Abstract
Subcritical water extraction (SWE) uses hot compressed water as an effective solvent for both polar and nonpolar compounds and has been developed as an environmentally benign extraction technology for natural materials. Polysaccharides as one of the main ingredients in Dendrobium plants showed obvious biological activity. Thus, SWE of polysaccharides obtained from Dendrobium nobile Lindl. was investigated in this work. The response surface methodology (RSM) was combined with a Box–Behnken design to evaluate the influence that the three independent variables had on the response. The optimal extraction conditions (determined via RSM) were 129.83 °C extraction temperature, 16.71 min extraction time, and 1.12 MPa extraction pressure. The maximum predicted polysaccharide yield was 20.67%, which corresponded well with the experiential extraction (21.88%). The polysaccharides obtained from either the stirring extraction, refluxing extraction, ultrasound extraction, or SWE methods were compared, and the extraction processes were modeled. The molecular weight, monosaccharide composition, and antioxidative activities of the polysaccharides were analyzed.
1. Introduction
Dendrobium nobile Lindl., Orchidaceae, is a herbal plant commonly used in Chinese traditional medicine and it is one of the several Dendrobium species that were specified in the Chinese Pharmacopeia.1,2 Previous research has shown that Dendrobium has applications as an agent in antioxidants, and also have anticancer, immunomodulatory, hepatoprotective, and neuroprotective activity.3 Many compounds have been extracted and isolated from the Dendrobium species to better understand these health functions,4 including polysaccharides,5,6 alkaloids,7,8 phenolics,9,10 phenanthrenes,11 and bibenzyls.12,13 Other chemical constituents were detected in Dendrobium officinale, such as phenols, acids, esters, and amides.14 Polysaccharides are considered to be one of the main active components in Dendrobium plants,15 demonstrating antitumor,16 antiviral,17 antihyperglycemic, and immunomodulatory activities.18,19 Moreover, polysaccharides as ideal biodegradable polymer materials can be used as drug carrier, food packaging, pharmaceutical preparations, and so forth.20,21
Most of the previous research pertaining to the compounds in the polysaccharides from D. nobile Lindl., have focused on their structural characteristics and pharmacological properties. Wang et al. reported that some of the D. nobile polysaccharides displayed remarkable immunomodulatory effects. Bioactive tests in vitro revealed that five water-soluble polysaccharides (DNP-W1B, DNP-W2, DNP-W3, DNP-W4, and DNP-W5) could stimulate ConA- and LPS-induced T and B lymphocyte proliferation.22−26 They also found that D. nobile polysaccharides have strong antitumor activities, and two water-soluble polysaccharides (DNP-W1 and DNP-W3) exhibited high antitumor activities against Sarcoma 180 in vivo and HL-60 in vitro. Polysaccharides were extracted thrice by distilled water for 2 h at 80 °C.27 Li et al. isolated and characterized a neutral polysaccharide (DNPE6(4)) from D. nobile Lindl and studied its anti-TMV and anti-CMV activities for the first time in vivo.28 Some others extracted and purified homogeneous heteropolysaccharides which could alleviate vinorelbine-induced decrease of macrophages in vivo29 and presented significant immune-modulating activities.30,31 Luo et al. extracted polysaccharides from the stem of the D. nobile Lindl. three times with hot water for 2 h each time, where the structure of the polysaccharides were characterized and the antioxidant activities were evaluated in vitro.32,33 They evaluated the mechanism of the antitumor activities and the immunomodulation effects of the four polysaccharide fractions taken from the D. nobile Lindl. in vivo.34 Pan et al. reported polysaccharides taken from four different Dendrobium species were extracted thrice with boiling distilled water, each time for 0.5 h, where their hypoglycemic and antioxidative activities were compared in vivo.35 Hot water extraction remains the most common extraction method for polysaccharides. This method is typically time-consuming, laborious, and has low selectivity and/or low extraction yields,36 making it necessary to bring forth novel extraction methods that would circumvent this.
Subcritical water extraction (SWE) uses hot compressed water as an effective solvent for polar and nonpolar compounds, where it is developed as an environmentally benign extraction technology for natural materials.37,38 Subcritical water is liquid water under pressure, at a temperature range between the usual boiling point (100 °C) and critical temperature (374.1 °C).39,40 At these conditions, the thermal motion of water molecules increases, thus changing its properties.41 Cooler water should extract more water-soluble organic compounds, whereas hotter temperature water should extract less soluble organic compounds. The pressure determines the state of the water by changing the density to enhance water solvency.42 SWE has been used to extract polyphenolic and antioxidant compounds from many natural products,43−45 although it has not been used as an extraction method for polysaccharides from the D. nobile Lindl. In this work, we proposed SWE as a feasible processing method for the extraction for polysaccharides from the D. nobile Lindl. The study used the Box–Behnken design (BBD) of response surface methodology (RSM) to optimize the extraction experiments. The polysaccharides were analyzed via UV–vis spectrophotometry. The extraction process was modeled and the polysaccharide’s antioxidative activities were analyzed.
2. Materials and Methods
2.1. Materials and Chemicals
The dry stem of the D. nobile Lindl. was purchased from Sichuan Wanan Industrial Development Co., Ltd. (Chengdu, China). The dry mushroom was obtain from Jiangnan biotechnology Co., Ltd. (Jiangsu, China). Reference standard glucose (GC purity > 99.5%) was purchased from Sigma-Aldrich Co., Ltd. (Beijing, China). The other chemicals were of analytical reagent grade.
2.2. Subcritical Water Extraction
SWE was performed on a stainless steel batch reactor (inner volume 100 mL), constructed in house. A schematic drawing of the SWE is presented in Figure 1. The dry stem of the D. nobile Lindl. was ground in a mill until the powder was less than 0.45 mm. The powder material and purified water were placed in the vessel at a solid to liquid ratio of 1:25 (g/mL). The vessel was then sealed and purged with inert N2. The extraction pressure (0.5–1.5 MPa) was controlled at a predetermined value. The reactor was heated to the experimental temperature (120–160 °C) by an electric heater. The mixture was agitated by a magnetic stirrer at 300 rpm. The pressure and temperature inside the reactor during each experiment were measured by the pressure gauge and the temperature controller. The extraction time was tracked after the interior temperature of the vessel reached the target level. After a predetermined treatment time (5–20 min), the vessel was quickly immersed into an ice bath to stop the extraction. The mixture in the vessel was paper-filtered and stored at 4 °C for further analysis.
Figure 1.
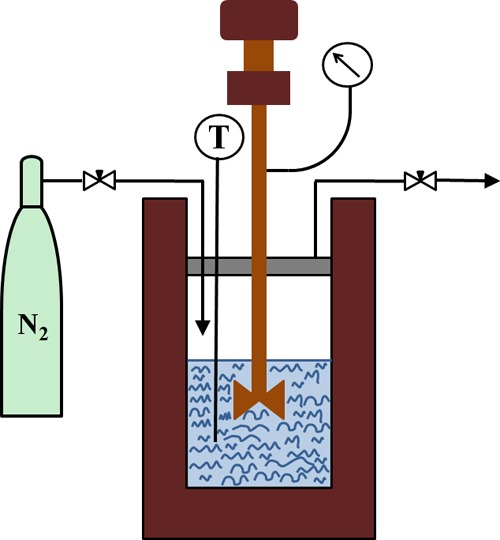
Schematic diagram of SWE.
