Abstract
Significance: Autologous platelet-rich plasma (PRP) has been suggested to be effective for wound healing. However, clinical evidence for its use in patients with diabetic ulcer remains inconsistent. The aim of this systematic review and meta-analysis was to evaluate the efficacy and safety of PRP in patients with diabetic ulcer.
Recent Advances: Relevant randomized controlled trials (RCTs) were identified via systematic search of PubMed, MEDLINE, EMBASE, the Cochrane Library, and Web of Knowledge databases. Results were pooled using a random-effects model. The primary outcome of the study was the healing rate of ulcers in patients with PRP, when compared with controls. Secondary outcomes included the percentage of ulcer area reduction, recurrence rate, and amputation rate.
Critical Issues: Eight RCTs that involved 431 participants were included. Compared with controls, PRP was associated with a significantly increased ratio of complete ulcer healing (odds ratio [OR] = 3.77, 95% confidence interval [CI] = 1.91–7.45, I2 = 42.2%) and reduced areas of ulcers (standard mean difference = 0.86, 95% CI = 0.27–1.45, I2 = 0.0%). No differences were observed between patients allocated to PRP or controls, in terms of the outcomes of recurrence rate (OR = 3.32, 95% CI = 0.41–27.18, I2 = 66.3%) or amputation rate (OR = 0.15, 95% CI = 0.15–1.28). The results of the trial sequence analyses revealed that the cumulative Z-curve crossed both the traditional boundary (p = 0.05) and trial sequential monitoring boundary.
Future Directions: Our findings suggest that PRP may improve ulcer healing without significant adverse effects for patients with diabetic ulcers.
Keywords: autologous platelet-rich plasma, PRP, diabetic ulcers, wound healing
Jiayuan Zhu, MD, PhD.

Bing Tang, MD, PhD.

Introduction
Diabetes has become a major public health problem worldwide, and the prevalence of diabetes, according to epidemiological studies, continuously increases worldwide. Indeed, the global prevalence of diabetes for adults within the age of 20–79 years has reached 8.2% (387 million people), while the total number of adults with diabetes in 2013 was 382 million.1 More importantly, this number is expected to reach 592 million in 2035.1 Therefore, the prevention of diabetes and its related complications has become a fundamental issue for both developed and developing countries. It has been reported that ∼15% of diabetic patients will have diabetic foot ulcers, and approximately a quarter of these patients will eventually undergo foot amputation.2 Chronic nonhealing diabetic foot ulcer has become an important cause to the morbidity and mortality for patients with diabetes.
Although standard treatments for ulcers have been well applied in patients with diabetic ulcers, such as revascularization, debridement, the use of antibiotics, offloading of the affected legs, and the intensive management of blood glucose,3,4 a considerable number of patients with diabetic ulcers would eventually undergo lower extremity amputation despite these treatments. The unsatisfying efficacy of these conventional treatments to patients with diabetic ulcers has been attributed to the lack of wound healing-related growth factors in these patients, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF).5 With the development of regenerative medicine, platelet-rich plasma (PRP) has been suggested to confer superior efficacy for wound healing to conventional therapies. PRP is a product derived from fresh whole blood that contains a high concentrate of platelets, which can release a variety of highly concentrative growth factors, including PDGF, transforming growth factor (TGF)-β, VEGF, epidermal growth factor (EGF), fibrinogen, osteocalcin, and insulin-like growth factor (IGF). It is noteworthy that these factors are essential for the regulation of important cellular processes involved in the healing of wounds, including cell proliferation, chemoattractant, and cell metabolism.6 Although the potential treatment efficacy of PRP has been proposed in patients with diabetic ulcers,7 previous clinical studies were generally of limited sample sizes, and the results of these studies were not always consistent. Therefore, in the present study, the investigators aimed to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to systematically evaluate the efficacy and safety of PRP for patients with diabetic ulcers.
Scope and Significance
The healing of diabetic ulcers remains a major clinical problem. In this review, the data showed that PRP may increase the incidence of complete wound healing compared with control group. Compared with previous reviews, our article is a unique meta-analysis that only focused RCTs on effectiveness and safety of PRP therapy on diabetic ulcers.
The result may also have some certain implications in the treatment of diabetic ulcers. The treatment of PRP may be an additional new therapy for diabetic ulcers in future.
Materials and Methods
Data sources and search strategy
Two researchers independently searched all references in the Cochrane Library, MEDLINE, EMBASE, PubMed, Scoups, and Web of Knowledge databases up to December 21, 2017. Additional trials were also searched for prescribing information documents of approved medications at relevant websites (Clinical Trials and Xue Glgoo). In addition, gray literatures that were nonconventional, difficult to locate and sometimes ephemeral were also searched. The following search terms were used: diabetic foot, diabetic feet, combined with PRP, AutoloGel, PRP, CT-102-activated platelet, and platelet gel. The search was limited to English language references. The authors were contacted when the study was unpublished or had inadequate data. There were no restrictions with respect to the date of publication or study settings.
