = 203; studies = 1), and 36 months (MD 5.17, 95% CI ‐9.20 to 19.55; participants = 364; studies = 2). Statistical heterogeneity was not present (Chi² = 0.31, df = 1, P = 0.58; I² = 0%). (Analysis 1.16).
1.16. Analysis.
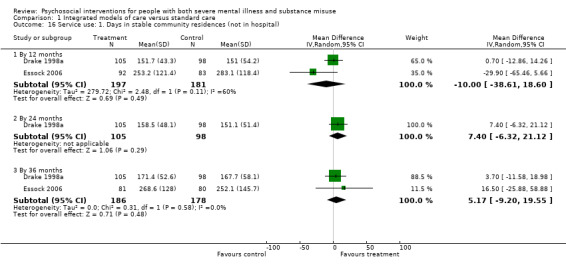
Comparison 1 Integrated models of care versus standard care, Outcome 16 Service use: 1. Days in stable community residences (not in hospital).
1.17 Service use: 2. Number hospitalised ‐ during the 36‐month study period
(Essock 2006) found no clear differences between treatment groups for the likelihood of hospitalisation by 36 months (treatment 42% hospitalised, control 48% hospitalised; RR 0.88, 95% CI 0.64 to 1.19; participants = 198; studies = 1) (Analysis 1.17 ).
1.17. Analysis.
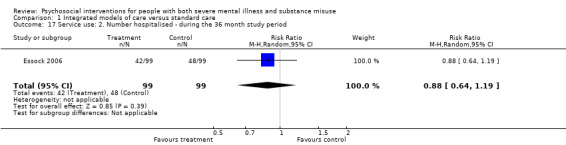
Comparison 1 Integrated models of care versus standard care, Outcome 17 Service use: 2. Number hospitalised ‐ during the 36 month study period.
1.18 Service use: 3. Relapse (hospitalisation days and crisis care) ‐ 36 months (skewed data)
Data for relapse (measured as average number of hospitalisation days and crisis care) were skewed and are presented in 'Other data' tables (Analysis 1.18).
1.18. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 18 Service use: 3. Relapse (hospitalisation days and crisis care) ‐ 36 months (skewed data).
| Service use: 3. Relapse (hospitalisation days and crisis care) ‐ 36 months (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Chandler 2006 | Treatment | 1.25 | 3.27 | 103 |
| Chandler 2006 | Control | 5.03 | 13.88 | 79 |
| Drake 1998a | Treatment | 10 | 31.62 | 105 |
| Drake 1998a | Control | 12.45 | 35.65 | 98 |
| Essock 2006 | Treatment | 23 | 68 | 99 |
| Essock 2006 | Control | 26 | 48 | 99 |
1.19 Service use: 4. Medication hours ‐ 36 months (skewed data)
Data reported by Chandler 2006 for average number of hours requiring medication were skewed and are presented in 'Other data' tables (Analysis 1.19).
1.19. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 19 Service use: 4. Medication hours ‐ 36 months (skewed data).
| Service use: 4. Medication hours ‐ 36 months (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Chandler 2006 | Treatment | 6.52 | 0.82 | 86 |
| Chandler 2006 | Control | 3.02 | 0.60 | 49 |
1.20 Service use: 5. Various measures (skewed data)
These data were also skewed and presented in 'Other data' tables (Analysis 1.20).
1.20. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 20 Service use: 5. Various measures (skewed data).
| Service use: 5. Various measures (skewed data) | |||||
|---|---|---|---|---|---|
| Study | Treatment | Mean | SD | N | Notes |
| Days institutionalised (hospital or incarcerated) ‐ 36 months (site 2) | |||||
| Essock 2006 | Integrated models of care | 139 | 262 | 48 | P = 0.02 using Mann Whitney U test (non‐parametric test). |
| Essock 2006 | TAU | 158 | 254 | 50 | |
| Days in hospital ‐ 36 months (site 2) | |||||
| Essock 2006 | Integrated models of care | 32 | 91 | 48 | P = 0.002 using Mann Whitney U test (non‐parametric test). |
| Essock 2006 | TAU | 41 | 60 | 50 | |
1.21 ‐ 1.24 Homelessness (skewed data)
Data regarding per cent of time on the street (Analysis 1.21) and time in independent housing (Analysis 1.22), average number of days in stable housing (Analysis 1.23) other various measures of homelessness (Analysis 1.24) were skewed and are presented in 'Other data' tables.
1.21. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 21 Homelessness: 1. Proportion of time on the street ‐ past 60 days.
| Homelessness: 1. Proportion of time on the street ‐ past 60 days | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 3 months | ||||
| Burnam 1995 | Treatment | 20.88 | 37.14 | 112 |
| Burnam 1995 | Control | 19.85 | 40.68 | 45 |
| 6 months | ||||
| Burnam 1995 | Treatment | 25.95 | 41.40 | 112 |
| Burnam 1995 | Control | 21.04 | 42.44 | 47 |
| 9 months | ||||
| Burnam 1995 | Treatment | 21.03 | 48.77 | 109 |
| Burnam 1995 | Control | 25.14 | 39.45 | 36 |
1.22. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 22 Homelessness: 2. Proportion of time in independent housing ‐ past 60 days.
| Homelessness: 2. Proportion of time in independent housing ‐ past 60 days | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 3 months | ||||
| Burnam 1995 | Treatment | 14.32 | 40.80 | 112 |
| Burnam 1995 | Control | 9.67 | 40.33 | 45 |
| 6 months | ||||
| Burnam 1995 | Treatment | 18.98 | 41.64 | 112 |
| Burnam 1995 | Control | 10.83 | 37.06 | 47 |
| 9 months | ||||
| Burnam 1995 | Treatment | 17.84 | 43.58 | 109 |
| Burnam 1995 | Control | 26.31 | 46.83 | 36 |
1.23. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 23 Homelessness: 3. Average number of days in stable housing (skewed data).
| Homelessness: 3. Average number of days in stable housing (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Morse 2006 | Treatment | 8.19 | 9.68 | 46 |
| Morse 2006 | Control | 5.02 | 8.62 | 49 |
| 12 months | ||||
| Morse 2006 | Treatment | 14.18 | 12.33 | 46 |
| Morse 2006 | Control | 11.34 | 12.04 | 49 |
| 18 months | ||||
| Morse 2006 | Treatment | 17.01 | 12.51 | 46 |
| Morse 2006 | Control | 10.55 | 12.87 | 49 |
| 24 months | ||||
| Morse 2006 | Treatment | 18.29 | 12.12 | 46 |
| Morse 2006 | Control | 12.59 | 13.27 | 49 |
1.24. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 24 Homelessness: 4. Various measures (skewed data).
| Homelessness: 4. Various measures (skewed data) | |||||
|---|---|---|---|---|---|
| Study | Treatment | Mean | SD | N | Notes |
| Time on streets (%) ‐ 3 months | |||||
| Burnam 1995 | Treatment | 24.77 | 42.21 | 54 | |
| Burnam 1995 | Control | 19.85 | 40.68 | 45 | |
| Time on streets (%) ‐ 6 months | |||||
| Burnam 1995 | Treatment | 29.67 | 44.86 | 45 | |
| Burnam 1995 | Control | 21.04 | 42.44 | 47 | |
| Time on streets (%) ‐ 9 months | |||||
| Burnam 1995 | Treatment | 19.73 | 47.28 | 43 | |
| Burnam 1995 | Control | 25.14 | 39.45 | 36 | |
| Days in stable community residence ‐ 24 months | |||||
| Essock 2006 | Treatment | 264.30 | 130.40 | 89 | |
| Essock 2006 | Control | 245.60 | 143.90 | 85 | |
| Time in independent housing in past 60 days ‐ 3 months | |||||
| Burnam 1995 | Treatment | 1.72 | 33.79 | 54 | |
| Burnam 1995 | Control | 9.67 | 40.33 | 45 | |
| Time in independent housing in past 60 days ‐ 6 months | |||||
| Burnam 1995 | Treatment | 35.20 | 48.78 | 45 | |
| Burnam 1995 | Control | 10.83 | 37.06 | 47 | |
| Time in independent housing in past 60 days ‐ 9 months | |||||
| Burnam 1995 | Treatment | 16.30 | 45.84 | 43 | |
| Burnam 1995 | Control | 26.31 | 46.83 | 36 | |
Comparison 2: Non‐integrated models of care Assertive community treatment/Intensive case management/Specialised management services versus standard care
See Table 2. Four trials reported useable data for this comparison (Bond 1991a; Bond 1991b; Jerrell 1995b; Lehman 1993).
2.1 Leaving the study early 1. Lost to treatment
Pooled results of Bond 1991a; Bond 1991b and Jerrell 1995b showed no clear difference between groups in numbers lost to treatment by six months (treatment 27% lost, control 22% lost; RR 1.23, 95% CI 0.73 to 2.06; participants = 134; studies = 3) Longer‐term evaluations at 12 months (treatment 28% lost, control 24% lost; RR 1.21, 95% CI 0.73 to 1.99; participants = 134; studies = 3), and 18 months (treatment 51% lost, control 37% lost; RR 1.35, 95% CI 0.83 to 2.19; participants = 134; studies = 3) also did not reveal any important difference between groups. Statistical heterogeneity was not present at six months, 12 months, 18 months, 24 months, 30 months or 36 months. (Analysis 2.1)
2.1. Analysis.
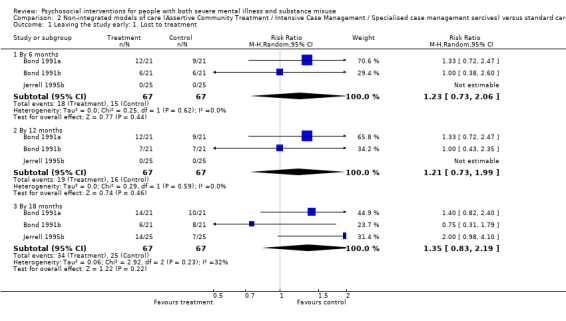
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 1 Leaving the study early: 1. Lost to treatment.
2.2 Leaving the study early 2. Lost to evaluation
We found no meaningful difference in the pooled results (Bond 1991b; Jerrell 1995b; Lehman 1993) for lost to evaluation by six months (treatment 10% lost, control 10% lost;(RR 1.00, 95% CI 0.38 to 2.60; participants = 121; studies = 3) and by 12 months (treatment 12% lost, control 12% lost; RR 1.00, 95% CI 0.43 to 2.35; participants = 121; studies = 3) Pooled results (Bond 1991b; Jerrell 1995b) at 18 months also revealed no major differences between treatment groups (treatment 43% lost, control 33% lost; RR 1.26, 95% CI 0.48 to 3.30; participants = 92; studies = 2). Statistical heterogeneity was not present at six, 12, or 18 months. (Analysis 2.2)
2.2. Analysis.
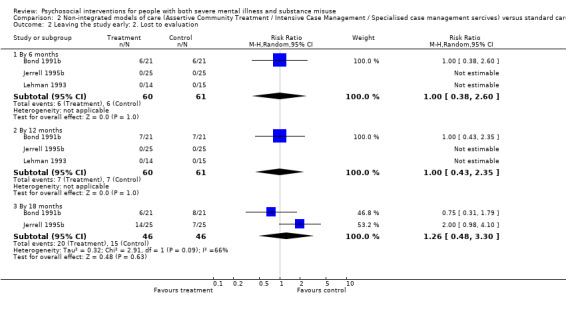
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 2 Leaving the study early: 2. Lost to evaluation.
2.3 ‐ 2.4 Substance use
Data for substance use Analysis 2.3, and Analysis 2.4, were skewed and are presented in 'Other data' tables.
2.3. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 3 Substance use: 1. Average scores on various scales (skewed data).
| Substance use: 1. Average scores on various scales (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Alcohol ‐ 6 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 2.99 | 3.09 | 24 |
| Jerrell 1995b | Control | 2.80 | 3.49 | 25 |
| Alcohol ‐ 12 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 2.03 | 2.82 | 24 |
| Jerrell 1995b | Control | 2.95 | 3.50 | 25 |
| Alcohol ‐ 18 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 1.90 | 2.88 | 10 |
| Jerrell 1995b | Control | 1.39 | 2.52 | 18 |
| Drugs ‐ 6 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 4.56 | 4.94 | 24 |
| Jerrell 1995b | Control | 4.84 | 5.69 | 25 |
| Drugs ‐ 12 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 3.74 | 4.19 | 24 |
| Jerrell 1995b | Control | 5.99 | 7.46 | |
| Drugs ‐ 18 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 2.70 | 4.11 | 10 |
| Jerrell 1995b | Control | 2.22 | 3.46 | 18 |
| General ‐ 12 months (ASI) | ||||
| Lehman 1993 | Treatment | 39.80 | 89.80 | 14 |
| Lehman 1993 | Control | 58.50 | 81.0 | |
2.4. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 4 Substance use: 2. Average score (USS, high = poor (1=client not abstinent, 5 client mets criteria for severe use) (skewed data).
| Substance use: 2. Average score (USS, high = poor (1=client not abstinent, 5 client mets criteria for severe use) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Morse 2006 | ||||
| Morse 2006 | Treatment (ACT) | 2.98 | 1.31 | 54 |
| Morse 2006 | Control | 2.93 | 1.19 | 49 |
| 12 months | ||||
| Morse 2006 | ||||
| Morse 2006 | Treatment (ACT) | 2.86 | 1.32 | 54 |
| Morse 2006 | Control (Standard care) | 2.78 | 1.18 | 49 |
| 18 months | ||||
| Morse 2006 | ||||
| Morse 2006 | Treatment (ACT) | 3.02 | 1.26 | 54 |
| Morse 2006 | Control (Standard care | 2.69 | 1.10 | 49 |
| 24 months | ||||
| Morse 2006 | ||||
| Morse 2006 | Treatment (ACT) | 2.70 | 1.28 | 54 |
| Morse 2006 | Control (Standard care) | 2.62 | 1.15 | 49 |
2.5 Mental state: Average scores on various scales (skewed data)
Mental state data from various scales were skewed and are presented in 'Other data' tables. (Analysis 2.5).
2.5. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 5 Mental state: 1. Average scores on various scales (skewed data).
| Mental state: 1. Average scores on various scales (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Depression symptoms ‐ 6 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 6.02 | 4.59 | 24 |
| Jerrell 1995b | Control | 8.11 | 4.73 | 25 |
| Depression symptoms ‐ 12 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 5.81 | 5.01 | 24 |
| Jerrell 1995b | Control | 7.78 | 5.04 | 25 |
| Depression symptoms ‐ 18 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 4.40 | 4.33 | 10 |
| Jerrell 1995b | Control | 8.42 | 5.99 | 18 |
| General ‐ average score ‐ 12 months (ASI) | ||||
| Lehman 1993 | Treatment | 0.30 | 0.22 | 14 |
| Lehman 1993 | Control | 0.25 | 0.22 | 15 |
| Manic symptoms ‐ 6 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 1.99 | 1.61 | 24 |
| Jerrell 1995b | Control | 2.05 | 1.55 | 25 |
| Manic symptoms ‐ 12 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 1.66 | 1.77 | 24 |
| Jerrell 1995b | Control | 2.47 | 1.98 | 25 |
| Manic symptoms ‐ 18 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 0.80 | 0.92 | 10 |
| Jerrell 1995b | Control | 2.60 | 1.42 | 18 |
| Schizophrenia symptoms ‐ 6 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 2.67 | 3.55 | 24 |
| Jerrell 1995b | Control | 4.60 | 5.25 | 25 |
| Schizophrenia symptoms ‐ 12 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 2.65 | 3.67 | 24 |
| Jerrell 1995b | Control | 4.93 | 5.39 | 25 |
| Schizophrenia symptoms ‐ 18 months (C‐DIS‐R) | ||||
| Jerrell 1995b | Treatment | 0.90 | 1.20 | 10 |
| Jerrell 1995b | Control | 4.11 | 5.17 | 18 |
2.6 ‐ 2.8 Global state: Foresnic measures (skewed data)
Data reported by Maloney 2006 on average number of arrests (Analysis 2.6), convictions (Analysis 2.7) and days in jail ( Analysis 2.8) were skewed and are presented in 'Other data' tables.
2.6. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 6 Global state: 1. Forensic measures ‐ number of arrests (skewed data).
| Global state: 1. Forensic measures ‐ number of arrests (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 0.36 | 0.50 | 58 |
| Maloney 2006 | Control | 0.58 | 1.00 | 43 |
| 18 months | ||||
| Maloney 2006 | Treatment | 0.46 | 1.12 | 58 |
| Maloney 2006 | Control | 0.20 | 0.41 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 0.23 | 0.59 | 58 |
| Maloney 2006 | Control | 0.34 | 0.81 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 0.36 | 0.78 | 58 |
| Maloney 2006 | Control | 0.51 | 0.88 | 43 |
2.7. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 7 Global state: 2. Forensic measures ‐ number of convictions (skewed data).
| Global state: 2. Forensic measures ‐ number of convictions (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 0.30 | 0.48 | 58 |
| Maloney 2006 | Control | 0.25 | 0.62 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 0.20 | 0.88 | 58 |
| Maloney 2006 | Control | 0.22 | 0.63 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 0.27 | 0.66 | 58 |
| Maloney 2006 | Control | 0.53 | 1.10 | 43 |
2.8. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 8 Global state: 3. Forensic measures ‐ days in jail (skewed data).
| Global state: 3. Forensic measures ‐ days in jail (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 10.91 | 18.50 | 58 |
| Maloney 2006 | Control | 12.90 | 30.12 | 43 |
| 18 months | ||||
| Maloney 2006 | Treatment | 7.25 | 14.55 | 58 |
| Maloney 2006 | Control | 20.92 | 39.46 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 8.80 | 20.91 | 58 |
| Maloney 2006 | Control | 7.28 | 14.07 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 20.84 | 34.05 | 58 |
| Maloney 2006 | Control | 24.59 | 38.27 | 43 |
2.9 Social functioning: 1. Average role functioning score (RFS, high = better functioning)
There was no clear difference between the psychosocial intervention and standard care group's average role functioning (RFS) scores (Jerrell 1995b) by six months (MD ‐0.78, 95% CI ‐2.91 to 1.35; participants = 50; studies = 1) or 12 months (MD 0.70, 95% CI ‐1.56 to 2.96; participants = 50; studies = 1), although by 18 months there was a clear difference, favouring the standard care group (MD ‐2.67, 95% CI ‐5.28 to ‐0.06; participants = 29; studies = 1). The average baseline means (SD) on the RFS were similar between groups so do not explain this difference: baseline treatment 9.46 (4.11) to 10.77 (2.36) at 18 months; and baseline control 10.03 (3.87) to 13.44 (4.78) at 18 months. Note that higher scores indicate better functioning. (Analysis 2.9).
2.9. Analysis.
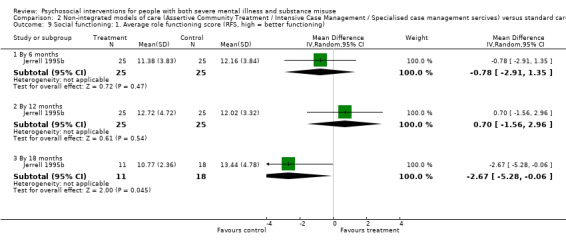
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 9 Social functioning: 1. Average role functioning score (RFS, high = better functioning).
2.10 Social functioning: 2. Average social adjustment score (RFS, high = better functioning)
There was no clear between treatment groups for average social adjustment scores (SAS) (Jerrell 1995b) by 6 months (MD ‐0.93, 95% CI ‐6.34 to 4.48; participants = 50; studies = 1), 12 months (MD 3.09, 95% CI ‐2.71 to 8.89; participants = 50; studies = 1) or 18 months (MD ‐3.75, 95% CI ‐10.12 to 2.62; participants = 29; studies = 1). (Analysis 2.10).
2.10. Analysis.
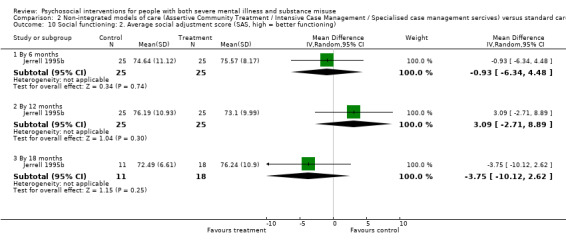
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 10 Social functioning: 2. Average social adjustment score (SAS, high = better functioning).
2.11 Quality of life/lfe satisfaction: Average score (QOLI, high = good) ‐ 12 months (skewed data)
Data for average life satisfaction (QOLI) were skewed so are presented in 'Other data' tables. (Analysis 2.11).
2.11. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 11 Quality of life/ life satisfaction: 1. Average score (QOLI, skewed data) ‐ 12 months.
| Quality of life/ life satisfaction: 1. Average score (QOLI, skewed data) ‐ 12 months | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Notes |
| Lehman 1993 | Treatment | 4.6 | 1.98 | 14 | QOLI min score=1 |
| Lehman 1993 | Control | 5.94 | 0.85 | 15 | |
2.12 Service use: Relapse (skewed data)
Bond 1991b reported % days in hospital but data were skewed and are presented in 'Other data' tables. (Analysis 2.12).
2.12. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 12 Service use: 1. Relapse (skewed data).
| Service use: 1. Relapse (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| % days in hospital | ||||
| Bond 1991b | Treatment | 13.9 | 38.80 | 14 |
| Bond 1991b | Control | 26.6 | 59.2 | 14 |
2.13 Service use: 1. Various measures ‐ 24 months (skewed data)
Data, reported by Morse 2006 relating to admissions and length of stay were all skewed data and are presented in 'Other data' tables. (Analysis 2.13). We found no pattern overall of one package of care favoured over another.
2.13. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 13 Service use: 2. Various measures ‐ 24 months (skewed data).
| Service use: 2. Various measures ‐ 24 months (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| State Operated admissions (site 1, Robert Young) | ||||
| Godley 1994 | Treatment | 0.21 | 0.43 | 14 |
| Godley 1994 | Control | 0.29 | 0.61 | 22 |
| State Operated admissions (site 2, Pilsen‐Little) | ||||
| Godley 1994 | Treatment | 1.53 | 1.46 | 17 |
| Godley 1994 | Control | 2.82 | 4.31 | 11 |
| State operated days admitted (site 1) | ||||
| Godley 1994 | Treatment | 8.36 | 22.36 | 14 |
| Godley 1994 | Control | 1.86 | 4.20 | 14 |
| State Operated days admitted (site 2) | ||||
| Godley 1994 | Treatment | 37.06 | 40.50 | 17 |
| Godley 1994 | Control | 76.91 | 110.34 | 11 |
| Private Hospital length of stay (site 1, days) | ||||
| Godley 1994 | Treatment | 8.36 | 22.36 | 14 |
| Godley 1994 | Control | 1.50 | 3.63 | 14 |
| Private Hospital length of stay (site 2, days) | ||||
| Godley 1994 | Treatment | 22.01 | 23.14 | 17 |
| Godley 1994 | Control | 17.55 | 18.04 | 11 |
2.14 Homlessness: Average number of days in stable housing (skewed data)
Morse 2006 reported average number of days in stable housing but data were skewed are presented in 'Other data' tables (Analysis 2.14).
2.14. Analysis.
Comparison 2 Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care, Outcome 14 Homelessness: 1. Average number of days in stable housing (skewed data).
| Homelessness: 1. Average number of days in stable housing (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Morse 2006 | Treatment | 5.77 | 7.42 | 54 |
| Morse 2006 | Control | 5.02 | 8.62 | 49 |
| 12 months | ||||
| Morse 2006 | Treatment | 13.87 | 11.66 | 54 |
| Morse 2006 | Control | 11.34 | 12.04 | 49 |
| 18 months | ||||
| Morse 2006 | Treatment | 18.19 | 11.61 | 54 |
| Morse 2006 | Control | 10.55 | 12.87 | 49 |
| 24 months | ||||
| Morse 2006 | Treatment | 17.78 | 12.68 | 54 |
| Morse 2006 | Control | 12.59 | 13.27 | 49 |
Comparison 3: Cognitive behavioural therapy versus standard care
See Table 3. Data for this comparison came from two trials (Edwards 2006; Naeem 2005).
3.1 Leaving the study early 1. Lost to treatment
We found that loss from treatment data (Edwards 2006; Naeem 2005) by three months were not clearly different (treatment 18%, control 23% lost; (RR 1.12, 95% CI 0.44 to 2.86; participants = 152; studies = 2). Statistical heterogeneity was not present (Chi² = 0.00, df = 1, P = 0.95; I² = 0%). (Analysis 3.1).
3.1. Analysis.
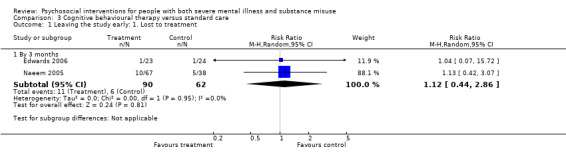
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 1 Leaving the study early: 1. Lost to treatment.
3.2 Leaving the study early: 2. Lost to evaluation
The number of participants lost to evaluation (Edwards 2006) after nine months were similar in each group (treatment 30%, control 29%; (RR 1.04, 95% CI 0.43 to 2.51; participants = 47; studies = 1). (Analysis 3.2).
3.2. Analysis.
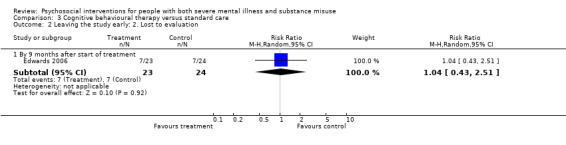
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 2 Leaving the study early: 2. Lost to evaluation.
3.3 Substance use: 1. Percentage of participants who used cannabis ‐ in last four weeks
Edwards 2006 reported % of participants using cannabis in the previous four weeks. No clear differences between treatment groups were found at three months assessment (treatment 57%, control 54%; RR 1.04, 95% CI 0.62 to 1.74; participants = 47; studies = 1) or at six months (RR 1.30, 95% CI 0.79 to 2.15; participants = 47; studies = 1). (Analysis 3.3).
3.3. Analysis.
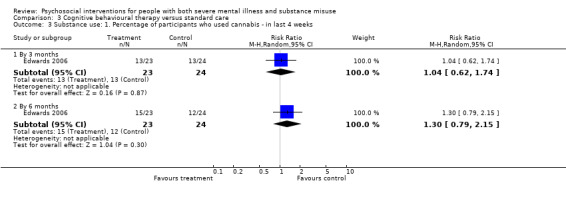
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 3 Substance use: 1. Percentage of participants who used cannabis ‐ in last 4 weeks.
3.4 Substance use: 2. Average score (various scales) (skewed data)
Various measures of substance use reporting skewed data are shown in 'Other data' tables. (Analysis 3.4).
3.4. Analysis.
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 4 Substance use: 2. Average score (various scales) (skewed data).
| Substance use: 2. Average score (various scales) (skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Time period | Mean | SD | N |
| CASUAS | |||||
| Edwards 2006 | Treatment | 3 months | 1.4 | 1.4 | 23 |
| Edwards 2006 | Control | 1.3 | 1.4 | 24 | |
| Edwards 2006 | Treatment | 6 months | 1.4 | 1.4 | 23 |
| Edwards 2006 | Control | 1.3 | 1.5 | 24 | |
| HONOS ‐ item 3 | |||||
| Naeem 2005 | Treatment | 3 months | 0.85 | 1.00 | 67 |
| Naeem 2005 | Control | 0.88 | 0.88 | 38 | |
| Naeem 2005 | |||||
| Naeem 2005 | |||||
3.5 Mental state: 1. Average insight score (Insight scale, low = poor) ‐ by three months
There was no clear difference between treatment groups on insight scores (Insight Scale) by three months (Naeem 2005) (MD 0.52, 95% CI ‐0.78 to 1.82; participants = 105; studies = 1). (Analysis 3.5).
3.5. Analysis.
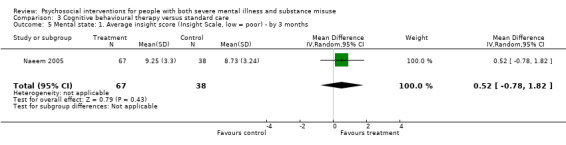
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 5 Mental state: 1. Average insight score (Insight Scale, low = poor) ‐ by 3 months.
3.6 Mental state: 2. Average score (various scales) (skewed data)
Various measures of mental state reporting skewed data are shown in 'Other data' tables. (Analysis 3.6).
3.6. Analysis.
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 6 Mental state: 2. Average score (various scales) (skewed data).
| Mental state: 2. Average score (various scales) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Anxiety symptoms ‐ 3 months (BAS) | ||||
| Naeem 2005 | Treatment | 4.41 | 2.95 | 67 |
| Naeem 2005 | Control | 4.31 | 3.41 | 38 |
| Depressive symptoms ‐ 3 months (BDI‐SF) | ||||
| Edwards 2006 | Treatment | 6.2 | 5.9 | 23 |
| Edwards 2006 | Control | 7.8 | 8.1 | 24 |
| Depressive symptoms ‐ 3 months (MADRS) | ||||
| Naeem 2005 | Treatment | 5.33 | 2.99 | 67 |
| Naeem 2005 | Control | 5.96 | 3.56 | 38 |
| Depressive symptoms ‐ 6 months (BDI‐SF) | ||||
| Edwards 2006 | Treatment | 7.5 | 6.3 | 23 |
| Edwards 2006 | Control | 6.3 | 7.2 | 24 |
| General symptoms total score ‐ 3 months (BPRS) | ||||
| Edwards 2006 | Treatment | 44.1 | 13.8 | 23 |
| Edwards 2006 | Control | 47.7 | 18.2 | 21 |
| General ‐ 3 months (CPRS) | ||||
| Naeem 2005 | Treatment | 24.26 | 12.17 | 67 |
| Naeem 2005 | Control | 24.46 | 13.85 | 38 |
| General ‐ 3 months (SCR) | ||||
| Naeem 2005 | Treatment | 5.2 | 3.67 | 67 |
| Naeem 2005 | Control | 4.9 | 4.3 | 38 |
| General symptoms total score ‐ 6 months (BPRS) | ||||
| Edwards 2006 | Treatment | 45.6 | 13.5 | 23 |
| Edwards 2006 | Control | 44.8 | 15.4 | 24 |
| Negative symptoms ‐ 3 months (SANS) | ||||
| Edwards 2006 | Treatment | 21.8 | 14.9 | 23 |
| Edwards 2006 | Control | 23.5 | 17 | 24 |
| Negative symptoms ‐ 6 months (SANS) | ||||
| Edwards 2006 | Treatment | 23.7 | 17.2 | 23 |
| Edwards 2006 | Control | 19.4 | 13.5 | 24 |
3.7 Social Functioning: 1. Average score (SOFAS, low = poor)
There was no clear difference between treatment groups in average social and occupational functioning scores (Edwards 2006) (SOFAS) by three months (MD ‐0.80, 95% CI ‐9.95 to 8.35; participants = 47; studies = 1) or by six months (MD ‐4.70, 95% CI ‐14.52 to 5.12; participants = 47; studies = 1). (Analysis 3.7).
3.7. Analysis.
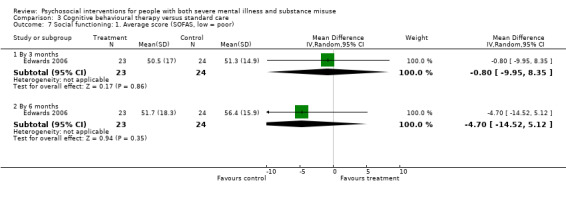
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 7 Social functioning: 1. Average score (SOFAS, low = poor).
3.8 Social Functioning: 2. Average score (HONOS) (skewed data)
Average HONOS scores were skewed and are presented in 'Other data' tables. (Analysis 3.8).
3.8. Analysis.
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 8 Social functioning: 2. Average score (HONOS) (skewed data).
| Social functioning: 2. Average score (HONOS) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 3 months | ||||
| Naeem 2005 | Treatment | 9.23 | 4.26 | 67 |
| Naeem 2005 | Control | 8.89 | 4.65 | 38 |
3.9 Service use: 1. Outpatient medication (SURS) (skewed data)
Outpatient medication data are shown in 'Other data' tables due to skewed data. (Analysis 3.9).
3.9. Analysis.
Comparison 3 Cognitive behavioural therapy versus standard care, Outcome 9 Service use: 1. Outpatient medication (SURS) (skewed data).
| Service use: 1. Outpatient medication (SURS) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 3 months | ||||
| Edwards 2006 | Treatment | 13.4 | 8.8 | 23 |
| Edwards 2006 | Control | 11.8 | 6.8 | 24 |
| 6 months | ||||
| Edwards 2006 | Treatment | 11.6 | 11.4 | 23 |
| Edwards 2006 | Control | 9.3 | 9.9 | 24 |
Comparison 4: Contingency management versus standard care
See Table 4. Four trials assessed this comparison (McDonell 2013; McDonell 2017; Petry 2013; Tracy 2007).
4.1 Leaving the study early: 1. Lost to treatment
No clear differences were found for lost to treatment by four to eight weeks (Petry 2013; Tracy 2007) (treatment 4%, control 21%; (RR 0.34, 95% CI 0.04 to 2.81; participants = 49; studies = 2). However, pooled results (McDonell 2013; McDonell 2017) reported that those assigned to the contingency management group were more likely not to complete the treatment period (leaving the study early) than participants in the standard care group at three months (treatment 48%, control 31% lost; (RR 1.55, 95% CI 1.13 to 2.11; participants = 255; studies = 2, Z = 2.76, P = 0.0057). (Analysis 4.1).
4.1. Analysis.
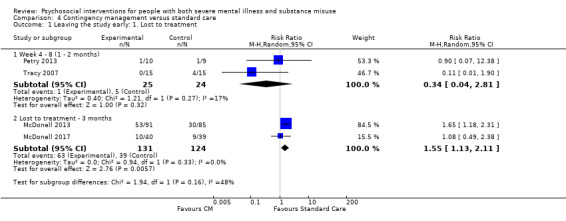
Comparison 4 Contingency management versus standard care, Outcome 1 Leaving the study early: 1. Lost to treatment.
4.2 Leaving the study early: 2. Lost to evaluation
No clear differences were found for lost to evaluation by six months (McDonell 2013; McDonell 2017) (treatment 33%, control 24%; (RR 1.36, 95% CI 0.91 to 2.02; participants = 255; studies = 2, P = 0.13). (Analysis 4.2).
4.2. Analysis.
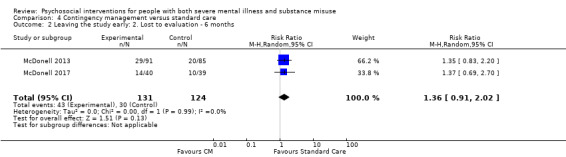
Comparison 4 Contingency management versus standard care, Outcome 2 Leaving the study early: 2. Lost to evaluation ‐ 6 months.
4.3 Substance use: 1. Stimulant‐positive urine test (higher = poor outcome)
Clearly more participants in the standard care group had stimulant‐positive urine tests compared to participants in the psychosocial group by 12 weeks (McDonell 2013) (treatment 10%, controls 25%; n = 176, (RR 0.34, 95% CI 0.17 to 0.68; participants = 176; studies = 1), Z = 3.04, P = 0.0024) but there was no difference at six months (treatment 54%, control 65%; n = 176, (RR 0.83, 95% CI 0.65 to 1.06; participants = 176; studies = 1), Z = 1.46, P = 0.14. (Analysis 4.3).
4.3. Analysis.
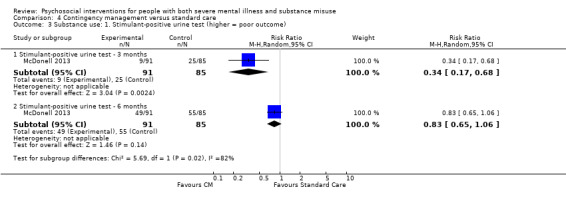
Comparison 4 Contingency management versus standard care, Outcome 3 Substance use: 1. Stimulant‐positive urine test (higher = poor outcome).
4.4 Substance use: 2 Average number of continuous days alcohol negative urine test (skewed data)
These data were skewed and are presented in 'Other data' tables. (Analysis 4.4).
4.4. Analysis.
Comparison 4 Contingency management versus standard care, Outcome 4 Substance use: 2. Number of continuous days alcohol negative urine tests, 3 months (skewed data).
| Substance use: 2. Number of continuous days alcohol negative urine tests, 3 months (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| McDonell 2017 | Treatment | 8.56 | 12.59 | 40 |
| McDonell 2017 | Control | 4.11 | 1.22 | 39 |
4.5 Substance use: 3. Injection use
Injection use during treatment was clearly lower in the psychosocial arm compared to the standard care arm at three months (McDonell 2013) (treatment 37%, control 66%; (RR 0.57, 95% CI 0.42 to 0.77; participants = 176; studies = 1), Z = 3.62, P < 0.001), but was not clearly different at the six‐month follow‐up (treatment 44%, control 56%; n = 107, (RR 0.78, 95% CI 0.53 to 1.15; participants = 107; studies = 1), Z = 1.24, P = 0.22). (Analysis 4.5).
4.5. Analysis.
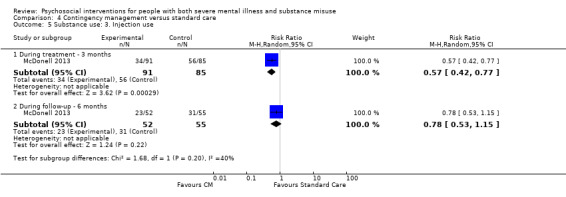
Comparison 4 Contingency management versus standard care, Outcome 5 Substance use: 3. Injection use.
4.6 Substance use: 4. Average scores on various measures (skewed data)
Average scores on various substance use measures were skewed and reported in 'Other data' tables. (Analysis 4.6).
4.6. Analysis.
Comparison 4 Contingency management versus standard care, Outcome 6 Substance use: 4. Average scores on various measures (skewed data).
| Substance use: 4. Average scores on various measures (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Stimulant use days ‐ 3 months (skewed data) | ||||
| McDonell 2013 | Treatment | 0.91 | 2.4 | 91 |
| McDonell 2013 | Control | 4.67 | 7.69 | 85 |
| McDonell 2017 | Treatment | 0.57 | 4.30 | 40 |
| McDonell 2017 | Control | 2.79 | 4.24 | 39 |
| Stimulant use days ‐ 6 months (skewed data) | ||||
| McDonell 2013 | Treatment | 1.83 | 4.94 | 52 |
| McDonell 2013 | Control | 3.65 | 7.15 | 55 |
| McDonell 2017 | Treatment | 0.34 | 3.84 | 30 |
| McDonell 2017 | Control | 1.95 | 3.84 | 30 |
| Days of alcohol ‐ 3 months | ||||
| McDonell 2013 | Treatment | 1.84 | 4.77 | 91 |
| McDonell 2013 | Control | 4.32 | 8.43 | 85 |
| McDonell 2017 | Treatment | 3.72 | 9.92 | 40 |
| McDonell 2017 | Control | 12.01 | 9.48 | 39 |
| Days of alcohol ‐ 6 months | ||||
| McDonell 2013 | Treatment | 3.6 | 7.92 | 52 |
| McDonell 2013 | Control | 4.21 | 7.86 | 55 |
| McDonell 2017 | Treatment | 3.32 | 7.67 | 30 |
| McDonell 2017 | Control | 10.73 | 7.61 | 30 |
| 5. Longest consecutive weeks of cocaine abstinence | ||||
| Petry 2013 | Treatment | 2.9 | 1.7 | 10 |
| Petry 2013 | Control | 0.6 | 1.7 | 9 |
4.7 Mental state: 1. Average scores (various scales) (skewed data)
Average scores on various mental state scales were skewed and reported in 'Other data' tables. (Analysis 4.7).
4.7. Analysis.
Comparison 4 Contingency management versus standard care, Outcome 7 Mental state: 1. Average scores (various scales) (skewed data).
| Mental state: 1. Average scores (various scales) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Brief Symptom Inventory ‐ 3 months | ||||
| McDonell 2013 | Treatment | 1.04 | 0.79 | 91 |
| McDonell 2013 | Control | 1.24 | 0.71 | 85 |
| Brief Symptom Inventroy ‐ 6 months | ||||
| McDonell 2013 | Treatment | 1.17 | 0.85 | 52 |
| McDonell 2013 | Control | 1.25 | 0.79 | 55 |
| PANSS, excitement scale ‐ 3 months | ||||
| McDonell 2013 | Treatment | 10.6 | 2.58 | 91 |
| McDonell 2013 | Control | 11.69 | 3.42 | 85 |
| PANSS, excitement scale ‐ 6 months | ||||
| McDonell 2013 | Treatment | 11.17 | 3.18 | 52 |
| McDonell 2013 | Control | 11.57 | 3.01 | 55 |
4.8 Service use: 1. Relapse (hospitalised ‐ six months post‐randomisation)
Relapse rates (hospitalised within 6 months after randomisation) were clearly lower in the psychosocial group compared to the standard care group (McDonell 2013) (treatment 2%, control 11%;(RR 0.21, 95% CI 0.05 to 0.93; participants = 176; studies = 1). (Analysis 4.8).
4.8. Analysis.
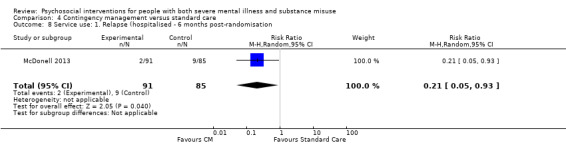
Comparison 4 Contingency management versus standard care, Outcome 8 Service use: 1. Relapse (hospitalised ‐ 6 months post‐randomisation.
Comparison 5: Motivational interviewing versus standard care
See Table 5. Data for this comparison came from nine trials (Baker 2002; Bechdolf 2011; Bonsack 2011; Graeber 2003; Graham 2016; Hickman 1997; Kavanagh 2004; Nagel 2009; Swanson 1999).
5.1 Leaving the study early: 1. Lost to treatment
Bonsack 2011 had an unusually long treatment period using motivational interviewing (six months). There were no differences in numbers of participants lost to treatment at three months (RR 0.89, 95% CI 0.30 to 2.61; participants = 62; studies = 1) or six months (RR 1.71, 95% CI 0.63 to 4.64; participants = 62; studies = 1). Analysis 5.1).
5.1. Analysis.
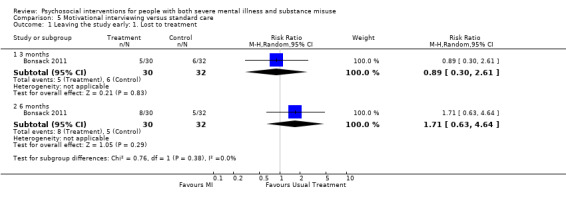
Comparison 5 Motivational interviewing versus standard care, Outcome 1 Leaving the study early: 1. Lost to treatment.
5.2 Leaving the study early: 2. Lost to evaluation
Pooled results from seven studies (Baker 2002; Bechdolf 2011; Graeber 2003; Graham 2016; Hickman 1997; Kavanagh 2004; Swanson 1999) revealed no difference in those lost to evaluation by three months (treatment 15% lost, control 15% lost; (RR 1.08, 95% CI 0.69 to 1.70; participants = 457; studies = 7). Similarly, six‐month data (Bechdolf 2011; Graeber 2003; Kavanagh 2004; Nagel 2009) (RR 0.85, 95% CI 0.29 to 2.53; participants = 164; studies = 4) and 12‐month data were not clearly different (RR 0.92, 95% CI 0.44 to 1.92; participants = 247; studies = 3) between motivational interviewing and the standard care group. Statistical heterogeneity was not present at three, six or 12 months. (Analysis 5.2).
5.2. Analysis.
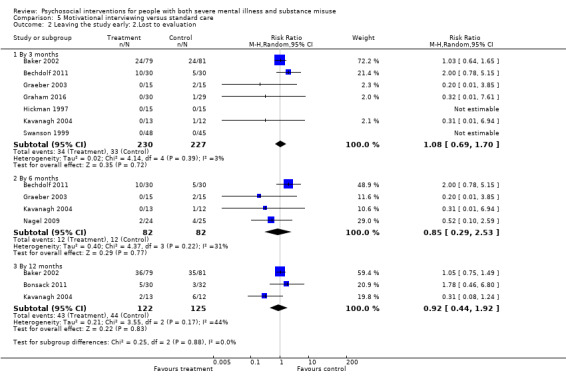
Comparison 5 Motivational interviewing versus standard care, Outcome 2 Leaving the study early: 2.Lost to evaluation.
5.3 Adverse event: 1. Death
We found no clear differences between the treatment groups in the likelihood of death due to all causes by 18 months (Nagel 2009) (treatment 4%, control 4%; RR 1.04, 95% CI 0.07 to 15.73; participants = 49; studies = 1). (Analysis 5.3).
5.3. Analysis.
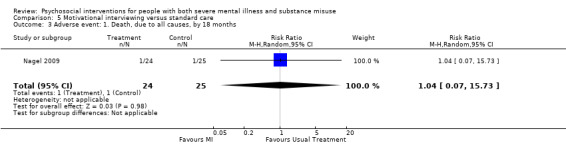
Comparison 5 Motivational interviewing versus standard care, Outcome 3 Adverse event: 1. Death, due to all causes, by 18 months.
5.4 Substance use: 1. Using substances ‐ by class of drug ‐ by about12 months
We found that alcohol dependence and abuse were not clearly different between treatment groups (Baker 2002) (treatment 39%, control 29%; (RR 1.35, 95% CI 0.62 to 2.92; participants = 52; studies = 1). Also, we found no clear differences in the likelihood of participants using amphetamine (treatment 9%, control 38%; (RR 0.24, 95% CI 0.03 to 1.92; participants = 19; studies = 1) or cannabis (treatment 50%, control 65%; (RR 0.77, 95% CI 0.49 to 1.21; participants = 62; studies = 1). (Analysis 5.4).
5.4. Analysis.
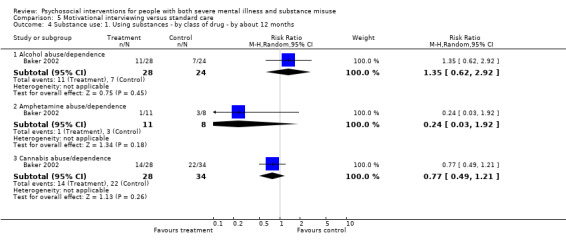
Comparison 5 Motivational interviewing versus standard care, Outcome 4 Substance use: 1. Using substances ‐ by class of drug ‐ by about 12 months.
5.5 Substance use: 2. Polydrug consumption levels ‐ by 12 months (OTI, high = poor)
Polydrug use was not found to be clearly different for three‐month (MD ‐0.41, 95% CI ‐0.91 to 0.09; participants = 89; studies = 1) and 12‐month (MD ‐0.07, 95% CI ‐0.56 to 0.42; participants = 89; studies = 1) evaluation data (OTI, high = poor) (Baker 2002). ( Analysis 5.5).
5.5. Analysis.
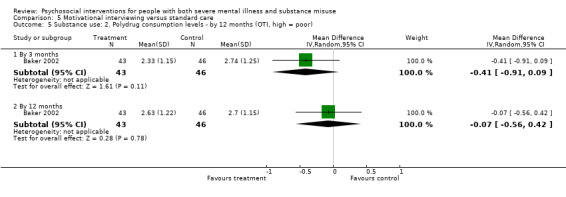
Comparison 5 Motivational interviewing versus standard care, Outcome 5 Substance use: 2. Polydrug consumption levels ‐ by 12 months (OTI, high = poor).
5.6 Substance use: 3. Any change ‐ not abstinent or not improved on all substances ‐ by 12 months
We found no clear difference (Kavanagh 2004) between treatment groups in the number of participants not abstaining or not improved on all substances by 12 months (treatment 38%, control 75%; n = 25, (RR 0.51, 95% CI 0.24 to 1.10; participants = 25; studies = 1). (Analysis 5.6).
5.6. Analysis.
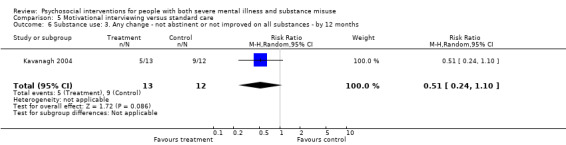
Comparison 5 Motivational interviewing versus standard care, Outcome 6 Substance use: 3. Any change ‐ not abstinent or not improved on all substances ‐ by 12 months.
5.7 Substance use: 4. Any change ‐ not abstaining from alcohol
Three‐month data reported by (Graeber 2003) did not reveal any clear difference between treatment groups for numbers of participants not abstaining from alcohol (treatment 40%, control 77%; n = 28, (RR 0.52, 95% CI 0.26 to 1.03; participants = 28; studies = 1). However, by six months we found data from this small study (Graeber 2003) showed clearly more participants in the standard care group were not abstaining from alcohol (treatment 42%, control 92%; (RR 0.36, 95% CI 0.17 to 0.75; participants = 28; studies = 1). (Analysis 5.7).
5.7. Analysis.
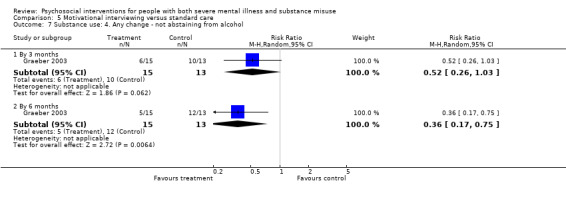
Comparison 5 Motivational interviewing versus standard care, Outcome 7 Substance use: 4. Any change ‐ not abstaining from alcohol.
5.8 Substance use: 5. Change in cannabis use from baseline (T0, lower scores indicate better outcome)
Change in cannabis use from baseline was lower in at three months (Bonsack 2011) (n = 62, MD ‐12.81 CI ‐23.05 to ‐2.57, P = 0.014), six months (n = 62, MD ‐9.64 CI ‐18.05 to ‐1.23, P = 0.025), but not at 12 months (n = 62, MD ‐5.82 CI ‐14.77 to 3.13). (Analysis 5.8).
5.8. Analysis.
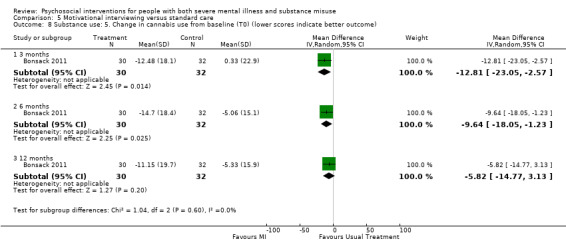
Comparison 5 Motivational interviewing versus standard care, Outcome 8 Substance use: 5. Change in cannabis use from baseline (T0) (lower scores indicate better outcome).
5.9 Substance use: 6. Cannabis consumption past 30 days ‐ average score (ASI, high = poor) (skewed data)
Average ASI scores for cannabis consumption were skewed and are presented in 'Other data' tables. (Analysis 5.9),
5.9. Analysis.
Comparison 5 Motivational interviewing versus standard care, Outcome 9 Substance use: 6. Cannabis consumption past 30 days (ASI, high = poor) (skewed data).
| Substance use: 6. Cannabis consumption past 30 days (ASI, high = poor) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| By 3 months | ||||
| Bonsack 2011 | Treatment | 4.6 | 9.4 | 30 |
| Bonsack 2011 | Control | 3.8 | 9.6 | 30 |
| By 6 months | ||||
| Bonsack 2011 | Treatment | 5.3 | 10.4 | 30 |
| Bonsack 2011 | Control | 4.43 | 8.4 | 30 |
5.10 Substance use: 7. Engagement with substance misuse treatment at three months (SATS, low = poor)
Data reported by (Graham 2016) showed engagement with substance misuse scores (SATS) at three months were not clearly different between groups (MD 0.30, 95% CI ‐0.47 to 1.07; participants = 59; studies = 1). (Analysis 5.10).
5.10. Analysis.
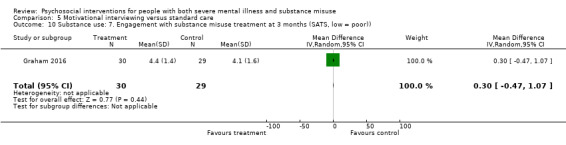
Comparison 5 Motivational interviewing versus standard care, Outcome 10 Substance use: 7. Engagement with substance misuse treatment at 3 months (SATS, low = poor)).
