Abstract
Objective: To assess the current evidence with regard to the effects of body weight–supported treadmill training (BWSTT) on cardiovascular and pulmonary function in people with spinal cord injury (SCI) with a focus on outcomes of heart rate (HR), blood pressure (BP), and respiratory parameters. Methods: A systematic literature search was conducted through MEDLINE/PubMed, the Cumulative Index to Nursing and Allied Health Literature, and Physiotherapy Evidence Database. Clinical trials involving adults with SCI and focusing on the effects of BWSTT on HR, BP, and respiratory measurements were included. The quality of included studies was assessed using the Downs and Black scale. The level of evidence of each study was identified using the Spinal Cord Injury Rehabilitation Evidence system. Results: Nine studies that met inclusion criteria were evaluated and included in this review. Overall, the quality index of all included studies was low. All studies scored less than 21 out of 28 on the Downs and Black scale. The level of evidence varied from level 2 to level 4. Level 4 evidence supports the use of BWSTT to decrease resting and exercise HR and improve heart rate variability. The use of BWSTT to improve respiratory parameters after SCI is supported by one study with level 2 evidence. The evidence that supports the use of BWSTT to improve resting BP is inconclusive. Conclusion: There has been low to moderate evidence to support the use of BWSTT in individuals with SCI to improve cardiovascular and pulmonary health. Future randomized controlled trials are needed to investigate the effect of BWSTT on cardiovascular and pulmonary function in people with SCI and compare BWSTT to other physical rehabilitation interventions.
Keywords: body weight–supported treadmill training, blood pressure, heart rate, locomotion training, respiratory parameters, spinal cord injury, walking training
Spinal cord injury (SCI) is a serious medical condition that leads to loss of or impairment in sensorimotor function, which negatively impacts the quality of life. Individuals with SCI are susceptible to various secondary complications, including deterioration in cardiovascular and respiratory health.1,2 It has been estimated that the prevalence rate of cardiovascular disease (CVD) in the SCI population (30%–50%) is significantly higher compared to the able-bodied population (5%–10%).3 A study reported that the SCI population has a 2.72 times higher risk of heart disease and a 3.72 times higher risk of stroke compared to the general population.4 In terms of morality after CVD, a cohort study that followed individuals with SCI for 5 years after discharge from inpatient rehabilitation found that 37% of individuals died due to cardiovascular causes.5
Physical inactivity is associated with a decrease in cardiovascular fitness (eg, elevated heart rate [HR]) and consequently an increased risk of CVD.6–8 Lack of physical activity or prolonged sitting observed in many SCI survivors due to limited functional mobility can contribute to elevated HR and other risk factors for CVD.3,6 Elevated resting HR is more common in individuals with SCI at T1 or below (paraplegia) as compared to those with cervical SCI (quadriplegia).8,9 Studies report that people with SCI, especially those with paraplegia, have higher resting HR compared to able-bodied people.7–10 Furthermore, the cardiac autonomic system can be altered after SCI due to the sedentary lifestyle and lack of mobility resulting from the injury.11 Heart rate variability (HRV) can be used as a sensitive measure to determine cardiac autonomic function. Reduced HRV after the injury is associated with an increased risk of heart diseases.12,13 Past studies show reduced HRV in individuals with SCI in comparison with able-bodied individuals.11,14 Physically inactive individuals with SCI have less HRV than those who are physically active.11
High blood pressure (BP) (ie, hypertension) is another factor that can increase the risk of cardiovascular disease.15 Hypertension is common in people living with long-term SCI, and it is more prevalent in people with paraplegia compared to quadriplegia.16–18 Previous studies report a high prevalence of hypertension among people with SCI, especially those with paraplegia, as compared to able-bodied people.16,19 Sedentary lifestyle after SCI plays an important role in the development of hypertension. Other risk factors such as obesity, hypercholesterolemia, diabetes, smoking, and age can also contribute to an increased risk of hypertension after SCI.16
