Abstract
Background:
Patent ductus arteriosus (PDA) is a common cause of morbidity in premature neonates. The purpose of this study was to compare the efficacy of oral ibuprofen and oral acetaminophen to closure of symptomatic PDA, in premature neonates with gestational age (GA) ≤32 weeks.
Materials and Methods:
This study was a randomized clinical trial with forty preterm neonates who were admitted in neonatal intensive care unit with symptomatic PDA and GA ≤32 weeks or birth body weight ≤1500 g. Twenty neonates received oral acetaminophen [Group A] and twenty neonates received oral ibuprofen [Group B] and compared with echocardiography finding each groups for closed PDA before and after treatment regiment.
Results:
Our results showed that the primary closure rate of PDA was 70% (95% confidence interval [CI]: 49.9%–90%) and 65% (95% CI: 54.3%–75.7%) in the acetaminophen and ibuprofen groups, respectively, and statistically no significant difference was observed between the two groups (P = 0.74).
Conclusion:
These findings suggest that there was no significant difference between the effectiveness of oral acetaminophen and oral ibuprofen on closing of PDA, but less adverse effects and contraindication for acetaminophen make it reasonable choice for the treatment of symptomatic PDA.
Keywords: Acetaminophen, ibuprofen, patent ductus arteriosus
INTRODUCTION
One of the common complications in preterm neonates suffering from respiratory distress syndrome (RDS) is patency of ductus arteriosus (PDA), and 60%–70% of preterm infants of <28 weeks' gestation receive medical or surgical therapy for a PDA.[1] Many studies have been shown that neonates with RDS and symptomatic PDA, had higher morbidity including respiratory failure, lower survival rate, and increased risk of intracranial hemorrhage, chronic lung disease and necrotizing enterocolitis (NEC).[2] In addition, the spontaneous PDA closure may occur in a high proportion (from 24% to 58%) of preterm neonates.[3,4,5] Several studies have been reported that the PDA may cause of severe hemodynamic and respiratory complications in the premature neonates. According to the results of many literatures, indomethacin and ibuprofen have been used to treat PDA.[3,6] Furthermore, surgical interventions have been recommended to treat the neonates who had not responded to the second medication therapy or those who suffer from symptoms of heart failure.[7] Many studies have shown the effect of ibuprofen on the closure of neonatal PDA.[8,9] Using ibuprofen has been associated with adverse effects and has contraindications such as gastrointestinal or brain hemorrhage, thrombocytopenia, coagulopathies, NEC, and renal failure.
Recently increasing reports that using of oral acetaminophen instead of ibuprofen for closure of PDA.[10,11] Acetaminophen acting was inhibition of prostaglandin synthetase by affecting on the peroxidase segment of the enzyme. Peroxidase is activated at a 10-fold lower concentration of peroxide than cyclooxygenase. Hypothetically, under these conditions, acetaminophen should be a more effective drug than cyclooxygenase inhibitors with less side effect.[12,13] Furthermore, when a neonate has a contraindication for ibuprofen, using of acetaminophen is the only option for treatment. Oral acetaminophen has advantages such as less expensive than the intravenous (IV) preparation.[14]
The aim of this study was to compare the efficacy of oral acetaminophen versus ibuprofen in the treatment of neonates complicated with PDA.
MATERIALS AND METHODS
This randomized, two-parallel group, active-controlled, double-blind, noninferiority trial was conducted at the Neonatal Intensive Care Unit of Alzahra and Shahid Beheshti Hospitals, two educational hospitals affiliated to Isfahan University of Medical Sciences, Isfahan, Iran, between January 2017 and October 2018.
The rate of closed PDA by oral ibuprofen was considered 80% that obtained in most previous studies.[14,15] According to statistical calculations 40 neonates were studied for current trial.
Preterm neonates with gestation <32 weeks or birth weight <1500 g; approved with significant PDA and <14 days after birth were included to study. Symptomatic PDA was defined with one of the clinical or biochemical signs with PDA or echocardiographic PDA features. The following three domains were considered: signs of significant left-to-right shunt including hyperdynamic pulsatile precordium, bounding peripheral pulses, and wide pulse pressure (defined as systolic blood pressure divided to diastolic blood pressure greater than >½ systolic blood pressure); signs of systemic poor perfusion including poor peripheral pulse volume, prolonged capillary refill time, decreased urine output, deranged renal function test, metabolic acidosis, and hypotension; and signs of pulmonary overperfusion including abnormal weight gain, increase in liver size, new onset or increase in ventilatory requirements that primarily involve positive end expiratory pressure, peak inspiratory pressure, and fraction of inspired oxygen (FiO2), respiratory acidosis, pulmonary crepitations, and hemorrhagic pulmonary edema (or) in the presence of any one of the below-mentioned echocardiographic signs suggestive of hemodynamic instability significance even in the absence mentioned clinical or biochemical sign.
