Abstract
Background
Healthcare-associated infections pose a major, yet often preventable risk to patient safety. Poor hand hygiene among healthcare personnel and unsanitary hospital environments may contribute to this risk in low-income settings. We aimed to describe hand hygiene behaviour and environmental contamination by season in a rural, sub-Saharan African hospital setting.
Methods
We conducted a concurrent triangulation mixed-methods study combining three types of data at a hospital in Madarounfa, Niger. Hand hygiene observations among healthcare personnel during two seasons contributed quantitative data describing hand hygiene frequency and its variability in relation to seasonal changes in caseload. Semistructured interviews with healthcare personnel contributed qualitative data on knowledge, attitudes and barriers to hand hygiene. Biweekly environmental samples evaluated microbial contamination from October 2016 to December 2017. Triangulation identified convergences, complements and contradictions across results.
Results
Hand hygiene compliance, or the proportion of actions (handrubbing or handwashing) performed out of all actions required, was low (11% during non-peak and 36% during peak caseload seasons). Interviews with healthcare personnel suggesting good general knowledge of hand hygiene contradicted the low hand hygiene compliance. However, compliance by healthcare activity was convergent with poor knowledge of precise hand hygiene steps and the motivation to prevent personal acquisition of infection identified during interviews. Contamination of environmental samples with gram-negative bacilli was high (45%), with the highest rates of contamination observed during the peak caseload season.
Conclusion
Low hand hygiene compliance coupled with high contamination rates of hospital environments may increase the risk of hospital-acquired infections in sub-Saharan African settings.
Keywords: gram-negative bacilli, hand hygiene, healthcare-associated infections, healthcare personnel, Niger
Introduction
Healthcare-associated infections (HAIs), or infections contracted while receiving medical treatment in a healthcare facility, pose a major, yet often preventable risk to patient safety1,2. HAIs can contribute to an increased risk of death,3 length of hospital stay4,5 and financial costs to health systems.6–8 Proper hand hygiene practices by healthcare personnel can effectively reduce the acquisition of HAIs by interrupting the transmission of dangerous microflora between patients.9,10 However, low hand hygiene compliance among healthcare personnel remains a problem in hospital environments, especially in low-income country (LIC) settings, where many barriers can contribute to poor hand hygiene practice.11
In an effort to improve hand hygiene practice by healthcare personnel, the WHO released Guidelines on Hand Hygiene in Health Care in 2009 with the aim to improve hand hygiene practices and reduce transmission of pathogenic microorganisms to patients.12 These guidelines defined clear procedures for proper handwashing with soap and water and the use of alcohol-based hand rub (ABHR) solutions. In addition, the guidelines introduced the ‘five moments for hand hygiene’ to define precise moments for hand hygiene based on an evidence-based model of the transmission of microorganisms by healthcare personnel hands.
Given the importance of hand hygiene to reduce HAIs, we conducted a mixed-methods study to describe hand hygiene compliance by healthcare personnel and to describe potential variation in hand hygiene practice between seasons. As the associated risks for children due to poor hand hygiene can be compounded by poor sanitation and environmental contamination by microflora within healthcare facilities, we also measured the risk of contamination by microflora within the hospital environment and explored the relationship between prevalence of microflora and corresponding hand hygiene activity by healthcare personnel.
Methods
Study design
This was a concurrent mixed-methods study applying three research methods to describe hand hygiene practices in a sub-Saharan African hospital setting.13 First, hand hygiene observations of healthcare personnel quantified the frequency of hygiene actions and variation in hand hygiene practice during changes in seasonal caseload. Second, qualitative individual interviews with healthcare personnel assessed their knowledge of, attitudes towards and barriers to hygiene behaviours. Third, environmental samples assessed bacterial contamination on surfaces throughout the hospital. Seasonal workload variability was defined as ‘peak’ from August to November and ‘non-peak’ from December to July. Hygiene resource availability was assessed using a cross-sectional audit conducted during the peak and non-peak caseload seasons.
