Larvae of the sponge R. odorabile survived exposure to high concentrations of petroleum hydrocarbons; however, their ability to settle and metamorphose was adversely affected at environmentally relevant concentrations, and these effects were paralleled by marked changes in sponge gene expression and preceded by disruption of the symbiotic microbiome. Given the ecological importance of sponges, uncontrolled hydrocarbon releases from shipping accidents or production could affect sponge recruitment, which would have concomitant consequences for reef ecosystem function.
KEYWORDS: sponge larvae, hydrocarbon toxicity, gene expression, microbial symbiosis
ABSTRACT
Accidental oil spills from shipping and during extraction can threaten marine biota, particularly coral reef species which are already under pressure from anthropogenic disturbances. Marine sponges are an important structural and functional component of coral reef ecosystems; however, despite their ecological importance, little is known about how sponges and their microbial symbionts respond to petroleum products. Here, we use a systems biology-based approach to assess the effects of water-accommodated fractions (WAF) of crude oil, chemically enhanced water-accommodated fractions of crude oil (CWAF), and dispersant (Corexit EC9500A) on the survival, metamorphosis, gene expression, and microbial symbiosis of the abundant reef sponge Rhopaloeides odorabile in larval laboratory-based assays. Larval survival was unaffected by the 100% WAF treatment (107 μg liter−1 polycyclic aromatic hydrocarbon [PAH]), whereas significant decreases in metamorphosis were observed at 13% WAF (13.9 μg liter−1 PAH). The CWAF and dispersant treatments were more toxic, with decreases in metamorphosis identified at 0.8% (0.58 μg liter−1 PAH) and 1.6% (38 mg liter−1 Corexit EC9500A), respectively. In addition to the negative impact on larval settlement, significant changes in host gene expression and disruptions to the microbiome were evident, with microbial shifts detected at the lowest treatment level (1.6% WAF; 1.7 μg liter−1 PAH), including a significant reduction in the relative abundance of a previously described thaumarchaeal symbiont. The responsiveness of the R. odorabile microbial community to the lowest level of hydrocarbon treatment highlights the utility of the sponge microbiome as a sensitive marker for exposure to crude oils and dispersants.
IMPORTANCE Larvae of the sponge R. odorabile survived exposure to high concentrations of petroleum hydrocarbons; however, their ability to settle and metamorphose was adversely affected at environmentally relevant concentrations, and these effects were paralleled by marked changes in sponge gene expression and preceded by disruption of the symbiotic microbiome. Given the ecological importance of sponges, uncontrolled hydrocarbon releases from shipping accidents or production could affect sponge recruitment, which would have concomitant consequences for reef ecosystem function.
INTRODUCTION
Tropical coral reefs are currently facing unprecedented declines due to global climate change and declining water quality (1). Natural hydrocarbon reservoirs are often found adjacent to coral reefs (2, 3), raising a unique conservation challenge as exploratory and extraction drilling are frequently undertaken in close proximity to these environmentally important biodiversity hot spots. Petroleum hydrocarbon exposures from shipping accidents (4, 5) and spills from coastal and offshore processing facilities can significantly impact coral reef communities over decadal time scales (6, 7). Two high-profile oil spills, the Montara well-head platform incident off northwest Australia (which released ∼4,500 m3 of medium crude oil into the Timor Sea) (8–10) and, shortly afterwards, the Macondo Deepwater Horizon incident (which released ∼780,000 m3 of light crude oil into the Gulf of Mexico) (11–14), emphasize the importance of understanding the effects of hydrocarbon spills and response interventions (e.g., application of chemical dispersants) on sessile reef invertebrates.
Marine sponges can occupy up to 80% of the available substrate and are ecologically important constituents of benthic environments as they provide habitat for a diverse array of epi- and endofauna, couple the benthic and pelagic zones by filtering large quantities of seawater, mediate biogeochemical fluxes, and facilitate consumption and release of nutrients (15–20). Sponges often host dense and diverse microbial communities which can comprise up to 35% of the host biomass and contribute to many aspects of the sponge’s physiology and ecology (21–23). Considering the functional importance of the microbiome for host health, sponges are often described as “holobionts,” indicating an interdependent consortium comprising the sponge host and the associated bacteria, archaea, unicellular algae, fungi, and viruses (24). In determining the sensitivity of marine sponges to environmental stressors, such as hydrocarbons, it is therefore necessary to consider the response of both the host and the symbiotic microbial community. To date, very little research has addressed how hydrocarbons and other petroleum products affect the sponge holobiont, particularly for early life history stages and processes (25–29).
Marine sponges often have decoupled life history stages, with the planktonic larvae of many species performing vertical migration to aid dispersal by optimizing exposure to water currents (30). This behavior may bring them into direct contact with water-soluble and entrained oil as well as with surface slicks following oil spills. Understanding the impact of hydrocarbon exposure on marine larvae is critical because the survival of early life history phases underpins reef recovery and resilience following disturbances (31, 32). A few field (5) and laboratory (29, 33–39) studies have described significant adverse effects of hydrocarbon exposure on the early life history stages of corals, with larval settlement generally considered to be one of the most sensitive early life history processes (29). Oil spill interventions often involve the application of large quantities of chemical dispersants (including surfactants) to promote oil solubility and reduce the impact of surface slicks (40). While dispersants have a lower toxicity than dissolved oil, they can increase the solubility of polycyclic aromatic hydrocarbons (PAHs) and therefore increase exposure to benthic and pelagic organisms (41). Despite the ecological importance of sponges, there is no available data on how they respond to dispersants, and only two studies have tested the impacts of oils or PAHs on sponge larvae, with contradictory results. While larvae of the encrusting sponge Crambe crambe were described as being sensitive to hydrocarbon exposure, with a nominal concentration of 0.5 μg liter−1 PAH mix (25) affecting metamorphosis, larvae of the demosponge Rhopaloeides odorabile were insensitive to condensate (liquid fraction from gas wells), with metamorphosis unaffected until dissolved total petroleum aromatic hydrocarbon (TPAH) concentrations exceeded 11,000 μg liter−1 (29).
Organisms cope with environmental stress by modifying their physiological functions and gene expression patterns to achieve cellular homeostasis (42). Although researchers have explored shifts in sponge gene expression in response to thermal stress (43–48), heavy metals (49, 50), and polychlorinated biphenyls (51), the molecular-level stress response of sponges to hydrocarbons has never been assessed. Similarly, a considerable body of research has evaluated how the sponge microbiome responds to various stressors, including temperature (52–54), carbonate chemistry (55), nutrients (56, 57), heavy metals (58–60), and sediments (61–64), but no studies have assessed how sponge symbionts respond to hydrocarbons. Interestingly, while many of these sponge microbiome studies report microbial community shifts with declining host health, others report remarkably stable microbial communities irrespective of host health or stressor level, indicating that the environmental sensitivity of sponge microbiomes is highly species and stressor specific. In addition, metaproteomic research has shown that while the genomic composition of the sponge microbiome may stay relatively stable upon initial exposure to environmental stress, expression of important symbiotic functions can be immediately affected, and this dysbiosis likely contributes to the overall host stress response (65).
The toxicity of crude oils extracted from the Northwest Shelf of Australia has been assessed for several temperate and tropical species (34, 66), yet the toxicity to sessile tropical reef sponges is unknown. To comprehensively explore the impacts of oil pollution on the larval sponge holobiont, we examined the acute toxicity of various concentrations of (i) water-accommodated fractions (WAFs) of crude oil, (ii) chemically enhanced WAFs (CWAFs) of crude oil, and (iii) dispersant to larvae of the abundant reef sponge Rhopaloeides odorabile. To quantify the holobiont stress response, we applied a multifaceted approach integrating standard ecotoxicological testing, larval settlement assays, multiplexed reverse transcription-quantitative PCR (mRT-qPCR) host gene expression analysis, and community profiling of the symbiotic microbial community. Identifying sensitive biological indicators for sponge stress responses to hydrocarbons will contribute to improving risk assessments and informing oil spill responses for the oil and gas industry, regulators, and spill responders.
RESULTS
To determine the larval sponge holobiont response to hydrocarbon exposure, a broad suite of response variables were measured, including survival, metamorphosis, host gene expression, and microbiome composition. The sensitivity of each of these parameters is summarized in Table 1. For ease of reference, the sensitivity of each of the endpoints is reported throughout the text as percent WAF or percent CWAF and total PAH (ΣPAH). The respective total petroleum hydrocarbons (TPH) and dispersant Corexit EC9500A concentrations can be found in Table 1.
TABLE 1.
Summary of response variables for each petroleum product treatment concentrationa
| Treatment and concn (%) |
ΣPAH (μg/liter) |
TPH (μg/liter) |
Corexit EC9500A (mg/liter)b |
Survival (%)c |
Metamorphosis (%)c |
Gene expression |
Sponge microbiome |
|---|---|---|---|---|---|---|---|
| WAF | |||||||
| 0 | 0 | 0 | ND | 100 | 31 ± 6 | ND | ND |
| 0.8 | 0.86 | 32.5 | ND | 100 | 25 ± 6 | ND | ND |
| 1.6 | 1.7 | 65.0 | ND | 100 | 28 ± 5 | X | ✓ |
| 3.1 | 3.3 | 126 | ND | 100 | 24 ± 8 | ND | ND |
| 6.3 | 6.8 | 256 | ND | 100 | 28 ± 2 | ND | ND |
| 13 | 13.9 | 528 | ND | 100 | 6.7 ± 3.9 | ND | ND |
| 25 | 26.8 | 1,015 | ND | 99 ± 1 | 8.0 ± 3.3 | ✓ | ✓ |
| 50 | 53.6 | 2,030 | ND | 100 | 1.3 ± 1.1 | ND | ND |
| 75 | 80.4 | 3,045 | ND | 100 | 4.0 ± 1.9 | ND | ND |
| 100 | 107.2 | 4,060 | ND | 100 | 2.7 ± 1.1 | ✓ | ✓ |
| CWAF | |||||||
| 0 | 0 | 0 | 0 | 100 | 31 ± 6 | ||
| 0.8 | 0.58 | 273.6 | 19 | 100 | 2.7 ± 2.2 | ND | ND |
| 1.6 | 1.2 | 547.2 | 38 | 100 | 9.3 ± 4.4 | X | ✓ |
| 3.1 | 2.2 | 1,060 | 74 | 100 | 1.3 ± 1.1 | ND | ND |
| 6.3 | 4.6 | 2,155 | 149 | 100 | 4.0 ± 1.9 | ND | ND |
| 13 | 9.4 | 4,446 | 308 | 100 | 2.7 ± 1.1 | ND | ND |
| 25 | 18.1 | 8,550 | 593 | 100 | 1.3 ± 1.1 | ✓ | ✓ |
| 50 | 36.2 | 17,100 | 1,186 | 0 | 0 | ✓≠ | X |
| 75 | 54.2 | 25,650 | 1,779 | 0 | 0 | ND | ND |
| 100 | 72.3 | 34,200 | 2,373 | 0 | 0 | ND | ND |
| Corexit | |||||||
| 0 | ND | ND | 0 | 100 | 31 ± 6 | ND | ND |
| 0.8 | ND | ND | 19 | 100 | 82.7 ± 4.4 | X | ND |
| 1.6 | ND | ND | 38 | 100 | 5.3 ± 1.1 | ✓≠ | ND |
| 3.1 | ND | ND | 74 | 4.0 ± 3.3 | 0 | ND | ND |
| 6.3 | ND | ND | 149 | 0 | 0 | ND | ND |
| Otherd | ND | ND | ≥308 | 0 | 0 | ND | ND |
Petroleum hydrocarbon analysis for total polycyclic aromatic hydrocarbons (ΣPAH) and total petroleum hydrocarbon (TPH) analysis can be found in Table S1 in the supplemental material. Light gray shading and X denote no significant difference; dark gray shading and a check mark (✓) denote a significant difference relative to levels in the control samples of the corresponding treatment (P < 0.05). ND, not done; ≠, gene expression change observed at 2 h, with no samples remaining to test at 24 h.
