Abstract
Flavokawains have a broad spectrum of biological activities; however, the herbicidal activity of these naturally occurring chalcones has been less investigated. Flavokawains and their analogues were prepared by the Claisen–Schmidt condensation reaction between xanthoxyline (or aromatic ketones) and a variety of aromatic and heteroaromatic aldehydes. These compounds were then evaluated for their inhibitory effect against representative dicot and monocot plants. Among 45 synthetic chalcones, derivatives containing phenoxyacetic acid, 4-(N,N-dimethylamino)phenyl, N-methylpyrrole, or thiophenyl groups inhibited the germination and growth of Chinese amaranth (Amaranthus tricolor L.) with moderate to high degrees compared to commercial butachlor. For barnyardgrass (Echinochloa crus-galli (L.) Beauv.), most of the thiophenyl chalcones interrupted shoot and root emergence. This finding highlighted the importance of functional groups on the herbicidal activity of chalcones. The level of inhibition also depended on the applied concentrations, plant species, and plant organs. (E)-2-(2-(3-Oxo-3-(thiophen-2-yl)prop-1-enyl)phenoxy)acetic acid (14f) was the most active compound among 45 derivatives. This chalcone could be a promising structure for controlling the germination and growth of weeds. The structure–activity relationship results provide useful information about the development of active chalconoids as novel natural product-like herbicides.
Introduction
Many farmers suffer from problems with weeds: they compete with crops for resources and decrease crop yields. Thus, in many parts of the world, agricultural production is insufficient to meet consumer demand. These problems have the greatest impact on undeveloped or developing countries, where agriculture is the main national income.1 Thus, finding an appropriate and effective way to eliminate undesirable plant species is crucially important. Generally, there are numerous methods of weeding, such as biological, chemical, manual, and mechanical.2−5 Among these, herbicides are still widespread.6 Moreover, since the discovery of the concept of allelopathy,7 agrochemists have been investigating the effect of allelochemicals on weeds and crop plants to produce natural product herbicides.8−10 Further, to discover new and effective weed killers, chemists have also formed derivatives of allelochemicals11−13 to find selective and eco-friendly herbicides.
Our research group has successfully isolated some allelochemicals—xanthoxyline (Figure 1), (±)-odorine, and oleuropein.14−16 These compounds had a great inhibitory effect on both tested monocot and dicot weeds. We are interested in adding functional groups to our natural herbicide, xanthoxyline, to chalcones, to form new herbicides, which are able to strongly and selectively inhibit weed germination and growth. We choose the chalcone group (Figure 1) because many reports have shown that chalcones exhibit strong pharmacological activity,17−20 usually with low toxicity.21 Especially, naturally occurring chalcones, for example, flavokawains A, B, and C, which can be modified from