The following echocardiographic features were considered as: A transductal diameter of ≥1.5 mm in the presence of one of the below-mentioned criteria, left atrial enlargement (left atrium: aortic root diameter ratio ≥1.4), ductal velocity <2 m/s, antegrade main pulmonary artery diastolic flow >20 cm/s, E wave: A wave ratio >1, isovolemic relaxation time ≤45 ms, and absent or reversed diastolic blood flow pattern in descending thoracic aorta.
Exclusion criteria were, presence of major congenital malformations or structural heart disease, contraindication for enteral feeding, contraindication for administration of any one of the study drugs such as blood urea >60 mg/dL, serum creatinine (Cr) level >1.6 mg/dL, platelet count <60,000/mm3, clinical bleeding from any site, deranged coagulogram, clinical or radiological evidence of necrotizing enterocolitis, intraventricular hemorrhage of moderate-to-severe grade severity (Grade III with or without intraparenchymal extension) or progression of intraventricular hemorrhage demonstrated in an earlier ultrasound, and an elevated alanine transaminase concentration.
We provided a detailed information and Written consent about the study objectives and side effects of treatment protocol. patient's enrollment were done after obtaining written consent from one of the parents. Parents had allowance to withdraw their neonate from the study at any time. The study protocol was approved by the Bioethics Committee of Isfahan University of Medical Sciences with study project number IR.MUI.REC.1396.3.996. Furthermore, the study protocol was registered at Iranian Registry of Clinical Trials with number IRCT20170801035441N1.
Study procedures and outcome evaluation
Patients were randomly allocated to two study groups, i.e., acetaminophen 15 mg/kg/6 h (eight doses) (Acetaminophen suspension, Hakim, 5 mL: 120 mg) or ibuprofen oral suspension (Ibuprofen suspension, Exir, 5 mL: 100 mg) administered through orogastric tubes at a dose of 10 mg/kg/dose followed by 5 mg/kg/dose after 24–48 h using block randomization with blocks of size four.
After the first course of treatment, if no response was found, the second course of treatment with the same drug was given for another 3 days. If no response was seen after two courses, the third course of treatment was started using the drug from another group. If no response with the third course was recorded, the fourth course with the same drug of the third course was started for the last plan of medical therapy. If the fourth course failed, surgery was performed only if the PDA was causing ventilation difficulties or heart failure.
For evaluating the main outcome, echocardiographic assessment was done until completion of the course or until the closure of the PDA, whichever is earlier. A PDA was considered as closed if there is no demonstrable open ductus, and the color Doppler demonstrated no flow across the ductus arteriosus region. After 24 h from the completion or earlier in case clinical signs appear, a repeat echocardiogram would be done to assess for reopening of PDA. Before the treatment course for machining the two groups, complete blood count, blood urea nitrogen (BUN), Cr, and brain sonography were done. In addition, the neonatologist was aware about using the drugs for the patients, whereas the pediatric cardiologist was blinded about it.
Statistical analysis
Quantitative and categorical data were presented as mean ± standard deviation and frequency (percentage). Normality of quantitative continuous data was evaluated using Shapiro–Wilk test and Q-Q plot. Baseline characteristics of the study participants were compared between the study groups using Chi-square test for categorical variables and two independent samples t-test or nonparametric Mann–Whitney U-test for quantitative variables. For tracking the noninferiority based on assumed noninferiority margin, the 95% confidence interval (CI) of the probability of closure of PDA was considered. Statistical analysis for comparing the main outcome between the two groups was conducted in the framework of intention to treat analysis.
RESULTS
Table 1 presents the basic and baseline clinical characteristics of study participants in the two groups. The two study groups are comparable statistically based on the evaluated baseline characteristics.
Table 1.
