Abstract
Background:
The platelet concentrates had been pioneered to be used in regenerative medicine since above a decade.
Aims and Objectives:
To compare the autologous platelet rich fibrin (PRF) and titanium prepared platelet rich fibrin (T-PRF) in the treatment of infrabony defects, clinically and radiographically and to compare the histologic difference between PRF and T-PRF by light microscopy and scanning electron microscopy (SEM).
Materials and Methods:
The present study is a split mouth randomised controlled trial study in which 20 sites were selected and randomly assigned equally into 10 sites each in group A [Test group=T-PRF] and group B [Control group=PRF]. Clinical parameters were evaluated at baseline,3 months and 9 months. Radiographic parameters were evaluated at baseline and 9 months. Histologic differences between light microscopy and SEM for both PRF and T-PRF was studied after sequential processing.
Results:
There was marked reduction in Probing Pocket depth and gain in Clinical Attachment Level in both the T-PRF and PRF groups from baseline to 9 months in intragroup comparisons. However, on intergroup comparisons, no statistical significance was seen. Radiographically, mean defect depths for both the groups showed statistically significant reduction from baseline values to 9 months on intragroup comparisons but not on intergroup comparisons. In-vitro evaluation, on both light and scanning electron microscopy, T-PRF showed denser fibril meshwork as compared to PRF.
Conclusion:
The clinical parameters and radiographic outcomes showed marked improvement at 9 months with both PRF and T-PRF in the treatment of infrabony defects from baseline values in intragroup comparison. However, statistically efficacy of T-PRF was not seen to be superior to that of PRF both clinically and radiographically. Histologic evaluation showed T-PRF had denser fibrils as compared to PRF in both light and scanning electron microscopy.
Keywords: Infrabony defect, platelet-rich fibrin, regeneration, titanium-prepared platelet-rich fibrin
INTRODUCTION
Periodontal regeneration is defined as reproduction or reconstitution of lost or injured part.[1] Currently, procedures that are used in periodontal regeneration offer limited potential toward attaining complete periodontal restoration.
In 1971, Robert Marx showed evidence that platelets are reservoirs of growth factors and cytokines which are the key factors for bone and soft-tissue regeneration.[2,3,4,5] Choukroun in 2001[6] prepared a second-generation platelet concentrate (platelet-rich fibrin [PRF]) which contains growth factors.[7,8] PRF clot forms a strong natural fibrin matrix which provides rapid wound healing.[9]
Despite many clinical trials, there is still a concern about health hazards of silica containing glass tubes used for the collection of blood for PRF.[10] Considering this, biocompatible titanium tubes were used for the preparation of titanium-prepared PRF (T-PRF) by Tunalı in 2013.[11] T-PRF is a platelet and leukocyte-rich fibrin similar to that obtained from classical PRF method. Histologically, T-PRF and PRF are almost similar.[12]
Numerous studies have proven the regenerative efficacy of PRF. However, there is limited literature on the clinical efficacy and histologic picture of T-PRF. Hence, this study was initiated.
MATERIALS AND METHODS
A randomized split-mouth study was conducted in the department of periodontology. Ethical clearance was obtained from institutional review board. Ten patients were recruited from the outpatient department.
Twenty sites were selected using a random sequence method and categorized as:
Group A (Test group) – 10 sites (T-PRF)
Group B (Control group) – 10 sites (PRF).
Verbal/written consent was taken. After Phase I therapy, patients were re-evaluated after 4 weeks. Patients with pocket depth ≥5 mm, with 2 or more pockets with three-walled defects, on both the sides, were included in the study.
Clinical evaluation was done presurgically [Figure 1], and at intervals of 3 months and 9 months’ postoperatively.
Figure 1.
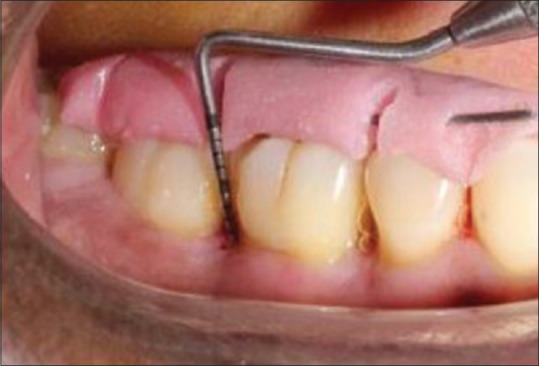
Probing depth at baseline of test group
The following clinical parameters were evaluated:
Inclusion criteria
Patients
In the age group of 35–55 years
Maintaining proper oral hygiene
Systemically healthy
Pocket depth ≥5 mmm.
