Abstract

The present study reports on CuO-assisted reduction of Cr(VI) under ambient conditions using sodium borohydride and its complete removal. The confirmation of the reductive removal of Cr(VI) was assisted by powder X-ray diffraction, Fourier transform infrared, scanning electron microscopy, mic absorption spectro, UV–vis, and UV–vis–diffuse reflectance spectroscopy techniques. The analysis revealed that the process involved adsorption of dichromate ion on the surface of copper oxide, reduction of Cr(VI), and precipitation of Cr(III) as its hydroxide. Cr(VI) reduction capacity of CuO was found to be around 27.2 mmol/(g h). The residue collected showed promising reusability for 3 to 4 cycles, and the exhausted residue was finally converted into a black composite, CuO/CuCr2O4. The composite showed positive response for the photodegradation of methyl orange. Thus, the current protocol proposed a complete package of cost-effective reduction of Cr(VI) to Cr(III), precipitation into its hydroxide, and the conversion of the residue into a photoactive composite.
1. Introduction
Hexavalent chromium is identified as one of the most abundant contaminants in water bodies1 due to the emanation of wastewaters from industries involving the processes such as electroplating, leather tanning, pigment processing, and wood preservation.2−4 Discharging the effluents containing chromium without pretreatment or improper treatment leads to contamination of water sources and pollutes the environment, which will eventually affect the health of living beings.5 The permissible level of residual Cr(VI) in water is reported to be 0.05 ppm.6 Cr(VI) contamination in the environment may cause health problems including allergic reactions, skin rashes, nose irritations and nosebleed, ulcers, weakened immune system, genetic material alteration, and kidney and liver damage.6,7 Hence, from environment safety and health points of view, it is essential to develop an effective Cr(VI) treatment and removal method.
The rich literature available on various methods such as chemical precipitation; reverse osmosis; ion exchange; foam flotation; electrolysis; adsorption; and chemical, biological, and photochemical reduction8 for the treatment and removal of Cr(VI) demonstrates the significance of the environmental problems due to its contamination. As Cr(III) is much less toxic, reduction of Cr(VI) to Cr(III) is considered to be a better option to take care of the Cr(VI) hazardousness.6,9 Reduction of Cr(VI) to Cr(III) is reported to be achieved chemically, biologically, and photochemically.10−15 Biotreatments are considered to be cost-effective and environmentally friendly; however, such methods are generally slow. Considering the chemical route, which is generally a viable method, most of the recent protocols involve costly chemicals such as Pd- and Pt-containing systems, complex catalyst preparation steps, and long duration.6,15,16 Such processes focus mainly on the reduction of Cr(VI) and in general have not addressed the secondary waste accumulation. The current study focused on the usage of a cost-effective material for the reduction of Cr(VI) and in addition addressed the transformation of accumulating secondary waste. The process involves reduction of Cr(VI) to Cr(III) in the presence of copper oxide and the conversion of the collected residue into a photoactive product CuO/CuCr2O4, which shows photcatalytic activity for degradation of organic dyes.
2. Results and Discussion
Chemical reduction of Cr(VI) using sodium borohydride in the presence of CuO as a catalyst is studied. The experiment is performed using both commercially available and freshly prepared CuO samples. The oxide is prepared by the combustion method. The powder X-ray diffraction (XRD) pattern given in Figure S1 shows pure CuO formation as per the standard data (JCPDS # 89-5895). The scanning electron microscopy (SEM) image shows formation of grains of different geometries and are of multiple sizes. The chemical conversion of Cr(VI) has been carried out at room temperature. Optimized experimental conditions are obtained by varying the parameters as given in the Experimental Section.
