Abstract
Silica nanomaterials (SNMs) and their composites have recently been investigated as scaffolds for bone tissue engineering. SNM scaffolds possess the ability to encourage bone cell growth and also allow the simultaneous delivery of biologically active biomolecules that are encapsulated in the mesopores. Their high mechanical strength, low cytotoxicity, ability to stimulate both the proliferation and osteogenic differentiation of progenitor cells make the SNMs appropriate scaffolds. Their physiochemical properties facilitate the cell spreading process, allow easy access to nutrients and help the cell-cell communication process during bone tissue engineering. The ability to deliver small biomolecules, such as dexamethasone, different growth factors, vitamins and mineral ions depends on the morphology, porosity, and crystallinity of SNMs and their composites with other polymeric materials. In this review, the abilities of SNMs to perform as suitable scaffolds for bone tissue engineering are comprehensively discussed.
Keywords: Mesoporous silica, Nanoparticles, Scaffolds, Bone tissue engineering, Biomolecule delivery
Graphical Abstract:

Application of mesoporous silica-based nanomaterials as scaffold in bone tissue engineering
1. Introduction
The capability of bone cells to undergo cell spreading, proliferation, migration, adhesion and differentiation is necessary for the regeneration of bone tissue [1]. It has been found that the choice of the scaffold material and its surface properties is critical for bone tissue engineering. Scaffolds can influence the cell division process [2, 3], cell-cell communication pathways [4], efficient delivery of nutrients [5] etc. Recently, nanotechnology has played a major role in designing new scaffolds, such as carbon-based nanomaterials [6, 7], metal nanoparticles [8], hydrogels [9, 10], hyaluronan [11], natural and synthetic polymers [12–21], and hybrid nanomaterials [22]. Silica nanomaterials (SNMs) have broad application in biomedical science [23, 24], drug delivery [25, 26], biosensors for detection of pathogens and viruses [27], aflatoxin in food products [28], cancer biomarkers [29], nanoflares for intracellular investigation [30]. Recently SNMs have demonstrated beneficial properties for bone tissue engineering because of their low cytotoxicity [31, 32], high porosity [33], high mechanical strength [34, 35], cost-effectiveness [36], and biocompatibility [37]. Crystallinity is a critical feature of SNMs that plays a key role in their biological response and activity. Many reports have indicated a direct relationship between low crystallinity of SNMs and their biological activity and biodegradability [38]. The main reason for this phenomenon at a molecular level is related to the number of siloxane rings in the structure of SNMs. Much evidence shows the harmful effect of siloxane rings on the biological activity of SNMs [39]. The main synthetic method of SNMs involves the treatment of silica at a high temperature, which leads to the formation of siloxane rings. SNMs that are prepared using low temperature methods are more appropriate than quartz nanoparticles that have a highly crystalline structure. Mesoporous SNMs show the best biodegradability and biocompatibility compared to other types of SMNs because of their low crystallinity [40]. The biocompatibility and biodegradability of SMNs is very important when they are used for bone repair and the engineering of bone tissue both in vivo and in vitro conditions [41]. Mesoporous SNMs possessing a uniform size and high porosity, high surface area and appropriate pore sizes, are ideal for growth and proliferation of bone cells [42–45]. There are many synthetic methods with simple and controllable approaches to tune the pore size of SNMs, some of which are summarized in Table 1.
Table 1.
Methods for synthesis of SNMs for obtaining uniformity of size and shape.
| Method | Precursor | Morphology | Pore diameter | Tempera ture (°C) | Advantage | Ref. |
|---|---|---|---|---|---|---|
| Single micelle-templating | 1,2-bis(trimethoxysilyl)ethane | Grapelike-extending micro-windows in a spherical structure | 0.5–1.2 nm | 100 | Uniform particle size | [49] |
| Tetraethyl orthosilicate (TEOS), Octadecyltrimethoxysilane (C18TMS) | Rattle-type SiO2/Fe2O3 nanospheres with core-shell | 1.8–25 nm | 550 | Facile synthetic method with tailored silica shell porosity | [50] | |
| Sodium silicate | Nanochannels in spherical structure | 5.5–7.5 nm | 560 | Different nanochannel directions with high capacity for drug loading | [51] | |
| Vesicle-templating | 3-aminopropyl-triethoxysilane (APES), TEOS | Spherical structure | 80–220 nm | 550 | High porosity and capacity of drug loading | [52] |
| TEOS | AuNPs@SiO2 yolk-shell spheres | 400–540 nm | 550 | Appropriate delivery of ibuprofen with potential applications in nanoreactors | [53] | |
| Microemuls ion | TEOS | Collapsed “kippah-like” | 1–6 nm | - | Ability to use as nanoreactors and scaffolds with drug delivery capabilities | [54] |
| Polymer beads-templating | TEOS, Polystyrene | Hollow spherical particles | 3 nm | 600 | Excellent particle uniformity for drug delivery-based scaffolds. | [55] |
The fabrication of SNMs with one-, two-, and three-dimensional structures is of great interest for the fundamental understanding of the roles of dimensionality and size in governing mechanical properties of the SNMs, in applications to tissue engineering. Hollow silica-nanotubes is another type of SNMs, which is attracting a great deal of attention in both fundamental and industrial studies. Silica nanotubes have novel properties, and could be used to confine molecules in their inner and outer spaces. They also have potential applications in fields such as electronics, optics, drug delivery, advanced catalysis, and energy storage/conversion, and could also be designed to mimic biological channels[46]. Recently, there has been a growing interest in silica nanofibers as well. These one-dimensional nanomaterials exhibit some novel physical and chemical properties due to their peculiar structure and size effects, and are of great importance in nano-devices and mesoscopic theoretical research. It has been known that silica nanofibers can be obtained by conventional techniques using the sol-gel process, and are affected by the composition of the sol and the ripening conditions. Silica nanofibers have also been obtained by the electrospinning technique and the sol-gel method. It could be possible to use a mixture of silica nanofibers with other different fibrous matrices for bone cell proliferation stimulation [47, 48]. From a structural aspect, it seems that silica nanotubes could be appropriate vehicles for drug delivery to bone cells, whereas silica nanofibers could be mixed with fibrous and polymeric matrices to improve the physicomechanical properties of the scaffolds.
The porosity of SNMs affects the degree of mineralization, access to nutrients and growth factors, and spreading or infiltration of bone cells [56]. The osteogenic regeneration process of bone cells growing on scaffolds to make new bone, is encouraged by the physicochemical properties of SNMs [57, 58]. The ability for controlled release of signaling molecules that have been loaded into SNMs also helps cell proliferation. Loading and release is governed by the physical structure and chemical interactions between the drug and the silica. Easy functionalization of the nanoparticle surface combined with good uniformity in shape and size, make it possible to use these nanomaterials in many experimental and clinical protocols [59, 60]. The release of growth factors such as bone morphogenetic protein-2 (rhBMP-2) has remarkable effects on the bone regeneration process. Controlled release of rhBMP-2 can be achieved by loading it into the mesoporous SNMs which are used as scaffolds. With conventional scaffolds, the uncontrolled release of this growth factor reduced the proliferation of bone cells. The size of the SNM mesopores can range from 5 nm to 30 nm in diameter [61–63]. The extended-time controlled release of fibroblast growth factor-18 (FGF-18) is another example of the use of SNMs scaffolds. Delivery of FGF-18 growth from SNMs scaffolds stimulated the proliferation process of bone cells more than the alkaline phosphatase activation and mineralization process.
In this review article, the important role of mesoporous SNMs for bone tissue engineering applications is critically discussed from two main aspects. Firstly, their capability for rapid and safe delivery of necessary growth factors and medicinal agents to bone cells is comprehensively discussed. Secondly, the use of silica scaffolds composed of SNMs (individual, functionalized, and composite forms) for growth and proliferation of bone cells in tissue engineering to replace missing or damaged bones.
2. Drug-delivery using silica nanomaterials in bone tissue engineering
2.1. Drug delivery
Dexamethasone is a corticosteroid that can play an effective stimulant role in bone tissue formation, and can be delivered in a controlled manner using SNMs as scaffolds. The application of dexamethasone combined with FGF-18 in a scaffold could be an improved method of encouraging new bone formation [64]. Delivery of anticancer drugs is another application of SNM-based scaffolds that could be used in the therapy of osteosarcoma. SNMs with uniform pore size allowed controlled release [65, 66].
Pinacidil is a cyanoguanidine drug that opens ATP-sensitive potassium channels, and has been used to enhance cell adhesion on scaffold networks. This drug enhances the viability of human embryonic stem cells (hESC), and can directly influence cell adhesion. Wang et al. [67] reported the preparation of modified silica nanoparticles (SNPs) in the presence of acrylic acid and oxygen gas using a plasma glow discharge method, that could be used as a filler for scaffolds. Carboxylic acid functional groups were introduced by a two-stage process involving the initial introduction of 3-aminopropyltriethoxysilane followed by a second step in which succinic acid was attached to the amino groups using (3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and and 1-hydroxybenzotriazole monohydrate. The carboxylic acid-modified spherical mesoporous SNMs were decorated by gelatin gels attached by hydrogen bonds to the COOH groups for controlled drug release. This process is shown in Figure 1
Figure 1.