Exclusion criteria
Pregnant and lactating females
Consuming tobacco in any form
Who have undergone periodontal surgical therapy in the past 6 months
Who have received antibiotic therapy in the previous 3 months.
Surgical procedure
Flap preparation
A full thickness mucoperiosteal flap was reflected exposing 1–2 mm of healthy bone.
Defect preparation
The defect was thoroughly debrided [Figure 2]. T-PRF was prepared [Figure 3]. T-PRF was placed in the defect and covered by T-PRF membrane in the test sites [Figure 4]. The T-PRF clot was cut longitudinally into two parts – one used as a graft and the second part as a membrane with the red blood cell (RBC) end of the membrane facing the defect like guided tissue regeneration (GTR). suturing was done [Figure 5].
Figure 2.
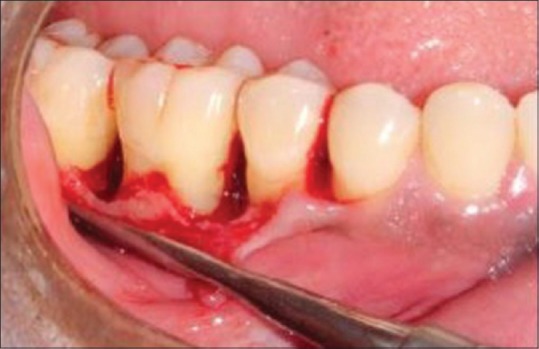
Incision, reflection, and debridement done. Postdebridement exposing the defect site (test group)
Figure 3.
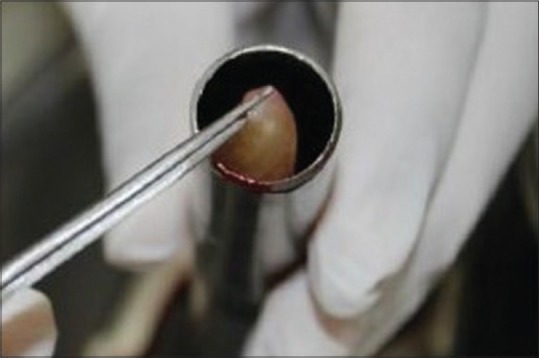
Preparation of titanium-prepared platelet-rich fibrin following centrifugation (test group)
Figure 4.
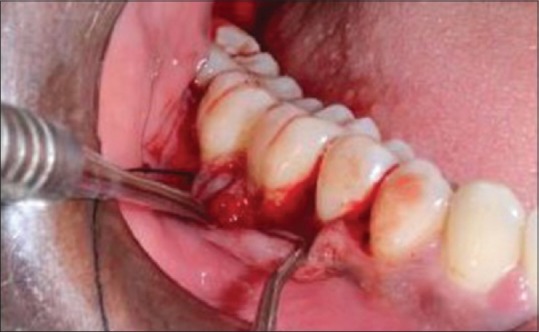
Placement of titanium-prepared platelet-rich fibrin as a Graft and membrane over the grafted site (test group)
Figure 5.
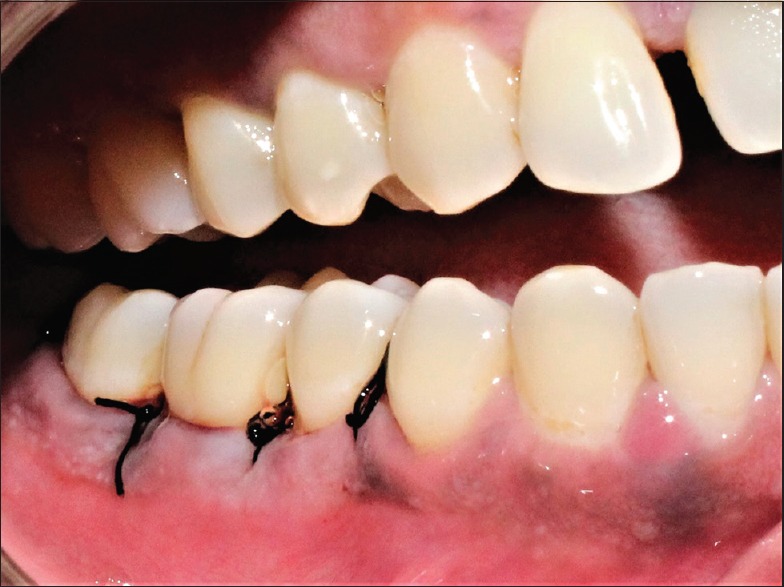
Interrupted sutures given using 4-0 silk suture (test group)
Coe-Pack was placed [Figure 6]. Postoperative instructions were given. Patients were prescribed antibiotics, analgesics, and 0.2% chlorhexidine mouthwash twice daily for 14 days. Patients were recalled at 3 and 9 months [Figures 7, 8]. Intraoral periapical radiographs were taken with paralleling technique and a radiographic grid of 1 mm2 framework on an intraoral periapical X-ray film. Grid measurement of infrabony defect on radiograph was measured using digital vernier caliper and divider set. Radiographic evaluation (i.e., defect depth) was done presurgically at baseline [Figure 9] and at 9 months’ postoperatively, for T-PRF [Figure 10]. Similar study design was used for PRF [Figures 11–16].
