Abstract
Background
Patient limitations guide selection of heart failure therapies, for which indications often specify New York Heart Association Class.
Objectives
To determine the extent of patient‐reported limitations during daily activities and compare to New York Heart Association class assigned by providers during the same visit, and to left ventricular ejection fraction (LVEF) group.
Methods and Results
While waiting for their appointment, 948 patients on return visits to an ambulatory HF clinic completed a written questionnaire assessing specific activity limitations, which were compared to physician‐assigned NYHA class during the same visit. Patient‐reported limitation to perform daily activity ranged from 25% for bathing to 61% for yardwork or housework and 71% for jogging or hurrying. Most patients who did not report limitations to perform daily life activities were correctly classified as NYHA I by the physicians (76%), but 12% of the 376 patients classified as NYHA I reported limitations to showering or bathing and 73% reported limitations while doing yardwork or house work. Limitation to walking was reported by 172 patients (50%) classified as class II. Limitations to walking one block were most common in patients with LVEF ≥40% compared to patients with LVEF <40%, and least commonly, in HF with better EF (improved from 31 ± 13 to 52 ± 7).
Conclusions
Activity limitations are commonly reported by ambulatory HF patients, but underestimated by physicians. It is not clear how this should guide therapy validated for NYHA class but focused activity questions may merit wider use to track limitations and improvement in ambulatory HF.
Keywords: cardiomyopathy, cardiovascular disease, heart failure
1. INTRODUCTION
Increasing penetration of recommended therapies for heart failure (HF) has improved outcomes,1 but morbidity and mortality remain substantial.2, 3 Heart failure guidelines mandate consideration not only for prolonging survival but also on improving quality of life (QOL),4, 5 upon which patients may place similar or greater value.6
A major contributor to QOL in HF is functional capacity, for which impairment can lead to loss of independence and reduced self‐esteem.7, 8 The New York Heart Association (NYHA) functional classification system9 is widely used in clinical practice, in clinical trials,10 in translation of trials into guideline recommendations for therapies,11 and in decisions about advanced HF therapies.12, 13 Understanding functional status and its improvement may be further enhanced by consideration of patient reported limitations to specific activities during daily life, as assessed by some of the questions in the Kansas City Cardiomyopathy Questionnaire.14, 15 Although NYHA classification has been revised several times since 1928,9 it still relies on physicians' subjective estimate of a patient's ability to carry out “ordinary” activity. Consequently, the decision of a physician to classify a patient per NYHA functional class will depend on both patient and physician interpretation of “ordinary physical activity” and how to grade limitations as slight or marked.16, 17 Although NYHA class remains a robust discriminator of clinical outcome, reproducibility is low when the same patients are evaluated by different physicians.14, 17, 18 In order to focus on patient‐centered outcomes,4 it would be potentially useful both in heart failure clinics and in clinical trials to consider patient's individual perceptions about limitations, adding information to frequently used NYHA classification. Although the nature of heart failure symptoms may be similar across ejection fraction (EF) groups, there are differences in demographics and comorbidities, such that the nature and degree of limitations may vary.19
We hypothesized that patients may report limitations to specific daily activity that is not well‐reflected in physician‐assigned NYHA classification. The major aim of the present study was to determine the extent of patient‐reported limitations during daily activities and to compare with the physician assignment of NYHA class on the same day. A further aim was to compare the frequency of perceived limitations across the HF different left ventricular ejection fraction (LVEF) types.
2. METHODS
2.1. Patient population and protocol
The present study is an observational cross‐sectional analysis of patients seen in the ambulatory HF clinic at Brigham and Women's Hospital. As part of a quality improvement initiative to improve communication about quality of life, patients were asked to complete a questionnaire, while waiting for routine clinical visit. The questionnaire was handed to the clinic physician beneath the standard registration/vital sign form as the visit began. The clinic visit and the associated clinical note were completed directly by attending physicians, who had finished formal training in heart failure. There were no specific instructions regarding how to review or incorporate the information in the clinical assessment or documentation.
