Abstract
Introduction
With an introduction of new ultrasonographic transducers, skin elastography may find an application in dermatology and aesthetic medicine enabling direct evaluation of various pathological or natural processes.
Aim
To verify which elastographic technique, strain elastography (SE) or shear wave elastography (SWE), is a better candidate for the reference method of facial skin elasticity examination and to determine normal ranges for elastographic parameters in various facial regions.
Material and methods
The study included 71 female volunteers (age: 40–67 years, mean: 52 ±7.5 years). All participants were subjected to SE and SWE of the skin in five anatomical regions: the forehead, suborbital regions, cheeks, nasolabial folds and chin. Reference ranges for elastographic parameters were defined as 95% confidence intervals and ±2 standard deviations and estimated by means of ROC analysis.
Results
Shear wave elastography parameters, but not SE indices, showed strong inverse correlations with the patient age. No significant correlations were found between SE and SWE parameters of the facial skin. In contrast to SWE, no significant correlations were observed between bilateral SE parameters. Based on these findings, SWE was chosen as the reference method to determine age-specific normative values for the elasticity of the facial skin. Reference and cut-off values of SWE parameters were defined for three age groups.
Conclusions
Shear wave elastography is suitable for the determination of elastographic parameters of normal facial skin, and can be used to determine reference ranges thereof. Elasticity of the facial skin decreases considerably with age, and this factor should be considered during determination of reference values for the elastographic parameters.
Keywords: shear wave elastography, strain elastography, skin elasticity, facial aging, aesthetic medicine
Introduction
Elastography, a sonographic technique of tissue strain evaluation, was first implemented to clinical practice in the 1990s [1]. Elastography measures the deformability of tissues caused by an external force, typically compression with an ultrasonographic transducer (strain elastography – SE), or the velocity of shear wave propagation within the tissue (shear wave elastography – SWE). Depending on the method, a digitally transformed result of elastography is expressed as a semiquantitative strain of the tissue of interest in relation to the strain of adjacent reference tissue (in SE), or the quantitative parameter of absolute tissue strain in kPa (in SWE) [2, 3].
Previous studies confirmed applicability of elastography in many medical disciplines, primarily as a method to distinguish between normal and pathologically altered tissues [4]. However, in the vast majority of these studies, elastography was used to examine deeper structures, such as internal organs, the vascular wall and musculotendinous system [5]. Elastographic assessment of the skin may be challenging. This results primarily from the superficial location of the skin, its relatively low thickness, multilayer structure and heterogeneous orientation of collagen fibers [6]. The skin, with an average thickness of 1–2 mm with its epidermis and hypodermis, comprises layers of different elasticity. As a result, elastographic parameters of the skin may be highly variable and poorly reproducible [7]. However, some of these limitations have been overcome in recent years due to the use of high-frequency ultrasonographic transducers suitable for the examination of superficially located regions of interest (ROIs) with small diameters [8].
Most previous studies using elastography for skin evaluation included patients with cancers [9, 10], connective tissue diseases or chronic systemic inflammation [11, 12]. These studies confirmed usefulness of elastography in differential diagnosis of proliferative processes and fibrosis taking place in the skin. However, to the best of our knowledge, aside from few experiments [7, 13, 14] none of the previous studies analyzed elastographic parameters of the normal skin or attempted to define reference values thereof. Elastographic parameters of the skin might serve as a measure of its true biological age, guiding the selection of tailored cosmetic-therapeutic procedures and parameters thereof [14]. Moreover, elastography might be used to monitor recovery of the skin after various procedures. However, all these applications of elastography necessitate the identification of reference ranges for facial skin elasticity.
Aim
The aim of this study was to verify which of currently available elastographic techniques, SE or SWE, is a better candidate for the reference method to determine facial skin elasticity and to determine normal ranges for elastographic parameters of the facial skin in five anatomical regions being the most common targets for aesthetic medical procedures.
Material and methods
The study protocol was approved by the Bioethical Committee at the Medical Centre of Postgraduate Education in Warsaw (5/PB-A/2018). All procedures were conducted in accordance with the Declaration of Helsinki in the tertiary University Hospital. The study included consecutive female volunteers referred to the hospital from the private aesthetic clinic before their facial treatment with high-intensity focused ultrasound (HIFU). Only patients aged at least 18 years at the time of enrollment were qualified to the study. Women with visible scars or other skin lesions on the face, present or past history of connective tissue diseases, other autoimmune disorders, diseases of the skin and subcutaneous tissue and/or peripheral blood vessels, allergy or atopy, surgery or trauma involving the face, as well as active current and past smokers and tanning bed users, were non-eligible for the study.