Nominal concentration.
Survival and metamorphosis were scored after 48 h (mean ± standard error).
Concentrations of 13, 25, 50, 75, and 100%.
SIMPER analysis of the genes from the control and the 25% WAF and CWAF exposures. Download Table S1, DOCX file, 0.02 MB (24.5KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Larval survival and metamorphosis.
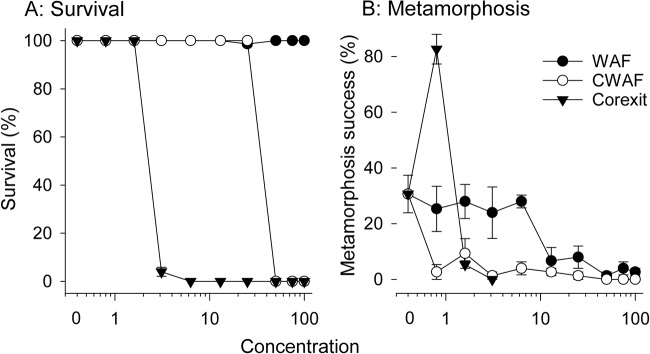
Larval survival was 100% in control samples and remained unaffected at all WAF concentrations including 100% (Table 1; Fig. 1A). In contrast, all larvae exposed to ≥50% CWAF were killed, as were all larvae exposed to ≥3.1% Corexit EC9500A (Table 1; Fig. 1A). Due to sharp drops from 100% to 0% survival for both CWAF and Corexit EC9500A treatments, 50% lethal concentration (LC50) values could not be calculated. The no-observed-effect concentration (NOEC) and lowest-observed-effect concentration (LOEC) for each treatment are reported in Table 2.
FIG 1.
Mean survival (A) and metamorphosis success (B) of sponge larvae exposed to WAFs, CWAsF, and Corexit EC9500A after 48 h versus concentrations of the treatments in percentages (n = 3 replicates per concentration ± standard error). Results are presented relative to percent treatment solution as the three solutions were prepared identically (corresponding ΣPAH, TPH, and Corexit EC9500A concentrations for each dilution are listed in Table 1).
TABLE 2.
Concentrations of total PAHs and dispersant with effects on survival and metamorphosis
| Response variable and parametera | WAF ΣPAH |
CWAF ΣPAH |
Corexit EC9500A |
|||
|---|---|---|---|---|---|---|
| Concn (μg/liter) | Treatment (%)d | Concn (μg/liter) | Treatment (%)d | Concn (mg/liter) | WAF treatment (%) | |
| Survival | ||||||
| LOEC | 18.1 | 25 | 38 | 1.6 | ||
| NOEC | >107 | 100 | 36.2 | 50 | 19 | 0.8 |
| Metamorphosis | ||||||
| LOEC | 14 | 13 | 0.58 | 0.8 | 38 | 1.6 |
| NOEC | 6.8 | 6.3 | <0.1 | 19 | 0.8 | |
| EC50 | 12 | 6.3–13b | NAc | NA | ||
Lowest-observed-effect concentration (LOEC) and no-observed-effect concentration (NOEC) for ΣPAH were calculated from one-way ANOVA (P < 0.01). EC50 settlement in sponge larvae was calculated from four-parameter logistic models (see Fig. S1 in the supplemental material).
Values represent the 95% confidence interval.
NA, not available. The EC50 could not be calculated due to limited data points on the slopes of dose-response curves.
Corresponding TPH concentrations can be read from Table 1.
Metamorphosis of sponge larvae (percent relative to 0% WAF controls) for ΣPAHs fitted to four-parameter logistic curve. Download FIG S1, PDF file, 0.1 MB (123.6KB, pdf) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
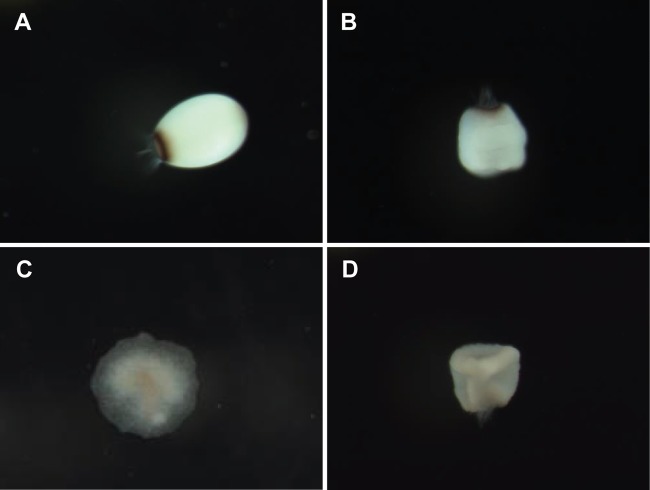
Metamorphosis of R. odorabile larvae was defined as the point at which the planktonic larvae (Fig. 2A) attached to the surface and underwent flattening of the entire body to form a disc-like morphology, with the center showing the remnants of the posterior larval pole (Fig. 2C) (30). Larval metamorphosis was 31% ± 6% in control treatments (Fig. 1B). The 13% WAF treatment caused significant (P < 0.01; analysis of variance [ANOVA], F9, 33 = 4.2) reductions in successful metamorphosis to 6.7% (Fig. 1B and Table 1). The 50% effective concentration (EC50) value for ΣPAHs in the WAF was 12 μg liter−1 (95% confidence interval [CI], 6.8 to 18 μg liter−1) (Table 2; see also Fig. S1 in the supplemental material). Larval metamorphosis was significantly reduced at all CWAF concentrations of ≥0.8% (P < 0.01; ANOVA, F9, 33 = 6.4) but the EC50 values for CWAF could not be calculated as there were limited data between minimum and maximum inhibition levels (Fig. S1). Larvae exposed to the higher CWAF concentrations mutated into irregular shapes and did not successfully metamorphose (Fig. 2B and D). The addition of Corexit EC9500A alone significantly inhibited larval metamorphosis to 5% at 38 mg liter−1 (P < 0.01; ANOVA, F9, 33 = 33.3), and this decreased to zero at higher Corexit EC9500A concentrations (Table 1), but interestingly metamorphosis was stimulated to 83% at 19 mg liter−1 (Fig. 1B; Table 1).
FIG 2.
Planktonic larvae in control (A) and 25% CWAF (B) treatments after 24 h of treatment exposure. Larvae under control conditions successfully settle and metamorphose (C), whereas larvae treated with 25% CWAF were deformed and did not successfully metamorphose (D). Approximate larval length is 270 ± 4.17 μm (113).
Host gene expression.
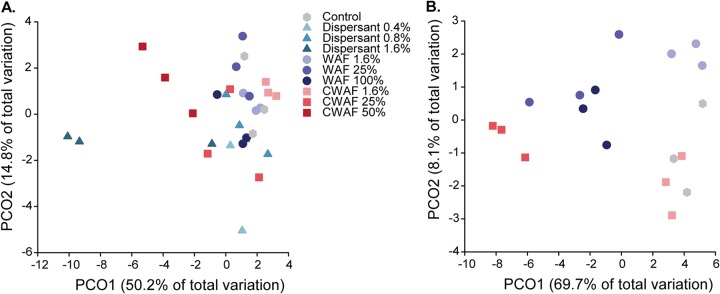
Larval gene expression was significantly affected by petroleum hydrocarbons after only 2 h exposure (permutational multivariate analysis of variance [PERMANOVA], pseudo-F9,20 = 4.31, P = 0.001) (Fig. 3A). The ordination demonstrates two clear patterns: first the separation of the 1.6% Corexit EC9500A (38 mg liter−1) treatment from all other samples and, second, a notable separation of samples in the 25% (18.1 μg liter−1 ΣPAH) and 50% (36.2 μg liter−1 ΣPAH) CWAF treatments from the controls (Fig. 3A). After 24 h, larvae from the 1.6% (1.7 μg liter−1 ΣPAH) WAF and 1.6% (1.2 μg liter−1 ΣPAH) CWAF treatments were not significantly different from those of the controls (P > 0.05); however, a significant difference was detected at 25% WAF (26.8 μg liter−1 ΣPAH; Monte Carlo P value [P(MC) = 0.012]) and 25% CWAF [18.1 μg l −1 ΣPAH; P(MC) = 0.001], also clearly separated in the ordination (Fig. 3B). Similarity percentage (SIMPER) analysis of samples from the 24-h exposure revealed that increased expression of heat shock protein 70 (HSP70) (29.56%), actin-related protein 2/3 (ARP2/3) complex (6.97%), profilin (6.13%), actin (5.57%), ferritin (5.57%), and HSP90 (5.26%) contributed most to the dissimilarity in expression profiles between samples in the control and 25% WAF (26.8 μg liter−1 ΣPAH) treatments (Table S1). Increased expression of HSP70 (26.38%), polyubiquitin (11.35%), ferritin (10.11%), profilin (6.92%), and HSP90 (6.82%) also contributed most to the dissimilarity in gene expression profiles between samples in the control and 25% CWAF (18.1 μg liter−1 ΣPAH) treatments after 24 h (Table S1). No significant differences in gene expression levels were evident between 25% WAF (26.8 μg liter−1 ΣPAH) and 100% WAF (107.2 μg liter−1 ΣPAH) (P > 0.05).
FIG 3.
PCO based on the Bray-Curtis similarity of gene expression values from 26 selected host genes after 2 h (A) and 24 h (B).
Microbial community analysis.
