Abstract
Objectives: To identify and describe the types and time course of dysphagia following cervical spinal cord injury (SCI). Methods: This was a prospective cohort study conducted in an SCI inpatient rehabilitation unit. Seventy-six individuals with SCI were enrolled. Inclusion criteria were age 18 years or older, admitted into SCI inpatient rehabilitation unit, and medically stable for participation in bedside swallow evaluation (BSE) and videofluoroscopic swallow study (VFSS). All participants first underwent a BSE, of whom 33 completed a VFSS. A follow-up BSE was conducted on individuals who tested positive on the initial BSE and continued to show signs of dysphagia. Diagnosis and type of dysphagia as well risk factors were collected. Results: Twenty-three out of 76 individuals with cervical SCI were diagnosed with dysphagia using the BSE. All participants with positive BSE and VFSS had pharyngeal dysfunction. For participants with a positive initial BSE and persisting dysphagia (n = 14), a follow-up BSE demonstrated resolution within 34 days. Risk factors associated with dysphagia were older age, nasogastric tube, invasive mechanical ventilation, tracheostomy, and pneumonia. Posterior spinal surgery was associated with a decreased risk of dysphagia. Conclusion: Dysphagia was present in 30% of individuals based on the initial BSE. All individuals with dysphagia demonstrated pharyngeal phase dysfunction on the VFSS. No participants experiencing dysphagia were missed on the BSE as confirmed by VFSS. In the subset of individuals who received a follow-up BSE, the time course of resolution of dysphagia was at most 34 days from initial BSE.
Keywords: bedside swallow evaluation, dysphagia, spinal cord injury, tetraplegia, videofluoroscopic swallow study
Dysphagia is a well-known secondary complication after acute cervical spinal cord injury (SCI). Dysphagia is characterized by a difficulty with swallowing and may be associated with pneumonia in this patient population.1–7 Prior studies have reported the incidence of dysphagia in acute cervical SCI to range between 17% and 41%.1,3,6–8 Previously identified risk factors for dysphagia in cervical SCI include presence of a tracheostomy, invasive mechanical ventilator use, presence of a nasogastric tube, halo vest fixation, older age, and an anterior cervical spinal surgical approach.2,3
Although the incidence and risk factors of dysphagia in acute cervical SCI have been reported, details on the types of dysphagia experienced in this population have not been described. Broadly, dysphagia is a swallowing disorder more frequently associated with individuals who have had a stroke or a brain injury and can be divided into three stages affecting the oral, pharyngeal, and esophageal phases.9,10 During the oral phase, food is chewed and propelled posteriorly by the tongue. Once the bolus is propelled past the faucial arches, the pharyngeal phase begins.10 During the pharyngeal phase, the bolus is moved through the pharynx and follows a progression of physiological movements: (1) velopharyngeal closure, (2) elevation and anterior movement of larynx and hyoid, (3) closure of the larynx, (4) cricopharyngeal opening, (5) tongue base and pharyngeal wall action, and (6) progressive contraction of the pharyngeal constrictors.10 Once the bolus passes through the cricopharyngeal sphincter, the esophageal phase begins. The bolus is moved through the cervical and thoracic esophagus by esophageal peristalsis.10
In patients with stroke and brain injury, dysphagia can be caused by dysfunction in all three phases of swallowing.11 Neville et al reported that abnormalities in the interaction between the pharynx and upper esophageal sphincter may contribute to the entry of food or liquid into the airway (aspiration) in individuals with cervical SCI.10,12 Furthermore, Logemann speculated that dysphagia in cervical SCI is primarily at the pharyngeal stage.10 However, there is a lack of evidence-based reports on characterization of dysphagia in persons with cervical SCI to date. Furthermore, there have been no studies to determine the time course of resolution of dysphagia in persons with SCI.
Dysphagia is commonly diagnosed by a bedside swallow evaluation (BSE), a flexible fiberoptic endoscopy evaluation of swallowing (FEES), or a videofluoroscopic swallow study (VFSS), with VFSS considered the gold standard diagnostic method.9 Previous studies have demonstrated that BSE is as good as VFSS in diagnosing dysphagia clinically in acute SCI.13 However, VFSS was identified as superior to BSE in determining different types of dysphagia and in guiding treatment and recommendations for food and liquid consistency.13
This study's objective was to investigate and characterize (a) the incidence and types of dysphagia using BSE and VFSS, (b) length of time to resolve dysphagia using a follow-up BSE, and (c) risk factors associated with dysphagia in individuals with acute cervical SCI. In addition, this study compared diagnostic tools (BSE and VFSS) to explore the specificity and sensitivity of the BSE compared to the VFSS.