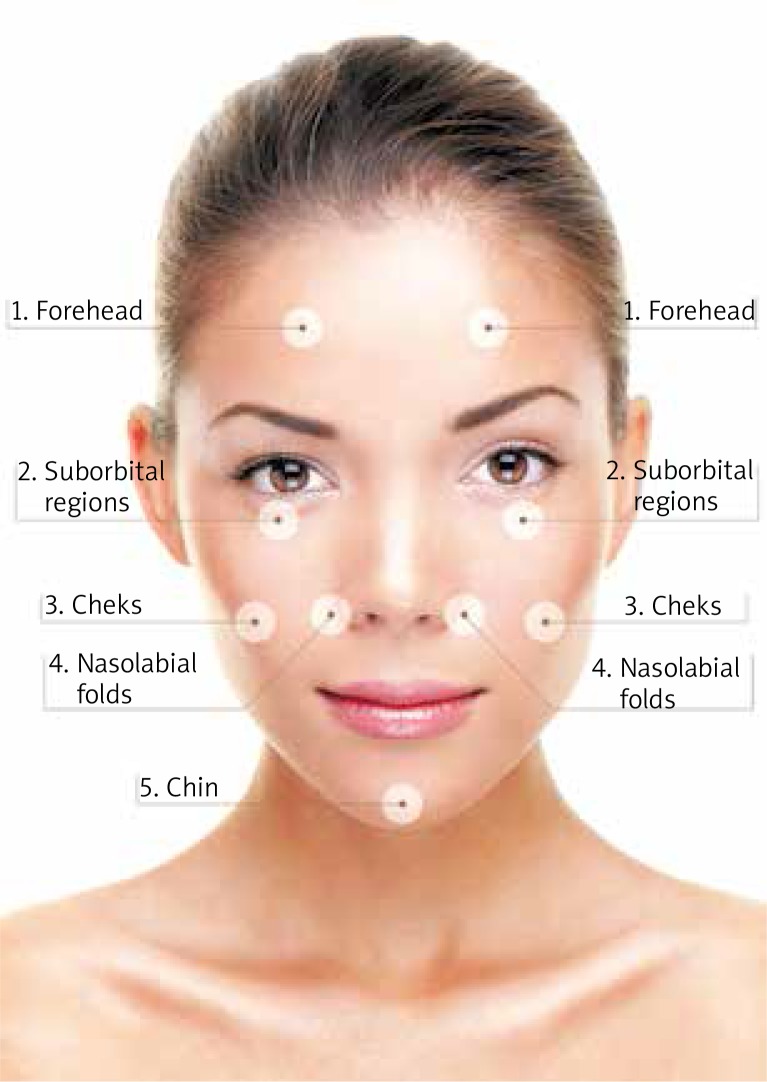
All participants were subjected to the ultrasonographic examination and elastography of the skin in five anatomical regions of the face i.e.: (1) the forehead on the right and left side, (2) suborbital regions (right and left), (3) cheeks (right and left), (4) nasolabial folds (right and left), and (5) chin (Figure 1). Each region was examined separately, with the patient in a supine position. Sonographic scans were obtained with Toshiba iAplio 900 ultrasonograph with a 5–18 MHz transducer. During examination, the face was covered with a hydrogel pad and a thick layer of gel. The transducer was placed perpendicularly to the skin, and transverse scans were obtained. Upon visualization of the area of interest, the thickness of the dermis and subcutaneous tissue in mm was measured. Then, SWE was performed, after stabilizing the elastographic image. Regions of interest was placed in the center of the screen, to cover a part of the examined structure. Three measurements were taken for each ROI and the average result was recorded. The reference value for the elasticity modulus was set at 100 kPa. The last stage of the examination was SE. Elasticity of the skin was estimated based on its deformability under a slight repeated compression with the transducer, with a soft tissue layer located directly beneath the superficial musculoaponeurotic system (SMAS) as the reference tissue.
Figure 1.
Anatomical regions subjected to elastographic examination
Statistical analysis
Normal distribution of the study variables was verified with Shapiro-Wilk test, and their statistical characteristics were presented as arithmetic means with 95% confidence intervals (95% CIs) and standard deviations (SDs), as well as medians and ranges. The power and direction of relationships between pairs of the variables were estimated on the basis of Spearman rank correlation coefficients (R). Intergroup comparisons were conducted with Kruskal-Wallis test with Dunn post-hoc tests. Reference ranges for elastographic parameters were defined with three methods, as 95% CIs, ±2 SD and on the basis of ROC analysis. During the latter, cut-off values of the elastographic parameters, which optimally distinguished between various groups were identified, along with their sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively) and the areas under ROC curves (AUC). All calculations were carried out with Statistica 10 package (StatSoft, United States) with a threshold of statistical significance set at p ≤ 0.05.
Results
The study included 71 women aged between 40 and 67 years (mean age: 52 ±7.5 years). The subjects were divided into three age groups: 40–49 years (n = 29, 40.8%), 50–59 years (n = 28, 39.4%), and 60 years and older (n = 14, 19.7%). Three subjects in every age group used hormone replacement therapy (HRT). Additionally 12 subjects in the youngest group used oral contraceptives, however 7 among them used pills irregularly due to timing errors or unwanted side effects.
