Abstract
Optical properties can be programmed on mesoscopic scales by patterning host materials while ordering their nanoparticle inclusions. While liquid crystals are often used to define the ordering of nanoparticles dispersed within them, this approach is typically limited to liquid crystals confined in classic geometries. In this work, the orientational order that liquid crystalline colloidal hosts impose on anisotropic nanoparticle inclusions is combined with an additive manufacturing method that enables engineered, macroscopic three-dimensional (3D) patterns of co-aligned gold nanorods and cellulose nanocrystals. These gels exhibit polarization-dependent plasmonic properties that emerge from the unique interaction between the host medium’s anisotropic optical properties defined by orientationally ordered cellulose nanocrystals, from the liquid crystal’s gold nanorod inclusions, and from the complexity of spatial patterns accessed with 3D printing. The gels’ optical properties that are defined by the interplay of these effects are tuned by controlling the gels’ order, which is tuned by adjusting the gels’ cellulose nanocrystal concentrations. Lithe optical responsiveness of these composite gels to polarized radiation may enable unique technological applications like polarization-sensitive optical elements.
1. Introduction
Additive manufacturing’s deft ability to locally tune material composition and structure has made it the focus of scientific studies to realize novel materials with previously inaccessible properties.1 Examples include the three-dimensional (3D) printing of buildings,2 alloyed objects,3 photovoltaics and other electronics,4,5 metamaterials,6 gels,7 microparticles and nanomaterials,8,9 optical elements,10,11 stimuli-responsive actuators and sensors,12 and even food.13 Although many innovative materials can be 3D printed, eliciting useful metamaterial properties through synergistic interactions of functional nanomaterial inclusions with their host remains a challenge. Both liquid-crystal (LC) and LC polymeric hosts with orientationally aligned plasmonic nanoinclusions, including LC elastomers, have been used to address this challenge. Prepared by fiber drawing,14 reorienting stress,15 and other methods,16 control over a metamaterial’s optical response in bulk or interfacial domains has been achieved. Additionally, hydrogels with isotropic or unaligned inclusions including plasmonic nanoparticles also have been characterized for diverse applications.17 Separately, extrusion-type direct-ink-writing (DIW) 3D printing methods have imparted orientational order to LCs in stimuli-responsive four-dimensional gels to enable “biomimetic” attributes.18,19 However, both alignment of LCs with embedded anisotropic plasmonic nanoparticles and patterning of orientational order through 3D printing have not been explored to date.
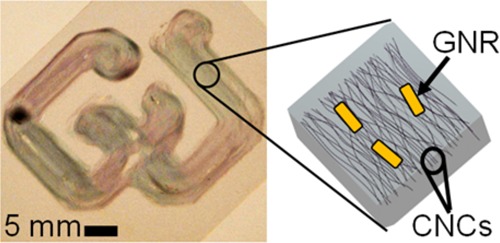
Capitalizing on the exquisite manufacturing control afforded by 3D printing methods, we report a new breed of gel-like composite materials with LC order and plasmonic properties endowed by synergistic guest–host colloidal interactions. We use LC colloidal hosts to orientationally order anisotropic nanoparticle inclusions to achieve gels with 3D patterns of co-aligned gold nanorods (GNRs) and cellulose nanocrystals (CNCs). While controlling the gels’ orientational order, we study the interplay of the GNRs’ polarization-dependent plasmonic properties with the optical and mechanical anisotropies of the host medium to characterize the composite gels’ optical properties. We demonstrate a suite of novel gels, which include hydrogels and aerogels, for applications that require optical control in spatially programmable, ultralight, and robust gels.
2. Methods
2.1. Metamaterial-Enabling Gel Constituents
Because mechanically robust gels and films with, for example, high optical transmission coefficients and low scattering can be made from cellulose20−22 and because well-characterized cellulosic gel chemistries exist in the literature,23−26 we have chosen to make strong and translucent gels using lyotropic CNCs, which are synthesized via a sulfuric acid-hydrolysis method.27 The partial replacement of primary alcohols with negatively charged sulfate ester moieties forms a stable aqueous CNC colloidal dispersion with intrinsic molecular charge and electrostatic charge-screened interactions in acidic and basic conditions. Moreover, the average measured length of the CNCs (∼300 nm, Figure 1a) is approximately equivalent to their average persistence length, which is a function of their average diameter (∼50 nm, Figure 1a) and crystalline cellulose fraction and distribution.28 Because of the CNCs’ stiffness and ability to form stable colloidal dispersions, monodomain alignment of lyotropic CNC dispersions can be achieved with relatively weak extrusion stresses, as we show in this work.
Figure 1.