The mixtures were combined and concentrated under the reduced pressure via a vacuum rotary evaporator at 65 °C. The mixtures were centrifuged at 4000 rpm for 10 min to obtain the supernatant. The supernatant was then precipitated by adding four times the volume of 95% alcohol, where the mixture was left to rest overnight at 4 °C. The precipitate was centrifuged at 4000 rpm for 10 min, washed with absolute ethanol, acetone, and diethyl ether, dissolved in water, and then the procedure was repeated. The crude polysaccharide was obtained via vacuum freeze-drying.
2.3. UV–Vis Spectrophotometry Determination
The phenol–sulfuric acid method was used to test the polysaccharide sample, as it is low cost and UV–vis spectrophotometry is easily available.46,47 Glucose (0.02–0.08 mg/mL) was used as the reference to obtain a calibration curve. The linear regression equation was: A488 = 9.6639C + 0.0016 (r = 0.9976), where A488 was the absorbance at 488 nm and C was the concentration of the glucose sample (mg/mL). One milliliter of the polysaccharide solution (0.20 mg/mL) was mixed thoroughly with 1 mL of the fresh 5% aqueous solution phenol in a test tube, then 5 mL of concentrated sulfuric acid (98%) was rapidly added to the mixture. The samples were vortexed for 30 s, placed in an oil bath, and then heated for 20 min at 100 °C for color development. The reference solutions were prepared via the same methods, although a 1 mL aliquot of the polysaccharide solution replaced the water. The samples were cooled to room temperature in a water bath, the mixtures were measured, and the absorbance was recorded at 488 nm on a UV–vis spectrophotometer (Shanghai Yoke instruments Co., Ltd., Shanghai, China). The total polysaccharide concentration was calculated with glucose as the standard.
2.4. Single Factor Experiments
The extraction temperature, solvent/solid ratio, extraction time, and pressure were the main factors influencing the SWE of the polysaccharides. During the single factor experiments, one factor was changed while the other factors were kept constant in each experiment, and all the experiments were repeated three times.
2.5. Experimental Design of RSM
2.5.1. Optimization of Process Parameters
The conditions for the SWE of the polysaccharides were further optimized from the D. nobile Lindl. The BBD serves as the RSM and was employed using a Design-Expert 7.1.6 Trial (State-Ease, Inc., Minneapolis MN, USA). BBD determined the maximum efficiency for an experiment involving three factors and three levels, the number of experiments conducted was less than a central composite design.48 The three independent variables, the extraction temperature (X1), extraction time (X2), and pressure (X3), were optimized by the BBD in order to obtain the maximum polysaccharide yield (Y). Each independent variable for X1, X2, and X3 were evaluated at three levels, see Table 1. The experimental design totaled 15 runs, including the three replicates at the center point. All experiments were performed at random. The experiments were performed in duplicates and the average polysaccharide yield was used as the response.
Table 1. Box–Behnken Design of Three Variables with Polysaccharide Yields.
| parameters and levels |
||||
|---|---|---|---|---|
| run | X1: temperature (°C) | X2: time (min) | X3: pressure (MPa) | polysaccharide yield (%) |
| 1 | 120 | 12.5 | 1.5 | 16.12 |
| 2 | 140 | 12.5 | 1.0 | 20.11 |
| 3 | 120 | 20.0 | 1.0 | 19.28 |
| 4 | 140 | 12.5 | 1.0 | 19.32 |
| 5 | 140 | 20.0 | 1.5 | 17.24 |
| 6 | 160 | 20.0 | 1.0 | 17.16 |
| 7 | 140 | 20.0 | 0.5 | 9.63 |
| 8 | 160 | 12.5 | 1.5 | 15.34 |
| 9 | 140 | 5.0 | 1.5 | 12.88 |
| 10 | 120 | 12.5 | 0.5 | 11.08 |
| 11 | 120 | 5.0 | 1.0 | 17.95 |
| 12 | 140 | 5.0 | 0.5 | 9.89 |
| 13 | 160 | 12.5 | 0.5 | 7.49 |
| 14 | 160 | 5.0 | 1.0 | 17.56 |
| 15 | 140 | 12.5 | 1.0 | 20.30 |
2.5.2. Statistical Analysis
The BBD experimental data were analyzed. Analysis of variance (ANOVA) was performed to determine the adequacy of the developed model and the statistical significance of the regression coefficients.49 The second-order polynomial coefficients were calculated and analyzed using a Design-Expert 7.1.6 Trial (State-Ease, Inc., Minneapolis MN, USA). Statistical significance was considered at P < 0.05.50
2.6. Modeling of the Extraction Process
The kinetic model enabled the exploration of the SWE process and predicted the experimental results.51 A pseudo-first-order kinetic model was employed to fit the experimental data. The rate constant was defined as the slope of the plot for the equation of the first-order reaction52,53
| 1 |
where C∞ was the equilibrium concentration of the polysaccharide in the solution (g/L) at infinite time (t = ∞), C was the concentration of the polysaccharide in the solution (g/L) at time t, kobs was the rate constant (min–1) of the extraction process, and a was the constant for the washing step of the polysaccharide extraction. If the sample was removed after each time interval, the total volume of solution could change. The maximum yield (Ym) of the given experiments replaced C∞, where eq 1 becomes54,55
| 2 |
Equation 2 was used to fit the experimental data and to obtain Ym and k values.
The stirring extraction, refluxing extraction, ultrasound extraction, and SWE were used to extract polysaccharides from the D. nobile Lindl. A series of experiments D. nobile Lindl. (particle sizes < 0.45 mm) were performed. They were prepared by water immersion to obtain a solvent/solid ratio of 1:25 (g/mL), where eight mixtures were extracted for 5, 10, 30, 50, 70, 110, 150, and 190 min. (1) The D. nobile Lindl. powder was then put in a conical flask with 60 °C water under magnetic stirring for the stirring reaction. (2) The D. nobile Lindl. powder was loaded in a round-bottom flask with water, and then heated to 90 °C in an oil bath under reflux for the refluxing extraction. (3) Ultrasound extraction was performed by placing the D. nobile Lindl. powder and water in an ultrasonic cleaner (KQ-300E, Kunshan ultrasonic instrument Co., Ltd., Kunshan, China) with a frequency of 40 KHz and a nominal power of 300 W at 60 °C. (4) The D. nobile Lindl. powder and water were placed in a high-pressure batch reactor for the SWE (see the section of 2.2). The operating pressure was 1.0 MPa and extraction temperature was 130 °C. Each sample was paper-filtered, the filtrate was obtained, and then analyzed.
2.7. Analysis of Molecular Weight and Monosaccharide Compositions
The molecular weights of the polysaccharides were determined by the gel permeation chromatography (GPC), using previous methodology,56 in combination with a Agilent 1200 HPLC equipped with a PL aquagel-OH MIXED-H 8 μm column (300 × 7.5 mm, Polymer Laboratories Ltd.). The detection was achieved with a Knauer differential refractometer. The column temperature was maintained at 30 °C. The eluent was 0.02 N NaCl in 5 mM sodium phosphate buffer (pH 7.50), with a flow rate of 0.60 mL/min. The PL pullulan polysaccharide standards (peak average molecular weights 738, 12 200, 100 000, 1 600 000, Polymer Laboratories Ltd.) were used to obtain the calibration curve. The polysaccharide sample was dissolved with 0.02 N NaCl in 5 mM sodium phosphate buffer (pH 7.50) with a concentration of 2 mg/mL and filtered through a 0.45 μm filter membrane prior to analysis. The molecular weight distribution of the samples was calculated according to the calibration curve.
