Abstract
Delayed wound healing in lymphedema is assumed to be caused by two reasons, pathophysiological and immunological effects of lymphedema. The aim of this review is to establish how impaired lymphatics alter wound healing pathophysiologically and immunologically, and to propose treatment modalities that can promote wound healing in lymphedema. Lymphaticovenular anastomoses (lymphovenous anastomoses [LVAs]) were performed on patients who had recurrent cellulitis several times with lymphorrhea and developed severe ulcers that were refractory to skin grafts, flaps, and conservative therapy. The lymphorrhea and the ulcer had healed by 4 weeks. Moreover, the lymphedema improved without compression therapy. Lymphedema is characterized pathophysiologically by localized peripheral edema that compresses the microvasculature and lymphatic vasculature and impairs tissue remodeling. Another suspected mechanism is an imbalance in the differentiation of participating immune cells. Profound suppression of T helper (Th)1 cells is likely to increase the risk of infection, and excessive differentiation of Th2 cells, including M2 macrophage polarization, may promote fibrosis, which disrupts the carefully orchestrated wound healing process. Although negative-pressure wound therapy is useful for the treatment of delayed wound healing in lymphedema, LVAs may be necessary to treat the fundamental problem of lymphedema. LVAs are considered to create a bypass to the lymph nodes through which dendritic cells (DCs) can transmit antigen information to T cells. LVAs are considered to neutralize chronic inflammation by allowing more DCs to return into the circulation, thereby improving wound healing.
Keywords: lymphedema, wound healing, immunology, lymphovenous anastomosis, chronic inflammation, immunodeficiency
Shuhei Yoshida, MD, PhD.

Introduction
The lymphatic system plays a crucial role in tissue homeostasis. Lymphedema is known to delay wound healing.1–4 If water and macromolecules are not cleared from the interstitium, the oncotic and hydrostatic pressure balance within the tissues is altered, and cell damage results contributing further to delayed wound healing.5 In addition to these pathophysiological mechanisms, it is suspected that the balance of immune cells involved in wound healing is disrupted in lymphedema.6
The aim of this report is to propose treatment modalities that can promote wound healing in lymphedema and to establish how impaired lymphatics alter wound healing pathophysiologically and immunologically.
Case Reports
Lymphovenous anastomosis
Various surgical approaches are being attempted for lymphedema treatment and a treatment algorithm based on the highest quality lymphedema research are developing.7 Lymphovenous anastomosis (LVA) is one of the promising surgical approaches.
Case 1
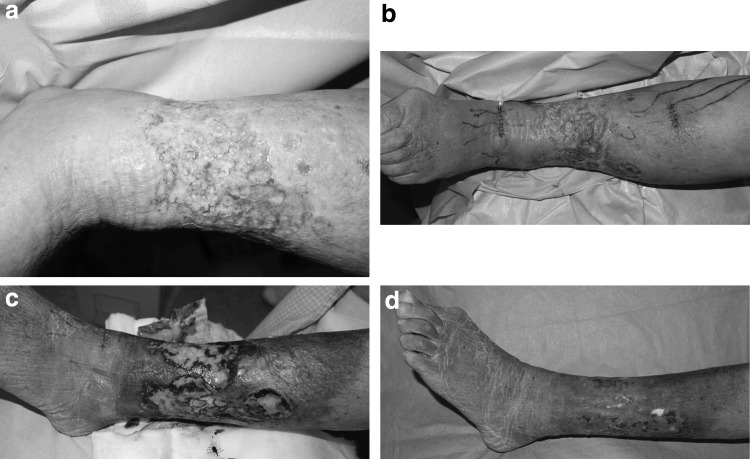
The patient was a 78-year-old woman who had first become aware of lymphedema in her leg several decades earlier and developed recurrent cellulitis with lymphorrhea. Compression had been performed by elastic bandage made of cotton combined with wound management. However, there had been no sign of healing. She presented to our clinic with a severe lymphorrhea at the site of an ulcer that measured 10 cm × 15 cm (Fig. 1a). Ultrasonography did not indicate venous ulcer resulting from venous thrombosis, varix, or reflux. The patient had no inoperable condition against LVAs, such as severe ischemia.
Figure 1.
(a) A 78-year-old woman with lymphedema with lymphorrhea. (b) Total four LVAs were created around the ulcer. (c) The lymphorrhea had resolved by 2 weeks postoperatively. (d) The ulcer had healed by 4 weeks. LVA, lymphovenous anastomosis.
