Abstract
Significance: Keloid scarring is a disfiguring fibroproliferative disorder that can significantly impair the quality of life in affected individuals. The mechanisms that initiate keloid scarring are incompletely understood, and keloids remain one of the most challenging skin conditions to treat. Keloids are unique to humans; thus, the lack of adequate animal models has hindered research efforts aimed at prevention and effective therapeutic intervention.
Recent Advances: In the absence of a suitable animal model, keloid researchers often rely on studying excised keloid scar tissue and keloid-derived cultured cells. Recently, in vivo models have been described that involve transplantation to mice of reconstructed skin containing keloid-derived fibroblasts and/or keratinocytes. These mouse–human hybrid animal models display some similarities with keloids and may enable investigation of novel therapies, although no model yet recapitulates all the features of human keloid scarring.
Critical Issues: Differences in skin physiology and modes of healing contribute to challenges in modeling keloids in laboratory animals. Furthermore, recent studies suggest that cells of the immune system contribute to keloid pathology. The need to use immunodeficient hosts for transplanted human keloid cells in recently described animal models precludes studying the role of the immune system in keloid scarring.
Future Directions: Future animal models may take advantage of humanized mice with immune systems reconstituted using human immune cells. Such models, when combined with grafted tissues prepared using keloid-derived cells, might enable investigation of complex interactions between systemic and local factors that combine to promote keloid scar formation and may aid in the development of novel therapies.
Keywords: : keloid, animal model, scar, wound healing, extracellular matrix, fibrosis
Dorothy M. Supp, PhD.

Scope and Significance
Keloid scarring is a disfiguring fibroproliferative disorder that can significantly impair the quality of life in affected individuals.1–4 Keloids result from an abnormal wound healing response, which involves excessive and prolonged deposition of extracellular matrix (ECM), particularly collagen. Despite decades of research that has advanced our understanding of wound healing, the mechanisms that initiate keloid scarring remain poorly understood. In contrast to normal scars, which stabilize over time, keloid scars are exuberant fibrous growths that extend beyond the original wound boundary and tend to grow indefinitely (Fig. 1), often impairing range of motion and interfering with normal daily activities. Keloid scars are firm, dense, and itchy and can be complicated by ulceration, bleeding, and infection.5 Keloids share several features with hypertrophic scars (HTS), another form of abnormal scarring. Until relatively recently, keloids and HTS were considered different manifestations of the same abnormal scarring process; currently, however, the more widely accepted viewpoint defines keloids and HTS as separate entities that have some common features, but which follow different clinical courses.6–8 Susceptibility to keloid scarring can run in families, suggesting a possible genetic predisposition in some keloid patients. Although recent studies have identified multiple loci that are strongly associated with keloid risk,9–11 no single causative gene has yet been identified. Keloids are more common in populations with darkly pigmented skin, such as African Americans, but it is not yet known whether this is due to pigmentation or common genetic ancestry.9 Furthermore, the possible influence of environmental factors on keloid risk has not been elucidated. Keloids can occur at any age but most commonly occur during the second and third decades of life5; it is not known whether this is due to hormonal influences or other factors. Although there are multiple therapies available for keloids, they remain one of the most challenging skin conditions to treat.12,13 There is currently no universally effective treatment, and most therapies are successful for only a subset of patients and have limited long-term success, with high recurrence rates. Development of improved treatments will require a deeper understanding of the molecular mechanisms that cause healing wounds to progress to keloid scars, as well as appropriate preclinical models for evaluation of novel therapies. This review discusses the complexities of modeling keloid scarring, summarizes currently available animal models, and describes new technologies that may improve future preclinical keloid models.
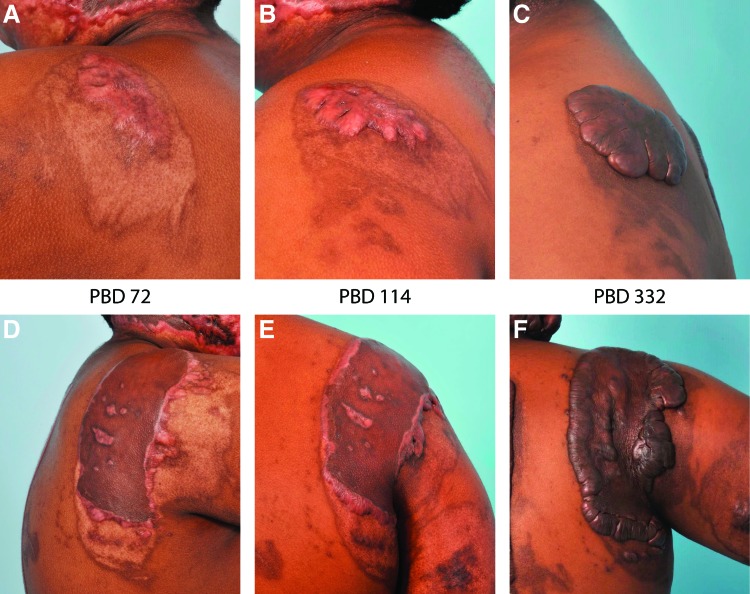
Figure 1.
Keloid scar development. Shown are photos of the same patient illustrating the rapid development of keloid lesions over time after skin injury. The patient, an African American male who sustained a 15% total body surface area burn at 15 years of age, developed widespread keloid lesions in both grafted and ungrafted burn wounds, as well as donor sites used for autograft harvesting. (A–C) Images of patient's left shoulder, showing a healed partial-thickness burn wound that was not grafted. Note the rapid development of large keloid with typical bulging appearance. (D–F) Images of patient's right shoulder; this deeper burn wound was treated with split-thickness skin autograft. Note the development of keloid scarring around the skin graft and within the grafted area where wounds appear to have occurred. Photographs were taken at PBD 72 (A, D), PBD 114 (B, E), and PBD 332 (C, F). PBD, postburn day. Color images are available online.
Translational Relevance
There is a paucity of high-quality clinical research evaluating keloid therapies. The evidence supporting many current therapies is based on low-quality studies, nonrandomized trials, and case reports, limiting the ability to make evidence-based decisions regarding therapy.13,14 Ideally, effective therapies would be based on a detailed understanding of the underlying pathology and would be developed and tested using preclinical animal models. However, keloid scarring is unique to humans, complicating the use of animals as models for preclinical studies. This has hindered translational research efforts aimed at development of effective strategies for keloid prevention and treatment.
Clinical Relevance
For functional analysis of the dynamic process of human wound healing, samples would ideally be obtained from uninjured skin and from wounds at multiple time points during healing. This is problematic for clinical studies of keloid scarring. How do researchers ethically sample normal tissue from a keloid patient if this will cause a new keloid to form at the biopsy site? If there is no family history or prior incidence of keloids, how can we predict who will form a keloid so that we can follow its early development and progression? For these reasons and others, animal models are critical.