Patients who did not provide information about limitations to perform daily life activities, those with unknown LVEF and those whose physicians did not assign a NYHA class in the chart were excluded from the present analysis.
2.2. Patient questionnaire
The two‐sided, single page, self‐administered questionnaire included questions about limitations to perform daily life activities, quality of life, symptoms, and patients' perception about clinical stability. To assess self‐perceived limitation to perform daily life activities, the questionnaire included the following question: “Please indicate how much you are limited by your heart failure (shortness of breath or fatigue) in your ability to do the following over the last 4 weeks?”. The activities listed included showering/bathing; walking one city block on level ground; yardwork, housework, or carrying groceries; climbing a flight of stairs (10 steps) without stopping; and hurrying or jogging (such as to catch a bus).15 The degree of each limitation was classified as not at all limited; slightly limited; moderately limited; quite a bit limited; or extremely limited. Additional questions related to recent hospitalizations, ICD shocks, orthopnea, and overall quality of life.
2.3. Clinical data
Patient demographics and clinical characteristics, including HF etiology and medical comorbidities, were assessed from clinical charts. NYHA functional class and current medications on the day of the clinic visit were updated in the electronic medical record by the attending physician, as part of routine assessment and included in our database.
LVEF was recorded from the most recent available echocardiogram within 18 months from the clinical visit. HF with reduced ejection fraction (HFrEF) was defined as LVEF <40%. HF with preserved ejection fraction (HFpEF) was identified by the clinical history of HF with left ventricle ejection fraction (LVEF) ≥40% without any previous echocardiogram with LVEF <40%. HF with better EF (HFbEF) was defined when prior echocardiographic EF had been <40% with subsequent improvement of at least 10% of LVEF in the present echocardiogram.20, 21
The Institutional Review Board of Brigham and Women's Hospital approved this retrospective observational study. No consents were obtained for the questionnaires, which were administered to patients as part of a clinical care and quality improvement initiative.
2.4. Statistical analysis
Initially, normality of distribution was tested, validating the use of parametric statistics. Continuous variables are presented as mean ± SD and categorical variables are presented as frequencies and percentages. Multiple‐group comparisons were performed using analysis of variance or chi‐square tests as appropriate. Post‐hoc correction for multiple comparisons between groups was performed using the Bonferroni method.
To compare patient‐perceived daily life activity limitations across LVEF‐HF types (HFrEF, HFpEF, and HFbEF), we performed multivariate logistic regression adjusted by age, body mass index, and comorbidities.
All statistical tests were two‐sided, and P values <.05 were considered to indicate statistical significance. Statistical analysis was performed using Stata version 14 (Stata Corp, College Station, TX).
3. RESULTS
A total of 1020 patients with HF completed a questionnaire on the day of their routine office visit. Patients were excluded for lack of recent measurement of LVEF (n = 33) or incomplete information about limitations to perform daily life activities (n = 39). The following analysis included the remaining 948 patients. The majority of patients were classified by physicians as NYHA I (n = 376, 40%); 343 were NYHA II (36%), 216 were NYHA III (23%), and 12 were NYHA IV (1%) (Figure 1).
Figure 1.
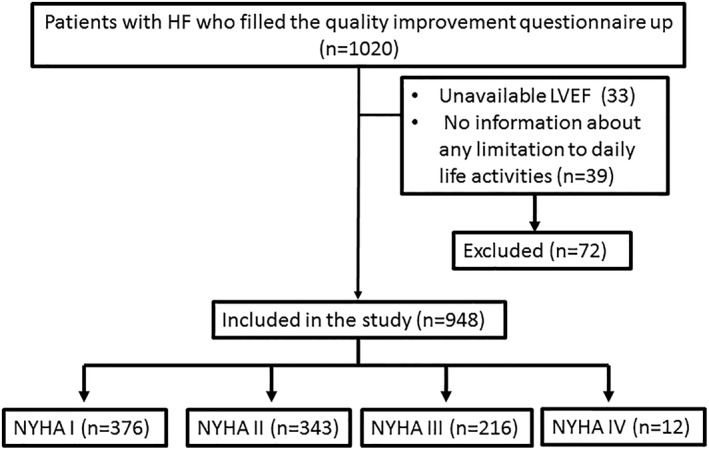
Patient's flow diagram. HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional classification
Patients classified as NYHA I by the HF physicians were younger, had lower body mass index, and presented less comorbidities as diabetes and chronic kidney disease than the ones classified as NYHA II or III (Table 1). The majority of patients (75%) reported limitations to daily life activities, most commonly to hurrying or catching a bus (71%). Limitation to walking a block was reported by 45%, and 25% perceived some limitation even to bathing.
