Abstract
Significance: Hypertrophic scars, keloids, and burn injuries of the skin have a significant impact on patients‘ lives and impact the health care system tremendously. Treating skin wounds and lesions can be challenging, with a variety of choices available for treatment. Scar and burn managements range from invasive, surgical options such as scar excision to less invasive, nonsurgical alternatives such as laser therapy or topical drug application.
Recent Advances: Laser treatment has become increasingly popular, with a growing body of research supporting its use for scars and burns. Numerous methods are available for the treatment of these skin diseases, including different nonsurgical laser therapies.
Critical Issues: To date, the optimal treatment method for scars, keloids, and burn injuries of the skin has not yet been established, although it is an area of increasing clinical concern.
Future Directions: This review provides an updated summary of the treatment of scars and burn wounds of the skin using different laser treatments, including the most recent technologies. It addresses their indications, mechanisms of action, differences, efficacies, and complications.
Keywords: critical review, laser technologies, hypertrophic scars, keloids, burn injuries of the skin
Geoffrey C. Gurtner, MD, FACS.

Scope and Significance
This review will focus on the different available laser therapies to give an up-to-date report for doctors, surgeons, and researchers. We give a short overview of the development of lasers, compare different laser treatments for scar types and burns, and show the most recent innovations in laser therapy. All addressed lasers in this review have multiple indications and show promising outcomes for the treatment of different scars and burns.
Translational Relevance
A multitude of treatments is available for the therapy of cutaneous scars and burn injuries, including laser technologies. By understanding the pathophysiology of cutaneous wounds and the use of different laser applications, nonsurgical laser therapies can be beneficial for the treatment of scars with the potential to improve clinical outcomes.
Clinical Relevance
Excessive scar formation after cutaneous wound healing results in poor functional and esthetic outcomes. The economic impact is greater when the costs of disability and revision surgeries due to dysfunctional tissue and disfiguring scars are included.1 The use of laser therapies can have the potential to treat cutaneous scars and burn injuries, resulting in improved outcomes and quality of life. The methods discussed in this review are aimed to give an updated summary of the treatment of scars and burn wounds of the skin using different laser treatments, including the most recent technologies.
Background
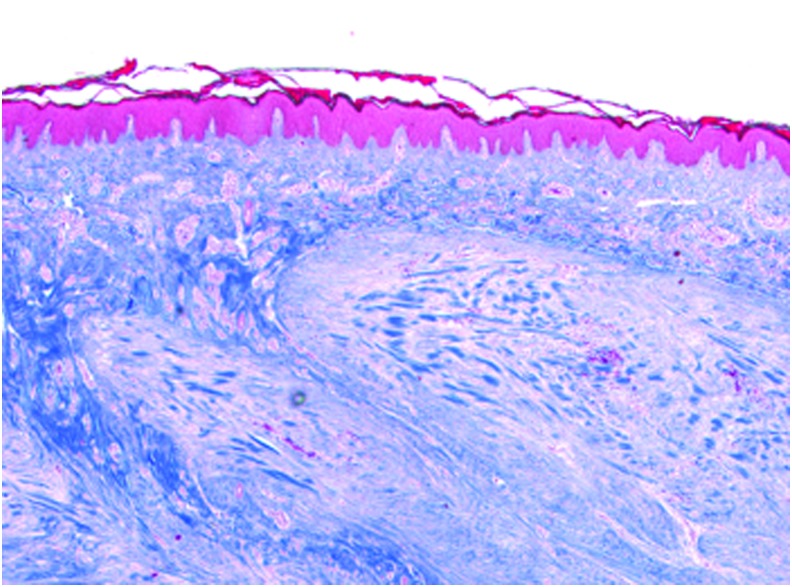
Cutaneous scar formation and burns represent a significant medical burden with billions of dollars spent annually on scar treatments in the United States. Hypertrophic scars (HTS) and keloids represent pathologic, fibrotic responses to cutaneous insult. The hallmark of both lesions is excessive deposition of collagen and other extracellular matrix (ECM) proteins within the skin, as well as proliferation of fibroblasts. In cases of HTS, the lesion typically does not extend beyond the margins of the original wound and growth is self-limiting with regression commonly observed within 2 years.2 Keloids are more aggressive, with advancing margins and minimal chance of spontaneous regression (Fig. 1).
Figure 1.
Histology of Keloid. Trichrome Stain. 10 × magnification. Color images are available online.