The paralysis of the respiratory musculature following SCI can result in an alteration of the mechanical properties of the lung and chest wall.20 Pulmonary function may decline after SCI because of decreased muscle strength of the diaphragm, respiratory, and abdominal muscles as well as a lack of sufficient mobility.21 Reduction in pulmonary function after SCI is reflected by decreases in spirometric and lung volume parameters and static mouth pressures.22 The weakness of respiratory muscles can result in an ineffective cough and a tendency toward mucus retention, which might increase susceptibility to respiratory diseases.22 Respiratory impairments are more severe in people with cervical injury compared to those with thoracic or lumbar injury due to a disruption in the function of the majority of the respiratory and abdominal muscles.22 Respiratory deficiency can lead to secondary problems such as respiratory infectious diseases, which are the leading causes of death in people with chronic SCI.23
Participating in regular exercise is necessary to prevent or reduce secondary complications after SCI. Aerobic exercise using arm cycling or functional electrical stimulation (FES) leg cycling has been utilized to improve cardiovascular and pulmonary health in the SCI population. However, exercise using these modalities has shown inconsistent results regarding its effects on cardiovascular and pulmonary health in the SCI population.24–27 Limitations of these modalities have been discussed in the literature.28–31 A major limitation of arm cycling or FES leg cycling exercise is the lack of sustainable activities of large leg muscles, leading to insufficient challenges to the cardiopulmonary system.32,33 As an alternative, body weight–supported treadmill training (BWSTT) is a tool that is widely used in the rehabilitation program for people with neurological conditions. Even though most studies have focused on investigating the effectiveness of BWSTT on motor function and concluded that it might be an appropriate intervention to improve walking ability, promising findings from previous studies have shown that regular walking training using BWSTT can help to improve cardiovascular and pulmonary health in the SCI population.34–42 Therefore, this systematic review aimed to qualitatively assess the current evidence with regard to the effects of walking training using BWSTT on cardiovascular and pulmonary health among people with SCI. The focus of the study is on the effects of BWSTT on HR and BP measurements and respiratory parameters.
Methods
Search strategy
A systematic literature search was conducted to examine the effect of BWSTT on measures of cardiovascular and pulmonary health in people with SCI. MEDLINE (PubMed), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Physiotherapy Evidence Database (PEDro) were searched. The search was limited to clinical trial studies in humans, with adult patients (≥18 years), and written in English. It was conducted for the time period of January 2004 until May 2018.
The search strategy included the terms for target population (spinal cord injury, paraplegia, quadriplegia or tetraplegia), outcome measures (heart rate, blood pressure, pulmonary function, respiratory function, and respiratory parameters), and intervention (locomotor training, walking training, gait training, or body weight support treadmill training). Different combinations of the terms were made using “AND” and “OR” in order to achieve a specific selection of literature (see Table 1).
Table 1.
Key words and combination of key word used in the search
| Population | Outcomes | Intervention |
|---|---|---|
|
|
|
Note: “AND” was used between terms in population, outcomes, and intervention columns. The terms in the columns are allied with “OR.”
Study eligibility criteria
Inclusion criteria were the following:
Studies with adult patients (≥18 years) with traumatic or nontraumatic SCI (cervical, thoracic, and lumbar), complete or incomplete lesions, and American Spinal Injury Association Impairment Scale (AIS) of A, B, C, or D.
Treadmill walking training, including manual-assisted BWSTT, robotic-assisted BWSTT, and treadmill walking training in water.
Studies focused on measurements of HR and BP and respiratory parameters after a course of treadmill walking training.
Clinical trial studies, including randomized controlled (RCTs), quasi-experimental, or pre-experimental trials.
Published in English.
Exclusion criteria were (a) overground walking training and (b) animal studies or studies on children.
Study selection
After removing duplicated studies, two independent researchers (R.A and A.A) screened the articles by reading titles and abstracts. Then, the full text of articles relevant to study objectives was read in detail to determine eligibility (see Figure 1). Disagreements on study selection were resolved by discussions between two researchers (R.A. and A.A)
Figure 1.
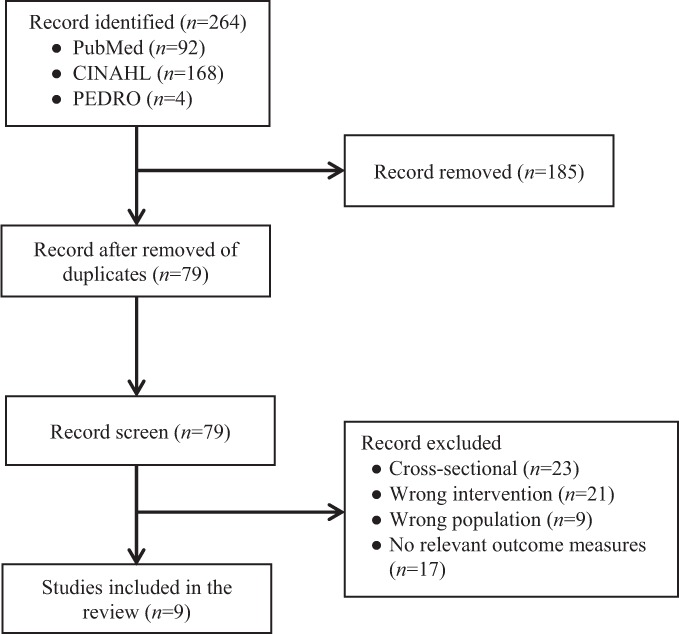
Flowchart of the literature search and selection process.
