Abstract
Objective: To inform on the interim results of the Remede d'Or study, which is a prospective, multicenter, single-blind, randomized, controlled clinical study on the safety and efficacy of RMD-G1, a topical carbopol-based hydrogel with a fibronectin matrix whose active pharmaceutical ingredient is erythropoietin (EPO), for treating diabetic foot ulcers (DFU).
Approach: The trial will comprise 20 patients with type 2 diabetes mellitus with neuroischemic DFUs who will be randomized into two groups: (1) a control group in which standard-of-care (SOC) will be used to treat the DFUs, and (2) a test group in which SOC and RMD-G1 will be used to treat the DFUs. On day 0, all participants will be randomized to receive either RMD-G1 and SOC treatment or SOC alone. The primary endpoint of the study is complete closure of the DFU within the 12-week study period following daily treatments and dressing changes.
Results: Interim results reveal that those DFUs which were treated with RMD-G1 responded positively: there was a significant reduction in the wound areas. In contrast, the condition of those DFUs which were treated with only SOC deteriorated.
Innovation: To date, no topical therapies with proven efficacy for treating DFUs exist. Topical application of EPO-based RMD-G1 in conjunction with SOC to a DFU accelerates their healing and closure.
Conclusions: The interim results of this trial indicate that topical RMD-G1 is a safe adjunctive therapy to SOC, which accelerates the closure of a DFU. RMD-G1 is safe pharmaceutical because EPO has a proven safety profile.
Keywords: diabetes, foot ulcers, erythropoietin, topical treatment, clinical trials
Saher Hamed, MD, PhD.

Introduction
Erythropoietin (EPO) is an approved drug which is widely used for treating anemia. There is growing evidence that both systemic administration and topical EPO application to skin wounds in animals with experimentally induced diabetes mellitus (DM) and in patients with DM accelerates the healing of these wounds. This accelerated wound healing is mediated by EPO because it concomitantly suppresses the inflammatory response and apoptosis and stimulates angiogenesis, reepithelialization, and collagen deposition.1 We have demonstrated that the beneficial therapeutic actions of topical EPO on diabetic wounds are boosted by incorporating a fibronectin (FN) matrix into the formulation because an FN matrix can (1) stabilize the provisional matrix (PM) in the wound bed,2,3 and (2) control the actions of full-length proteins by preventing them from exerting random and indiscriminate local effects on the wound.4,5
Diabetic foot ulcers (DFUs) create a significant public health problem because they are common and serious complications of DM. In addition, the costly management of this disabling and recurring manifestation is a therapeutic and societal challenge.6–8 In the United States, the direct cost of care of DFUs is between $9 and $13 billion.9–11 It has been reported that up to half of all costs of inpatient care related to diabetic patients can be directly attributed to DFUs.12 The direct medical costs of care and treating DFUs also incur considerable indirect economic costs to the patients, their families, and society through mortality, disability, lost income, and decreased societal contributions.13
Several treatments for DFUs have been tried with varying degrees of success, and none can be recommended for various reasons, which include safety, efficacy, and cost. To date, no devices or systemic or topical therapies with proven efficacy for treating DFUs exist.14 Therefore, a safe and cost-effective treatment, which does not increase the workload of care staff, is easy to use, and is well received by patients, is needed.
Remedor Biomed has developed a patented technology, RMD-G1, which comprises EPO as the active pharmaceutical ingredient (API) in a carbopol-based hydrogel with an FN matrix. Recently, Remedor Biomed announced the initiation of its first Remede d'Or study, which was a multicenter, single-blind, randomized, controlled clinical trial and whose aim was to evaluate the safety and efficacy of topical RMD-G1 treatment for DFUs. This study is an exploratory proof-of-concept study on RMD-G1 treatment for DFU and the purpose of this report is to describe the epidemiology of DFUs, outline the rationale of RMD-G1 treatment for DFUs, describe the study's protocol, and present the interim results of the trial.
