Abstract
We review a case of a 22-year-old healthy man who underwent a 5-h maxillofacial surgery while continuously supine with foam pads placed prophylactically over elevated heels. Immediately after surgery, Stage 1 pressure injuries appeared on the left lateral heel and right lateral ankle, despite the absence of local pressure to these areas. Both lesions eventually resolved. Eight months later, a Doppler evaluation was performed of the patient's lower extremities, the peroneal artery and its tributaries were marked, and the intraoperative positioning was simulated to determine if a wedge at the back of the calf could have obstructed blood flow in these vessels. In this position, the feet naturally abducted so that the lateral calcaneal and posterior malleolar arteries became positioned immediately underneath the wedge. We propose a vascular mechanism of pressure injury development, postulating that some heel pressure injuries are not the result of localized pressure but rather angiosomal ischemia, based on the observation that the anatomical pattern of these lesions frequently follow the distribution of a named vessel. We hypothesize that in this case, intraoperative positioning along with permissive hypotension may have occluded arterial or venous flow to the relevant angiosomes, causing an ischemia reperfusion injury to the downstream tissues.
Keywords: angiosomes, deep tissue injury, pressure injury, ischemia reperfusion, pressure ulcer
Caroline E. Fife, MD.

We report a recent intraoperative case that suggests a new mechanism of lower extremity pressure injury formation.
A 22-year-old healthy White athletic man with a muscular build (body mass index: 28) underwent a 5-h maxillofacial operation to repair maxillary hypoplasia with apertognathia, mandibular hyperplasia, and macroglossia. A segmental Le Fort I osteotomy, bilateral mandibular vertical ramus osteotomies, and partial tongue reduction were performed with the patient under general anesthesia, in addition to local injections of bupivacaine hydrochloride and epinephrine. He was admitted in his usual good state of health. He was in the supine position throughout the procedure, with his heels elevated by wedges placed at the posterior calf behind the heels, bilaterally. A bordered foam dressing was placed prophylactically on both heels. Intraoperative records show that his mean arterial pressure (MAP) was maintained at ∼60 mmHg, except for an ∼20-min period when it dropped slightly, <60 mmHg. He experienced minimal blood loss, vital signs were stable before and after surgery, and his hemoglobin and hematocrit were 16.3 g/dL and 45.9%, postoperatively.
Immediately upon recovery from anesthesia, the patient reported excruciating pain in his left heel. Examination revealed a triangular nonblanching Stage 1 pressure injury over the lateral aspect of the left heel (Fig. 1). He also had a smaller Stage 1 pressure injury of the right lateral malleolus (Fig. 2), which was not painful. The following day, the hospital Wound and Ostomy nurse observed the painful lesion on the left lateral heel blanched with pressure, which, per pressure injury guidelines,1 was not recorded as a pressure injury. Within 48 h, the left lateral heel discoloration and the right ankle lesion almost resolved (Fig. 3). The lateral heel was painful for ∼3 more weeks, with reduced sensitivity to touch for ∼6 weeks, confirming recoverable injury to the sural nerve consistent with neuropraxia. Review of medical records and discussion with the surgeon confirmed that the patient had been in the supine position throughout the operation and was never turned on either side. Hereunder, we explore and theorize a pathophysiological vascular mechanism that resulted in the development of Stage 1 pressure injuries on the lateral heel and lateral malleolus within the 5-h time frame of the operative procedure.
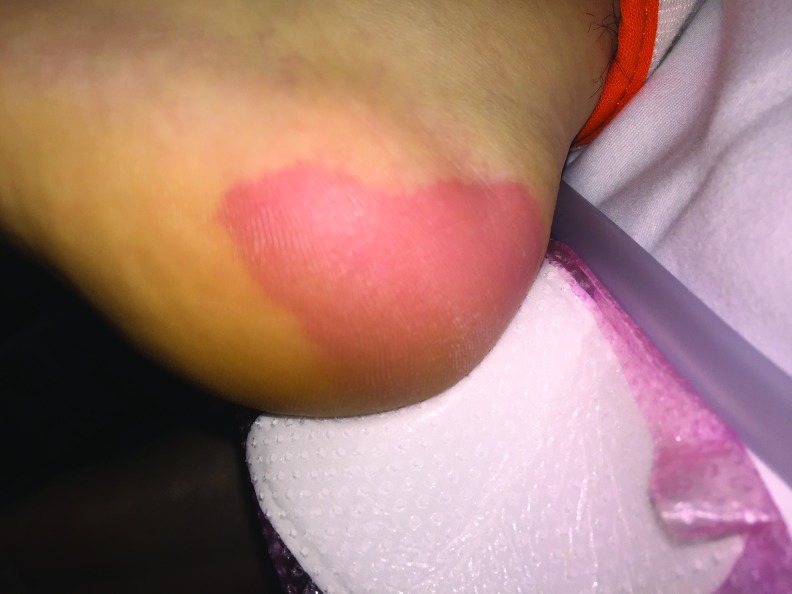
Figure 1.