The samples were hydrolyzed with 1.0 M H2SO4 at 105 °C for 2.5 h for the monosaccharide composition analysis. Following hydrolysis, the samples were filtered and diluted to 50-fold, and then analyzed via high-performance anion-exchange chromatography (HPAEC) using a Dionex ICS3000 gradient pump, amperometric detector, AS50 autosampler, a Carbopac PA-20 column (4 × 250 mm, Dionex), and a guard PA-20 column (3 × 30 mm, Dionex). Monosaccharides were separated in carbonate-free 18 mM NaOH under a N2 atmosphere with a postcolumn addition of 0.3 M NaOH at a rate of 0.5 mL/min. The running time was 45 min, followed by 10 min elution with 0.2 M NaOH to wash the column. An additional 15 min elution was performed with 18 mM NaOH to re-equilibrate the column. The calibration was performed with standard solutions of l-rhamnose, l-arabinose, d-glucose, d-xylose, d-mannose, d-galactose, glucuronic acid, and galacturonic acid.
2.8. Antioxidant Activity Assay
2.8.1. Hydroxyl Radical Scavenging Ability
The hydroxyl radical (•OH) scavenging ability of the purified polysaccharides was measured in accordance with previous methods.56 Various concentrations (0–2 mg/mL) were incubated with 2 mmol/L FeSO4 (1 mL), 0.1% H2O2 (1.0 mL), and 1 mL of 6 mmol/L salicylic acid (dissolved with alcohol) for 30 min at 37 °C. The hydroxyl radical was detected by monitoring the absorbance at 510 nm. The hydroxyl radical scavenging effect was calculated as follows: scavenging effect (%) = [1 – (Abs. of sample – Abs. of control)/Abs. of blank] × 100%. Salicylic acid was substituted with distilled water for the control. Ascorbic acid was used as the positive control. Lentinan extracted from mushroom was used as a comparison of other polysaccharide extracts.
2.8.2. ABTS Radical Scavenging Activity
The antioxidant activity was determined via 2,2′-azinobis[3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS) radical cations as reported by Fan et al.,57 with some modifications. The ABTS radical cation was produced by reacting 5 mL of 7 mmol/L ABTS diammonium salt with 0.088 mL of 140 mmol/L potassium persulfate. The mixture was placed in the dark for 24 h. When ready for use, the ABTS solution was diluted with distilled water to an absorbance of 0.70 ± 0.02 at 734 nm. Each sample (0.2 mL) with various concentrations (0.0625–2 mg/mL) was added to 3 mL of the diluted ABTS solution and mixed vigorously. The reaction mixture was allowed to stand at 25 °C for 60 min. The absorbance at 734 nm was quickly recorded. The ABTS scavenging effect was calculated as follows: effect (%) = [1 – (Abs. of sample – Abs. of control)/Abs. of blank] × 100%. Ascorbic acid was used as the positive control. Distilled water was substituted for the ABTS-diluted solution for the control. Distilled water was used instead of the sample for the blank. Lentinan extracted from mushroom was used as a comparison of other polysaccharide extracts.
3. Results and Discussion
3.1. Single Factor Experimental Analysis
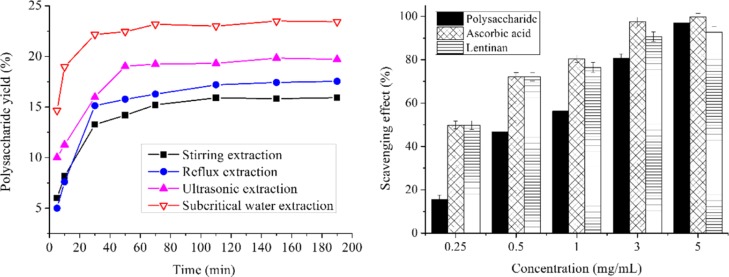
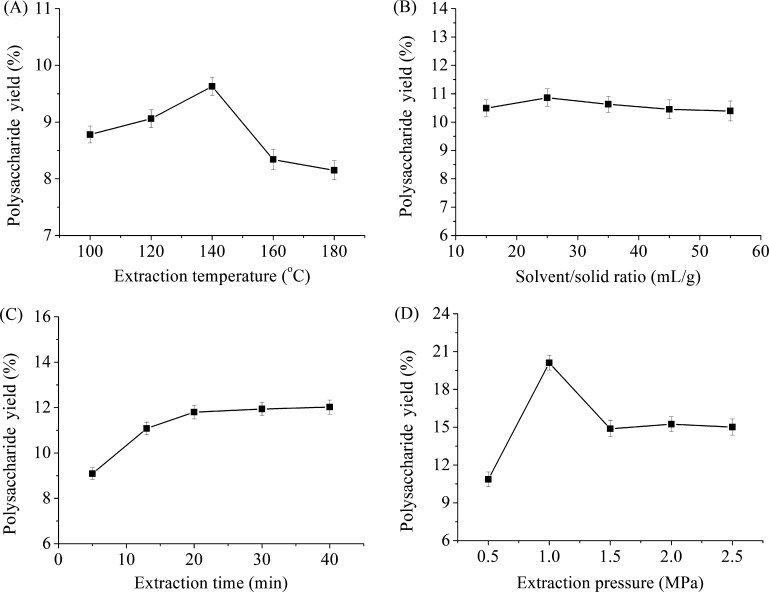
Effects of extraction temperature (°C), solvent/solid ratio (mL/g), extraction time (min), and extraction pressure (MPa) on polysaccharide yield (%) are shown in Figure 2. In Figure 2A, extractions are carried over varied intervals from 100 to 180 °C, while other extraction variables are set as follows: solvent/solid ratio at 15:1 (mL/g), extraction time at 12.5 min, and extraction pressure at 0.5 MPa. Results demonstrate that the maximum polysaccharide yield (9.63%) was obtained at 140 °C, from which point the yield began to decrease because of the partial degradation of polysaccharides. It is also observed in the SWE of other natural product.58,59 The effect of the solvent/solid ratio on the polysaccharide yield was explored from 15:1 to 55:1 (mL/g) with an extraction temperature of 140 °C, extraction time at 12.5 min, and extraction pressure at 0.5 MPa. As shown in Figure 2B, the increased solvent/solid ratio had no obvious effect on polysaccharides yield. This indicates that solvent/solid ratio at 25:1 (mL/g) is sufficient to obtain high polysaccharides yield. Thus, the solvent/solid ratio was not a variable tested in future experiments. It is known that a short extraction time is beneficial to reduce the costs for industrial applications. As shown in Figure 2C, the maximum polysaccharide yield was obtained at 11.80% during a 12.5 min extraction interval, from which point the yield began to increase slightly. This might be explained by the research that active component yields will not continue to increase once equilibrium is reached.60 Hence, 12.5 min was a suitable time for the extraction of polysaccharides from D. nobile Lindl. As detailed in Figure 2D (all other conditions fixed as described above), polysaccharide yields increased with increasing extraction pressure to 1.0 MPa, reaching their maximum yields (20.11%) when the temperature was 140 °C, solvent/solid ratio was 25:1 (mL/g), and time was 12.5 min. Yield of polysaccharides dropped when the pressure was more than 1.5 MPa, it may due to the destruction of the cellular structures of D. nobile Lindl. by heat and pressure and some dissolution of unfavorable ingredients.61 Thus, extraction pressure of 1.0 MPa was applicable. According to the results of a single-factor study, an extraction temperature of 120–160 °C, under an extraction time of 5–20 min, and an extraction pressure of 0.5–1.5 MPa were adopted for RSM experiments.
Figure 2.
Influence of different extraction factors on polysaccharide yield ((A) extraction temperature; (B) solvent/solid ratio; (C) extraction time; and (D) extraction pressure). Values are means ± SD, n = 3.