Based on these findings, we made a diagnosis of lymphedema with lymphorrhea and intractable skin ulcer. The lower extremity lymphedema (LEL) index obtained before LVA was 271.8 LVA under local anesthesia was planned. Indocyanine green (ICG) lymphography was performed by injecting 0.1–0.2 mL of ICG dye (Diagnogreen® 0.5%; Daiichi Pharmaceuticals, Tokyo, Japan) subcutaneously into the first interdigital space and into the posterolateral condylar region on the day before surgery to visualize lymphatics through a Photodynamic Eye system (Hamamatsu Photonics, Hamamatsu, Japan).
The LVAs were performed by using an operating microscope. The lymphatic vessels had enlarged to 0.8–1.0 mm, indicating an increase in internal pressure. No signs of venous insufficiency or hypertension were observed in the subcutaneous vein. The lymphatic vessels were anastomosed end to end with the veins using 12-0 nylon suture. After the anastomosis, lymph was observed to be flowing from the lymphatic vessels to the veins. Two anastomoses were created in the distal region and two in the proximal region of the ulcer (Fig. 1b). The number of anastomosis was the maximum we were able to perform LVAs for the patient in the limited operation time, around 3 h, at one time. We allowed the patient to walk immediately after surgery, however, the patient was prohibited for 1 week from keeping her foot down for more than 1 h, concerning about wound healing from the LVAs itself.
Case 2
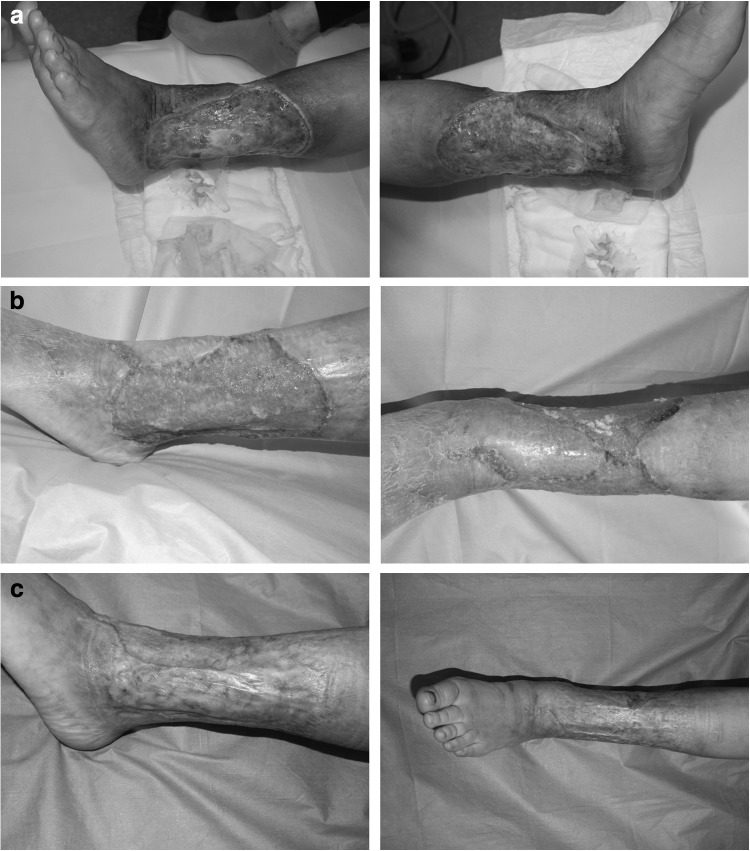
The patient was a 45-year-old woman who had been aware of left leg lymphedema for a few decades and had recurrent cellulitis several times a year with lymphorrhea. A decade earlier, she had developed severe ulcers that were refractory to skin grafts, flaps, and conservative therapy. Compression had been performed by elastic bandage made of cotton combined with wound management. However, there had been no sign of healing. She visited our clinic with severe lymph leak in the area of an ulcer that measured 10 cm × 15 cm and involved both sides of her left leg (Fig. 2a). Based on the findings from ultrasonography and ICG, we made a diagnosis of lymphedema with lymphorrhea and an intractable skin ulcer. The LEL index obtained before LVA was 263. LVAs under local anesthesia was performed. Six LVAs were performed, comprising three in the distal region and three in the proximal region of the ulcer.
Figure 2.
(a) A 45-year-old woman with lymphedema with lymphorrhea. (b) After six LVAs, the lymphorrhea had resolved by 2 weeks postoperatively, at which time the ulcer was starting to show well-vascularized granulation tissue. (c) The wound healed by 10 weeks after LVA.