Irrespective of the anatomical region of the face, SWE parameters of the dermis and subcutaneous tissue showed strong inverse correlations with the patient age. Moreover, the age correlated positively (at a threshold of statistical significance) with SE parameters for the dermis of the right forehead and for subcutaneous tissue of the right nasolabial fold. Aside from two exceptions (SE parameters for the dermis in the left suborbital region and for subcutaneous tissue of the left nasolabial fold), no significant correlations were found between patients’ body mass index (BMI) and the elastographic indices (Table 1).
Table 1.
Spearman rank correlation coefficients (R) between SE and SWE elastographic parameters, age and BMI of the study subjects and ultrasonographically determined thickness of the dermis and subcutaneous tissue
| Variable | Age [years] | BMI [kg/m2] | Thickness [mm] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative strain, SE | Strain, SWE (kPa) | Relative strain, SE | Strain, SWE (kPa) | Relative strain, SE | Strain, SWE (kPa) | |||||||
| R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | |
| Forehead: | ||||||||||||
| Dermis (R) | 0.239 | 0.045 | –0.804 | < 0.001 | 0.257 | 0.053 | –0.019 | 0.886 | 0.174 | 0.146 | –0.160 | 0.182 |
| Subcutaneous tissue (R) | –0.009 | 0.940 | –0.543 | < 0.001 | 0.113 | 0.404 | 0.123 | 0.362 | –0.146 | 0.225 | 0.091 | 0.449 |
| Dermis (L) | 0.119 | 0.323 | –0.799 | < 0.001 | 0.088 | 0.515 | 0.059 | 0.662 | 0.060 | 0.617 | 0.019 | 0.877 |
| Subcutaneous tissue (L) | –0.046 | 0.707 | –0.579 | < 0.001 | 0.072 | 0.598 | 0.113 | 0.403 | 0.227 | 0.059 | –0.163 | 0.174 |
| Suborbital region: | ||||||||||||
| Dermis (R) | –0.006 | 0.961 | –0.709 | < 0.001 | 0.120 | 0.375 | 0.055 | 0.683 | 0.106 | 0.380 | –0.026 | 0.830 |
| Subcutaneous tissue (R) | 0.089 | 0.459 | –0.643 | < 0.001 | 0.057 | 0.676 | 0.011 | 0.933 | 0.239 | 0.046 | 0.323 | 0.006 |
| Dermis (L) | –0.199 | 0.095 | –0.694 | < 0.001 | –0.295 | 0.026 | 0.066 | 0.628 | 0.175 | 0.146 | 0.189 | 0.115 |
| Subcutaneous tissue (L) | –0.098 | 0.415 | –0.663 | < 0.001 | –0.103 | 0.446 | 0.058 | 0.666 | –0.025 | 0.839 | 0.026 | 0.829 |
| Cheek: | ||||||||||||
| Dermis (R) | 0.012 | 0.920 | –0.645 | < 0.001 | 0.002 | 0.986 | –0.040 | 0.767 | –0.060 | 0.620 | –0.125 | 0.298 |
| Subcutaneous tissue (R) | –0.019 | 0.876 | –0.537 | < 0.001 | 0.018 | 0.896 | 0.021 | 0.878 | 0.095 | 0.432 | 0.189 | 0.114 |
| Dermis (L) | –0.200 | 0.095 | –0.608 | < 0.001 | –0.012 | 0.930 | –0.031 | 0.819 | 0.052 | 0.667 | 0.058 | 0.632 |
| Subcutaneous tissue (L) | –0.048 | 0.692 | –0.421 | < 0.001 | 0.059 | 0.660 | –0.029 | 0.829 | 0.221 | 0.064 | 0.148 | 0.216 |
| Nasolabial fold: | ||||||||||||
| Dermis (R) | –0.173 | 0.150 | –0.815 | < 0.001 | 0.134 | 0.320 | –0.052 | 0.700 | –0.265 | 0.026 | –0.104 | 0.388 |
| Subcutaneous tissue (R) | 0.237 | 0.046 | –0.790 | < 0.001 | –0.040 | 0.766 | –0.065 | 0.629 | 0.251 | 0.035 | –0.118 | 0.327 |
| Dermis (L) | –0.161 | 0.181 | –0.807 | < 0.001 | –0.117 | 0.386 | –0.056 | 0.680 | –0.037 | 0.764 | 0.199 | 0.098 |
| Subcutaneous tissue (L) | –0.037 | 0.757 | –0.751 | < 0.001 | –0.304 | 0.021 | –0.031 | 0.817 | 0.131 | 0.281 | –0.156 | 0.198 |
| Chin: | ||||||||||||
| Dermis | –0.104 | 0.389 | –0.829 | < 0.001 | 0.254 | 0.056 | –0.139 | 0.302 | 0.000 | 0.999 | 0.061 | 0.611 |
| Subcutaneous tissue | 0.031 | 0.800 | –0.711 | < 0.001 | 0.211 | 0.116 | –0.134 | 0.321 | –0.078 | 0.516 | 0.049 | 0.687 |
With few exceptions, elastographic parameters of the dermis and subcutaneous tissue did not correlate significantly with ultrasonographically determined thickness of these layers. The only statistically significant associations were observed between SE parameters and thickness of the dermis of the right nasolabial fold (inverse correlation), SE parameters and thickness of subcutaneous tissue in the same area (positive correlation), and SE/SWE parameters and thickness of subcutaneous tissue in the right suborbital region (positive correlations) (Table 1).