Table 1.
Patients demographics and clinical characteristics accordingly to NYHA functional classification
| All patients | NYHA I | NYHA II | NYHA III | NYHA IV | P value between NYHA (I‐III) groups | |
|---|---|---|---|---|---|---|
| Number of patients (%) | 948 (100.0) | 376 (40) | 343 (36) | 216 (23) | 12 (1) | |
| Demographics | ||||||
| Age (years) | 57 ± 16 | 52 ± 16* | 58 ± 15* | 61 ± 15* | 62 ± 10 | <.001 |
| BMI (kg/m2) | 30 ± 7 | 29 ± 6* | 30 ± 6* | 31 ± 8* | 30 ± 11 | <.001 |
| Female, n (%) | 379 (40) | 141 (37) | 143 (42) | 90 (42) | 5 (42) | .65 |
| LVEF (%) | 42 ± 16 | 46 ± 15* | 40 ± 16** | 39 ± 18** | 39 ± 17 | <.001 |
| Comorbidities | ||||||
| Diabetes mellitus (%) | 210 (22) | 48 (13)* | 89 (26)** | 66 (31)** | 7 (58) | <.001 |
| Obstructive lung disease (%) | 149 (16) | 32 (8)* | 60 (17)** | 50 (23)** | 7 (58) | <.001 |
| Chronic kidney disease (%) | 133 (14) | 25 (7)* | 58 (17)** | 45 (21)** | 5 (42) | <.001 |
| ICM (%) | 156 (16) | 34 (9)* | 79 (23)** | 41 (19)** | 1 (8) | <.001 |
| Hypertension (%) | 432 (46) | 139 (37)* | 160 (47)* | 124 (57)* | 8 (67) | .003 |
| Atrial fibrillation/flutter (%) | 260 (26) | 65 (17)* | 100 (29)** | 79 (37)** | 5 (42) | <.001 |
| Therapies | ||||||
| ICD (%) | 244 (39) | 77 (31)* | 105 (44)** | 59 (44)** | 2 (20) | .012 |
| CRTD (%) | 95 (20) | 17 (10)* | 41 (22)** | 34 (31)** | 3 (30) | <.001 |
| ACE inhibitors/ARBs (%) | 678 (72) | 263 (70) | 253 (74) | 153 (71) | 9 (75) | .70 |
| Beta‐blockers (%) | 753 (79) | 276 (73)* | 289 (84)** | 177 (82)** | 11 (92) | .002 |
| Diuretic agents (%) | 544 (57) | 141 (37)* | 220 (64)* | 172 (80)* | 11 (92) | <.001 |
| Digoxin (%) | 216 (23) | 56 (15)* | 80 (23)* | 75 (35)* | 5 (42) | <.001 |
| Warfarin (%) | 307 (32) | 85 (23)* | 122 (36)** | 93 (43)* | 7 (58) | <.001 |
| Amiodarone (%) | 102 (11) | 29 (8)*** | 48 (14)** | 22 (10) | 3 (25) | .019 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CRT, cardiac resynchronization therapy; LVEF, left ventricle ejection fraction; ICD, implantable cardioverter defibrillator; ICM, ischemic cardiomyopathy.
*P < .05 vs all other groups; **P < .05 vs NYHA I; ***P < .05 vs NYHA II.
