Abstract
Background: Catheter-associated urinary tract infection (CAUTI) is associated with increased morbidity and mortality and influences the quality of life of patients with spinal cord injury (SCI). Objectives: This clinical review aims to highlight the unique surveillance, prevention, diagnosis, and management challenges of CAUTI in the SCI population. Methods: Narrative review of the current literature on catheter use in persons with SCI was conducted to determine gaps in knowledge and opportunities for improvement. Results: Surveillance of CAUTI is challenging in the SCI population as the ability to detect symptoms used to diagnose CAUTI (ie, suprapubic pain, dysuria) is impaired. In terms of prevention of CAUTI, current strategies refocus on appropriate catheter insertion and care and early removal of catheters, which is not always feasible for persons with SCI. Prophylactic antibiotics, nutraceuticals, and coated catheters show limited efficacy in infection prevention. Diagnosing CAUTI after SCI is challenging, often resulting in an overdiagnosis of CAUTI when truly asymptomatic bacteriuria exists. In the management of CAUTI in patients with SCI, the use of multiple antibiotics over time in an individual increases the rate of multidrug-resistant organisms; therefore, the exploration of novel non-antibiotic treatments is of importance. The patient experience should be at the center of all these efforts. Conclusion: Better diagnostic tools or biomarkers are needed to define true CAUTI in people with SCI. SCI-specific evidence to inform catheter management and CAUTI treatment guidelines is needed, with the goal to minimize catheter-related harm, reduce antibiotic resistance, and improve satisfaction and overall quality of life for SCI patients.
Keywords: catheter-associated urinary tract infection (CAUTI), neurogenic bladder, spinal cord injury (SCI), urinary catheters
Catheter-associated urinary tract infection (CAUTI) has been a salient topic in recent years due to the economic burden of the condition and the health burden to the patient.1–4 Diseases of the genitourinary tract (of which a large proportion are CAUTI) are frequently cited as the most common reasons for health care use (emergency room and inpatient visits), and septicemia (of which urosepsis is included) is among the leading causes of death for persons with SCI.4,5 Even though the importance of recognizing and treating true CAUTI is clear, distinguishing this condition from asymptomatic bacteriuria (ASB; a positive urine culture without clinical signs or symptoms of infection) is particularly challenging in the setting of spinal cord injury (SCI). Often after SCI with subsequently associated neurogenic bladder, bladder instrumentation with either indwelling or intermittent catheterization is necessary to safely and effectively eliminate urine and maintain continence, leading to high rates of ASB that do not need to be treated.6 However, the development of even nonspecific symptoms in the setting of a positive urine culture will likely result in a diagnosis of CAUTI, raising the possibility of missing other clinically meaningful infections. Thus, SCI creates unique challenges to surveilling, preventing, diagnosing, and managing CAUTI. This narrative review will explore these challenges and offer solutions.
Methods
The objective of this narrative review is to examine the current body of literature guiding surveilling, preventing, diagnosing, and managing CAUTI in the SCI population. A PubMed query using the article medical subject headings (MESH) key words (spinal cord injury, catheter-associated urinary tract infection, neurogenic bladder, urinary catheters) was completed, and the articles were reviewed by two of the authors for their relevance. A synthesis of the results is provided below
Results
Surveillance of CAUTI
Challenge: Current quality measures only include indwelling transurethral (Foley) catheters in catheter surveillance and CAUTI metrics. People with SCI use multiple catheter types for bladder drainage, so there is an incomplete picture of the scope of the problem in this population. Previous interventions aimed at reducing the incidence of CAUTI in the acute care setting have focused on catheter surveillance and often the removal of indwelling catheters as soon as possible. Early catheter removal is not always the best solution in the SCI population, and there is some concern that the current metric incentivizes removing Foley catheters in patients with SCI without an adequate backup plan for bladder drainage. Inadequate bladder drainage in this population can lead to autonomic dysreflexia, kidney failure, and sepsis. Bladder management after SCI poses significant psychosocial challenges to the patient that must be factored into the decision-making process.