Figure 6.
Placement of periodontal dressing (test group)
Figure 7.
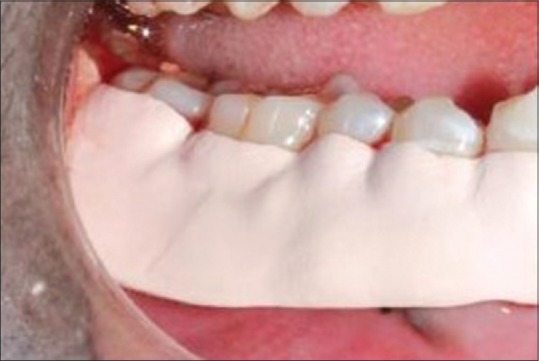
Probing depth after 3 months of test site
Figure 8.
Probing depth after 9 months shows a reduction in the pocket probing depth (test site)
Figure 9.
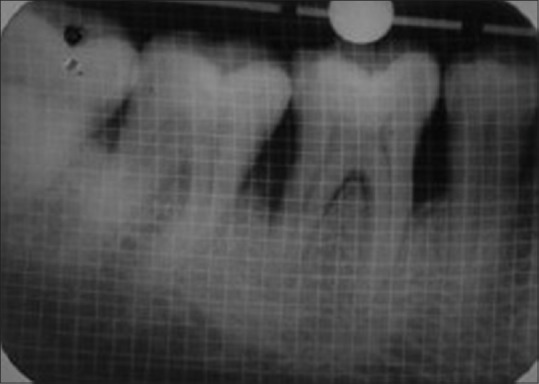
Baseline preoperative radiograph of defect site of test group
Figure 10.
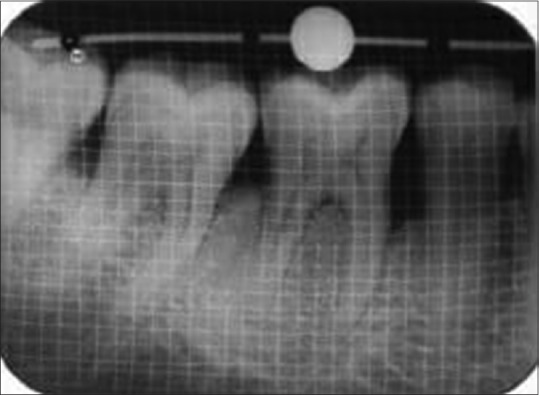
9 months’ postoperative radiograph showed increase in the height of alveolar bone (test group)
Figure 11.
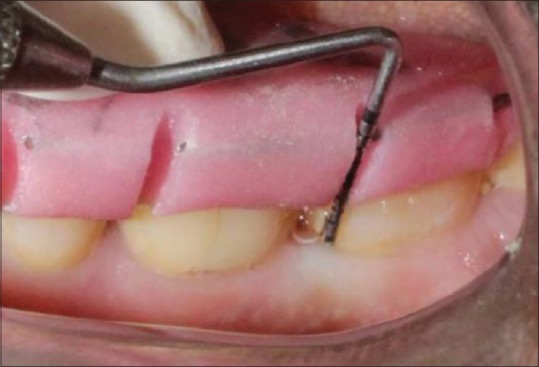
Probing depth at baseline for control group
Figure 16.