Results
Case 1
The lymphorrhea had resolved by 2 weeks postoperatively (Fig. 1c) and the ulcer had healed by 4 weeks. The wound management was performed by elastic bandage made of cotton. Moreover, the lymphedema in her leg improved postoperatively without compression therapy (Fig. 1d). LEL index obtained 6 months after LVA was 250. There has been no recurrence of the ulcer or lymphorrhea, and cellulitis since the surgery.
Case 2
The lymphorrhea had resolved by 2 weeks postoperatively, at which time the ulcer was starting to show well-vascularized granulation tissue (Fig. 2b). The wound management was performed by elastic bandage made of cotton. We then performed skin grafting. The wound healed by 10 weeks after LVA (Fig. 2c). Moreover, the leg lymphedema improved postoperatively without compression therapy. The ulcer, lymphorrhea, and cellulitis have not recurred since LVA. LEL index obtained 6 months after LVA was 237.
Discussion
Pathophysiological effects of lymphedema on wound healing
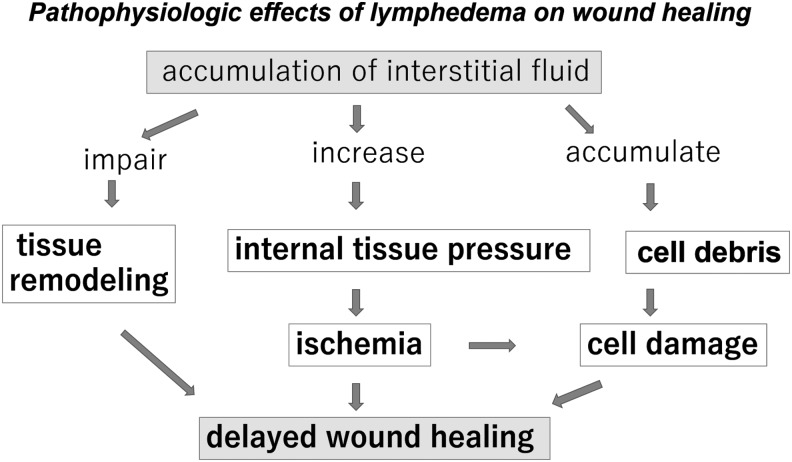
If water and macromolecules are not cleared from the interstitium, the balance between oncotic and hydrostatic pressure within the tissues is altered, resulting in cell damage. Swelling of the interstitium leads to disruption of the normal pathways for distribution of nutrients to cells, which impairs normal wound healing9 (Fig. 3).
Figure 3.
The flowchart shows pathophysiological effects of lymphedema on wound healing. It is mainly composed of three flows; impairment of tissue remodeling, increased internal tissue pressure leading to microcirculatory ischemia, and accumulation of cell debris.
Immunological effects of lymphedema on wound healing
Immunodeficiency in lymphedema
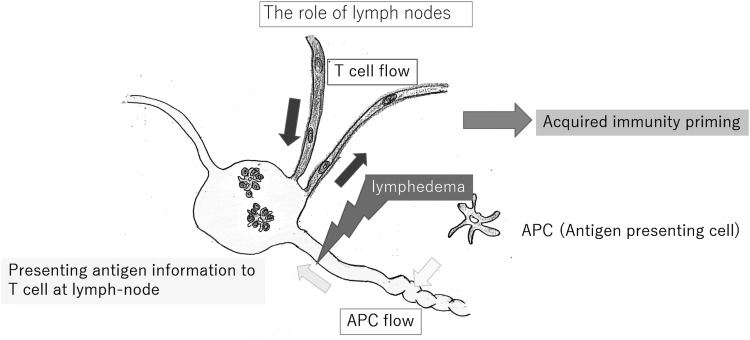
It is widely accepted that lymph nodes are organs that provide a specialized microenvironment for the meeting of migratory immune cells, especially lymphocytes and antigen-presenting cells, including dendritic cells (DCs). If the lymphatic route is blocked, the immune system would be unaware of an inflammatory process occurring in the afferent tissue and remain unengaged, resulting in immune ignorance10 (Fig. 4).
Figure 4.
DCs exist in peripheral tissues and entry into the lymphatic capillaries. The lymph nodes coordinate cell trafficking from two sources: the blood vessels, through which the majority of lymphocytes enter the lymph nodes, and the lymphatic vessels, which transport interstitial fluid, including DCs. The intensive defense provided by acquired immunity requires functional lymphatic organs. Lymphedema is a state in which the lymphatic conduits are impaired, expression of acquired immunity is impossible in the presence of lymphedema. DC, dendritic cell.