Data extraction
The study information was extracted by two reviewers (R.A. and A.A.) independently. The following data were extracted from included studies: author's name, year, study design, sample size, participants' characteristics (gender, age, injury level on the SCI, grade based on AIS, and time since injury), intervention program (length and frequency), and outcomes related to measurements of HR, BP, and respiratory parameters. There were no disagreements between the reviewers regarding data extraction.
Assessment of quality study and level of evidence
Included studies were assessed using the modified Downs and Black scale to determine their methodological quality. The Downs and Black scale is composed of 27 questions to assess the quality of a study across five categories, including reporting (10 questions), external validity (3 questions), internal validity (bias and confounding; 13 questions), and power (one question) (see Appendix).43 The total score of the scale ranges from 0 to 28. A higher score indicates a better quality of methodology. From the percentage of derived total score, studies were classified as high (>75%), moderate (50%–74%), or low quality (<50%). The level of evidence of each study was identified using the Spinal Cord Injury Rehabilitation Evidence (SCIRE) system, a five-level system that differentiates between studies of differing quality and incorporates the types of research designs commonly used in rehabilitation research (see Table 2).13 This scale has been used in published systematic review studies of exercise training in people with SCI.44,45 Two researchers evaluated and scored studies independently. In case of disagreement between two evaluators, a consensus was made through discussion.
Table 2.
Level of evidence and criteria based on the Spinal Cord Injury Rehabilitation Evidence (SCIRE) system
| Level of evidence | Criteria |
|---|---|
| Level 1 |
|
| Level 2 (n = 2) |
|
| Level 3 |
|
| Level 4 (n = 7) |
|
| Level 5 |
|
Note: The PEDro scale is developed by the Physiotherapy Evidence Database to determine the quality of clinical trials (high quality = score 6–10, fair quality = 4–5, poor quality = score ≤ 3). RCT = randomized controlled trail.
Results
An overview of the results of the literature search and screening process is provided in Figure 1. The electronic database search retrieved 264 articles. Removal of duplicates within and between the individual databases left 79 articles for further examination. Of the 79 retrieved articles, 53 articles were excluded after screening for titles and abstracts. The remaining 26 articles were screened by reading the full text to determine eligibility. Specific reasons for article exclusion are presented in Figure 1. Nine articles were evaluated in detail and are included in this review study.
A summary of the study designs, participants' characteristics, interventions, and outcome measures for each of the nine studies reviewed is provided in Table 3. The number of participants in the included studies ranged from 6 to 52 participants; a total of 121 participants took part in those studies. They included individuals with incomplete SCI, complete SCI, or both. The duration of interventions ranged from 2 to 5 times per week for 4 weeks to 6 months. Seven studies examined the effects of walking training on HR measurements.34–40 Four studies examined the effects of walking training on BP measurements.34–36,40 Three studies examined the effects of walking training on respiratory parameters.36,41,42
Table 3.
Summary of the studies, participant characteristics, interventions, outcome measures, and main findings (N = 9)
| Author | Study design | Evidence level | Participants | Intervention | Outcome measures | Main findings |
|---|---|---|---|---|---|---|
| Ditor et al, 200434 | Single group, pre- and posttest | Level 4 | 8 individuals with chronic cervical SCI | BWSTT with manual assistance |
|
|
| [6 men, 2 women; mean age, 27.6 y; SCI level: C4-C5; AIS: B-C; mean postinjury, 9.6 y] | [3×/wk for 6 mo] | |||||
| Ditor et al, 200535 | Single group, pre- and posttest | Level 4 | 6 individuals with chronic SCI | BWSTT with manual assistance |
|
|
| [4 men, 2 women; mean age, 37.77±15.4 y; SCI level: C4-T12; AIS: A-B; mean postinjury, 7.67±9.4 y] | [3×/wk for 4 mo] | |||||
| Soyupek et al, 200936 | Single group, pre- and posttest | Level 4 | 8 individuals with SCI | BWSTT with manual assistance |
|
|
| [6 men, 2 women; mean age, 40.75±13.93 y; SCI level: C6-L1; AIS: B-D] | ||||||
| [5×/wk for 6 wk] | ||||||
| Hoekstra et al, 201337 | Single group, pre- and posttest | Level 4 | 10 individuals with chronic SCI | BWSTT with robotic assistance | Cardiopulmonary fitness, including:
Walking training intensity:
|
|
| [4 men, 6 women; mean age, 49±14 y; SCI level: C3-L2; AIS: C-D; mean postinjury, 9±10 y] | [2–3×/wk; total of 24 sessions] | |||||
| Millar et al, 200938 | Randomized crossover | Level 2 | 6 individuals with chronic SCI | Group 1: BWSTT with manual assistance |
|
|
| [6 men; mean age, 37.1±7.7 y; SCI level: C5-T10; AIS: A-C; mean postinjury, 5.0±4.4 y] | Group 2: Passive HUTT | |||||
| [3×/wk for 4 wk] |
Table 3.