5.11 Substance use: 8. Average scores (OTI, high = poor) (skewed data)
Average substance use scores on the Opiate Treatment Index (OTI) (Analysis 5.11) are reported in 'Other data' tables due to skewed data.
5.11. Analysis.
Comparison 5 Motivational interviewing versus standard care, Outcome 11 Substance use: 8. Average scores (OTI, high = poor) (skewed data).
| Substance use: 8. Average scores (OTI, high = poor) (skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Time period | Mean | SD | N |
| Alcohol | |||||
| Baker 2002 | Treatment | 3 months | 2.55 | 5.29 | 28 |
| Baker 2002 | Control | 1.51 | 2.05 | 23 | |
| Baker 2002 | Treatment | 6 months | 4.26 | 6.59 | 28 |
| Baker 2002 | Control | 4.19 | 7.74 | 23 | |
| Baker 2002 | Treatment | 12 months | 2.98 | 3.11 | 28 |
| Baker 2002 | 1.83 | 3.54 | 23 | ||
| Amphetamine | |||||
| Baker 2002 | Treatment | 3 months | 0.32 | 1.05 | 11 |
| Baker 2002 | Control | 0.01 | 0.03 | 8 | |
| Baker 2002 | Treatment | 6 months | 0.14 | 0.31 | 11 |
| Baker 2002 | Control | 0.17 | 0.35 | 8 | |
| Baker 2002 | Treatment | 12 months | 0.06 | 0.17 | 11 |
| Baker 2002 | 0.03 | 0.05 | 8 | ||
| Cannabis | |||||
| Baker 2002 | Treatment | 3 months | 2.52 | 6.10 | 28 |
| Baker 2002 | Control | 3.44 | 4.69 | 34 | |
| Baker 2002 | Treatment | 6 months | 4.77 | 8.00 | 28 |
| Baker 2002 | Control | 3.89 | 6.22 | 34 | |
| Baker 2002 | Treatment | 12 months | 5.81 | 7.76 | 28 |
| Baker 2002 | 4.47 | 6.09 | 34 | ||
| Polydrug use | |||||
| Baker 2002 | Treatment | 3 months | 2.74 | 1.25 | 43 |
| Baker 2002 | Control | 2.33 | 1.15 | 46 | |
| Baker 2002 | Treatment | 6 months | 2.74 | 1.14 | 43 |
| Baker 2002 | Control | 2.52 | 1.28 | 46 | |
| Baker 2002 | Treatment | 12 months | 2.63 | 1.22 | 43 |
| Baker 2002 | Control | 2.7 | 1.15 | 46 | |
6.12 Substance use: 9. Other measures of alcohol use (various scales, skewed data)
Other measures of alcohol use reported by (Bonsack 2011; Graeber 2003; Graham 2016; Hickman 1997) were skewed and are presented as 'Other data tables'. (Analysis 5.12).
5.12. Analysis.
Comparison 5 Motivational interviewing versus standard care, Outcome 12 Substance use: 9. Other measures of alcohol use (skewed data).
| Substance use: 9. Other measures of alcohol use (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Alcohol volume (AUI subscale) | ||||
| Hickman 1997 | Treatment | 18.1 | 9.93 | 15 |
| Hickman 1997 | Control | 14.6 | 13.6 | 15 |
| Drinking days ‐ by 6 months | ||||
| Graeber 2003 | Treatment | 3.10 | 6.24 | 15 |
| Graeber 2003 | Control | 12.73 | 8.25 | 13 |
| Alcohol consumption, last 30 days (ASI) ‐ 3 months | ||||
| Bonsack 2011 | Treatment | 6.2 | 9.1 | 30 |
| Bonsack 2011 | Control | 5.13 | 8.2 | 30 |
| Alcohol consumption, last 30 days (ASI) ‐ 6 months | ||||
| Bonsack 2011 | Treatment | 5.57 | 8.5 | 30 |
| Bonsack 2011 | Control | 6.43 | 10 | 30 |
| Alcohol use (AUDIT) ‐ 3 months | ||||
| Graham 2016 | Treatment | 15.11 | 7.71 | 9 |
| Graham 2016 | Control | 13.09 | 7.92 | 11 |
| Alcohol use (CAUS) ‐ 3 months | ||||
| Graham 2016 | Treatment | 2.25 | 1.22 | 12 |
| Graham 2016 | Control | 2.18 | 0.98 | 11 |
| Drug use (CDUS) ‐ 3 months | ||||
| Graham 2016 | Treatment | 1.89 | 0.90 | 18 |
| Graham 2016 | Control | 2.41 | 1.06 | 17 |
| Drug use (SDS) ‐ 3 months | ||||
| Graham 2016 | Treatment | 4.64 | 4.18 | 14 |
| Graham 2016 | Control | 5.31 | 3.68 | 13 |
5.13 Mental state: 1. Average score (SCL‐90‐R, high = poor) ‐ by three months
We found that three‐month data by Hickman 1997 revealed no clear differences between treatment groups in average general severity scores (MD ‐0.19, 95% CI ‐0.59 to 0.21; participants = 30; studies = 1), average positive distress symptoms scores (MD ‐0.19, 95% CI ‐0.66 to 0.28; participants = 30; studies = 1), or average total positive symptoms scores (MD ‐4.20, 95% CI ‐18.72 to 10.32; participants = 30; studies = 1) as measured by the SCL‐90. (Analysis 5.13).
5.13. Analysis.
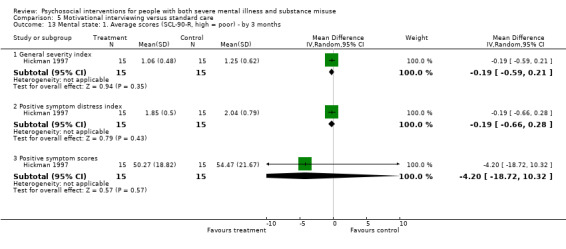
Comparison 5 Motivational interviewing versus standard care, Outcome 13 Mental state: 1. Average scores (SCL‐90‐R, high = poor) ‐ by 3 months.
5.14 Mental state: 2. Average score (PANSS negative symptoms, high = poor)
Average PANSS negative symptom scores reported by (Bonsack 2011) were not clearly different between treatment groups at three months (MD ‐0.10, 95% CI ‐2.06 to 1.86; participants = 62; studies = 1) or six months (MD 0.00, 95% CI ‐1.80 to 1.80; participants = 62; studies = 1). (Analysis 5.14).
5.14. Analysis.
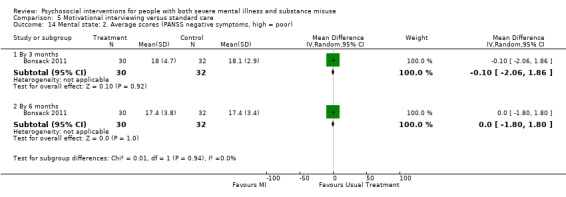
Comparison 5 Motivational interviewing versus standard care, Outcome 14 Mental state: 2. Average scores (PANSS negative symptoms, high = poor).
5.15 Mental state: 3. Average score (PANSS positive symptoms, high = poor)
Average PANSS positive symptom scores reported by (Bonsack 2011) were not clearly different between treatment groups at three months (MD ‐0.30, 95% CI ‐2.55 to 1.95; participants = 62; studies = 1) or six months (MD ‐0.10, 95% CI ‐2.58 to 2.38; participants = 62; studies = 1). ( Analysis 5.15).
5.15. Analysis.
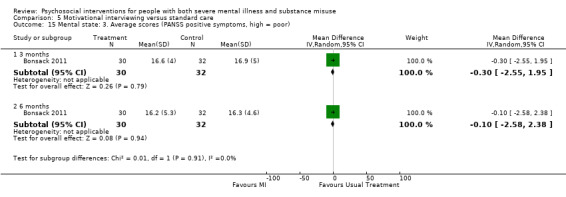
Comparison 5 Motivational interviewing versus standard care, Outcome 15 Mental state: 3. Average scores (PANSS positive symptoms, high = poor).
5.16 Mental state: 4. Average score (HADS and BSI, high = poor) (skewed data)
Mental state data reported by Baker 2002; Graham 2016 were skewed and are presented in 'Other data' tables, Analysis 5.16.
5.16. Analysis.
Comparison 5 Motivational interviewing versus standard care, Outcome 16 Mental state: 4. Average score (HADS, BSI, high = poor) (skewed data) ‐ by 3 months.
| Mental state: 4. Average score (HADS, BSI, high = poor) (skewed data) ‐ by 3 months | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| HADS‐A, Anxiety | ||||
| Graham 2016 | Treatment | 7.3 | 5.3 | 24 |
| Graham 2016 | Control | 8.1 | 5.3 | 23 |
| HADS‐D, Depression | ||||
| Graham 2016 | Treatment | 5.4 | 3.8 | 24 |
| Graham 2016 | Control | 7.3 | 4.1 | 23 |
| BSI | ||||
| Baker 2002 | Treatment | 1.16 | 0.92 | 56 |
| Baker 2002 | Control | 1.23 | 0.86 | 56 |
5.17 Global state: Average score (GAF, low = poor)
Average GAF scores reported by (Bonsack 2011) were not clearly different between treatment groups at three months (MD ‐0.40, 95% CI ‐3.53 to 2.73; participants = 51; studies = 1), six months (MD ‐1.00, 95% CI ‐4.81 to 2.81; participants = 49; studies = 1) or 12 months (MD 2.30, 95% CI ‐1.30 to 5.90; participants = 54; studies = 1). (Analysis 5.17).
5.17. Analysis.
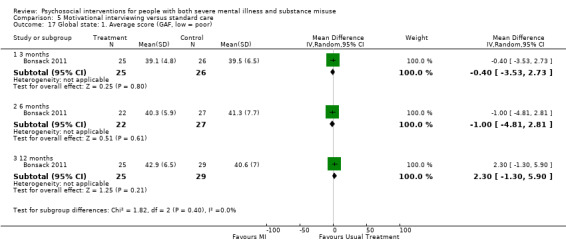
Comparison 5 Motivational interviewing versus standard care, Outcome 17 Global state: 1. Average score (GAF, low = poor).
5.18 Global state: Forensic measures ‐ average number of crimes (skewed data)
Average number of crimes reported at six and 12 months were skewed and are presented in 'Other data' tables (Analysis 5.18).
5.18. Analysis.
Comparison 5 Motivational interviewing versus standard care, Outcome 18 Global state: 2. Forensic measures ‐ average number of crimes (skewed data).
| Global state: 2. Forensic measures ‐ average number of crimes (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Baker 2002 | Treatment | 0.33 | 1.04 | 52 |
| Baker 2002 | Control | 0.36 | 0.85 | 50 |
| 12 months | ||||
| Baker 2002 | Treatment | 0.23 | 0.61 | 52 |
| Baker 2002 | Control | 0.30 | 0.93 | 50 |
5.19 Social functioning: 1. Average score (OTI, high = poor)
Average OTI social functioning scores reported by (Baker 2002) did not reveal any clear differences between treatment groups by six months (MD ‐0.71, 95% CI ‐2.76 to 1.34; participants = 102; studies = 1), or by 12 months (MD ‐1.42, 95% CI ‐3.35 to 0.51; participants = 102; studies = 1). (Analysis 5.19).
5.19. Analysis.
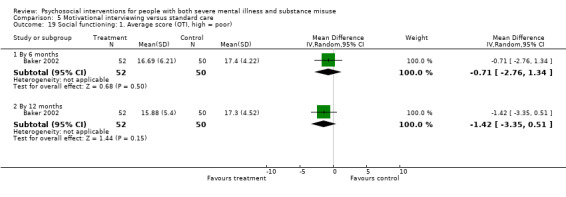
Comparison 5 Motivational interviewing versus standard care, Outcome 19 Social functioning: 1. Average score (OTI, high = poor).
5.20 Social functioning: 2. Average score (SOFAS, high = poor)
Social occupational functioning (SOFAS) scores reported by (Bonsack 2011) were not clearly different between treatment groups at three months (MD 0.10, 95% CI ‐3.02 to 3.22; participants = 62; studies = 1), six months (MD ‐0.10, 95% CI ‐3.51 to 3.31; participants = 62; studies = 1) or 12 months (MD 2.70, 95% CI ‐1.08 to 6.48; participants = 62; studies = 1). (Analysis 5.20).
5.20. Analysis.
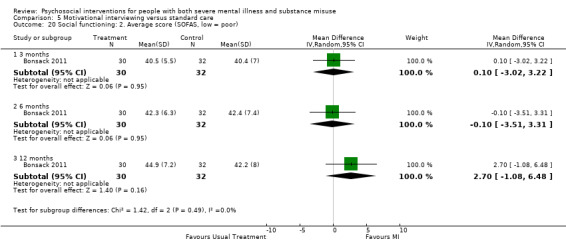
Comparison 5 Motivational interviewing versus standard care, Outcome 20 Social functioning: 2. Average score (SOFAS, low = poor).
5.21 Service use: 1. Relapse (hospital readmission by 12 months)
We found no clear differences in hospital readmissions by 12 months (Bonsack 2011) (treatment 30%, control 34%) (RR 0.87, 95% CI 0.42 to 1.80; participants = 62; studies = 1). (Analysis 5.21).
5.21. Analysis.
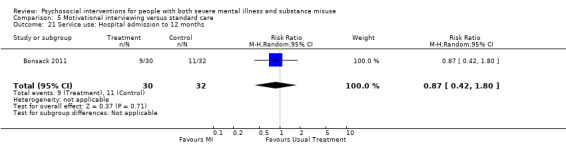
Comparison 5 Motivational interviewing versus standard care, Outcome 21 Service use: Hospital admission to 12 months.
6.22 Service use: 2. Lost to first aftercare appointment
We found participants in Motivational interviewing group were more likely to attend their first aftercare appointment (Swanson 1999) (treatment 58%, control 84%; n = 93, (RR 0.69, 95% CI 0.53 to 0.90; participants = 93; studies = 1), compared with those receiving standard care. (Analysis 5.22).
5.22. Analysis.
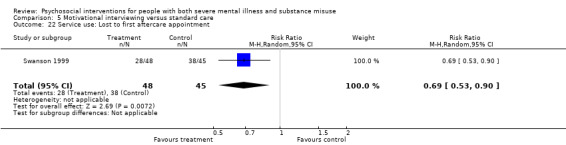
Comparison 5 Motivational interviewing versus standard care, Outcome 22 Service use: Lost to first aftercare appointment.
Comparison 6: Skills training versus standard care
See Table 6. Data for this comparison came from five trials (Bogenschutz 2014; Eack 2015; Hellerstein 1995; Jerrell 1995a; Rosenblum 2014).
6.1 Leaving the study early: 1. Lost to treatment
Our analyses found that the pooled results of Bogenschutz 2014 and Rosenblum 2014 showed no clear difference in lost to treatment between skills training and standard care groups at three months (treatment 16%, control 19%, (RR 0.97, 95% CI 0.68 to 1.36; participants = 461; studies = 3). Data reported by Hellerstein 1995 and Jerrell 1995a showed a 51% greater likelihood that participants would be lost to treatment from the standard care group by six months which was clearly more than the skills training group (treatment 16%, control 31%; (RR 0.49, 95% CI 0.24 to 0.97; participants = 94; studies = 2), although this clear difference was not present at 12 months (treatment 33%, control 33%; (RR 1.42, 95% CI 0.20 to 10.10; participants = 122; studies = 3). By contrast, at 18 months we found a clear difference between the treatment groups where participants given skills training were twice as likely to be lost to treatment (treatment 59%, control 24%; (RR 2.60, 95% CI 1.36 to 4.97; participants = 75; studies = 2) (Analysis 6.1).
6.1. Analysis.
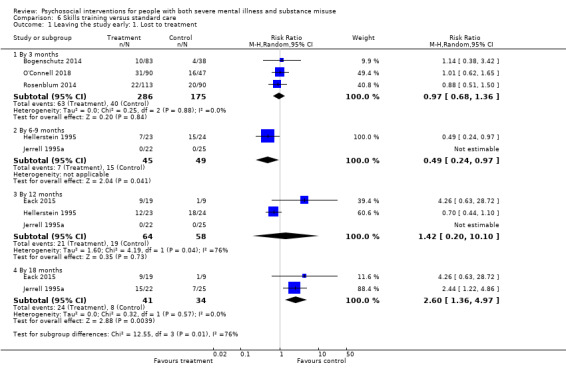
Comparison 6 Skills training versus standard care, Outcome 1 Leaving the study early: 1. Lost to treatment.
6.2 Lost to evaluation
Data reported by (Bogenschutz 2014) showed no clear differences in lost to evaluation between groups at six months (RR 0.92, 95% CI 0.40 to 2.08; participants = 121; studies = 1), nine months (RR 1.05, 95% CI 0.47 to 2.33; participants = 121; studies = 1) and 12 months (RR 1.14, 95% CI 0.55 to 2.36; participants = 121; studies = 1). Analysis 6.2.
6.2. Analysis.
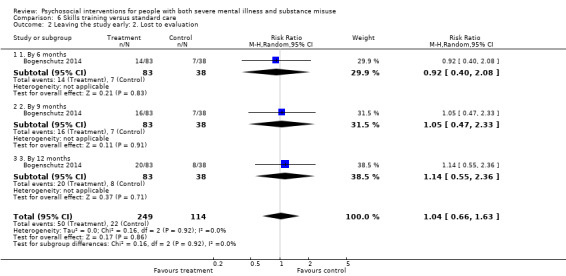
Comparison 6 Skills training versus standard care, Outcome 2 Leaving the study early: 2. Lost to evaluation.
6.3 Adverse event: 1. Death
We found Bogenschutz 2014 showed no clear difference between skills training and standard care with regards to the likelihood of participants dying by 12 months of treatment (treatment 1.2% died, control 8% died) (RR 0.15, 95% CI 0.02 to 1.42; participants = 121; studies = 1). Analysis 6.3.
6.3. Analysis.
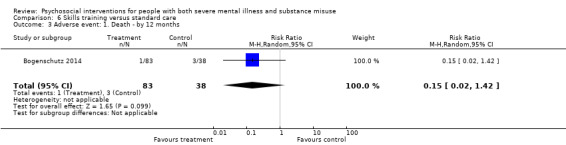
Comparison 6 Skills training versus standard care, Outcome 3 Adverse event: 1. Death ‐ by 12 months.
6.4 Substance use: 1. Alcohol use: proportion days abstinent from alcohol (TLFB method)
Proportion of days abstinent from alcohol was clearly higher in the skills training group compared to standard care group at three months (MD 0.02, 95% CI 0.00 to 0.04; participants = 107; studies = 1), but was clearly lower at six months (MD ‐0.05, 95% CI ‐0.07 to ‐0.03; participants = 100; studies = 1), and there was no difference between treatment groups at nine months (MD 0.01, 95% CI ‐0.01 to 0.03; participants = 98; studies = 1) or 12 months (MD 0.03, 95% CI 0.00 to 0.06; participants = 93; studies = 1). (Analysis 6.4).
6.4. Analysis.
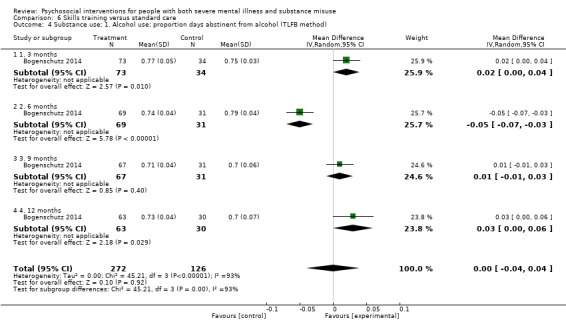
Comparison 6 Skills training versus standard care, Outcome 4 Substance use: 1. Alcohol use: proportion days abstinent from alcohol (TLFB method).
6.5 ‐ 6.6 Substance use: (skewed data)
Average scores of various substance use scales (Analysis 6.5) or average days used (Analysis 6.6) were skewed and reported in 'Other data' tables.
6.5. Analysis.
Comparison 6 Skills training versus standard care, Outcome 5 Substance use: 2. Average scores (C‐DIS‐R) (skewed data).
| Substance use: 2. Average scores (C‐DIS‐R) (skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Time period | Mean | SD | N |
| Alcohol | |||||
| Jerrell 1995a | Treatment | 6 months | 0.99 | 1.92 | 21 |
| Jerrell 1995a | Control | 2.80 | 3.49 | 25 | |
| Jerrell 1995a | Treatment | 12 months | 2.24 | 2.83 | 21 |
| Jerrell 1995a | Control | 2.95 | 3.50 | 25 | |
| Jerrell 1995a | Treatment | 18 months | 1.43 | 2.70 | 7 |
| Jerrell 1995a | Countrol | 1.39 | 2.52 | 18 | |
| Drugs | |||||
| Jerrell 1995a | Treatment | 6 months | 1.85 | 2.74 | 21 |
| Jerrell 1995a | Control | 4.84 | 5.69 | 25 | |
| Jerrell 1995a | Treatment | 12 months | 3.52 | 3.53 | 21 |
| Jerrell 1995a | Control | 5.99 | 7.46 | 25 | |
| Jerrell 1995a | Treatment | 18 months | 1.43 | 2.70 | 7 |
| Jerrell 1995a | Control | 2.22 | 3.46 | 18 | |
6.6. Analysis.
Comparison 6 Skills training versus standard care, Outcome 6 Sustance use: 3. Average days used (skewed data).
| Sustance use: 3. Average days used (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Any alcohol in last 30 days, at follow‐up (6 months) | ||||
| Rosenblum 2014 | Treatment | 3.1 | 6.4 | 91 |
| Rosenblum 2014 | Control | 6.1 | 8.8 | 70 |
| Any illicit drug in last 30 days, at follow‐up (6 months) | ||||
| Rosenblum 2014 | Treatment | 5.5 | 9.2 | 91 |
| Rosenblum 2014 | Control | 8 | 10.6 | 70 |
6.7 Social Functioning: 1. Average score (RFS, high = better functioning)
Average role functioning scores reported in (Jerrell 1995a) showed no clear differences between treatment groups by six months (MD 0.61, 95% CI ‐1.63 to 2.85; participants = 47; studies = 1), 12 months (MD 1.07, 95% CI ‐1.15 to 3.29; participants = 47; studies = 1) or 18 months (MD ‐2.55, 95% CI ‐6.24 to 1.14; participants = 25; studies = 1). (Analysis 6.7).
6.7. Analysis.
Comparison 6 Skills training versus standard care, Outcome 7 Social functioning: 1. Average score (RFS, high = better functioning).
6.8 Social Functioning: 2. Average score (SAS, high = better functioning)
No clear differences between treatment groups were observed in social adjustment, measured as average SAS score by (Jerrell 1995a) at six months (MD ‐0.92, 95% CI ‐6.58 to 4.74; participants = 47; studies = 1), 12 months (MD 2.58, 95% CI ‐3.39 to 8.55; participants = 47; studies = 1) or 18 months (MD ‐4.66, 95% CI ‐15.29 to 5.97; participants = 25; studies = 1). (Analysis 6.8).
6.8. Analysis.
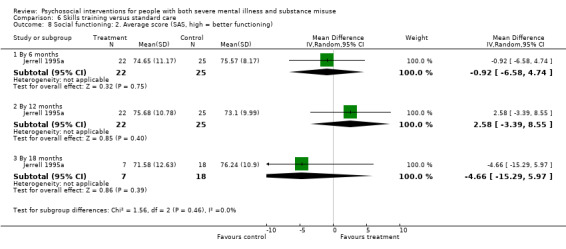
Comparison 6 Skills training versus standard care, Outcome 8 Social functioning: 2. Average score (SAS, high = better functioning).
6.9 Service use: 1. Average days in hospital (skewed data)
Data from Hellerstein 1995 for days in hospital were skewed and are presented in 'Other data' tables. (Analysis 6.9).
6.9. Analysis.
Comparison 6 Skills training versus standard care, Outcome 9 Service use: 1. Days in hospital (skewed data).
| Service use: 1. Days in hospital (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Hellerstein 1995 | Treatment | 5.4 | 10.4 | 16 |
| Hellerstein 1995 | Control | 3.6 | 6.7 | 13 |
Comparison 7: Cognitive behavioural therapy + motivational interviewing versus standard care
See Table 7. Data for this comparIson came from nine trials (Baker 2006; Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Bellack 2006; Gouzoulis‐Mayfrank 2015; Hjorthoj 2013; Kemp 2007; Madigan 2013).
7.1 Leaving the study early: 1. Lost to treatment
We found that the results from Baker 2006 indicated that clearly more participants assigned to CBT+ MI group were lost to treatment by three months (treatment 12%, control 0%; n = 130, RR 17.00 95% CI 1.00 to 288.56; participants = 130; studies = 1). In contrast, Madigan 2013 reported no group difference in lost to treatment by three months (treatment 29%, control 24%; n = 88, RR 1.19 95% CI 0.56 to 2.55; participants = 88; studies = 1), as did Gouzoulis‐Mayfrank 2015 (treatment 30%, control 44%, n = 100, RR 0.68 95% CI 0.40 to 1.15; participants = 100; studies = 1). Combined, participants in the CBT + MI groups were no more likely to be lost to treatment by three months than participants in the standard care groups (treatment 23%, control 20%; RR 1.20, 95% CI 0.44 to 3.30; participants = 318; studies = 3), but there was considerable statistical heterogeneity (Chi² = 6.71, df = 2, P = 0.03; I² = 70%). Six month‐data reported in (Barrowclough 2010; Barrowclough 2014; Bellack 2006; Gouzoulis‐Mayfrank 2015; Hjorthoj 2013) revealed no difference between CBT + MI group and standard care for loss to treatment (treatment 29%, control 28%; RR 0.90, 95% CI 0.66 to 1.22; participants = 815; studies = 5, P = 0.48). Similarly, we found nine‐ to 10‐month data (Barrowclough 2001; Barrowclough 2014; Hjorthoj 2013) were not clearly different (treatment 25%, control 32%; (RR 0.80, 95% CI 0.52 to 1.22; participants = 211; studies = 3), nor were 12‐month data (Barrowclough 2010) (treatment 17.7%, control 17.8%; RR 0.99, 95% CI 0.62 to 1.59; participants = 327; studies = 1). Statistical heterogeneity was not present at six or nine to 10 months). (Analysis 7.1).
7.1. Analysis.
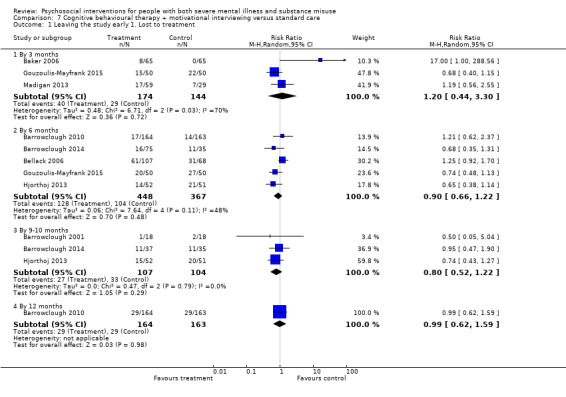
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 1 Leaving the study early 1. Lost to treatment.
7.2 Leaving the study early: 2. Lost to evaluation
We found all lost to evaluation data to be equivocal between CBT + MI and standard care by three months (Baker 2006; Gouzoulis‐Mayfrank 2015) (treatment 17% lost, control 23% lost; RR 0.75, 95% CI 0.46 to 1.21; participants = 230; studies = 2), by six months (Baker 2006; Barrowclough 2014; Bellack 2006; Gouzoulis‐Mayfrank 2015; Kemp 2007) (treatment 21% lost, control 26% lost; RR 0.80, 95% CI 0.57 to 1.12; participants = 469; studies = 5), at nine months (Barrowclough 2001; Barrowclough 2014) (treatment 24%, control 26%; RR 0.82, 95% CI 0.46 to 1.47; participants = 146; studies = 2), 12‐14 months (Baker 2006; Barrowclough 2001; Barrowclough 2014; Gouzoulis‐Mayfrank 2015; Madigan 2013) (treatment 34%, control 34%; RR 0.99, 95% CI 0.73 to 1.34; participants = 464; studies = 5), 18 months (Barrowclough 2001; Barrowclough 2010); (treatment 20%, control 22%; RR 0.92, 95% CI 0.61 to 1.38; participants = 363; studies = 2), and 24 months (Barrowclough 2010) (treatment 21%, control 28%; (RR 0.76, 95% CI 0.52 to 1.11; participants = 327; studies = 1). Statistical heterogeneity was not present for any of the above subgroup analyses. (Analysis 7.2).
7.2. Analysis.
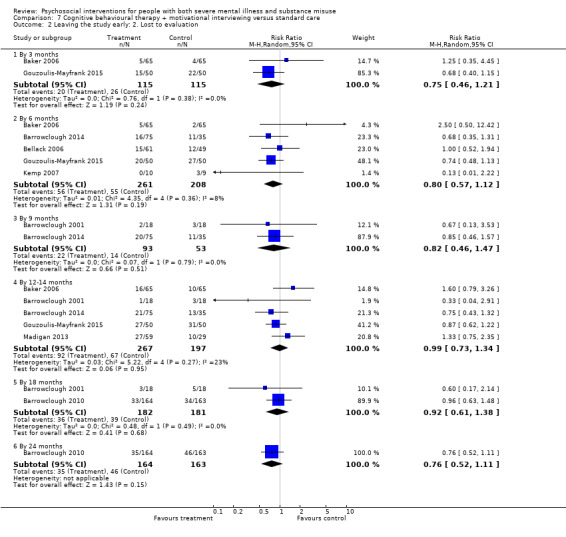
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 2 Leaving the study early: 2. Lost to evaluation.
7.3 Adverse event: 1. Death ‐ by about one year
Pooled results from (Baker 2006; Barrowclough 2001; Barrowclough 2010; Barrowclough 2014) found no clear difference in numbers of participants dying in either the CBT + MI groups or standard care groups by about one year (treatment 1.9%, control 3.2%; n = 603, RR 0.60, 95% CI 0.20 to 1.76; participants = 603; studies = 4). Statistical heterogeneity was not present (Chi² = 2.94, df = 3, P = 0.40; I² = 0%). (Analysis 7.3).
7.3. Analysis.
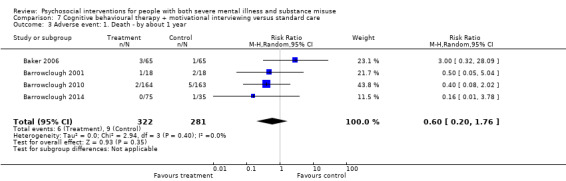
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 3 Adverse event: 1. Death ‐ by about 1 year.
7.4 Adverse event: 2. Death or hospitalisation by 24 months
Similarly, there was no clear difference for the likelihood of participants hospitalised or dying versus alive and not admitted to hospital by 24 months (Barrowclough 2010) (treatment 23%, control 20%; n = 326, RR 1.15, 95% CI 0.76 to 1.74; participants = 326; studies = 1). (Analysis 7.4).
7.4. Analysis.
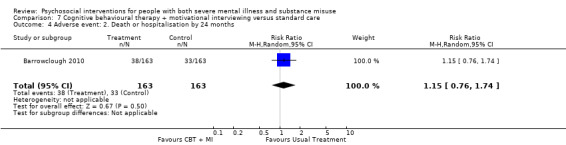
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 4 Adverse event: 2. Death or hospitalisation by 24 months.
7.5 Substance use: 1. Average number of different drugs used during past month
Polydrug usage was not clearly different between CBT + MI and standard care groups by three months (Baker 2006) (MD 0.37, 95% CI ‐0.01 to 0.75; participants = 119; studies = 1), or by six months (MD 0.19, 95% CI ‐0.22 to 0.60; participants = 119; studies = 1). (Analysis 7.5).
7.5. Analysis.
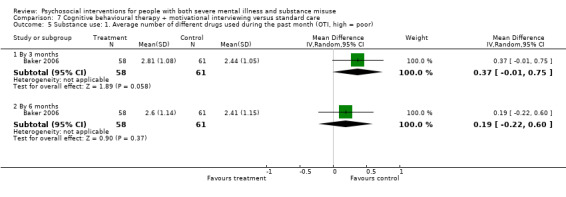
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 5 Substance use: 1. Average number of different drugs used during the past month (OTI, high = poor).
7.6 Substance use: 2. Cannabis use last 30 days
Moreover, cannabis use in the last 30 days was not clearly different between the two treatment groups at three months the end of treatment (MD ‐0.20, 95% CI ‐2.54 to 2.14; participants = 54; studies = 1), or at 12 months (MD ‐0.30, 95% CI ‐2.84 to 2.24; participants = 42; studies = 1). (Analysis 7.6).
7.6. Analysis.
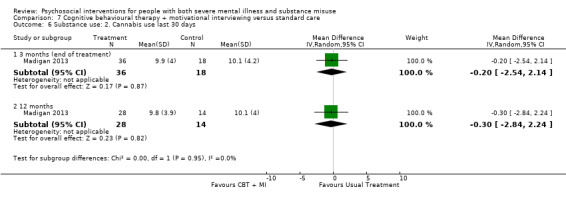
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 6 Substance use: 2. Cannabis use last 30 days.
7.7 Substance use: 3. Clinically important change ‐ change in main substance use, abstinent or large decrease
Gouzoulis‐Mayfrank 2015 reported data that showed no clear difference between CBT + MI and standard care groups for numbers of participants with a large decrease in main substance use or were abstinent (baseline to three months) (RR (Non‐event) 0.67, 95% CI 0.43 to 1.04; participants = 100; studies = 1). (Analysis 7.7).
7.7. Analysis.
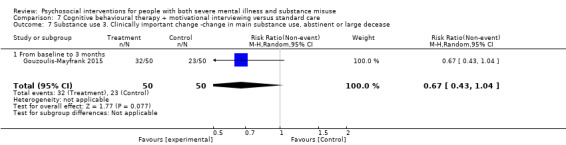
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 7 Substance use 3. Clinically important change ‐change in main substance use, abstinent or large decease.
7.8 ‐ 7.9 Substance use: skewed data
Average scores of various substance use measures that reported skewed data are shown in 'Other data' tables (Analysis 7.8; Analysis 7.9).
7.8. Analysis.
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 8 Substance use: 4. Averages of various measures (skewed data).
| Substance use: 4. Averages of various measures (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Alcohol ‐ estimated daily consumption ‐ past month ‐ 3 months | ||||
| Baker 2006 | Treatment | 4.92 | 4.69 | 21 |
| Baker 2006 | Control | 3.35 | 4.10 | 31 |
| Alcohol ‐ estimated daily consumption ‐ past month ‐ 6 months | ||||
| Baker 2006 | Treatment | 3.73 | 4.07 | 21 |
| Baker 2006 | Control | 2.52 | 4.20 | 31 |
| Alcohol ‐ estimated daily consumption ‐ past month ‐ 12 months | ||||
| Baker 2006 | Treatment | 3.58 | 4.80 | 18 |
| Baker 2006 | Control | 2.19 | 3.04 | 28 |
| Alcohol ‐ frequency per month by 6 months | ||||
| Kemp 2007 | Treatment | 3.1 | 3.8 | 10 |
| Kemp 2007 | Control | 2.3 | 3.8 | 6 |
| Alcohol quantity per session ‐ 6 months | ||||
| Kemp 2007 | Treatment | 4.7 | 2.9 | 10 |
| Kemp 2007 | Control | 3.6 | 2.8 | 6 |
| Alcohol Assessment ‐ AUDIT ‐ 6 months | ||||
| Kemp 2007 | Treatment | 9.3 | 6.9 | 10 |
| Kemp 2007 | Control | 4.8 | 5 | 6 |
| General ‐ ASI score ‐ 6 months | ||||
| Bellack 2006 | Treatment | 72.90 | 249 | 61 |
| Bellack 2006 | Control | 74.10 | 155 | 49 |
| Drug abuse screening test ‐ DAST‐10 by 6 months | ||||
| Kemp 2007 | Treatment | 1.8 | 1.9 | 10 |
| Kemp 2007 | Control | 3.5 | 2.4 | 6 |
| Amphetamine‐ estimated daily consumption ‐ past month ‐ 6 months | ||||
| Baker 2006 | Treatment | 0.19 | 0.41 | 11 |
| Baker 2006 | Control | 1.47 | 2.28 | 9 |
| Amphetamine‐ estimated daily consumption ‐ past month ‐ 3 months | ||||
| Baker 2006 | Treatment | 0.34 | 0.53 | 11 |
| Baker 2006 | Control | 0.25 | 0.57 | 9 |
| Amphetamine‐ estimated daily consumption ‐ past month ‐ 12 months | ||||
| Baker 2006 | Treatment | 0.14 | 0.26 | 9 |
| Baker 2006 | Control | 0.01 | 0.25 | 8 |
| Cannabis‐ estimated daily consumption ‐ past month ‐ 3 months | ||||
| Baker 2006 | Treatment | 5.09 | 7.21 | 39 |
| Baker 2006 | Control | 5.66 | 8.72 | 34 |
| Cannabis‐ estimated daily consumption ‐ past month ‐ 6 months | ||||
| Baker 2006 | Treatment | 5.37 | 11.75 | 39 |
| Baker 2006 | Control | 4.67 | 8.68 | 34 |
| Cannabis‐ estimated daily consumption ‐ past month ‐ 12 months | ||||
| Baker 2006 | Treatment | 8.53 | 14.59 | 29 |
| Baker 2006 | Control | 4.12 | 6.51 | 29 |
| cannabis ‐ days of use last month by 6 months | ||||
| Hjorthoj 2013 | Treatment | 12 | 11.44 | 38 |
| Hjorthoj 2013 | Control | 15.6 | 12.58 | 30 |
| Cannabis ‐ days of use last month by 10‐12 months | ||||
| Hjorthoj 2013 | Treatment | 11.1 | 11.19 | 37 |
| Hjorthoj 2013 | Control | 13.7 | 13.31 | 31 |
| Cannabis ‐ joints last 30 days by 6 months | ||||
| Hjorthoj 2013 | Treatment | 28.6 | 38.75 | 38 |
| Hjorthoj 2013 | Control | 47.1 | 58.26 | 30 |
| Kemp 2007 | Treatment | 38.8 | 113.1 | 10 |
| Kemp 2007 | Control | 25.3 | 61.1 | 6 |
| Cannabis ‐ joints last 30 days by 10 months | ||||
| Hjorthoj 2013 | Treatment | 26.8 | 38.21 | 37 |
| Hjorthoj 2013 | Control | 44.6 | 58.46 | 31 |
| Proportion of days abstinence from all substances last 90 days by 6 months | ||||
| Barrowclough 2010 | Treatment | 34.27 | 34.01 | 147 |
| Barrowclough 2010 | Control | 30.06 | 35.18 | 148 |
| Proportion of days abstinence from all substances last 90 days by 12 months | ||||
| Barrowclough 2010 | Treatment | 40.24 | 36.18 | 137 |
| Barrowclough 2010 | Control | 34.51 | 34.18 | 136 |
| Proportion of days abstinence from all substances last 90 days by 18 months | ||||
| Barrowclough 2010 | Treatment | 38.17 | 37.23 | 129 |
| Barrowclough 2010 | Control | 38.47 | 34.94 | 127 |
| Proportion of days abstinence from all substances last 90 days by 24 months | ||||
| Barrowclough 2010 | Treatment | 44.25 | 38.36 | 130 |
| Barrowclough 2010 | Control | 37.18 | 36.89 | 117 |
7.9. Analysis.
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 9 Substance use: 5. Average change in % days abstinent during & after treatment.
| Substance use: 5. Average change in % days abstinent during & after treatment | |||||
|---|---|---|---|---|---|
| Study | Treatment Group | Median | range | N | Notes |
| Barrowclough 2001 | CBT + MI | 19.99 | ‐25.6 to 83.4 | 17 | U = 86.5 P < 0.03 Non‐parametric analysis. Data summed over 4 time periods (to 12 months) and subtracted from baseline. |
| Barrowclough 2001 | TAU | ‐6.52 | ‐67.9‐53.2 | 15 | |
7.10 Mental state: 1. Average score (PANSS total, high = poor)
No clear differences were found between CBI + MI and standard care groups for average total PANSS scores by six months (Hjorthoj 2013; Kemp 2007) (n = 78, MD 0.99 CI ‐5.91 to 7.89), nine to 10 months (Barrowclough 2001; Hjorthoj 2013) (n = 92, MD ‐5.01 CI ‐11.25 to 1.22), 12 months (Barrowclough 2010) (n = 274, MD 2.52 CI ‐0.68 to 5.72) and by 24 months (Barrowclough 2010) (n = 247, MD 2.71 CI ‐0.58 to 6.00,). (Analysis 7.10).
7.10. Analysis.
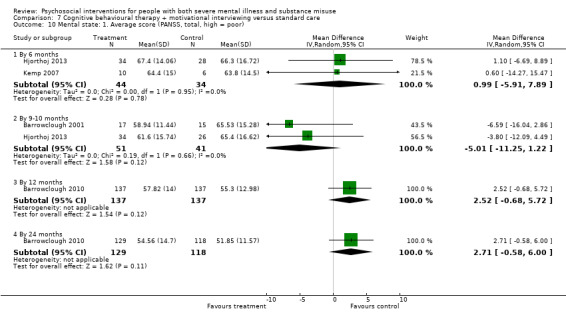
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 10 Mental state: 1. Average score (PANSS, total, high = poor).
7.11 Mental state: 2. Average score (PANSS positive, high = poor)
No clear differences were found between CBI+MI and standard care groups for average PANSS positive subscale scores at 12 months (MD 0.03, 95% CI ‐1.18 to 1.24; participants = 274; studies = 1) or 24 months (MD 0.52, 95% CI ‐0.80 to 1.84; participants = 247; studies = 1). (Analysis 7.11).
7.11. Analysis.
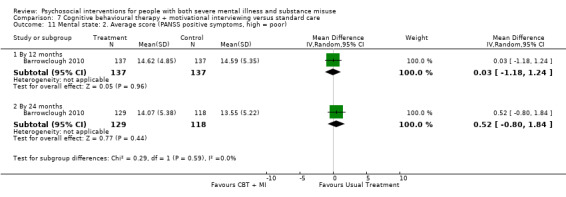
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 11 Mental state: 2. Average score (PANSS positive symptoms, high = poor).
7.12 Mental state: 3. Average score (PANSS negative, high = poor)
No clear differences were found between CBI + MI and standard care groups for average PANSS negative subscale scores at 12 (MD 0.39, 95% CI ‐0.65 to 1.43; participants = 274; studies = 1) or 24 months (MD 0.16, 95% CI ‐0.84 to 1.16; participants = 247; studies = 1). (Analysis 7.12).
7.12. Analysis.
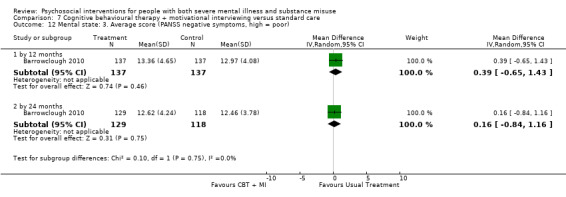
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 12 Mental state: 3. Average score (PANSS negative symptoms, high = poor).
7.13 Mental state: 4. Average score (various scales, high = poor) (skewed data)
Average scores for other measures of mental state that reported skewed data are presented in 'Other data' tables. (Analysis 7.13).
7.13. Analysis.
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 13 Mental state: 4. Average score (various scales, high = poor)) (skewed data).
| Mental state: 4. Average score (various scales, high = poor)) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Depressive symptoms ‐ 3 months (BDI‐11) | ||||
| Baker 2006 | Treatment | 16.93 | 12.51 | 58 |
| Baker 2006 | Control | 10.93 | 10.43 | 61 |
| Depressive symptoms ‐ 6 months (BDI‐11) | ||||
| Baker 2006 | Treatment | 14.10 | 11.38 | 58 |
| Baker 2006 | Control | 9.92 | 9.29 | 61 |
| Depressive symptoms ‐ 12 months (BDI‐11) | ||||
| Baker 2006 | Treatment | 17.14 | 13.20 | 44 |
| Baker 2006 | Control | 9.68 | 10.30 | 53 |
| Depressive symptoms ‐ 3 months Calgary Depression Scale | ||||
| Madigan 2013 | Treatment | 4.4 | 4.3 | 40 |
| Madigan 2013 | Control | 4.6 | 4.8 | 20 |
| Depressive symptoms ‐ 12 months Calgary Depression Scale | ||||
| Madigan 2013 | Treatment | 4.3 | 4.4 | 33 |
| Madigan 2013 | Control | 4.3 | 4.2 | 11 |
| General symptoms total score ‐ 6 months (BPRS) | ||||
| Baker 2006 | Treatment | 35.47 | 9.34 | 58 |
| Baker 2006 | Control | 34.52 | 8.53 | 61 |
| General symptoms total score ‐ 12 months (BPRS) | ||||
| Baker 2006 | Treatment | 35.43 | 8.59 | 44 |
| Baker 2006 | Control | 32.58 | 8.19 | 53 |
| General symptoms total score ‐ 3 months (BPRS) | ||||
| Baker 2006 | Treatment | 35.31 | 9.04 | 58 |
| Baker 2006 | Control | 34.46 | 11.24 | 61 |
| General symptoms ‐ 18 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 21.13 | 6.39 | 15 |
| Barrowclough 2001 | Control | 30.07 | 8.17 | 14 |
| Barrowclough 2014 | Treatment (long) | 35.1 | 9.0 | 23 |
| Barrowclough 2014 | Control | 31.6 | 8.5 | 21 |
| Total score ‐ 3 months (PANSS) | ||||
| Gouzoulis‐Mayfrank 2015 | Treatment | 65.36 | 28.26 | 50 |
| Gouzoulis‐Mayfrank 2015 | Control | 77.42 | 31.55 | 50 |
| Total score ‐ 6 months (PANSS) | ||||
| Gouzoulis‐Mayfrank 2015 | Treatment | 61.92 | 26.823 | 50 |
| Gouzoulis‐Mayfrank 2015 | Control | 77.9 | 33.254 | 50 |
| Total score ‐ 12 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 56.88 | 14.23 | 17 |
| Barrowclough 2001 | Control | 63.40 | 17.96 | 15 |
| Total score ‐ 18 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 52.20 | 11.12 | 15 |
| Barrowclough 2001 | Control | 58.50 | 15.04 | 14 |
| Manic symptoms ‐ 3 months (BPRS) | ||||
| Baker 2006 | Treatment | 6.43 | 2.46 | 58 |
| Baker 2006 | Control | 7.39 | 3.51 | 61 |
| Manic symptoms ‐ 6 months (BPRS) | ||||
| Baker 2006 | Treatment | 6.38 | 2.23 | 58 |
| Baker 2006 | Control | 6.57 | 3.56 | 61 |
| Manic symptoms ‐ 12 months (BPRS) | ||||
| Baker 2006 | Treatment | 6.07 | 1.63 | 44 |
| Baker 2006 | Control | 6.18 | 2.32 | 53 |
| Negative symptoms ‐ 3 months (BPRS) | ||||
| Baker 2006 | Treatment | 6.24 | 2.10 | 58 |
| Baker 2006 | Control | 6.48 | 2.47 | 61 |
| Negative symptoms ‐ 6 months (BPRS) | ||||
| Baker 2006 | Treatment | 6.00 | 1.52 | 58 |
| Baker 2006 | Control | 6.08 | 1.54 | 61 |
| Negative symptoms ‐ 12 months (BPRS) | ||||
| Baker 2006 | Treatment | 6.86 | 1.36 | 44 |
| Baker 2006 | Control | 6.58 | 2.35 | 53 |
| Negative symptoms ‐ 6 months (PANSS) | ||||
| Hjorthoj 2013 | Treatment | 16.3 | 5.92 | 34 |
| Hjorthoj 2013 | Control | 15.3 | 5.35 | 28 |
| Negative symptoms ‐ 9‐10 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 12.47 | 4.12 | 17 |
| Barrowclough 2001 | Control | 16.20 | 4.87 | 15 |
| Hjorthoj 2013 | Treatment | 14.9 | 5.2 | 34 |
| Hjorthoj 2013 | Control | 15.2 | 6.15 | 26 |
| Negative symptoms ‐ 12 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 12.65 | 4.97 | 17 |
| Barrowclough 2001 | Control | 14.67 | 6.02 | 15 |
| Negative symptoms ‐ 18 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 10.27 | 2.25 | 15 |
| Barrowclough 2001 | Control | 15.50 | 5.71 | 14 |
| Barrowclough 2014 | Treatment | 13.7 | 3.9 | 23 |
| Barrowclough 2014 | Control | 15.2 | 6.5 | 21 |
| Negative symptoms ‐ 3 months (SANS) | ||||
| Madigan 2013 | Treatment | 3.8 | 2.4 | 40 |
| Madigan 2013 | Control | 3.2 | 2.3 | 20 |
| Negative symptoms ‐ 12 months (SANS) | ||||
| Madigan 2013 | Treatment | 4.6 | 3 | 32 |
| Madigan 2013 | Control | 4.8 | 3.2 | 19 |
| Positive symptoms ‐ 6 months (PANSS) | ||||
| Hjorthoj 2013 | Treatment | 15.6 | 5.09 | 34 |
| Hjorthoj 2013 | Control | 17 | 5.01 | 28 |
| Positive symptoms ‐ 9‐10 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 15.29 | 4.69 | 17 |
| Barrowclough 2001 | Control | 16.40 | 4.29 | 15 |
| Hjorthoj 2013 | Treatment | 14.4 | 5.22 | 34 |
| Hjorthoj 2013 | Control | 16.2 | 4.99 | 26 |
| Positive symptoms ‐ 12 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 13.35 | 4.57 | 17 |
| Barrowclough 2001 | Control | 16.07 | 5.54 | 15 |
| Positive symptoms ‐ 18 months (PANSS) | ||||
| Barrowclough 2001 | Treatment | 13.87 | 4.27 | 15 |
| Barrowclough 2001 | Control | 12.93 | 4.23 | 14 |
| Barrowclough 2014 | Treatment (Long) | 13.0 | 4.6 | 24 |
| Barrowclough 2014 | Control | 12.7 | 5.1 | 21 |
| Positive symptoms ‐ 3 months (SAPS) | ||||
| Madigan 2013 | Treatment | 4.8 | 3.7 | 42 |
| Madigan 2013 | Control | 5.1 | 4.1 | 22 |
| Positive symptoms ‐ 12 months (SAPS) | ||||
| Madigan 2013 | Treatment | 4.9 | 4 | 32 |
| Madigan 2013 | Control | 5.1 | 4.2 | 17 |
7.14 Global state: 1. Average score (GAF, low = poor)
Average global assessment scores for functioning (GAF) were not clearly different by three months (Baker 2006; Gouzoulis‐Mayfrank 2015; Madigan 2013) (MD ‐0.17, 95% CI ‐2.79 to 2.46; participants = 277; studies = 3), six months (MD 1.65, 95% CI ‐2.46 to 5.76; participants = 219; studies = 2), 12 months (Baker 2006; Barrowclough 2001; Barrowclough 2010; Madigan 2013) (MD 1.24, 95% CI ‐1.86 to 4.34; participants = 445; studies = 4), 18 months (MD 4.57, 95% CI ‐3.07 to 12.21; participants = 72; studies = 2) or 24 months (MD ‐0.21, 95% CI ‐2.93 to 2.51; participants = 234; studies = 1). Lower scores indicate poorer functioning. Statistical heterogeneity was not present at any time period. (Analysis 7.14).
7.14. Analysis.
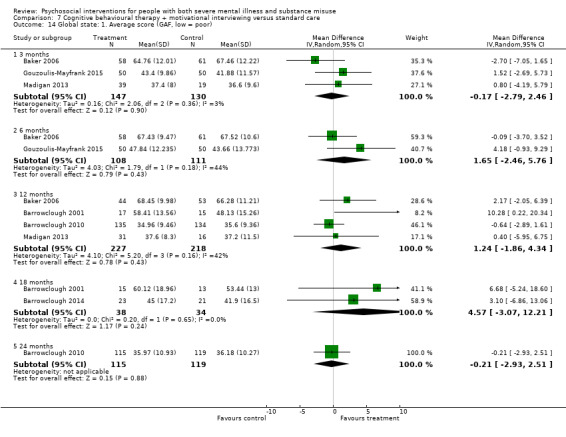
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 14 Global state: 1. Average score (GAF, low = poor).
