Abstract
Background:
Changes in the levels of C-reactive protein (CRP), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) observed during periodontal disease were linked with vascular manifestations. Recent studies showed that the beta-blocker propranolol reduces the pathological parameters associated with certain molecules at sites of bone injury. Hence, in this study, we evaluated the activity of propranolol on hematological parameters and systemic concentrations of inflammatory proteins in a model of experimental periodontitis.
Materials and Methods:
Periodontal disease was induced in rats. After euthanasia, the number of inflammatory cells in each rat was quantified using histopathological assays. In addition, hematological parameters were quantitated using automated analysers, cytokine levels were determined using an enzyme-linked immunosorbent assay, and CRP levels were determined using a high-sensitivity immunoturbidimetric assay.
Results:
Low doses of propranolol suppressed the systemic production of CRP, TNF-α, and IL-6; however, the hematological parameters were not affected.
Conclusions:
β-adrenergic activation indirectly contributes to the pattern of systemic inflammatory molecules observed in periodontal disease. These molecules may initiate cardiovascular diseases as a consequence of periodontitis.
Keywords: Adrenergic system, C-reactive protein, cytokines, experimental periodontitis, vascular lesions
INTRODUCTION
Periodontal disease is a common inflammatory disease that affects the tissues surrounding teeth; it is caused by an uncontrolled immunological activation of bacterial infections affecting soft and hard tissues, leading to destruction of the periodontium.[1,2] The disease is progressive and affects certain structures around the teeth, such as the periodontal ligament and bone matrix.[3] Furthermore, an inflammatory reaction is trigged by periodontal pathogens. The host's immune response regulates both homeostasis and immunity, which is mediated by immune signaling,[1,3,4,5] especially that related to oxidative balance.[3] Periodontitis is related to an increased risk of developing several chronic inflammatory diseases, such as cardiovascular disease (CVD), metabolic syndrome, diabetes, and rheumatoid arthritis.[6,7,8] Mounting evidence indicates that inflammatory mechanisms play a considerable role in atherogenesis and CVD.[9] In this sense, immunological activation may lead to the synthesis of mediators in periodontal tissues.[10,11] The release of mediators in chronic periodontal diseases depends on several factors, including persistent infection.[12] Thus, the upregulation of inflammatory factors during periodontal diseases is associated with the concomitant development of several diseases with systemic features, including CVD.[13,14]
Recent studies demonstrated a significant reduction in C-reactive protein (CRP) and tumor necrosis factor α (TNF-α) levels after periodontal care in individuals with CVD.[9] A signaling mechanism linked the amplification of osteoclastogenesis and its inflammatory mediators to the induction of β-adrenergic receptors.[15,16] Adrenoceptors are cell membrane receptors that are expressed in several tissues throughout the body. Their activation regulates the activity of several organs, including the heart, lungs, skin, and brain,[17] as well as osteoclasts.[18] Our group recently demonstrated that blockage of adrenoceptors with propranolol, a selective β-adrenoceptor antagonist, is important for the regulation of local inflammatory mediators; they contribute to tissue injury by reducing RANK-L expression, and consequently, osteoclastogenesis in periodontal disease.[19] Propranolol decreased cell migration, activation, and proliferation in experimental studies on cancer. These effects have been attributed to the inhibition of cAMP/protein kinase (PK) A and Ras signaling, induction of the mitogen-activated PK pathway, and some transcriptional factors, resulting in increased gene expression.[20,21,22] Furthermore, β-adrenergic receptors appear to be associated with increased expression of cytokines in microglial cells.[23] However, blocking of these receptors with propranolol may reduce sympathetic hyperactivity and decrease disease onset in a model of acute traumatic coagulopathy.[24] As CVDs are associated with an increase in the amount of circulating cytokines and acute-phase proteins, the aim of this study was to determine the effect of propranolol on hematological parameters, systemic cytokines, and CRP levels in an experimental model of periodontitis.
MATERIALS AND METHODS
Animals
Ninety-day-old Wistar rats weighing 235 ± 22 g at the beginning of the study were used (n = 30). The animals were divided into five groups with equal numbers per group (n = 6). The protocol used in this study was approved by the Institutional Committee for Animal at University of Uberaba (protocol #048/2009).