Study setting
The study was carried out in the inpatient therapeutic feeding centre of Madarounfa Health District of the Maradi region of south-central Niger. In collaboration with the Ministry of Health of Niger, Médecins Sans Frontières (MSF) has provided paediatric care in the Madarounfa Health District since 2001. In 2016, over 4800 children were treated for complicated severe acute malnutrition (SAM) in the MSF inpatient therapeutic feeding centre. The management of complicated SAM in this setting generally observes an important seasonal increase in caseload from August to November (peak caseload season) due to household food shortages before the annual harvest and increases in infectious illnesses during the rainy season.
Hand hygiene observations
Hand hygiene observations were conducted according to the procedures for direct observation or audit described in the WHO Hand Hygiene Technical Reference Manual12 in December 2016 (non-peak caseload season) and September 2017 (peak caseload season).
Two study nurses independent from the medical team were trained and supervised by a study doctor, who established the location plan and timetable of the observations. They observed healthcare personnel during their usual care activities and, using standardised forms, recorded 200 hand hygiene opportunities, defined as a moment when a hand hygiene action is necessary (whether the indication that motivates this action is single or multiple). Corresponding actions were assessed according to the type of action (categorised as ‘handrubbing with ABHR solution or handwashing with water and soap’, ‘gloves only’ implying wearing gloves without hand hygiene action or ‘no action’) and the thoroughness of the action (categorised as ‘correct’ or ‘incorrect’ according to the step-by-step procedures described in the WHO guidelines12). Actions were further characterised by personnel type (e.g. doctor, nurse, nutrition assistant, hygienist, lab technician), corresponding moment(s) according to WHO’s five ‘moments’ for hand hygiene12 (e.g. before touching patient, before clean/aseptic procedure, after bodily fluid exposure risk, after touching patient, after touching a patient’s surroundings) and the type of care provided (e.g. clinical exam/routine monitoring, IV placement/blood draws, therapeutic measures, food/medication preparation, cleaning/adjusting equipment).
Qualitative individual interviews
Semistructured interviews with a total of 57 healthcare personnel were conducted from October to November 2017 during the peak caseload season. Prior to implementation, a guideline composed of open-ended questions on knowledge, attitudes and barriers to hand hygiene was developed. The guideline was tested during a 4 d pilot period before data collection, when questions and transcripts were assessed for data quality, monitored for relevance to predefined themes and, if necessary, reformulated if misunderstood. Interviews with all participants were conducted face-to-face in the local language and audio-recorded.
Environmental sampling
Environmental samples were collected on a randomly selected day once every 2 wk for 15 mo. In the triage and admission area, samples were collected on height boards and weight scales. In the intensive care and nutrition units, samples were collected from three randomly selected child-occupied beds. Hospital carts, oxygen concentrators and water faucets were sampled if present. Additionally, caretakers of the children from the three randomly selected beds in all three units had their dominant hands sampled. All surfaces were systematically sampled with sterile swabs moistened with saline. The surfaces of the beds, weight scales and height boards were sampled in the centre of a 10x10 cm square. Faucets were swabbed on the tap and spouts. The dominant hand of caretakers was swabbed on the palm of the hand, fingertips and between the fingers. The swabs were processed at the laboratory the same day where they were inoculated on McConkey culture media, which is selective for gram-negative bacilli, and incubated for 24 to 48 h at 35°C. Predominant gram-negative bacilli were identified by conventional biochemical tests.
Data analysis
Hand hygiene compliance was defined as the proportion of observed hand hygiene opportunities that resulted in ‘handrubbing with ABHR solution’ or ‘handwashing with water and soap’.12 Hand hygiene compliance was calculated by caseload season (peak vs non-peak) and further stratified by personnel type, WHO hand hygiene moment and type of care provided.
Individual interview audio recordings were transcribed and translated verbatim from Hausa (or local languages) into French narrative text. Textual responses generated from the open-ended questions underwent a manual word-by-word inductive and deductive review to identify predefined and emergent thematic areas considering importance relative to the study aims when deeming emergent themes to be salient.14,15 Exemplar quotations were presented in text to illustrate key findings.