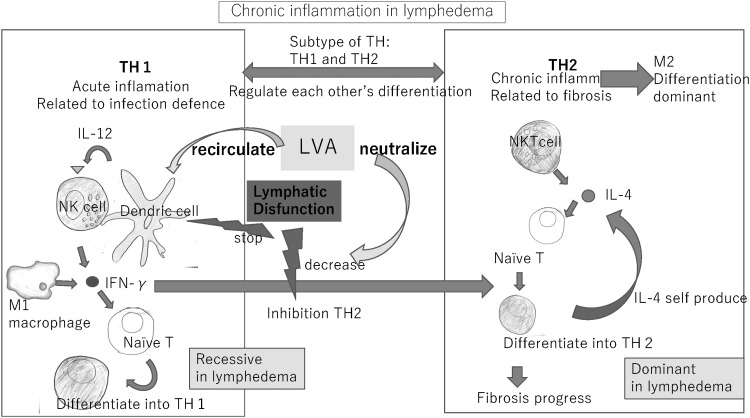
Chronic inflammation in lymphedema
The T helper (Th)1 and 2 cell subsets and macrophages are related such that they mutually regulate their differentiation. Differentiation of Th1 cells from naive T cells is driven mainly by interferon-gamma (IFN-γ). IFN-γ inhibits differentiation of naive T cells to Th2 cells. When the influence of IFN-γ is weak, the natural killer T cells, are assumed to produce interleukin (IL)-4 without being affected by DCs. IL-4 causes naive T cells to differentiate into Th2 cells that promote chronic inflammation with the help of M2 macrophages that promote fibrosis. Th2 self-produces IL-4, which creates a state of Th2 and M2 dominance through positive feedback.11 In lymphedema region, the activity of DCs is suppressed, which decreases the ability of IFN-γ to inhibit differentiation of Th2 cells. Fibrosis is promoted in response to chronic inflammation driven by Th2 cells and M2 macrophages (Fig. 5).
Figure 5.
The Th1 and 2 cell subsets and macrophages are regulated during their differentiation. In lymphedema, the activity of DCs in the lymphatic tissues is suppressed, which decreases the ability of IFN-γ to inhibit differentiation of Th2 cells. Fibrosis is promoted in response to chronic inflammation driven by Th2 cells and M2 macrophages. LVA is considered to neutralize chronic inflammation by allowing more DCs to return into the circulation, thereby improving wound healing. IFN-γ, interferon-gamma; Th, T helper.
Role of macrophages in wound healing
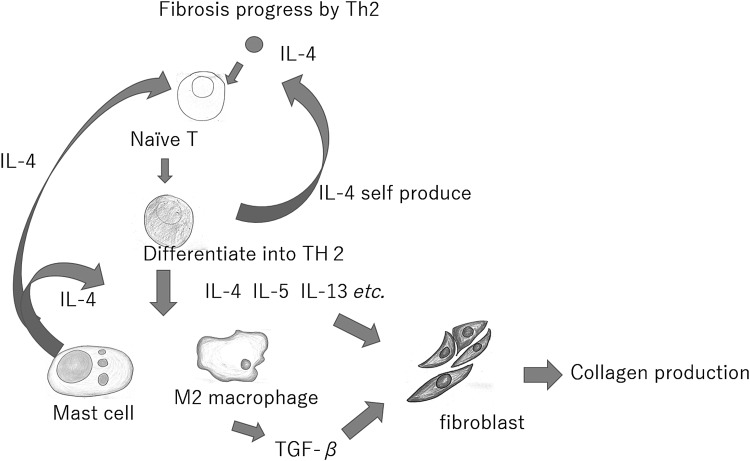
Polarization of M1 to M2 is a vital step in wound healing and can be amplified by cytokines, in particular IL-4,12 as well as the increased numbers of M2 then result in elevated levels of IL-10, transforming growth factor-β, and IL-12 (Fig. 6). M1 and M2 are both critical for natural wound healing.13
Figure 6.
The differentiation shift of T cells results in fibroblasts activated by IL-13 from Th2, and these activated fibroblasts produce an abundance of collagen. These events promote fibrotic remodeling by producing profibrotic cytokines, such as IL-13, or by indirectly promoting differentiation of monocytes toward alternatively activated macrophages. Mast cells regulate proliferation of fibroblasts through heterotypic cell–cell contact and secretion of IL-4. IL, interleukin.
