Abstract
Background
Association between plasminogen activator inhibitor‐1 (PAI‐1) rs1799889 polymorphism and venous thromboembolism (VTE) were explored by many previous studies, yet the findings of these studies were conflicting.
Hypothesis
PAI‐1 rs1799889 polymorphism may serve as a genetic marker of VTE. We aimed to better clarify the relationship between PAI‐1 rs1799889 polymorphism and VTE in a larger combined population by performing a meta‐analysis.
Methods
Literatures were searched in Pubmed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI). We used Review Manager to combine the results of individual studies.
Results
Forty‐eight studies involving 14 806 participants were eligible for inclusion. Combined results revealed that PAI‐1 rs1799889 polymorphism was significantly associated with VTE in Caucasians (dominant comparison: odds ratio [OR] 1.20, 95% confidence interval [CI] 1.09‐1.32; recessive comparison: OR 0.84, 95% CI 0.76‐0.94; allele comparison: OR 1.08, 95% CI 1.02‐1.15) and East Asians (dominant comparison: OR 1.60, 95% CI 1.17‐2.19; allele comparison: OR 1.53, 95% CI 1.21‐1.93). Further analyses obtained similar significant associations in these with deep vein thrombosis (DVT) and these with Factor V Leiden mutation.
Conclusions
Our findings supported that PAI‐1 rs1799889 polymorphism may serve as one of the predisposing factors of VTE in both Caucasians and East Asians, especially in these with DVT and these with Factor V Leiden mutation.
Keywords: meta‐analysis, plasminogen activator inhibitor‐1 (PAI‐1), polymorphism, venous thromboembolism (VTE)
1. INTRODUCTION
Venous thromboembolism (VTE) is a common and severe thrombotic disorder. Its age‐ and sex‐adjusted incidence rate is estimated to be around 1.2‐1.4 per 1000 person‐years, and its 30‐day mortality rate could reach up to 10%.2, 3 Previous studies demonstrated that agedness, major surgical operations, cancer, gestation, and sequential oral contraceptives could increase susceptibility to VTE.4, 5 Nevertheless, many people who were exposed to these risk factors did not ultimately develop VTE, which suggested that genetic factors were also involved in its development.
Plasminogen activator inhibitor‐1 (PAI‐1) is a serine protease inhibitor, and it is involved in the regulation of fibrinolysis and thrombosis via inhibiting biological activities of tissue plasminogen activator (t‐PA) and urokinase plasminogen activator (u‐PA).6 Previous basic researches showed that blockage of PAI‐1 could lead to thrombus degradation, whereas activation of PAI‐1 could accelerate thrombus formation.7, 8 So if a gene polymorphism could alter the expression level or protein structure of PAI‐1, it is possible that this polymorphism may also affect individual susceptibility to thrombotic disorders like VTE.
In recent years, many investigations reported findings regarding potential associations between PAI‐1 rs1799889 A/G polymorphism and VTE.9, 10, 11, 12 Nevertheless, these findings were somehow inconsistent. In this meta‐analysis, we aimed to better clarify the relationship between PAI‐1 rs1799889 A/G polymorphism and VTE. We will also perform comprehensive analyses to investigate the effects of ethnic background, type of disease, and established risk factors of VTE (Factor V Leiden mutation, cancer status, and recent major surgery) on genetic association between PAI‐1 rs1799889 A/G polymorphism and VTE.
2. MATERIALS AND METHODS
This meta‐analysis was written in accordance with PRISMA guideline.13 We also created an Open Science Framework (osf.io) account to make this meta‐analysis more publicly available.
2.1. Literature search and inclusion criteria
We searched PubMed, Web of Science, Embase, and CNKI using the following key words: “plasminogen activator inhibitor‐1,” “PAI‐1,” “plasminogen activator inhibitor 1,” “PAI1,” “serpin family E member 1,” “SERPINE1,” “polymorphism,” “variant,” “variation,” “mutation,” “SNP,” “venous thromboembolism,” “VTE,” “deep vein thrombosis,” “DVT,” “pulmonary embolism,” and “PE.” The latest literature searching update was conducted in June 2019.