Methods
Participants
Individuals with acute cervical SCI were recruited from a community hospital's acute inpatient SCI rehabilitation unit. This project was reviewed and approved by the Research and Human Subjects Review Committee at the study site. Consents were signed by either the participant, legal authorized representative, or two witnesses at time of enrollment. The inclusion criteria for this study were (1) age 18 years or older, (2) admission to the SCI rehabilitation unit, and (3) medically stable to participate in BSE and subsequent VFSS. The exclusion criteria were (1) oral or nasal intubation, (2) known swallowing dysfunction prior to SCI, and (3) any significant cognitive deficits impairing the participant's ability to follow instructions during BSE or VFSS. Seventy-six participants were enrolled in the study.
Data collection
Demographic data including age, gender, and race were collected; also collected were injury characteristics including the American Spinal Injury Association Impairment Scale (AIS), length of acute inpatient rehabilitation stay, time from injury, invasive mechanical ventilator dependence, presence of a nasogastric tube, history of pneumonia, history of bronchoscopy, history of re-intubation, presence of a halo vest, presence of cervical collar, presence of tracheostomy, and approach for surgical spine fusion (Table 1). Every participant was asked to undergo BSE within 24 hours of admission to acute rehabilitation and VFSS within 72 hours of BSE.
Table 1.
Demographic data for individuals with and without dysphagia based on bedside evaluation
| Demographics | Dysphagia | χ2/t | p | |
|---|---|---|---|---|
| Yes | No | |||
| Age mean (±SD) | 48 (19) | 39 (17) | t(74) = −2.1 | .04 |
| Time from injury, days | 22 (15) | 30 (43) | t(71.5) = 1.2 | .22 |
| Length of stay, days | 48 (20) | 39 (18) | t(74) = −1.8 | .08 |
| High tetraplegiaa, Y (N) | 19 (4) | 37 (16) | χ2(1) = 1.4 | .24 |
| Complete (Incomplete) SCI | 8 (15) | 25 (28) | χ2(1) = 1.0 | .32 |
| Gender, M (F) | 21(2) | 42 (11) | ** | .32 |
| Race | ** | .89 | ||
| African America | 1 | 2 | ||
| Asian | 3 | 5 | ||
| Caucasian | 12 | 25 | ||
| Hispanic | 4 | 9 | ||
| Other/Unknown | 3 | 12 | ||
Note: Double asterisks represent comparisons using Fisher's exact test.
aHigh tetraplegia was defined as an injury at or above C4.
For individuals who had any abnormal findings at the initial BSE assessment and who continued to have clinical signs of dysphagia during their hospitalization, a follow-up BSE was conducted to re-evaluate them for any improvement in their swallowing function. To closely examine the time course of resolution of dysphagia, the participants were re-evaluated using a BSE as soon as the clinicians felt the participants were ready to be advanced to a regular diet; these data were used to assess the time course of dysphagia.
Evaluation procedures
Bedside swallow evaluation
BSE is the preferred initial screening method for dysphagia in individuals with SCI; it is less costly, less invasive, and does not include radiation exposure as compared to VFSS. A speech language pathologist (SLP) experienced with BSE evaluated all participants for dysphagia. BSE was performed on participants while they were in a hospital bed or wheelchair based on individual comfort and medical precautions. Spine precautions related to the presence of a halo vest, use of a soft or hard cervical collar, and head of bed positioning (greater than 30 degrees) were taken into consideration. If the head of bed needed to be positioned under 30 degrees, the BSE was performed in the bed. If the participant had a tracheostomy and/or was invasive mechanical ventilator-dependent, a licensed respiratory care practitioner assisted with oxygen saturation monitoring, cuff deflation, suctioning, and invasive mechanical ventilator changes during BSE. In addition to interpreting the patients' performance on the BSE, the SLP was responsible for administering the food, deciding when the patients should be suctioned, and determining when the cuff should be deflated.