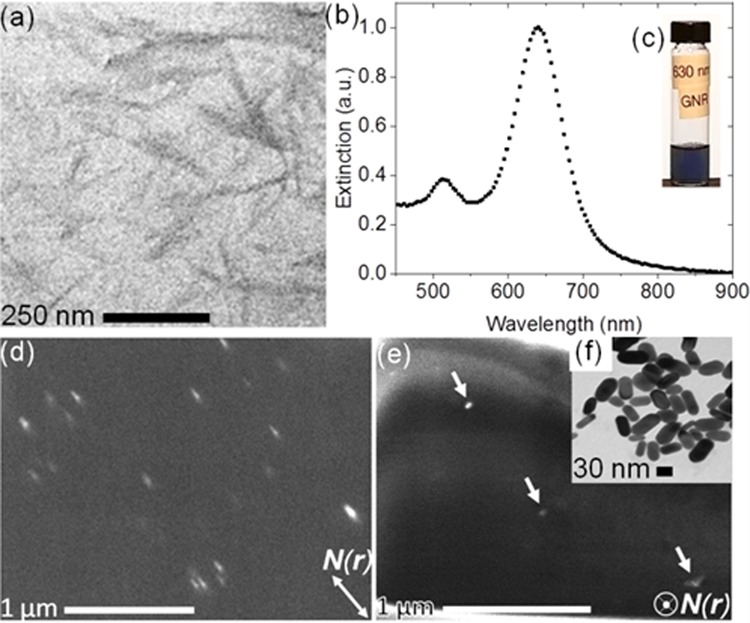
(a) Transmission electron microscopy (TEM) micrograph demonstrating CNCs’ anisotropy, which enables guest–host alignment in a gel. (b) Spectral characterization of GNR inclusions with transverse (∼530 nm) and longitudinal (∼630 nm) surface plasmon resonance (SPR) extinction peaks. An aqueous dispersion of GNRs as viewed with the background white light is shown in the inset (c). (d) A scanning electron microscopy (SEM) micrograph of the top surface of a gel with the GNRs’ longitudinal axis aligned with the local in-plane director N(r), as shown. Guest GNRs (white rods) are aligned by host CNCs (dark background) and constrained within the same gel. The gel with GNRs viewed in the plane of their transverse axes, as indicated by N(r), is shown in the micrograph (e). The imaged ends of the GNRs (white dots) are indicated by the white arrows. The inset (f) shows a TEM micrograph of GNRs drop-cast on a substrate.
CNCs can provide orientational ordering to composite gels. Even if no chemical reaction occurs with the CNCs’ surface functional groups, the CNCs can become mechanically constrained about their sol-phase ordering directions within the gel matrix. Additionally, spectral characterization of GNRs dispersed in deionized water reveals their anisotropy characterized by a transverse surface plasmon resonance (TSPR) extinction peak at ∼530 nm and a longitudinal surface plasmon resonance (LSPR) extinction peak at ∼630 nm. The extinction spectrum is shown in Figure 1b, while the GNRs dispersed in water are shown with white background illumination in Figure 1c. As shown in Figure 1d, the GNRs’ rodlike shape allows them to be aligned with the local in-plane director N(r) defined by the CNC alignment. Co-alignment of their longitudinal axes occurs via steric interactions within LC media such as lyotropic CNCs. Secondary-electron detection reveals a stronger signal from the GNRs than from the background CNCs, due to a larger cross-section of secondary-electron generation for the gold. The GNRs appear as white rods surrounded by a dark background in the SEM micrographs of Figure 1d. Meanwhile, a perspective orthogonal to the plane of Figure 1d is shown in Figure 1e. Therein, as indicated by N(r), the plane defined by the GNRs’ transverse axes is demonstrated by the ends of the rodlike GNRs. These hemispherical-like ends are indicated by the white arrows and appear as white dots. Both transverse and longitudinal profiles of the GNRs are shown in inset 1(f), which was captured using TEM. The GNRs appear dark, while the Formvar background appears bright because of the differences between the GNRs’ and CNCs’ electron transmission (see the Supporting Information for preparation and measurement details). The GNRs’ anisotropy demonstrated in inset Figure 1f is also visible with alignment shown by Figure 1 d,e, demonstrating that the GNRs are aligned along the local director field N(r) that is defined by the colloidal CNCs in the composite gel. The GNRs’ alignment and mechanical constraint enable the polarization-dependent excitement of each GNR’s two distinct plasmonic extinction modes within the bulk host medium.29−32
2.2. Manufacturing Procedures
We realize metamaterial gels whose polarization-dependent optical extinction is enabled by the orientational alignment of anisotropic GNRs by CNCs. Steric interactions among CNCs and GNRs minimize each particle’s excluded volume and maximize the total system entropy through orientational alignment that can be understood by extending the theoretical framework developed by Onsager.33 Although Liu et al.31 employed a static process to co-align GNRs and CNCs throughout a macroscopic sample specimen, we use a kinetic extrusion process, direct-ink writing (DIW) 3D printing, to spatially align the GNRs’ longitudinal axis with the flow of extruded CNCs. We use the critical concentrations found by Liu et al. as references for our kinetic experimental paradigm to form liquid crystals of CNCs and align GNRs. We also adopt our colloidal system to the two different experimental procedures described below.
2.2.1. Procedure 1
Cross-linked poly(acrylamide) (PAM) has been chosen as our gels’ polymer matrix to host CNCs and GNRs. PAM-CNC-GNR hydrogels are prepared by photoinitiated radical polymerization. The chemical structures of the acrylamide (AAm) monomers, photoinitiator 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (Irgacure-2959), base catalyst N,N,N′,N′-tetramethylethane-1,2-diamine (TEMED), and cross-linker N,N′-methylenebis(acrylamide) (MBAA) are presented in Figure 2a. Aqueous colloidal dispersions of CNCs and GNRs were mixed with AAm, Irgacure-2959, TEMED, and MBAA before printing. The resultant sol was printed with a commercially available fused-filament-fabrication 3D printer that was retrofitted into a DIW printer. The extrusion printing process and alignment of CNCs with weak extrusion stresses are shown schematically in Figure 2b. Therein, the direction of the flow with respect to the printing substrate is determined by the ink nozzle’s translational velocity parallel to the substrate. After printing, Procedure 1’s chemistry grants the formulator enhanced control over the hydrogel’s degree of cross-linking and elasticity by means of timed exposure to ultraviolet (UV) light. A schematic of post-printing UV-gelation is presented in Figure 2c. The chemical structure of the final hydrogel, with cross-linking both within and between gel filaments, is shown schematically in Figure 2d.