(Top) The synthesis procedure of carboxylic acid functionalized mesoporous SNMs. (a) The synthesis of these materials in three steps, fabrication of mesoporous SNMs, modification of their surface with amino functional groups using 3-aminopropyltriethoxysilane (APTES), and finally addition of carboxylic acid groups using (3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCl), 1-hydroxybenzotriazole monohydrate (HOBT) and succinic acid. (b, c) and (e, f) Transmission electron microscopy (TEM) of unmodified and modified SNMs at different resolutions, respectively. (d) and (g) Scanning electron microscopy (SEM) images of unmodified and modified SNMs. (h) and (i) Average sizes of unmodified and modified SNMs and their particle size distribution. (j) Zeta potential of unmodified and modified SNMs. (k) Infrared spectroscopy of unmodified and modified SNMs. (l) Thermogravimetry of unmodified and modified SNMs. (Bottom-left) Confocal laser microscopy images of cells on surface rhodamine phalloidin fluorescence. The adhesion of bone stem cells without pinacidil drug (a) and with drug (b). (Bottom-right) The interaction between gelatin functional groups and modified SNM surface functional groups. Figures reproduced with permission from ref [67]. Copyright 2018 Wiley.
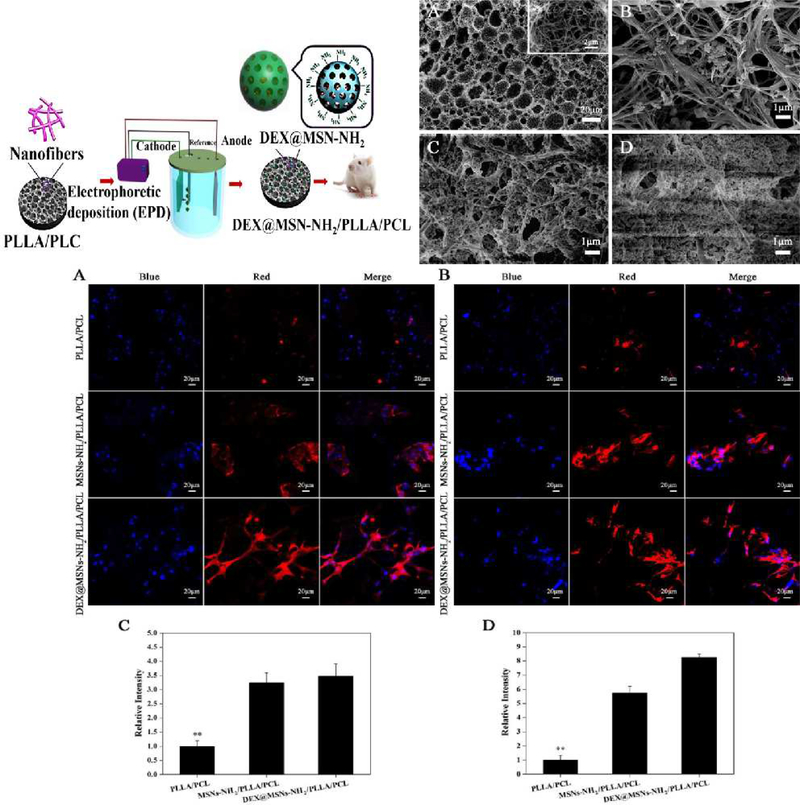
Qiu and co-workers [68] developed a composite material formed from poly(L-lactic acid)/poly(ε–caprolactone) (PLLA/PCL) and mesoporous SNPs to form scaffolds that could deliver dexamethasone. The nanofibrous morphology of these scaffolds was obtained using an electrophoretic method (Figure 2). The controlled release of dexamethasone allowed good osteoblastic differentiation of the cells as shown by an increase in alkaline phosphatase activity and increased osteocalcin expression, which allowed the rapid remineralization of defective bone tissue. The SNP surface was functionalized with amino groups for loading of dexamethasone into the scaffold. The Brunauer–Emmett–Teller (BET) test for the SNPs showed a high surface area and suitable pore diameters (972.58 m2/g and 2.97 nm). The porous interconnected network morphology of their scaffolds was controlled by regulation of the voltage in the electrophoretic synthesis method (Figure 2). The amount of dexamethasone drug loading was 0.078 μg/mg. 12% of the initial concentration of dexamethasone was released in first hour in both in vivo and in vitro tests. 40% of the initial concentration was released in the first day, while the remaining drug was released constantly for four weeks. Enzyme-biodegradability tests using proteinase K showed that 46.2% of the initial weight was degraded in 16 days.
Figure 2.
(Top-left) The electrophoretic method for fabrication of MSNs-NH2/PLLA/PCL scaffold for delivery of dexamethasone. (Top-right) SEM images showing the effect of varying the voltage in the electrophoretic method on the porosity of MSNs-NH2/PLLA/PCL scaffolds. (A) SEM image of microstructure of scaffold; (B) fabrication process of the scaffold at 1 Volt, (C) 3 Volts and (D) 5 Volts. (Bottom) Osteocalcin expression analysis for the modified and unmodified mesoporous SNMs showing the effect of SNMs on the differentiation process of bone stem cells after (A, C) 14 days and (B, D) 21 days. Figure reproduced with permission from ref [68]. Copyright 2016 American Chemical Society.
2.2. Growth factor-delivery
Because of the prominent role of different growth factors to induce osteogenic programming and differentiation, these biomolecules have attracted much attention in bone tissue engineering. Growth factors trigger signal transduction via causing type I or type II phosphorylation of the receptors. BMP-2 acts as a potent osteogenic transcription factor in osteoblastic cells [69–74]. However one of the most important side effects of BMP-2, is that an excessive amount of this protein can delay the fusion process of defective or damaged bone tissue. Another side effect of the uncontrolled presence of BMP-2 growth factor is the formation of ectopic bone tissue due to unwanted leakage from loaded implants. Osteolysis and suppression of bone formation can occur as result of too high a rate BMP-2 release. The formation of low-quality bone if BMP-2 is present in excessive amounts remains an unsolved challenge. Another side effect can be local inflammation that produces a seroma or urogenital complications [75–82]. Therefore, the development of the scaffolds or materials such as SNMs with the ability to release BMP-2 in a controlled manner is important in bone tissue engineering.
Neumann et al. [83] developed a method using amino-functionalized nanoporous SNPs as a biocompatible and biodegradable material for the immobilization of BMP-2 in bone tissue. They used these materials to encourage osteoblastic differentiation of adipose-derived human mesenchymal stem cells (adMSC). Their results showed that the diameter of the spherical SNPs was between 30 nm and 50 nm, and the high specific surface area was 1345 m2/g. They achieved immobilization of BMP-2 on the functionalized SNPs via electrostatic, hydrogen bonding and hydrophobic interactions between the nanoparticles and the BMP-2 protein structure. The type of stem cells can be an important factor to ensure success in this approach. NIH3T3 and adMSC cells showed suitable viability, while the HepG2 cells did not.
Cui et al. simulated the BMP-2 growth factor by designing the P28 peptide molecule (P28: S[PO4]DDDDDDDKIPKASSVPTELSAISTLYL) [84] that had a structure similar to BMP-2, and investigated its osteogenic differentiation effects on bone tissue. They used hollow mesoporous SNPs for controlled release of P28 during bone tissue growth on “true bone ceramic” (TBC). IN addition to in vitro they tested the scaffold for the treatment of defective rabbit bone in vivo. After 6 and 12 weeks good results were obtained. The fabrication process of the scaffold is shown in Figure 3 [85].
Figure 3.
(Top) The fabrication of TBC/hollow-mesoSNPs/P28 porous scaffolds. (Bottom) Imaging of MC3T3-E1 stem cells after 6 weeks and 12 weeks with and without hollow meso SNPs using Masson’s trichrome and HE staining technique. Figure reproduced with permission from ref [85]. Copyright 2017 American Chemical Society.
2.3. Simultaneous delivery of drugs & growth factors
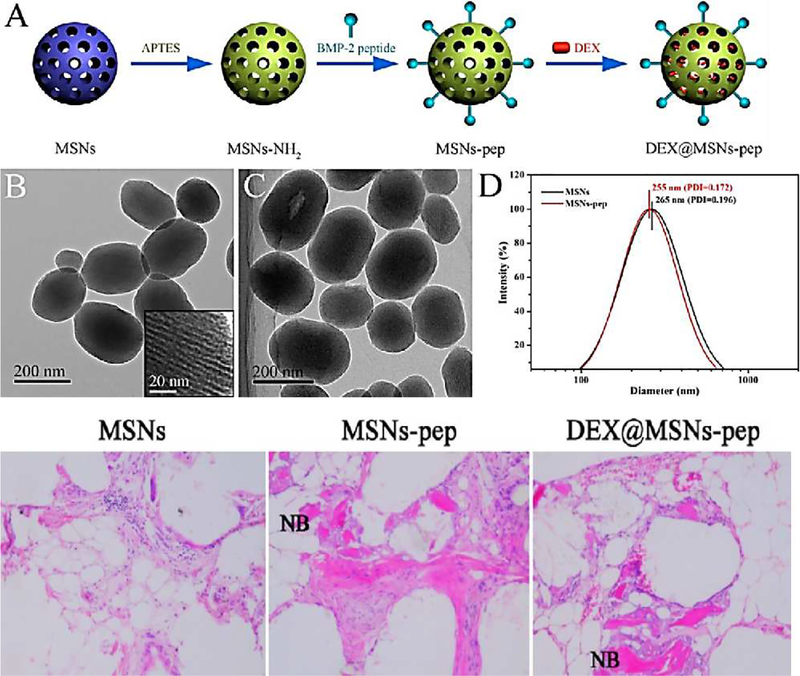
The simultaneous delivery of dual combinations of both drugs and growth factors has shown the best results in bone tissue engineering. The simultaneous release of both dexamethasone and BMP-2 requires a special design for scaffold materials. Zhou et al. developed a novel generation of scaffolds with the ability for controlled release of dexamethasone and BMP-2. They prepared aminated mesoporous SNPs with amino groups that could interact with the BMP-2, while the mesopores of the SNPs could be loaded with dexamethasone. Using this strategy, the controlled simultaneous delivery of a drug and a growth factor was possible. The method of synthesis of this material is shown in Figure 4 [86].