Strain elastography and SWE parameters of the dermis in all examined regions except for the right nasolabial fold correlated positively with respective elastographic characteristics of subcutaneous tissue in the same areas. However, correlations between SWE parameters of the dermis and subcutaneous tissue were markedly stronger than in the case of SE results (Table 2).
Table 2.
Spearman rank correlation coefficients (R) between SE and SWE parameters for the dermis and subcutaneous tissue in various facial regions
| Variable | Relative strain, SE | Strain, SWE [kPa] | ||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| Forehead: | ||||
| Right | 0.331 | 0.005 | 0.748 | < 0.001 |
| Left | 0.518 | < 0.001 | 0.808 | < 0.001 |
| Suborbital region: | ||||
| Right | 0.547 | < 0.001 | 0.887 | < 0.001 |
| Left | 0.480 | < 0.001 | 0.849 | < 0.001 |
| Cheek: | ||||
| Right | 0.608 | < 0.001 | 0.762 | < 0.001 |
| Left | 0.539 | < 0.001 | 0.682 | < 0.001 |
| Nasolabial fold: | ||||
| Right | 0.040 | 0.740 | 0.826 | < 0.001 |
| Left | 0.362 | 0.002 | 0.800 | < 0.001 |
| Chin: | ||||
| 0.696 | < 0.001 | 0.821 | < 0.001 | |
Bilateral SWE parameters for the dermis and subcutaneous tissue of the forehead, suborbital regions, cheeks and nasolabial folds correlated positively with each other. For SE parameters, significant bilateral correlations were found solely for the cheek dermis and subcutaneous tissue of the nasolabial folds (Table 3).
Table 3.
Spearman rank correlation coefficients (R) between SE and SWE parameters of facial skin determined on the right and left side
| Variable | Relative strain, SE | Strain, SWE [kPa] | ||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| Forehead: | ||||
| Dermis | 0.028 | 0.814 | 0.903 | < 0.001 |
| Subcutaneous tissue | 0.031 | 0.798 | 0.865 | < 0.001 |
| Suborbital region: | ||||
| Dermis | 0.150 | 0.212 | 0.925 | < 0.001 |
| Subcutaneous tissue | 0.164 | 0.173 | 0.888 | < 0.001 |
| Cheek: | ||||
| Dermis | 0.383 | 0.001 | 0.917 | < 0.001 |
| Subcutaneous tissue | 0.206 | 0.084 | 0.768 | < 0.001 |
| Nasolabial fold: | ||||
| Dermis | 0.163 | 0.174 | 0.871 | < 0.001 |
| Subcutaneous tissue | 0.473 | < 0.001 | 0.900 | < 0.001 |
Except for SWE and SE parameters of the dermis in the left suborbital region and the left cheek, no statistically significant correlations were found between elastographic parameters measured with the two elastographic methods (Table 4).
Table 4.
Spearman rank correlation coefficients (R) between elastographic parameters of the facial skin determined by means of SE and SWE
| Variable | Right side | Left side | ||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| Forehead: | ||||
| Dermis | –0.137 | 0.255 | –0.090 | 0.454 |
| Subcutaneous tissue | 0.028 | 0.815 | 0.137 | 0.258 |
| Suborbital region: | ||||
| Dermis | 0.132 | 0.271 | 0.238 | 0.046 |
| Subcutaneous tissue | 0.113 | 0.348 | 0.132 | 0.274 |
| Cheek: | ||||
| Dermis | 0.039 | 0.744 | 0.281 | 0.018 |
| Subcutaneous tissue | 0.018 | 0.881 | –0.087 | 0.470 |
| Nasolabial fold: | ||||
| Dermis | 0.160 | 0.183 | 0.078 | 0.519 |
| Subcutaneous tissue | –0.140 | 0.245 | –0.034 | 0.776 |
| Chin: | ||||
| Dermis | R = 0.151, | P = 0.209 | ||
| Subcutaneous tissue | R = –0.049, | P = 0.683 | ||
Based on the abovementioned findings, we have chosen SWE as the reference method to determine normative values for the elasticity of the facial skin. Since SWE parameters correlated inversely with the age of the study subjects, the reference values were defined for three age groups. To confirm the appropriateness of the cut-off values for age, we compared SWE parameters for the three groups; the study groups differed significantly in terms of all elastographic characteristics (p < 0.001). The age-specific reference values for the elastographic parameters of the facial skin, estimated with various methods, are listed in Tables 5 and 6.
Table 5.