Overview
Human versus animal wound healing
It is not known why humans form keloid scars, but animals do not. Keloid etiology is complex, and there are numerous physiological differences between humans and rodents, the most commonly used research animals, as well as differences in pathology and responses to injury.15 Anatomically, rodent skin is thinner and is more loosely attached to the underlying fascial tissue. Skin contraction plays a larger role in wound healing in rodents, whereas granulation tissue formation and reepithelialization constitute the primary mode of healing in human wounds.16 Mechanical forces are known to regulate profibrotic gene expression in human fibroblasts, resulting in the upregulation of genes encoding ECM proteins, such as collagen and fibronectin, and profibrotic cytokines, such as transforming growth factor beta 1 (TGF-β1).17 Differences in skin tension during wound healing may underlie, in part, the different physiological responses to wounding in human versus murine skin. Human skin, which is tightly adhered to the underlying fascia, experiences higher levels of mechanical stress during healing than murine skin, which is tightly attached to the panniculus carnosus but more loosely adhered to underlying fascia, resulting in protection from excess mechanical load during healing. Differences in density and size of hair follicles, which can contribute stem cells to aid in wound healing, as well as immunological differences also contribute to different wound healing processes in rodents and humans.16,18 Additionally, the microbiome has been shown to influence wound healing, but there are differences in the microbiomes of mice and humans.19,20 Despite these numerous differences, mice are commonly used in wound healing research, and although they can form thickened scars under certain experimental circumstances, keloids have never been observed in murine skin.
Fibrotic wound healing in animals
Pigs are increasingly used in wound healing studies, as pig skin more closely resembles human skin in thickness and hair density.16,21,22 Some pig breeds develop raised scars resembling HTS under certain wounding conditions; thus, despite their relatively high cost, pigs have become a useful model for HTS. Deep wounds (full thickness or deep partial thickness) in the female red Duroc pig were shown to heal with characteristics similar to human HTS.23,24 These include contracted, thickened, hyperpigmented scars with abnormal gene expression patterns similar to the alterations observed in human HTS, including increased TGF-β1 and collagen type 1 and decreased decorin, although the levels of expression varied over time after wounding.24,25 Histologically, deep excisional wounds in the female red Duroc pig model display disorganized collagen fibers, collagen nodules, presence of myofibroblasts, and elevated mast cell counts similar to human HTS.26 However, in the pig model, the timing of these features appears compressed relative to human HTS.26 A recent innovation of this model from Powell's laboratory involves a full-thickness burn followed by grafting with meshed split thickness skin autograft in the female red Duroc pig, which was found to more closely model human burn scars compared with excisional wounds.27,28 Unfortunately, features observed in human keloids but not HTS, such as continued growth beyond the wound margin, have not yet been reported in any porcine wound model.
The only animal, aside from humans, known to naturally develop extreme fibroproliferative scarring is the horse. Limb wounds in horses, in contrast to wounds in other areas of the body, can develop a type of scarring known as exuberant granulation tissue (EGT) or “proud flesh,” which is similar in some respects to human keloid scars.29 Wounds on the distal limbs of horses are characterized by complications not encountered in trunk wounds, such as minimal soft tissue coverage around the wound, reduced blood supply, frequent movement of the injured limb, and greater risk of microbial contamination. Additionally, leg wounds have reduced contraction and slower rates of wound reepithelialization.30 Loss of tissue in leg wounds is common, complicating primary closure and resulting in healing by secondary intention, which contributes to the deposition of excessive granulation tissue.31 Similar to keloids in humans, EGT in horses is raised and extends beyond the wound bed, resembling benign tumor-like growths, and fibroblasts of EGT display overproduction of ECM.31 In contrast to keloids, which form after wound reepithelialization is complete and have an intact (although aberrant) epidermis, EGT frequently occurs before completion of wound reepithelialization. The wound margins may display a hyperplastic epidermis, but ulceration of the central portion of the wound is common. As an animal model for human pathology, horses are less than ideal due to high costs for veterinary care and housing, long life span, large size, and paucity of species-specific reagents compared with rodent models. However, as the only other known species to exhibit extreme fibroproliferative healing, comparative studies of EGT and keloid scarring may provide insights that can ultimately benefit both species.
Experimental animal models of dermal fibrosis
Although keloid-like scarring has not been induced in rodent models to date, manipulation of the murine wound to reduce contraction and promote healing via reepithelialization, as in human wounds, can enable generation of scars exhibiting some features of human HTS.15 To create murine wound healing model that more closely resembles human wound healing, Gurtner's laboratory created a model in which splints were fastened to the margins of excisional wounds in mice.32,33 The splints counteracted wound contraction, increasing the role of reepithelialization in wound closure, more analogous to healing in human skin.32 This group later used biomechanical loading devices for application of mechanical stress during healing of incisional wounds to mimic the forces experienced by human wound healing under tension.34 Mechanical loading during the proliferative phase of healing resulted in scars more closely resembling human HTS, emphasizing the role of mechanical stress in HTS formation.34
A reproducible model of raised scarring was developed in rabbits35 and has been subsequently utilized in numerous studies for preclinical analysis of anti-scar therapies. This model involves full-thickness excisional wounds (≥7 mm diameter) created on the ventral ear surface down to the level of cartilage, including removal of the perichondrium.36 Wounds of this size and depth display delayed reepithelialization, resulting in thickened scars, similar to human HTS, which may persist for weeks to months.36 The efficacy of silicone gel sheeting, a commonly used intervention for HTS and keloids, was tested in rabbit ear wounds and was shown to significantly reduce scar elevation in this model.37 The mechanism of action appeared to involve increased epidermal hydration due to occlusion, which reduced the keratinocyte activation and decreased profibrotic paracrine signaling resulting in lower dermal ECM production.38,39 However, when analyzed in numerous clinical trials, the benefits of silicone sheeting have been less clear.40 This exemplifies important differences between preclinical studies in animal models and human clinical trials that complicate clinical translation of many promising therapies. Animal models can be highly uniform and reproducible, enabling preclinical studies with tightly controlled treatment regimens and strictly defined outcome measures. In contrast, human trials can be limited by subject heterogeneity, susceptibility to bias, and uncertainty regarding patient compliance. For anti-scar therapies, the numerous differences between humans and animals, detailed above, and inadequacies of current models further complicate clinical translation.