Diagnosis of dysphagia was made if the SLP observed any dysfunction of oral, pharyngeal, and/or esophageal phases of swallow during BSE. Oral dysfunction would be diagnosed if there was poor bolus control, mastication, or inability to move the bolus from anterior to posterior oral cavity. Pharyngeal dysfunction was diagnosed if there was laryngeal elevation/excursion, wet vocal quality, and/or throat clearing or coughing, while esophageal dysfunction included no strictures, dysmotility, or stasis noted in the esophagus. Participants diagnosed with dysphagia were placed on modified diets under the guidance of the SLP in conjunction with the treating physician. Food consistency modifications included dysphagia pureed (applesauce consistency), dysphagia ground (ground beef consistency), or mechanical soft (blended or finely chopped consistency) diets. Liquid consistency modifications included ice chips, thin liquids, thickened liquids, carbonated liquids, and no liquids (dry tray).
Videofluoroscopic swallow study
VFSS provides direct visualization of the anatomy and physiology of swallowing under fluoroscopy. Foods and liquids of different consistency were made radiopaque by adding barium. Participants with dysphagia on VFSS were further characterized based on swallowing dysfunction and presence of the following: oral dysphagia, delay in pharyngeal peristalsis, vallecular pooling, pyriform sinus pooling, decreased laryngeal elevation, penetration of test material into the larynx, and aspiration of test material past the true vocal cords. The evaluation was completed with the 5-minute standard time allotted for VFSS. The radiology department's protocol at the test institution did not fully capture the distal esophagus on VFSS, so the study was unable to fully evaluate esophageal phase dysfunction.
Data analysis
Demographics were compared between individuals with and without dysphagia (Table 1). Continuous variables (age, time from injury, and length of stay) were compared using t tests. Levene's test for equality of variances was used and corrected for when comparing time from injury. Chi-square test was used for categorical variables (high tetraplegia and completeness of injury); high tetraplegia was defined as a C4 or higher level of injury. Fisher's exact test was used to compare race subgroups and gender for individuals with and without dysphagia.
Chi-square test or Fisher's exact test was used to determine associations between a diagnosis of dysphagia and potential risk factors, which included the following: presence of a tracheostomy, invasive mechanical ventilator dependence, presence of a nasogastric tube, high tetraplegia, completeness of SCI, history of pneumonia, history of bronchoscopy, history of re-intubation, presence of cervical collar, presence of halo, concurrent brain injury, surgery (anterior and/or posterior versus no surgery), and surgical approach (anterior surgery or posterior surgery versus no surgery). Estimates of relative risk and effect size (Cramer's V) were also calculated for each comparison (Table 2).
Table 2.
Risk factor analysis for dysphagia
| Risk factors | Dysphagia | RR | χ2(1) | p | V | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Presence of tracheostomy (n = 76) |
Yes | 17 | 23 | 2.6 | 6.0 | .014 | .28 |
| No | 6 | 30 | |||||
| Invasive mechanical ventilator dependence (n = 76) | Yes | 15 | 21 | 2.1 | 4.2 | .040 | .24 |
| No | 8 | 32 | |||||
| Presence of NG tube (n = 76) | Yes | 4 | 1 | 3.0 | ** | .027 | .29 |
| No | 19 | 52 | |||||
| High tetraplegia (n = 76) | Yes | 19 | 37 | 1.7 | 1.4 | .244 | .13 |
| No | 4 | 16 | |||||
| Complete SCI (n = 76) | Yes | 8 | 25 | .69 | 1.0 | .317 | .12 |
| No | 15 | 28 | |||||
| History of pneumonia (n = 67) | Yes | 14 | 11 | 3.4 | 11 | .001 | .41 |
| No | 7 | 35 | |||||
| History of bronchoscopy (n = 67) | Yes | 4 | 4 | 1.7 | ** | .247 | .15 |
| No | 17 | 42 | |||||
| History of re-intubation (n = 68) | Yes | 3 | 2 | 2.1 | ** | .167 | .18 |
| No | 18 | 45 | |||||
| Presence of cervical collar (n = 73) | Yes | 14 | 33 | 1.1 | .07 | .796 | .03 |
| No | 7 | 19 | |||||
| Presence of halo (n = 76) | Yes | 2 | 1 | 2.3 | ** | .216 | .16 |
| No | 21 | 52 | |||||
| Concurrent brain injury (n = 76) | Yes | 10 | 17 | 1.4 | .91 | .340 | .11 |
| No | 13 | 36 | |||||
| Spinal surgerya (n = 76) | Yes | 17 | 46 | .58 | ** | .195 | .16 |
| No | 6 | 7 | |||||
Table 2.