While the frequency and degree of limitations were reported as higher by patients with higher NYHA designation (Figure 2), under‐classification of NYHA by physicians was common.
Figure 2.

Degree of limitations to perform daily activities reported by ambulatory heart failure patients. Panel A includes all patients (NYHA I to IV; n = 948) and panels B, C, and D include patients designated as NYHA class I (n = 376), II (n = 343), and III (n = 216), respectively. Colors indicate the perceived degree of limitation for each activity
Almost 12% of patients classified as NYHA I by HF physicians reported limitations to showering and bathing, and 20% of them presented limitations to walk one block on level ground. Among patients designated as class II, 52% described limitation, while walking a block and 73% perceived limitations, while doing housework or yardwork.
When patients were distributed accordingly to LVEF group (Table 2), most of them were classified as HFrEF (n = 400, LVEF: 26 ± 7%), followed by HFbetterEF EF (n = 382, LVEF: 52 ± 7%) and HFpEF (n = 166, LVEF: 60 ± 7%). Patients with HFbetterEF reported the fewest limitations to daily life activities after adjusting for age, gender, and comorbidities (Figure 3). Although patients with HFpEF presented higher frequency of limitations when compared to patients with HFrEF in the univariate analysis, the significance of this comparison disappeared in the regression model adjusted for demographic characteristics and comorbidities.
Table 2.
Patients demographics and comorbidities accordingly to left ventricular ejection fraction subtype
| HFrEF | HFbEF | HFpEF | P value between LVEF groups | |
|---|---|---|---|---|
| Number of patients (%) | 400 (42) | 382 (40) | 166 (18) | |
| Demographics | ||||
| Age (years) | 52 ± 14* | 54 ± 16* | 64 ± 16* | <.001 |
| BMI (kg/m2) | 29 ± 7 | 30 ± 7 | 30 ± 8 | .34 |
| Female, n (%) | 111 (28) | 175 (46) | 93 (56) | .65 |
| LVEF (%) | 26 ± 7* | 52 ± 8* | 60 ± 7* | <.001 |
| Comorbidities | ||||
| Diabetes mellitus (%) | 100 (25) | 58 (15) ** *** | 52 (31) | <.001 |
| Obstructive lung disease (%) | 57 (14) | 57 (15) | 35 (21) | .11 |
| Chronic kidney disease (%) | 67 (17) | 33 (9)** *** | 33 (20) | <.001 |
| ICM (%) | 102 (25)* | 54 (14)* | 0 (00)* | <.001 |
| Hypertension (%) | 166 (42)*** | 163 (43)*** | 103 (62)** | <.001 |
| History of atrial fibrillation/flutter (%) | 112 (28) | 85 (22) *** | 53 (32) | .038 |
Abbreviations: BMI: body mass index; ICM: ischemic cardiomyopathy; HFbEF: Heart failure with better ejection fraction; HFpEF: Heart failure with reduced ejection fraction; HFrEF: Heart failure with reduced ejection fraction; LVEF: left ventricle ejection fraction.
*P < .05 vs all other groups; **P < .05 vs HFrEF; ***P < .05 vs HFpEF.
Figure 3.

Percentage of patients reporting limitations to perform daily life activities accordingly to left ventricular ejection fraction subtype. *P < .01 vs HFpEF & HFrEF. P values from multivariate logistic regression adjusted by age, body mass index, and comorbidities. HFrEF, heart failure with reduced ejection fraction (n = 400); HFbetterEF, heart failure with better ejection fraction (n = 382); HFpEF, heart failure with preserved ejection fraction (n = 166)
4. DISCUSSION
Ambulatory patients seen for routine visits in HF clinic reported substantial limitations to daily activities that were often not reflected in the NYHA class as assessed by experienced heart failure clinicians. Patients with HFpEF and HFrEF were more limited in their activities than patients whose previously low‐ejection fraction had improved (HFbetterEF).