Figure 4.
(Top) (A) Schematic illustration of synthetic method for aminated mesoporous SNPs; (B, C) TEM images of mesoporous SNPs and BMP-2 loaded nanoparticles; (D) the size distribution of SNPs before (black) and after (red) the modification process. (Bottom) The progress of new bone (NB) formation after 3 weeks for unmodified (left), after loading BMP-2 growth factor (middle), and after simultaneous loading of both dexamethasone and BMP-2 in mesoporous SNPs (right). Figure reproduced with permission from ref [86]. Copyright 2015 American Chemical Society.
Yao et al. fabricated gelatin nanofibrous mesoporous silicate-based scaffolds for dual delivery of BMP-2 and deferoxamine. Deferoxamine can activate hypoxia-inducible factor-1 alpha (HIF-1α) that can trigger angiogenic signaling and help bone regeneration [87, 88]. Deferoxamine molecules showed a cooperative influence on the activity of BMP-2 growth factor in bone cell growth [89].
2.4. Delivery of small molecules and nucleic acids
Kim et al. designed a scaffold system with the ability for siRNA gene delivery into bone osteoblastic stem cells using mesoporous silica nanoshells (SNSs). Silencing of the Pelkho-1 gene led to up-regulation of the expression of osteoblastic transcription factors (Runx2 and Smad2) as shown in Figure 5 [90].
Figure 5.
Schematic of (a) the fabrication process of SNSs; (b) the efficient uptake of siRNA loaded SNSs into cells; (c) controlled release of siRNA and Plekho-1 gene silencing; (d) up-regulation of osteoblastic transcription factors. Figure reproduced with permission from ref [90]. Copyright 2016 Wiley.
Controlled delivery of small molecules can be useful for different reasons such as encouraging the differentiation of cells, or fighting infections at tissue-scaffold junctions. Vitamin D3 has significant effects on alkaline phosphatase activity and calcium deposition, and therefore plays a key role in bone cell growth. However due to the lipophilic nature of vitamin D3, conventional scaffolds show difficulty in controlling the rate of release. Sumathra et al. reported novel scaffolds for the loading and delivery of vitamin D3 using cellulose/hydroxyapatite/mesoporous silica nanoparticles (C/HAp/mesoporous SNPs). The proliferation and adhesion of bone stem cells (MG63), and the osteoinductive activation of alkaline phosphatase were stimulated, leading them to propose that the vitamin D3-loaded scaffolds could be used for treatment of bone defects [91].
2.5. Antibacterial agent-delivery
Bacterial infection is a common complication in bone repair procedures [92, 93]. Generally, skin pathogens such as Gram-positive (Staphylococcus aureus, S, epidermis and Propionibacterium acnes) and Gram-negative (Pseudomonas aeruginosa) bacteria can cause osteomyelitis. Antibiotic-based therapies are frequently ineffective because of the accumulation of biofilms. Typically, these biofilms are composed of lipopolysaccharides, lipids, various proteins and DNA molecules in which the bacteria are embedded [94–97]. Among non-antibiotic anti-infective agents, silver ions (Ag+) have gained prominence because of their high efficiency and unique mechanism for killing the bacteria. Silver ions can interfere with the DNA and membranes of the bacterial cells and subsequently kill them [98, 99]. The construction of Ag+ and silver nanoparticle-based scaffolds is relatively straight-forward, and they have been applied in many studies. Scaffolds combining collagen with silver-loaded SNPs have been used to control the delivery of silver ions during bone tissue engineering both in vivo and in vitro [98, 100].
Ma et al. [101] described platelet-derived growth factor BB (PDGF-BB)/Ag encapsulated SNPs (P-AGMesoporous SNPs) as an anti-bacterial scaffold for bone tissue engineering. The scaffold allowed growth of bone marrow stromal cells (BMSC), with osteoinductive regeneration, antibacterial and provascularization properties. The constant release of Ag+ ions from the scaffold after 14 days provided a long-lasting anti-infective capability. The expression levels of vascular endothelial growth factor (VEGF), HIF-1α, HGF and ANG-1 were up-regulated. The anti-infective effects of this scaffold against Escherichia coli, Pseudomonas aeruginosa and Candida sporogenes microbial cells were shown. The scaffold showed good antibacterial properties and after 24 h all of the bacteria were destroyed.
In Table 2 the critical effective features of SNMs for drug-delivery in bone tissue engineering are summarized, and the advantage of each morphology, size and other physiochemical properties of the scaffolds are discussed.
Table 2.
Summary of the application of SNM-scaffolds in drug delivery in bone tissue engineering.
| Materials | Delivered agent (drug, growth factor etc.) | Average size of SNPs | Experimental trial | Advantage | Cell type | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Spherical SNP | Ag+ | 126 nm | In vitro | Preserves viability, Low toxicity, Biocompatibility | Human bone marrow-mesenchymal stem cells (hBM-MSCs) and human adipose-derived stem cells (hASCs) | [102] |
| 2 | Mesoporous SNP | Calcium, phosphorus and silicon ions | 50–100 nm | In vivo | Osteoinductivity, Biodegradability, Constant rate of release | hBM-MSCs | [103] |
| 3 | poly(-caprolactone)/SNP (PCL/SNPs) composite (MCM-41 and SBA-15) | Dexamethasone | - | In vitro | More efficient, Tunable loading yield for drug, Adjustable release kinetics | - | [104] |
| 4 | Porous mesoporous bioactive glass | Dexamethasone, boron ions, Li+, Sr+, Cu+, bone morphogenetic protein, Co2+, SiO44−, Ca2+ | 300 – 500 μ,m | in vitro and in vivo | Efficient improvement in proliferation and bone-related gene expression (Col I and Runx2), enhanced alkaline phosphatase release, synergistic effect of therapeutic ions and growth factors, effects of Co2+ ions on HIF-1a expression and bone related gene expression of human bone marrow stromal cells (BMSCs) and proliferation, differentiation, vascular endothelial growth factor (VEGF) secretion, excellent anti-bacterial properties. | hBM-MSCs | [105] |
2.6. Anti-cancer agent delivery
In the case of cancer treatment, SNMs have also been widely used as drug release platforms in bone regeneration strategies. On one hand, they can elicit a multifunctional action (inherent bioactivity and local therapeutic action of the drug). On the other hand, anti-cancer medications can be incorporated into the pores of the SNMs and released to the target tissue. The internalization of SNMs could also be enhanced through surface-functionalization with various types of organic and bioorganic ligands. Additionally, SNMs have shown the ability to bind and penetrate into the targeted cancer cells, and are well tolerated, as demonstrated by serological, hematological, and histopathological examinations of blood samples and mouse tissues. Recent reports have shown that SNMs preferentially accumulate in experimental tumors. Finally, the drug delivery capability of MSNs can be demonstrated by monitoring the tumor growth in mice treated with drug‐loaded SNMs. These results suggest that SNMs are biocompatible, preferentially accumulate in tumors, and effectively deliver anti-cancer drugs to tumors and suppress tumor growth[106].
3. Silica-containing substrates and matrices, as scaffolds for bone tissue engineering
3.1. Polymeric and fibrous matrices
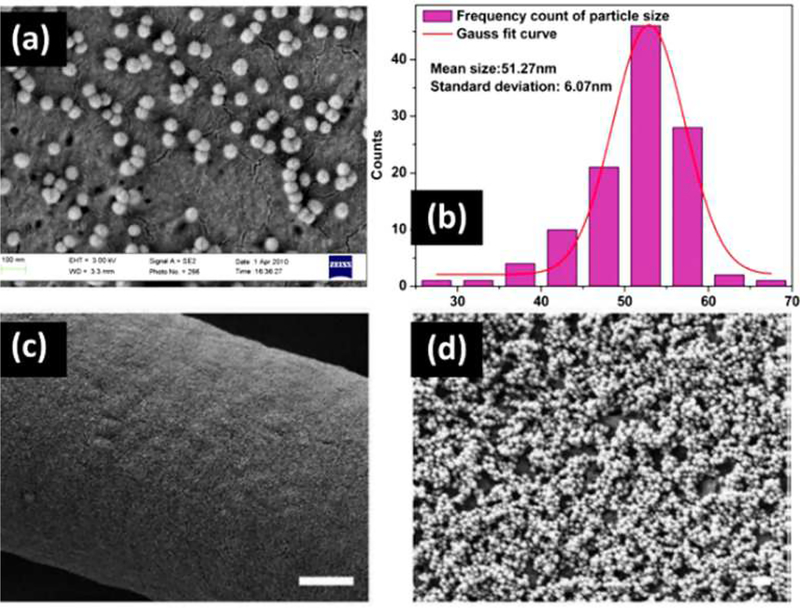
SNMs can be used as active nanoscale components of the extracellular matrix to stimulate osteoblastic functions. They should be well-distributed onto the surface of different three-dimensional scaffolds in order to ensure positive interactions between the SNMs and the cells [107]. Improved cell growth was obtained when the particle size was reduced from ~100 nm to ~10 nm. Additionally, cell adhesion was enhanced when nanostructures are used as extracellular matrix components. SNMs play the role of a molecular sieve, and can provide suitable water storage and release during the cell growth process. In order to prepare an appropriate SNM matrix for cell scaffolds, several methods have been investigated in recent years. Fibrous, polymeric, inorganic, and hydrogel substrates have been studied as a basis for a SNM matrix [33, 107, 108]. Various physicochemical strategies such as electrostatic assembly to form multilayer films, SNMs matrix peptide-modulation, electrospinning and sol–gel combinations, and vitamin-modification, have been employed for this goal. Several techniques such as phase separation, beam lithography, chemical etching, polymer demixing, and photolithography have been studied to obtain more efficient cell scaffolds using SNPs. However, researchers still continue to look for new approaches to precisely control the dimensions, structure, and orientation of these nanostructures. SNPs were electrostatically assembled onto three-dimensional polycaprolactone (PCL) fibrous matrices [107]. Multilayer films were evenly coated by PCL and SNPs based on opposite electrostatic charges and assembled to each other. This method produced uniformly sized SNPs, well dispersed in the matrix, increased surface roughness, and a significant decrease in the water contact angle (Figure 6). The cytotoxicity of SNPs and fibrous matrices towards fibroblast cells, and cell proliferation over three days were monitored. For silica coated samples, the viability (V) was partially decreased, but seeding number (SN) was four times greater (V: > 90%, SN: > 40,000). The average cell number for a monolayer system was two times greater than for a trilayer, and for a 5-layer fibrous control system, after seven days. Therefore, it seems that by assembling a higher number of layers of PCL fibrous SNPs matrix, the viability of the osteoblast cells was decreased. A possible explanation is because the pores of SNPs are blocked in 3 and 5 layer systems. The PCL fibers were well-coated by SNMs due to the strong interaction between silicon and oxygen atoms [62].