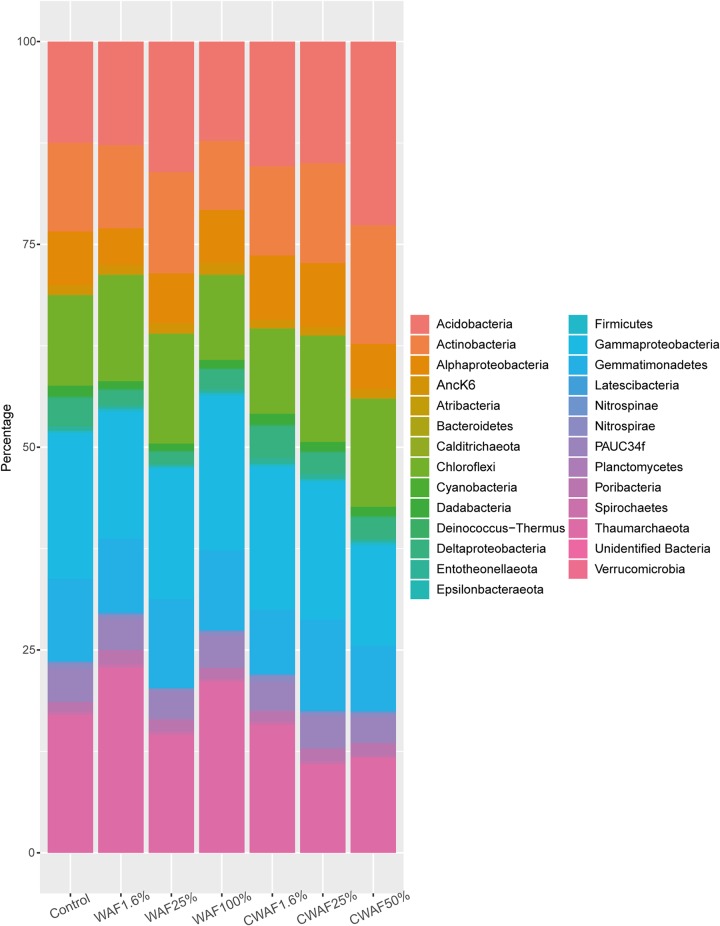
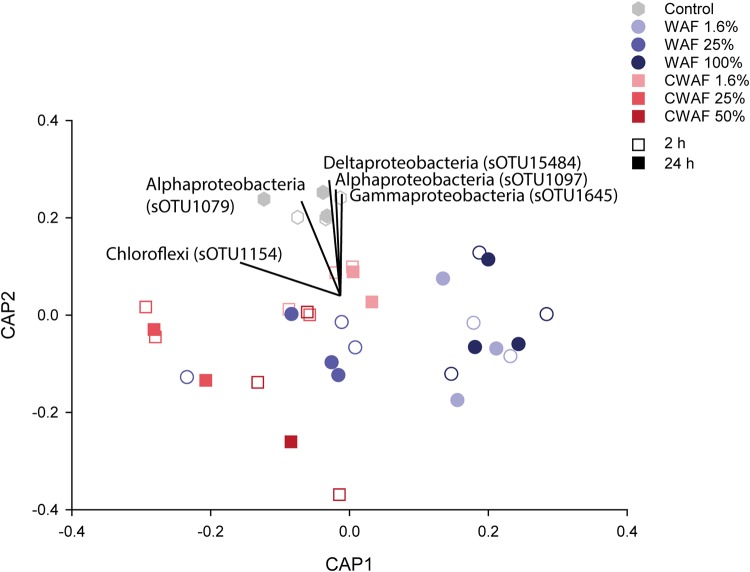
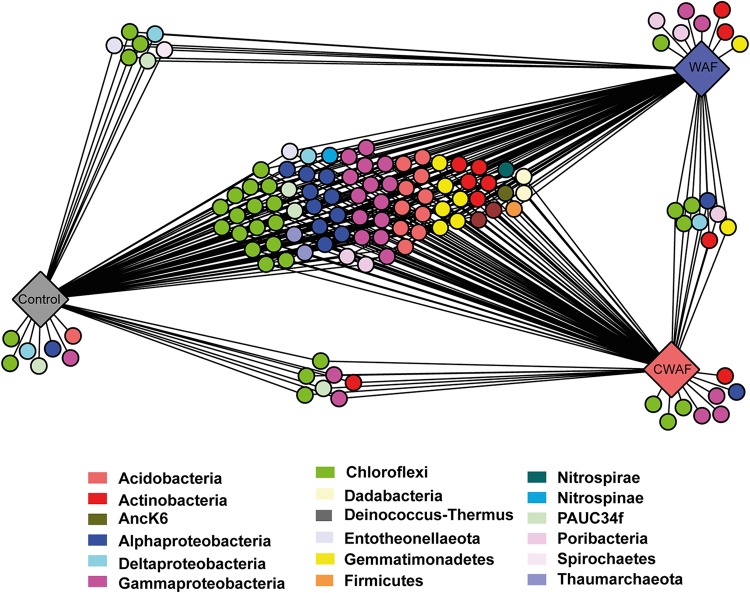
The R. odorabile microbiome is dominated by Gammaproteobacteria, Thaumarchaea, Acidobacteria, Gemmatimonadetes, Chloroflexi, PAUC34f, and Actinobacteria (Fig. 4). The microbiome was significantly affected by hydrocarbon treatment (PERMANOVA, pseudo-F6 = 1.655, P = 0.0438) (Fig. 4 and 5), with the microbial communities of sponge larvae exposed to WAF treatments of 1.6% (P = 0.0378), 25% (P = 0.0325), and 100% (P = 0.0258) all significantly different from those of the control samples. In contrast, the microbiome of CWAF-exposed larvae was only significantly different from that of the controls at 1.6% (P = 0.0171) and 25% (P = 0.0383) CWAF. While samples exposed to 50% CWAF were not significantly different, they clustered further from control samples in the ordination than the other two CWAF treatments (Fig. 5). The nonsignificant result likely reflects lower replication with this treatment (n = 4) (Table S2). A significant difference between time points was also observed (PERMANOVA, pseudo-F6 = 2.9448, P = 0.01), but no interaction between treatment and time was identified (PERMANOVA, pseudo-F6 = 0.9951, P = 0.1734), with treatment differences more distinct than those of time (Fig. 5). A previously described R. odorabile thaumarchaeal symbiont (sub-operational taxonomic unit 137 [sOTU137]) (67) also significantly decreased in abundance across all hydrocarbon treatments (ANOVA, F6 = 2.45, P = 0.04). A decrease in the relative abundances of Thaumarchaea was evident in sponges exposed to treatments of 25% CWAF and above, and a decrease in Gammaproteobacteria was detected at 50% CWAF (Fig. 4). In contrast, an increase in the relative abundance of Acidobacteria was evident in the microbiome of sponges exposed to the 50% CWAF treatment (Fig. 4). To identify specific microbial sOTUs primarily responsible for driving differences in community composition between control and WAF- and CWAF-treated samples, Cytoscape network analysis was performed using the 100 most abundant sOTUs in each treatment data set (i.e., control, WAF, and CWAF). While many of the dominant sOTUs were present across all treatments, seven OTUs were exclusively present in control samples, eight OTUs were exclusive to samples in the WAF treatment, and eight were exclusive to samples in the CWAF treatment, with an additional eight OTUs being shared between the WAF- and CWAF-treated samples but absent from the controls (Fig. 6; see Table S3 for sOTU details). Treatment-specific OTUs spanned multiple bacterial phyla and classes (Fig. 6; Table S3).
FIG 4.
Stacked bar chart depicting the relative abundance of each bacterial phyla, plus class for Proteobacteria, associated with each treatment.
FIG 5.
CAP analysis based on Bray-Curtis similarity of the OTUs derived from 16S rRNA gene sequencing of the Rhopaloeides odorabile larval microbiome from each treatment after 2 and 24 h.
FIG 6.
Cytoscape networks created using the 100 most abundant OTUs from each treatment.
EMP sample IDs for the WAF and CWAF exposure experiments. Download Table S2, DOCX file, 0.02 MB (21.9KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxonomic assignment of the most abundant sOTUs that were exclusive to a treatment. Symbionts of sponges are indicated by an asterisk (*) whereas symbionts of coral species are indicated by a plus sign (+). Download Table S3, DOCX file, 0.02 MB (21.9KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
General.
Sponges perform a range of important functional roles in marine systems (15), particularly on coral reefs where they process large volumes of seawater and efficiently remove the particulate and dissolved organic carbon (68, 69). The current study showed that R. odorabile larvae can survive high concentrations of petroleum hydrocarbons, but their ability to undergo successful settlement, crucial for recruitment, is affected at moderate concentrations of PAHs. This effect was exacerbated by the addition of the dispersant Corexit EC9500A. Effects on host gene expression and the associated microbiome were evident at sublethal concentrations of PAHs, in both the presence and absence of dispersant, providing valuable insights into stress response pathways. Considering the sensitivity of the symbiotic microbial community, assessment of the microbiome represents a promising indicator for monitoring sublethal stress responses in this sponge species.
Larval survival and settlement.
Although concentrations of PAHs are low in pristine coral reef ecosystems (70), the concentrations found in tropical and subtropical marine environments can be as high as 34.4 μg liter−1 in areas with no obvious signs of contamination (71–73). However, after large-scale accidental releases, such as the Deep Water Horizon spill, PAH concentrations reached ≥189 μg liter−1 (74), and even higher levels have been detected following bilge water discharges (e.g., 13,700 μg liter−1) (72). While R. odorabile larvae in this study were able to survive high concentrations of petroleum hydrocarbons, they lost the ability to settle and metamorphose at environmentally relevant concentrations (e.g., 13.9 to 26.8 μg liter−1).
The high tolerance of R. odorabile larvae to light crude WAFs from the Northwest Shelf of Australia is consistent with previous work showing high survival of the same species to WAFs of condensate (derived from a lighter Western Australian condensate) (29). Larval metamorphosis was more sensitive to the light crude oil in the present study (NOEC = 14 μg liter−1 ΣPAH) than to condensate exposures (NOEC = 121 μg liter−1 ΣPAH). These concentrations of PAHs (≥189 μg liter−1) were less than the concentrations identified in seawater following the Deep Water Horizon spill (74). However, comparing sensitivities of marine species to petroleum hydrocarbons between studies is notoriously difficult due to differences in exposure methodologies and in the ways in which hydrocarbon concentrations are measured and expressed (75, 76). For instance, the discrepancy in sensitivities between the two R. odorabile studies could be attributed to the WAFs from the current study having been prepared with more energy (a greater vortex), which would result in more whole-oil droplets in suspension (entrained oil, measured as TPH). These higher-energy WAF preparations are generally considered more toxic than lower-energy WAF preparations (77). The only other study to examine effects of PAHs on sponges found inhibition of metamorphosis of Crambe crambe larvae at only 0.5 μg liter−1 ΣPAH (25). The sensitivity of R. odorabile is more consistent with the sensitivity of coral larvae to condensate/light crude (29, 33), fuel oil (39), and individual PAHs (78); however, the disparate sensitivities of the only two sponge species analyzed to date highlight the need for standardized and comparative studies to establish relative species sensitivities of sponge larvae to oil pollution.