3.2. Optimization of SWE Conditions
3.2.1. Box–Behnken Design
The Box–Behnken statistical design with 3 factors (temperature, time, and pressure), over 3 levels and 15 runs, was selected for the optimization test. The observed responses (polysaccharide yield) are given in Table 1. The batch runs were performed with the BBD-designed experimental conditions, to visualize the effects that the independent factors had on the responses and on the results, for each experimental condition.62 As the extraction temperature increased, the time and the pressure enhanced the polysaccharide yield. The results showed a wide range of differences between the polysaccharide yield. The maximum polysaccharide yield of 20.3% was achieved at 140 °C, for 12.5 min, and with 1.0 MPa (under conditions of run 15). The response given in Table 1 correlated to the three independent variables using a polynomial equation. The least squares regression was used to fit the data to eq 3. The best fit models in the coded factors were follows63
| 3 |
where Y represented the multiple response, A was the temperature, B was the time, and C was the pressure.
The three-dimensional response surfaces were constructed from the BBD data. Figure 3 shows the interactions between the two variables by maintaining the third variable at level zero. The three-dimensional plots provided a visual interpretation of the interaction between two variables and facilitated the quantification of the optimum experimental conditions.64 As seen in Figure 3a,c, the extraction temperature exhibited an important effect on the polysaccharide yield. The polysaccharide yield increased as the temperature increased to nearly 130 °C but as the temperature rose further, there was a gradual decrease in the polysaccharide yield because high temperature affected the polarity of subcritical water. This affected the solubility of the polysaccharides, which could result in the destruction of the polysaccharide’s structure and lead to degradation.42 Morales et al. also noticed the degradation of polysaccharides obtained from mushrooms.65 This indicated that temperature plays an important role in the SWE extraction of polysaccharide. The effect that the extraction time had on the polysaccharide yield is presented in Figure 3a,b. The extraction yield increased when the time increased from 5 to 16.71 min, although increasing the time further only increased the extraction yield slightly. Extraction time did not demonstrate a significant effect on the polysaccharide yield. Figure 3b,c shows that the polysaccharide yield increased as the extraction pressure increased to the optimum level (1.12 MPa), and then decreased as the factor increased further. The higher pressure more easily destroyed the plant cell wall, which would increase the diffusion of the polysaccharides, although a much higher pressure caused the material to become too compact and the material in the middle of the vessel could not be in full contact with the water, thus decreasing the extraction yield.42
Figure 3.
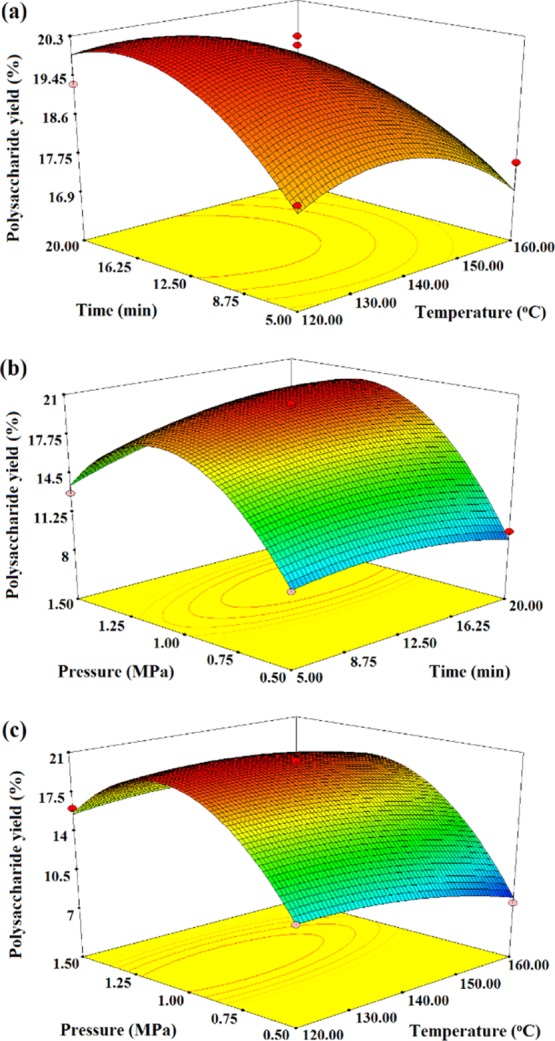
Response surfaces obtained from the BBD to the (a) temperature and time; (b) time and pressure; and (c) temperature and pressure.
The optimum conditions obtained by the BBD were as follows: temperature = 129.83 °C, time = 16.71 min, and pressure = 1.12 MPa. The maximum predicted that the polysaccharide yield was 20.67%, which corresponded fairly well to the real extraction yield (21.88%). This result indicated that the developed mathematical model was both accurate and adequate for predicting the polysaccharide extraction yield.
3.2.2. Analysis of Variance
The ANOVA test results are presented in Table 2. The model was determined to be adequate. The model F value of 46.83 demonstrated that the model was significant. There was a 0.03% chance that this large of a model F value could be due to noise. The values of “Prob > F” less than 0.0500 (0.0003) indicated that the model terms were significant. The model’s determination coefficient R2 was 0.9883, suggesting that 98.83% of the variability in the response was explained by the model. The lack of fit (2.89) was not significant because the P-value was greater than 0.0500 (0.2673).66 The P-value was used as a tool to confirm the significance of each coefficient, which could indicate the pattern of the interaction between the variables.64 The smaller the P-value was, the more significant the corresponding coefficient was. X1, X3, X2X3, and X32 were significant (P < 0.05), while X2, X1X2, X1X3, X12, and X22 were not significant (P > 0.05). The model obtained fit well with the BBD data.
Table 2. ANOVA of the Quadratic Model for the Polysaccharide Yield.
| source | sum of squares | degrees of freedom | mean square | F value | p-value Prob > F |
|---|---|---|---|---|---|
| model | 243.13 | 9 | 27.01 | 46.83 | 0.0003 |
| X1 | 5.92 | 1 | 5.92 | 10.26 | 0.0239 |
| X2 | 3.16 | 1 | 3.16 | 5.48 | 0.0663 |
| X3 | 68.97 | 1 | 68.97 | 119.57 | 0.0001 |
| X1X2 | 0.75 | 1 | 0.75 | 1.30 | 0.3064 |
| X1X3 | 1.97 | 1 | 1.97 | 3.42 | 0.1236 |
| X2X3 | 5.34 | 1 | 5.34 | 9.25 | 0.0287 |
| X12 | 3.07 | 1 | 3.07 | 5.33 | 0.0690 |
| X22 | 3.77 | 1 | 3.77 | 6.53 | 0.0509 |
| X32 | 155.52 | 1 | 155.52 | 269.60 | <0.0001 |
| residual | 2.88 | 5 | 0.58 | ||
| lack of fit | 2.34 | 3 | 0.78 | 2.89 | 0.2673 |
| pure error | 0.54 | 2 | 0.27 | ||
| total | 246.01 | 14 |
3.3. Extraction with Different Methods
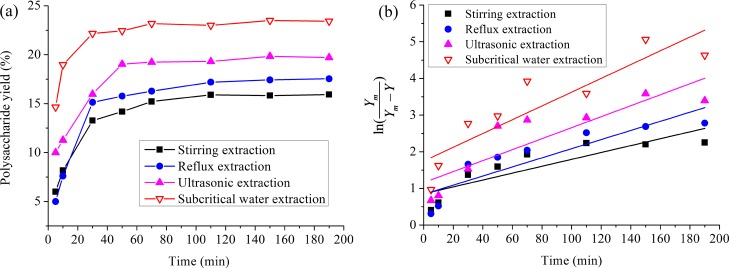
Figure 4 shows the
extraction time-dependent yield data (a) and the 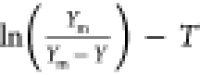 data (b) of the D. nobile Lindl. polysaccharide over the stirring extraction, the refluxing
extraction, the ultrasound extraction, and the SWE methods. As shown
in Figure 4a, the polysaccharide
yields rose observably as the extraction time increased from 5 to
30 min, although the yields did not have a clear change at 50 min.
