Abstract
Introduction
Adipokines are regulatory molecules which act as mediators of the inflammatory, fibrotic and metabolic processes by interacting with the immune system.
Aim
We hypothesized that chemerin and visfatin by pro-inflammatory properties play a significant role in inflammation in systemic sclerosis. To address this hypothesis, we determined serum chemerin and visfatin levels in SSc patients, compared with the control group and defined the correlations with clinical and laboratory parameters in SSc patients.
Material and methods
The study included 48 Caucasian female patients with SSc and 38 healthy subjects of the control group. Serum concentrations of selected adipokines were measured using commercially available ELISA Kits.
Results
Patients with SSc had higher chemerin levels (209.38 ±55.35 ng/ml) than the control group (182.71 ±33.94 ng/ml) and the difference was statistically significant (Z = 2.14, p = 0.032). The highest chemerin levels were found in dcSSc patients (242.46 ±95.82 ng/ml). We indicated a positive correlation of chemerin and visfatin with levels of inflammatory markers: CRP (r = 0.35, p = 0.013 for chemerin; r = 0.41, p = 0.003 for visfatin) and ESR (r = 0.31, p = 0.03 for chemerin; r = 0.30, p = 0.03 for visfatin). What is more, chemerin manifested a statistically significant positive correlation with the concentration of complement component C3 (r = 0.47, p = 0.001) and C4 (r = 0.29, p = 0.049), whereas visfatin correlated with C4 levels (r = 0.32, p = 0.029).
Conclusions
The results of our study indicate that chemerin and visfatin as pro-inflammatory cytokines might represent new markers corresponding with inflammation in systemic sclerosis and might reflect the bridge between metabolism, inflammation and potentially, chemerin may also link inflammation with skin and lung fibrosis.
Keywords: visfatin, chemerin, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a chronic, multi-organ connective tissue disease of uncertain etiology which affects the skin and internal organs, particularly the lungs, heart and gastrointestinal tract. It is characterized by widespread vascular injury, inflammation, fibrosis and autoimmune disturbances [1]. Recently adipocytokines have attracted much attention in terms of autoimmune disorders as inflammatory molecules involved in pathological cytokine networks [2]. In SSc, several adipocytokines have been reported to be implicated in the development of immune abnormalities, fibrosis as well as microangiopathy [3–8].
Adipocytokines, or adipokines, is a general term for bioactive proteins secreted by adipose tissue and mediators linking adipose tissue, inflammation and immunity. Thus, in addition to energy homeostasis, they participate in inflammatory processes, and emerging evidence indicates that some have also immunomodulating, pro- or anti-fibrotic or even angiogenic properties [9–13].
Chemerin is an adipocytokine that emerged in 1997 and was originally isolated from inflamed biological fluids in the context of psoriasis and hypothesized to be involved in cell-cell or cell-extracellular matrix interactions [14]. It is a 16-kDa protein acting as tazarotene-induced gene 2 (TIG2; also known as retinoic acid receptor responder gene 2, RARRES2) [14]. Chemerin and its receptors are expressed in adipose tissue, dendritic cells and macrophages [15]. Recently, it has been reported to have a number of physiological and pathophysiological actions [16]. Chemerin has a dual (“chimeric”) effect on inflammation: pro-inflammatory through ChemR23 receptor activation or anti-inflammatory via CMKLR1 (chemokine-like receptor 1), thus exhibits two opposite actions [17, 18]. On the one hand, it exerts a chemotactic function for immune cells (leukocytes, immature dendritic cells and macrophages), promoting cellular migration in inflammatory conditions [2, 18–22], on the other hand, it inhibits pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) [17, 18]. Chemerin is thought to be involved in the chronic inflammatory process, including psoriasis, lichen planus, SLE and rheumatoid arthritis (RA) [20, 23–26]. Moreover, chemerin has been found to mediate dysregulated angiogenesis and may be assumed as a pro-angiogenic factor that plays an important role in tumor invasion and metastasis by promoting angiogenesis [15, 18, 21, 27].
Visfatin, also known as nicotinamide phosphoribosyltransferase (NAMPT), is a 52 kDa adipocytokine, which was first found in 1994 and was initially recognized as a pre-B-cell colony enhancing factor (PBEF), which enhances differentiation of B-cell precursors in synergy with IL-7 and stem cell factor [28]. Later, in 2004, it was identified as a key regulator of metabolism and insulin resistance [29]. It is secreted by adipocytes and macrophages infiltrating the adipose tissue [30–32]. It is also produced by several tissues, including skeletal muscle, liver, and bone marrow, basically by immune cells such as neutrophils and lymphocytes [28]. It functions as an immunomodulatory and proinflammatory cytokine that stimulates monocytes to produce pro-inflammatory cytokines such as IL-1, IL-6, IL-8 and TNF-α and induces chemotaxis of activated mononuclears [10, 19, 31, 33, 34]. Reflecting its pro-inflammatory properties, an upregulation of visfatin was reported in the inflammatory condition such as inflammatory bowel disease, Behçet disease and RA [5, 8, 10, 11, 22, 35–38]. Moreover, there is a large body of evidence showing that visfatin might attenuate angiogenesis and endothelial cell reparation [29, 39] and the potential role of visfatin in organ fibrosis was reported [11–13].
Taking these data into consideration, we hypothesized that chemerin and visfatin may be potentially involved in the inflammatory mechanisms of SSc pathogenesis.
Aim
Thus, to further explore this hypothesis, in the present study, we measured the serum concentrations of visfatin and chemerin in patients with SSc and in healthy controls to further investigate the role of these molecules in SSc.
Material and methods
Patients
A total of 48 Caucasian female patients with SSc (aged 34–84 years, 62 ±10.6years), fulfilling the American College of Rheumatology (ACR) and/or EULAR classification criteria were included in the study [40, 41]. The control group included 38 healthy subjects, matched with patients for sex, age, BMI and race (aged 36–88; 56.3 ±9.7 years) (Table 1). Patients and healthy controls were voluntarily recruited and informed consent was obtained from all participants. The study was approved by the Bioethics Committee. Patients with overlap syndromes, kidney disease, diabetes mellitus, thrombosis, metabolic syndrome/hyperlipidemia, pregnancy, neoplastic diseases and those with habitual cigarette smoking and alcohol drinking were excluded from the study.
Table 1.
Demographic data of SSc patients and healthy controls
| Parameter | SSc patients(n = 48) | Healthy controls(n = 38) |
|---|---|---|
| Gender, female : male ratio | 48 : 0 | 38 : 0 |
| Age. mean (SD), range [years] | 62.6 (10.5), 34–84 | 56.3 (9.7), 36–88 |
| BMI, mean (SD) | 28.07 (11.49) | 25.51 (3.38) (p = 0.212)* |
| Total cholesterol, mean (SD) [mg/dl] | 197.62 (34.3) | 202.06 (30.02) (Z = –0.634; p = 0.525)* |
| LDL, mean (SD) [mg/dl] | 115.13 (28.07) | 116.34 (27.14) (Z = –0.091; p = 0.927)* |
| HDL, mean (SD) [mg/dl] | 57.39 (19.69) | 62.79 (12.8) (Z = –1.895; p = 0.058)* |
| TG, mean (SD) [mg/dl] | 127.0 (44.1) | 104.36 (35.4) (Z = 2.6305; p = 0.008)* |
| eGFR [ml/min] | 81.51 (21.02) | Not estimated |
BMI – body mass index, LDL – low-density lipoprotein, HDL – high-density lipoprotein, TG – triglycerides, eGFR – estimated glomerular filtration rate (normal value ≥ 90 ml/min).
Mann-Whitney U test.
Clinical assessment
According to the criteria proposed by LeRoy et al., patients were classified as having either limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc) [42]. Disease onset was defined as the first clinical symptom of SSc other than Raynaud phenomenon (RP). Disease duration was defined as the interval between onset and time of blood sampling. The disease activity was assessed according to the European Scleroderma Study Group (EScSG) disease activity index score for SSc (Valentini disease activity index) as active or inactive [43]. Skin thickness of all patients was evaluated using a modified Rodnan total skin thickness score (mRSS) [44]. Microvascular complications such as digital ulcers and osteolysis of the distal phalanges of the fingers (acroosteolysis) were also evaluated. Nailfold capillaroscopy was performed to estimate SSc microangiopathy according to the criteria proposed by Cutolo et al. [45]. Patients with ground glass opacification, centrilobular nodules or honeycomb picture in high-resolution computed tomography (HRCT) were regarded to have lung involvement. Lung function parameters were assessed by spirometry (forced vital capacity – FVC; total lung capacity – TLC) in 41 of SSc patients and percentage diffusing capacity for carbon monoxide (%DLCO) was examined in 26 individuals from the SSc group. Pulmonary artery pressure and valvular insufficiency were assessed by colour Doppler echocardiography (ECO). The pulmonary arterial hypertension was defined as right ventricular systolic pressure (RVSP) ≥ 35 mm Hg in Doppler echocardiography and was determined only at rest [46].