Risk factor analysis for dysphagia
| Risk factors | Dysphagia | RR | χ2(1) | p | V | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Anterior spinal surgery (n = 40) | Yes | 12 | 15 | .96 | .01 | .919 | .02 |
| No | 6 | 7 | |||||
| Posterior spinal surgery (n = 28) | Yes | 1 | 14 | .14 | ** | .029 | .46 |
| No | 6 | 7 | |||||
Note: Double asterisks represent comparisons using Fisher's exact test. RR = relative risk; NG = nasogastric; V = Cramer's V.
aTwenty-one participants (4 with dysphagia) had both anterior and posterior spinal surgery.
Individuals who agreed to undergo the VFSS were used to test the reliability of the BSE compared to the VFSS (Table 3). Specificity, sensitivity, positive predictive power, and negative predictive power were calculated for the comparison. Chi-square test, t test, Fisher's exact test, and Cramer's V were calculated using SPSS version 24, whereas relative risk, specificity, sensitivity, positive predictive power, and negative predictive power were calculated in Excel.
Table 3.
Comparison between bedside swallow evaluation (BSE) and videofluoroscopic swallow study (VFSS)
| VFSS | ||||||
|---|---|---|---|---|---|---|
| + | − | Total | Specificity | 100% | ||
| BSE | + | 14 | 3 | 17 | Sensitivity | 84.2% |
| − | 0 | 16 | 16 | PPV | 82.4% | |
| Total | 14 | 19 | NPV | 100% | ||
Note: NPV = negative predictive value; PPV = positive predictive value.
Results
Seventy-six individuals with acute cervical SCI were enrolled. The average age of participants with dysphagia diagnosed with BSE and without dysphagia was 48 ± 19 years and 39 ± 17 years, respectively. There was a significant difference in the age of participants who presented with and without dysphagia, as diagnosed by the BSE [t(74) = −2.1, p = 0.04] (Table 1). There were no significant differences in time from injury, length of stay, high tetraplegia, completeness of injury, gender, or race (p > .08) (Table 1). Levene's test for equality of variance was significant for time from injury [F(1, 74) = 5.4, p = .02] but not for age or length of stay; time from injury [t(71.5) =1.2, p = .22].
Twenty-three participants (30.2%) were diagnosed with pharyngeal dysphagia based on the BSE. Significant risk factors associated with dysphagia were presence of tracheostomy [χ2(1) = 6.0, p = 0.014, Cramer's V (V) = .28, relative risk (RR) = 2.6], invasive mechanical ventilator dependence [χ2(1) = 4.2, p = .040, V = .24, RR = 2.1], presence of nasogastric tube (Fisher's exact test, p = .027, V = .29, RR = 3.0), and history of pneumonia [χ2(1) = 11, p = 0.001, V = .41, RR = 3.4] (Table 2). High tetraplegia, complete SCI, history of bronchoscopy, history of reintubation, presence of a cervical collar, presence of halo, concurrent brain injury, surgery compared to no surgery, and anterior cervical spinal surgery were not significantly associated with dysphagia (p > .17). A posterior surgical approach was statistically significant but had a fractional relative risk of presenting with dysphagia (Fisher's exact test, p = .029, V = .46, RR = .14).
Of the 76 participants who underwent BSE, 23 were diagnosed with dysphagia and 53 without. Of the 23 diagnosed with dysphagia on BSE, 17 agreed to proceed with VFSS. Of these participants, 14 were found to have dysphagia. Six out of the 23 participants diagnosed with dysphagia did not complete the VFSS (5 declined and 1 did not tolerate the procedure) (Figure 1). Thirty-six out of the 53 participants who were not diagnosed with dysphagia declined to do the VFSS due to concerns about radiation exposure and anxiety. All participants who did not receive a dysphagia diagnosis based on the BSE were placed on a regular diet without further complications. A total of 33 participants completed the VFSS on an average of 1.4 days after the initial BSE.
Figure 1.
