Abstract
Fascioliasis occurs on all inhabited continents. It is caused by Fasciola hepatica and Fasciola gigantica, trematode parasites with complex life cycles, and primarily affects domestic livestock. Humans become infected after ingestion of contaminated food (typically wild aquatic vegetables) or water. Fascioliasis may be difficult to diagnose as many symptoms are non-specific (e.g. fever, abdominal pain and anorexia). Treatment options are limited, with older effective therapies such as emetine and bithionol no longer used due to safety issues and unavailability, and most common anthelminthics having poor efficacy. Clinical trials conducted over a 25-year period, together with numerous case reports, demonstrated that triclabendazole has high efficacy in the treatment of human fascioliasis in adults and children and in all stages and forms of infection. Triclabendazole was approved for human use in Egypt in 1997 and in France in 2002 and a donation program for the treatment of fascioliasis in endemic countries was subsequently established by the manufacturer and administered by the World Health Organization. Here the published data on triclabendazole in the treatment of human fascioliasis are reviewed, with a focus on more recent data, in light of the 2019 US Food and Drug Administration approval of the drug for use in human infections.
Keywords: fascioliasis, neglected diseases, review, triclabendazole
Introduction
In February 2019, the US Food and Drug Administration (FDA) approved triclabendazole (Egaten, Novartis Pharmaceuticals, East Hanover, NJ, USA) for the treatment of human fascioliasis.1 Triclabendazole has been used in many other parts of the world, following approval in Egypt in 1997 and France in 2002, through a donation program established under the guidance of the World Health Organization’s (WHO) Department of Control of Neglected Tropical Diseases. Here we review the history of triclabendazole in the treatment of human fascioliasis, with a focus on more recent published studies.
Fascioliasis is caused by two species of flukes (Fasciola hepatica and Fasciola gigantica), with complex life cycles involving freshwater snails as well as mammalian hosts, that mainly infect goats, cattle and sheep, but can infect humans after ingestion of larval flukes (metacercariae) in contaminated water or aquatic vegetables.2F. hepatica grows to 30 mm and is found in Europe, the Americas, Asia, Africa and Oceania. The larger (75 mm) F. gigantica is observed primarily in western Africa and much of Asia, with both species in parts of Asia and southern and eastern Africa.3,4
Fascioliasis is considered to be one of the most widespread foodborne trematode infections (Figure 1).5,6 Considered a neglected tropical disease,6,7 human fascioliasis occurs frequently where consumption of wild aquatic vegetables is common or through contaminated water, notably in Bolivia, Peru, Cuba, China, Iran, Vietnam and Egypt.2 Fascioliasis in humans has also been reported in many European countries (notably Turkey, Spain and France), with occasional cases in the USA.2 Residents of more than 70 countries are at risk of fascioliasis; in 2005 there were an estimated 2.6 million infections worldwide, with 1.3 million in Latin America and 1.1 million in Africa.5 School-age children are disproportionately affected in endemic areas. In both children and adults, fascioliasis can be a serious infection, with high fever, enlarged tender liver, anaemia and neurological sequelae.8,9 Natural disasters, particularly flooding, may increase the transmission of fascioliasis, leading to outbreaks. Reported deaths are rare, but can occur.9 Fascioliasis contributes to huge financial losses in the livestock industry and is associated with significant illness and morbidity, mainly in lower-income farming communities.2
Figure 1.
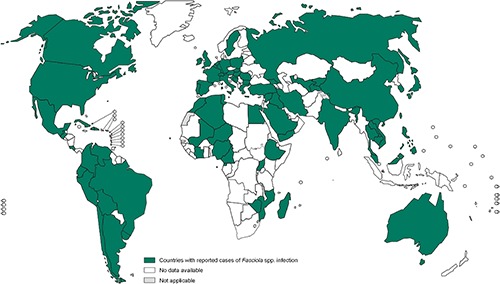
Worldwide distribution of fascioliasis according to the WHO, based on data for the latest year available.5
Typical cases of human fascioliasis can be broadly divided into two stages.3 The acute stage may last for 3–5 months and is considered to be associated with the migration of flukes into the bile ducts. It may be accompanied by fever, anorexia, abdominal pain, gastrointestinal disturbances, urticaria, ascites, hepatomegaly and splenomegaly, anaemia and eosinophilia. Respiratory symptoms, although rare, may also occur.10–13