To be included in this meta‐analysis, some criteria must be met: (a) About PAI‐1 rs1799889 A/G polymorphism and VTE in human beings; (b) providing distributions of genotypes or alleles in cases and controls; (c) Full text in English or native language of the authors (Chinese) is retrievable. Studies were deemed to be ineligible for inclusion if: (a) Not about PAI‐1 rs1799889 A/G polymorphism and VTE; (b) studies that were not carried out in humans; (c) case reports or case series; (d) reviews and comments. If we found repeated publications during literature searching, only the most comprehensive study was included for analyses.
2.2. Data extraction and quality assessment
Following information was extracted by two authors: the last name of the first author and publication year, country of the principal investigator and ethnicity of study participants, type of disease, total sample size of each study, and the distribution of PAI‐1 rs1799889 A/G polymorphism in cases and controls. We also calculated the probability value (P value) of Hardy‐Weinberg equilibrium.
The authors used Newcastle‐Ottawa scale (NOS) to assess the quality of eligible studies.14 The score range of NOS is between zero and nine, when a study got a score of seven or more, we considered that the methodology of this study is good.
Two authors extracted data and assessed quality of eligible publications. The authors wrote to the leadings authors for additional information if essential information was found to be incomplete.
2.3. Statistical analyses
Review Manager was used to combine the results of eligible studies. Z test was employed to assess whether PAI‐1 rs1799889 A/G polymorphism was significantly associated with VTE, with the statistical significance P level set at .05. We used I 2 statistics to assess between‐study heterogeneities. We used Random‐effect models (DerSimonian‐Laird method) to combine the results if I 2 is larger than 50%. Otherwise, fixed‐effect models (Mantel‐Haenszel method) were used to combine the results. We also conducted subgroup analyses by ethnicity of participants, type of disease and whether the study subjects had established risks of VTE. We examined the stability of combined results by deleting one study each time and combining the results of the remaining studies. We used funnel plots to estimate whether our combined results may be influenced by publication biases.
This article does not contain any studies with human participants or animals performed by any of the authors, thus ethical approval is not required.
3. RESULTS
3.1. Characteristics of included studies
One thousand eight hundred and twenty‐nine studies were identified by our comprehensive literature searching. One hundred and thirty‐three studies were retrieved for eligibility assessment after exclusion of irrelevant and duplicate articles. Another eighty‐five articles were further excluded by us because these articles did not meet the inclusion criteria that were set forth for this meta‐analysis. Totally forth‐eight studies containing 5731 cases and 9075 controls were ultimately included in this meta‐analysis (see Figure 1). Table 1 presented essential data extracted from included studies.
Figure 1.