Study selection
Two reviewers independently screened the titles and abstracts according to the inclusion criteria. RCTs of adults with diabetic ulcers, who received treatment with PRP as identified by an expert advisory panel, were included. In brief, the preparation of PRP8,9 was relatively simple. This mainly included three steps. First, blood, including autologous blood or homologous, is collected. Second, the collected blood is centrifuged to separate the platelet from the rest of the blood components. Third, PRP is extracted into a sterile small tube. The platelets should be active before the growth factors are released from PRP. There are mainly two methods to activate the platelets. For the first method, a platelet activator is added, which is a combination of thrombin and 10% calcium chloride (CaCl2). This would allow the platelet to release growth factors (platelet releasate). For the second method, platelet lyses (lysate) are activated by freezing. The final product is applied locally to the ulcers as a gel or solution. Participants were seen at fixed time intervals, appointments were scheduled for those allocated to PRP or conventional treatment. The wounds area was estimated by considering the wound as an ellipsis whose diameters were the largest and shortest dimensions of the wound. Wounds area was measured at the beginning of treatment (initial area) and the end point (final area); the “reduction rate” was calculated as [final area (mm2) − initial area (mm2)/initial area (mm2)]. In addition, RCTs that compared the therapy with the control group (standard care, platelet poor plasma therapy, or placebo) were included. Reviews, case reports, meeting abstracts, nonrandomized clinical trials, clinical control trials, or overlapping publications were excluded. Full-text articles were retrieved and reviewed when a decision on inclusion could not be made solely based on the abstracts. When the two researchers were not in agreement, a consensus was made through a senior researcher to solve the disagreement.
Data extraction and quality assessment
Two reviewers independently extracted study characteristics pertaining to the study design, number of patients, age, gender, ulcer duration, follow-up, location, attrition, study setting, treatment regimens, outcome measures, and adverse events from the eligible references. Any disagreement was also solved by consensus through a senior researcher. The quality of included studies was evaluated using the modified Jadad scale.10,11 The quality evaluation criteria included randomized methods, allocation concealment, blinding, withdrawal/drop out conditions, and the application of an intension-to-treatment (ITT) analysis. A score of 1–3 indicated low quality, while a score of 4–7 indicated high quality.
Data synthesis and analysis
The statistical analysis was conducted using STATA version 12.0 (Statacorp LP, College Station, TX). Continuous data were reported as standard mean difference (SMD) with 95% confidence interval (CI), while dichotomous data were reported as odds ratio (OR) with 95% CI. Pooled estimates of SMD or OR were calculated using a random-effects model (M-H, heterogeneity), in which both within-study and between-study variations were considered. Heterogeneity among studies was assessed using the Cochrane Q test and by calculating the I2 statistics. For the Q statistic, p < 0.10 was considered statically significant. The I2 statistic indicated the percentage of the observed between-study variability caused by the heterogeneity. Studies with an I2 value of <25%, ∼50%, ∼75%, and ∼100% were considered to have mild, moderate, large, and extreme heterogeneity.12 Metaregression with restricted maximum likelihood estimation was performed to assess for potentially important covariates that might exert a substantial impact on between-study heterogeneity.13 If no significant covariates were found to exert a substantial impact, a sensitivity analysis was performed to evaluate for the robustness of the primary outcome.14 In addition, publication bias was assessed quantitatively using Egger's test.15,16 A p-value <0.05 was considered statistically significant.
Trial sequential analysis
In the meta-analyses, repeated test of accumulating data would bring about increased risk of producing a type I error, which would lead to a false-positive or false-negative conclusion. Therefore, trial sequential analysis (TSA) was applied to correct these random errors. This method has been suggested to be useful for identifying whether the present evidence is reliable and conclusive.17 TSA mainly depends on the diversity-adjusted required information size (RIS) with adjusted threshold for statistical significance, which constructs trial sequential monitoring boundaries.18,19 When the cumulative Z-curve surpasses the trial monitoring boundary or futility zone, the conclusion is often considered as reliable and further studies are not likely to change it. If the trial sequential monitoring boundary is not crossed, more RCTs are needed to confirm the reliability of the conclusion.20 The diversity-adjusted RIS was calculated with an overall 5% risk of a type I error and a power of 80% (two-sided). Relative risk reduction is a parameter calculated by dividing the absolute risk reduction by the control event rate.21 TSA was conducted using TSA Version 0.9 Beta.
Results
Search results
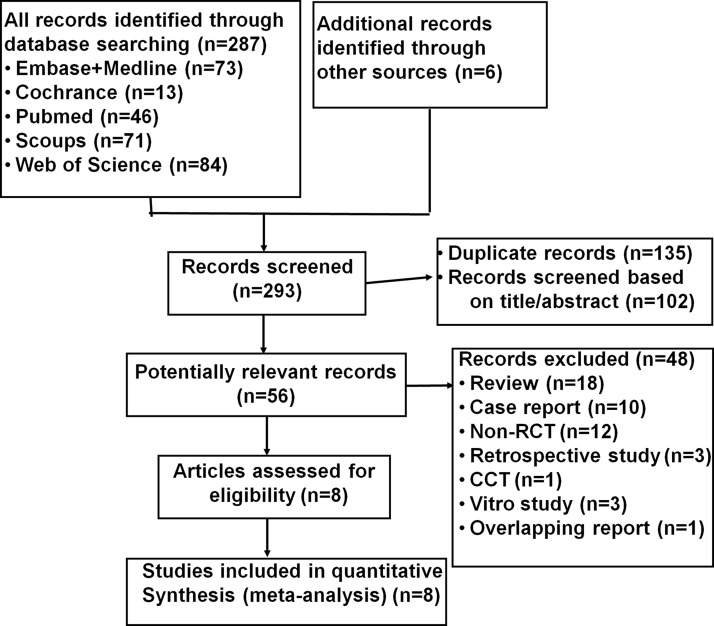
The process of the study selection is presented in Fig. 1. A total of 293 records were initially identified. After the exclusion of duplicates and screening via the titles and abstracts, 56 potential articles were identified. After reviewing the full text, 48 articles were excluded for various reasons (Fig. 1). Finally, the remaining eight articles22–29 were included and analyzed.