xanthoxyline,22−25 have shown a wide range of biological activities. Besides, several reports26−29 on the herbicidal activities of Kava root (Piper methysticum L.) revealed that a series of kava lactones, which have a similar chemical structure to chalcone, highly inhibited seed germination and seedling growth of tested plants such as lettuce, radish, and barnyardgrass. Our recent work30 also indicated that trans-cinnamaldehyde, a chalcone-related compound, greatly suppressed shoot and root growth of Chinese amaranth and barnyardgrass. However, to the best of our knowledge, the weed control ability of flavokawains and related chalcones has not yet been investigated. Even though the herbicidal activities of some simple trans-chalcones (or 1,3-diphenyl-2-propen-1-one) (Figure 1) have been reported,31−38 there is a small variety of substituents on the rings of tested chalcones. Therefore, more work is needed to explore the chemical clues that provide herbicidal activity. Herein, we have designed and synthesized flavokawains and a series of their analogues. To maximize the probability of discovering the active compounds, we have explored a variety of aromatic aldehydes, containing either electron-withdrawing or electron-donating groups, as well as heteroaromatic aldehydes. Then, we investigated the structure–activity relationship (SAR) of these chalcones against representative monocot (barnyardgrass; Echinochloa crus-galli (L.) Beauv.) and dicot (Chinese amaranth; Amaranthus tricolor L.) plants.30 We aimed to find promising chalcone structures with good weed control property, so that the gained knowledge could be applied to find novel herbicides among natural compounds or their analogues.
Figure 1.
Chemical structures of xanthoxyline, chalcones, and trans-chalcone.
Results and Discussion
Synthesis and Herbicidal Activity of Flavokawains A, B, and C and Their Analogues on Chinese Amaranth
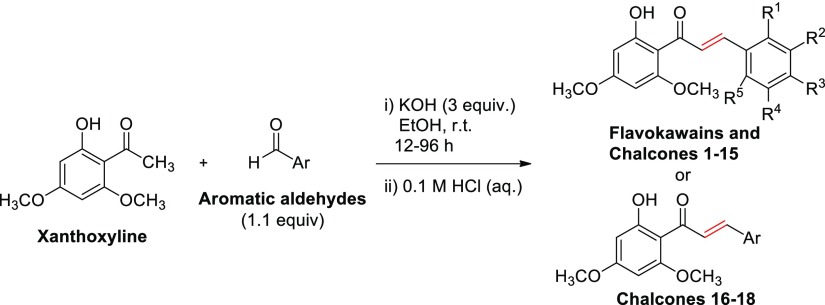
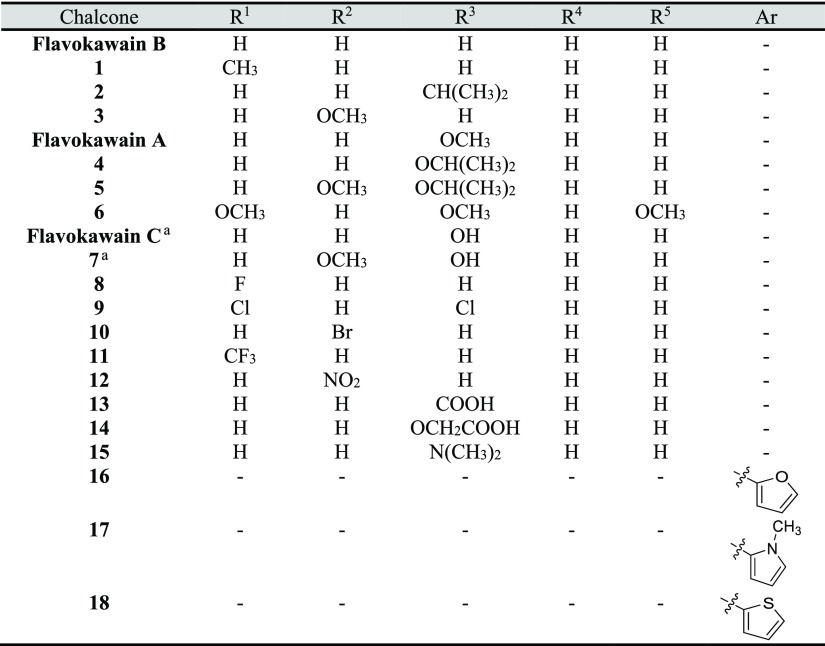
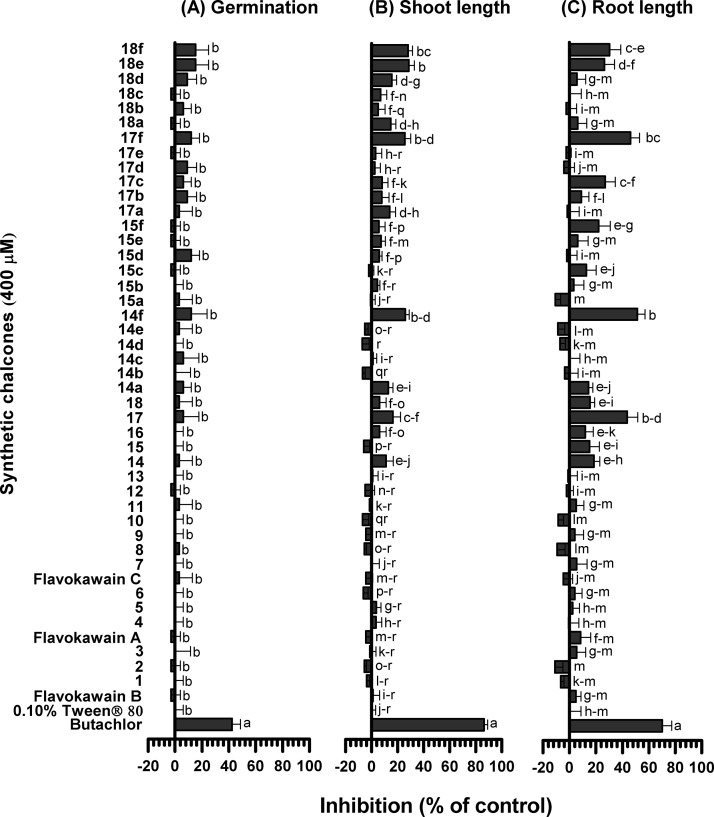
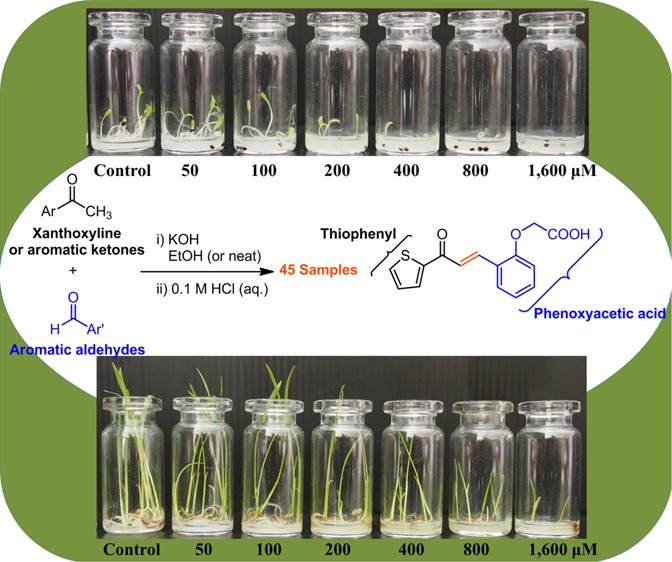
Three flavokawains (A, B, and C) and 18 chalcones containing both electron-withdrawing and electron-donating substituents as well as some heteroaromatic rings (Table 1) were used for the preliminary screening. These chalcones were prepared in low to high yields (see the Supporting Information) by the Claisen–Schmidt condensation reaction between xanthoxyline and a variety of aromatic aldehydes. Flavokawains A and B, together with chalcones 1–6 and chalcones 8–18, were prepared in ethanol media at room temperature.25 However, flavokawain C and chalcone 7, which is composed of a hydroxyl group on ring B, were prepared by grinding a mixture of neat starting materials and base in a mortar.39 These 21 compounds were initially evaluated for their inhibitory activities on seed germination and seedling growth of Chinese amaranth, a model for dicot plants.30,40
Table 1. Synthesis of Xanthoxyline-Derived Chalcones.
General Procedure B: grinding (neat) at room temperature for 20 min or until the reaction was completed (monitored by TLC).