Both types of lesions are commonly thought to arise from fibroblast overactivity during the proliferative phase of wound healing in a process driven by IL-6, IL-10 and TGF-β.3 It has more recently been proposed that HTS and keloids are manifestations of endothelial dysfunction during the early, inflammatory stage of wound healing.4 These pathologic scars are only seen following injuries that penetrate at least to the depth of the reticular dermis, suggesting that any insult causing chronic inflammation within this tissue layer could lead to the development of HTS or keloid. Recruitment of deep dermal fibroblasts, a resident fibroblast subpopulation with diminished collagenase activity, then leads to increased production of ECM and inflammatory cytokines.5 Indeed, it has long been understood that neutralization of proinflammatory factors such as transforming growth factor beta (TGF-β) can help attenuate scar formation.6 On histology, HTS are characterized by an accumulation of wavy collagen (type III predominates) bundles running parallel to the epithelial surface, while keloids contain sheets of loosely organized collagen I and III.7
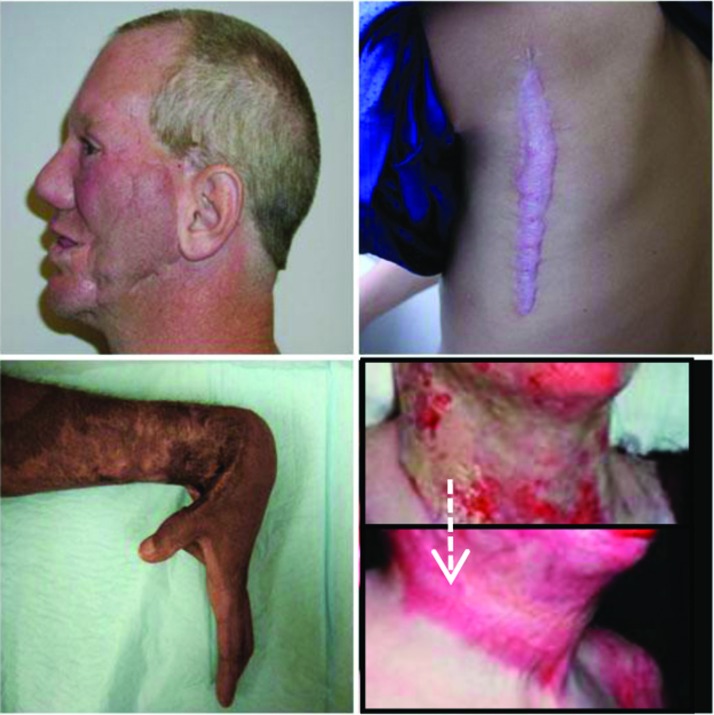
Many HTS result from burns, and some 32–72% of patients with burn injury will go on to develop HTS.8 Approximately half a million cases of burn injury are reported annually in the United States.9 Advances in the acute care of burn patients have pushed survival rates for even the most severe burns involving 80% of the total body surface area above 50%.10 As a result, many more patients go on to experience the sequelae of serious, life-threatening burn injuries such as HTS (Fig. 2).
Figure 2.
Excessive scar formation. Color images are available online.
Risk factors for HTS include dark skin, female gender, young age, burn site on neck and upper limb, multiple surgical procedures, time to healing, and burn severity.8 Contracture, in which scar tissue involving a joint can grow to limit its range of motion, is another common complication of burns, along with pruritus, chronic pain, and cosmetic disfigurement.
Among subjective measures of scar severity, the Vancouver Scar Scale (VSS), developed in 1990, is perhaps most widely used. Four criteria: pigmentation, vascularity, pliability and scar height are assessed independently, with increasing score representing more advanced disease (Table 1).11 Normal skin receives a score of zero. More recently the Patient and Observer Scar Assessment Scale (POSAS) in which the lesion is also scored by the patient with added criteria for pain and pruritus has also entered into regular use (Table 2).12
Table 1.
Vancouver scar scale
| Vancouver Scar Scale | |||||||
|---|---|---|---|---|---|---|---|
| Pigmentation | Vascularity | Pliability | Height | ||||
| Normal | 0 | Normal | 0 | Normal | 0 | Flat, Normal | 0 |
| Hypopigmentation | 1 | Pink | 1 | Supple | 1 | <2 mm | 1 |
| Mixed | 2 | Red | 2 | Yielding | 2 | 2–5 mm | 2 |
| Hyperpigmentation | 3 | Purple | 3 | Firm | 3 | >5 mm | 3 |
| Banding | 4 | ||||||
| Contracture | 5 | ||||||
Table 2.