Environmental contamination was defined as the proportion of environmental samples positive for gram-negative bacilli, which account for a large proportion of HAIs in hospital settings.16–18 All cultures were stratified according to the hospital unit and the type of sample surface. The overall proportion of positive samples was plotted over time to explore temporal trends in environmental contamination throughout the year. Positive cultures were identified by bacterial species; species that comprised at least 2% of the total sample were reported.
All results were triangulated in a matrix to identify whether results converged (agreement), were complementary (different but complement), were contradictory (disagreement) or were not applicable.13
Ethical considerations
Ethical approval for the study was provided by the Comité Consultatif National d’Ethique in Niger and the Comité de Protection des Personnes, Ile-de-France. Written informed consent for qualitative data collection was obtained from all interview participants.
Results
Patient admissions, personnel and hygiene resources in the peak and non-peak caseload periods are shown in Table 1. The number of patients doubled from non-peak to peak season (71 vs 165). ABHR stations tripled between non-peak and peak seasons (6 vs 19).
Table 1.
Description of hospital environment in Madarounfa Hospital, Niger, December 2016 and September 2017
| Non-peak caseload December 2016 | Peak caseload September 2017 | |
|---|---|---|
| Number of patients/number of beds (n/n) | ||
| Intensive care | 5/12 | 21/22 |
| Nutrition unit | 24/27 | 105/97 |
| Paediatric ward | 26/26 | 28/26 |
| Neonatal ward | 16/12 | 11/8 |
| Hygiene resources | ||
| Soap available/handwashing stations available for caretakers (n/n) | 0/24 | 3/20 |
| Soap available/handwashing stations reserved for healthcare personnel (n/n) | 1/9 | 4/8 |
| Alcohol-based hand rub stations (n) | 6 | 19 |
| Toilets* (n) | 9 | 8 |
| Bathing areas* (n) | 9 | 7 |
*One reserved for use only by health personnel.
Hand hygiene observations
Hand hygiene compliance among all healthcare personnel was low during both seasons (Figure 1). We observed higher compliance during the peak season (37%) vs the non-peak season (11%), but less thorough hand hygiene with fewer correctly performed actions during the peak season (28/71, 39%) compared with the non-peak season (16/22, 73%).
Figure 1.
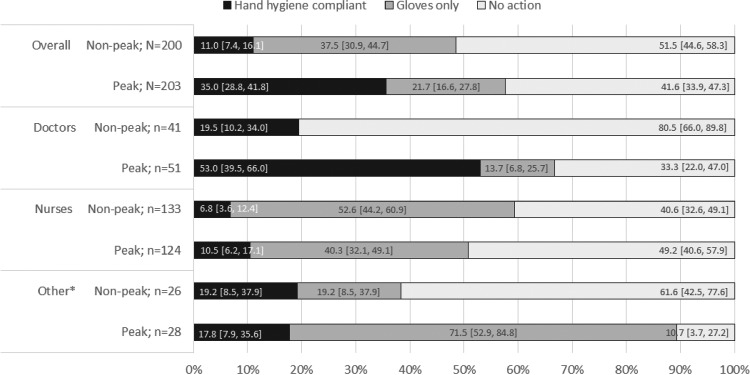
Hand hygiene compliance by type of healthcare personnel. *Includes nutrition assistants, hygienists and lab technicians.
Compliance varied according to the WHO hand hygiene moments (Figure 2a). In both seasons, compliance was lowest before touching a patient and before aseptic procedure than after bodily fluid exposure risk, after touching a patient and touching a patient’s surroundings. However, moments with lower hand hygiene compliance appeared to observe higher glove usage in place of hand hygiene actions. Compliance by healthcare activity is shown in Figure 2b. During both seasons, the highest percentage of hand hygiene opportunities with ‘no action’ were food/medication preparation (71% non-peak, 68% peak) and cleaning/adjusting equipment (50% non-peak, 42% peak). Gloves used as a substitute for hand hygiene were frequent across all activities in both seasons, particularly IV placement/blood draws (53% non-peak, 46% peak) and therapeutic measures (65% non-peak, 30% peak).
Figure 2.