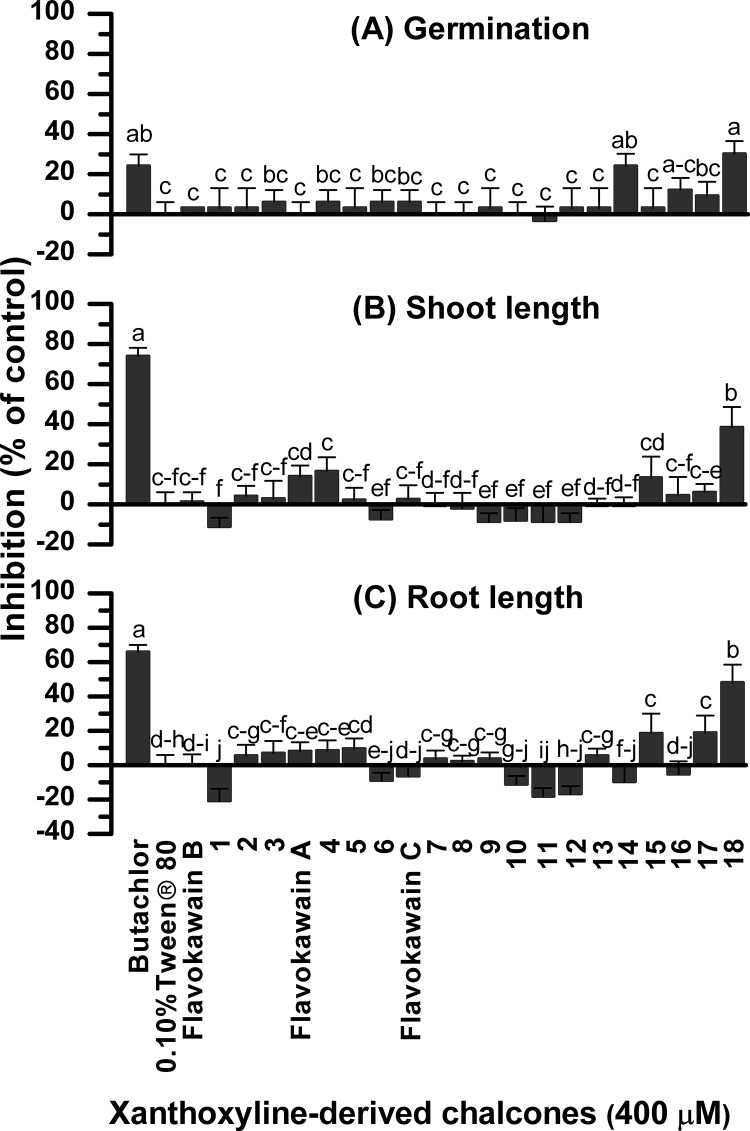
As most tested chalcones were low-polarity compounds, polysorbate 80 or Tween 80 was used as a surfactant to enhance the solubility of chalcones in aqueous media.30,40 For this reason, a 0.10% (v/v) aqueous solution of Tween 80 was then used as a negative control experiment for all bioassays. A commercial herbicide, butachlor,41 was used as a reference. The preliminary results showed that at 400 μM, all flavokawains had no effect on seed germination and seedling growth of Chinese amaranth (Figure 2). Among the 18 flavokawain derivatives, chalcone 14, which contained a phenoxyacetic acid ring, slightly suppressed the germination. A thiophenyl derivative 18 also showed strong inhibition of both germination and seedling emergence. Derivatives comprising a (N,N-dimethyl)aminophenyl group (compound 15) and a N-methylpyrrole group (compound 17) significantly inhibited root elongation. In contrast, compounds containing methyl and trifluoromethyl groups (chalcones 1 and 11) slightly promoted root growth of Chinese amaranth. However, other derivatives containing both electron-withdrawing (including halogens) and electron-donating substituents at ring B had no significant effect on the germination and growth.
Figure 2.
Inhibitory effects of 3 flavokawains and 18 related chalcones on seed germination (A) and shoot (B) and root (C) growth of Chinese amaranth. Aqueous solutions of Tween 80 and butachlor were used as negative and positive references, respectively. Different letters in each graph indicate significant differences (p < 0.05) between treatments. Error bars represent the standard error of an average of four replicates.
Synthesis and Herbicidal Activity of Other Related Chalcones on Chinese Amaranth
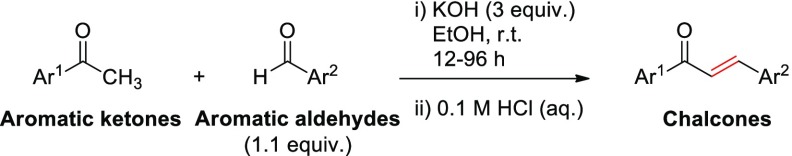
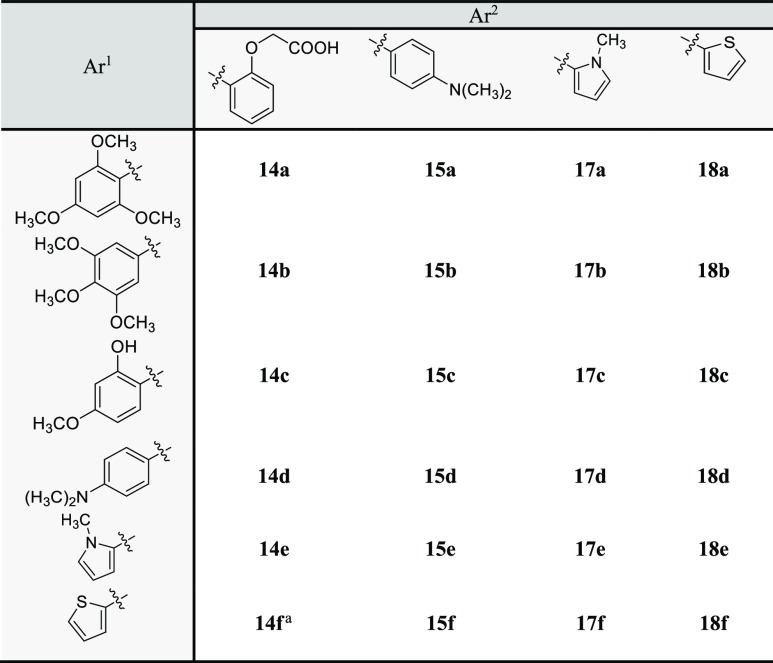
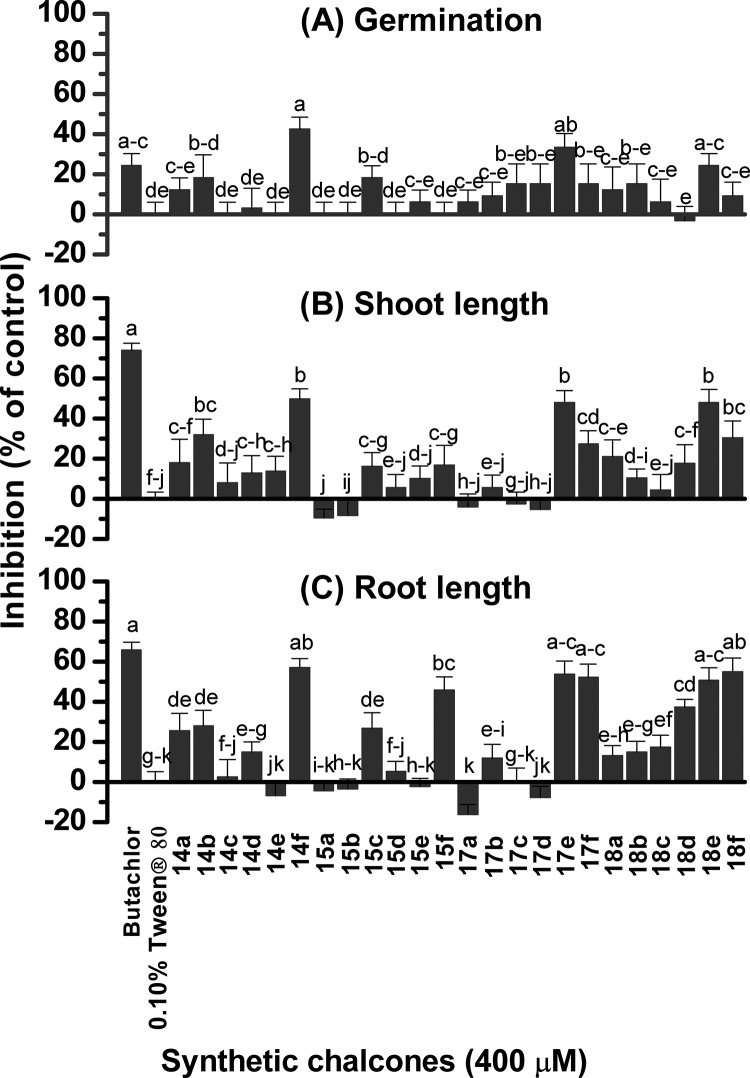
Results from the previous section indicated that the most active chalcones generally possess phenoxyacetic acid, 4-(N,N-dimethylamino)phenyl, N-methylpyrrole, or thiophenyl groups. To extensively investigate the relationship between the structural characteristics of the synthetic chalcones and their herbicidal activities, chalcones 14a–18f in which ring B or both rings had those functional groups were then prepared (Table 2). The yields ranged from moderate to high. The herbicidal potential of these 24 chalcones at 400 μM on Chinese amaranth was then evaluated (Figure 3): chalcone 18a slightly inhibited shoot growth of the plant. Compounds 14a, 15c, 15f, 18c, and 18d suppressed root growth, while compounds 14b, 17f, and 18f inhibited both shoot and root growths. Chalcones 14f, 17e, and 18e significantly inhibited seed germination (43%), shoot (50%), and root growth (57%) of Chinese amaranth, where the metric refers to the fraction of seeds that were inhibited by the most active herbicide 14f. The effect of 14f was quite close to the reference herbicide, butachlor, especially for seed germination. However, other chalcones had no harmful effect on Chinese amaranth.