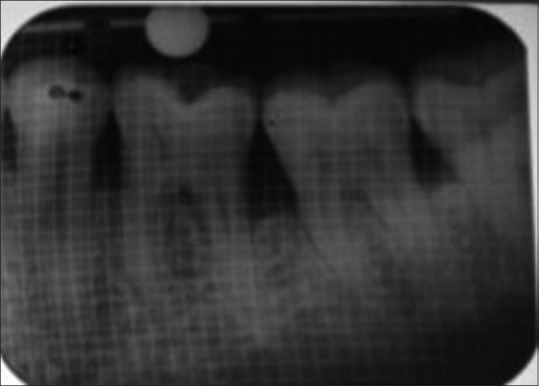
Nine months’ postoperative radiograph of defect site of control group
Figure 12.
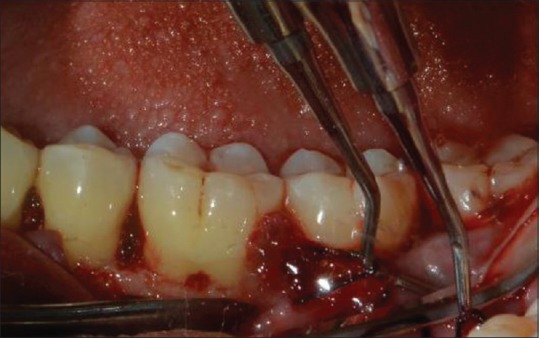
Placement of platelet-rich fibrin in the defect site (control site)
Figure 13.
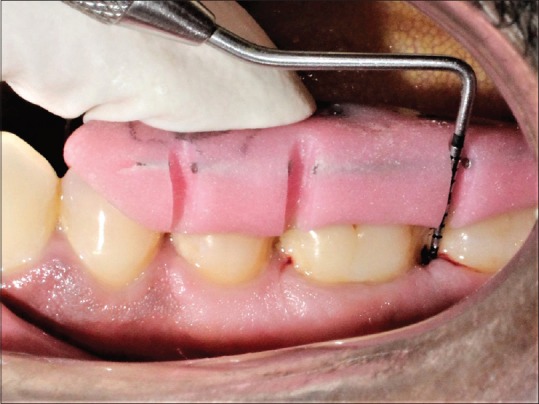
Probing depth after 3 months for control group
Figure 14.
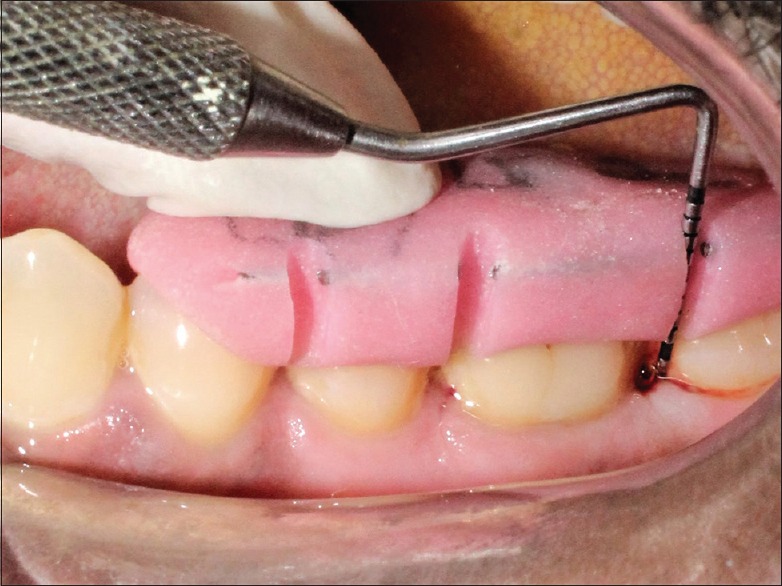
Probing depth after 9 months for control group
Figure 15.
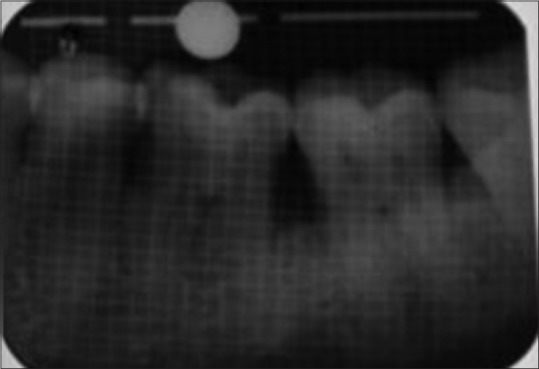
Baseline preoperative radiograph of defect site of control group
For the in vitro study, the fibrin clots derived after centrifugation was used for light microscopy and scanning electron microscopy analysis.