A mouse model involving orthotopic grafting of human skin was developed in Tredget's laboratory that has similarities to human HTS.41,42 This model involves grafting of full-thickness or split-thickness normal human abdominal skin obtained from elective cosmetic surgery to full-thickness excisional wounds on the backs of athymic mice. Approximately 1 month after grafting, the human skin grafts harden and the upper layer peels off; by 2 months after grafting, the grafts appear reddish, firm, and raised compared with surrounding mouse skin, reminiscent of human HTS.41 The hypertrophy was observed to be greater in grafts of full-thickness human skin compared with split-thickness skin; this was attributed to injury caused by dermatome preparation of the split-thickness skin, which may have initiated proinflammatory profibrotic responses in the graft.41 Similarities with human HTS included increased levels of collagen type 1 alpha 1 (COL1A1), TGF-β1, and connective tissue growth factor compared with normal human skin, as well as increased numbers of mast cells, macrophages, and alpha smooth muscle actin-positive cells.41 In addition, whorled collagen fibers and decreased levels of decorin compared with normal skin were observed.42 These studies demonstrate that under specific conditions, this chimeric model can recapitulate many of the features of HTS in a convenient small animal model, using a human tissue source that is more readily available than HTS or keloid scar.
It is important to note that none of these models mimic features that distinguish keloids from HTS, such as continued growth beyond the boundaries of the initial wound. This supports the concept that keloids and HTS represent distinct clinical entities and suggests that critical components present in humans, but not in animals, are missing from these models.
Discussion: Current Status of Keloid-Specific Models
Keloid scar implantation models
Because keloid scarring is a uniquely human trait and, as described above, no single causative gene has yet been identified, keloid-specific models involve cells and/or tissue derived from keloid patients. Studies by Shetlar and colleagues dating back to the 1980s described implantation of keloid scar tissue into subcutaneous pockets in immunodeficient mice, with tissue integrity reportedly maintained from 60 to >240 days without rejection.43,44 The implants vascularized quickly, and remodeling of the edges of the implants was observed in addition to the reduction of implant size over time.44 A follow-up study also demonstrated a reduction in weight of implanted tissue over time, as well as a significant reduction in chondroitin-4-sulfate levels, particularly 80 days postimplantation.45 Normal human skin implants also decreased in size but to a lesser degree than the scar implants. The authors speculated that the reduction in implant size over time might have been due to rejection, which may have been greater for the keloid tissue compared with normal skin,45 although there was no histological analysis shown to support the immune rejection. Nevertheless, the authors asserted that the model could be useful for testing of therapeutic agents in relatively short-term studies. They subsequently tested the effects of oral pirfenidone, an antifibrotic drug, using this model; the weight of all implants decreased with time, as observed in prior studies, but weights of implants in the pirfenidone-treated animals were significantly lower than untreated controls.46 They also tested injection of triamcinolone, which is a common treatment for keloid scars, but had difficulty in injecting the drug directly into the subcutaneously implanted keloid tissue. Subcutaneous injection near the implants caused a reduction in size of the tissue but also caused a decrease in body weight of the treated mice.46 Another group of investigators undertook a similar study using mice harboring subcutaneous implants of deepithelialized keloid tissue pieces to investigate intralesional triamcinolone in addition to four other pharmaceutical agents.47 In that study, the implants initially increased in size, and then decreased, with no differences observed among any of the treatment groups and controls. The authors of that study found that collagen organization of the implanted tissue was similar to the original tissue, but several of the implants were enclosed in a “pseudocapsule” that may have interfered with drug exposure.47
There are several limitations inherent to models that rely on implanted keloid scar tissue. Because they depend on the availability of freshly excised scar tissue, they are limited to studies of keloids treated by surgical excision, which are likely to be older larger scars. Thus, these studies cannot be used to investigate early events in the development of keloid scars or preventative strategies. Although not explicitly stated in all publications describing these models, they generally involve the implantation of dermal tissue with the epidermis removed47; thus, dermal–epidermal interactions cannot be studied. Furthermore, because only a small piece of keloid tissue can be implanted, any regional differences present in the keloid48 but not represented by the implanted tissue may affect the results. These models involve the implantation of keloid tissue into subcutaneous locations43–46 rather than orthotopic grafting, which may result in different responses to investigational therapies. Additionally, keloids are somewhat age-dependent, occurring most often in people between the ages of 10 and 30 years5; this age dependence and the potential role of hormonal status are not considered in these implantation models. Importantly, because immunodeficient host animals must be used to prevent the rejection of implanted human cells, the role of the immune system in keloid scarring cannot be studied. A more recent publication reported the implantation of deepidermalized keloid tissue into subcutaneous pockets in immunocompetent animals.49 The authors reported that keloid tissue pieces persisted for up to 4 months after implantation and expressed human genes, including TGF-β1 and vascular endothelial growth factor. Interestingly, xenogeneic skin grafts became necrotic within 5 days, in contrast to the subcutaneously implanted keloid tissue, suggesting that the subcutaneous pocket provided a privileged environment for engraftment.49 Unfortunately, few experimental details were provided in that study, making replication by other investigators difficult.
Tissue-engineered keloid models
Several keloid models have been described that involve engineered tissues prepared using keloid-derived cells, which can be studied in vitro or in vivo after orthotopic grafting or implantation in mice. One of the first reports of organotypic culture to study keloid pathology involved a “raft” culture system, which is an artificial tissue consisting of fibroblasts embedded in a collagen gel with keratinocytes seeded on the surface.50 In studies of raft cultures containing keloid or normal fibroblasts and normal keratinocytes, wounding of the rafts in vitro resulted in increased collagen deposition, with higher levels observed in rafts containing keloid fibroblasts compared with normal fibroblasts.50 A similar study was performed in our laboratory utilizing engineered skin substitutes (ESS), which were originally developed as an adjunctive therapy for long-term wound closure in patients with very large full-thickness burns.51 ESS are prepared using a bovine collagen-based scaffold seeded with primary fibroblasts and overlain with primary keratinocytes. During in vitro culture at the air–liquid interface, which promotes epidermal stratification, fibroblasts begin to remodel the dermal matrix and replace the bovine collagen in the dermal scaffold with newly synthesized human collagen.52 Remodeling continues after transplantation to wounds, with peak expression of type 1 collagen occurring from 2 to 4 weeks after grafting.52 When ESS were prepared using keloid-derived fibroblasts and keratinocytes, COL1A1 and COL1A2, as well as periostin (POSTN), a matricellular protein overexpressed in keloid fibroblasts,53 were expressed at higher levels than in ESS composed of normal cells.54 Wounding of keloid ESS in vitro resulted in increased deposition of newly synthesized collagen and increased POSTN expression compared with ESS containing normal fibroblasts and keratinocytes, suggesting that this model recapitulates a keloid-like cellular phenotype.54