Statistical characteristics of SWE parameters for the facial skin, along with proposed reference ranges
| Age [years] | Statistical characteristics | Reference ranges | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min. | Max. | –95% CI | +95% CI | –2 SD | +2 SD | |
| Forehead – dermis: | |||||||||
| 40–49 | 17.7 | 1.8 | 17.4 | 14.6 | 22.0 | 17.2 | 18.1 | 14.1 | 21.2 |
| 50–59 | 14.3 | 1.2 | 14.0 | 13.0 | 17.0 | 14.0 | 14.7 | 11.9 | 16.8 |
| ≥ 60 | 11.5 | 1.7 | 11.8 | 9.0 | 15.0 | 10.9 | 12.2 | 8.1 | 14.9 |
| Forehead – subcutaneous tissue: | |||||||||
| 40–49 | 15.5 | 2.9 | 15.0 | 11.0 | 21.0 | 14.8 | 16.3 | 9.7 | 21.3 |
| 50–59 | 12.7 | 2.1 | 12.0 | 9.0 | 19.0 | 12.2 | 13.3 | 8.6 | 16.9 |
| ≥ 60 | 10.7 | 1.7 | 11.0 | 8.0 | 14.0 | 10.0 | 11.3 | 7.2 | 14.1 |
| Suborbital region – dermis: | |||||||||
| 40–49 | 17.9 | 2.0 | 18.0 | 13.0 | 21.0 | 17.4 | 18.4 | 14.0 | 21.8 |
| 50–59 | 12.7 | 2.5 | 12.6 | 7.0 | 20.0 | 12.0 | 13.3 | 7.7 | 17.6 |
| ≥ 60 | 11.7 | 1.9 | 11.0 | 8.8 | 16.0 | 11.0 | 12.5 | 7.8 | 15.6 |
| Suborbital region – subcutaneous tissue: | |||||||||
| 40–49 | 16.4 | 2.8 | 16.0 | 8.0 | 25.0 | 15.6 | 17.1 | 10.7 | 22.0 |
| 50–59 | 11.7 | 2.5 | 11.0 | 7.0 | 19.0 | 11.1 | 12.4 | 6.8 | 16.7 |
| ≥ 60 | 10.6 | 2.1 | 10.0 | 8.0 | 16.0 | 9.8 | 11.5 | 6.3 | 14.9 |
| Cheek – dermis: | |||||||||
| 40–49 | 16.6 | 2.5 | 16.1 | 10.0 | 21.0 | 16.0 | 17.3 | 11.7 | 21.6 |
| 50–59 | 13.2 | 2.4 | 13.0 | 9.0 | 22.0 | 12.5 | 13.8 | 8.4 | 18.0 |
| ≥ 60 | 11.4 | 2.0 | 11.0 | 7.8 | 16.0 | 10.7 | 12.2 | 7.4 | 15.5 |
| Cheek – subcutaneous tissue: | |||||||||
| 40–49 | 13.9 | 3.0 | 14.0 | 8.0 | 21.0 | 13.1 | 14.6 | 7.8 | 19.9 |
| 50–59 | 11.5 | 2.0 | 11.0 | 8.0 | 19.8 | 11.0 | 12.0 | 7.5 | 15.5 |
| ≥ 60 | 10.0 | 2.3 | 9.4 | 6.0 | 15.6 | 9.1 | 10.9 | 5.4 | 14.5 |
| Nasolabial fold – dermis: | |||||||||
| 40–49 | 18.9 | 1.5 | 19.0 | 17.0 | 23.0 | 18.5 | 19.3 | 16.0 | 21.8 |
| 50–59 | 15.3 | 1.5 | 15.0 | 12.0 | 20.0 | 14.9 | 15.7 | 12.3 | 18.3 |
| ≥ 60 | 11.7 | 1.8 | 11.0 | 9.0 | 16.0 | 11.0 | 12.4 | 8.0 | 15.4 |
| Nasolabial fold – subcutaneous tissue: | |||||||||
| 40–49 | 9.3 | 1.4 | 9.0 | 7.0 | 13.0 | 8.9 | 9.7 | 6.6 | 12.1 |
| 50–59 | 6.2 | 0.9 | 6.0 | 5.0 | 8.0 | 6.0 | 6.4 | 4.4 | 8.0 |
| ≥ 60 | 5.3 | 0.7 | 5.0 | 4.0 | 6.4 | 5.0 | 5.6 | 3.9 | 6.7 |
| Chin – dermis: | |||||||||
| 40–49 | 19.3 | 1.7 | 19.0 | 16.0 | 25.0 | 18.7 | 20.0 | 15.8 | 22.8 |
| 50–59 | 15.3 | 1.1 | 15.0 | 13.0 | 18.0 | 14.9 | 15.7 | 13.0 | 17.6 |
| ≥ 60 | 8.9 | 1.7 | 9.2 | 5.5 | 11.0 | 7.9 | 9.8 | 5.5 | 12.2 |
| Chin – subcutaneous tissue: | |||||||||
| 40–49 | 15.4 | 2.2 | 15.0 | 11.0 | 21.0 | 14.6 | 16.3 | 10.9 | 19.9 |
| 50–59 | 12.6 | 1.8 | 13.0 | 8.8 | 18.0 | 11.9 | 13.3 | 9.0 | 16.2 |
| ≥ 60 | 8.5 | 1.3 | 9.0 | 6.0 | 10.0 | 7.8 | 9.2 | 6.0 | 11.0 |
Table 6.