Chemical dispersion of the light crude oil by the dispersant Corexit EC9500A markedly increased the apparent toxicity of the treatments, causing total larval mortality and reduced metamorphosis at 50% and 13% CWAFs, respectively (compared with >100% and 50% for WAFs). This increase in toxicity is likely due to changes in the chemical composition of the test solutions, with CWAF containing >10-fold more TPHs than WAF, as well as the Corexit EC9500A itself. The lowest CWAF concentration 0.8% (0.58 μg liter−1 ΣPAH; 19 mg liter−1 Corexit) caused significant inhibition of metamorphosis, while metamorphosis was reduced at only 1.6% (38 mg liter−1) Corexit EC9500A solution alone, indicating that the combined effect of oil and dispersant was responsible for this higher larval sensitivity. Similar increases in toxicity of oil in the presence of dispersant have been observed for other marine species, including corals (34, 79–81). Sponge larval metamorphosis had a similar sensitivity to Corexit E9500A (LOEC = 38 mg liter−1) as larvae from multiple coral species (LOEC of 5 to 70 mg liter−1) (33, 82–84) (EC50 = 14 mg liter−1) (85). Intriguingly, the lowest exposure of Corexit EC9500A (19 mg liter−1) caused a large increase in settlement and metamorphosis (Table 1 and Fig. 1B). The most parsimonious explanation for this result is that, at this concentration, the dispersant mimics an external chemical inducer or internal signaling molecule that initiates metamorphosis. However, it may also be a sublethal stress response as thermal stress has been shown to increase settlement in this species (86). This type of response has not been reported for coral larvae over a wider range of exposures to five dispersants, including Corexit EC9500A (85), and further investigation is warranted as control of larval settlement in sponges may be useful for in vitro studies or reef restoration practices.
Gene expression.
Larval gene expression patterns were significantly affected at 26.8 μg liter−1 ΣPAH in the WAF treatment and at 18.1 μg liter−1 ΣPAH in the CWAF treatment. Host gene expression was disrupted by WAF and CWAF concentrations 2- to 4-fold lower than those causing larval mortality. Heat shock protein 70 (HSP70) contributed most to the differences between the control and the WAF and CWAF treatments, and HSP70 and HSP90 combined were responsible for 35% of the variation in expression, a stress response consistent with what has been observed for this species following exposure to elevated temperature (45). A similar molecular-level response has also been observed in corals, with increased expression of both HSP70 and HSP90 in Acropora tenuis larvae exposed to anthracene (78). Similarly, HSP70 was significantly upregulated in the coral Pocillopora damicornis when it was exposed to WAFs (87); and although expression levels were not quantified, HSP70 was identified via RT-PCR in the adult coral Stylophora pistillata exposed to five different WAF concentrations yet was undetectable in the control treatment (88). Other toxicants, such as heavy metals, induce a similar cellular stress response in reef taxa, with an upregulation of HSP70 identified in corals (89), ascidians (90), and sponges (91). Here, we observed changes in host gene expression profiles at sublethal concentrations of both WAFs and CWAFs. Given the sensitivity of HSP70 in multiple taxa exposed to various contaminants (78, 87), this gene represents a strong general bioindicator candidate for use to detect sublethal stress responses in marine species exposed to oil and pollution generally.
Sponge microbiome.
The R. odorabile larval microbiome was highly sensitive to hydrocarbon exposure, with a shift in the microbiome occurring at concentrations as low as 1.7 μg liter−1 ΣPAH in the WAF treatment and 1.2 μg liter−1 ΣPAH in the CWAF treatment. Sponge symbionts undertake a broad range of metabolic functions, including carbon, nitrogen, and sulfur metabolism, vitamin synthesis, production of bioactive metabolites, and nutrient transport (92–94); hence, microbial shifts or loss of key symbionts can have adverse impacts on the holobiont (52, 65, 95). Of particular interest for R. odorabile larvae exposed to hydrocarbons was the significant reduction in a putatively ammonia-oxidizing thaumarchaeal symbiont (67). The sensitivity of the R. odorabile thaumarchaeal symbiont is consistent with recent analyses showing that ammonia-oxidizing archaea are ∼1,000 times more sensitive to hydrocarbon contamination than heterotrophic bacteria (96). However, it could also be that this symbiont is particularly sensitive to environmental perturbation as previous research has demonstrated that it is highly sensitive to heavy metal contamination (60). Several microbial OTUs were identified as being exclusive to WAF (n = 8) or CWAF (n = 8) treatments, and these OTUs spanned multiple taxa, including Gammaproteobacteria, Alphaproteobacteria, Chloroflexi, Gemmatimonadetes, Poribacteria, and Actinobacteria (see Table S3 in the supplemental material). Interestingly, OTUs exclusive to WAF or CWAF treatments shared highest percent similarity to other sponge- or coral-associated bacteria. However, despite being among the 100 most abundant OTUs, taxa that were exclusive to the WAF and CWAF treatments comprised <1% of the total microbial community. It is likely that these OTUs are exceptionally rare (and therefore undetectable) in the sponge microbiome under control conditions but become selected for in the WAF and CWAF treatments. Alternatively, these novel microorganisms may have been acquired from the surrounding seawater as a low abundance of sponge-specific microbes has been previously detected within the rare seawater biosphere (97). Future studies should employ metagenomic approaches to determine whether these symbionts have the genomic potential to degrade hydrocarbons as previous studies of seawater (98–100), sediments (101–103), sand (104), biofilms (98), phytoplankton (105), mussels (106), sponges (106), and corals (107) have all shown increased relative abundances of putative hydrocarbon degraders following oil exposure.
Several recent studies have highlighted the potential for microorganisms to act as sensitive markers for environmental disturbance in reef ecosystems (reviewed in reference 108). In particular, sponge symbionts have been described as sublethal stress indicators for elevated seawater temperature (52, 53, 65) and copper contamination (60). This high environmental sensitivity supports the diagnostic value of the sponge microbiome and highlights how coral reef monitoring initiatives could be enhanced by incorporating assessments of sponge symbionts. The coral microbiome has also been shown to shift after exposure to crude oil, including higher relative abundances of putative hydrocarbon degraders such as Pseudomonas, Pseudoalteromonas, and Alteromonas versus a dominance of Vibrio in corals not exposed to oil (109). However, given Santos et al. used a longer exposure time (4 to 16 weeks) and did not perform chemical analysis, it remains unknown whether the coral microbiome is as responsive to WAFs as the sponge-larval microbiome.
Larval R. odorabile can survive high concentrations of WAFs; however, a loss of critical biological function is detected at spill-relevant ΣPAH concentrations, as evidenced by adverse effects on metamorphosis, settlement, host gene expression, and the microbiome. Clearly, exposure to petroleum hydrocarbons from accidental releases or spills has the potential to negatively impact sponge recruitment to adult populations, which can have adverse consequences for the ecology of reef systems. The identification of toxic thresholds (NOEC = 6.9 μg liter−1 ΣPAH) and effective concentrations (EC50 = 12 μg liter−1 ΣPAH) for sponge larval settlement for light crude oil adds to the very limited data available on coral reef-associated taxa. This study also revealed changes in sponge larval gene expression upon PAH exposure, particularly, increased expression of the HSP70 and HSP90 genes, which is consistent with reports for other marine species (78, 87). Importantly, the sponge microbiome proved to be the most sensitive indicator of sublethal stress following exposure to petroleum hydrocarbons and Corexit EC9500A. To better understand the consequences of this microbial dysbiosis (such as the reduced relative abundance of the dominant thaumarchaeal symbiont in PAH exposed sponges), future research should employ metagenomic and metatranscriptomic approaches to validate the link between disruption of key microbial pathways and host health. Finally, the clearly distinct microbial communities that develop in sponge larvae from the WAF, CWAF, and Corexit EC9500A treatments highlight the diagnostic utility of the R. odorabile microbiome as a sensitive in situ marker for exposure to hydrocarbon contamination. Monitoring of the R. odorabile microbiome has the potential to provide regulators and industry with an early indication of oil contamination on coral reefs.
MATERIALS AND METHODS
Preparation of WAFs and CWAFs.
A sample of light crude oil (36.1° American Petroleum Institute [API] gravity) from Barrow Island (northwest Western Australia) was provided by Chevron Australia, and the dispersant Corexit EC9500A was provided by the Australian Maritime Safety Authority. Water-accommodated fractions (WAFs) and chemically enhanced water-accommodated fractions (CWAFs) were prepared from the crude oil as previously described (110, 111). Briefly, the WAF was prepared by adding 1,600 ml of filtered (0.45-μm pore size) seawater (36 practical salinity units [PSU], pH 8.1) to a solvent-rinsed 2-liter glass aspirator bottle and mixed using a magnetic stirrer to generate a 20 to 25% vortex. Crude oil (40 ml) was subsequently added to the center of the vortex to achieve a concentration of 25 ml liter−1, the aspirator was loosely capped, and fluids were mixed for 18 h in darkness. To prepare CWAF, 4 ml of the dispersant Corexit EC9500A (1:10 dispersant/oil) was gently added to the top of the vortexing mixture described above and allowed to mix for 18 h (112). The WAFs and CWAFs were allowed to settle for 6 h before immediate water sampling for chemical analyses and applications in the larval assays. Dilutions of the 100% WAF and CWAF (100, 75, 50, 25, 12.5, 6.25, 3.13, 1.56, 0.78, and 0 % [vol/vol]) were prepared using filtered (0.45-μm pore size) seawater to mimic dilution in the water column (112). A separate solution of Corexit EC9500A was prepared in the same way by applying 4 ml of dispersant to 1,600 ml of filtered seawater, mixing, settling, and diluting as described above. Total petroleum hydrocarbons were analyzed by gas chromatography flame ionization detection (Queensland Government Forensic and Scientific Services [QHFSS] method 16308), and PAHs were analyzed by gas chromatography-mass spectrometry (QHFSS method 16647) at the National Association of Testing Authorities (NATA)-accredited Queensland Government Forensic and Scientific Services (Archerfield, Queensland, Australia). The 100% WAF and 100% CWAF contained 107 and 72 μg liter−1 total polycyclic aromatic hydrocarbons (ΣPAHs), respectively, and the total petroleum hydrocarbon (TPH) concentrations in the 100% WAF and the 100% CWAF were 1 and 2 orders of magnitude higher than the concentration of ΣPAHs, respectively (Table 1; see Table S4 in the supplemental material), indicating the presence of oil droplets in both preparations.
Concentrations of TPH and ΣPAH in the 100% WAF and CWAF of Barrow Island oil used in the current study. RL is the reporting limit. Download Table S4, DOCX file, 0.01 MB (15KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sponge collection and larval culture.
Rhopaloeides odorabile is a common gonochoristic Great Barrier Reef (GBR) sponge that broods tufted parenchymella larvae that are released during the Austral summer (113). Seven female sponges were collected from Davies Reef, central GBR, Australia (18°50.558′S, 147°37.618′E) and transported to the Australian Institute of Marine Science (AIMS). Sponges were maintained in flowthrough aquaria which allowed the controlled collection of larvae over several hours during their afternoon release. Larvae were collected using larval traps according to established methods (30, 114) and were pooled prior to being used in experimental assays.
Larval settlement assays.