2.1. Reduction of Cr(VI)
Initially, 100 ppm Cr(VI) solution is treated with 12.5 mL of 0.5 M sodium borohydride solution in the presence of 0.1 g of CuO. The pH of the Cr(VI) solution is not adjusted and is noted to be 4.8 at room temperature (35 °C). The solution is sampled at regular intervals and analyzed by UV–vis spectroscopy. Disappearance of the characteristic absorbance peak at 352 nm, which corresponds to the ligand-to-metal charge transfer transition6 in the spectrum of potassium dichromate is considered to be the index of conversion of Cr(VI). Profiles shown in Figure S2i confirm the complete conversion of Cr(VI) in a span of 15 min (the experiment was carried out till 120 min). In the case of the solutions whose pH is adjusted to 2 and 10, the process of complete reduction of Cr(VI) requires a duration of 30 min (Figure S2ii,iii). The observations suggest that the current protocol could be applied to liquid environments having a wide range of pH values. As there is no significant effect of pH on the reduction process, all of the successive experiments have been carried out without adjusting the pH. It is to be noted here that in most of the reductive removal protocols reported, reduction is carried out under highly acidic conditions.17−19 Control experiments carried out in the presence of sodium borohydride and CuO separately indicate no significant conversion of Cr(VI) (Figure S3), thus providing evidence for the catalytic activity of CuO. Decrease in the amount of the catalyst to 50 and 25 mg resulted in complete conversion in 30 and 240 min, respectively. When the amount of the catalyst is reduced further to 2 mg, incomplete conversion is noticed even after longer reaction duration. The effect of concentration of dichromate solution also shows difference in reaction duration: in the presence of 100 mg of CuO, complete conversion of 20 ppm of potassium dichromate takes place within a minute of mixing, 100 ppm requires 15 min, and increasing the concentration to 500 ppm increases the duration to 135 min. The conversion experiment carried out using lower concentrations of sodium borohydride, viz., 0.25, 0.125, and 0.0635 M (CuO, 100 mg; dichromate solution, 100 ppm) shows that the first two concentrations resulted in complete conversion during 15 min of reaction, while in the presence of 0.0635 M sodium borohydride, the duration has gone up to 30 min. Thus, the optimal experimental conditions for the conversion of 100 ppm dichromate solution in 15 min are found to be CuO, 100 mg; NaBH4, 0.125 M; and pH, 4.8. As Cr(VI) is found to be stable over a wide range of pH values,8 current study’s observation of the reduction of Cr(VI) at different pH conditions in the range 2–10 provides an advantage in the treatment of Cr(VI) in aqueous media of different pH values. The activity of commercial CuO is found to be similar to that of freshly prepared CuO, which practically gives an additional advantage.
The diphenylamine test confirms the complete reduction of Cr(VI) to Cr(III) (Figure 1). The initial dichromate solution before the reduction procedure gives a bluish violet or purple color with diphenylamine (Figure 1a), which is due to the oxidation of diphenylamine by dichromate.20 However, the solution obtained after the reduction process does not show any color change (Figure 1b) with diphenylamine, indicating the absence of Cr(VI). Atomic absorption spectroscopy (AAS) analysis of the liquid sample after the completion of the process shows the presence of only trace amount of Cr (∼0.5 ppm). This could be due to complete reduction of Cr(VI) to Cr(III), which is less mobile in nature21 and precipitates as hydroxide.
Figure 1.

Confirmation of Cr(VI) conversion by the diphenylamine test (a) before the reduction reaction and (b) after the reaction.
The experiment is performed under similar conditions using simulated tannery wastewater containing Fe3+, Al3+, Na+, Cl–, and SO42– ions22 to study the effect of the presence of other ions on the reduction process. The results given in Figure 2 confirm the absence of the interference. A complete reduction of Cr(VI) is noticed within a span of 15 min.
Figure 2.
UV–vis spectra indicating reduction of Cr(VI) in a simulated tannery water sample.