To generate a keloid animal model, ESS were prepared using keloid-derived fibroblasts and keratinocytes or normal skin-derived fibroblasts and keratinocytes and, after the 2-week incubation period, ESS were grafted to 2 × 2 cm full-thickness wounds in immunodeficient Foxn1nu−/− mice. After 12 weeks, keloid ESS were significantly thicker than ESS prepared using normal cells and displayed densely packed, thick disorganized collagen fibers (Fig. 2). This engineered skin model was used to investigate the differences between fibroblasts isolated from different regions in keloid scars.55 The dermal component of ESS prepared with keloid keratinocytes and fibroblasts from the deep reticular dermis of keloid scars was significantly thicker than the dermis of control ESS containing normal cells and expressed significantly increased COL1A1 levels. In contrast, ESS prepared with keloid keratinocytes and superficial, papillary dermal fibroblasts of keloid scars was not thicker than controls but significantly increased in area with time after grafting compared with control normal ESS and ESS prepared using deep keloid fibroblasts.55 In all previous studies using nonkeloid cells, the ESS contracted after transplantation to mice; the grafts generally contract to 40–60% of their original area by 6–8 weeks postgrafting, after which point the grafts area stabilizes.56,57 ESS prepared using keloid keratinocytes and superficial keloid fibroblasts initially decreased in area, but at about 4 weeks after transplantation, the grafts increased in area, in some cases exceeding the area of the original graft.55 This phenomenon was attributed to the loose-skinned nature of the mouse model; instead of bulging over the wound margin, as might occur in humans, which are tight-skinned, the grafts displace surrounding mouse skin in this loose-skinned model. The results suggest that this model recapitulates some of the features of keloid scars and can be used to investigate novel therapies for keloid scar suppression. The phenotypes observed with deep versus superficial keloid fibroblasts allowed us to propose a model that describes the different contributions of these cell populations in the development of bulging keloid scars (Fig. 3).55 This model is consistent with the increased migration rate of keloid keratinocytes, which was observed in other in vitro studies.53
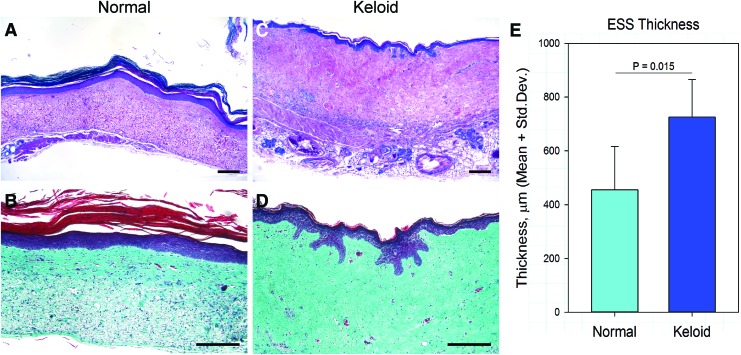
Figure 2.
Normal and keloid ESS in vivo. (A–D) Shown are histological sections of normal (A, B) and keloid (C, D) ESS 12 weeks after transplantation to mice. Sections in the top panels (A, C) were stained with Tango stain, similar to hematoxylin; bottom panels (B, D) show Masson's trichrome-stained sections. Note the densely packed disorganized collagen fibers in the keloid ESS. Scale bars for all sections, 200 μm. (E) Quantitation of the total thickness of the grafted tissue demonstrated that ESS prepared with keloid cells were significantly thicker than ESS prepared using normal cells. ESS, engineered skin substitutes. Color images are available online.
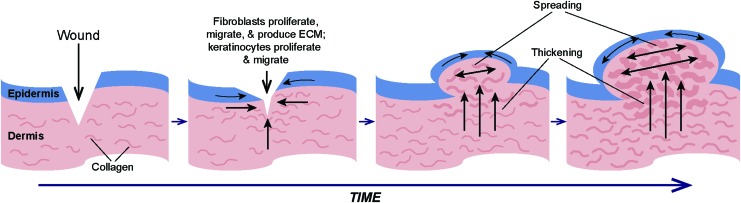
Figure 3.
Model for the development of bulging keloid scars. Analysis of deep versus superficial keloid fibroblasts in engineered skin grafted to mice suggested that deep fibroblasts contribute to graft thickening, whereas superficial fibroblasts induce a spreading phenotype.55 Other studies described the migratory phenotype of keloid keratinocytes.53,68 Together, these observations contributed to the model illustrated here. Shown at the left is a schematic diagram of a cross-section of skin following a wound. During wound healing and over time, fibroblasts proliferate, migrate, and deposit ECM to form granulation tissue over which keratinocytes migrate to close the wound. For reasons that have yet to be identified, cells in scars that progress to keloids fail to respond to “stop” signals, and proliferation, ECM production, and migration continue unchecked. Continued production of ECM in fibroblasts in the deep dermis contributes to thickening of the lower dermis, while fibroblasts in the upper dermis exhibit a spreading phenotype, causing an increase in area. With increasing time after injury, the combination of deep dermal thickening and superficial spreading results in a bulging phenotype. Figure adapted with permission from Supp et al.55 ECM, extracellular matrix. Color images are available online.
A similar model was described that utilized a plasma/fibrin gel as the dermal scaffold.58 In this model, thrombin was added to human plasma containing dermal fibroblasts, and the mixture was dispensed into a polyethylene ring that served as a frame during polymerization. Keratinocytes were seeded atop this structure, and the constructs were incubated in vitro for 2 weeks before transplanting to full-thickness wounds in immunodeficient mice.58 The constructs were affixed to wounds by suturing through the polyethylene frame; the frames prevented initial contraction but detached 2 weeks after transplantation. The transplants were found to stably engraft for up to 18 weeks, and skin substitutes containing keloid fibroblasts and keratinocytes displayed increased collagen density and increased size of collagen bundles compared with controls containing normal cells.58 The keloid skin substitutes were thicker than normal controls and displayed increased expression of COL1A1 and plasminogen activator inhibitor-1. A unique feature of this model is that there is no exogenous collagen; all collagen deposited in the constructs is produced by the cells seeded within the skin substitute.58
More recently, a model was described that involved transplantation of keloid fibroblasts and keratinocytes to mice utilizing a chamber graft model.59 In this model, human skin fibroblasts and keratinocytes were mixed in a chamber that was sutured to wounds in mice, and the cells self-organized into reconstituted skin analogs in vivo.59 Keloid scar cells and “white” scar cells (presumably, nonkeloid scars) were compared with normal skin cells using this model.59 The chambers were implanted in highly immunodeficient NOD/Shi-scid/IL-2RγKO mice (NOG mice), which lack all T cell, B cell, and NK cell activity and have reduced macrophage function. Reconstituted skin implants consisting of keloid-derived cells were significantly thicker at 12 weeks after implantation compared with those prepared using either “white” scar cells or normal cells.59 In addition, the keloid reconstituted skin displayed a keloid-like disorganization of collagen fibers and expressed high levels of the protein versican. Unlike keloid scars in humans, invasion of surrounding tissue was not observed, and the authors speculated that this might be due to the features of the human skin microenvironment, such as tension, which are absent in the mouse model.59 This is consistent with the results observed in our keloid ESS model, with keloid grafts displacing rather than growing over adjacent mouse skin.