4.1. Symptom burden in patients with heart failure
The penetrance of guideline‐recommended therapies was high in the present cohort, although the timing of the study preceded the introduction of newer therapies that may further improve functional classification.11, 22, 23, 24 The burden of activity limitation was higher than expected for an ambulatory middle‐aged HF population, with more than half of patients reporting limitations to climbing one flight of stairs, 30% of patients reporting limitations to dressing without stopping, and 25% presenting limitations to bathing. Skalska et al25 reported a high dependence on assistance for bathing (28%) in a HF cohort, but their patients were much older (mean age 80 years) than our population with mean age of 57 years.
4.2. Disparity between patient‐reported limitations and assigned NYHA class
NYHA classification has been widely used in clinical trials as both an enrollment criterion and as an outcome measurement. It has consistently provided reliable discrimination for risk of hospitalizations and death.14 Our study confirms that higher NYHA classification discriminates between patients with greater vs fewer limitations to routine activities. However, NYHA classification was not well calibrated to patient‐reported activity limitations, as 10% patients classified as NYHA I described limitations performing low‐intensity activities as showering and dressing, while most class II patients reported limitations when climbing one flight of stairs and during housework or yardwork. Alternatively, 5% of patients classified as NYHA III perceived no limitation to hurrying and jogging, which are moderate to high‐intensity activities. As patients tend to avoid activities that are difficult to perform,25 their general perception of ability and their reports to physicians may underestimate their actual limitation for specified activities.
Discrepancies in classifying patients as NYHA II or III has been previously described, not only for differing perceptions between physicians regarding the same patient,14 but also by comparisons of NYHA classification with a more objective measurement as “how far a patient can walk”.16 While quantitative classification has been established by Weber et al26 for clinical class and peak oxygen consumption, there is currently no standard approach of questioning to be consistently employed by physicians to calibrate the NYHA classification with specific activities.
It is not known how the accelerating time demands of ambulatory follow‐up clinics have affected the disparity between patient and physician perception of limitation. After the in‐depth evaluation as a new patient, returning patients may have decreased their activity gradually to match their disease progression, failing to appreciate the cumulative degree of limitation. However, there is likely also an unacknowledged patient‐provider collusion to focus on positive information. The patients may be reluctant to “disappoint” their physician but also resistant to negative news, new prescriptions, or new proscriptions. On their side of the collusion, physicians may consciously or unconsciously communicate their pleasure at favorable information and their time conflict when encountering new issues that warrant additional attention during a busy clinic where other patients are waiting.
4.3. Perceived limitations and LVEF subtype
In our study, patients with HFpEF and HFrEF perceived similar limitations to perform daily life activities after adjusting for age, gender, and comorbidities. Depending on the details of the populations studied, QOL impairment has been shown to be similar or slightly different between HFrEF and HFpEF groups.19, 27 This may reflect the characteristic pathophysiology of each of these LVEF HF subtypes,28, 29, 30, 31, 32, 33, 34, 35 and the fact that HFpEF in general encompasses older patients in whom age and accumulating comorbidities play an increasing role in limitation.36 In our study, the difference between limitations reported by patients with HFrEF and HFpEF disappeared when the regression model was adjusted by age, body mass index, and comorbidities.
More recently, HFbetterEF has been described, beginning with low LVEF and improving to LVEF >0.40 or 0.50, depending on the study.21, 37 The associated perception of better quality of life and less exercise limitation may reflect better circulatory reserve and also relative framing where they compare their current state to previously more severe limitations. Although patients with HFbetterEF have better survival38 and biochemical profile than patients with HFpEF and HFrEF, persistent abnormalities in the neurohormonal profile indicate that HFbEF is rarely truly “recovered.”21 Our study expanded these findings, showing that patients with HFbEF still present substantial limitations to daily life activities, as almost 20% of them reported limitations to dressing and the majority reported inability to jogging or hurrying as to catch a bus.
4.4. Study limitations
The present study analyzed patient self‐perceived limitations to daily life activity. Factors of resilience, spiritualism, duration of disease, and previous experience and expectations of disease progression39 could have influenced these perceptions.40, 41 These variables were not addressed, and we cannot ascertain if they could have impacted patient perceptions of limitations or discussions with their doctors who assigned the NYHA designation.