Solution: Refine current CAUTI metrics to better address quality, safety, and psychosocial concerns for urinary care after neurogenic bladder (such as with SCI) and provide far-reaching neurogenic bladder education.
The National Healthcare Safety Network CAUTI outcome measure is the ratio of number of observed health care–associated CAUTIs among patients in inpatient care locations (acute care general hospitals, long-term acute care hospitals, rehabilitation hospitals, oncology hospitals, and behavior health hospitals) to the number of predicted health care–associated CAUTIs over the data period, based on the national CAUTI baseline.7 The definition of “indwelling catheter” narrowly includes only intraurethral Foley catheters; the measure explicitly excludes suprapubic catheters, condom catheters, in and out catheterizations (commonly referred to as an intermittent catheterization program, or ICP in the rehabilitation medicine literature), and nephrostomy tubes. The definition of CAUTI used in the metric includes suprapubic pain and dysuria as diagnostic criteria. Saint et al8 described surveillance bundles that were shown to reduce the rates of CAUTI in the acute care setting. A primary component of this bundle includes avoiding use of indwelling catheters and using alternative methods such as condom catheters.
The measure as currently written accurately captures the scope of the problem in the general acute inpatient population; but given the diversity of catheter type and chronic nature of catheter use in persons with SCI, further refinement is necessary for the metric to truly promote quality care and patient safety in this population. Even though there is evidence that a focus on sterile catheter insertion is helpful in delaying the onset of bacteriuria (which may reduce CAUTI rates) with short-term catheter use,9 once a very basic level of hygiene is achieved (daily meatal care, cleansing with basic tap water before insertion), catheter insertion and maintenance strategies have failed to produce substantial reduction in CAUTI rates for long-duration catheter use.10,11 Jamison et al12 performed a Cochrane review in 2013 to determine the efficacy of indwelling urethral catheters, suprapubic catheters, ICP, and condom catheters for long-term bladder management in persons with neurogenic bladder, and they found no evidence of superiority of one bladder management type over the other. There have been several studies that show clean ICP is non-inferior to performing ICP under sterile procedure and is more cost-effective and convenient for the patient.13 ICP is not without its own risks, however, including the risk for urethral strictures, false passages, incontinence, bladder distention related to insufficient or ineffective catheterization, and an increased reliance on caregivers.14 Bladder management with condom catheters or other external collection systems may be an option in patients without neurogenic bladder, but it can lead to unsafe pressures that promote bladder distention (which leaves a patient more susceptible to infection) and renal impairment in patients with neurogenic bladder, such as after SCI. In additional to the medical and quality considerations, the Consortium for Spinal Cord Medicine clinical practice guidelines for bladder management after SCI also highlight important psychosocial considerations, including adequate planning for transitions of care from hospital to home, body image issues, and difficulty with ICP due to dexterity, spasticity, and/or reliable caregiver support.14 Therefore, in persons with SCI and other conditions resulting in neurogenic bladder, the decision on a long-term bladder management strategy is a multifactorial and dynamic process, with patient quality of life at the center (Figure 1).15,16
Figure 1.
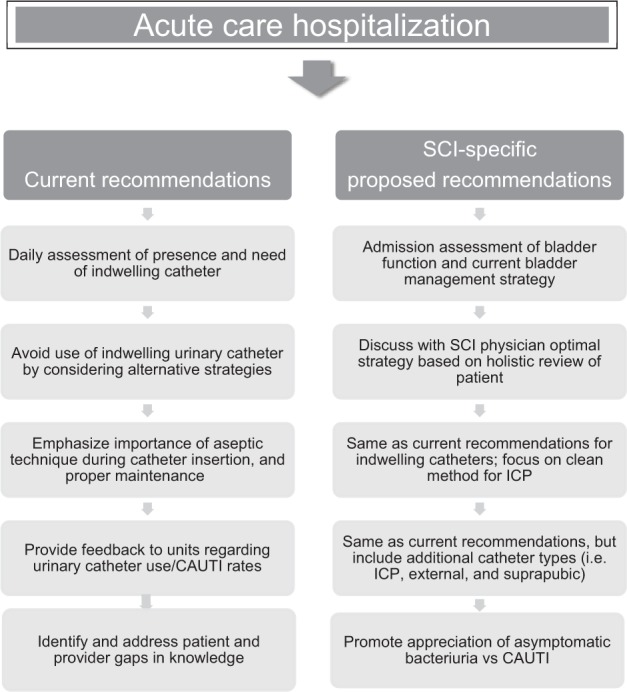
Catheter-associated urinary tract infection (CAUTI) bundle tailored to spinal cord injury (SCI) population. ICP = intermittent catheterization program.