Experimental design and periodontitis induction
Experimental periodontitis was induced after relaxation and sedation of the animals. Animals were submitted to general anesthesia with xylazine (10 mg/kg intraperitoneally) and ketamine (50 mg/kg intraperitoneally), after anesthesia, sterile 4/0 silk ligatures (Ethicon, Johnson e Johnson, São Paulo, SP, Brazil) were placed around of the first molars of each animal. The ligatures were left in position for the entire experimental period so inflammation could be constantly induced by bacterial colonization of the surrounding tissue. Twenty-four hours after ligation, the animals were randomly assigned to the following groups: healthy animals that received vehicle (control, without disease), sick animals that received vehicle, and sick animals that received propranolol (0.1, 5, and 20 mg/[kg • d]), previously standardized concentrations for low, medium, and high dosage.[24] For sample size calculation (n = 6), we considered the probability P = 0.5 at which some event occurs (increase and/or decrease); thus, for P = (0.5)events, P = (0.5)6 means P = 0.01 (P < 0.05).[25]
Hematological parameters
Thirty days after ligature placement, rats were anesthetized using an intraperitoneal injection of 40 mg/kg sodium pentobarbital (Nembutal®, 50 mg/mL; Dainippon Pharmaceutical, Osaka, Japan) to collect blood from their ophthalmic plexuses. The blood was separated into two tubes. One tube contained ethylenediaminetetraacetic acid (0.3 mg/mL) (Sigma-Aldrich, St. Louis, MO) for the measurement of hematological parameters, and the other did not contain any anticoagulant for protein quantification from serum. Rats were finally euthanized by administration of an overdose of anesthesia. Blood counts were measured using an automated analyser (ABX MICROS 60; Horiba ABX Diagnostics; France), and the differential leukocyte counts were obtained manually by smearing blood onto glass slides and dyeing with a panoptic stain (Newprov Products Laboratory LTDA, Brazil).
Histological procedures and histometric analyses
The mandible was dissected, fixed in 10% buffered neutral formalin for 48 h, and decalcified in 10% ethylenediaminetetraacetic acid for 3 months, followed by dehydration, diaphanization, and paraffinization. Each sample was subsequently sliced into 6-μm sections; when the furcation area was completely evident, the first and last sections were excluded. Sections were mounted on glass slides and stained with hematoxylin and eosin for histometric analysis.[19,26] Images were evaluated using the ImageJ software (Rasband, WS; ImageJ, US National Institutesof Health, Bethesda, MD, USA) package to calculate the number of cells per unit area. The area analyzed was standardized by random insertion of a square (51.25 × 10-3 mm2) into the area evaluated, to achieve a total area of 10.25 × 10-1 mm2 per animal.
Cytokine detection
Serum levels of TNF-α and interleukin 6 (IL-6) were determined by an enzyme-linked immunosorbent assay as per the manufacturer's instructions (R and D Systems, Minneapolis, MN, United States). Cytokine concentration was expressed in pg/mL. Results were calculated using the standard curves for each assay.
Estimation of C-reactive protein concentration
Whole blood was centrifuged at 1831 g for 10 min, and serum CRP was evaluated using an immunoturbidimetric CRP-latex high-sensitivity assay (Denka Seiken, Tokyo, Japan) (lower limit ≈ 0.1 mg/L; upper limit = 10 mg/L). An internal quality control was applied for all laboratory tests.[27]
Statistical analysis
The Prism 4.0 software package was used (GraphPad, La Jolla, CA, USA). Normality (Kolmogorov–Smirnov test) and homogeneous variance tests (Bartlett's test) were applied to all variables. Parametric tests were conducted for cases with normal distributions and homogeneous variance, and the results were expressed as mean ± standard deviation. Nonparametric tests were used for cases with non-Gaussian distributions, and the results were expressed as median, maximum, and minimum values. Data with P ≤ 0.05 were considered significant.
RESULTS
Experimental periodontitis induced by ligature placement
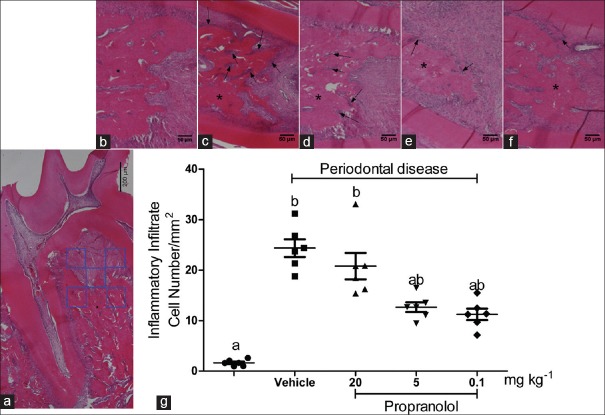
An area of 51.25 × 10-3 mm2 per histological section (a total area of 10.25 × 10-1 mm2 per animal) was evaluated to verify the increase in cell number [inflammatory infiltrate; Figure 1a]. The number of cells by area of inflammatory infiltrate increased significantly in the untreated and 20 mg/kg propranolol group compared to that in the other groups [P < 0.05; Figure 1b–g].