Figure 6. SEM images of synthesized SNPs.
(a) histogram of particle size distribution measured from SEM images, (c,d) SEM images of coated fiber surface with 5 layers of SNPs, (e) atomic force microscopic (AFM) image of coated fiber surface with 5 layers of SNPs, (f) evolution of water contact angle with contact time for three-dimensional fibrous matrix as control, and coated fiber surface with 1 layer, 3 layers and 5 layers of SNPs. The scale bar for SEM image c is 200 nm. Figure reproduced with permission from ref [107]. Copyright 2014 Wiley.
SNMs can be used in various forms in different polymeric matrices such as PCL, polyhydroxybutyrate (PHB) and a mixture of both polymers, to produce an integrated polymeric matrix with high stiffness and flexibility. In fiber-SNP composite scaffolds, interface interactions between the organic and inorganic phases are lower due to greater heterogeneity and particle aggregation onto the surface of the strands. Therefore smoother surfaces cause more positive interactions between mesoporous SNMs and organic fibers. In this regard, one report compared the efficiency of SNPs in a polymeric matrix as SNP-coated fibers (P5S1N) and sol–gel derived silica (P5S1S), prepared by electrospinning and sol–gel methods respectively [33]. Different performance and biological response were observed using P5S1S and P5S1N for bone tissue engineering by culturing MG-63 osteoblast-like cells on polymer-silica scaffolds. The results implied that P5S1S enhanced the properties of the PCL/PHB fibrous matrix by improved distribution of the inorganic phase onto the surface of the fibers. As can be seen in Figure 7, smoother polymeric fibers with good uniformity were obtained by using P5S1S in comparison with P5S1N (a,b). Thermal resistance of both samples was also compared by using thermogravimetric analysis (TGA) and the results revealed that the SNPs were removed from the fiber surface at lower temperatures. It seems that, uniform distribution of silica using the sol–gel method causes good interfacial adhesion in the silanol form. In addition, particle agglomeration may cause the pores to be blocked, resulting in poor water diffusion. MG-63 cells cultured on P5S1S exhibited better growth, viability and alkaline phosphatase (ALP) activity (Figure 7) [107].
Figure 7.
SEM images of (a) P5S1S, (b) P5S1N and (c) MG-63 cells cultured on P5S1S after 7 days cultivation. (d) thermal resistance behavior (TGA curves) of P5S1S and P5S1N; (e) MG-63 cell viability; and (f) ALP activity on P5S1S and P5S1N scaffolds. Figure reproduced with permission from ref [33]. Copyright 2015 Elsevier.
In order to inhibit the aggregation of SNPs and provide more active porous sites, the surface of SNPs can be functionalized with various bio-compatible compounds. Since, SNPs have a high surface energy, they are likely to be agglomerated in the NP form. Therefore, for tissue engineering purposes, the surface of SNPs is often modified before addition to polymeric and fibrous matrices. In this way, the physiochemical properties, such as hydrophilicity, bioactivity, thermal resistance and flexibility can be improved [107, 108]. In one recent report, vitamin B1 (thiamine) was used for surface modification of the SNPs before insertion into PCL strands. From an organic chemistry aspect, covalent binding between the amine groups of thiamine and the hydroxyl groups of SNPs was used for the surface attachment. As a comparative criterion, mechanical treatment of both neat PCL and thiamine-modified SNPs inserted into PLC matrix, was investigated, and results showed that the strain value of PCL/SNPs was significantly improved [33].
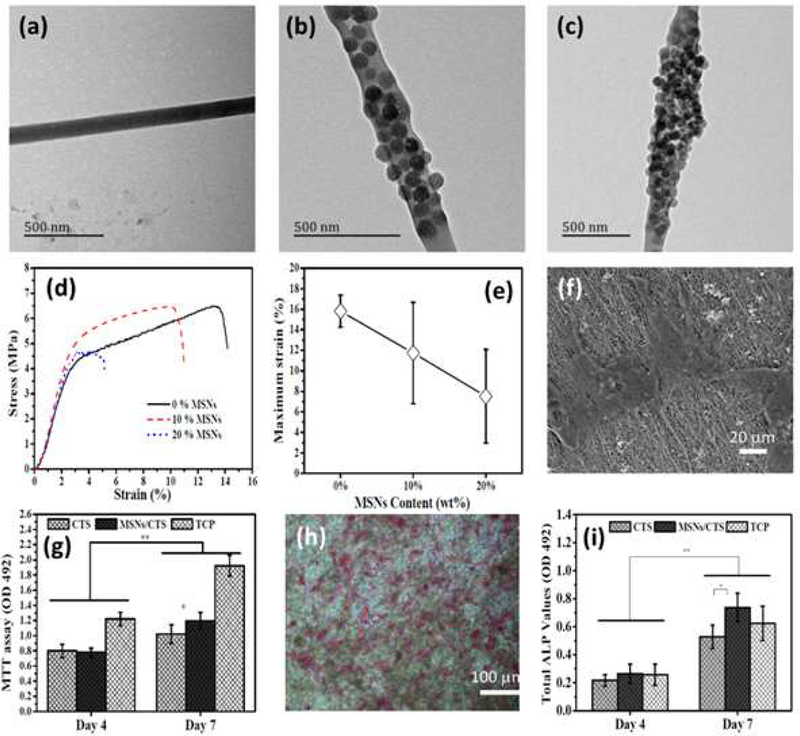
Since, pure PCL is a polymeric matrix with poor mechanical properties and low bioactivity, researchers have made efforts to improve its stability. Macroporous SNMs can maintain the structural stability of polymeric and fibrous scaffolds, and promote cell adhesion and proliferation in tissue engineering. In one report, Pickering high internal phase emulsion templates and three-dimensional printing was used to produce silicate porous scaffolds with well-defined pores [108]. The viscosity of the Pickering emulsion was modified with SNMs to improve the printing capability. In some cases, the SNMs for bone tissue engineering applications have been provided by natural resources such as marine diatoms. Diatomite is a natural deposit formed from diatom skeletons, and is a cheap resource of biogenic silica with relatively high abundance. Raw diatomite contains several inorganic and organic contaminants; therefore, purification processes are needed to obtain pure silica. Raw diatomite is initially purified under acidic conditions, then treated under alkaline conditions to produce the diatom NPs [109]. Other natural resources like chitosan can also be used as fibrous scaffolds for bone tissue engineering applications. Since, fibrous chitosan strands do not have sufficient strength to be employed as scaffolds, their mechanical properties must be enhanced by SNPs. In comparison with other types of modifying agents, SNMs can form strong bonds with polymeric fibrous strands. Recently, Li et al. [110] have demonstrated an improvement in resistance to stress in chitosan nanofibers containing bioactive ceramic SNPs, used for cultivation of MC3T3-E1 osteoblast cells. As shown in Figure 8, the effect of SNPs on the morphology and mechanical properties of the chitosan nanofiber scaffolds was investigated. TEM images show that SNPs were well-coated on the chitosan fibers when 10 wt% of SNPs was used (Figure 8a–c). It seems that aggregation onto the fiber surface occurred in the samples with > 20 wt% of SNPs. In addition, the mechanical properties of the fibrous scaffolds were improved when the SNP concentration was lower than 10 wt%, and then declined at higher percentages. Thus, the optimum coating was 10 wt% chitosan-SNPs because particle aggregation occurred at values higher than 10 %wt of SNPs. Aggregation increases the distance between the fiber strands and reduces the tensile resistance of the fibrous matrix (Figure 8d and e). Fig 10f shows the polygonal morphology of osteoblast cells after seven days culture on the SNP-modified chitosan scaffold, using SEM imaging. Therefore, osteoblast cells grow well on the SNP-containing chitosan scaffold (Figure 8g and h). Quantitative evaluation of the ALP activity disclosed that after seven days cultivation, there was a significant difference between SNP-containing chitosan and uncoated chitosan matrices (Figure 8i) [110].
Figure 8.
TEM images of (a) chitosan fiber, (b) SNPs-chitosan 10 wt% and (c) SNPs-chitosan 20 wt%, (d) typical tensile stress-strain curves of uncoated and SNPs-coated chitosan nanofibers with different percentages of SNPs, (e) the effects of SNPs coating on maximum strain of SNPs-containing chitosan scaffolds, (f) SEM image of MC3T3-E1 osteoblast cells cultured on SNPs-chitosan 10 wt% scaffold after 7 days, (g) histogram of MC3T3-E1 osteoblast cells proliferation by MTT test, (h) ALP staining of MC3T3-E1 osteoblasts cells attached to SNPs-containing chitosan scaffold after a 7-day cultivation, (i) quantitatively measured ALP activity after 4 and 7 days. Figure reproduced with permission from ref [110]. Copyright 2016 RSC Advances 2015
Figure 10.