Table 2. Synthesis of Chalcones 14a–18f.
General Procedure B: grinding (neat) at room temperature for 20 min or until the reaction was completed (monitored by TLC).
Figure 3.
Inhibitory effects of 24 synthetic chalcones on seed germination (A) and shoot (B) and root (C) growth of Chinese amaranth. Aqueous solutions of Tween 80 and butachlor were used as negative and positive controls, respectively. Different letters in each graph indicate significant differences (p < 0.05) between treatments. Vertical bars represent the standard error of an average of four replicates.
Herbicidal Activity of All Synthesized Chalcones on Barnyardgrass
For monocots, we used barnyardgrass as a model. All 45 chalcones could not affect the germination (Figure 4). However, chalcones 14a, 17a, 18a, and 18d slightly inhibited the shoot growth and compounds 17, 15f, and 17c slightly inhibited the root length. Chalcones 14f, 17f, 18e, and 18f inhibited both shoot and root elongation. Analogues 18e and 18f showed the highest harmful activity against shoot emergence by ∼29%, while derivative 14f strongly suppressed the root length by 51%. Also, butachlor exhibited the largest inhibitory activity on the tested plant. However, other chalcones had no significant effect.
Figure 4.
Inhibitory effects of 3 flavokawains and 42 synthetic chalcones on seed germination and shoot and root growth of barnyardgrass. Aqueous solutions of Tween 80 and butachlor were used as negative and positive controls, respectively. Different letters in each graph indicate significant differences (p < 0.05) between treatments. Horizontal bars represent the standard error of an average of four replicates.
Herbicidal Activity of Chalcone 14f on Chinese Amaranth and Barnyardgrass
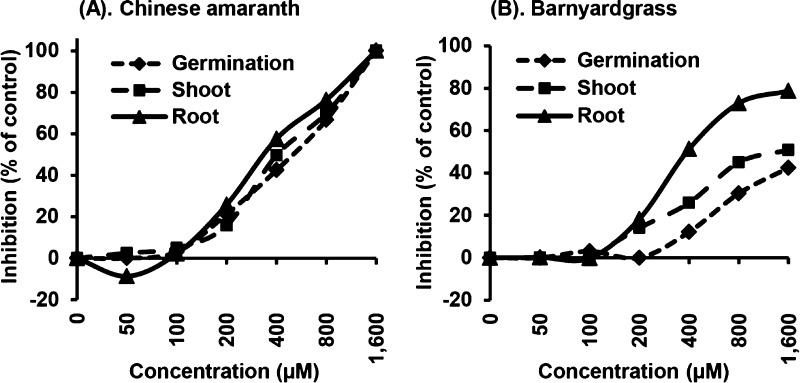
Compound 14f showed the highest potential to become a chalconoid herbicide. Thus, we measured the effect of concentrations on its inhibitory activity. The applied concentrations ranged from 50 to 1600 μM. At the highest concentration, this chalcone completely inhibited the germination of Chinese amaranth (Figure 5A) and highly reduced seed germination (42%), shoot (51%), and root length (79%) of barnyardgrass (Figure 5B). The degree of inhibition decreased at lower concentrations. Chalcone 14f could suppress germination and growth of both plants with concentrations as low as 200 μM.