Figure 1.
Experimental periodontitis was induced by ligature placement. (a) Panoramic image of histological sections. The quadrants indicate the area of analysis per image and the asterisk indicates the alveolar bone. (b-f) Representative images of each group (sham-ligated and ligated with vehicle, 20, 5, and 0.1 mg/[kg•d] propranolol, respectively). The arrows indicate the inflammatory infiltrate. (g) Comparison of proportions of the infiltrated cells by area evaluated between the different groups. The letters a, b, and c represent statistical differences among groups (P < 0.05)
Plasma concentrations of tumour necrosis factor-α, interleukin-6, and C-reactive protein
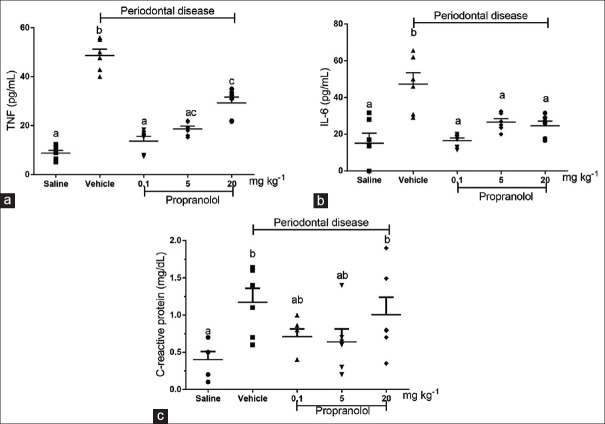
Systemic levels of TNF-α, IL-6, and CRP were determined [Figure 2]. We observed a significant increase (P < 0.05) in the plasma concentration of TNF-α in animals with experimental periodontitis (48.59 ± 3.59 pg/mL), compared to that in animals without periodontitis (8.83 ± 2.07 pg/mL) [Figure 2a]. The same pattern was observed for IL-6 (15.04 ± 5.48 pg/mL in the group without periodontitis and 47.25 ± 6.22 pg/mL in the group with periodontitis). TNF-α production in animals with periodontitis treated with 0.1 mg/kg propranolol (13.67 ± 1.93 pg/mL) or 5 mg/kg propranolol (18.67 ± 1.14 pg/mL) was strongly and significantly lower than that in animals with periodontitis not treated with propranolol (P < 0.05). Rats treated with 20 mg/kg propranolol also showed significantly lower TNF-α production than the untreated disease group, although this effect was notably less evident than in low-propranolol groups [Figure 2a]. Regarding IL-6 production, all the groups treated with different doses of propranolol showed significantly lower IL-6 production than the untreated group (47.25 ± 6.22 pg/mL): 16.48 ± 1.42 pg/mL in the group treated with 0.1 mg/kg, 26.53 ± 1.93 pg/mL in the group treated with 5 mg/kg, and 24.58 ± 2.50 pg/mL in the group treated with 20 mg/kg propranolol [Figure 2b]. As shown in Figure 2c, animals with experimental periodontitis had higher concentrations of CRP (1.175 ± 0.142 ng/mL) than animals without periodontitis (0.400 ± 0.096 ng/mL). CRP production in animals with periodontitis treated with low concentrations of propranolol (0.1 and 5 mg/kg) did not differ significantly from that in the control group (P < 0.05). In contrast, animals with periodontitis treated with high doses of propranolol showed CRP levels similar to that of the untreated animals.
Figure 2.