Clinical Problem Addressed
The incidence of DM has nearly quadrupled in the past three decades, and the number of diabetic patients has risen to over 420 million among the world's adult population,15 and this number is predicted to reach 640 million by 2040.16 Within the diabetic population, nearly 25% of all diabetic patients will develop a DFU during their lifetime.6,17,18
DFUs are typically categorized as neuropathic, ischemic, or neuroischemic ulcers. Of the three types, the most severe adverse outcomes occur with an ischemic DFU, because healing time, ulcer recurrence, risk of amputation, and mortality are the largest.19 The incidence of hospitalization of diabetic patients with a DFU is high because of infection and many of these patients require a limb amputation.9,10,20–22 Moreover, it has been resolved that 55% of patients with DM who had a lower extremity amputation will require a subsequent amputation within 3 years.23,24 The risk for mortality of a diabetic patient with a DFU is 2.5 times higher than that of a diabetic patient without a DFU. In addition, up to 70% of patients may die within 5 years after amputation.15,25
Risk factors for a DFU can be divided to three groups: pathophysiological variations, anatomic deformities, and trauma. Pathophysiological variations happen at the molecular level leading to complications comprising peripheral vascular disease, peripheral neuropathy, a compromised immune system, and defective wound healing. Neuroarthropathy contributes to foot deformity, leading to high plantar pressures and increased risk of skin rupture. These risk factors do not classically occur independently, but rather in combination, further increasing the risk of ulceration. Finally, external influences, such as acute or lasting trauma, are often the originating factors in the development of DFUs.
Development of a DFU is also exacerbated by defective wound healing due to poor blood flow to the DFU and depletion of growth factors and cytokines, which delays its healing and closure. DFUs have an extended inflammatory phase with impaired neovascularization and fibroblast dysfunction and are characterized by degradation of the extracellular matrix and impeded formation of the PM.26,27
The management of DFUs is multidisciplinary. The existing guidelines for managing a DFU include standard-of-care (SOC) treatment, which comprises blood glucose control, treatment of comorbidities, local wound care with efficient debridement, cleansing, control of infection, offloading, vascular evaluation, and revascularization if required, the use of wound dressing types that maintain a moist environment, and increasing patient's awareness to prevention and treatments.28 In addition, the intricate challenges of the ulcer environment, including ischemia, hypoxia, oxidative stress, microbial infection, as well as the role played by inflammatory cells have to be considered. Unfortunately, the outcomes are usually unsatisfactory when using these management strategies.8,29–31 Therefore, there is a need to complement SOC treatment with therapies that promote skin regeneration, accelerate wound healing, restore skin function, and maintain the efficacy of any applied or administered drug in the DFU environment.
Materials and Methods
RMD-G1 patented technology and intended use
RMD-G1 is a topical EPO-containing (2,000 IU/g) carbopol-based hydrogel with an FN matrix. The product is viscous, slightly transparent, has an odor similar to that of benzyl alcohol, and is slightly acidic (pH = 6.25). The API in RMD-G1 is epoetin alfa, which is sourced from Janssen Cilag (EPREX; Janssen Cilag). RMD-G1 is manufactured by Remedor Biomed under good manufacturing practice conditions, stored between 2°C and 8°C, and has a stable shelf-life of 24 months from the manufacturing date. For the Remede d'Or study, RMD-G1 was packed in a sterile container closure system of 2-mL volume aluminum pharmaceutical-grade tubes 11/55 mm (Tubex GmbH, Germany).
RMD-G1 is indicated for treating DFUs in adult patients with DM. RMD-G1 is an adjunct treatment, and not a substitute for good diabetic wound care, which includes initial debridement, wound cleansing, pressure relief, and infection control. In the trial, RMD-G1 will be applied daily onto a clean wound at 0.25 g/cm2 wound surface. After its application, the wound will be covered by a dressing to prevent leakage of the gel and contamination of the wound area.
The Remede d'Or study
In February 2015, Remedor Biomed obtained permission from the Israeli Ministry of Health to conduct a clinical trial to test the safety and efficacy of topical RMD-G1 on DFUs in a multicenter, single-blind, randomized, controlled clinical trial. To this end, this study will enroll 20 patients with DFUs at five investigational sites in Israel. All the patients will be randomized into two groups of 10 patients each: (1) a control group in which SOC will be used to treat the DFUs and (2) a test group in which SOC and RMD-G1 will be used to treat the DFUs.
The primary outcome of the study is complete wound closure. The study protocol was constructed by a six-person board of experts, some of whom are the study coordinators at each investigation site.