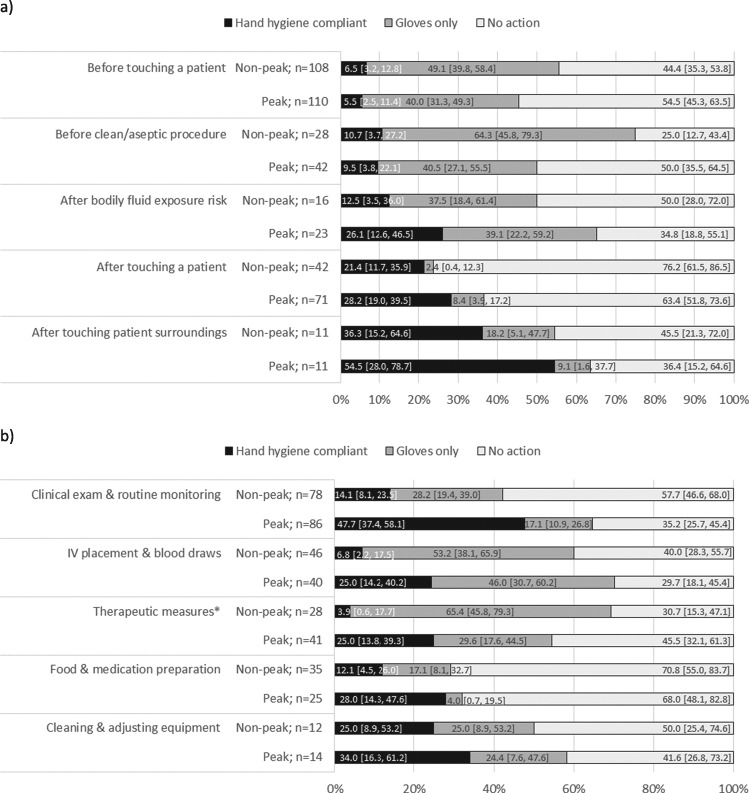
Hand hygiene compliance according to (a) WHO hand hygiene moment and (b) type of healthcare activity. Several moments and activities may exist per opportunity. *Includes injections, transfusions and placement of nasogastric or Foley tube.
Qualitative individual individuals
Personnel were well informed that hand hygiene was important to prevent the transmission of infection, understood what constituted sufficient hand sanitation12 and generally at what points hand hygiene was necessary.12
To prevent infection, good hygiene is critical. For hospital personnel and caregivers, we must wash our hands, which I think helps control infection. This is because you can contract a germ from one place and transmit it to someone else. When you touch a child, you must sterilize your hands before you touch another child. (Doctor 2)
This knowledge, however, was coupled with a lack of precision in specific steps to promote good practice and a number of inaccuracies and misconceptions about hand hygiene. Replacing sanitation actions with gloves was commonly described as acceptable. Non-medical activities resulting in patient contact were often omitted as a critical point requiring hand hygiene:
Handwashing must be done after any contact with biological fluid or medical equipment; it should normally be done between patient examinations, after removing gloves and even before putting gloves on. There are several techniques for handwashing … (Nurse 10)
Attitudes towards hand hygiene by health personnel were found to be motivated by personal welfare, where good hygiene was necessary to prevent the personal acquisition of infection. Hand hygiene as a mechanism to reduce infection in patients was infrequently mentioned as a motivating factor.
At the hospital, I have to be careful to protect myself because there are a lot of risks. You know that we are always surrounded by children who have many different diseases. So if we touch a child, each time we must wash our hands with chlorine. (Nutrition Assistant 4)
Furthermore, health personnel expressed that motivation to comply with correct hand hygiene practice was dependent on the context of the patient’s condition, type of care and the total patient caseload. These conditions were subject to individual interpretation.
It is case by case, some cases you can wash your hands, but in other cases we must use ABHR solution. The ABHR solution is used to sterilise our hands and after we take off our gloves we also use the ABHR solution. (Doctor 3)
A major barrier to hand hygiene compliance was highly demanding work contexts, including high caseloads, emergency situations and caring for patients with complicated conditions. Personnel understood hand hygiene remained important during these moments, but often omitted correct practice given the urgency of the situation.