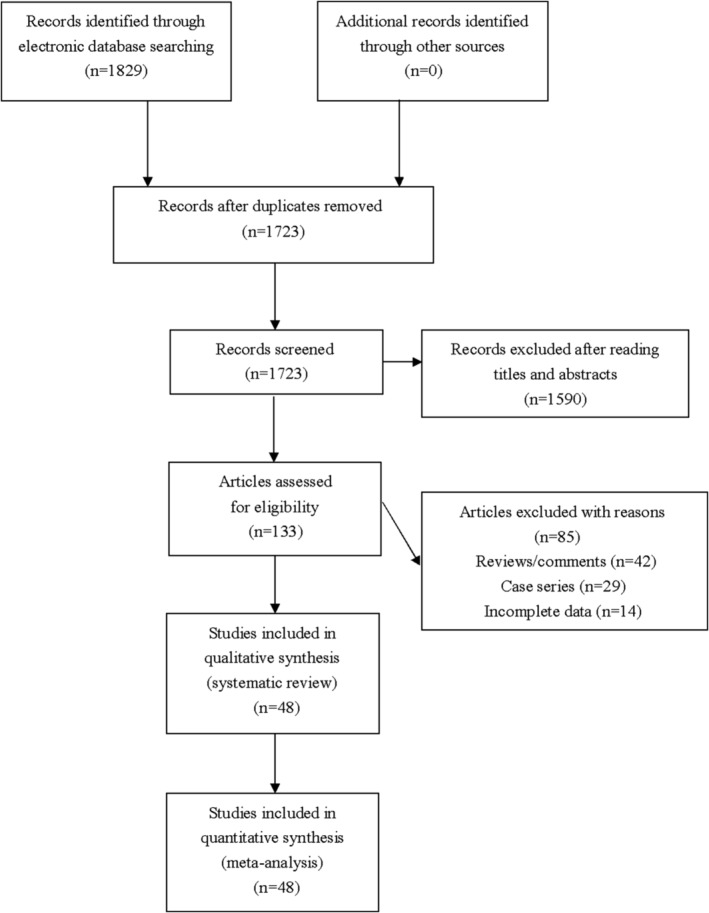
Flowchart of study selection for the present study
Table 1.
The characteristics of included studies for PAI‐1 rs1799889 A/G polymorphism and VTE
| Genotype distribution (AA/AG/GG) | ||||||||
|---|---|---|---|---|---|---|---|---|
| First author, year | Country | Ethnicity | Type of disease | Sample size | Cases | Controls | P‐value for HWE | NOS score |
| Akar 2000 | Turkey | Caucasian | DVT | 136/113 | 38/77/21 | 28/57/28 | 0.925 | 7 |
| Akhter 2010 | India | West Asian | DVT | 110/110 | 48/54/8 | 29/56/25 | 0.838 | 8 |
| Alfirevic 2010 | Croatia | Caucasian | VTE | 100/105 | 31/52/17 | 33/55/17 | 0.457 | 7 |
| Arslan 2011 | Turkey | Caucasian | DVT | 33/33 | 14/19/0 | 12/18/3 | 0.305 | 7 |
| Barcellona 2013 | Italy | Caucasian | VTE | 402/466 | 72/265/65 | 61/305/100 | <0.001 | 7 |
| Bedencic 2008 | Slovenia | Caucasian | VTE | 295/223 | 93/157/45 | 63/115/45 | 0.569 | 7 |
| Bezgin 2018 | Turkey | Caucasian | VTE | 310/287 | 74/160/76 | 81/137/69 | 0.460 | 7 |
| Bezgin 2018 | Turkey | Caucasian | DVT | 247/287 | 59/130/58 | 81/137/69 | 0.460 | 7 |
| Bezgin 2018 | Turkey | Caucasian | PE | 20/287 | 5/11/4 | 81/137/69 | 0.460 | 7 |
| Chen 2005 | China | East Asian | DVT | 120/120 | 46/55/19 | 42/51/27 | 0.135 | 8 |
| Cushman 2004 | USA | Caucasian | VTE | 138/469 | 37/68/33 | 119/248/102 | 0.202 | 9 |
| D'Amico 2015 | Italy | Caucasian | VTE | 243/622 | NA | NA | NA | 7 |
| Eroglu 2006 | Turkey | Caucasian | DVT | 45/80 | 10/30/5 | 33/41/6 | 0.161 | 7 |
| Espinosa 2002 | Spain | Caucasian | VTE | 38/100 | 8/19/11 | 21/52/27 | 0.662 | 7 |
| Farajzadeh 2014 | Iran | West Asian | VTE | 193/500 | 50/83/60 | 353/91/56 | <0.001 | 8 |
| Ferrara 2013 | Italy | Caucasian | DVT | 168/70 | 139/27/2 | 62/7/1 | 0.158 | 7 |
| Folsom 2003 | USA | Mixed | VTE | 308/640 | 77/160/68 | 173/326/141 | 0.590 | 8 |
| Giannaki 2013 | Greece | Caucasian | VTE | 102/102 | NA | NA | NA | 7 |
| Grubic 1996 | Slovenia | Caucasian | DVT | 83/50 | 25/50/8 | 15/29/6 | 0.160 | 8 |
| Gu 2014 | China | East Asian | VTE | 198/212 | 146/35/17 | 123/55/34 | <0.001 | 8 |
| Hasan 2006 | Egypt | Caucasian | DVT | 48/40 | 20/22/6 | 8/16/16 | 0.292 | 7 |
| Kaya 2013 | Turkey | Caucasian | VTE | 80/79 | 19/43/18 | 29/36/14 | 0.628 | 7 |
| Kotwal 2013 | USA | Mixed | PE | 12/12 | NA | NA | NA | 7 |
| Kuhli‐Hattenbach 2017 | Germany | Caucasian | VTE | 25/241 | 6/16/3 | 56/122/63 | 0.837 | 7 |
| Kumari 2014 | India | West Asian | VTE | 93/102 | 31/39/23 | 27/53/22 | 0.674 | 7 |