Methods
This prospective, multicenter, single-blind, randomized, controlled study is recruiting diabetic patients with a DFU whose wound size ranges between 2 and 15 cm2. The study is conducted at five selected clinical sites in Israel, all of which have diabetology, vascular surgery, and rehabilitation units. This study is registered with ClinicalTrials.gov, number NCT02361931.
Study population
The eligible participants are outpatients who are older than 18 years with type 2 DM and grade 1 and grade 2 noninfected neuroischemic DFUs according to the Wagner Diabetic Foot Ulcer Grade Classification system.32,33 The participants are assessed for diabetic control, as measured by a blood glycated hemoglobin level (HbA1c) of ≤10% and agreed to wear an offloading system every day throughout the treatment period.
Additional inclusion criteria are:
the DFU is at least 3 months old; the DFU has been documented for at least 4 weeks and has not shown signs of healing despite SOC treatment;
the DFU is not infected and the patient does not have osteomyelitis;
the presence of neuropathy, as confirmed by a 10 g Semmes-Weinstein monofilament for pressure perception;
the presence of moderate blood perfusion in the affected limb as defined by an ankle brachial index (ABI) of >0.4;
no surgical revascularization of the limb with the DFU was done in the previous 2 months.
The exclusion criteria were (1) patients with documented hypersensitivity to any of the components of RMD-G1, (2) patients with severe renal failure (defined as requirement for dialysis), (3) patients with a systemic infection, (4) patients with active Charcot's neuroarthropathy, as determined by clinical and/or radiographic examinations, and (5) patients with an active neoplastic condition, which is being treated by radiotherapy, chemotherapy, hormonal therapy, or immunosuppressant agents.
Interventions
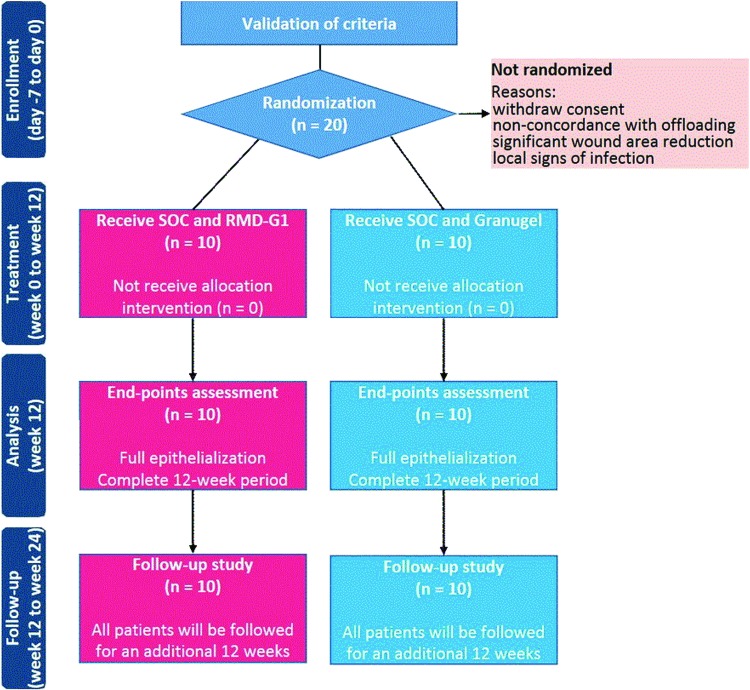
The DFUs of the participants who fulfilled the inclusion criteria (day 14), will be treated for 2 weeks (run-in period) with SOC treatment. The participant's ABI, ankle systolic blood pressure, and, if applicable, systolic toe blood pressure will be measured at baseline. On day 0, all participants will be assessed to confirm (1) concordance with the offloading system, (2) diabetic control (HbA1c ≤10%), and (3) no signs of local infection or osteomyelitis. Participants who demonstrate significant wound area regression during the run-in period will not be introduced into the study (Fig. 1).
Figure 1.
Anticipated flow of participants through each phase of the trial. SOC, standard of care. Color images are available online.