Figure 2.
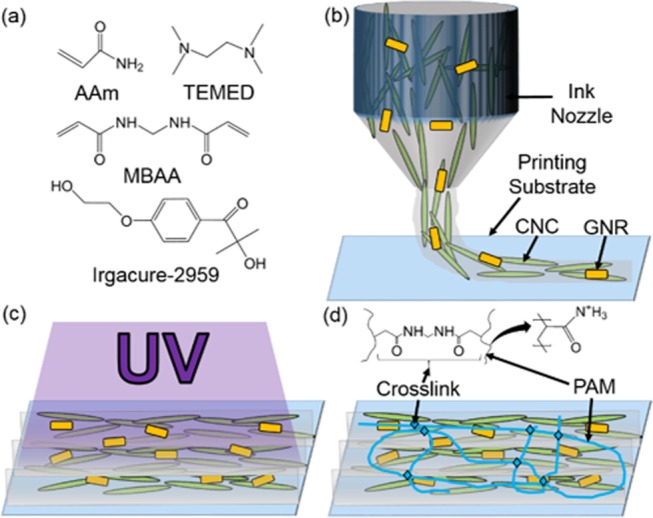
Schematics depicting manufacturing Procedure 1 (not to scale). (a) Structures of the chemicals that are mixed with GNRs and CNCs to make the gel precursor. (b) CNCs and GNRs are orientationally aligned along the flow of the gel precursor by a DIW 3D printing process. (c) Gelation of the printed sol with UV light. (d) PAM chains are cross-linked at points along each polymer’s length.
2.2.2. Procedure 2
Manufacturing of a hydrogel with Procedure 1 is tenable because the concentration of used CNCs (21–22 wt %) yields a suitably viscous sol that maintains an object’s shape and director alignment during and after printing and before cross-linking. However, when printing with CNC concentrations less than ∼20 wt %, low viscosities diminish both an object’s well-defined orientational ordering and its geometric stability as its profile slowly collapses and flows under surface-tension and gravitational forces. Procedure 2 addresses these concerns by tuning the viscosity to an appropriate range (10–20 Pa s) before cross-linking so that precursors with CNC concentrations less than 20 wt % can be easily printed. While Procedure 1 limits GNRs and CNCs to co-axial alignment, Procedure 2 enables the tuning of guest–host alignment. As CNC concentrations increase up to 20 wt %, the following alignment regimes in hydrogels become practicable: (1) isotropic CNC and GNR organization, (2) nematic CNC and isotropic GNR organization, and (3) nematic CNC and GNR organization. We found that a gel precursor with a final CNC concentration of 0.76 wt % yielded a gel with the properties of (1) and, after evaporation over tens of minutes, the properties of (2). Condition (3) was realized at ∼10 wt % CNCs, though the critical concentration differentiating (2) and (3) has not been determined (see the Supporting Information for optical characterization of these regimes).
To implement Procedure 2, the precursor is oligomerized before printing. Aqueous colloidal dispersions of CNCs and GNRs were mixed with AAm, Irgacure-2959, and TEMED and then were exposed to UV radiation to tune the sol’s viscosity. The viscous sol with added cross-linker MBAA was printed, as depicted in Figure 2b. Subsequent cross-linking of PAM oligomers within the printed object occurred with UV exposure, as illustrated in Figure 2c. Because polymer chains with terminal, radical moieties exist before cross-linking occurs and persist thereafter for extended durations,34−38 only the ends of PAM chains are cross-linked whereby still longer chains are formed. The final cross-linked hydrogel is illustrated in Figure 2d.