Cut-off values for the SWE parameters of the facial skin optimally distinguishing between patients of various age, along with their sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively), and the areas under ROC curves (AUC)
| Age [years] | Cut-off [KPa] | Sensitivity | Specificity | PPV | NPV | AUC | –95% CI | +95% CI |
|---|---|---|---|---|---|---|---|---|
| Forehead – dermis: | ||||||||
| < 50 vs. ≥ 50 | 16.0 | 0.879 | 0.881 | 0.836 | 0.914 | 0.959 | 0.932 | 0.985 |
| < 60 vs. ≥ 60 | 13.0 | 1.000 | 0.750 | 0.942 | 1.000 | 0.954 | 0.915 | 0.994 |
| Forehead – subcutaneous tissue: | ||||||||
| < 50 vs. ≥ 50 | 13.2 | 0.741 | 0.810 | 0.729 | 0.819 | 0.835 | 0.767 | 0.902 |
| < 60 vs. ≥ 60 | 11.0 | 0.956 | 0.464 | 0.879 | 0.722 | 0.856 | 0.785 | 0.926 |
| Suborbital region – dermis: | ||||||||
| < 50 vs. ≥ 50 | 15.4 | 0.897 | 0.905 | 0.867 | 0.927 | 0.958 | 0.928 | 0.989 |
| < 60 vs. ≥ 60 | 7.0 | 1.000 | 0.000 | 0.803 | 0.809 | 0.735 | 0.883 | |
| Suborbital region – subcutaneous tissue: | ||||||||
| < 50 vs. ≥ 50 | 14.9 | 0.793 | 0.869 | 0.807 | 0.859 | 0.906 | 0.855 | 0.957 |
| < 60 vs. ≥ 60 | 9.5 | 0.939 | 0.321 | 0.849 | 0.563 | 0.794 | 0.711 | 0.878 |
| Cheek – dermis: | ||||||||
| < 50 vs. ≥ 50 | 15.0 | 0.828 | 0.821 | 0.762 | 0.873 | 0.886 | 0.829 | 0.943 |
| < 60 vs. ≥ 60 | 10.8 | 0.921 | 0.393 | 0.861 | 0.550 | 0.828 | 0.751 | 0.904 |
| Cheek – subcutaneous tissue: | ||||||||
| < 50 vs. ≥ 50 | 14.0 | 0.534 | 0.917 | 0.816 | 0.740 | 0.777 | 0.698 | 0.856 |
| < 60 vs. ≥ 60 | 9.5 | 0.930 | 0.536 | 0.891 | 0.652 | 0.786 | 0.684 | 0.887 |
| Nasolabial fold – dermis: | ||||||||
| < 50 vs. ≥ 50 | 17.0 | 1.000 | 0.905 | 0.879 | 1.000 | 0.975 | 0.951 | 0.999 |
| < 60 vs. ≥ 60 | 12.0 | 1.000 | 0.679 | 0.927 | 1.000 | 0.958 | 0.921 | 0.996 |
| Nasolabial fold – subcutaneous tissue: | ||||||||
| < 50 vs. ≥ 50 | 7.5 | 0.897 | 0.976 | 0.963 | 0.932 | 0.983 | 0.967 | 0.998 |
| < 60 vs. ≥ 60 | 5.0 | 1.000 | 0.107 | 0.820 | 1.000 | 0.887 | 0.835 | 0.940 |
| Chin – dermis: | ||||||||
| < 50 vs. ≥ 50 | 18.0 | 0.897 | 0.952 | 0.929 | 0.930 | 0.984 | 0.963 | 1.000 |
| < 60 vs. ≥ 60 | 13.0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Chin – subcutaneous tissue: | ||||||||
| < 50 vs. ≥ 50 | 15.0 | 0.690 | 0.952 | 0.909 | 0.816 | 0.899 | 0.827 | 0.971 |
| < 60 vs. ≥ 60 | 11.0 | 0.982 | 1.000 | 1.000 | 0.933 | 0.990 | 0.969 | 1.000 |
Discussion
During the first stage of our analysis, we verified if elastographic parameters of the skin were modulated by age of the study subjects. Previous studies demonstrated unequivocally that aging is associated with a significant decrease in skin elasticity, either determined by elastography or examined with other methods [6, 15]. Our results confirmed these observations, interestingly however, statistically significant inverse correlations between the age and skin elasticity were found solely for SWE, rather than for SE parameters.