Static WAF and CWAF exposures were conducted in 7-ml glass vials made up to 6.5 ml with 10 dilutions of either WAF, CWAF, or Corexit EC9500A and containing 25 larvae. Three replicate vials were used for each of the treatment concentrations. Vials were sealed with caps leaving an ∼0.5-ml headspace that enabled oxygen exchange (O2 concentrations maintained at >7.5 mg liter−1 over the 24-h exposure). Vials were transferred to an incubator shaker with 40 μE of light over a 12-h/12-h cycle at ∼60 rpm to maintain gentle water movement. Vials were removed after 24 h of exposure, and the larvae and treatment solutions from individual vials were transferred directly into individual six-well cell culture plates (12 ml; Nunc, NY, USA) that had been immersed in flowthrough aquaria for 48 h to develop an early microbial biofilm required for successful settlement (115). Metamorphosis was assessed after 48 h and scored as positive if larvae had firmly attached to the surface and undergone flattening of the body to form a disc-like morphology, with the center showing the remnants of the posterior larval pole (Fig. 2C) (30).
Additional experiments were completed to examine changes in host gene expression and the symbiotic microbial community following exposure to hydrocarbon treatments during the larval swimming phase. This series of exposures included a control and three WAF/CWAF treatment dilutions (100%, 25%, and 1.6%), with three replicate vials maintained for each concentration. In addition, due to insufficient larval numbers, microbial assays did not contain the Corexit EC9500A treatment. Experimental hydrocarbon treatments were prepared, and treatment exposures were conducted, according to the same procedures outlined above, excluding the settlement assays. Gene expression and microbiome changes were assessed 2 h and 24 h after treatment exposure. At the end of each exposure period, larvae were removed from the treatments, rinsed in filtered seawater, immersed in liquid nitrogen, and stored at –80°C.
Host mRT-qPCR analysis.
To investigate the expression profiles of 26 selected host genes in larvae exposed to three concentrations of WAF, CWAF, and Corexit EC9500A, we developed a multiplexed reverse transcription-quantitative PCR (mRT-qPCR) assay using a GenomeLab GeXP Genetic Analysis System (Beckman Coulter, Fullerton, CA). Experiments were conducted on pooled larvae for each treatment replicate, as previously described (45). Briefly, this method allows the sensitive and simultaneous detection of target genes in multiplexed reactions, with cDNA synthesis performed with target-specific primers and subsequent amplification with universal primers, removing the documented bias of PCR efficiency variation between genes. The set of 26 genes were selected based on their known or putative roles in the cell stress response and cellular homeostasis-related processes as previously described (44) (Table S5). Kanamycin (Kanr) was used as an internal control. Following the procedures of Webster and colleagues (45), mRNA was extracted from all larval sponge samples using a Dynabeads oligo(dT) kit (Invitrogen). Integrity of the mRNA was measured using an ND-1000 spectrophotometer (NanoDrop Technologies) with ratios of 260 nm/280 nm between 1.8 and 2 as the criteria. mRNA was reverse transcribed into cDNA and PCR amplified in 20-μl reaction mixtures containing 4 μl of PCR buffer (5×), 4 μl of MgCl2 (25 mM), 0.7 μl of Thermo-Start DNA polymerase (ABgene), 8.7 μl of cDNA, and 2 μl of forward primer (200 nM). The PCR thermal cycling protocol included 10 min at 95°C followed by 35 cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 70°C. PCR products were analyzed on an automated capillary electrophoresis sequencer CEQ 8800 Genetic Analysis System (Beckman-Coulter). Electropherograms were inspected for erroneous amplification products with a GenomeLab 178 Genetic Analysis System, version 10.0.29, software, and reproducibility was assessed by overlaying graphs from independent runs. Automatic filters were created to exclude false signals due to shoulder peaks, high homology, or alternative transcripts. Filtered positive data were imported and binned following a range extension of 2 bp in GenomeLab eXpress Profiler software. Finally, an expression stability measure according to Vandesompele et al. (116) for each of the 26 genes of interest was established in the GeNorm VBA applet for Microsoft Excel, and all positive amplicons were normalized against the geometric mean of the most stable pair of reference genes (RGs) (YWHAY and YWHAZ) in Excel. The geometric mean was calculated by averaging the Kanr normalized peak area of the RG pair, and peak areas of all other genes of interest were divided by this geometric mean. Gene expression data for both time points can be found in Data Set S1.
List of genes used in the multiplexed RT-qPCR assay. Data are from reference 45. Download Table S5, DOCX file, 0.01 MB (14.2KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gene expression data from the mRT-qPCR assay from both time points used as input for the SIMPER analysis. Download Data Set S1, XLSX file, 0.03 MB (28.8KB, xlsx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DNA extraction, sequencing, and processing for microbial community profiling.
Genomic DNA was extracted from pooled larvae using a PowerSoil high-throughput 96-well DNA isolation kit (MoBio Laboratories, Inc.), according to the manufacturer’s protocol. As part of the Earth Microbiome Project (EMP) (117), samples were sent to the University of Colorado, Boulder, CO, where 16S rRNA genes were PCR amplified and sequenced on an Illumina HiSeq 2500 platform using bacterial primers 515F/806R and standard protocols (118).
Quality-filtered, demultiplexed fastq sequences were denoised by collaborators at the sponge microbiome project using Deblur (119). Briefly, to create the deblurred BIOM table input, sequences were trimmed to 100 bp, and the number of minimum reads was 25. Taxonomy was added using Qiime, the Ribosomal Database Project (RDP) Classifier, and Greengenes, version 13.8 (120). Samples from the current study (Table S1) were extracted from the larger BIOM table, and sOTUs were reclassified using the SILVA database (version 132), using a minimum cutoff of 60% similarity. Singletons and doubletons, i.e., sOTUs formed by one or two sequences, respectively, across all samples, were removed from the data set. Several samples were removed from the analysis due to low numbers of sequence reads, resulting in <3 replicates per time point for some treatments (Table S2).
Data analyses.
Inhibition of metamorphosis (inhibition percent relative to 0% WAF control) was calculated from treatment data as follows: inhibition (%) = 100 × [(% metamorphosiscontrol − % metamorphosistreatment)/% metamorphosiscontrol]. The concentrations of PAHs and TPHs that inhibited 50% of metamorphosis (EC50) were calculated from concentration-response curves (four-parameter logistic models) fitted to the percent inhibition and from concentration data of each treatment using the program GraphPad Prism (version 6; San Diego, CA, USA). Analysis of variance (ANOVA) was performed to identify treatments which caused significant (P < 0.05) inhibition of metamorphosis in comparison to that of control treatments (NCSS, version 9; NCSS, Kaysville, UT).
Principal coordinate analysis (PCO) was used to visually compare larval gene expression patterns among treatments, and canonical analysis of principal coordinates (CAP) was used to visually compare microbial community patterns among treatments and time points. PERMANOVA, using 9,999 permutations, was used to test differences in both gene expression levels and microbial community structures between treatments. Samples from the two time points were combined for the microbial analysis due to the low replication levels with some treatments, with time included in the model. Where pairwise comparisons resulted in insufficient unique permutations, Monte Carlo P values were used. Similarity percentage (SIMPER) analysis was used to determine genes that contributed to differences in expression patterns and OTUs that contributed to differences in microbial community structure. The distribution of the 100 most abundant sOTUs across larval treatments was visualized using Cytoscape, version 3.2.1 (www.cytoscape.org) (121). To minimize the number of nodes in the Cytoscape network, 0 and 1.6% WAF treatments were pooled and assigned to the control group, and 25 and 100% WAF treatments were pooled and assigned to the WAF group. Given the increased toxicity of CWAFs, the control group was made up only of the 0% CWAF treatment, whereas the CWAF group was made up of the 1.6, 25, and 50% CWAF treatments combined. All statistical analyses were based on Bray-Curtis distances of square root-transformed data and were performed using PRIMER 6/PERMANOVA+, version 1.0.2 (Plymouth, United Kingdom).
Data availability.
Gene expression data for both time points can be found in Data Set S1. Processed sequences and metadata are available at http://qiita.microbio.me/ under study identification number 10793, and the deblurred BIOM table can be accessed through the GigaScience repository (https://doi.org/10.5524/100332) using sample identification numbers from Table S2.
ACKNOWLEDGMENTS
We thank the crew of the RV Cape Ferguson. We thank Rebecca Fisher for her discussions and advice on statistical analyses.
This study was funded by the Australian Institute of Marine Science.
REFERENCES
- 1.Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough JM, Morrison TH, Palumbi SR, van Nes EH, Scheffer M. 2017. Coral reefs in the Anthropocene. Nature 546:82. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 2.Hovland M. 1990. Do carbonate reefs form due to fluid seepage. Terra Nova 2:8. doi: 10.1111/j.1365-3121.1990.tb00031.x. [DOI] [Google Scholar]
- 3.O’Brien GW, Glenn KC. 2005. Natural hydrocarbon seepage, sub-seafloor geology and eustatic sea-level variations as key determiners of the nature and distribution of carbonate build-ups and other benthic habitats in the Timor Sea, p 31–42. In Russel BC, Larson HK, Glasby CJ, Wilan RC, Martin J (ed), The Beagle, records of the museums and art galleries of the Northern Territory. Museum and Art Gallery of the Northern Territory, Darwin, Australia. [Google Scholar]
- 4.Haapkylä J, Ramade F, Salvat B. 2007. Oil pollution on coral reefs: a review of the state of knowledge and management needs. Vie Milieu 57:95–111. [Google Scholar]
- 5.Loya Y, Rinkevich B. 1980. Effects of oil pollution on coral reef communities. Mar Ecol Prog Ser 2:167–180. doi: 10.3354/meps003167. [DOI] [Google Scholar]
- 6.Guzman HM, Burns KA, Jackson J. 1994. Injury, regeneration and growth of Caribbean reef corals after a major oil spill in Panama. Mar Ecol Prog Ser 105:231–241. doi: 10.3354/meps105231. [DOI] [Google Scholar]
- 7.Jackson JBC, Cubit JD, Keller BD, Batista V, Burns K, Caffey HM, Caldwell RL, Garrity SD, Getter CD, Gonzalez C, Guzman HM, Kaufmann KW, Knap AH, Levings SC, Marshall MJ, Steger R, Thompson RC, Weil E. 1989. Ecological effects of a major oil spill on Panamanian coastal marine communities. Science 243:37–44. doi: 10.1126/science.243.4887.37. [DOI] [PubMed] [Google Scholar]
- 8.Storrie J. 2011. Montara wellhead platform oil spill—a remote area response, abstr 159. Int Oil Spill Conf Proc, Portland, Oregon, 23 to 26 May 2011.
- 9.Heyward A, Jones RJ, Meeuwig J, Burns K, Radford B, Colquhoun J, Cappo M, Case M, O’Leary R, Fisher R, Meekan M, Stowar M. 2012. Montara: 2011 offshore banks assessment survey. Report for PTTEP Australasia (Ashmore Cartier) Pty. Ltd. Australian Institute of Marine Science, Townsville, Australia. [Google Scholar]
- 10.Short M. 2011. Montara well head platform spill—Australia’s first offshore oiled wildlife response, abstr 208. Int Oil Spill Conf Proc, Portland, Oregon, 23 to 26 May 2011.