Furthermore, the current definition of CAUTI used in surveillance is problematic in the SCI population. Due to impaired sensation below the neurologic level of injury, many people with SCI cannot appreciate suprapubic pain or dysuria. If their bladder is managed by an indwelling catheter, they will not appreciate frequency or urgency; in addition, the rates of asymptomatic bacteriuria are very high in these cases, making the diagnosis of true infection even more difficult.6 Given this, a greater focus on catheter-related harms, rather than just infection, is warranted. Multiple authors have described other noninfectious catheter harms, which include asymptomatic bacteria associated with inappropriate antibiotic use, trauma, patient suffering, and the cost to society.17–19 Although immobility is often included as a catheter-associated harm in the non-SCI population, properly managed indwelling catheters can afford many persons with SCI greater freedom of mobility, further highlighting the nuances of choosing a bladder drainage strategy in this population.
Interventions (including provider and patient education) aimed at minimizing unnecessary urine culture collection and highlighting the importance of adequate bladder drainage in the setting of neurogenic bladder and proper catheter management could simultaneously improve performance on the CAUTI metric and improve patient safety. Figure 1 describes current CAUTI bundle surveillance strategies and summarizes the SCI-specific suggestions to enhance these strategies.
Prevention of CAUTI
Challenge: Preventing CAUTI in a high-risk population such as in persons with SCI has been a popular area of research. Prophylactic antibiotics, nutraceuticals, and varying catheter materials have been examined for their potential in reducing infection after SCI.
Solution: Understand the strengths and limitations of the current evidence and provide recommendations for further studies.
Given that CAUTI has a high incidence and leads to significant morbidity amongst people with SCI and indwelling catheters, pharmacologic methods of prevention would be desirable. Prophylactic antibiotics have been explored, most recently in a retrospective study looking at long-term use of nitrofurantoin. This study showed a significantly decreased incidence of UTI and mean days between positive urine cultures in participants with long-term nitrofurantoin use versus those without.20 Ciprofloxacin was shown in a small, randomized controlled trial to prevent UTI/CAUTI after SCI, but this report from 1994 must be balanced with newer evidence about the significant harms of fluoroquinolone use.21,22
In regard to nutraceuticals, cranberry supplements have been studied extensively, with conflicting randomized controlled trials showing reduction in UTI versus no benefit in SCI.23 A Cochrane review examining the topic could not endorse cranberry use as an effective prophylactic measure.24 Phé et al25 performed an open-label feasibility study of using D-mannose as a preventative CAUTI measure in 22 neurogenic bladder patients with multiple sclerosis who had reported recurrent CAUTIs; a significant reduction in the monthly rate of UTIs was seen.
Bonfill et al26 assessed the efficacy of silver alloy–coated urinary catheters as a preventative measure for CAUTI after SCI in a small randomized controlled trial; the results did not support their routine use. Cardenas et al27 explored whether intermittent catheterization with a low friction hydrophilic-coated catheter delays the onset of the first symptomatic UTI and reduces the number of symptomatic UTIs in patients with acute SCI compared with intermittent catheterization (IC) with standard, uncoated catheters. The investigation found a significant delay of onset to the first symptomatic CAUTI among participants who utilized the hydrophilic-coated catheters and a decrease in the incidence of antibiotic-treated symptomatic UTIs. However, this study was not blinded, and UTI was diagnosed by a patient-reported inventory of symptoms utilizing a nonstandard definition (which included foul smelling and/or cloudy urine among the diagnostic criteria).