Patient and observer scar assessment scale
| Patient Scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 = No; Yes = 10 | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Is the scar painful? | ||||||||||
| Is the scar itching? | ||||||||||
| Is the color of the scar different? | ||||||||||
| Is the scar more stiff? | ||||||||||
| Is the thickness of the scar different? | ||||||||||
| Is the scar irregular? | ||||||||||
| 1 = As Normal Skin; Very Different To Normal Skin = 10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Overall opinion of the scar | ||||||||||
| Observer Scale | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 = Normal Skin; Worst Scar Imaginable = 10 | |||||||||||||||
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Category | ||||
| Vascularity | Pale | Pink | Red | Purple | Mix | ||||||||||
| Pigmentation | Hypo | Hyper | Mix | ||||||||||||
| Thickness | Thicker | Thinner | |||||||||||||
| Relief | More | Less | Mix | ||||||||||||
| Pliability | Supple | Stiff | Mix | ||||||||||||
| Surface area | Expansion | Contraction | Mix | ||||||||||||
| Overall opinion | |||||||||||||||
Attempts to establish an objective scale of scar measurement that can accurately predict which lesions will progress to become HTS have been proposed, but at present, there is no “gold standard” scale for the evaluation of scars.13 Of the criteria commonly evaluated, pliability and erythema, but not pigmentation, correlated significantly with clinical evaluation of hypertrophy.2
Current recommendations for treatment of HTS include multimodal combination therapy with steroid injections, 5-fluorouracil and laser treatment.14 Many such regimens are justified empirically and there is a lack of evidence-based algorithms.15 Research in molecular biology and regenerative medicine addressed to specific inflammatory mediators or cell-cell interactions is ongoing, but clinical applications in the treatment of HTS remain limited.1 To date, there are no adequately powered randomized control trials to evaluate whether lasers work to improve hypertrophic burn scars. There remains a need to better characterize the myriad emerging laser and light-based therapies, which are increasingly available and relatively noninvasive.
Overview
Historical background on laser
The word laser is an acronym for “light amplification by stimulated emission of radiation.” An external energy source, or pump, is used to stimulate an active medium housed within a resonant chamber. Active media may be liquid, solid, or gas and are selected based on their ability to emit radiation of desired wavelengths. Lasers are distinguished as radiation sources by their emission being monochromatic (single, fixed wavelength), unidirectional and perfectly in-phase, or coherent.
Crucially, these special properties allow for the precise application of a predictable amount of energy. Lasers vary broadly in their design and purpose, but certain parameters bear special medical significance. Beam energy can be applied either continuously or in a pulsatile manner depending upon the desired effect. Medical lasers are characterized as either ablative or nonablative, with the former disrupting the epidermis and the latter leaving the outermost layer of skin intact en route to targets or chromophores, in the dermis. The operative principle of laser-based therapy is photothermolysis. Energy absorption by chromophores within the skin generates heat, leading to targeted destruction and a local inflammatory response. Interruption of the microvasculature starves the tissue of oxygen, limiting cellular proliferation and protein deposition, and therefore attenuates scar formation. Synthesis of new collagen and reorganization of fiber bundles lead to better approximation of healthy, prewound tissue.16
While the theoretical underpinnings of laser technology were first described in the work of Albert Einstein,17 the practical history of lasers began in the early 1950 s when the first “stimulated” emissions of microwave radiation (Microwave Amplification by Stimulated Emission of Radiation, or MASER) were generated by J.P. Gordon and C.H. Townes at Bell Telephone Laboratories. Publishing in Nature in 1960, Dr. T. H. Maiman at Hughes Aircraft Company presented the first working laser using a synthetic ruby crystal stimulated by high-intensity flash lamps, which generated millisecond pulses of coherent 694 nm (near infrared) ruby laser light.18 It was not until 1962 that the first medical application for laser was reported by Goldman, when he used a ruby laser in dermatology.19
1964 saw the development of both the carbon dioxide (CO2) and neodymium:yttrium aluminum Garnet (Nd:YAG) lasers. The CO2 proved useful in surgery on highly vascularized organs, owing to its focused beam and high degree of absorption by water. Because soft tissue consists mostly of water, the CO2 laser beam cuts tissue like a scalpel following immediate hemostasis. Further discoveries in fiberoptics allowed for the transmission of far-infrared laser beams, which broadened the utility of CO2 lasers to include endoscopic surgery.
In 1986, the U.S. Food and Drug Administration approved the pulsed dye laser (PDL) for treatment of cutaneous vascular disorders. The PDL was based on the concept of selective photothermolysis introduced by Anderson and Parrish.