On day 0, all participants will be randomized to receive either RMD-G1 treatment as an adjunctive therapy to SOC (test group) or SOC alone (control group) for their DFUs. Granugel® (Convatec, United Kingdom), which is a moisturizing gel usually prescribed by doctors as part of SOC of DFUs, will be used in the SOC treatment. The DFUs will be treated and the dressing changes will be done daily in the outpatient departments, at each patient's home, or at each clinic visit for up to 12 weeks or until complete wound closure. All treatments and dressing changes will be conducted by the investigating team and community nurses, who will be trained by the investigator.
Local wound treatment will follow the global consensus guidelines on the diabetic foot,8 with any debridement carried out by standard techniques on day 0 and will be documented in the case report form. Any hyperkeratosis will be removed. Demographic parameters, the participant's medical, surgical, and DFU history and a detailed wound description (location, duration, surrounding skin condition, and state of the wound bed) will be documented by the investigator at the start of the trial. Patient and wound assessments will be performed weekly by the investigator during the 12-week treatment period, and will include a clinical examination, a planimetric record (wound area tracing), and digital imaging of the wound. Toxicology screening, which will include a complete blood count, urinalysis, and electrocardiography will be performed on day 0 and during weeks 5, 9, and 12 of the study period. Follow-up visits will also include evaluation of the tolerability to RMD-G1 treatment, occurrence of local adverse events, and determination of the participant's quality of life. After the 12-week study period or on documented full healing with confirmation of wound closure, the participants will be entered into a 12-week follow-up period, in which the wound area will be measured and toxicology screening will be done during weeks 5, 9, and 12 of the follow-up period.
Outcomes
The primary endpoint of the study is the 75% closure or more of the DFU, which is defined as 75% epithelialization with no secretions within the 12-week study period. Safety and tolerability will be assessed based on reported adverse events.
The secondary endpoints, which are related to the efficacy of treatment and will be assessed at each scheduled visit, are (1) the time to reach complete wound closure (days), (2) absolute wound area regression (AWAR, cm2), (3) relative wound area regression (RWAR, %), (4) the mean rate of wound closure (cm2/day), and (5) the number of participants with a wound surface area regression ≥50% and ≥75% by week 4. Other secondary outcomes are the local tolerability of RMD-G1, the occurrence of infection and any other local adverse event, and the participant's quality of life, which will be assessed by the QoL-SF-12 questionnaire.34,35 Since its introduction in 1996,34 the 12-item short-form (SF-12) is widely used as a generic measure of the quality of life, and the efficacy of this tool to assess the quality of life of patients with diabetic foot disease has been demonstrated.35
Randomization, blinding, and allocation
The DFUs of the eligible participants who fulfilled the inclusion criteria will be randomly assigned (1:1) to either a treatment with RMD-G1 and SOC or a control (SOC alone). The randomization was performed through a computer-generated concealed block randomization procedure by an independent external contract research organization (CRO), which was blind to treatment assignment.
Additionally, analysis of all outcomes will be conducted at the end of the study by a CRO experienced in similar trials. Participants, caregivers, and persons in charge of data collection, but not clinical investigators will be masked to group assignment during the entire study period. Group assignments will not be disclosed to the CRO before the clinical database had been registered and frozen and all planned analyses had been completed.
The appearance, form, and packaging of RMD-G1 and Granugel are identical. Before the start of the clinical study, an expert team examined the two packages and found no distinctive features. Treatment allocation was done by Trialog Clinical Trials Ltd., Israel, which specializes in drug storage and distribution quality assurance and has no contact with the study participants or the clinical centers. The investigator receives the number of the allocated participant by email and RMD-G1 and Granugel are labeled by participant number so only the investigator, but not the patient, knows the content of each container.
Ethics
This trial is being conducted according to the European good clinical practice recommendations, the principles of the declaration of Helsinki (1975), and the specific regulations of the Israeli Ministry of Health. Remedor Biomed received approval to conduct the trial from the Israeli Ministry of Health as well as from Ethics Committees for the designated investigational sites (Protocol 0252, Approval no. 2015/2316). Before inclusion, each participant signed a consent form to participate in the investigation following a verbal explanation of the protocol.