2.2. Kinetics and Analysis of the Resultant Residue
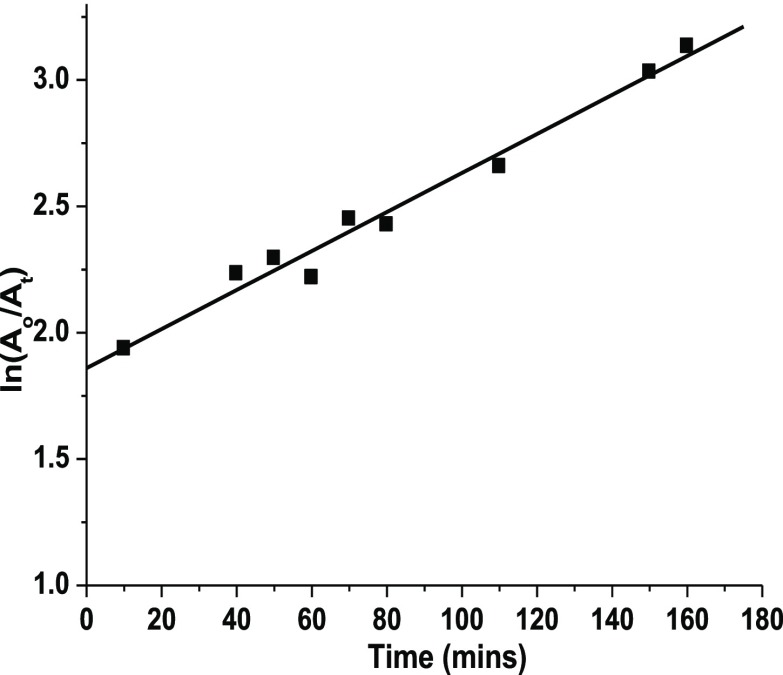
The kinetics of the process of reduction of Cr(VI) is monitored by the change in intensity of the absorption peak at 352 nm as a function of time. The plot shown in Figure 3 displays a linear correlation between ln(Ao/At) and time. The experimental conditions are as follows: potassium dichromate solution, 500 ppm; CuO, 100 mg; NaBH4, 0.125 M; and pH, 4.8. A pseudo-first-order model is applied to demonstrate the kinetics of the reduction of Cr(VI) in the presence of CuO. The rate constant is determined to be 7.7 × 10–3 min–1.
Figure 3.
Plot of ln(Ao/At) vs time for the reduction of Cr(VI).
The pH of the solution is measured as 10.2 after the addition of sodium borohydride to the mixture of CuO and dichromate solution (15 min stirring). The increase in the pH during the process helps in the precipitation of Cr(III) as its hydroxide. It is noted that Cr(OH)3 predominates in the pH range 6–12.23 The residue collected after the reaction is analyzed by the powder X-ray diffraction technique, and the pattern is compared with that of pure copper oxide (Figure 4). Close examination specifies the presence of metallic Cu and Cr(OH)3·3H2O along with a small amount of CuO in the residue. The peaks appearing at 2θ = (43.28, 50.44°) and 2θ = 36.48° in Figure 4ii confirm the presence of metallic Cu and Cr(OH)3·3H2O, respectively. The reduction of Cr(VI) is also confirmed by the presence of hydroxide of Cr(III) as revealed in the powder XRD pattern in Figure 4ii. Such a possibility of formation of Cr(III) hydroxide and its precipitation on the surface of the adsorbent is reported.21
Figure 4.
Powder XRD patterns of (i) pure CuO and (ii) CuO after the reaction ((a) Cu, (b) Cr(OH)3·3H2O, (c) CuO).
It is to be noted that the current protocol does not require addition of sodium hydroxide separately as reported in most of the reduction and precipitation procedures wherein for the precipitation of Cr(III), sodium hydroxide is added.17−19 The increase in the pH of the dichromate solution from 4.8 to 10.2 after the process could be due to the hydrolysis of sodium borohydride in the presence of copper oxide, which results in the generation of hydrogen needed for the reduction reaction as given below.24
Under this condition, Cr(III), which is formed by the reduction of Cr(VI), subsequently gets precipitated out as its hydroxide. The processes of adsorption of the dichromate ion on the surface of CuO and its reduction and precipitation as hydroxide are monitored by infrared spectral analysis on the samples collected as given below.
-
1.
CuO immersed in dichromate solution, stirred for 15 min, residue collected after drying as such (C1)
-
2.
CuO immersed in dichromate solution, stirred for 15 min, residue washed and dried (C2)
-
3.
CuO immersed in dichromate solution, sodium borohydride added, stirred for 15 min, residue collected after drying as such (C3)
-
4.
CuO immersed in dichromate solution, sodium borohydride added, stirred for 15 min, residue washed and dried (C4)
The IR spectra of C1–C4 are given in Figure 5. CuO peaks appear in the 440–590 cm–1 region.25 The spectra of both C1 and C2 show similar features before and after washing the residue. This specifies the attachment of dichromate ions to the catalyst surface (spectrum of dichromate is given in Figure 5ii for comparison). In the spectrum of C3 (Figure 5iii), along with other species, the presence of Cr(OH)3·xH2O is identified based on the bands appearing at around 3335, 1640, 939, and 520 cm–1.26 Such features completely disappear in the spectrum of C4 (Figure 5iii), which represents the residue collected after washing. This observation confirms the absence of interaction of Cr(III) hydroxide with the catalyst surface; thus, in the initial step, adsorption of the dichromate ion on the surface of the metal oxide takes place, which further gets reduced to Cr(III) upon introduction of sodium borohydride, and finally the ion is precipitated out as its hydroxide.