Basic and baseline clinical characteristics of study participants in the study groups
| Variables | Ibuprofen | Acetaminophen | P¥ |
|---|---|---|---|
| Sex (%) | |||
| Female | 55 | 61 | 0.70 |
| Male | 45 | 38 | |
| Method of delivery (%) | |||
| Caesarian | 50 | 55 | 0.75 |
| Vaginal delivery | 50 | 45 | |
| Cardiac anomaly (%) | |||
| No anomaly | 60 | 70 | 0.40 |
| ASD | 30 | 15 | |
| AVSD | 5 | 0 | |
| LVH-ASD-PH | 0 | 5 | |
| LVH | 0 | 5 | |
| VSD | 5 | 5 | |
| Cardiac murmur (%) | |||
| 2/6 | 50 | 46 | 0.66 |
| 3/3 | 35 | 46 | |
| 4/6 | 15 | 6 | |
| Type of feeding (%) | |||
| PO | 80 | 64 | 0.46 |
| NPO | 20 | 35 | |
| Gestational age (weeks) | 30.80 (1.99) | 30.35 (2.13) | 0.49 |
| Weight (g) | 1230.53 (182.1) | 1125.78 (200.06) | 0.10 |
| Apgar score at minute 1 | 6.37 (0.72) | 6.30 (0.82) | 0.81 |
| Apgar score at minute 5 | 7.94 (0.25) | 8.20 (0.63) | 0.21 |
¥Resulted from independent samples t-test for continuous variables and Chi-square or Fisher’s exact test for categorical variables. ASD=Atrial septal defect; AVSD=Atrioventricular septal defect; LVH=Left ventricular hypertrophy; PH=Pulmonary hypertension; VSD=Ventricular septal defect; PO= Per os (taken through the mouth); NPO= nil per os(Nothing by mouth)
Our results showed that the primary closure rate of PDA was 70% (95% CI: 49.9%–90%) and 65% (95% CI: 54.3%–75.7%) in the acetaminophen and ibuprofen groups, respectively, and statistically no significant difference was observed between two groups (P = 0.74). In the acetaminophen group, in the second course, the closure rate was 83.3% (95% CI: 76.02%–90.5%), whereas in the ibuprofen group, it was 71.4% (95% CI: 61.30%–81.5%) and the observed difference was not statistically significant (P = 0.56).
In the acetaminophen group, in the first treatment course from twenty patients six cases did not treat with acetaminophen, in the second course form six patients four cases treated with repeated dose of acetaminophen but two cases did not treat the PDA, and in the third and fourth course for two patients the drug changed to ibuprofen and just one of them had clinical response.
In the ibuprofen group, in the first treatment course form twenty patients, five cases did not treat the PDA. In the second course form five patients, three cases treated with second course of ibuprofen and in the third and fourth course for two patients the drug changed to acetaminophen and just one of them was treated. After the completion of the fourth course of treatment, one case from each group remained with opened PDA [Algorithm 1].
Algorithm 1.
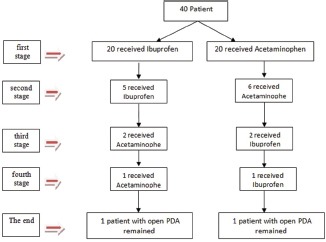
The process of treatment in the study groups and switching the patients to different treatment options during the study course
The mean hart rate in both groups before and after treatment and the results of comparing the mean change between groups. Although both groups showed significant decrease at the end of treatment (P < 0.001), the mean changes were significantly different between the groups [Table 2].
Table 2.
Comparison of heart rate of the patients among the studied drugs
| Variable | Ibuprofen | Acetaminophen | PϮ |
|---|---|---|---|
| Before treatment | 156.7±8.33 | 153.45±10.48 | 0.28 |
| After treatment | 148.35±6.19 | 145.2±8.3 | 0.18 |
| Mean change from baseline | −8.35±5.56 | −8.25±7.54 | 0.96 |
| P¥ | <0.001 | <0.001 |
¥Resulted from paired samples test and; ϮResulted from independent samples t-test
No short-term complication was reported in the acetaminophen group; however, in the ibuprofen group, one of the neonates had no contraindication regarding using ibuprofen, but experienced gastrointestinal bleeding.
DISCUSSION
The PDA was closed due to increased neonatal O2sat in systemic circulation after birth and decreased synthesized endogenous prostaglandins from cyclooxygenase and or peroxidase pathway.