In chronic disease, adult flukes are resident in the bile ducts. The presence of the parasite, together with the resulting inflammation and epithelial hyperplasia, may lead to biliary obstruction. Biliary colic, epigastric pain, jaundice and right upper quadrant abdominal tenderness may occur during this phase of infection. Hepatic enlargement may occur, associated with splenomegaly or ascites.11,12,14,15 Gallstones are commonly found.10,16 Flukes may have long lifespans in humans: chronic disease may persist for >10 y.12 Rarely, Fasciola spp. may be found in other organs, including the abdominal wall, lungs, heart, brain, skin, muscles and the genitourinary system. Such cases, known as ectopic fascioliasis, may present with a variety of symptoms, depending on the location, sometimes mimicking tumours or abscesses.17
Diagnosis
Fascioliasis can be challenging to diagnose, as many clinical features of the disease (e.g. gastrointestinal symptoms and eosinophilia) are non-specific.18 Diagnosis is generally based on a combination of clinical symptomatology, laboratory evaluations, imaging (most commonly ultrasonography or computed tomography scanning), serological testing or coprologic antigen tests and detection of Fasciola spp. eggs in faeces or duodenal aspirates, using for example the Kato–Katz method.3,19 Detection of Fasciola spp. eggs, while providing a definitive diagnosis, is considered to be possible only in chronic disease (where adult flukes are present in the biliary tract), and acute fascioliasis remains more difficult to identify,2,20,21 although serology is useful and imaging may be more sensitive and specific than in chronic disease.12
Treatment
The treatment of human fascioliasis is based on the use of anthelminthics to kill flukes complemented by symptomatic treatment to relieve abdominal pain, and potentially the use of antispasmodics to treat biliary colic, which may result when dead or dying flukes accumulate in the biliary ducts and cause drainage obstruction.3
Triclabendazole
Triclabendazole (6-chloro-5-(2,3-dichlorophenoxy)-2-(methylthio)-1H-benzimidazole) is a benzimidazole derivative of ampholytic character (Figure 2). It was originally developed and marketed by Ciba as Fasinex to treat fascioliasis in domestic livestock and has been in veterinary use since 1983.22 Following cessation of commercial production of bithionol, development of triclabendazole for human use was initiated in the 1990s via a collaboration between the WHO and Ciba (the manufacturer of Fasinex at the time).
Figure 2.
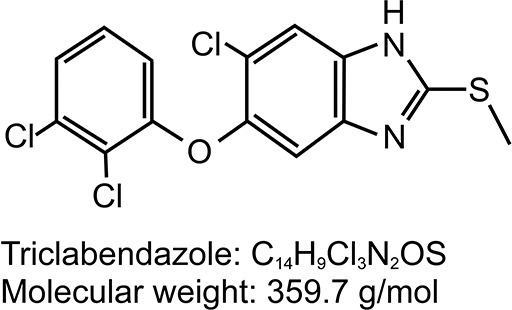
Structure and chemical formula of triclabendazole.
Pharmacodynamics and pharmacokinetics
Triclabendazole is a narrow-spectrum anthelminthic, with activity against Fasciola (F. hepatica and F. gigantica) and Paragonimus spp. It is effective against Fasciola spp. at all stages of infection.14,23 The mechanism of action of triclabendazole has not been fully elucidated and may involve multiple targets, including tegumental disruption via inhibition of microtubule-based processes or adenylate cyclase activity.24 The sulphoxide metabolite appears to have a delayed but more potent effect on parasite motility than triclabendazole itself,25 and it is probable that the drug acts principally through this metabolite, which is largely predominant in human plasma following pre-systemic biotransformation of triclabendazole.26,27 As a result, pharmacokinetic investigations have relied mainly on the determination of plasma concentrations of the sulphoxide metabolite.
Pharmacokinetic parameters were similar in healthy volunteers and fascioliasis patients.28 Two open-label, non-randomized studies, one performed in Peru and the other in Europe, compared administration under fed and fasting conditions in fascioliasis patients, with similar results in each study27 (unpublished data, Novartis). Absorption was rapid: tmax [time to reach the maximum serum concentration] of 2–3 h for the parent compound). The mean maximum serum concentration (Cmax) and median tmax of sulphoxide metabolite were increased from 2 h (fasting) to 4 h (fed) and 15.8 μmol/L (fasting) to 38.6 μmol/L (fed condition), respectively. Overall, food resulted in about 2.2-fold increase in the AUC of sulphoxide metabolite.27 The mean elimination half-life of the sulphoxide metabolite from plasma is approximately 11 h in the fed state. The enhanced exposure to the drug under fed conditions led to a recommendation that triclabendazole should be administered with food.
No excretion studies were conducted in humans; however, in animals, the drug is largely excreted via the biliary tract in the faeces (approximately 90%), together with the sulphoxide and subsequently the sulphone metabolite. Less than 10% of an oral dose is excreted in the urine.26
No significant safety issues related to drug–drug interactions were observed from clinical studies and post-marketing safety data (unpublished data, Novartis). This could potentially be attributed to the single-dose administration of triclabendazole.