More recently, so-called fractional lasers, which ablate the skin in a grid-like pattern of miniscule columns called microscopic treatment zones (MTZs) and leave intervening tissue unharmed, have entered use. Fractional laser platforms can either be ablative or nonablative. In vitro studies suggest that skin treated with fractional Er:YAG laser expresses heightened levels of inflammatory cytokines such as IL-6, IL-8, and IL-24, as well as various chemokines (like CXCLs) and protein-digesting MMPs.20
Discussion
Different laser therapies and their applications
Pulsed dye laser
The PDL functions by the excitation of a liquid dye medium, emitting photons of a wavelength absorbed avidly by the hemoglobin molecule (585 nm). This modality is therefore well suited to targeted remodeling of cutaneous vascular lesions. It was eventually applied to burn patients with hyperemic and pruritic scars and showed promising results.16,21 PDL is thought to attenuate the formation of HTS by targeting their developing microvasculature.22 Furthermore, PDL treatment is associated with decreased TGF-β expression, fibroblast proliferation, and collagen type III deposition.23 Special care must be taken in treating darker skin where epidermal melanin competes with hemoglobin for laser absorption.24
PDL has shown promise25 in the treatment of scars and some have suggested it to be the first-line treatment and prophylaxis cases of keloids and HTS at some centers.26 The development of long-PDL provided another treatment modality with comparable efficacy in HTS reduction, but significantly less risk of posttreatment purpura.27 A systematic review of PDL treatment of HTS supports the use of 585 and 595 nm devices with consistent low to moderate improvement seen across included study populations.25 Another systematic review of eight randomized controlled trials using 585 nm PDL for the treatment of HTS and keloid found that PDL was superior to conventional treatment in improving scar appearance.28
PDL treatment has long been used with favorable results in the setting of burn injury. The use of PDL on scars at the seams of newly healed skin grafts in pediatric burn patients helps to reduce erythema, scar height and tissue elasticity.29 Facial burns that lead to HTS can be particularly difficult to treat, with excision sometimes resulting in unavoidable disfigurement. In a study of 57 patients with hypertrophic facial burn scars, PDL treatment was used in place of surgical excision. Of these patients, 34 (60%) received additional treatment with Z-plasty to relieve scar tension. Favorable outcomes were achieved, ranging from reversal of HTS to treatment of stable erythema, depending upon time from initial burn injury to laser treatment.16 Scars can be “rehabilitated” through laser treatments and smaller procedures, while the use of larger surgical treatments may create deformities that are worse than the original scar.16
As the HTS develops and thickens, some hypothesize that the underlying microvasculature is better protected and less accessible to the PDL, and therefore recommend that PDL treatment begins as soon as possible following discovery of the lesion.30 The energy required for effective treatment increases with scar thickness. By titrating up laser energy until pinpoint bleeding is observed, clinicians can ensure that the beam penetrates to the level of the dermal blood vessels.31
Ablative fractional laser therapies have shown to improve the uptake of topical medical drugs into the stratum corneum, epidermis, and dermis.32,33 Studies in animal models suggest that pretreatment with CO2 and erbium:yttrium-aluminum-garnet (Er:YAG) lasers can increase the permeability and depth of penetration of topically applied drugs.34 In a prospective case series of 15 patients with HTS, fractional ablative CO2 laser treatment combined with topical application of 20 mg/mL triamcinolone acetonide was successful in reducing scar burden, principally by the improvement of scar texture.35 One major limitation of the existing studies on laser-assisted drug delivery (LAD) is their performance on animal models, but additional long-term studies in humans, including a large number of patients, are still missing.
Recent research suggests that the coagulation zone around laser treatment sites in fractional ablative lasers may serve as a reservoir for topical drugs.32 Tissue in this zone is thicker and tighter adjacent to the skin that was actually treated with a fractional ablative laser. Because of this tightness, the coagulation zone is thought to have a lower diffusity than the treated area surrounding it, which appears to make it serve as a storage depot for secondary drug delivery. This might postitively influence the treated skin exclusively without having a systemic effect.32,35
Interestingly, researchers have applied a similar pretreatment principle to sequential laser therapy. One group describes a laser “drilling” technique performed with pulsatile CO2 laser followed by a second “deep” pass with a continuous fractional CO2 device.36 This regimen is thought to increase the penetration depth of the CO2 laser in the treatment of HTS. VSS and UNC Scar Scale Score were significantly decreased in the treated cohort of 158 patients with HTS.