Laboratory measurements
As part of routine patient assessment: 1) the presence of ANAs and their specificity including anticentromere antibodies (ACAs), anti-topoisomerase 1 antibodies (anti-topo I, Scl-70), anti-RNA polymerase I or III antibodies, anti- U3- and U1-RNP, PM-Scl and anti-Ku antibodies was determined in the blood by means of IIF on HEp-2 cells and/or immunoblot analysis, 2) serum levels of C3 and C4 complement were measured, 3) CRP and ESR values were assessed, 4) estimated glomerular filtration rate (eGFR) was calculated from routine creatinine measurements using Modification of Diet in Renal Disease equation [47]. The lipid profile, including total cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL) and triglycerides (TG) levels was analyzed among patients and healthy controls.
Serum concentrations of adipokines were measured using commercially available ELISA Kits: visfatin (Nampt/Visfatin human EIA KIT immunoGen, Poland) and chemerin (Human chemerin Elisa, BioVendor, Czech Republic) according to the manufacturer’s instructions. The material for the study was fasting peripheral blood drawn for blood clot in the morning. The samples were allowed to clot for 30 min and centrifuged for 15 min at 1000 × g. Obtained sera were stored at –70°C immediately after collection until further analysis. The concentration level of cytokines was calculated using an appropriate standard curve generated by the reader ELISA ELX 800 Bio-tek Instruments, USA.
Statistical analysis
The results were analyzed statistically using STATISTICA 10.0 PL software. Equality of distribution for each variable within normal distribution groups was tested using the Lilliefors version of the Kolmogorov-Smirnov test as well as the Shapiro-Wilk test. Pairs of independent groups were compared using the Mann-Whitney U test. The relationship between adipokines and patient characteristics were correlated by Spearman’s rank correlation test, where p < 0.05 was considered as statistically significant.
Results
Clinical and laboratory characteristics of SSc patients
Detailed clinical and laboratory characteristics of SSc patients and healthy controls are summarized in Tables 1 and 2.
Table 2.
Clinical and laboratory parameters of patients with systemic sclerosis (SSc) (n = 48)
| Parameter | Value |
|---|---|
| Disease type, n (%): | |
| lcSSc | 42 (87.5) |
| dcSSc | 6 (12.5) |
| Duration of Raynaud’s phenomenon, mean (SD) [years] | 16.91 (9.3) |
| Disease duration, mean (SD) [years]: | 12.85 (7.63) |
| lcSSc (n); mean ± SD (range) | 42; 12.98 ±7.76 (1.5–35) |
| Early lcSSc (n); mean ± SD (range) | 8; 2.93 ±1.34 (1.5–5) |
| Late lcSSc (n); mean ± SD (range) | 34; 15.11 ±6.74 (5–35) |
| dcSSc (n); mean ± SD (range) | 42; 11.5 ±7.89 (2–25) |
| Early dcSSc (n); mean ± SD | 1; 1 ±2 |
| Late dcSSc (n); mean ± SD (range) | 5; 13.4 ±7.12 (6–25) |
| Active disease, n (%) | 9 (18.7) |
| Inactive disease, n (%) | 39 (81.25) |
| History of digital ulcers, n (%): | |
| Active digital ulcers | 13 (27) |
| No active digital ulcers | 35 (73) |
| Acroosteolysis, n (%) | 13 (27) |
| Early pattern of microangiopathy, n (%) | 10 (20.8) |
| Active pattern of microangiopathy, n (%) | 8 (16.6) |
| Late pattern of microangiopathy, n (%) | 20 (41.6) |
| mRSS, mean (SD), (range) | 10 (6.2), (2–28) |
| Antinuclear antibodies, n (%): | |
| ACA | 26 (54.1) |
| Topo-1 | 17 (35.4) |
| Anti-RNA polymerase III | 2 (4.1) |
| Other (anti-fibrillarin, ANA-speckled pattern) | 3 (6.25) |
| ILD, n (%) | 43 (89.5) |
| DLCO, mean (SD), (range) | 84.4 (19.1), (41–114) |
| DLCO: | |
| ≥ 80% | 17 |
| < 80% | 9 |
| FVC, mean (SD), (range) | 97.9 (23.2), (50–142) |
| FVC: | |
| ≥ 80% | 35 |
| < 80% | 6 |
| PH (RVSP ≥ 35 mm Hg), n (%) | 11 (22.9) |
| Esophageal involvement, n (%) | 35 (72.9) |
| ECG changes, n (%) | 6 (12.5) |
| Heart valves abnormalities, n (%) | 31 (64.5) |
| Myalgia, n (%) | 10 (20.8) |
| Arthralgia, n (%) | 41 (85.6) |
| ESR, mean (SD), (range) [mm/h] | 26.065 (15.6), (7–80) |
| CRP, mean (SD), (range) [mg/l] | 7.94 (17.2), (0.5–88.6) |
| Complement C3, mean (SD) (range): | 1.10 (0.20), (0.73–1.47) |
| Low level (n)/in normal range (n) | 11/37 |
| Complement C4, mean (SD) (range): | 0.24 (0.08), (0.06–0.58) |
| Low level (n)/in normal range (n) | 4/44 |
lcSSc – limited cutaneous systemic sclerosis, dcSSc – diffuse cutaneous systemic sclerosis, ACA – anti-centromere antibodies, Topo-1 – anti-topoisomerase 1 antibodies, ANA – antinuclear antibodies, mRSS – modified Rodnan skin score, ILD – interstitial lung disease, DLCO – diffusing capacity for carbon monoxide, FVC – Forced Vital Capacity, TLC – total lung capacity, PH – pulmonary arterial pressure, ECG – electrocardiogram, ESR – erythrocyte sedimentation rate, CRP – C-reactive protein, SD – standard deviation.
Out of 48 patients, 42 had lcSSc and 6 had dcSSc. Mean disease duration for the whole SSc group was 12.85 ±7.63 years. In the dcSSc group, the majority of patients (n = 5/6) was in the late disease period (mean disease duration 13.4 ±7.12 years, range: 6–25 years). Similarly, in the lcSSc group, most patients (n = 34/42) have late disease with mean duration of 15.11 ±6.74 years (range: 5–35).
Since serum chemerin levels are reported to be significantly elevated in patients with chronic kidney disease [48, 49], we estimated the glomerular filtration rate among the SSc group. The mean value of eGFR was slightly decreased in SSc patients (81.51 ±21.02 ml/min/1.73 m2). In the dcSSc group, 3/6 patients while in the lcSSc group, 10/42 individuals had eGFR < 90 ml/min/1.73 m2. In the whole SSc group, chemerin levels did not correlate with eGFR values (r = –0.19, p = 0.14); however, in the early lcSSc group (n = 8), a significant negative correlation has been found between chemerin levels and eGFR (r = –0.88, p = 0.018). Then, we classified SSc patients into two groups according to a significant decrease in eGFR. Eight SSc patients had significantly decreased eGFR < 60 ml/min/1.73 m2 (all with lcSSc); however, patients with decreased eGFR < 60 ml/min/1.73 m2 had comparable serum chemerin levels (210.19 ±63.16 ng/ml) than those with values ≥ 60 ml/min/1.73 m2 (204.43 ±52.44 ng/ml), (Z = 0.31; p = 0.74). Importantly, serum chemerin levels did not correlate with eGFR in either SSc patients with eGFR < 60 ml/min/1.73 m2 (r = 0.13, p = 0.075) or in SSc patients with eGFR ≥ 60 ml/min/1.73 m2 (r = –0.29, p = 0.08). Since the renal function seems to not affect serum chemerin levels in our SSc patients with eGFR < 60 ml/min/1.73 m2, we did not exclude those 8 patients with eGFR < 60 ml/min/1.73 m2 from the following analyses. Controls with eGFR < 90 ml/min/1.73 m2 were excluded from the study.