In summary, trials examining prophylactic antibiotics, nutraceuticals, and varying catheter materials for the prevention of UTI/CAUTI have been limited by small sample sizes, bias due to study design, and varying definitions of UTI/CAUTI, therefore providing a weak base of support to any of these methods for CAUTI prevention in SCI. In regard to prophylactic antibiotic use, more convincing evidence for their use would be needed to counter balance their adverse effects and potential for promoting of antibiotic resistance. Further research should focus on prospective, randomized controlled trials of potential pharmaceutical and nutraceutical agents using a broadly accepted definition of CAUTI (such as one provided by the Infectious Diseases Society of America).28
Diagnosis and management of CAUTI
Challenge: Diagnosis of CAUTI is especially challenging after SCI due to the atypical signs and symptoms of infection. This leads to overdiagnosis of CAUTI when ASB truly exists and subsequent overtreatment with antibiotics. Rates of multidrug-resistant organisms in persons with SCI are high. As diagnostically challenging a condition CAUTI is to providers, it poses a significant burden to persons with SCI.
Solution: Develop a more robust evidence base to guide efficient and accurate diagnosis of CAUTI after SCI. A better understanding of the pathogenesis of the transition from asymptomatic bacteriuria to symptomatic, tissue-invasive urinary tract infection in SCI will be essential to finding biomarkers to support the diagnosis of CAUTI. Explore and expand the role of non-antibiotic treatments for CAUTI, such as bacteriophages. Explore the perspective of the SCI patient around goals of treatment, issues with medication adherence, and bladder management burden.
Achieving an accurate diagnosis of CAUTI based on clinical practice guidelines has been shown to be a cognitively taxing activity for providers who are assessing non-SCI patients; the challenge is even greater in diagnosing CAUTI in persons with SCI.29 Persons with SCI often do not present with typical signs and symptoms (dysuria, suprapubic pain, frequency) due to lack of sensation and bladder management strategy, which makes the diagnosis is this patient population more difficult. The Infectious Diseases Society of America guidelines for CAUTI recognize this and include SCI-specific symptoms such as increased spasticity and autonomic dysreflexia in their decision algorithms,28 but limited evidence on sensitivity/specificity of these symptoms exists. Cloudy or foul smelling urine are often considered to be symptoms of CAUTI, but no studies have delineated the clinical significance of these findings, even if they are of recent onset.28
The rate of ASB in persons with SCI is very high, given how common bladder instrumentation is in this patient population, and ASB does not require antibiotic treatment (except in pregnancy and prior to urologic procedures).30 However, a wide gap remains between guidelines and actual practice for urine testing and subsequent bacteriuria management. For many clinicians, translating ASB and CAUTI guidelines to the bedside proves difficult when faced with a “positive” urinalysis or urine culture result.31,32 In a small pilot study, Skelton et al demonstrated that a positive urine culture drove antibiotic use (including a 35% rate of unnecessary treatment for ASB) in the SCI population.3
Given how subjective the current definition of CAUTI currently is, studies into objective measures such as infection biomarkers have been pursued to aid in diagnosis. Nanda et al33 conducted a systematic review of the available literature in 2009 and found that procalcitonin showed promise in aiding efficient diagnosis and initiation of treatment of UTI and pyelonephritis in the pediatric population, but there was limited evidence in adults. Interleukin-6 showed some promise in aiding to distinguish UTI from pyelonephritis in adults but needed further validation. The authors commented on the paucity of evidence on the efficacy of any biomarker in special populations such as SCI.