Efficacy and safety studies in humans
Triclabendazole treatment of human fascioliasis has been studied over a period of almost 30 year using a variety of doses and regimens in a wide range of geographic areas. Most studies were non-comparative, given the lack of effective alternative treatments and that use of placebo controls would not be considered ethically acceptable (although one placebo-controlled study with nitazoxanide in fascioliasis has been conducted).29 Two relatively recent studies compared triclabendazole with artemisinin derivatives.30,31
Early clinical development of triclabendazole
Clinical development of triclabendazole in humans was initiated by the WHO, with the support of the then-sponsor Ciba, starting a clinical trials program in the treatment of human fascioliasis and paragonimiasis. This program, conducted between 1989 and 1992, included six trials in fascioliasis (two performed in Bolivia and one each in Chile, Peru, Cuba and Iran).32 The studies all had similar designs (open label and uncontrolled, although three of the studies assessed different triclabendazole doses) and the same efficacy endpoint: parasitological cure, based on the absence of Fasciola spp. eggs in faecal samples at Day 60. All patients had to be egg-positive at baseline. Table 1 shows cure rates in these studies by dose regimen. These studies established that a triclabendazole dose of 10 mg/kg (10 mg/kg single dose or two 5 mg/kg doses) was effective (with cure rates ranging from 14/20 [70%] to 22/22 [100%]) and well tolerated in adult and paediatric patients; a total dose of 20 mg/kg (10 mg/kg doses given on Days 1 and 3) also showed very high cure rates and was well tolerated.
Table 1.
Rates of egg clearance by dose regimen in Ciba/WHO studies and Egyptian government studies
| Study location/date | Triclabendazole dose regimen | Cure ratea, n/N (%) | |
|---|---|---|---|
| CIBA/WHO studies | |||
| Bolivia (paediatric) 1991–1992 | 5 mg/kg single dose postprandial | 10/20 (50) | |
| 10 mg/kg single dose postprandial | 17/20 (85) | ||
| 2×5 mg/kg postprandial, 1 d | 20/20 (100) | ||
| Iran 1989–1991 | 5 mg/kg daily for 3 d fasting | 16/17 (94) | |
| 10 mg/kg single dose postprandial | 14/20 (70) | ||
| 10 mg/kg single dose fasting | 13/17 (76) | ||
| 2×5 mg/kg 1 d fasting | 9/14 (64) | ||
| Bolivia 1990–1992 | 10 mg/kg single dose postprandial | 22/22 (100) | |
| Cuba 1990–1992 | 10 mg/kg single dose fasting | 12/14 (86) | |
| Chile 1990–1992 | 10 mg/kg single dose fasting | 19/24 (79) | |
| Peru 1991 | 10 mg/kg fasting on Day 1, then 10 mg/kg postprandial Day 3 | 9/10 (90) | |
| 10 mg/kg postprandial on Day 1, then 10 mg/kg fasting Day 3 | 12/12 (100) | ||
| Egyptian government studies | |||
| Egypt | 10 mg/kg single dose postprandial | 23/25 (92) | |
| 2×5 mg/kg postprandial, 1 d (6 h apart) | 24/25 (96) | ||
| Egypt | 10 mg/kg single doseb | 24/30 (80) | |
| 2×5 mg/kg, 1 db | 26/30 (87) | ||
| Egypt | 10 mg/kg single dose postprandial | 22/25 (88) | |
aCure rate: absence of eggs in faeces (duodenal drainage in the paediatric study in Bolivia) at 60 d (90 d in Peru 1991 study).
bRelationship to food not specified.
References: Laburte et al.32, unpublished data from Novartis.
In addition, three studies were performed in Egypt in 1996, with analysis and reporting done by the investigators under supervision of the Egyptian Ministry of Health. All patients received 10 mg/kg triclabendazole, either as a single dose or as two 5 mg/kg doses given on the same day: in two studies doses were given under fed conditions and in the other the relationship to food was not reported. Cure rates (absence of eggs in faeces at 60 d) in these studies ranged from 24/30 (80%) to 24/25 (96%) (Table 1).
Also in 1996, a Ciba-sponsored open-label, non-comparative trial evaluated the use of triclabendazole to control a fascioliasis epidemic that was reported in La Palma, Pinar del Río, West Cuba in 1995.10 Eighty-two adult or adolescent patients were treated with two 10 mg/kg doses of triclabendazole, given after food, 12 h apart. All patients had faecal Fasciola spp. eggs at baseline. Three patients were lost to follow-up and 79 completed the study. At Day 60, 71/77 (92%) patients no longer had faecal Fasciola spp. eggs. Regulatory approval in Egypt (1997) and France (2002) for the use of triclabendazole in the treatment of human fascioliasis were obtained on the basis of these studies and the dosing regimen established in them.
Human use following initial development and registration
Since the initial studies in humans, many case reports, case series and studies with triclabendazole in the treatment of human fascioliasis in a wide range of countries have been published. These include data on the treatment of adults, adolescents and children, but treatment has not been specifically studied in elderly patients or patients with renal or hepatic impairment. Also, there are no studies on its use in pregnancy or lactation. Clinical studies (Table 2) include two randomized trials, one comparing triclabendazole with artesunate in 100 patients in Vietnam30 and the other comparing two doses of triclabendazole in 84 paediatric patients in Peru,33 a study with 90 paediatric patients in Bolivia,34 a study in Egypt with 134 patients,35 a study with 165 patients in Iran36 and a trial with 40 patients in Egypt, of whom 16 were treated with triclabendazole.31
Table 2.