In view of chemerin and visfatin as bioactive proteins secreted by adipose tissue and key regulators of metabolism, we assessed the lipid profile in SSc patients, compared it to healthy controls and analysed a potential correlation with these adipocytokines. No statistically significant difference was found according to BMI (p = 0.177), total cholesterol, LDL and HDL; whereas TG concentrations were significantly elevated in SSc patients when compared with healthy controls (Table 1). Concerning the lipid profile, we found a statistically significant positive correlation between chemerin and LDL values (r = 0.31; p = 0.03), visfatin and TG values (r = 0.31; p = 0.02) and a significant negative correlation was observed between visfatin levels and HDL (r = –0.34; p = 0.017), while in the control group, no significant correlations were found except of close to a significant positive correlation between chemerin and HDL (r = 0.30; p = 0.06) (Table 3).
Table 3.
Adipokines correlation with clinical and laboratory parameters in SSc patients with use of Spearman’s rank correlation test
| Disease characteristic | Chemerin | Visfatin | ||||
|---|---|---|---|---|---|---|
| Total SSc group | lcSSc | dcSSc | Total SSc group | lcSSc | dcSSc | |
| RP |
r = 0.21 p = 0.15 |
– | – |
r = –0.02 p = 0.25 |
– | – |
| Disease duration |
r = 0.07 p = 0.613 |
r = –0.02 p = 0.872 |
r = 0.371 p = 0.468 |
r = –0.17 p = 0.26 |
r = –0.14 p = 0.34 |
r = –0.60 p = 0.208 |
| mRSS |
r = –0.03 p = 0.80 |
r = 0.02 p = 0.859 |
r = –0.82 p = 0.04 |
r = 0.09 p = 0.53 |
r = 0.15 p = 0.32 |
r = –0.41 p = 0.41 |
| Disease activity index |
r = –0.27 p = 0.066 |
r = –0.22 p = 0.16 |
r = –0.20 p = 0.693 |
r = –0.053 p = 0.715 |
r = –0.064 p = 0.684 |
r = –0.23 p = 0.451 |
| Total cholesterol |
r = 0.09 p = 0.548 |
– | – |
r = –0.14 p = 0.335 |
– | – |
| HDL |
r = –0.13 p = 0.37 |
– | – |
r = –0.37 p = 0.009 |
– | – |
| LDL |
r = 0.15 p = 0.325 |
– | – |
r = 0.08 p = 0.60 |
– | – |
| TGL |
r = 0.3 p = 0.037 |
– | – |
r = 0.34 p = 0.019 |
– | – |
| BMI |
r = –0.07 p = 0.634 |
– | – |
r = 0.08 p = 0.574 |
– | – |
RP – Raynaud phenomenon – duration, HDL – high-density lipoprotein, LDL – low-density lipoprotein, TGL – triglycerides, BMI – body mass index.
Serum levels of chemerin and visfatin in SSc patients and the control group
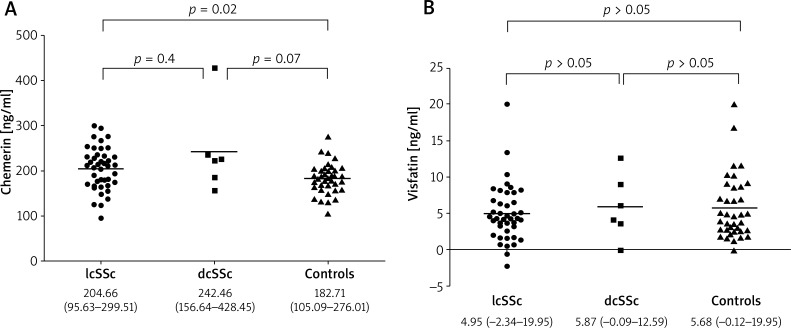
Serum chemerin levels in SSc patients were significantly higher (209.38 ±55.35 ng/ml) than in the control group (182.71 ±33.94 ng/ml), (p = 0.032) (Table 4). The highest chemerin levels were found in dcSSc patients (242.46 ±95.82 ng/ml) and this was close to statistically significance when compared to healthy controls (p = 0.07). Patients with lcSSc have significantly higher serum chemerin (204.65 ±47.00) than the control group (p = 0.02) but comparable with the dcSSc subtype (p = 0.4). In contrast, we did not find any differences in visfatin concentrations in SSc patients when compared to controls as well as between lcSSc and dcSSc groups (Figures 1 A and B).
Table 4.
Adipokines levels in SSc patients compared to the control group with use of Mann-Whitney U test
| Cytokine | SSc patients | Control group | Z | P-value |
|---|---|---|---|---|
| Mean ± SD [ng/ml] | Mean ± SD [ng/ml] | |||
| Chemerin | 209.38 ±55.35 | 182.71 ±33.94 | 2.14 | 0.032 |
| Visfatin | 5.06 ±3.94 | 5.68 ±4.39 | –0.301 | 0.763 |
Figure 1.
Chemerin (A) and visfatin (B) levels in dcSSc, lcSSc patients and the control group; data are given as medium and range
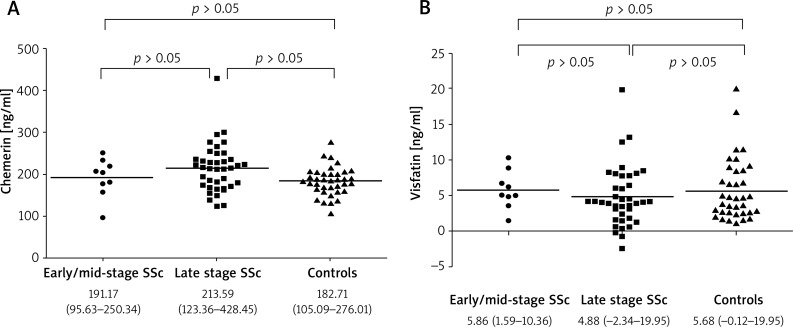
Figure 2.
Chemerin (A) and visfatin (B) levels in SSc patients according to disease duration and in the control group; data are given as medium and range
Correlation of serum chemerin and visfatin levels with clinical characteristics of SSc patients
Correlation with disease duration
Since previous reports suggested that chemerin levels correlate well with duration of the disease in SSc patients, we investigated a potential correlation of serum chemerin and visfatin with time from disease onset.
The mean value of disease duration for our SSc group was 12.85 (1.5–35) years, for patients with lcSSc (n = 42) – 12.98 (1.5–35) years and for dcSSc (n = 6) the mean value was 11.5 (2–25) years. Neither chemerin nor visfatin levels correlated with disease duration in the total SSc group (r = 0.07, p = 0.613 and r = –0.17, p = 0.26, respectively). Similarly, when divided according to the disease subtype (lcSSc and dcSSc), no correlations were found between serum chemerin or visfatin and time from the disease onset (Table 3). Next, we classified SSc patients according to disease duration into early/mid-stage (< 6 years; 9 patients, including 1 patient with dcSSc and 8 with lcSSc) and late stage (≥ 6 years; 39 patients, including 34 with lcSSc and 5 with dcSSc) as described previously [5, 50, 51]. Serum chemerin as well as visfatin levels did not differ significantly between SSc patients with early/mid-stage and those with the late stage of the disease (p = 0.35 and p = 0.16, respectively) (Figures 2 A and B). Moreover, there was no correlation of both chemerin and visfatin with the time of disease duration either in patients with early/mid-stage or late stage of the disease (r = 0.80; p = 0.08 for visfatin in early/mid-stage; r = –0.11; p = 0.77 for chemerin in early/mid-stage; r = –0.14; p = 0.37 for visfatin in late stage; r = –0.10; p = 0.52 for chemerin in late disease), (data not shown).
Correlation with skin and lung fibrosis
Next, we analyzed the possible association of serum chemerin and visfatin with parameters reflecting dermal and pulmonary fibrosis, including modified Rodnan total skin thickness score (mRSS), presence of ILD on lung HRCT, pulmonary function tests, including forced vital capacity (FVC), as well as percentage diffusing capacity for carbon monoxide (%DLCO).
The mean value of mRSS in the total SSc group was 10 ±6.2 and ranged from 2 to 28. We also divided patients into two groups based on the severity of skin involvement: those with mRSS value < 14 and those with mRSS ≥ 14 (the mRSS cut off value was chosen based on EScSG disease activity index score).
Scleroderma-related interstitial lung disease, based on HRCT findings, was present in 43 patients (89.5%), 9/26 patients had decreased %DLCO value (< 80%), 6/41 had lowered %FVC (< 80%) and in 12/38 decreased TLC (< 80%) was found.