There have been successful interventions aimed at improving the accuracy of diagnosis of CAUTI in non-SCI populations.8,31 Trautner et al34–36 have designed and validated an intervention to decrease guideline-discordant management of catheter-associated ASB in veterans in hospitals and nursing homes. This intervention, entitled Kicking CAUTI: The No Knee-Jerk Antibiotics Campaign, encourages clinicians to stop and think before ordering urine cultures and prescribing antibiotics for CAUTI. In a trial involving two Veterans Health Administration medical centers, one intervention, and one control site, the intervention significantly decreased inappropriate screening for and treatment of ASB by 71% and 75%, respectively. Of note, the intervention had greater positive impact in long-term care than in acute care; long-term care is a difficult setting for diagnosis of CAUTI because many residents are not able to communicate the presence or absence of urinary symptoms. Therefore, this successful intervention provides a starting point for improving diagnostic efficacy of CAUTI in people with SCI, but the intervention will need tailoring to fit the needs to SCI patients and providers.
Persons with SCI are exposed to many courses of antibiotics over the course of their lifetime — some necessarily, some unnecessarily. This can lead to the emergence of multidrug-resistant organisms, which have been shown to be very prevalent in the SCI population. Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis are among the most commonly isolated species.37,38 Thus, antibiotic-sparing treatment options should be considered for both prevention and treatment of CAUTI in SCI. One of these novel treatments include bacteriophages. Bacteriophage (often called phage) are viruses that infect only bacteria, and each strain of phage typically kills only a single species of bacteria. Phage hold considerable promise for treating the multidrug-resistant, biofilm-associated pathogens that cause CAUTI and occasionally urosepsis in persons with SCI. Phage can kill multidrug-resistant bacteria by mechanisms different from those of antibiotics, thus antibiotic resistance does not create phage resistance among bacteria.39–41 When antibiotic-resistant bacteria become resistant to phage, in some cases the bacterial sensitivity to antibiotics is restored.42–46 Some bacteriophages produce depolymerase enzymes that enable them to disrupt the extracellular polysaccharide matrix of biofilms.47 Our work demonstrates that phage are effective against pathogenic Pseudomonas aeruginosa biofilms on urinary catheters.48 The high level of specificity of phage may allow targeting of the pathogen causing CAUTI while avoiding harm to other species in the host microbiome.49,50 Thus phage specificity confers a marked advantage over broad-spectrum antibiotics, particularly in terms of subsequent risk of Clostridiodes difficile colitis; antibiotic use is a known risk factor for recurrent C. difficile infections in veterans with SCI.51 We are currently studying bacteriophage as a means to treat CAUTI in SCI both in vitro52 and in an animal model (manuscript under preparation), but phage therapy under compassionate use protocols has had recent successes in several non-SCI cases. For example, Aslam et al53 described a successful outcome for a multidrug-resistant Staphylococcus aureus left ventricular device infection using a combination of three phages intravenously (AB-SA01, 3 × 10 9 plaque-forming units) every 12 hours for 28 days in addition to antibiotics (cefazolin 2 g IV every 8 hours and minocycline 100 mg orally twice daily). Schooley et al54 described successful treatment of an Acinetobacter baumannii pancreatic pseudocyst using several phage cocktails administered intraabdominally and intravenously over the course of 18 weeks. Dedrick et al55 described eradication of a disseminated Mycobacterium abscessus infection in a pediatric patient with cystic fibrosis with an intravenous three-phages cocktail (109 plaque forming units per dose of each phage) every 12 hours for at least 32 weeks.
Patient-centered care is one of the Institute of Medicine's six quality aims.56 Thus, it is important to include patients in every step of intervention development. We theorize that an intervention to promote antibiotic stewardship in the SCI population cannot be successful without patient engagement. Patient engagement includes (a) understanding patient attitudes about antibiotics and the importance of adhering to medications as prescribed and (b) understanding how this population prefers to receive health care education. We are currently conducting a mixed-methods study utilizing qualitative semistructured interviews and a medication adherence survey of SCI patients to further explore this topic.