We also verified if elasticity of the dermis and subcutaneous tissue correlated with thickness of these two skin layers. A few previous studies demonstrated that a relatively low thickness of the skin may hinder application of ultrasonographic elastography in determination of its elasticity [7, 16]. However, aside from few exceptions, we did not find statistically significant associations between ultrasonographically-determined thickness of the skin layers and their SE or SWE parameters. Therefore, skin thickness does not seem to be a confounder for the presented reference values of elastographic parameters.
During the next stage of statistical analysis, we verified consistency between elastographic parameters of the dermis and subcutaneous tissue in each anatomical region. As mentioned previously, sparse available evidence suggests that elasticity of individual skin layers varies and should be determined separately [13, 17]. Nevertheless, we assumed that if the skin and fat in a given person/given anatomical region are more elastic, this will refer to both these layers. Based on this assumption, we considered a significant correlation between the elasticity of the dermis and subcutaneous tissue in a given region as a measure of the elastographic technique’s accuracy. As expected, we found that SWE parameters of the dermis correlated positively with respective elastographic characteristics of subcutaneous tissue and statistically significant correlations existed between either SE or SWE parameters for these two layers. As considerably stronger correlations were observed for SWE parameters, we considered this as another argument for selection of shear wave elastography as the reference method for determining facial skin elasticity.
Finally, we checked if bilateral elastographic parameters correlated significantly with each other. While the correlations between all right- and left-sided SWE parameters turned out to be highly significant, aside from few exceptions, we did not find statistically significant relationships between the results of SE for the right and left side of the face. Previous studies did not demonstrate asymmetry in SWE parameters of the skin in various anatomical regions [8]. Perhaps, the lack of significant correlations as documented hereby between right- and left-sided SE parameters of the facial skin resulted from a measurement bias, most likely associated with poor reproducibility of this technique. Low reproducibility of SE parameters for the facial skin may be linked with the fact that in this method, elasticity is determined in relation to the strain of a reference soft tissue underneath. Except for few anatomical regions, the soft tissue layer under the facial skin is very thin and therefore, elastographic measurement may be inadvertently extended onto an underlying bone. A few previous studies showed that this may be a source of a considerable bias during SE-based measurements [16, 18].
During the last stage of the analysis we verified if SE and SWE elastographic parameters for a given region correlated with each other. Aside from two exceptions, we did not find statistically significant correlations between the results of SE and SWE, which does not seem to be surprising in view of the findings presented above. In this study, the results of SE not only did not correlate with respective SWE parameters, but also were not associated with age (an established determinant of skin elasticity) and showed significant bilateral differences. Owing to these potential drawbacks of SE, we have eventually chosen SWE as the reference method to determine the elasticity of the facial skin. Considering statistically significant correlations between SWE parameters and age, we have defined the reference values for three age groups: 40 to 49 years, 50 to 59 years, and 60 years and older (Table 5). The appropriateness of such stratification was verified based on the intergroup comparison of SWE parameters. The reference values were estimated with three methods, as 95% Cis, ±2 SD and based on ROC analysis. The normal ranges determined as ±2 SD seem to be more applicable from a clinical perspective, since limits for consecutive age groups are wider and slightly overlap, leaving some space for likely individual variability in elastographic parameters. However, also the cut-off values determined by means of ROC analysis accurately distinguished between various age groups on the basis of some selected SWE parameters (Table 6). The appropriateness of this approach was confirmed by AUC values ≥ 0.95 and very high sensitivity, specificity, PPV and NPV levels.
The principal aim of this study was to determine reference ranges for the elastographic parameters of the facial skin in women of various age. To the best of our knowledge, normative values for the elastographic parameters of the skin, either on the face or in other anatomical regions, were not a subject of any published study. Few previous studies involving elastography of the facial skin included patients with various pathologies, primarily malignancies and connective tissue diseases [9, 12]; even if these studies included control groups, they were too small to define universal reference values applicable for everyday clinical practice. The presented reference values may find an application in many medical disciplines. In this study, we analyzed the elasticity of the facial skin in patients qualified for medical aesthetic procedures. We assumed that these baseline elastographic parameters might be later used to monitor the rate of skin recovery in the early and late post-procedural period. However, the reference values of elastographic parameters of the facial skin might be also applied in other clinical disciplines, e.g. rheumatology. The degree of skin fibrosis is an essential component of many scoring systems used to assess the severity of some connective tissue diseases, e.g. systemic sclerosis [7]. Therefore, SWE might constitute a non-invasive alternative for skin biopsy, used currently in this indication, especially that previous studies demonstrated that elastographic parameters of the skin in patients with systemic sclerosis differ markedly from those determined in healthy controls [19, 20].
Determination of the reference ranges for any parameter requires control for potential confounders. To avoid a selection bias, our study included solely healthy women. Subjects with factors that influence the elasticity of facial skin were not eligible. However, we included patients using hormone replacement therapy and oral contraceptives, considering that this treatment is frequent among patients of age groups studied. The number of patients using HRT in each group was too small to assess its influence on skin elasticity. Moreover, the influence of oral contraceptives among youngest volunteers was not assessed due to a limited sample and irregularity of pill use. It will probably require more attention in future studies.