Levels of serum chemerin and visfatin were not associated with the severity of skin involvement as assessed by mRSS – the values were comparable between patients with mRSS < 14 and those with mRSS ≥ 14 (p = 0.80 for chemerin and p = 0.53 for visfatin), (data not shown). Moreover, serum chemerin and visfatin did not correlate with the mRSS value in the total SSc group (r = –0.03; p = 0.80 for chemerin; r = 0.09; p = 0.53 for visfatin) and in patients with lcSSc (r = 0.02; p = 0.85 for chemerin; r = 0.15; p = 0.32 for visfatin); however there was a significant inverse correlation between serum chemerin and mRSS in those with dcSSc (r = –0.82; p = 0.04) while no correlation was found for visfatin in this group (r = –0.41; p = 0.41) (Table 3).
Similarly, when considering pulmonary involvement, we did not observe any statistically significant associations between chemerin or visfatin levels and the presence of ILD on lung HRCT (Table 5). Chemerin serum levels as well as values of visfatin were not significantly different in patients with decreased %FVC values than in those patients with normal %FVC. Patients with SSc with decreased %DLCO values had comparable chemerin or visfatin levels when compared to SSc individuals with normal %DLCO (Table 5). Of note, impairment of the pulmonary function due to fibrosis in SSc progresses with disease duration. Notably, when we divided patients into two groups: an early/mid-stage and late disease, chemerin positively correlated with FVC value in late stage disease (r = 0.42; p = 0.01). Moreover, in patients with late disease we found an almost significant inverse correlation of serum chemerin and the presence of ILD on lung HRCT (r = –0.31; p = 0.058) (Table 6).
Table 5.
Associations of serum chemerin and visfatin with characteristics of pulmonary involvement in SSc patients; Mann-Whitney U test; data are given as mean ± SD
| Parameter | Clinical parameterTotal SSc group | Z | P-value | |
|---|---|---|---|---|
| ILD positive (n = 43) | ILD negative (n = 5) | |||
| Chemerin [ng/ml] | 210.666 ±57.091 | 180.757 ±45.070 | –1.023 | 0.306 |
| Visfatin [ng/ml] | 5.092 ±4.105 | 3.779 ±2.169 | –0.578 | 0.563 |
| Decreased %DLCO (n = 9) | %DLCO in normal range (n = 17) | |||
| Chemerin [ng/ml] | 208.99 ±41.77 | 225.65 ±68.07 | 0.395 | 0.692 |
| Visfatin [ng/ml] | 4.96 ±4.53 | 5.66 ±5.07 | 0.131 | 0.895 |
| Decreased FVC (n = 6) | FVC in normal range (n = 35) | |||
| Chemerin [ng/ml] | 199.61 ±37.36 | 212.83 ±62.37 | 0.398 | 0.690 |
| Visfatin [ng/ml] | 4.70 ±4.18 | 5.06 ±4.30 | 0.225 | 0.821 |
| PH positive (n = 11) | PH negative (n = 35) | |||
| Chemerin [ng/ml] | 207.84 ±56.66 | 210.31 ±55.97 | 0.257 | 0.796 |
| Visfatin [ng/ml] | 6.67 ±6.62 | 4.41 ±2.64 | –0.952 | 0.340 |
ILD – interstitial lung disease, DLCO – diffusing capacity for carbon monoxide, FVC – forced vital capacity, TLC – total lung capacity, PH – pulmonary arterial pressure.
Table 6.
Correlations between serum chemerin and visfatin with parameters of pulmonary involvement in SSc patients with use of Spearman’s rank correlation test
| Parameter of pulmonary involvement | Chemerin | Visfatin | ||||
|---|---|---|---|---|---|---|
| Total SSc group | lcSSc | dcSSc | Total SSc group | lcSSc | dcSSc | |
| Presence of ILD |
r = –0.155 p = 0.301 |
r = 0.160 p = 0.322 |
N/A* |
r = 0.089 p = 0.554 |
r = 0.086 p = 0.596 |
N/A* |
| %DLCO |
r = 0.212 p = 0.297 |
r = 0.107 p = 0.632 |
r = 0.4 p = 0.6 |
r = –0.09 p = 0.660 |
r = –0.158 p = 0.481 |
r = 0.8 p = 0.2 |
| %FVC |
r = 0.241 p = 0.127 |
r = 0.215 p = 0.205 |
r = 0.1 p = 0.872 |
r = 0.034 p = 0.832 |
r = –0.07 p = 0.666 |
r = 0.7 p = 0.188 |
| RVSP |
r = –0.04 p = 0.79 |
r = 0.08 p = 0.621 |
r = –0.82 p = 0.041 |
r = 0.143 p = 0.339 |
r = 0.194 p = 0.229 |
r = –0.207 p = 0.693 |
| Early/mid–stage SSc | Late SSc | Early/mid–stage SSc | Late SSc | |||
| Presence of ILD |
r = –0.410 p = 0.272 |
r = –0.313 p = 0.058 |
r = 0.41 p = 0.272 |
r = 0.033 p = 0.843 |
||
| %DLCO | N/A** |
r = 0.178 p = 0.404 |
N/A ** |
r = 0.05 p = 0.814 |
||
| %FVC |
r = –0.542 p = 0.265 |
r = 0.422 p = 0.011 |
r = –0.6 p = 0.208 |
r = 0. 114 p = 0.512 |
||
| RVSP |
r = 0.125 p = 0.766 |
r = –0.059 p = 0.723 |
r = 0.755 p = 0.03 |
r = 0.025 p = 0.879 |
||
All patients with dcSSc had ILD on HRCT.
Only 2 patients with early/mid-stage disease had estimated %DLCO, lcSSc – limited cutaneous systemic sclerosis, dcSSc – diffuse cutaneous systemic sclerosis, ILD – interstitial lung disease, DLCO – diffusing capacity for carbon monoxide, FVC – forced vital capacity, TLC – total lung capacity, RVSP – right ventricular systolic pressure.
Correlation with vascular involvement
In our SSc patient group, 11 (22.9%) had elevated pulmonary artery pressure as assessed by Doppler echocardiography, including 9 individuals with limited disease. None of the SSc patients had renal crisis. Active digital ulcers were present in 13 (27%) and acroosteolysis in 13 (27%) of SSc subjects. Based on criteria proposed by Cutolo et al. [45], an early capillaroscopic pattern was found in 10 (20.8%), active pattern in 8 (16.6%) and late pattern in 20 (41.6%) SSc patients. An increased value of sPAP (≥ 35 mm Hg) on ECO was present in 11 (22.9%) patients, among them 9 subjects with lcSSc.
According to the clinical complication of SSc microangiopathy, we did not find any association between analyzed adipocytokines serum levels and the presence of active digital ulcers, as well as no substantial differences were observed according to the presence or absence of acroosteolysis. No significant differences in the levels of chemerin and visfatin were found between the three capillaroscopic patterns (early, active and late).
Chemerin concentrations negatively correlated with the PAH value in the group of dcSSc patients (r = –0.82, p = 0.04), while for lcSSc, no substantial correlation has been observed (r = 0.08; p = 0.62). In contrast, serum visfatin showed a positive correlation with PAH in early disease (r = 0.75; p = 0.03) (Table 6).
Correlation of serum chemerin and visfatin levels with inflammatory markers in SSc patients
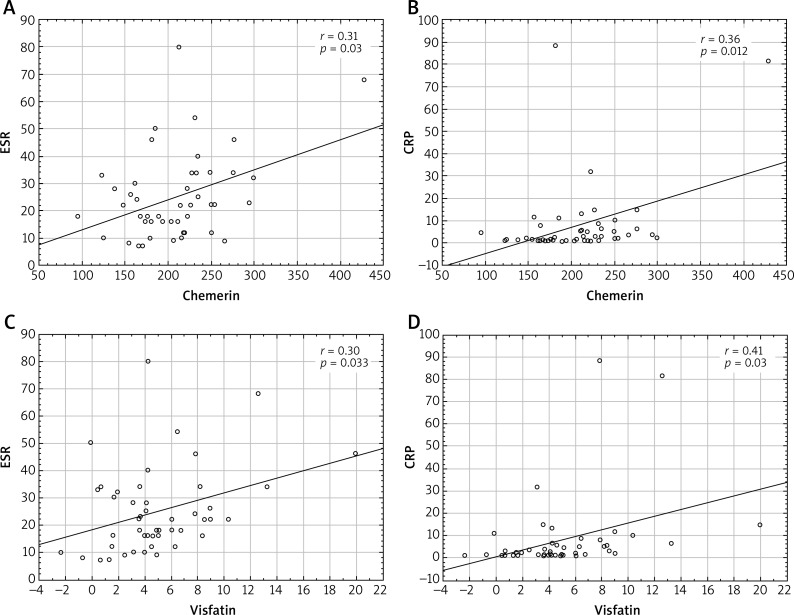
Consistently with the potential role in the regulation of inflammatory processes, we correlated chemerin and visfatin serum levels with serum markers of inflammation in SSc patients. Interestingly, chemerin levels positively correlated with both C-reactive protein (CRP) (r = 0.36; p = 0.012) and ESR (r = 0.31; p = 0.03). We also indicated a significant positive correlation of visfatin with CRP (r = 0.41, p = 0.003) and ESR (r = 0.30, p = 0.03), (Figure 3).