Fractional Ablative CO2 laser
Evidence suggests that CO2 lasers may be effective in the treatment of older, mature scars resistant to treatment by PDL. The fractional 10,600 nm CO2 laser, which ablates skin and scar tissue in a columnar pattern to a depth of several millimeters, destroys disorganized ECM proteins leading to reepithelialization and the deposition of new collagen in physiologic orientation.37 Even thick HTS resulting from severe burns can be targeted in this manner. FXCO2 has also proven to be safe and effective in the pediatric population. In a study of 47 patients 6–16 years of age, treatment with CO2 laser conferred improvement in VSS total score as assessed by both physician and parent, as well as decreased scar thickness measured by ultrasound.38
In the treatment of widespread hypertrophic burn scars, FXCO2 laser offers the advantage of fewer treatment sessions compared with nonablative modalities.13 In a study of 100 patients with either burn or traumatic scars, Keen et al. observed excellent response to CO2 laser treatment with a mean of six sessions.39 Treatment of keloids proves more challenging. Among 15 patients with hypertrophic and keloidal scars, only the HTS showed significant improvement after three CO2 fractional laser sessions.40 However, one published case series, including eight patients with 12 total keloids using superpulsed CO2 laser treatment, reported good results with no recurrence.41 In a recent split-scar, evaluator-blinded study of patients with recent surgical scars of the head and neck who underwent ablative FXCO2 therapy, patients, but not physician observers, saw improvement after three sessions.42 Of note, significant improvement was seen in itch score and global patient evaluation; however, observer POSAS score was no better in the treated half of the scar than the untreated half.42
Other groups have reported good results with FXCO2 in the treatment of HTS. Among 10 patients with hypertrophic burn scars who underwent treatment with fractional CO2 laser therapy, VSS, POSAS score, and quality of life measures all showed significant improvement within 6 months of treatment.43 Strikingly, scar firmness was observed to decrease by 30% after a single session. When 40 patients with HTS were treated over 4 monthly sessions with fractional CO2 laser, there was a statistically significant improvement as measured by VSS.44 Skin biopsy taken before treatment and then again 3 months afterward showed increased epidermal thickness along with thinning of the stratum corneum and replacement of irregular collagen bands with organized new collagen fibrils.44 TGF-β1 expression after laser therapy was significantly decreased.44
Another study of 10 patients with mature, full-thickness, hypertrophic burn scars treated with a fractional CO2 laser gives further insight into the mechanism of treatment.45 Not only was significant improvement seen as evaluated with VSS and POSAS but also reverse transcription polymerase chain reaction analysis of fresh tissue samples (obtained before the initial treatment and again 48 h after the first treatment) showed that types I and III procollagen mRNA levels were greatly diminished after treatment.45 Furthermore, FXCO2-treated skin shows an increase in the ratio of type III to type I collagen after 2 months, suggesting that collagenogenic processes are first suppressed and then “reset,” with subsequent collagen deposition more closely mirroring the subtype proportions seen in healthy skin.45
MMP-1 was also significantly upregulated after treatment, as was expression of miR-18α and miR-19α.45 Metalloproteinase activity likely explains at least, in part, the ability of CO2 laser to increase lesion pliability for both HTS and keloid.46 Changes to collagen organization as well as increased expression of matrix metalloproteinase 9 (MMP-9) have been seen on histology.46 Conversely, profibrotic growth factors, namely TGF-β2, -β3, and βFGF, are seen to be downregulated.45
As FXCO2 treatment enters into greater use and various treatment protocols develop, the need for scientific work, which better characterizes tissue-level effects of laser, has become clear. FXCO2 treatment parameters such as MTZ density, energy level, and the number of sequential pulses, sometimes called “pulse stacking,” can be adjusted with unclear implications for the treatment outcome. In a study of Red Duroc pigs with full-thickness burn wounds, eschar excision and split-thickness skin autograft were performed.47 Three months later, scars were treated with a fractional CO2 laser with 70mJ of energy delivered as either a single pulse or stacked for three consecutive pulses. Esthetic and functional criteria of the skin such as erythema and transepidermal water loss were recorded before treatment and then at regular intervals in the days following FXCO2 therapy. Triple stacking of FXCO2 pulses was found to cause only minor increases in MTZ depth and width. Somewhat surprisingly, reepithelialization of the skin was observed by 48 h in both treatment protocols, with local inflammation markers returning to baseline by 1 week postprocedure.
As mentioned previously, contracture remains a feared and debilitating sequela of pathologic scarring, which involves a joint. In a published case series of two pediatric patients, one group reports success in treating restrictive pediatric scar contractures with an ablative microfractionated 600 nm CO2 laser.48 Both patients demonstrated subjective and objective improvements in range of motion and function, and no operative complications were reported. In a study of red duroc pigs, who received skin autograft following third-degree burn, treatment with PDL, fractional CO2, or PDL & fractional CO2 resulted in significantly less contraction versus skin graft-only controls.49 There were no statistically significant differences in contraction among laser therapy groups, although fractional CO2 showed a slight advantage in reduction of erythema.49
FXCO2 therapy has also been proposed as an adjunct in treatment of burn HTS hypopigmentation. In this study, LAD allows synthetic alpha-melanocyte-stimulating hormone analogs to cross the epidermis, leading to melanogenesis restoration of skin pigmentation.50
Summary
The efficacy, ease of use and minimal side effect profile of laser and light-based therapies virtually ensure that they will take on an increasingly central role in the treatment of HTS, keloids, and burns. The great range of laser platforms available make this a highly versatile modality (Table 3) that can be tailored to each lesion and even account for differences in patient skin type.