7.15 Global state: 2. Forensic measures ‐ arrests reported ‐ by six months
The number of reported arrests (Bellack 2006) were not clearly different between the CBT + MI and standard care group by six months (treatment 13%, control 27%; (RR 0.49, 95% CI 0.22 to 1.10; participants = 110; studies = 1). (Analysis 7.15).
7.15. Analysis.
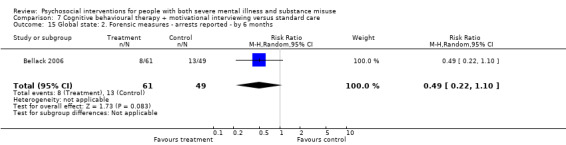
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 15 Global state: 2. Forensic measures ‐ arrests reported ‐ by 6 months.
7.16 Social functioning: 1. Average score (SFS, low = poor)
Barrowclough 2001 reported on social functioning using average SFS scores. There was no clear difference between CBT+ MI and standard care group scores by nine months (end of treatment) (MD 5.01, 95% CI ‐0.55 to 10.57; participants = 32; studies = 1). However, by 12 months (three months following end of treatment) results favoured the CBT+ MI group (high scores = better) (MD 7.27, 95% CI 0.86 to 13.68; participants = 32; studies = 1). (Analysis 7.16).
7.16. Analysis.
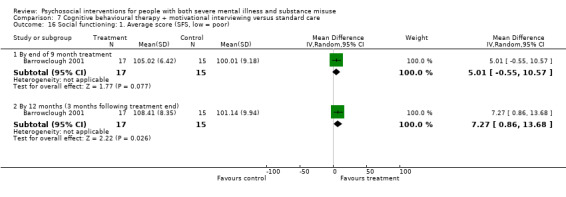
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 16 Social functioning: 1. Average score (SFS, low = poor).
7.17 Quality of life/ life satisfaction: 1. Average score (BQOL, general life satisfaction, low = poor) ‐ by six months
Average general life satisfaction scores (BQOL) were higher for the CBT + MI group (Bellack 2006) by six months (MD 0.58, 95% CI 0.00 to 1.16; participants = 110; studies = 1, P = 0.049), although confidence intervals crossed the line of no effect. Differences in baseline means (SD) did not account for this finding (treatment 4.25 (1.65) to 4.79 (1.66) at 6 months, and control 3.96 (1.58) to 4.21 (1.43) at 6 months). Lower scores indicate less life satisfaction. (Analysis 7.17).
7.17. Analysis.
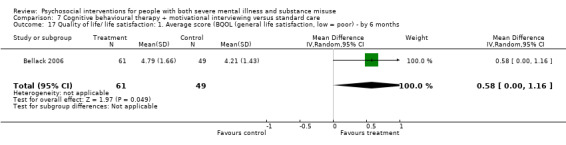
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 17 Quality of life/ life satisfaction: 1. Average score (BQOL (general life satisfaction, low = poor) ‐ by 6 months.
7.18 Quality of life/ life satisfaction: 2. Average score (BQOL, overall quality of life, low = poor) ‐ by six months
No clear differences between treatment groups were found in overall quality of life scores (BQOL) by six months (Bellack 2006) (MD ‐0.02, 95% CI ‐0.61 to 0.57; participants = 110; studies = 1). (Analysis 7.18).
7.18. Analysis.
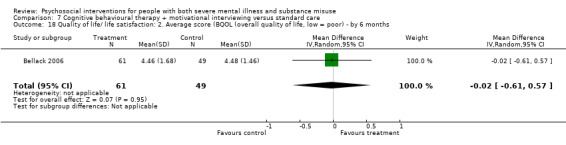
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 18 Quality of life/ life satisfaction: 2. Average score (BQOL (overall quality of life, low = poor) ‐ by 6 months.
7.19 Quality of life/ life satisfaction: 3. Average score (WHOQOL, BREF, higher scores = better QoL) ‐ by six months
No clear differences between treatment groups were found in WHOQOL Bref scores by six months (Kemp 2007) (MD ‐15.70, 95% CI ‐36.19 to 4.79; participants = 16; studies = 1). (Analysis 7.19).
7.19. Analysis.
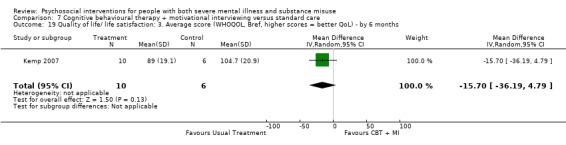
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 19 Quality of life/ life satisfaction: 3. Average score (WHOQOL, Bref, higher scores = better QoL) ‐ by 6 months.
7.20 Quality of life/ life satisfaction: 4. Average score (MANSA, higher scores = better QoL)
No clear differences between treatment groups were found in quality of life scores using the MANSA by six months (Hjorthoj 2013) (MD ‐2.70, 95% CI ‐7.01 to 1.61; participants = 64; studies = 1) or by 10 months (MD 0.90, 95% CI ‐3.73 to 5.53; participants = 61; studies = 1). (Analysis 7.20).
7.20. Analysis.
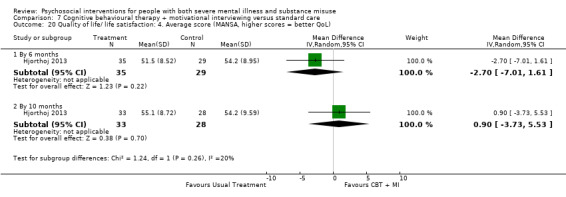
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 20 Quality of life/ life satisfaction: 4. Average score (MANSA, higher scores = better QoL).
7.21 Quality of life/ life satisfaction: 5. Average score (CSQ, client satisfaction, higher = good)
One study (Hjorthoj 2013) reported client satisfaction using average CSQ score, there was a clear difference in scores, favouring the CBT + MI group by 10 months (MD 6.40, 95% CI 3.87 to 8.93; participants = 62; studies = 1, P < 0.001). (Analysis 7.21).
7.21. Analysis.
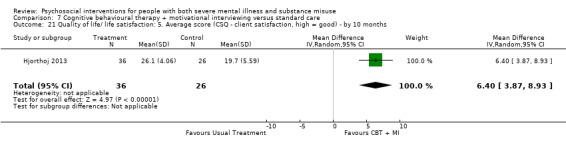
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 21 Quality of life/ life satisfaction: 5. Average score (CSQ ‐ client satisfaction, high = good) ‐ by 10 months.
7.22 Service use: 1. Relapse (hospitalised)
We were only able to include limited data for relapse and found no major difference in the likelihood of relapse (measured by number of hospitalisations) between groups (Barrowclough 2001; Barrowclough 2014) by end of nine‐month treatment phase (treatment 17% relapsed, control 28% relapsed; RR 0.58, 95% CI 0.29 to 1.16; participants = 107; studies = 2), or by 12 months (three months after treatment finished (Barrowclough 2001) (treatment 33%, control 67%; (RR 0.50, 95% CI 0.24 to 1.04; participants = 36; studies = 1), or 18 months (nine months after treatment finished) (Barrowclough 2001; Barrowclough 2014) (treatment 20%, control 33%; (RR 0.61, 95% CI 0.34 to 1.10; participants = 105; studies = 2). (Analysis 7.22).
7.22. Analysis.
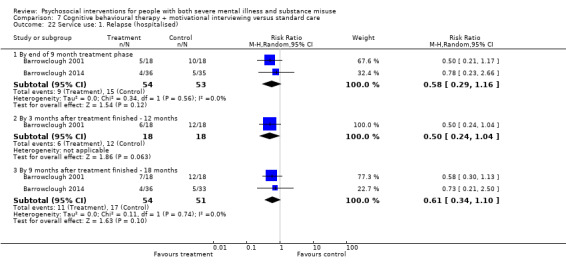
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 22 Service use: 1. Relapse (hospitalised).
7.23 Economic outcomes: 1. Diret cost in US$ (BQOL, money subscale) (skewed data)
The average direct cost subscale of the BQOL at six months reported by Bellack 2006 was skewed and is presented in 'Other data' tables (Analysis 7.23).
7.23. Analysis.
Comparison 7 Cognitive behavioural therapy + motivational interviewing versus standard care, Outcome 23 Economic outcomes: 1. Direct cost in US$ (BQOL, money subscale) (skewed data).
| Economic outcomes: 1. Direct cost in US$ (BQOL, money subscale) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Bellack 2006 | Treatment | 329 | 649 | 61 |
| Bellack 2006 | Control | 180 | 201 | 49 |
Comparison 8: Cognitive behavioural therapy + psychological rehabilitation versus standard care
8.1 ‐ 8.3 Global state: Forensic measures
See also Table 8.
All outcome data regarding forensic measures were skewed data and are reported in 'Other data' tables (Maloney 2006). There was no real indication that the number of arrests was less in the cognitive behavioural therapy + psychosocial rehabilitation group over all the time periods (Analysis 8.1), and this also applied to the number of convictions (Analysis 8.2). The number of days in jail for each group was also not really noticeably different (Analysis 8.3). It should be stressed that all data were skewed and not re‐analysed, and are merely reported again in this review.
8.1. Analysis.
Comparison 8 Cognitive behavioural therapy + psychosocial rehabilitation versus standard care, Outcome 1 Global state: 1. Forensic measures ‐ number of arrests (skewed data).
| Global state: 1. Forensic measures ‐ number of arrests (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 0.60 | .84 | 18 |
| Maloney 2006 | Control | 0.58 | 1.00 | 43 |
| 18 months | ||||
| Maloney 2006 | Treatment | 0.31 | 0.60 | 18 |
| Maloney 2006 | Control | 0.20 | 0.41 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 0.17 | 0.51 | 18 |
| Maloney 2006 | Control | 0.34 | 0.81 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 0.56 | 1.29 | 18 |
| Maloney 2006 | Control | 0.51 | 0.88 | 43 |
8.2. Analysis.
Comparison 8 Cognitive behavioural therapy + psychosocial rehabilitation versus standard care, Outcome 2 Global state: 2. Forensic measures ‐ number of convictions (skewed data).
| Global state: 2. Forensic measures ‐ number of convictions (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 0.22 | 0.44 | 18 |
| Maloney 2006 | Control | 0.25 | 0.62 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 0.33 | 1.19 | 18 |
| Maloney 2006 | Control | 0.22 | 0.63 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 0.44 | 1.20 | 18 |
| Maloney 2006 | Control | 0.53 | 1.10 | 43 |
8.3. Analysis.
Comparison 8 Cognitive behavioural therapy + psychosocial rehabilitation versus standard care, Outcome 3 Global state: 3. Forensic measures ‐ days in jail (skewed data).
| Global state: 3. Forensic measures ‐ days in jail (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 8.78 | 16.88 | 18 |
| Maloney 2006 | Control | 12.90 | 30.12 | 43 |
| 18 months | ||||
| Maloney 2006 | Treatment | 19.19 | 30.01 | 18 |
| Maloney 2006 | Control | 20.92 | 39.46 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 4.67 | 18.59 | 18 |
| Maloney 2006 | Control | 7.28 | 14.07 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 19.33 | 34.49 | 18 |
| Maloney 2006 | Control | 24.59 | 38.27 | 43 |
Comparison 9: Combined cognitive behavioural therapy + intensive case management versus standard care
9.1 ‐ 9.3 Global state: Forensic measures
See Table 9.
All outcome data regarding forensic measures were skewed data and are reported in 'Other data' tables (Maloney 2006). There is some indication that the number of arrests was less in the cognitive behavioural therapy + intensive case management group over all the time periods (Analysis 9.1), and this also applied to the number of convictions (Analysis 9.2). However, the number of days in jail for each group was not noticeably different (Analysis 9.3). It should be stressed that all data were skewed and not re‐analysed, merely reported again in this review.
9.1. Analysis.
Comparison 9 Cognitive behavioural therapy + intensive case management versus standard care, Outcome 1 Global state: 1. Forensic measures‐ number of arrests (skewed data).
| Global state: 1. Forensic measures‐ number of arrests (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 0.11 | 0.33 | 16 |
| Maloney 2006 | Control | 0.58 | 1.00 | 43 |
| 18 months | ||||
| Maloney 2006 | Treatment | 0.07 | 0.28 | 16 |
| Maloney 2006 | Control | 0.20 | 0.41 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 0.31 | 0.63 | 16 |
| Maloney 2006 | Control | 0.34 | 0.81 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 0.15 | 0.38 | 16 |
| Maloney 2006 | Control | 0.51 | 0.88 | 43 |
9.2. Analysis.
Comparison 9 Cognitive behavioural therapy + intensive case management versus standard care, Outcome 2 Global state: 2. Forensic measures ‐ number of convictions (skewed data).
| Global state: 2. Forensic measures ‐ number of convictions (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 0.11 | 0.33 | 16 |
| Maloney 2006 | Control | 0.25 | 0.62 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 0.23 | 0.44 | 16 |
| Maloney 2006 | Control | 0.22 | 0.63 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 0.15 | 0.38 | 16 |
| Maloney 2006 | Control | 0.53 | 1.10 | 43 |
9.3. Analysis.
Comparison 9 Cognitive behavioural therapy + intensive case management versus standard care, Outcome 3 Global state: 3. Forensic measures ‐ days in jail (skewed data).
| Global state: 3. Forensic measures ‐ days in jail (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 12 months | ||||
| Maloney 2006 | Treatment | 17.44 | 39.13 | 16 |
| Maloney 2006 | Control | 12.90 | 30.12 | 43 |
| 18 months | ||||
| Maloney 2006 | Treatment | 15.15 | 29.59 | 16 |
| Maloney 2006 | Control | 20.92 | 39.46 | 43 |
| 24 months | ||||
| Maloney 2006 | Treatment | 13.46 | 31.76 | 16 |
| Maloney 2006 | Control | 7.28 | 14.07 | 43 |
| 30 months | ||||
| Maloney 2006 | Treatment | 14.08 | 24.29 | 16 |
| Maloney 2006 | Control | 24.59 | 38.27 | 43 |
Comparison 10: Sensitivity analyses
All of the included studies were described as randomised and random sequence generation was judged as at low or unclear risk of bias for all included trials. Therefore, we did not undertake the anticipated sensitivity analysis. There were only two comparisons (Analysis 7.14; Analysis 5.2) where four or more studies were reported for a comparison and sensitivity analyses were undertaken for these.
Analysis 10.2 grouped studies investigating motivational interviewing plus cognitive behavioural therapy according to risk of bias for allocation concealment and Analysis 10.1 grouped studies investigating motivational interviewing according to diagnostic entry criteria (mixed diagnoses versus schizophrenia only trials) for the short to medium term (three to six months). Neither of these analyses altered the overall result.
10.2. Analysis.
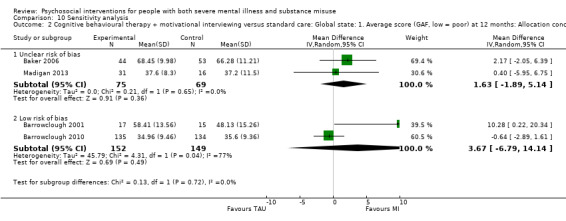
Comparison 10 Sensitivity analysis, Outcome 2 Cognitive behavioural therapy + motivational interviewing versus standard care: Global state: 1. Average score (GAF, low = poor) at 12 months: Allocation concealment.
10.1. Analysis.
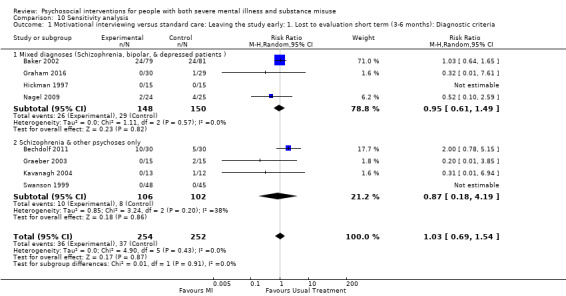
Comparison 10 Sensitivity analysis, Outcome 1 Motivational interviewing versus standard care: Leaving the study early: 1. Lost to evaluation short term (3‐6 months): Diagnostic criteria.
Discussion
Summary of main results
Comparison 1: Integrated models of care versus standard care
Please see Table 1. Overall, there was low‐quality evidence of no difference between integrated models of care and standard care in terms of numbers lost to treatment or deaths by 36 months, although individually some studies (Burnam 1995; Essock 2006) showed some effect for retaining participants in evaluation during the early stages of each study. At the end of each treatment period differences were no longer apparent. All four studies had sample sizes greater than 100 participants, drawn from homeless (Burnam 1995), forensic (Chandler 2006) and community populations (Drake 1998a; Essock 2006). Modified scales were used by Burnam 1995, precluding inclusion.
There was low‐quality evidence of no difference in alcohol or substance use between integrated models of care and standard care in terms of, or not, of remission by 36 months.
Moreover, there was low‐quality evidence of no difference between integrated models of care and standard care in terms of average general global functioning scores and moderate‐quality evidence of no difference for quality of life scores.
Outcome measures of jail and hospital days, arrests and hours of medication service were all skewed in Chandler 2006. This resulted in attrition being the only clear outcome measure which could be analysed. We were able to include data from Essock 2006 and Drake 1998a. They provided both treatment and controls groups with a certain level of integrated care, the difference being that the assertive community treatment (ACT) teams provided most outpatient services themselves, while standard care (standard case management) brokered services to other clinicians. The null results found in this review suggest that providing services by the same team may not be crucial to successful integration of services, although readers are advised that the quality of evidence is low overall.
Morse 2006 was a three‐arm study with about 50 people in each arm. Interesting data were presented for important outcomes but all were continuous and skewed. None gave the impression of a real difference occurring between the psychosocial interventions and the standard care. Again, considering the huge effort that must have gone into the integrated assertive community treatment and ACT, this might indicate how difficult this group of people are to treat, or how standard care has as good an effect as anything in terms of substance misuse and general housing outcomes.
Comparison 2: Non‐integrated models of care ‐ assertive community treatment only (ACTO) or intensive case management or specialised case management services versus standard care
Please see Table 2. There was very low‐quality evidence of no difference between assertive community treatment or intensive case management and standard care in terms of being lost to treatment by 12 months. Death was not measured in any of the trials. Alcohol or drug use as data were skewed or not reported.
Moreover, there was very low‐quality evidence of no difference between treatment groups in terms of mental state, average general global functioning or general life satisfaction.
The results showed no support for retaining participants at any time period. We were only able to include little data as attrition rates were high (Bond 1991a), adapted scales were used, and the data were skewed or reporting was unclear (Bond 1991b; Lehman 1993; Jerrell 1995b). The role functioning (RFS) data provided by Jerrell 1995b by the end of the study (18 months) favoured the Twelve‐Step recovery control group, with a small but significant difference. The social adjustment scores were similar between groups.
One small study (Godley 1994) presents data by site and, clearly, practice by site does differ considerably. There is not really a clear pattern in the data suggesting an effect, and where there is some suggestion of a difference between groups the data are based on very few people.
Comparison 3: Cognitive behavioural therapy (CBT) versus standard care
Please see Table 3. There was low‐quality evidence of no difference between CBT and standard care in terms of numbers lost to treatment by three months. Death was not measured in any of the trials. Neither trial reported alcohol use separately, so this effect could not be estimated and evidence for substance use by six months was very low quality.
There was low‐quality evidence of no difference between CBT and standard care for mental state (BPRS) at six months, and evidence was very low for global functioning at six months. No study reported life satisfaction.
Support for retention in CBT was from the pooled results of Edwards 2006 and Naeem 2005. One study (Edwards 2006) found a 30% increased likelihood of cannabis use by those in the treatment group after 10 weekly sessions of CBT. No other differences were observed on measures of substance use or mental state and functioning, but again much of the data were unusable.
Comparison 4: Contingency management versus standard care
Please see Table 4. There was moderate‐quality evidence between contingency management and standard care in terms of numbers lost to treatment by three months. Death was not measured in any of the trials. There was also no useable data available for alcohol use (data skewed) and no clear difference between treatment groups for substance use (stimulant‐positive urine tests) by six months.
Moreover, there was low‐quality evidence of no difference between contingency management and standard care for mental state (number hospitalised) at six months. Neither trial reported on global assessment of functioning and general life satisfaction.
McDonell 2013 reported fewer participants with a stimulant‐positive urine at the end of treatment (three months) for the contingency management arm compared to standard care. However, by six months (three months post‐treatment) this was no longer significant. Moreover, they also reported less injection use at the end of treatment (three months) for the active arm compared to standard care, and again this was no longer significant at six months. Over the six‐month trial, fewer participants in the contingency managed arm were hospitalised compared to standard care. Petry 2013 reported longer consecutive weeks of cocaine abstinence in 10 participants receiving contingency management compared to treatment as usual (TAU) (n = 9). However, less than half the expected samples were remitted and when missing samples were not included, no between‐group differences were apparent. McDonell 2017 reported longer durations of continuous alcohol abstinence in the contingent intervention group at three months, however, these differences were not statistically different at the three‐month post‐treatment follow‐up.
Comparison 5: Motivational interviewing (MI) versus standard care
Please see Table 5. There was very low‐quality evidence of no difference between motivational interviewing and standard care in terms of numbers lost to treatment (six months), lost to evaluation (12 months) or deaths (18 months). There was very low‐quality evidence of not abstaining from alcohol (six months) or polydrug use (12 months).
There was very low‐quality evidence of no difference between motivational interviewing and standard care in terms of mental state (Symptom Checklist 90 (SCL‐90), three months) and average global functioning at 12 months. None of the trials measured general life satisfaction.
Some support was found for the effectiveness of motivational interviewing in reducing substance use, even though studies were generally small, interventions brief, and follow‐up times shorter than for other comparisons. Graeber 2003 found that there was more likelihood that participants in the treatment group would abstain from alcohol after only three sessions of motivational interviewing; by three months and six months this increased. Bonsack 2011 reported that individual sessions of motivational interviewing for up to six months reduced the number of joints consumed at three and six months, but not at 12 months follow‐up. Similarly, participants in the treatment group of Kavanagh 2004 showed they were more likely to be abstaining or had improved on all substances by 12 months after three hours of motivational interviewing over six to nine sessions. More participants in the treatment group of Swanson 1999 attended their first aftercare appointment after one 15‐minute and one one‐hour session. Graham 2016 reported marginal improvements in engagement in treatment at three months after a brief (two‐week) inpatient intervention. Bechdolf 2011 also reported higher chances of attending outpatients over a period of six months. In contrast, Baker 2002 reported little differences between groups after one 45‐minute session, which was more apparent at 12 months than at three months when the treatment showed some benefit. Hickman 1997 showed little difference in mental state scores after one brief session. The results indicate that multiple sessions of motivational interviewing may lead to short‐term reductions in substance use and increased attendance at outpatient appointments.
Comparison 6: Skills training versus standard care
Please see Table 6. There was very low‐quality evidence of no difference between skills training and standard care in terms of numbers lost to treatment by 12 months and low‐quality evidence of no difference for death. There were no useable data for substance use by 12 months as the data were skewed. No clear differences were reported in days abstinent from alcohol.
Moreover, there was very low‐quality evidence of no difference between skills training and standard care for relapse (in mental state symptoms) at eight months and no differences for global state at 12 months. No trial reported on general life satisfaction.
Pooled results from Bogenschutz 2014 and Rosenblum 2014 reported no differences in lost to treatment at three months. Pooled results of Hellerstein 1995 and Jerrell 1995a showed that control group participants were more likely to be lost from the study at six to nine months. However, by 18 months Jerrell 1995a and Eack 2015 reported that participants in their treatment programme were more likely to be lost. Hellerstein 1995 offered their treatment group a same site co‐ordinated treatment approach and their control group were offered the same treatment, which was not case co‐ordinated. In the largest study to date, Rosenblum 2014 found those in the double‐trouble in recovery group self‐reported fewer days using alcohol or drugs over 30 days compared to waiting list controls. However, the data were skewed and effects were not apparent at both study sites. Eack 2015 also reported reductions in alcohol use in the last 30 days (but not cannabis use) during cognitive enhancement therapy in a small pilot study. In contrast, Bogenschutz 2014 reported no differences in abstinence from alcohol or drugs in individuals following a 12 weeks of a 12‐step facilitation therapy.
Comparison 7: Cognitive behavioural therapy + motivational interviewing (CBT + MI) versus standard care
Please see Table 7. There was low‐quality evidence of no difference between cognitive behavioural therapy + motivational interviewing and standard care in terms of numbers lost to treatment or deaths by 12 months. All the data for alcohol use were skewed and evidence of no differences for drug use by six months was low quality.
There was very low‐quality evidence of no difference between cognitive behavioural therapy + motivational interviewing and standard care in terms of mental state (relapse) and average global state (GAF scores) at 12 months. Moreover, there was low‐quality evidence for no difference in quality of life scores at six months between treatment arms.
We found some support for the effectiveness of cognitive behavioural therapy + motivational interviewing over standard care, yet, findings were inconsistent and, again, much data were unable to be used from all seven eligible studies. The Barrowclough 2001 was a small study but showed an increased likelihood of relapse in the control group up until 18 months. Global functioning was slightly lower in the control group by nine months, although this difference was not sustained at later time periods (up to 18 months). Gouzoulis‐Mayfrank 2015 reported transient large decreases in substance use at three months in treated participants compared to standard care, but the sample size was small and attrition was high (> 50% by 12 months). Bellack 2006 showed slightly decreased general life satisfaction scores and a 51% increased likelihood of being arrested in their reasonably sized control group by six months. By contrast, Baker 2006 showed that participants were more likely to leave the treatment group by three months. The treatment group also seemed to have a slightly higher mean number of drugs used by three months; this difference was not apparent by six months, and Madigan 2013 showed no difference in cannabis use at three or six months. Moreover, the phase‐specific study by Barrowclough 2014 found no evidence that brief or long‐term cognitive behavioural therapy + motivational interviewing therapy conferred benefit over standard care in reducing frequency or amount of cannabis use at nine months or 18 months following a first episode of psychosis. The largest study to date (Barrowclough 2010) reported no differences between interventions and death or hospitalised versus not admitted to hospital and alive by 24 months. Nor did this study report any differences in substance use, mental state (Positive and Negative Syndrome Scale (PANSS)), or other outcomes. Hjorthoj 2013 reported higher satisfaction scores by 10 months, but no differences were reported in quality of life scores or other outcomes.
Further research is required to determine whether long‐term cognitive behavioural therapy combined with motivational interviewing is useful and cost‐effective.
Comparison 8: Cognitive behavioural therapy + psychological rehabilitation versus standard care
Please see Table 8. All of the outcomes for this comparison were very‐low quality or the outcome was not measured. It is problematic to interpret the skewed data and all data were from one study (Maloney 2006) allocating less than 100 people to this comparison. It is feasible that a subtle difference between treatment groups could not be highlighted because of the limited power of the trial but, from what data we have, there is no indication that the number of arrests is less in the cognitive behavioural therapy plus psychosocial rehabilitation group over all the time periods. This also applies to the number of convictions and the number of days in jail.
Comparison 9: Cognitive behavioural therapy + intensive case management versus standard care
Please see Table 9. All of the outcomes for this comparison were very‐low quality or the outcome was not measured. Again Maloney 2006 reports useful outcomes relating to functioning in society but again the data are skewed and difficult to interpret. Unlike the preceding comparison, however, there is a suggestion that there may be some positive effect for people allocated to the cognitive behavioural therapy + intensive case management group. The number of arrests is less in the cognitive behavioural therapy + intensive case management group over all time periods, and this also applies to the number of convictions at 12 and 30 months. However, the number of days in jail for each group is not noticeably different. This may give some hope that the very intensive approach does have some benefit in terms of these important outcomes but, again, these findings from such a small study should be replicated before making any change in policy. Economic analyses would also be of interest for this package of care that is likely to be expensive.
Sensitivity analysis
Sensitivity analyses were conducted to ascertain if there were substantial differences in the results when lesser quality trials were excluded. There were relatively few trials to conduct the sensitivity analysis due to the small numbers of trials in each intervention and the large number of outcome measures at variable time points. There was no indication that trials of lesser quality or those recruiting patients with severe mental illness other than schizophrenia influenced the overall outcomes in this review.
Overall completeness and applicability of evidence
Many of the included studies were described as pilot studies which included small samples sizes. Eighteen trials involved more than 100 participants and three of these involved more than 200 participants after randomisation (Barrowclough 2010; Drake 1998a; Rosenblum 2014). However, the overall power for a particular common outcome and comparison was low due to the variety of interventions and outcomes measured.
Examination of the summary of findings indicates that several critical or important outcomes were not measured by any of the studies, and therefore no power exists. Future research could examine these comparisons in order to bring to light any potential benefits in the management of patients with a dual diagnosis.
The majority of studies presented medium‐term data; with six months to one year follow‐up. This is a reasonable length of time to assess differences in the intervention effects. Longer‐term studies (one to three years) employed integrated and assertive community care interventions. These types of studies are important to engage patients in treatment programmes that help recovery from serious mental illness.
All study participants had a diagnosis of severe mental illness and substance misuse. Participants were from a wide range of settings, so the results of this review will be applicable to similar patients, particularly those in the USA (25 trials). Some generalisation can be assumed for the UK (five trials) and Australia (six trials) for cognitive behavioural therapy and motivational interviewing as the studies from these areas examined these interventions. Integrated, non‐integrated, and skills‐training intervention findings may apply elsewhere only if the intervention is delivered in a similar manner. However, as there are differences between the USA (Bond 2017) and other countries' services, including education and training of health service staff, generalisation to other areas must be interpreted with caution (Donald 2005; Lowe 2004; Tyrer 2004). This is also true for resource‐constrained settings. We did not identify any trials from low‐ or middle‐income countries.
Quality of the evidence
Primary outcome measures selected for this review were: remaining in treatment, substance use, and mental state. Pooled results demonstrated no consistent evidence to support any one treatment intervention over standard care. Some support for motivational interviewing was found from individual studies for substance use reduction. When motivational interviewing was offered in conjunction with cognitive behavioural therapy there was little support for improved mental state. These findings suggest that motivational interviewing is a crucial component to the effectiveness of treatment with cognitive behavioural therapy. However, it was challenging to identify the key aspects of each intervention given that these are mostly complex, multi‐faceted interventions. Little attention was paid to reporting the fidelity of the delivery of each intervention so quality was difficult to gauge.
Missing outcomes or too few data
Out of the primary outcome measures, studies only reported numbers lost to treatment clearly enough to allow pooling of results in each of the comparisons. Often the other primary outcome measures (substance use, mental state) were reported as continuous rather than binary data and much of these data were problematic. With this particular population, skewed data may be unavoidable and, as such, is problematic to present and manage in a meta‐analysis. However, opportunities were missed to report simple and useful binary outcomes.
Potential biases in the review process
It is possible that we failed to identify small negative trials, and we would be most interested if readers know of these. We endeavoured to reduce this potential bias by conducting a wide search, duplicate extraction, multiple checking, and handsearching key references and journals. We also contacted many of the authors of these trials over the years, and for this review we asked if they knew of any recently completed or ongoing trials. The introduction of web sites and journals to register trials hopefully will reduce the 'file drawer' phenomenon, as negative trials are less likely to be published.
A limitation of this review is that there was substantial variation between studies as to what constituted standard care, in addition to some differences between the interventions themselves. For example, fidelity, duration, and intensity of treatment conditions varied, furthermore the outcome reporting periods also differed. This resulted in difficulties in grouping and interpreting data. There was a high volume of problematic data due to skew, use of non‐validated scales, or unclear reporting. Further high‐quality randomised trials are required which employ large samples, use validated and clinically relevant measures, and present data in a way that can be incorporated into a meta‐analysis.
It is possible that our consideration of these data have been biased by our foreknowledge of the past work (Cleary 2008; Hunt 2013; Ley 2000). It is difficult to know what to do about this except to state that we do make every effort to be open to any new information or interpretation.
Agreements and disagreements with other studies or reviews
The findings of this review agree with other narrative syntheses of the literature, which have come to the same conclusions. There is little evidence from trials to support any one psychosocial treatment over another to reduce substance use or improve mental state for people with a serious mental illness (Baker 2012; Cleary 2009a; Crockford 2017; Dixon 2010; Drake 1998b; Hjorthoj 2009; Horsfall 2009; NICE 2011).
Authors' conclusions
Implications for practice.
This review is larger than the previous or original review (41 as opposed to 32 and six studies, respectively), although all three have similar results. The findings reveal no compelling evidence to support any one psychosocial treatment to reduce substance use or to improve mental state for people with severe mental illnesses. Some support for substance use reduction came from one small study assessing motivational interviewing, where more participants receiving this treatment abstained from alcohol. Further, more participants receiving motivational interviewing attended their first aftercare appointment. In combination with cognitive behavioural therapy, motivational interviewing also improved mental state, life satisfaction and social functioning. Little support was found for integrated, non‐integrated, or skills‐training programmes being superior to standard care. A recent study (McDonell 2013) reported reduced stimulant use in homeless people randomised to contingency management. This intervention was combined in another study (Bellack 2006) with motivational interviewing and cognitive behavioural therapy, with some positive outcomes.
However, methodological difficulties exist which hinder pooling and interpreting results and include high attrition rates; varying fidelity of interventions; varying outcome measures, settings and samples (sample size, participant level of substance use, motivation to change, diagnoses, age, gender, cultural, socioeconomic and contextual influences); and, in some cases, comparison groups may have received higher levels of treatment than usual standard care. Therefore, it is not yet possible to reach clear conclusions, although it is pleasing to see that the field is developing with an increase in high‐quality randomised controlled trials offering high‐fidelity programmes and reporting more usable data. However, the largest trial to date (Barrowclough 2010) did not find that motivational interviewing combined with cognitive behavioural therapy significantly improved patient outcomes.
1. For people with severe mental illness and substance misuse problems, and their carers
People with both severe mental health and substance misuse problems should be aware that at present there is little evidence to support any particular psychosocial intervention over another or over standard care. This does not mean that particular treatments do not help, but that data are few and the little supportive evidence found in these studies need further studies to support their use. No‐one can suggest to people entering a service that one form of support should really take precedence over another.
2. For clinicians
Clinicians need to keep up‐to‐date on the latest research findings in this area because as new trials are published, the evidence base should rapidly build to support particular interventions for this challenging group of patients. Interventions for substance reduction may need to be further developed and adapted for people with a serious mental illness. Clinicians who seek to offer existing interventions over and above standard care should take the opportunity to work with trial researchers to generate useful data.
3. For policy makers and commissioners of care
Developments in specific treatments and in models of service delivery are still taking place. While there is no evidence that the innovative integrated services that have been developed in the USA are helpful, conversely there is also no convincing evidence that they lead to a worse outcome. The development of such services may be unlikely in other countries, such as the UK where the general policy is to build on the existing links and to use mainstream services as far as possible (Haddock 2014; Seivewright 2005). This may be a function of methodological problems within the studies or it may be that there is, in fact, no effect. Policies in this difficult area are needed. These policies should be either based on good evidence or in their implementation should generate the relevant evidence.
Implications for research.
1. General
1.1 Reporting of outcome measures
Only validated and non‐adapted scales should be used in future trials. Clear reporting of data during treatment and at various follow‐up periods with an indication that they meet the assumptions of the analyses undertaken would be helpful. Wherever possible, dichotomous data should be reported in addition to continuous data, as the use of outcomes such as retention in treatment, relapse, hospitalisation and abstinence rates are relevant to the topic and are preferable to reporting skewed data (Jones 2004).
1.2 Methodology
Clear and strict adherence to the CONSORT statement (Altman 2001; Begg 1996; Moher 1998; Turpin 2005) for methodology and all outcomes should be the goal of future trials. A full description of the number of participants lost to treatment and evaluation after the randomisation process should be completed at each time point for both treatment arms. A clear description of the randomisation process and blinding is also not difficult and is now necessary. The use of intention‐to‐treat analysis can assist with minimising bias resulting from missing data. Double‐blind evaluation of outcomes of psychosocial interventions is not possible due to the nature of the intervention. However, researchers should take every precaution to minimise the effect of bias by at least using raters blind to group assignment.
2. Specific
Consistent with our suggestions for more high‐quality randomised controlled trials, other recently published reviews advocate a need for more consistent and methodologically rigorous trials on this topic to test both individual components and integrated programme (Bennett 2017; Boniface 2018; De Witte 2014; Donald 2005; Drake 2004; Lubman 2010; Mueser 2005; Murthy 2012; Tiet 2007). Also worth noting are recent treatment recommendations and guidelines on psychosocial interventions for substance reduction modified for people with a mental illness (Baandrup 2016; Baker 2012; Crockford 2017; Dixon 2010; Galletly 2016; Hasan 2015; Kelly 2012; NICE 2011; Work Group 2007; Ziedonis 2005).
Future high‐quality trials in this area will contribute to the growing body of data and will allow future reviews to tease out findings. Assessing brief interventions (such as motivational interviewing) over standard care will allow the identification of cost‐effective and easy‐to‐implement components that can be quickly integrated into standard care. New trials should aim to recruit sufficiently large sample sizes and collect data that can be reported and, if appropriate, synthesised in meta‐analyses. Informed consent of participants should include statements that all anonymous data will be publicly available. The use of measurement scales should be of clinical value, in common use, and have demonstrated reliability and validity. We suggest a design for a future trial with the key methodological points highlighted in Table 11. Future reviews may explore differences between subgroups (determined a priori), such as differences between levels of substance use (misuse versus dependence), differences between substances used, and differences between age groups (for example, first‐episode schizophrenia versus older patients).
2. Suggested design for trial.
| Methods | Allocation: centralised sequence generation with table of random numbers or computer‐generated code, stratified by severity of substance use. Sequence concealed until interventions assigned. Blinding: those recruiting and assigning participants, those assessing outcomes will be blind to treatment allocation. Duration: minimum of 1 year. |
| Participants | Diagnosis: severe mental illness based on a diagnosis of schizophrenia, schizoaffective disorder, and other psychotic disorders. N = 440* recruited to obtain a minimum sample of 280 at 12 months given the high drop‐out rate for some of the outcome measures. Age: adults 18‐55 years. Sex: men and women. Setting: hospital and community. |
| Interventions | 1. Standard care plus 3‐5 sessions of motivational interviewing + 3 months of weekly CBT. 2. Standard care plus one motivational interview. |
| Outcomes | Lost to treatment. Death. Substance use: number of patients using substances, OTI. Mental state: BPRS, PANSS. Relapse: number of patients readmitted to hospital over a specified follow‐up period. Quality of life: BQOL. Functioning: GAF. Arrests, number homeless. |
| Notes | * size of study to detect a 10% difference in improvement with 80% certainty. If scales are used to measure outcome then there should be binary cut‐off points, defined before study start, of clinically important improvement. |
BPRS: Brief Psychiatric Rating Scale BQOL: Brief Quality of Life Scale CBT: Cognitive behavioural therapy GAF: Global Assessment of Functioning OTI: Opiate Treatment Index PANSS: Positive & Negative Syndrome Scale for schizophrenia
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2019 | New citation required but conclusions have not changed | Results from 2018 search added to review. Overall conclusions have not changed with the addition of new data. |
| 2 May 2018 | New search has been performed | Search updated, 113 records identitified for screening. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 7 April 2014 | Amended | Correction to number of new studies added. |
| 26 September 2013 | New citation required but conclusions have not changed | Review is substantially updated with the addition of 7 new trials, conclusions remain similar to previous versions of this review. |
| 2 July 2013 | New search has been performed | Update with 7 new trials. Complete revision of previous review with 'Risk of bias' added for all current and previous trials and full GRADE assessments done on all comparisons. |
| 20 March 2009 | Amended | New plain language summary added. |
| 26 April 2008 | Amended | Converted to new review format. |
Notes
There are no published notes to communicate.
Acknowledgements
This update builds on earlier versions and we would like to thank Ann Ley, David Jeffery and Stuart McLaren for their past reviews of this topic (Ley 2000) and Garry Walter and Sandra Matheson for their contribution to the 2008 update of this review (Cleary 2008) and Raj Sitharthan for his contribution to the 2013 update of this review (Hunt 2013).
Jonathan Pushpa‐Rajah and Corey W Joseph peer reviewed the 2013 update. We thank them for this and their helpful comments. Nandi Siegfried is grateful for the support from the NIHR Cochrane Incentive Scheme, 2012, towards her involvement in the 2013 update.
We would like to thank authors who kindly sent their unpublished data or ongoing studies for inclusion in this and previous review. We would also like to thank Clive Adams, John Rathbone, Claire Irving and Tessa Grant for their editorial assistance and Farhad Shakranek for conducting the updated search for the current review. Carrie Brooke‐Sumner was supported by a South African Medical Research Council intramural career development award.
Appendices
Appendix 1. Search methods
Search for 2007 version
Electronic searching
1.1 We searched the Cochrane Schizophrenia Group Trials Register (May 2006) using the phrase:
[((*polydrug* or *substanc* or *alcoh* or *tranquiliz* or *narcot* or * abus* or *opiat* or *street drug* or *solvent* or *inhalan* or *intoxi*) in REFERENCE) or ((substance abus* or drug abus* or *alcohol*) in STUDY)]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
1.2 We searched the Cochrane Schizophrenia Group Trials Register (August 2005) using the phrase:
[and (polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* and diagnos*)]
1.3 We searched the Cochrane Schizophrenia Group Trials Register (April, 2002) using the phrase:
[(*substance abuse* or *substance*) in abstract or title or index terms of REFERENCE] or [Substance Abuse* IN HEALTH CARE CONDITION]
1.4 We searched the Cochrane Schizophrenia Group Trials Register (August and December, 2001) using the phrase:
[(*substance abuse* or *substance*) in abstract or title or index terms of REFERENCE] or [Substance Abuse* IN HEALTH CARE CONDITION]
1.5 We searched the Cochrane Schizophrenia Group Trials Register (April and August 2000) using the phrase:
(polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or "street drug*" or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*)) or (dual* and diagnos*) or substance‐abuse or drug‐dependence or alcoholism or alcohol‐abuse or drug‐abuse‐prevention or "drug use" or "drug user" or "drug users" or "drug misuse" or "drug misuser" or "drug misusers" or "drug dependant" or "drug dependence" or "drug addict" or "drug addicts" or "drug addiction" or "drug habit" or "drug habits" or "drug withdrawal" or "drug rehabilitation" or "non‐prescription drugs" or "non‐prescription drug" or "illegal drug" or "illegal drugs" or "illicit drug" or "illicit drugs" or "intoxicating drug" or "intoxicating drugs" or "drug intoxication".
1.6 We searched the Cochrane Schizophrenia Group Trials Register (August 1998) using the phrase:
[and (polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* and diagnos*)]
1.7 We searched The Cochrane Library (Issue 3, 1998) using the Cochrane Schizophrenia Group's terms for schizophrenia combined with the phrase:
[and (polydrug* or alcohol* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or misus* or depend* or addict* or illegal* or illicit* or habit* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* and diagnos*)]
1.8 We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2006 Issue 2) using the phrase:
[polydrug* or tranquiliz* or narcot* or opiat* or ((substanc* or alcohol* or solvent* or inhalan* or intoxi*) near/4 (abus* or misus*)) or "street drug*" for publication dates 2001‐2006]
1.9 We searched Biological Abstracts (1985 to February 1998) using the Cochrane Schizophrenia Group's terms for both randomised controlled trials and schizophrenia combined with the phrase:
[and (drug* or polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* near diagnos*)]
1.9 We searched CINAHL on Silver Platter (1982 to February 1998) using the Cochrane Schizophrenia Group's terms for both randomised controlled trials and schizophrenia combined with the phrase:
[and (drug* or polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* near diagnos*) or explode "substance‐abuse"/ all subheadings/ all age subheadings or explode "drug‐dependence"/ all subheadings/ all age subheadings or explode "alcoholism"/ all topical subheadings / all age subheadings]
1.10 We searched EMBASE (January 1980 to February 1998) using the Cochrane Schizophrenia Group's terms for both randomised controlled trials and schizophrenia combined with the phrase:
[and (polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* near diagnos*) or explode "substance‐abuse"/ all subheadings or explode "drug‐dependence"/ all subheadings or explode "alcohol‐abuse"/ all subheadings or explode "alcoholism"/ all subheadings]
1.11 We searched MEDLINE on Silver Platter (January 1966 to February 1998) using the Cochrane Schizophrenia Group's terms for both randomised controlled trials and schizophrenia combined with the phrase:
[and (drug* or polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or usin* or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or dual* near diagnos*)or explode "substance‐abuse"/ all subheadings or explode "drug‐dependence"/ all subheadings or explode "alcohol‐abuse"/ all subheadings or explode "alcoholism"/ all subheadings]
1.12 We searched PsycLIT on Silver Platter (January 1974 to February 1998) using the Cochrane Schizophrenia Group's terms for both randomised controlled trials and schizophrenia combined with the phrase:
[and (drug* or polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* near diagnos*) or explode "alcoholism" or explode "drug‐abuse" or explode "alcohol‐abuse" or "drug‐abuse‐prevention" in de]
1.13 We searched Sociofile on Silver Platter (January 1974 to February 1998) using the Cochrane Schizophrenia Group's terms for both randomised controlled trials and schizophrenia combined with the phrase:
[and (drug* or polydrug* or substance* or alcohol* or tranquil* or chemical* or narcotic* or opiat* or street drug* or solvent* or inhalant* or psychotropic* or intoxica*) and (abus* or use* or misus* or using or utiliz* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or rehab* or intoxica* or non‐prescri*) or (dual* near diagnos*)or explode "substance‐abuse" or explode "alcoholism" or explode "drug‐abuse" or explode "drug‐addiction" or explode "detoxification"]
1.14 We also searched CINAHL, EBM Reviews‐Cochrane Register of Controlled Trials, and PsycINFO (December 2006) using the phrase:
diagnosis‐dual‐psychiatry.mp (CINAHL, PsychINFO); dual diagnosis.mp (EBM Reviews); and schizo$ and (randomised or randomized) and (drug or substance).mp (Ovid Medline). Other searches included the phrases; (RCT or randomised or randomized) and (motivational or CBT or program or services) on (Ovid Medline)
1.14 ISI database ‐ Social Sciences Citation Index We sought each of the studies which went into the pool as a cited reference on the above database. Reports of articles that had cited these studies were then inspected in order to identify further trials.
1.15 We hand searched Schizophrenia Bulletin (January 1990 ‐ December 1998) and British Journal of Psychiatry (October 1989 ‐ December 1998).
2. Additional search strategy by current authors
2.1 For the 2007 update, we performed an additional search in 2006‐2007. The previous draft (2004) yielded 13 studies, and the updated search yielded an additional 12 randomised controlled trials (n=25). The Cochrane Schizophrenia Group Trials Register 19‐05‐06 found 65 new references since the last search on 02‐11‐2004. CENTRAL: The Cochrane Librarys register of Clinical Trials Substance Misuse Search 19‐05‐2006. It is worth noting that the word 'drug' generates a large number of false positives. It also makes the search technically difficult on large databases such as Medline and Embase. We undertook the EMBASE search without the word 'drug', as were searches of The Cochrane Library and the Cochrane Schizophrenia Group's Register.
We undertook a further final search using the same search strategy as above in March 2007 during the study write‐up by one of the authors [GH]. In addition, we searched for all authors listed in the included trials using Ovid Medline, PubMED and ISI Web of Science (citation search).
2.2 Reference lists We inspected reference lists of all the studies and unpublished reports to identify any further relevant trials [MC, GH, SM] up until April 2007.
2.3 Personal contact We contacted the authors of all studies initially selected for inclusion in order to identify further relevant trials. We simultaneously conducted a Delphi survey of opinion of informed professionals in the UK who had knowledge and experience of people with severe mental illness and substance misuse. As part of the recruitment letter, individuals were asked if they had conducted or knew of any ongoing, published or unpublished trials. We contacted authors, during November and December 2006, of research studies rated at risk of low, medium or high risk bias (A, B or C) to ascertain whether they knew of any relevant published, unpublished or currently ongoing trials [MC, GH, SM].
Search for 2013 version
Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group Trials Register (July 2012) using the phrase:
[((*polydrug* or *substanc* or *alcoh* or *tranquiliz* or *narcot* or * abus* or *opiat* or *street drug* or *solvent* or *inhalan* or *intoxi*) in title, abstract and indexing terms REFERENCE) or ((*substance abus* or drug abus* or *alcohol* or *cannabis*) in health care conditions of STUDY)].
The Cochrane Schizophrenia Group Trials Register is compiled by systematic searches of major databases, handsearches of relevant journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
Appendix 2. Data collection methods (2004 and 2007)
2.1. Selection of trials In the initial draft of this review (2004), AL performed the search for trials (original version of review) and the preliminary update was carried out by MF. Nandi Siegfried (NS) inspected both included and excluded citations, in order to cross check according to the inclusion criteria. For the (2007) update, JW performed the search, updated by GH. We read the titles, abstracts and descriptor terms of all downloaded material from the electronic searches and discarded irrelevant reports. We (MC, GH, and SM) independently inspected all citations to establish relevance of the article and whether or not it should be acquired. Where doubt remained we sought further information from authors and added these trials to the list of those awaiting assessment.
2.2. Assessment of methodological quality We allocated trials to one of three categories described in the Cochrane Reviewers' Handbook (Higgins 2006) This system is based on the evidence of a strong relationship between the potential for bias in the results and allocation concealment (Schulz 1995). We included only those trials that stated they were randomised with randomisation methods described or implied (categories A or B, not C). When trials were included in the review, but provided no usable data for the analysis, we allocated these to Category D and did not assign them to an intervention. Where disagreement occurred regarding which category a trial should be allocated, we attempted to resolve this by discussion. If doubt remained we contacted authors for further information.
In this review, we included randomised controlled trials that did not ensure blinded participants, clinicians and outcome raters due to the nature of some self‐report measures and the psychosocial treatments involved. However, we noted those randomised controlled trials which did not specify or which stated specifically that raters were not blinded due to the possibility of bias in these studies (see Characteristics of Included Studies Table). We were not blinded to the names of the authors, institutions, journal of publication or results of the trials.
2.3. Data extraction We (MC, GH and SM) extracted data from the selected trials independently. Again, we resolved any disputes by discussion. When it was not possible to extract data, or further information was needed, we attempted to contact the authors.
2.4. Data synthesis 4.1 Data analysis We analysed data using RevMan, The Cochrane Collaboration software for preparing and maintaining Cochrane reviews.
2.5 Incomplete data Except for the analysis of lost to treatment, we did not use data from studies where overall attrition was greater than 50% because of the strong likelihood of bias.
2.6 Dichotomous/binary data Due to expected high heterogeneity between studies, we used a standard estimation of the random effects risk ratio (RR) with the 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate.
2.7 Continuous data 2.7.1 Summary statistic: again, due to expected high heterogeneity, we estimated the weighted mean difference (MD) between groups using a random effects model.
2.7.2 Skewed data: continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards are applied to all data: (a) standard deviations and means were reported in the paper or were obtainable from the authors; and (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); (c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases, skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. Many of the studies included in this review reported skewed data that fell outside of the above standards and as such were unable to be included in the analysis. We have, however, included skewed data in tables and have reported any significant results from non‐parametric tests (e.g. Mann‐Whitney) applied by the authors.
2.7.3 Valid scales: continuous data from rating scales were included only if the measuring instrument had been described in a peer‐reviewed journal and the instrument was either self‐report or completed by an independent rater or relative (not the therapist). It has been shown that the use of rating scales which have not been described in a peer‐reviewed journal (Marshall 2000) are associated with bias, therefore we excluded the results of such scales.
2.7.4 Change data: when continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), there is no way of telling whether data are non‐normally distributed (skewed) or not. It is thus preferable to use endpoint data, which typically cannot have negative values.
2.7.5 Cluster trials Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a unit‐of‐analysis error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes Type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect=1+ (m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intra‐class correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
2.8 Investigation for heterogeneity Consideration of all the included studies within any comparison was undertaken to judge clinical heterogeneity. Visual inspection of graphs was used to investigate the possibility of statistical heterogeneity. This was supplemented employing, the I‐squared statistic which provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was >75%, this was interpreted as evidence of high levels of heterogeneity (Higgins 2003).
2.9 Addressing publication bias We aimed to enter data from all included trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. We were unable to undertake this due to the very limited number of trials included in the analysis.
2.10 General Where possible, we entered data into RevMan in such a way that the area to the left of the 'line of no effect' indicated a 'favourable' outcome for the psychosocial interventions. Where this was not possible, we labelled the graphs in MetaView accordingly so that the direction of any effects was clear.