An excruciatingly painful, but blanching, pressure injury on the left lateral heel pictured here immediately after a maxillofacial operation. Note that a foam pad placed prophylactically before surgery was removed so that the heel can be examined.
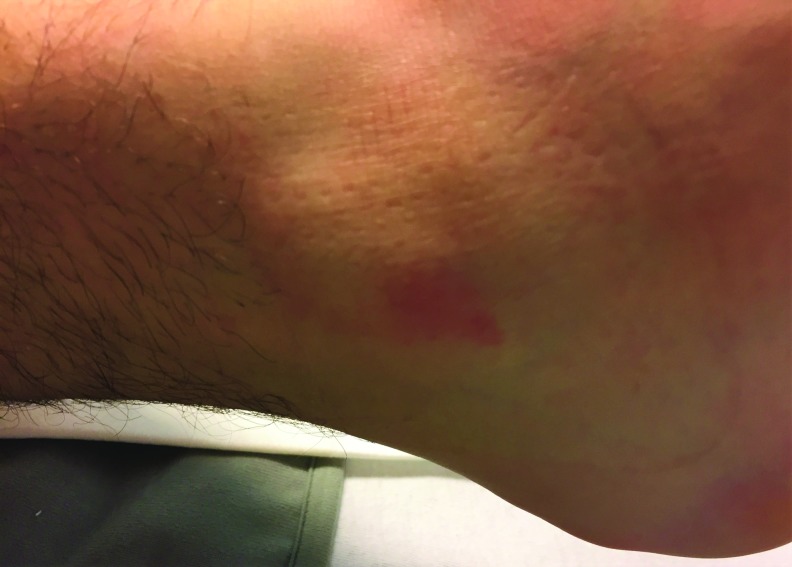
Figure 2.
A small pressure injury on the right lateral malleolus immediately after a maxillofacial operation.
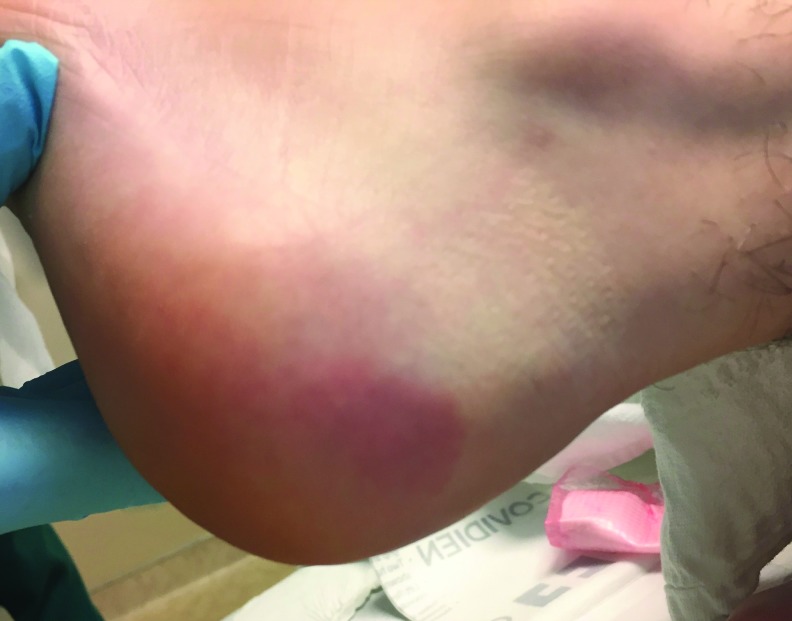
Figure 3.