(a) Synthetic route for surface modification of SNPs and cross-linking to the hydrogel matrix, SEM images of (b) prepared SNPs, (c) silica-modified hydrogel scaffold (Scale bar = 20 ‐m) and (d) the magnified image of silica-modified hydrogel scaffold. Figure reproduced with permission from ref [114]. Copyright 2016 Wiley.
3.2. Hydrogel matrices
In hydrogel matrices, SNMs have also been used to enhance the scaffold properties and improve bone cell cultivation. The incorporation of cell-adhesion peptide sequences into the porous SNPs can increase the capture of growth factors, and allow sustained cell stimulation properties for tissue engineering purposes [111]. This occurs by formation of an integrin cell-peptide cross-linked network within the hydrogel substrates. In a hydrogel matrix, binding ligands increase the interactions between the nano-components (and the loaded growth factors or drugs such as dexamethasone or morphogenetic peptides), and the superficial receptors on the cells. In addition, using a silica-composite hydrogel matrix for tissue engineering can produce healthier cultured bone cells, that do not undergo differentiation to tumor cells or any unwanted cell type [112, 113]. In this regard, Luo et al. reported a study in which a bone-forming peptide was incorporated into the SNPs on an alginate hydrogel substrate to improve cell viability and proliferation properties. In this study, hMSC bone cells were mixed with usnic acid (UA), alginate (RA), bone forming peptide-1 (pep-RA) and peptide-loaded SNP-modified alginate (pep@MSNs-RA) hydrogel matrices, as four different substrates, and then they were injected into the mice. Next, the samples were removed at 2 and 4 weeks post-implantation, and compared with each other using various assays. Figure 9 shows that desirable blood vessel formation occurred for RA, pep-RA and pep@MSNs-RA. The effects of the peptide carried by SNPs were investigated via Alizarin Red-S staining and cell microscopy. Both ALP activity and proliferation were higher for RA-based cultivation matrices compared to UA [112].
Figure 9.
(a) Bone tissues from cultivation of hMSC cell line mixed with various hydrogel matrices, after 2 and 4 weeks. As can be seen, a uniform and well-mixed tissue was observed for silica-modified matrix due to the appropriate hydrophobic interactions. (b) The capability of peptide carried by SNPs, compared with silica-free matrices, by Alizarin Red-S (ARS) staining after 2 and 4 weeks cultivation. Figure reproduced with permission from ref [112]. Copyright 2018 Elsevier.
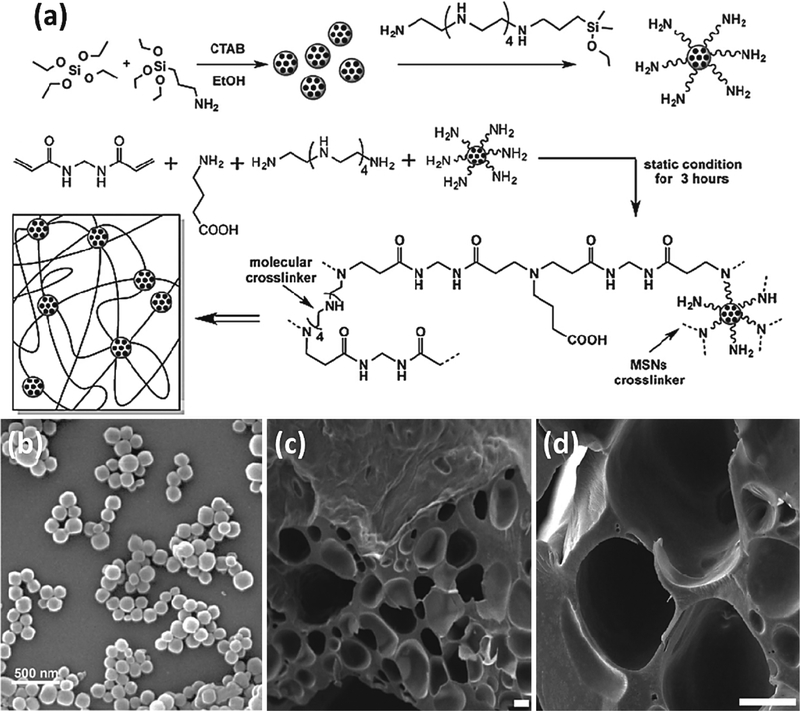
Surface polymerization of SNPs is also another efficient strategy to prepare hydrogel-based scaffolds for tissue engineering studies. Reportedly, polyamidoamines were covalently attached onto the surface of SNPs to improve the mechanical properties of the hydrogel matrix [113]. The surface of SNPs was initially modified with tetraethyl orthosilicate (TEOS) and 3-aminopropyltriethoxysilane (APTES) to create amino groups onto the surface as chemical attachment sites. The hydrophobic interactions between cells and SNPs were also increased using this surface modification. The amine-modified SNPs were chemically attached to the hydrogel structure by cross-linking. To prepare a SNP-modified hydrogel matrix, γ-aminobutyric was used due to its hydrophilicity and biocompatibility. The synthetic route and SEM images of the modified SNPs and silica-hydrogel scaffold are shown in Figure 10 [114]. One advantage is that hydrogel matrices can store and release a large volume of water through their swelling ability. Therefore, a synergistic effect occurs between SNPs and hydrogel matrices due to the water absorbance ability of SNPs, functioning as mesoporous molecular sieves. Glutaraldehyde was also used as a cross-linker to covalently link the SNPs to the hydrogel matrix [115]. It was shown that the cross-linking strategy did not affect the SNPs interaction with the matrix.
3.3. Inorganic matrices
Mesoporous bioactive glass NPs have been used as inorganic scaffolds for tissue engineering applications. The mineralization of bone, gene expression, and cell proliferation can be increased by release of inorganic ions. These bioactive elements such as calcium, cerium, gallium, copper, cobalt, lithium, boron, zinc and manganese have been doped into the SNP pores giving controlled released during the cell culture process. In addition to cell adhesion and proliferation, an antibacterial property is observed with manganese release. Different amounts of manganese were doped into the SNPs, and the effect on bone cells was investigated in vitro, as recently reported by Nawaz et al. [116]. Aluminum oxide NPs were also used to enhance NIH 3T3 cell viability, with antioxidant activity and low cytotoxicity [117]. The mechanical properties of organic-inorganic hybrid scaffolds can also be improved using alumina NPs due to their mechanical strength.
3. Discussion:
In this review, the substantial advantages of SNM scaffolds, for bone tissue engineering have been comprehensively discussed. Their advantages include, the ability to contain and release large volumes of water, the presence of a large active surface area for covalent bonding, low toxicity, good biocompatibility, and ability to act as carriers for drugs, growth factors and essential minerals. These nanomaterials have been used as tools in cell culture studies due to their ability to improve the physical, mechanical and chemical properties of the extracellular matrix. Cell proliferation is improved by the use of different matrices containing SNMs in various cell culture protocols. SNMs have also been successfully applied as three-dimensional scaffolds, where they improve the wettability and roughness of the fibrous matrices. Numerous experiments investigating the possible cytotoxicity of SNMs have shown that, not only are they non-toxic for bone cells, but they also are suitable for cell culture studies. Additionally, the nanoscale surface structure of SNMs can effectively enhance osteoblast attachment and proliferation. Moreover, the alkaline phosphatase activity of bone cells growing in SNM-modified matrices is higher than in control matrices. The mechanical strength and resistance to stress is increased in silica-modified matrices, and the polymer strands and fibers are more flexible, with improved resistance against tension. Mechanical pressure is directly transmitted through the SNMs, and is therefore better distributed throughout the whole scaffold. SNMs can enhance the stiffness and strength of fibrous matrices due to the improved distribution of inorganic materials, and a better organic–inorganic interface is achieved in these hybrid structures. Another advantage is the possibility to use silica from natural resources such as diatomite, which is a cheap and abundant source of biogenic silica, and can be easily converted to silica NPs with controlled size and morphology.
4. Conclusions
Recently, many studies have demonstrated that the silica nanomaterials scaffolds have many beneficial properties for bone tissue engineering. Their specific physiochemical properties such as high mechanical strength, low cytotoxicity, good biocompatibility and the ability to simultaneous delivery of small biomolecules and essential minerals lead to encourage bone cell growth during bone tissue engineering and make them appropriate scaffolds. Overall, all the above studies taken together, demonstrate the great potential of silica-modified scaffolds for bone tissue engineering.
Table 3.
Brief overview of the application of SNMs as scaffolds for bone tissue engineering.
| Matrix and scaffold | Preparation method | Examined cell line | Experi mental system | Advantages | Ref. | |
|---|---|---|---|---|---|---|
| 1 | Polyhydroxybutyrate/poly(ε-caprolactone)/silica nanoparticles (PHB/PCL/SNPs) | Electrospinning, Sol-gel, Dispersion electrospinning | MG-63 | In vitro | Enhanced viability, ALP activity | [33] |
| 2 | Bone forming peptide/SNPs | Covalent cross-linking | stem cells (hMSCs) | In vitro & In vivo | Enhanced cell adhesion, Survivability, Proliferation, Expansion and osteogenesis | [112] |
| 3 | Polyamidoamine/SNPs | Covalent cross-linking | mBM-MSC | In vitro & In vivo | Enhanced viability, biocompatibility, proliferation, Mechanical properties, | [114] |
| 4 | Poly(l-lactide acid)/silica nanoparticles (PLLA/SNPs) | Double sonication | Simulated body fluid (SBF) | In vitro | Enhanced mechanical properties, | [113] |
| 5 | Polylactic acid/silica nanoparticles (PLA/SNPs) | Melt mixing | MC3T3-E1 | In vitro | Enhanced viability, proliferation and thermomechanical properties, Biodegradability | [111] |
| 6 | collagen-chitosan/SNPs | in situ gelation (Stober method) | Simulated body fluid (SBF) | In vitro | Enhanced viability, Low toxicity | [118] |
| 7 | Polycaprolactone Fibrous/SNPs | Electrostatic layer-by-layer self-assembly | hFOB 1.19 | In vitro | Enhanced cell attachment, proliferation, and ALP | [58] |
Highlights.