Appendix 3. Search methods (2012)
Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group Trials Register (July 2012) using the phrase:
[((*polydrug* or *substanc* or *alcoh* or *tranquiliz* or *narcot* or * abus* or *opiat* or *street drug* or *solvent* or *inhalan* or *intoxi*) in title, abstract and indexing terms REFERENCE) or ((*substance abus* or drug abus* or *alcohol* or *cannabis*) in health care conditions of STUDY)].
The Cochrane Schizophrenia Group Trials Register is compiled by systematic searches of major databases, handsearches of relevant journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
Searching other resources
1. Reference lists
We searched all references of articles selected for inclusion, major review articles (Baker 2012; Dixon 2010; Drake 2008; Dutra 2008; Horsfall 2009; Kelly 2012) as well as recent guidelines (NICE 2011) on this topic for further relevant trials.
2. Journal databases
Two further searches were completed (8 October 2012 and 15 January 2013) by the principal reviewer (GEH) using the Cochrane Database of Systematic Reviews, MEDLINE (daily update, PREMEDLINE), and PsycINFO. A separate search for randomised trials using contingency management was completed as this was an additional intervention category for this update. We also searched MEDLINE for recent articles (2008 to 2013) by the first authors of all included studies in order to get a more complete list of recent publications.
We also did 'forward' searches to identify trials that cited previously included RCTs using Web of Science and Scopus. Scopus was used to identify trials that cited the most recent version of this review (Cleary 2008) up to 15 February 2013.
3. Trials registries
In addition, web sites and journals that list ongoing trials in the USA, UK, Australia and various European countries were searched for RCTs through the Cochrane Schizophrenia Group Trials Register. The principal researcher (GEH) searched www.clinicaltrials.gov for protocols of current and previously included studies for proposed outcome measures to assess selective reporting bias.
4. Personal contact
We contacted the first author (or corresponding author) of newly included studies for this update regarding their knowledge of ongoing or unpublished trials.
Additional searches
Similar search methods were conducted by the reviewers as int previous update (Appendix 1 sections 1.3, 1.5, 1.6‐1.8) using Ovid Medline (Daily update), PsycINFO, CINAHL, Cochrane, Scopus and ISI Web of Science for recent updates for authors listed on ongoing trials, included studies and for articles citing our previous review (Cleary 2008) between 2008 and 2013. For an example of an advanced search for RCTs involving psychosocial interventions for dual diagnosis patients between 1980 and 2008 see Cleary 2009b.
Appendix 4. Data collection methods (2012)
1. Selection of studies
GEH inspected all citations from the new electronic search and identified relevant abstracts, full text articles and trials against the inclusion criteria. To ensure reliability, KM inspected all full text articles for inclusion. Where there were uncertainties or disagreements, two additional authors provided resolution (NS and MC). Where disputes could not be resolved, these studies remained as awaiting assessment or ongoing studies and we contacted the authors for clarification.
1. Extraction
GEH and KM extracted data from the included studies. We resolved disputes by discussion and adjudication from the other review authors (NS and MC) when necessary. If it was not possible to extract data or if further information was needed, we attempted to contact the authors. We extracted data presented only in graphs and figures whenever possible, but the data were included only if two review authors independently had the same result. When further information was necessary, we contacted authors of studies in order to obtain missing data or for clarification of methods.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and
the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally the measuring instrument should either be: i) a self‐report or ii) completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we have noted whether or not this is the case in Characteristics of included studies.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we used mean differences (MD) rather than standardised mean differences throughout (Higgins 2011, Chapter 9.4.5.2).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion:
standard deviations and means are reported in the paper or obtainable from the authors;
when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996));
if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2SD > (S ‐ S min), where S is the mean score and S min is the minimum score.
Endpoint scores on scales often have a finite start and endpoint and these rules can be applied. We entered skewed endpoint data from studies of fewer than 200 participants as 'other data; within Data and analyses rather than into a statistical analysis. Skewed data pose less of a problem when looking at mean if the sample size is large; we entered such endpoint data into the syntheses.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not; we entered skewed change data into analyses regardless of size of study.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (for example, mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into ’clinically improved’ or ’not clinically improved’. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS) (Overall 1962) or the PANSS (Kay 1986; Kay 1987) this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the treatment intervention. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (for example, 'Not improved') we reported data where the left of the line indicates an unfavourable outcome. This was noted in the relevant graphs.
Assessment of risk of bias in included studies
GEH worked independently by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This new set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article, such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain additional information.
We noted the level of risk of bias in the text of the review.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The Number Needed to Treat or Harm (NNT or H) statistic with its CIs is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' tables, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We would prefer not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
None of the presently included trials used cluster randomisation. For the purposes of future updates of this review, where clustering is not accounted for in primary studies we planned to present data in a table with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, should we include cluster RCTs, we will seek to contact first authors of studies to obtain intra‐class correlation coefficients for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we plan to present these data as if from a non‐cluster randomised study but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intra‐class correlation coefficient (ICC) (design effect = 1 + (m ‐ 1)*ICC) (Donner 2002). If the ICC is not reported it was assumed to be 0.1 (Ukoumunne 1999).
If we had identified cluster trials, we would have analysed them taking into account intra‐class correlation coefficients and relevant data documented in the report. Synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
None of the presently included studies employed a cross‐over trial design. For the purposes of future updates of the review, a major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (for example, pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we proposed to only use the data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data are binary we simply added these and combined them within the two‐by‐two table. If data were continuous we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for we would not reproduce these data or use them within the analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would address this within the 'Summary of findings' tables by down‐rating quality. Finally, we would also downgrade quality within the 'Summary of findings' tables should loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome is between 0 and 50% and where these data are not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention to treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes the rate of those who stay in the study ‐ in that particular arm of the trial ‐ was used for those who did not. We undertook a sensitivity analysis testing how prone the primary outcomes are to change when data only from people who complete the study to that point were compared to the intention to treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome is between 0% and 50%, and data only from people who complete the study to that point are reported, we reproduced these.
3.2 Standard deviations
If standard deviations are not reported, we first tried to obtain the missing values from the authors. If not available, where there are missing measures of variance for continuous data but an exact standard error and confidence intervals available for group means, and either a P value or t value available for differences in mean, we can calculate them according to the rules described in the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011). That is, when only the standard error (SE) is reported, standard deviations (SDs) are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis by excluding the imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data have been used in the trial, if less than 50% of the data have been assumed we would present and use these data and indicate that they are the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on: i) magnitude and direction of effects, and ii) strength of evidence for heterogeneity (for example, P value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic was interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for the heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10 of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not plan to use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. As no meta‐analyses of more than five studies were undertaken, we did not conduct funnel plot analysis.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the random‐effects model for all analyses. The reader is, however, able to choose to inspect the data using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses ‐ only primary outcomes
1.1 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of psychosocial interventions for people with schizophrenia in general. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we have reported this. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed studies outside of the company of the rest to see if homogeneity was restored. For this review we decided that should this occur, with data contributing to the summary finding of no more than around 10% of the total weighting, we would present the data. If not, then we did not pool the data and discussed the issues. We know of no supporting research for this 10% cut‐off, but we use prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity is obvious we simply stated hypotheses regarding these for future reviews or versions of this review. We do not anticipate undertaking analyses relating to these.
Sensitivity analysis
We conducted sensitivity analyses on outcomes of comparisons with four or more trials where studies with different quality were combined to ascertain if there were substantial differences in the results when lesser quality trials or those comprising patients with schizophrenia (or other psychoses) were compared to trials of higher quality or using mixed diagnostic groups. We applied all sensitivity analyses to the primary outcomes based on randomised sequence, allocation concealment and blinding of outcome measurement. We only conducted sensitivity analyses to comparisons with four or more studies as analyses with less than four trials would provide unclear decisions on whether there have been any possible biases in the estimate of effects.
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way so as to imply randomisation. For the primary outcomes we included these studies and if there was no substantive difference when the implied randomised studies were added to those with a better description of randomisation then we entered all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data) we compared the findings of the primary outcomes when we used our assumptions and when we used data only from people who completed the study to that point. If there was a substantial difference, we reported the results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing standard deviation (SD) data (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumptions and when we used data only from people who completed the study to that point. A sensitivity analysis was undertaken testing how prone results were to change when completer‐only data were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
A sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials was not needed for this update as there were no cluster randomised trials.
If we noted substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome but presented them separately.
Appendix 5. Previous plain language summaries
Dual diagnosis is the name often given to people who have a severe mental health problem and a drug and/or alcohol problem as well. While the number of people with these problems varies, in some urban areas it can be over 50% of all those with mental health difficulties. Although individuals may feel they are self‐medicating when using these substances, drugs and alcohol can have a detrimental effect on the symptoms of their illness, the way their medication works and their interaction with the wider world. They can also make people more vulnerable to suicide, hepatitis C, HIV and homelessness, and can cause them to be aggressive or to do something that moves them into the criminal justice system.
People who have a substance abuse problem but no mental health problem can be helped by a variety of interventions that look at their motivation for change (motivational interviewing ‐ MI), how to adapt their behaviour by improving coping strategies (cognitive behavioural therapy ‐ CBT), a supportive approach similar to the one used by Alcoholics Anonymous and skills training. These are all examples of psychosocial interventions. However, using these interventions on people with mental health problems is more complex, because it is unclear whether the intervention for the substance abuse should be offered with that for the mental health problem and whether the same team should be responsible for both (integrated intervention).
This review attempts to assess all trials using psychosocial interventions compared to care as usual where they are used to help those who have a substance abuse problem and a severe mental illness. Thirty‐four studies were identified containing a total of 3397 people. Three trials were based in a hospital, 21 in the community, eight in hospital and the community and two in the community and in jail. They used different psychosocial interventions, with four trials using integrated models of care, four using non‐integrated, seven combining MI and CBT, two using CBT, eight using MI, two using contingency management and two using skills training. Trials lasted from three months to three years. No trial showed any definitive difference between the psychosocial intervention and the usual treatment, although the difference in the study designs made it difficult to compare one trial to another. There are also problems caused by high dropout rates, differences in the outcome measures and dependability in the way psychological interventions were delivered. To allow more thorough assessment of whether psychosocial interventions work for people with substance abuse problems and severe mental illnesses, more high quality trials are needed which address these problems.
(Plain language summary prepared for this review by Janey Antoniou of RETHINK, UK www.rethink.org and updated by the authors)
Psychosocial interventions for people with both severe mental illness and substance misuse
‘Dual diagnosis’ is the term used to describe people who have a mental health problem and also have problems with drugs or alcohol. In some areas, over 50% of all those with mental health difficulties will have problems with drugs or alcohol. For people with mental illness, substance misuse often has a negative and damaging effect on the symptoms of their illness and the way their medication works. They may become aggressive or engage in activities that are illegal. Substance misuse can also increase risk of suicide, hepatitis C, HIV, relapse, incarceration and homelessness. People who have substance misuse problems but no mental illness can be treated via a variety of psychosocial interventions. These include motivational interviewing, or MI, that looks at people’s motivation for change; cognitive behavioural therapy, or CBT, which helps people adapt their behaviour by improving coping strategies; a supportive approach similar to that pioneered by Alcoholics Anonymous; family psycho‐education observing the signs and effects of substance misuse; and group or individual skills training. However, using these interventions for people with dual diagnosis is more complex.The aim of this review was to assess the effects of psychosocial interventions for substance reduction in people with a serious mental illness compared to care as usual or standard care. A search for studies was carried out in July 2012; 32 studies were included in the review with a total of 3165 people. These studies used a variety of different psychosocial interventions (including CBT, MI, skills training, integrated models of care). In the main, evidence was graded as low or very low quality and no study showed any great difference between psychosocial interventions and standard care. There was no compelling evidence to support any one psychosocial treatment over another. However, differences in study designs made comparisons between studies problematic. Studies also had high numbers of people leaving early, differences in outcomes measured, and differing ways in which the psychosocial interventions were delivered. More large scale, high quality and better reported studies are required to address these shortcomings. This will better address whether psychosocial interventions are effective and good for people with mental illness and substance misuse problems.
This plain language summary has been written by a consumer, Ben Gray from RETHINK.
Data and analyses
Comparison 1. Integrated models of care versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Lost to treatment ‐ by 36 months | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.82, 1.45] |
| 2 Leaving the study early: 2. Lost to evaluation | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 By 3 months | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.27, 1.08] |
| 2.2 By 6 months | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.27, 1.73] |
| 2.3 By 9 months | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.49, 1.19] |
| 2.4 By 12 months | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.29] |
| 2.5 By 24 months | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.47, 2.12] |
| 2.6 By 36 months | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.66] |
| 3 Adverse event: 1. Death ‐ by 36 months | 2 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.39, 3.57] |
| 4 Substance use: 1. Clinically important change (not in remission) ‐ by 36 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Alcohol use | 1 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.84, 1.56] |
| 4.2 Drug use | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.25] |
| 5 Substance use: 2. Average score for progress towards recovery (SATS, low = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 By 6 months | 1 | 203 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.28, 0.42] |
| 5.2 By 36 months | 1 | 203 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.41, 0.63] |
| 6 Substance use: 3a. Alcohol use: average score (AUS) (skewed data) | Other data | No numeric data | ||
| 6.1 6 months | Other data | No numeric data | ||
| 6.2 12 months | Other data | No numeric data | ||
| 6.3 18 months | Other data | No numeric data | ||
| 6.4 24 months | Other data | No numeric data | ||
| 6.5 30 months | Other data | No numeric data | ||
| 6.6 36 months | Other data | No numeric data | ||
| 7 Substance use: 3b. Alcohol use: average number of days using in last 6 months (skewed data) | Other data | No numeric data | ||
| 7.7 6 month | Other data | No numeric data | ||
| 7.8 12 month | Other data | No numeric data | ||
| 7.9 18 month | Other data | No numeric data | ||
| 7.10 24 month | Other data | No numeric data | ||
| 7.11 30 month | Other data | No numeric data | ||
| 7.12 36 month | Other data | No numeric data | ||
| 8 Substance use: 4a. Drug use: average score (DUS) (skewed data) | Other data | No numeric data | ||
| 8.1 6 months | Other data | No numeric data | ||
| 8.2 12 months | Other data | No numeric data | ||
| 8.3 18 months | Other data | No numeric data | ||
| 8.4 24 months | Other data | No numeric data | ||
| 8.5 30 months | Other data | No numeric data | ||
| 8.6 36 months | Other data | No numeric data | ||
| 9 Substance use: 4b. Drug use: average number of days using in last 6 months (skewed data) | Other data | No numeric data | ||
| 9.7 6 months | Other data | No numeric data | ||
| 9.8 12 months | Other data | No numeric data | ||
| 9.9 18 months | Other data | No numeric data | ||
| 9.10 24 months | Other data | No numeric data | ||
| 9.11 30 months | Other data | No numeric data | ||
| 9.12 36 months | Other data | No numeric data | ||
| 10 Substance use: 5. Avearage score (SATS, low = poor) (skewed data) | Other data | No numeric data | ||
| 10.1 6 months | Other data | No numeric data | ||
| 10.2 12 months | Other data | No numeric data | ||
| 10.3 18 months | Other data | No numeric data | ||
| 10.4 24 months | Other data | No numeric data | ||
| 10.5 30 months | Other data | No numeric data | ||
| 10.6 36 months | Other data | No numeric data | ||
| 11 Substance use: 6. Average score ( USS, high = poor) 1=client not abstinent, 5 client mets criteria for severe use) (skewed data) | Other data | No numeric data | ||
| 11.1 6 months | Other data | No numeric data | ||
| 11.2 12 months | Other data | No numeric data | ||
| 11.3 18 months | Other data | No numeric data | ||
| 11.4 24 months | Other data | No numeric data | ||
| 12 Mental state: 1. Average score (BPRS, high = poor) (skewed data) | Other data | No numeric data | ||
| 12.1 6 months | Other data | No numeric data | ||
| 12.2 12 months | Other data | No numeric data | ||
| 12.3 18 months | Other data | No numeric data | ||
| 12.4 24 months | Other data | No numeric data | ||
| 12.5 30 months | Other data | No numeric data | ||
| 12.6 36 months | Other data | No numeric data | ||
| 13 Global state: 1. Average score (GAF, low = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 By 6 months | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐1.58, 3.78] |
| 13.2 By 12 months | 1 | 171 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐2.07, 3.47] |
| 13.3 By 18 months | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.58, 3.58] |
| 13.4 By 24 months | 1 | 166 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐1.18, 4.58] |
| 13.5 By 30 months | 1 | 164 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.56, 2.36] |
| 13.6 By 36 months | 1 | 170 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.47, 3.27] |
| 14 Global state: 2. Forensic measures (skewed data) | Other data | No numeric data | ||
| 14.1 Arrests ‐ 36 months | Other data | No numeric data | ||
| 14.2 Convictions ‐ 36 months | Other data | No numeric data | ||
| 14.3 Felony ‐ 36 months | Other data | No numeric data | ||
| 14.4 Hospital or jail ‐ 3 months | Other data | No numeric data | ||
| 14.5 Jail days ‐ 36 months | Other data | No numeric data | ||
| 15 Quality of life/ life satisfaction: 1. Average general score (QOLI, range 1‐7, low = poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 By 6 months | 2 | 361 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.41, 0.20] |
| 15.2 By 12 months | 2 | 372 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.28, 0.32] |
| 15.3 By 18 months | 2 | 377 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.27, 0.44] |
| 15.4 By 24 months | 2 | 370 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.29, 0.33] |
| 15.5 By 30 months | 2 | 366 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.27, 0.32] |
| 15.6 By 36 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.18, 0.38] |
| 16 Service use: 1. Days in stable community residences (not in hospital) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 By 12 months | 2 | 378 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐38.61, 18.60] |
| 16.2 By 24 months | 1 | 203 | Mean Difference (IV, Random, 95% CI) | 7.40 [‐6.32, 21.12] |
| 16.3 By 36 months | 2 | 364 | Mean Difference (IV, Random, 95% CI) | 5.17 [‐9.20, 19.55] |
| 17 Service use: 2. Number hospitalised ‐ during the 36 month study period | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.19] |
| 18 Service use: 3. Relapse (hospitalisation days and crisis care) ‐ 36 months (skewed data) | Other data | No numeric data | ||
| 19 Service use: 4. Medication hours ‐ 36 months (skewed data) | Other data | No numeric data | ||
| 20 Service use: 5. Various measures (skewed data) | Other data | No numeric data | ||
| 20.1 Days institutionalised (hospital or incarcerated) ‐ 36 months (site 2) | Other data | No numeric data | ||
| 20.2 Days in hospital ‐ 36 months (site 2) | Other data | No numeric data | ||
| 21 Homelessness: 1. Proportion of time on the street ‐ past 60 days | Other data | No numeric data | ||
| 21.1 3 months | Other data | No numeric data | ||
| 21.2 6 months | Other data | No numeric data | ||
| 21.3 9 months | Other data | No numeric data | ||
| 22 Homelessness: 2. Proportion of time in independent housing ‐ past 60 days | Other data | No numeric data | ||
| 22.1 3 months | Other data | No numeric data | ||
| 22.2 6 months | Other data | No numeric data | ||
| 22.3 9 months | Other data | No numeric data | ||
| 23 Homelessness: 3. Average number of days in stable housing (skewed data) | Other data | No numeric data | ||
| 23.1 6 months | Other data | No numeric data | ||
| 23.2 12 months | Other data | No numeric data | ||
| 23.3 18 months | Other data | No numeric data | ||
| 23.4 24 months | Other data | No numeric data | ||
| 24 Homelessness: 4. Various measures (skewed data) | Other data | No numeric data | ||
| 24.3 Time on streets (%) ‐ 3 months | Other data | No numeric data | ||
| 24.4 Time on streets (%) ‐ 6 months | Other data | No numeric data | ||
| 24.5 Time on streets (%) ‐ 9 months | Other data | No numeric data | ||
| 24.6 Days in stable community residence ‐ 24 months | Other data | No numeric data | ||
| 24.7 Time in independent housing in past 60 days ‐ 3 months | Other data | No numeric data | ||
| 24.8 Time in independent housing in past 60 days ‐ 6 months | Other data | No numeric data | ||
| 24.9 Time in independent housing in past 60 days ‐ 9 months | Other data | No numeric data |
1.11. Analysis.
Comparison 1 Integrated models of care versus standard care, Outcome 11 Substance use: 6. Average score ( USS, high = poor) 1=client not abstinent, 5 client mets criteria for severe use) (skewed data).
| Substance use: 6. Average score ( USS, high = poor) 1=client not abstinent, 5 client mets criteria for severe use) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| 6 months | ||||
| Morse 2006 | Treatment (IACT) | 3.15 | 1.09 | 46 |
| Morse 2006 | ||||
| Morse 2006 | Control | 2.93 | 1.19 | 49 |
| 12 months | ||||
| Morse 2006 | Treatment (IACT) | 3.07 | 0.96 | 46 |
| Morse 2006 | ||||
| Morse 2006 | Control | 2.78 | 1.18 | 49 |
| 18 months | ||||
| Morse 2006 | Treatment (IACT) | 2.83 | 1.30 | 46 |
| Morse 2006 | ||||
| Morse 2006 | Control | 2.69 | 1.10 | 49 |
| 24 months | ||||
| Morse 2006 | Treatment (IACT) | 2.76 | 1.11 | 46 |
| Morse 2006 | ||||
| Morse 2006 | Control | 2.62 | 1.15 | 49 |
Comparison 2. Non‐integrated models of care (Assertive Community Treatment / Intensive Case Management / Specialised case management sercives) versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Lost to treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 By 6 months | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.73, 2.06] |
| 1.2 By 12 months | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.73, 1.99] |
| 1.3 By 18 months | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.83, 2.19] |
| 2 Leaving the study early: 2. Lost to evaluation | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 By 6 months | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.38, 2.60] |
| 2.2 By 12 months | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.43, 2.35] |
| 2.3 By 18 months | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.48, 3.30] |
| 3 Substance use: 1. Average scores on various scales (skewed data) | Other data | No numeric data | ||
| 3.1 Alcohol ‐ 6 months (C‐DIS‐R) | Other data | No numeric data | ||
| 3.2 Alcohol ‐ 12 months (C‐DIS‐R) | Other data | No numeric data | ||
| 3.3 Alcohol ‐ 18 months (C‐DIS‐R) | Other data | No numeric data | ||
| 3.4 Drugs ‐ 6 months (C‐DIS‐R) | Other data | No numeric data | ||
| 3.5 Drugs ‐ 12 months (C‐DIS‐R) | Other data | No numeric data | ||
| 3.6 Drugs ‐ 18 months (C‐DIS‐R) | Other data | No numeric data | ||
| 3.7 General ‐ 12 months (ASI) | Other data | No numeric data | ||
| 4 Substance use: 2. Average score (USS, high = poor (1=client not abstinent, 5 client mets criteria for severe use) (skewed data) | Other data | No numeric data | ||
| 4.1 6 months | Other data | No numeric data | ||
| 4.2 12 months | Other data | No numeric data | ||
| 4.3 18 months | Other data | No numeric data | ||
| 4.4 24 months | Other data | No numeric data | ||
| 5 Mental state: 1. Average scores on various scales (skewed data) | Other data | No numeric data | ||
| 5.1 Depression symptoms ‐ 6 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.2 Depression symptoms ‐ 12 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.3 Depression symptoms ‐ 18 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.4 General ‐ average score ‐ 12 months (ASI) | Other data | No numeric data | ||
| 5.5 Manic symptoms ‐ 6 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.6 Manic symptoms ‐ 12 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.7 Manic symptoms ‐ 18 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.8 Schizophrenia symptoms ‐ 6 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.9 Schizophrenia symptoms ‐ 12 months (C‐DIS‐R) | Other data | No numeric data | ||
| 5.10 Schizophrenia symptoms ‐ 18 months (C‐DIS‐R) | Other data | No numeric data | ||
| 6 Global state: 1. Forensic measures ‐ number of arrests (skewed data) | Other data | No numeric data | ||
| 6.1 12 months | Other data | No numeric data | ||
| 6.2 18 months | Other data | No numeric data | ||
| 6.3 24 months | Other data | No numeric data | ||
| 6.4 30 months | Other data | No numeric data | ||
| 7 Global state: 2. Forensic measures ‐ number of convictions (skewed data) | Other data | No numeric data | ||
| 7.1 12 months | Other data | No numeric data | ||
| 7.2 24 months | Other data | No numeric data | ||
| 7.3 30 months | Other data | No numeric data | ||
| 8 Global state: 3. Forensic measures ‐ days in jail (skewed data) | Other data | No numeric data | ||
| 8.1 12 months | Other data | No numeric data | ||
| 8.2 18 months | Other data | No numeric data | ||
| 8.3 24 months | Other data | No numeric data | ||
| 8.30 30 months | Other data | No numeric data | ||
| 9 Social functioning: 1. Average role functioning score (RFS, high = better functioning) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 By 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐2.91, 1.35] |
| 9.2 By 12 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.56, 2.96] |
| 9.3 By 18 months | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐2.67 [‐5.28, ‐0.06] |
| 10 Social functioning: 2. Average social adjustment score (SAS, high = better functioning) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 By 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐6.34, 4.48] |
| 10.2 By 12 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 3.09 [‐2.71, 8.89] |
| 10.3 By 18 months | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐10.12, 2.62] |
| 11 Quality of life/ life satisfaction: 1. Average score (QOLI, skewed data) ‐ 12 months | Other data | No numeric data | ||
| 12 Service use: 1. Relapse (skewed data) | Other data | No numeric data | ||
| 12.5 % days in hospital | Other data | No numeric data | ||
| 13 Service use: 2. Various measures ‐ 24 months (skewed data) | Other data | No numeric data | ||
| 13.1 State Operated admissions (site 1, Robert Young) | Other data | No numeric data | ||
| 13.2 State Operated admissions (site 2, Pilsen‐Little) | Other data | No numeric data | ||
| 13.3 State operated days admitted (site 1) | Other data | No numeric data | ||
| 13.4 State Operated days admitted (site 2) | Other data | No numeric data | ||
| 13.5 Private Hospital length of stay (site 1, days) | Other data | No numeric data | ||
| 13.6 Private Hospital length of stay (site 2, days) | Other data | No numeric data | ||
| 14 Homelessness: 1. Average number of days in stable housing (skewed data) | Other data | No numeric data | ||
| 14.1 6 months | Other data | No numeric data | ||
| 14.2 12 months | Other data | No numeric data | ||
| 14.3 18 months | Other data | No numeric data | ||
| 14.4 24 months | Other data | No numeric data |
Comparison 3. Cognitive behavioural therapy versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Lost to treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 By 3 months | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.44, 2.86] |
| 2 Leaving the study early: 2. Lost to evaluation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 By 9 months after start of treatment | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.43, 2.51] |
| 3 Substance use: 1. Percentage of participants who used cannabis ‐ in last 4 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 By 3 months | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.62, 1.74] |
| 3.2 By 6 months | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.79, 2.15] |
| 4 Substance use: 2. Average score (various scales) (skewed data) | Other data | No numeric data | ||
| 4.2 CASUAS | Other data | No numeric data | ||
| 4.3 HONOS ‐ item 3 | Other data | No numeric data | ||
| 5 Mental state: 1. Average insight score (Insight Scale, low = poor) ‐ by 3 months | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.78, 1.82] |
| 6 Mental state: 2. Average score (various scales) (skewed data) | Other data | No numeric data | ||
| 6.1 Anxiety symptoms ‐ 3 months (BAS) | Other data | No numeric data | ||
| 6.2 Depressive symptoms ‐ 3 months (BDI‐SF) | Other data | No numeric data | ||
| 6.3 Depressive symptoms ‐ 3 months (MADRS) | Other data | No numeric data | ||
| 6.5 Depressive symptoms ‐ 6 months (BDI‐SF) | Other data | No numeric data | ||
| 6.6 General symptoms total score ‐ 3 months (BPRS) | Other data | No numeric data | ||
| 6.7 General ‐ 3 months (CPRS) | Other data | No numeric data | ||
| 6.8 General ‐ 3 months (SCR) | Other data | No numeric data | ||
| 6.9 General symptoms total score ‐ 6 months (BPRS) | Other data | No numeric data | ||
| 6.11 Negative symptoms ‐ 3 months (SANS) | Other data | No numeric data | ||
| 6.12 Negative symptoms ‐ 6 months (SANS) | Other data | No numeric data | ||
| 7 Social functioning: 1. Average score (SOFAS, low = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 By 3 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐9.95, 8.35] |
| 7.2 By 6 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐14.52, 5.12] |
| 8 Social functioning: 2. Average score (HONOS) (skewed data) | Other data | No numeric data | ||
| 8.1 3 months | Other data | No numeric data | ||
| 9 Service use: 1. Outpatient medication (SURS) (skewed data) | Other data | No numeric data | ||
| 9.1 3 months | Other data | No numeric data | ||
| 9.2 6 months | Other data | No numeric data |
Comparison 4. Contingency management versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Lost to treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Week 4 ‐ 8 (1 ‐ 2 months) | 2 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 2.81] |
| 1.2 Lost to treatment ‐ 3 months | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.13, 2.11] |
| 2 Leaving the study early: 2. Lost to evaluation ‐ 6 months | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.91, 2.02] |
| 3 Substance use: 1. Stimulant‐positive urine test (higher = poor outcome) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Stimulant‐positive urine test ‐ 3 months | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.17, 0.68] |
| 3.2 Stimulant‐positive urine test ‐ 6 months | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.06] |
| 4 Substance use: 2. Number of continuous days alcohol negative urine tests, 3 months (skewed data) | Other data | No numeric data | ||
| 5 Substance use: 3. Injection use | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 During treatment ‐ 3 months | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.77] |
| 5.2 During follow‐up ‐ 6 months | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.15] |
| 6 Substance use: 4. Average scores on various measures (skewed data) | Other data | No numeric data | ||
| 6.1 Stimulant use days ‐ 3 months (skewed data) | Other data | No numeric data | ||
| 6.2 Stimulant use days ‐ 6 months (skewed data) | Other data | No numeric data | ||
| 6.3 Days of alcohol ‐ 3 months | Other data | No numeric data | ||
| 6.4 Days of alcohol ‐ 6 months | Other data | No numeric data | ||
| 6.5 5. Longest consecutive weeks of cocaine abstinence | Other data | No numeric data | ||
| 7 Mental state: 1. Average scores (various scales) (skewed data) | Other data | No numeric data | ||
| 7.1 Brief Symptom Inventory ‐ 3 months | Other data | No numeric data | ||
| 7.2 Brief Symptom Inventroy ‐ 6 months | Other data | No numeric data | ||
| 7.3 PANSS, excitement scale ‐ 3 months | Other data | No numeric data | ||
| 7.4 PANSS, excitement scale ‐ 6 months | Other data | No numeric data | ||
| 8 Service use: 1. Relapse (hospitalised ‐ 6 months post‐randomisation | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.05, 0.93] |
Comparison 5. Motivational interviewing versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Lost to treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.30, 2.61] |
| 1.2 6 months | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.63, 4.64] |
| 2 Leaving the study early: 2.Lost to evaluation | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 By 3 months | 7 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.69, 1.70] |
| 2.2 By 6 months | 4 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.29, 2.53] |
| 2.3 By 12 months | 3 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.44, 1.92] |
| 3 Adverse event: 1. Death, due to all causes, by 18 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 15.73] |
| 4 Substance use: 1. Using substances ‐ by class of drug ‐ by about 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Alcohol abuse/dependence | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.92] |
| 4.2 Amphetamine abuse/dependence | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 1.92] |
| 4.3 Cannabis abuse/dependence | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.21] |
| 5 Substance use: 2. Polydrug consumption levels ‐ by 12 months (OTI, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 By 3 months | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.91, 0.09] |
| 5.2 By 12 months | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.56, 0.42] |
| 6 Substance use: 3. Any change ‐ not abstinent or not improved on all substances ‐ by 12 months | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.10] |
| 7 Substance use: 4. Any change ‐ not abstaining from alcohol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 By 3 months | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.03] |
| 7.2 By 6 months | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.75] |
| 8 Substance use: 5. Change in cannabis use from baseline (T0) (lower scores indicate better outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐12.81 [‐23.05, ‐2.57] |
| 8.2 6 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐18.05, ‐1.23] |
| 8.3 12 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐5.82 [‐14.77, 3.13] |
| 9 Substance use: 6. Cannabis consumption past 30 days (ASI, high = poor) (skewed data) | Other data | No numeric data | ||
| 9.1 By 3 months | Other data | No numeric data | ||
| 9.2 By 6 months | Other data | No numeric data | ||
| 10 Substance use: 7. Engagement with substance misuse treatment at 3 months (SATS, low = poor)) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.47, 1.07] |
| 11 Substance use: 8. Average scores (OTI, high = poor) (skewed data) | Other data | No numeric data | ||
| 11.1 Alcohol | Other data | No numeric data | ||
| 11.2 Amphetamine | Other data | No numeric data | ||
| 11.3 Cannabis | Other data | No numeric data | ||
| 11.4 Polydrug use | Other data | No numeric data | ||
| 12 Substance use: 9. Other measures of alcohol use (skewed data) | Other data | No numeric data | ||
| 12.1 Alcohol volume (AUI subscale) | Other data | No numeric data | ||
| 12.2 Drinking days ‐ by 6 months | Other data | No numeric data | ||
| 12.3 Alcohol consumption, last 30 days (ASI) ‐ 3 months | Other data | No numeric data | ||
| 12.4 Alcohol consumption, last 30 days (ASI) ‐ 6 months | Other data | No numeric data | ||
| 12.5 Alcohol use (AUDIT) ‐ 3 months | Other data | No numeric data | ||
| 12.6 Alcohol use (CAUS) ‐ 3 months | Other data | No numeric data | ||
| 12.7 Drug use (CDUS) ‐ 3 months | Other data | No numeric data | ||
| 12.8 Drug use (SDS) ‐ 3 months | Other data | No numeric data | ||
| 13 Mental state: 1. Average scores (SCL‐90‐R, high = poor) ‐ by 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 General severity index | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.59, 0.21] |
| 13.2 Positive symptom distress index | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.66, 0.28] |
| 13.3 Positive symptom scores | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐18.72, 10.32] |
| 14 Mental state: 2. Average scores (PANSS negative symptoms, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 By 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.06, 1.86] |
| 14.2 By 6 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.80, 1.80] |
| 15 Mental state: 3. Average scores (PANSS positive symptoms, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.55, 1.95] |
| 15.2 6 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.58, 2.38] |
| 16 Mental state: 4. Average score (HADS, BSI, high = poor) (skewed data) ‐ by 3 months | Other data | No numeric data | ||
| 16.1 HADS‐A, Anxiety | Other data | No numeric data | ||
| 16.2 HADS‐D, Depression | Other data | No numeric data | ||
| 16.3 BSI | Other data | No numeric data | ||
| 17 Global state: 1. Average score (GAF, low = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 3 months | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐3.53, 2.73] |
| 17.2 6 months | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐4.81, 2.81] |
| 17.3 12 months | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐1.30, 5.90] |
| 18 Global state: 2. Forensic measures ‐ average number of crimes (skewed data) | Other data | No numeric data | ||
| 18.1 6 months | Other data | No numeric data | ||
| 18.2 12 months | Other data | No numeric data | ||
| 19 Social functioning: 1. Average score (OTI, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 By 6 months | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.76, 1.34] |
| 19.2 By 12 months | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐3.35, 0.51] |
| 20 Social functioning: 2. Average score (SOFAS, low = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐3.02, 3.22] |
| 20.2 6 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.51, 3.31] |
| 20.3 12 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐1.08, 6.48] |
| 21 Service use: Hospital admission to 12 months | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.42, 1.80] |
| 22 Service use: Lost to first aftercare appointment | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.53, 0.90] |
Comparison 6. Skills training versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Lost to treatment | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 By 3 months | 3 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.36] |
| 1.2 By 6‐9 months | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.24, 0.97] |
| 1.3 By 12 months | 3 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.20, 10.10] |
| 1.4 By 18 months | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.36, 4.97] |
| 2 Leaving the study early: 2. Lost to evaluation | 1 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.66, 1.63] |
| 2.1 1. By 6 months | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.40, 2.08] |
| 2.2 2. By 9 months | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.47, 2.33] |
| 2.3 3. By 12 months | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.55, 2.36] |
| 3 Adverse event: 1. Death ‐ by 12 months | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.42] |
| 4 Substance use: 1. Alcohol use: proportion days abstinent from alcohol (TLFB method) | 1 | 398 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 4.1 1. 3 months | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.02 [0.00, 0.04] |
| 4.2 2. 6 months | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.07, ‐0.03] |
| 4.3 3. 9 months | 1 | 98 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 4.4 4. 12 months | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] |
| 5 Substance use: 2. Average scores (C‐DIS‐R) (skewed data) | Other data | No numeric data | ||
| 5.1 Alcohol | Other data | No numeric data | ||
| 5.2 Drugs | Other data | No numeric data | ||
| 6 Sustance use: 3. Average days used (skewed data) | Other data | No numeric data | ||
| 6.1 Any alcohol in last 30 days, at follow‐up (6 months) | Other data | No numeric data | ||
| 6.2 Any illicit drug in last 30 days, at follow‐up (6 months) | Other data | No numeric data | ||
| 7 Social functioning: 1. Average score (RFS, high = better functioning) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 By 6 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐1.63, 2.85] |
| 7.2 By 12 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐1.15, 3.29] |
| 7.3 By 18 months | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐2.55 [‐6.24, 1.14] |
| 8 Social functioning: 2. Average score (SAS, high = better functioning) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 By 6 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐6.58, 4.74] |
| 8.2 By 12 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐3.39, 8.55] |
| 8.3 By 18 months | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐4.66 [‐15.29, 5.97] |
| 9 Service use: 1. Days in hospital (skewed data) | Other data | No numeric data |
Comparison 7. Cognitive behavioural therapy + motivational interviewing versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early 1. Lost to treatment | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 By 3 months | 3 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.44, 3.30] |
| 1.2 By 6 months | 5 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 1.3 By 9‐10 months | 3 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.22] |
| 1.4 By 12 months | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.59] |
| 2 Leaving the study early: 2. Lost to evaluation | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 By 3 months | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.46, 1.21] |
| 2.2 By 6 months | 5 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.57, 1.12] |
| 2.3 By 9 months | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.47] |
| 2.4 By 12‐14 months | 5 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.34] |
| 2.5 By 18 months | 2 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.61, 1.38] |
| 2.6 By 24 months | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.52, 1.11] |
| 3 Adverse event: 1. Death ‐ by about 1 year | 4 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.20, 1.76] |
| 4 Adverse event: 2. Death or hospitalisation by 24 months | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.76, 1.74] |
| 5 Substance use: 1. Average number of different drugs used during the past month (OTI, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 By 3 months | 1 | 119 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.01, 0.75] |
| 5.2 By 6 months | 1 | 119 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.22, 0.60] |
| 6 Substance use: 2. Cannabis use last 30 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 3 months (end of treatment) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐2.54, 2.14] |
| 6.2 12 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.84, 2.24] |
| 7 Substance use 3. Clinically important change ‐change in main substance use, abstinent or large decease | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.04] |
| 7.1 From baseline to 3 months | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.04] |
| 8 Substance use: 4. Averages of various measures (skewed data) | Other data | No numeric data | ||
| 8.1 Alcohol ‐ estimated daily consumption ‐ past month ‐ 3 months | Other data | No numeric data | ||
| 8.2 Alcohol ‐ estimated daily consumption ‐ past month ‐ 6 months | Other data | No numeric data | ||
| 8.3 Alcohol ‐ estimated daily consumption ‐ past month ‐ 12 months | Other data | No numeric data | ||
| 8.4 Alcohol ‐ frequency per month by 6 months | Other data | No numeric data | ||
| 8.5 Alcohol quantity per session ‐ 6 months | Other data | No numeric data | ||
| 8.6 Alcohol Assessment ‐ AUDIT ‐ 6 months | Other data | No numeric data | ||
| 8.7 General ‐ ASI score ‐ 6 months | Other data | No numeric data | ||
| 8.8 Drug abuse screening test ‐ DAST‐10 by 6 months | Other data | No numeric data | ||
| 8.9 Amphetamine‐ estimated daily consumption ‐ past month ‐ 6 months | Other data | No numeric data | ||
| 8.10 Amphetamine‐ estimated daily consumption ‐ past month ‐ 3 months | Other data | No numeric data | ||
| 8.11 Amphetamine‐ estimated daily consumption ‐ past month ‐ 12 months | Other data | No numeric data | ||
| 8.12 Cannabis‐ estimated daily consumption ‐ past month ‐ 3 months | Other data | No numeric data | ||
| 8.13 Cannabis‐ estimated daily consumption ‐ past month ‐ 6 months | Other data | No numeric data | ||
| 8.14 Cannabis‐ estimated daily consumption ‐ past month ‐ 12 months | Other data | No numeric data | ||
| 8.16 cannabis ‐ days of use last month by 6 months | Other data | No numeric data | ||
| 8.17 Cannabis ‐ days of use last month by 10‐12 months | Other data | No numeric data | ||
| 8.18 Cannabis ‐ joints last 30 days by 6 months | Other data | No numeric data | ||
| 8.19 Cannabis ‐ joints last 30 days by 10 months | Other data | No numeric data | ||
| 8.20 Proportion of days abstinence from all substances last 90 days by 6 months | Other data | No numeric data | ||
| 8.21 Proportion of days abstinence from all substances last 90 days by 12 months | Other data | No numeric data | ||
| 8.22 Proportion of days abstinence from all substances last 90 days by 18 months | Other data | No numeric data | ||
| 8.23 Proportion of days abstinence from all substances last 90 days by 24 months | Other data | No numeric data | ||
| 9 Substance use: 5. Average change in % days abstinent during & after treatment | Other data | No numeric data | ||
| 10 Mental state: 1. Average score (PANSS, total, high = poor) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 By 6 months | 2 | 78 | Mean Difference (IV, Random, 95% CI) | 0.99 [‐5.91, 7.89] |
| 10.2 By 9‐10 months | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐5.01 [‐11.25, 1.22] |
| 10.3 By 12 months | 1 | 274 | Mean Difference (IV, Random, 95% CI) | 2.52 [‐0.68, 5.72] |
| 10.4 By 24 months | 1 | 247 | Mean Difference (IV, Random, 95% CI) | 2.71 [‐0.58, 6.00] |
| 11 Mental state: 2. Average score (PANSS positive symptoms, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 By 12 months | 1 | 274 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.18, 1.24] |
| 11.2 By 24 months | 1 | 247 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.80, 1.84] |
| 12 Mental state: 3. Average score (PANSS negative symptoms, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 by 12 months | 1 | 274 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.65, 1.43] |
| 12.2 by 24 months | 1 | 247 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.84, 1.16] |
| 13 Mental state: 4. Average score (various scales, high = poor)) (skewed data) | Other data | No numeric data | ||
| 13.1 Depressive symptoms ‐ 3 months (BDI‐11) | Other data | No numeric data | ||
| 13.2 Depressive symptoms ‐ 6 months (BDI‐11) | Other data | No numeric data | ||
| 13.3 Depressive symptoms ‐ 12 months (BDI‐11) | Other data | No numeric data | ||
| 13.4 Depressive symptoms ‐ 3 months Calgary Depression Scale | Other data | No numeric data | ||
| 13.5 Depressive symptoms ‐ 12 months Calgary Depression Scale | Other data | No numeric data | ||
| 13.6 General symptoms total score ‐ 6 months (BPRS) | Other data | No numeric data | ||
| 13.7 General symptoms total score ‐ 12 months (BPRS) | Other data | No numeric data | ||
| 13.8 General symptoms total score ‐ 3 months (BPRS) | Other data | No numeric data | ||
| 13.9 General symptoms ‐ 18 months (PANSS) | Other data | No numeric data | ||
| 13.10 Total score ‐ 3 months (PANSS) | Other data | No numeric data | ||
| 13.11 Total score ‐ 6 months (PANSS) | Other data | No numeric data | ||
| 13.12 Total score ‐ 12 months (PANSS) | Other data | No numeric data | ||
| 13.13 Total score ‐ 18 months (PANSS) | Other data | No numeric data | ||
| 13.14 Manic symptoms ‐ 3 months (BPRS) | Other data | No numeric data | ||
| 13.15 Manic symptoms ‐ 6 months (BPRS) | Other data | No numeric data | ||
| 13.16 Manic symptoms ‐ 12 months (BPRS) | Other data | No numeric data | ||
| 13.17 Negative symptoms ‐ 3 months (BPRS) | Other data | No numeric data | ||
| 13.18 Negative symptoms ‐ 6 months (BPRS) | Other data | No numeric data | ||
| 13.19 Negative symptoms ‐ 12 months (BPRS) | Other data | No numeric data | ||
| 13.20 Negative symptoms ‐ 6 months (PANSS) | Other data | No numeric data | ||
| 13.21 Negative symptoms ‐ 9‐10 months (PANSS) | Other data | No numeric data | ||
| 13.22 Negative symptoms ‐ 12 months (PANSS) | Other data | No numeric data | ||
| 13.23 Negative symptoms ‐ 18 months (PANSS) | Other data | No numeric data | ||
| 13.24 Negative symptoms ‐ 3 months (SANS) | Other data | No numeric data | ||
| 13.25 Negative symptoms ‐ 12 months (SANS) | Other data | No numeric data | ||
| 13.26 Positive symptoms ‐ 6 months (PANSS) | Other data | No numeric data | ||
| 13.27 Positive symptoms ‐ 9‐10 months (PANSS) | Other data | No numeric data | ||
| 13.28 Positive symptoms ‐ 12 months (PANSS) | Other data | No numeric data | ||
| 13.29 Positive symptoms ‐ 18 months (PANSS) | Other data | No numeric data | ||
| 13.30 Positive symptoms ‐ 3 months (SAPS) | Other data | No numeric data | ||
| 13.31 Positive symptoms ‐ 12 months (SAPS) | Other data | No numeric data | ||
| 14 Global state: 1. Average score (GAF, low = poor) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 3 months | 3 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐2.79, 2.46] |
| 14.2 6 months | 2 | 219 | Mean Difference (IV, Random, 95% CI) | 1.65 [‐2.46, 5.76] |
| 14.3 12 months | 4 | 445 | Mean Difference (IV, Random, 95% CI) | 1.24 [‐1.86, 4.34] |
| 14.4 18 months | 2 | 72 | Mean Difference (IV, Random, 95% CI) | 4.57 [‐3.07, 12.21] |
| 14.5 24 months | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐2.93, 2.51] |
| 15 Global state: 2. Forensic measures ‐ arrests reported ‐ by 6 months | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.10] |
| 16 Social functioning: 1. Average score (SFS, low = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 By end of 9 month treatment | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 5.01 [‐0.55, 10.57] |
| 16.2 By 12 months (3 months following treatment end) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 7.27 [0.86, 13.68] |
| 17 Quality of life/ life satisfaction: 1. Average score (BQOL (general life satisfaction, low = poor) ‐ by 6 months | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.58 [0.00, 1.16] |
| 18 Quality of life/ life satisfaction: 2. Average score (BQOL (overall quality of life, low = poor) ‐ by 6 months | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.61, 0.57] |
| 19 Quality of life/ life satisfaction: 3. Average score (WHOQOL, Bref, higher scores = better QoL) ‐ by 6 months | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐15.70 [‐36.19, 4.79] |
| 20 Quality of life/ life satisfaction: 4. Average score (MANSA, higher scores = better QoL) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 By 6 months | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐7.01, 1.61] |
| 20.2 By 10 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐3.73, 5.53] |
| 21 Quality of life/ life satisfaction: 5. Average score (CSQ ‐ client satisfaction, high = good) ‐ by 10 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 6.40 [3.87, 8.93] |
| 22 Service use: 1. Relapse (hospitalised) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 By end of 9 month treatment phase | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.29, 1.16] |
| 22.2 By 3 months after treatment finished ‐ 12 months | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 22.3 By 9 months after treatment finished ‐ 18 months | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.34, 1.10] |
| 23 Economic outcomes: 1. Direct cost in US$ (BQOL, money subscale) (skewed data) | Other data | No numeric data | ||
| 23.1 6 months | Other data | No numeric data |
Comparison 8. Cognitive behavioural therapy + psychosocial rehabilitation versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Forensic measures ‐ number of arrests (skewed data) | Other data | No numeric data | ||
| 1.1 12 months | Other data | No numeric data | ||
| 1.2 18 months | Other data | No numeric data | ||
| 1.3 24 months | Other data | No numeric data | ||
| 1.4 30 months | Other data | No numeric data | ||
| 2 Global state: 2. Forensic measures ‐ number of convictions (skewed data) | Other data | No numeric data | ||
| 2.1 12 months | Other data | No numeric data | ||
| 2.2 24 months | Other data | No numeric data | ||
| 2.3 30 months | Other data | No numeric data | ||
| 3 Global state: 3. Forensic measures ‐ days in jail (skewed data) | Other data | No numeric data | ||
| 3.1 12 months | Other data | No numeric data | ||
| 3.2 18 months | Other data | No numeric data | ||
| 3.3 24 months | Other data | No numeric data | ||
| 3.30 30 months | Other data | No numeric data |
Comparison 9. Cognitive behavioural therapy + intensive case management versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Forensic measures‐ number of arrests (skewed data) | Other data | No numeric data | ||
| 1.1 12 months | Other data | No numeric data | ||
| 1.2 18 months | Other data | No numeric data | ||
| 1.3 24 months | Other data | No numeric data | ||
| 1.4 30 months | Other data | No numeric data | ||
| 2 Global state: 2. Forensic measures ‐ number of convictions (skewed data) | Other data | No numeric data | ||
| 2.1 12 months | Other data | No numeric data | ||
| 2.2 24 months | Other data | No numeric data | ||
| 2.3 30 months | Other data | No numeric data | ||
| 3 Global state: 3. Forensic measures ‐ days in jail (skewed data) | Other data | No numeric data | ||
| 3.1 12 months | Other data | No numeric data | ||
| 3.2 18 months | Other data | No numeric data | ||
| 3.3 24 months | Other data | No numeric data | ||
| 3.4 30 months | Other data | No numeric data |
Comparison 10. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Motivational interviewing versus standard care: Leaving the study early: 1. Lost to evaluation short term (3‐6 months): Diagnostic criteria | 8 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.69, 1.54] |
| 1.1 Mixed diagnoses (Schizophrenia, bipolar, & depressed patients ) | 4 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.49] |
| 1.2 Schizophrenia & other psychoses only | 4 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.18, 4.19] |
| 2 Cognitive behavioural therapy + motivational interviewing versus standard care: Global state: 1. Average score (GAF, low = poor) at 12 months: Allocation concealment | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Unclear risk of bias | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 1.63 [‐1.89, 5.14] |
| 2.2 Low risk of bias | 2 | 301 | Mean Difference (IV, Random, 95% CI) | 3.67 [‐6.79, 14.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baker 2002.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 12 months. Setting: psychiatric hospital. Location: Hunter region, NSW, Australia. |
|
| Participants | Diagnosis: 37% schizophrenia with SCID abuse or dependence (alcohol 54%, cannabis 51%, amphetamine 22%, benzodiazepine 11%).* N = 160. Age: mean ˜ 31 years. Sex: 120 M, 40 F. Ethnicity: not reported. Inclusion criteria: acute in‐patient, SCID abuse or dependence for alcohol, cannabis or amphetamine, self‐report of hazardous use during last month of one or more of these drug types on OTI. | |
| Interventions | 1. Psychosocial intervention: Motivational interviewing (MI): Individual 30‐45 minutes of MI plus standard care. N = 79. 2. Standard care: standard care plus informed that they were using substances at hazardous level and should reduce their consumption. N = 81. | |
| Outcomes | Leaving the study early: lost to evaluation.
Substance use: SCID ‐ alcohol, amphetamine and cannabis**, OTI ‐ polydrug use.