| Kupeli 2011 | Turkey | Caucasian | VTE | 80/103 | 24/38/18 | 28/57/28 | 0.925 | 7 |
| Kupeli 2011 | Turkey | Caucasian | PE | 51/103 | 15/27/9 | 28/57/28 | 0.925 | 7 |
| Lichy 2007 | Germany | Caucasian | VTE | 76/195 | 21/40/15 | 61/91/43 | 0.413 | 7 |
| Mansilha 2005 | Portugal | Caucasian | DVT | 81/88 | 22/40/19 | 21/39/28 | 0.311 | 7 |
| Meglic 2003 | Slovenia | Caucasian | VTE | 30/53 | 7/16/7 | 14/29/10 | 0.464 | 8 |
| Morange 2000 | France | Caucasian | VTE | 168/214 | 50/79/39 | 50/105/59 | 0.804 | 7 |
| Oguzulgen 2009 | Turkey | Caucasian | PE | 143/181 | 36/63/44 | 46/94/41 | 0.595 | 7 |
| Onur 2012 | Turkey | Caucasian | VTE | 28/50 | 12/10/6 | 17/19/14 | 0.093 | 8 |
| Ozkan 2012 | Turkey | Caucasian | VTE | 158/134 | 91/56/11 | 66/57/11 | 0.789 | 8 |
| Pop 2014 | Romania | Caucasian | DVT | 168/162 | 51/71/46 | 38/95/29 | 0.025 | 7 |
| Prabhudesai 2017 | India | West Asian | VTE | 87/251 | 23/38/26 | 82/132/37 | 0.170 | 8 |
| Ridker 1997 | USA | Mixed | VTE | 121/495 | 36/59/26 | 133/247/115 | 0.988 | 7 |
| Ringelstein 2012 | Germany | Caucasian | VTE | 136/1054 | 44/72/20 | 326/521/207 | 0.964 | 8 |
| Ringwald 2009 | Germany | Caucasian | DVT | 50/85 | 11/29/10 | 21/42/22 | 0.915 | 8 |
| Russo 2015 | Italy | Caucasian | VTE | 113/101 | 26/68/19 | 31/51/19 | 0.807 | 7 |
| Sartori 1998 | Sweden | Caucasian | DVT | 70/100 | 21/42/7 | 26/50/24 | 0.997 | 7 |
| Sartori 2003 | Italy | Caucasian | DVT | 73/76 | 29/34/10 | 23/42/11 | 0.244 | 7 |
| Schenk 2008 | Germany | Caucasian | VTE | 69/238 | 23/41/5 | 66/122/50 | 0.645 | 7 |
| Seguí 2000 | Spain | Caucasian | DVT | 190/93 | NA | NA | NA | 7 |
| Stegnar 1998 | Slovenia | Caucasian | VTE | 158/145 | 46/88/24 | 38/76/31 | 0.541 | 7 |
| Tàssies 2000 | Spain | Caucasian | VTE | 59/100 | 17/29/13 | 27/52/21 | 0.662 | 7 |
| Vesa 2016 | Romania | Caucasian | DVT | 127/114 | 42/51/34 | 26/66/22 | 0.089 | 8 |
| Visanji 2000 | UK | Caucasian | VTE | 99/99 | 39/45/15 | 26/43/30 | 0.196 | 7 |
| Visanji 2000 | UK | Caucasian | PE | 28/99 | 12/13/3 | 26/43/30 | 0.196 | 7 |
| Vuckovic 2018 | Serbia | Caucasian | VTE | 100/100 | NA | NA | NA | 8 |
| Yioti 2013 | Greece | Caucasian | VTE | 38/44 | NA | NA | NA | 7 |
| Zhou 2005 | China | East Asian | DVT | 29/24 | 8/17/4 | 6/14/4 | 0.392 | 7 |
Abbreviations: DVT, deep vein thrombosis; HWE, Hardy‐Weinberg equilibrium; NA, not available; NOS, Newcastle‐Ottawa scale; PAI‐1, plasminogen activator inhibitor‐1; PE, pulmonary embolism; VTE, venous thromboembolism.