Efficacy outcomes in clinical studies with triclabendazole in human chronic fascioliasis
| Study | Location/date/population | Triclabendazole regimen | Cure rate, n/N (%)/time point |
|---|---|---|---|
| Maco et al.33 | Peru/2001–2006/children 2–16 y, faecal egg positive | Two 7.5 mg/kg doses 12 h apart10 mg/kg single dose | 44/44 (100)/60 d 38/40 (95)/60 d |
| Keiser et al.31 | Egypt/2007–2010/adults and children, faecal egg positive, previously failed artemisinin treatment | 10 mg/kg single dose Two 10 mg/kg doses 1 d aparta | 11/16 (69)/28 d 3/4 (75)/28 d |
| El-Morshedy et al.35 | Egypt/NS/adults and children 2–60 y, faecal egg positive | 10 mg/kg single dose Two 10 mg/kg doses 1 d apart | 54/68 (79.4)/35 d 62/66 (93.9)/35 d |
| Talaie et al.36 | Iran/NS/adults and children 10–65 y, faecal egg positiveb | 10 mg/kg single dose Two 10 mg/kg doses 1 d apart Three 10 mg/kg doses 1 d apart | 7/7 (100)/60 d 9/9 (100)/60 d 9/9 (100)/60 dc |
| Villegas et al.34 | Bolivia/2008/children 5–14 y identified as faecal egg positive in a community screening programme | 10 mg/kg single dose Two 10 mg/kg doses 3 months apartd | 70/90 (77.8)/3 months 18/20 (90.0)/2 months |
aPatients who failed to respond to a single 10 mg/kg dose were re-treated with two doses given 1 d apart.
bStudy included patients who were faecal egg positive (‘cases’) or faecal egg negative but with characteristic signs or symptoms and laboratory data (‘suggestive cases’); cure rates for cases are shown here.
cIncludes two patients who were egg positive at Days 14 and 30. These patients received a further 10 mg/kg dose of triclabendazole on Day 30 and were egg-free at Day 60.
dPatients who were egg positive at 3 months were re-treated with a second 10 mg/kg dose; cure rate 2 months after second dose.
NS: not specified.
Hien et al.30 describe the only reported randomized trial comparing triclabendazole (two doses of 10 mg/kg, 12 h apart) with another therapy (artesunate 4 mg/kg once daily for 10 d). Faecal egg counts were not used for diagnosis (which was based on serology, imaging, clinical signs and symptoms and eosinophilia) or efficacy assessment, as the study focused on patients with acute stage disease. The primary efficacy parameter was resolution of abdominal pain at Day 10; the secondary endpoint was complete response at 3 months (defined as resolution of symptoms, normalization of eosinophilia and improvement in ultrasound appearance). Each component of complete response was also summarised separately at 3 months. The proportion of patients with resolution of abdominal pain at Day 10 was higher with artesunate than triclabendazole (50/50 [100%] vs 44/50 [88%], p<0.05). At 3 months, however, the rate of complete response (although low in both groups) was significantly higher in the triclabendazole group (18/50 [36%] vs 5/50 [10%] with artesunate, p<0.01), as was the rate of resolution of eosinophilia (21/50 [42%] vs 8/50 [16%], p<0.01). The rate of resolution of symptoms was also higher with triclabendazole (46/50 [92%] vs 38/50 [76%], p=0.05) and the rate of improvement of ultrasound appearance was similar in the two groups (35/50 [70%] and 33/50 [66%]). The authors considered it likely that the low rate of complete response was due to the low rate of resolution of eosinophilia, itself the result of high rates of co-infection with other parasites in the study population.
Table 2 summarises efficacy outcomes in other studies: faecal egg counts were used to assess efficacy, except in a subgroup of patients with acute fascioliasis in the study by Talaie et al.,36 who were diagnosed on the basis of clinical signs or symptoms and laboratory findings. Cure rates after treatment with triclabendazole doses of 10 mg/kg (repeated in some cases) or 15 mg/kg ranged from 11/16 (69%) to 44/44 (100%).
In patients with acute fascioliasis in the study by Talaie et al.,36 cure was defined as the absence of faecal Fasciola spp. eggs, a decrease in or complete disappearance of clinical signs and symptoms and immunoglobulin G levels ≤128 mg/dl. In this study, patients received either one, two or three daily 10 mg/kg doses of triclabendazole. Cure rates at 60 d were 23/36 (63.9%), 24/35 (68.6%) and 23/36 (63.9%), respectively.