Social functioningr OTI .*** Unable to use Adverse event: death (not reported by group). Substance use: OTI ‐ alcohol, cannabis, and amphetamine (data skewed). Mental state: BSI (data skewed). Forensic: OTI ‐ crime (data skewed). |
|
| Notes | Not ITT analysis. * Some participants were dependent on more than one of these substances. Paid AUD$20 each assessment. ** Data reported on subset who participated in all 3 evaluation points and also met SCID abuse/dependence criteria at start of study. *** Data reported on subset who participated in evaluation at 6 and 12 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors state: Quote: Interviewers formally blind to patient group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up 44% (71/160) 1 year. Number of lost to follow‐up reported, but no reasons for missing data provided. Full ITT analysis with imputed data for all missing values not reported. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes of interest fully reported for each intervention. No protocol available. |
| Other bias | Low risk | No details. No evidence of other bias are occurring. |
Baker 2006.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 12 months. Setting: community. Location: Hunter region, NSW, Australia. |
|
| Participants | Diagnosis: 75% ICD‐10 schizophrenia or schizoaffective disorder with SCID‐1 diagnosis of abuse or dependence past 12 months (alcohol 69%, cannabis 74%, amphetamine 42%).* N = 130. Age: mean 29 years. Sex: 102 M, 28 F. Ethnicity: not reported. Inclusion criteria: SCID abuse or dependence for alcohol, cannabis or amphetamine during preceding month, age at least 15 years, ability to speak English, having a confirmed ICD‐10 psychotic disorder, no organic brain impairment, and not intending to move from area within 12 months. | |
| Interventions | 1. Psychosocial intervention: CBT + MI (10 weekly one‐hour sessions). N = 65. 2. Standard care: standard care plus self‐help books. N = 65. | |
| Outcomes | Leaving the study early: lost to evaluation.
Adverse event: death.
Substance use: OTI (polydrug use only).
Global state: GAF. Unable to use Leaving the study early: lost to treatment (no control group data). Substance use: OTI (alcohol, cannabis, amphetamine ‐ skewed data). Mental state: BPRS, BDI‐II (data skewed). |
|
| Notes | Not ITT analysis. Authors report that a separate ITT analysis was run with similar results. *Some participants were dependent on more than one of these. Participants paid AUD $20 for each assessment interview. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants drew a card from an envelope but no details provided regarding the generation of the random sequence or whether cards were shuffled beforehand. |
| Allocation concealment (selection bias) | Unclear risk | Patients drew a card from an envelope. No further details provided so it is unclear if envelope was opaque and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blind so detection bias rated as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up 20% (26/130) 1 year. Number and reason for missing data clearly reported in flow sheet. Missing outcome data balanced across groups. Full ITT analysis with imputed data for all missing values not reported. |
| Selective reporting (reporting bias) | Unclear risk | In the report, the results are fully reported. There is no protocol. |
| Other bias | Low risk | Funded by public institution. No evidence other biases are occurring. |
Barrowclough 2001.
| Methods | Allocation: randomised.
Design: single‐centre (three sites).
Duration: 12, 18* months. Setting: own homes. Location: Tameside & Glossop, Stockport and Oldham, UK. |
|
| Participants | Diagnosis: ICD‐10 & DSM‐IV schizophrenia or schizoaffective disorder with DSM‐IV substance abuse or dependence. N = 36. Age: 18‐65 years, mean ˜ 31 years. Sex: 33 M, 3 F. Ethnicity: white European. Inclusion criteria: current substance abuse, in current contact with mental health services, min. 10 hours face‐to‐face contact with the caregiver per week, no organic brain disease or other serious medical illness or learning disability. | |
| Interventions | 1. Psychosocial intervention: MI with annualised individual CBT for the participant and CBT for family/caregiver for 9 months (plus routine care with family support worker). N = 18. 2. Standard care: routine care plus family support worker. N = 18. | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation.
Adverse events: death.
Mental state: PANSS, relapse: number of participants experiencing relapse.
Global state: GAF.
Social functioning: SFS. Unable to use Substance use: ASI ‐ % days abstinent (no mean/SD). Mental state: PANSS (some data skewed). Relapse: duration of relapse (only median and range supplied). Other: SFS 18‐month (only adjusted means reported). |
|
| Notes | Part ITT analysis. *18‐month data (see secondary reference Haddock et al 2003). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list stratified for sex and three types of substance use. |
| Allocation concealment (selection bias) | Low risk | Allocated by third party. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters independent and blind so detection bias rated as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 22% (8/36) 18 months. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No selective reporting evident between study protocol (N0244032344) and published article. |
| Other bias | Low risk | Funded by public institution (local health authorities). No evidence other bias occurring. |
Barrowclough 2010.
| Methods | Allocation: randomised. Design: multi‐centre (six large NHS mental health trusts). Duration: 24 months. Setting: Community (most patients received home treatment). Location: London, Lancashire and Manchester, UK. |
|
| Participants | Diagnosis: ICD‐10 & DSM‐IV non‐affective psychotic disorder (schizophrenia, schizoaffective etc) and DSM‐IV diagnosis of dependence on or abuse of drugs, alcohol or both. N = 327 Age: 17‐67 years, mean ˜38. Sex: 283 M, 44 F. Ethnicity: 81% (n = 266) white. Inclusion criteria: English speaking, fixed abode, and no history of organic factors implicated in the aetiology of psychotic symptoms. |
|
| Interventions |
1. Psychosocial intervention: CBT + MI (up to 26 individual therapy sessions delivered over 12 months (manual based) plus routine care. N = 164.* 2. Standard care: routine care plus access to community‐based rehabilitation activities. N = 163. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Adverse event: death. Mental state: PANSS. Global state: GAF. Substance use: Inventory of drug use consequences, days abstinent, readiness to change. Service use: relapse (admissions last 12 months), hospitalisation (for psychosis) or death versus not admitted and alive. Unable to use Substance use: proportion days abstinent from all substances (skewed data). |
|
| Notes | *One case was misdiagnosed (affective) and excluded from the analysis (CBT + MI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, algorithm taking into account substance type (alcohol alone, drugs alone, or alcohol and drugs) and NHS trust. |
| Allocation concealment (selection bias) | Low risk | Researcher not involved in the study generated sequence. Remote independent service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded.It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the primary outcome of hospital admission, data were obtained from participant psychiatric case notes and is unlikely to be affected by blinding. For other outcomes involving self‐report, precautions were taken to maintain the blindness. Throughout the trial, 135 breaks in the blindness of an assessor were reported in total. However, only one assessment was completed unblinded; in all other cases a new “blind” assessor was allocated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 25% (81/327) 2 years. Flow sheet provided describing reasons for incomplete data and deaths. Evenly balanced between treatment groups. No missing values for primary outcome measure (re‐hospitalisation/or death). |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest fully reported and these match the trial protocol. |
| Other bias | Low risk | Authors independent of funding, No input from funding sources on protocol. |
Barrowclough 2014.
| Methods | Allocation: randomised. Design: multiple centres. Duration: 18 months (up to 9 months post treatment). Setting: community. Location: Manchester and Lancaster, England. |
|
| Participants | Diagnosis: DSM‐IV non‐affective psychotic disorder (schizophrenia, N = 76, 69%) and cannabis abuse or dependence. N = 110. Age: 24 (range 16‐35 years). Sex: 98 M, 12 F. Ethnicity: white (N = 102, 93%). Inclusion criteria: early intervention centre referrals with recent cannabis misuse in the last 3 months. |
|
| Interventions |
1. Psychosocial intervention: CBT + MI: Brief MI + CBT, 12 sessions of integrated MI‐CBT over 4.5 months. N = 38. 2. Psychosocial intervention: CBT + MI: Long‐term MI + CBT, 24 sessions of integrated MI ‐ CBT over 9 months. N = 37.* 3. Standard care: TAU. N = 35. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Substance use: proportion days abstinent from cannabis, all substances, Global state: GAF. Relapse: in previous 9 months, number of hospitalisations and time to first hospitalisation. Unable to use Mental State: PANSS (skewed data), |
|
| Notes | Funded by NIHS. Trial identifier: ISRCTN88275061. “ReCAP” Rethinking Choices After Psychosis *For some of the analyses, the brief‐ and long‐term groups were combined. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised permuted blocks |
| Allocation concealment (selection bias) | Low risk | Allocated through an independent remote service (centralised) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were aware of receipt of allocation to MI‐CBT, or long MI‐CBT or standard care. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was number of days abstinent from cannabis and the outcome assessors were housed in different locations and participants and care coordinators were reminded not to divulge information that might lead to 'unblinding'. However, the actual outcome was done via self‐report and there is a chance that participants may report based on social desirability bias. We judged this to be low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition for brief MI‐CBT was 12/38 (31.6%); attrition for long MI‐CBT was 9/37 (24.3%); attrition in TAU was 13/35 (37.1%). Attrition for the combined MI‐CBT (21/75, 28%) and TAU less than 10% which is acceptable for low risk. |
| Selective reporting (reporting bias) | Low risk | No selective reporting evident between study protocol (ISRCTN88275061) and published article. |
| Other bias | Low risk | UK National Institute for Health Research |
Bechdolf 2011.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 6 months.
Setting: Inpatient, some of the treatment could be as outpatient. Location: Cologne, Germany. |
|
| Participants | Diagnosis: ICD‐10 schizophrenia or schizoaffective disorder (n = 50, 83.3%) or substance‐induced psychosis (n = 10) with substance abuse or dependence. N = 60. Age: mean 31.5 years. Sex: 43 M, 17 F. Ethnicity: not reported. Inclusion criteria: German speaking and excluded if they were close to discharge. | |
| Interventions | 1. Psychosocial intervention: MI (4 individual sessions of 50 minutes each given as an inpatient) plus routine care. N = 30. 2. Standard care: routine care plus 4 sessions of non‐specific supportive sessions (Supportive Therapy, ST). N = 30. | |
| Outcomes | Leaving the study early: lost to evaluation (3 and 6 months). Unable to use Mental state: PANSS (no means or SDs reported). Global state: GAF (no means or SDs reported). Substance use: ASI alcohol, cannabis consumption (skewed data). Service use: relapse (hospitalisations) (not reported). |
|
| Notes | All analyses were performed by intention‐to‐treat conditions. Text was translated from German to English by internal translators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified for gender and SUD, single and multiple. Sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Researcher not involved in the study generated sequence. Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blinded so detection bias was rated as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 25% (15/60) 6 months. Missing data have been imputed using appropriate methods (LOCF), but these can still have unclear risk effects. |
| Selective reporting (reporting bias) | Unclear risk | Rating of mental state not fully reported (numerical data not provided for PANSS, GAF) both secondary outcomes reported as not significantly different between groups over time. |
| Other bias | Low risk | None according to authors. |
Bellack 2006.
| Methods | Allocation: randomised (adaptive urn procedure).
Design: single‐centre.
Duration: 6 months.
Setting: community clinics and Veterans Affairs Medical Center. Location: Baltimore, Md, USA. |
|
| Participants | Diagnosis: 38% DSM‐IV schizophrenia or schizoaffective disorder, 55% major affective disorder. DSM‐IV substance abuse or dependence (predominate drug of abuse was 69% cocaine, 25% opiates, 7% cannabis). N = 175.** Mean age: 43 years. Sex: 111 M, 64 F. Ethnicity: 75% African American. Inclusion criteria: meeting criteria for severe and persistent mental illness and current dependence on cocaine, heroin or cannabis. | |
| Interventions | 1. Psychosocial intervention: CBT + MI: BTSAS: Behavioural Treatment for Substance Abuse in severe and persistent mental illness (SPMI). BTSAS consisted of motivational interviewing at baseline, 3 and 6 months and includes MI and CBT approaches. N=61.* 2. Standard care: routine care which included Supportive Treatment for Addiction Recovery (STAR) which includes some psycho education and group discussion regarding substance misuse. N=49. | |
| Outcomes | Leaving the study early: lost to treatment, **lost to evaluation.
Global state: forensic measures (arrests by 6 months). Quality of life / life satisfaction: BQOL. Unable to use Substance use: urinalysis (no means, SDs or time period given). Mental state: ASI (data skewed). Hospitalisation. (psychiatric and substance use admissions combined). Other: SFS (only 1 subscale score used), BQOL money subscale (data skewed). |
|
| Notes | Not ITT analysis. *Participants paid for clean urine test average payment per person USD 60. ** n = 175 randomised, however 46 patients failed to initiate treatment and 19 failed to become engaged (analysis was based on subset of 110 patients who were engaged in treatment). Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using an adaptive urn procedure adjusted for sex, psychiatric diagnosis, drug of choice and number of substance use disorders. Separate randomisation was conducted for participants from community clinics and VA centre. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was urinalysis results so the review authors judge that this outcome is not likely to be influenced by a lack of blinding. Moreover, raters were blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 25% (27/110) 6 months of "engaged" participants. 46 patients failed to initiate treatment and 19 failed to become engaged (analysis was based on subset of 110 patients who were engaged in treatment) so ITT analysis was not completed. Missing data were not balanced across interventions. Missing outcomes are enough to induce clinically relevant bias in observed effect size. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. Author states there were conflicting data on substance use between self‐report, drug screens and clinical ratings (SCID) of dependence. |
| Other bias | Low risk | Supported by NIDA grant. |
Bogenschutz 2014.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 12 weeks with follow up 9 months post‐treatment. Setting: outpatient dual diagnosis programme. Location: Albuquerque, NM, USA. |
|
| Participants | Diagnosis: serious mental illness (Schizophrenia/psychotic, 18%, Bipolar, 36% and Major depression, 46%) with alcohol dependence or abuse. N = 121. Age: ˜ 42 years. Sex: 64 M 57 F. Ethnicity: Hispanic (39%), white (48%) and other (13%). Inclusion criteria: attending outpatient dual diagnosis program having a psychotic or major affective disorder and alcohol abuse or dependence active within the last month. |
|
| Interventions |
1. Psychosocial intervention: Skills training: 12‐step facilitation (TSF) for the dually diagnosed for 12 weeks. Several topics were covered including social skills training to help patients tolerate group meetings. N=83. 2. Standard care: described as treatment as usual (TAU). N = 38. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Adverse event: death. Substance use: alcohol abstinence for a given period, drinks per drinking day using time‐line follow back (TLFB) to confirm self‐report of substance use. Unable to use Mental State: Brief Symptom Inventory (BSI) (baseline only reported). Service use: 12‐step attendance on drinking. |
|
| Notes | Trial identifier, NCT00583440 Attendees received $40 for baseline interview, and 40$ for each follow‐up assessment at 12, 24, 36 and 48 weeks and $10 for brief assessments (weeks 4 and 8). Funded by NIAAA. There was limited 12‐step participation in both groups due to limited availability of 12‐step meetings. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn‐randomisation procedure with 2:1 ratio using block randomisation (although this is not reported as computerised or with a real urn). |
| Allocation concealment (selection bias) | Unclear risk | No further details regarding concealment. If a real urn, foreknowledge of the next allocation cannot be concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were aware of their allocation. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Substance use by self‐report of abstinent from alcohol or number of drinks per drinking day for each period. Unclear if raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up was 14/121 (12%) after 12 weeks of treatment and 28/121 (23%) at 9 months post‐treatment. |
| Selective reporting (reporting bias) | Unclear risk | No selective reporting evident between study protocol (NCT00583440) and published article. Blood alcohol levels were measured at each visit but not reported or used in data analysis so rated unclear. |
| Other bias | Low risk | Funded by NIAAA. |
Bond 1991a.
| Methods | Allocation: randomised.
Design: single‐centre (two sites, see Bond‐Evansville).
Duration: 18 months.
Setting: community centre. Location: Anderson, SC, USA. |
|
| Participants | Diagnosis: 57% DSM‐III‐R schizophrenia with co‐occurring substance disorder. N = 42.* Age: 18 ‐ 45 years, mean ˜ 30 years. Sex: 14 M, 7 F (only 21 received treatment). Ethnicity: 14% black. Inclusion criteria: chronic, DSM‐III‐R or documented evidence of substance abuse / dependence, extensive hospital/crisis service use over previous year. | |
| Interventions | 1. Psychosocial intervention: non‐integrated ACT. Includes reference groups to encourage attendance at CMHC'S, peer support group meetings several times/week, not focused exclusively on substance abuse, used successful members as role models, also home and community visits throughout project period. N = 21. 2. Standard care: routine care. N = 21.** | |
| Outcomes | Leaving the study early: lost to treatment. No other usable data (57% lost to follow‐up). | |
| Notes | ITT analysis. * Control group same as Bond‐Evansville 91. Only 21 received treatment (all figures given are for those 21 who received treatment). ** 4 received assistance from a case management program. Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if raters were blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 57% (24/42 18 months and 50% (21/42) did not receive treatment as planned and no reasons given for missing or lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol to compare outcomes reported. In the published auricles, means and SD were not reported, but were kindly provided by the author. |
| Other bias | Low risk | No evidence other biases are occurring. |
Bond 1991b.
| Methods | Allocation: randomised.
Design: single‐centre (two sites). Duration: 18 months. Setting: community centre. Location: Evansville, Indiana, USA. |
|
| Participants | Diagnosis: 53% DSM‐III‐R schizophrenia/schizoaffective disorder with co‐occurring substance disorder. N = 42.* Age: 18 ‐ 45 years, mean ˜ 30 years. Sex: 23 M, 7 F. Ethnicity: 26 white. Inclusion criteria: chronic, DSM‐III‐R or documented evidence of substance abuse / dependence, extensive hospital/crisis service use over previous year. | |
| Interventions | 1. Psychosocial intervention: non‐integrated ACT with emphasis on replacement activities (e.g. employment), stressed assistance in medication and money management, applied principles of individualised planning and client choice. N = 21. 2. Standard care: routine care. N = 21.** | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Unable to use Substance use: DAPS (adapted version of this scale, not peer‐reviewed). Service use: relapse (hospitalisation) ‐ number of days by 6 months (data skewed), number of days at > 6 months (no SD). Other: LSC (adapted version of this scale, not peer reviewed scale), medication compliance: > 50% loss. |
|
| Notes | ITT analysis. *Only 30 received treatment (all figures given are for those 30 who received treatment). ** 4 received assistance from a case management program. Control group same as Bond‐Anderson 91. Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if raters were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 33% (14/42) 18 months. No ITT analysis and no reasons given for missing or lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol to compare outcomes reported. In the published auricles, means and SD were not reported, but were kindly provided by the author. |
| Other bias | Unclear risk | No evidence other biases are occurring. |
Bonsack 2011.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 12 months (6 months post treatment). Setting: inpatient and outpatient. Location: Lausanne Switzerland. |
|
| Participants | Diagnosis: DSM‐IV for schizophrenia, schizoaffective, schizotypal and brief psychotic disorder (n = 57, 92%) and cannabis misuse, 82% (n = 50) met criteria for cannabis dependence. N = 62. Age: mean 26.4 years (range 18‐35 years). Sex: 54 M, 8 F. Ethnicity: not stated. Inclusion criteria: current cannabis use (current alcohol or other drugs excluded) and good command of French. |
|
| Interventions |
1. Psychosocial intervention: MI (individual sessions and optional group sessions for up to 6 months). N = 30. 2. Standard care: TAU which included case management, early intervention and mobile team when needed. N = 32. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Substance use: change in cannabis use from baseline. Mental state: PANSS (total, positive, negative symptoms). Global state: Global Assessment of functioning (GAF) Social functioning: Social and Occupational Social Functioning Scale (SOFAS) Service use: Hospital readmissions (12 months). Unable to use Substance use: Cannabis and alcohol use (ASI) (skewed data). |
|
| Notes | Authors have kindly provided further data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, blocks of 8. |
| Allocation concealment (selection bias) | Unclear risk | Numbered sealed envelopes held by administration staff not involved with the research. Remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blinded so detection risk rated as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 13% (8/62) 1 year. Used ITT and replaced missing values with LOCF. Missing values balanced across treatments, however can still have unclear risk with imputations. |
| Selective reporting (reporting bias) | Unclear risk | Reports fully all outcomes of interest (means, SD and n) mentioned in the Methods. No protocol. |
| Other bias | Low risk | No evidence of other bias occurring. |
Burnam 1995.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 9 months.
Setting: community, residential. Location: West Los Angeles county, Ca, USA. |
|
| Participants | Diagnosis: schizophrenia and or major affective disorder with co‐occurring substance disorder.* N = 276 (132 were included in analysis). Age: mean ˜ 37 years. Sex: 232 M, 44 F. Ethnicity: 58% white. Inclusion criteria: homeless, substance abuse within past year. | |
| Interventions | 1. Psychosocial intervention: integrated mental health and substance use treatment. Residential: educational groups, 12‐step programmes including AA or NA, discussion groups, individual counselling, case management, psychiatric consultation, ongoing medication management, general community activities. N = 67. 2. Psychosocial intervention: non‐residential: above model operating 1‐9 PM 5 days/week, more case management for basic needs. N = 144.** 3. Standard care: routine care with no special intervention but free to access other services (shelters, mental health clinics, AA groups). N = 65. | |
| Outcomes | Leaving the study early: lost to evaluation. Unable to use Substance use: level of alcohol in previous 30 days (modified measure used). Mental state: SCL‐90 and PERI ( modified version of scales used). Homelessness: number of days living in independent housing (data skewed). |
|
| Notes | ITT analysis. * Schizophrenia, 6%; major affective disorder, 60%, both 34%. ** Only residential and control group data used. Non residential intervention did not meet a priori category. Authors kindly provided further data. Participants paid $10 for each assessment interview. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description. Assignment to non‐residential group was set at twice that of the other groups requiring a larger sample size. |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if raters were independent or blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 38% (50/132) 9 months. No reasons given for dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No protocol to compare outcomes reported. In the published articles, means and SD were not reported, but were kindly provided by the author. |
| Other bias | Low risk | No evidence other biases are occurring. |
Chandler 2006.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 36 months.
Setting: community and jail. Location: Alameda county, San Francisco, Ca, USA. |
|
| Participants | Diagnosis: current serious mental illness and substance use disorder.* N = 182. Age: 18‐78 years. Sex: 131M, 51F. Ethnicity: 66% African American. Inclusion criteria: current serious mental illness and substance use disorder, US resident, not sentenced to prison, not on parole, not currently enrolled in another program, GAF ≤ 50, English or Spanish speaking, have at least 2 jail episodes in 2 years prior. | |
| Interventions | 1. Psychosocial intervention: integrated mental health and substance use treatment. In custody standard care + brief aftercare + Integrated Dual Disorders Treatment. Post‐custody; MI, substance abuse counselling, group treatment oriented to both disorder, family psycho‐education regarding dual disorders, multidisciplinary team, integrated substance abuse specialists, stage wise interventions, time unlimited services, outreach etc. N = 103. 2. Standard care: service as usual. In custody standard care + usual post‐custody services + 60 days of post‐release case management and housing assistance. N = 79. | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Unable to use Global state: forensic measures ‐ arrests, convictions, felonies, jail days, hours of medication services (data skewed). Service use: relapse (hospitalisation) (data skewed). |
|
| Notes | * Schizophrenia, schizoaffective disorder or psychosis 56%, major depression 26%, bipolar 10%, other 8%. Not ITT analysis. Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, computer‐generated in blocks of two, then blocks of three. |
| Allocation concealment (selection bias) | Low risk | Randomisation used an algorithm [template] supplied by the evaluator. A research assistant maintained the data which documented eligibility and randomisation, and they were reviewed weekly by the evaluator (Personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcomes: clinician‐/participant‐mediated ‐ rating ‐ unclear. Secondary outcomes: some clinician‐/participant‐mediated ‐rating ‐ unclear. Some outcomes were administrative which would have a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 37% (68/182) 3 years. Did not employ direct evaluation of substance use to assess the impact of the intervention. Unknown attrition rate among controls no interview was conducted leading to high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Relied on administrative data to analyse results not taking into consideration moving out of area, recovered or death. Controls had less follow‐up time post‐release due to randomisation procedure. |
| Other bias | Unclear risk | Study was funded by a State grant to evaluate service in Alameda county. |
Drake 1998a.
| Methods | Allocation: randomised.
Design: multi‐centre (7 sites: 2 urban and 5 rural).
Duration: 36 months.
Setting: community. Location: New Hampshire, USA. |
|
| Participants | Diagnosis: 53% DSM‐III‐R schizophrenia with active DSM‐III‐R substance use disorder (73% alcohol abuse, 42% drug abuse).* N = 223. Age: 18 ‐ 60 years, mean ˜ 34 years. Sex: 165 M, 58 F. Ethnicity: 96% white. Inclusion criteria: active DSM‐III‐R substance use disorder in past 6 months; no other medical conditions or mental retardation. | |
| Interventions | 1. Psychosocial intervention: integrated ACT: community‐based, high intensity, direct substance abuse treatment by team members, use of stage‐wise dual‐disorder model, dual‐disorder treatment groups and exclusive team focus on patients for those with dual disorders. Caseload ˜ 12. N = 109. 2. Standard care: standard case management: community‐based, team working with client's support system and vigorously addressing co‐occurring substance use. Caseload ˜ 25. N = 114. | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation.
Adverse event: death.
Substance use: SATS, Not in remission, progress towards recovery.
Homelessness: number of days living in stable community residences. Quality of life/life satisfaction: QOLI (General Life Satisfaction Scale). Unable to use Substance use: AUS, DUS, no of days when misusing (data skewed). Mental state: BPRS (data skewed). Service use: Relapse (hospitalisation) (data skewed). Quality of life / life satisfaction: QOLI (subscales). |
|
| Notes | Not ITT analysis. Authors have kindly provided further data. *Some participants had more than one dependence. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome: clinician‐/participant‐mediated rating ‐ unclear. Secondary outcomes: some are clinician‐/participant‐mediated – rating – unclear. Interviewer blind so detection bias was rated as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 9% (20/223) 3 years. Number of lost to follow‐up is reported, but reasons for missing data are not reported for each group. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes of interest are reported. No protocol to compare results. |
| Other bias | Low risk | No evidence other biases are occurring. |
Eack 2015.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 18 months. Setting: outpatients. Location: Pittsburgh, PA, USA. |
|
| Participants | Diagnosis: Schizophrenia, N = 17 or schizoaffective disorder (n = 14) and 29 (94%) with SCID substance abuse or dependence with moderate to higher addiction severity for cannabis or alcohol on the Addiction Severity Index. N = 31. Age: mean 38 years (range 18‐ 60 years). Sex: 22 M, 9 F. Ethnicity: not reported. Inclusion criteria: patients with schizophrenia and SUD were stable on antipsychotic medication, IQ > 80 and not dependent on cocaine or opioids. |
|
| Interventions |
1. Psychosocial intervention: routine care plus Cognitive Enhancement Therapy (CET) with individual and 45 group training sessions in social cognition and 60 hours of computer‐assisted training in attention and problem solving and additional psycho‐educational content on substance use. N = 22. 2. Standard care: routine care only, N = 9 |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Substance use: alcohol or cannabis use last 30 days (unable to use analysis due to small participant number). Mental State: BPRS, GAF (unable to use, composite scores only). |
|
| Notes | Trial identifier: NCT01292577. Participants received payment for research assessments. Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequences were generated using R computer software (author note via email) |
| Allocation concealment (selection bias) | Low risk | Assignments were maintained by an independent data manager and concealed from data collectors and testers (author note via email) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Particpants and providers would be aware of the group allocation. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were conducted by trained raters and assessors were blinded to treatment allocation. Substance use was by last 30 days recall so was by self‐report and participants were aware of their allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large difference between groups for attrition with CET group = 47% (9/19) and TAU = 20% (1/9). Although, this is reported in the text as not significantly different (P = 0.148). |
| Selective reporting (reporting bias) | Low risk | NCT01292577. No indication of selective reporting bias. |
| Other bias | Low risk | Funding by NIH |
Edwards 2006.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 6 months.
Setting: community. Location: Melbourne, Victoria, Australia. |
|
| Participants | Diagnosis: 72% DSM‐IV schizophrenia/schizophreniform, 11% affective psychosis, 17% NOS/delusional /other actively using cannabis.
N = 47.
Age: mean ˜ 21 years.
Sex: 34 M, 13 F. Ethnicity: not stated. Inclusion criteria: DSM‐IV diagnosis of a psychotic disorder, informed consent for research participation, adequate English language comprehension and patients continuing to use cannabis at 10 weeks post‐initial clinical stabilisation. |
|
| Interventions | 1. Psychosocial intervention: cannabis‐focused intervention (cannabis and psychosis therapy, CAP) for individuals with first‐episode psychosis. CAP consisted of a cognitive‐behavioural‐oriented program delivered in weekly sessions by trained clinicians over 3 months. N = 23. 2. Standard care: involving psycho‐education plus standard Early Psychosis Prevention and Intervention Centre (EPPIC) care. EPPIC includes case management, regular psychiatric review and medication, access to mobile assessment and treatment, family work, group programs, and a prolonged recovery clinic. N = 24. | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation.
Substance use: % of patients using cannabis in the last 4 weeks.
Social functioning: SOFAS. Unable to use Substance use: RTCQ‐C (adapted scale), CASUAS (modified SCAN) (all data skewed). Mental state: BDI‐SF, SANS (all data skewed), BPRS (some data skewed, unvalidated subscales). Service use: outpatient attendance and medication: SURS (data skewed). |
|
| Notes | ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised codes were computer‐generated and placed in sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were managed by a non‐clinical member of the research team. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters independent and blind to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 30% (14/47) 9 months. Missing data were handled using LOCF and analyses of cases with complete data were also undertaken. Due to positive skewness some variables were transformed (untransformed scores are displayed). The LOCF can still produce unclear risk of bias effects |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes of interest are reported. No protocol to make judgement on selective reporting. |
| Other bias | Low risk | Funded by the Victorian Government Dept of Human Services. No information available. No evidence of other bias occurring. |
Essock 2006.
| Methods | Allocation: randomised.
Design: multi‐centre, 2 urban sites.
Duration: 36 months.
Setting: community (two sites). Location: Bridgeport and Hartford, Connecticut, USA. |
|
| Participants | Diagnosis: 76% DSM‐III‐R schizophrenia, 17% mood disorder with co‐occurring DSM‐III‐R substance use disorder ( 74% alcohol abuse, 81% other substances).* N = 198. Age: mean ˜ 37 years. Sex: 142 M, 56 F. Ethnicity: 55%, African American, 27% White, 14% Hispanic, 4% other. Inclusion criteria: major psychotic disorder and active substance use disorder within past 6 months, high service use in the past two years, homelessness or unstable housing, poor independent living skills, no pending legal charges, no medical conditions or mental retardation that would preclude participation, if inpatient, discharge scheduled. | |
| Interventions | 1. Psychosocial intervention: integrated ACT with a direct substance use component. N = 99. 2. Standard care: standard case management.** N = 99. | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation.
Adverse event: death.
Service use: relapse (number of patients hospitalised during study).
Global state: GAS (see GAF).
Quality of life/life satisfaction: QOLI (General Life Satisfaction Scale). Homelessness: number of days living in stable community residences. Unable to use Substance use: AUS, DUS, SATS, number of days using in the past 6 months (skewed data). Mental state: Expanded BPRS Hospitalisation: days in hospital and days in hospital or in jail (skewed data). |
|
| Notes | Not ITT analysis. * Some participants had more than one dependence. *Participants paid US $15 for each interview and additional $5 for each urine and saliva sample. ** Refer to correspondence regarding clinical case management team (Kanter 2006). Authors kindly provided additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using separate computer‐generated randomisation stream for each site. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 14% (27/198) 3 years. Insufficient reporting of missing data (numbers and reasons for missing data are reported for the total sample, not for each intervention group). Seven randomised participants were lost for administrative reasons, but their intervention allocation was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Listed outcomes of interest are fully reported for each site, one site had better outcomes than the other. Author provided further data of combined sites for each treatment. |
| Other bias | Low risk | Public funded. No further details. No evidence other biases are occurring. |
Godley 1994.
| Methods | Allocation: part randomised within larger study.
Design: multi‐centre (6 sites, 4 sites not randomised).
Duration: 24 months.
Setting: community (6 sites, 4 sites not randomised). Location: Illinois, USA. |
|
| Participants | Diagnosis: DSM‐III R major psychiatric and substance abuse/dependence disorder. N = 97 (2 randomised sites). Age: mean ˜ 35 years. Sex: 77 M, 20 F. Ethnicity: white 38%, Hispanic 38%, other 24%. Inclusion criteria: DSM‐III R major psychiatric and substance abuse/dependence disorder. | |
| Interventions | 1. Psychosocial intervention: specialised case management services for mentally ill substance abusers (N = 52). 2. Standard care: standard care (N = 45). | |
| Outcomes | No usable data*: Substance use: drug and alcohol questionnaire (unpublished scale). Global state: Area of Difficulty Checklist (unpublished scale), Vocational Outcomes Form (adapted scale), GAF (data not reported). Homelessness: number of state‐operated facility admissions, number of state‐operated facility days, and average length of stay (data skewed). | |
| Notes | Not ITT analysis. *No usable data, only skewed data reported for the 2 randomised sites. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if raters were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 37% (36/97) 1 year, >51% at 18 and 24 months for randomised groups. Insufficient reporting of missing data (numbers and reasons for missing data are reported not for each intervention group). |
| Selective reporting (reporting bias) | High risk | Results are presented for each site, not combined for each treatment. Combined data from randomised and non‐randomised sites. Unusable. |
| Other bias | Low risk | No details. |
Gouzoulis‐Mayfrank 2015.
| Methods | Allocation: randomised. Design: single‐centre. Duration: follow‐up at 3, 6 and 12 months. Setting: inpatient and outpatient. Location: Cologne, Germany. |
|
| Participants | Diagnosis: DSM‐IV schizophrenia, schizophreniform or schizoaffective disorder and substance misuse or dependence. N = 100. Age: 31 years. Sex: 84 M, 16 F. Ethnicity: not reported. Inclusion criteria: voluntary inpatients with schizophrenia and substance misuse or dependence |
|
| Interventions |
1. Psychosocial intervention: integrated treatment, 6‐ minute group therapy sessions once per week stating in hospital and continued as outpatient with extra 90‐minute sessions in manual based CBT plus psycho‐education of substance use. N = 50. Duration of treatment was not set 2. Standard care: TAU. N = 50. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Global state: GAF Unable to use (Skewed data) Substance use: use of cannabis and alcohol in previous month (T1‐T3) (3, 6 months); abstinent or change in intensity of the main substance use (non‐standard, self‐rating with small samples sizes) Mental State: PANSS (total) Service use: relapse (readmission to hospital (number of re‐admissions)) (skewed data) Quality of life/life satisfaction: Satisfaction questionnaire was not standardised. |
|
| Notes | Trial identifier: U1111‐1119‐5851 ITT analysis (LOCF) T3 (12‐month) outcome had high attrition rate (> 50%), not used in mean difference analyses Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes prepared with papers with sequential numbers and placed in box and shaken before pulled out alternately to create two groups. |
| Allocation concealment (selection bias) | Low risk | Researcher not involved in the trial prepared sealed envelopes with group allocation which was concealed from researcher allocating patients to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers would be aware of assignment given it was ward‐dependent. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was based on self‐report of use in past 30 days. Urine samples were done but not reported how these were included into outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This was > 50% at 12 months and close to 50% at 3 months (although similar between groups). LOCF may not be best use over time |
| Selective reporting (reporting bias) | Unclear risk | The article reports on the same outcomes as on the registry DRKS 00000671. However, the authors state that the primary outcome was measured using multimodal parameters including urine samples. However, these do not appear to be incorporated into the final outcome which measured % change in abstinence based on days of use pre month x mean daily dose (this is assumed to be self‐report). We would expect that the urine results should be reported in the article. |
| Other bias | Low risk | Nil noted. Funded as part of doctoral scholarship at University Clinic |
Graeber 2003.
| Methods | Allocation: randomised (in a yoked fashion).
Design: single‐centre.
Duration: 6 months.
Setting: VA medical centre. Location: Albuquerque, New Mexico, USA. |
|
| Participants | Diagnosis: 100% DSM‐IV schizophrenia and met criteria for an alcohol use disorder within the 3‐month period prior to study enrolment; patients with additional non‐alcohol substance use (except active intravenous drug abuse) were eligible for protocol enrolment. N = 30. Age: mean ˜ 42.87 years. Sex: 29 M, 1 F. Ethnicity: 40% White, 40% Hispanic, 20%, African American. Inclusion criteria: as above. | |
| Interventions | 1. Psychosocial intervention: three‐session MI intervention, focused on personal choice and responsibility and de‐emphasised labelling, with the therapist assuming a directive and client‐centred style. N = 15. 2. Standard care: three‐session Educational Treatment ‐ didactic, focused on the material being delivered with the therapist assuming a directive interpersonal style. N = 15. | |
| Outcomes | Leaving the study early: lost to evaluation.
Substance use: abstinence rates. Unable to use Substance use: BDP (data skewed). |
|
| Notes | Not ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in a yoked fashion, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Raters not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 7% (2/30) 6 months. No missing data for primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | Pilot study, no protocol to make judgement on selective reporting. |
| Other bias | Low risk | No evidence other bias are occurring. With only two therapists, each nested within a single treatment does not allow treatment effects to be independent of therapists. |
Graham 2016.
| Methods | Allocation: randomised. Design: Single National Health Service (NHS) Trust. Duration: 2‐week intervention with 3‐month follow‐up. Setting: inpatient and community. Location: Birmingham and West Midlands, UK. |
|
| Participants | Diagnosis: ICD‐10 Schizophrenia (n = 36), Schizoaffective (N = 5), Bipolar disorder (N = 17) psychosis (N = 1) and abusing alcohol or drugs over the past three months based on DSM‐IV criteria for substance related disorder. N = 59. Age: 38 years. Sex: 50 M 9 F. Ethnicity: Caucasian (48%), Asian (17%), Black (25%), mixed (10%) Inclusion criteria: New admissions with DSM or ICD diagnosis of psychosis and DSM substance related disorder over the last 6 months. |
|
| Interventions |
1. Psychosocial intervention: Brief integrated MI consisting of 4‐6 (15‐30 minute) sessions over 2 weeks. N = 30. 2. Standard care: TAU. N = 29. |
|
| Outcomes | Leaving the study early: lost to evaluation. Substance use: number of substances used last 30 days (none vs. one or more). engagement Substance Abuse Treatment Scale (SATs). Unable to use Substance use: number of days substances used and severity of substance use, AUDIT, SDS (skewed data) Mental State: HADS (skewed data) |
|
| Notes | Trial identifier: ISRCTN43548483 Funded by Institute for Health Research Authors have kindly provided further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as 'randomisation'. Authors confirmed randomised via computer (string was derived in Stats Direct Software) |
| Allocation concealment (selection bias) | Low risk | Stated as "central" and by email which implies a separation between the allocation sequence and the person who is allocating the patient (The researcher had to apply for a group allocation, automated via email ‐ assumption) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers would be aware of what types of intervention they received. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The primary outcome was based on 'observable behaviours' using the Substance Addiction Treatment Scale (SATS) reported by the primary clinician. The trial is reported as 'independent rater blinded' but it would seem likely that the primary clinician would be aware of the allocation as it was likely to be disclosed by the patient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% (1/59) at 3 months. Raw SATS data (primary outcome) for each group were provided by the author. |
| Selective reporting (reporting bias) | Low risk | Report outcomes compare well to protocol registered on ISRCTN 43548483 |
| Other bias | Low risk | Nil noted. Funded by NIHR. |
Hellerstein 1995.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 8 months.
Setting: community, outpatient; Beth Israel Medical Center. Location: New York, NY, USA. |
|
| Participants | Diagnosis: RDC schizophrenia with 74% DSM‐III‐R psychoactive substance abuse/dependence. N = 47. Age: 18‐50 years, mean ˜ 32 years. Sex: 36 M, 11 F. Ethnicity: 43% African American, 32% Hispanic. Inclusion criteria: psychoactive substance abuse/dependence, desire for substance abuse treatment, no life‐threatening medical illness or need for long‐term hospitalisation. | |
| Interventions | 1. Psychosocial intervention: skills training ‐ group outpatient psychotherapy and psycho‐education plus drug treatment all at same site, twice weekly. N = 23. 2. Standard care: comparable levels of psychiatric care and substance abuse treatment from separate sites without formal case co‐ordination. N = 24. | |
| Outcomes | Leaving the study early: lost to treatment. Unable to use Substance use: ASI‐drug (change data). Mental state: ASI‐psychiatric (change data). Service use: relapse (days in hospital) (data skewed). |
|
| Notes | ITT analysis. Further data collected and mentioned in 2001 paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if raters were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 47% (22/47) 4 months, 64% (30/47) 8 months. Reasons for dropping out of study or not starting (non‐starters, n = 18) not given. |
| Selective reporting (reporting bias) | Unclear risk | Raw means were not reported for primary outcome, only change from baseline scores. |
| Other bias | Low risk | No evidence other bias occurring. |
Hickman 1997.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 3 months.
Setting: community. Location: Indiana University, In, USA. |
|
| Participants | Diagnosis: 80% DSM ‐IV schizophrenia or schizoaffective disorder with co‐occurring substance disorder. N = 30. Age: mean ˜ 37 years. Sex: 26 M, 4 F. Ethnicity: 97% white. Inclusion criteria: recent alcohol abuse, leading to need for treatment. | |
| Interventions | 1. Psychosocial intervention: routine care (see below) plus a brief MI, structured one‐time presentation of personal feedback on alcohol intake. N = 15. 2. Standard care: routine care alone (involving pharmacotherapy, case management services, substance abuse treatment groups). N = 15. | |
| Outcomes | Leaving the study early: lost to evaluation.
Mental state: SCL‐90‐R. Unable to use Substance use: self‐report alcohol volume in prior 3 months, AUI subscale (data skewed). |
|
| Notes | ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Randomised from the group of people attending relevant treatment programs at the time the experiment was conducted. |
| Allocation concealment (selection bias) | High risk | No details provided. Comment: "Procedure selection was modified to allow for participant refusal and to minimise disruption to an existing treatment program." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if raters were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 0% (0/30) 3 months. No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | PhD thesis, study did not undergo peer review via publication. No protocol to make judgement on selective reporting. |
| Other bias | Low risk | No evidence other bias occurring. |
Hjorthoj 2013.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 10 months: 6 months treatment, 4‐month follow‐up.
Setting: Hospital, community and early intervention services. Location: Copenhagen, Denmark. |
|
| Participants | Diagnosis: ICD‐10 schizophrenia spectrum psychosis (F2) and ICD‐10 cannabis use disorder.
N = 103.
Age: mean ˜ 21 years (range 17‐42).
Sex: 78 M, 25 F. Ethnicity: Not stated. Inclusion criteria: cannabis use, those dependent on alcohol, opioids or cocaine were excluded. |
|
| Interventions |
1. Psychosocial intervention: CBT + MI, CapOpus consisted of individual and group based motivational interviewing and CBT with 24 sessions, 1‐2 weekly over 6 months and incorporates both family and case manager. N = 52. 2. Standard care: TAU. N = 51. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Substance use: number of cannabis‐using days in the past month. Quality of life/life satisfaction: Client satisfaction (CSQ). Unable to use Substance use: days cannabis use, joints/30 days (skewed data). Mental state: PANSS 6 months and 10 months (data skewed). |
|
| Notes | ITT analysis, missing data were estimated for each analysis using log‐linear replacement. Authors kindly provided additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated random sequence stratified by cannabis use per day and type of TAU (some had case management or ACT depending on referral source), block size varied between 6, 8 and 10. |
| Allocation concealment (selection bias) | Low risk | Researcher not involved in the study generated the sequence, was known only to the Copenhagen Trial unit. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 34% (35/103) 10 months. Detailed flow chart provided, reasons for missing values are provided for each group. Full ITT analysis provided with missing values handled by multiple imputations, which can bias studies so rated unclear. |
| Selective reporting (reporting bias) | Low risk | Outcomes fully reported and were not different to trial protocol. |
| Other bias | Low risk | No evidence other bias occurring. |
Jerrell 1995a.
| Methods | Allocation: randomised.
Design: multi‐centre (2 sites, see Jerrell‐Calif 95b).
Duration: 18 months.
Setting: community. Location: South Carolina, USA. |
|
| Participants | Diagnosis: 62% DSM‐III‐R schizophrenia with co‐occurring substance disorder. N = 47. Age: 18‐59 years, mean ˜ 34 years. Sex: 33 M, 14 F. Ethnicity: 64% white. Inclusion criteria: substance abuse disorder, previous inpatient or residential psychiatric treatment, plus either poor work/life skill history last 2 years, history of intervention by mental health authorities or police for inappropriate social behaviour. | |
| Interventions | 1. Psychosocial intervention: behavioural skills programme: psycho‐educational approach with self‐management skills, repeated practice and reinforcement. Weekly group sessions with two licensed clinicians. N = 22. 2. Standard care: TAU plus 12‐step recovery programme: clinical staff (some 'recoverers') offered mock AA meetings within the Mental Health Centre, took or referred clients to community AA meetings, facilitated a sponsor relationship and provided counselling. N = 25. | |
| Outcomes | Leaving the study early: lost to treatment.
Social functioning: RFS (SAS‐SMI) Social Adjustment Scale. Unable to use Substance use: C‐DIS‐R (data skewed and no author analysis of randomised cohort). Mental state: C‐DIS‐R (data skewed and no author analysis of randomised cohort). Social functioning: SLS (not peer‐reviewed scale). |
|
| Notes | Part ITT analysis. Data reported are for randomised cohort only ‐ kindly supplied by the authors. Control group same as Jerrell‐Calif 95b. Participants paid for each assessment interview. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised (using the urn method) balanced for age, ethnicity, diagnosis, substance use severity and level of psychiatric functioning. Non‐randomised sample was excluded from this analysis (data provided by author). |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if raters were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 47% (22/47) 18 months. No ITT analysis with missing values imputed. No reasons given for lost to evaluation. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Low risk | No evidence other bias occurring. |
Jerrell 1995b.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 18 months.
Setting: community. Location: South Carolina, USA. |
|
| Participants | Diagnosis: 72% DSM‐III‐R schizophrenia with co‐occurring substance disorder. N = 50. Age: 18‐59 years, mean ˜ 33 years. Sex: 38 M, 12 F. Ethnicity: 66% white. Inclusion criteria: substance abuse disorder, previous inpatient or residential psychiatric treatment, plus either poor work/life skill history last 2 years, history of intervention by mental health authority or police for inappropriate social behaviour. | |
| Interventions | 1. Psychosocial intervention: routine care plus intensive case management: intensive assistance with housing, daily living, legal problems, money management, personal relationships and leisure activities. N = 25. 2. Standard care: routine care plus 12‐step recovery programme: clinical staff (some 'recoverers') offered mock AA meetings within the Mental Health Centre, took or referred clients to community AA meetings, facilitated a sponsor relationship and provided counselling. N = 25. | |
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation.
Social functioning: RFS SAS‐SMI Social Adjustment Scale. Unable to use Substance use: C‐DIS‐R (data skewed and no author analysis of randomised cohort). Mental state: C‐DIS‐R (data skewed and no author analysis of randomised cohort). Social functioning: SLS (not peer‐reviewed scale). |
|
| Notes | Part ITT analysis. Data reported are for randomised cohort only ‐ kindly supplied by the authors. Control group same as Jerrell‐Calif 95a. Participants paid for each assessment interview. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised (using the urn method) balanced for age, ethnicity, diagnosis, substance use severity and level of psychiatric functioning. Non‐randomised sample was excluded from this analysis (data provided by author). |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if raters were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 42% (21/50) 18 months. No ITT analysis with missing values imputed. No reasons given for lost to evaluation. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Low risk | No evidence other bias occurring. |
Kavanagh 2004.
| Methods | Allocation: randomised. Design: single‐centre (3 hospital sites, Royal Brisbane, Logan or Wolston Park). Duration: 12 months. Setting: hospital and community. Location: Brisbane, Qld, Australia. |
|
| Participants | Diagnosis: 100% DSM‐IV psychotic disorder with a current DSM‐IV substance use disorder (88% alcohol, 76% cannabis, 12% inhalants, 8% cocaine or heroin). N = 25. Age: 17‐31 years, mean: 23 years. Sex: 15 M, 10 F. Ethnicity: 84% Anglo‐Saxon. Inclusion criteria: 16‐35 years, consensus diagnosis of a DSM‐IV psychotic disorder; a current DSM‐IV substance use disorder; < 3 years since the first psychotic episode, less than 3 previous episodes of psychosis, able to converse in English without an interpreter, no diagnosis of developmental disability or amnesic disorder, not currently receiving other treatment for substance abuse, and, not currently taking heroin or methadone. | |
| Interventions | 1. Psychosocial intervention: routine care plus Start Over and Survive (SOS). Brief motivational intervention comprising 3 hours of individual treatment over 6‐9 sessions usually completed within 7‐10 days as an inpatient. N = 13. 2. Standard care: comprised pharmacotherapy, access to in‐patient programmes and aftercare involving either case management or general practice consultations. N = 12. | |
| Outcomes | Leaving the study early: lost to evaluation. Substance use: number of participants abstinent or improved on all substances at 12 months. | |
| Notes | ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutations table for each site. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 32% (8/25) 1 year. One SC participant had baseline data carried forward as missing data at 3, 6, and 12 month follow‐ups. |
| Selective reporting (reporting bias) | Unclear risk | Pilot study, no protocol. Five SOS participants did not proceed beyond initial rapport building stage so two analyses were done; one with all SOS subjects (n=13) and another one including SOS treated (n=8) |
| Other bias | Low risk | No evidence other bias occurring. |
Kemp 2007.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 6 months.
Setting: community based early intervention programme. Location: Western Sydney, NSW, Australia. |
|
| Participants | Diagnosis: DSM‐IV psychotic illness and current alcohol or cannabis use based on AUDIT or DAST scores.
N = 19.
Age: mean ˜ 21 years, range 17‐25 years.
Sex: 13 M, 3 F (3, Unknown). Ethnicity: not stated. Inclusion criteria: young English speaking, living within the area health sector and not homeless. |
|
| Interventions |
1. Psychosocial intervention: MI + CBT, Stop using stuff (SUS) manualised 4‐6 hours. N = 10. 2. Standard care: TAU, standard care included case management and has a significant focus on substance reduction. N = 9. |
|
| Outcomes | Leaving the study early: lost to evaluation. Mental state: PANSS total. Unable to use Mental State: DASS (some data skewed). Substance use: AUDIT, DAST‐10 frequency and quantity of cannabis or alcohol use (skewed data). |
|
| Notes | Authors have kindly provided additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, cards were shuffled, numbered and placed in sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Numbered sealed envelopes with the allocation placed into a box by a third person. Envelopes were then drawn in order from the box each time a patient was randomised. Unclear whether envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Measures that were clinician‐rated were all performed by the clinician providing the treatment and were therefore not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 16% (3/19) 6 months. Three patients dropped out of the TAU group, no explanation was given. No ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Unclear risk | Had extreme baseline imbalance in substance use (AUDIT), DASS and self‐efficacy score. This could be due to low subject numbers recruited for study. |
Lehman 1993.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 12 months. Setting: community. Location: Baltimore, Md, USA. | |
| Participants | Diagnosis: 67% DSM‐III‐R schizophrenia /schizoaffective disorder with co‐occurring substance disorder. N = 29.* Age: 18‐40 years, mean ˜ 31 years. Sex: 22 M, 7 F. Ethnicity: 70% Afro‐American. Inclusion criteria: current substance abuse or dependence disorder. | |
| Interventions | 1. Psychosocial intervention: routine care plus intensive case management: educational group sessions on substance abuse/mental illness (5 hours per week), experiential "rap" session, on‐site self‐help group, off‐site self‐help group (AA/NA), social activities. 1:15 staff‐patient ratio. N = 14. 2. Standard care: CMHC‐based, psychosocial rehabilitation services, routine outpatient services, supported housing if needed, no organised substance abuse treatment. 1:25 staff‐patient ratio. N = 15. | |
| Outcomes | Leaving the study early: lost to evaluation. Unable to use Lost to treatment: data reporting unclear. Substance use: ASI‐alcohol, ASI‐drug (data skewed). Mental state: ASI‐psychiatric (data skewed). Relapse: days in hospital (data skewed). Other: QOLI (Life satisfaction) (all data skewed). |
|
| Notes | ITT analysis. * Data reported in this review are based only on those who had current (past 30 days) substance use disorders (29 out of 54). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using the urn method. No further details. |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blindness not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 0% (0/29) 1 year. No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Low risk | No evidence other bias occurring. |
Madigan 2013.
| Methods | Allocation: randomised.
Design: single‐centre (3 sites).
Duration: 12 months.
Setting: inpatients and community. Location: Dublin, Ireland. |
|
| Participants | Diagnosis: DSM‐IV diagnosis of psychosis (schizophrenia, n = 38; other psychosis, n = 30) major depression (n = 6) and bipolar disorder (n = 14) and DSM‐IV current cannabis dependence.