- 11.McNutt MK, Camilli R, Crone TJ, Guthrie GD, Hsieh PA, Ryerson TB, Savas O, Shaffer F. 2012. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc Natl Acad Sci U S A 109:20260–20267. doi: 10.1073/pnas.1112139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atlas RM, Hazen TC. 2011. Oil biodegradation and bioremediation: a tale of the two worst spills in U.S. history. Environ Sci Technol 45:6709–6715. doi: 10.1021/es2013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White HK, Hsing P-Y, Cho W, Shank TM, Cordes EE, Quattrini AM, Nelson RK, Camilli R, Demopoulos AWJ, German CR, Brooks JM, Roberts HH, Shedd W, Reddy CM, Fisher CR. 2012. Impact of the Deepwater Horizon oil spill on a deep-water coral community in the Gulf of Mexico. Proc Natl Acad Sci U S A 109:20303–20308. doi: 10.1073/pnas.1118029109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher CR, Hsing P-Y, Kaiser CL, Yoerger DR, Roberts HH, Shedd WW, Cordes EE, Shank TM, Berlet SP, Saunders MG, Larcom EA, Brooks JM. 2014. Footprint of Deepwater Horizon blowout impact to deep-water coral communities. Proc Natl Acad Sci U S A 111:11744–11749. doi: 10.1073/pnas.1403492111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell JJ. 2008. The functional roles of marine sponges. Estuar Coast Shelf Sci 79:341–353. doi: 10.1016/j.ecss.2008.05.002. [DOI] [Google Scholar]
- 16.Southwell MW, Weisz J, Martens CS, Lindquist N. 2008. In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol Oceanogr 53:986–996. doi: 10.4319/lo.2008.53.3.0986. [DOI] [Google Scholar]
- 17.Maldonado M, Ribes M, van Duyl FC. 2012. Nutrient fluxes through sponges: biology, budgets, and ecological implications. Adv Mar Biol 62:113–182. doi: 10.1016/B978-0-12-394283-8.00003-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, Blasiak LC, Karolin JO, Powell RJ, Geddes CD, Hill RT. 2015. Phosphorus sequestration in the form of polyphosphate by microbial symbionts in marine sponges. Proc Natl Acad Sci U S A 112:4381–4386. doi: 10.1073/pnas.1423768112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado M, Aguilar R, Bannister RJ, Bell JJ, Conway KW, Dayton PK, Díaz C, Gutt J, Kelly M, Kenchington ELR, Leys SP, Pomponi SA, Rapp HT, Rützler K, Tendal OS, Vacelet J, Young CM. 2015. Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles, and conservation concerns, p 1–39. In Ross S, Bramanti L, Gori A, Orejas C (ed), Marine animal forests. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 20.de Goeij JM, van Oevelen D, Vermeij MJ, Osinga R, Middelburg JJ, de Goeij AF, Admiraal W. 2013. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110. doi: 10.1126/science.1241981. [DOI] [PubMed] [Google Scholar]
- 21.Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas T, Moitinho-Silva L, Lurgi M, Björk JR, Easson C, Astudillo-García C, Olson JB, Erwin PM, López-Legentil S, Luter H, Chaves-Fonnegra A, Costa R, Schupp PJ, Steindler L, Erpenbeck D, Gilbert J, Knight R, Ackermann G, Victor Lopez J, Taylor MW, Thacker RW, Montoya JM, Hentschel U, Webster NS. 2016. Diversity, structure and convergent evolution of the global sponge microbiome. Nat Commun 7:11870–11812. doi: 10.1038/ncomms11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster NS, Thomas T. 2016. The sponge hologenome. mBio 7:e00135. doi: 10.1128/mBio.00135-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster NS, Taylor MW. 2012. Marine sponges and their microbial symbionts: love and other relationships. Environ Microbiol 14:335–346. doi: 10.1111/j.1462-2920.2011.02460.x. [DOI] [PubMed] [Google Scholar]
- 25.Cebrian E, Uriz MJ. 2007. Contrasting effects of heavy metals and hydrocarbons on larval settlement and juvenile survival in sponges. Aquat Toxicol 81:137–143. doi: 10.1016/j.aquatox.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Batista D, Tellini K, Nudi AH, Massone TP, Scofield A. d L, Wagener A. d L R. 2013. Marine sponges as bioindicators of oil and combustion derived PAH in coastal waters. Mar Environ Res 92:234–243. doi: 10.1016/j.marenvres.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Mahaut ML, Basuyaux O, Baudiniere E, Chataignier C, Pain J, Caplat C. 2013. The porifera Hymeniacidon perlevis (Montagu, 1818) as a bioindicator for water quality monitoring. Environ Sci Pollut Res Int 20:2984–2992. doi: 10.1007/s11356-012-1211-7. [DOI] [PubMed] [Google Scholar]
- 28.Vad J, Kazanidis G, Henry L-A, Jones DOB, Tendal OS, Christiansen S, Henry TB, Roberts JM. 2018. Potential impacts of offshore oil and gas activities on deep-sea sponges and the habitats they form. Adv Mar Biol 79:33–60. doi: 10.1016/bs.amb.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Negri AP, Brinkman DL, Flores F, Botté ES, Jones RJ, Webster NS. 2016. Acute ecotoxicology of natural oil and gas condensate to coral reef larvae. Sci Rep 6:21153. doi: 10.1038/srep21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whalan S, Ettinger-Epstein P, Battershill C, de Nys R. 2008. Larval vertical migration and hierarchical selectivity of settlement in a brooding marine sponge. Mar Ecol Prog Ser 368:145–154. doi: 10.3354/meps07573. [DOI] [Google Scholar]
- 31.Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL. 1999. Patterns of recruitment and abundance of corals along the Great Barrier Reef. Nature 397:59–63. doi: 10.1038/16237. [DOI] [Google Scholar]
- 32.Richmond RH. 1993. Coral reefs: present problems and future concerns resulting from anthropogenic disturbance. Am Zool 33:524–536. doi: 10.1093/icb/33.6.524. [DOI] [Google Scholar]
- 33.Goodbody-Gringley G, Wetzel DL, Gillon D, Pulster E, Miller A, Ritchie KB. 2013. Toxicity of Deepwater Horizon source oil and the chemical dispersant, Corexit 9500, to coral larvae. PLoS One 8:e45574. doi: 10.1371/journal.pone.0045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negri AP, Heyward AJ. 2000. Inhibition of fertilization and larval metamorphosis of the coral Acropora millepora (Ehrenberg, 1834) by petroleum products. Marine Pollution Bull 41:420–427. doi: 10.1016/S0025-326X(00)00139-9. [DOI] [Google Scholar]
- 35.Mercurio P, Negri AP, Burns KA, Heyward AJ. 2004. The ecotoxicology of vegetable versus mineral based lubricating oils: 3. Coral fertilization and adult corals. Environ Pollut 129:183–194. doi: 10.1016/j.envpol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Te F. 1991. Effects of two petroleum products on Pocillopora damicornis planulae. Pacific Sciences 45:290–298. [Google Scholar]
- 37.Villanueva RD, Montano MNE, Yap HT. 2008. Effects of natural gas condensate—water accommodated fraction on coral larvae. Mar Pollut Bull 56:1422–1428. doi: 10.1016/j.marpolbul.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Villanueva RD, Yap HT, Montano M. 2011. Reproductive effects of the water-accommodated fraction of a natural gas condensate in the Indo-Pacific reef-building coral Pocillopora damicornis. Ecotoxicol Environ Saf 74:2268–2274. doi: 10.1016/j.ecoenv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Nordborg FM, Flores F, Brinkman DL, Agusti S, Negri AP. 2018. Phototoxic effects of two common marine fuels on the settlement success of the coral Acropora tenuis. Sci Rep 8:8635. doi: 10.1038/s41598-018-26972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council. 2005. Oil spill dispersants: efficacy and effects. The National Academies Press, Washington, DC. [Google Scholar]
- 41.Prince RC. 2015. Oil spill dispersants: boon or bane? Environ Sci Technol 49:6376–6384. doi: 10.1021/acs.est.5b00961. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann GE, Todgham AE. 2010. Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72:127–145. doi: 10.1146/annurev-physiol-021909-135900. [DOI] [PubMed] [Google Scholar]
- 43.López-Legentil S, Song B, Mcmurray SE, Pawlik JR. 2008. Bleaching and stress in coral reef ecosystems: hsp70 expression by the giant barrel sponge Xestospongia muta. Mol Ecol 17:1840–1849. doi: 10.1111/j.1365-294X.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- 44.Pantile R, Webster NS. 2011. Strict thermal threshold identified by quantitative PCR in the sponge Rhopaloeides odorabile. Mar Ecol Prog Ser 431:97–105. doi: 10.3354/meps09128. [DOI] [Google Scholar]
- 45.Webster N, Pantile R, Botté E, Abdo D, Andreakis N, Whalan S. 2013. A complex life cycle in a warming planet: gene expression in thermally stressed sponges. Mol Ecol 22:1854–1868. doi: 10.1111/mec.12213. [DOI] [PubMed] [Google Scholar]
- 46.Guzman C, Conaco C. 2016. Gene expression dynamics accompanying the sponge thermal stress response. PLoS One 11:e0165368. doi: 10.1371/journal.pone.0165368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller WEG, Koziol C, Kurelec B, Dapper J, Batel R, Rinkevich B. 1995. Combinatory effects of temperature stress and nonionic organic pollutants on stress protein (hps70) gene expression in the freshwater sponge Ephydatia Fluviatilis. Environ Toxicol Chem 14:1203–1208. doi: 10.1002/etc.5620140712. [DOI] [Google Scholar]
- 48.Koziol C, Batel R, Arinc E, Schröder HC, Müller W. 1997. Expression of the potential biomarker heat shock protein 70 and its regulator, the metazoan DnaJ homolog, by temperature stress in the sponge Geodia cydonium. Mar Ecol Prog Ser 154:261–268. doi: 10.3354/meps154261. [DOI] [Google Scholar]
- 49.Schröder HC, Efremova SM, Margulis BA, Guzhova IV, Itskovich VB, Müller W. 2006. Stress response in Baikalian sponges exposed to pollutants. Hydrobiologia 568:277–287. doi: 10.1007/s10750-006-0302-1. [DOI] [Google Scholar]
- 50.Schröder HC, Hassanein HMA, Lauenroth S, Koziol C, Mohamed T-A, Lacorn M, Steinhart H, Batel R, Müller W. 1999. Induction of DNA strand breaks and expression of HSP70 and GRP78 homolog by cadmium in the marine sponge Suberites domuncula. Arch Environ Contam Toxicol 36:47–55. doi: 10.1007/s002449900441. [DOI] [PubMed] [Google Scholar]
- 51.Wiens M, Koziol C, Hassanein HMA, Batel R, Schröder HC, Müller W. 1998. Induction of gene expression of the chaperones 14–3-3 and HSP70 by PCB 118 (2,3′,4,4′,5-pentachlorobiphenyl) in the marine sponge Geodia cydonium: novel biomarkers for polychlorinated biphenyls. Mar Ecol Prog Ser 165:247–257. doi: 10.3354/meps165247. [DOI] [Google Scholar]
- 52.Webster NS, Cobb RE, Negri AP. 2008. Temperature thresholds for bacterial symbiosis with a sponge. ISME J 2:830–842. doi: 10.1038/ismej.2008.42. [DOI] [PubMed] [Google Scholar]