Effects of propranolol administration on systemic levels of pro-inflammatory cytokines and C-reactive protein. Thirty days after periodontitis induction and treatment with propranolol, serum levels of tumor necrosis factor-α (a), interleukin-6 (b), and C-reactive protein (c), were estimated. The letters a, b, and c represent statistically signifi cant differences among groups (P < 0.05). ANOVA followed by Bonferroni's test was performed to compare groups
Effects of propranolol on hematological parameters from animals with experimental periodontitis
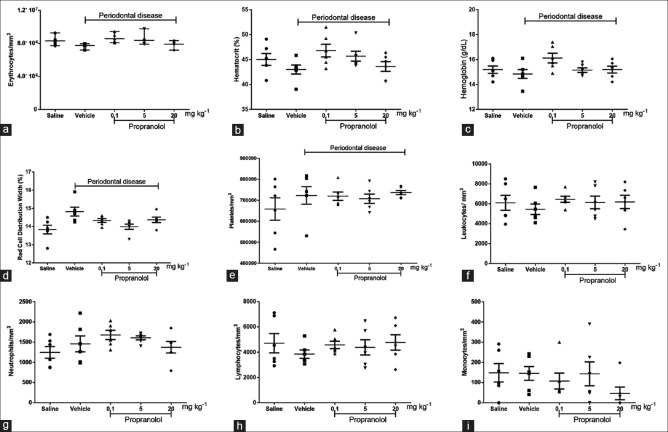
We also assessed differences in the counts of erythrocytes, platelets, total leukocytes, neutrophils, lymphocytes, and monocytes in the blood of animals in the different groups in this study. We observed a decrease in the number of erythrocytes (6.81%), hematocrit (4.49%), hemoglobin (30%), leukocytes (10.65%), and lymphocytes (18.25%) in animals with induced periodontitis compared to the negative control. An increase in red blood cell distribution width (by 7.16%), platelets (by 9.85%), and neutrophils (by 17.01%) was observed. The absolute values of monocyte counts in animals with induced periodontitis treated with 20 mg/kg of propranolol were lower than those in animals of other groups. None of the variations described for hematological parameters were statistically significant (P > 0.05). The individual results are shown in Figure 3a–i.
Figure 3.
Evaluation of hematological parameters. As depicted above, erythrocytes (a), hematocrit (b), hemoglobin (c), red cell distribution width (d), platelets (e), total leukocytes (f), neutrophils (g), lymphocytes (h), and monocytes (i) were quantified. The erythrocytes, hematocrit, hemoglobin, and red cell distribution width were evaluated automatically (ABX MICROS 60, Horiba ABX Diagnostics; France). The total number of leukocytes was counted with a Neubauer chamber, and the differential cell count (100 cells in total) was obtained from the stained blood smears. ANOVA and Bonferroni's test were used for group comparison
DISCUSSION
In this study, we observed that the production of pro-inflammatory factors decreased in our model of periodontal disease when the animals were treated with propranolol. Notably, the therapeutic dosage of propranolol varies and depends mainly on the objectives of drug therapy. Thus, in this study, we used three propranolol concentrations, the lowest of which does not alter cardiac parameters and is close to the dose proposed for the initial treatment of some hemangiomas;[28] the other doses are therapeutic doses capable of interfering with cardiac function or are above the therapeutic dose.[18] We focused on the pro-inflammatory proteins CRP, IL-6, and TNF-α, as their levels increase systemically after induction of experimental periodontal disease, and as they may be associated with CVDs.[29,30,31,32,33] Although few studies, including the present study, support these observations, there is a growing interest in developing methods for modulating the production and secretion of pro-inflammatory molecules during periodontal diseases to reduce the incidence of human CVDs.[34]
Our current data suggest that inhibition of the inflammatory process triggered by CRP, IL-6, and TNF-α, either specifically in the periodontium or systemically using β-blockers, reduces the severity of periodontal diseases, and consequently, the risk of CVDs. In the present study it was found that the effects of propranolol are dependent on drug concentration, and the same has been demonstrated by other researchers.[19,23,35] Smaller doses of propranolol have shown good tolerability and exceptional antagonistic action in the treatment of infantile hemangioma.[28,36] Given the limitation of the experimental model, the clinical effects of the different parameters that propranolol can act should be better evaluated. Despite evidence of a decrease in negative effects in the case of higher concentrations (5 mg/[kg • d]), propranolol does interact with the cardiovascular system,[19] leading to changes that are expected and already exploited for therapeutic use in humans, such as induction of bradycardia. At even higher concentrations (20 mg/[kg•d]), propranolol not only negatively affects the cardiovascular system but also does not effectively reduce bone resorption.[19]
We believe that adrenergic blocking of inflammation and regulation of the expression of genes involved in inflammatory responses, such as nuclear factor-κB, is also dose dependent.[19,23,24] Thus, dysregulation of the transcription of pro-inflammatory proteins[19] associated with the systemic effects of high doses of propranolol (20 mg/[kg•d]) potentiates negative effects, as shown in our study.