N = 88.
Age: mean ˜ 28 years.
Sex: 69 M, 19 F. Ethnicity: Not stated (homogenous group). Inclusion criteria: without learning disability or organic brain damage. |
|
| Interventions |
1. Psychosocial intervention: CBT + MI group sessions once per week for 12 weeks and invited back 6 weeks later (week 18) for a booster session. Interventions were held in community setting. N = 59.* 2. Standard care: TAU included care from multi‐disciplinary team, 5 patients had counselling for opiate more than one year prior to the present trial. N = 29. |
|
| Outcomes | Leaving the study early: lost to treatment (3 months), lost to evaluation (9 months). Substance use: frequency of cannabis use last 30 days. Global state: GAF global functioning. Quality of life/life satisfaction: subjective quality of life (WHOQOL, BREF). Unable to use Mental State: SANS. SAPS (positive, negative), Calgary Depression Scale for Schizophrenia (skewed data). |
|
| Notes | * Note: 2:1 randomisation to CBT/MI arm. A token voucher was given to participants to cover costs of attendance of assessments. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block randomised, 2:1 (CBT/MI:TAU) ratio. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was conducted by a researcher uninvolved in the provision or assessment of interventions. Concealment not described in sufficient detail to allow a definite judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters of clinical outcomes blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 42% (37/88) 1 year. Similar reasons for missing data across groups. Missing values were not imputed for ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Low risk | No evidence other bias occurring. |
Maloney 2006.
| Methods | Allocation: randomised. Design: single‐centre (9 jails operated by LA county Sheriff's Dept). Duration: 30 months. Setting: jail and community. Location: Los Angeles, Ca, USA. | |
| Participants | Diagnosis: assessed by the MINI Plus 46% bipolar disorders, 27% psychotic disorders, 21.5% depressive disorders, 6% other with co‐occurring substance disorder. Primary substance use diagnoses as assessed by the CASAD, were: 51.8% cocaine dependence/abuse, 18.5% alcohol dependence/abuse, 4.4% opoid dependence, and 3.7% other. N = 135. Age: mean ˜ 36 years. Sex: 135 F. Ethnicity: 51% Black, 33% non‐Hispanic White, 12% Hispanic. Inclusion criteria: inmates who had a major Axis 1 mental illness and co‐occurring substance use disorder, age 18‐50, history of at least 2 arrests, homeless or at risk of homelessness. | |
| Interventions | 1. Psychosocial intervention: (CBT + psychosocial rehabilitation) ‐ the Intensive Jail (IJ) group received a minimum of 3 weeks of specialised treatment during their qualifying incarceration. Housed in a mental observation dormitory, IJ was a hybrid of psychosocial rehabilitation, cognitive‐behavioural, and harm‐reduction approaches. Individual supportive counselling twice per week, crisis intervention services and a series of 12 to 15 one‐hour groups per week. Groups included 12‐step sobriety maintenance, relapse prevention, symptom and medication management, crime prevention, parenting, and independent living. N = 18. 2. Psychosocial intervention: the Intensive Community Treatment (ICM). ICM treatment approach provided intensive case management. Transition planning, which began while participants were still in custody and continued after release, consisted of housing placement, pursuit of public entitlements, linkage to psychiatric care, and transportation to pharmacy and residential placement post‐release. In addition, on‐going consultations to ensure continuity of care, and interventions aimed at substance recovery maintenance, relapse prevention, psychiatric stability and reduction of re‐arrests were provided. IC treatment was carried out in conjunction with a number of community agencies, including those specializing in dual recovery, rehabilitation, housing, entitlement benefits, and childcare and parenting etc. N = 58. 3. Psychosocial intervention: (CBT + ICM) ‐ Combined treatment (COB) group received both intensive jail and intensive community interventions as described above. N = 16. 4. Standard care: included psychiatric medication evaluation and follow‐up, recreation therapy and stress management groups, parenting classes, drug education, academic education and religious services. N = 43. | |
| Outcomes | No usable data*: Leaving the study early: lost to treatment, lost to evaluation (no breakdown by IJ, IC, COB treatment arms). Global state: forensic measures ‐ number of arrests and convictions for new offences, number of days in jail (skewed data), severity of offence (no data provided). | |
| Notes | Not ITT analysis. * No usable data, only skewed data reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, computer‐generated procedure with no matching procedures. Participants were randomly assigned to one of four groups. Group sizes were not equivalent due to early jail releases, which necessitated the discontinuation of new participants being randomly assigned to the treatment groups after May 2003. |
| Allocation concealment (selection bias) | High risk | Randomisation and participants’ assignment to treatment groups were implemented by the treatment team’s researchers. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Raters were not blinded so detection risk of bias was considered high for these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 14% (19/135) 30 months. No ITT analysis. Due to early jail releases there was unequal discontinuation of new participants randomly assigned to the treatment groups after May 2003. These participants were not included in the analysis. |
| Selective reporting (reporting bias) | High risk | No usable data, many of the outcomes reported for site, not by treatment arm. |
| Other bias | Low risk | No evidence other bias occurring. |
McDonell 2013.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 6 months.
Setting: community. Location: Seattle, Washington, USA. |
|
| Participants | Diagnosis: Schizophrenia spectrum (n = 69, 39%), bipolar disorder (n = 60, 34%) and major depression (n = 47, 27%) and dependence on stimulants (cocaine, amphetamine, methamphetamine).
N = 176.
Age: mean 43 years.
Sex: 115 M, 61 F. Ethnicity: 54% White, 30% Africian American, 16% other. Inclusion criteria: used stimulants in the last 30 days. Exclusion criteria were organic brain disorder, dementia or medical disorders or psychiatric symptoms severe enough to compromise safe participation. Patients were excluded if current participation in methadone maintenance. |
|
| Interventions |
1. Psychosocial intervention: Contingency Management (CM) for 3 months with urine submission for 3 months. Negative urine results reinforced with selecting prizes or varying value (given a message of “well done” with no financial reward, or with a financial reward of 1 dollar, $20, or $80). A positive urine was not reinforced with a chance for a selecting a prize. N = 91. 2. Standard care: TAU plus submission of urine for 3 months but received the reinforcement their "yoked" partner in the active arm received, i.e., they were non‐contingently reinforced. |
|
| Outcomes | Leavind the study early: lost to treatment, lost to evaluation.* Substance use: stimulant‐negative urine test, (3 and 6 months), injection drug use (3 and 6 months). Service use: hospitalised (6 months after randomisation). Unable to use Substance use: stimulant use days, alcohol use days (skewed data). Mental state: Brief Symptoms Inventory (data skewed), PANSS excitement subscore (data skewed). |
|
| Notes | Authors kindly provided additional data and comments on the randomisation procedure. * There was a high attrition rate (see attrition bias) with imputed values for ITT analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using an urn randomised procedure balancing groups on gender, substance use severity, mood vs psychotic disorder and psychiatric hospitalisation in the past year. Further correspondence verified a computer programme was used for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Researcher not involved in the study generated the sequence. No further details on concealment prior to allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was urinalysis results so the review authors judge that this outcome is not likely to be influenced by blinding. Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 28% (49/176) completed at least one assessment by 6 months. Only 42% (n = 38) completed 12 weeks of CM and 65% (n = 55) completed TAU. Imputed values were used for missing data. |
| Selective reporting (reporting bias) | Low risk | The study protocol (NCT00809770) is available and all of the study's specified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Other bias | Low risk | Five patients were forced into CM (to start yoking procedure) and two were administratively removed with unknown consequences. |
McDonell 2017.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 7 months (1 month induction, 3 months treatment, 3 month follow‐up). Setting: outpatients and community. Location: Seattle, WA, USA. |
|
| Participants | Diagnosis: serious mental illness DSM‐IV‐TR (schizophrenia, N = 24; bipolar, N = 29 and major depression, N = 26) and alcohol dependence. N = 79. Age: 45 years. Sex: 50 M, 29 F. Ethnicity: Caucasian (N = 44) African American (N = 23), other (N = 12). Inclusion criteria: Alcohol use on > 4 days of the last 30 and enrolment in outpatient addiction group treatment; those with comorbid drug dependence were excluded. There was a 4 week induction period to increase retention. |
|
| Interventions |
1. Psychosocial intervention: Contingency management (CM, 12 weeks) with three urine samples per week reinforced with selecting prizes for varying magnitudes of reinforcement for testing negative for alcohol, attending treatment sessions or refraining from alcohol use for one week. Prizes ranged from zero value (“good job”), small ($1), large ($20) and jumbo ($80). N = 40. 2. Standard care: TAU in addition to 12 weeks of psycho‐education and non‐non contingent reinforcement of submitted urine samples or treatment attendance. N = 39. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation Unable to use Substance use: negative urine sample tests of alcohol, self‐reported alcohol use (skewed data) Mental State: PANSS. BSI (Brief Symptom Inventory)(omnibus data provided by author not broken down by treatment group) |
|
| Notes | Trial identifier: NCT01567943 Patients received $20‐30 for interviews. Funded by NIAAA, USA Authors have kindly provided further data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn‐randomisation procedure generated by computer (author's note) |
| Allocation concealment (selection bias) | Low risk | Randomised by a study staff member who concealed from other research staff (author's note) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were aware of the group into which they were allocated and the consequence of their actions on the contingency management. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Urine samples were taken so the outcome was blinded. PANSS raters were not blinded (author's note via email) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 10/40 (25%) CM arm and 9/39 (23%) in non‐CM arm at 3 months. Attrition of primary outcome (alcohol abstinence based on urine test) in first 12 weeks was 14/40 (35%) CM and 10/39 (26%) in non‐CM arm as they missed at least 3 weeks (9 consecutive visits). ITT analysis used but may introduce its own bias. |
| Selective reporting (reporting bias) | Low risk | The protocol matches the report NCT01567943 [secondary outcomes are not reported in terms of magnitude and direction, but only based on significance] |
| Other bias | Low risk | Funding was from the National Institute on Alcohol Abuse and Alcoholism and all authors declared receipt of research funding from pharmaceutical companies. |
Morse 2006.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 24 months. Setting: community. Location: St. Louis, Mo, USA. | |
| Participants | Diagnosis: DSM‐IV 48% schizophrenia, 19% schizoaffective disorder, 11% atypical psychotic disorder, 11% bipolar disorder, 9% major depression‐recurrent disorder, 2% other. All had one or more substance use disorders; 46% substance dependence disorder for alcohol and/or drugs; 64% substance abuse disorder for alcohol and/or drugs, 40% an alcohol‐only diagnosis, 18% drug‐only diagnosis, 42% had both drug and alcohol disorders ‐ cocaine most frequently used drug (34%) cannabis (19%).** N = 196.* Age: 18 ‐ 66 years, mean ˜ 40 years. Sex: 119 M, 30 F. Ethnicity: 73% Afro‐American, 25% Caucasian, 2% other. Inclusion criteria: homeless, severe mental illness, DSM‐IV substance use disorder, and not currently enrolled in an intensive case management program. | |
| Interventions | 1. Psychosocial intervention: Integrated Assertive Community Treatment. N = 46. 2. Psychosocial intervention: Assertive Community Treatment Team only (ACTO). Referred clients to other community providers for outpatient or individual substance abuse services and to 12‐step groups. N = 54. 3. Standard care: routine care, provided with a list of community agencies (mental health and substance abuse treatment) and staff provided linkage assistance to facilitate access. N = 49. | |
| Outcomes | Unable to use*** Leaving the study early: lost to treatment, lost to evaluation (not reported by group). Substance use: USS (data skewed), number of days using substances (unclear measure). Mental state: BPRS (averaged item scores reported, not totals). Homelessness: number of days in stable housing (data skewed), client satisfaction (not peer‐reviewed scale). | |
| Notes | Not ITT analysis.
* Figures are based on the 149 who received treatment. ** Participants paid USD $5 for short and $10 for long interview. *** No usable data, only skewed data reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised (no further description). |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if raters were independent or blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 24% (47/196) 2 years. Full ITT analysis not reported. |
| Selective reporting (reporting bias) | High risk | Lost to treatment and evaluation not reported by group. No usable data, only skewed data are reported. |
| Other bias | Low risk | No evidence other bias occurring. |
Naeem 2005.
| Methods | Allocation: randomised. Design: multi‐centre (6 sites, Belfast, Glasgow, Hackney, Newcastle, Southhampton and Swansea). Duration: 3 months. Setting: inpatients and community. Location: Southhampton and Newcastle‐upon‐Tyne, UK. | |
| Participants | Diagnosis: 100% ICD‐10 schizophrenia with co‐occurring mild to moderate substance abuse according to HoNOS Item 3* (alcohol 74%, drug use problem 26%**). N = 105. Age: 18 ‐ 65 years. Sex: 87 M, 18 F. Ethnicity: 90% Caucasian. Inclusion criteria: patients with schizophrenia, age 18‐65 years; receiving treatment within mental health services. | |
| Interventions | 1. Psychosocial intervention: CBT plus psycho‐education: 6 sessions over 3 months and carers offered 3 sessions along with carer‐oriented information. N = 67.*** 2. Standard care: Routine care. N = 38. | |
| Outcomes | Leaving the study early: lost to treatment.
Mental state: Insight Scale. Unable to use Substance use: HoNOS item 3 (data skewed). Mental state: BSA, SCR, CPRS, MADRS (all data skewed). Other: HoNOS ‐ general functioning (data skewed). |
|
| Notes | ITT analysis. *Patients were excluded if they met a diagnosis of drug or substance misuse dependence. **Based on those participants who provided details (n = 70/105). ***There was a 2:1 ratio to include more participants into the CBT arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised; random numbers computer‐generated/sealed envelopes stratified by site. Ratio of 2:1 for CBT:TAU. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes opened at the time of treatment allocation. Unclear if envelopes were sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 14% (15/105) at 3 months. No reasons are given for withdrawals; 10 participants dropped out of treatment versus 5 participants from TAU. Missing values were imputed for full ITT analysis but can have unclear bias effects. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Low risk | No evidence other bias occurring. |
Nagel 2009.
| Methods | Allocation: randomised.
Design: single‐centre (3 remote settings).
Duration: 18 months.
Setting: community. Location: Northern Territory, Australia. |
|
| Participants | Diagnosis: DSM‐IV schizophrenia (39%, n = 19), major depression (45%, n = 22), substance‐induced psychosis (10%, n = 50 and bipolar disorder (6%, n = 30 and substance use (alcohol and cannabis) with psychological dependence.
N = 49.*
Age: mean ˜ 21 years.
Sex: 28 M, 21 F. Ethnicity: Indigenous Aboriginal. Inclusion criteria: current patients with chronic mental illness attending a community health centre were recruited as well as their carers. Patients with organic mental illness, intellectual disability, and age less than 18 were excluded. |
|
| Interventions |
1. Psychosocial intervention: MI (early intervention group), two brief sessions (1 hr) spaced 2‐6 weeks apart. Session two involved carers and 2 psycho‐education videos presented. Followed up at 6, 12 and 18 months. N = 24. 2. Standard care: TAU, (late intervention group) had treatment (MI) after the 6 month assessment (T1) and then followed up at 12 months (T2, 6 months F/up) and 18 months (T3, 1 yr). N = 25. |
|
| Outcomes | Leaving the study early: lost to evaluation (6 months). Adverse event: death (all causes). Unable to use Mental state: HoNOS, LSP‐16, K‐10 (no SD, n). Substance use: SDS alcohol, cannabis (no means, SD, n). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, random number sequence. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Raters were not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 29% (14/49) 18 months. Full ITT was conducted but this can still have unclear bias effects. |
| Selective reporting (reporting bias) | Unclear risk | The original protocol (NCR00192582) could not be accessed as only the final article was listed. Selective reporting was unclear. |
| Other bias | Unclear risk | Follow‐up data at 12 and 18 months confounded, as both groups received treatment. Only T1 data are informative regarding group differences. |
O'Connell 2018.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 3 months, follow‐up to 1 year. Setting: outpatients. Location: New Haven CT, USA. |
|
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia spectrum or affective disorder with psychotic features and co‐occurring substance use or dependence as determined by Dartmouth Assessment of Lifestyle Instrument (DALI). N = 137. Age: 38 years. Sex: 91 M, 46 F. Ethnicity: African‐American (58%), white (20%), other (22%). Inclusion criteria: At least one hospital admission in last year and eligible for public sector care (no private insurance). |
|
| Interventions |
1. Psychosocial intervention: standard care plus Manualised Skills training for persons with co‐occurring disorders. N = 48. 2. Psychosocial intervention: standard care plus Manualised Skills training for persons with co‐occurring disorders plus peer‐led social engagement programme. N = 42. 3. Standard care: TAU, standard care plus transportation vouchers. N = 47. |
|
| Outcomes | Leaving the study early: lost to treatment at 3 months (subject numbers by treatment group provided by author email). Unable to use Lost to evaluation, at 9 months (greater than 50% lost to treatment). Substance use: alcohol use last 30 days at 3 and 9 months. Days drank alcohol, experienced problems (skewed data). Mental State: PANSS, SFS (Social Functioning Scale), skewed data. Readmission to hospital, 6 month and 1 year, no Ns provided. |
|
| Notes | No trial identifier found. Participats paid $25 per interview. Funded by NIDA Authors have kindly provided further data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported (reported only as 'randomised'). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were aware of their group allocation. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measures were administered by trained research assistants but no report regarding attempt to blind them to group allocation. All outcomes were by self‐report and we judged this to be at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition was 56/137 (41%). There is no flow diagram, but the three‐month loss of individuals is reported as not significantly different between any of the 3 groups. |
| Selective reporting (reporting bias) | High risk | No report of registration and protocol was not seen. Data unusable or published in a form not able to use in meta‐analysis as attrition was not reported by treatment arm and Ns not provided for follow‐up assessments. |
| Other bias | Low risk | Funded by National Institute on Drug Abuse. |
Petry 2013.
| Methods | Allocation: randomised. Design: single‐centre. Duration: 8 weeks (56 days). Setting: outpatient. Location: New Britain, CT, USA. |
|
| Participants | Diagnosis: serious mental illness (major depression, 47%), bipolar (37%) and schizophrenia (16%) and cocaine dependence. N = 19. Age: 42 years. Sex: M F, not reported. Ethnicity: White (68%). Exclusion criteria: excluded if they were in recovery from pathological gambling. |
|
| Interventions |
1. Psychosocial intervention: Contingency Management comprised of receiving $1 for each provided sample plus draws to win prizes (varied from zero value, $20 up to $100) based on number of consecutive negative urine samples for cocaine, N = 10. 2. Standard care: TAU, plus $1 for each urine sample provided twice per week. N = 9. |
|
| Outcomes | Leaving the study early: lost to treatment. Unable to use Substance use (skewed data): proportion of samples cocaine negative, consecutive weeks cocaine negative (more than half the samples were not submitted). Mental State: BSI (not reported as means, SD, N). |
|
| Notes | Trial identifier: NCT01478815 Participants received $10 for baseline and $20 gift cards for post‐treatment evaluation. Funded by NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised program used for stratification and assumed to be randomised by computer. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were aware of their group allocation. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Substance use by urine analysis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall lost to follow‐up at 8 weeks was 2/19 (11%), 1 participant from each group was lost to follow‐up. The primary outcome was proportion of cocaine‐negative urine samples and longest duration of cocaine abstinence. Less than half of the expected samples were remitted (in both groups) by 8 weeks. Although analyses were completed on submitted and expected samples based on positive or negative assumptions, attrition for the primary outcome was rated high risk as the proportion varied depending on how the missing samples were considered. |
| Selective reporting (reporting bias) | Low risk | NCT01478815. No indication of selective reporting |
| Other bias | Low risk | NIH funded |
Rosenblum 2014.
| Methods | Allocation: randomised. Design: multiple sites. Duration: 3 and 6 months. Setting: outpatient and residential. Location: Grand Rapids, MI and New York, NY, USA. |
|
| Participants | Diagnosis: serious mental illness (major depression, bipolar disorder, N = 97 and schizophrenia, N = 59) and lifetime history of substance misuse determined by Modified Simple Screening instrument for Substance Abuse. N = 203. Age: 42 years. Sex: 138 M, 65 F. Ethnicity: Hispanic (13%), Black (32%) white (52%), other (5%). Inclusion criteria: Those not meeting current misuse (alcohol > 4 drinks per day or illicit use last 90 days) were excluded. |
|
| Interventions |
1. Psychosocial intervention: peer‐supported 12‐step self‐help program called Double Trouble in Recovery (DTR), comprising one group meeting per week with a peer facilitator to help develop new skills and coping behaviours, N = 113. 2. Standard care: TAU, received standard residential or day patient treatment and asked not to attend DTR meetings until after follow‐up interview, N = 163. |
|
| Outcomes | Leaving the study early: lost to treatment, lost to evaluation. Substance use: number of day any alcohol or drug use past 30 days. Mental State: (QoL unable to use; not standard instrument). Medication Adherence (MARS). |
|
| Notes | Trial identifier: NCT01333280 139 excluded after randomisation as they did not meet recent substance misuse criteria. Primary psychiatric diagnoses not reported. Attendees received $30 for baseline interview, $5 per meeting attended and $50‐100 for follow‐up interviews. Funded by NIDA. One author is director of NGO that receives sales from distribution of DTR material. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table using block randomisation. However, within the 2 regions participants in the two groups differed at baseline on PTSD and bipolar diagnoses. [this issue is addressed in the new ROB2.0 as if randomisation was done appropriately but did not create 2 identical groups then it may indicate that the sequence was not successful (or could be a chance finding)] |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by sealed envelopes (assumed to be consecutively numbered and opaque) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were aware of their group allocation. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome of substance use was by self‐report but was validated by saliva testing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 20% in the DTR group and 22% in the control condition with no significant difference between groups. We judged this to be low risk |
| Selective reporting (reporting bias) | Low risk | NCT01333280. No indication of selective reporting. |
| Other bias | Low risk | NIDA funding |
Swanson 1999.
| Methods | Allocation: randomised.
Design: single‐centre (two inner city hospitals).
Duration: not stated; time to first appointment after discharge.
Setting: hospital. Location: New York, NY, USA. |
|
| Participants | Diagnosis: schizophrenia, psychosis, or schizo‐affective disorder (45% of sample have substance abuse problems).* N = 93. Age: mean ˜ 34 years. Sex: 60 M, 33 F. Ethnicity: 46% African‐American, 45% Hispanic. Inclusion criteria: voluntary inpatients with no organic brain disease or other serious medical illness, learning disability or deafness. | |
| Interventions | 1. Psychosocial intervention: routine care plus 15 minute motivational interview at start of hospitalisation. Another one hour motivational interview 1 or 2 days before discharge. N = 48. 2. Standard care: routine care. N = 45. | |
| Outcomes | Leaving the study early: lost to evaluation. Service use: attendance at first aftercare appointment following hospital discharge. | |
| Notes | Not ITT analysis. *Data given only for those with substance abuse problems. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised (using random number table). |
| Allocation concealment (selection bias) | High risk | Therapist consulted a random number table to determine group assignment. May have influenced section bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if raters independent or blind to allocation. Primary outcome measure was time to next appointment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 0%, (0/93) 3 months. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'yes' or 'no' as no protocol was available. |
| Other bias | Low risk | No evidence other bias occurring. |
Tracy 2007.
| Methods | Allocation: randomised.
Design: single‐centre.
Duration: 1 month.
Setting: community. Location: New Haven Connecticut, USA. |
|
| Participants | Diagnosis: Met current or lifetime DSM‐IV Axis 1 psychiatric disorder* and had a co‐occurring current diagnosis of cocaine or alcohol abuse or dependence. N = 30. Age: not stated. Sex: gender not stated. Inclusion criteria: homeless or seeking shelter at least 18 years of age in addition to current SUD and psychiatric diagnosis. | |
| Interventions |
1. Psychosocial intervention: Contingency management: Low‐cost contingency management with variable ratio reinforcement where patients received reinforcers** contingent upon demonstrating abstinence from both alcohol and cocaine, as verified by breathalyser and cocaine‐free urine specimens. N = 15. 2. Standard care: TAU, assessment only. N = 15. |
|
| Outcomes | Leaving the study early: lost to treatment (4 weeks). Unable to use Substance use: mean and SDs not provided for alcohol and substance use; percentages (and Chi2 statistics) are provided for some outcomes but not subject number (N) for each group. |
|
| Notes | * Specific diagnoses are not reported, unknown seriousness or duration of psychiatric illness. ** Reinforcers were redeemable prizes ranging in value from no prize, $1, $20 and $100. All participants received $30 for the screening, baseline and termination interviews and $5 for each weekly assessment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a table. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐/participant‐mediated and participants and personnel not blinded. It is not possible to blind a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if raters were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: 13% (4/30) 1 month. Four patients in the TAU (assessment only) group did not complete the study, no further details are provided. Analyses adjusted for greater cocaine use using covariate. |
| Selective reporting (reporting bias) | High risk | Gender, specific diagnoses and age not reported for participants. Mean and SDs not provided for alcohol and substance use; percentages (and Chi2 statistics) are provided for some outcomes but not participant number (N) for each group. |
| Other bias | Low risk | No evidence other biases are occurring. |
F = Female, M = Male, N = Number ITT ‐ Intention‐to‐treat analysis
LOCF ‐ Last observation carried forward
RDC ‐ Research diagnostic criteria SCID ‐ Structured Clinical Interview for Diagnosis
Type of care AA ‐ Alcoholics Anonymous ACT ‐ Assertive Community Treatment CBT ‐ Cognitive Behaviour Therapy NA ‐ Narcotics Anonymous
For full list of diagnostic scales and abbreviations see Table 10.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Audier 2011 | Allocation: not randomised. Intervention: money for taking medication; not SUD focused. |
| Bachmann 1997 | Allocation: not randomised. |
| Bagoien 2013 | Allocation: randomised. Participants: not severely mentally ill; not all participants had a current drug and alcohol use problem. |
| Barkhof 2013 | Allocation: not randomised |
| Barrowclough 2006b | Allocation: randomised. Participants: people with severe mental illness; substance misuse not identified as the primary problem. |
| Battersby 2013 | Allocation: randomised. Participants: veterans who misused alcohol (AUDIT > 8) and who also had a psychiatric or medical condition. Not all participants had a serious psychotic illness (many had PTSD, panic or GAD). |
| Bechdolf 2004 | Allocation: randomised. Participants: people with schizophrenia and a primary diagnosis of drug and alcohol dependence were excluded; no information provided on any participants with a dual diagnosis. |
| Beebe 2012 | Allocation: randomised. Participants: people with schizophrenia and co‐occurring substance use disorders. Intervention: MI to increase exercise, not to reduce substance use. |
| Bell 2011 | Allocation: randomised. Intervention: cognitive remediation, not SUD focused. |
| Bennett 2001 | Allocation: not a randomised trial. |
| Bowen 2000 | Allocation: randomised. Participants: not severely mentally ill (panic disorder with or without agoraphobia). |
| Brooner 2013 | Allocation: randomised. Participants: received routine methadone maintenance, low percentage of participants had a comorbid psychotic disorder (< 5%). |
| Brown 2015 | Allocation: randomised. Participants: adolescents (aged 13‐17) with psychiatric comorbidity, non‐adult population. Patients were excluded it they had a psychotic disorder. |
| Carey 2004 | Allocation: randomised. Participants: people with severe mental illness; not all patients had a current drug and alcohol use problem. |
| Castle 2002 | No usable data. Email from author confirmed study abandoned; not enough participants to publish the results. |
| Clarke 2000 | Allocation: randomised. Participants: people with severe mental illness; not all participants had a current drug and alcohol use problem. |
| Clausen 2016 | Allocation: not randomised |
| de Waal 2015 | Allocation: randomised. Intervention: to reduce victimisation in patients with a dual diagnosis. Not a intervention to reduce SUD. |
| DeMarce 2008 | Allocation: randomised. Participants: substance abusers with co‐occurring psychiatric disorder. No description of seriousness of psychiatric diagnoses. Intervention: participants received a behavioural continuing care adherence intervention involving contracting, prompting and reinforcing attendance or standard treatment. |
| Drake 2004a | Allocation: original sample randomised. Participants: people with severe mental illness and substance misuse. Interventions: psychosocial. Outcomes: no usable data (results from control group not reported). |
| Drake 2006 | Allocation: original sample randomised. Participants: people with severe mental illness and substance misuse. Interventions: psychosocial. Outcomes: no usable data (results from control group not reported). |
| Drebing 2005 | Allocation: randomised. Participants: only 11% (n = 2) of the veterans had a psychotic diagnosis, 74% had an affective disorder and 58% had an anxiety disorder. Did not fulfil the criteria for serious mental illness. |
| Drebing 2007 | Allocation: randomised. Participants: only 9% of the veterans had a psychotic diagnosis, 80% had major depression and 53% were given a PTSD diagnosis. |
| Eberhard 2009 | Allocation: randomised. Participants: ˜40% had affective disorder, ˜23% anxiety disorder and ˜13% PTSD, 7% personality disorder. All patients were non‐psychotic. |
| Faber 2012 | Allocation: subanalysis from a larger randomised trial. Participants: first‐episode psychosis, but substance use was not an inclusion criteria. Intervention: not a psychosocial intervention focused on reducing substance use. |
| Fiszdon 2016 | Allocation: randomised. Participants: people meeting criteria for substance abuse in last 30 days excluded, not a dual diagnosis population. |
| Gaughran 2017 | Allocation: randomised care coordinators (n = 104) and the patients they cared for (406). Participants: outpatients with a psychotic disorder; Not all participants had a SUD. Intervention: to improve lifestyle choices, improve diet and reduce smoking (IMPACT health promotion). Where applicable, intervention targeted alcohol, cannabis and other illegal substances. |
| Gleesen 2009 | Allocation: randomised. Participants: young people with first‐episode psychosis; not all patients had a current or past drug or alcohol use problem. Intervention: individual and family CBT focused on relapse prevention, not SUD focused. |
| Goldstein 2005 | Allocation: randomised. Participants: only two participants had a diagnosis of schizophrenia. |
| Harrison 2017 | Allocation: not randomised. |
| Havassy 2000 | Allocation: randomised. Participants: people with severe mental illness and substance misuse. Interventions: two case0management programmes; no specific substance misuse treatment. |
| Herman 2000 | Allocation: randomised. Participants: people with severe mental illness and substance misuse. Interventions: psychosocial. Outcomes: no usable data. |
| Hulse 2002 | Allocation: randomised. Participants: not all severely mentally ill (10% psychotic); 26% did not have a dual diagnosis. |
| ISRCTN58667926 | Allocation: randomised. Participants: serious mental illness; substance use disorder not listed as an inclusion criteria. |
| James 2004 | Allocation: not randomised (alternate allocation). |
| Jerrell 2000 | Allocation: randomised. Participants: people with severe mental illness and a co‐occurring substance disorder. Interventions: day treatment integrating mental health and substance use symptoms; community group meetings, skill‐building, 12‐step groups, relapse prevention skills and case management versus treatment as usual. Outcomes: no usable data ‐ control group data not available. |
| Kelly 2002 | Allocation: not randomised. |
| Kidorf 2013 | Allocation: randomised. Participants: attending methadone clinic who also had a psychiatric disorder; none were diagnosed with schizophrenia. Many had PTSD or antisocial personality disorder. Intervention: not a SUD psychosocial intervention |
| Killackey 2013 | Allocation: randomised. Participants: young people with first episode psychosis; not all participants had a SUD Intervention: vocational intervention (supported employment), not a SUD intervention |
| Lozano 2013 | Allocation: not randomised. |
| Magura 2003 | Allocation: not a randomised trial. |
| Mangrum 2006 | Allocation: randomised. Participants: co‐occurring severe and persistent mental illness and substance use disorders. Interventions: integrated versus parallel treatment. Outcomes: no usable data (not broken down by site, only 2 of 3 sites were randomly assigned). |
| Martino 2000 | Allocation: randomised. Participants: people with psychotic or mood disorders and concurrent DSM‐IV substance‐related disorders. Interventions: pre‐admission motivational interview versus standard pre‐admission interview. Outcomes: no usable data. |
| Martino 2006 | Allocation: randomised. Participants: dually diagnosed psychotic and drug‐related disordered patients. Interventions: pre‐admission motivational interview versus standard preadmission interview. Outcomes: no usable data. |
| McGurk 2009 | Allocation: randomised. Intervention: cognitive remediation, not a SUD focused intervention. |
| Mercer 1997 | Allocation: not randomised. |
| Mueser 2001 | Allocation: not a randomised trial. |
| Mueser 2009 | Allocation: randomised. Participants: family members of patients with a dual diagnosis. Interventioin: family education programme, did not directly involve patients. |
| NCT00043693 | Allocation: randomised. Participants: family members of patients with a dual diagnosis. Intervention: family education programme, did not directly involve patients. |
| NCT00316303 | Allocation: randomised. Participants: patients with psychosis and substance use disorder. Intervention: focused on reducing harm (HIV, other risk behaviours); not specific for reducing substance use. |
| NCT00447720 | Allocation: randomised. Intervention: educational programme for case managers. |
| NCT00495911 | Allocation: randomised. Participants: people with first‐episode psychosis;SUD was not listed as an inclusion criteria. |
| NCT01361698 | Allocation: randomised. Participants: substance use not an inclusion criteria. Intervention: recovery focused (increased adherence); not specifically focused on SUD. |
| NCT02264327 | Allocation: randomised. Participants: not patient focused. Intervention: training MI to staff in increasing MI knowledge and skill retention over time |
| Noordraven 2017 | Allocation: randomised. Intervention: financial incentives for improving adherence; not a psychosocial intervention to reduce substance use |
| Nuijten 2012 | Allocation: randomised. Participants: inpatients and outpatients with serious mental illness and SUD. Interventioin: integrated treatment based on hospital status; inpatient or outpatient. No TAU group. |
| Nuttbrock 1998 | Allocation: quasi‐randomised (treatment facilities retained the final acceptance). |
| Odom 2005 | No useable data. Email from author confirmed results not published, supervisor left |
| Penn 2000 | Allocation: quasi‐randomised (alternate allocation). |
| Petersen 2006 | Allocation: randomised. Participants: first‐episode schizophrenia spectrum disorder; not all patients had substance use or dependence (146 vs 401 patients). Interventioin: enriched assertive community treatment vs standard care. |
| Petrakis 2005 | Allocation: randomised. Participants: patients with an Axis I psychiatric disorder and comorbid alcohol dependence. Interventions: naltrexone versus disulphiram; not psychosocial. |
| Ries 2004 | Allocation: randomised. This single‐centre, two‐armed, community‐based RCT was conduced in the USA. Participants: 41 people with severe mental illness Intervention: 1) Contingency Management of supplementary social security income or food voucher with a motivational message, or 2) treatment as usual. Outcome: data were not reported per group for loss to treatment and no means, SD or sample sizes were reported for number of weeks of substance use. The trial was excluded due to a lack of usable data. |
| Rosenheck 1998 | Allocation: randomised. Participants: high users of inpatient services. Interventions: intensive psychiatric community care; no specific substance misuse treatment programme. |
| Rowe 2007 | Allocation: randomised. Participants: only 38% had a psychotic disorder and 30% did not a dual diagnosis. |
| Sacks 2004 | Allocation: randomised. Participants: only 63% of sample had a serious mental illness and no data provided separately for this group. |
| Sacks 2008 | Allocation: randomised. Participants: majority of participants were diagnosed with major depression (65%) with other co‐morbidities (PTSD or anxiety) and history of alcohol or substance use. 27% had bipolar disorder and no mention of other psychotic disorders. |
| Sacks 2011 | Allocation: randomised. Participants: diagnosed with HIV/AIDS and co‐occurring mental and SUD.High percentage of homelessness, incarceration and IV drug use. Intervention: aftercare focused to improve health status focusing on AIDS issues and adhere to AIDS medication. Not a psychosocial intervention to reduce substance use. |
| Santa Ana 2007 | Allocation: not a randomised trial, based on consecutive admissions. |
| Schmitz 2002 | Allocation: randomised. Participants: bipolar disorder exclusively; excluded due to none having a diagnosis of schizophrenia. |
| Shorey 2015 | Allocation: randomised. Participants: veterans with a primary alcohol use disorder, the majority did not have a psychotic disorder. |
| Sigmon 2000 | Allocation: not randomised. |
| Sitharthan 1999 | Participants: study was discontinued, no participants were recruited. |
| Smeerdijk 2012 | Allocation: randomised. Participants: carers of people with a dual diagnosis. Interventioin: MI for family carers, no direct contact with patients. |
| Smelson 2007 | Allocation: randomised. Intervention: designed to facilitate outpatient engagement, not psychosocial intervention to reduce substance use. |
| Somers 2015 | Allocation: randomised. Participants: homeless mentally ill adults, not all had a SUD, Intervention: housing support was provided based on need, intervention was not SUD focused. |
| Stain 2016 | Allocation: randomised, single‐blind trial Participants: young people with ultra‐high risk of developing psychosis; not all patients had a current or past drug or alcohol use problem. |
| Steadman 2005 | Allocation: not randomised. |
| Swanson 2000 | Allocation: randomised. Participants: people with psychotic or major mood disorders and history of hospital recidivism (50% substance misuse). Interventions: involuntary out‐patient commitment. No specific substance misuse treatment. |
| Tantirangsee 2015 | Allocation: randomised. Participants: not all participants had a drug or alcohol substance use disorder. Intervention: to reduce tobacco smoking. |
| Teague 1995 | Allocation: not a randomised trial. |
| Thornicroft 2007 | Allocation: randomised. Participants: case managers of people with a dual diagnosis. Intervention: did not include direct patient contact. |
| Timko 2004 | Allocation: quasi‐randomised (not all patients randomly allocated to community treatment were placed there). |
| Tyrer 2011 | Allocation: randomised. Participants: people with comorbid substance misuse and psychosis. Interventions: nidotherapy is not a specific SUD focused intervention; nidotherapy was developed mainly for people with personality disorders. |
| Weiss 2000 | Allocation: not randomised (sequential allocation). |
| Weiss 2007 | Allocation: randomised. Participants: bipolar disorder exclusively; excluded due to none having a diagnosis of schizophrenia. |
| Weiss 2009 | Allocation: randomised. Participants: bipolar disorder exclusively; excluded due to none having a diagnosis of schizophrenia. |
| Woolderink 2015 | Intervention: evaluation of E‐mental health intervention for children of parents with a mental illness unrelated to SUD |
AUDIT: Alcohol Use Disorders Identification Test; CBT: cognitive behavioural therapy; IV: intravenous; DSM‐IV: Diagnostic & Statistical Manual of Mental Disorders 4th edition; MI: motivational interviewing; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; SD: standard deviation; SUD: substance use disorder; TAU: treatment as usual.
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12616000275460.
| Methods | Allocation: randomised Location Nedlands, Western Australia |
| Participants | Inpatients with both a psychiatric disorder and substance use disorder |
| Interventions | Brief Infromation Pack (BIP) intervention vs. Engagement Assistant Referral Therapy (EART) |
| Outcomes | Change in mental state as assessed by the BSI. |
| Notes | 2016, not yet recruiting |
Gurevich 2015.
| Methods | Allocation: randomised. Location Moscow |
| Participants | Comorbid schizophrenia and alcohol dependence, N = 154 |
| Interventions | Psychotheraeutic program, no further details in Abstract |
| Outcomes | Reduced craving for alcohol |
| Notes | Report in Russian, requires translation into English language |
Meister 2010.
| Methods | German dissertation. Requires translation. Allocation: randomised. Design: cluster‐RCT. |
| Participants | Young people with first episode psychosis and substance misuse. N = 65. |
| Interventions | 1. Group‐based motivational behavioural therapy (MOVE); N = 36, 9 clusters. 2. TAU: Supportive treatment for addiction recovery (STAR); N = 29, 7 clusters. |
| Outcomes | Days of alcohol and substance use, quality of life and client satisfaction. |
| Notes | To be assessed, PhD dissertation needs translation from German to English. Supervisor (M Lambert) sent e‐mail, no reply. |
NCT01883791.
| Methods | Allocation: randomised Location: UCLA, Los Angeles, CA |
| Participants | Psychotic disorder with any past use of cannabis or stimulants and at least one heavy drinking day in the past year, n = 188 out of 715 patients with a mental health |
| Interventions | Screening, brief intervention and referral to treatment (SBIRT) |
| Outcomes | Reductions in substance use, ASSIST, AUDIT, DAST and increased of quality of life |
| Notes | Emailed contact person, Mitchell Karno. |
NCT02214667.
| Methods | Allocation: randomised. Location: University of North Carolina, Chapel Hill and Boston University |
| Participants | Jail detainees with drug or alcohol abuse/dependence and a serious mental illness |
| Interventions | Dual‐Diagnosis Motivational interviewing (DDMI) and integrated group therapy (IGT) vs TAU. |
| Outcomes | Substance use, mental state with 6‐month follow‐up |
| Notes | 2017, not yet recruiting. Contact person: RA van Dorn |
NCT02319746.
| Methods | Allocation: randomised location: Madrid, Spain |
| Participants | People with first episode psychosis and regular cannabis user |
| Interventions | CBT for cannabis cessation plus pharmacological treatment |
| Outcomes | Cannabis cessation, Mental state (PANSS, HDRS, YMRS, HAM‐A), psychosocial functioning (FAST) |
| Notes | Contact person: Gonzalez‐Ortega |
NCT02670902.
| Methods | Allocation: randomised Location: New York University |
| Participants | Individuals with co‐occurring substance use and mental health problems leaving residential treatment settings |
| Interventions | Critical time intervention‐residential (CTI‐R) vs usual discharge services‐residential |
| Outcomes | Completed treatment, Satisfaction, relapsed, substance use (ASI) |
| Notes | Contact person: J. Manuel |
NCT03007940.
| Methods | Allocation: cluster‐randomised (wait list) control group design Location: Darmouth college, Madison Wisconsin |
| Participants | People with co‐occurring substance use and mental health disorders |
| Interventions | Integrated service |
| Outcomes | Change in dual diagnosis capability in addiction treatment, change in ASI |
| Notes | No contact person listed. Start date 2015‐ 2019 |
AUDIT: Alcohol Use Disorders Identification Test; BSI: Brief Symptom Inventory;; CBT: cognitive behavioural therapy; HAM‐D:Hamilton Rating Scale for Depression; PANSS: Positive and Negative Syndrome Scale; RCT: randomised controlled trial; TAU: treatment as usual
Characteristics of ongoing studies [ordered by study ID]
Bennett 2007.
| Trial name or title | Alcohol use disorders in schizophrenia, NCT00280813. |
| Methods | Allocation: randomised. Location: Maryland, USA. |
| Participants | Expected total enrolment, n = 62. |
| Interventions | Behavioural treatment (CBT+) for alcohol abuse in schizophrenia (BTAAS). |
| Outcomes | Symptom ratings addiction severity, quality of life |
| Starting date | 2007 |
| Contact information | Melanie Bennett, Mbennett@psych.umaryland.edu |
| Notes | Sent e‐mail to authors: confirmed trial has been completed, 2013; no reply from recent email June 25 2018. |
CIRCLE trial.
| Trial name or title | Contingency intervention for reduction of cannabis in early psychosis. |
| Methods | Allocation: randomised. Design: single‐centre, community. Location: London, UK. |
| Participants | First episode psychosis and problematic cannabis use. Expected: N = 68. |
| Interventions | Contingency management versus TAU. |
| Outcomes | Relapse |
| Starting date | 28/11/2011 to 2016. Study completed. |
| Contact information | Dr Meghan Craig, Prof Sonia Johnson and Luke Sheridan Rains University College London |
| Notes | Sent e‐mail to Dr Luke Sheridan Rains (June 2018), reports the study is currently under peer review |
NCT00783185.
| Trial name or title | Dual diagnosis ‐ comparison of specialized treatment versus unspecified treatment. |
| Methods | Allocation: randomised. location: University of Konstanz, Germany. |
| Participants | Diagnoses: schizophrenia and diagnoses with psychotic features; and misuse of cannabis during 12 months preceding hospital admission. Estimated N = 50. |
| Interventions | Group educational programme to reduce consumption of cannabis. |
| Outcomes | Mental health (PANSS) and cannabis consumption and detection (urine samples) |
| Starting date | Jan 2006; estimated completion dated Jan 2010. |
| Contact information | Hans Watzl |
| Notes | E‐mail sent to author, no reply. Email sent 2018 bounced. No publication found. |
NCT00798109.
| Trial name or title | Effect of motivational therapy on schizophrenia with cannabis misuse (SCHIZOCAN). |
| Methods | Allocation: randomised. location: Paris, France. |
| Participants | Diagnosis: schizophrenia and cannabis abuse/dependence. Estimated n = 330. |
| Interventions | Motivational therapy (MI) vs TAU. |
| Outcomes | Non‐specified. |
| Starting date | Nov 2008; estimated completion date Sept 2011. |
| Contact information | Caroline Dubertret |
| Notes | E‐mailed authors, no reply 2013 or June 2018 |
Verstappen 2007.
| Trial name or title | NTR1083. Effects and indicators of CBT for cannabis use in psychosis. |
| Methods | Allocation: randomised. Location: Maastricht, the Netherlands. |
| Participants | Target number of participants, n = 48. |
| Interventions | MI+CBT versus TAU. |
| Outcomes | Cannabis use and psychotic symptoms |
| Starting date | 2007‐2011 |
| Contact information | Cecile Henequet |
| Notes | E‐mail sent, no reply from author. 2018; no email address available. No publication found. |
CBT: cognitive behavioural therapy; MI: motivational interviewing; PANSS: Positive and Negative Syndrome Scale; TAU: treatment as usual
Differences between protocol and review
1. In 2013, we have added contingency management to the list of interventions as there were no studies with usable data until then. The authors feel that this is an area of research of fundamental value that merits inclusion as indicated by several ongoing trials using this intervention, and should not be overlooked.
2. The cochrane Schizophrenia group currently excludes studies that do not involve any participants with schizophrenia in reviews, and those studies that solely recruit patients with major depressive disorder or bipolar disorder are no longer included. Consequently, search terms for depression and bipolar disorder have been dropped. We removed two studies from this 2013 update which were included in prior review updates as they did not conform to this new guideline.
3. Where the protocol did not define the primary outcome of interest, this update has specified primary and secondary outcomes ‐ this decision was not influenced by examination of the results to date.
4. The addition of 'Summary of findings' tables using GRADE criteria were included in this update and 2013 to conform to the new format of Cochrane Reviews.
5. The Cochrane Schizophrenia group maintains a method template. We have used the latest version of this template and updated our methods section and naming of outcomes. This does not involve changes to the methodology but minor revisions such as clarification of outcome presentation and updating of references. Previous method templates can be found in Appendices.
Contributions of authors
Current update (2018)
Glenn E. Hunt ‐ searching, trial selection, contacting authors, data extraction and entry, 'Risk of bias' assessment, review writing. Corresponding author.
Nandi Siegfried ‐ protocol production, advice, 'Risk of bias' assessment, formulating and construction of GRADE 'Summary of findings' tables, review writing.
Kirsten Morley ‐ trial selection, data extraction and entry, review writing.
Carrie Brooke‐Sumner ‐ data extraction and 'Risk of bias' table entry.
Michelle Cleary ‐ advice, trial selection, data extraction, review writing.
Previous update of review (2013)
Glenn E. Hunt ‐ grant writing, searching, trial selection, contacting authors, data extraction and entry, review writing. Corresponding author.
Nandi Siegfried ‐ protocol production, grant writing, advice, trial selection, formulating and construction of GRADE 'Summary of findings' tables, review writing.
Kirsten Morley ‐ trial selection, data extraction and entry, review writing.
Raj Sitharthan‐ advice, review writing.
Michelle Cleary ‐ grant writing, advice, trial selection, data extraction, review writing.
Previous update of review (2008)
Michelle Cleary ‐ grant writing, searching, trial selection, data extraction and entry, review writing.
Glenn Hunt ‐ grant writing, searching, trial selection, data extraction and entry, review writing.
Sandra Matheson ‐ searching, citation ordering, data extraction and entry, review writing.
Nandi Siegfried ‐ protocol production, advice, review writing.
Garry Walter ‐ grant writing, review writing.
Initial draft of this review (2004)
Ann Ley ‐ protocol production, searching, citation ordering, data extraction and entry, review writing.
David Jeffery ‐ grant writing, protocol production, trial selection, data extraction, review writing. Corresponding author.
Stuart McLaren ‐ grant writing, protocol production, trial selection and advice.
Nandi Siegfried ‐ protocol production, trial selection and advice.
Sources of support
Internal sources
-
Sydney South West Area Mental Health Service, NSW, Australia.
2008
-
Sydney Local Health District, NSW, Australia.
2013, 2018
-
South African Medical Research Council intramural career development award, South Africa.
2018
External sources
-
McGeorge Bequest, University of Sydney, NSW, Australia.
2007
-
NIHR Cochrane Incentive Scheme, UK.
2012
Declarations of interest
None. The authors are employed as follows.
Glenn E Hunt, BA, MSc, PhD Principal Research Fellow/Associate Professor, Discipline of Psychiatry and Addiction Medicine, University of Sydney and Research Unit, Concord Centre for Mental Health, Sydney Local Health District, Hospital Road, Concord, NSW, 2139, Australia.
Nandi Siegfried, MBChB, MPH (Hons), DPhil (Oxon), FCPHM Honorary Associate Professor, Department of Psychiatry and Mental Health, University of Cape Town, Groote Schuur Hospital, Cape Town, South Africa, and Chief Specialist Scientist, Alcohol, tobacco and other Drug Research Unit, South African Medical Research Council, Francie Van Zijl Drive, Parow Valley, South Africa.
Kirsten Morley, BPsych (Hons), PhD NSW Health EMC Fellow/Associate Professor, NHMRC Center of Research Excellence in mental health and substance use, Addiction Medicine, University of Sydney, Camperdown, NSW, 2006, Australia
Carrie Brooke‐Sumner, BSc(Hons), MSc, PhD Post‐Doctoral Fellow, Alcohol, tobacco and other Drug Research Unit, South African Medical Research Council, Francie Van Zijl Drive, Parow Valley, South Africa.