This was most likely due to the attainment of dynamic equilibrium
between the internal and external particles during the period between
50 and 190 min. The extraction time at their maximum polysaccharide
yields were approximately the same (50 min), although the polysaccharide
yields were different. The polysaccharide yields decreased in the
following order: SWE > ultrasonic extraction > refluxing extraction
> stirring extraction. The corresponding polysaccharide yield at 190
min was 23.42, 19.72, 17.54, and 15.93%. The results indicated that
the polysaccharide did not reach sufficient extraction levels via
the latter three methods. The plot for eq 2 is presented in Figure 4b, where Table 3 provides the experimental estimate for the k-value of the kinetic parameters, which was highest for
SWE, followed by ultrasound extraction and refluxing extraction, and
the stirring extraction had the lowest yield. The results confirmed
that the SWE at a certain pressure and temperature could change the
water density, thus improving the solvency of the water and gaining
additional water-soluble organic compounds.42 The SWE of the D. nobile Lindl. polysaccharide
was highly effective. The R2 value in Table 3 suggests that the
model correlated well with all of the experimental data, indicating
that the model was appropriate for the analysis of extraction processes
on natural extracts.
data (b) of the D. nobile Lindl. polysaccharide over the stirring extraction, the refluxing
extraction, the ultrasound extraction, and the SWE methods. As shown
in Figure 4a, the polysaccharide
yields rose observably as the extraction time increased from 5 to
30 min, although the yields did not have a clear change at 50 min.
This was most likely due to the attainment of dynamic equilibrium
between the internal and external particles during the period between
50 and 190 min. The extraction time at their maximum polysaccharide
yields were approximately the same (50 min), although the polysaccharide
yields were different. The polysaccharide yields decreased in the
following order: SWE > ultrasonic extraction > refluxing extraction
> stirring extraction. The corresponding polysaccharide yield at 190
min was 23.42, 19.72, 17.54, and 15.93%. The results indicated that
the polysaccharide did not reach sufficient extraction levels via
the latter three methods. The plot for eq 2 is presented in Figure 4b, where Table 3 provides the experimental estimate for the k-value of the kinetic parameters, which was highest for
SWE, followed by ultrasound extraction and refluxing extraction, and
the stirring extraction had the lowest yield. The results confirmed
that the SWE at a certain pressure and temperature could change the
water density, thus improving the solvency of the water and gaining
additional water-soluble organic compounds.42 The SWE of the D. nobile Lindl. polysaccharide
was highly effective. The R2 value in Table 3 suggests that the
model correlated well with all of the experimental data, indicating
that the model was appropriate for the analysis of extraction processes
on natural extracts.
Figure 4.
Time-dependent yield data (a) and 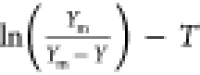 data (b) of polysaccharide from D. nobile Lindl. at different conditions.
data (b) of polysaccharide from D. nobile Lindl. at different conditions.
Table 3. k and R2 Values for Different Extraction Methods.
| extraction methods | stirring extraction | reflux extraction | ultrasonic extraction | SWE |
|---|---|---|---|---|
| k (1/min) | 0.00938 | 0.01243 | 0.01498 | 0.01879 |
| R2 | 0.86688 | 0.89110 | 0.88137 | 0.89913 |
3.4. Molecular Weight and Monosaccharide Composition of Polysaccharides
The average molecular weights of the D. nobile Lindl. polysaccharides obtained via different extraction methods were calculated at 117.09, 86.72, 103.46, and 85.72 kDa, and the chromatograms are shown in Figure S1 (see Supporting Information). The polysaccharides had no absorbance at 280 nm, suggesting that these polysaccharides did not contain protein. As seen in Table 4, the polysaccharides extracted via the SWE have the lowest Mw 85.72 × 103. This demonstrated that the polysaccharides from the SWE had the highest antioxidant activity. Zha et al. indicated that higher antioxidant activities of polysaccharides are found as the molecular weight decreases.67 The polysaccharide that was extracted via SWE had the lowest Mw/Mn value (60.37), indicating that the polysaccharide had a relatively low index of polydispersity and a relatively narrow molecular weight distribution. The molecular weight changes could due to the hot compressed water extraction.
Table 4. Molecular Weight and Monosaccharide Composition of Polysaccharide from D. nobile Lindl. with Different Extraction Methods.
| sample | stirring extraction | reflux extraction | ultrasonic extraction | SWE |
|---|---|---|---|---|
| Mw × 103 (g/mol) | 117.09 | 86.72 | 103.46 | 85.72 |
| Mn × 103 (g/mol) | 0.815 | 0.48 | 0.97 | 1.42 |
| arabinose (%) | 1.03 | 1.83 | 0.97 | 1.05 |
| galactose (%) | 1.45 | 2.43 | 1.5 | 1.6 |
| glucose (%) | 54.28 | 46.98 | 54.84 | 48.63 |
| mannose (%) | 43.25 | 48.76 | 42.7 | 48.73 |
HPAEC analysis of the monosaccharide composition demonstrated that the polysaccharide that was extracted by the SWE was composed of l-arabinose, d-galactose, d-glucose, and d-mannose with a 1.05:1.6:48.63:48.73 ratio. Figure S2 (see Supporting Information) and Table 4 show that the monosaccharide composition of the D. nobile Lindl. polysaccharide obtained via various extraction methods were similar.
3.5. Antioxidant Activity Results
3.5.1. Scavenging Effect on the Hydroxyl Radical
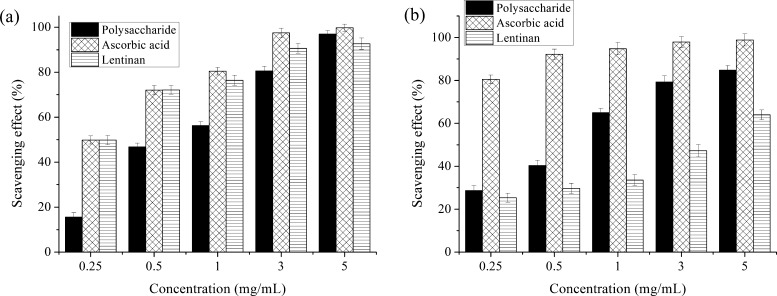
The hydroxyl radical is the most active of the oxygen radicals, where it induces severe damage to the adjacent biomolecules.68 As seen in Figure 5a, the hydroxyl radical scavenging activity of the polysaccharide is measured at various concentrations (0.25–5 mg/mL), with ascorbic acid as the positive control. These results suggested that the polysaccharide activity gradually rose as the concentrations increased. The ascorbic acid showed an excellent scavenging effect within 0.25–5 mg/mL. The polysaccharide and lentinan extracted from mushroom both exhibited high scavenging effects on the hydroxyl radical. The effect obtained 96.98 and 92.70% at 5 mg/mL, respectively, which was close to the ascorbic acid (99.74%). The results indicated that the D. nobile Lindl. polysaccharide displayed a strong scavenging power for the hydroxyl radical.
Figure 5.