Despite the biological effects of propranolol, the mechanism of action of this β-blocker remains unclear. Further studies are required to determine whether the signaling characterized by the activation of several inflammatory molecules in periodontitis is affected by propranolol in periodontal disease.
Regarding adrenergic receptor ligands and periodontal diseases, we have previously established that the β-blocker propranolol suppresses the local inflammatory markers and bone resorption by inhibiting osteoclastogenesis.[19] Herein, we have further demonstrated that this suppression is reflected in the type of cells present in inflammatory infiltrates and in the systemic levels of pro-inflammatory proteins involved in cardiovascular alterations. In agreement with this result, the present study also indicates that the decrease in bone resorption observed after treatment with propranolol is dose dependent. Other side effects of propranolol have been observed and include bradycardia, hypotension, hyperglycemia, rash, gastrointestinal discomfort or reflux, fatigue, and bronchospasm.[37]
Furthermore, the results presented here can act as a basis for new clinical studies that seek to relate inflammatory diseases, including periodontitis, to the manifestations of CVD. In addition, our results demonstrate that β-adrenergic receptors are involved in the signaling and potentiation of mediators related to CVD and are dose dependently controlled by propranolol. Overall, β-adrenergic blockade indirectly contributes to the pattern of systemic inflammatory molecules that are involved in CVD progression because of experimental periodontitis.
CONCLUSION
Together, the data support the conclusion that, the β-adrenergic activation indirectly contributes to the pattern of systemic inflammatory molecules observed in periodontal disease. These molecules may initiate cardiovascular diseases as a consequence of periodontitis.
Financial support and sponsorship
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) – Research Productivity Fellowship to MHN and JTCN and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pihlstrom BL, Tabak L. The national institute of dental and craniofacial research: Research for the practicing dentist. J Am Dent Assoc. 2005;136:728–37. doi: 10.14219/jada.archive.2005.0256. [DOI] [PubMed] [Google Scholar]
- 2.Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol. 1986;13:418–30. doi: 10.1111/j.1600-051x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 3.Kanzaki H, Wada S, Narimiya T, Yamaguchi Y, Katsumata Y, Itohiya K, et al. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front Physiol. 2017;8:351. doi: 10.3389/fphys.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues WF, Miguel CB, Mendes NS, Freire Oliveira CJ, Ueira-Vieira C. Association between pro-inflammatory cytokine interleukin-33 and periodontal disease in the elderly: A retrospective study. J Indian Soc Periodontol. 2017;21:4–9. doi: 10.4103/jisp.jisp_178_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–48. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 7.Nibali L, Tatarakis N, Needleman I, Tu YK, D’Aiuto F, Rizzo M, et al. Clinical review: Association between metabolic syndrome and periodontitis: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:913–20. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- 8.Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26:315–21. doi: 10.1016/j.tem.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Koppolu P, Durvasula S, Palaparthy R, Rao M, Sagar V, Reddy SK, et al. Estimate of CRP and TNF-alpha level before and after periodontal therapy in cardiovascular disease patients. Pan Afr Med J. 2013;15:92. doi: 10.11604/pamj.2013.15.92.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renvert S, Lindahl C, Roos-Jansåker AM, Lessem J. Short-term effects of an anti-inflammatory treatment on clinical parameters and serum levels of C-reactive protein and proinflammatory cytokines in subjects with periodontitis. J Periodontol. 2009;80:892–900. doi: 10.1902/jop.2009.080552. [DOI] [PubMed] [Google Scholar]
- 11.Han DH, Shin HS, Kim MS, Paek D, Kim HD. Group of serum inflammatory markers and periodontitis-metabolic syndrome coexistence in Koreans. J Periodontol. 2012;83:612–20. doi: 10.1902/jop.2011.110304. [DOI] [PubMed] [Google Scholar]