Conclusion
Future studies should focus on SCI patient and provider attitudes about diagnosing ASB and CAUTI, knowledge of clinical practice guidelines concerning management of ASB and UTI, and barriers to enacting the guidelines in clinical practice. Understanding these gaps and designing an intervention to address them have the potential to make the approach to diagnosis and management of ASB/UTI in the SCI population more systematic, evidence-based, and patient-centered, while also decreasing antibiotic overuse.
Acknowledgments
Funding disclosure: This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413, Drs. Skelton-Dudley and Trautner), HSR&D Career Development Award 1 IK2 HX002484-02 (Skelton-Dudley), and VA RR&D I01RX002595-01A2 (Trautner).
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
REFERENCES
- 1.Agency for Healthcare Research and Quality; Eliminating CAUTI: Interim Data Report. Executive Summary. Published 2013. Accessed September 19, 2019. https://www.ahrq.gov/professionals/quality-patient-safety/cusp/cauti-interim/cauti-interim1.html. [Google Scholar]
- 2.Mody L, Meddings J, Edson BS et al. Enhancing resident safety by preventing healthcare-associated infection: A national initiative to reduce catheter-associated urinary tract infections in nursing homes. Clin Infect Dis. 2015;61(1):86–94. doi: 10.1093/cid/civ236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skelton F, Grigoryan L, Holmes SA, Poon IO, Trautner B. Routine urine testing at the spinal cord injury annual evaluation leads to unnecessary antibiotic use: A pilot study and future directions. Arch Phys Med Rehabil. 2018;99(2):219–225. doi: 10.1016/j.apmr.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skelton F, Salemi JL, Akpati L et al. Genitourinary complications are a leading and expensive cause of emergency department and inpatient encounters for persons with spinal cord injury. Arch Phys Med Rehabil. 2019;100(9):1614–1621. doi: 10.1016/j.apmr.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Spinal Cord Injury Statistical Center Spinal Cord Injury Facts and Figures at a Glance: 2018. Accessed September 19, 2018. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202018.pdf.
- 6.Nicolle LE, Gupta K, Bradley SF et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of Americaa. Clin Infect Dis. 2019;68(10):e83–e110. doi: 10.1093/cid/ciy1121. [DOI] [PubMed] [Google Scholar]
- 7.National Quality Forum National Healthcare Safety Network (NHSN) Catheter-associated Urinary Tract Infection (CAUTI) outcome measure. 2017 Accessed October 17, 2018. http://www.qualityforum.org/QPS/MeasureDetails.aspx?standardID=1121&print=0&entityTypeID=1.
- 8.Saint S, Greene MT, Krein SL et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111–2119. doi: 10.1056/NEJMoa1504906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moola S, Konno R. A systematic review of the management of short-term indwelling urethral catheters to prevent urinary tract infections. JBI Libr Syst Rev. 2010;8(17):695–729. doi: 10.11124/01938924-201008170-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fasugba O, Koerner J, Mitchell BG, Gardner A. Systematic review and meta-analysis of the effectiveness of antiseptic agents for meatal cleaning in the prevention of catheter-associated urinary tract infections. J Hosp Infect. 2017;95(3):233–242. doi: 10.1016/j.jhin.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Huang K, Liang J, Mo T, Zhou Y, Ying Y. Does periurethral cleaning with water prior to indwelling urinary catheterization increase the risk of urinary tract infections? A systematic review and meta-analysis. Am J Infect Control. 2018;46(12):1400–1405. doi: 10.1016/j.ajic.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2013;(11) doi: 10.1002/14651858.CD004375.pub4. CD004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy LM, Cleary J, Ahern S et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865–870. doi: 10.1111/j.1532-5415.1995.tb05528.x. [DOI] [PubMed] [Google Scholar]
- 14.Consortium for Spinal Cord Medicine Bladder management for adults with spinal cord injury: A clinical practice guideline for health-care providers. J Spinal Cord Med. 2006;29(5):527–573. [PMC free article] [PubMed] [Google Scholar]