Figure 1.
Flowchart of the database search and study identification.
Characteristics of the included studies
Tables 1 and 2 summarize the characteristics of the eight included studies,22–29 which involved 431 participants and were all published in the English language. These trials were published from 1992 to 2017, with sample sizes ranging from 13 to 117. All trials22–29 included patients with diabetic ulcers. Treatment duration ranged from 5 to 20 weeks, and the median was 12 weeks. The PRP therapies for diabetic ulcers included autologous PRP and homologous PRP. In five trials (62.5%),22–24,28,29 autologous PRP was applied, while in the remaining three trials (37.5%),25–27 homologous PRP was used. All autologous or homologous PRPs were activated using a platelet activator, which was a combination of thrombin and 10% CaCl2. Follow-up durations ranged from 5 to 120 weeks, and the median duration was 20 weeks.
Table 1.
Summary of participants in included studies
| Study ID | Design | No. of Patients (Male/Female) | Age, Year (±SD) | Proportion of Women | Ulcer's Duration | Follow-up (Week) | Location | Autologous/Homologous PRP |
|---|---|---|---|---|---|---|---|---|
| Driver (2006) | RCT | 72 (59M/13F) | 56.9 ± 9.7 | 18.1% | Not specified | 24 | Foot ulcer | Autologous PRP |
| Saldalamacchia (2004) | RCT | 14 (6M/8F) | 59.6 ± 8.4 | 57.1% | Not specified | 5 | Foot ulcer | Autologous PRP |
| Steed (1992) | RCT | 13 (9M/4F) | 56.6 ± 12.3 | 30.8% | 15.2 ± 14.7 months | 20 | Foot ulcer | Homologous PRP |
| Li (2015) | RCT | 117 (75M/42F) | 62.8 ± 11.6 | 35.9% | 26.5 ± 46.1 days | 12 | Diabetic ulcer | Autologous PRP |
| Steed (1996) | RCT | 36 | Not specified | Not specified | 15.5 ± 14.5 months | 120 | Foot ulcer | Homologous PRP |
| Ahmed (2017) | RCT | 56 (38M/18F) | 46.5 ± 17.0 | 32.1% | 12.0 ± 2.1 weeks | 12 | Foot ulcer | Autologous PRP |
| Holloway (1993) | RCT | 81 | Not specified | Not specified | Not specified | 20 | Foot ulcer | Homologous PRP |
| Friese (2007) | RCT | 42 | Not specified | Not specified | Not specified | 25 | Foot ulcer | Autologous PRP |
PRP, platelet-rich plasma; RCT, randomized controlled trial.
Table 2.
Summary of participants in included studies
| Study ID | Attrition | ITT/PP Analysis | Study Setting | Treatment | PRP Method | Jadad Score |
|---|---|---|---|---|---|---|
| Driver (2006) | 44.4% | ITT analysis | United States | Group 1: application of PG on the ulcer twice a week for 12 weeks Group 2: application of normal saline gel |
PRP releasate | 7 |
| Saldalamacchia (2004) | None | N/A | Italy | Group 1: application of PG on the ulcer once a week for 5 weeks Group 2: standard care |
PRP releasate | 2 |
| Steed (1992) | None | N/A | United States | Group 1: application of CT-102 on the ulcer once a week for 20 weeks Group 2: application of saline gel |
PRP releasate | 3 |
| Li (2015) | 12.0% | ITT analysis | China | Group 1: application of PG on the ulcer once 2 weeks for 12 weeks Group 2: usual care |
PRP releasate | 5 |
| Steed (1996) | None | N/A | United States | Group 1: application of CT-102 on the ulcer once per day for 20 weeks Group 2: application of saline guaze |
PRP releasate | 3 |
| Ahmed (2017) | None | N/A | Egypt | Group 1: application of PG on the ulcer twice a week for 12 weeks Group 2: standard care |
PRP releasate | 2 |
| Holloway (1993) | 13.6% | ITT analysis | United States | Group 1: topical application of CT-102 on the ulcer once daily for 20 weeks Group 2: placebo |
PRP releasate | 5 |
| Friese (2007) | Group 1: application of PRP on the DFU every 2 weeks for 12 weeks. Group 2: cleaning, polyurethane foam dressing applied and changed as needed, debridement, and offloading. |
PRP releasate | Low |
DFU, diabetic foot ulcer; ITT, intension-to-treatment; N/A, not applicable; PG, platelet gel.