The left lateral heel pressure injury on postoperative day 2. The discoloration is nearly resolved here, but the area remained painful for 3 weeks.
Discussion
The heels are believed to be at risk for pressure injury due to the lack of soft tissue overlying the calcaneus1; thus, heels are normally elevated for offloading purposes during operative procedures. The malleolus is at similar risk, since it also overlies a bony prominence. Therefore, the location of these intraoperative pressure injuries is not surprising, except that they developed without any pressure being applied to the lateral heel or the lateral malleolus. If pressure injuries are localized damage to the skin and soft tissue from prolonged pressure,2 these lesions defy this explanation and, perhaps, were of vascular nature.
Both lesions follow the angiosomal distribution of named vessels. The importance of angiosomes, three-dimensional blocks of tissue supplied by a main source artery and its accompanying vein(s), in the pathophysiology of lower extremity ulceration and their treatment through revascularization are well established.3,4 The pattern of tissue injury over the left lateral heel (Fig. 1) is best explained as ischemia in the distribution of the lateral calcaneal branch of the peroneal artery.4 This would account for the pattern of injury appearing as a well demarcated “triangle” over the lateral heel. If the ischemic insult occurred at the capillary level, then the margins would not be so perfectly demarcated and would only affect the tissue at the point of contact with the support surface, similar to the diffuse circle of red that occurs on the calf after keeping one's leg crossed for too long.
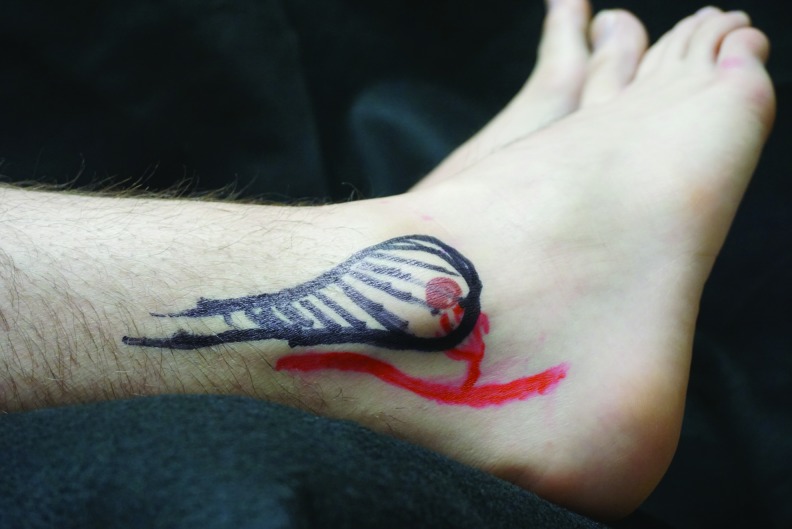
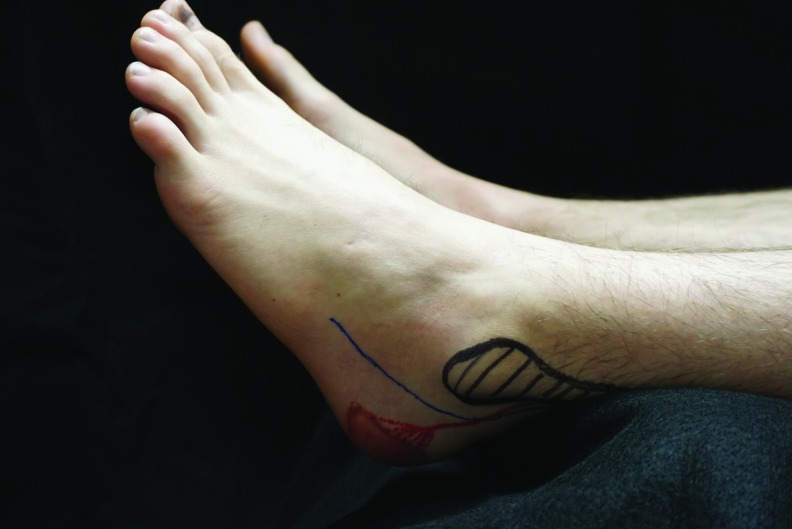
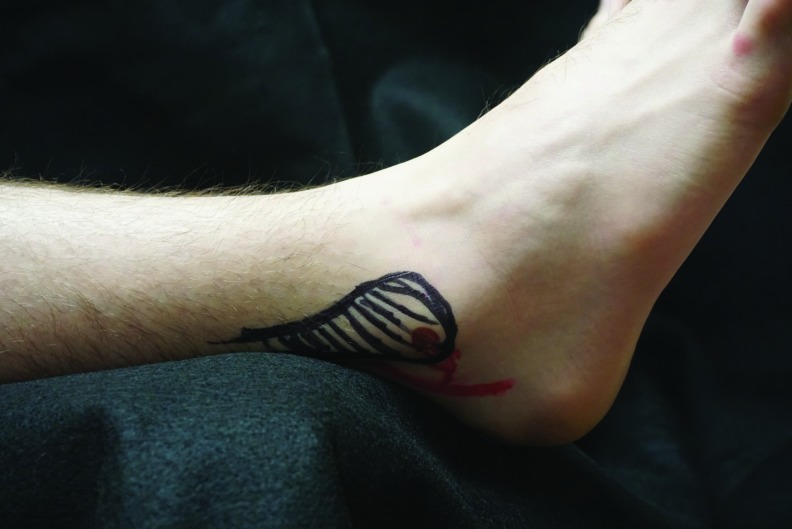