Patients filled the questionnaires while waiting for their office visits. Attending physicians had access to the answered questionnaires, which were expected to influence their decisions when classifying NYHA in patient notes. There was no report about how frequently physicians had read or used the information provided by the questionnaires or whether this information influenced their clinical assessment. However, considering that the activity information was provided in real time to the physicians on the same day, the discrepancies between their assessments and the patient descriptions are even more notable.
Results of the present study arise from a HF referral center, where all the physicians providing assessment and care are HF specialists with access to heart transplants and implants of ventricular assist devices when needed. These results may not be generalizable to other care settings, particularly to general medicine clinics where multiple different diseases are assessed simultaneously.
5. CONCLUSIONS
The discrepancy between patient perception of activity limitation and the physician estimate of NYHA class suggests that routine outpatient evaluation should incorporate either patient reporting via a separate interface or physician elicitation of specific activity performance as suggested by Albert et al42 Discussing the specific activities most important to patients would clearly advance the agenda to render more patient‐centered care.
It is less clear whether reclassification of functional level according to specific activities should refine the selection of current therapies according to the NYHA class designated in the trials that provided the evidence for approval, reimbursement, and guideline recommendations. New trials designed to demonstrate improvement in functional capacity may need to isolate the questions about specific activities from the integrated summary of questionnaires such as the Kansas City Cardiomyopathy Questionnaire4 or use the Duke activity status index43 within which these activity questions are currently embedded. It is possible that such refinement would enhance recognition of symptomatic benefit or impairment for daily activities during adjustment of therapies. As the choice and titration of therapies become increasingly complex, more precise elicitation and tracking of activity limitations may help guide future interventions indicated to improve functional capacity and quality of life.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
ACKNOWLEDGEMENTS
Authors report no relationship with industry in relation to the topic of this manuscript.
Castro RRT, Joyce E, Lakdawala NK, et al. Patients report more severe daily limitations than recognized by their physicians. Clin Cardiol. 2019;42:1181–1188. 10.1002/clc.23269
REFERENCES
- 1. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998‐2008. Jama. 2011;306:1669‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123‐1133. [DOI] [PubMed] [Google Scholar]
- 4. Spertus JA. Evolving applications for patient‐centered health status measures. Circulation. 2008;118:2103‐2110. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776‐803. [DOI] [PubMed] [Google Scholar]
- 6. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant: Off Pub Int Soc Heart Transplant. 2001;20:1016‐1024. [DOI] [PubMed] [Google Scholar]
- 7. Paul S, Sneed N. Patient perceptions of quality of life and treatment in anoutpatient congestive heart failure clinic. Congest heart fail 2002;8:74‐9. [DOI] [PubMed] [Google Scholar]
- 8. Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985;55:1037‐1042. [DOI] [PubMed] [Google Scholar]
- 9. Dolgin M, Committee NYHAC. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels/the Criteria Committee of the new York Heart Association. Vol 9th Boston, MA: Little, Brown and Company; 1994. [Google Scholar]
- 10. Hawwa N, Vest AR, Kumar R, et al. Comparison between the Kansas City cardiomyopathy questionnaire and New York heart association in assessing functional capacity and clinical outcomes. J Card Fail. 2016;23:280‐285. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282‐e293. [DOI] [PubMed] [Google Scholar]
- 12. Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant: Official Publ Int Soc Heart Transplant. 2013;32:157‐187. [DOI] [PubMed] [Google Scholar]
- 13. Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant: Official Publ Int Soc Heart Transpl. 2016;35:1‐23. [DOI] [PubMed] [Google Scholar]
- 14. Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart & Lung: J Critical Care. 2002;31:262‐270. [DOI] [PubMed] [Google Scholar]