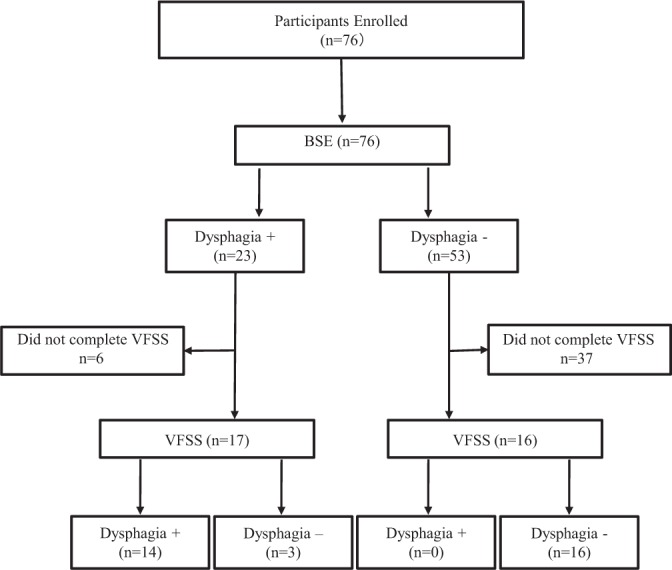
Flow diagram showing study participation. BSE = bedside swallow evaluation; dysphasia + = diagnosed with dysphasia; dysphasia - = not diagnosed with dysphagia; VFSS = videofluoroscopic swallow study.
When comparing BSE versus VFSS as the gold standard diagnostic tool, sensitivity of BSE was 84.2% and specificity was 100% (Table 3). The positive predictive value of BSE was 82.4%, and the negative predictive value was 100%. Diet recommendations made based on BSE were altered after completion of VFSS in 7 participants out of the 33 who completed the VFSS. Liquid recommendations made based on BSE were altered after completion of VFSS in 10 participants of the 33 who completed the VFSS.
Of the 14 participants found to have dysphagia on VFSS, dysfunction was found in the following swallowing mechanisms (Table 4): oral phase (n = 0, 0%), decreased pharyngeal peristalsis (n = 7,50.0%), decreased laryngeal elevation (n = 12,85.7%), vallecular pooling (n = 12,85.7%), pooling in the pyriform sinus (n = 8,57.1%), penetration (n = 4,28.6%), and aspiration (n = 3,21.4%). Of the participants with aspiration on VFSS, silent aspiration was observed in two out of three participants (66.7%).
Table 4.
Swallowing dysfunction characteristics
| VFSS swallowing phase dysfunction | n | % |
|---|---|---|
| Oral | 0 | 0.0 |
| Pharyngeal peristalsis | 7 | 50.0 |
| Laryngeal elevation | 12 | 85.7 |
| Vallecular pooling | 12 | 85.7 |
| Pyriform sinus pooling | 8 | 57.1 |
| Penetration | 4 | 28.6 |
| Aspiration | 3 | 21.4 |
| Silent aspiration | 2 of 3 | 66.7 |
Note: Fourteen participants received the videofluoroscopic swallow study (VFSS), which excluded the esophageal phase due to the testing institution's radiological protocol. Some participants had multiple phases of dysfunction based on the VFSS.
Fourteen participants who were initially diagnosed with dysphagia based on the BSE completed a follow-up BSE assessment. This group was composed of 11 participants who completed the VFSS and three additional participants who had declined to complete the VFSS. Based on the VFSS, three participants were found not to have dysphagia and therefore were removed from this analysis. In this sample of 11 participants, the median time to resolve dysphagia from initial BSE was 14 days (range, 6–34 days). Table 5 summarizes the sample's time to resolve dysphagia and includes subgrouping based on diet type and liquid type.
Table 5.
Time to resolve dysphagia from initial BSE for participants who tested positive on the initial BSE and completed a follow-up BSE
| Participants, n | Time to resolution, days | |||
|---|---|---|---|---|
| Median | Range | |||
| Whole group | 11 | 14 | 6–34 | |
| Diet type | Liquid type | |||
| NPO | 5 | 12 | 6–23 | |
| Carbonate | 1 | 6 | ||
| Ice chips | 2 | 14–23 | ||
| No liquids | 2 | 11–12 | ||
| Dysphagia puree | Thin | 1 | 12 | 12 |
| Dysphagia ground | Thin | 2 | 23.5 | 13–34 |
| Mechanical soft | 3 | 22 | 20–24 | |
| All liquids | 2 | 20–22 | ||
| Thin | 1 | 24 | ||
Note: Whole group and subgroups based on diet type and liquid type are summarized. BSE = bedside swallow evaluation; NPO = nothing by mouth.