3.2. Meta‐analysis results
PAI‐1 rs1799889 A/G polymorphism was found to be significantly associated with VTE in Caucasians (dominant comparison: AA vs AG + GG, odds ratio [OR] 1.20, 95% confidence interval [CI] 1.09‐1.32; recessive comparison: GG vs AA + AG, OR 0.84, 95% CI 0.76‐0.94; allele comparison: A vs G, OR 1.08, 95% CI 1.02‐1.15) and East Asians (dominant comparison: AA vs AG + GG, OR 1.60, 95% CI 1.17‐2.19; allele comparison: A vs G, OR 1.53, 95% CI 1.21‐1.93). Further analyses revealed similar significant associations in the DVT (recessive model: GG vs AA + AG, OR 0.73, 95% CI 0.53‐0.99; allele model: A vs G, OR 1.13, 95% CI 1.02‐1.25) subgroup, yet no any positive results regarding PE were detected in this meta‐analysis (see Table 2). We also performed stratified analyses to explore the effects of established risk factors of VTE on observed genetic associations between PAI‐1 rs1799889 A/G polymorphism and VTE, and we found positive results in these with Factor V Leiden mutation, whereas no any significant associations were detected in these with cancer or these who recently had a major surgery operation.
Table 2.
Results of overall and subgroup analyses for PAI‐1 rs1799889 A/G polymorphism and VTE
| Dominant comparison (AA vs AG + GG) | Recessive comparison (GG vs AA + AG) | Over‐dominant comparison (AG vs AA + GG) | Allele comparison (A vs G) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Sample size | P value | OR (95%CI) | I 2 statistic | P value | OR (95%CI) | I 2 statistic | P value | OR (95%CI) | I 2 statistic | P value | OR (95%CI) | I 2 statistic |
| Overall | 5731/9075 | 0.30 | 1.10 (0.92‐1.32) | 78% | 0.11 | 0.87 (0.73‐1.03) | 65% | 0.57 | 1.04 (0.92‐1.17) | 55% | 0.21 | 1.04 (0.98‐1.10) | 55% |
| Caucasian | 4460/6609 | 0.0002 | 1.20 (1.09–1.32) | 49% | 0.003 | 0.84 (0.76‐0.94) | 35% | 0.73 | 1.02 (0.93‐1.11) | 21% | 0.01 | 1.08 (1.02‐1.15) | 31% |
| East Asian | 347/356 | 0.004 | 1.60 (1.17–2.19) | 34% | 0.06 | 0.71 (0.50‐1.02) | 21% | 0.28 | 0.83 (0.60‐1.16) | 38% | 0.0004 | 1.53 (1.21–1.93) | 49% |
| West Asian | 483/963 | 0.66 | 0.75 (0.20‐2.80) | 96% | 0.54 | 1.36 (0.51‐3.65) | 91% | 0.80 | 1.12 (0.47‐2.66) | 92% | 0.56 | 0.75 (0.28‐1.99) | 97% |
| DVT | 1778/1645 | 0.06 | 1.16 (0.99‐1.36) | 36% | 0.05 | 0.73 (0.53–0.99) | 55% | 0.84 | 1.02 (0.88‐1.17) | 46% | 0.02 | 1.13 (1.02–1.25) | 47% |
| PE | 254/682 | 0.47 | 1.14 (0.80‐1.61) | 0% | 0.44 | 0.75 (0.36‐1.57) | 65% | 0.41 | 0.88 (0.64‐1.20) | 0% | 0.57 | 1.11 (0.77‐1.60) | 55% |