Because first-line treatment of PDA recommended ibuprofen, few studies on evaluating the efficacy of acetaminophen in the treatment of PDA have been done.[13]
Acetaminophen was first proposed as an alternative to ibuprofen by Hammerman et al. in 2011, that recommended for the treatment of PDA.[16] That study showed that acetaminophen was effective on closure of PDA in premature infants. After that, several articles have demonstrated the efficacy of different drugs on the closure of PDA.[9,12,16,17,18] The aim of this study was to compare the efficacy of oral ibuprofen versus oral acetaminophen on the closure of PDA in premature neonates with gestational age (GA) ≤32 W.
Linder et al. discussed about the effects of indomethacin and ibuprofen on the closure of PDA. In this article, they reported that the effects of drugs that they examined are similar for the mentioned patients, and indomethacin imposed more side effects compared to ibuprofen.[19] El-Mashad et al. bilirubin compared indomethacin, ibuprofen, and acetaminophen side effects. They found elevated Serum blood level level of BUN and Cr in neonates witch treated with indomethacin and also elevated level of ibuprofen group.[20] Whereas, all the studied drugs equally suitable to close the PDA, but in our study, we recorded certain complications with ibuprofen. One case in the ibuprofen group suffered gastrointestinal bleeding followed by the first course of treatment and did not respond to the first step of treatment, but in the second course with acetaminophen, the PDA was closed. Al-Lawama et al. investigated the efficacy of oral acetaminophen compared to oral ibuprofen for the treatment of PDA. In this study, they found no significant difference in the mortality or primary closure rates between the two groups. Moreover, there was no significant difference in the short-term neonatal outcomes. The findings are consistent with our findings.[13] Bardanzellu et al. reported that oral ibuprofen can effectively close PDA, but it was associated with some adverse effects that made restricted indication for used for every neonate.[21] Huang et al. concluded that acetaminophen may confer comparable treatment for the closure of PDA as ibuprofen although acetaminophen may cause lower risk of adverse events.[22]
In the study of Asbagh et al. after prescribed oral acetaminophen (15 mg/kg/6 h for 48 h), twelve (75%) of the neonates were treated successfully. The rate of PDA closure in this study is similar to our result of first course of treatment in the acetaminophen group.[23]
In a study by Sinha et al., ten preterm neonates with PDA with a GA of 33–27 weeks were prescribed acetaminophen at 15 mg/kg/8 h for 48 h. The results indicated that the PDA was closed after 72 h in all neonates. In addition, no adverse effects were observed.[17] In another study by Oncel et al., ten neonates with PDA were treated with acetaminophen at 15 mg/kg/18 h intervals for 72 h. PDA was closed in every ten neonates.[12] According to the findings of these studies, using of acetaminophen with longer interval or high dose can be effective, but in our study, acetaminophen was used at 15 mg/kg/6 h for eight doses. In a study by Aly et al., neonates with PDA were treated with ibuprofen with the first dose of 10 mg/kg and followed by two doses of 5 mg/kg at 24 h intervals, and the rate of PDA closure was 83% of the infants.[24] In a study by Aly et al. neonates with PDA were treated with ibuprofen with the first dose of 10 mg/kg and followed by two doses of 5 mg/kg at 24 h intervals, and the rate of PDA closure was 83% of the patients.[24] we used similar protocol to administration of ibuprofen in our study and we got near same results with two courses of treatment with ibuprofen (90% closure). The results of a meta-analysis by Mitra et al. showed that high dose of oral ibuprofen was associated with a higher closure chance of symptomatic PDA versus standard doses of IV ibuprofen or IV indomethacin. The results of a meta-analysis by Mitra et al. showed that high dose of oral ibuprofen was associated with a higher closure chance of symptomatic PDA versus standard doses of IV ibuprofen or indomethacin.[25] Another meta-analysis by Hossain et al. reported that efficacy of oral versus IV route of acetaminophen had no statistically significant difference for closure of PDA.[26] according to above results of these meta-analysis we decided to compare efficacy of oral acetaminophen with oral ibuprophen on closer of PDA. In addition we made four course of treatment with two course of each drug to determine the effect of combined two drugs for cases that not responded to first course of treatment.