Future prospects
Like the implantation models, the keloid engineered skin models are limited in that immunodeficient mice are required to enable engraftment of human cells in vivo. Thus, the role of the immune system in keloid scarring cannot be investigated using these models. There is mounting evidence of immune cell dysregulation in keloid scarring. Keloid scars have been found to have increased numbers of T cells, B cells, and mast cells compared with normal skin.60–62 Additionally, “alternatively activated” (M2) macrophages were elevated in keloid scars compared with normal skin and normal scar tissue.61,62 Macrophages in keloid scars were shown to express higher levels of interleukin 10, interleukin 12, and TGF-β1, consistent with the M2 phenotype.62 M2 macrophages are considered to be anti-inflammatory and are associated with wound closure, in contrast to “classically activated” (M1) macrophages, which are proinflammatory and highly phagocytic and are associated with the early phases of wound healing.63 M2 macrophages stimulate fibroblast proliferation, differentiation, and collagen production,63 which not only promote wound closure but may also lead to excessive scarring if unchecked; studies suggest that this may occur in keloids.61,62 In addition to differences in resident immune cell populations in keloid scars, circulating CD14+ monocytes, the precursors of macrophages, isolated from peripheral blood of keloid patients were found to stimulate the proliferation of fibroblasts to a greater degree than monocytes from nonkeloid controls.64 These findings suggest that immune cell contributions to keloid pathology should not be overlooked in development and use of keloid animal models.
Even if immunocompetent mice could be used in keloid models, they would likely be inadequate due to numerous differences between the immune systems of humans and mice.18 For example, the balance of neutrophils and lymphocytes is different, with human blood being very neutrophil-rich compared with mouse blood.18 There are also numerous differences in T cell development, regulation, and activation between humans and mice.18 Future keloid models might benefit from the use of “humanized” mice harboring components of the human immune system. Although several models that involve transplantation of human tissues to mice have been referred to as “humanized,” more broadly this term has been used to describe immunodeficient mice that possess immune systems reconstituted with human cells.65,66 These models have been used to study numerous human diseases, including infectious diseases and cancer.65–67 Humanized mice are generated using severely immunodeficient recipients harboring multiple mutations that result in lack of adaptive immunity and compromised innate immunity; further immune suppression can be achieved using sublethal doses of radiation.66 Multiple approaches can be used to reconstitute the recipient's immune system with human cells. The first involves injection of peripheral blood leukocytes, which results in rapid engraftment of T cells.66 This model is most useful for short-term studies of T cell function due to the development of xenogeneic graft-versus-host disease (GVHD), which may occur in as little as 1–2 months. This may be an unsuitable period for studies of keloid scarring. In a second model, called the bone marrow/liver/thymus or “BLT” model, human fetal liver and thymus tissue pieces are implanted under the kidney capsule of immunodeficient mice, which are also injected with fetal liver hematopoietic stem cells.67 This model permits the development of all human hematopoietic cell lineages, although these mice are also susceptible to GVHD. The use of these mice may be limited due to ethical considerations regarding the use of human fetal tissue in their generation; regulations regarding fetal tissue research vary, and this work may be prohibited by law in some locations. A third model involves intravenous infusion of CD34+ hematopoietic stem cells from bone marrow or cord blood, which enables engraftment of a virtually complete human immune system, including cells of the myeloid lineage.66 Investigation of implanted native keloid tissue or engineered keloid grafts using mice with humanized immune systems might enable the identification of key immune cell populations involved in keloid pathology. Ideally, for studies of human keloid development, humanized mice would be reconstituted with hematopoietic stem cells from keloid-susceptible individuals (Fig. 4). The requirement for highly enriched hematopoietic stem cell populations for generation of fully humanized mice is currently an obstacle to adoption of this approach for studies of human keloid scarring. However, this is a rapidly progressing field, and new developments and further refinements are expected that will increase the utility of humanized mice and enable their future use for keloid animal models. As we learn more about the role of the immune system from keloid clinical studies and from existing mouse models, we may be better equipped to engineer humanized mice using the most relevant immune cell components. In addition, continued research into the genetic basis for keloid scarring may uncover specific genes that predispose individuals to keloid scarring; this knowledge could then be incorporated into humanized mouse models using transgenic or gene-targeting technologies.
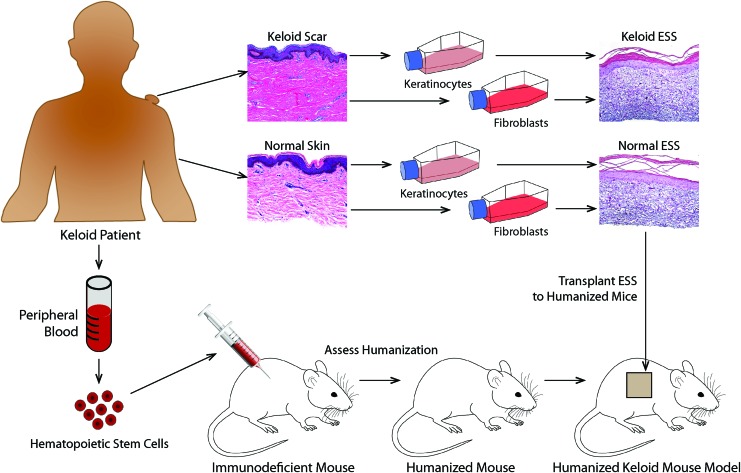
Figure 4.
Use of humanized mice to study keloid scar development. This schematic diagram illustrates the potential use of humanized mice as hosts for grafting of ESS prepared using keloid-derived or normal skin-derived fibroblasts and keratinocytes. Humanized mice are prepared by injection of CD34+ hematopoietic stem cells into severely immunodeficient mice (see text for details). Resulting mice harbor immune systems reconstituted by human cells, enabling studies of the human immune response in a mouse experimental model. Grafting of ESS containing keloid-derived cells to humanized mice can permit investigation of the role of the immune system in keloid scar development, which is currently not possible using standard immunodeficient mouse hosts. ESS containing normal cells can be compared with ESS containing keloid-derived cells to determine the relative contribution(s) of skin cells and immune cells in keloid pathology. This diagram shows images of ESS prepared using primary keratinocytes and fibroblasts cultured from keloid scar and normal skin. Melanocytes69 and microvascular endothelial cells70 have also been used in preparation of ESS; thus, this model can be used to study the relative roles of numerous different cell types in keloid pathology. Comparison of mice humanized with keloid patient-derived hematopoietic stem cells versus normal donor stem cells can be used to identify specific components of the immune system involved in keloid development. Currently, isolation of sufficient numbers of hematopoietic stem cells from peripheral blood is an obstacle to implementation of such a model, but future developments aimed at expansion of this population and improved methods for stem cell recovery are expected to enable such studies in the near future. Color images are available online.