Summary of the studies, participant characteristics, interventions, outcome measures, and main findings (N = 9) (CONT.)
| Author | Study design | Evidence level | Participants | Intervention | Outcome measures | Main findings |
|---|---|---|---|---|---|---|
| Stevens et al, 201539 | Single group, pre- and posttest | Level 4 | 11 individuals with chronic incomplete SCI | Underwater treadmill training |
|
|
| [7 men and 4 women; mean age, 48±12 y; SCI level: C2-L2; AIS: C-D; mean postinjury, 5±8 y] | [3×/wk for 8 wk] | |||||
| Carvalho et al, 200540 | Single group, pre- and posttest | Level 4 | 12 individuals with complete cervical SCI | BWSTT with manual assistance and NES |
|
|
| [12 men; mean age, 33.8±33.73 y; SCI level: C4-C7; AIS: A; median postinjury, 77.58 months] | [2×/wk for 3 months] | |||||
| Terson de Paleville et al, 201341 | Single group, pre- and posttest | Level 4 | 8 individuals with chronic SCI | BWSTT with manual assistance |
|
|
| [7 men, 1 woman; mean age, 37±18 y; SCI level: C3-T12; AIS: A-D; mean postinjury, 25±12 months] | [5×/wk; total of 62±10 sessions] | |||||
| Tiftik et al, 201542 | Quasi-experimental study | Level 2 | 52 individuals with SCI [Group A: 19 men and 7 women; mean age, 31.2±12.7 y; SCI level: 7 C1-C8, 6 T1-T12, 13 L1-S4/5; mean postinjury, 10.6±13.5 months] | Divided into two groups: |
|
|
| Group A: BWSTT + rehabilitation program Group B: rehabilitation program alone | ||||||
| [Group B: 21 men and 5 women; mean age, 35.6±15.0 y; SCI level: 10 C1-C8, 9 T1-T12, 7 L1-S4/5; mean postinjury, 14.5±12.5 months] | ||||||
| [3 sessions/wk for 4 wk] |
Note: AIS = American Spinal Injury Association Impairment Scale; BP = blood pressure; BPV = blood pressure variability, BWSTT = body weight–supported treadmill training; EMF = electromyography; FEV1 = forced expiratory volume 1 second; FVC = forced vital capacity; HF = high-frequency power; HR = heart rate; HRV = heart rate variability; HUTT = head-uptilt training; IC = inspiratory capacity; LF = low-frequency power; METs = metabolic equivalents; MEPT = maximum expiratory pressure task; NES = neuromuscular electrical stimulation; SBP = systolic blood pressure.
Quality assessment for included studies
The results of the quality review are presented in Table 4. Overall, the quality index of all included studies was low. All studies scored less than 21 out of 28 on the Downs and Black scale. All studies fulfilled most of the criteria for reporting. The objectives of the study, the main outcomes, the characteristics of the patients, the interventions of interest, and the main findings were clearly described in these studies.34–42 However, all studies34–37,39–42 except one38 were rated poorly on the measurements of the internal validity (bias and confounding) because of lack of control group and randomization. Two studies were scored poorly on the measurement of the external validity because only men were included, and this might have an influence on the generalizability of findings.38,40 All studies did not report estimation of sample size; thus, they were rated poorly on the measurements of the power.34–42
Table 4.