Figure 5.
Inhibitory effects of chalcone 14f on seed germination, shoot, and root growths of (A) barnyardgrass and (B) Chinese amaranth. Aqueous solutions of Tween 80 were used as negative control.
From the above results, the most active synthetic chalcones generally contained phenoxyacetic acid, 4-(N,N-dimethylamino)phenyl, N-methylpyrrole, or thiophenyl rings. Some herbicides bearing similar functional groups have been reported previously, for example, phenoxy,42 aminobenzene,43 pyrrole,44 and thiophene45 herbicides. Even though we should not speculate that the herbicidal potential and the mode of action of any compounds depend purely on a single functional group, these structures are still worth mentioning in order to understand a relationship between substituents and a trend of activity of those compounds. Apart from those active structures, Groth and co-workers38 demonstrated that certain polyhydroxy chalcones, such as okanin (Figure 6), could affect plant growth by selective inhibition of C4 phosphoenolpyruvate carboxylase (PEPC). Apparently, the potency and selectivity of those active chalcones relied upon the quantity and position of hydroxyl groups on the chalcone rings with a higher number of hydroxyls leading to greater effect. This was quite different from our result that the hydroxyl-bearing compounds such as flavokawain C and chalcone 7 showed no activity. In terms of unreactive groups, we found that certain substituents, such as CH3, CF3, OCH3, isopropyl, isopropoxy, NO2, and halo groups, showed very low inhibitory activity toward tested plants. This was similar to Gomes’s work37 that, among their 13 synthesized chalcones, simple trans-chalcone (Figure 1) was the most active compound against the initial growth of sesame (Sesamum indicum L.) and brachiaria (Urochloa decumbens (Stapf) R. D. Webster). Other chalcones bearing either electron-donating groups (OH, N(CH3)2, or OCH3) or electron-withdrawing groups (NO2 or Cl) showed only low to moderate effects. Apart from the previously mentioned chalcones, 2,4′-dimethoxychalcone (Figure 6) was reported to exhibit the allelopathic effect against two weed species: Mimosa pudica and Senna obtusifolia.31 However, there was only chalcone in this study; therefore, the relationship between structure and activity of chalcones was unable to compare.
Figure 6.
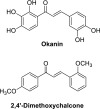
Structures of chalcone herbicides discussed here.
We showed that some synthesized chalcones at a concentration of 400 μM inhibited the growth of a crop (Chinese amaranth) and a weed (barnyardgrass): the detrimental effects of these chalcones were concentration- and species-dependent. Similar results have been reported, for example, Chen and co-workers32 evaluated the effect of a trans-chalcone (Figure 1) on the growth of 20 annual plant species and found that this simple chalcone suppressed the growth of those plants with a wide range of sensitivities depending on doses and plant species. In addition, Díaz-Tielas and co-workers36 found that at concentrations of 12.5–400 μM, the compound was effective against a variety of crops and weeds.
The mode of action of chalcones is not yet fully understood. Chen et al.33,34 found that trans-chalcone inhibited lignin biosynthesis in maize (Zea mays L. cv. Yellowcorn) and soybean (Glycine max L. cv. Harosoy 63). Lignin is a complex phenolic polymer found in the integral xylem and cell walls. It enhances cell wall rigidity, promotes minerals transport, and confers resistance against various stresses.46 As it plays an important role in the growth and development of plants, interference with lignin biosynthesis can seriously affect growth. Diaz-Tielas et al.35 suggested that this simple chalcone could disrupt the root growth of Arabidopsis thaliana by altering the mitochondrial membrane potential and inducing programmed cell death. Moreover, as previous mentioned, Groth and co-workers38 found that hydroxychalcones affected plant development by inhibition of C4 PEPC, a key enzyme for carbon fixation.
Although our work here did not determine the mode of action of chalcone 14f against the growth and development of the C4 plants (Chinese amaranth and barnyardgrass), its mechanism could be attributed to a similar mechanism. However, in general, chalcones have a wide variety of structures which result in a diverse range of biological activities. Therefore, other mechanisms of chalcone herbicide actions should be explored and investigated in order to understand the advantages of those active compounds, as well as their functional groups.
We observed that most synthesized chalcones exhibited more severe effects on Chinese amaranth than barnyardgrass. This confirmed our former reports14,30 that allelochemicals, such as xanthoxyline and trans-cinnamaldehyde, showed a stronger inhibitory activity against dicots than monocots. We note that barnyardgrass was more resistant to the applied chemicals than Chinese amaranth, but it is possible that the chalcones were more specific to Chinese amaranth than barnyardgrass.
Conclusions
In summary, a series of novel flavokawain analogues were prepared in reasonable yields. The first SAR studies of this group of compounds were carried out against model monocot and dicot plants. Herbicidal activities of these chalconoids rely upon the groups added to rings A and B, the applied concentrations, plant species, and organs. Derivatives containing phenoxyacetic acid, 4-(N,N-dimethylamino)phenyl, N-methylpyrrole, or especially thiophenyl functional groups showed promising inhibitory activity. Chalcone 14f (thiophenyl group on ring A and phenoxyacetic acid group on ring B) was the most potent compound among those synthesized chalcones and flavokawains. This study gives us a chemical clue that might boost herbicidal activity. At the highest applied concentration, chalcone 14f clearly suppressed germination and growth more than at the lower concentrations. Among the tested species, barnyardgrass was more tolerant to the applied chemicals than Chinese amaranth and shoots were more tolerant than roots. Further intensive investigation is still needed to understand the mode of action of reactive chalcones and to develop potential natural product-based herbicides.