Table 3.
Overview of different scar types and commonly used laser treatments
| Scar Type | Characteristics | Commonly Used Lasers |
|---|---|---|
| Traumatic | Pink | PDL |
| Hypertrophic | Raised, erythematous | PDL, CO2 |
| Restricted to wound boundaries | ||
| Can be symptomatic | ||
| Keloid | Raised, firm, dark purple-reddish | PDL, CO2 |
| Overgrow outside of original wound margin | ||
| Often symptomatic | ||
| Hypertrophic burns | Contract, stiff, firm | Fractional lasers |
| Hyperpigmentation, altered texture | ||
| Often symptomatic |
PDL, pulsed dye laser.
The level of evidence for laser therapies in managing HTS, keloids, and burns remains low. Additional research in the efficacy of multiple quantifiable parameters, like scar texture, recurrence rates, and adverse effects, is necessary to establish guidelines. Updated data on scar, keloid, and burn management using laser technologies will benefit both surgeons and patients in making optimal decisions regarding evidence-based strategies for the treatment of HTS, keloids, and burn injuries of the skin.
Take-Home Messages.
Scars and burn injuries of the skin are prevalent and highly impact the patients' lives as well as the health care system.
Laser treatments have the potential to treat scars, keloids, and burn injuries of the skin, with a growing body of research supporting its use for scars and burns.
Despite recent advances in laser technologies, there still is a lack of evidence-based strategies for their treatment of scar and burn injuries of the skin.
The adequate use of laser treatments for scars can have the potential to reduce health care costs and improve the quality of scars.
Acknowledgments and Funding Sources
None declared.
Abbreviations and Acronyms
- CO2
carbon dioxide
- ECM
extracellular matrix
- Er:YAG
erbium:yttrium-aluminum-garnet
- FGF
fibroblast growth factor
- FXCO2
Fractional Ablative CO2 laser
- HTS
hypertrophic scars
- IL
interleukin
- LAD
laser-assisted drug delivery
- MASER
microwave amplification by stimulated emission of radiation
- MMP
matrix metalloproteinase
- MTZ
microscopic treatment zone
- Nd:YAG
neodymium:yttrium aluminum garnet
- PDL
pulsed dye laser
- POSAS
patient and observer scar assessment scale
- TGF-β
transforming growth factor beta
- VSS
Vancouver Scar Scale
Author Disclosure and Ghostwriting
G.G. has no disclosures relevant to this topic. B.K., Z.S.B., D.C.W., and J.S.F. have no potential conflict of interests, affiliations, or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this study. The content of this article was expressly written by the authors listed. No ghostwriters were involved in the writing of this article. No competing financial interest exists.
About the Authors
Britta Kuehlmann, MD, PhD, is a Plastic and Reconstructive Surgeon and postdoctoral research fellow at Stanford University. Her research interests include foreign body responses, capsular fibrosis around implants, and skin regeneration. Zachary Stern-Buchbinder, MS, is a medical student studying wound healing and foreign body responses. Derrick C. Wan, MD, FACS, is an Associate Professor in the Department of Surgery at Stanford University. He runs an NIH-funded laboratory that focuses on regulation of stem cell biology and their use for soft tissue reconstruction. Friedstat Jonathan S, MD, practices at Massachusetts General Hospital, Harvard Medical School, and specializes in burn injury, burn reconstruction, burns, and critical care. His main research interest is in outcomes research for burn reconstruction patients. Geoffrey C. Gurtner, MD, FACS, is the Johnson and Johnson Professor of Surgery and Materials Science Engineering at Stanford University. He also serves as the Associate Chairman for Research in the Department of Surgery. Dr. Gurtner runs an NIH- and DoD-funded laboratory that seeks to elucidate the human response to injury for the promotion of tissue repair and regeneration.