The quality of included studies based on the modified Down and Black scale
| Authors | Reporting | External validity-bias | Internal validity-bias | Internal validity-confounding | Power | Total score | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | |
| Ditor et al, 200434 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Ditor et al, 200535 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 |
| Soyupek et al, 200936 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Hoekstra et al, 201337 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Millar et al, 200938 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 15 |
| Stevens et al, 201539 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Carvalho et al, 200540 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Terson de Paleville et al, 201341 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Tiftik et al, 201542 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
Note: 1 = “yes”; 0 = “no” or “unable to determine”
Levels of evidences for included studies
The level of evidence varied from level 2 to level 4 (see Table 3). Two studies included a control group.38,42 One of the studies was a randomized crossover study design with small sample size and was classified as level 2.38 The other study was a prospective study with a control group (ie, quasi-experimental study design) and was also categorized as level 2.42 The remaining seven studies used a single group, pre- and post-test study design and were placed at level 4.34–37,39–41 The sample sizes of all studies34–41 were small (range, 6–12) except one study42 that included 52 participants.
Discussion
To the best of our knowledge, this review is the first to systematically synthesize the evidence regarding the effects of BWSTT program on measurements of HR, BP, and respiratory parameters in people with SCI. An exhaustive search found nine studies that met inclusion/exclusion criteria. Given the heterogeneous nature of the SCI population and low research quality, there is weak to moderate evidence to support the effects of BWSTT on improving HR measures and respiratory parameters; however, the evidence regarding BP measures is still lacking or needed.
Heart rate measurements
Changes in HR were measured pre- and post-BWSTT in seven single group studies.34–40 One study40 included only individuals with motor complete cervical SCI (ie, quadriplegia) and found no changes in resting HR after BWSTT (two sessions per week for 3 months). On the contrary, another study34 that included both motor incomplete and complete cervical SCI reported a significant decrease in resting HR after 6 months of BWSTT with three sessions per week. Two studies with mixed SCI lesion levels (ie, quadriplegia and paraplegia)36,37 also reported a significant decrease in resting HR after 6 weeks or 24 sessions over 10 to 16 weeks of BWSTT. Two other studies with mixed SCI lesion levels35,38 reported a nonsignificant decrease in resting HR after 4 months or 4 weeks of BWSTT. Exercise HR was measured in two studies37,39 with incomplete and mixed SCI lesion levels and was significantly decreased after 24 sessions of BWSTT.
The intensity and duration of BWSTT may influence HR adaptation. One study38 found no significant decrease in resting HR, but the duration of this study was very short (4 weeks of BWSTT) as compared to the other studies34,36,37 (8 weeks to 6 months of BWSTT). Authors of this study did not provide information about the intensity of training. Also, it might be challenging to find a significant change in HR after 4 weeks of exercise. It has been shown that at least 6 to 8 weeks of walking training would be ideal for producing HR adaptation.36,37 Furthermore, moderate to high intensity of arm aerobic exercise for 8 weeks has been shown to improve cardiopulmonary fitness in individuals with SCI.46 Future studies in walking training should focus on the impact of the training intensity on HR outcomes.
Completeness of injury (incomplete or complete SCI) might also have an influence on HR response to walking training. In two studies35,40 that included only individuals with motor complete injuries, BWSTT did not produce a significant decrease in resting HR. In Ditor's study,35 only participants who had HR response (ie, increased HR) during walking training showed a decrease in resting HR. It seems that HR response and voluntary contraction of leg muscles during BWSTT are essential factors for inducing HR adaptation.35 A repetitive and intensive locomotor training has been shown to improve the activity of leg muscles even in persons with motor complete SCI.47,48 It is still unknown how significantly increased activity of leg muscles after a course of walking training can contribute to HR adaptation.
Compared to arm cycling exercise, walking training can trigger the activity of larger muscles in the body (ie, leg and trunk muscles) through activation of central pattern generators located within the spinal cord.49–51 In addition, being in upright posture during walking training can provide greater stress and challenge to the cardiovascular system. It has been shown that in individuals with SCI, HR and oxygen uptake were significantly higher during BWSTT in comparison to exercise in a sitting position.32,33 Consequently, walking exercise might induce a greater positive adaptation in HR than conventional exercise approaches such as cycling or FES leg cycling exercise.52 However, the efficacy of walking training compared to other exercise approaches has not been studied.