Quality assessment of the included studies
According to the modified Jadad Rating Scales,10 three studies (37.5%)22,24,25 were of high quality (scores ≥4), and five studies (62.5%)23,26–29 were of low quality (scores ≤3) (Table 2).
Efficacy outcomes
Primary efficacy outcome (complete ulcer healing rate)
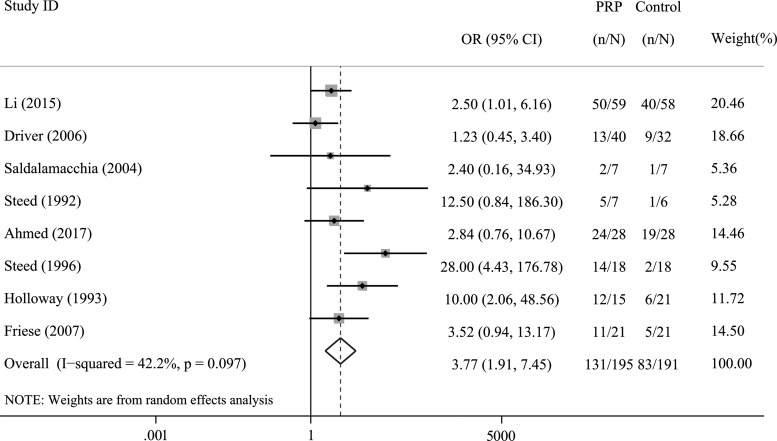
Eight trials, which involved 431 participants, specified this outcome. Compared with the control treatment, PRP significantly increased the number of patients with ulcer healing (OR = 3.77, 95% CI = 1.91–7.45, I2= 42.2%; Fig. 2).
Figure 2.
Forest plot for the meta-analysis of the rate of complete ulcer healing in patients allocated to the PRP group and control group. CI, confidence interval; PRP, platelet-rich plasma.
Sources of heterogeneity and metaregression
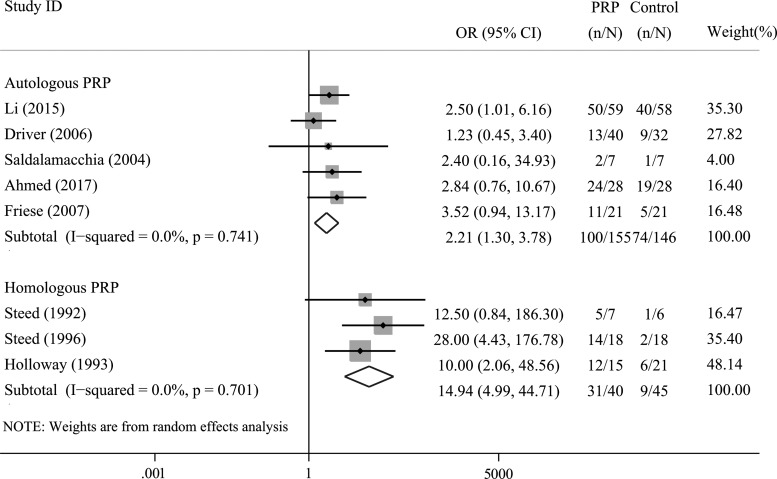
A subgroup analysis was performed according to the different PRPs used. Four trials applied autologous PRP, while three trials applied homologous PRP. The results revealed that both autologous (OR = 2.21, 95% CI = 1.30–3.78, I2= 0.0%) and homologous PRPs significantly improved the rate of complete ulcer healing in these patients (OR = 14.94, 95% CI = 4.99–44.71, I2= 0.0%; Fig. 3). Since there were <10 trials, it was not suitable to conduct a metaregression analysis.
Figure 3.
Subgroup analysis of the rate of complete ulcer healing in patients allocated to the PRP group and control group according to the PRP used.
Sensitivity analysis
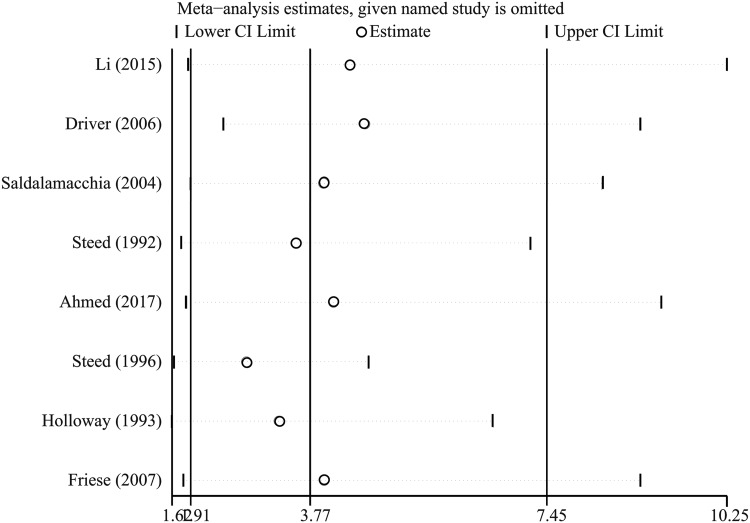
The sensitivity analysis (Fig. 4) revealed that omitting either of the included studies one at a time did not significantly change the results.