References
- 1. Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 2007;4:e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliveira GV, Chinkes D, Mitchell C, Oliveras G, Hawkins HK, Herndon DN. Objective assessment of burn scar vascularity, erythema, pliability thickness, and planimetry. Dermatol Surg 2005;31:48–58 [DOI] [PubMed] [Google Scholar]
- 3. Berman B, Maderal A, Raphael B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol Surg 2017;43 Suppl 1:S3–S18 [DOI] [PubMed] [Google Scholar]
- 4. Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis - Keloids and hypertrophic scars may be vascular disorders. Med Hypotheses 2016;96:51–60 [DOI] [PubMed] [Google Scholar]
- 5. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016;388:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor β. Lancet 1992;339:213–214 [DOI] [PubMed] [Google Scholar]
- 7. Wolfram D, Tzankov A, Pulzl P, Piza-Katzer H. Hypertrophic scars and keloids—a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 2009;35:171–181 [DOI] [PubMed] [Google Scholar]
- 8. Lawrence JW, Mason ST, Schomer K, Klein MB. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res 2012;33:136–146 [DOI] [PubMed] [Google Scholar]
- 9. American Burn Association. Burn Injury Fact Sheet. http://ameriburn.org/wp-content/uploads/2017/12/nbaw-factsheet_121417-1.pdf (last accessed November27, 2018)
- 10. Saffle JR. Predicting outcomes of burns. N Engl J Med 1998;338:387–388 [DOI] [PubMed] [Google Scholar]
- 11. Sullivan TA, Smith J, Kermode J, Mclver E, Courtemanche D. Rating the burn scar. J Burn Care Rehabil 1990;11:256–260 [DOI] [PubMed] [Google Scholar]
- 12. Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004;113:1960–1965 [DOI] [PubMed] [Google Scholar]
- 13. Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg 2014;40:825–831 [DOI] [PubMed] [Google Scholar]
- 14. Mokos ZB, Jovic A, Grgurevic L, et al. Current therapeutic approach to hypertrophic scars. Front Med (Lausanne) 2017;4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 2014;72:S198–S201 [DOI] [PubMed] [Google Scholar]
- 16. Donelan MB, Parrett BM, Sheridan RL. Pulsed dye laser therapy and z-plasty for facial burn scars: the alternative to excision. Ann Plast Surg 2008;60:480–486 [DOI] [PubMed] [Google Scholar]
- 17. Einstein A. Zur quantentheorie der strahlung. Phys Z 1917;18:121–128 [Google Scholar]
- 18. Korrapati PS, Karthikeyan K, Satish A, Krishnaswamy VR, Venugopal JR, Ramakrishna S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater Sci Eng C Mater Biol Appl 2016;67:747–765 [DOI] [PubMed] [Google Scholar]
- 19. Choy D. History of lasers in medicine. Thorac Cardiovasc Surg 1988;36:114–117 [DOI] [PubMed] [Google Scholar]
- 20. Schmitt L, Amann PM, Marquardt Y, et al. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med Sci 2017;32:805–814 [DOI] [PubMed] [Google Scholar]
- 21. Parrett BM, Donelan MB. Pulsed dye laser in burn scars: current concepts and future directions. Burns 2010;36:443–449 [DOI] [PubMed] [Google Scholar]
- 22. Reiken SR, Wolfort SF, Berthiaume F, Compton C, Tompkins RG, Yarmush ML. Control of hypertrophic scar growth using selective photothermolysis. Lasers Surg Med 1997;21:7–12 [DOI] [PubMed] [Google Scholar]
- 23. Kuo YR, Jeng SF, Wang FS, et al. Flashlamp pulsed dye laser (PDL) suppression of keloid proliferation through down-regulation of TGF-beta1 expression and extracellular matrix expression. Lasers Surg Med 2004;34:104–108 [DOI] [PubMed] [Google Scholar]
- 24. Tong AK, Tan OT, Boll J, Parrish JA, Murphy GF. Ultrastructure: effects of melanin pigment on target specificity using a pulsed dye laser (577 nm). J Invest Dermatol 1987;88:747–752 [DOI] [PubMed] [Google Scholar]
- 25. Vrijman C, Van Drooge A, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol 2011;165:934–942 [DOI] [PubMed] [Google Scholar]
- 26. Alster TS, Handrick C. Laser treatment of hypertrophic scars, keloids, and striae. Semin Cutan Med Surg 2000;19:287–292 [DOI] [PubMed] [Google Scholar]
- 27. Bellew SG, Weiss MA, Weiss RA. Comparison of intense pulsed light to 595-nm long-pulsed pulsed dye laser for treatment of hypertrophic surgical scars: a pilot study. J Drugs Dermatol 2005;4:448–452 [PubMed] [Google Scholar]
- 28. de las Alas JM, Siripunvarapon AH, Dofitas BL. Pulsed dye laser for the treatment of keloid and hypertrophic scars: a systematic review. Expert Rev Med Devices 2012;9:641–650 [DOI] [PubMed] [Google Scholar]