Discussion
Dysphagia, a complication after cervical SCI, is clinically important but may not be addressed as a priority in individuals with acute SCI due to treatment for other secondary complications of SCI including respiratory failure, hypotension, neurogenic bladder, neurogenic bowel, or sepsis. The incidence of dysphagia in this study was 30% based on the initial BSE, which is similar to incidences found in prior studies.1,2,3,5 Risk factors associated with dysphagia in this study were presence of tracheostomy, invasive mechanical ventilator dependence, presence of nasogastric tube, history of pneumonia, and older age. Posterior spinal surgery was associated with a decreased risk of dysphagia. Factors without a significant association to dysphagia in our sample included high tetraplegia, complete SCI, history of bronchoscopy, history of reintubation, use of cervical collar, anterior surgical approach, and presence of halo. Similar to previous literature, individuals in the dysphagia group were significantly older than the individuals who did not present with dysphagia.14 The BSE was again confirmed to be an accurate screening tool relative to the VFSS with high sensitivity and specificity (84.2% and 100%, respectively).
Classically, dysphagia has been divided into three phases: (1) oral phase, (2) pharyngeal phase, and (3) esophageal phase.10 Individuals with cerebral cortex hemispheric stroke typically have swallowing dysfunction of the oral phase, although all phases may be affected.10 Logemann10 described the types of swallowing problems that may occur with individuals with SCI; these swallowing difficulties are typically “pharyngeal in nature” and may include “a delay in triggering the pharyngeal swallow, reduced laryngeal elevation and anterior movement causing reduced cricopharyngeal opening, reduced tongue base motion, and unilateral or bilateral pharyngeal wall dysfunction.”10(p318) In addition, it was noted that there may be “a tendency for poor laryngeal movement and consequent reduced cricopharyngeal opening” that may occur especially for patients with cervical injuries at C4, 5, or 6.
In this study, none of the participants demonstrated oral phase dysphagia on VFSS. All participants with dysphagia on VFSS showed pharyngeal phase dysfunction with decreased pharyngeal peristalsis and decreased laryngeal elevation. Pooling in the valleculae and decreased cricopharyngeal opening, resulting in pooling in the pyriform sinuses, was also observed in the majority of participants with dysphagia on VFSS. The association between tracheostomy and dysphagia in this study may indicate that there may have been direct laryngeal damage from tracheostomy. A study by Ceriana et al15 noted that dysphagia in persons with tracheostomy showed the following on VFSS: incomplete backward epiglottis folding (48%), pharyngeal retention (32%), penetration (33%), and aspiration (28%). Patients on mechanical ventilation usually have inflated tracheostomy cuff, and mechanical ventilation was identified as a risk factor for dysphagia in this study. However, contrary to prior reports that dysphagia in SCI is associated with anterior cervical fusion as it may also interfere with laryngeal movement, this study did not find anterior cervical fusion to be a significant risk factor.
Contrary to a prior study that did not find association between nasogastric (NG) tube and dysphagia in persons with stroke, all individuals with an NG tube presented with pharyngeal dysphagia in this study; the pathophysiology for pharyngeal dysfunction remains unclear.16 In young persons with normal swallow, swallowing transit time was longer with NG tube, but the NG tube did not seem to affect the swallowing function, namely “adequacy of bolus containment, pharyngeal clearance, and airway protection.”17(p159) However, the NG tube seems to cause slowing of pharyngeal response and duration of pharyngeal transit and earlier laryngeal elevation, which may be protective on the respiratory tract.17 Given small number of participants who had an NG tube (n = 3) and agreed to do VFSS, we could not clarify pathophysiology of dysphagia that may be caused by the NG tube.