Histological procedures for light microscopy[16]
The T-PRF and PRF clots were fixed in 10% neutrally buffered formalin for 24 h at room temperature followed by dehydration in increasing gradients of alcohol (70%, 95%, and 100%). They were then placed in xylene before paraffin inclusion. Successive sections of 4 μ were performed along the center of the long axis of clots and then stained with hematoxylin and eosin. They were viewed in ×40 magnification under a light microscope.
Histological procedure for scanning electron microscopy[16]
The morphologic evaluation of the T-PRF and PRF clots was conducted with a scanning electron microscope (QUANTA 400). The clots were fixed in 2.5% glutaraldehyde for 24 h and treated for gradual desiccation and subsequently examined under a scanning electron microscope. Photographs were taken at 20 kV using a magnification from ×2000.
Platelet-rich fibrin preparation protocol
During surgery, 9 ml of blood was collected from antecubital vein in a plain vacutainer tube and immediately centrifuged at 3000 rpm for 10 min, at room temperature in a table-top centrifuge, REMI-R4C.[4]
Titanium-prepared platelet-rich fibrin preparation protocol
Using a similar protocol, titanium tubes were used to procure T-PRF at 2800 rpm for 12 min.[16]
Defect depth
Occlusal stents were made with acrylic and ligature wire, and a round metal ball was inserted into it. It provided a fixed reference point and fixed angulations for the clinical measurements of the infrabony defects. The ball used in stent acted as a guidance for the measurement of defect depth radiographically.[17]
Statistical analysis
Data analysis was done using Windows-based “MedCalc Statistical Software” Version 17.8.2 (MedCalcSoftware, Ostend, Belgium; http://www.medcalc.org; 2017). Data analysis was done P < 0.05 was taken for statistical significance.
The software for the calculations used was G Power 3.0.10 software. The α error was set at 5% with the power of the study at 80%.
Measurement data for probing depth, RAL, plaque index, gingival index, and defect depth are expressed as means with standard deviation and standard error of mean. Ninety-five percent confidence intervals are also presented as applicable for the mean values. Change from baseline data is presented as means with percentage means. Data for all parameters were tested using Shapiro–Wilk test for normal distribution. P < 0.05 was taken for statistical significance.
RESULTS
The reduction in PPD at 3 months was 32.79% and 42.62% at 9 months for group PRF. It was 33.33% and 43.86% at 3 and 9 months for group T-PRF. It was not statistically significant on intergroup comparison [Graph 1 and Table 1].
Graph 1.
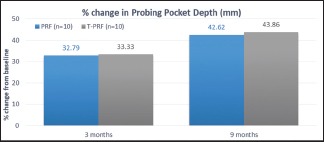
Percentage change in probing pocket depth
Table 1.
Mean reduction and percentage change in probing pocket depth (mm) amongst study group
| PERIOD | GROUP PRF | GROUP T-PRF | UNPAIRED ‘t’ TEST | |||
|---|---|---|---|---|---|---|
| MEAN CHANGE | % CHANGE | MEAN CHANGE | % CHANGE | ‘t’ | ‘p’ | |
| 3 MONTHS | 2.00 | 32.79 | 1.90 | 33.33 | 0.171 | 0.866 |
| 9 MONTHS | 2.60 | 42.62 | 2.50 | 43.86 | 0.231 | 0.820 |
The reduction in RAL at 3 and 9 months was 31.15% and 40.98% for group PRF and 31.58% and 42.11% at 3 and 9 months for group T-PRF [Graph 2 and Table 2]. It was not statistically significant on intergroup comparison.
Graph 2.
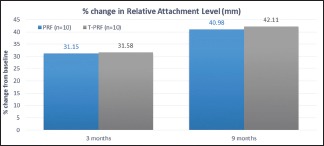
Percentage change in relative attachment level
Table 2.
Mean reduction and percentage change in relative attachment level (ral) (mm) amongst study groups
| PERIOD | GROUP PRF | GROUP T-PRF | UNPAIRED ‘t’ TEST | |||
|---|---|---|---|---|---|---|
| MEAN CHANGE | % CHANGE | MEAN CHANGE | % CHANGE | ‘t’ | ‘p’ | |
| 3 MONTHS | 1.90 | 31.15 | 1.80 | 31.58 | 0.172 | 0.866 |
| 9 MONTHS | 2.50 | 40.98 | 2.40 | 42.11 | 0.176 | 0.862 |
The reduction in defect depth at 9 months was 26.23% for group PRF and 23.74% for group T-PRF which was not statistically significant [Graph 3 and Table 3].