- 53.Lemoine N, Buell N, Hill A, Hill M. 2007. Assessing the utility of sponge microbial symbiont communities as models to study global climate change: a case study with Halichondria bowerbanki, p 419–425. In Custodio MR, Lobo-Hajdu G, Hajdu E, Muricy G (ed), Porifera research: biodiversity, innovation and sustainability. Museu Nacional, Rio de Janeiro, Brazil. [Google Scholar]
- 54.López-Legentil S, Erwin PM, Pawlik JR, Song B. 2010. Effects of sponge bleaching on ammonia-oxidizing archaea: distribution and relative expression of ammonia monooxygenase genes associated with the barrel sponge Xestospongia muta. Microb Ecol 60:561–571. doi: 10.1007/s00248-010-9662-1. [DOI] [PubMed] [Google Scholar]
- 55.Morrow KM, Bourne DG, Humphrey C, Botté ES, Laffy P, Zaneveld JR, Uthicke S, Fabricius K, Webster NS. 2014. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J 9:894–908. doi: 10.1038/ismej.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luter HM, Gibb K, Webster NS. 2014. Eutrophication has no short-term effect on the Cymbastela stipitata holobiont. Front Microbiol 5:216. doi: 10.3389/fmicb.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simister R, Taylor MW, Tsai P, Webster N. 2012. Sponge-microbe associations survive high nutrients and temperatures. PLoS One 7:e52220. doi: 10.1371/journal.pone.0052220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian R-M, Wang Y, Bougouffa S, Gao Z-M, Cai L, Zhang W-P, Bajic VB, Qian P-Y. 2014. Effect of copper treatment on the composition and function of the bacterial community in the sponge Haliclona cymaeformis. mBio 5:e01980-14. doi: 10.1128/mBio.01980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selvin J, Shanmugha Priya S, Seghal Kiran G, Thangavelu T, Sapna Bai N. 2009. Sponge-associated marine bacteria as indicators of heavy metal pollution. Microbiol Res 164:352–363. doi: 10.1016/j.micres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Webster NS, Webb RI, Ridd MJ, Hill RT, Negri AP. 2001. The effects of copper on the microbial community of a coral reef sponge. Environ Microbiol 3:19–31. doi: 10.1046/j.1462-2920.2001.00155.x. [DOI] [PubMed] [Google Scholar]
- 61.Luter HM, Whalan S, Webster NS. 2012. Thermal and sedimentation stress are unlikely causes of brown spot syndrome in the coral reef sponge, Ianthella basta. PLoS One 7:e39779. doi: 10.1371/journal.pone.0039779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pineda MC, Duckworth A, Webster N. 2016. Appearance matters: sedimentation effects on different sponge morphologies. J Mar Biol Ass 96:481–492. doi: 10.1017/S0025315414001787. [DOI] [Google Scholar]
- 63.Pineda M-C, Strehlow B, Sternel M, Duckworth A, Haan J, Jones R, Webster NS. 2017. Effects of sediment smothering on the sponge holobiont with implications for dredging management. Sci Rep 7:5156. doi: 10.1038/s41598-017-05243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pineda M-C, Strehlow B, Sternel M, Duckworth A, Jones R, Webster NS. 2017. Effects of suspended sediments on the sponge holobiont with implications for dredging management. Sci Rep 7:4925. doi: 10.1038/s41598-017-05241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan L, Liu M, Simister R, Webster NS, Thomas T. 2013. Marine microbial symbiosis heats up: the phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J 7:991–1002. doi: 10.1038/ismej.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neff JM, Ostazeski S, Gardiner W, Stejskal I. 2000. Effects of weathering on the toxicity of three offshore Australian crude oils and a diesel fuel to marine animals. Environ Toxicol Chem 19:1809–1821. doi: 10.1002/etc.5620190715. [DOI] [Google Scholar]
- 67.Webster NS, Watts JEM, Hill RT. 2001. Detection and phylogenetic analysis of novel crenarchaeote and euryarchaeote 16S ribosomal RNA gene sequences from a Great Barrier Reef Sponge. Mar Biotechnol (NY) 3:600–608. doi: 10.1007/s10126-001-0065-7. [DOI] [PubMed] [Google Scholar]
- 68.McMurray SE, Pawlik JR, Finelli CM, El-Sabaawi R. 2017. Demography alters carbon flux for a dominant benthic suspension feeder, the giant barrel sponge, on Conch Reef, Florida Keys. Funct Ecol 31:2188–2198. doi: 10.1111/1365-2435.12908. [DOI] [Google Scholar]
- 69.Perea-Blázquez A, Davy SK, Bell JJ. 2012. Estimates of particulate organic carbon flowing from the pelagic environment to the benthos through sponge assemblages. PLoS One 7:e29569. doi: 10.1371/journal.pone.0029569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kroon FJ, Berry KLE, Brinkman DL, Kookana R, Leusch FDL, Melvin SD, Neale PA, Negri AP, Puotinen M, Tsang JJ, van de Merwe JP, Williams M. 22 November 2019 Sources, presence, and potential effects of contaminants of emerging concern in the marine environments of the Great Barrier Reef and Torres Strait, Australia. Sci Total Environ doi: 10.1016/j.scitotenv.2019.135140. [DOI] [PubMed] [Google Scholar]
- 71.D'Costa A, Shyama SK, Praveen Kumar MK. 2017. Bioaccumulation of trace metals and total petroleum and genotoxicity responses in an edible fish population as indicators of marine pollution. Ecotoxicol Environ Saf 142:22–28. doi: 10.1016/j.ecoenv.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 72.Baum G, Kegler P, Scholz-Bottcher BM, Alfiansah YR, Abrar M, Kunzmann A. 2016. Metabolic performance of the coral reef fish Siganus guttatus exposed to combinations of water borne diesel, an anionic surfactant and elevated temperature in Indonesia. Mar Pollut Bull 110:735–746. doi: 10.1016/j.marpolbul.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 73.Cheng JO, Cheng YM, Chen TH, Hsieh PC, Fang MD, Lee CL, Ko FC. 2010. A preliminary assessment of polycyclic aromatic hydrocarbon distribution in the kenting coral reef waters of southern Taiwan. Arch Environ Contam Toxicol 58:489–498. doi: 10.1007/s00244-009-9411-y. [DOI] [PubMed] [Google Scholar]
- 74.Diercks A-R, Highsmith RC, Asper VL, Joung D, Zhou Z, Guo L, Shiller AM, Joye SB, Teske AP, Guinasso N, Wade TL, Lohrenz SE. 2010. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophys Res Lett 37:L20602. doi: 10.1029/2010GL045046. [DOI] [Google Scholar]
- 75.Redman AD, Parkerton TF. 2015. Guidance for improving comparability and relevance of oil toxicity tests. Mar Pollut Bull 98:156–170. doi: 10.1016/j.marpolbul.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 76.Turner NR, Renegar DA. 2017. Petroleum hydrocarbon toxicity to corals: a review. Mar Pollut Bull 119:1–16. doi: 10.1016/j.marpolbul.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 77.French-McCay DP. 2002. Development and application of an oil toxicity and exposure model. Environ Toxicol Chem 21:2080–2094. doi: 10.1002/etc.5620211011. [DOI] [PubMed] [Google Scholar]
- 78.Overmans S, Nordborg M, Díaz-Rúa R, Brinkman DL, Negri AP, Agustí S. 2018. Phototoxic effects of PAH and UVA exposure on molecular responses and developmental success in coral larvae. Aquat Toxicol 198:165–174. doi: 10.1016/j.aquatox.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Shafir S, Rijn JV, Rinkevich B. 2007. Short and long term toxicity of crude oil and oil dispersants to two representative coral species. Environ Sci Technol 41:5571–5574. doi: 10.1021/es0704582. [DOI] [PubMed] [Google Scholar]
- 80.Epstein N, Bak RPM, Rinkevich B. 2000. Toxicity of third generation dispersants and dispersed Egyptian crude oil on Red Sea coral larvae. Marine Pollution Bull 40:497–503. doi: 10.1016/S0025-326X(99)00232-5. [DOI] [Google Scholar]
- 81.Lane A, Harrison PL. Effects of oil contaminants on survivorship of larvae of the scleractinian reef coras Acropora tenuis, Goniastrea aspera and Platygyra sinensis from the Great Barrier Reef, p 403–408. 2000. In Moosa MK, Soemodihardjo S, Soegiarto A, Romimohtarto K, Nontji A, Soekarno Suharsono (ed), Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23 to 27 October 2000. Ministry of Environment, Indonesian Institute of Sciences, Bali, Indonesia. [Google Scholar]
- 82.Frometa J, DeLorenzo ME, Pisarski EC, Etnoyer PJ. 2017. Toxicity of oil and dispersant on the deep water gorgonian octocoral Swiftia exserta, with implications for the effects of the Deepwater Horizon oil spill. Mar Pollut Bull 122:91–99. doi: 10.1016/j.marpolbul.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 83.DeLeo DM, Ruiz-Ramos DV, Baums IB, Cordes EE. 2016. Response of deep-water corals to oil and chemical dispersant exposure. Deep Sea Res Part 2 Top Stud Oceanogr 129:137–147. doi: 10.1016/j.dsr2.2015.02.028. [DOI] [Google Scholar]
- 84.Studivan MS, Hatch WI, Mitchelmore CL. 2015. Responses of the soft coral Xenia elongata following acute exposure to a chemical dispersant. Springerplus 4:80. doi: 10.1186/s40064-015-0844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Negri AP, Luter HM, Fisher R, Brinkman DL, Irving P. 2018. Comparative toxicity of five dispersants to coral larvae. Sci Rep 8:3043. doi: 10.1038/s41598-018-20709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whalan S, Webster NS, Negri AP. 2012. Crustose coralline algae and a cnidarian neuropeptide trigger larval settlement in two coral reef sponges. PLoS One 7:e30386. doi: 10.1371/journal.pone.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rougee L, Downs CA, Richmond RH, Ostrander GK. 2006. Alteration of normal cellular profiles in the scleractinian coral (Pocillopora damicornis) following laboratory exposure to fuel oil. Environ Toxicol Chem 25:3181–3187. doi: 10.1897/05-510r2.1. [DOI] [PubMed] [Google Scholar]
- 88.Shafir S, Van Rijn J, Rinkevich B. 2003. The use of coral nubbins in coral reef ecotoxicology testing. Biomol Eng 20:401–406. doi: 10.1016/s1389-0344(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 89.Venn AA, Quinn J, Jones R, Bodnar A. 2009. P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquat Toxicol 93:188–195. doi: 10.1016/j.aquatox.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Agell G, Turon X, De Caralt S, Lopez-Legentil S, Uriz MJ. 2004. Molecular and organism biomarkers of copper pollution in the ascidian Pseudodistoma crucigaster. Mar Pollut Bull 48:759–767. doi: 10.1016/j.marpolbul.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Müller WEG, Batel R, Lacorn M, Steinhart H, Simat T, Lauenroth S, Hassanein H, Schroder H. 1998. Accumulation of cadmium and zinc in the marine sponge Suberites domuncula and its potential consequences on single-strand breaks and on expression of heat-shock protein: a natural field study. Mar Ecol Prog Ser 167:127–135. doi: 10.3354/meps167127. [DOI] [Google Scholar]