How could ischemia to the left calcaneal artery occur if the heel was elevated while in a supine position? When lying supine, both feet naturally abduct due to external rotation of the hips so that the pressure from an elevating wedge resets on the posterior Achilles tendon, slightly lateral to and just above the curvature of the heel. We hypothesized that this positioning could apply pressure on the peroneal artery either at the origin of the lateral calcaneal artery, or at the origin of the posterior lateral malleolar artery, perhaps sufficient to cause an ischemia reperfusion injury to the tissue supplied by these vessels, without any intense or prolonged pressure being applied directly to the area, particularly if the MAP is reduced. To test this hypothesis, 8 months postoperative, we performed a Doppler evaluation of the patient's feet and marked the position of these vessels and other anatomical landmarks. We positioned him approximately as he was during surgery to determine whether a wedge at the back of the calf could have obstructed flow in these small vessels (Figs. 4–7). We confirmed that the patient's heavy muscular legs would cause significant compressive force against the wedge and the calcaneal and posterior malleolar arteries would be positioned underneath the wedge. Taking further into account permissive hypotension during surgery, it seems feasible that pressure injuries to the lateral heel and the lateral malleolus could occur without any pressure having been applied directly to those areas, simply by occluding the arterial flow to the relevant angiosome.4
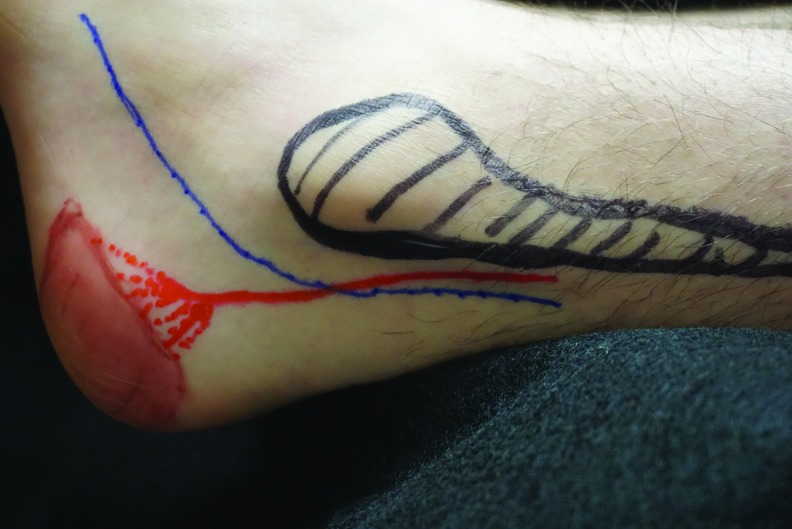
Figure 4.
The left lateral calcaneal artery angiosome when the foot is abducted and externally rotated. The approximate location of the original heel pressure injury is depicted in brown ink, the lateral calcaneal artery in red ink, the sural nerve in blue, and the fibula in black.
Figure 5.