In relation to cardiac autonomic function, one study34 showed an improvement in HRV, as reflected by a significant reduction in the ratio of low-frequency power to high-frequency power, in individuals with incomplete cervical SCI after 6 months of BWSTT. Two studies35,38 reported a trend toward an improvement in HRV after 4 weeks or 4 months of BWSTT. The duration of one of these studies that found nonsignificant improvement in HRV after training was very short (ie, 4 weeks).38 It might be difficult to find a significant improvement in HRV within a short term of training. However, this study has noted a significant improvement in HR complexity after training, which is an indirect measurement of cardiac autonomic function.38 The other study included only individuals with motor complete SCI, and only participants who showed HR response (ie, increased HR during training) to BWSTT improved their HRV after 4 months of training.35
The findings of these studies34,36,37,39 suggest that BWSTT might result in decreased resting and exercise HR and improved cardiac autonomic function in individuals with SCI. These studies that support the use of BWSTT in improving HR measurements had a single group, pre- and post-test study design. Therefore, level 4 evidence is given for the use of BWSTT to improve HR measurements following SCI.
Blood pressure measurements
Four studies34–36,40 investigated the effects of BWSTT on changes in BP measurements in individuals with SCI. Two studies35,36 included individuals with cervical, thoracic, or lumbar SCI, and the other two studies34,40 targeted only individuals with cervical SCI. In the studies with mixed SCI levels, no significant changes in resting BP were observed in individuals with motor incomplete SCI after 6 weeks of BWSTT36 or in motor complete SCI after 4 months of BWSTT.35 In the studies of cervical SCI, one study observed no significant change in resting BP in motor incomplete SCI after 6 months of BWSTT.34 Another study with motor complete cervical SCI reported a significant increase in resting BP after 3 months of BWSTT combined with neuromuscular electrical stimulation.40 The authors of this study suggested that the increase in resting BP might be due to improvement in sympathetic activity after the BWSTT.40
It is well known that regular exercise at moderate/high intensity can lead to a decrease in resting BP.53,54 Past studies indicated that regular exercise might decrease resting BP in people with paraplegia (SCI at T1 or below) for whom the prevalence rate of hypertension is high.6,55 However, people with cervical SCI (quadriplegia) tend to have abnormally lower resting BP compared to able-bodied individuals and those with thoracic or lumbar SCI (paraplegia) due to impaired cardiovascular autonomic function after SCI.16–18 Additional reduction in BP after a period of exercise in people with quadriplegia might provoke symptoms of hypotension.40 A physical exercise program of FES leg cycling for several months increased resting BP in people with quadriplegia.56 The use of BWSTT in people with quadriplegia may or may not lead to an increase in resting BP, as shown in two reviewed studies.34,40 Studies that included participants with quadriplegia and paraplegia35,36 were therefore inconclusive in terms of changes in BP due to the fact that resting BP may change in opposite directions in these populations.
As discussed previously, the four studies34–36,40 that reported outcomes in resting BP after BWSTT were inconclusive due to their heterogeneity in terms of study participants and the response of the participants. In addition, all four studies had small sample sizes, ranging from 6 to 12 participants. This limited number of studies, small sample sizes, different levels of SCI, and variable responses to walking exercise allow no conclusions to be drawn regarding the effects of BWSTT on resting BP. Future clinical trials need to include large sample sizes and focus on either paraplegia or quadriplegia.
Two studies34,35 also measured BP variability pre- and post-BWSTT training. One study34 reported a significant decrease in low-frequency systolic BP (SBP) in individuals with incomplete cervical SCI after 6 months of BWSTT. The other study from the same investigation team found no significant changes in measurements of BP variability in individuals with motor complete cervical or thoracic SCI after 4 months of BWSTT.35 No conclusion can be made at present.
Respiratory parameters
Only three out of nine studies that were included in this review measured pulmonary function as an outcome.36,41,42 In all three studies, there were improvements in some of the respiratory parameters after a course of walking training. Soyupek et al36 found significant increases in forced vital capacity (FVC) and inspiratory capacity (IC) in individuals with motor incomplete SCI after 6 weeks of BWSTT. Another prospective study with a control group showed that vital capacity (VC), VC%, FVC, forced expiratory volume in one second (FEV1), and forced expiratory flow rate 25% to 75% were significantly increased in participants who received 4 weeks of BWSTT (the experimental group) but not in those who received standard rehabilitation program (the control group).42 Both groups showed a significant increase in maximum voluntary ventilation.42 However, this study did not use a random procedure in assigning the participants into one of two groups.
In addition to measuring pulmonary function, a study explored the underlying mechanisms of improvement in pulmonary function after walking training.41 They reported significant increases in FVC, FEV1, and maximum expiratory pressure after 62±10 sessions of BWSTT. Also, the amplitude and motor unit recruitment of all respiratory muscles during respiratory tasks significantly increased after training as compared to baseline measures. The findings of the study suggest that BWSTT could induce neuroplasticity in spinal neural circuitry that is responsible for the activation of respiratory muscles.