Figure 4.
Sensitivity analysis for the rate of complete ulcer healing in patients allocated to the PRP group and control group.
Publication bias
The Egger's test revealed no evidence of significant publication bias among the included trials for the meta-analysis of the ratio of complete ulcer healing (p = 0.071).
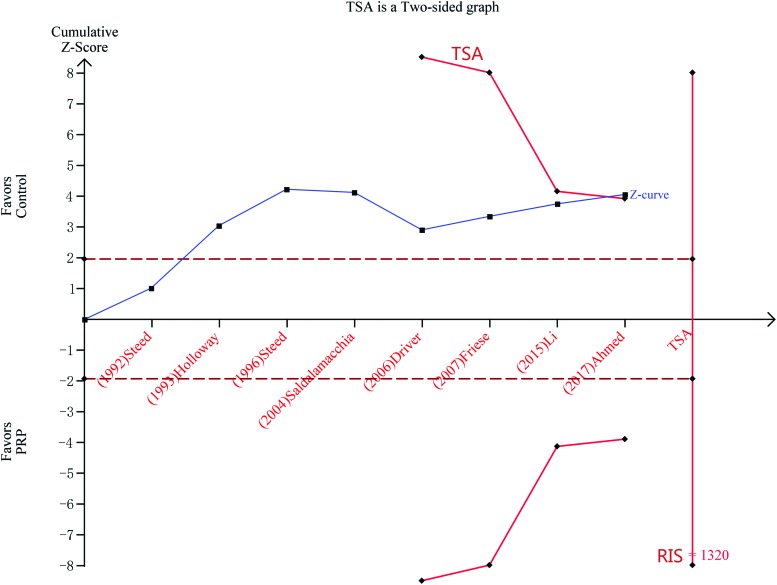
Trials sequential analysis
TSA was conducted using random effects (Biggerstaff-Tweedie method), and the cumulative Z-curve crossed the conventional boundary for benefit, but not the RIS boundary (Fig. 5).
Figure 5.
TSA of the included studies. A two-sided graph is plotted by TSA, where the dotted lines represent the conventional significance boundaries, the blue line indicates the cumulative Z-score, and the red lines shows the α-spending boundary and required information size. TSA, trial sequential analysis. Color images are available online.
Secondary efficacy outcomes
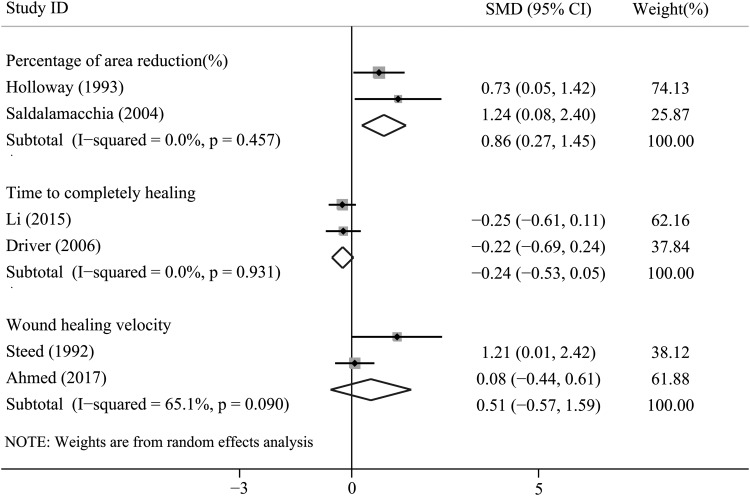
Time to complete ulcer healing
Three RCTs reported data on this outcome. Two trials provided detailed data. Time to complete ulcer healing of one trial conducted by Driver was 42.9 ± 18.3 days for PRP group and 47.4 ± 22 days for control group. Time to complete ulcer healing of one trial conducted by Li et al. was 36 ± 40 days for PRP group and 45 ± 31.1 days for control group. No significant difference was detected for the outcome of time to complete ulcer healing between the two groups (SMD = −0.24, 95% CI = −0.53 to 0.05, I2= 0.0%; Fig. 6).
Figure 6.
Forest plot for the meta-analyses of secondary outcomes: (1) percentage of ulcer area reduction; (2) time to complete ulcer healing; and (3) ulcer healing velocity. SMD, standardized mean difference.
Percentage of ulcer area reduction
Four trials specified this outcome. However, only two trials provided detailed data. Treatment with PRP significantly reduced the area of diabetic ulcer, when compared with controls (SMD = 0.86, 95% CI = 0.27–1.45, I2= 0.0%; Fig. 6).
Ulcer healing velocity
Three trials specified information on ulcer healing velocity. Merely two trials reported detailed data. The data revealed that there was no significant difference between the PRP group and controlled group in terms of the velocity of ulcer healing (SMD = 0.51, 95% CI = −0.57 to 1.59, I2= 65.1%; Fig. 6).