Figure 3.
Correlation of chemerin and visfatin levels with inflammatory markers (ESR and CRP) in SSc patients; Spearman’s rank correlation test
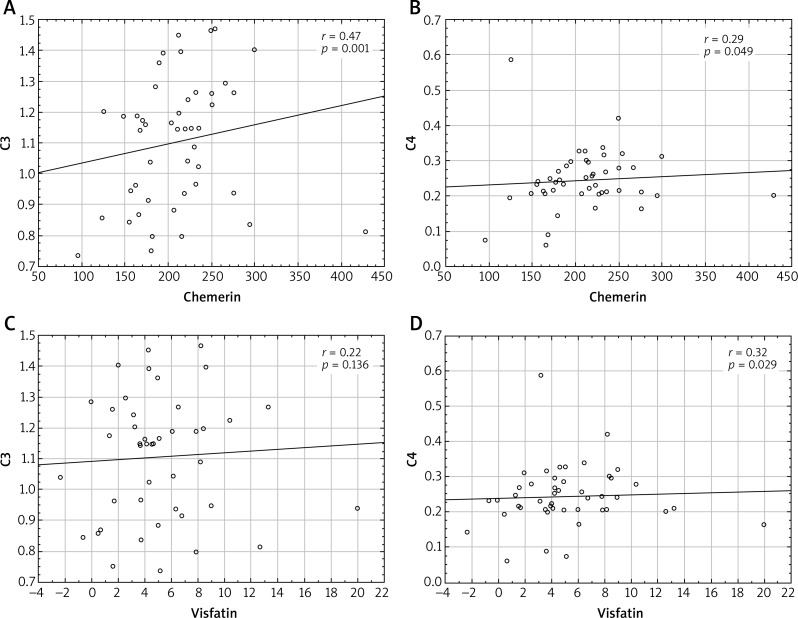
What is more, chemerin manifested a strong positive correlation with the concentration of complement component C3 (r = 0.47, p = 0.001) and C4 (r = 0.29, p = 0.049), whereas visfatin levels positively correlated with serum C4 (r = 0.32, p = 0.029), but not with C3 component (Figure 4).
Figure 4.
Correlation of chemerin and visfatin levels with complement component C3 and C4 in SSc patients; Spearman’s rank correlation test
Correlation with disease activity
Nine (18.7%) patients had active disease according to Valentini activity index [43]. A comparison made among patients with SSc stratified according to the disease activity did not show statistically significant differences in either chemerin or visfatin levels between active patients and those with inactive disease (209.28 ±91.16 ng/ml vs. 214.88 ±43.91 ng/ml for chemerin; 5.36 ±4.20 ng/ml vs. 5.28 ±4.36 for visfatin; p > 0.05 for both comparisons), (data not shown); however with the use of Spearman’s rank correlation test, there was a close to significant negative correlation between chemerin serum levels and disease activity index (r = –0.27, p = 0.066) (Table 4).
Discussion
In recent years, there have been more and more data on the role of adipocytokines in autoimmune responses. In the last decade, two relatively novel adipocytokines, chemerin and visfatin, were suggested to potentially contribute to the development of rheumatic inflammatory conditions due to their pro-inflammatory and immunomodulating properties as well as potential pro-fibrotic and angiogenic activity; however there are only scarce data in the literature on the significance of visfatin and chemerin in systemic sclerosis [2, 5, 21].
Our study sought to compare serum chemerin and visfatin levels in SSc patients and healthy controls and to determine whether chemerin and visfatin are associated with clinical profiles of the patients. Additionally, due to the fact that chemerin and visfatin seem to have a role in inflammatory and immune responses we aimed to assess if their values might be associated with inflammatory markers of the disease. Moreover, since some factors, including BMI and renal function (eGFR), might influence chemerin or visfatin levels in serum [2, 52], all patients and subjects from the control group were adjusted for age and sex, and did not differ significantly in BMI and lipid profile, except for higher TG values among SSc patients. In the SSc group, serum chemerin levels did not correlate with eGFR even in those patients with a significantly impaired renal function (eGFR < 60/ml/1.73 m2), which is in opposite to the study by Masui et al. [5]. Thus, we did not exclude those patients from following analyses. The analyzed laboratory data were adjusted to these demographic parameters to facilitate comparison of the evaluated groups (patients and controls).
The main findings of the present study are as follows: 1) patients with SSc have higher serum chemerin levels than healthy controls, whereas visfatin levels did not differ significantly; 2) the highest chemerin levels were found in patients with a diffuse clinical subtype of the disease (dcSSc); 3) among dcSSc patients, serum chemerin levels inversely correlated with the severity of skin involvement and the presence of PH; 4) in patients with late disease, chemerin levels positively correlated with %FVC and there was a negative correlation with the presence of lung fibrosis; 5) visfatin positively correlated with the presence of PH in patients with early disease; 6) both chemerin and visfatin levels positively correlated with markers of systemic inflammation CRP and ESR as well as with serum complement component C3 and C4.
In the present study, patients with SSc displayed significantly higher serum chemerin when compared to the control group and the highest chemerin values were found among patients with a diffuse subtype of the disease. Our findings are in opposite to results of Akamata et al. [2] who reported comparable serum chemerin levels in SSc patients and healthy individuals as well as no significant difference between dcSSc and lcSSc patients. Moreover, in contrast to our results, authors showed the lowest chemerin levels in two dcSSc patients. Our notion is also in conflict with the study of Chighizola et al. [21] who reported significantly lower chemerin levels in dcSSc patients compared to controls whereas patients with lcSSc presented similar serum chemerin to healthy subjects.
The discrepancy between studies related to chemerin might result from the different proportion of disease subtypes and different time of disease duration among SSc patients in particular reports, since in our study, the number of dcSSc patients was relatively low – only 6 from 48 patients had dcSSc, while in this of Akamata et al. [2], the dcSSc : lcSSc ratio was significantly higher – 34 : 30. Moreover, in the study of Akamata et al. [2], the mean time of disease duration was 6 years (2–13.5), whereas our patients had an average value of disease duration of 12.85 years, ranging from 1.5 to 35 and were mostly in late stage of the disease (early/mid-stage : late SSc ratio was 9 : 39). Of note, the correlation between serum chemerin and disease duration has been suggested in previous reports and it was particularly significant when considering patients with dcSSc [2, 21]. Akamata et al. [2] reported significantly decreased serum chemerin levels in early dcSSc compared with late-stage dcSSc and in the study by Chighizola et al. [21], dcSSc patients with disease duration below 36 months presented lower levels of serum chemerin compared to those with dcSSc diagnosis longer than 36 months. Unfortunately, we did not confirm such correlation either in the total SSc group or among patients with a diffuse disease subtype.
According to visfatin, our observations are in the line with those of Masui et al. [5] and Ozgen et al. [53], who found no differences in serum visfatin levels among total SSc, dcSSc, lcSSc and healthy controls. Importantly previous studies have reported that serum visfatin levels were increased in rheumatoid arthritis (RA) [36, 53] and active Behçet disease (BD) patients [53], but not in systemic lupus erythematosus (SLE) [53, 54]. These findings may result from differences in the cytokine secretion profile of the particular diseases, since RA and BD are Th1-mediated diseases, while SLE and SSc are Th2-mediated pathologies [53].
In case of visfatin serum levels, we also showed no correlation with time from disease onset, which is in opposite to the report of Masui et al. [5], who found that serum visfatin levels are comparable to normal during early- and mid-stage dcSSc, but are significantly increased in late-stage dcSSc. Once again, it may be a consequence of different proportions of disease subtypes and disease duration as discussed before.
Keeping in mind that both adipocytokines may be involved in fibrotic and angiogenic responses, which are key events in SSc pathogenesis, we looked forward to their potential contribution to clinical characteristics of SSc patients.