Statistical analysis
At the end of the study, all data will be presented in terms of descriptive statistics by treatment group and in total. Descriptive statistics for numerical data will be presented as mean, standard deviation, median, and ranges. Descriptive statistics for categorical data will be frequencies and percentages. AWAR and RWAR between group comparisons at each week and at the end of treatment will be evaluated by the Mann–Whitney U test. For the 50% and 75% RWAR, an exact Fisher test will be used. A sensibility analysis will be used to compare last RWAR using an univariate generalized linear model procedure, including treatment and areas categories (<5 or ≥5 cm2) as fixed factors. A p-value <0.05 will be regarded as indicating a significant value. No adjustment of alpha risk will be applied to compensate for multiplicity tests. The analysis will be done using SPSS software (IBM, Inc.).
Results
Up to the date of writing this article, 13 of the 15 screened individuals with DFU have been enrolled. The 13 eligible individuals were randomly assigned to treatment, 6 to SOC alone (control) and 7 to the RMD-G1 and SOC treatment. Of the enrolled 13 individuals, 5 patients treated with SOC alone and 5 patients treated with RMD-G1 and SOC have completed the study.
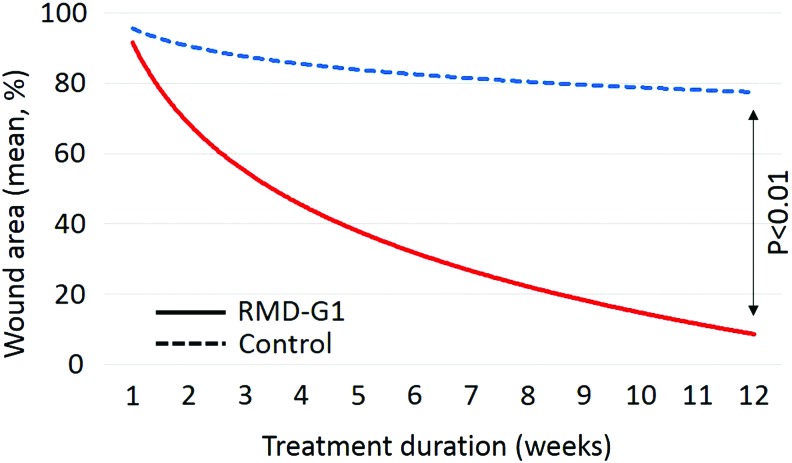
Analysis of the 10 individuals who completed the study revealed that DFUs treated with RMD-G1 and SOC responded positively. Specifically, there was a significant reduction in the wound areas (p < 0.01) (Fig. 2), an increased closure rate of the DFUs, and complete wound closure of those DFUs, which were treated with RMD-G1 and SOC, as compared with those DFUs, which were treated with SOC alone. Additionally, the condition of the DFUs treated with only SOC deteriorated. The data obtained from ten patients who completed the study are presented in Table 1. Moreover, no adverse events related to RMD-G1 treatment have been reported.
Figure 2.
Wound area changes during the treatment. The results are presented as approximated mean of percentage of wound area regression for five patients treated with SOC and RMD-G1 (solid line) and five patients treated with SOC alone (dashed line). Color images are available online.
Table 1.
The main clinical parameters of ten patients
| RMD-G1 Group (n = 5) | Control Group (n = 5) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Gender (M/F) | 4/1 | 2/3 | ||
| Age (year) | 66.0 ± 9.4 | — | 69.6 ± 7.8 | — |
| Body weight (kg) | 88.6 ± 9.4 | 89.7 ± 10.2 | 80.9 ± 15.9 | 77.6 ± 11.5 |
| BMI | 30.2 ± 3.6 | — | 29.8 ± 4.2 | — |
| ABI | 0.9 ± 0.1 | — | 0.9 ± 0.2 | — |
| Wound area (sq.cm) | 5.0 ± 4.9 | 0.8 ± 1.6* | 7.5 ± 5.5 | 5.9 ± 4.6 |
| Time to closure (weeks) | — | 7.2 ± 0.5 | — | — |
| HbA1c (%) | 8.0 ± 1.1 | 8.4 ± 0.3 | 7.7 ± 1.6 | 8.2 ± 1.4 |
| RBC count (108/μL) | 4.5 ± 0.9 | 4.5 ± 0.8 | 4.0 ± 0.3 | 3.9 ± 0.3 |
| Hemoglobin levels (g/dL) | 12.6 ± 1.3 | 12.3 ± 1.1 | 11.2 ± 0.8 | 10.8 ± 0.5 |
| HCT (%) | 38.6 ± 4.8 | 38.2 ± 3.9 | 34.2 ± 1.5 | 33.4 ± 2.2 |
Patients DFUs were treated with topical RMD-G1 and SOC (RMD-G1 group) or with SOC alone (control group) in the Remede d'Or study.
p < 0.01 as compared before and after the treatment.