There are always risks [for infection], especially with new admissions often in emergencies. When patients are brought to us with complicated conditions, we use gloves in bulk. However, in urgent situations, sometimes we provide care [without gloves or washing] and wash our hands after, where we have already spread infection without knowing. (Doctor 1)
Environmental sampling
Gram-negative bacilli were identified in nearly half of environmental samples (282/622, 45%) and caretakers’ hands (157/335, 47%) (Table 2). Contamination of both environmental surfaces and caretakers’ hands was highest in November 2016 (Figure 3), the period that corresponds to the end of the peak caseload season. Reduction in the proportion of positive samples immediately after the peak caseload season was more pronounced in environmental surfaces (Figure 3a) compared with caretakers’ hands (Figure 3b). A number of opportunistic pathogens were present (Supplementary Tables 1 and 2): Acinetobacter baumannii constituted 16% of the total surfaces bacteria and 15% of the bacteria on caretakers’ hands, while Pseudomonas spp. constituted 9% of the total surfaces bacteria as well as on caretakers’ hands. Enterobacter cloacae, a member of the normal faecal flora, was also present, constituting 4% of all bacteria identified on surfaces and 9% on caretakers’ hands.
Table 2.
Prevalence of gram-negative bacilli contamination in Madarounfa Hospital by service unit and surface type, October 2016–December 2017; n/N (%)
| Admission | Nutrition | Intensive care | |
|---|---|---|---|
| Environmental surface | |||
| Height board | 24/39 (62) | 1/1 (100) | - |
| Weight scale | 26/49 (53) | 6/7 (86) | 4/7 (57) |
| Bed | 31/107 (29) | 98/223 (44) | 44/89 (49) |
| Trolley | 0/12 (0) | 1/7 (14) | 5/11 (46) |
| Oxygen concentrator | 4/12 (33) | 2/3 (67) | 9/17 (53) |
| Sink faucet | 16/23 (70) | 8/9 (89) | 3/6 (50) |
| Total | 101/242 (42) | 116/250 (46) | 65/130 (50) |
| Caretakers’ hands | |||
| Total | - | 114/248 (46) | 43/87 (49) |
Figure 3.
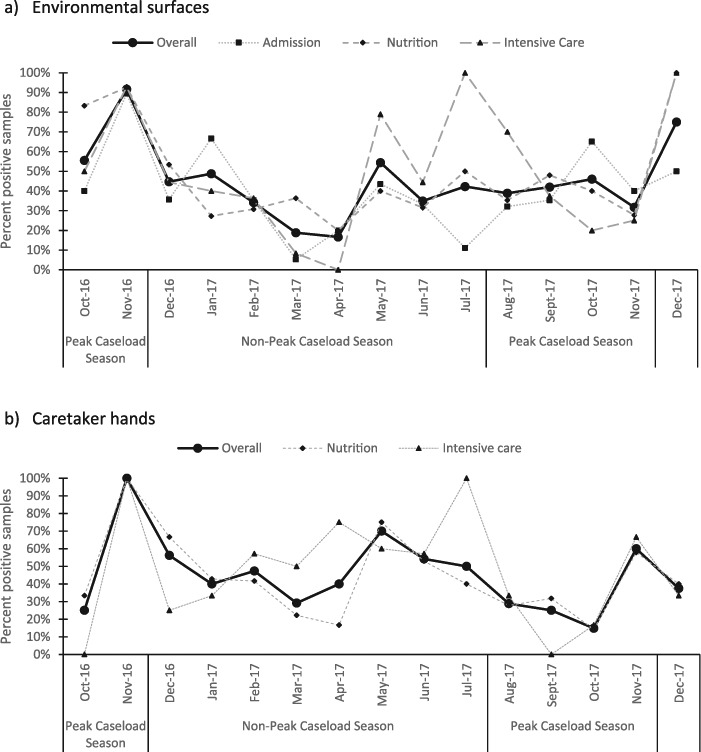
Prevalence of samples gram-negative bacilli contamination in Madarounfa Hospital on (a) environmental surfaces and (b) caretakers’ hands, October 2016–December 2017.