In a study of Asadpour et al. compared oral acetaminophen with oral ibuprofen on closure of PDA in premature neonates. This study concluded equal or better results in acetaminophen group, but in our study, the result of the first course of treatment did not demonstrate that acetaminophen was more potent than ibuprofen but in side effects or in failer the treatment with ibuprofen choose of acetaminophen is reasonable.[27] Based on our findings, one of the parameters that could compare the effect of acetaminophen and ibuprofen on closure of PDA plus echocardiographic finding is heart rate. According to our results, each drug had the same influence on heart rate and there was no statistically significant difference between the two drugs. Our previous study was compared the therapeutic effects of low dose IV acetaminophen and oral ibuprofen, demonstrated no statistical difference between two groups.[28] In the present study, the use of oral acetaminophen was compared with oral ibuprofen, which showed the same effect.
Limitation
The small number of patients in our study is the main limitation and therefore multicenter studies are needed to collect adequate numbers of cases to resolve it.
CONCLUSION
These findings suggest that there was no significant difference between the effectiveness of oral acetaminophen and oral ibuprofen on closing of PDA, but less adverse effects and contraindication for acetaminophen make it reasonable choice for the treatment of symptomatic PDA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the Pediatric Department of Shahid Beheshti and Al-Zahra Hospitals for their generous support and assistance with this study.
REFERENCES
- 1.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–81. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 2.Solanky J, Agrawal HK. Effect of oral ibuprofen and oral acetaminophen in the treatment of symptomatic patent ductus arteriosus in premature infants: A hospital based study. Indian J Res. 2017;6:75–7. [Google Scholar]
- 3.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2013;4:Cd003481. doi: 10.1002/14651858.CD003481.pub5. [DOI] [PubMed] [Google Scholar]
- 4.Dani C, Bertini G, Corsini I, Elia S, Vangi V, Pratesi S, et al. The fate of ductus arteriosus in infants at 23-27 weeks of gestation: From spontaneous closure to ibuprofen resistance. Acta Paediatr. 2008;97:1176–80. doi: 10.1111/j.1651-2227.2008.00871.x. [DOI] [PubMed] [Google Scholar]
- 5.Katakam LI, Cotten CM, Goldberg RN, Dang CN, Smith PB. Safety and effectiveness of indomethacin versus ibuprofen for treatment of patent ductus arteriosus. Am J Perinatol. 2010;27:425–9. doi: 10.1055/s-0029-1243371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lago P, Bettiol T, Salvadori S, Pitassi I, Vianello A, Chiandetti L, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: A randomised controlled trial. Eur J Pediatr. 2002;161:202–7. doi: 10.1007/s00431-002-0915-y. [DOI] [PubMed] [Google Scholar]
- 7.Olgun H, Ceviz N, Kartal İ, Caner İ, Karacan M, Taştekin A, et al. Repeated courses of oral ibuprofen in premature infants with patent ductus arteriosus: Efficacy and safety. Pediatr Neonatol. 2017;58:29–35. doi: 10.1016/j.pedneo.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Alipour MR, Mozaffari Shamsi M, Namayandeh SM, Pezeshkpour Z, Rezaeipour F, Sarebanhassanabadi M. The effects of oral ibuprofen on medicinal closure of patent ductus arteriosus in full-term neonates in the second postnatal week. Iran J Pediatr. 2016;26:e5807. doi: 10.5812/ijp.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amoozgar H, Ghodstehrani M, Pishva N. Oral ibuprofen and ductus arteriosus closure in full-term neonates: A prospective case-control study. Pediatr Cardiol. 2010;31:40–3. doi: 10.1007/s00246-009-9542-y. [DOI] [PubMed] [Google Scholar]
- 10.Nadir E, Kassem E, Foldi S, Hochberg A, Feldman M. Acetaminophen treatment of patent ductus arteriosus in preterm infants. J Perinatol. 2014;34:748–9. doi: 10.1038/jp.2014.96. [DOI] [PubMed] [Google Scholar]
- 11.Terrin G, Conte F, Oncel MY, Scipione A, McNamara PJ, Simons S, et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: A systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2016;101:F127–36. doi: 10.1136/archdischild-2014-307312. [DOI] [PubMed] [Google Scholar]