Role of T lymphocytes in wound healing
T lymphocytes migrate into the wound after inflammatory cells and macrophages following injury during the proliferative phase.14 A Th1/Th2 paradigm in tissue fibrosis has been established. It is widely accepted that Th1/Th2 cells are involved in wound healing.
Immunological effects of lymphedema on wound healing
The differentiation status of macrophages and T lymphocytes in lymphedema and wound healing could be expected to be very similar, and seems to be beneficial to wound healing. However, this is not the case in reality. It is conceivable that excessive and endless fibrotic remodeling has deleterious effects on wound healing. It may be that the imbalance in differentiation between Th1 and Th2 is excessively increased in lymphedema. Differentiation of Th1 cells is excessively suppressed in lymphedema, which renders the host prone to severe infection and may cause depression of wound healing activation, whereas intensive differentiation of Th2 cells causes progression of fibrosis, leading to disruption of the wound healing process.
Treatment of wound healing in lymphedema
There are various options for wound treatment for the treatment of lymphedema.15–17 Negative-pressure wound therapy (NPWT) and LVA are considered to be effective treatments for intractable ulcers associated with lymphedema. However, NPWT does not address the pathophysiological and immunological problems associated with lymphedema, and the fundamental problem of delayed wound healing remains after the ulcer has healed.
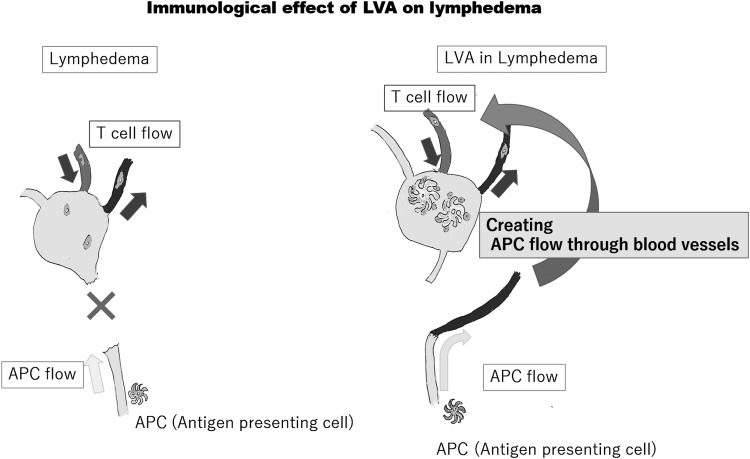
LVA creates the bypass between lymphatics and veins, which makes it possible for DCs to recirculate through blood vessels. LVA is considered to create a bypass to the lymph nodes through which DCs can transmit antigen information to T cells from the blood circulation (Fig. 7, right).
Figure 7.
The lymphatic vessels transport not only interstitial fluid but also antigen information to the lymph nodes. From the viewpoint of acquired immunity, LVA is considered to create a bypass to the lymph nodes through which DCs can transmit antigen information to T cells from the blood circulation.
Although there has been little clinical case, adipose-derived stem cells are expected as the treatment for lymphedema by creating direct lymphatic routes, and wound regeneration.18
Conclusion
Immunological disorders that arise in association with lymphedema are assumed to be factors impairing wound healing. Excessive chronic inflammation and fibrosis may have a chaotic effect on the process of wound healing. LVA is considered to neutralize chronic inflammation by allowing more DCs to return into the circulation, thereby improving wound healing. So far, we have not experienced the remarkable recurrence of lymphedema or ulcers after LVAs. However, true long-term outcomes on LVAs are still unknown. We need to continue careful observation about LVAs.
Key Findings.
Delayed wound healing in lymphedema is assumed to be caused by two reasons, pathophysiologic and immunologic effects of lymphedema.
The pathophysiologic reason is composed of impaired tissue remodeling impairs from excessive accumulation of interstitial fluid, microvasculature ischemia caused by internal tissue pressure, and cell damage by accumulation of cell debris.
The immunologic reason is imbalanced polarization of Th cells and macrophages in lymphedema.
LVA is considered to neutralize the imbalanced polarization, thereby improving wound healing.
Acknowledgments and Funding Sources
All authors declare that there were no funding sources for this study and they approved the final article.