Scavenging effect of polysaccharide, ascorbic acid, and lentinan on hydroxyl radical (a) and ABTS radical (b). Values are means ± SD, n = 3.
3.5.2. Scavenging Effect on ABTS Radical
The ABTS assay is often used when evaluating the total antioxidant power of single compounds and complex mixtures of natural products.69 As Figure 5b shows, the polysaccharide, ascorbic acid, and lentinan extracted from mushroom demonstrated dose-dependent activities. The rate of polysaccharides, ascorbic acid, and lentinan scavenged ABTS radicals was 84.76, 98.85, and 63.96% at 5 mg/mL, respectively. The ABTS radical scavenging activity of the polysaccharide was inferior than the ascorbic acid but superior than lentinan. These results suggested that polysaccharide from different natural sources showed different radical scavenging abilities. In a word, the D. nobile Lindl. polysaccharide could be explored as novel potential antioxidants because of the remarkable antioxidant activity with a scavenging range between 28.66 and 84.76%.
4. Conclusions
SWE is a green process that was demonstrated to be an excellent method for D. nobile Lindl polysaccharide extraction. Single factor experiments and BBD of RSM were used to identify the optimal experimental conditions to be a time and pressure of 129.83 °C, 16.71 min, and 1.12 MPa. The maximal polysaccharide yield reached 21.88%. The polysaccharide was quantitatively determined via UV–vis spectrophotometry, where the results were compared with polysaccharides obtained by other methods (stirring extraction, refluxing extraction, ultrasound extraction, and SWE methods). The extraction processes were modeled and analyzed. The model had a good correlation between the experimental data and was determined to be fit for the comparison of the polysaccharide extraction. The polysaccharide extracted via SWE was composed of l-arabinose, d-galactose, d-glucose, and d-mannose at a 1.05:1.6:48.63:48.73 ratio. The average molecular weight was 85.72 kDa. The antioxidant activity assay demonstrated that the SWE had little effect on the medicinal properties of the polysaccharide. The rate of polysaccharide scavenging of hydroxyl radicals and the ABTS radical at 5.00 mg/mL were 96.98 and 84.76%. These results demonstrated that the SWE was an efficient and quick method for extracting polysaccharides and has potential as a useful technique for extracting other natural extracts.
Acknowledgments
The authors are extremely grateful to the Fundamental Research Funds for the Central Universities (2018ZY05), National Natural Science Foundation of China (21808014), and Beijing excellent talent training (Youth backbone individual project 2017).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02550.
Additional images of GPC of polysaccharides and high-performance anion-exchange chromatographies of monosaccharide composition (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang Y.; Wang H.; Mei N.; Ma C.; Lou Z.; Lv W.; He G. Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol. 2018, 107, 230–235. 10.1016/j.ijbiomac.2017.08.175. [DOI] [PubMed] [Google Scholar]
- Xu J.; Han Q.-B.; Li S.-L.; Chen X.-J.; Wang X.-N.; Zhao Z.-Z.; Chen H.-B. Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. 10.1007/s11101-013-9310-8. [DOI] [Google Scholar]
- Ng T. B.; Liu J.; Wong J. H.; Ye X.; Wing Sze S. C.; Tong Y.; Zhang K. Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. 10.1007/s00253-011-3829-7. [DOI] [PubMed] [Google Scholar]
- Teixeira da Silva J. A.; Ng T. B. The medicinal and pharmaceutical importance of Dendrobium species. Appl. Microbiol. Biotechnol. 2017, 101, 2227–2239. 10.1007/s00253-017-8169-9. [DOI] [PubMed] [Google Scholar]
- Li X.-L.; Xiao J.-J.; Zha X.-Q.; Pan L.-H.; Asghar M.-N.; Luo J.-P. Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohydr. Polym. 2014, 106, 247–254. 10.1016/j.carbpol.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Huang K.; Li Y.; Tao S.; Wei G.; Huang Y.; Chen D.; Wu C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. 10.3390/molecules21060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J.; Jiang L. S.; Zhang Y.; Tian Y.; Li L. S.; Lu Y. L.; Yang W. J.; Shi J. S. Dendrobium nobile Lindl. Alkaloids Decreases the Level of Intracellular beta-Amyloid by Improving Impaired Autolysosomal Proteolysis in APP/PS1 Mice. Front. Pharmacol. 2018, 9, 1479. 10.3389/fphar.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Wu Q.; Liu H.; Ling H.; He Y.; Wang C.; Wang Z.; Lu Y.; Lu Y. Alkaloids of dendrobium nobile lindl. Altered hepatic lipid homeostasis via regulation of bile acids. J. Ethnopharmacol. 2019, 241, 111976. 10.1016/j.jep.2019.111976. [DOI] [PubMed] [Google Scholar]
- Klongkumnuankarn P.; Busaranon K.; Chanvorachote P.; Sritularak B.; Jongbunprasert V.; Likhitwitayawuid K. Cytotoxic and antimigratory activities of phenolic compounds from Dendrobium brymerianum. Evid. Based Complement. Alternat. Med. 2015, 2015, 350410. 10.1155/2015/350410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Zhang S.; Gao B.; Qian Z.; Liu J.; Wu S.; Si J. Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of Dendrobium catenatum flowers and selection of quality control herb-markers. Food Res. Int. 2019, 123, 732–745. 10.1016/j.foodres.2019.05.040. [DOI] [PubMed] [Google Scholar]
- Hwang J. S.; Lee S. A.; Hong S. S.; Han X. H.; Lee C.; Kang S. J.; Lee D.; Kim Y.; Hong J. T.; Lee M. K.; Hwang B. Y. Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Biorg. Med. Chem. Lett. 2010, 20, 3785–3787. 10.1016/j.bmcl.2010.04.054. [DOI] [PubMed] [Google Scholar]
- Chaotham C.; Pongrakhananon V.; Sritularak B.; Chanvorachote P. A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 2014, 34, 1931–1938. [PubMed] [Google Scholar]
- Zhang Y.-Y.; Wang P.; Song X.-Q.; Zuo W.-J.; Wang H.; Chen L.-L.; Mei W.-L.; Dai H.-F. Chemical constituents from Dendrobium hainanense. J. Asian Nat. Prod. Res. 2019, 21, 873–880. 10.1080/10286020.2018.1475476. [DOI] [PubMed] [Google Scholar]
- Tang H.; Zhao T.; Sheng Y.; Zheng T.; Fu L.; Zhang Y. Dendrobium officinale Kimura et Migo: a review on its ethnopharmacology, phytochemistry, pharmacology, and industrialization. Evid. Based Complement. Alternat. Med. 2017, 2017, 7436259. 10.1155/2017/7436259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakova V.; Bonte F.; Lobstein A. Dendrobium: sources of active ingredients to treat age-related pathologies. Aging Dis 2017, 8, 827. 10.14336/ad.2017.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wang J. H.; Luo J. P.; Zhang Q.; Lu J. The Structure-Activity Relationship and Molecular Mechanism of Anti-tumor Polysaccharide Isolated from Dendrobium Nobile Lindl. Curr. Top. Nutraceutical Res. 2019, 17, 153–163. [Google Scholar]