Experimental Section
Chemicals and Instrument
Butachlor (N-(butoxymethyl)-2-chloro-N-(2,6-diethylphenyl)acetamide) (60% w/v) was purchased from Sinon Corporation (Bangkok, Thailand). Tween 80 and all aldehydes used in this study were purchased from Sigma-Aldrich (Singapore). All ketones were purchased from Tokyo Chemical Industry (TCI, Tokyo, Japan) and Sigma-Aldrich (Singapore). Xanthoxyline starting material was isolated from a crude extract of dried fruits of Zanthoxylum limonella.14 All solvents were purified by distillation before use. Melting points were recorded on a Gallenkamp meting point apparatus. Infrared (IR) spectra were recorded on a PerkinElmer 8900 at the Department of Chemistry, Faculty of Science, KMITL. 1H and 13C NMR spectra were recorded on a Bruker AVANCE III HD (500 MHz) at The Equipment Center, Chulalongkorn University, using residual protonated chloroform (CDCl3, 7.27 ppm for 1H NMR and 77.00 ppm for 13C NMR), residual protonated methanol (CD3OD, 3.31 ppm for 1H NMR and 49.00 ppm for 13C NMR), or residual protonated dimethyl sulfoxide (DMSO-d6, 2.50 ppm for 1H NMR and 39.52 ppm for 13C NMR) as internal standard. High-resolution mass spectra were recorded on a Bruker micrOTOF II mass spectrometer at the Faculty of Science, Ramkhamhaeng University, and a Bruker Daltonics (micrOTOF) at the Faculty of Science, Mahidol University.
Synthesis of Flavokawains and Related Chalcones
A total of 45 chalcones with different substituents (electron-donating or electron-withdrawing groups) were synthesized by the Claisen–Schmidt reaction with slight modifications to the procedures in literature as follows.
General Procedure A25
To a mixture of xanthoxyline (1.0 mmol, or other aromatic ketones) and aromatic aldehydes (1.1 mmol) in ethanol (20 ml), potassium hydroxide (3.0 mmol) was slowly added. The mixture was continuously stirred at ambient temperature, until xanthoxyline (or the ketone) was completely consumed [monitored by thin-layer chromatography (TLC), usually 12–96 h]. The mixture was then acidified with 1 N HCl (aq) to pH 4–6. In the case of precipitated chalcones, they were filtered and recrystallized from methanol. In the other cases, the acidified mixtures were extracted with ethyl acetate (three times), and then the combined organic layers were concentrated in vacuo to afford crude products. These crude mixtures were purified through silica gel column chromatography (EtOAc/hexane) to obtain the pure compounds.
General Procedure B39
A mixture of aromatic ketones (1.0 mmol), aromatic aldehydes (1.1 mmol), and potassium hydroxide (3.0 mmol) was ground or crushed with a mortar and pestle at room temperature for 20 min or until the reaction was completed (monitored by TLC). To this mixture, 5 mL of cold distilled water was added. Then, the mixture was transferred to a 50 ml beaker and acidified with 1 N HCl (aq) to pH 4–6. The precipitated solids were filtered, dissolved in methanol, and recrystallized from the methanol to obtain the pure chalcones.
Preparation of Aqueous Solutions of Butachlor at 400 μM
Butachlor (40 μmol) was well mixed with distilled water in a 100 mL volumetric flask to produce a 400 μM solution of butachlor.
Aqueous Solution (0.10% (v/v)) of Tween 80
Tween 80 (0.1 mL) and distilled water were thoroughly mixed in a 100 mL volumetric flask to produce a stock solution of 0.10% (v/v) Tween 80.
Aqueous Solutions of Flavokawains and Related Chalcones at 400 μM
Following our previous work,30 40 μmol of pure chalcone and 0.1 mL of Tween 80 were thoroughly mixed in a 100 mL beaker followed by adding 40 mL of distilled water. This solution was then transferred to a 100 mL volumetric flask. The volume of the solution was adjusted by adding distilled water to produce a 400 μM solution of chalcone containing 0.10% (v/v) Tween 80.
Aqueous Solutions of Chalcone 14f at 50, 100, 200, 400, 800, and 1600 μM
Stock solutions of compound 14f at 1600 μM were prepared using the same procedure as the 400 μM solution. This stock solution was then diluted with 0.10% (v/v) solution of Tween 80 to produce 800, 400, 200, 100, and 50 μM solutions.
Tested Plants
Chinese amaranth (A. tricolor L.) and barnyardgrass (E. crus-galli (L.) Beauv.) were tested. Seeds of Chinese amaranth were purchased from Thai Seed & Agriculture Co. Ltd. (Bangkok, Thailand) and seeds of barnyardgrass were collected from paddy fields in Phitsanulok Province (16°48′57″N 100°15′49″E, Thailand). Both plants showed >80% germination.
Seed Germination and Seedling Growth Bioassay30
Following our previous work,30 to a small vial (45 mm × 20 mm) lined with germination paper, 0.5 mL aqueous solution of Tween 80 (or aqueous solutions of chalcones) was added. Ten seeds of the tested plant were placed onto the paper and the vials were then sealed with Parafilm. These vials were kept in a growth chamber (cool white 840 Climacell 707, Munich, Germany) at 28–30 °C with a photoperiod of 12 h (light intensity 100 μmol m–2·s–1) and 80% relative humidity. Each treatment was replicated four times. After 7 days, the germinated seeds were counted, and both shoot and root lengths were measured. Inhibition of germination and early growth of the tested plants was then calculated:
Statistical Analysis
For data analysis, a completely randomized design with four replications was used. Control experiments were conducted under the same conditions but without chalcones. Analysis of variance was used for all bioassays, and treatments were compared using Tukey’s studentized range test at a probability level of p ≤ 0.05.
Acknowledgments
This study was funded by King Mongkut’s Institute of Technology Ladkrabang (New Lecturer Mentoring Program, grant number KREF165906) and National Science and Technology Development Agency (NSTDA, grant number FDA-CO-2561-7195-TH). The authors would also like to thank the Department of Chemistry, Faculty of Science, KMITL for laboratory facilities. The authors thank Dr John Morris, KMITL Research and Innovation Services (KRIS), for assistance with the manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03144.
Percent yields, melting points, retardation factor (Rf), IR spectra, 1H NMR spectra, 13C NMR spectra, and high-resolution mass spectra of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yaduraju N. T.; Rao A. N.. Implications of weeds and weed management on food security and safety in the Asia-Pacific region. In 24th Asian-Pacific Weed Science Society Conference, the Role of Weed Science in Supporting Food Security by 2020; Bakar B. H., Denny K., Soekisman T., Eds.; Asian-Pacific Weed Science Society in Collarboration with Weed Science Society of Indonesia and Padjadjaran University: Bandung, Indonesia, 2013; pp 13–30.