| Factor V Leiden | 993/1870 | 0.01 | 1.29 (1.06‐1.57) | 5% | 0.06 | 0.68 (0.46‐1.02) | 65% | 0.92 | 0.99 (0.83‐1.19) | 0% | 0.03 | 1.24 (1.02‐1.52) | 60% |
| Cancer | 264/297 | 0.91 | 1.03 (0.57‐1.87) | 57% | 0.46 | 0.81 (0.45‐1.43) | 0% | 0.91 | 0.98 (0.70‐1.38) | 31% | 0.46 | 1.10 (0.85‐1.42) | 50% |
| Surgery | 91/121 | 0.41 | 1.28 (0.71‐2.32) | 0% | 0.41 | 0.73 (0.35‐1.54) | 0% | 1.00 | 1.00 (0.52‐1.91) | 0% | 0.72 | 1.08 (0.71‐1.64) | 0% |
Note: The values in bold represent there is statistically significant differences between cases and controls.
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; NA, not available; OR, odds ratio; PAI‐1, plasminogen activator inhibitor‐1; PE, pulmonary embolism; VTE, venous thromboembolism.
3.3. Sensitivity analyses
We examined the stability of combined results by deleting one study each time and combining the results of the remaining studies. The trends of associations remained consistent in sensitivity analyses, which indicated that the combined results were statistically stable.
3.4. Publication biases
Funnels plots were employed to estimate whether our combined results may be influenced by publication biases. Funnel plots of every comparison were symmetrical, which indicated that the combined results were unlikely to be seriously impacted by overt publication biases (see Figure S1).
4. DISCUSSION
The A/G variant of rs1799889 polymorphism is associated with a guanosine insertion at the −675 site of the PAI.15 Past pre‐clinical studies also demonstrated that the transcriptional activity of A allele was significantly higher than that of the G allele.16, 17 So theoretically, it is possible that carriers of the A allele were more prone to thrombotic disorders compared to carriers of the G allele. Recently, many genetic association studies assessed association between PAI‐1 rs1799889 A/G polymorphism and VTE, yet the findings were somehow conflicting. Thus, this meta‐analysis was performed by us to more comprehensively analyze relationship between rs1799889 polymorphism and VTE. The combined results demonstrated that PAI‐1 rs1799889 A/G polymorphism was significantly associated with VTE in both Caucasians and East Asians. Further analyses obtained similar positive findings in these with DVT and these with Factor V Leiden mutation. The trends of associations remained consistent in sensitivity analyses, which indicated that the combined results were stable.