Safety outcomes
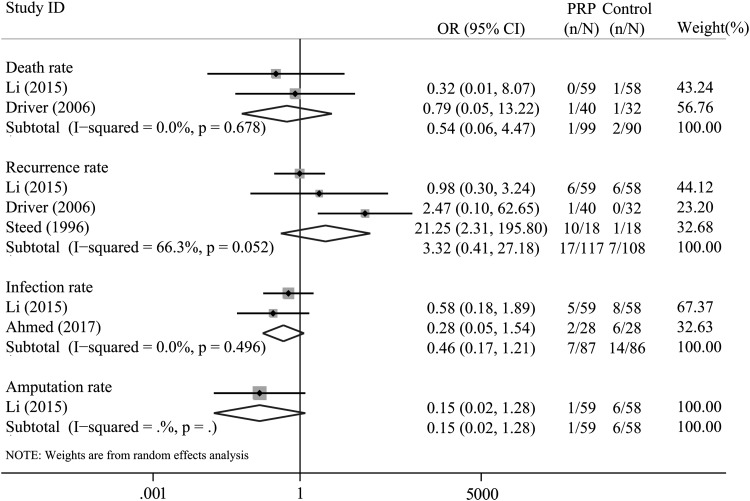
Mortality
Merely two trials22,24 reported the mortality outcome of patients. The overall pooled results did not reveal a statistically significant difference in terms of the mortality rate between these groups (OR = 0.54, 95% CI = 0.06–4.47, I2= 0.0%; Fig. 7).
Figure 7.
Forest plot for the meta-analysis of safety outcomes between PRP versus the controlled group: (1) death rate; (2) recurrence rate; (3) infection rate; and (4) amputation rate.
Recurrence rate
Three trials specified the number of recurrences. There was no significant difference in the risk of recurrence rate detected between the two groups (OR = 3.32, 95% CI = 0.41–27.18, I2= 66.3%, Fig. 7).
Infection rate
Two trials23,24 specified data on infection. There was no difference in infection rate for patients included in the two groups (OR = 0.46, 95% CI = 0.17–1.21, I2= 0.0%; Fig. 7).
Amputation rate
Merely one trial specified the number of amputations. There was no difference between the two groups in terms of the rate of amputation in patients from each group (OR = 0.15, 95% CI = 0.15–1.28, I2: No Application; Fig. 7).
Quality of life
This was not reported in any of the trials.
Discussion
Based on the results of the meta-analyses, the systematic review of available RCTs indicated that for patients with diabetic ulcers, the use of PRP therapy, when compared with controls, are associated with a significantly improved rate of complete ulcer healing without significant increased risk of adverse events. Moreover, subgroup analyses revealed that the benefits of PRP-based therapy on ulcer healing in patients with diabetes were consistent, according to the different PRPs used. Taken together, these results suggest that PRP may become a promising treatment strategy for patients with diabetic ulcers.
The results of this meta-analysis are generally consistent with previous reviews on this topic. An earlier review conducted by Villela and Santos 30 assessed the efficacy of PRP for diabetic ulcers, and this indicated similar results in the aspect of the efficacy of PRP. However, their results were influenced by one trial, which should have been excluded. Picard et al.31 included five RCTs to assess the influence of PRP on the healing rate of diabetic ulcers. The reviewer did not perform a meta-analysis, and included one quasirandom clinical trial that acted as an RCT. Martinez-Zapata et al.32 performed a rigorous meta-analysis of two RCTs to investigate the efficacy of autologous PRP for diabetic ulcers. Their results revealed that autologous PRP may make an increase in the incident of healing in people with diabetic ulcers. However, the quality of evidence was low, because the two included trials were too small and the result was significantly influenced by Li et al.33 In the present study, an updated meta-analysis of RCTs was performed, which focused on both the efficacy and safety outcomes of PRP in patients with diabetic ulcers. The potential heterogeneity may have been decreased by limiting the design of the included studies and etiologies of the ulcers.
In the overview for safety, the present findings revealed that the use of PRP was not associated with significantly affected risk of adverse events in patients with diabetic ulcers. These results were consistent with previous studies.32,34 However, the follow-up duration of one trial28 was as short as 5 weeks, which may be too short for evaluating the potential influence of PRP on risk of adverse events. In addition, incidences of adverse events were inconsistently reported, according to the included studies. Indeed, the majority of studies did not undertake a systematic approach to evaluate or record adverse events. None of the trials evaluated quality of life as a safety outcome.
PRP includes many growth factors, which have capacities to promote tissue regeneration and repair. PRP activated by thrombin could release 5–8 accelerated wound healing growth factors, such as TGF, PDGF, IGF, VEGF, EGF, and so on. These growth factors play important roles in the formation, proliferation, and differentiation of cells, increase collagen synthesis, and activate macrophages and other growth factors. The increased concentration of these growth factors in PRP can significantly stimulate the proliferation of fibroblasts and the expression of type I collagen.35 Besides, PRP could stimulate granulation, angiogenesis, cell proliferation, and promote reepithelialization in vivo.36 In additon, PRP may have some additional advantages, which may make these suitable for clinical use, such as low-cost and minimally invasive.37 However, in view of the fact that only pilot RCTs of relatively low quality were available, more large-scale RCTs are needed to confirm these present results before PRP-based therapy for diabetic ulcer could be clinically applied.