Regarding cutaneous signs of fibrosis, we did not find any correlation between either chemerin or visfatin and the severity of skin involvement (mRSS score) in the total SSc group; however, when considering only patients with a diffuse disease subtype (dcSSc), serum chemerin inversely correlated with the intensity of skin fibrosis. In this aspect, we find data from a previous study by Akamata et al. [2] somewhat inconsistent, since authors reported a positive correlation between serum chemerin levels and mRSS in the dcSSc group, but concluded that the reduction of serum chemerin may largely contribute to the initiation of fibrosis in dcSSc, what indicates a rather inverse correlation and fits well with our findings. Consequently, a negative correlation between chemerin and mRSS suggests that down-regulation of chemerin may play some role in the developmental process of skin sclerosis, particularly in dcSSc. In favor of this suggestion is the fact that chemerin was found downregulated in fibroblasts surrounded with thickened collagen bundles in dcSSc lesional skin and it resulted from autocrine TGF-β stimulation and Fli1 deficiency [2]. Thus, chemerin might have an anti-fibrotic effect on SSc dermal fibroblasts. Of note, in previous reports, chemerin was reduced particularly in the early phase of dcSSc when fibrotic changes are rapidly progressive [2, 21]. Unfortunately, we were unable to face with this notion since the number of patients with early/mid-stage dcSSc in our study was too low (n = 1) to reach the statistical power for the correlation with the late-stage group (n = 5).
According to pulmonary fibrosis, we did not find any correlation between serum chemerin or visfatin with the presence of ILD on HRCT, %FVC on lung function tests or %DLCO in the total SSc group. Similarly, in the report by Chighizola et al. [21], no significant difference emerged when chemerin levels were compared between patients with and those without ILD. This is also in line with Akamata et al. [2] who found no significant correlation of serum chemerin levels with %VC or %DLCO.
Of note, impairment of the pulmonary function due to fibrosis in SSc progresses with disease duration. Consistently with this notion, we found that in our SSc patients with the late stage of the disease chemerin positively correlated with FVC value and there was an almost significant inverse correlation with presence of ILD on lung HRCT. Increased chemerin may therefore potentially play a protective role in lung fibrosis. This can be explained by in vitro studies on a mice model, which showed that CMKLR1 chemoattractant receptor for chemerin is expressed on NK cells and other immune cells implicated in pulmonary fibrosis [55]. In CMKLR1-deficient mice, neutrophilic infiltration of the airways was significantly increased and during the fibrotic phase, CMKLR1-deficient mice developed more severe pulmonary fibrosis as measured by lung collagen content and histopathology. Thus, the protective role of chemerin in lung fibrosis may be due to dampening the initial inflammatory response and by recruiting anti-fibrotic leukocytes [55].
For serum visfatin, in compliance with our results, Masui et al. [5] detected no significant correlation with either skin or interstitial lung disease. However, since authors observed significantly elevated serum visfatin in late-stage dcSSc that is generally characterized by the spontaneous resolution of skin sclerosis, they suggested that visfatin elicits the anti-fibrotic effect on SSc dermal fibroblasts [5]. Indeed, in vitro experiments showed that visfatin reverses the pro-fibrotic phenotype of SSc dermal fibroblasts suppressing COL1A2 mRNA expression and increasing MMP-1 mRNA levels, in a dose-dependent manner [5]. Importantly, visfatin also reversed the pro-fibrotic phenotype of normal dermal fibroblasts stimulated with TGF-β [5]. However, these in vitro observations of Masui et al. [5] need further evaluation, since in a couple of other fibrotic disorders, the profibrotic effect of visfatin has been well documented [11, 12]. Serum visfatin levels correlated with fibrosis scores in patients with chronic hepatitis C and visfatin expression was positively associated with the fibrosis stage in non-alcoholic fatty liver disease [11, 12]. Moreover, visfatin induced the expression of basic fibroblast growth factor (bFGF) in rat hepatic stellate cells [56] and promoted the proliferation and the synthesis of type I and type III collagen in rat cardiac fibroblasts [13]. Thus, taken together visfatin might have a dual effect on fibrosis in a context-dependent manner. Whether its levels influence skin sclerosis in SSc and the detailed mechanism remains to be elucidated.
Since the elevated expression of chemerin was reported in dermal small blood vessels, suggesting the contribution of the activated chemerin/ChemR23 pathway to the development of SSc vasculopathy [2], and there is a large body of evidence showing that visfatin is a potential new player in the development of endothelial dysfunction [29, 39, 56, 57], we further examined their potential contribution to the vascular symptoms reflecting SSc-related microangiopathy.
As for cutaneous vascular symptoms, in contrast to Akamata et al. [2], who reported higher serum chemerin levels in SSc patients with digital ulcers, we did not find any associations between either chemerin or visfatin and the presence of active digital ulcerations. Moreover, in our SSc group no correlation has been found with severity of capillary damage assessed on nailfold video-capillaroscopy (NVC). Our observations fit well with another previous study of Chighizola et al. [21] who found comparable chemerin levels when patients were grouped upon capillaroscopic patterns or the presence of digital ulcers.
An important novel finding of our study is that chemerin concentrations negatively correlated with PH estimated on ECO by elevated right ventricular systolic pressure (RVSP), but only in the group of dcSSc patients, whereas serum visfatin showed a positive correlation with PH in early/mid-stage disease, mostly in lcSSc individuals (8 patients with lcSSc and 1 patient with dcSSc). To the best of our knowledge, there is only one anecdotic reference in the literature concerning chemerin and PH among SSc patients [2]. Similarly to our results, Akamata et al. showed that chemerin did not affect the presence of elevated RVSP in the total SSc group, but the authors did not consider lcSSc and dcSSc separately [2]. Such consideration seems justified in view of different pulmonary hypertension mechanisms in lcSSc and dcSSc. PH due to vasculopathy of the small pulmonary arteries (group 1; pulmonary arterial hypertension, PAH) most commonly occurs in patients with limited cutaneous disease and is more common than in dcSSc; conversely, PH secondary to pulmonary fibrosis occurs more frequently in dcSSc versus lcSSc [58–60]. Taking this aspect into consideration, our observations suggest that down-regulation of chemerin may play some role in the developmental process of ILD-related PH in dcSSc patients, rather than PAH. In line with this suggestion is our notion of inverse correlation between chemerin and lung involvement in SSc patients with the late stage of the disease. However, chemerin receptor ChemR23 is widely expressed in the media of rat pulmonary arteries and in vitro results on rat models suggested that chemerin could directly participate in in-vivo pulmonary vascular remodeling and PAH development particularly in conditions where endothelin-1 (ET-1) is up-regulated since it potentiates ET-1-induced vasoconstriction [61, 62].
On the other hand, based on our observation, visfatin seems to be rather associated with induction of PAH in lcSSc patients. In favor of this suggestion is that in patients with idiopathic PAH, visfatin positively correlated with PAH and with pulmonary vascular resistance [63]. Moreover, in vitro studies established the influence of visfatin on several biological processes involved in pulmonary vascular remodeling [64]. Plasma, mRNA and protein levels of visfatin were increased in the lungs and isolated pulmonary artery endothelial cells from patients with pulmonary arterial hypertension, as well as in lungs of rodent models of pulmonary hypertension. Visfatin activity promoted human pulmonary artery smooth muscle cell proliferation via a paracrine effect [65]. Therefore, visfatin may play an important role in PAH pathobiology by promoting pulmonary vascular remodeling during pulmonary hypertension development and pharmacological inhibition of visfatin activity attenuated experimental PH [65]. Despite this appealing biological plausibility, further studies are needed to ascertain whether they have a relevant role in the pathophysiology of PAH and whether it is possible that similar effects could occur in the context of SSc-related PAH.
Interestingly, reflecting their role in inflammatory processes, we showed that both chemerin and visfatin levels positively correlated with parameters of systemic inflammation, i.e. CRP and ESR in patients with SSc. Data concerning this issue are scarce. Only one previous study analyzed the potential association of visfatin with inflammation in SSc and on the contrary, showed that the levels of high-sensitivity CRP and ESR did not correlate with serum visfatin levels in dcSSc and lcSSc [5]. However, our results might be supported by findings from other inflammatory conditions [31, 36–38]. The visfatin level was positively correlated with the CRP level as well as tissue inflammation in the BD and RA group [25, 26, 53, 66–68]. Visfatin has well-documented pro-inflammatory and immunomodulatory effects [10, 35]. It stimulates monocytes to produce pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α [69].