The study described by Villegas et al.34 is of particular interest. The Bolivian northern Altiplano region has a very high endemicity of fascioliasis, with the highest prevalence observed in children.37 Following consultation with experts from the WHO, the Bolivian Ministry of Health decided to implement large-scale deployment of triclabendazole in this region, without individual diagnosis, in 2008. In a pilot study performed prior to implementation of this policy, asymptomatic schoolchildren were tested for Fasciola spp. carriage by faecal egg counts.34 Those with positive results were treated with triclabendazole (10 mg/kg single dose, repeated if necessary). The single dose resulted in a cure rate of 70/90 (78%) at 3 months, and 18/20 (90%) of the patients who required re-treatment were cured at 2 months (Table 2). The policy of preventive triclabendazole treatment for fascioliasis was subsequently implemented by the Bolivian government and 71 456 patients were treated in 2008 and 223 946 in 2009.38
In addition to these clinical studies, numerous case reports and case series have described triclabendazole treatment of human fascioliasis, as reviewed in Keiser et al.14 Across the studies, case series and case reports from a very wide range of geographical areas, successful treatment of all forms and stages of fascioliasis has been described. In addition to acute and chronic stages of infection, asymptomatic chronic fascioliasis was the subject of case reports39–42 as well as studies by El-Morshedy et al.35 and Villegas et al.34 A small number of case reports have also described the efficacy of triclabendazole in ectopic fascioliasis.17,42–44
In most reports, parasites were not identified to the species level, but given the geographical distribution of each species, it is likely that F. hepatica was involved in most cases. In one published study performed in China,45F. gigantica was identified using serology and molecular techniques, and improvement of clinical signs and symptoms was reported in all patients. In one of the Egyptian government studies, both F. hepatica and F. gigantica infections were treated; the cure rate for F. gigantica was lower, but only 12 such infections were treated. Successful treatment of F. gigantica was also described in case reports.46
Safety and tolerability
Based on the available data, triclabendazole appears to be well tolerated in the treatment of fascioliasis. The most common adverse events (AEs) associated with treatment appear to be related to the expulsion of dead or dying flukes from the hepatobiliary system. These AEs, characterized as biliary colic, include abdominal, hypochondrial and epigastric pain, often accompanied by sweating, in some cases with associated obstructive jaundice and elevations in serum levels of hepatic enzymes, most commonly alkaline phosphatase.10,31,33–36 The timing of these events (biliary colic symptoms tend to occur 3–7 days post-therapy, with elevations of hepatic enzymes, where present, generally occurring around 7 days post-treatment), indicates that parasite expulsion is the most likely cause rather than a direct relationship to triclabendazole. Triclabendazole Cmax occurs 4–10 hour post-ingestion, whereas expulsion of parasites typically happens 48 h after dosing.10 Among other AEs commonly reported during the studies, urticaria, pruritus and rash may be related to fascioliasis, as they commonly occur in infected patients. Other relatively common AEs were headache, dizziness and vertigo.
Treatment failure and resistance
Triclabendazole resistance has been widely reported in fascioliasis affecting livestock.23 In human infections, a small number of cases in which patients failed to respond to multiple treatment courses with recommended doses of triclabendazole have been reported.47–49 These cases occurred in areas where resistance in livestock infections is common. The chronology with regard to the evolution of symptoms, treatment details and evaluation of response to treatment suggest that some of these cases may indicate reinfection rather than treatment failures, but treatment failure due to resistance could not be ruled out. Although resistance to triclabendazole in livestock infections is well established, it appears to be rare and sporadic in human fascioliasis.
Other treatments
Most anthelminthics, such as praziquantel, albendazole and metronidazole, show little efficacy in fascioliasis.10,50 Emetine has been reported to be effective,51 but the use of emetine (and dehydroemetine) is limited by safety issues, principally cardiotoxicity.39 Prior to the development of triclabendazole, bithionol was widely used,11 but it requires a lengthy treatment course, is associated with tolerability issues11 and cure rates vary considerably.52–54 Bithionol is no longer commercially available.
Other compounds that have shown some efficacy but have not been investigated further are metronidazole55 and nitazoxanide.29,56,57,58 Artemisinin derivatives have also been investigated in human fascioliasis, but efficacy was not optimal and/or required rescue with triclabendazole.30,31
Discussion
Human fascioliasis has been reported on all inhabited continents but is most common in certain regions, notably specific areas of Peru, Bolivia, Egypt, Iran and Vietnam. Despite the worldwide distribution of fascioliasis, treatment options were limited prior to the development of triclabendazole, and remain so, as no other therapies have demonstrated similar, adequate efficacy against fascioliasis.
On the basis of studies in the 1990s performed by the WHO in collaboration with Ciba, studies by the Egyptian government and subsequent published studies, case series and case reports, triclabendazole is highly effective in the treatment of human fascioliasis. The early studies established that a 10 mg/kg single dose, given postprandially, is usually effective, however, patients need to be followed up for confirmation of clinical improvement. In case of treatment failure with a 10 mg/kg single dose, two additional doses of 10 mg/kg can be given 12–24 h apart. These studies were the basis of regulatory approvals in Egypt in 1997 and France in 2002 and are reflected in the current WHO recommendations. The recent approval by the US FDA recommends a total dose of 20 mg/kg (two 10 mg/kg doses given 12 h apart); this is based primarily on more recent studies.30,31,33 Studies in paediatric patients suggest that the same treatment regimens should be used in children of ≥6 y of age and is well tolerated.33,34
Triclabendazole has been shown in clinical studies to be effective in the treatment of chronic and acute forms of fascioliasis and in both F. hepatica and F. gigantica infections. Triclabendazole treatment was generally well tolerated, with most AEs probably related to the expulsion of dead or dying flukes from the biliary tract. Although resistance to triclabendazole in livestock infections is well established (as might be expected following its extensive use in veterinary medicine), it appears to be rare and sporadic in human fascioliasis.