Graph 3.
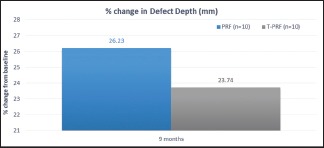
Percentage change in defect depth
Table 3.
Mean reduction and percentage change in defect depth (mm) amongst study groups
| PERIOD | GROUP PRF | GROUP T-PRF | UNPAIRED ‘t’ TEST | |||
|---|---|---|---|---|---|---|
| MEAN CHANGE | % CHANGE | MEAN CHANGE | % CHANGE | ‘t’ | ‘p’ | |
| 9 MONTHS | 1.60 | 26.23 | 1.35 | 23.74 | 0.778 | 0.447 |
In light microscopy, the PRF sample showed an organized network of fibers [Figure 17]. In T-PRF, the sections showed a highly organized structure of fibrin and cellular components [Figure 18]. Both the fibrin and cellular components appeared denser than PRF.
Figure 17.
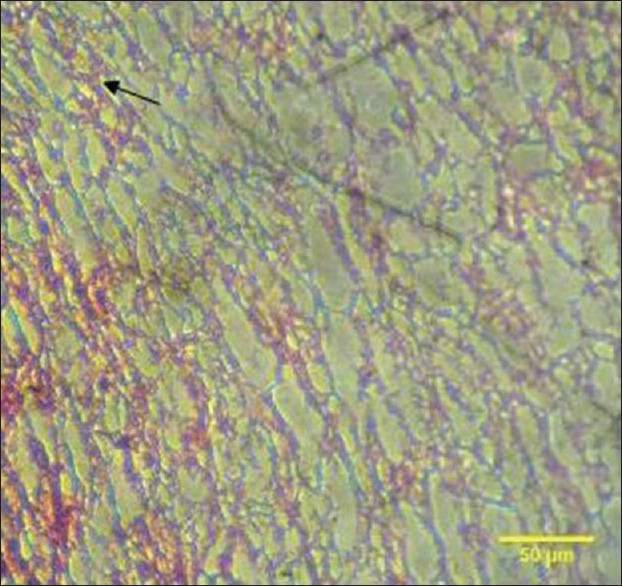
The arrow shows light microscopic analysis of platelet-rich fibrin clot showing a highly organized network of fibers. The fibrin is prominent and highly distinguishable. Dense cellular components are also observed
Figure 18.
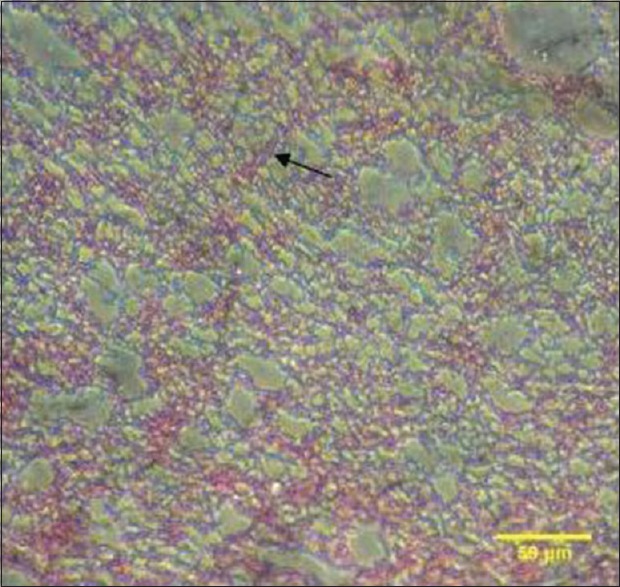
The arrow shows light microscopic analysis of titanium-prepared platelet-rich fibrin clot showing a well-organized structure of fibrin and cellular components. Both the fibrin and cellular components appeared denser and thicker as compared to platelet-rich fibrin
Scanning electron microscopy showed that PRF fibrin matrix appeared organized with line-like structures [Figure 19] whereas T-PRF fibrin matrix appeared more organized, mature, and thicker [Figure 20].
Figure 19.