The present meta-analysis has limitations, which should be noted when interpreting these results. First, only references in the English language were included. This could produce potential publication bias. Since there were <10 trials, a funnel plot was not plotted. However, the Egger's test performed revealed no significant publication bias. Second, the included studies were of relatively poor quality, which may limit the grade of the evidence. Third, most of the included trials have inadequate sample sizes to detect the significant outcome. Therefore, TSA was used to correct these random errors, to test for more reliable and conclusive evidences. Fourth, three studies22,24,25 were with biases, which was due to dropouts, missing data, or protocol violations, and these may influence the reliability of the final conclusion. However, the results of three trials22,24,25 used the ITT analysis, which was more conservative38 and reduced the influence of the above bias.39 Finally, the heterogeneity among trials was high in the present meta-analysis. Although subgroup analyses were performed to investigate the potential source of high heterogeneity, the results revealed that the efficacy of PRP on primary outcome was consistent in the subgroup analyses, in which different PRPs were used. The potential source of heterogeneity could not be determined. However, as RCTs, each study balanced these potential factors, which may potentially affect the outcomes. The treatment group and control group were mostly treated under equal conditions in each study. Hence, clinical heterogeneity was considered to be minimal. At last, but not least, as we all know, the clinical outcomes of diabetic ulcers were related to some independent factors, such as ischemia, depth, size, and location of diabetic ulcers. Owing to the lack of the data of ischemia and location of diabetic ulcers, we fail to understand whether PRP is applied to treat ischemia ulcers. In our future study, we should consider these related issues.
Conclusion
Limited evidence from pilot RCTs suggests that PRP may be an effective therapy for diabetic ulcer, which is not associated with significant increased risk of adverse events. Compared with control therapy, PRP-based therapy could improve the complete healing of diabetic ulcers. However, due to the relatively low quality of the included trials, high-quality RCTs are needed to confirm the reliability of this conclusion. In addition, more studies that report the safety outcomes and quality of life of patients with diabetic ulcers receiving PRP therapy should be conducted.
Acknowledgments
We express our gratitude to Dr. Beishan Li for the suggestions to improve the quality of this review. This work was supported by research grant 81272096 (J.Y.Z.), 81471875 (J.Y.Z.), 81571908 (B.T.), and 81501675 (Z.C.H.) from National Natural Science Foundation of China, research grant 2014A030313099 (B.T.) and 2014A030310117 (Z.C.H.) from Guangdong Provincial Natural Science Foundation of China, research grant 2016B090916001 (J.Y.Z.) and 2015A020212009 (Z.C.H.) from Science and Technology Planning Project of Guangdong Province, research grant 201508020111 (J.Y.Z.) from Guangzhou City Science and Technology Program of China, and research grant 2013001 (J.Y.Z.) and 2017013 (Z.C.H.) from the Sun Yat-sen University Clinical Research 5010 Program.
Abbreviations and Acronyms
- CI
confidence interval
- EGF
epidermal growth factor
- IGF
insulin-like growth factor
- ITT
intension-to-treatment
- OR
odds ratio
- PDGF
platelet-derived growth factor
- PRP
platelet-rich plasma
- RCTs
randomized controlled trials
- RIS
required information size
- SMD
standard mean difference
- TGF
transforming growth factor
- TSA
trial sequential analysis
- VEGF
vascular endothelial growth factor
Authors' Contributions
J.-Y.Z. and B.T. designed the research. Z.-C.H., S.-Q.Q, B.T., J.-Y.Z., J.Z. X.-L.C., P.W., S.-B.W., F.S., and Y.-X.D performed the experiment. Z.-C.H. and S.-Q.Q wrote the article. B.T., J.-Y.Z. Z.-C.H., and S.-Q.Q researched data. J.-Y.Z., B.T. and J.W. reviewed/edited the article.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Zhicheng Hu, MD, PhD, an associate chief physician in the First Affiliated Hospital of Sun Yat-sen University, who published seven SCI articles until now, including two articles of J Invest Dermatol, two articles of Br J Surg, and one article of J Am Coll Surg, Plast Reconstr Surg, and Clin Exp Dermatol. Shanqiang Qu, MD, Xiaoling Cao, MD, Peng Wang, MD, Shaobin Huang, MD, and Yunxian Dong, MD, are PhD candidates of the First Affiliated Hospital of Sun Yat-sen University. Jian Zhang, MD, and Fen Shi, MD, are residents of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University. Jun Wu, MD, PhD, is the academic leader of the Burn Department in the First Affiliated Hospital of Sun Yat-sen University and the editor in chief of Burns and Trauma. Bing Tang, MD, PhD, is a professor and chief physician in the First Affiliated Hospital of Sun Yat-sen University. Jiayuan Zhu, MD, PhD, is a professor, chief physician, and Vice Director of Surgery in the First Affiliated Hospital of Sun Yat-sen University. He published 12 SCI articles, including J Invest Dermatol, Br J Surg, J Am Coll Surg, J Biol Chem, Plast Reconstr Surg, and so on. He also hosts three projects on the Natural Science Foundation of China and four major projects at the provincial foundation, and so on.