Summary
Keloids remain a challenging problem for patients, clinicians, and researchers. Development of improved animal models will certainly benefit the keloid research community. In the meantime, the field can benefit from carefully designed preclinical studies involving cells and tissues isolated from keloid scars. As with clinical trials, careful study design is critically important for preclinical experiments involving tissue samples obtained from patients. Table 1 lists some aspects of preclinical studies that should be considered for the performance of well-designed in vitro and animal studies.
Table 1.
Considerations for preclinical keloid studies
| Contact institutional review board (IRB) to determine if patient consent is required for collection of keloid scar tissue. • Informed consent is required if protected health information (PHI) is collected or if patient can be identified by information collected. • An IRB protocol is necessary if you are required to obtain informed consent, even if discarded tissue is collected. • An IRB protocol may not be required if samples are de-identified, but check with your local IRB first to make sure. |
| Collect as much demographic and medical information on keloid patient as possible for every sample collected. • Patient age, race, sex, general health, and single/multiple scars • Scar etiology: cause, duration, and prior treatments • Family history |
| Confirm keloid diagnosis before initiating experiments. • View clinical photos • Examine histological sections |
| Carefully document scar characteristics. • Size, shape, thickness, pigmentation, ulceration, and infection • Locations of biopsies |
| Include normal controls in experiments and use “matched” controls whenever possible. • Ideally, nonlesional skin from keloid patient (although this may not be truly “normal” if patient predisposed to keloid formation). • For unrelated normal skin controls, try to match age, race, sex, and body site. |
| Always use multiple biological replicates. • “Biological” replicates are from different individuals; do not confuse with “technical” replicates. ○ Biological replicates help control for person-to-person variability. ○ Technical replicates help control for experiment-to-experiment variability. • Perform a power analysis to ensure sample size is large enough to detect a significant difference if one exists. |
| For mouse studies, select mouse strain(s) carefully. • Strain-specific differences may affect experimental outcomes. • Outbred mice may exhibit more mouse-to-mouse variability in phenotype compared with inbred mice. |
Despite some of the limitations outlined in this review, the models involving engineered or reconstituted skin containing keloid or normal cells can be used to investigate novel therapies for keloid suppression because different phenotypes are observed when grafts are prepared using keloid versus normal cells. However, because these models do not recapitulate all the features of human keloid scar, therapies developed using current models may not all translate to human studies. Raised scarring can be induced in some animal models, as detailed above, but these lesions do not display specific critical features of keloid scars, such as continued growth beyond the wound margin. Thus, there are significant factors specific to keloid pathology that have not yet been identified or modeled in animals. Currently, the best animal model to study keloid scarring is the human. High-quality well-controlled clinical trials for keloid therapies are required to unequivocally demonstrate the safety and efficacy of therapeutic interventions but unfortunately are lacking for many current treatment options.14 Thus, many clinicians rely on anecdotal evidence or personal experience for selection of appropriate therapeutic approaches. Clinical researchers must invest the time and resources required to perform well-designed trials for keloid treatments; widespread adoption of any specific therapy must be supported by data, which can only be generated by careful evaluation in clinical trials.
Take-Home Messages:
Keloids are considered an extreme form of abnormal fibroproliferative scarring. Keloids can develop relatively rapidly after wounding in susceptible individuals. These lesions tend to grow indefinitely and extend beyond the boundary of the original wound. This distinguishes keloids from HTS, which do not extend beyond the original wound margin.
Keloid scars are extremely resistant to treatment. Although many different treatment options currently exist, most are successful for only a subset of keloid patients, and recurrence rates for most therapies, including surgical excision, are very high. There have been relatively few well-designed controlled clinical studies for most keloid therapies. There is a need for the development and validation of effective therapeutic interventions and preventative strategies.
Keloid scars are unique to humans. This may be due to differences in skin physiology, modes of wound healing, and immune system function between laboratory animals, such as rodents, and humans. Because animals do not get keloid scars, there is no accepted animal model for keloid scarring. This has hindered research aimed at understanding the underlying molecular mechanisms of keloid pathology and evaluation of novel therapies.
Although wounds in some experimental animal models, including mice, rabbits, and pigs, can generate thickened scars under specific circumstances that resemble HTS, keloid scarring has not been observed in these animals. Horses develop a type of proliferative scarring, called EGT, which shares many features with human keloid scarring. However, horses are less than ideal animal models, due in part to prohibitive costs and paucity of species-specific reagents, and dissimilarities between keloids and EGT suggest differences in underlying pathologies.
In the absence of an animal model, researchers have utilized keloid tissue samples and cultured primary cells isolated from excised keloids for preclinical research studies. Early keloid animal models involved subcutaneous implantation of keloid tissue into immunodeficient mice. These models were limited by availability of excised scar tissue, the requirement for immunodeficient mouse hosts to prevent immune rejection of human tissue, and subcutaneous location of implants.
Current animal models involve the production of engineered skin-like tissues fabricated using keloid-derived fibroblasts and keratinocytes. After orthotopic grafting to mice, these engineered keloid tissues exhibit some features of human keloids, such as excess collagen production and thickening. However, they do not increase in size to the same extent as human keloids and do not extend beyond the original wound boundary. Like implantation models, the engineered skin models are limited by the requirement for immunodeficient mice to enable engraftment of human cells.
Future keloid animal models may make use of humanized mice, which are genetically immunodeficient mice that have immune systems reconstituted using human cells. The use of humanized mice for grafting of engineered keloid tissues might one day enable the investigation of the relative contributions of the immune system and skin cells in keloid pathology.
Acknowledgments and Funding Sources
Keloid research in Dr. Supp's laboratory has been supported by the Medical Research Grants #85300 and #85500 from the Shriners Hospitals for Children (SHC). D.M.S. thanks the patients of SHC—Cincinnati and the University of Cincinnati Medical Center for generous donation of tissue samples for use in this research; the surgeons at these institutions for assistance with sample collection; and the SHC—Cincinnati Clinical Research Core for assistance in enrolling and consenting patients. All tissue samples were obtained with the approval of the University of Cincinnati Institutional Review Board.
Abbreviations and Acronyms
- COL1A1
collagen type 1 alpha 1
- ECM
extracellular matrix
- EGT
exuberant granulation tissue
- ESS
engineered skin substitutes
- GVHD
graft-versus-host disease
- HTS
hypertrophic scar
- IRB
institutional review board
- PBD
postburn day
- PHI
protected health information
- POSTN
periostin
- TGF-β1
transforming growth factor beta 1
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
About the Author
Dorothy M. Supp, PhD, is a Senior Investigator in the Research Department at the Shriners Hospitals for Children—Cincinnati, a pediatric burn hospital, and an Adjunct Research Professor in the Department of Surgery, Division of Plastic, Reconstructive and Hand Surgery/Burn Surgery at the University of Cincinnati College of Medicine. Dr. Supp's laboratory focuses on translational research in the fields of wound healing and tissue engineering. Current research interests include the development of improved therapies for keloid and hypertrophic scarring, development of next-generation skin substitutes, and ex vivo gene therapy for epidermolysis bullosa.