Abbreviations and Acronyms
- DC
dendritic cell
- ICG
indocyanine green
- IFN-γ
interferon-gamma
- IL
interleukin
- LEL
lower extremity lymphedema
- LVA
lymphovenous anastomosis
- NPWT
negative-pressure wound therapy
- Th
T helper
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Authors
Shuhei Yoshida, MD, PhD, is an assistant professor at The International Center for Lymphedema, Hiroshima University Hospital. Isao Koshima, MD, PhD, is a professor at The International Center for Lymphedema, Hiroshima University Hospital. Yuichi Hamada, MD, PhD, is a director at the Department of Plastic and Reconstructive Surgery, Japanese Red Cross Fukuoka Hospital. Ayano Sasaki, MD, is a fellow at the Department of Plastic and Reconstructive Surgery, Hiroshima University. Yumio Fujioka, MD, is a fellow at the Department of Plastic and Reconstructive Surgery, Hiroshima University. Shogo Nagamatsu, MD, PhD, is an assistant professor at the Department of Plastic and Reconstructive Surgery, Hiroshima University. Kazunori Yokota, MD, PhD, is a professor at the Department of Plastic and Reconstructive Surgery, Hiroshima University. Mitsunobu Harima, MD, is an assistant professor at the Department of Plastic and Reconstructive Surgery, Tokyo University. Shuji Yamashita, MD, PhD, is a lecturer at the Department of Plastic and Reconstructive Surgery, Tokyo University.
References
- 1. Komatsu E, Nakajima Y, Mukai K, et al. Lymph drainage during wound healing in a hindlimb lymphedema mouse model. Lymphat Res Biol 2017;15:32–38 [DOI] [PubMed] [Google Scholar]
- 2. Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care 2017;30:305–318 [DOI] [PubMed] [Google Scholar]
- 3. Kimura T, Sugaya M, Blauvelt A, et al. Delayed wound healing due to increased interleukin-10 expression in mice with lymphatic dysfunction. J Leukoc Biol 2013;94:137–145 [DOI] [PubMed] [Google Scholar]
- 4. Jones RE, Russell RD, Huo MH. Wound healing in total joint replacement. Bone Joint J 2013;95-B(11 Suppl A):144–147 [DOI] [PubMed] [Google Scholar]
- 5. Mallon EC, Ryan TJ. Lymphedema and wound healing. Clin Dermatol 1994;12:89–93 [DOI] [PubMed] [Google Scholar]
- 6. Hancock DG, Potezny TM, White PM. Immune regulation by the peripheral lymphatics and its implications for wound healing and infection control in lymphoedema. Wound Pract Res 2016;24:76–83 [Google Scholar]
- 7. Carl HM, Walia G, Bello R, et al. Systematic review of the surgical treatment of extremity. J Reconstr Microsurg 2017;33:412–425 [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto T, Matsuda N, Todokoro T, et al. Lower extremity lymphedema (LEL) index: a simple method for severity evaluation of lower extremity lymphedema. Ann Plast Surg 2011;67:637–640 [DOI] [PubMed] [Google Scholar]
- 9. Eleanor CM, Terence JR. Lymphedema and wound healing. Clin Dermatol 1994;12:89–93 [DOI] [PubMed] [Google Scholar]
- 10. Lakkis FG, Arakelov A, Konieczny BT, e al. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med 2000;6:686–688 [DOI] [PubMed] [Google Scholar]
- 11. Kenneth M, Mowat A, Casey W, et al. Part IV. The adaptive immune response. In: Toledo M, Bochicchio A, Acevedo-Quinones C, eds. Janeway's Immunobiology, 9th ed. New York: Garland Science, 2016:345–531 [Google Scholar]
- 12. Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 2016;73:3861–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Girish JK, Sufan C. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ 2017;62:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fishel RS, Barbul A, Beschorner WE, et al. Lymphocyte participation in wound healing: morphologic assessment using monoclonal antibodies. Ann Surg 1987;206:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuomo R, Nisi G, Grimaldi L, et al. Use of ultraportable vacuum therapy systems in the treatment of venous leg ulcer. Acta Biomed 2017;88:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mancini S, Cuomo R, Poggialini M, et al. Autolytic debridement and management of bacterial load with an occlusive hydroactive deressing impregnated with polyhexamethylene biguanide. Acta Biomed 2018;88:409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cuomo R, D'Aniello C, Grimaldi L, et al. EMLA and lidocaine spray: a comparison for surgical debridement in venous leg ulcers. Adv Wound Care (New Rochelle) 2015;4:358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida S, Hamuy R, Hamada Y, et al. Adipose-derived stem cell transplantation for therapeutic lymphangiogenesis in a mouse secondary lymphedema model. Regen Med 2015;10:549–562 [DOI] [PubMed] [Google Scholar]