Triangulation
Low compliance of hand hygiene actions identified through hand hygiene observations and high contamination by gram-negative bacilli confirmed through environmental sampling were contradicted by interviews suggesting good general knowledge of the importance and practice of hand hygiene among healthcare personnel. However, low compliance for particular hand hygiene moments and activities seen in the hand hygiene observations were convergent with the lack of knowledge regarding the precise steps necessary for hand hygiene identified through interviews. Higher hand hygiene compliance after touching a patient, after touching their surroundings and during non-medical activities seen in the hand hygiene observations were convergent with a motivation to prevent the personal acquisition of infection identified during interviews. Low overall hand hygiene compliance from hand hygiene observations and high contamination on surfaces, caretakers’ hands and during the peak season from environmental sampling were complementary to personnel expressing highly demanding work contexts as a barrier to faithful hand hygiene practice from individual interviews.
Discussion
This mixed-methods study found low hand hygiene compliance by healthcare personnel in a rural hospital in Niger, with higher hand hygiene compliance in the peak caseload season compared with the non-peak season and during medical activities compared with activities requiring minimal or no contact with the patient such as food and medication preparation. Low hand hygiene compliance observed through the standardised audit was consistent with results from qualitative interviews indicating a lack of precise knowledge regarding specific steps to promote good hygiene practice. A high proportion of environmental surfaces and caretakers’ hands were contaminated with opportunistic pathogens, with the highest levels of contamination found during the peak caseload season.
Hand hygiene compliance observed in this setting was consistent with reports from other sub-Saharan African hospital settings, where compliance ranged from 9% to 34%.19–21 Although no international standards exist for hand hygiene compliance by healthcare personnel, compliance in sub-Saharan African settings has been lower than observations in high-income country contexts, where compliance has been reported to be as high as 65% to 94%.22,23 In our study, hygiene practices were observed to be low during medical care, but additional observations at high-risk transmission events such as after latrine use and before meals suggest personal practice is even lower (results not shown). Hand hygiene in this setting is especially important as our results from environmental sampling indicated a high contamination with gram-negative bacilli on surfaces (42% to 50%) in service units and caretakers’ hands (46%), particularly during the peak caseload season. This rate is substantially higher compared with the 26% to 38% found in a similar study in Iran.24 In rural Niger, poor hand hygiene compliance by healthcare personnel, high bacterial contamination and the peak caseload season may compound the risk of transmission and lead to high rates of HAIs.
We found higher hand hygiene compliance in the peak caseload season compared with the non-peak season. This unexpected finding may have been due to the increased availability of handwashing and ABHR stations. This finding suggests that access to ABHR stations may compensate for other barriers to good practice associated with increased caseloads and may result in higher compliance even during the peak contamination/caseload season. Our rural hospital experienced challenges maintaining a consistent supply of soap at handwashing stations during both observation periods; an increase in ABHR stations during the peak season may have facilitated an increase in hand hygiene compliance. This result is consistent with Munoz-Price et al., who found that greater access to ABHR dispensers increased the frequency of hand hygiene among anaesthesiologists in operating rooms in the USA.25 Whitby et al. also found that increasing access to ABHR stations in Australian hospitals significantly improved hand hygiene by healthcare personnel, provided that programmes were accompanied with behaviour modification reinforcement.26 However, in addition to poor resource availability, a number of other factors (e.g. increased yields in patient caseloads, false perceptions of hand hygiene) can also contribute to poor hand hygiene practice.
Due to the diversity of factors that may facilitate the spread of HAIs, a package of strategies incorporating more than one intervention may be necessary to increase hygiene compliance within hospital settings. The WHO has recommended a comprehensive hand hygiene improvement strategy that includes five parallel interventions to ‘change the behaviour of individual healthcare workers to optimize compliance with hand hygiene at the recommended moments and improve patient safety’.12 However, a Cochrane review conducted in high-income countries found that this proposed strategy only marginally increased hand hygiene compliance, suggesting an optimal combination of interventions has yet to be identified.27 Furthermore, the proposed WHO strategy does not address the additional risks from high bacterial contamination and fluctuations in seasonal caseloads as identified in our study. Additional research may help to identify operational strategies that can promote improved hygiene in low-resource environments.