ABI, ankle brachial index; BMI, body mass index; DFU, diabetic foot ulcers; HbA1C, blood glycated hemoglobin level; HCT, hematocrit level; RBC, red blood cell; SOC, standard of care.
Discussion
Delayed healing of a neuroischemic DFU has been related to prolonged local inflammatory response, an unstable PM, increased degradation of the extracellular matrix, lack of growth factors and their receptors that are crucial for healing, fibroblast dysfunction, impaired neovascularization, increased oxidative stress, and cellular apoptosis in the wound bed, all of which collectively hinder reepithelialization and wound closure.1,26,27
EPO is a well-known glycoprotein hormone, which is primarily produced by the tubular cells of the kidney. EPO is widely known for regulating the red blood cell mass by stimulating differentiation and proliferation of precursor cells and hindering apoptosis of erythroid cells in the bone marrow. Millions of people have received EPO since its market approval by the United States Food and Drug Administration in 1989 as a treatment of anemia in patients with chronic kidney disease and later on as a treatment for chemotherapy-associated anemia. EPO has also nonhematopoietic cellular targets in the skin. Growing studies in experimental healthy and diabetic animals have demonstrated that systemic or topical treatment with EPO onto acute and chronic wounds and burns is safe and effective.1 Recently, the molecular mechanisms of EPO action in wound repair have been elucidated. EPO acts on all cutaneous cells that are involved in the wound healing process by promoting cellular differentiation and proliferation, exerting cytoprotective actions, and inhibiting inflammation and apoptosis due to the presence of EPO receptors in these cells.1
We have previously reported that topical EPO application on cutaneous wounds in rats, mice, and pigs with experimentally induced DM accelerates their healing by stimulating angiogenesis, reepithelialization, and collagen deposition, and suppressing the inflammatory response and apoptosis.1,4,5,36 It has been reported that treatment of skin wounds with topical EPO encouraged the formation of granulation tissue and reepithelialization of chronic wounds in three patients37 and the complete healing of DFUs in three adult patients with DM and glycemic control.38 Interestingly, we have also demonstrated that the beneficial actions of topical EPO on diabetic wounds are boosted by FN. Increasing evidence suggests that FN levels and secretion into the wound bed are decreased in ischemic ulcers due to (1) impaired neovascularization and vascular occlusion, and (2) prolonged inflammatory response that degrades the extracellular matrix, including FN.39,40 By adding an FN matrix to a topical EPO formulation, we were able to boost local EPO distribution and therefore focus EPO's effects onto the wound surface.1,4
Innovation
Growing evidence suggests that topical EPO is safe and beneficial for diabetic wound treatment. To our knowledge, Remede d'Or study is the first controlled trial to assess the safety and efficacy of a topical EPO-containing hydrogel for treating DFUs. Topical RMD-G1 is a patented technology which comprises EPO as the API in a carbopol-based hydrogel with an FN-matrix. Preliminary data revealed that the wound areas are lower and the closure rates are faster of those DFUs which were treated with RMD-G1 and SOC than those DFUs which were treated with SOC alone.
Key Findings.
Our study is the first controlled trial to assess the safety and efficacy of a topical EPO-containing hydrogel (RMD-G1) as an adjunctive therapy to SOC for treating DFUs.
The DFUs of two diabetic patients who were treated with RMD-G1 and SOC completely closed within the 12-week treatment period.
No adverse events were reported following daily treatment of DFUs with RMD-G1 and SOC.
Topical EPO can be an easy, a safe and an effective method for the treatment of DFUs.