- Barberi P. Weed management in organic agriculture: are we addressing the right issues?. Weed Res. 2002, 42, 177–193. 10.1046/j.1365-3180.2002.00277.x. [DOI] [Google Scholar]
- Bayer D. E.Weed management. In Rice Production; Luh B. S., Ed.; Springer Science+Business Media: New York, 1991; Vol. 1, pp 287–309. [Google Scholar]
- Iderawumi A. M.; Friday C. E. Characteristics effects of weed on growth performance and yield of maize (Zea mays). Biomed. J. Sci. Tech. Res. 2018, 7, 5880–5883. 10.26717/bjstr.2018.07.001495. [DOI] [Google Scholar]
- Tu M.; Hurd C.; Randall J. M.; Conservancy T. N.. Weed Control Methods Handbook: Tools & Techniques for Use in Natural Areas; All U.S. Government Documents (Utah Regional Depository), 2001. [Google Scholar]
- Gianessi L. P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. 10.1002/ps.3598. [DOI] [PubMed] [Google Scholar]
- Willis R. J.The History of Allelopathy; Springer Science & Business Media: Dordrecht, The Natherlands, 2007; p 316. [Google Scholar]
- Duke S. O.; Romagni J. G.; Dayan F. E. Natural products as sources for new mechanisms of herbicidal action. Crop Protect. 2000, 19, 583–589. 10.1016/s0261-2194(00)00076-4. [DOI] [Google Scholar]
- Macias F. A.Allelopathy in the search for natural herbicide models. Allelopathy; American Chemical Society, 1994; Vol. 582, pp 310–329. [Google Scholar]
- Vyvyan J. R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. 10.1016/s0040-4020(02)00052-2. [DOI] [Google Scholar]
- Abe M.; Nishikawa K.; Fukuda H.; Nakanishi K.; Tazawa Y.; Taniguchi T.; Park S.-Y.; Hiradate S.; Fujii Y.; Okuda K.; Shindo M. Key structural features of cis-cinnamic acid as an allelochemical. Phytochemistry 2012, 84, 56–67. 10.1016/j.phytochem.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Yang X.-F.; Lei K.; Kong C.-H.; Xu X.-H. Effect of allelochemical tricin and its related benzothiazine derivative on photosynthetic performance of herbicide-resistant barnyardgrass. Pestic. Biochem. Physiol. 2017, 143, 224–230. 10.1016/j.pestbp.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Zhang J.-H.; Yang L.; Ruan X.; Sheng Z.-L.; Yu M.-F.; Zheng B.-S.; Zhang J.-Y.; Li X.-X.; Zhao Y.-X.; Wang Q. Synthesis and herbicidal activity of 4, 8-DHT and its derivates. Ind. Crops Prod. 2018, 111, 755–767. 10.1016/j.indcrop.2017.11.051. [DOI] [Google Scholar]
- Charoenying P.; Teerarak M.; Laosinwattana C. An allelopathic substance isolated from Zanthoxylum limonella Alston fruit. Sci. Hortic. 2010, 125, 411–416. 10.1016/j.scienta.2010.04.045. [DOI] [Google Scholar]
- Teerarak M.; Charoenying P.; Laosinwattana C. Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol. Plant. 2012, 34, 1277–1285. 10.1007/s11738-011-0923-5. [DOI] [Google Scholar]
- Teerarak M.; Laosinwattana C.; Charoenying P. Evaluation of allelopathic, decomposition and cytogenetic activities of Jasminum officinale L. f. var. grandiflorum (L.) Kob. on bioassay plants. Bioresour. Technol. 2010, 101, 5677–5684. 10.1016/j.biortech.2010.02.038. [DOI] [PubMed] [Google Scholar]
- de Mello M. V. P.; Abrahim-Vieira B. d. A.; Domingos T. F. S.; de Jesus J. B.; de Sousa A. C. C.; Souza A. M. T. d.; Souza A. M. T. d. A comprehensive review of chalcone derivatives as antileishmanial agents. Eur. J. Med. Chem. 2018, 150, 920–929. 10.1016/j.ejmech.2018.03.047. [DOI] [PubMed] [Google Scholar]
- Gomes M.; Muratov E.; Pereira M.; Peixoto J.; Rosseto L.; Cravo P.; Andrade C.; Neves B. Chalcone derivatives: promising starting points for drug design. Molecules 2017, 22, 1210. 10.3390/molecules22081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra D. K.; Bharti S. K.; Asati V. Anti-cancer chalcones: structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. 10.1016/j.ejmech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Singh P.; Anand A.; Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 2014, 85, 758–777. 10.1016/j.ejmech.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Phang C.-W.; Karsani S. A.; Malek S. N. A. Induction of apoptosis and cell cycle arrest by flavokawain C on HT-29 human colon adenocarcinoma via enhancement of reactive oxygen species generation, upregulation of p21, p27, and Gadd153, and inactivation of inhibitor of apoptosis proteins. Pharmacogn. Mag. 2017, 13, S321. 10.4103/0973-1296.210180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu N.; Ho W.; Yeap S.; Akhtar M.; Abdullah M.; Omar A.; Alitheen N. The flavokawains: uprising medicinal chalcones. Cancer Cell Int. 2013, 13, 102. 10.1186/1475-2867-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeck P.; Bandeira Falcão C. A.; Leal P. C.; Yunes R. A.; Filho V. C.; Torres-Santos E. C.; Rossi-Bergmann B. Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg. Med. Chem. 2006, 14, 1538–1545. 10.1016/j.bmc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Boeck P.; Leal P. C.; Yunes R. A.; Filho V. C.; López S.; Sortino M.; Escalante A.; Furlán R. L. E.; Zacchino S. Antifungal activity and studies on mode of action of novel xanthoxyline-derived chalcones. Arch. Pharm. 2005, 338, 87–95. 10.1002/ardp.200400929. [DOI] [PubMed] [Google Scholar]