In comparison to exercises in sitting position, upright posture during walking training can trigger the activity of trunk muscles including abdominal muscles, which play an important role in respiration.49–51 It is also a highly effective stressor of the cardiopulmonary system since the lungs need to work harder to deliver more oxygen to larger working muscles.57 Furthermore, it has been shown that walking training increases the connectivity of neural spinal circuity between motor cortex and leg muscles.58 The increased muscle activity of inspiratory and expiratory muscles after BWSTT may be explained by neuroplastic changes within the neural spinal circuity that controls respiration.41 All of those factors might induce greater adaptive changes in pulmonary function. Based on this information mentioned, the use of BWSTT to improve pulmonary function after SCI is supported by one study42 with level 2 evidence.
Conclusion
To maintain cardiovascular and pulmonary health after SCI, it is crucial for people with SCI to engage in regular physical activity. There is some evidence that the use of BWSTT as an exercise in individuals with SCI has positive effects on cardiovascular and pulmonary health by improving resting and exercise HR and respiratory parameters. No clear evidence is currently available about which type of physical rehabilitation produces the best results. In addition, it is not clear whether BWSTT leads to a better outcome in cardiovascular and pulmonary function compared to conventional physical rehabilitation methods, such as arm cycling or FES leg cycling exercise. Furthermore, current studies have not provided information about the effects of the intensity level of walking training on these outcome measurements. Because of limited studies, further investigations are necessary. Future randomized controlled trial studies are needed to investigate the effects of BWSTT on cardiovascular and pulmonary health compared to other physical rehabilitation interventions. Further studies also should investigate the influence of walking training intensity (ie, walking speed or amount of body weight support) on cardiovascular or pulmonary health.
Acknowledgments
The authors declare no conflicts of interest.
APPENDIX Modified Downs and Black Checklist for the Assessment of the Methodological Quality of Studies
| Item | Criteria | Possible answers |
|---|---|---|
| Reporting | ||
| 1 | Is the hypothesis/aim/objective of the study clearly described? | Yes = 1 |
| No = 0 | ||
| 2 | Are the main outcomes to be measured clearly described in the Introduction or Methods section? | Yes = 1 |
| No = 0 | ||
| 3 | Are the characteristics of the patients included in the study clearly described? | Yes = 1 |
| No = 0 | ||
| 4 | Are the interventions of interest clearly described? | Yes = 1 |
| No = 0 | ||
| 5 | Are the distributions of principal confounders in each group of subjects to be compared clearly described? | Yes = 2 |
| Partially = 1 | ||
| No = 0 | ||
| 6 | Are the main findings of the study clearly described? | Yes = 1 |
| No = 0 | ||
| 7 | Does the study provide estimates of the random variability in the data for the main outcomes? | Yes = 1 |
| No = 0 | ||
| 8 | Have all important adverse events that may be a consequence of the intervention been reported? | Yes = 1 |
| No = 0 | ||
| 9 | Have the characteristics of patients lost to follow-up been described? | Yes = 1 |
| No = 0 | ||
| 10 | Have actual probability values been reported (eg, 0.035 rather than <0.05) for the main outcomes except where the probability value is less than 0.001? | Yes = 1 |
| No = 0 | ||
| External validity | ||
| 11 | Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 12 | Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 13 | Were the staff, places, and facilities where the patients were treated representative of the treatment the majority of patients receive? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| Internal validity – bias | ||
| 14 | Was an attempt made to blind study subjects to the intervention they have received? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 15 | Was an attempt made to blind those measuring the main outcomes of the intervention? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 16 | If any of the results of the study were based on “data dredging,” was this made clear? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 17 | In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies is the time period between the intervention and outcome the same for cases and controls? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| Item | Criteria | Possible answers |
|---|---|---|
| 18 | Were the statistical tests used to assess the main outcomes appropriate? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 19 | Was compliance with the intervention/s reliable? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 20 | Were the main outcome measures used accurate (valid and reliable)? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| Internal validity – confounding | ||