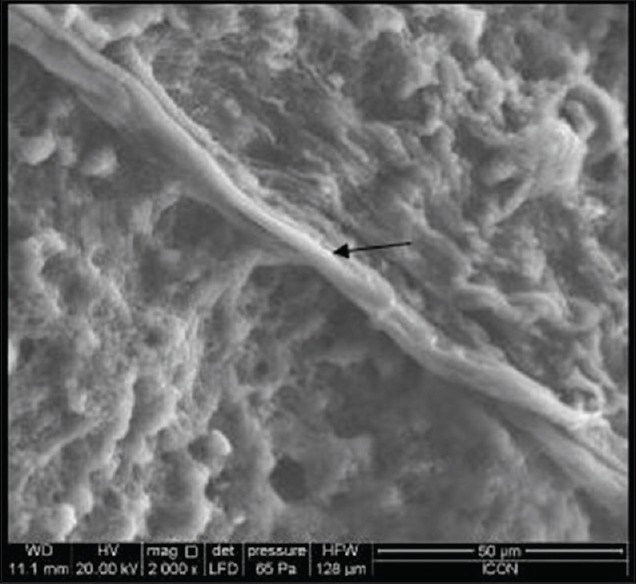
The arrow shows scanning electron microscopic showing platelet-rich fibrin matrix as organized and mature line-like structures. The presence of both thin and thick fibrils are seen
Figure 20.

The arrow shows scanning electron microscopic showing titanium-prepared platelet-rich fibrin matrix as organized, mature, and thicker (arrow)
DISCUSSION
There has not been any biomaterial which is considered as gold standard for the treatment of infrabony defects.[18] Hence, there is always a quest for an ideal biomaterial. PRF has been the most vividly researched biomaterial and was first developed as a biomaterial in France in 2001.[19]
There have been a lot of successful clinical trials that have been reported with PRF in regenerative treatment.[10,20] O’Connell[20] in a study described that contact with silica particle could not be avoided. The tubes were coated with dense silica particles which would sediment with the RBCs, a minute portion of which were suspended colloidally in buffy coat, fibrin, and platelet poor plasma layers, and thus reach the patient.
Many authors have discussed the biocompatibility and hemocompatibility of titanium.[21] Tunalı et al.[11] in their study, found that the structure of PRF and T-PRF was very similar histologically. The fibrin clot of T-PRF was denser.[11] PRF/T-PRF was used as a GTR membrane in the present study for the prevention of an epithelial migration.
In our study, the reduction in PPD in both the groups from baseline to 9 months was seen (intragroup comparison). On intergroup comparison, there was no statistically significant difference. This result is in accordance with Chatterjee et al.[22] and Sharma and Pradeep.[8]
In intragroup comparison, statistically significant gain in the attachment levels was seen at 3 and 9 months in control and the test groups. However, on intergroup comparison, there was no statistical difference. This was in accordance with a study conducted by Chatterjee et al.[22]
PRF is a reservoir of growth factors and cytokines, has shown to decrease matrix metalloproteinases-8 and interleukin-1 β but increase in tissue inhibitor of metalloproteinases-1 levels, thereby promoting periodontal soft-tissue healing.[23] It has also been reported that PRF induces a significant and continuous stimulation of proliferation in all cell types of the periodontium except epithelial cells.[24]
Both the groups showed statistically significant reduction in the defect depth from baseline values to 9 months on intragroup comparisons which were similar to a study conducted by Chatterjee et al.[22] Sharma and Pradeep.[8] On intergroup comparisons, both the groups showed statistically insignificant mean and percentage change reduction.
This depth reduction can be attributed to the property of PRF.[25] PRF stimulates the production of osteoprotegerin which in turn causes proliferation of osteoblasts.[26]
Uneventful healing in patients was observed which was in agreement with another previous study by Sharma and Pradeep.[8] Thus, supporting the fact that PRF enhances wound healing.