Michelle Cleary, RN, BHlthSc (Nurs), MHlth Sc (Nurs), PhD Professor, School of Nursing, College of Health and Medicine, University of Tasmania, Lilyfield, NSW, 2040, Australia.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Baker 2002 {published data only}
- Baker A, Lewin T, Reichler H, Clancy R, Carr V, Garrett R, et al. Evaluation of a motivational interview for substance use within psychiatric inpatient services. Addiction 2002;97(10):1329‐38. [CSzG: 8308] [DOI] [PubMed] [Google Scholar]
- Baker A, Lewin T, Reichler H, Clancy R, Carr V, Garrett R, et al. Motivational interviewing among psychiatric inpatients with substance use disorders. Acta Psychiatrica Scandinavica 2002;106(3):233‐40. [CSzG: 16812] [DOI] [PubMed] [Google Scholar]
Baker 2006 {published data only}
- Baker A, Bucci S, Kay‐Lambkin F. Intervention for alcohol, cannabis and amphetamine use among people with a psychotic illness. NDARC Technical Report No. 193. Sydney: National Drug and Alcohol Research Centre, 2004:1‐100. [CSzG: 16861; ISBN:1 887027 84 7] [Google Scholar]
- Baker A, Bucci S, Lewin TJ, Kay‐Lambkin F, Constable PM, Carr VJ. Cognitive‐behavioural therapy for substance use disorders in people with psychotic disorders ‐ Randomised controlled trial. British Journal of Psychiatry 2006;188(5):439‐48. [CSzG: 13856] [DOI] [PubMed] [Google Scholar]
- Baker A, Bucci S, Lewin TJ, Richmond R, Carr VJ. Comparisons between psychosis samples with different patterns of substance use recruited for clinical and epidemiological studies. Psychiatry Research 2005;134(3):241‐50. [CSzG: 16810] [DOI] [PubMed] [Google Scholar]
Barrowclough 2001 {published data only}
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O'Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy and family intervention for patients with comorbid schizophrenia and substance use disorders. American Journal of Psychiatry 2001;158(10):1706‐13. [CSzG: 7863] [DOI] [PubMed] [Google Scholar]
- Haddock G. Dual diagnosis project: evaluation of family support and cognitive behaviour therapy for recent onset schizophrenia sufferers with substance misuse problems. National Research Register 2001; Vol. 1. [CSzG: 5489; N0244032344]
- Haddock G, Barrowclough C, Tarrier N, Moring J, O'Brien R, Schofield N, et al. Cognitive‐behavioural therapy and motivational intervention for schizophrenia and substance misuse: 18‐month outcomes of a randomised controlled trial. British Journal of Psychiatry 2003;183(November):418‐26. [CSzG: 11201] [DOI] [PubMed] [Google Scholar]
Barrowclough 2010 {published data only}
- Barrowclough C. A pilot study to assess the impact of motivational intervention on schizophrenia patients with associated substance misuse. National Research Register 2004; Vol. 3:1‐4. [CSzG: 12949]
- Barrowclough C. A randomized controlled trial of integrated motivational interviewing and cognitive behavior therapy (mi‐cbt) for people with a schizophrenia diagnosis and substance misuse ‐ the MIDAS trial. Schizophrenia Bulletin 2011;37 Suppl 1:295. [CSzG: 22830] [Google Scholar]
- Barrowclough C. An evaluation of motivational (MI) plus cognitive therapy (CBT) for schizophrenia and substance misuse. http://www.controlled‐trials.com 2003. [CSzG: 14484]
- Barrowclough C. An evaluation of motivational interventions (MI) plus cognitive behaviour therapy (CBT) for schizophrenia and substance misuse ‐ MIDAS. National Research Register 2004; Vol. 1. [CSzG: 12947]
- Barrowclough C, Eisner E, Bucci S, Emsley R, Wykes T. The impact of alcohol on clinical outcomes in established psychosis: a longitudinal study. Addiction 2014;109(8):1297‐305. [DOI] [PubMed] [Google Scholar]
- Barrowclough C, Emsley R, Eisner E, Beardmore R, Wykes T. Does change in cannabis use in established psychosis affect clinical outcome?. Schizophrenia Bulletin 2013;39(2):339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowclough C, Haddock G, Beardmore R, Conrod P, Craig T, Davies L, et al. Evaluating integrated MI and CBT for people with psychosis and substance misuse: recruitment, retention and sample characteristics of the MIDAS trial. Addictive Behaviors 2009;34(10):859‐66. [CSzG: 18777] [DOI] [PubMed] [Google Scholar]
- Barrowclough C, Haddock G, Fitzsimmons M, Johnson R. Treatment development for psychosis and co‐occurring substance misuse: a descriptive review. Journal of Mental Health 2006;15(6):619‐32. [CSzG: 16834] [Google Scholar]
- Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ 2010;341(7784):6325. [CSzG: 21934; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowclough C, Meier P, Beardmore R, Emsley R. Predicting therapeutic alliance in clients with psychosis and substance misuse. Journal of Nervous and Mental Disease 2010;198(5):373‐7. [CSzG: 20696] [DOI] [PubMed] [Google Scholar]
- Berry K, Gregg L, Hartwell R, Haddock G, Fitzsimmons M, Barrowclough C. Therapist‐client relationships in a psychological therapy trial for psychosis and substance misuse. Drug and Alcohol Dependence 2015;152:170‐6. [CSzG: 29829] [DOI] [PubMed] [Google Scholar]
- Haddock G, Beardmore R, Earnshaw P, Fitzsimmons M, Nothard S, Butler R, et al. Assessing fidelity to integrated motivational interviewing and CBT therapy for psychosis and substance use: the MI‐CBT fidelity scale (MI‐CTS). Journal of Mental Health 2012;21(1):38‐48 2012;21(1):38‐48. [DOI] [PubMed] [Google Scholar]
- Hartley S, Haddock G, Barrowclough C. Anxiety and depression and their links with delusions and hallucinations in people with a dual diagnosis of psychosis and substance misuse: A study using data from a randomised controlled trial. Behaviour Research and Therapy 2012;50(1):65‐71. [CSzG: 23617] [DOI] [PubMed] [Google Scholar]
- Johnson R. MIDAS ‐ mental health clinical trials ‐ information for service users and carers. http://www.controlled‐trials.com/ 2005. [CSzG: 12916]
- Shabaruddin FH, Davies LM. Cost of delivering psychiatric inpatient care to mental health patients with psychosis and co‐occuring substance use: A UK based study. Value in Health 2011;14:A291. [CSzG: 23430] [Google Scholar]
Barrowclough 2014 {published data only}
- Barrowclough C, Lobbanl F, Warburton J, Choudhry I, Gregg L, Wood H, et al. Helper recap: Rethinking choices after psychosis ‐ a phase specific psychological therapy for people with problematic cannabis use following a first episode of psychosis. Early Intervention in Psychiatry 2010;4 Suppl 1:161. [CSzG: 23469] [Google Scholar]
- Barrowclough C, Marshall M, Gregg L, Fitzsimmons M, Tomenson B, Warburton J, et al. A phase‐specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: a randomized controlled trial. Psychological Medicine 2014;44(13):2749‐61. [CSzG: 29048] [DOI] [PubMed] [Google Scholar]
- Berry K, Gregg L, Lobban F, Barrowclough C. Therapeutic alliance in psychological therapy for people with recent onset psychosis who use cannabis. Comprehensive Psychiatry 2016;67:73‐80. [CSzG: 33604] [DOI] [PubMed] [Google Scholar]
- Berry K, Palmer T, Gregg L, Barrowclough C, Lobban F. Attachment and therapeutic alliance in psychological therapy for people with recent onset psychosis who use cannabis. Clinical Psychology & Psychotherapy 2018;25(3):440‐5. [CSzG: 37966] [DOI] [PubMed] [Google Scholar]
- Worrell L. Helper programme (substance misuse)‐ a phase‐specific psychological therapy for people with problematic cannabis use following a first episode of psychosis (recap). http://public.ukcrn.org.uk 2011. [CSzG: 23027]
Bechdolf 2011 {published data only}
- Bechdolf A, Pohlmann B, Guttgemanns J, Geyer C, Lindner K, Ferber C, et al. State‐dependent motivational interviewing for people with schizophrenia and substance use : Results of a randomised controlled trial [Motivationsbehandlung für patienten mit der doppeldiagnose psychose und sucht]. Nervenarzt 2011;83(7):888‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellack 2006 {published data only}
- Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental iIlness. Archives of General Psychiatry 2006;63(4):426‐32. [CSzG: 12628] [DOI] [PubMed] [Google Scholar]
- Bennett M, Bellack A, Dixon L, Brown C. The effects of a group based behavioral approach to substance misuse and schizophrenia. Schizophrenia Bulletin 2011;1:296. [CSzG: 22833] [Google Scholar]
- Brown CH, Bennett ME, Li L, Bellack AS. Predictors of initiation and engagement in substance abuse treatment among individuals with co‐occurring serious mental illness and substance use disorders. Addictive Behaviors 2011;36(5):439‐47. [CSzG: 22844] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00295139. Behavioral treatment of drug abuse in SPMI patients. http://www.clinicaltrials.gov 2006. [CSzG: 14598]
Bogenschutz 2014 {published data only}
- Bogenschutz MP, Rice SL, Tonigan JS, Vogel HS, Nowinski J, Hume D, et al. 12‐step facilitation for the dually diagnosed: a randomized clinical trial. Journal of Substance Abuse Treatment 2014;46(4):403‐11. [CSzG: 29165; NCT00583440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 1991a {published and unpublished data}
- Bond GR, McDonel EC, Miller LD, Pensec M. Assertive community treatment and reference groups: an evaluation of their effectiveness for young adults with serious mental illness and substance abuse problems. Psychosocial Rehabilitation Journal 1991;15(2):31‐43. [CSzG: 16926] [Google Scholar]
Bond 1991b {published and unpublished data}
- Bond GR, McDonel EC, Miller LD, Pensec M. Assertive community treatment and reference groups: an evaluation of their effectiveness for young adults with serious mental illness and substance abuse problems. Psychosocial Rehabilitation Journal 1991;15(2):31‐43. [CSzG: 16926] [Google Scholar]
Bonsack 2011 {published and unpublished data}
- Bonsack C. Motivational intervention to reduce cannabis use in young people with psychosis: A randomized controlled trial. Early Intervention in Psychiatry 2010;4 Suppl 1:159. [CSzG: 23471] [DOI] [PubMed] [Google Scholar]
- Bonsack C, Manetti SG, Favrod J, Montagrin Y, Besson J, Bovet P, et al. Motivational intervention to reduce cannabis use in young people with psychosis: A randomized controlled trial. Psychotherapy and Psychosomatics 2011;80(5):287‐97. [CSzG: 23093] [DOI] [PubMed] [Google Scholar]
Burnam 1995 {published and unpublished data}
- Burnam MA, Morton SC, McGlynn EA, Peterson LP, Stecher BM, Hayes C, et al. An experimental evaluation of residential and nonresidential treatment for dually diagnosed homeless adults. Journal of Addictive Diseases 1995;14(4):111‐34. [CSzG: 8751] [DOI] [PubMed] [Google Scholar]
- McGlynn EA, Boynton J, Morton SC, Stecher BM, Hayes C, Vaccaro JV, et al. Treatment for the dually diagnosed homeless: program models and implementation experience: Los Angeles. Alcohol Treatment Quarterly 1993;10(3‐4):171‐86. [CSzG: 4118] [Google Scholar]
Chandler 2006 {published data only}
- Chandler D, Spicer G. Integrated treatment for jail recidivists with co‐occurring psychiatric and substance use disorders. Community Mental Health Journal 2006;42(4):405‐25. [CSzG: 16816] [DOI] [PubMed] [Google Scholar]
- Drake RE, Morrissey JP, Mueser KT. The challenge of treating forensic dual diagnosis clients: comment on "Integrated treatment for jail recidivists with co‐occurring psychiatric and substance use disorders". Community Mental Health Journal 2006;42(4):427‐32. [CSzG: 16818] [DOI] [PubMed] [Google Scholar]
Drake 1998a {published and unpublished data}
- Clark RE, Teague GB, Ricketts SK, Bush PW, Xie H, McGuire TG, et al. Cost‐effectiveness of assertive community treatment versus standard case management for persons with co‐occurring severe mental illness and substance use disorders. Health Services Research 1998;33(5):1285‐8. [CSzG: 5636] [PMC free article] [PubMed] [Google Scholar]
- Clarke RE. Family support and substance use outcomes for persons with mental illness and substance use. Schizophrenia Bulletin 2001;27(1):93‐101. [CSzG: 5884] [DOI] [PubMed] [Google Scholar]
- Drake RE. Ten year follow up of dual diagnosis treatment. CRISP database accessed 10/05/02 2002.
- Drake RE, McHugo GJ, Clark RE, Teague GB, Xie H, Miles K. Assertive community treatment for patients with co‐occurring severe mental illness and substance use disorder: a clinical trial. American Journal of Orthopsychiatry 1998;68(2):201‐15. [CSzG: 4974] [DOI] [PubMed] [Google Scholar]
- McHugo GJ, Drake RE, Teague GB, Xie H. Fidelity to assertive community treatment and client outcomes in the New Hampshire dual disorders study. Psychiatric Services 1999;50(6):818‐24. [CSzG: 5155] [DOI] [PubMed] [Google Scholar]
- Xie H, Drake R, McHugo G. Are there distinctive trajectory groups in substance abuse remission over 10 years? An application of the group‐based modeling approach. Administration and Policy in Mental health and Mental Health Services 2006;33(4):423‐32. [CSzG: 17219] [DOI] [PubMed] [Google Scholar]
- Xie H, McHugo GJ, Fox MB, Drake RE. Substance abuse relapse in a ten‐year prospective follow‐up of clients with mental health and substance use disorders. Psychiatric Services 2005;56(10):1282‐7. [CSzG: 16831] [DOI] [PubMed] [Google Scholar]
Eack 2015 {published and unpublished data}
- Eack SM, Hogarty SS, Bangalore SS, Keshavan MS, Cornelius JR. Patterns of substance use during Cognitive Enhancement Therapy: an 18‐month randomized feasibility study. Journal of Dual Diagnosis 2016;12:74‐82. [CSzG: 33634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty SS, Greenwald DP, Litschge MY, McKnight SA, Bangalore SS, et al. Cognitive Enhancement Therapy in substance misusing schizophrenia: Results of an 18‐month feasibility trial. Schizophrenia Research 2015;161(2‐3):478‐83. [CSzG: 29308] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty SS, Greenwald DP, Litschge MY, McKnight SA, Bangalore SS, et al. Cognitive Enhancement Therapy in substance misusing schizophrenia: results of an 18‐month feasibility trial. Schizophrenia Bulletin 2015;41:S310. [CSzG: 29436] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtalik JA, Eack SM. Fronto‐limbic neuroplasticity effects of Cognitive Enhancement Therapy during emotion regulation in schizophrenia patients who abuse cannabis. Schizophrenia Bulletin 2015;41:S243. [CSzG: 29719] [Google Scholar]
- Wojtalik JA, Hogarty SS, Cornelius JR, Phillips ML, Keshavan MS, Newhill CE, et al. Cognitive Enhancement Therapy improves frontolimbic regulation of emotion in alcohol and/or cannabis misusing schizophrenia: a preliminary study. Frontiers in Psychiatry 2016;6:186. [CSzG: 33452] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2006 {published data only}
- Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, et al. Randomized controlled trial of a cannabis‐focused intervention for young people with first‐episode psychosis. Acta Psychiatrica Scandinavica 2006;114(2):109‐17. [CSzG: 13211] [DOI] [PubMed] [Google Scholar]
Essock 2006 {published data only}
- Essock SM, Mueser KT, Drake RE, Covell NH, McHugo GJ, Frisman LK, et al. Comparison of ACT and and standard case management for delivering integrated treatment for co‐occurring disorders. Psychiatric Services 2006;57(2):185‐96. [CSzG: 12680] [DOI] [PubMed] [Google Scholar]
- Kanter J. Clinical case management, case management and ACT. Psychiatric Services 2006; Vol. 57, issue 4:578. [DOI] [PubMed]
- Manuel JI. A longitudinal analysis of psychiatric medication adherence and provider continuity among individuals with co‐occurring disorders. Dissertation Abstracts International Section A: Humanities and Social Sciences 2009;69(10A):4126. [Google Scholar]
- Manuel JI, Covell NH, Jackson CT, Essock SM. Does assertive community treatment increase medication adherence for people with co‐occurring psychotic and substance use disorders?. Journal of the American Psychiatric Nurses Association 2011;17(1):51‐6. [CSzG: 22855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Godley 1994 {published data only}
- Godley SH, Hoewing‐Roberson R, Godley MD. Final MISA Report. Bloomington, IN: Lighthouse Institute, 1994. [CSzG: 970] [Google Scholar]
Gouzoulis‐Mayfrank 2015 {published and unpublished data}
- Gouzoulis‐Mayfrank E, Konig S, Koebke S, Schness T, Schmitz‐Buhl M, Daumann J. Trans‐sector integrated treatment in psychosis and addiction. Deutsches Arzteblatt International 2015;112(41):683‐91. [CSzG: 33168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- U1111‐1119‐5851. Evaluation of an integrative therapy approach for the comorbidity of psychosis and addiction. http://www.drks.de/DRKS00000671 2011. [CSzG: 22748]
Graeber 2003 {published data only}
- Graeber DA, Moyers TB, Griffith G, Guajardo E, Tonigan S. A pilot study comparing motivational interviewing and an educational intervention in patients with schizophrenia and alcohol use disorders. Community Mental Health Journal 2003;39(3):189‐202. [CSzG: 9901] [DOI] [PubMed] [Google Scholar]
Graham 2016 {published and unpublished data}
- Graham HL, Birchwood M, Griffith E, Freemantle N, McCrone P, Stefanidou CA, et al. A pilot study to assess the feasibility and impact of a brief motivational intervention on problem drug and alcohol use in adult mental health inpatient units: study protocol for a randomized controlled trial. Trials 2014;15(1):308. [CSzG: 29050] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham HL, Copello A, Griffith E, Freemantle N, McCrone P, Clarke L, et al. Pilot randomised trial of a brief intervention for comorbid substance misuse in psychiatric in‐patient settings. Acta Psychiatrica Scandinavica 2016;133(4):298‐309. [CSzG: 33324] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN43548483. A pilot study to assess the feasibility and impact of a motivational intervention (MI) on problem drug and alcohol use in adult mental health inpatient units. http://isrctn.org/ISRCTN43548483 2014. [CSzG: 28909] [DOI] [PMC free article] [PubMed]
Hellerstein 1995 {published data only}
- Hellerstein DJ, Rosenthal RN, Miner CR. A prospective study of integrated outpatient treatment for substance‐abusing schizophrenic patients. American Journal on Addictions 1995;4(1):33‐42. [CSzG: 5029] [Google Scholar]
- Hellerstein DJ, Rosenthal RN, Miner CR. Integrating services for schizophrenia and substance abuse. Psychiatric Quarterly 2001;72(4):291‐306. [CSzG: 8206] [DOI] [PubMed] [Google Scholar]
Hickman 1997 {unpublished data only}
- Hickman ME. The effects of personal feedback on alcohol intake in dually diagnosed clients: an empirical study of William R. Miller's motivational enhancement therapy. Unpublished thesis. University Graduate School, Dept. Counseling Psychology, Indiana University 1997. [CSzG: 4153]
Hjorthoj 2013 {published and unpublished data}
- Fohlmann AH, Hjorthoej C, Larsen A, Nordentoft M. CapOpus. randomized clinical trial: Specialized addiction treatment (MI & CBT) versus treatment as usual for young patients with cannabis abuse and psychosis. Early Intervention in Psychiatry 2010;4 Suppl 1:159. [CSzG: 23478] [Google Scholar]
- Fohlmann AH, Hjorthoej C, Larsen A, Nordentoft M. CapOpus. randomized clinical trial: Specialized addiction treatment (MI & CBT) versus treatment as usual for young patients with cannabis abuse and psychosis. Early Intervention in Psychiatry 2010;4 Suppl 1:160. [CSzG: 23478] [Google Scholar]
- Hjorthaj CR. Validity of self‐reported cannabis use as measured by the timeline follow‐back instrument in a trial randomizing people with comorbid cannabis use disorder and schizophrenia spectrum disorder. Early Intervention in Psychiatry 2010;4 Suppl 1:160. [CSzG: 23483] [Google Scholar]
- Hjorthoj C, Fohlmann A, Larsen AM, Madsen MT, Vesterager L, Gluud C, et al. Design paper: the CapOpus trial: a randomized, parallel‐group, observer‐blinded clinical trial of specialized addiction treatment versus treatment as usual for young patients with cannabis abuse and psychosis. Trials 2008;9:42. [CSzG: 16594] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthoj CR, Fohlmann A, Larsen A‐M, Madsen MTR, Vesterager L, Gluud C, et al. Interim analysis of the CapOpus trial: a randomized, parallel‐group, observer‐blinded clinical trial of specialized addiction treatment versus treatment as usual for young patients with cannabis abuse and psychosis. Schizophrenia Research 2010;117(2‐3):190. [CSzG: 20563] [Google Scholar]
- Hjorthoj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M. Correlations and agreement between delta‐9‐tetrahydrocannabinol (THC) in blood plasma and timeline follow‐back (TLFB)‐assisted self‐reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clinical trial. Addiction 2011;107(6):1123‐31. [CSzG: 23547; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hjorthoj CR, Orlovska S, Fohlmann A, Nordentoft M. Psychiatric treatment following participation in the CapOpus randomized trial for patients with comorbid cannabis use disorder and psychosis. Schizophrenia Research 2013;151(1‐3):191‐6. [CSzG: 28381] [DOI] [PubMed] [Google Scholar]
- Hjorthoj CR, Vesterager L, Nordentoft M. Test‐retest reliability of the Danish adult reading test in patients with comorbid psychosis and cannabis‐use disorder. Nordic Journal of Psychiatry 2013;67(3):159‐63. [CSzG: 28766; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hjorthøj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychological Medicine 2013;43(7):1499‐510. [CSzG: 28754; PUBMED: 23040144] [DOI] [PubMed] [Google Scholar]
- NCT00484302. Cannabis and psychosis randomized clinical trial: specialized addiction treatment versus treatment as usual for young patients with cannabis abuse and psychosis. http://www.clinicaltrials.gov 2007. [CSzG: 15056] [DOI] [PMC free article] [PubMed]
- Nordentoft M, Hjorthoj C, Fohlmann A. Capopus trial: An observer‐blinded RCT of specialized addiction treatment versus standard treatment for young patients with cannabis. Proceedings of the 17th European Psychiatric Association, EPA Congress; 2009 Jan 24‐28; Lisbon Portugal 2009;24:S1178. [CSzG: 20894] [Google Scholar]
- Toftdahl NG, Nordentoft M, Hjorthoj C. The effect of changes in cannabis exposure on psychotic symptoms in patients with comorbid cannabis use disorder. Journal of Dual Diagnosis 2016;12(2):129‐36. [CSzG: 33821] [DOI] [PubMed] [Google Scholar]
Jerrell 1995a {unpublished data only}
- Jerrell JM. Cost‐effective treatment for persons with dual disorders. New Directions for Mental Health Services 1996;70(Summer):79‐91. [CSzG: 16821] [DOI] [PubMed] [Google Scholar]
- Jerrell JM, Hu TW, Ridgely MS. Cost‐effectiveness of substance disorder interventions for people with severe mental illness. Journal of Mental Health Administration 1994;21(3):283‐97. [CSzG: 1166] [DOI] [PubMed] [Google Scholar]
- Jerrell JM, Ridgely MS. Comparative effectiveness of three approaches to serving people with severe mental illness and substance abuse disorders. Journal of Nervous and Mental Disease 1995;183(9):566‐76. [CSzG: 3727] [DOI] [PubMed] [Google Scholar]
- Jerrell JM, Ridgely MS. Impact of robustness of program implementation on outcomes of clients in dual diagnosis programs. Psychiatric Services 1999;50(1):109‐12. [CSzG: 16917] [DOI] [PubMed] [Google Scholar]
Jerrell 1995b {unpublished data only}
- Jerrell JM, Ridgely MS. Comparative effectiveness of three approaches to serving people with severe mental illness and substance abuse disorders. Journal of Nervous and Mental Disease 1995;183(9):566‐76. [CSzG: 3727] [DOI] [PubMed] [Google Scholar]
Kavanagh 2004 {published data only}
- Kavanagh DJ, Young R, White A, Saunders JB, Wallis J, Shockley N, et al. A brief motivational intervention for substance misuse in recent‐onset psychosis. Drug and Alcohol Review 2004;23(2):151‐5. [CSzG: 8309] [DOI] [PubMed] [Google Scholar]
Kemp 2007 {published and unpublished data}
- Kemp R, Harris A, Vurel E, Sitharthan T. Stop using stuff: trial of a drug and alcohol intervention for young people with comorbid mental illness and drug and alcohol problems. Australasian Psychiatry 2007;15(6):490‐3. [CSzG: 15549; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lehman 1993 {published data only}
- Lehman AF, Herron JD, Schwartz RP, Myers CP. Rehabilitation for adults with severe mental illness and substance use disorders: a clinical trial. Journal of Nervous and Mental Disease 1993;181(2):86‐90. [CSzG: 1646] [DOI] [PubMed] [Google Scholar]
Madigan 2013 {published data only}
- Madigan K, Brennan D, Lawlor E, Turner N, Kinsella A, O'Connor JJ, et al. A multi‐center, randomized controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Schizophrenia Research 2013;143(1):138‐42. [CSzG: 25073] [DOI] [PubMed] [Google Scholar]
Maloney 2006 {unpublished data only}
- Maloney MP. Reducing criminal recidivism in jail‐incarcerated mothers with co‐occurring disorders. Manuscript kindly provided by Dr Maloney ‐ no further details given.
McDonell 2013 {published and unpublished data}
- Angelo FN, McDonell MG, Lewin MR, Srebnik D, Lowe J, Roll J, et al. Predictors of stimulant abuse treatment outcomes in severely mentally ill outpatients. Drug and Alcohol Dependence 2013;131(1‐2):162‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. American Journal of Psychiatry 2013;170(1):94‐101. [CSzG: 24857] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, McDonell MG, McPherson S, Srebnik D, Angelo F, Roll JM, et al. An economic evaluation of a contingency‐management intervention for stimulant use among community mental health patients with serious mental illness. Drug and Alcohol Dependence 2015;153:293‐9. [CSzG: 29903] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00809770. Contingency management of psychostimulant abuse in the severely mentally ill. ClinicalTrials.gov 2008. [CSzG: 17380]
- Weiss RD. Contingency management for patients with serious mental illness and stimulant dependence. American Journal of Psychiatry 2013;170(1):6‐8. [DOI] [PubMed] [Google Scholar]
McDonell 2017 {published and unpublished data}
- Lowe JM, McDonell MG, Leickly E, Angelo FA, Vilardaga R, McPherson S, et al. Determining ethyl glucuronide cutoffs when detecting self‐reported alcohol use in addiction treatment patients. Alcoholism: Clinical and Experimental Research 2015;39(5):905‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Leickly E, McPherson S, Skalisky J, Hirchak K, Oluwoye O, et al. Pretreatment ethyl glucuronide levels predict response to a contingency management intervention for alcohol use disorders among adults with serious mental illness. American Journal on Addictions 2017;26(7):673‐5. [CSzG: 36340] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Leickly E, McPherson S, Skalisky J, Srebnik D, Angelo F, et al. A randomized controlled trial of ethyl glucuronide‐ based contingency management for outpatients with co‐occurring alcohol use disorders and serious mental Illness. American Journal of Psychiatry 2017;174(4):370‐7. [CSzG: 35589] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Skalisky J, Leickly E, McPherson S, Battalio S, Nepom JR, et al. Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients. Drug and Alcohol Dependence 2015;157:184‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01567943. Contingency management of alcohol abuse in the severely mentally ill. http://ClinicalTrials.gov/show/NCT01567943 2012. [CSzG: 23792]
- Oluwoye O, Leickly E, Skalisky J, McPherson S, Hirchak K, Srebnik D, et al. Serious mental illness in heavy drinkers is associated with poor treatment outcomes in outpatients with co‐occurring disorders. International Journal of Mental Health Addiction 2018;16(3):672‐9. [CSzG: 37902] [PMC free article] [PubMed] [Google Scholar]
- Skalisky J, Leickly E, Oluwoye O, McPherson SM, Srebnik D, Roll JM, et al. Prevalence and correlates of cannabis use in outpatients with serious mental illness receiving treatment for alcohol use disorders. Cannabis and Cannabinoid Research 2017;2(1):133‐8. [CSzG: 36516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morse 2006 {published data only}
- Calsyn RJ, Yonker RD, Lemming MR, Morse GA, Klinkenberg WD. Impact of assertive community treatment and client characteristics on criminal justice outcomes in dual disorder homeless individuals. Criminal Behaviour and Mental Health 2005;15(4):236‐48. [CSzG: 16813] [DOI] [PubMed] [Google Scholar]
- Fletcher TD, Cunningham JL, Calsyn RJ, Morse GA, Klinkenberg WD. Evaluation of treatment programs for dual disorder individuals: modeling longitudinal and mediation effects. Administration and Policy in Mental Health 2008;35(4):319‐36. [CSzG: 16624; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morse GA, Calsyn RJ, Klinkenberg WD, Cunningham J, Lemming MR. Integrated treatment for homeless clients with dual disorders: a quasi‐experimental evaluation. Journal of Dual Diagnosis 2008;4(3):219‐37. [CSzG: 19618] [Google Scholar]
- Morse GA, Calsyn RJ, Klinkenberg WD, Helminiak TW, Wolff N, Drake RE, et al. Treating homeless clients with severe mental illness and substance use disorders: costs and outcomes. Community Mental Health Journal 2006;42(4):377‐404. [CSzG: 13863] [DOI] [PubMed] [Google Scholar]
Naeem 2005 {published data only}
- Naeem F, Kingdon D, Turkington D. Cognitive behaviour therapy for schizophrenia in patients with mild to moderate substance misuse problems. Cognitive Behaviour Therapy 2005;34(4):207‐15. [CSzG: 12597] [DOI] [PubMed] [Google Scholar]
- Turkington D, Kingdon D, Turner T. Effectiveness of a brief cognitive‐behavioural therapy intervention in the treatment of schizophrenia. British Journal of Psychiatry 2002;180:523‐7. [CSzG: 8740] [DOI] [PubMed] [Google Scholar]
Nagel 2009 {published data only}
- Nagel T, Robinson G, Condon J, Trauer T. Approach to treatment of mental illness and substance dependence in remote indigenous communities: results of a mixed methods study. Australian Journal of Rural Health 2009;17(4):174‐82. [CSzG: 18782; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Connell 2018 {published and unpublished data}
- O'Connell MJ, Flanagan EH, Delphin‐Rittmon ME, Davidson L. Enhancing outcomes for persons with co‐occurring disorders through skills training and peer recovery support. Journal of Mental Health 2018;in press:(online 10 March 2017). [CSzG: 35896; DOI: 10.1080/09638237.2017.1294733] [DOI] [PubMed] [Google Scholar]
Petry 2013 {published data only}
- NCT01478815. Contingency management for persons with severe mental illness. http://ClinicalTrials.gov/show/NCT01478815 2011. [CSzG: 23332]
- Petry NM, Alessi SM, Rash Carla J. A randomized study of contingency management in cocaine‐dependent patients with severe and persistent mental health disorders. Drug and Alcohol Dependence 2013;130:234‐7. [CSzG: 28275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenblum 2014 {published data only}
- Rosenblum A, Matusow H, Fong C, Vogel H, Uttaro T, Moore TL, et al. Efficacy of dual focus mutual aid for persons with mental illness and substance misuse. Drug and Alcohol Dependence 2014;135:78‐87. [CSzG: 27823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swanson 1999 {published data only}
- Pantalon MV, Swanson AJ. Use of the University of Rhode Island Change Assessment to measure motivational readiness to change in psychiatric and dually diagnosed individuals. Psychology of Addictive Behaviors 2003;17(2):91‐7. [CSzG: 9830] [DOI] [PubMed] [Google Scholar]
- Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. Journal of Nervous and Mental Disease 1999;187(10):630‐5. [CSzG: 9248] [DOI] [PubMed] [Google Scholar]
Tracy 2007 {published data only}
- Tracy K, Babuscio T, Nich C, Kiluk B, Carroll KM, Petry NM, et al. Contingency management to reduce substance use in individuals who are homeless with co‐occurring psychiatric disorders. American Journal of Drug and Alcohol Abuse 2007;33(2):253‐8. [CSzG: 14807; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Audier 2011 {published data only}
- Audier C, Mulder CL, Staring A, Hoorn BE, Hakkaart‐vanRoijen L, Blanken P. Money for medication: A randomized controlled study on the effectiveness of financial incentives to improve medication adherence in patients with a psychotic disorder and co‐morbid substance abuse. Schizophrenia Bulletin 2011;1:294‐5. [Google Scholar]
Bachmann 1997 {published data only}
- Bachmann KM, Moggi F, Hirsbrunner HP, Donati R, Brodbeck J, Hirsbrunner H. An integrated treatment program for dually diagnosed patients. Psychiatric Services 1997;48(3):314‐6. [DOI] [PubMed] [Google Scholar]
Bagoien 2013 {published data only}
- Bagoien G, Bjorngaard J, Ostensen C, Romundstad P, Morken G. Motivational interviewing to patients with comorbid substance use admitted to an acute psychiatric department. Psychiatrische Praxis 2011;38:S10. [Google Scholar]
- Bagoien G, Bjorngaard JH, Ostensen C, Reitan SK, Romundstad P, Morken G. The effects of motivational interviewing on patients with comorbid substance use admitted to a psychiatric emergency unit ‐ a randomised controlled trial with two year follow‐up. BMC Psychiatry 2013;13:93. [PUBMED: 23517244] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkhof 2013 {published data only}
- Barkhof E, Meijer CJ, Sonneville LM, Linszen DH, Haan L. The effect of motivational interviewing on medication adherence and hospitalization rates in nonadherent patients with multi‐episode schizophrenia. Schizophrenia Bulletin 2013;39(6):1242‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrowclough 2006b {published data only}
- Barrowclough C, Haddock G, Lobban F, Jones S, Siddle R, Roberts C, et al. Group cognitive‐behavioural therapy for schizophrenia. British Journal of Psychiatry 2006;189:527‐32. [DOI] [PubMed] [Google Scholar]
Battersby 2013 {published data only}
- Battersby MW, Beattie J, Pols RG, Smith DP, Condon J, Blunden S. A randomised controlled trial of the Flinders Program of chronic condition management in Vietnam veterans with co‐morbid alcohol misuse, and psychiatric and medical conditions. Australian and New Zealand Journal of Psychiatry 2013;47(5):451‐62. [DOI] [PubMed] [Google Scholar]
Bechdolf 2004 {published data only}
- Bechdolf A, Knost B, Kuntermann C, Schiller S, Klosterkotter J, Hambrecht M, et al. A randomized comparison of group cognitive‐behavioural therapy and group psychoeducation in patients with schizophrenia. Acta Psychiatrica Scandinavica 2004;110(1):21‐8. [DOI] [PubMed] [Google Scholar]
Beebe 2012 {published data only}
- Beebe LH, Smith K, Burk R, McIntyre K, Dessieux O, Tavakoli A, et al. Motivational intervention increases exercise in schizophrenia and co‐occurring substance use disorders. Schizophrenia Research 2012;135(1‐3):204‐5. [DOI] [PubMed] [Google Scholar]
Bell 2011 {published data only}
- Bell MD. Cognitive remediation with work therapy in the initial phase of substance abuse treatment. Schizophrenia Bulletin 2011;Suppl 1:296. [Google Scholar]
Bennett 2001 {published data only}
- Bennett ME, Bellack AS, Gearon JS. Treating substance abuse in schizophrenia. An initial report. Journal of Substance Abuse Treatment 2001;20:163‐75. [DOI] [PubMed] [Google Scholar]
Bowen 2000 {published data only}
- Bowen RC, D'Arcy C, Keegan D, Senthilselvan A. A controlled trial of cognitive behavioural treatment of panic in alcoholic inpatients with comorbid panic disorder. Addictive Behaviours 2000;25(4):593‐7. [DOI] [PubMed] [Google Scholar]
Brooner 2013 {published data only}
- Brooner RK, Kidorf MS, King VL, Peirce J, Neufeld K, Stoller K, et al. Managing psychiatric comorbidity within versus outside of methadone treatment settings: a randomized and controlled evaluation. Addiction (Abingdon, England) 2013;108(11):1942‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015 {published data only}
- Brown RA, Abrantes AM, Minami H, Prince MA, Bloom EL, Apodaca TR, et al. Motivational interviewing to reduce substance use in adolescents with psychiatric comorbidity. Journal of Substance Abuse Treatment 2015;59:20‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carey 2004 {published and unpublished data}
- Carey KB, Purnine DM, Maisto SA, Carey MP. Enhancing readiness‐to‐change substance abuse in persons with schizophrenia. A four‐session motivation‐based intervention. Behaviour Modification 2001;25(3):331‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gordon CM, Schroder KE, Vanable PA. Reducing HIV‐risk behavior among adults receiving outpatient psychiatric treatment: Results from a randomized controlled trial. Journal of Consulting and Clinical Psychology 2004;72(2):252‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2002 {published and unpublished data}
- ACTRN12610000249055. A randomised control trial of a group based intervention and relapse prevention package for substance misuse and psychosis. Australian New Zealand Clinical Trials Registry 2010.
- Castle DJ, James W, Koh G, Kisely N, Preston N. An RCT A group‐based intervention for substance abuse in schizophrenia. Schizophrenia Research 2003;60:276‐7. [Google Scholar]
- Castle DJ, James W, Koh G, Wightman P, Kisely S, Spencer C, et al. Substance use in schizophrenia: why do people use, and what can be done about it?. Schizophrenia Research 2002;53(3 Suppl 1):178. [Google Scholar]
Clarke 2000 {published data only}
- Clarke GN, Herinckz HA, Kinney RF, Paulson RI, Cutler DL, Lewis K, et al. Psychiatric hospitalizations, arrests, emergency room visits, and homelessness of clients with serious and persistent mental illness: Findings from a randomized trial of two ACT programs vs. usual care. Mental Health Services Research 2000;2(3):155‐64. [DOI] [PubMed] [Google Scholar]
Clausen 2016 {published data only}
- Clausen H, Ruud T, Odden S, Saltytė Benth J, Heiervang KS, Stuen HK, et al. Hospitalisation of severely mentally ill patients with and without problematic substance use before and during Assertive Community Treatment: an observational cohort study. BMC Psychiatry 2016;16:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMarce 2008 {published data only}
- DeMarce JM, Lash SJ, Stephens RS, Grambow SC, Burden JL. Promoting continuing care adherence among substance abusers with co‐occurring psychiatric disorders following residential treatment. Addictive Behaviors 2008;33(9):1104‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Waal 2015 {published data only}
- Waal MM, Kikkert MJ, Blankers M, Dekker JJ, Goudriaan AE. Self‐wise, Other‐wise, Streetwise (SOS) training: a novel intervention to reduce victimization in dual diagnosis psychiatric patients with substance use disorders: protocol for a randomized controlled trial. BMC Psychiatry 2015;15:267. [NTR 4472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drake 2004a {published data only}
- Drake RE, Xie X, McHugo GJ, Shumway M. Three‐year outcomes of long‐term patients with co‐occurring bipolar and substance use disorders. Biological Psychiatry 2004;56(November 15):749‐56. [DOI] [PubMed] [Google Scholar]
Drake 2006 {published data only}
- Drake RE, McHugo GJ, Xie H, Fox M, Packard J, Helmsetter B. Ten‐year recovery outcomes for clients with co‐occurring schizophrenia and substance use disorders. Schizophrenia Bulletin. 2006;32(3):464‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drebing 2005 {published data only}
- Drebing CE, Ormer EA, Krebs C, Rosenheck R, Rounsaville B, Herz L, et al. The impact of enhanced incentives on vocational rehabilitation outcomes for dually diagnosed veterans. Journal of Applied Behavior Analysis 2005;38(3):359‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drebing 2007 {published data only}
- Drebing CE, Ormer EA, Mueller L, Hebert M, Penk WE, Petry NM, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. Journal of Rehabilitation Research and Development 2007;44(6):851‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eberhard 2009 {published data only}
- Eberhard S, Nordstrom G, Hoglund P, Ojehagen A. Secondary prevention of hazardous alcohol consumption in psychiatric out‐patients: a randomised controlled study. Social Psychiatry and Psychiatric Epidemiology 2009;12:1013‐21. [DOI] [PubMed] [Google Scholar]
Faber 2012 {published data only}
- Faber G, Smid HG, Gool AR, Wunderink L, Bosch RJ, Wiersma D. Continued cannabis use and outcome in first‐episode psychosis: Data from a randomized, open‐label, controlled trial. Journal of Clinical Psychiatry 2012;73(5):632‐8. [DOI] [PubMed] [Google Scholar]
Fiszdon 2016 {published data only}
- Fiszdon JM, Kurtz MM, Choi J, Bell MD, Martino S. Motivational interviewing to increase cognitive rehabilitation adherence in schizophrenia. Schizophrenia Bulletin 2016;42(2):327‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaughran 2017 {published data only}
- Gaughran F, Stahl D, Ismail K, Atakan Z, Lally J, Gardner‐Sood P, et al. Improving physical health and reducing substance use in psychosis‐‐randomised control trial (IMPACT RCT): study protocol for a cluster randomised controlled trial. BMC Psychiatry 2013;13:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner‐Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT). BMC Psychiatry 2017;17(1):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Greenwood K, Atakan Z, Sood P, Ohlsen R, Papanastasiou E, et al. The impact study ‐ motivating a change in health behaviour. European Psychiatry. 2011; Vol. 26, issue (Suppl 1):2151.
Gleesen 2009 {published data only}
- Alvarez‐Jimenez M, Gleeson J, Cotton S, Wade D, Gee D, Pearce T, et al. Duration of untreated psychosis, insight and adherence with cognitive‐behavioural therapy in first‐episode psychosis. Early Intervention in Psychiatry 2008;2(Suppl 1):A101. [Google Scholar]
- Cotton SM, Gleeson JF, Alvarez‐Jimenez M, McGorry PD. Quality of life in patients who have remitted from their first episode of psychosis. Schizophrenia Research 2010;121(1‐3):259‐65. [DOI] [PubMed] [Google Scholar]
- Gleeson J, Alvarez‐Jimenez M, Killackey E, McGorry P. Two year outcomes from the episode ii trial. Early Intervention in Psychiatry 2010;4(Suppl 1):24. [Google Scholar]
- Gleeson J, Cotton S, Alvarez‐Jimenez M, Wade D, Gee D, Crisp K, et al. An RCT of relapse prevention therapy for first‐episode psychosis patients. Early Intervention in Psychiatry 2008;2(Suppl 1):A20. [Google Scholar]
- Gleeson J, Wade D, Castle D, Gee D, Crisp K, Pearce T, et al. The EPISODE II trial of cognitive and family therapy for relapse prevention in early psychosis: rationale and sample characteristics. Journal of Mental Health 2008;17(1):19‐32. [Google Scholar]
- Gleeson J, Wade D, Mcgorry P, Albiston D, Castle D, Gilbert M, et al. Episode II: prevention of relapse following early psychosis. Schizophrenia Research 2004;70(1):61‐2. [Google Scholar]
- Gleeson JF, Cotton SM, Alvarez‐Jimenez M, Wade D, Crisp K, Newman B, et al. Family outcomes from a randomized control trial of relapse prevention therapy in first‐episode psychosis. Journal of Clinical Psychiatry 2010;71(4):475‐83. [DOI] [PubMed] [Google Scholar]
- Gleeson JF, Cotton SM, Alvarez‐Jimenez M, Wade D, Gee D, Crisp K, et al. A randomized controlled trial of relapse prevention therapy for first‐episode psychosis patients. Journal of Clinical Psychiatry 2009;70(4):477‐86. [ACTRN126050005144606] [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Blanch C, Gleeson JF, Koval P, Cotton SM, McGorry PD, Alvarez‐Jimenez M. Social functioning trajectories of young first‐episode psychosis patients with and without cannabis misuse: a 30‐month follow‐up study. PLOS One 2015;10(4):e0122404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman B. EPISODE II: Prevention of relapse following early psychosis. Australian New Zealand Clinical Trials Registry 2003.
Goldstein 2005 {published data only}
- Goldstein G, Haas GL, Shemansky WJ, Barnett B, Salmon‐Cox S. Rehabilitation during alcohol detoxication in comorbid neuropsychiatric patients. Journal of Rehabilitation Research and Development 2005;42(2):225‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harrison 2017 {published data only}
- Harrison J, Curtis A, Cousins L, Spybrook J. Integrated dual disorder treatment implementation in a large state sample. Community Mental Health Journal 2017;53(3):358‐66. [DOI] [PubMed] [Google Scholar]
Havassy 2000 {published data only}
- Havassy BE, Shropshire MS, Quigley LA. Effects of substance dependence on outcomes of patients in a randomized trial of two case management models. Psychiatric Services 2000;51(5):639‐44. [DOI] [PubMed] [Google Scholar]
Herman 2000 {published and unpublished data}
- Herman SE, BootsMiller B, Jordan L, Mowbray CT, Brown G, Deiz N, et al. Immediate outcomes of substance abuse treatment within a state psychiatric hospital. Journal of Mental Health Administration 1997;24(2):126‐38. [DOI] [PubMed] [Google Scholar]
- Herman SE, Frank KA, Mowbray CT, Ribisl K, Davidson WS, BootsMiller B, et al. Longitudinal effects of integrated treatment on alcohol use for persons with serious mental illness and substance use disorders. Journal of Behavioral Health Services and Research 2000;27(3):286‐302. [DOI] [PubMed] [Google Scholar]
Hulse 2002 {published data only}
- Hulse GK, Tait RJ. Six‐month outcomes associated with a brief alcohol intervention for adult in‐patients with psychiatric disorders. Drug and Alcohol Review 2002;21(2):105‐12. [DOI] [PubMed] [Google Scholar]
ISRCTN58667926 {published data only}
- ISRCTN58667926. Impact ‐ improving physical health and reducing substance use in severe mental illness: randomised controlled trial of a health promotion intervention. http://public.ukcrn.org.uk/ 2011.
James 2004 {published data only}
- James W, Preston NJ, Koh G, Spencer C, Kisely SR, Castle DJ. A group intervention which assists patients with dual diagnosis reduce their drug use: a randomized controlled trial. Psychological Medicine 2004;34(6):983‐90. [DOI] [PubMed] [Google Scholar]
Jerrell 2000 {published data only}
- Jerrell JM, Wilson JL, Hiller DC. Issues and outcomes in integrated treatment programs for dual disorders. Journal of Behavioral Health Services & Research 2000;27(3):303‐13. [DOI] [PubMed] [Google Scholar]
Kelly 2002 {published data only}
- Kelly JF, McKellar JD, Moos R. Major depression in patients with substance use disorders: relationship to 12‐step self‐help involvement and substance use outcomes. Addiction 2003;98(4):499‐508. [DOI] [PubMed] [Google Scholar]
Kidorf 2013 {published data only}
- Kidorf M, Brooner RK, Gandotra N, Antoine D, King V L, Peirce J, et al. Reinforcing integrated psychiatric service attendance in an opioid‐agonist program: A randomized and controlled trial. Drug and Alcohol Dependence 2013;133(1):30‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, King VL, Peirce J, Gandotra N, Ghazarian S, Brooner RK. Substance use and response to psychiatric treatment in methadone‐treated outpatients with comorbid psychiatric disorder. Journal of Substance Abuse Treatment 2015;51:64‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Killackey 2013 {published data only}
- Allott KA, Chinnery GL, Cotton SM, Jackson HJ, Killackey EJ. Does individual placement and support compensate for neurocognitive deficit in first‐episode psychosis?. Early Intervention in Psychiatry 2012;6:14. [Google Scholar]
- Arnold C, Allott K, Farhall J, Killackey E, Cotton S. Neurocognitive and social cognitive predictors of cannabis use in first‐episode psychosis. Schizophrenia Research 2015;168(1‐2):231‐7. [DOI] [PubMed] [Google Scholar]
- Caruana E, Cotton S, Killackey E, Allott K. The relationship between cognition, job complexity, and employment duration in first‐episode psychosis. Psychiatric Rehabilitation Journal 2015;38(3):210‐7. [DOI] [PubMed] [Google Scholar]
- Cotton S, Allott K, Chinnery G, Jackson H, Killackey E. The relationship between vocational functioning and quality of life in patients with first episode psychosis. Early Intervention in Psychiatry 2014;8:81. [Google Scholar]
- Herniman SE, Allott KA, Killackey E, Hester R, Cotton SM. The effect of comorbid depression on facial and prosody emotion recognition in first‐episode schizophrenia spectrum. Journal of Affective Disorders 2017;208:223‐9. [DOI] [PubMed] [Google Scholar]
- Karambelas GJ, Cotton SM, Farhall J, Killackey E, Allott KA. Contribution of neurocognition to 18‐month employment outcomes in first‐episode psychosis. Early Intervention in Psychiatry 2019;13(3):453‐60. [DOI: 10.1111/eip.12504] [DOI] [PubMed] [Google Scholar]
- Killackey E, Allott K, Cotton S, Chinnery G, Jackson H. Baseline to 18 months: Main results from a randomized controlled trial of individual placement and support for young people with first‐episode psychosis. Early Intervention in Psychiatry 2014;8:152. [DOI] [PubMed] [Google Scholar]
- Killackey E, Allott K, Cotton SM, Chinnery G, Jackson HJ, Baksheev G. The role of neurocognition within a randomised controlled trial of Individual Placement and Support for first‐episode psychosis. Schizophrenia Bulletin 2013;39:S264‐5. [Google Scholar]
- Killackey E, Allott K, Cotton SM, Jackson H, Scutella R, Tseng YP, et al. A randomized controlled trial of vocational intervention for young people with first‐episode psychosis: method. Early Intervention in Psychiatry 2013;7(3):329‐37. [ACTRN12608000094370] [DOI] [PubMed] [Google Scholar]
- Killackey E, Jackson H, Allott K, Cotton S. Vocational recovery in first episode psychosis: Preliminary results from a large randomised controlled trial of individual placement and support. Schizophrenia Research 2014;153(Suppl. 1):S282. [Google Scholar]
- Killackey EJ, Allott KA, Cotton SM, Chinnery GL, Sun P, Collins Z, et al. Vocational recovery in first‐episode psychosis: First results from a large randomized controlled trial of ips. Early Intervention in Psychiatry 2012;6:13. [Google Scholar]
- On ZX, Cotton S, Farhall J, Killackey E, Allott K. Relationship between duration of untreated psychosis and neurocognition and social cognition in first‐episode psychosis. Schizophrenia Research 2016;176(2‐3):529‐32. [DOI] [PubMed] [Google Scholar]
Lozano 2013 {published data only}
- Lozano BE, LaRowe SD, Smith JP, Tuerk P, Roitzsch J. Brief motivational feedback may enhance treatment entry in veterans with comorbid substance use and psychiatric disorders. American Journal on Addictions 2013;22(2):132‐5. [DOI] [PubMed] [Google Scholar]
Magura 2003 {published data only}
- Magura S, Laudet AB, Mahmood D, Rosenblum A, Vogel HS, Knight EL. Role of self‐help processes in achieving abstinence among dually diagnosed persons. Addictive Behaviours 2003;28(3):399‐413. [DOI] [PubMed] [Google Scholar]
Mangrum 2006 {published data only}
- Mangrum LF, Spence RT, Lopez M. Integrated versus parallel treatment of co‐occurring psychiatric and substance use disorders. Journal of Substance Abuse Treatment 2006;30(1):79‐84. [DOI] [PubMed] [Google Scholar]
Martino 2000 {published and unpublished data}
- Martino S, Carroll KM, O'Malley SS, Rounsaville BJ. Motivational interviewing with psychiatrically ill substance abusing patients. American Journal on Addictions 2000;9(1):88‐91. [DOI] [PubMed] [Google Scholar]
Martino 2006 {published data only}
- Martino S, Carroll KM, Nich C, Rounsaville BJ. A randomized controlled pilot study of motivational interviewing for patients with psychotic and drug use disorders. Addiction 2006;101(10):1479‐92. [DOI] [PubMed] [Google Scholar]
McGurk 2009 {published data only}
- McGurk SR, Mueser KT, Derosa TJ, Wolfe R. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophrenia Bulletin 2009;35(2):319‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercer 1997 {published data only}
- Mercer McFadden C, Drake RE, Brown NB, Fox RS. The Community Support Program demonstrations of services for young adults with severe mental illness and substance use disorders, 1987‐1991. Psychiatric Rehabilitation Journal 1997;20(3):13‐24. [Google Scholar]
Mueser 2001 {published data only}
- Mueser KT, Essock SM, Drake RE, Wolfe RS, Frisman L. Rural and urban differences in patients with a dual diagnosis. Schizophrenia Research 2001;48(1):93‐107. [DOI] [PubMed] [Google Scholar]
Mueser 2009 {published data only}
- Mueser K, Glynn SM, Xie H, Zarate R, Cather C, Fox L, et al. Family psychoeducation with patients who have co‐occurring substance use disorders and severe mental illness. Schizophrenia Research 2010;117(2‐3):121. [Google Scholar]
- Mueser KT, Glynn SM, Cather C, Xie H, Zarate R, Smith LF, et al. A randomized controlled trial of family intervention for co‐occurring substance use and severe psychiatric disorders. Schizophrenia Bulletin 2013;39(3):658‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Glynn SM, Cather C, Zarate R, Fox L, Feldman J, et al. Family intervention for co‐occurring substance use and severe psychiatric disorders: participant characteristics and correlates of initial engagement and more extended exposure in a randomized controlled trial. Addictive Behaviors 2009;34(10):867‐77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00043693 {published data only}
- NCT00043693. Family intervention for SMI and substance use disorder. http://www.clinicaltrials.gov 2002.
NCT00316303 {published data only}
- NCT00316303. Effectiveness of the screen, test, immunize, reduce risk, and refer (stirr) intervention for people with both a mental and substance abuse disorder. http://clinicaltrials.gov 2009.
NCT00447720 {published data only}
- NCT00447720. HIV prevention among substance abusing SMI. http://www.clinicaltrials.gov 2007.
NCT00495911 {published data only}
- NCT00495911. A randomized controlled trial of individual therapy for first episode psychosis. http://www.clinicaltrials.gov 2007.