Most clinical trials with triclabendazole in the treatment of human fascioliasis have employed open-label, non-comparative designs (although in many studies, some randomised, different dose regimens were compared). This was mainly due to the lack of effective active comparators. Ethical considerations preclude placebo controls in studies in fascioliasis, although one placebo-controlled study with nitazoxanide has been reported.29 In this study, only 1/16 (6.25%) placebo-treated patients stopped excreting eggs at 90 d (as compared with 32/65 [49%] nitazoxanide-treated patients). This suggests that only a small placebo response can be expected in fascioliasis and that the use of placebo controls in studies with triclabendazole would be have been of little value. The only prospective randomized comparative trial with triclabendazole, which used artesunate as a comparator, was unusual in that egg counts were not used to assess efficacy (patients appeared to have the acute form of fascioliasis) and complete response rates at 60 d were low for both compounds (for reasons discussed previously) but significantly higher with triclabendazole than artesunate.30
Triclabendazole is the only treatment for fascioliasis recommended by the WHO and the Pan American Health Organization,59 and is the drug of choice recommended by the US Centers for Disease Control and Prevention.60 Following initial approvals in Egypt and France, a donation program for triclabendazole for the treatment of fascioliasis in endemic countries, under the guidance of the WHO’s Department of Control of Neglected Tropical Diseases, was established. Between 2006 and 2016, the manufacturer (Novartis) donated triclabendazole to the WHO as treatment for 1.15 million patients.61 The recent approval by the US FDA makes the drug readily available in the USA and further demonstrates the continued importance of triclabendazole for the treatment of fascioliasis.
Acknowledgements
None.
Authors’ contributions
All authors reviewed and assessed the literature and company data. All authors contributed to writing the manuscript. All authors read and approved the final manuscript. PG and DH are guarantors of the paper.
Funding
This work was supported by Novartis Pharma AG.
Competing interests
All authors are employed by Novartis, the funding source.
Ethical approval
Not required.
References
- 1. US Food and Drug Administration Drug Trials Snapshots: EGATEN. https://www.fda.gov/drugs/drug-trials-snapshots-egaten [accessed 29 April 2019].
- 2. Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology. 2018;145(13Special Issue):1665–1699. [DOI] [PubMed] [Google Scholar]
- 3. Mas-Coma S, Bargues MD, Valero MA. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology. 2014;141(14):1918–1946. [DOI] [PubMed] [Google Scholar]
- 4. Mas-Coma S, Valero MA, Bargues MD. Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009;69:41–146. [DOI] [PubMed] [Google Scholar]
- 5. Furst T, Keiser J, Utzinger J. Global burden of human food borne trematodiasis: a systematic review and meta analysis. Lancet Infect Dis. 2012;12(3):210–221. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization Neglected tropical diseases. https://www.who.int/foodborne_trematode_infections/en/ [accessed 29 April 2019].
- 7. Center for Drug Evaluation and Research/Center for Biologics Evaluation and Research Tropical disease priority review vouchers. Guidance for industry. October 2016. https://www.fda.gov/media/72569/download [accessed 30 April 2019].