3. Results and Discussion
3.1. Selective Optical Extinction in Patterned Hydrogels
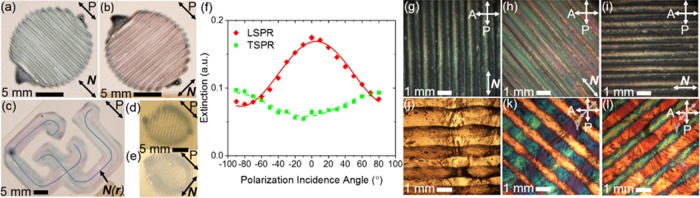
Color modulation is apparent to the naked eye upon exposure of PAM-CNC-GNR gels to white, linearly polarized light. Although the blueish appearance originating from the LSPR is excited by polarized light with polarization P that is parallel to the LC director N in Figure 3a, the TSPR’s reddish appearance is excited when uniform N is rotated orthogonal to P, as demonstrated by Figure 3b. In addition to hydrogels with average, uniform director N, hydrogels with a continuously changing director in a single-layer object have been printed, as shown in Figure 3c. Therein, the LSPR and TSPR are observed by the blueish and reddish colors at positions where N(r) ∥ P and N(r) ⊥ P, respectively, where r represents the two-dimensional spatial coordinates in the plane of the flat sample. The hydrogels in Figure 3d,e also demonstrate consistent geometric profiles though they are made with lower CNC concentrations than those in Figure 3a–c. All single-layer hydrogels have an equivalent concentration of GNRs; however, the optical density of GNRs is decreased in Figure 3d,e because of thinner gel layers than those layers shown in Figure 3a–c. Additionally, while the hydrogel in Figure 3d is isotropic, Figure 3e’s hydrogel possesses guest–host alignment, as indicated by N. For those cases with guest–host alignment in single-layer hydrogels, to demonstrate that the longitudinal axes of the GNRs are aligned with the printing direction as described by the director N, Figure 3f plots the LSPR and TSPR extinction peaks as a function of the angle θ between the incident polarization P and the director N. Both LSPR and TSPR extinction data exhibit cos2 θ extinction behavior, as demonstrated by the LSPR’s solid red and TSPR’s dashed green best-fit curves. At 0°, N ∥ P such that the LSPR extinction peak predominates. Meanwhile, at ± 90°, N ⊥ P such that the TSPR extinction peak predominates. To understand the degree of the GNRs’ alignment with N, the scalar order parameters for GNR inclusions in hydrogels manufactured with procedure 1 and 2 were measured as S1 = 0.45 ± 0.13 and S2 = 0.34 ± 0.07, respectively, while the maximum dichroic ratios were found to be R1 = 3.2 ± 1.2 and R2 = 2.6 ± 0.46, respectively. Normalized extinction coefficients were estimated to be ∼2.8 OD mm–1 at the TSPR peak and ∼3.4 OD mm–1 at the LSPR peak (see the Supporting Information for measurement and calculation procedures).
Figure 3.
Optically anisotropic metamaterial hydrogels. (a–c) Single-layer hydrogels printed via Procedure 1. (a) With N ∥ P, as shown by the double-headed arrows, the LSPR mode predominates, giving a blueish hue. (b) The disc in (a) rotated in-plane by 90° such that N ⊥ P with the TSPR predominating, giving a reddish hue. (c) The “CU” logo with continuously changing N(r), as indicated by the blue curves, simultaneously exhibits extinction from the LSPR and TSPR modes. (d, e) Single-layer disks printed using Procedure 2. (d) Isotropic organization of both CNCs and GNRs. (e) Guest–host alignment with incident light of polarization P, experiencing TSPR extinction. (f) Analysis of GNR alignment with average N in a single-layer disc prepared according to Procedure 2. The LSPR extinction peak predominates when N ∥ P (0°), while the TSPR extinction peak predominates when N ⊥ P (±90°). Both extinction-peak maxima demonstrate that the GNRs’ longitudinal axes are aligned with the printing direction and N. (g) The birefringent disc from (a) and (b) under polarizing optical microscopy (POM) with N ∥ P results in a minimum transmitted intensity, where the transmission axes of the polarizer P and analyzer A are as shown. (h) With N rotated 45° from either P or A, the birefringent disc exhibits peak transmitted intensity of the incident white light. (i) With N ∥ A under POM, an intensity minimum occurs again. The POM micrographs in (g)–(i) indicate that average director N is parallel with the printing direction, which can be seen as the striping in each image. POM analysis provides wavelength-independent evidence of the CNCs’ N alignment with the direction of printing. (j) A two-layer hydrogel micrograph captured with brightfield microscopy. The horizontal stripes represent the top layer that rests on the bottom layer, which is printed vertically. (k) The sample in (j) under POM and a full-wave retardation plate with slow-axis γ at 530 nm. Bottom (blue-appearing) and top (yellow-appearing) layers have a uniform intralayer N with orthogonal interlayer alignment. For a layer exhibiting a blue color, N ∥ γ, while for a layer exhibiting a yellow color, N ⊥ γ. (l) The sample in (k) with reversed color schemes demonstrating an in-plane rotation of 90°.
The average CNC director N alignment, which is analyzed independently from the average GNR alignment within a hydrogel, can be understood qualitatively with polarizing optical microscopy (POM) micrographs, as shown in Figure 3g–i. Intensity minima are observed whenever N ∥ P or N ∥ A, where A and P are the analyzer’s and polarizer’s transmission axes, as shown in Figure 3g,i, respectively. Additionally, an intensity maximum is achieved when the sample is at a 45° displacement from either P or A. The multiplicity of colors shown in Figure 3h is a result of the material’s varying thickness but homogeneous composition in the plane orthogonal to N or slight director relaxation after extrusion but before cross-linking. The observed POM intensity minima and maximum suggest that, for single-layer hydrogels with a sufficiently large CNC concentration, PAM-CNC-GNR hydrogels have a CNC director that is, on average, parallel to the printing direction within the plane of the flat hydrogel. Moreover, in this case, the alignment of GNRs with CNCs demonstrates that the existence of GNR alignment and of GNR-polarization-sensitive optical properties may be understood generally by the co-existing CNC alignment.