Abbreviations and Acronyms
- ABI
ankle brachial index
- API
active pharmaceutical ingredient
- AWAR
absolute wound area regression
- CRO
contract research organization
- DFU
diabetic foot ulcers
- DM
diabetes mellitus
- EPO
erythropoietin
- FN
fibronectin
- HbA1C
blood glycated hemoglobin level
- PM
provisional matrix
- RWAR
relative wound area regression
- SOC
standard of care
Acknowledgments And Funding Sources
The authors thank the investigators for their commitment, time, and effort, and all members who contributed to make the start of the trial possible. This study is sponsored by Remedor Biomed Ltd. All authors approved the final article.
Author Disclosure and Ghostwriting
This study was funded by Remedor Biomed Ltd. of which S.H. is the founder. No competing financial interests exist for Y.U., A.S., S.A., P.Y.L., and L.T. Moreover, M.B., M.S., and Y.S. are employees/consultants of Remedor Biomed Ltd. The article was written by S.H. and all authors approved the final submitted version. No ghostwriters were used to write this article.
About the Authors
Saher Hamed, MD, PhD, is a medical doctor and recognized researcher fellowship trained at the Israeli Institute of Technology—Technion. Dr. Hamed is actively engaged in research on chronic wounds. He has authored numerous significant peer-reviewed publications. He is the founder of Remedor, and inventor of RMD-G1 technology. Mark Belokopytov, PhD, is the clinical director of Remedor. Yehuda Ullmann, MD, PhD, is an associate professor and the Director of the Department of Plastic and Reconstructive Surgery in Rambam Health Care Campus, Haifa, Israel. Muhammad Safadi, PhD, is a pharmaceutical chemistry expert and senior consultant of Chemical, Manufacturing, and Control of Remedor. Yafit Stark, PhD, is a pharmaceutical clinical expert, and senior consultant of clinical affairs of Remedor. Aziz Shoufani, MD, is a plastic and reconstructive surgeon, and the Director of the Department of Plastic & Reconstructive Surgery in the Emek Medical Center, Afula, Israel. Sadanori Akita, MD, PhD, is an associate professor of Plastic and Reconstructive Surgery, of Nagasaki University, Japan. Paul Y. Liu, MD, FACS, is the Chief, Department of Plastic Surgery, Rhode Island Hospital, and the Director of Plastic Surgery Residency at Brown University, United States of America. Luc Teot, MD, is an associate professor of Plastic Surgery at Montpellier University Hospital, France.
References
- 1. Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmoulière A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen 2014;22:23–33 [DOI] [PubMed] [Google Scholar]
- 2. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002;115:3861–3863 [DOI] [PubMed] [Google Scholar]
- 3. Grinnell F. Fibronectin and wound healing. J Cell Biochem 1984;26:107–116 [DOI] [PubMed] [Google Scholar]
- 4. Hamed S, Ullmann Y, Egozi D, et al. . Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol 2011;6:1365–1374 [DOI] [PubMed] [Google Scholar]
- 5. Hamed S, Ullmann Y, Egozi D, et al. . Topical erythropoietin treatment accelerates the healing of cutaneous burn wounds in diabetic pigs through an aquaporin-3-dependent mechanism. Diabetes 2017;66:2254–2265 [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 [DOI] [PubMed] [Google Scholar]
- 7. Petrakis I, Kyriopoulos IJ, Ginis A, Athanasakis K. Losing a foot versus losing a dollar; a systematic review of cost studies in diabetic foot complications. Expert Rev Pharmacoecon Outcomes Res 2017;17:165–180 [DOI] [PubMed] [Google Scholar]
- 8. Bakker K, Apelqvist J, Lipsky BA, et al. . The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev 2016;32:2–6 [DOI] [PubMed] [Google Scholar]
- 9. Skrepnek GH, Mills JL, Sr., Armstrong DG. A diabetic emergency one million feet long: disparities and burdens of illness among diabetic foot ulcer cases within emergency departments in the United States, 2006–2010. PLoS One 2015;10:e0134914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hicks CW, Selvarajah S, Mathioudakis N, et al. . Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg 2016;33:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes care 2014;37:651–658 [DOI] [PubMed] [Google Scholar]