To better understand the combined results of this meta‐analysis, some points should be considered. Firstly, the etiology of VTE is complex, so we recommend future studies to conduct haplotype analyses and investigate gene‐gene interactions to more precisely analyze the effects of genetics on disease susceptibility.18 Secondly, environmental factors may also affect relationship between PAI‐1 rs1799889 A/G polymorphism and PAI. Unfortunately, the majority of eligible publications only focused on genetic associations, so we could not estimate genetic‐environmental interactions in this meta‐analysis.19 Thirdly, this meta‐analysis was designed to assess associations between all PAI‐1 polymorphisms and VTE. Nevertheless, only rs1799889 polymorphism was analyzed by us because no any other PAI‐1 polymorphisms were studied by at least two different studies. Fourthly, it should be noted that in 2014, Wang et al also performed a meta‐analysis to investigate association between rs1799889 polymorphism and VTE.20 Since many related articles were published after this meta‐analysis, an updated study like ours is warranted. The sample size of the current meta‐analysis was around 50% larger than that of the previous meta‐analysis (14 806 subjects vs 9254 subjects), so our work should be considered as a valuable supplement to pre‐existing literatures. Consistent with findings of the previous meta‐analysis, we also confirmed that rs1799889 polymorphism was associated with an elevated susceptibility to VTE in both Caucasians and East Asians. The results of these two meta‐analyses indicated that PAI‐1 rs1799889 polymorphism might serve as one of the predisposing factors of VTE. Subgroup analyses by established risk factors of VTE were also further performed by us. Nevertheless, since these analyses were only based on limited number of participants, the findings of these analyses should be considered as only exploratory, and further experimental studies should try to confirm these results. Besides, more stratified analyses should also be conducted by future meta‐analyses if there are sufficient data to support additional analyses for other established risk factors of VTE. Fifthly, although we also conducted subgroup analyses by type of disease in this meta‐analysis, it is noteworthy that studies only focused on PE were scare, so the results of subgroup analyses by type of disease should also be taken as exploratory. Future studies are still needed to confirm these findings.
Some limitations of this meta‐analysis should also be mentioned. Firstly, the results regarding associations between polymorphisms in PAI‐1 rs1799889 polymorphism and VTE were based on combining unadjusted findings of eligible publications due to lack of raw data.21 Secondly, gray literatures were not searched. So although funnel plots of every comparison were symmetrical, it is still possible that the combined results may be affected by publication biases.22, 23
5. CONCLUSIONS
In summary, the combined results of this meta‐analysis proved that PAI‐1 rs1799889 A/G polymorphism may serve as one of the predisposing factors of VTE in both Caucasians and East Asians, especially in these with DVT and these with Factor V Leiden mutation. Further studies with larger sample sizes still need to verify our findings. Besides, given that the pathogenesis of VTE is complex, despite our comprehensive analyses, we still recommend further studies to explore gene‐gene interactions and gene‐environmental interactions in the development of VTE.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
AUTHOR CONTRIBUTIONS
Guangbin Huang and Xuejun Deng conceived of the study, participated in its design. Guangbin Huang and Pan Wang conducted the systematic literature review. Tao Li performed data analyses. Guangbin Huang and Xuejun Deng drafted the manuscript. All authors have read and approved the final manuscript.
Supporting information
Figure S1 Funnel plots.
ACKNOWLEDGMENTS
None.