Bone tissue engineering uses scaffolds to encourage growth of new bone cells
Silica nanomaterials have multifunctional applications in this field.
They can deliver drugs, growth factors, antimicrobials or combinations
Specific surface features stimulate bone cell proliferation and differentiation
Their mechanical properties are better than other scaffolds
Acknowledgements
The Authors are grateful for financial support from the Immunology Research Center, Tabriz University of Medical Sciences.
Funding and conflicts of interest
MRH was supported by US NIH Grants R01AI050875 and R21AI121700. MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kasemo B, Biological surface science, Surf. Sci 500 (2002) 656–677. [Google Scholar]
- [2].Grasman JM, Zayas MJ, Page RL, Pins GD, Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries, Acta Biomater. 25 (2015) 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Domingues-Faria C, Vasson MP, Goncalves-Mendes N, Boirie Y, Walrand S, Skeletal muscle regeneration and impact of aging and nutrition, Ageing. Res. Rev 26 (2016) 22–36. [DOI] [PubMed] [Google Scholar]
- [4].Grellier M, Bordenave L, Amedee J, Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering, Trends. biotechnol 27 (2009) 562–571. [DOI] [PubMed] [Google Scholar]
- [5].Botchwey EA, Dupree MA, Pollack SR, Levine EM, Laurencin CT, Tissue engineered bone: Measurement of nutrient transport in three-dimensional matrices, J. Biomed. Mater. Res. A 67 (2003) 357–367. [DOI] [PubMed] [Google Scholar]
- [6].Eivazzadeh-Keihan R, Maleki A, de la Guardia M, Bani MS, Chenab KK, Pashazadeh-Panahi P, Baradaran B, Mokhtarzadeh A, Hamblin MR, Carbon based nanomaterials for tissue engineering of bone: building new bone on small black scaffolds: A review, J. Adv. Res 18 (2019) 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ku SH, Lee M, Park CB, Carbon-based nanomaterials for tissue engineering, Adv. Helthc. Mater 2 (2013) 244–260. [DOI] [PubMed] [Google Scholar]
- [8].Marsich E, Bellomo F, Turco G, Travan A, Donati I, Paoletti S, Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: preparation, characterization and biological properties, J. Mater. Sci.: Mater. Med 24 (2013) 1799–1807. [DOI] [PubMed] [Google Scholar]
- [9].Lee KY, Mooney DJ, Hydrogels for tissue engineering, Chem. Rev 101 (2001) 1869–1880. [DOI] [PubMed] [Google Scholar]
- [10].Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK, Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach, ACS nano 9 (2015) 3109–3118. [DOI] [PubMed] [Google Scholar]
- [11].Allison DD, Grande-Allen KJ, Hyaluronan: a powerful tissue engineering tool, Tissue Eng. 12 (2006) 2131–2140. [DOI] [PubMed] [Google Scholar]
- [12].Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS, Polymeric scaffolds in tissue engineering application: a review, Int. J. Polym. Sci. 2011 (2011). [Google Scholar]
- [13].Sabir MI, Xu X, Li L, A review on biodegradable polymeric materials for bone tissue engineering applications, J. Mater. Sci 44 (2009) 5713–5724. [Google Scholar]
- [14].Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK, Alginate composites for bone tissue engineering: a review, Int. J. Biol. Macromol 72 (2015) 269–281. [DOI] [PubMed] [Google Scholar]
- [15].Zhao W, Jin X, Cong Y, Liu Y, Fu J, Degradable natural polymer hydrogels for articular cartilage tissue engineering, J. Chem. Technol. Biotechnol 88 (2013) 327–339. [Google Scholar]
- [16].Guo B, Ma PX, Synthetic biodegradable functional polymers for tissue engineering: a brief review, Sci. China Chem 57 (2014) 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pina S, Oliveira JM, Reis RL, Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review, Adv. Mater 27 (2015) 1143–1169. [DOI] [PubMed] [Google Scholar]
- [18].Kretlow JD, Mikos AG, Mineralization of synthetic polymer scaffolds for bone tissue engineering, Tissue Eng. 13 (2007) 927–938. [DOI] [PubMed] [Google Scholar]
- [19].Liu X, Holzwarth JM, Ma PX, Functionalized synthetic biodegradable polymer scaffolds for tissue engineering, Macromol. Biosci 12 (2012) 911–919. [DOI] [PubMed] [Google Scholar]
- [20].Chen G, Sato T, Ushida T, Ochiai N, Tateishi T, Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen, Tissue Eng. 10 (2004) 323–330. [DOI] [PubMed] [Google Scholar]
- [21].Song J, Malathong V, Bertozzi CR, Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone, JACS. 127 (2005) 3366–3372. [DOI] [PubMed] [Google Scholar]
- [22].Jia X, Kiick KL, Hybrid multicomponent hydrogels for tissue engineering, Macromol. Biosci 9 (2009) 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI, Mesoporous silica nanoparticles in biomedical applications, chem. Soc. Rev 41 (2012) 2590–2605. [DOI] [PubMed] [Google Scholar]
- [24].Baino F, Fiorilli S, Vitale-Brovarone C, Bioactive glass-based materials with hierarchical porosity for medical applications: review of recent advances, Acta Biomater. 42 (2016) 18–32. [DOI] [PubMed] [Google Scholar]
- [25].Chen JF, Ding HM, Wang JX, Shao L, Preparation and characterization of porous hollow silica nanoparticles for drug delivery application, Biomaterials 25 (2004) 723–727. [DOI] [PubMed] [Google Scholar]
- [26].Li ZZ, Wen LX, Shao L, Chen JF, Fabrication of porous hollow silica nanoparticles and their applications in drug release control, J. Control. Release 98 (2004) 245–254. [DOI] [PubMed] [Google Scholar]
- [27].Mokhtarzadeh A, Eivazzadeh-Keihan R, Pashazadeh P, Hejazi M, Gharaatifar N, Hasanzadeh M, Baradaran B, de la Guardia M, Nanomaterial-based biosensors for detection of pathogenic virus, Trends Anal. Chem 97 (2017) 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eivazzadeh-Keihan R, Pashazadeh P, Hejazi M, de la Guardia M, Mokhtarzadeh A, Recent advances in nanomaterial-mediated bio and immune sensors for detection of aflatoxin in food products, Trends Anal. Chem 87 (2017) 112–128. [Google Scholar]
- [29].Eivazzadeh-Keihan R, Pashazadeh-Panahi P, Baradaran B, Maleki A, Hejazi M, Mokhtarzadeh A, de la Guardia M, Recent advances on nanomaterial based electrochemical and optical aptasensors for detection of cancer biomarkers, Trends Anal. Chem 100 (2018) 103–115. [Google Scholar]
- [30].Chenab KK, Eivazzadeh-Keihan R, Maleki A, Pashazadeh-Panahi P, Hamblin MR, Mokhtarzadeh A, Biomedical applications of nanoflares: Targeted intracellular fluorescence probes, Nanomedicine: NBM. 17 (2019) 342–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gu W, Wu C, Chen J, Xiao Y, Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration, Int. J. Nanomedicine 8 (2013) 2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tavakol S, Nikpour MR, Hoveizi E, Tavakol B, Rezayat SM, Adabi M, Abokheili SS, Jahanshahi M, Investigating the effects of particle size and chemical structure on cytotoxicity and bacteriostatic potential of nano hydroxyapatite/chitosan/silica and nano hydroxyapatite/chitosan/silver; as antibacterial bone substitutes, J. Nanoparticle Res 16 (2014) 2622. [Google Scholar]
- [33].Ding Y, Yao Q, Li W, Schubert DW, Boccaccini AR, Roether JA, The evaluation of physical properties and in vitro cell behavior of PHB/PCL/sol–gel derived silica hybrid scaffolds and PHB/PCL/fumed silica composite scaffolds, Colloid Surface. B 136 (2015) 93–98. [DOI] [PubMed] [Google Scholar]
- [34].Zhou J, Lin H, Fang T, Li X, Dai W, Uemura T, Dong J, The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone, Biomaterials 31 (2010) 1171–1179. [DOI] [PubMed] [Google Scholar]
- [35].Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, Kang Y, Yang Y, Khademhosseini A, Vascularized bone tissue engineering: approaches for potential improvement, Tissue Eng. Part B Rev. 18 (2012) 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shabafrooz V, Mozafari M, Vashaee D, Tayebi L, Electrospun nanofibers: from filtration membranes to highly specialized tissue engineering scaffolds, J. Nanosci. Nanotechnol 14 (2014) 522–534. [DOI] [PubMed] [Google Scholar]
- [37].Fernandes JS, Gentile P, Pires RA, Reis RL, Hatton PV, Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue, Acta Biomater. 59 (2017) 2–11. [DOI] [PubMed] [Google Scholar]
- [38].Hajiali F, Tajbakhsh S, Shojaei A, Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review, Polym rev. 58 (2018) 164–207. [Google Scholar]