Aspiration was found in 21.4% (3 out of 14) of the participants who completed VFSS and tested positive for dysphagia. Of these, two out of three demonstrated silent aspiration without overt signs (coughing choking or gagging) and symptoms or findings of aspiration (pulmonary complications, leukocytosis, elevated temperature, or difficulty breathing). This suggests that clinicians should be cognizant of aspiration as a major risk factor and know how to identify covert signs of silent aspiration or less obvious signs, such as runny nose, increased saliva production, watery eyes, and multiple swallows on a single bolus. Addressing dysphagia may decrease the risk of aspiration pneumonia, which may lead to improved long-term outcomes (ie, functional independence and life expectancy).18
Additionally, Logemann mentioned that tracheostomy and/or ventilator dependence makes BSE difficult and therefore subsequent VFSS is required; however, no literature or data were referenced to support this assertion.10 We found that BSE can be performed accurately at the bedside to screen for dysphagia regardless of invasive mechanical ventilator dependence or tracheostomy. VFSS confirmed findings of dysphagia on BSE and provided additional information on the phase of swallowing affected. In addition, VFSS provided clarification on consistency of food and liquids that were appropriate. After VFSS, different diet recommendations were implemented in 7 of the 33 participants (21.2%) evaluated using VFSS. Different liquid recommendations were made in 10 of the 33 participants (30.3%) evaluated using VFSS.
In a subsample of the participants (n = 11) initially diagnosed with dysphagia using the BSE, the time to resolve dysphagia ranged from 6 to 34 days, with the median time to resolve dysphagia being 14 days from initial BSE (Table 5). Albeit a small sample, participant diet type distributed such that a nothing-by-mouth (NPO) diet had a median time to resolve dysphagia of 11.5 days, dysphagia puree had a median time to resolve of 12 days, dysphagia ground diet had a median time to resolve of 23.5 days, and a mechanically soft diet had time median time to resolve of 22 days. Although participants on NPO diets may be thought to have the greatest dysphagia severity and therefore time to resolve dysphagia, it was the experience of the study SLP that an NPO diet may be related to other issues (eg, poor lung health or impaired coordination), which, if resolved, could resolve the dysphagia. Therefore, diet types of dysphagia puree, dysphagia ground, and mechanical soft swallowing impairments may be more related to pharyngeal dysfunction. Greater sample sizes would be needed to clarify how diet type is related to time to resolve dysphagia and dysphagia severity.
This study was limited in that not all participants were willing to undergo VFSS, primarily citing concerns for radiation exposure. Patients underwent VFSS within 72 hours after completion of BSE; this may be too long to determine whether dysphagia is present and whether it resolved within that time frame. Another limitation of this study was the inability to fully evaluate esophageal phase dysfunction. This was in part due to the radiology department's protocol at the test institution, which did not fully capture the distal esophagus on VFSS.
Future studies should address therapeutic interventions that can be implemented to treat dysphagia in individuals in SCI. Given the preponderance of pharyngeal phase dysphagia identified in this study, directed therapies may improve dysphagia more effectively. The Masako maneuver and Shaker technique are two validated exercises that improve the duration and amplitude of laryngeal elevation.19,20
Conclusion
Dysphagia was present in 30% of individuals with acute tetraplegia based on the initial BSE. Risk factors for dysphagia included presence of tracheostomy, invasive mechanical ventilator dependence, presence of nasogastric tube, and history of pneumonia. No participants with dysphagia were missed on the BSE, as confirmed by VFSS. Thus, the BSE is an appropriate screening tool for individuals with tetraplegia. Videofluoroscopy provides additional information, compared to the BSE, for tolerance to various food and liquid consistencies, which may suggest that VFSS is a better tool for assessing liquid and diet recommendations. All participants in this study with dysphagia demonstrated pharyngeal phase dysfunction, which has not been reported previously. Most importantly, dysphagia in persons with SCI seems to resolve within 1 month of diagnosis in rehabilitation setting. Therefore, providers should be vigilant to screen for dysphagia in persons with acute SCI, and future projects should focus on therapeutic interventions to address pharyngeal dysphagia in individuals with SCI in acute setting to avoid secondary complications, such as pneumonia.
Acknowledgments
Dr Shem reports that this study was funded by a field-initiated grant (#H133G080165) from the National Institute on Disability and Rehabilitation Research (NIDRR).