The right posterior lateral malleolar artery when the foot is abducted and externally rotated. The approximate location of the original ankle pressure injury is depicted in brown ink, the posterior lateral malleolar artery in red ink, and the fibula in black.
Figure 6.
The left lateral calcaneal artery angiosome when the foot relaxes naturally. Note that if a wedge is placed behind the heels, as the foot abducts, the weight of the leg rests on the branches of the peroneal artery such as the lateral calcaneal artery.
Figure 7.
The right lateral calcaneal artery angiosome when the foot relaxes naturally. The approximate locations of the pressure injuries have been marked with light brown ink. Note that if a wedge is placed behind the heels, as the foot abducts, the weight of the leg rests on the branches of the peroneal artery such as the posterior lateral malleolar artery.
Ulcerations of the lateral malleolus have been described as a result of ischemia to the peroneal artery supplying this angiosome.4 It has long been assumed that the “intense and prolonged pressure” that is the proximate cause of tissue damage (and is thus now termed “injury,” in the physical sense) is local.2 We postulate that, in some cases, it is due to ischemia of the vascular supply to an angiosome at a point proximal to the affected area causing an ischemia reperfusion “injury.” The peroneal artery is most vulnerable to occlusion from external pressure applied to the lower leg at the anatomic area posterior to the fibula and anterior to the Achilles in the curvature of the posterior heel,4 where wedges or pillows would be placed. Elevating the heels should protect them from physical injury caused by contact with the operating table, but not from ischemia due to occlusion of blood flow at a more proximal point.
In this case report, both lower extremity pressure injuries resolved completely. Thus, it is fair to ask whether a reversible ischemia reperfusion injury to the skin and subcutaneous tissues are an acceptable price to pay for a safer, less bloody operative field during maxillofacial surgery. However, we cannot debate which trade-offs may or may not be acceptable until we have a correct understanding of the mechanism by which such pressure injuries occur.
Current theory suggests some pressure injuries—but not all—are likely caused by capillary occlusion due to pressure occurring directly over the bony interface.5 We postulate that the limitation of blood flow does occur, but it does not have to be due to direct pressure. We theorize that some pressure injuries may be the result of angiosomal ischemia, based on the anatomical pattern of these lesions frequently following the distribution of a named vessel.3,4 The recoverable phenomenon previously known as “Stage 1 pressure ulcers” (now Stage 1 pressure injuries),2 the pathology of which have remained elusive,1 can now be explained as ischemia reperfusion injuries. Ischemia reperfusion injury is a mechanism that also explains the commonly observed phenomenon of intense pain with some pressure injuries, since nerves are often supplied by the same vessels,6 and the long-term or permanent loss of sensation among patients who suffer significant tissue necrosis.
Angiosomal ischemia has been demonstrated in many patients with peripheral arterial disease who develop lower extremity ulceration. Direct endovascular revascularization, based on angiosomal anatomy, has been demonstrated to be the most effective way of healing ischemic ulcers.3,7 Patients with chronic peripheral arterial disease can develop collateral circulation to compensate for the loss of pulsatile flow, making the tissue less dependent on a single vessel and the anatomical distribution of ischemia less defined. However, in our case, without the mitigating impact of collateral blood supply and the absence of any underlying disease,8 we hypothesize that the acute occlusion of one of these relatively small diameter vessels could result in a clearly angiosomal pattern of injury. The lateral calcaneal artery is a terminal branch of the peroneal artery. Collateral circulation of the lateral malleolar network is formed primarily from branches of the peroneal artery and occlusion of the vessel before the perforating branch would affect the entire lateral malleolar network.
Where vascular ischemia is the final common pathway for tissue destruction, prevention protocols should target risk factors such as hypotension, hypoalbuminemia, hypoxia, anemia, and reduced cardiac output,9,10 all of which are modifiable determinants of angiosomal perfusion. For patients undergoing prolonged surgical interventions, it may be appropriate to consider intraoperative repositioning when feasible, and to consider vascular anatomy when positioning offloading wedges or pillows. If the ischemic insult originates within a named vessel (either artery or vein) proximal to the visible area of skin change, and is associated with a low MAP, no foam pad placed over the area of visible skin change will mitigate it. Based on our personal experience in clinical practice, single-use prophylactic foam dressings can contribute half a million dollars each year to a single hospital's budget. Perhaps, long-term investment is the purchase of higher quality padding for operating room tables may be better able to reduce mechanical loading to large areas of the body. In addition, we need a better mechanism for noninvasively assessing the perfusion of high-risk areas. If the angiosomal theory for pressure injury formation has merit, it has significant implications for pressure injury prevention protocols both inside and outside the operating room.