- 92.Fan L, Reynolds D, Liu M, Stark M, Kjelleberg S, Webster NS, Thomas T. 2012. Functional equivalence and evolutionary convergence in complex communities of microbial sponge symbionts. Proc Natl Acad Sci U S A 109:E1878–E1887. doi: 10.1073/pnas.1203287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hentschel U, Piel J, Degnan SM, Taylor MW. 2012. Genomic insights into the marine sponge microbiome. Nat Rev Microbiol 10:641–654. doi: 10.1038/nrmicro2839. [DOI] [PubMed] [Google Scholar]
- 94.Moitinho-Silva L, Seridi L, Ryu T, Voolstra CR, Ravasi T, Hentschel U. 2014. Revealing microbial functional activities in the Red Sea sponge Stylissa carteri by metatranscriptomics. Environ Microbiol 16:3683–3698. doi: 10.1111/1462-2920.12533. [DOI] [PubMed] [Google Scholar]
- 95.Pita L, Rix L, Slaby BM, Franke A, Hentschel U. 2018. The sponge holobiont in a changing ocean: from microbes to ecosystems. Microbiome 6:46. doi: 10.1186/s40168-018-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Urakawa H, Rajan S, Feeney ME, Sobecky PA, Mortazavi B. 2019. Ecological response of nitrification to oil spills and its impact on the nitrogen cycle. Environ Microbiol 21:18–33. doi: 10.1111/1462-2920.14391. [DOI] [PubMed] [Google Scholar]
- 97.Taylor MW, Tsai P, Simister RL, Deines P, Botte E, Ericson G, Schmitt S, Webster NS. 2013. “Sponge-specific” bacteria are widespread (but rare) in diverse marine environments. ISME J 7:438–443. doi: 10.1038/ismej.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salerno JL, Little B, Lee J, Hamdan LJ. 2018. Exposure to crude oil and chemical dispersant may impact marine microbial biofilm composition and steel corrosion. Front Mar Sci 5:196. doi: 10.3389/fmars.2018.00196. [DOI] [Google Scholar]
- 99.Dubinsky EA, Conrad ME, Chakraborty R, Bill M, Borglin SE, Hollibaugh JT, Mason OU, M Piceno Y, Reid FC, Stringfellow WT, Tom LM, Hazen TC, Andersen GL. 2013. Succession of hydrocarbon-degrading bacteria in the aftermath of the deepwater horizon oil spill in the Gulf of Mexico. Environ Sci Technol 47:10860–10867. doi: 10.1021/es401676y. [DOI] [PubMed] [Google Scholar]
- 100.Hazen TC, Dubinsky EA, DeSantis TZ, Andersen GL, Piceno YM, Singh N, Jansson JK, Probst A, Borglin SE, Fortney JL, Stringfellow WT, Bill M, Conrad ME, Tom LM, Chavarria KL, Alusi TR, Lamendella R, Joyner DC, Spier C, Baelum J, Auer M, Zemla ML, Chakraborty R, Sonnenthal EL, D'haeseleer P, Holman H-YN, Osman S, Lu Z, Van Nostrand JD, Deng Y, Zhou J, Mason OU. 2010. Deep-Sea oil plume enriches indigenous oil-degrading bacteria. Science 330:204–208. doi: 10.1126/science.1195979. [DOI] [PubMed] [Google Scholar]
- 101.Bacosa HP, Erdner DL, Rosenheim BE, Shetty P, Seitz KW, Baker BJ, Liu Z. 2018. Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil. ISME J 12:2532–2543. doi: 10.1038/s41396-018-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orcutt BN, Joye SB, Kleindienst S, Knittel K, Ramette A, Reitz A, Samarkin V, Treude T, Boetius A. 2010. Impact of natural oil and higher hydrocarbons on microbial diversity, distribution, and activity in Gulf of Mexico cold-seep sediments. Deep Sea Res Part 2 Top Stud Oceanogr 57:2008–2021. doi: 10.1016/j.dsr2.2010.05.014. [DOI] [Google Scholar]
- 103.Handley KM, Piceno YM, Hu P, Tom LM, Mason OU, Andersen GL, Jansson JK, Gilbert JA. 2017. Metabolic and spatio-taxonomic response of uncultivated seafloor bacteria following the Deepwater Horizon oil spill. ISME J 11:2569–2583. doi: 10.1038/ismej.2017.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kappell AD, Wei Y, Newton RJ, Van Nostrand JD, Zhou J, McLellan SL, Hristova KR. 2014. The polycyclic aromatic hydrocarbon degradation potential of Gulf of Mexico native coastal microbial communities after the Deepwater Horizon oil spill. Front Microbiol 5:205–205. doi: 10.3389/fmicb.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson H, Angelova A, Bowler B, Jones M, Gutierrez T. 2017. Enhanced crude oil biodegradative potential of natural phytoplankton-associated hydrocarbonoclastic bacteria. Environ Microbiol 19:2843–2861. doi: 10.1111/1462-2920.13811. [DOI] [PubMed] [Google Scholar]
- 106.Rubin-Blum M, Antony CP, Borowski C, Sayavedra L, Pape T, Sahling H, Bohrmann G, Kleiner M, Redmond MC, Valentine DL, Dubilier N. 2017. Short-chain alkanes fuel mussel and sponge Cycloclasticus symbionts from deep-sea gas and oil seeps. Nat Microbiol 2:17093. doi: 10.1038/nmicrobiol.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Dahash LM, Mahmoud HM. 2013. Harboring oil-degrading bacteria: a potential mechanism of adaptation and survival in corals inhabiting oil-contaminated reefs. Mar Pollut Bull 72:364–374. doi: 10.1016/j.marpolbul.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 108.Glasl B, Webster NS, Bourne DG. 2017. Microbial indicators as a diagnostic tool for assessing water quality and climate stress in coral reef ecosystems. Mar Biol 164:91. doi: 10.1007/s00227-017-3097-x. [DOI] [Google Scholar]
- 109.Fragoso Ados Santos H, Duarte GAS, Rachid C, Chaloub RM, Calderon EN, Marangoni L, Bianchini A, Nudi AH, do Carmo FL, van Elsas JD, Rosado AS, Castro CBE, Peixoto RS. 2015. Impact of oil spills on coral reefs can be reduced by bioremediation using probiotic microbiota. Sci Rep 5:18268. doi: 10.1038/srep18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aurand D, Coelho G (ed). 2005. Cooperative aquatic toxicity testing of dispersed oil and the “Chemical response to oil spills: ecological effects research forum (CROSERF).” Technical Report 07-03 Ecosystem Management and Associates, Inc, Lusby, MD. [Google Scholar]
- 111.Singer MM, Aurand D, Bragin GE, Clark JR, Coelho GM, Sowby ML, Tjeerdema RS. 2000. Standardization of the preparation and quantitation of water-accommodated fractions of petroleum for toxicity testing. Mar Pollut Bull 40:1007–1016. doi: 10.1016/S0025-326X(00)00045-X. [DOI] [Google Scholar]
- 112.Barron MG, Ka'aihue L. 2003. Critical evaluation of CROSERF test methods for oil dispersant toxicity testing under subarctic conditions. Mar Pollut Bull 46:1191–1199. doi: 10.1016/S0025-326X(03)00125-5. [DOI] [PubMed] [Google Scholar]
- 113.Whalan S, Battershill C, de Nys R. 2007. Sexual reproduction of the brooding sponge Rhopaloeides odorabile. Coral Reefs 26:655–663. doi: 10.1007/s00338-007-0236-8. [DOI] [Google Scholar]
- 114.Whalan S, Ettinger-Epstein P, de Nys R. 2008. The effect of temperature on larval pre-settlement duration and metamorphosis for the sponge, Rhopaloeides odorabile. Coral Reefs 27:783–786. doi: 10.1007/s00338-008-0400-9. [DOI] [Google Scholar]
- 115.Whalan S, Webster NS. 2014. Sponge larval settlement cues: the role of microbial biofilms in a warming ocean. Sci Rep 4:4072. doi: 10.1038/srep04072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gilbert JA, Jansson JK, Knight R. 2014. The Earth Microbiome project: successes and aspirations. BMC Biol 12:69. doi: 10.1186/s12915-014-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amir A, McDonald D, Navas-Molina JA, Kopylova E, Morton JT, Zech Xu Z, Kightley EP, Thompson LR, Hyde ER, Gonzalez A, Knight R. 2017. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2:e00191-16. doi: 10.1128/mSystems.00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moitinho-Silva L, Nielsen S, Amir A, Gonzalez A, Ackermann GL, Cerrano C, Astudillo-Garcia C, Easson C, Sipkema D, Liu F, Steinert G, Kotoulas G, McCormack GP, Feng G, Bell JJ, Vicente J, Bjork JR, Montoya JM, Olson JB, Reveillaud J, Steindler L, Pineda MC, Marra MV, Ilan M, Taylor MW, Polymenakou P, Erwin PM, Schupp PJ, Simister RL, Knight R, Thacker RW, Costa R, Hill RT, Lopez-Legentil S, Dailianis T, Ravasi T, Hentschel U, Li Z, Webster NS, Thomas T. 2017. The sponge microbiome project. Gigascience 6:1–7. doi: 10.1093/gigascience/gix077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lopes CT, Franz M, Kazi F, Donaldson SL, Morris Q, Bader GD. 2010. Cytoscape Web: an interactive web-based network browser. Bioinformatics 26:2347–2348. doi: 10.1093/bioinformatics/btq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SIMPER analysis of the genes from the control and the 25% WAF and CWAF exposures. Download Table S1, DOCX file, 0.02 MB (24.5KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Metamorphosis of sponge larvae (percent relative to 0% WAF controls) for ΣPAHs fitted to four-parameter logistic curve. Download FIG S1, PDF file, 0.1 MB (123.6KB, pdf) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EMP sample IDs for the WAF and CWAF exposure experiments. Download Table S2, DOCX file, 0.02 MB (21.9KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxonomic assignment of the most abundant sOTUs that were exclusive to a treatment. Symbionts of sponges are indicated by an asterisk (*) whereas symbionts of coral species are indicated by a plus sign (+). Download Table S3, DOCX file, 0.02 MB (21.9KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Concentrations of TPH and ΣPAH in the 100% WAF and CWAF of Barrow Island oil used in the current study. RL is the reporting limit. Download Table S4, DOCX file, 0.01 MB (15KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of genes used in the multiplexed RT-qPCR assay. Data are from reference 45. Download Table S5, DOCX file, 0.01 MB (14.2KB, docx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gene expression data from the mRT-qPCR assay from both time points used as input for the SIMPER analysis. Download Data Set S1, XLSX file, 0.03 MB (28.8KB, xlsx) .
© Crown copyright 2019.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Gene expression data for both time points can be found in Data Set S1. Processed sequences and metadata are available at http://qiita.microbio.me/ under study identification number 10793, and the deblurred BIOM table can be accessed through the GigaScience repository (https://doi.org/10.5524/100332) using sample identification numbers from Table S2.