Of note, our notion concerning chemerin is novel and has not been previously reported in SSc; however this is not unique for SSc, since a positive correlation between chemerin serum levels and CRP in patients with type 2 diabetes and metabolic syndrome has been notified [70, 71]. Moreover, the decrease in chemerin serum levels was associated with the drop in ESR and CRP as well as overall disease activity (DAS28) in RA patients after anti-TNF therapy [72]. Importantly, chemerin does not seem to correlate with CRP in healthy, non-obese individuals [73]. This indicates pro-inflammatory activity of chemerin in pathological conditions. In fact, chemerin and ChemR23 receptor have been found to activate MAPK pathways and up-regulate TNF-α, IL-1β, IL-6, and MMPs [74]. This adipocytokine is implicated in chemotaxis of immune cells, including plasmacytoid dendritic cells, macrophages and NK cells [19]. In psoriatic skin lesions, fibroblast-derived chemerin largely contributes to the recruitment of plasmacytoid dendritic cells producing a large amount of IFN-α [20]. In inflammatory arthritis, the concentration of chemerin in synovial fluid is elevated and chemerin activates synovial fibroblasts and promotes joint inflammation and injury [25, 26]. On the other hand, chemerin may also have its own anti-inflammatory effects [75] and consistently, significantly elevated levels of key pro-inflammatory and pro-fibrotic cytokines (TNF-α, IFN-γ, IL-10, IL-17, and TGF-β) have been observed in interstitial lung tissue in CMKLR1-deficient mice [55].
Regarding inflammatory markers, to the best of our knowledge, we are the first to report that both chemerin and visfatin levels positively correlated with serum complement component C3 and C4. The complement system response to inflammation and infection is significant in innate and adaptive immune mechanisms and has been implicated in pathogenesis of systemic sclerosis (SSc) [76].
Taken together, our results as well as literature data from other inflammatory conditions suggest that both adipocytokines may represent new markers corresponding with inflammation in systemic sclerosis and might reflect the bridge between metabolism, inflammation and potentially, chemerin may also link inflammation with fibrosis. Since C3 and C4 status may indicate endothelium activation or damage, our observations might also suggest that chemerin and visfatin reflect vascular inflammation in SSc. Moreover, the positive correlation of chemerin and visfatin with ESR, C3 and C4 may suggest their potential role as markers of overall disease activity.
It has to be noted that our study has several limitations. First, it was a single-center investigation with a small and homogenous sample size. Another potential drawback of the study may be a selection bias. When interpreting our negative findings, we also need to consider the low statistical power, so we cannot fully exclude the possibility of a type II error.
Conclusions
Significantly higher serum chemerin in patients with SSc and the positive correlation of chemerin and visfatin with systemic inflammatory markers indicate that both adipocytokines may represent new markers corresponding with inflammation in systemic sclerosis and might reflect the bridge between metabolism and inflammation. The highest chemerin levels in our patients with the diffuse subtype suggest that chemerin might be specifically associated with this subtype of the disease. Consistently, when considering patients with diffuse disease subtype (dcSSc), serum chemerin inversely correlated with the intensity of skin fibrosis and there was a tendency toward the inverse correlation with interstitial lung disease in our SSc patients with late stage of the disease. Thus, potentially, chemerin may link inflammation with fibrosis: down-regulation of chemerin may play some role in the developmental process of skin sclerosis, particularly in dcSSc and increased chemerin may potentially play a protective role in lung fibrosis. Since chemerin concentrations negatively correlated with pulmonary hypertension (PH) estimated on ECO in dcSSc patients, whereas serum visfatin showed a positive correlation with PH in early/mid-stage disease, mostly in lcSSc individuals, chemerin and visfatin might be differentially involved in the development of pulmonary hypertension in SSc. Down-regulation of chemerin may play some role in the ILD-related PH in dcSSc patients, whereas visfatin seems to be rather associated with induction of PAH in lcSSc patients. Further studies are necessary to investigate multifunctional properties of these adipokines in such complex diseases like SSc.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Distler JH, Gay S, Distler O. Angiogenesis and vasculogenesis in systemic sclerosis. Rheumatology (Oxford) 2006;45:iii26–7. doi: 10.1093/rheumatology/kel295. [DOI] [PubMed] [Google Scholar]
- 2.Akamata K, Asano Y, Taniguchi T, et al. Increased expression of chemerin in endothelial cells due to Fli 1 deficiency may contribute to the development of digital ulcers in systemic sclerosis. Rheumatology. 2015;54:1308–16. doi: 10.1093/rheumatology/keu479. [DOI] [PubMed] [Google Scholar]
- 3.Masui Y, Asano Y, Shibata S, et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. J Eur Acad Dermatol Venereol. 2012;26:354–60. doi: 10.1111/j.1468-3083.2011.04077.x. [DOI] [PubMed] [Google Scholar]
- 4.Masui Y, Asano Y, Takahashi T, et al. Clinical significance of monitoring serum adiponectin levels during intravenous pulse cyclophosphamide therapy in interstitial lung disease associated with systemic sclerosis. Mod Rheumatol. 2013;23:323–9. doi: 10.1007/s10165-012-0660-7. [DOI] [PubMed] [Google Scholar]
- 5.Masui Y, Asano Y, Shibata S, et al. A possible contribution of visfatin to the resolution of skin sclerosis in patients with diffuse cutaneous systemic sclerosis via a direct anti-fibrotic effect on dermal fibroblasts and Th1 polarization of the immune response. Rheumatology. 2013;52:1239–44. doi: 10.1093/rheumatology/ket010. [DOI] [PubMed] [Google Scholar]
- 6.Masui Y, Asano Y, Akamata K, et al. Serum resistin levels: a possible correlation with pulmonary vascular involvement in patients with systemic sclerosis. Rheumatol Int. 2014;34:1165–70. doi: 10.1007/s00296-013-2880-3. [DOI] [PubMed] [Google Scholar]
- 7.Aozasa N, Asano Y, Akamata K, et al. Serum apelin levels: clinical association with vascular involvements in patients with systemic sclerosis. J Eur Acad Dermatol Venereol. 2013;27:37–42. doi: 10.1111/j.1468-3083.2011.04354.x. [DOI] [PubMed] [Google Scholar]
- 8.Toyama T, Asano Y, Takahashi T, et al. Clinical significance of serum retinol binding protein-4 levels in patients with systemic sclerosis. J Eur Acad Dermatol Venereol. 2013;27:337–44. doi: 10.1111/j.1468-3083.2011.04413.x. [DOI] [PubMed] [Google Scholar]
- 9.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–25. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 11.Huang JF, Huang CF, Yu ML, et al. Serum visfatin is correlated with disease severity and metabolic syndrome in chronic hepatitis C infection. J Gastroenterol Hepatol. 2011;26:530–5. doi: 10.1111/j.1440-1746.2010.06438.x. [DOI] [PubMed] [Google Scholar]
- 12.Kukla M, Ciupińska-Kajor M, Kajor M, et al. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol J Pathol. 2010;61:147–53. [PubMed] [Google Scholar]
- 13.Yu XY, Qiao SB, Guan HS, et al. Effects of visfatin on proliferation and collagen synthesis in rat cardiac fibroblasts. Horm Metab Res. 2010;42:507–13. doi: 10.1055/s-0030-1249059. [DOI] [PubMed] [Google Scholar]
- 14.Nagpal S, Patel S, Jacobe H, et al. Tazarotene-induced gene 2 (tig2), a novel retinoid-responsive gene in skin. J Invest Dermatol. 1997;109:91–5. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 15.Bozaoglu K, Bolton J, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocronology. 2007;148:4687–94. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy AJ, Yang P, Read C, et al. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J Am Heart Assoc. 2016;5:e004421. doi: 10.1161/JAHA.116.004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittamer V, Franssen J, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–85. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur J, Adya R, Tan BK, et al. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin- induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–8. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 19.Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–8. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Albanesi C, Scarponi C, Pallotta S, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–58. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chighizola CB, Raschi E, Privitera D, et al. Serum chemerin in systemic sclerosis: a novel marker of early diffuse disease? Clin Exp Rheumatol. 2017;35:223–4. [PubMed] [Google Scholar]
- 22.Ernst MC, Sinal CJ. ChemerIn: at the crossroads of inflammation and obesity. Trends Endocrinol Metabol. 2010;21:660–7. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Vermi W, Leonardi S, Morassi M, et al. Cutaneous distribution of plasmocytoid dendritic cell in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology. 2009;214:877–86. doi: 10.1016/j.imbio.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Parolini S, Santoro A, Marcenaro E, et al. The role of chemerin in colonization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–32. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko K, Miyabe J, Takayasu A, et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ter. 2011;13:R158. doi: 10.1186/ar3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisinger K, Bauer S, Schäffler A, et al. Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp Mol Pathol. 2012;92:90–6. doi: 10.1016/j.yexmp.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang N, Wang QJ, Feng YY, et al. Overexpression of chemerin was associated with tumor angiogenesis and poor clinical outcome in squamous cell carcinoma of the oral tongue. Clin Oral Investig. 2014;18:997–1004. doi: 10.1007/s00784-013-1046-8. [DOI] [PubMed] [Google Scholar]