- Li Z.; Shi J.; Hu D.; Song B. A polysaccharide found in Dendrobium nobile Lindl stimulates calcium signaling pathway and enhances tobacco defense against TMV. Int. J. Biol. Macromol. 2019, 137, 1286. 10.1016/j.ijbiomac.2019.06.179. [DOI] [PubMed] [Google Scholar]
- Yang L.-C.; Lu T.-J.; Hsieh C.-C.; Lin W.-C. Characterization and immunomodulatory activity of polysaccharides derived from Dendrobium tosaense. Carbohydr. Polym. 2014, 111, 856–863. 10.1016/j.carbpol.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Lee C.-T.; Kuo H.-C.; Chen Y.-H.; Tsai M.-Y. Current Advances in the Biological Activity of Polysaccharides in Dendrobium with Intriguing Therapeutic Potential. Curr. Med. Chem. 2018, 25, 1663–1681. 10.2174/0929867324666170227114648. [DOI] [PubMed] [Google Scholar]
- Gao S.; Tang G.; Hua D.; Xiong R.; Han J.; Jiang S.; Zhang Q.; Huang C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. 10.1039/c8tb02491j. [DOI] [PubMed] [Google Scholar]
- Lv D.; Zhu M.; Jiang Z.; Jiang S.; Zhang Q.; Xiong R.; Huang C. Green Electrospun Nanofibers and Their Application in Air Filtration. Macromol. Mater. Eng. 2018, 303, 1800336. 10.1002/mame.201800336. [DOI] [Google Scholar]
- Wang J.-H.; Zha X. Q.; Pan L. H.; Luo J. P. Structural Characterization of an Immunoactive Polysaccharide DNP-W1B from Dendrobium nobile Lindl. Chem. Res. Chin. Univ. 2013, 34, 881–885. 10.7503/cjcu20120883. [DOI] [Google Scholar]
- Wang J.-H.; Zha X.-Q.; Luo J.-P.; Yang X.-F. An acetylated galactomannoglucan from the stems of Dendrobium nobile Lindl. Carbohydr. Res. 2010, 345, 1023–1027. 10.1016/j.carres.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Wang J.-H.; Luo J.-P.; Yang X.-F.; Zha X.-Q. Structural analysis of a rhamnoarabinogalactan from the stems of Dendrobium nobile Lindl. Food Chem. 2010, 122, 572–576. 10.1016/j.foodchem.2010.03.012. [DOI] [Google Scholar]
- Wang J.-H.; Zuo S. R.; Luo J. P. Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl. Molecules 2017, 22, 611. 10.3390/molecules22040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-H.; Luo J.-P.; Zha X.-Q. Structural features of a pectic polysaccharide from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 81, 1–7. 10.1016/j.carbpol.2010.01.040. [DOI] [Google Scholar]
- Wang J.-H.; Luo J.-P.; Zha X.-Q.; Feng B.-J. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 114–118. 10.1016/j.carbpol.2009.07.032. [DOI] [Google Scholar]
- Li Z.; Shi J.; Hu D.; Song B. A polysaccharide found in Dendrobium nobile Lindl stimulates calcium signaling pathway and enhances tobacco defense against TMV. Int. J. Biol. Macromol. 2019, 137, 1286–1297. 10.1016/j.ijbiomac.2019.06.179. [DOI] [PubMed] [Google Scholar]
- Wu Y.-G.; Wang K.-W.; Zhao Z.-R.; Zhang P.; Liu H.; Zhou G.-J.; Cheng Y.; Wu W.-J.; Cai Y.-H.; Wu B.-L.; Chen F.-Y. A novel polysaccharide from Dendrobium devonianum serves as a TLR4 agonist for activating macrophages. Int. J. Biol. Macromol. 2019, 133, 564–574. 10.1016/j.ijbiomac.2019.04.125. [DOI] [PubMed] [Google Scholar]
- He T.-B.; Huang Y.-P.; Yang L.; Liu T.-T.; Gong W.-Y.; Wang X.-J.; Sheng J.; Hu J.-M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. 10.1016/j.ijbiomac.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Wu F.; Zhou C.; Zhou D.; Ou S.; Huang H. Structural characterization of a novel polysaccharide fraction from Hericium erinaceus and its signaling pathways involved in macrophage immunomodulatory activity. J. Funct. Foods 2017, 37, 574–585. 10.1016/j.jff.2017.08.030. [DOI] [Google Scholar]
- Luo A.; He X.; Zhou S.; Fan Y.; He T.; Chun Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. 10.1016/j.ijbiomac.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Luo A.; He X.; Zhou S.; Fan Y.; Luo A.; Chun Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. 10.1016/j.carbpol.2009.10.033. [DOI] [Google Scholar]
- Luo A.; Fan Y. Immune stimulating activity of water-soluble polysaccharide fractions from Dendrobium nobile Lindl. Afr. J. Pharm. Pharmacol. 2011, 5, 625–631. 10.5897/ajpp11.169. [DOI] [Google Scholar]
- Pan L.-H.; Li X.-F.; Wang M.-N.; Zha X.-Q.; Yang X.-F.; Liu Z.-J.; Luo Y.-B.; Luo J.-P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. 10.1016/j.ijbiomac.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Kanmaz E. Ö. Subcritical water extraction of phenolic compounds from flaxseed meal sticks using accelerated solvent extractor (ASE). Eur. Food Res. Technol. 2014, 238, 85–91. 10.1007/s00217-013-2088-5. [DOI] [Google Scholar]
- Yan J.-K.; Wu L.-X.; Cai W.-D.; Xiao G.-S.; Duan Y.; Zhang H. Subcritical water extraction-based methods affect the physicochemical and functional properties of soluble dietary fibers from wheat bran. Food Chem. 2019, 298, 124987. 10.1016/j.foodchem.2019.124987. [DOI] [PubMed] [Google Scholar]
- Chen H.-m.; Fu X.; Luo Z.-g. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. 10.1016/j.foodchem.2014.07.078. [DOI] [PubMed] [Google Scholar]
- Nkurunziza D.; Pendleton P.; Chun B. S. Optimization and kinetics modeling of okara isoflavones extraction using subcritical water. Food Chem. 2019, 295, 613–621. 10.1016/j.foodchem.2019.05.129. [DOI] [PubMed] [Google Scholar]
- Islam M. N.; Shin M.-S.; Jo Y.-T.; Park J.-H. TNT and RDX degradation and extraction from contaminated soil using subcritical water. Chemosphere 2015, 119, 1148–1152. 10.1016/j.chemosphere.2014.09.101. [DOI] [PubMed] [Google Scholar]
- Wang X.; Chen Q.; Lü X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocolloids 2014, 38, 129–137. 10.1016/j.foodhyd.2013.12.003. [DOI] [Google Scholar]
- Chao Z.; Ri-fu Y.; Tai-qiu Q. Ultrasound-enhanced subcritical water extraction of polysaccharides from Lycium barbarum L. Sep. Purif. Technol. 2013, 120, 141–147. 10.1016/j.seppur.2013.09.044. [DOI] [Google Scholar]
- Cheigh C.-I.; Yoo S.-Y.; Ko M.-J.; Chang P.-S.; Chung M.-S. Extraction characteristics of subcritical water depending on the number of hydroxyl group in flavonols. Food Chem. 2015, 168, 21–26. 10.1016/j.foodchem.2014.07.047. [DOI] [PubMed] [Google Scholar]
- Kim S.-W.; Ko M.-J.; Chung M.-S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Cleaner Prod. 2019, 231, 1192–1199. 10.1016/j.jclepro.2019.05.280. [DOI] [Google Scholar]