Figure 5.

Infrared spectra of residues C1, C2 (i), potassium dichromate (ii), and C3, C4 (iii).
2.3. Reaction Pathway and Reusability of the Catalyst
Cr(VI) exists in different forms in aqueous solution depending on the pH of the medium. Dichromate (Cr2O72–) and hydrogen chromate (HCrO4–) ions exist in equilibrium in the pH range 2.0–6.0. The predominant form at higher pH is (CrO42–).27 As it can be seen from the reactions given below, the reduction of these different species essentially requires protons and electrons12,28
| 1 |
| 2 |
| 3 |
In the current protocol, the required species such as electrons and protons for the reduction of Cr(VI) are chemically generated during CuO-assisted hydrolysis of sodium borohydride. The control experiments (Section 2.3), reduction of Cr(VI) to Cr(III), and its precipitation as its hydroxide as evidenced from the diphenylamine test, AAS, and powder XRD analyses, provide the information that the copper oxide acts as a catalyst in the process of hydrolysis of sodium borohydride and thus assisted in the generation of the required active species. Copper oxide aids in discharging electrons from BH4–, and the H+ ions are offered by water, which is the medium in the hydrolysis reaction.29 The presence of metallic copper in the residue (Figure 4) collected after the conversion shows simultaneous reduction of both Cr(VI) and Cu(II). This suggests a probable pathway of reduction of CuO to Cu via Cu2O in the presence of sacrificial NaBH4. This in turn aids the conversion of Cr(VI) to Cr(III) (Figure 6).
Figure 6.

Reaction pathway.
Interestingly, the collected residue also shows activity. The residue collected by centrifugation is dried and tested. Reduction in the amount of the residue is noticed in each cycle: the amounts after first, second, and third cycles are 67, 57, and 51 mg, respectively. The UV–vis absorbance profiles compiled in Figure S4 show the activities of the residues upon treating with 100 ppm dichromate solution. First two cycles show the completion of the conversion in 15 min, and the third cycle indicates 30 min of duration due to relatively lesser amount of the residue (51 mg). Since the collected residue is a mixture of Cu, Cr(OH)3·3H2O, and CuO, the species that are responsible for the activity in the recycling process could be metallic copper and copper oxide. The activity of metallic copper is tested separately under similar experimental conditions. The results (Figure S4iv) confirm that the freshly formed copper during the reduction process further acts as the catalyst and thus the resultant residue can be reused till it gets exhausted and leads to affordable reduction of Cr(VI).
2.4. Conversion of the Residue into a Photoactive Composite and the Advantage of the Current Protocol
The residues collected in the treatment of 500 ppm (CR500) and 100 ppm (CR100) dichromate solutions were dissolved in dilute nitric acid, and the nitrate mixtures were combusted separately using urea as a fuel. The black mass obtained was subjected to powder X-ray diffraction analysis. The results confirmed the formation of a composite of CuO and CuCr2O4 (Figure 7i). The residues collected from the reactions of both CR100 and CR500 show similar product formation. The plots of % reflectance vs wavelength data (Figure 7ii) given in the range 200–800 nm show almost 90% absorbance of the incident radiation. Table 1 shows the low color coordinates L*a*b* of CR100 and CR500 samples, which characterize black hue (Figure 7ii inset).30 Different morphologies of the products are inferred from the SEM images (Figure 7ii inset) of the composites. Figure 7iii shows the absorbance vs wavelength spectra of the composites obtained using CR100 and CR500. Comparison of the spectra with that of pure CuO proves the formation of the composite. It is evident that the formation of composite with CuCr2O4 influences the absorption edge of CuO. The absorption edge of the composites is red-shifted compared to that of CuO. Similar observation is made by Mageshwari et al. in the case of a CuCr2O4-embedded CuO nanocomposite.31 The photoactivity of the composite is tested for the degradation of methyl orange (MO). During irradiation, the MO solution turns colorless, and the UV–vis spectra recorded initially, after 90 and 120 min, are given in Figure 8. The disappearance of the characteristic azo band of methyl orange appearing at around 465 nm confirms the degradation of the dye at around 120 min. The advantages of the current protocol include usage of a simple cost-effective oxide, reusability of the resultant residue as a catalyst, and conversion of the exhausted residue into a photoactive composite. Thus, the current protocol demonstrates a route toward possible alleviation of secondary pollution.