Multilayer hydrogels, like the two-layer lattice shown with brightfield microscopy in Figure 3j, also have been characterized. Either Procedure 1 or 2 can be used to generate multilayer structures with the additional step of partial cross-linking of each layer after completion and before writing the next, upper layer. For the two-layer hydrogel in Figure 3j, prior to the addition of the top layer, the bottom layer was partially cross-linked by exposure to UV light (2 mW/cm2 at 275 nm) for 2 min. The extra UV-exposure step ensures that intralayer CNC-GNR alignment and optical properties are preserved within the multilayer gel while enabling the second layer to be cross-linked to the first in the final UV-exposure step. Uniform intralayer CNC director alignment is demonstrated in Figure 3k,l, using a full-wave plate with 530 nm retardation along the slow-axis γ. For Figure 3k,l, the blue-appearing color indicates parallel alignment of a layer’s average director N with γ. Conversely, the yellow-appearing color indicates that a layer’s N is aligned orthogonal to γ. In Figure 3k, the director is rotated by 90° from the first (blue) to the second (yellow) layer. Meanwhile, Figure 3l displays the same sample as in Figure 3k but rotated in-plane by 90°. After this rotation, the first layer now appears yellow while the second appears blue. Through the consistency of each layer’s coloration, Figure 3k,l reveals that each intralayer director is preserved on average despite the presence of an additional layer above or below the other layer.
The samples in Figure 3a–c,g–i are printed with Procedure 1. Those in Figure 3d,e,j–l are printed with Procedure 2 using 9.4 wt % CNCs with 10.2 wt % AAm (Figure 3e), 0.76 wt % CNCs (Figure 3d), and 10 wt % CNCs (Figure 3j–l). Figure 3f was generated with data from a single-layer disc prepared with Procedure 2 (9.4 wt % CNCs with 10.2 wt % AAm). All single-layer gels have an equivalent GNR concentration. All printed samples have an individual layer thickness of ∼0.35 mm.
3.2. Hydrogel-to-Aerogel Solvent Exchange and Optical Comparison
The PAM-CNC-GNR gels’ solvent does not need to be water; though hydrogels are a convenient embodiment of the aligned PAM-CNC-GNR gel system, gels with nonaqueous solvents present further opportunities to control light.
By adjusting the solvent’s refractive index, the plasmonic extinction peaks can be shifted because of a different refractive index contrast between the surface of the GNRs and their surrounding media.39 Spectral analysis of a gel’s extinction peaks before and after conversion from a hydrogel to an aerogel is shown in Figure 4a. The extinction peaks’ blueshift, especially of the LSPR mode, may be qualitatively observed by comparison between the hydrogel’s and aerogel’s colors in Figure 4b,c, respectively, which portray the corner of one gel in the shape of CU, whose full extent is visible in Figure 4d,e.
Figure 4.
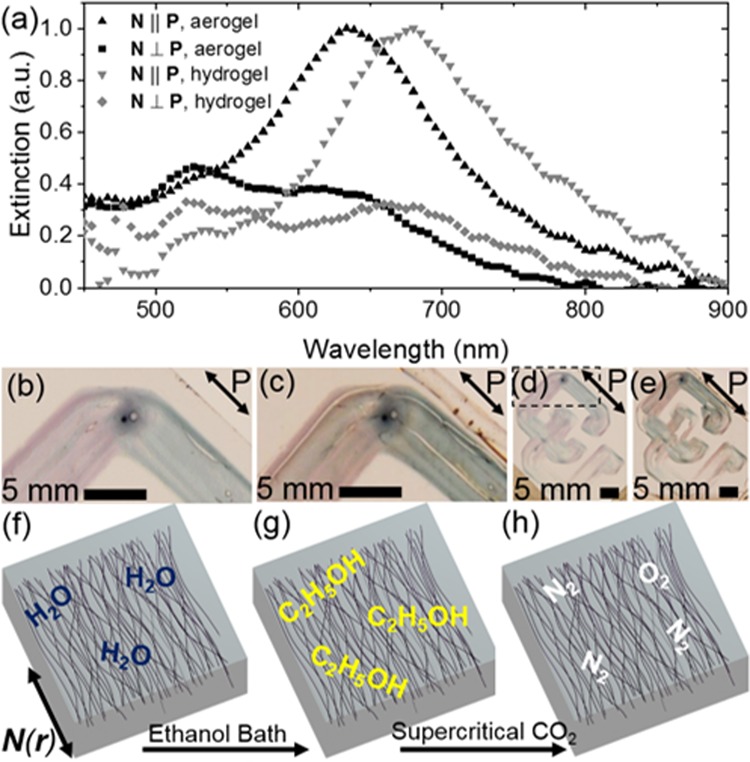
(a) Spectral extinction peaks (LSPR: N ∥ P, TSPR: N ⊥ P), with incident polarization P from a hydrogel and its subsequent aerogel. (b) A subsection of the CU-shaped plasmonic hydrogel exhibiting the reddish and bluish appearing TSPR and LSPR absorption modes, respectively. (c) The same section is shown in (b) after the hydrogel becomes an aerogel. (d) The entire CU-shaped hydrogel whose subsection in (b) is indicated by the dashed rectangle. (e) The entire CU-shaped aerogel. (f) The printed hydrogel’s guest–host alignment is represented by the black curves with director field N(r). (g) An alcogel with ethanol. (h) An aerogel with preserved structure and N(r) formed by supercritical carbon dioxide drying.
The procedure to form aerogels from hydrogels is presented schematically in Figure 4f–h, wherein the printed hydrogel (Figure 4f) is immersed in an absolute ethanol bath to yield an alcogel (Figure 4g). Direct evaporation of a liquid solvent under ambient conditions can collapse a gel’s network because of surface-tension forces from the retreating liquid during evaporation. Because ethanol is miscible with liquid carbon dioxide while water is not, the water-to-ethanol solvent exchange functions as an intermediate step for supercritically dried aerogels. Supercritical carbon dioxide can transition seamlessly to a gaseous state without a discrete phase transition; thus, in the absence of surface tension, its conversion into a gas does not collapse or otherwise damage the gel’s solid network.40 The resultant aerogel (Figure 4h) maintains the solid network’s structural alignment and optical anisotropy present in both its hydrogel and alcogel states.