In light microscopy, T-PRF appears denser in both fibrin network and cellular components as compared to PRF. In scanning electron microscopy, T-PRF appears to show denser fibers and denser infiltration of cells indicating the presence of dense fibrin matrix.[27] The histologic picture is in accordance with Tunalı et al.[16]
CONCLUSION
Results of the study show that both PRF and T-PRF are equally efficacious as regenerative material. However, T-PRF was more biocompatible as it did not contain silica particles and also histologically it had denser fibrin meshwork as compared to PRF which is giving it an added advantage over PRF. Further research with a bigger sample size is required to prove the clinical and histologic efficacy of T-PRF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.4th ed. Chicago, III: The American Academy of Periodontology; 2001. The American Academy of Periodontology. Glossary of Periodontal Terms. [Google Scholar]
- 2.Giannobile W. The potential role of growth and differentiation factors in periodontal regeneration. J Periodontol. 1996;67:545–53. [PubMed] [Google Scholar]
- 3.Deodhar AK, Rana RE. Surgical physiology of wound healing: A review. J Postgrad Med. 1997;43:52–6. [PubMed] [Google Scholar]
- 4.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Nevins ML, Reynolds MA. Tissue engineering with recombinant human platelet-derived growth factor BB for implant site development. Compend Contin Educ Dent. 2011;32:18, 20–7. [PubMed] [Google Scholar]
- 6.Choukroun J, Adda F, Schoeffler C, Vervelle A. An opportunity in perio-implantology: The PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 7.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51–5. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: A randomized controlled clinical trial. J Periodontol. 2011;82:1705–12. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 9.Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J Clin Periodontol. 2011;38:925–32. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 10.Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J Periodontol. 2009;80:244–52. doi: 10.1902/jop.2009.080253. [DOI] [PubMed] [Google Scholar]
- 11.Tunalı M, Özdemir H, Küçükodacı Z, Akman S, Fıratlı E. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): A new platelet concentrate. Br J Oral Maxillofac Surg. 2013;51:438–43. doi: 10.1016/j.bjoms.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Takemoto S, Yamamoto T, Tsuru K, Hayakawa S, Osaka A, Takashima S. Platelet adhesion on titanium oxide gels: Effect of surface oxidation. Biomaterials. 2004;25:3485–92. doi: 10.1016/j.biomaterials.2003.10.070. [DOI] [PubMed] [Google Scholar]
- 13.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 14.Silness J, Loe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 15.Pihlstrom BL. Measurement of attachment level in clinical trials: Probing methods. J Periodontol. 1992;63:1072–7. doi: 10.1902/jop.1992.63.12s.1072. [DOI] [PubMed] [Google Scholar]
- 16.Tunalı M, Özdemir H, Küçükodacı Z, Akman S, Yaprak E, Toker H, et al. A novel platelet concentrate: Titanium-prepared platelet-rich fibrin. Biomed Res Int. 2014;2014:209548. doi: 10.1155/2014/209548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glickman I, Smulow JB. Philadelphia: Saunders; 1974. Periodontal Disease: Clinical, Radiographic, and Histopathologic Features. [Google Scholar]
- 18.Greenwell H. Committee on Research, Science and Therapy. American Academy of Periodontology. Position paper: Guidelines for periodontal therapy. J Periodontol. 2001;72:1624–8. doi: 10.1902/jop.2001.72.11.1624. [DOI] [PubMed] [Google Scholar]
- 19.Choukroun J, Adda F, Schoeffer C, Vervelle A. PRF: An opportunity in perio-implantology. Implantodontie. 2000;42:55–62. [Google Scholar]
- 20.O’Connell SM. Safety issues associated with platelet-rich fibrin method. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:587. doi: 10.1016/j.tripleo.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson AS, Bjursten LM, Ericson LE, Thomsen P. Hollow implants in soft tissues allowing quantitative studies of cells and fluid at the implant interface. Biomaterials. 1988;9:86–90. doi: 10.1016/0142-9612(88)90076-2. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee A, Pradeep AR, Garg V, Yajamanya S, Ali MM, Priya VS. Treatment of periodontal intrabony defects using autologous platelet-rich fibrin and titanium platelet rich fibrin: A randomized, clinical, comparative study. J Investig Clint Dent. 2017;8:e12231. doi: 10.1111/jicd.12231. [DOI] [PubMed] [Google Scholar]
- 23.Eren G, Tervahartiala T, Sorsa T, Atilla G. Cytokine (interleukin-1beta) and MMP levels in gingival crevicular fluid after use of platelet-rich fibrin or connective tissue graft in the treatment of localized gingival recessions. J Periodontal Res. 2016;51:481–8. doi: 10.1111/jre.12325. [DOI] [PubMed] [Google Scholar]
- 24.Chung-Hung T, Shih-Ya S, Jiing-Huei Z, Yu-Chao C. Platelet-rich fibrin modulates cell proliferation of human periodontally related cells in vitro. J Dent Sci. 2009;4:130–5. [Google Scholar]
- 25.Marx RE, Carlson ER. Tissue banking safety: Caveats and precautions for the oral and maxillofacial surgeon. J Oral Maxillofac Surg. 1993;51:1372–9. doi: 10.1016/s0278-2391(10)80144-2. [DOI] [PubMed] [Google Scholar]
- 26.Chang IC, Tsai CH, Chang YC. Platelet-rich fi brin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblasts. J Biomed Mater Res A. 2010;95:327–32. doi: 10.1002/jbm.a.32839. [DOI] [PubMed] [Google Scholar]
- 27.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]