- 15.Sugimura T, Arnold E, English S, Moore J. Chronic suprapubic catheterization in the management of patients with spinal cord injuries: Analysis of upper and lower urinary tract complications. BJU Int. 2008;101(11):1396–1400. doi: 10.1111/j.1464-410X.2007.07404.x. [DOI] [PubMed] [Google Scholar]
- 16.Katsumi HK, Kalisvaart JF, Ronningen LD, Hovey RM. Urethral versus suprapubic catheter: Choosing the best bladder management for male spinal cord injury patients with indwelling catheters. Spinal Cord. 2010;48(4):325–329. doi: 10.1038/sc.2009.134. [DOI] [PubMed] [Google Scholar]
- 17.Fakih MG, Gould CV, Trautner BW et al. Beyond infection: Device utilization ratio as a performance measure for urinary catheter harm. Infect Control Hosp Epidemiol. 2016;37(3):327–333. doi: 10.1017/ice.2015.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leuck AM, Wright D, Ellingson L, Kraemer L, Kuskowski MA, Johnson JR. Complications of Foley catheters--Is infection the greatest risk? J Urol. 2012;187(5):1662–1666. doi: 10.1016/j.juro.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 19.Saint S, Trautner BW, Fowler KE et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018;178(8):1078–1085. doi: 10.1001/jamainternmed.2018.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chew AB, Suda KJ, Patel UC et al. Long-term prescribing of nitrofurantoin for urinary tract infections (UTI) in veterans with spinal cord injury (SCI) J Spinal Cord Med. 2018:1–9. doi: 10.1080/10790268.2018.1488096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biering-Sørensen F, Høiby N, Nordenbo A, Ravnborg M, Bruun B, Rahm V. Ciprofloxacin as prophylaxis for urinary tract infection: Prospective, randomized, cross-over, placebo controlled study in patients with spinal cord lesion. J Urol. 1994;151(1):105–108. doi: 10.1016/s0022-5347(17)34882-6. [DOI] [PubMed] [Google Scholar]
- 22.LeMaire SA, Zhang L, Luo W et al. Effect of ciprofloxacin on susceptibility to aortic dissection and rupture in mice. JAMA Surg. 2018;153(9) doi: 10.1001/jamasurg.2018.1804. e181804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess MJ, Hess PE, Sullivan MR, Nee M, Yalla SV. Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord. 2008;46(9):622–626. doi: 10.1038/sc.2008.25. [DOI] [PubMed] [Google Scholar]
- 24.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD001321.pub5. CD001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phé V, Pakzad M, Haslam C et al. Open label feasibility study evaluating D-mannose combined with home-based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourol Urodyn. 2017;36(7):1770–1775. doi: 10.1002/nau.23173. [DOI] [PubMed] [Google Scholar]
- 26.Bonfill X, Rigau D, Esteban-Fuertes M et al. Efficacy and safety of urinary catheters with silver alloy coating in patients with spinal cord injury: A multicentric pragmatic randomized controlled trial. The ESCALE trial. Spine J. 2017;17(11):1650–1657. doi: 10.1016/j.spinee.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Cardenas DD, Moore KN, Dannels-McClure A et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: A prospective, randomized, multicenter trial. PM R. 2011;3(5):408–417. doi: 10.1016/j.pmrj.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Hooton TM, Bradley SF, Cardenas DD et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 29.Naik AD, Skelton F, Amspoker AB, Glasgow RA, Trautner BW. A fast and frugal algorithm to strengthen diagnosis and treatment decisions for catheter-associated bacteriuria. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174415. e0174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolle LE, Bradley S, Colgan R et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 31.Lo E, Nicolle LE, Coffin SE et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(suppl 2):S32–47. [PubMed] [Google Scholar]
- 32.Loeb M, Brazil K, Lohfeld L et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669. doi: 10.1136/bmj.38602.586343.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanda N, Juthani-Mehta M. Novel biomarkers for the diagnosis of urinary tract infection--a systematic review. Biomark Insights. 2009;4:111–121. doi: 10.4137/bmi.s3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trautner BW, Bhimani RD, Amspoker AB et al. Development and validation of an algorithm to recalibrate mental models and reduce diagnostic errors associated with catheter-associated bacteriuria. BMC Med Inform Decis Mak. 2013;13:48. doi: 10.1186/1472-6947-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trautner BW, Grigoryan L, Petersen NJ et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120–1127. doi: 10.1001/jamainternmed.2015.1878. [DOI] [PubMed] [Google Scholar]