- 12. Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis. Dermatol Surg 2001;27:347–351 [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Game FL, Hinchliffe RJ, Apelqvist J, et al. . A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2012;28:119–141 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Global report on diabetes. 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf (last accessed June17, 2018)
- 16. International Diabetes Federation. IDF diabetes atlas 7th ed. 2015. http://diabetesatlas.org/IDF_Diabetes_Atlas_8e_interactive_EN/ (last accessed June9, 2018)
- 17. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 18. Boulton AJ, Armstrong DG, Albert SF, et al. . Comprehensive foot examination and risk assessment a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes care 2008;31:1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Technol 2011;5:1591–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 2010;52:17S–22S [DOI] [PubMed] [Google Scholar]
- 21. Skrepnek GH, Mills JL, Armstrong DG. Foot-in-wallet disease: tripped up by “cost-saving” reductions? Diabetes Care 2014;37:e196–e197.r [DOI] [PubMed] [Google Scholar]
- 22. Pandian G, Hamid F, Hammond M. Rehabilitation of the patient with peripheral vascular disease and diabetic foot problems. In: DeLisa J, Gans BM, eds. Rehabilitation Medicine: Principles and Practice, 3rd ed. Philadelphia, PN: Lippincott-Raven 1998:1517–1544 [Google Scholar]
- 23. Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev 2008;24:S3–S6 [DOI] [PubMed] [Google Scholar]
- 24. Armstrong DG, Lavery LA, Harkless LB, Van Houtum WH. Amputation and reamputation of the diabetic foot. J Am Podiatr Med Assoc 1997;87:255–259 [DOI] [PubMed] [Google Scholar]
- 25. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 2016;33:1493–1498 [DOI] [PubMed] [Google Scholar]
- 26. Lazaro JL, Izzo V, Meaume S, Davies AH, Lobmann R, Uccioli L. Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. J Wound Care 2016;25:277–287 [DOI] [PubMed] [Google Scholar]
- 27. Dinh T, Tecilazich F, Kafanas A, et al. . Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012;61:2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frykberg RG, Banks J. Management of diabetic foot ulcers: a review. Fed Pract 2016;33:16–23 [PMC free article] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence (NICE). Diabetic foot problems: prevention and management. NICE Guideline [NG19]. 2015. https://nice.org.uk/guidance/ng19/chapter/Introduction (last accessed June28, 2018) [PubMed]
- 30. Lavery LA, Davis KE, Berriman SJ, et al. . WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen 2016;24:112–126 [DOI] [PubMed] [Google Scholar]
- 31. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015 based on the IWGDF Guidance Documents. Diabetes Metab Res Rev 2016;32:7–15 [DOI] [PubMed] [Google Scholar]
- 32. Wagner FW. Supplement: algorithms of foot care. In: Levin ME, O'Neal LW, eds. The Diabetic Foot, 3rd ed. St. Louis, MO: CV Mosby 1983:291–302 [Google Scholar]
- 33. Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24:84–88 [DOI] [PubMed] [Google Scholar]
- 34. Ware J , Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996:34:220–223 [DOI] [PubMed] [Google Scholar]
- 35. Wukich DK, Sambenedetto TL, Mota NM, Suder NC, Rosario BL. Correlation of SF-36 and SF-12 component scores in patients with diabetic foot disease. J Foot Ankle Surg 2016;55:693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Invest Dermatol 2010;130:287–294 [DOI] [PubMed] [Google Scholar]
- 37. Bader A, Lorenz K, Richter A, et al. . Interactive role of trauma cytokines and erythropoietin and their therapeutic potential for acute and chronic wounds. Rejuvenation Res 2011;14:57–66 [DOI] [PubMed] [Google Scholar]
- 38. Gunter CI, Kern1 L, Giri1 S, Machens HG, Bader A. First results on three patients treated with topical recombinant human erythropoietin (rhEPO) to improve wound healing in diabetic foot ulcers. J Transplant Stem Cel Biol 2015;2:4–7 [Google Scholar]
- 39. Labat-Robert J, Leutenegger M, Llopis G, Ricard Y, Derouette JC. Plasma and tissue fibronectin in diabetes. Clin Physiol Biochem 1984;2:39–48 [PubMed] [Google Scholar]
- 40. Wysocki AB, Grinnell F. Fibronectin profiles in normal and chronic wound fluid. Lab Invest 1990:63:825–831 [PubMed] [Google Scholar]