- 12.Oncel MY, Yurttutan S, Uras N, Altug N, Ozdemir R, Ekmen S, et al. An alternative drug (paracetamol) in the management of patent ductus arteriosus in ibuprofen-resistant or contraindicated preterm infants. Arch Dis Child Fetal Neonatal Ed. 2013;98:F94. doi: 10.1136/archdischild-2012-302044. [DOI] [PubMed] [Google Scholar]
- 13.Al-Lawama M, Alammori I, Abdelghani T, Badran E. Oral paracetamol versus oral ibuprofen for treatment of patent ductus arteriosus. J Int Med Res. 2018;46:811–8. doi: 10.1177/0300060517722698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagheri MM, Niknafs P, Sabsevari F, Torabi MH, Bahman Bijari B, Noroozi E, et al. Comparison of oral acetaminophen versus ibuprofen in premature infants with patent ductus arteriosus. Iran J Pediatr. 2016;26:e3975. doi: 10.5812/ijp.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Sundaram V, Yadav R, Oleti TP, Murki S, Krishna A, et al. Oral paracetamol versus oral ibuprofen for closure of haemodynamically significant patent ductus arteriosus in preterm neonates (<32 weeks): A blinded, randomised, active-controlled, non-inferiority trial. BMJ Paediatr Open. 2017;1:e000143. doi: 10.1136/bmjpo-2017-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: A surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128:e1618–21. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R, Negi V, Dalal SS. An interesting observation of PDA closure with oral paracetamol in preterm neonates. J Clin Neonatol. 2013;2:30–2. doi: 10.4103/2249-4847.109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asadpour N. Comparison of the effect of oral acetaminophen and ibuprofen on patent ductus arteriosus closure in premature infants referred to Hajar hospital in Shahrekord in 2016-2017. Clin Neonatol. 2018;7:224–30. [Google Scholar]
- 19.Linder N, Bello R, Hernandez A, Rosen C, Birk E, Sirota L, et al. Treatment of patent ductus arteriosus: Indomethacin or ibuprofen? Am J Perinatol. 2010;27:399–404. doi: 10.1055/s-0029-1243315. [DOI] [PubMed] [Google Scholar]
- 20.El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr. 2017;176:233–40. doi: 10.1007/s00431-016-2830-7. [DOI] [PubMed] [Google Scholar]
- 21.Bardanzellu F, Neroni P, Dessì A, Fanos V. Paracetamol in patent ductus arteriosus treatment: Efficacious and safe? Biomed Res Int. 2017;2017:1438038. doi: 10.1155/2017/1438038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Wang F, Wang K. Paracetamol versus ibuprofen for the treatment of patent ductus arteriosus in preterm neonates: A meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2018;31:2216–22. doi: 10.1080/14767058.2017.1338263. [DOI] [PubMed] [Google Scholar]
- 23.Akbari Asbagh P, Zarkesh MR, Nili F, Nayeri FS, Tofighi Naeem A. Prophylactic teatment with oral paracetamol for patent ductus arteriosus in preterm infants: A randomized clinical trial. Tehran University Medical J. 2015;73:86–92. [Google Scholar]
- 24.Aly H, Lotfy W, Badrawi N, Ghawas M, Abdel-Meguid IE, Hammad TA. Oral ibuprofen and ductus arteriosus in premature infants: A randomized pilot study. Am J Perinatol. 2007;24:267–70. doi: 10.1055/s-2007-976550. [DOI] [PubMed] [Google Scholar]
- 25.Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: A systematic review and meta-analysis. JAMA. 2018;319:1221–38. doi: 10.1001/jama.2018.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain J, Shabuj M. Oral paracetamol versus intravenous paracetamol in the closure of patent ductus arteriosus: A proportion meta-analysis. J Clinical Neonatology. 2018;7:121–4. [Google Scholar]
- 27.Asadpour N, Harandi P, Hamidi M, Malek Ahmadi M, Malekpour-Tehrani A. Comparison of the effect of oral acetaminophen and ibuprofen on patent ductus arteriosus closure in premature infants referred to hajar hospital in Shahrekord in 2016-2017. J Clin Neonatol. 2018;7:224–30. [Google Scholar]
- 28.Ghaderian M, Armanian AM, Sabri MR, Montaseri M. Low-dose intravenous acetaminophen versus oral ibuprofen for the closure of patent ductus arteriosus in premature neonates. J Res Med Sci. 2019;24:13. doi: 10.4103/jrms.JRMS_631_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