Figure 7.
(i) Powder XRD patterns [*, CuO; #, CuCr2O4] and (ii) UV–vis–diffuse reflectance spectra (reflectance vs wavelength) (inset pictures, SEM images) of black composites obtained using (a) 100 ppm Cr and (b) 500 ppm Cr solutions. (iii) Absorbance vs wavelength spectra of (a) CuO, (b) CuO–CuCr2O4 composite (Cr, 100 ppm), and (c) CuO–CuCr2O4 composite (Cr, 500 ppm).
Table 1. CIE L*a*b* Colorimetric Data for the Black Residue.
| color coordinates |
|||
|---|---|---|---|
| sample | L* | a* | b* |
| CR100 | 37.8 | 0.34 | 0.60 |
| CR500 | 38.0 | 0.53 | 1.79 |
Figure 8.
Time-dependent UV–vis absorption spectra of MO dye solution in the presence of the composite.
A survey of reports on reductive removal of Cr(VI) proposes various approaches. The protocol involving extraction, reduction, and precipitation requires emulsion liquid membrane for extraction, ferrous salt for reduction, and alkali for precipitation and heating at 100 °C for an efficient process.17 Mu et al. have reported the removal of Cr(VI) via reduction and adsorption using core–shell Fe@Fe2O3.32 The experiment was performed under an Ar atmosphere involving 100 mL of 8 ppm Cr(VI) solution and 0.015 g of core–shell Fe@Fe2O3 at room temperature. Ali et al. studied the removal of Cr(VI) using iron nanoparticles supported on a porous cation-exchange resin.15 The process involves a separate procedure for the preparation of iron nanoparticles and their dispersion on a polymer support. In another approach, Pd supported on amine-functionalized SiO2 yielded efficient conversion of Cr(VI).6 However, the method comprises costly chemicals and tedious catalyst preparation. Lu et al. studied natural clino-pyrrhotite for reductive removal of Cr(VI).33 For effective removal of 1 μmol Cr(VI), 0.22 g of the catalyst is used, and the formation of a larger quantity of sludge is unavoidable. Such reported approaches resulted in successful conversion of Cr(VI) to Cr(III). Yet, in most of the protocols, costly chemicals, tedious procedure of nanoparticle or supported material preparation, and experimentation under an inert atmosphere are involved. In addition, accumulation of converted Cr(III) as hydroxide as sludge is inevitable.
The current procedure involves less costly chemicals, simple process, reusability of the catalytic system, collection of the converted Cr(III), and its transformation into a useful composite, which eases the sludge accumulation. Reusability and conversion of the collected residue into a useful material may certainly help in effective purification of Cr(VI)-contaminated aqueous medium.
3. Conclusions
The current investigation brings out a facile protocol for the complete removal of hazardous Cr(VI). The reduction process applies cost-effective copper oxide and can be carried out in aqueous media with a wide pH range (2–10). The collected residue after recycling is successfully converted into a photoactive composite, CuO/CuCr2O4. Testing on simulated tannery wastewater indicates the absence of interference due to the presence of other ions. Such an attempt to convert the accumulating residue into a useful product is significant from the secondary pollution point of view.
4. Experimental Section
4.1. Synthesis of CuO
Reagents used: Cu(NO3)2·3H2O (S.D. Fine chemicals Ltd., 99%), NH2CONH2 (S.D. Fine chemicals Ltd., 99%), and NaBH4 (S.D. Fine chemicals Ltd., 98%).
CuO was prepared by the combustion method. The required amount of Cu(NO3)2·3H2O was dissolved in minimum quantity of water, to that, urea was added as fuel, and the mixture was kept for combustion at 500 °C for 15 min. The collected solid was kept for calcination at 750 °C for 2 h.