References
- 1. Van Loey NEE, Van Son MJM. Psychopathology and psychological problems in patients with burn scars. Am J Clin Dermatol 2003;4:245–272 [DOI] [PubMed] [Google Scholar]
- 2. Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 2006;297:433–438 [DOI] [PubMed] [Google Scholar]
- 3. Balci DD, Inandi T, Dogramaci CA, Celik E. DLQI scores in patients with keloids and hypertrophic scars: a prospective case control study. J Dtsch Dermatol Ges 2009;7:688–692 [DOI] [PubMed] [Google Scholar]
- 4. Furtado F, Hochman B, Ferrara SF, et al. What factors affect the quality of life of patients with keloids? Rev Assoc Med Bras 2009;55:700–704 [DOI] [PubMed] [Google Scholar]
- 5. Chike-Obi CJ, Cole PD, Brissett AE. Keloids: pathogenesis, clinical features, and management. Semin Plast Surg 2009;23:178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burd A, Huang L. Hypertrophic response and keloid diathesis: two very different forms of scar. Plast Reconstr Surg 2005;116:150e–157e [DOI] [PubMed] [Google Scholar]
- 7. De FB, Garbi C, Santoriello M, Santillo A, Wilson RR. Differential apoptosis markers in human keloids and hypertrophic scars fibroblasts. Mol Cell Biochem 2009;327:191–201 [DOI] [PubMed] [Google Scholar]
- 8. Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns 2014;40:1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velez Edwards DR, Tsosie KS, Williams SM, Edwards TL, Russell SB. Admixture mapping identifies a locus at 15q21.2-22.3 associated with keloid formation in African Americans. Hum Genet 2014;133:1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hellwege JN, Russell SB, Williams SM, Edwards TL, Velez Edwards DR. Gene-based evaluation of low-frequency variation and genetically-predicted gene expression impacting risk of keloid formation. Ann Hum Genet 2018;82:206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santos-Cortez RLP, Hu Y, Sun F, et al. Identification of ASAH1 as a susceptibility gene for familial keloids. Eur J Hum Genet 2017;25:1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Love PB, Kundu RV. Keloids: an update on medical and surgical treatments. J Drugs Dermatol 2013;12:403–409 [PubMed] [Google Scholar]
- 13. Trace AP, Enos CW, Mantel A, Harvey VM. Keloids and hypertrophic scars: a spectrum of clinical challenges. Am J Clin Dermatol 2016;17:201–223 [DOI] [PubMed] [Google Scholar]
- 14. Durani P, Bayat A. Levels of evidence for the treatment of keloid disease. J Plast Reconstr Aesthet Surg 2008;61:4–17 [DOI] [PubMed] [Google Scholar]
- 15. Zomer HD, Trentin AG. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 2018;90:3–12 [DOI] [PubMed] [Google Scholar]
- 16. Nuutila K, Katayama S, Vuola J, Kankuri E. Human wound-healing research: issues and perspectives for studies using wide-scale analytic platforms. Adv Wound Care (New Rochelle) 2014;3:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang JH, Thampatty BP, Lin JS, Im HJ. Mechanoregulation of gene expression in fibroblasts. Gene 2007;391:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–2738 [DOI] [PubMed] [Google Scholar]
- 19. Scales BS, Huffnagle GB. The microbiome in wound repair and tissue fibrosis. J Pathol 2013;229:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCoy KD, Geuking MB, Ronchi F. Gut microbiome standardization in control and experimental mice. Curr Protoc Immunol 2017;117:23.1.1–23.1.13 [DOI] [PubMed] [Google Scholar]
- 21. Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J 2015;56:127–138 [DOI] [PubMed] [Google Scholar]
- 22. Debeer S, Le Luduec JB, Kaiserlian D, et al. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol 2013;23:456–466 [DOI] [PubMed] [Google Scholar]
- 23. Zhu KQ, Engrav LH, Gibran NS, et al. The female, red Duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns 2003;29:649–664 [DOI] [PubMed] [Google Scholar]
- 24. Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen 2004;12:305–319 [DOI] [PubMed] [Google Scholar]
- 25. Zhu KQ, Engrav LH, Tamura RN, et al. Further similarities between cutaneous scarring in the female, red Duroc pig and human hypertrophic scarring. Burns 2004;30:518–530 [DOI] [PubMed] [Google Scholar]
- 26. Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns 2006;32:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blackstone BN, Kim JY, McFarland KL, et al. Scar formation following excisional and burn injuries in a red Duroc pig model. Wound Repair Regen 2017;25:618–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeBruler DM, Blackstone BN, McFarland KL, et al. Effect of skin graft thickness on scar development in a porcine burn model. Burns 2018;44:917–930 [DOI] [PubMed] [Google Scholar]
- 29. Theoret CL, Wilmink JM. Aberrant wound healing in the horse: naturally occurring conditions reminiscent of those observed in man. Wound Repair Regen 2013;21:365–371 [DOI] [PubMed] [Google Scholar]
- 30. Theoret CL, Barber SM, Moyana TN, Gordon JR. Expression of transforming growth factor beta(1), beta(3), and basic fibroblast growth factor in full-thickness skin wounds of equine limbs and thorax. Vet Surg 2001;30:269–277 [DOI] [PubMed] [Google Scholar]
- 31. Theoret CL, Olutoye OO, Parnell LK, Hicks J. Equine exuberant granulation tissue and human keloids: a comparative histopathologic study. Vet Surg 2013;42:783–789 [DOI] [PubMed] [Google Scholar]
- 32. Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 2004;12:485–492 [DOI] [PubMed] [Google Scholar]
- 33. Hu MS, Cheng J, Borrelli MR, et al. An improved humanized mouse model for excisional wound healing using double transgenic mice. Adv Wound Care (New Rochelle) 2018;7:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–3261 [DOI] [PubMed] [Google Scholar]
- 35. Morris DE, Wu L, Zhao LL, et al. Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg 1997;100:674–681 [DOI] [PubMed] [Google Scholar]
- 36. Kloeters O, Tandara A, Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen 2007;15 Suppl 1:S40–S45 [DOI] [PubMed] [Google Scholar]
- 37. Saulis AS, Chao JD, Telser A, Mogford JE, Mustoe TA. Silicone occlusive treatment of hypertrophic scar in the rabbit model. Aesthet Surg J 2002;22:147–153 [DOI] [PubMed] [Google Scholar]
- 38. Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg 2008;61:1219–1225 [DOI] [PubMed] [Google Scholar]