- 12.Glurich I, Grossi S, Albini B, Ho A, Shah R, Zeid M, et al. Systemic inflammation in cardiovascular and periodontal disease: Comparative study. Clin Diagn Lab Immunol. 2002;9:425–32. doi: 10.1128/CDLI.9.2.425-432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tehrani DM, Gardin JM, Yanez D, Hirsch CH, Lloyd-Jones DM, Stein PK, et al. Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: Cardiovascular health study. Atherosclerosis. 2013;231:246–51. doi: 10.1016/j.atherosclerosis.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent F, Romagna C, Laurent Y, Chaux-Bodard AG, Veyre S, Hemar J, et al. Relationship between coronary artery disease and periodontal disease. What the cardiologist must know? Ann Cardiol Angeiol (Paris) 2007;56:297–302. doi: 10.1016/j.ancard.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi T, Tsuboi T, Arai M, Togari A. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol. 2001;61:579–86. doi: 10.1016/s0006-2952(00)00591-8. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008;8:94–104. [PubMed] [Google Scholar]
- 17.Guimarães S, Moura D. Vascular adrenoceptors: An update. Pharmacol Rev. 2001;53:319–56. [PubMed] [Google Scholar]
- 18.Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch Biochem Biophys. 2009;482:96–103. doi: 10.1016/j.abb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues WF, Madeira MF, da Silva TA, Clemente-Napimoga JT, Miguel CB, Dias-da-Silva VJ, et al. Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br J Pharmacol. 2012;165:2140–51. doi: 10.1111/j.1476-5381.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–74. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Ma QY, Hu HT, Zhang M. B2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 2010;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 22.Thaivalappil S, Bauman N, Saieg A, Movius E, Brown KJ, Preciado D. Propranolol-mediated attenuation of MMP-9 excretion in infants with hemangiomas. JAMA Otolaryngol Head Neck Surg. 2013;139:1026–31. doi: 10.1001/jamaoto.2013.4773. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Li J, Sheng X, Zhao H, Cao XD, Wang YQ, et al. Beta-adrenoceptor mediated surgery-induced production of pro-inflammatory cytokines in rat microglia cells. J Neuroimmunol. 2010;223:77–83. doi: 10.1016/j.jneuroim.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Yu WK, Lin ZL, Tan SJ, Bai XW, Ding K, et al. Impact of β-adrenoceptor blockade on systemic inflammation and coagulation disturbances in rats with acute traumatic coagulopathy. Med Sci Monit. 2015;21:468–76. doi: 10.12659/MSM.893544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: A brief survey. Am J Physiol. 1990;258:L148–56. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- 26.Napimoga MH, Benatti BB, Lima FO, Alves PM, Campos AC, Pena-Dos-Santos DR, et al. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009;9:216–22. doi: 10.1016/j.intimp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues WF, Miguel CB, Napimoga MH, Oliveira CJ, Lazo-Chica JE. Establishing standards for studying renal function in mice through measurements of body size-adjusted creatinine and urea levels. Biomed Res Int. 2014;2014:872827. doi: 10.1155/2014/872827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verburg-van Kemenade BM, Van der Aa LM, Chadzinska M. Neuroendocrine-immune interaction: Regulation of inflammation via G-protein coupled receptors. Gen Comp Endocrinol. 2013;188:94–101. doi: 10.1016/j.ygcen.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–37. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 30.Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: The heart of the matter. J Am Dent Assoc. 2006;137(Suppl):14S–20S. doi: 10.14219/jada.archive.2006.0402. [DOI] [PubMed] [Google Scholar]
- 31.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: New insights from an old molecule. QJM. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 32.Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183–93. doi: 10.1536/jhj.45.183. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JD, Zimomra ZR, Stewart LT. Beta-adrenergic receptor activation primes microglia cytokine production. J Neuroimmunol. 2013;254:161–4. doi: 10.1016/j.jneuroim.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Saffi MA, Furtado MV, Montenegro MM, Ribeiro IW, Kampits C, Rabelo-Silva ER, et al. The effect of periodontal therapy on C-reactive protein, endothelial function, lipids and proinflammatory biomarkers in patients with stable coronary artery disease: Study protocol for a randomized controlled trial. Trials. 2013;14:283. doi: 10.1186/1745-6215-14-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santosh PJ, Bell L, Lievesley K, Singh J, Fiori F. Paradoxical physiological responses to propranolol in a Rett syndrome patient: A case report. BMC Pediatr. 2016;16:194. doi: 10.1186/s12887-016-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 37.Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: Early experience at a tertiary vascular anomalies center. Laryngoscope. 2010;120:676–81. doi: 10.1002/lary.20807. [DOI] [PubMed] [Google Scholar]