4.2. Characterization
The phase formation and the precipitated product of Cr(III) were identified by powder XRD analysis at room temperature using an X-ray diffractometer (Bruker D8 Advanced) with Cu Kα radiation in the scan range of 10–70°. Fourier transform infrared (FT-IR) analysis (JASCO FT-IR 4100) was carried out using the KBr pellet technique. UV–vis spectra and UV–vis–near-infrared absorption characteristics were obtained using a Jasco spectrophotometer (model V-560). Atomic absorption spectroscopic analysis of the filtrate was carried out using Varian AA240.
4.3. Chemical Conversion of Cr(VI) to Cr(III) and Combustion of the Resultant Residue
To 25 mL of dichromate solution (source: K2Cr2O7) taken in a beaker, 100 mg of CuO was introduced and kept in the dark for 30 min for equilibration to enable adsorption and desorption processes (the pH of the solution was noted as 4.8). The freshly prepared sodium borohydride solution (0.5 M) was introduced and stirred (200 rpm). The solution was sampled after centrifugation and analyzed by UV–visible spectroscopy. The parameters varied were as follows: amount of the catalyst (100, 50, 25, and 2 mg); pH (2, 4.8, and 10); concentration of dichromate solution (20, 100, and 500 ppm), and the concentration of sodium borohydride (0.25, 0.125, and 0.0635 M). Control experiments were carried out with CuO and NaBH4 separately. The residue collected after the reduction of Cr(VI) was dissolved in 1:1 nitric acid. The nitrate mixture was combusted using urea as a fuel in a furnace preheated at 500 °C.
4.4. Photocatalytic Activity of the Composite
To 25 mL (10 ppm) of methyl orange solution taken in a photoreactor tube, 0.1 g of the composite powder was introduced. The suspension was stirred in the dark under ambient conditions for 30 min to ensure the adsorption–desorption equilibrium between the dye molecule and the composite powder. To the reaction mixture, 100 μL of hydrogen peroxide (30%) was added and irradiated with UV radiation of wavelength 365 nm. Small aliquots were collected, centrifuged, and analyzed by UV–vis spectroscopy to determine the degradation of the dye.
Acknowledgments
The authors thank VIT University for providing all of the required facilities to carry out the work and duly acknowledge Sanjukta Biswal and Pavithra. V for technical help.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b01452.
Powder X-ray diffraction pattern of CuO; UV–vis spectra of chromium samples collected at regular intervals under different pH conditions; UV–visible spectra recorded for the reduction of Cr(VI); UV–vis absorbance plots illustrating the reusability of the collected residue and reduction of Cr(VI) solution in the presence of metallic copper (PDF)
Author Present Address
† Department of Chemistry, IIT Kanpur, Kanpur, Uttar Pradesh 208016, India (A.G.).
The authors declare no competing financial interest.
Supplementary Material
References
- Yadav M.; Xu Q. Catalytic chromium reduction using formic acid and metal nanoparticlesimmobilized in a metal–organic framework. Chem. Commun. 2013, 49, 3327–3329. 10.1039/c3cc00293d. [DOI] [PubMed] [Google Scholar]
- Prabhakaran S. K.; Vijayaraghavan K.; Balasubramanian R. Removal of Cr(VI) Ions by Spent Tea and Coffee Dusts: Reduction to Cr(III) and Biosorption. Ind. Eng. Chem. Res. 2009, 48, 2113–2117. 10.1021/ie801380h. [DOI] [Google Scholar]
- Kotaś J.; Stasicka Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. 10.1016/S0269-7491(99)00168-2. [DOI] [PubMed] [Google Scholar]