- [39].Shadjou N, Hasanzadeh M, Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress, Mater. Sci. Eng. C 55 (2015) 401–409. [DOI] [PubMed] [Google Scholar]
- [40].Erol M, Mouriňo V, Newby P, Chatzistavrou X, Roether J, Hupa L, Boccaccini AR, Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering, Acta Biomater. 8 (2012) 792–801. [DOI] [PubMed] [Google Scholar]
- [41].Fu Q, Rahaman MN, Fu H, Liu X, Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation, J. Biomed. Mater. Res. B 95 (2010) 164–171. [DOI] [PubMed] [Google Scholar]
- [42].Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI, Nel AE, Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model, ACS nano 5 (2011) 4131–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lin YS, Haynes CL, Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles, Chem. Mater 21 (2009) 3979–3986. [Google Scholar]
- [44].Tabasi O, Falamaki C, Khalaj Z, Functionalized mesoporous silicon for targeted-drug-delivery, Colloid Surface. B 98 (2012) 18–25. [DOI] [PubMed] [Google Scholar]
- [45].Wu SH, Mou CY, Lin HP, Synthesis of mesoporous silica nanoparticles, Chem. Soc. Rev 42 (2013) 3862–3875. [DOI] [PubMed] [Google Scholar]
- [46].Tao Z, Toms BB, Goodisman J, Asefa T, Mesoporosity and functional group dependent endocytosis and cytotoxicity of silica nanomaterials, Chem. Res. Toxicol 22 (2009) 1869–1880. [DOI] [PubMed] [Google Scholar]
- [47].Harada M, Adachi M, Surfactant-Mediated Fabrication of Silica Nanotubes, Adv. mater 12 (2000) 839–841. [Google Scholar]
- [48].Choi SS, Lee SG, Im SS, Kim SH, Joo YL, Silica nanofibers from electrospinning/sol-gel process, J. Mater. Sci. Lett 22 (2003) 891–893. [Google Scholar]
- [49].Liu J, Yang Q, Zhang L, Yang H, Gao J, Li C, Organic-inorganic hybrid hollow nanospheres with microwindows on the shell, Chem. Mater 20 (2008) 4268–4275. [Google Scholar]
- [50].Yi DK, Lee SS, Papaefthymiou GC, Ying JY, Nanoparticle architectures templated by SiO2/Fe2O3 nanocomposites, Chem. Mater 18 (2006) 614–619. [Google Scholar]
- [51].Yeh YQ, Chen BC, Lin HP, Tang CY, Synthesis of hollow silica spheres with mesostructured shell using cationic− anionic-neutral block copolymer ternary surfactants, Langmuir 22 (2006) 6–9. [DOI] [PubMed] [Google Scholar]
- [52].Han L, Gao C, Wu X, Chen Q, Shu P, Ding Z, Che S, Anionic surfactants templating route for synthesizing silica hollow spheres with different shell porosity, Solid State Sci. 13 (2011) 721–728. [Google Scholar]
- [53].Liu J, Qiao SZ, Budi Hartono S, Lu GQ, Monodisperse yolk–shell nanoparticles with a hierarchical porous structure for delivery vehicles and nanoreactors, Angew. Chem. Int. Ed. Engl 49 (2010) 4981–4985. [DOI] [PubMed] [Google Scholar]
- [54].Kao KC, Tsou CJ, Mou CY, Collapsed (kippah) hollow silica nanoparticles, Chem. Comm 48 (2012) 3454–3456. [DOI] [PubMed] [Google Scholar]
- [55].Qi G, Wang Y, Estevez L, Switzer AK, Duan X, Yang X, Giannelis EP, Facile and scalable synthesis of monodispersed spherical capsules with a mesoporous shell, Chem. Mater 22 (2010) 2693–2695. [Google Scholar]
- [56].Ravichandran R, Gandhi S, Sundaramurthi D, Sethuraman S, Krishnan UM, Hierarchical mesoporous silica nanofibers as multifunctional scaffolds for bone tissue regeneration, J. Biomater. Sci. Polym. Ed 24 (2013) 1988–2005. [DOI] [PubMed] [Google Scholar]
- [57].Kuo YC, Wang CC, Surface modification with peptide for enhancing chondrocyte adhesion and cartilage regeneration in porous scaffolds, Colloids Surface. B 84 (2011) 63–70. [DOI] [PubMed] [Google Scholar]
- [58].Liu X, Ma PX, Polymeric scaffolds for bone tissue engineering, Ann. Biomed. Eng 32 (2004) 477–486. [DOI] [PubMed] [Google Scholar]
- [59].Perez RA, Won JE, Knowles JC, Kim HW, Naturally and synthetic smart composite biomaterials for tissue regeneration, Adv. Drug Deliv. Rev 65 (2013) 471–496. [DOI] [PubMed] [Google Scholar]
- [60].Bharti C, Nagaich U, Pal AK, Gulati N, Mesoporous silica nanoparticles in target drug delivery system: A review, Int. J. Pharm. Investig 5 (2015) 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Laflamme C, Rouabhia M, Effect of BMP-2 and BMP-7 homodimers and a mixture of BMP-2/BMP-7 homodimers on osteoblast adhesion and growth following culture on a collagen scaffold, Biomed. Mater 3 (2008) 015008. [DOI] [PubMed] [Google Scholar]
- [62].Chen SH, Wang XL, Xie XH, Zheng LZ, Yao D, Wang DP, Leng Y, Zhang G, Qin L, Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: an in vitro efficacy study, Acta Biomater. 8 (2012) 3128–3137. [DOI] [PubMed] [Google Scholar]
- [63].Liu X, Zhu L, Zhao T, Lan J, Yan W, Zhang H, Synthesis and characterization of sulfonic acid-functionalized SBA-15 for adsorption of biomolecules, Micropor. Mesopor. Mat 142 (2011) 614–620. [Google Scholar]
- [64].Peyressatre M, Prével C, Pellerano M, Morris MC, Targeting cyclin-dependent kinases in human cancers: from small molecules to peptide inhibitors, Cancers 7 (2015) 179–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wu C, Fan W, Chang J, Functional mesoporous bioactive glass nanospheres: synthesis, high loading efficiency, controllable delivery of doxorubicin and inhibitory effect on bone cancer cells, J. Mater. Chem B 1 (2013) 2710–2718. [DOI] [PubMed] [Google Scholar]
- [66].Andronescu E, Ficai M, Voicu G, Ficai D, Maganu M, Ficai A, Synthesis and characterization of collagen/hydroxyapatite: magnetite composite material for bone cancer treatment, J. Mater. Sci.: Mater. Med 21 (2010) 2237–2242. [DOI] [PubMed] [Google Scholar]
- [67].Wang N, Ma M, Luo Y, Liu T, Zhou P, Qi S, Xu Y, Chen H, Mesoporous Silica Nanoparticles-Reinforced Hydrogel Scaffold Together with Pinacidil Loading to Improve Stem Cell Adhesion, Chem. Nano. Mat 4 (2018) 631–641. [Google Scholar]
- [68].Qiu K, Chen B, Nie W, Zhou X, Feng W, Wang W, Chen L, Mo X, Wei Y, He C, Electrophoretic deposition of dexamethasone-loaded mesoporous silica nanoparticles onto poly (l-lactic acid)/poly (ε-caprolactone) composite scaffold for bone tissue engineering, ACS Appl. Mater. Interfaces 8 (2016) 4137–4148. [DOI] [PubMed] [Google Scholar]
- [69].Shi Y, Massagué J, Mechanisms of TGF-β signaling from cell membrane to the nucleus, cell 113 (2003) 685–700. [DOI] [PubMed] [Google Scholar]
- [70].ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ, Controlling cell fate by bone morphogenetic protein receptors, Mol. Cell. Endocrinol 211 (2003) 105–113. [DOI] [PubMed] [Google Scholar]
- [71].Jason JY, Barnes AP, Hand R, Polleux F, Ehlers MD, TGF-β signaling specifies axons during brain development, Cell 142 (2010) 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12, Mol. Cell. Biol 20 (2000) 8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Takazawa Y, Tsuji K, Nifuji A, Kurosawa H, Ito Y, Noda M, An osteogenesis-related transcription factor, core-binding factor A1, is constitutively expressed in the chondrocytic cell line TC6, and its expression is upregulated by bone morphogenetic protein-2, J. Endocrinol 165 (2000) 579–586. [DOI] [PubMed] [Google Scholar]
- [74].Banerjee C, Javed A, Choi J-Y, Green J, Rosen V, Van Wijnen AJ, Stein JL, Lian JB, Stein GS, Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype, Endocrinology 142 (2001) 4026–4039. [DOI] [PubMed] [Google Scholar]
- [75].McKay W, Science-based assessment: accelerating product development of combination medical devices, NMAB Roundtable on Biomedical Engineering Materials and Applications. Washington DC, 2003. [Google Scholar]
- [76].Burkus JK, Sandhu HS, Gornet MF, Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft, Spine 31 (2006) 775–781. [DOI] [PubMed] [Google Scholar]
- [77].James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering, Stem cells Transl. Med 1 (2012) 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aghaloo T, Jiang X, Soo C, Zhang Z, Zhang X, Hu J, Pan H, Hsu T, Wu B, Ting K, A study of the role of nell-1 gene modified goat bone marrow stromal cells in promoting new bone formation, Mol. Ther 15 (2007) 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shahlaie K, Kim KD, Occipitocervical fusion using recombinant human bone morphogenetic protein-2: adverse effects due to tissue swelling and seroma, Spine 33 (2008) 2361–2366. [DOI] [PubMed] [Google Scholar]
- [80].Robin BN, Chaput CD, Zeitouni S, Rahm MD, Zerris VA, Sampson HW, Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study, Spine 35 (2010) E1350–E1354. [DOI] [PubMed] [Google Scholar]