- 8. Lopez M, White AC Jr, Cabada MM. Burden of Fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg. 2012;86(3):481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mas-Coma S, Agramunt VH, Valero MA. Neurological and ocular fascioliasis in humans. Adv Parasitol. 2014;84:27–149. [DOI] [PubMed] [Google Scholar]
- 10. Millán JC, Mull R, Freise S et al. The efficacy and tolerability of triclabendazole in Cuban patients with latent and chronic Fasciola hepatica infection. Am J Trop Med Hyg. 2000;63(5–6):264–269. [DOI] [PubMed] [Google Scholar]
- 11. Arjona R, Riancho JA, Aguado JM et al. Fascioliasis in developed countries: a review of classic and aberrant forms of the disease. Medicine (Baltimore). 1995;74(1):13–23. [DOI] [PubMed] [Google Scholar]
- 12. Marcos LA, Terashima A, Gotuzzo E. Update on hepatobiliary flukes: fascioliasis, opisthorchiasis and clonorchiasis. Curr Opin Infect Dis. 2008;21(5):523–530. [DOI] [PubMed] [Google Scholar]
- 13. Kaya M, Beştaş R, Cetin S. Clinical presentation and management of Fasciola hepatica infection: single-center experience. World J Gastroenterol. 2011;17(44):4899–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keiser J, Engels D, Büscher G et al. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs. 2005;14(12):1513–1526. [DOI] [PubMed] [Google Scholar]
- 15. Goral V, Senturk S, Mete O et al. A case of biliary Fascioliasis by Fasciola gigantica in Turkey. Korean J Parasitol. 2011;49(1):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hawramy TA, Saeed KA, Qaradaghy SH et al. Sporadic incidence of fascioliasis detected during hepatobiliary procedures: a study of 18 patients from Sulaimaniyah governorate. BMC Res Notes. 2012;5:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musa D, Godbole G, Chiodini PL et al. Unusual case of a lung abscess. BMJ Case Rep 2013;2013: bcr2012008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabada MM, White AC Jr. New developments in epidemiology, diagnosis, and treatment of fascioliasis. Curr Opin Infect Dis. 2012;25(5):518–522. [DOI] [PubMed] [Google Scholar]
- 19. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 20. Marcos LA, Tagle M, Terashima A et al. Natural history, clinicoradiologic correlates, and response to triclabendazole in acute massive fascioliasis. Am J Trop Med Hyg. 2008;78(2):222–227. [PubMed] [Google Scholar]
- 21. Fica A, Dabanch J, Farias C et al. Acute fascioliasis—clinical and epidemiological features of four patients in Chile. Clin Microbiol Infect. 2012;18(1):91–96. [DOI] [PubMed] [Google Scholar]
- 22. McCarthy JS, Moore TA. Drugs for helminths. In: Bennet JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 8th edn., Vol. 1 Philadelphia: Saunders; 2015, p. 519–27.e3. [Google Scholar]
- 23. Fairweather I. Triclabendazole progress report, 2005–2009: an advancement of learning? J Helminthol. 2009;83(2):139–150. [DOI] [PubMed] [Google Scholar]
- 24. Kelley JM, Elliott TP, Beddoe T et al. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol. 2016;32(6):458–469. [DOI] [PubMed] [Google Scholar]
- 25. Stitt AW, Fairweather I. The effect of the sulphoxide metabolite of triclabendazole (Fasinex®) on the tegument of mature and immature stages of the liver fluke, Fasciola hepatica. Parasitology. 1994;108(5):555–567. [DOI] [PubMed] [Google Scholar]
- 26. Hennessy DR, Lacey E, Steel JW et al. The kinetics of triclabendazole disposition in sheep. J Vet Pharmacol Ther. 1987;10(1):64–72. [DOI] [PubMed] [Google Scholar]
- 27. Lecaillon JB, Godbillon J, Campestrini J et al. Effect of food on the bioavailability of triclabendazole in patients with fascioliasis. Br J Clin Pharmacol. 1998;45(6):601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. el-Tantawy WH, Salem HF, Mohammed Safwat NAS. Effect of fascioliasis on the pharmacokinetic parameters of triclabendazole in human subjects. Pharm World Sci. 2007;29(3):190–198. [DOI] [PubMed] [Google Scholar]
- 29. Favennec L, Jave Ortiz J, Gargala G et al. Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Aliment Pharmacol Ther. 2003;17(2):265–270. [DOI] [PubMed] [Google Scholar]
- 30. Hien TT, Truong NT, Minh NH et al. A randomized controlled pilot study of artesunate versus triclabendazole for human fascioliasis in central Vietnam. Am J Trop Med Hyg. 2008;78(3):388–392. [PubMed] [Google Scholar]
- 31. Keiser J, Sayed H, el-Ghanam M et al. Efficacy and safety of artemether in the treatment of chronic fascioliasis in Egypt: exploratory phase-2 trials. PLoS Negl Trop Dis. 2011;5(9):e1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laburte C, Mott K, Mull R, Severne Y In: Angelico M, Rocchi G, editors. Infectious diseases and public healthTriclabendazole for fascioliasis: efficacy and tolerability of a single dose treatment. L’Aquila, Italy: Balaban, 1998; p. 325–333. [Google Scholar]
- 33. Maco V, Marcos L, Delgado J et al. Efficacy and tolerability of two single-day regimens of triclabendazole for fascioliasis in Peruvian children. Rev Soc Bras Med Trop. 2015;48(4):445–453. [DOI] [PubMed] [Google Scholar]
- 34. Villegas F, Angles R, Barrientos R et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl Trop Dis. 2012;6(8):e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. el-Morshedy H, Farghaly A, Sharaf S et al. Triclabendazole in the treatment of human fascioliasis: a community-based study. East Mediterr Health J. 1999;5(5):888–894. [PubMed] [Google Scholar]
- 36. Talaie H, Emami H, Yadegarinia D et al. Randomized trial of a single, double and triple dose of 10 mg/kg of a human formulation of triclabendazole in patients with fascioliasis. Clin Exp Pharmacol Physiol. 2004;31(11):777–782. [DOI] [PubMed] [Google Scholar]
- 37. Parkinson M, O’Neill SM, Dalton JP. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiol Infect. 2007;135(4):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization Neglected tropical diseases. https://www.who.int/neglected_diseases/fascioliasis_treatment_bolivia_2009/en/ [accessed 30 April 2019].