4. Conclusions
We have developed a plasmonic metamaterial whose guest–host optical interactions cause polarization-dependent extinction of incident light. Capitalizing on 3D printing, we have programmed spatially complex optical extinction behavior across the lateral extent of each printed layer. Moreover, we have shown that the direct-ink-writing 3D printing method enables macroscopic physical scaling in the gel’s lateral spatial extent and of the number of layers. Selective alignment and layering permit the formulator to tune the optical properties of the gel. By converting the printed hydrogel into an aerogel, the plasmonic resonances blueshift while the aerogel’s selective extinction of polarized radiation suggests polarization-sensitive and ultralight materials’ applications.41 Our work propounds the usage of these composite gels as hosts for upconverting plasmonic nanoparticles, nanoantennas, or both. Mechanically and electrically coupled via elastic assembly, such novel guest particles could enhance both optical absorption by intrinsic plasmonic resonances and the resultant fluorescence of higher-energy photonic signals.42 We hypothesize that an additional possibility to extend this work would be to actuate the gels photomechanically by inclusion of azobenzene-surface-functionalized plasmonic nanoparticles within the gel’s network.43−45 Finally, this guest–host LC gel architecture may be suitable as a template for high-efficiency advanced electronics, such as pn-junctions in photovoltaics, wherein aberrant excitonic carrier depletion is decreased by increasing electrode and recombination area and proximity.46
Acknowledgments
We acknowledge discussions with H. Mundoor, E. Abraham, and K. McCarthy. We thank Tomoko Borsa at the Nanomaterials Characterization Facility, University of Colorado Boulder for assistance with FIB-SEM measurements. A.J.H. thanks the National Renewable Energy Laboratory for hosting and the Office of Science Graduate Student Research Program for support while the manuscript was written. This research was supported by the U.S. Department of Energy, ARPA-E award DE-AR0000743. Publication of this article was funded by the University of Colorado Boulder Libraries Open Access Fund.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02418.
Preparation of gold nanorods; preparation of cellulose nanocrystals; preparation of hydrogels: Procedure 1; preparation of hydrogels: Procedure 2; 3D printing equipment design; 3D printing procedures and parameters; preparation of aerogels; optical imaging and spectroscopy; degree of order of plasmonic inclusions, spectral data collection, and analysis methodology; and electron microscopy (PDF)
Author Present Address
⊥ Physics Department, King Faisal University, Hofuf 31982, Saudi Arabia (G.H.S.).
Author Present Address
∥ Department of Physics, Cornell University, 109 Clark Hall, Ithaca, New York 14853, United States (Q.L.).
The authors declare no competing financial interest.
Supplementary Material
References
- Ngo T. D.; Kashani A.; Imbalzano G.; Nguyen K. T. Q.; Hui D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Composites, Part B 2018, 143, 172–196. 10.1016/j.compositesb.2018.02.012. [DOI] [Google Scholar]
- Wu P.; Wang J.; Wang X. A Critical Review of the Use of 3-D Printing in the Construction Industry. Autom. Constr. 2016, 68, 21–31. 10.1016/j.autcon.2016.04.005. [DOI] [Google Scholar]
- Frazier W. E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. 10.1007/s11665-014-0958-z. [DOI] [Google Scholar]
- Hübler A.; Trnovec B.; Zillger T.; Ali M.; Wetzold N.; Mingebach M.; Wagenpfahl A.; Deibel C.; Dyakonov V. Printed Paper Photovoltaic Cells. Adv. Energy Mater 2011, 1, 1018–1022. 10.1002/aenm.201100394. [DOI] [Google Scholar]
- Lewis J. A.; Ahn B. Y. Three-Dimensional Printed Electronics. Nature 2015, 518, 42–43. 10.1038/518042a. [DOI] [PubMed] [Google Scholar]