- Zhao T.; Luo Y.; Zhang X.; Zhang W.; Qu H.; Mao G.; Zou Y.; Wang W.; Li Q.; Chen Y.; Feng W.; Yang L.; Wu X. Subcritical water extraction of bioactive compounds from Radix Puerariae and optimization study using response surface methodology. Chem. Eng. Commun. 2019, 206, 1218–1227. 10.1080/00986445.2018.1555529. [DOI] [Google Scholar]
- Dubois M.; Gilles K. A.; Hamilton J. K.; Rebers P. A.; Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. 10.1021/ac60111a017. [DOI] [Google Scholar]
- Swami R.; Singh I.; Jeengar M. K.; Naidu V. G. M.; Khan W.; Sistla R. Adenosine conjugated lipidic nanoparticles for enhanced tumor targeting. Int. J. Pharm. 2015, 486, 287–296. 10.1016/j.ijpharm.2015.03.065. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. Application of Box-Behnken design for fabrication of titanium alloy and 304 stainless steel joints with silver interlayer by diffusion bonding. Mater. Des. 2015, 77, 161–169. 10.1016/j.matdes.2015.04.003. [DOI] [Google Scholar]
- Gu T.; Chen Z.; Jiang X.; Zhou L.; Liao Y.; Duan M.; Wang H.; Pu Q. Synthesis and inhibition of N-alkyl-2-(4-hydroxybut-2-ynyl) pyridinium bromide for mild steel in acid solution: Box-Behnken design optimization and mechanism probe. Corros. Sci. 2015, 90, 118–132. 10.1016/j.corsci.2014.10.004. [DOI] [Google Scholar]
- Wang Z.; Zhao Y.; Su T. Extraction and antioxidant activity of polysaccharides from Rana chensinensis skin. Carbohydr. Polym. 2015, 115, 25–31. 10.1016/j.carbpol.2014.08.082. [DOI] [PubMed] [Google Scholar]
- Fan X.-D.; Hou Y.; Huang X.-X.; Qiu T.-Q.; Jiang J.-G. Ultrasound-Enhanced Subcritical CO2Extraction of Lutein fromChlorella pyrenoidosa. J. Agric. Food Chem. 2015, 63, 4597–4605. 10.1021/acs.jafc.5b00461. [DOI] [PubMed] [Google Scholar]
- Yi S.; Sun S.; Deng Y.; Ye Y.; Jian X. Removal and recovery of CI reactive red 195 from effluent by solvent extraction using reverse micelles. Text. Res. J. 2015, 85, 1095–1103. 10.1177/0040517514559584. [DOI] [Google Scholar]
- Torun M.; Dincer C.; Topuz A.; Sahin–Nadeem–Nadeem H.; Ozdemir F. Aqueous extraction kinetics of soluble solids, phenolics and flavonoids from sage (Salvia fruticosa Miller) leaves. J. Food Sci. Technol. 2015, 52, 2797–2805. 10.1007/s13197-014-1308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Liu C.; Rong Y.; Rong L.. Extraction of Mogroside and Limonin with Different Extraction Methods and its Modeling. Int. J. Food Eng. 2012, 8 (2). 10.1515/1556-3758.2361 [DOI] [Google Scholar]
- Liu J.; Chen P.; He J.; Deng L.; Wang L.; Lei J.; Rong L. Extraction of oil from Jatropha curcas seeds by subcritical fluid extraction. Ind. Crops Prod. 2014, 62, 235–241. 10.1016/j.indcrop.2014.08.039. [DOI] [Google Scholar]
- Liu J.; Chen P.; Rong L.; Lei J. D.; He J.; Deng L.; Wang L. Cascade Extraction and Separation of the Active Constituents from. Res. Dev., Jpn. 2015, 22, 109–117. 10.15261/serdj.22.109. [DOI] [Google Scholar]
- Fan Y.; He X.; Zhou S.; Luo A.; He T.; Chun Z. Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int. J. Biol. Macromol. 2009, 45, 169–173. 10.1016/j.ijbiomac.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Xiao S.; Xi X.; Tang F.; Dai J.; Liu J.; Lei J.; Wang L. Subcritical Water Extraction of Ursolic Acid from Hedyotis diffusa. Appl. Sci. 2017, 7, 187. 10.3390/app7020187. [DOI] [Google Scholar]
- Liu J.; Chen P.; Yao W.; Wang J.; Wang L.; Deng L.; He J.; Zhang G.; Lei J. Subcritical water extraction of betulinic acid from birch bark. Ind. Crops Prod. 2015, 74, 557–565. 10.1016/j.indcrop.2015.05.064. [DOI] [Google Scholar]
- Herodež Š. S.; Hadolin M.; Škerget M.; Knez Ž. Solvent extraction study of antioxidants from Balm (Melissa officinalis L.) leaves. Food Chem. 2003, 80, 275–282. 10.1016/s0308-8146(02)00382-5. [DOI] [Google Scholar]
- Koomyart I.; Nagamizu H.; Khuwijitjaru P.; Kobayashi T.; Shiga H.; Yoshii H.; Adachi S. Subcritical Water Treatment for Producing Seasoning From Semidried Isada Krill. J. Food Process Eng. 2014, 37, 567–574. 10.1111/jfpe.12110. [DOI] [Google Scholar]
- Jung K.-W.; Park D.; Hwang M.; Ahn K. Decolorization of Acid Orange 7 by an electric field-assisted modified orifice plate hydrodynamic cavitation system: Optimization of operational parameters. Ultrason. Sonochem. 2015, 26, 22–29. 10.1016/j.ultsonch.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandham K.; Sivakumar V.; Prakash Maran J. Optimization of process parameters in electrocoagulation treating chicken industry wastewater to recover hydrogen gas with pollutant reduction. Renewable Energy 2015, 80, 101–108. 10.1016/j.renene.2015.01.030. [DOI] [Google Scholar]
- Zhang Q.; Bian Y.; Shi Y.; Zheng S.; Gu X.; Zhang D.; Zhu X.; Wang X.; Jiang D.; Xiong Q. An economical and efficient technology for the extraction of resveratrol from peanut (Arachis hypogaea) sprouts by multi-stage countercurrent extraction. Food Chem. 2015, 179, 15–25. 10.1016/j.foodchem.2015.01.113. [DOI] [PubMed] [Google Scholar]
- Morales D.; Smiderle F. R.; Villalva M.; Abreu H.; Rico C.; Santoyo S.; Iacomini M.; Soler-Rivas C. Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. 10.1016/j.jff.2019.103446. [DOI] [Google Scholar]
- Trindade A. S. N.; Dantas A. F.; Lima D. C.; Ferreira S. L. C.; Teixeira L. S. G. Multivariate optimization of ultrasound-assisted extraction for determination of Cu, Fe, Ni and Zn in vegetable oils by high-resolution continuum source atomic absorption spectrometry. Food Chem. 2015, 185, 145–150. 10.1016/j.foodchem.2015.03.118. [DOI] [PubMed] [Google Scholar]
- Zha X.-Q.; Li X. L.; Zhang H. L.; Cui S. H.; Liu J.; Wang J. H.; Pan L. H.; Luo J. P. Pectinase hydrolysis of Dendrobium huoshanense polysaccharide and its effect on protein nonenzymatic glycation. Int. J. Biol. Macromol. 2013, 61, 439–447. 10.1016/j.ijbiomac.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Yang B. Y.; Wu Y. M.; Liu Y.; Gu X.; Zhang H.; Wang C. J.; Cao H. Z.; Huang L. J.; Wang Z. F. Structural characterization and antioxidant activities of kappa-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. 10.1016/j.foodchem.2015.01.105. [DOI] [PubMed] [Google Scholar]
- Li Y. J.; Lin D.; Jiao B.; Xu C.; Qin J.; Ye G.; Su G. Purification, antioxidant and hepatoprotective activities of polysaccharide from Cissus pteroclada Hayata. Int. J. Biol. Macromol. 2015, 77, 307–313. 10.1016/j.ijbiomac.2015.03.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.