Our data highlight a number of key programmatic modifications that may be considered in LIC settings to facilitate hand hygiene compliance. Increasing the availability of ABHR solution and/or access to a safe, continuous water supply and soap at the point of patient care may make hygiene actions more feasible. Annual training for healthcare personnel to improve their understanding of the necessary moments for hand hygiene and emphasising that gloves are not a replacement for hand hygiene may support improved knowledge of when hand hygiene is necessary and how it is performed correctly. Implementation of monthly routine monitoring and performance feedback for hand hygiene, as recommended by the WHO Guidelines,12 may also encourage healthcare personnel to adopt hand hygiene into routine behaviour.
This study had a number of strengths and limitations. We triangulated data from environmental sampling, hand hygiene observations and qualitative interviews to more fully describe contamination and drivers of poor hand hygiene within the study setting; we also considered differences in hand hygiene practice by caseload seasonality. The comparison between peak and non-peak caseload seasons provided new information on how seasonal differences in caseloads may influence hand hygiene practices by healthcare personnel and the risk of HAIs. Qualitative data collection was nevertheless limited to the peak season, preventing the exploration of seasonal variation in personnel perceptions of hand hygiene knowledge and practice. Personnel were, however, probed to discuss narratively how variation in hospital contexts affected their hand hygiene behaviour.
Conclusion
We found low hand hygiene compliance by healthcare personnel coupled with high environmental contamination rates in a rural hospital in Niger. Improved access to hand hygiene resources and regular disinfection of high-risk contamination surfaces should be prioritised to control the transmission of opportunistic bacteria throughout hospital facilities. Collectively, these improvements could have important implications for reducing the risk of HAIs and improving patient safety in sub-Saharan Africa hospital settings.
Supplementary Material
Acknowledgements
We thank all of the health centre staff, families and children who participated in this study and our field research teams.
Authors’ contributions
C. Langendorf, C. Marquer and S. Isanaka contributed to the conception and design of the study. CL, CM, F. Berthé and F. Nackers collected the data. K. Tang contributed to the statistical analysis and revision of the manuscript. All authors contributed to the interpretation of the data, critically reviewed the manuscript for important intellectual content and approved the final manuscript.
Funding
This study was funded by Médecins Sans Frontières—Operational Center Paris. The funder helped to design and plan the study, and participated in the writing of this report, but had no role in data collection and analysis.
Competing interests
There are no potential conflicts of interest relevant to this article.
Ethical approval
Ethical approval for the study was provided by the Comité Consultatif National d’Ethique in Niger and the Comité de Protection des Personnes, Ile-de-France.
References
- 1. Pittet D, Donaldson L. Clean care is safer care: a worldwide priority. Lancet. 2005;366:1246–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allegranzi B, Pittet D. Preventing infections acquired during health-care delivery. Lancet. 2008;372(9651):1719–1720. [DOI] [PubMed] [Google Scholar]
- 3. Klevens R, Edwards J, Richards C, Horan T, Pollock D, Cado D. Estimating health care-associated infections and deaths in. Public Health Rep. 2007;122(April):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1994;271(20):1598–1601. [DOI] [PubMed] [Google Scholar]
- 5. Appelgren P, Hellström I, Weitzberg E, Söderlund V, Bindslev L, Ransjö U. Risk factors for nosocomial intensive care infection: a long-term prospective analysis. Acta Anaesthesiol Scand. 2001;45(6):710–719. [DOI] [PubMed] [Google Scholar]
- 6. Zimlichman E, Henderson D, Tamir O et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173(22):2039–2046. [DOI] [PubMed] [Google Scholar]