- 39. Gallant-Behm CL, Mustoe TA. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen 2010;18:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013;CD003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J, Ding J, Jiao H, et al. Human hypertrophic scar-like nude mouse model: characterization of the molecular and cellular biology of the scar process. Wound Repair Regen 2011;19:274–285 [DOI] [PubMed] [Google Scholar]
- 42. Momtazi M, Kwan P, Ding J, et al. A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen 2013;21:77–87 [DOI] [PubMed] [Google Scholar]
- 43. Shetlar MR, Shetlar CL, Hendricks L, Kischer CW. The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med 1985;179:549–552 [DOI] [PubMed] [Google Scholar]
- 44. Kischer CW, Pindur J, Shetlar MR, Shetlar CL. Implants of hypertrophic scars and keloids into the nude (athymic) mouse: viability and morphology. J Trauma 1989;29:672–677 [DOI] [PubMed] [Google Scholar]
- 45. Shetlar MR, Shetlar CL, Kischer CW, Pindur J. Implants of keloid and hypertrophic scars into the athymic nude mouse: changes in the glycosaminoglycans of the implants. Connect Tissue Res 1991;26(1–2):23–36 [DOI] [PubMed] [Google Scholar]
- 46. Chen J, Roop DR. Genetically engineered mouse models for skin research: taking the next step. J Dermatol Sci 2008;52:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waki EY, Crumley RL, Jakowatz JG. Effects of pharmacologic agents on human keloids implanted in athymic mice. A pilot study. Arch Otolaryngol Head Neck Surg 1991;117:1177–1181 [DOI] [PubMed] [Google Scholar]
- 48. Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol 2011;164:83–96 [DOI] [PubMed] [Google Scholar]
- 49. Park TH, Rah DK, Chang CH, Kim SY. Establishment of patient-derived keloid xenograft model. J Craniofac Surg 2016;27:1670–1673 [DOI] [PubMed] [Google Scholar]
- 50. Torkian BA, Yeh AT, Engel R, Sun CH, Tromberg BJ, Wong BJ. Modeling aberrant wound healing using tissue-engineered skin constructs and multiphoton microscopy. Arch Facial Plast Surg 2004;6:180–187 [DOI] [PubMed] [Google Scholar]
- 51. Boyce ST, Simpson PS, Rieman MT, et al. Randomized, paired-site comparison of autologous engineered skin substitutes and split-thickness skin graft for closure of extensive, full-thickness burns. J Burn Care Res 2017;38:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klingenberg JM, McFarland KL, Friedman A, Boyce ST, Aronow BJ, Supp DM. Engineered human skin substitutes undergo large-scale genomic reprogramming and normal skin-like maturation following transplantation to athymic mice. J Invest Dermatol 2010;130:587–601 [DOI] [PubMed] [Google Scholar]
- 53. Hahn JM, Glaser K, McFarland KL, Aronow BJ, Boyce ST, Supp DM. Keloid-derived keratinocytes exhibit an abnormal gene expression profile consistent with a distinct causal role in keloid pathology. Wound Rep Regen 2013;21:530–544 [DOI] [PubMed] [Google Scholar]
- 54. Supp DM, Glaser K, Hahn JM, McFarland KL, Boyce ST. Abnormal responses of keloid tissue to wounding identified using in vitro model system. Eplasty 2012;12:e19. [PMC free article] [PubMed] [Google Scholar]
- 55. Supp DM, Hahn JM, Glaser K, McFarland KL, Boyce S. Deep and superficial keloid fibroblasts contribute differentially to tissue phenotype in a novel in vivo model of keloid scar. Plast Reconstr Surg 2012;129:1259–1271 [DOI] [PubMed] [Google Scholar]
- 56. Supp DM, Boyce ST. Overexpression of vascular endothelial growth factor accelerates early vascularization and improves healing of genetically modified cultured skin substitutes. J Burn Care Rehabil 2002;23:10–20 [DOI] [PubMed] [Google Scholar]
- 57. Powell HM, Supp DM, Boyce ST. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials 2008;29:834–843 [DOI] [PubMed] [Google Scholar]
- 58. Lee YS, Hsu T, Chiu WC, et al. Keloid-derived, plasma/fibrin-based skin equivalents generate de novo dermal and epidermal pathology of keloid fibrosis in a mouse model. Wound Repair Regen 2016;24:302–316 [DOI] [PubMed] [Google Scholar]
- 59. Sunaga A, Kamochi H, Sarukawa S, et al. Reconstitution of human keloids in mouse skin. Plast Reconstr Surg Glob Open 2017;5:e1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boyce DE, Ciampolini J, Ruge F, Murison MS, Harding KG. Inflammatory-cell subpopulations in keloid scars. Br J Plast Surg 2001;54:511–516 [DOI] [PubMed] [Google Scholar]
- 61. Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol 2012;167:1053–1066 [DOI] [PubMed] [Google Scholar]
- 62. Jin Q, Gui L, Niu F, et al. Macrophages in keloid are potent at promoting the differentiation and function of regulatory T cells. Exp Cell Res 2018;362:472–476 [DOI] [PubMed] [Google Scholar]
- 63. Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci 2017;18:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liao WT, Yu HS, Arbiser JL, et al. Enhanced MCP-1 release by keloid CD14+ cells augments fibroblast proliferation: role of MCP-1 and Akt pathway in keloids. Exp Dermatol 2010;19:e142–e150 [DOI] [PubMed] [Google Scholar]
- 65. Yong KSM, Her Z, Chen Q. Humanized mice as unique tools for human-specific studies. Arch Immunol Ther Exp (Warsz) 2018;66:245–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Walsh NC, Kenney LL, Jangalwe S, et al. Humanized mouse models of clinical disease. Annu Rev Pathol 2017;12:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Skelton JK, Ortega-Prieto AM, Dorner M. A Hitchhiker's guide to humanized mice: new pathways to studying viral infections. Immunology 2018;154:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hahn JM, McFarland KL, Combs KA, Supp DM. Partial epithelial-mesenchymal transition in keloid scars: regulation of keloid keratinocyte gene expression by transforming growth factor-β1. Burns Trauma 2016;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boyce ST, Lloyd CM, Kleiner MC, Swope VB, Abdel-Malek Z, Supp DM. Restoration of cutaneous pigmentation by transplantation to mice of isogeneic human melanocytes in dermal-epidermal engineered skin substitutes. Pigment Cell Melanoma Res 2017;30:531–540 [DOI] [PubMed] [Google Scholar]
- 70. Fratianne RB, Brandt CP. Improved survival of adults with extensive burns. J Burn Care Rehabil 1997;18:347–351 [DOI] [PubMed] [Google Scholar]