- 36.Trautner BW, Petersen NJ, Hysong SJ, Horwitz D, Kelly PA, Naik AD. Overtreatment of asymptomatic bacteriuria: Identifying provider barriers to evidence-based care. Am J Infect Control. 2014;42(6):653–658. doi: 10.1016/j.ajic.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Fitzpatrick MA, Suda KJ, Safdar N et al. Changes in bacterial epidemiology and antibiotic resistance among veterans with spinal cord injury/disorder over the past 9 years. J Spinal Cord Med. 2017:1–9. doi: 10.1080/10790268.2017.1281373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suda KJ, Patel UC, Sabzwari R et al. Bacterial susceptibility patterns in patients with spinal cord injury and disorder (SCI/D): An opportunity for customized stewardship tools. Spinal Cord. 2016;54(11):1001–1009. doi: 10.1038/sc.2016.38. [DOI] [PubMed] [Google Scholar]
- 39.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1(2):111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen RC, Pfrunder-Cardozo KR, Meinel D, Egli A, Hall AR. Associations among antibiotic and phage resistance phenotypes in natural and clinical Escherichia coli isolates. MBio. 2017;8(5) doi: 10.1128/mBio.01341-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sybesma W, Zbinden R, Chanishvili N et al. Bacteriophages as potential treatment for urinary tract infections. Front Microbiol. 2016;7:465. doi: 10.3389/fmicb.2016.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres-Barcelo C, Arias-Sanchez FI, Vasse M, Ramsayer J, Kaltz O, Hochberg ME. A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106628. e106628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep. 2016;6 doi: 10.1038/srep26717. 26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres-Barcelo C, Hochberg ME. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 2016;24(4):249–256. doi: 10.1016/j.tim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Yosef I, Manor M, Kiro R, Qimron U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 2015;112(23):7267–7272. doi: 10.1073/pnas.1500107112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rios AC, Moutinho CG, Pinto FC et al. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol Res. 2016;191:51–80. doi: 10.1016/j.micres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Abedon ST. Ecology of anti-biofilm agents I: Antibiotics versus bacteriophages. Pharmaceuticals (Basel) 2015;8(3):525–558. doi: 10.3390/ph8030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao KS, Lehman SM, Tweardy DJ, Donlan RM, Trautner BW. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J Appl Microbiol. 2012;113(6):1530–1539. doi: 10.1111/j.1365-2672.2012.05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan BK, Abedon ST, Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013;8(6):769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 50.Galtier M, De Sordi L, Maura D et al. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ Microbiol. 2016;18(7):2237–2245. doi: 10.1111/1462-2920.13284. [DOI] [PubMed] [Google Scholar]
- 51.Ramanathan S, Johnson S, Burns SP et al. Recurrence of Clostridium difficile infection among veterans with spinal cord injury and disorder. Am J Infect Control. 2014;42(2):168–173. doi: 10.1016/j.ajic.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Mapes AC, Trautner BW, Liao KS, Ramig RF. Development of expanded host range phage active on biofilms of multi-drug resistant. Bacteriophage. 2016;6(1) doi: 10.1080/21597081.2015.1096995. e1096995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aslam S, Pretorius V, Lehman SM, Morales S, Schooley RT. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J Heart Lung Transplant. 2019;38(4):475–476. doi: 10.1016/j.healun.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Schooley RT, Biswas B, Gill JJ et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61(10) doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dedrick RM, Guerrero-Bustamante CA, Garlena RA et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25(5):730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.US Institute of Medicine Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. Committee on Quality of Health Care in America. [Google Scholar]