NCT01361698 {published data only}
- NCT01361698. Illness Management and Recovery (IMR) in Danish community mental health centres. http://ClinicalTrials.gov/show/NCT01361698 2011. [DOI] [PMC free article] [PubMed]
NCT02264327 {published data only}
- NCT02264327. Phase II Motivational Interviewing: an experiential online training tool. http://ClinicalTrials.gov/show/NCT02264327 2014.
Noordraven 2017 {published data only}
- Noordraven EL, Wierdsma AI, Blanken P, Bloemendaal AF, Staring AB, Mulder CL. Financial incentives for improving adherence to maintenance treatment in patients with psychotic disorders (Money for Medication): a multicentre, open‐label, randomised controlled trial. Lancet Psychiatry 2017;4(3):199‐207. [NTR2350] [DOI] [PubMed] [Google Scholar]
Nuijten 2012 {published data only}
- Nuijten M, Blanken P, Hoorn B, Brink W, Hendriks V. A randomised controlled trial of outpatient versus inpatient integrated treatment of dual diagnosis patients: A failed but informative study. Mental Health and Substance Use 2012;5(2):132‐47. [Google Scholar]
Nuttbrock 1998 {published data only}
- Nuttbrock L, Ng‐Mak DS, Rahav M, Rivera JJ. Pre‐ and post‐admission attrition of homeless, mentally ill chemical abusers referred to residential treatment programs. Addiction 1997;92(10):1305‐16. [PubMed] [Google Scholar]
- Nuttbrock LA, Rahav M, Rivera JJ, Ng‐Mak DS, Link BG. Outcomes of homeless mentally ill chemical abusers in community residences and a therapeutic community. Psychiatric Services 1998;49(1):68‐76. [DOI] [PubMed] [Google Scholar]
- Rahav M, Rivera JJ, Collins J, Ng‐Mak D, Sturz EL, Struening EL, et al. Bringing experimental research designs into existing treatment programs: the case of community‐based treatment of the dually diagnosed. In: Fletcher BW//Inciardi JA//Horton AM editor(s). Drug Abuse Treatment: the Implementation of Innovative Approaches. Westport, Connecticut, USA: Greenwood Press, 1994:79‐93. [Google Scholar]
- Rahav M, Rivera JJ, Nuttbrock L, Ng Mak D. Characteristics and treatment of homeless, mentally ill, chemical‐abusing men. Journal of Psychoactive Drugs 1995;27(1):93‐103. [DOI] [PubMed] [Google Scholar]
Odom 2005 {published and unpublished data}
- Odom AE. A randomized study of integrated outpatient treatment and assertive community treatment for patients with comorbid mental illness and substance use disorders: comparing treatment outcome for domiciled and homeless patients [PhD thesis]. New School University, 2005. [Google Scholar]
Penn 2000 {published data only}
- Brooks AJ, Penn PE. Comparing treatments for dual diagnosis: Twelve‐step and self‐management and recovery training. American Journal of Drug and Alcohol Abuse 2003;29(2):359‐83. [DOI] [PubMed] [Google Scholar]
- Penn PE, Brooks AJ. Five years, twelve steps, and REBT in the treatment of dual diagnosis. Journal of Rational‐Emotive & Cognitive‐Behavior Therapy 2000;18(4):197‐208. [Google Scholar]
Petersen 2006 {published data only}
- Petersen L, Jeppesen P, Thorup A, Ohlenschlaeger J, Christensen T, Krarup G, et al. Substance abuse in first‐episode schizophrenia‐spectrum disorders. Schizophrenia Research 2006;86 Suppl 1:S44. [Google Scholar]
- Petersen L, Jeppesen P, Thorup A, Ohlenschlaeger J, Krarup G, Ostergard T, et al. Substance abuse and first‐episode schizophrenia‐spectrum disorders The Danish OPUS trial. Early Intervention in Psychiatry 2007;1(1):88‐96. [DOI] [PubMed] [Google Scholar]
- Petersen L, Nordentoft M, Bertelsen M, Thorup A. Does cannabis abuse determine outcome among patients with first episode psychosis. Results from the OPUS‐trial. Schizophrenia Bulletin 2007;33(2):453. [Google Scholar]
Petrakis 2005 {published data only}
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll C, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry 2005;57(10):1128‐37. [DOI] [PubMed] [Google Scholar]
Ries 2004 {published data only}
- Ries RK, Dyck DG, Short R, Srebnik D, Fisher A, Comtois KA. Outcomes of managing disability benefits among patients with substance dependence and severe mental illness. Psychiatric Services 2004;55(4):445‐7. [DOI] [PubMed] [Google Scholar]
Rosenheck 1998 {published data only}
- Rosenheck RA, Neale MS. Cost‐effectiveness of intensive psychiatric community care for high users of inpatient services. Archives of General Psychiatry 1998;55(5):459‐66. [DOI] [PubMed] [Google Scholar]
Rowe 2007 {published data only}
- Rowe M, Bellamy C, Baranoski M, Wieland M, O'Connell MJ, Benedict P, et al. A peer‐support, group intervention to reduce substance use and criminality among persons with severe mental illness. Psychiatric Services 2007;58(7):955‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sacks 2004 {published data only}
- Sacks S, Sacks JY, McKendrick K, Banks S, Stommel J. Modified TC for MICA offenders: crime outcomes. Behavioral Sciences and the Law 2004;22(4):477‐501. [DOI] [PubMed] [Google Scholar]
Sacks 2008 {published data only}
- Sacks JY, Sacks S, McKendrick K, Banks S, Schoeneberger M, Hamilton Z, et al. Prison therapeutic community treatment for female offenders: profiles and preliminary findings for mental health and other variables (crime, substance use and HIV risk). Journal of Offender Rehabilitation 2008;46(3‐4):233‐61. [Google Scholar]
- Sullivan CJ, McKendrick K, Sacks S, Banks S. Modified therapeutic community treatment for offenders with MICA disorders: substance use outcomes. American Journal of Drug and Alcohol Abuse 2007;33(6):823‐32. [DOI] [PubMed] [Google Scholar]
Sacks 2011 {published data only}
- Sacks S, McKendrick K, Vazan P, Sacks JY, Cleland CM. Modified therapeutic community aftercare for clients triply diagnosed with HIV/AIDS and co‐occurring mental and substance use disorders. AIDS Care 2011;23(12):1676‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santa Ana 2007 {published data only}
- Santa Ana EJ, Wulfert E, Nietert PJ. Efficacy of group motivational interviewing (GMI) for psychiatric inpatients with chemical dependence. Journal of Consulting and Clinical Psychology 2007;75(5):816‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmitz 2002 {published data only}
- Schmitz JM, Averill P, Sayre S, McCleary P, Moeller FG, Swann A. Cognitive‐behavioral treatment of bipolar disorder and substance abuse: A preliminary randomized study. Addictive Disorders and Their Treatment 2002;1(1):17‐24. [Google Scholar]
Shorey 2015 {published data only}
- Shorey RC, Martino S, Lamb KE, LaRowe SD, Santa Ana EJ. Change talk and relatedness in group motivational interviewing: a pilot study. Journal of Substance Abuse Treatment 2015;51:75‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sigmon 2000 {published data only}
- Sigmon SC, Steingard S, Badger GJ, Anthony SL, Higgins ST. Contingent reinforcement of marijuana abstinence among individuals with serious mental illness: a feasibility study. Experimental & Clinical Psychopharmacology 2000;8(4):509‐17. [DOI] [PubMed] [Google Scholar]
Sitharthan 1999 {published data only}
- Sitharthan T, Singh S, Kranitis P, Currie J, Freeman P, Murugesan G, et al. Integrated drug and alcohol intervention: development of an opportunistic intervention program to reduce alcohol and other substance use among psychiatric patients. Australian and New Zealand Journal of Psychiatry 1999;33(5):676‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smeerdijk 2012 {published data only}
- NCT01167556. Family motivational intervention in schizophrenia. http://ClinicalTrials.gov/show/NCT01167556 2006.
- Smeerdijk M, Keet R, Haan L, Schippers G, Linszen D. Family motivation intervention in early onset psychosis and cannabis abuse: a randomized clinical trial. Proceedings of the 12th International Congress on Schizophrenia Research; 2009 Mar 28‐Apr 1; San Diego, CA. Oxford Univ Press, 2009:371.
- Smeerdijk M, Keet R, Dekker N, Raaij B, Krikke M, Koeter M, et al. Motivational interviewing and interaction skills training for parents to change cannabis use in young adults with recent onset schizophrenia. Early Intervention in Psychiatry 2010;4 Suppl 1:6. [Google Scholar]
- Smeerdijk M, Keet R, Dekker N, van RB, Krikke M, Koeter M, et al. Motivational interviewing and interaction skills training for parents to change cannabis use in young adults with recent‐onset schizophrenia: a randomized controlled trial. Psychological Medicine 2012;42(8):1627‐36. [DOI] [PubMed] [Google Scholar]
- Smeerdijk M, Keet R, Haan L, Barrowclough C, Linszen D, Schippers G. Feasibility of teaching motivational interviewing to parents of young adults with recent‐onset schizophrenia and co‐occurring cannabis use. Journal of Substance Abuse Treatment 2014;46(3):340‐5. [DOI] [PubMed] [Google Scholar]
- Smeerdijk M, Keet R, Raaij B, Koeter M, Linszen D, Haan L, et al. Motivational interviewing and interaction skills training for parents of young adults with recent‐onset schizophrenia and co‐occurring cannabis use: 15‐month follow‐up. Psychological Medicine 2015;45(13):2839‐48. [DOI] [PubMed] [Google Scholar]
Smelson 2007 {published data only}
- NCT00137267. A brief community linkage intervention for dually diagnosed individuals. https://www.ClinicalTrials.gov/ct/show/ 2005.
- Smelson D, Kalman D, Losonczy MF, Kline A, Sambamoorthi U, Hill LS, et al. A brief treatment engagement intervention for individuals with co‐occurring mental illness and substance use disorders: results of a randomized clinical trial. Community Mental Health Journal 2012;48(2):127‐32. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Losonczy MF, Ziedonis D, Sussner BD, Castles‐Fonseca K, Rodrigues S, et al. A brief community linkage intervention for veterans with a persistent mental illness and a co‐occurring substance abuse disorder. European Journal of Psychiatry 2012;21(2):143‐52. [NCT00137267] [Google Scholar]
Somers 2015 {published data only}
- Cheung A, Somers JM, Moniruzzaman A, Patterson M, Frankish CJ, Krausz M, et al. Emergency department use and hospitalizations among homeless adults with substance dependence and mental disorders. Addiction Science and Clinical Practice 2015;10:17. [ISRCTN575077 and 66721740] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel F, Moniruzzaman A, Somers J, Frankish J, Strehlau V, Schutz C, et al. A longitudinal study of suicidal ideation among homeless, mentally ill individuals. Social Psychiatry and Psychiatric Epidemiology 2016;51(1):107‐14. [DOI] [PubMed] [Google Scholar]
- Palepu A, Patterson M L, Moniruzzaman A, Frankish C J, Somers J. Housing first improves residential stability in homeless adults with concurrent substance dependence and mental disorders. American Journal of Public Health 2013;103(2 (Suppl.)):e30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezansoff SN, Moniruzzaman A, Fazel S, McCandless L, Procyshyn R, Somers JM. Housing first improves adherence to antipsychotic medication among formerly homeless adults with schizophrenia: results of a randomized controlled trial. Schizophrenia Bulletin 2017;43(4):852‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JM Patterson M L Moniruzzaman A Currie L Rezansoff S N Palepu A, et al. Vancouver At Home: Pragmatic randomized trials investigating Housing First for homeless and mentally ill adults. Trials 2013;14(1):365. [ISRCTN57595077] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JM, Moniruzzaman A, Palepu A. Changes in daily substance use among people experiencing homelessness and mental illness: 24‐month outcomes following randomization to Housing First or usual care. Addiction (Abingdon, England) 2015;110(10):1605‐14. [DOI] [PubMed] [Google Scholar]
- Somers JM, Moniruzzaman A, Patterson M, Currie L, Rezansoff SN, Palepu A, et al. A randomized trial examining Housing First in Congregate and Scattered Site Formats. PLOS One 2017;12(1):e0168745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JM, Rezansoff SN, Moniruzzaman A, Palepu A, Patterson M. Housing First reduces re‐offending among formerly homeless adults with mental disorders: results of a randomized controlled trial. PLOS One 2013;8(9):e72946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stain 2016 {published data only}
- Stain HJ, Bucci S, Baker AL, Carr V, Emsley R, Halpin S, et al. A randomised controlled trial of cognitive behaviour therapy versus non‐directive reflective listening for young people at ultra high risk of developing psychosis: The detection and evaluation of psychological therapy (DEPTh) trial. Schizophrenia Research 2016;176(2‐3):212‐9. [DOI] [PubMed] [Google Scholar]
- Stain HJ, Halpin SA, Baker AL, Startup M, Carr VJ, Schall U, et al. Impact of rurality and substance use on young people at ultra high risk for psychosis. Early Intervention in Psychiatry 2018;12(6):1173‐80. [DOI: 10.1111/eip.12437] [DOI] [PubMed] [Google Scholar]
Steadman 2005 {published data only}
Swanson 2000 {published data only}
- Swanson JW, Swartz MS, Borum R, Hiday VA, Wagner R, Burns BJ. Involuntary out‐patient commitment and reduction of violent behaviour in persons with severe mental illness. British Journal of Psychiatry 2000;176:324‐31. [DOI] [PubMed] [Google Scholar]
Tantirangsee 2015 {published data only}
- ACTRN12612001059853. The effectiveness of brief‐alcohol, smoking, substance involvement screening test (brief‐assist) linked brief intervention (bi) for substance abuse among schizophrenic patients. http://www.anzctr.org.au/ACTRN12612001059853.aspx 2012.
- Tantirangsee N, Assanangkornchai S, Marsden J. Effects of a brief intervention for substance use on tobacco smoking and family relationship functioning in schizophrenia and related psychoses: a randomised controlled trial. Journal of Substance Abuse Treatment 2015;51:30‐7. [DOI] [PubMed] [Google Scholar]
- Tantirangsee N, Assanangkornchai S, Marsden J. Sy02‐1the association between clinical improvement in substance‐related problems and psychiatric symptoms in psychosis: a post‐hoc analysis. Alcohol and Alcoholism 2014;49(Suppl. 1):i3‐4. [Google Scholar]
- Tantirangsee N, Assanangkornchai S, Marsden J. Sy03‐1‐2isam fellowship the association between clinical improvement in substance‐related problems and psychiatric symptoms in psychosis: a post‐hoc analysis. Alcohol and Alcoholism 2014;49(Suppl. 1):i4‐5. [Google Scholar]
Teague 1995 {published data only}
- Teague GB, Drake RE, Ackerson TH. Evaluating use of continuous treatment teams for persons with mental illness and substance abuse. Psychiatric Services 1995;46(7):689‐95. [DOI] [PubMed] [Google Scholar]
Thornicroft 2007 {published data only}
- Craig TKJ, Johnson, S, McCrone P, Afuwape S, Hughes E, Gournay K, et al. Integrated care for co‐occurring disorders: psychiatric symptoms, social functioning, and service costs at 18 months. Psychiatric Services 2008;59(3):276‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hughes E, Wanigaratne S, Gournay K, Johnson S, Thornicroft G, Finch E, et al. Training in dual diagnosis interventions (the COMO Study): randomised controlled trial. BMC Psychiatry 2008;8:12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Thornicroft G, Afuwape S, Leese M, White IR, Hughes E, et al. Effects of training community staff in interventions for substance misuse in dual diagnosis patients with psychosis (COMO study): cluster randomised trial. British Journal of Psychiatry 2007;191(November):451‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thornicroft G. Prevention of admission to psychiatric hospital. A randomised controlled trial of service use, health and social care outcomes of a community mental health team intervention specific to dual diagnosis (psychosis and substance misuse) patients. http://www.controlled‐trials.com. United Kingdom, 2007.
Timko 2004 {published data only}
- Timko C, Chen S, Sempel J, Barnett P. Dual diagnosis patients in community or hospital care: one‐year outcomes and health care utilization and costs. Journal of Mental Health 2006;15(2):163‐77. [Google Scholar]
- Timko C, Sempel JM. Short‐term outcomes of matching dual diagnosis patients' symptom severity to treatment intensity. Journal of Substance Abuse Treatment. 2004;26(3):209‐18. [DOI] [PubMed] [Google Scholar]
Tyrer 2011 {published data only}
- Ranger M, Tyrer P, Miloseska K, Fourie H, Khaleel I, North B, et al. Cost‐effectiveness of nidotherapy for comorbid personality disorder and severe mental illness: randomized controlled trial. Epidemiologia e Psichiatria Sociale 2009;18(2):128‐36. [MEDLINE: ] [PubMed] [Google Scholar]
- Tyrer P, Miloseska K, Whittington C, Ranger M, Khaleel I, Crawford M, et al. Nidotherapy in the treatment of substance misuse, psychosis and personality disorder: Secondary analysis of a controlled trial. Psychiatrist 2011;35(1):9‐14. [Google Scholar]
Weiss 2000 {published data only}
- Weiss RD, Griffin ML, Greenfield SF, Najavits LM, Wyner D, Soto JA, et al. Group therapy for patients with bipolar disorder and substance dependence: results of a pilot study. Journal of Clinical Psychiatry 2000;61(5):361‐7. [DOI] [PubMed] [Google Scholar]
Weiss 2007 {published data only}
- Weiss RD, Griffin ML, Kolodziej ME, Greenfield SF, Najavits LM, Daley DC, et al. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. American Journal of Psychiatry 2007;164(1):100‐7. [DOI] [PubMed] [Google Scholar]
Weiss 2009 {published data only}
- Weiss RD, Griffin ML, Jaffee WB, Bender RE, Graff FS, Gallop RJ, et al. A "community‐friendly" version of integrated group therapy for patients with bipolar disorder and substance dependence: a randomized controlled trial. Drug & Alcohol Dependence 2009;104(3):212‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, R. Modifying group therapy for bipolar substance abusers. NCT00227838. http://www.clinicaltrials.gov 2005.
Woolderink 2015 {published data only}
- Woolderink M. (Economic) Evaluation of E‐mental health interventions for children of parents with mental illness (e^3 COPMI). http://www.trialregister.nl/trialreg/index.asp 2009.
- Woolderink M, Bindels JA, Evers SM, Paulus AT, Asselt AD, Schayck OC. An online health prevention intervention for youth with addicted or mentally ill parents: experiences and perspectives of participants and providers from a randomized controlled trial. Journal of Medical Internet Research 2015;17(12):e274. [NTR 1982] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12616000275460 {published data only}
- Solar A, Chamber J. A clinical study comparing more time intensive therapy with experienced alcohol and drug nurse clinicians to a Brief Information Pack for patients on a mental health unit and alcohol and drug disorders in linking to the community alcohol and drug rehabilitation service of their choice. International Clinical Trials Registry Platform 2016;1:1‐2. [ACTRN126160000275460] [Google Scholar]
Gurevich 2015 {published data only}
- Gurevich GL, Agibalova TV, Rychkova OV, Buzik OZ. Pychotherapy in patients with alcohol dependence and comorbid endogenous pathology. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova 2015;115(4 Pt 2):28‐33. [DOI] [PubMed] [Google Scholar]
Meister 2010 {published data only}
- Meister K, Rietschel L, Burlon M, Jannsen H, Bock T, Wegscheider K, et al. A cluster‐randomized, parallel group, observer blind trial of a group based motivational‐behavioural therapy for young people with psychosis and substance use disorder. Early Intervention in Psychiatry 2010;4 Suppl 1:163. [Google Scholar]
- Meister, K. Comorbid psychosis and addiction. Epidemiology, explanatory models and evaluation of motivational behavioural group therapy [Komorbidität psychose und sucht. Epidemiologie, erklärungsmodelle und evaluation einer motivational‐verhaltenstherapeutischen gruppentherapie]. Dissertation, University of Hamburg 2010.
NCT01883791 {published data only}
- NCT01883791. Screening, Brief Intervention and Referral to Treatment for substance abuse in mental health treatment settings (SBIRT in MH). https://ClinicalTrials.gov/show/NCT01883791 2017.
- Spear SE, Karno M, Glasner‐Edwards S, Rawson R, Saitz R, Dominguez B. Applying SBIRT to new settings: Preliminary findings of substance use disorder risk in community mental health settings. Drug and Alcohol Dependence 2015;156:e209. [Google Scholar]
NCT02214667 {published data only}
- NCT02214667. Treating co‐occurring substance use and mental disorders among jail inmates. http://Clinicaltrials.gov/show/NCT02214667 2015.
- Dorn RA, Desmarais SL, Rade CB, Burris EN, Cuddeback GS, Johnson KL, et al. Jail‐to‐community treatment continuum for adults with co‐occurring substance use and mental disorders: study protocol for a pilot randomized controlled trial. Trials 2017;18(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02319746 {published data only}
- Gonzalez‐Ortega I, Echeburua E, Garcia‐Alocen A, Vega P, Gonzalez‐Pinto A. Cognitive behavioral therapy program for cannabis use cessation in first‐episode psychosis patients: study protocol for a randomized controlled trial. Trials 2016;17:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02319746. Cognitive behavioral therapy program to first‐episode psychosis patients and cannabis abuse. https://Clinicaltrials.gov/ct2/show/NCT02319746 2014.
NCT02670902 {published data only}
- NCT02670902. Intervention for persons leaving residential substance abuse treatment. https://ClinicalTrials.gov/show/NCT02670902 2016.
NCT03007940 {published data only}
- NCT03007940. Using NIATx strategies to implementiIntegrated services in routine care. https://ClinicalTrials.gov/show/NCT03007940 2016.
References to ongoing studies
Bennett 2007 {published data only}
- Bennett M, Bellack AS, Dixon L. Treatment of alcohol use disorders in people with severe mental illness. Schizophrenia Bulletin 2007;33(2):421. [Google Scholar]
- NCT00280813. Treatment of alcohol use disorders in schizophrenia. http://www.clinicaltrials.gov 2006.
CIRCLE trial {published data only}
- ISRCTN33576045. Contingency intervention for reduction of cannabis in early psychosis ‐circle. http://www.controlled‐trials.com 2011.
- ISRCTN33576045. Randomised controlled trial of the clinical and cost‐effectiveness of a contingency management intervention for reduction of cannabis use and of relapse in early psychosis. Circle. http://public.ukcrn.org.uk/ 2012.
- Johnson S, Sheridan Rains L, Marwaha S, Strang J, Craig T, Weaver T, et al. A randomised controlled trial of the clinical and cost‐effectiveness of a contingency management intervention compared to treatment as usual for reduction of cannabis use and of relapse in early psychosis (CIRCLE): a study protocol for a randomised controlled trial. Trials 2016;17(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00783185 {published data only}
- NCT00783185. Dual diagnosis (psychosis and cannabis misuse): comparison of specialized treatment versus unspecified treatment. http://www.clinicaltrials.gov 2008.
NCT00798109 {published data only}
- NCT00798109. Effect of motivational therapy on schizophrenia with cannabis misuse. http://www.clinicaltrials.gov 2008.
Verstappen 2007 {published data only}
- Verstappen N, Henquet C. Effects and indicators of CBT for cannabis use in psychosis [Studie naar 'Behandeling cananbisgebruik en psychose']. http://www.trialregister.nl/trialreg/index.asp 2007.
Additional references
Addington 1992
- Addington D, Addington J, Maticka‐Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophrenia Research 1992;6(3):201‐8. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [TP020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2001
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trial: explanation and elaboration. Annals of Internal Medicine 2001;134(8):663‐94. [DOI] [PubMed] [Google Scholar]
Andreasen 1982
- Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry 1982;39:784‐8. [DOI] [PubMed] [Google Scholar]
Appleby 1997
- Appleby L, Dyson V, Altman E, Luchins DJ. Assessing substance use in multiproblem patients: reliability and validity of the Addiction Severity Index in a mental hospital population. Journal of Nervous & Mental Disease 1997;185(3):159‐65. [DOI] [PubMed] [Google Scholar]
Asberg 1978
- Asberg M, Montgomery SA, Perris C, et al. A comprehensive psychopathological rating scale. Acta Psychiatrica Scandinavica Supplement 1978;271:5‐27. [DOI] [PubMed] [Google Scholar]
Baandrup 2016
- Baandrup L, Østrup Rasmussen J, Klokker L, Austin S, Bjornshave T, Fuglsang Bliksted V, et al. Treatment of adult patients with schizophrenia and complex mental health needs‐A national clinical guideline. Nordic Journal of Psychiatry 2016;70(3):231‐40. [DOI] [PubMed] [Google Scholar]
Baker 2012
- Baker AL, Hiles SA, Thornton LK, Hides L, Lubman DI. A systematic review of psychological interventions for excessive alcohol consumption among people with psychotic disorders. Acta Psychiatrica Scandinavica 2012;126(4):243‐55. [DOI] [PubMed] [Google Scholar]
Barrowclough 2006a
- Barrowclough C, Haddock G, Fitzsimmons M, Johnson R. Treatment development for psychosis and co‐occurring substance misuse: A descriptive review. Journal of Mental Health 2006;15(6):619‐32. [Google Scholar]
Beck 1972
- Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technique. Postgraduate Medicine 1972;52(6):81‐5. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Bennett 2017
- Bennett ME, Bradshaw KR, Catalano LT. Treatment of substance use disorders in schizophrenia. American Journal of Drug & Alcohol Abuse 2017;43(4):377‐90. [DOI] [PubMed] [Google Scholar]
Birchwood 1990
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale: the development and validation of a scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry 1990;157(December):853‐9. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bogenschutz 2006
- Bogenschutz MP, Geppert CM, George J. The role of twelve‐step approaches in dual diagnosis treatment and recovery. American Journal on Addictions 2006;15(1):50‐60. [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Bond 1988
- Bond GR, Miller LD, Krumwied RD, Ward RS. Assertive case management in three CMHCs: a controlled study. Hospital and Community Psychiatry 1988;39(4):411‐8. [DOI] [PubMed] [Google Scholar]
Bond 1990
- Bond GR, Witheridge TF, Dincin J, Wasmer D, Webb J, DeGraaf‐Kaser R. Assertive community treatment for frequent users of psychiatric hospitals in a large city: a controlled study. American Journal of Community Psychology 1990;18(6):865‐91. [DOI] [PubMed] [Google Scholar]
Bond 2015
- Bond GR, Drake RE. The critical ingredients of assertive community treatment. World Psychiatry 2015;14(2):240‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 2017
- Bond GR, Drake RE. New directions for psychiatric rehabilitation in the USA. Epidemiology & Psychiatric Science 2017;26(3):223‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boniface 2018
- Boniface S, Malet‐Lambert I, Coleman R, Deluca P, Donoghue K, Drummond C, et al. The effect of brief interventions for alcohol among people with comorbid mental health conditions: A systematic review of randomized trials and narrative synthesis. Alcohol & Alcoholism 2018;53(3):282‐93. [DOI] [PubMed] [Google Scholar]
Brunette 2018
- Brunette MF, Mueser KT, Babbin S, Meyer‐Kalos P, Rosenheck R, Correll CU, et al. Demographic and clinical correlates of substance use disorders in first episode psychosis. Schizophrenia Research 2018;194:4‐12. [DOI] [PubMed] [Google Scholar]
Burns 2007
- Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatrica Scandinavica 2007;116(6):403‐18. [DOI] [PubMed] [Google Scholar]
Callaghan 2012
- Callaghan RC, Cunningham JK, Allebeck P, Arenovich T, Sajeev G, Remington G, et al. Methamphetamine use and schizophrenia: a population‐based cohort study in California. American Journal of Psychiatry 2012;169(4):389‐96. [DOI] [PubMed] [Google Scholar]
Carey 1996
- Carey KB, Cocco KM, Simons JS. Concurrent validity of clinicians' rating of substance abuse among psychiatric outpatients. Psychiatric Services 1996;47(8):842‐7. [DOI] [PubMed] [Google Scholar]
Carra 2009
- Carra G, Johnson S. Variations in rates of comorbid substance use in psychosis between mental health settings and geographical areas in the UK. A systematic review. Social Psychiatry & Psychiatric Epidemiology 2009;44(6):429‐47. [DOI] [PubMed] [Google Scholar]
Chanut 2005
- Chanut F, Brown TG, Dongier M. Motivational interviewing and clinical psychiatry. Canadian Journal of Psychiatry 2005;50(9):548‐54. [DOI] [PubMed] [Google Scholar]
Cleary 2009a
- Cleary M, Hunt GE, Matheson S, Walter G. Psychosocial treatments for people with co‐occurring severe mental illness and substance misuse: systematic review. Journal of Advanced Nursing 2009;65(2):238‐58. [DOI] [PubMed] [Google Scholar]
Cleary 2009b
- Cleary M, Hunt GE, Horsfall J. Conducting efficient literature searches. Strategies for mental health nurses. Journal of Psychosocial Nursing & Mental Health Services 2009;47(11):34‐41. [DOI] [PubMed] [Google Scholar]
Corse 1995
- Corse SJ, Hirschinger NB, Zanis D. The use of the Addiction Severity Index with people with severe mental illness. Psychiatric Rehabilitation Journal 1995;19(1):9‐18. [Google Scholar]
Crockford 2017
- Crockford D, Addington D. Canadian Schizophrenia Guidelines: Schizophrenia and Other Psychotic Disorders with Coexisting Substance Use Disorders. Canadian Journal of Psychiatry 2017;62(9):624‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darke 1991
- Darke S, Ward J, Hall W, Heather N, Wodak A. The Opiate Treatment Index (OTI) Researchers Manual. Technical report No. 11. Sydney: National Drug & Alcohol Research Centre, 1991. [Google Scholar]
Darke 1992
- Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multi‐dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. British Journal of Addiction 1992;87(5):733‐42. [DOI] [PubMed] [Google Scholar]
David 1992
- David A, Buchanan A, Reed A, Almeida O. The assessment of insight in psychosis. British Journal of Psychiatry 1992;161(November):599‐602. [DOI] [PubMed] [Google Scholar]
De Witte 2014
- Witte NA, Crunelle CL, Sabbe B, Moggi F, Dom G. Treatment for outpatients with comorbid schizophrenia and substance use disorders: a review. European Addiction Research 2014;20(3):105‐14. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Derogatis 1973
- Derogatis LR, Lipman RS, Covi L. SCL‐90: An outpatient psychiatric rating scale: Preliminary report. Psychopharmacology Bulletin 1973;9(1):13‐27. [PubMed] [Google Scholar]
Derogatis 1975
- Derogatis LR, Yevzeroff H, Wittelsberger B. Social class, psychological disorder, and the nature of the psych0pathologic indicator. Journal of Consulting and Clinical Psychology 1975;43(2):183‐91. [DOI] [PubMed] [Google Scholar]
Derogatis 1983a
- Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychological Medicine 1983;13(3):595‐605. [PubMed] [Google Scholar]
Derogatis 1983b
- Derogatis LR. SCL‐90‐R: Administration, Scoring & Procedures Manual‐II. Towson, MD: Clinical Psychometric Research, 1983. [Google Scholar]
Dieterich 2017
- Dieterich M, Irving CB, Bergman H, Khokhar MA, Park B, Marshall M. Intensive case management for severe mental illness. Cochrane Database of Systematic Reviews 2017;1:CD007906. [DOI: 10.1002/14651858.CD007906.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Dixon 2010
- Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, et al. The 2009 schizophrenia PORTpsychosocial treatment recommendations and summary statements. Schizophrenia Bulletin 2010;36(1):48‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dohrenwend 1980
- Dohrenwend BP, Shrout PE, Egri G, et al. Nonspecific psychological distress and other dimensions of psychopathology. Measures for use in the general population. Archives of General Psychiatry 1980;37(11):1229‐36. [DOI] [PubMed] [Google Scholar]
Donald 2005
- Donald M, Downer J, Kavanagh D. Integrated versus non‐integrated management and care for clients with co‐occurring mental health and substance use disorders: a qualitative systematic review of randomised controlled trials. Social Science and Medicine 2005;60(6):1371‐83. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Drake 1993
- Drake RE, Bartels SJ, Teague GB, Noordsy DL, Clark RE. Treatment of substance abuse in severely mentally ill patients. Journal of Nervous and Mental Disease 1993;181(10):606‐11. [DOI] [PubMed] [Google Scholar]
Drake 1996
- Drake RE, Mueser KT, Clarke RE, Wallach MA. The course, treatment, and outcome of substance disorder in persons with severe mental illness. American Journal of Orthopsychiatry 1996;66(1):42‐51. [DOI] [PubMed] [Google Scholar]
Drake 1998b
- Drake RE, Mercer‐McFadden C, Mueser KT, McHugo GJ, Bond GR. A review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophrenia Bulletin 1998;24(4):589‐608. [DOI] [PubMed] [Google Scholar]
Drake 2004
- Drake RE, Mueser KT, Brunette MF, McHugo GJ. A review of treatments for people with severe mental illnesses and co‐occurring substance use disorders. Psychiatric Rehabilitation Journal 2004;27(4):360‐74. [DOI] [PubMed] [Google Scholar]
Drake 2008
- Drake RE, O'Neal EL, Wallach MA. A systematic review of psychosocial research on psychosocial interventions for people with co‐occurring severe mental and substance use disorders. Journal of Substance Abuse and Treatment 2008;34(1):123‐38. [DOI] [PubMed] [Google Scholar]
DSM III‐R
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (DSM III‐R). Diagnostic & Statistical Manual of Mental Disorders (DSM III‐R). 3rd Edition. Washington D.C: American Psychiatric Association, 1987. [Google Scholar]
DSM‐IV
- APA 1994. Diagnostic & Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: APA, 1994. [Google Scholar]
Dutra 2008
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta‐analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry 2008;165(2):179‐87. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JP, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766‐71. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Galletly 2016
- Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Australian and New Zealand Journal of Psychiatry 2016;50(5):410‐72. [DOI] [PubMed] [Google Scholar]
Goldman 1992
- Goldman HH, Skodal AE, Lave TR. Revising Axis V for DSM‐IV: a review of measures of social functioning. Americal Journal of Psychiatry 1992;149(9):1148‐56. [DOI] [PubMed] [Google Scholar]
Green 1987
- Green RS, Gracely EJ. Selecting a rating scale for evaluating services to the chronically mentally ill. Community Mental Health Journal 1987;23(2):91‐102. [DOI] [PubMed] [Google Scholar]
Green 2007
- Green AI, Drake RE, Brunette MF, Noordsy DL. Schizophrenia and co‐occurring substance use disorder. Americal Journal of Psychiatry 2007;164(3):402‐8. [DOI] [PubMed] [Google Scholar]
Gregg 2007
- Gregg L, Barrowclough C, Haddock G. Reasons for increased substance use in psychosis. Clinical Psychology Review 2007;27(4):494‐510. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [DOI] [PubMed] [Google Scholar]
Haddock 2014
- Haddock G, Eisner E, Boone C, Davies G, Coogan C, Barrowclough C. An investigation of the implementation of NICE‐recommended CBT interventions for people with schizophrenia. Journal of Mental Health 2014;23(4):162‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23(February):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartz 2014
- Hartz SM, Pato CN, Medeiros H, Cavazos‐Rehg P, Sobell JL, Knowles JA, et al. Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry 2014;71(3):248‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasan 2015
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia. Part 3: Update 2015 Management of special circumstances: Depression, Suicidality, substance use disorders and pregnancy and lactation. World Journal of Biological Psychiatry 2015;16(3):142‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hjorthoj 2009
- Hjorthoj C, Fohlmann A, Nordentoft M. Treatment of cannabis use disorders in people with schizophrenia spectrum disorders ‐ a systematic review. Addictive Behaviors 2009;34(6‐7):520‐5. [DOI] [PubMed] [Google Scholar]
Hodgins 1992
- Hodgins DC, el‐Guebaly N. More data on the Addiction Severity Index. Reliability and validity with the mentally ill substance abuser. Journal of Nervous & Mental Disease 1992;180(3):197‐201. [DOI] [PubMed] [Google Scholar]
Horn 1987
- Horn JL, Wanberg KW, Foster FM. The Alcohol Use Inventory. Minneapolis, MN: National Computer Systems, 1987. [Google Scholar]
Horsfall 2009
- Horsfall J, Cleary M, Hunt GE, Walter G. Psychosocial treatments for people with co‐occurring severe mental illnesses and substance use disorders (dual diagnosis): a review of empirical evidence. Harvard Review of Psychiatry 2009;17(1):24‐34. [DOI] [PubMed] [Google Scholar]
Hunt 2002
- Hunt GE, Bergen J, Bashir M. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival 4 years after a relapse. Schizophrenia Research 2002;54(3):253‐64. [DOI] [PubMed] [Google Scholar]
Hunt 2016
- Hunt GE, Malhi GS, Cleary M, Lai HMX, Sitharthan T. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990‐2015: Systematic review and meta‐analysis. Journal of Affective Disorders 2016;206:331‐49. [DOI] [PubMed] [Google Scholar]
Hunt 2018
- Hunt GE, Large M, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990‐2017: Systematic review and meta‐analysis. Drug and Alcohol Dependence 2018;191:234‐58. [DOI] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
ICD‐10
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva: World Health Organization, 1992. [Google Scholar]
Jones 2004
- Jones C, Cormac I, Silveira da Mota Neto JI, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000524] [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behavioural therapy versus other psychosocial treatments for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008712.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapur 2005
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis ‐ linking biology, pharmacology and phenomenology of psychosis. Schizophrenia Research 2005;79:59‐68. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [DOI] [PubMed] [Google Scholar]
Kelly 2012
- Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addictive Behaviors 2012;37(1):11‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khokhar 2018
- Khokhar JY, Dwiel LL, Henricks AM, Doucette WT, Green AI. The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophrenia Research 2018;194:78‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koob 2010
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35(1):217‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2012a
- Lai HM, Sitharthan T, Huang QR. Exploration of the comorbidity of alcohol use disorders and mental health disorders among inpatients presenting to all hospitals in New South Wales, Australia. Substance Abuse 2012;33(2):138‐45. [DOI] [PubMed] [Google Scholar]
Lai 2012b
- Lai HM, Sitharthan T. Exploration of the comorbidity of cannabis use disorders and mental health disorders among inpatients presenting to all hospitals in New South Wales, Australia. American Journal of Drug & Alcohol Abuse 2012;38(6):567‐74. [DOI] [PubMed] [Google Scholar]
Lai 2015
- Lai HM, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990‐2014: A systematic review and meta‐analysis. Drug and Alcohol Dependence 2015;154:1‐13. [DOI] [PubMed] [Google Scholar]
Lappin 2016
- Lappin JM, Roxburgh A, Kaye S, Chalmers J, Sara G, Dobbins T, et al. Increased prevalence of self‐reported psychotic illness predicted by crystal methamphetamine use: Evidence from a high‐risk population. International Journal of Drug Policy 2016;38:16‐20. [DOI] [PubMed] [Google Scholar]
Large 2014
- Large M, Mullin K, Gupta P, Harris A, Nielssen O. Systematic meta‐analysis of outcomes associated with psychosis and co‐morbid substance use. Australian & New Zealand Journal of Psychiatry 2014;48(5):418‐32. [DOI] [PubMed] [Google Scholar]
Larsen 1979
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Evaluation and Program Planning 1979;2(2):197‐207. [DOI] [PubMed] [Google Scholar]
LeDuc 1995
- LeDuc PA, Mittleman G. Schizophrenia and psychostimulant abuse: a review and re‐analysis of clinical evidence. Psychopharmacology 1995;121:407‐27. [DOI] [PubMed] [Google Scholar]
Lehman 1988
- Lehman AF. A quality of life interview for the chronically mentally ill. Evaluation and Program Planning 1988;11(1):51‐62. [Google Scholar]
Lehman 1995
- Lehman AF. Evaluating Quality of Life for Persons with Severe Mental Ilness: Assessment Toolkit. Cambridge, Mass: The Evaluation Center at Health Services Research Institute, 1995. [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [PUBMED: 16905632] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187(October):366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2004
- Lowe AL, Abou‐Saleh MT. The British experience of dual diagnosis in the national health service. Acta Neuropsychiatrica 2004;16(1):41‐6. [DOI] [PubMed] [Google Scholar]
Lubman 2010
- Lubman DI, King JA, Castle DJ. Treating comorbid substance use disorders in schizophrenia. International Review of Psychiatry 2010;22(2):191‐201. [DOI] [PubMed] [Google Scholar]
Lukoff 1986
- Lukoff D, Nuechterlein KH, Ventura J. Manual for expanded Brief Psychiatric Rating Scale (BPRS). Schizophrenia Bulletin 1986;12(4):594‐602. [Google Scholar]
Marshall 1998
- Marshall M, Gray A, Lockwood A, Green R. Case management for people with severe mental disorders. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000050] [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176(March):249‐52. [DOI] [PubMed] [Google Scholar]
Mas‐Esposito 2015
- Mas‐Exposito L, Amador‐Campos JA, Gomez‐Benito J, Mauri‐Mas L, Lalucat‐Jo L. Clinical case management for patients with schizophrenia with high care needs. Community Mental Health Journal 2015;51(2):165‐70. [DOI] [PubMed] [Google Scholar]
McHugo 1995
- McHugo GJ, Drake RE, Burton HL, Ackerson TH. A scale for assessing the stage of substance abuse treatment in persons with severe mental illness. Journal of Nervous and Mental Disease 1995;183(12):762‐7. [DOI] [PubMed] [Google Scholar]
McKetin 2013
- McKetin R, Lubman DI, Baker AL, Dawe S, Ali RL. Dose‐related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry 2013;70(3):319‐24. [DOI] [PubMed] [Google Scholar]
McLellan 1980
- McLellan AT, Luborsky L, Woody GE, O'Brien C. An improved evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Disease 1980;168(1):26‐33. [DOI] [PubMed] [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment 1992;9(3):199‐213. [DOI] [PubMed] [Google Scholar]
Mihalopoulos 1999
- Mihalopoulos C, McGorry PD, Carter RC. Is phase‐specific community ‐oriented treatment of early psychosis an economically viable method of improving outcome?. Acta Psychiatrica Scandinavica 1999;100(1):47‐55. [DOI] [PubMed] [Google Scholar]
Miller 1987
- Miller WR, Marlatt AG. Brief Drinker Profile (BDP) Interview Booklet. Odessa, FL: Psychological Assessment Resources, Inc, 1987. [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Montgomery 1978
- Montgomery SA, Asberg M, Taylor P, Montgomery D. Development of a schizophrenia scale sensitive to change. Neuropharmacology 1978;17(12):1061‐3. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(April):382‐9. [DOI] [PubMed] [Google Scholar]
Morgan 2017
- Morgan VA, Waterreus A, Carr V, Castle D, Cohen M, Harvey C, et al. Responding to challenges for people with psychotic illness: Updated evidence from the Survey of High Impact Psychosis. Australian & New Zealand Journal of Psychiatry 2017;51(2):124‐40. [DOI] [PubMed] [Google Scholar]
Mueser 1995
- Mueser KT, Drake RE, Clark RE, McHugo GJ, Mercer‐McFadden C, Ackerson TH. Evaluating Substance Abuse in Persons with Severe Mental Illness. Cambridge, MA: Human Services Research Institute, 1995. [Google Scholar]
Mueser 2004
- Mueser KT. Clinical interventions for severe mental illness and co‐occurring substance use disorder. Acta Neuropsychiatrica 2004;16(1):26‐35. [DOI] [PubMed] [Google Scholar]
Mueser 2005
- Mueser KT, Drake RE, Sigmon SC, Brunette MF. Psychosocial interventions of adults with severe mental illnesses and co‐occurring substance use disorders: a review of specific interventions. Journal of Dual Diagnosis 2005;1(2):57‐82. [Google Scholar]
Mueser 2013
- Mueser KT, Deavers F, Penn DL, Cassisi JE. Psychosocial treatments for schizophrenia. Annual Review of Clinical Psychology 2013;9:465‐97. [DOI] [PubMed] [Google Scholar]
Murthy 2012
- Murthy P, Chand P. Treatment of dual diagnosis disorders. Current Opinion in Psychiatry 2012;25(3):194‐200. [DOI] [PubMed] [Google Scholar]
NICE 2011
- NICE. Psychosis with coexisting substance misuse. Assessment and management in adults and young people. National Clinical Guideline 120. London: British Psychological Society and the Royal College of Psychiatrists, 2011:1‐322. [http://www.nice.org.uk/CG120] [PubMed] [Google Scholar]
Norman 1996
- Norman RM, Malla AK, Cortese L, Diaz F. A study of the interrelationship between and comparative interrater reliability of the SAPS, SANS and PANSS. Schizophrenia Research 1996;19(1):73‐85. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10(3):799‐812. [Google Scholar]
Pettersen 2014
- Pettersen H, Ruud T, Ravndal E, Havnes I, Landheim A. Engagement in assertive community treatment as experienced by recovering clients with severe mental illness and concurrent substance use. International Journal of Mental Health Systems 2014;8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pluddemann 2013
- Pluddemann A, Dada S, Parry CD, Kader R, Parker JS, Temmingh H, et al. Monitoring the prevalence of methamphetamine‐related presentations at psychiatric hospitals in Cape Town, South Africa. African Journal of Psychiatry 2013;16(1):45‐9. [DOI] [PubMed] [Google Scholar]
Priebe 1999
- Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). International Journal of Social Psychiatry 1999;45(1):7‐12. [DOI] [PubMed] [Google Scholar]
Rector 2012
- Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. Journal of Nervous & Mental Disease 2012;200(10):832‐9. [DOI] [PubMed] [Google Scholar]
Regier 1990
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. JAMA 1990;264(19):2511‐8. [PubMed] [Google Scholar]
Rollnick 1992
- Rollnick S, Heather N, Gold R, Hall W. Development of a short 'readiness to change' questionnaire for use in brief opportunistic interventions among excessive drinkers. British Journal of Addiction 1992;87(5):743‐54. [DOI] [PubMed] [Google Scholar]
Romer Thomsen 2018
- Romer Thomsen K, Thylstrup B, Pedersen MM, Pedersen MU, Simonsen E, Hesse M. Drug‐related predictors of readmission for schizophrenia among patients admitted to treatment for drug use disorders. Schizophrenia Research 2018;195:495‐500. [DOI] [PubMed] [Google Scholar]
Sara 2015
- Sara GE, Large MM, Matheson SL, Burgess PM, Malhi GS, Whiteford HA, et al. Stimulant use disorders in people with psychosis: a meta‐analysis of rate and factors affecting variation. Australian & New Zealand Journal of Psychiatry 2015;49(2):106‐17. [DOI] [PubMed] [Google Scholar]
Saunders 1993
- Saunders JB, Aasland OG, Babor TF de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption‐‐II. Addiction 1993;88(6):791‐804. [DOI] [PubMed] [Google Scholar]
Schmidt 2011
- Schmidt LM, HesseM, Lykke J. The impact of substance use disorders on the course of schizophrenia ‐ a 15‐year follow‐up study: dual diagnosis over 15 years. Schizophrenia Research 2011;130(1‐3):228‐33. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seibyl 1993
- Seibyl JP, Satel SL, Dominic A, Southwick SM, Krystal JH, Charney DS. Effects of cocaine on hospital course in schizophrenia. Journal of Nervous and Mental Disease 1993;181(1):30‐7. [DOI] [PubMed] [Google Scholar]
Seivewright 2005
- Seivewright N, McMahon C, Egleston P. Stimulant use still going strong: revisiting ....misuse of amphetamines and related drugs. Advances in Psychiatric Treatment 2005;11(July):262‐9. [Google Scholar]
Shokraneh 2017
- Shokraneh F, Adams CE. Study‐based registers of randomized controlled trials: Starting a systematic review with data extraction or meta‐analysis. BioImpacts 2017;7(4):209‐17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegfried 1998
- Siegfried N. A review of comorbidity: major mental illness and problematic substance use. Australian and New Zealand Journal of Psychiatry 1998;32(5):707‐17. [DOI] [PubMed] [Google Scholar]
Skevington 2004
- Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL‐BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research 2004;13(2):299‐310. [DOI] [PubMed] [Google Scholar]
Spitzer 1990
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM‐III‐R ‐ Patient Edition (SCID‐P). Washington, DC: American Psychiatric Press, 1990. [Google Scholar]
Stein 1980
- Stein LI, Test MA. Alternative to mental hospital treatment: 1 Conceptual model, treatment program, and clinical evaluation. Archives of General Psychiatry 1980;37(4):392‐7. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D (editors) on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Thoma 2015
- Thoma N, Pilecki B, McKay D. Contemporary cognitive behavior therapy: A review of theory, history, and evidence. Psychodynamic Psychiatry 2015;43(3):423‐62. [DOI] [PubMed] [Google Scholar]
Thompson 2000
- Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophrenia Research 2000;42(3):240‐7. [DOI] [PubMed] [Google Scholar]
Tiet 2007
- Tiet QQ, Mausbach B. Treatment for patients with dual diagnosis: a review. Alcoholism: Clinical and Experimental Research 2007;31(4):513‐36. [DOI] [PubMed] [Google Scholar]
Todd 2004
- Todd J, Green G, Harrison M, Ikuesan BA, Self C, Baldacchino A, et al. Defining dual diagnosis of mental illness and substance misuse: some methodological issues. Journal of Psychiatric and Mental Health Nursing 2004;11(1):48‐54. [DOI] [PubMed] [Google Scholar]
Tsuang 2006
- Tsuang J, Fong TW, Lesser I. Psychosocial treatment of patients with schizophrenia and substance abuse disorders. Addictive Disorders and Their Treatment 2006;5(2):53‐66. [Google Scholar]
Turpin 2005
- Turpin DL. CONSORT and QUOROM guidelines for reporting randomized clinical trials and systematic reviews. American Journal of Orthodontics and Dentofacial Orthodapedics 2005;128(6):681‐6. [DOI] [PubMed] [Google Scholar]
Tyrer 1984
- Tyrer P, Owen RT, Cicchetti DV. The brief scale for anxiety: a subdivision of the Comprehensive Psychopathological Rating Scale. Journal of Neurology, Neurosurgery and Psychiatry 1984;47(9):970‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyrer 2004
- Tyrer P, Weaver T. Desperately seeking solutions: the search for appropriate treatment for comorbid substance misuse and psychosis. Psychiatric Bulletin 2004;28(1):1‐2. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Warren 2007
- Warren JI, Stein JA, Grella CE. Role of social support and self‐efficacy in treatment outcomes among clients with co‐occurring disorders. Drug and Alcohol Dependence. 2007;89(2‐3):267‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wieduwilt 1999
- Wieduwilt KM, Jerrell JM. The reliability and validity of the SAS‐SMI. Journal of Psychiatric Research 1999;33(2):105‐12. [DOI] [PubMed] [Google Scholar]
Wing 1990
- Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry 1990;47(6):589‐93. [DOI] [PubMed] [Google Scholar]
Wing 1996
- Wing JK, Curtis R, Beevor A. The Health of the Nation Outcome Scales. London: Royal College of Psychiatrists, 1996. [Google Scholar]
Work Group 2007
- Work Group on Substance Use Disorders, Kleber HD, Weiss RD, Anton RF, Rounsaville BJ, George TP, Strain EC, et al. Treatment of patients with substance use disorders, second edition. American Psychiatric Association. Americal Journal of Psychiatry 2007;164 Suppl:5‐123. [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Young 1978
- Young RC, Briggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133(November):429‐35. [DOI] [PubMed] [Google Scholar]
Zanis 1997
- Zanis DA, McLellan AT, Corse S. Is the Addiction Severity Index a reliable and valid assessment instrument among clients with severe and persistent mental illness and substance abuse disorders?. Community Mental Health Journal 1997;33(3):213‐27. [DOI] [PubMed] [Google Scholar]
Ziedonis 2005
- Ziedonis DM, Smelson D, Rosenthal RN, Batki SL, Green AI, Henry RJ, et al. Improving the care of individuals with schizophrenia and substance use disorders: consensus recommendations. Journal of Psychiatric Practice 2005;11(5):315‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cleary 2008
- Cleary M, Hunt GE, Matheson SL, Siegfried N, Walter G. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001088.pub2] [DOI] [PubMed] [Google Scholar]
Hunt 2013
- Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001088.pub3] [DOI] [PubMed] [Google Scholar]
Ley 2000
- Ley A, Jeffery DP, McLaren S, Siegfried N. Treatment programmes for people with both severe mental illness and substance misuse. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001088] [DOI] [PubMed] [Google Scholar]