Huang G, Wang P, Li T, Deng X. Genetic association between plasminogen activator inhibitor‐1 rs1799889 polymorphism and venous thromboembolism: Evidence from a comprehensive meta‐analysis. Clin Cardiol. 2019;42:1232–1238. 10.1002/clc.23282
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Wilbur J, Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am Fam Phys. 2012;86:913‐919. [PubMed] [Google Scholar]
- 2. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5:692‐699. [DOI] [PubMed] [Google Scholar]
- 3. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158:585‐593. [DOI] [PubMed] [Google Scholar]
- 4. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160:809‐815. [DOI] [PubMed] [Google Scholar]
- 5. Streiff MB. Predicting the risk of recurrent venous thromboembolism (VTE). J Thromb Thrombolysis. 2015;39:353‐366. [DOI] [PubMed] [Google Scholar]
- 6. Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIa, PAI‐1 and alpha‐antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007;5:812‐817. [DOI] [PubMed] [Google Scholar]
- 7. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor‐1 (PAI‐1): a key factor linking fibrinolysis and age‐related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72‐e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Westrick RJ, Eitzman DT. Plasminogen activator inhibitor‐1 in vascular thrombosis. Curr Drug Targets. 2007;8:966‐1002. [DOI] [PubMed] [Google Scholar]
- 9. Bezgin T, Kaymaz C, Akbal Ö, Yılmaz F, Tokgöz HC, Özdemir N. Thrombophilic gene mutations in relation to different manifestations of venous thromboembolism: a single tertiary center study. Clin Appl Thromb Hemost. 2018;24:100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Amico M, Pasta F, Pasta L. Thrombophilic genetic factors PAI‐1 4G‐4G and MTHFR 677TT as risk factors of alcohol, cryptogenic liver cirrhosis and portal vein thrombosis, in a Caucasian population. Gene. 2015;568:85‐88. [DOI] [PubMed] [Google Scholar]
- 11. Onur E, Kurdal AT, Tugrul B, et al. Is genetic screening necessary for determining the possibility of venousthromboembolism in cancer patients? Med Princ Pract. 2012;21:160‐163. [DOI] [PubMed] [Google Scholar]
- 12. Vuckovic BA, Djeric MJ, Tomic BV, Djordjevic VJ, Bajkin BV, Mitic GP. Influence of decreased fibrinolytic activity and plasminogen activator inhibitor‐1 4G/5G polymorphism on the risk of venous thrombosis. Blood Coagul Fibrinolysis. 2018;29:19‐24. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264‐269. [DOI] [PubMed] [Google Scholar]
- 14. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 15. Matsubara Y, Murata M, Isshiki I, et al. Genotype frequency of plasminogen activator inhibitor‐1 (PAI‐1) 4G/5G polymorphism in healthy Japanese males and its relation to PAI‐1 levels. Int J Hematol. 1999;69:43‐47. [PubMed] [Google Scholar]
- 16. Hoekstra T, Geleijnse JM, Schouten EG, Kluft C. Diurnal variation in PAI‐1 activity predominantly confined to the 4G‐allele of the PAI‐1 gene. Thromb Haemost. 2002;88:794‐798. [PubMed] [Google Scholar]
- 17. Burzotta F, Di Castelnuovo A, Amore C, et al. 4G/5G promoter PAI‐1 gene polymorphism is associated with plasmatic PAI‐1 activity in Italians: a model of gene‐environment interaction. Thromb Haemost. 1998;79:354‐358. [PubMed] [Google Scholar]
- 18. Oliveira‐Paula GH, Lacchini R, Tanus‐Santos JE. Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide. 2017;63:39‐51. [DOI] [PubMed] [Google Scholar]
- 19. Ma WQ, Han XQ, Wang X, Wang Y, Zhu Y, Liu NF. Associations between XRCC1 gene polymorphisms and coronary artery disease: a meta‐analysis. PLoS One. 2016;11:e0166961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Wang C, Chen N, et al. Association between the plasminogen activator inhibitor‐1 4G/5G polymorphism and risk of venous thromboembolism: a meta‐analysis. Thromb Res. 2014;134:1241‐1248. [DOI] [PubMed] [Google Scholar]
- 21. Xie X, Shi X, Liu M. The roles of TLR gene polymorphisms in atherosclerosis: a systematic review and meta‐analysis of 35,317 subjects. Scand J Immunol. 2017;86:50‐58. [DOI] [PubMed] [Google Scholar]
- 22. Liu A, Wan A, Feng A, Rui R, Zhou B. ICAM‐1 gene rs5498 polymorphism decreases the risk of coronary artery disease. Medicine (Baltimore). 2018;97:e12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta‐analysis. Lipids Health Dis. 2018;17:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Funnel plots.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