Our study was not free from other potential limitations. The first was a relatively small sample size, especially in the context of age group analysis. This probably resulted in a number of exceptions that we observed during statistical analysis. Nevertheless, to the best our knowledge, this was the largest elastographic study of the normal facial skin that has been conducted thus far. Second, it cannot be excluded that elastographic parameters determined in this study were influenced by some non-identified confounders. We tried to overcome this potential bias, enrolling solely the patients who satisfied possibly the most exhaustive list of inclusion and exclusion criteria. Finally, the findings presented herein might be biased due to some drawbacks inherent in the elastographic examination of thin, superficially located tissues, such as the skin, or due to the subjective selection of ROI. We did our best to neutralize these potential limitations, using a high-frequency transducer and a hydrogel pad, reducing the diameter of ROI and repeating each measurement to achieve better reproducibility.
Conclusions
The SWE is a reliable method for the determination of elastographic parameters of normal facial skin, and can be used to determine reference ranges thereof. Elasticity of the facial skin decreases considerably with age, and this factor should be considered during determination of reference values for the elastographic parameters.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ophir J, Céspedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 2.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85:1435–45. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 5.Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–9. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Luo CC, Qian LX, Li GY, et al. Determining the in vivo elastic properties of dermis layer of human skin using the supersonic shear imaging technique and inverse analysis. Med Phys. 2015;42:4106–15. doi: 10.1118/1.4922133. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Ma C, Liang X, et al. Reproducibility analysis on shear wave elastography (SWE)-based quantitative assessment for skin elasticity. Medicine (Baltimore) 2017;96:e6902. doi: 10.1097/MD.0000000000006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang X, Yan F, Yang Y, et al. Quantitative assessment of healthy skin elasticity: reliability and feasibility of shear wave elastography. Ultrasound Med Biol. 2017;43:445–52. doi: 10.1016/j.ultrasmedbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Dasgeb B, Morris MA, Mehregan D, Siegel EL. Quantified ultrasound elastography in the assessment of cutaneous carcinoma. Br J Radiol. 2015;88:20150344. doi: 10.1259/bjr.20150344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botar-Jid CM, Cosgarea R, Bolboacă SD, et al. Assessment of cutaneous melanoma by use of very- high-frequency ultrasound and real-time elastography. AJR Am J Roentgenol. 2016;206:699–704. doi: 10.2214/AJR.15.15182. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Cardones AR, Doherty J, et al. Preliminary results on the feasibility of using ARFI/SWEI to assess cutaneous sclerotic diseases. Ultrasound Med Biol. 2015;41:2806–19. doi: 10.1016/j.ultrasmedbio.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu KH, Bhatia K, Chu W, et al. Shear wave elastography: a new quantitative assessment of post-irradiation neck fibrosis. Ultraschall Med. 2015;36:348–54. doi: 10.1055/s-0034-1366364. [DOI] [PubMed] [Google Scholar]
- 13.Chartier C, Mofid Y, Bastard C, et al. High-resolution elastography for thin-layer mechanical characterization: toward skin investigation. Ultrasound Med Biol. 2017;43:670–81. doi: 10.1016/j.ultrasmedbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Alfageme Roldán F. Elastography in dermatology. Actas Dermosifiliogr. 2016;107:652–60. doi: 10.1016/j.ad.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Edwards C, Marks R. Evaluation of biomechanical properties of human skin. Clin Dermatol. 1995;13:375–80. doi: 10.1016/0738-081x(95)00078-t. [DOI] [PubMed] [Google Scholar]
- 16.Deffieux T, Gennisson JL, Larrat B, et al. The variance of quantitative estimates in shear wave imaging: theory and experiments. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59:2390–410. doi: 10.1109/TUFFC.2012.2472. [DOI] [PubMed] [Google Scholar]
- 17.Osanai O, Ohtsuka M, Hotta M, et al. A new method for the visualization and quantification of internal skin elasticity by ultrasound imaging. Skin Res Technol. 2011;17:270–7. doi: 10.1111/j.1600-0846.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y, Zhu QL, Liu H, et al. A preliminary study of acoustic radiation force impulse quantification for the assessment of skin in diffuse cutaneous systemic sclerosis. J Rheumatol. 2015;42:449–55. doi: 10.3899/jrheum.140873. [DOI] [PubMed] [Google Scholar]
- 19.Iagnocco A, Kaloudi O, Perella C, et al. Ultrasound elastography assessment of skin involvement in systemic sclerosis: lights and shadows. J Rheumatol. 2010;37:1688–91. doi: 10.3899/jrheum.090974. [DOI] [PubMed] [Google Scholar]
- 20.Di Geso L, Filippucci E, Girolimetti R, et al. Reliability of ultrasound measurements of dermal thickness at digits in systemic sclerosis: role of elastosonography. Clin Exp Rheumatol. 2011;29:926–32. [PubMed] [Google Scholar]