- Chen J. H.; Hsu K. C.; Chang Y. M. Surface Modification of Hydrophobic Resin with Tricaprylmethylammonium Chloride for the Removal of Trace Hexavalent Chromium. Ind. Eng. Chem. Res. 2013, 52, 11685–11694. 10.1021/ie401233r. [DOI] [Google Scholar]
- Tahiri S.; Albizane A.; Messaoudi A.; Azzi M.; Bennazha J.; Younssi S. A.; Bouhria M. Thermal behavior of chrome shavings and of sludges recovered after digestion of tanned solid wastes with calcium. Waste Manage. 2007, 27, 89–95. 10.1016/j.wasman.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Celebi M.; Yurderi M.; Bulut A.; Kaya M.; Zahmakiran M. Palladium nanoparticles supported on amine-functionalized SiO2 for the catalytic hexavalent chromium reduction. Appl. Catal., B 2016, 180, 53–64. 10.1016/j.apcatb.2015.06.020. [DOI] [Google Scholar]
- Elliott D. W.; Zhang W. Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. 10.1021/es0108584. [DOI] [PubMed] [Google Scholar]
- Dinker M. K.; Kulkarni P. S. Recent Advances in Silica-Based Materials for the Removal of Hexavalent Chromium: A Review. J. Chem. Eng. Data. 2015, 60, 2521–2540. 10.1021/acs.jced.5b00292. [DOI] [Google Scholar]
- Miretzky P.; Fernandez Cirelli A. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. 10.1016/j.jhazmat.2010.04.060. [DOI] [PubMed] [Google Scholar]
- Zhang H.-K.; Lu H.; Wang J.; Zhou J.-T.; Sui M. Cr(VI) Reduction and Cr(III) Immobilization by Acinetobacter sp. HK-1 with the Assistance of a Novel Quinone/Graphene Oxide Composite. Environ. Sci. Technol. 2014, 48, 12876–12885. 10.1021/es5039084. [DOI] [PubMed] [Google Scholar]
- Wang N.; Zhu L.; Deng K.; She Y.; Yu Y.; Tang H. Visible light photocatalytic reduction of Cr(VI) on TiO2 in situ modified with small molecular weight organic acids. Appl. Catal., B 2010, 95, 400–407. 10.1016/j.apcatb.2010.01.019. [DOI] [Google Scholar]
- Yang L.; Xiao Y.; Liu S.; Li Y.; Cai Q.; Luo S.; Zeng G. Photocatalytic reduction of Cr(VI) on WO3 doped long TiO2 nanotube arrays in the presence of citric acid. Appl. Catal., B 2010, 94, 142–149. 10.1016/j.apcatb.2009.11.002. [DOI] [Google Scholar]
- Gherbi R.; Nasrallah N.; Amrane A.; Maachi R.; Trari M. Photocatalytic reduction of Cr(VI) on the new hetero-system CuAl2O4/TiO2. J. Hazard. Mater. 2011, 186, 1124–1130. 10.1016/j.jhazmat.2010.11.105. [DOI] [PubMed] [Google Scholar]
- Meichtry J. M.; Colbeau-Justin C.; Custo G.; Litter M. I. TiO2- photocatalytic transformation of Cr(VI) in the presence of EDTA: Comparison of different commercial photocatalysts and studies by Time Resolved Microwave Conductivity. Appl. Catal., B 2014, 144, 189–195. 10.1016/j.apcatb.2013.06.032. [DOI] [Google Scholar]
- Ali S. W.; Mirza M. L.; Bhatti T. M. Removal of Cr(VI) using iron nanoparticles supported on porous cation-exchange resin. Hydrometallurgy 2015, 157, 82–89. 10.1016/j.hydromet.2015.07.013. [DOI] [Google Scholar]
- Chen X.; Kuo D.-H. Nano flower Bimetal CuInOS Oxysulfide Catalyst for the Reduction of Cr(VI) in the Dark. ACS Sustainable Chem. Eng. 2017, 5, 4133–4143. 10.1021/acssuschemeng.7b00107. [DOI] [Google Scholar]
- Kulkarni P. S.; Kalyani V.; Mahajani V. V. Removal of Hexavalent Chromium by Membrane-Based Hybrid Processes. Ind. Eng. Chem. Res. 2007, 46, 8176–8182. 10.1021/ie070592v. [DOI] [Google Scholar]
- Gheju M.; Balcu I. Hexavalent chromium reduction with scrap iron in continuous-flow system. Part 2: Effect of scrap iron shape and size. J. Hazard. Mater. 2010, 182, 484–493. 10.1016/j.jhazmat.2010.06.058. [DOI] [PubMed] [Google Scholar]