- 39. Apt W, Aguilera X, Vega F et al. Treatment of human chronic fascioliasis with triclabendazole: drug efficacy and serologic response. Am J Trop Med Hyg. 1995;52(6):532–535. [DOI] [PubMed] [Google Scholar]
- 40. Incani RN, Vieira JM, Pacheco M et al. Human infection by Fasciola hepatica in Venezuela: report of a geriatric case. Invest Clin. 2003;44(3):255–260. [PubMed] [Google Scholar]
- 41. Curtale F, Hassanein YA, Savioli L. Control of human fascioliasis by selective chemotherapy: design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile Delta, Egypt. Trans R Soc Trop Med Hyg. 2005;99(8):599–609. [DOI] [PubMed] [Google Scholar]
- 42. Dauchy FA, Vincendeau P, Lifermann F. Eight cases of fascioliasis: clinical and microbiological features. Med Mal Infect. 2006;36(1):42–46. [DOI] [PubMed] [Google Scholar]
- 43. Marcos LA, Légua P, Sánchez J et al. Cervical tumor caused by the sexually mature stage of Fasciola hepatica. Trans R Soc Trop Med Hyg. 2009;103(3):318–320. [DOI] [PubMed] [Google Scholar]
- 44. Tanir G, Karaman A, Tüfekçı SB et al. A case of ectopic intraabdominal fascioliasis presented with acute abdomen. Turk J Gastroenterol. 2011;22(3):347–350. [DOI] [PubMed] [Google Scholar]
- 45. Chen JX, Chen MX, Ai L et al. An outbreak of human fascioliasis gigantica in southwest China. PLoS One. 2013;8(8):e71520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramachandran J, Ajjampur SS, Chandramohan A et al. Cases of human fascioliasis in India: tip of the iceberg. J Postgrad Med. 2012;58(2):150–152. [DOI] [PubMed] [Google Scholar]
- 47. Winkelhagen AJ, Mank T, Vries PJ et al. Apparent triclabendazole-resistant human Fasciola hepatica infection, the Netherlands. Emerg Infect Dis 2012;18(6):1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cabada MM, Lopez M, Cruz M et al. Treatment failure after multiple courses of triclabendazole among patients with fascioliasis in Cusco, Peru: a case series. PLoS Negl Trop Dis. 2016;10(1):e0004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gil LC, Díaz A, Rueda C et al. Fascioliasis hepática humana: resistencia al tratamiento con triclabendazol. Rev Med Chile. 2014;142(10):1330–1333. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization report of the who informal meeting on use of triclabendazole in fascioliasis control. https://www.who.int/neglected_diseases/preventive_chemotherapy/WHO_CDS_NTD_PCT_2007.1.pdf [accessed 30 April 2019].
- 51. Hardman EW, Jones RLH, Davies AH. Fascioliasis—a large outbreak. Br Med J. 1970;3:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farid Z, Kamal M, Woody J. Treatment of acute toxaemic fascioliasis. Trans R Soc Trop Med Hyg. 1988;82(2):299. [DOI] [PubMed] [Google Scholar]
- 53. Bassiouny HK, Soliman NK, el-Daly SM, Badr NM. Human fascioliasis in Egypt: effect of infection and efficacy of bithionol treatment. J Trop Med Hyg. 1991;94(5):333–337. [PubMed] [Google Scholar]
- 54. Yazgan-Aksoy D, Kerimoğlu U, Oto A et al. Fasciola hepatica infection: clinical and computerized tomographic findings of ten patients. Turk J Gastroenterol. 2006;17(1):40–45. [PubMed] [Google Scholar]
- 55. Mansour-Ghanaei F, Shafaghi A, Fallah M. The effect of metronidazole in treating human fascioliasis. Med Sci Monit. 2003;9(10):127–130. [PubMed] [Google Scholar]
- 56. Rossignol JF, Abaza H, Friedman H. Successful treatment of human fascioliasis with nitazoxanide. Trans R Soc Trop Med Hyg. 1998;92(1):103–104. [DOI] [PubMed] [Google Scholar]
- 57. Zumaquero-Ríos JL, Sarracent-Pérez J, Rojas-García R. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanide. PLoS Negl Trop Dis. 2013;7(11):e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lukambagire AH, Mchaile DN, Nyindo M. Diagnosis of human fascioliasis in Arusha region, northern Tanzania by microscopy and clinical manifestations in patients. BMC Infect Dis. 2015;15:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pan American Health Organization general information: fascioliasis. http://www.paho.org/hq/index.php?option=com_content&view=article&id=5758&Itemid=4153&lang=en [accessed 30 April 2019].
- 60. Centers for Disease Control and Prevention Parasites – Fasciola – Resources for health professionals. https://www.cdc.gov/parasites/fasciola/health_professionals/index.html#tx [accessed 30 April 2019].
- 61. Access to Medicine Foundation. Access to medicine index2016. https://accesstomedicineindex.org/media/atmi/Access-to-Medicine-Index-2016.pdf [accessed 30 April 2019].