- 7. Stone PW, Hedblom EC, Murphy DM, Miller SB, Homan L. The economic impact of infection control: making the business case for increased infection control resources. Am J Infect Control. 2005;33:542–547. [DOI] [PubMed] [Google Scholar]
- 8. Arefian H, Vogel M, Kwetkat A, Hartmann M. Economic evaluation of interventions for prevention of hospital acquired infections: A systematic review. PLoS One. 2016;11(1):e0146381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenthal VD, Guzman S, Safdar N. Reduction in nosocomial infection with improved hand hygiene in intensive care units of a tertiary care hospital in Argentina. Am J Infect Control. 2005;33(7):392–397. [DOI] [PubMed] [Google Scholar]
- 10. Pittet D, Allegranzi B, Sax H et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6:641–652. [DOI] [PubMed] [Google Scholar]
- 11. Allegranzi B, Nejad SB, Combescure C et al. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organisation (WHO) WHO guidelines on hand hygiene in health care: first global patient safety challenge clean care is safer care. World Health 2009;30(1):270. [PubMed] [Google Scholar]
- 13. Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks, CA: Sage, 2008. [Google Scholar]
- 14. Priest H, Roberts P, Woods L. An overview of three different approaches to the interpretation of qualitative data. Part 1: Theoretical issues. Nurse Res 2002;10(1):43. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 16. Gaynes R, Edwards JR. National Nosocomial Infections Surveillance System JR, system NNIS. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005;41(6):848–854. [DOI] [PubMed] [Google Scholar]
- 17. Anton Y. Peleg and DCH. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu C-J, Lee H-C, Lee N-Y et al. Predominance of gram-negative bacilli and increasing antimicrobial resistance in nosocomial bloodstream infections at a university hospital in southern Taiwan, 1996-2003. J Microbiol Immunol Infect 2006;39(2):135–143. [PubMed] [Google Scholar]
- 19. Saito H, Inoue K, Ditai J et al. Alcohol-based hand rub and incidence of healthcare associated infections in a rural regional referral and teaching hospital in Uganda (“WardGel” study). Antimicrob Resist Infect Control. 2017;6(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdella NM, Tefera MA, Eredie AE, Landers TF, Malefia YD, Alene KA. Hand hygiene compliance and associated factors among health care providers in Gondar University hospital, Gondar, north West Ethiopia. BMC Public Health. 2014;14(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holmen IC, Seneza C, Nyiranzayisaba B, Nyiringabo V, Bienfait M, Safdar N. Improving hand hygiene practices in a rural Hospital in sub-Saharan Africa. Infect Control Hosp Epidemiol. 2016;37(7):834–839. [DOI] [PubMed] [Google Scholar]
- 22. Linam WM, Margolis PA, Atherton H, Connelly BL. Quality-improvement initiative sustains improvement in Pediatric health care worker hand hygiene. Pediatrics 2011;128(3):e689–698. [DOI] [PubMed] [Google Scholar]
- 23. Price L, Roome K, Lisa R et al. Toward improving the World Health Organization fifth moment for hand hygiene in the prevention of cross-infection. Am J Infect Control. 2016;44(6):631–635. [DOI] [PubMed] [Google Scholar]
- 24. Ayatollahi AA, Amini A, Rahimi S, Takrami SR, Darsanaki RK, Nezhad MS. Prevalence of gram-negative bacilli isolated from the equipment and surfaces in hospital wards of Golestan Province, north of Iran. Eur J Microbiol Immunol 2017;7(4):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munoz-Price LS, Patel Z, Banks S et al. Randomized crossover study evaluating the effect of a hand sanitizer dispenser on the frequency of hand hygiene among anesthesiology staff in the operating room. Infect Control Hosp Epidemiol 2014;35(6):717–720. [DOI] [PubMed] [Google Scholar]
- 26. Whitby M, McLaws ML, Slater K, Tong E, Johnson B. Three successful interventions in health care workers that improve compliance with hand hygiene: Is sustained replication possible? Am J Infect Control. 2008;36(5):349–355. [DOI] [PubMed] [Google Scholar]
- 27. Moralejo D, El Dib R, Prata RA, Barretti P, Corrêa I. Improving adherence to standard precautions for the control of health care-associated infections. Cochrane Database Syst Rev [Internet]. John Wiley & Sons, Ltd; 2018. Available from: 10.1002/14651858.CD010768.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