- 28.Samal B, Sun Y, Stearns G, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–7. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai S. Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr Pharm Des. 2009;15:20–8. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuhara A, Matsuda M, Nishizawa M, et al. VisfatIn: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 31.Berndt J, Klöting NM, Kralisch S, et al. Plasma visfatin concentrations and fat depot-scientific mRNA expression in humans. Diabetes. 2005;54:2911–6. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 32.Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-B colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–9. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 33.Garten A, Petzold S, Schuster S, et al. Nampt and its potential role in inflammation and type 2 diabetes. Handb Exp Pharmacol. 2011;203:147–64. doi: 10.1007/978-3-642-17214-4_7. [DOI] [PubMed] [Google Scholar]
- 34.Garten A, Schuster S, Penke M, et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–46. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 35.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–8. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 36.Otero M, Lago R, Gomez R, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–27. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye SQ, Simon BA, Maloney JP, et al. Pre-B cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171:361–70. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 39.Imail S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and aging. J Diabetes Obetes Metab. 2013;15:26–33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masi AT, Rodnan GP, Medsger TA, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 41.Van Der Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann Rheum Dis. 2013;72:1747–55. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 42.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 43.Valentini G, Della Rossa A, Bombardieri S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis. 2001;60:592–8. doi: 10.1136/ard.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clements P, Lachenbruch P, Seibold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 45.Cutolo M, Pizzorni C, Sulli A. Nailfold video-capillaroscopy in systemic sclerosis. Z Rheumatol. 2004;63:457–62. doi: 10.1007/s00393-004-0673-5. [DOI] [PubMed] [Google Scholar]
- 46.Voilliot D, Magne J, Dulgheru R, et al. Determinants of exercise- induced pulmonary arterial hypertension in systemic sclerosis. Int J Cardiol. 2014;173:373–9. doi: 10.1016/j.ijcard.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 47.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 48.Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91:159–63. doi: 10.1016/j.diabres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Pfau D, Bachmann A, Lössner U, et al. Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care. 2010;33:171–3. doi: 10.2337/dc09-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noda S, Asano Y, Akamata K, et al. A possible contribution of altered cathepsin B expression to the development of skin sclerosis and vasculopathy in systemic sclerosis. PLoS One. 2012;7:e32272. doi: 10.1371/journal.pone.0032272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noda S, Asano Y, Takahashi T, et al. Decreased cathepsin V expression due to Fli1 deficiency contributes to the development of dermal fibrosis and proliferative vasculopathy in systemic sclerosis. Rheumatology. 2013;52:790–9. doi: 10.1093/rheumatology/kes379. [DOI] [PubMed] [Google Scholar]
- 52.Garland JS. Elevated body mass index as a risk factor for chronic kidney disease: current perspectives. Diabetes Metab Syndr Obes. 2014;7:347–55. doi: 10.2147/DMSO.S46674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozgen M, Kocca SS, Askoy K, et al. Visfatin levels and intima-media thicknesses in rheumatic diseases. Clin Rheumatol. 2011;30:757–63. doi: 10.1007/s10067-010-1649-2. [DOI] [PubMed] [Google Scholar]
- 54.Vadacca M, Margiotta D, Rigon A, et al. Adipokines and systemic lupus erythematosus: relationship with metabolic syndrome and cardiovascular disease risk factors. J Rheumatol. 2009;36:295–7. doi: 10.3899/jrheum.080503. [DOI] [PubMed] [Google Scholar]
- 55.Monnier J, Lewen S, Carlson J, Zabel B. Pathogenic role for chemerin receptor CMKLR1 in experimental pulmonary fibrosis. J Immunol. 2013;190(Suppl. 1):58.23. [Google Scholar]
- 56.Yuan C, Liang NL, Liu XJ, Yang L. Effect of visfatin on the expression of basic fibroblast growth factor in rat hepatic stellate cells Sichuan. Da Xue Xue Bao Yi Xue Ban. 2010;41:617–20. [PubMed] [Google Scholar]
- 57.Wang P, Yang X, Zhang Z, et al. Depletion of NAD pool contributes to impairment of endothelial progenitor cell mobilization in diabetes. Metabolism. 2016;65:852–62. doi: 10.1016/j.metabol.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Vallejo S, Romacho T, Angulo J, et al. Visfatin impairs endothelium-dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS One. 2011;6:e27299. doi: 10.1371/journal.pone.0027299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Launay D, Sobanski V, Hachulla E, Humbert M. Pulmonary hypertension in systemic sclerosis: different phenotypes. Eur Respir Rev. 2017;26:170056. doi: 10.1183/16000617.0056-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweiss N, Linda Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep. 2010;12:8–18. doi: 10.1007/s11926-009-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanthazi A, Jespers P, Vegh G, et al. Chemerin alone or added to endothelin-1 stimulates rat pulmonary vascular smooth muscle cells proliferation, migration and resistance to apoptosis. Am J Respir Crit Care Med. 2018;197:A7680. [Google Scholar]
- 62.Hanthazi A, Gomart S, Gaudreau Ménard C, et al. Chemerin potentiates pulmonary artery reactivity to endothelin-1. Eur Respir J. 2015;46:PA2440. [Google Scholar]
- 63.Kochetkova EA, Nevsorova VA, Ugay LG, et al. Issues of adipokine regulation in idiopathic pulmonary arterial hypertension and systemic osteopenia. Kardiologiia. 2018;2:17–23. doi: 10.18087/cardio.2018.2.10078. [DOI] [PubMed] [Google Scholar]
- 64.Santos M, Reis A, Gonçalves F, et al. Adiponectin levels are elevated in patients with pulmonary arterial hypertension. Clin Cardiol. 2014;37:21–5. doi: 10.1002/clc.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Sysol JR, Singla S, et al. Nicotinamide phosphoribosyltransferase promotes pulmonary vascular remodeling and is a therapeutic target in pulmonary arterial hypertension. Circulation. 2017;135:1532–46. doi: 10.1161/CIRCULATIONAHA.116.024557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asano Y, Ihn H, Yamane K, et al. Impaired Smad7-Smurf-mediated negative regulation of TGF-beta signaling in scleroderma fibroblasts. J Clin Invest. 2004;113:253–64. doi: 10.1172/JCI16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nowell MA, Richards PJ, Fielding CA, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–95. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 68.Jurcovicová J, Stofková A, Skurlová M, et al. Alterations in adipocyte glucose transporter GLUT4 and circulating adiponectin and visfatin in rat adjuvant induced arthritis. Gen Physiol Biophys. 2010;29:79–84. [PubMed] [Google Scholar]
- 69.Berndt J, Klöting NM, Kralisch S, et al. Plasma visfatin concentrations and fat depot-scientific mRNA expression in humans. Diabetes. 2005;54:2911–6. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 70.Lachine N, Elnekiedy A, Megallaa M, et al. Serum chemerin and high-sensitivity C reactive protein as markers of subclinical atherosclerosis in Egyptian patients with type 2 diabetes. Ther Adv Endocrinol Metab. 2016;7:47–56. doi: 10.1177/2042018816637312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zylla S, Pietzner M, Kühn JP, et al. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity (Silver Spring) 2017;25:468–75. doi: 10.1002/oby.21735. [DOI] [PubMed] [Google Scholar]
- 72.Herenius MM, Oliveira AS, Wijbrandts CA, et al. Anti-TNF therapy reduces serum levels of chemerin in rheumatoid arthritis: a new mechanism by which anti-TNF might reduce inflammation. PLoS One. 2013;8:e57802. doi: 10.1371/journal.pone.0057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maghsoudi Z, Kelishadi R, Hosseinzadeh-Attar MJ. Association of chemerin levels with anthropometric indexes and C-reactive protein in obese and non-obese adolescents. ARYA Atheroscler. 2015;11(Suppl 1):102–8. [PMC free article] [PubMed] [Google Scholar]
- 74.Kaur J, Adya R, Tan BK, et al. Identification of chemerin receptor (chemr23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–8. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 75.Yamawaki H, Kameshima S, Usui T, et al. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem Biophys Res Commun. 2012;423:152–7. doi: 10.1016/j.bbrc.2012.05.103. [DOI] [PubMed] [Google Scholar]
- 76.Liu Z, Tang Q, Wen J, et al. Elevated serum complement factors 3 and 4 are strong inflammatory markers of the metabolic syndrome development: a longitudinal cohort study. Sci Rep. 2016;6:18713. doi: 10.1038/srep18713. [DOI] [PMC free article] [PubMed] [Google Scholar]