Abbreviation Used
- MAP
mean arterial pressure
Author Disclosure and Ghostwriting
There was no financial support for this study, and the authors have no conflicts of interest to declare. The authors thank Kristen Eckert (Strategic Solutions, Inc., Cody, WY) for her assistance in writing and editing the article.
About the Authors
Caroline E. Fife, MD, has been a certified wound specialist since 1998 and is the current medical director of the St. Luke's Wound Care Clinic in The Woodlands, Texas. She is the chief medical officer of Intellicure, a Texas-based software company, which since 2000 has provided a specialty-specific electronic medical record system to wound and hyperbaric centers across the United States. Dr. Fife is also the executive director of the U.S. Wound Registry, a nonprofit organization which develops and reports wound care quality measures to facilitate physician participation in the Merit-based Incentive Payment System (MIPS). The USWR also provides data for comparative effectiveness studies in wound care. Efthymios Gkotsoulias, DPM, is a foot and ankle surgeon at Baylor College of Medicine practicing in the north Houston area. His main focus is diabetic foot infections, limb salvage/preservation, reconstructive surgery of the foot and ankle, and revision surgery. He completed a 3-year surgical residency at Gundersen Medical Foundation in La Crosse Wisconsin serving as cochief resident. He received his doctorate of podiatric medicine degree from Scholl College of Podiatric Medicine at Rosalind Franklin University of Medicine and Science.
References
- 1. Langemo D. Heel Pressure Ulcers: 2014 International Pressure Ulcer Prevention & Treatment guidelines. National Pressure Ulcer Advisory Panel, 2014. www.npuap.org/wp-content/uploads/2015/02/4.-Preventing-Treating-Heel-Ulcers-D-Langemo.pdf (last accessed March22, 2018)
- 2. The National Pressure Ulcer Advisory Panel—NPUAP. National Pressure Ulcer Advisory Panel (NPUAP) announces a change in terminology from pressure ulcer to pressure injury and updates the stages of pressure injury. NPUAP, 2016. www.npuap.org/national-pressure-ulcer-advisory-panel-npuap-announces-a-change-in-terminology-from-pressure-ulcer-to-pressure-injury-and-updates-the-stages-of-pressure-injury (last accessed March1, 2018)
- 3. Varela C, Aci NF, Haro JD, et al. The role of foot collateral vessels on ulcer healing and limb salvage after successful endovascular and surgical distal procedures according to an angiosome model. Vasc Endovasc Surg 2010;44:654–660 [DOI] [PubMed] [Google Scholar]
- 4. Alexandrescu V, Hubermont G. Primary infragenicular angioplasty for diabetic neuroischemic foot ulcers following the angiosome distribution: a new paradigm for the vascular interventionist? Diabetes Metab Syndr Obes 2011;4:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Pressure Ulcer Advisory Panel. Deep tissue injury. 2012. www.npuap.org/wp-content/uploads/2012/01/DTI-white-Paper.pdf (last accessed March9, 2018)
- 6. Loerakker S, Manders E, Strijkers GJ, et al. The effects of deformation, ischemia, and reperfusion on the development of muscle damage during prolonged loading. J Appl Physiol (1985) 2011;111:1168–1177 [DOI] [PubMed] [Google Scholar]
- 7. Biancari F, Juvonen T. Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2014;47:517–522 [DOI] [PubMed] [Google Scholar]
- 8. Murrant CL. Structural and functional limitations of the collateral circulation in peripheral arterial disease. J Physiol 2008;586:5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox J. Pressure ulcer development and vasopressor agents in adult critical care patients: a literature review. Ostomy Wound Manage 2013;59:50–54, 56–60. [PubMed] [Google Scholar]
- 10. Terekeci H, Kucukardali Y, Top C, Onem Y, Celik S, Oktenlic C. Risk assessment study of the pressure ulcers in intensive care unit patients. Eur J Intern Med 2009;20:394–397 [DOI] [PubMed] [Google Scholar]