- Chanda D.; Shigeta K.; Gupta S.; Cain T.; Carlson A.; Mihi A.; Baca A. J.; Bogart G. R.; Braun P.; Rogers J. A. Large-Area Flexible 3D Optical Negative Index Metamaterial Formed by Nanotransfer Printing. Nat. Nanotechnol. 2011, 6, 402–407. 10.1038/nnano.2011.82. [DOI] [PubMed] [Google Scholar]
- Tian K.; Bae J.; Bakarich S. E.; Yang C.; Gately R. D.; Spinks G. M.; In Het Panhuis M.; Suo Z.; Vlassak J. J. 3D Printing of Transparent and Conductive Heterogeneous Hydrogel–Elastomer Systems. Adv. Mater. 2017, 29, 1604827 10.1002/adma.201604827. [DOI] [PubMed] [Google Scholar]
- Martinez A.; Lee T.; Asavei T.; Rubinsztein-Dunlop H.; Smalyukh I. I. Three-Dimensional Complex-Shaped Photopolymerized Microparticles at Liquid Crystal Interfaces. Soft Matter 2012, 8, 2432–2437. 10.1039/c2sm07125h. [DOI] [Google Scholar]
- Wang X.; Jiang M.; Zhou Z.; Gou J.; Hui D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos., Part B 2017, 110, 442–458. 10.1016/j.compositesb.2016.11.034. [DOI] [Google Scholar]
- Squires A. D.; Constable E.; Lewis R. A. 3D Printed Terahertz Diffraction Gratings and Lenses. J. Infrared, Millimeter, Terahertz Waves 2015, 36, 72–80. 10.1007/s10762-014-0122-8. [DOI] [Google Scholar]
- Thiele S.; Arzenbacher K.; Gissibl T.; Giessen H.; Herkommer A. M. 3D-Printed Eagle Eye: Compound Microlens System for Foveated Imaging. Sci. Adv. 2017, 3, e1602655 10.1126/sciadv.1602655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X.; Roach D. J.; Wu J.; Hamel C. M.; Ding Z.; Wang T.; Dunn M. L.; Qi H. J. Advances in 4D Printing: Materials and Applications. Adv. Funct. Mater. 2018, 29, 1805290 10.1002/adfm.201805290. [DOI] [Google Scholar]
- Godoi F. C.; Prakash S.; Bhandari B. R. 3D Printing Technologies Applied for Food Design: Status and Prospects. J. Food Eng. 2016, 179, 44–54. 10.1016/j.jfoodeng.2016.01.025. [DOI] [Google Scholar]
- Yang H.; Liu J-J.; Wang Z-F.; Guo L-X.; Keller P.; Lin B-P.; Sun Y.; Zhang X-Q. Near-Infrared-Responsive Gold Nanorod/Liquid Crystalline Elastomer Composites Prepared by Sequential Thiol-Click Chemistry. Chem. Commun. 2015, 51, 12126–12129. 10.1039/C5CC02599K. [DOI] [PubMed] [Google Scholar]
- Pletsch H.; Tebbe M.; Dulle M.; Forster B.; Fery A.; Forster S.; Greiner A.; Agarwal S. Reversible Gold Nanorod Alignment in Mechano-Responsive Elastomers.. Polymer 2015, 66, 167–172. 10.1016/j.polymer.2015.04.037. [DOI] [Google Scholar]
- Hsu S-W.; Rodarte A. L.; Som M.; Arya G.; Tao A. R. Colloidal Plasmonic Nanocomposites: From Fabrication to Optical Function. Chem. Rev. 2018, 118, 3100–3120. 10.1021/acs.chemrev.7b00364. [DOI] [PubMed] [Google Scholar]
- Thoniyot P.; Tan M. J.; Karim A. A.; Young D. J.; Loh X. J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-C.; Zhang Y. S.; Akpek A.; Shin S. R.; Khademhosseini A. 4D Bioprinting: The Next-Generation Technology for Biofabrication Enabled by Stimuli-Responsive Materials. Biofabrication 2017, 9, 012001 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- Gladman A. S.; Matsumoto E. A.; Nuzzo R. G.; Mahadevan L.; Lewis J. A. Biomimetic 4D Printing. Nat. Mater. 2016, 15, 413–418. 10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- De La Cruz J. A.; Liu Q.; Senyuk B.; Frazier A. W.; Peddireddy K.; Smalyukh I. I. Cellulose-Based Reflective Liquid Crystal Films as Optical Filters and Solar Gain Regulators. ACS Photonics 2018, 5, 2468–2477. 10.1021/acsphotonics.8b00289. [DOI] [Google Scholar]
- Liu Q.; Frazier A. W.; Zhao X.; De La Cruz J. A.; Hess A. J.; Yang R.; Smalyukh I. I. Flexible Transparent Aerogels as Window Retrofitting Films and Optical Elements with Tunable Birefringence. Nano Energy 2018, 48, 266–274. 10.1016/j.nanoen.2018.03.029. [DOI] [Google Scholar]
- Hess A. J.; Liu Q.; Smalyukh I. I.. Bacterial Cellulose Gels, Process for Producing and Methods of Use. (Regents of the University of Colorado). U.S. Patent US2019/0055373 A12019.