- 15. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245‐1255. [DOI] [PubMed] [Google Scholar]
- 16. Raphael C, Briscoe C, Davies J, et al. Limitations of the New York heart association functional classification system and self‐reported walking distances in chronic heart failure. Heart. 2007;93:476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227‐1234. [DOI] [PubMed] [Google Scholar]
- 18. Severo M, Gaio R, Lourenco P, Alvelos M, Bettencourt P, Azevedo A. Indirect calibration between clinical observers—application to the New York heart association functional classification system. BMC Res Notes. 2011;4:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis EF, Lamas GA, O'Meara E, et al. Characterization of health‐related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83‐91. [DOI] [PubMed] [Google Scholar]
- 20. Himelman RB, Cassidy MM, Landzberg JS, Schiller NB. Reproducibility of quantitative two‐dimensional echocardiography. Am Heart J. 1988;115:425‐431. [DOI] [PubMed] [Google Scholar]
- 21. Basuray A, French B, Ky B, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380‐2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54‐61. [DOI] [PubMed] [Google Scholar]
- 23. Zugck C, Martinka P, Stöckl G. Ivabradine treatment in a chronic heart failure patient cohort: symptom reduction and improvement in quality of life in clinical practice. Adv Ther. 2014;31:961‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMurray JJ, Packer M, Desai AS, et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail. 2014;16:817‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skalska A, Wizner B, Wiecek A, et al. Reduced functionality in everyday activities of patients with self‐reported heart failure hospitalization—population‐based study results. Int J Cardiol. 2014;176:423‐429. [DOI] [PubMed] [Google Scholar]
- 26. Edmonds PM, Rogers A, Addington‐Hall JM, McCoy A, Coats AJ, Gibbs JS. Patient descriptions of breathlessness in heart failure. Int J Cardiol. 2005;98:61‐66. [DOI] [PubMed] [Google Scholar]
- 27. Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213‐1223. [DOI] [PubMed] [Google Scholar]
- 28. Joyce E, Chung C, Badloe S, et al. Variable contribution of heart failure to quality of life in ambulatory heart failure with reduced, better, or preserved ejection fraction. JACC Heart Fail. 2016;4:184‐193. [DOI] [PubMed] [Google Scholar]
- 29. Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015;119:739‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Esposito F, Mathieu‐Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769‐781. [DOI] [PubMed] [Google Scholar]
- 32. Wilson JR, Rayos G, Yeoh TK, Gothard P, Bak K. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation. 1995;92:47‐53. [DOI] [PubMed] [Google Scholar]
- 33. Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87:470‐475. [DOI] [PubMed] [Google Scholar]
- 34. Kono Y, Yamada S, Iwatsu K, et al. Predictive value of functional limitation for disease severity in patients with mild chronic heart failure. J Cardiol. 2012;60:411‐415. [DOI] [PubMed] [Google Scholar]
- 35. Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatric Cardiol. 2015;12:294‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17:527‐532. [DOI] [PubMed] [Google Scholar]
- 38. Nadruz W Jr, West E, Santos M, et al. Heart failure and midrange ejection fraction: implications of recovered ejection fraction for exercise tolerance and outcomes. Circ Heart Fail. 2016;9:e002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kind P, Dolan P. The effect of past and present illness experience on the valuations of health states. Med Care. 1995;33:As255‐As263. [PubMed] [Google Scholar]
- 40. Mark DB. Assessing quality‐of‐life outcomes in cardiovascular clinical research. Nat Rev Cardiol. 2016;13:286‐308. [DOI] [PubMed] [Google Scholar]
- 41. Wilson IB, Cleary PD. Linking clinical variables with health‐related quality of life. A conceptual model of patient outcomes. Jama. 1995;273:59‐65. [PubMed] [Google Scholar]
- 42. Albert N, Trochelman K, Li J, Lin S. Signs and symptoms of heart failure: are you asking the right questions? Am J Critical Care: Off Publ, Am Associat Critical‐Care Nurses. 2010;19:443‐452. [DOI] [PubMed] [Google Scholar]
- 43. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self‐administered questionnaire to determine functional capacity (the Duke activity status index). Am J Cardiol. 1989;64:651‐654. [DOI] [PubMed] [Google Scholar]