- 29. Bailey JK, Burkes SA, Visscher MO, et al. Multimodal quantitative analysis of early pulsed-dye laser treatment of scars at a pediatric burn hospital. Dermatol Surg 2012;38:1490–1496 [DOI] [PubMed] [Google Scholar]
- 30. Brewin MP, Lister TS. Prevention or treatment of hypertrophic burn scarring: a review of when and how to treat with the pulsed dye laser. Burns 2014;40:797–804 [DOI] [PubMed] [Google Scholar]
- 31. Willows BM, Ilyas M, Sharma A. Laser in the management of burn scars. Burns 2017;43:1379–1389 [DOI] [PubMed] [Google Scholar]
- 32. Waibel JS, Rudnick A, Shagalov DR, Nicolazzo DM. Update of ablative fractionated lasers to enhance cutaneous topical drug delivery. Adv Ther 2017;34:1840–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL). Lasers Surg Med 2014;46:462–469 [DOI] [PubMed] [Google Scholar]
- 34. Sklar LR, Burnett CT, Waibel JS, Moy RL, Ozog DM. Laser assisted drug delivery: a review of an evolving technology. Lasers Surg Med 2014;46:249–262 [DOI] [PubMed] [Google Scholar]
- 35. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med 2013;45:135–140 [DOI] [PubMed] [Google Scholar]
- 36. Lei Y, Li SF, Yu YL, Tan J, Gold MH. Clinical efficacy of utilizing Ultrapulse CO2 combined with fractional CO2 laser for the treatment of hypertrophic scars in Asians-A prospective clinical evaluation. J Cosmet Dermatol 2017;16:210–216 [DOI] [PubMed] [Google Scholar]
- 37. Hultman CS, Friedstat JS, Edkins RE, Cairns BA, Meyer AA. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg 2014;260:519–532 [DOI] [PubMed] [Google Scholar]
- 38. Zadkowski T, Nachulewicz P, Mazgaj M, et al. A new CO2 laser technique for the treatment of pediatric hypertrophic burn scars: an observational study. Medicine (Baltimore) 2016;95:e5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keen A, Sheikh G, Hassan I, et al. Treatment of post-burn and post-traumatic atrophic scars with fractional CO2 laser: experience at a tertiary care centre. Lasers Med Sci 2018;33:1039–1046 [DOI] [PubMed] [Google Scholar]
- 40. El-Zawahry BM, Sobhi RM, Bassiouny DA, Tabak SA. Ablative CO2 fractional resurfacing in treatment of thermal burn scars: an open-label controlled clinical and histopathological study. J Cosmet Dermatol 2015;14:324–331 [DOI] [PubMed] [Google Scholar]
- 41. Scrimali L, Lomeo G, Nolfo C, et al. Treatment of hypertrophic scars and keloids with a fractional CO2 laser: a personal experience. J Cosmet Laser Ther 2010;12:218–221 [DOI] [PubMed] [Google Scholar]
- 42. Buelens S, Van Hove AS, Ongenae K, et al. Fractional Carbon Dioxide Laser of Recent Surgical Scars in the Head and Neck Region: A Split-Scar, Evaluator-Blinded Study. Dermatol Surg 2017;43 Suppl 1:S75–S84 [DOI] [PubMed] [Google Scholar]
- 43. Poetschke J, Dornseifer U, Clementoni MT, et al. Ultrapulsed fractional ablative carbon dioxide laser treatment of hypertrophic burn scars: evaluation of an in-patient controlled, standardized treatment approach. Lasers Med Sci 2017;32:1031–1040 [DOI] [PubMed] [Google Scholar]
- 44. Makboul M, Makboul R, Abdelhafez AH, Hassan SS, Youssif SM. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-beta1 expression in hypertrophic scar. J Cosmet Dermatol 2014;13:169–179 [DOI] [PubMed] [Google Scholar]
- 45. Qu L, Liu A, Zhou L, et al. Clinical and molecular effects on mature burn scars after treatment with a fractional CO(2) laser. Lasers Surg Med 2012;44:517–524 [DOI] [PubMed] [Google Scholar]
- 46. Azzam OA, Bassiouny DA, El-Hawary MS, El Maadawi ZM, Sobhi RM, El-Mesidy MS. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci 2016;31:9–18 [DOI] [PubMed] [Google Scholar]
- 47. DeBruler DM, Blackstone BN, Baumann ME, et al. Inflammatory responses, matrix remodeling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg Med 2017;49:675–685 [DOI] [PubMed] [Google Scholar]
- 48. Krakowski AC, Goldenberg A, Eichenfield LF, Murray JP, Shumaker PR. Ablative fractional laser resurfacing helps treat restrictive pediatric scar contractures. Pediatrics 2014;134:e1700–e1705 [DOI] [PubMed] [Google Scholar]
- 49. Bailey JK, Blackstone BN, DeBruler DM, et al. Effects of early combinatorial treatment of autologous split-thickness skin grafts in red duroc pig model using pulsed dye laser and fractional CO2 laser. Lasers Surg Med 2018;50:78–87 [DOI] [PubMed] [Google Scholar]
- 50. Carney BC, McKesey JP, Rosenthal DS, Shupp JW. Treatment strategies for hypopigmentation in the context of burn hypertrophic scars. Plast Reconstr Surg Glob Open 2018;6:e1642. [DOI] [PMC free article] [PubMed] [Google Scholar]