- Zhou C.; Wu Q.; Lei T.; Negulescu I. I. Adsorption Kinetic and Equilibrium Studies for Methylene Blue Dye by Partially Hydrolyzed Polyacrylamide/Cellulose Nanocrystal Nanocomposite Hydrogels. Chem. Eng. J. 2014, 251, 17–24. 10.1016/j.cej.2014.04.034. [DOI] [Google Scholar]
- Yang X.; Bakaic E.; Hoare T.; Cranston E. D. Injectable Polysaccharide Hydrogels Reinforced with Cellulose Nanocrystals: Morphology, Rheology, Degradation, and Cytotoxicity. Biomacromolecules 2013, 14, 4447–4455. 10.1021/bm401364z. [DOI] [PubMed] [Google Scholar]
- Yang J.; Han C-R.; Duan J-F.; Xu F.; Sun R-C. Mechanical and Viscoelastic Properties of Cellulose Nanocrystals Reinforced Poly(ethylene glycol) Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207. 10.1021/am4001997. [DOI] [PubMed] [Google Scholar]
- Abitbol T.; Johnstone T.; Quinn T. M.; Gray D. G. Reinforcement with Cellulose Nanocrystals of Poly(vinyl alcohol) Hydrogels Prepared by Cyclic Freezing and Thawing. Soft Matter 2011, 7, 2373–2379. 10.1039/c0sm01172j. [DOI] [Google Scholar]
- Giese M.; Blusch L. K.; Khan M. K.; MacLachlan M. J. Functional Materials from Cellulose-Derived Liquid-Crystal Templates. Angew. Chem., Int. Ed. 2015, 54, 2888–2910. 10.1002/anie.201407141. [DOI] [PubMed] [Google Scholar]
- Lahiji R. R.; Xu X.; Reifenberger R.; Raman A.; Rudie A.; Moon R. J. Atomic Force Microscopy Characterization of Cellulose Nanocrystals.. Langmuir 2010, 26, 4480–4488. 10.1021/la903111j. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Yuan Y.; Smalyukh I. I. Electrically and Optically Tunable Plasmonic Guest–Host Liquid Crystals with Long-Range Ordered Nanoparticles. Nano Lett. 2014, 14, 4071–4077. 10.1021/nl501581y. [DOI] [PubMed] [Google Scholar]
- Sheetah G. H.; Liu Q.; Smalyukh I. I. Self-Assembly of Predesigned Optical Materials in Nematic Codispersions of Plasmonic Nanorods. Opt. Lett. 2016, 41, 4899–4902. 10.1364/OL.41.004899. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Campbell M. G.; Evans J. S.; Smalyukh I. I. Orientationally Ordered Colloidal Co-Dispersions of Gold Nanorods and Cellulose Nanocrystals. Adv. Mater. 2014, 26, 7178–7184. 10.1002/adma.201402699. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Senyuk B.; Tang J.; Lee T.; Qian J.; He S.; Smalyukh I. I. Plasmonic Complex Fluids of Nematiclike and Helicoidal Self-Assemblies of Gold Nanorods with a Negative Order Parameter. Phys. Rev. Lett. 2012, 109, 088301 10.1103/PhysRevLett.109.088301. [DOI] [PubMed] [Google Scholar]
- Onsager L. The Effects of Shape on the Interaction of Colloidal Particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. 10.1111/j.1749-6632.1949.tb27296.x. [DOI] [Google Scholar]
- Burtscher P. Stability of Radicals in Cured Composite Materials. Dent. Mater. 1993, 9, 218–221. 10.1016/0109-5641(93)90064-W. [DOI] [PubMed] [Google Scholar]
- Tanaka H.; Sato T.; Otsu T. A Study on Long-Lived Propagating Polymer Radicals of Acrylamide Derivatives at Room Temperature. Makromol. Chem. 1979, 180, 267–269. 10.1002/macp.1979.021800128. [DOI] [Google Scholar]
- Rintoul I.; Wandrey C. Limit of Applicability of the Monomer-Enhanced Mechanism for Radical Generation in Persulfate Initiated Polymerization of Acrylamide. Lat. Am. Appl. Res. 2010, 40, 365–372. [Google Scholar]
- Kattner H.; Buback M. EPR Study of Backbiting in the Aqueous-Solution Polymerization of Acrylamide. Macromol. Rapid Commun. 2015, 36, 2186–2191. 10.1002/marc.201500479. [DOI] [PubMed] [Google Scholar]
- Rodrigues E. J. D. R.; Neto R. P. C.; Sebastiao P. J. O.; Tavares M. I. B. Real-Time Monitoring by Proton Relaxometry of Radical Polymerization Reactions of Acrylamide in Aqueous Solution. Polym. Int. 2018, 67, 675–683. 10.1002/pi.5546. [DOI] [Google Scholar]
- Mock J. J.; Smith D. R.; Schultz S. Local Refractive Index Dependence of Plasmon Resonance Spectra from Individual Nanoparticles. Nano Lett. 2003, 3, 485–491. 10.1021/nl0340475. [DOI] [Google Scholar]
- Brinker C. J.; Scherer G. W.. Sol–gel Science: The Physics and Chemistry of Sol–gel Processing; Academic Press: New York, 1990; pp 453–505. [Google Scholar]
- White T. J.; Broer D. J. Programmable and Adaptive Mechanics with Liquid Crystal Polymer Networks and Elastomers. Nat. Mater. 2015, 14, 1087–1098. 10.1038/nmat4433. [DOI] [PubMed] [Google Scholar]
- Ackerman P. J.; Mundoor H.; Smalyukh I. I.; van de Lagemaat J. Plasmon–Exciton Interactions Probed Using Spatial Coentrapment of Nanoparticles by Topological Singularities. ACS Nano 2015, 9, 12392–12400. 10.1021/acsnano.5b05715. [DOI] [PubMed] [Google Scholar]
- Hosono N.; Yoshikawa M.; Furukawa H.; Totani K.; Yamada K.; Watanabe T.; Horie K. Photoinduced Deformation of Rigid Azobenzene-Containing Polymer Networks. Macromolecules 2013, 46, 1017–1026. 10.1021/ma302157u. [DOI] [Google Scholar]
- Yuan Y.; Abuhaimed G. N.; Liu Q.; Smalyukh I. I. Self-Assembled Nematic Colloidal Motors Powered by Light. Nat. Commun. 2018, 9, 5040 10.1038/s41467-018-07518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi W. C.; O’Neill M.; Aldred M. P.; Kitney S. P.; Vlachos P.; Kelly S. M. Distributed Bilayer Photovoltaics Based on Nematic Liquid Crystal Polymer Networks. Chem. Mater. 2007, 19, 5475–5484. 10.1021/cm071727q. [DOI] [Google Scholar]
- Das S.; Chakraborty P.; Nandi A. K. Mechanically Robust Hybrid Hydrogels for Photovoltaic Applications. Macromol. Symp. 2016, 369, 119–124. 10.1002/masy.201600046. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.