- Gheju M.; Balcu I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. 10.1016/j.jhazmat.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Raj G.Advanced Practical Inorganic Chemistry; Goel Publishing Hose: New Delhi, 2002. [Google Scholar]
- Luo P.; Zhang J.-S.; Zhang B.; Wang J.-H.; Zhao Y.-F.; Liu J.-D. Preparation and Characterization of Silane Coupling Agent Modified Halloysite for Cr(VI) Removal. Ind. Eng. Chem. Res. 2011, 50, 10246–10252. 10.1021/ie200951n. [DOI] [Google Scholar]
- Sahu S. K.; Meshram P.; Pandey B. D.; Kumar V.; Mankhand T. R. Removal of chromium(III) by cation exchange resin, Indion 790 for tannery waste treatment. Hydrometallurgy 2009, 99, 170–174. 10.1016/j.hydromet.2009.08.002. [DOI] [Google Scholar]
- Stanin F. T.; Pirnie M.. The Transport and the Fate of Cr(VI) in the Environment. In Cr(VI) Hand Book; Guertin J. S., Avakian C. P., Jacobs J. A., Eds.; CRC Press, 2004. [Google Scholar]
- Demirci U. B.; Miele P. Reaction mechanisms of the hydrolysis of sodium borohydride: A discussion focusing on cobalt-based catalysts. C. R. Chim. 2014, 17, 707–716. 10.1016/j.crci.2014.01.012. [DOI] [Google Scholar]
- Gupta V. K.; Chandra R.; Tyagi I.; Verma M. Removal of hexavalent chromium ions using CuO nanoparticles for water purification applications. J. Colloid Interface Sci. 2016, 478, 54–62. 10.1016/j.jcis.2016.05.064. [DOI] [PubMed] [Google Scholar]
- Zhang H. L.; Liang S. T.; Luo M. T.; Ma M. G.; Fan P. P.; Xu H. B.; Li P.; Zhang Y. Preparation and color performance control of Cr2O3 green pigment through thermal decomposition of chromium hydroxide precursor. Mater. Lett. 2014, 117, 244–247. 10.1016/j.matlet.2013.12.010. [DOI] [Google Scholar]
- Nematollahzadeh A.; Seraj S.; Mirzayi B. Catecholamine coated maghemite nanoparticles for the environmental remediation: Hexavalent chromium ions removal. Chem. Eng. J. 2015, 277, 21–29. 10.1016/j.cej.2015.04.135. [DOI] [Google Scholar]
- Park D. H.; Yun Y.-S.; Lim S.-R.; Park J. M.. Kinetic Analysis and Mathematical Modeling of Cr(VI) Removal in a Differential Reactor Packed with Ecklonia Biomass. J. Microbiol. Biotechnol. 2006, 16, 1720–−1727.http://www.koreascience.or.kr/article/JAKO200604623664711.page. [Google Scholar]
- Mandlimath T. R.; Gopal B. Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-aminophenol. J. Mol. Catal. A: Chem. 2011, 350, 9–15. 10.1016/j.molcata.2011.08.009. [DOI] [Google Scholar]
- Costa G.; Della V. P.; Ribeiro M. J.; Oliveira A. P. N.; Monrós G.; Labrincha J. A. Synthesis of black ceramic pigments from secondary raw materials. Dyes Pigm. 2008, 77, 137–144. 10.1016/j.dyepig.2007.04.006. [DOI] [Google Scholar]
- Mageshwari K.; Sathyamoorthy R.; Lee J. Y.; Park J. Novel CuCr2O4 embedded CuO nanocomposites for efficient photodegradation of organic dyes. Appl. Surf. Sci. 2015, 353, 95–102. 10.1016/j.apsusc.2015.06.027. [DOI] [Google Scholar]
- Mu Y.; Ai Z.; Zhang L.; Song F. Insight into Core–Shell Dependent Anoxic Cr(VI) Removal with Fe@Fe2O3 Nanowires: Indispensable Role of Surface Bound Fe(II). ACS Appl. Mater. Interfaces 2015, 7, 1997–2005. 10.1021/am507815t. [DOI] [PubMed] [Google Scholar]
- Lu A.; Zhong S.; Chen J.; Shi J.; Tang J.; Lu A. Removal of Cr(VI) and Cr(III) from Aqueous Solutions and Industrial Wastewaters by Natural Clino-pyrrhotite. Environ. Sci. Technol. 2006, 40, 3064–3069. 10.1021/es052057x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.