REFERENCES
- 1.Abel R, Ruf S, Spahn B. Cervical spinal cord injury and deglutition disorders. Dysphagia. 2004;19(2):87–94. doi: 10.1007/s00455-003-0511-y. [DOI] [PubMed] [Google Scholar]
- 2.Chaw E, Shem K, Castillo K, Wong SL, Chang J. Dysphagia and associated respiratory considerations in cervical spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(4):291–299. doi: 10.1310/sci1804-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirshblum S, Johnston MV, Brown J, O'Connor KC, Jarosz P. Predictors of dysphagia after spinal cord injury. Arch Phys Med Rehabil. 1999;80(9):1101–1105. doi: 10.1016/s0003-9993(99)90068-0. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos SM, Chen JC, Feldenzer JA, Bucci MN, McGillicuddy JE. Anterior cervical osteophytes as a cause of progressive dysphagia. Acta Neurochir (Wien) 1989;101(1–2):63–65. doi: 10.1007/BF01410071. [DOI] [PubMed] [Google Scholar]
- 5.Shem K, Castillo K, Wong SL, Chang J, Kolakowsky-Hayner S. Dysphagia and respiratory care in individuals with tetraplegia: Incidence, associated factors, and preventable complications. Top Spinal Cord Inj Rehabil. 2012;18(1):15–22. doi: 10.1310/sci1801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin JC, Yoo JH, Lee YS, Goo HR, Kim DH. Dysphagia in cervical spinal cord injury. Spinal Cord. 2011;49(9):1008–1013. doi: 10.1038/sc.2011.34. [DOI] [PubMed] [Google Scholar]
- 7.Wolf C, Meiners TH. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord. 2003;41(6):347–353. doi: 10.1038/sj.sc.3101440. [DOI] [PubMed] [Google Scholar]
- 8.Wise MF, Milani JC. Incidence of swallowing problems in quadriplegics [abstract]. Abstracts Digest; American Spinal Injury Association, 13th Annual Scientific Meeting; March 20–22, 1987; Boston. Atlanta: ASIA; 1987. p. 30. [Google Scholar]
- 9.Logemann JA. Evaluation and treatment of swallowing disorders. Am J Speech Lang Pathol. 1994;3(3):41–44. [Google Scholar]
- 10.Logemann JA. Evaluation and Treatment of Swallowing Disorders. Austin, TX: PRO-ED; 1998. [Google Scholar]
- 11.González-Fernández M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: An overview. Curr Phys Med Rehabil Rep. 2013;1(3):187–196. doi: 10.1007/s40141-013-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neville AL, Crookes P, Velmahos GC, Vlahos A, Theodorou D, Lucas CE. Esophageal dysfunction in cervical spinal cord injury: A potentially important mechanism of aspiration. J Trauma. 2005;59(4):905–911. doi: 10.1097/01.ta.0000188086.02488.b1. [DOI] [PubMed] [Google Scholar]
- 13.Shem KL, Castillo K, Wong SL, Chang J, Kao MC, Kolakowsky-Hayner SA. Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289. doi: 10.1016/j.pmrj.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Iruthayarajah J, McIntyre A, Mirkowski M, Welch-West P, Loh E, Teasell R. Risk factors for dysphagia after a spinal cord injury: A systematic review and meta-analysis. Spinal Cord. 2018;56(12):1116–1123. doi: 10.1038/s41393-018-0170-3. [DOI] [PubMed] [Google Scholar]
- 15.Ceriana P, Carlucci A, Schreiber A et al. Changes of swallowing function after tracheostomy: A videofluoroscopy study. Minerva Anestesiol. 2015;81(4):389–397. [PubMed] [Google Scholar]
- 16.Wang T-G, Wu M-C, Chang Y-C, Hsiao T-Y, Lien I-N. The effect of nasogastric tubes on swallowing function in persons with dysphagia following stroke. Arch Phys Med Rehabil. 2006;87:1270–1273. doi: 10.1016/j.apmr.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Huggins PS, Tuomi SK, Young C. Effects of nasogastric tubes on the young, normal swallowing mechanism. Dysphagia. 1999;14(3):157–161. doi: 10.1007/PL00009598. [DOI] [PubMed] [Google Scholar]
- 18.Warner FM, Tong B, Jutzeler CR, Cragg JJ, Scheuren PS, Kramer JLK. Journal club: Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology. 2017;89(7):e76–e78. doi: 10.1212/WNL.0000000000004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babu S, Balasubramaniam RK, Varghese A. Effect of modified shaker exercise on the amplitude and duration of swallowing sounds: Evidence from cervical auscultation. Rehabil Res Pract. 2017;2017 doi: 10.1155/2017/6526214. 6526214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byeon H. Effect of the Masako maneuver and neuromuscular electrical stimulation on the improvement of swallowing function in patients with dysphagia caused by stroke. J Phys Ther Sci. 2016;28(7):2069–2071. doi: 10.1589/jpts.28.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]