- Seo Y. H.; Oh Y. J. Synthesis of flavokawain B and its anti-proliferative activity against gefitinib-resistant non-small cell lung cancer (NSCLC). Bull. Korean Chem. Soc. 2013, 34, 3782–3786. 10.5012/bkcs.2013.34.12.3782. [DOI] [Google Scholar]
- Van T.; Xuan T.; Minh T.; Quan N. Isolation and purification of potent growth inhibitors from Piper methysticum root. Molecules 2018, 23, 1907. 10.3390/molecules23081907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan T. D.; Tsuzuki E.; Terao H.; Matsuo M.; Khanh T. D. Identification of potential allelochemicals from Kava (Piper methysticum L.) root. Allelopathy J. 2003, 12, 197–203. [Google Scholar]
- Xuan T. D.; Elzaawely A. A.; Fukuta M.; Tawata S. Herbicidal and fungicidal activities of lactones in Kava (Piper methysticum). J. Agric. Food Chem. 2006, 54, 720–725. 10.1021/jf0519461. [DOI] [PubMed] [Google Scholar]
- Xuan T. D.; Yuichi O.; Junko C.; Tsuzuki E.; Hiroyuki T.; Mitsuhiro M.; Khanh T. D.; Hong N. H. Kava root (Piper methysticum L.) as a potential natural herbicide and fungicide. Crop Protect. 2003, 22, 873–881. 10.1016/s0261-2194(03)00083-8. [DOI] [Google Scholar]
- Chotsaeng N.; Laosinwattana C.; Charoenying P. Inhibitory effects of a variety of aldehydes on Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. Molecules 2018, 23, 471. 10.3390/molecules23020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt H. R.; Santos L. S.; Souza Filho A. P. S. Atividade alelopática de chalcona sintética, de seus precursores e de cetonas e aldeídos relacionados. Planta Daninha 2007, 25, 747–753. 10.1590/s0100-83582007000400011. [DOI] [Google Scholar]
- Chen W. J.; Yun M. S.; Deng F.; Yogo Y. Effects of root-applied naringenin and chalcone on the growth of annual plants. Weed Biol. Manag. 2004, 4, 235–238. 10.1111/j.1445-6664.2004.00143.x. [DOI] [Google Scholar]
- Chen W.; Yun M.-S.; Deng F.; Yogo Y. The rates of maize growth and lignin biosynthesis change after root-applied chalcone. Weed Biol. Manag. 2005, 5, 118–122. 10.1111/j.1445-6664.2005.00165.x. [DOI] [Google Scholar]
- Chen W.-J.; Yun M.-S.; Deng F.; Yogo Y. Chalcone suppresses lignin biosynthesis in illuminated soybean cells. Weed Biol. Manag. 2011, 11, 49–56. 10.1111/j.1445-6664.2011.00404.x. [DOI] [Google Scholar]
- Diaz-Tielas C.; Graña E.; Sotelo T.; Reigosa M. J.; Sánchez-moreiras A. M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant Cell Environ. 2012, 35, 1500–1517. 10.1111/j.1365-3040.2012.02506.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Tielas C.; Sotelo T.; Graña E.; Reigosa M. J.; Sánchez-Moreiras A. M. Phytotoxic potential of trans-chalcone on crop plants and model species. J. Plant Growth Regul. 2014, 33, 181–194. 10.1007/s00344-013-9360-6. [DOI] [Google Scholar]
- Gomes A. S.; Oliveira S. C. C.; Mendonça I. S.; Silva C. C. d.; Guiotti N. X.; Melo L. R.; Silva W. A.; Borghetti F. Potential herbicidal effect of synthetic chalcones on the initial growth of sesame, Sesamum indicum L., and brachiaria, Urochloa decumbens (Stapf) RD Webster. Iheringia, Ser. Bot. 2018, 73, 46–52. 10.21826/2446-8231201873106. [DOI] [Google Scholar]
- Nguyen G. T.; Erlenkamp G.; Jäck O.; Küberl A.; Bott M.; Fiorani F.; Gohlke H.; Groth G. Chalcone-based selective inhibitors of a C4 plant key enzyme as novel potential herbicides. Sci. Rep. 2016, 6, 27333. 10.1038/srep27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar M.; Reddy T. P.; Dadhich A. S.; Ram B.; Balram B. Synthesis, characterisation and antibacterial evaluation of chalcone derivatives linked with 2-trifluoromethyl furan. Der Pharma Chem. 2015, 7, 177–183. [Google Scholar]
- Chotsaeng N.; Laosinwattana C.; Charoenying P. Herbicidal activities of some allelochemicals and their synergistic behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. 10.3390/molecules22111841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z.; Zhou F.; Wei S. Synthesis and herbicidal activities of benzothiazole N,O-acetals. Bioorg. Med. Chem. Lett. 2015, 25, 4065–4068. 10.1016/j.bmcl.2015.08.051. [DOI] [PubMed] [Google Scholar]
- Tayeb W.; Chaieb I.; Hammami M.. Environmental fate and effects of 2, 4-dichlorophenoxyacetic herbicide. In Herbicides: Properties, Crop Protection and Environmental Hazards; Piotrowsky K. P., Ed.; Nova Sciences Publisher: New York, 2011; pp 161–187. [Google Scholar]
- Travlos I. S.; Gkotsi T.; Roussis I.; Kontopoulou C.-K.; Kakabouki I.; Bilalis D. J. Effects of the herbicides benfluralin, metribuzin and propyzamide on the survival and weight of earthworms (Octodrilus complanatus). Plant Soil Environ. 2017, 63, 117–124. 10.17221/811/2016-PSE. [DOI] [Google Scholar]
- Bauer K.; Hoffmann M. G. Synthesis and herbicidal activity of novel 2,3-dibromo-5- trifluoromethyl-4-pyrrolecarboxylate derivatives. Pestic. Sci. 1994, 42, 25–28. 10.1002/ps.2780420105. [DOI] [Google Scholar]
- Lewkowski J.; Malinowski Z.; Matusiak A.; Morawska M.; Rogacz D.; Rychter P. The effect of new thiophene-derived aminophosphonic derivatives on growth of terrestrial plants: a seedling emergence and growth test. Molecules 2016, 21, 694. 10.3390/molecules21060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Luo L.; Zheng L. Lignins: biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. 10.3390/ijms19020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.