- [81].James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C, A review of the clinical side effects of bone morphogenetic protein-2, Tissue Eng. Part B Rev 22 (2016) 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen D, Zhao M, Mundy GR, Bone morphogenetic proteins, Growth factors 22 (2004) 233–241. [DOI] [PubMed] [Google Scholar]
- [83].Neumann A, Christel A, Kasper C, Behrens P, BMP2-loaded nanoporous silica nanoparticles promote osteogenic differentiation of human mesenchymal stem cells, RSC Adv. 3 (2013) 24222–24230. [Google Scholar]
- [84].Cui W, Sun G, Qu Y, Xiong Y, Sun T, Ji Y, Yang L, Shao Z, Ma J, Zhang S, Repair of rat calvarial defects using Si-doped hydroxyapatite scaffolds loaded with a bone morphogenetic protein-2-related peptide, J. Orthop. Res 34 (2016) 1874–1882. [DOI] [PubMed] [Google Scholar]
- [85].Cui W, Liu Q, Yang L, Wang K, Sun T, Ji Y, Liu L, Yu W, Qu Y, Wang J, Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration, ACS Biomater. Sci. Eng 4 (2017) 211–221. [DOI] [PubMed] [Google Scholar]
- [86].Zhou X, Feng W, Qiu K, Chen L, Wang W, Nie W, Mo X, He C, BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells, ACS Appl. Mater. Interfaces 7 (2015) 15777–15789. [DOI] [PubMed] [Google Scholar]
- [87].Maes C, Carmeliet G, Schipani E, Hypoxia-driven pathways in bone development, regeneration and disease, Nat. Rev. Rheumatol 8 (2012) 358–366 [DOI] [PubMed] [Google Scholar]
- [88].Ceradini DJ, Gurtner GC, Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue, Trends. Cardiovas. Med 15 (2005) 57–63. [DOI] [PubMed] [Google Scholar]
- [89].Yao Q, Liu Y, Selvaratnam B, Koodali RT, Sun H, Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering, J. Control. Release 279 (2018) 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kim JJ, Singh RK, Patel KD, Kim HW, Delivery of Small Genetic Molecules through Hollow Porous Nanoparticles Silences Target Gene and in Turn Stimulates Osteoblastic Differentiation, Part. Part. Sys. Char 33 (2016) 878–886. [Google Scholar]
- [91].Sumathra M, Munusamy MA, Alarfaj AA, Rajan M, Osteoblast response to Vitamin D3 loaded cellulose enriched hydroxyapatite Mesoporous silica nanoparticles composite, Biomed. Pharmacother 103 (2018) 858–868. [DOI] [PubMed] [Google Scholar]
- [92].Johnson CT, García AJ, Scaffold-based anti-infection strategies in bone repair, Ann. Biomed. Eng 43 (2015) 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mouriño V, Boccaccini AR, Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds, J. R. Soc. Interface 7 (2009) 209–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N, Applying insights from biofilm biology to drug development—can a new approach be developed?, Nat. Rev. Drug Discov 12 (2013) 791–808. [DOI] [PubMed] [Google Scholar]
- [95].Lazzarini L, Mader JT, Calhoun JH, Osteomyelitis in long bones, JBJS, 86 (2004) 2305–2318. [DOI] [PubMed] [Google Scholar]
- [96].Lew DP, Waldvogel FA, Osteomyelitis, The Lancet 364 (2004) 369–379. [DOI] [PubMed] [Google Scholar]
- [97].Trampuz A, Zimmerli W, Diagnosis and treatment of infections associated with fracture-fixation devices, Injury 37 (2006) S59–S66. [DOI] [PubMed] [Google Scholar]
- [98].Marambio-Jones C, Hoek EM, A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment, J. Nanopart. Res 12 (2010) 1531–1551. [Google Scholar]
- [99].Kargozar S, Montazerian M, Hamzehlou S, Kim HW, Baino F, Mesoporous bioactive glasses (MBGs): promising platforms for antibacterial strategies, Acta Biomater. 81 (2018) 1–19. [DOI] [PubMed] [Google Scholar]
- [100].Mandal A, Meda V, Zhang W, Farhan K, Gnanamani A, Synthesis, characterization and comparison of antimicrobial activity of PEG/TritonX-100 capped silver nanoparticles on collagen scaffold, Colloid. Surface. B 90 (2012) 191–196. [DOI] [PubMed] [Google Scholar]
- [101].Rokavec M, Kaller M, Horst D, Hermeking H, Pan-cancer EMT-signature identifies RBM47 down-regulation during colorectal cancer progression, Sci. Rep 7 (2017) 4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tarpani L, Morena F, Gambucci M, Zampini G, Massaro G, Argentati C, Emiliani C, Martino S, Latterini L, The influence of modified silica nanomaterials on adult stem cell culture, Nanomaterials 6 (2016) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yang H.y., Niu L.n., Sun J.l., Huang X.q., Pei D.d., Huang C, Tay FR, Biodegradable mesoporous delivery system for biomineralization precursors, Int. J. Nanomedicine 12 (2017) 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].De Matos M, Piedade A, Alvarez-Lorenzo C, Concheiro A, Braga M, De Sousa H, Dexamethasone-loaded poly (ɛ-caprolactone)/silica nanoparticles composites prepared by supercritical CO2 foaming/mixing and deposition, Int. J. Pharm 456 (2013) 269–281. [DOI] [PubMed] [Google Scholar]
- [105].Wu C, Miron R, Sculean A, Kaskel S, Doert T, Schulze R, Zhang Y, Proliferation, differentiation and gene expression of osteoblasts in boron-containing associated with dexamethasone deliver from mesoporous bioactive glass scaffolds, Biomaterials 32 (2011) 7068–7078. [DOI] [PubMed] [Google Scholar]
- [106].Kargozar S, Mozafari M, Hamzehlou S, Kim HW, Baino F, Mesoporous bioactive glasses (MBGs) in cancer therapy: Full of hope and promise, Mater. 251 (2019) 241–246. [Google Scholar]
- [107].Tang Y, Zhao Y, Wang X, Lin T, Layer-by-layer assembly of silica nanoparticles on 3D fibrous scaffolds: Enhancement of osteoblast cell adhesion, proliferation, and differentiation, J. Biomed. Mater. Res. A 102 (2014) 3803–3812. [DOI] [PubMed] [Google Scholar]
- [108].Yang T, Hu Y, Wang C, Binks BP, Fabrication of hierarchical macroporous biocompatible scaffolds by combining pickering high internal phase emulsion templates with three-dimensional printing, ACS Appl. Mater. Interfaces 9 (2017) 22950–22958. [DOI] [PubMed] [Google Scholar]
- [109].Le TDH, Bonani W, Speranza G, Sglavo V, Ceccato R, Maniglio D, Motta A, Migliaresi C, Processing and characterization of diatom nanoparticles and microparticles as potential source of silicon for bone tissue engineering, Mater. Sci. Eng. C 59 (2016) 471–479. [DOI] [PubMed] [Google Scholar]
- [110].Li K, Sun H, Sui H, Zhang Y, Liang H, Wu X, Zhao Q, Composite mesoporous silica nanoparticle/chitosan nanofibers for bone tissue engineering, Rsc Adv. 5 (2015) 17541–17549. [Google Scholar]
- [111].Georgiopoulos P, Kontou E, Meristoudi A, Pispas S, Chatzinikolaidou M, The effect of silica nanoparticles on the thermomechanical properties and degradation behavior of polylactic acid, J. Biomater. Appl 29 (2014) 662–674. [DOI] [PubMed] [Google Scholar]
- [112].Luo Z, Zhang S, Pan J, Shi R, Liu H, Lyu Y, Han X, Li Y, Yang Y, Xu Z, Time-responsive osteogenic niche of stem cells: a sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation, Biomaterials 163 (2018) 25–42. [DOI] [PubMed] [Google Scholar]
- [113].Guerzoni S, Deplaine H, El Haskouri J, Amorós P, Pradas MM, Edlund U, Ferrer GG, Combination of silica nanoparticles with hydroxyapatite reinforces poly (L-lactide acid) scaffolds without loss of bioactivity, J. Bioact. Compat. Pol 29 (2014) 15–31. [Google Scholar]
- [114].Fiorini F, Prasetyanto EA, Taraballi F, Pandolfi L, Monroy F, López-Montero I, Tasciotti E, De Cola L, Nanocomposite hydrogels as platform for cells growth, proliferation, and chemotaxis, Small 12 (2016) 4881–4893. [DOI] [PubMed] [Google Scholar]
- [115].Sani F, Mehdipour F, Talaei-Khozani T, Sani M, Razban V, Fabrication of platelet-rich plasma/silica scaffolds for bone tissue engineering, Bioinspir. Biomim. Nan 7 (2017) 74–81. [Google Scholar]
- [116].Nawaz Q, Rehman MAU, Burkovski A, Schmidt J, Beltrán AM, Shahid A, Alber NK, Peukert W, Boccaccini AR, Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications, J. Mater. Sci.: Mater Med 29 (2018) 64. [DOI] [PubMed] [Google Scholar]
- [117].Karunakaran G, Suriyaprabha R, Rajendran V, Kannan N, Effect of contact angle, zeta potential and particles size on the in vitro studies of Al2O3 and SiO2 nanoparticles, IET Nanobiotechnol. 9 (2014) 27–34. [DOI] [PubMed] [Google Scholar]
- [118].Lewandowska-Łańcucka J, Fiejdasz S, Rodzik Ł, Kozieł M, Nowakowska M, Bioactive hydrogel-nanosilica hybrid materials: a potential injectable scaffold for bone tissue engineering, Biomed. Mater 10 (2015) 015020. [DOI] [PubMed] [Google Scholar]