References
- 1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–149 [DOI] [PubMed] [Google Scholar]
- 2. Tzeng YS, Deng SC, Wang CH, Tsai JC, Chen TM, Burnouf T. Treatment of nonhealing diabetic lower extremity ulcers with skin graft and autologous platelet gel: a case series. Biomed Res Int 2013;2013:837620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guest JF, Fuller GW, Vowden P. Diabetic foot ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J 2018;15:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen 2016;24:112–126 [DOI] [PubMed] [Google Scholar]
- 5. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg 2003;90:133–146 [DOI] [PubMed] [Google Scholar]
- 6. Houdek MT, Wyles CC, Stalboerger PG, Terzic A, Behfar A, Moran SL. Collagen and fractionated platelet-rich plasma scaffold for dermal regeneration. Plast Reconstr Surg 2016;137:1498–1506 [DOI] [PubMed] [Google Scholar]
- 7. Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diabetes Metab Res Rev 2000;16 Suppl 1:S84–S92 [DOI] [PubMed] [Google Scholar]
- 8. Kakudo N, Kushida S, Kusumoto K. Platelet-rich plasma: the importance of platelet separation and concentration. Plast Reconstr Surg 2009;123:1135–1136 [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Wang C, Liu H, Liu G, Ran X. Antibacterial effect of autologous platelet-rich gel derived from subjects with diabetic dermal ulcers in vitro. J Diabetes Res 2013;2013:269527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 11. Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord 2001;12:232–236 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen NH, Singh S. A primer on systematic reviews and meta-analyses. Semin Liver Dis 2018;38:103–111 [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004;23:1663–1682 [DOI] [PubMed] [Google Scholar]
- 14. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008;37:1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gjerdevik M, Heuch I. Improving the error rates of the Begg and Mazumdar test for publication bias in fixed effects meta-analysis. BMC Med Res Methodol 2014;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75 [DOI] [PubMed] [Google Scholar]
- 18. Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009;38:276–286 [DOI] [PubMed] [Google Scholar]
- 20. Wu XD, Liu MM, Liang X, Hu N, Huang W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Clin Nutr 2018;37:505–515 [DOI] [PubMed] [Google Scholar]
- 21. Barratt A, Wyer PC, Hatala R, et al. ; Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 1. Relative risk reduction, absolute risk reduction and number needed to treat. CMAJ 2004;171:353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Driver VR. A prospective, randomized, controlled, blinded, multicenter pivotal trial of a platelet rich plasma gel* versus control when added to the standard of care in the treatment of non-healing diabetic foot ulcers. Diabetes 2006;55(Suppl 1):A25 [Google Scholar]
- 23. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg 2017;38:206–211 [DOI] [PubMed] [Google Scholar]
- 24. Li L, Chen D, Wang C, et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: a prospective, randomized clinical trial. Wound Repair Regen 2015;23:495–505 [DOI] [PubMed] [Google Scholar]
- 25. Holloway GA, Steed DL, Demarco MJ, Moosa. A randomized, controlled dose response trial of activated platelet supernatant, topical CT-102 in chronic, nonhealing diabetic wounds. Wounds 1993;5:198–206 [Google Scholar]
- 26. Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double-blind trial in healing chronic diabetic foot ulcers. CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992;15:1598–1604 [DOI] [PubMed] [Google Scholar]
- 27. Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair Regen 1996;4:230–233 [DOI] [PubMed] [Google Scholar]
- 28. Saldalamacchia G, Lapice E, Cuomo V, et al. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovas Dis 2004;14:395–396 [DOI] [PubMed] [Google Scholar]
- 29. Friese G, Herten M, Scherbaum WA. The use of autologous platelet concentrate activated by autologous thrombin (APC+) is effective and safe in the treatment of chronic diabetic foot ulcers: a randomized controlled trial. Fifth International Symposium Diabetic Foot Noordwijkerhout, The Netherlands 2007 [Google Scholar]
- 30. Villela DL, Santos V. Evidence on the use of platelet-rich plasma for diabetic ulcer: a systematic review. Growth Factors 2010;28:111–116 [DOI] [PubMed] [Google Scholar]
- 31. Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen 2015;23:638–643 [DOI] [PubMed] [Google Scholar]
- 32. Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;(5):CD006899. doi: 10.1002/14651858.CD006899.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li L, Wang C, Wang Y, et al. [Impact of topical application of autologous platelet-rich gel on medical expenditure and length of stay in hospitals in diabetic patients with refractory cutaneous ulcers]. Sichuan Da Xue Xue Bao Yi Xue Ban 2012;43:762–765 [PubMed] [Google Scholar]
- 34. Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2012;10:CD006899. doi: 10.1002/14651858.CD006899.pub2 [DOI] [PubMed] [Google Scholar]
- 35. Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med 2012;29:32–36 [DOI] [PubMed] [Google Scholar]
- 36. Pietramaggiori G, Scherer SS, Mathews JC, et al. Quiescent platelets stimulate angiogenesis and diabetic wound repair. J Surg Res 2010;160:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeRossi R, Coelho AC, Mello GS, et al. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras 2009;24:276–281 [DOI] [PubMed] [Google Scholar]
- 38. Porta N, Bonet C, Cobo E. Discordance between reported intention-to-treat and per protocol analyses. J Clin Epidemiol 2007;60:663–669 [DOI] [PubMed] [Google Scholar]
- 39. Molenberghs G, Thijs H, Jansen I, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics 2004;5:445–464 [DOI] [PubMed] [Google Scholar]