| 21 | Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 22 | Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited over the same period of time? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 23 | Were study subjects randomized to intervention groups? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 24 | Was the randomized intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 25 | Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| 26 | Were losses of patients to follow-up taken into account? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
| Power | ||
| 27 | Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%? | Yes = 1 |
| No = 0 | ||
| Unable to determine = 0 | ||
REFERENCES
- 1.Kostovski E, Iversen PO, Hjeltnes N. [Complications of chronic spinal cord injury] Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2010;130(12):1242–1245. doi: 10.4045/tidsskr.09.0055. [DOI] [PubMed] [Google Scholar]
- 2.Sezer N, Akkus S, Ugurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6(1):24–33. doi: 10.5312/wjo.v6.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: An overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–152. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 4.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: Results from a national population health survey. Neurology. 2013;81(8):723–728. doi: 10.1212/WNL.0b013e3182a1aa68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterthun R, Post MW, van Asbeck FW, van Leeuwen CM, van Koppenhagen CF. Causes of death following spinal cord injury during inpatient rehabilitation and the first five years after discharge. A Dutch cohort study. Spinal Cord. 2014;52(6):483–488. doi: 10.1038/sc.2014.28. [DOI] [PubMed] [Google Scholar]
- 6.Flank P, Fahlstrom M, Bostrom C, Lewis JE, Levi R, Wahman K. Self-reported physical activity and risk markers for cardiovascular disease after spinal cord injury. J Rehabil Med. 2014;46(9):886–890. doi: 10.2340/16501977-1857. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki M, Irizawa M, Komura T et al. Daily energy expenditure in active and inactive persons with spinal cord injury. J Human Ergol. 1992;21(2):125–133. [PubMed] [Google Scholar]
- 8.Lee YH, Lee JH, Kim SH et al. Hemodynamic adaptations to regular exercise in people with spinal cord injury. Ann Rehabil Med. 2017;41(1):25–33. doi: 10.5535/arm.2017.41.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid A, Huonker M, Barturen JM et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol (Bethesda, Md: 1985) 1998;85(2):635–641. doi: 10.1152/jappl.1998.85.2.635. [DOI] [PubMed] [Google Scholar]
- 10.Hjeltnes N, Wallberg-Henriksson H. Improved work capacity but unchanged peak oxygen uptake during primary rehabilitation in tetraplegic patients. Spinal Cord. 1998;36(10):691–698. doi: 10.1038/sj.sc.3100687. [DOI] [PubMed] [Google Scholar]
- 11.Serra-Ano P, Montesinos LL, Morales J et al. Heart rate variability in individuals with thoracic spinal cord injury. Spinal Cord. 2015;53(1):59–63. doi: 10.1038/sc.2014.207. [DOI] [PubMed] [Google Scholar]
- 12.Liao D, Cai J, Rosamond WD et al. Cardiac autonomic function and incident coronary heart disease: A population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Larson MG, Venditti FJ, Jr. et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 14.Bunten DC, Warner AL, Brunnemann SR, Segal JL. Heart rate variability is altered following spinal cord injury. Clin Auton Res. 1998;8(6):329–334. doi: 10.1007/BF02309623. [DOI] [PubMed] [Google Scholar]
- 15.Alwan A, Armstrong T, Bettcher D . Raised blood pressure (hypertension): A major risk factor for CVDs. In: Mendis S, Puska P, Norrving B, editors. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva, Switzerland: World Health Organization; 2011. pp. 38–39. [Google Scholar]
- 16.Adriaansen JJ, Douma-Haan Y, van Asbeck FW et al. Prevalence of hypertension and associated risk factors in people with long-term spinal cord injury living in the Netherlands. Disabil Rehabil. 2017;39(9):919–927. doi: 10.3109/09638288.2016.1172349. [DOI] [PubMed] [Google Scholar]
- 17.Wecht JM, Zhu C, Weir JP, Yen C, Renzi C, Galea M. A prospective report on the prevalence of heart rate and blood pressure abnormalities in veterans with spinal cord injuries. J Spinal Cord Med. 2013;36(5):454–462. doi: 10.1179/2045772313Y.0000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu C, Galea M, Livote E, Signor D, Wecht JM. A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. J Spinal Cord Med. 2013;36(5):463–475. doi: 10.1179/2045772313Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Increased cardiovascular disease risk in Swedish persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42(5):489–492. doi: 10.2340/16501977-0541. [DOI] [PubMed] [Google Scholar]
- 20.Fugl-Meyer AR. Effects of respiratory muscle paralysis in tetraplegic and paraplegic patients. Scand J Rehabil Med. 1971;3(4):141–150. [PubMed] [Google Scholar]
- 21.Halar EM, Bell KR. In mobility and inactivity: Physiological and functional changes, prevention and treatment. In: Delisa JA, editor. Physical Medicine and Rehabilitation. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 1447–1467. [Google Scholar]
- 22.Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166(3):129–141. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Garshick E, Kelley A, Cohen SA et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warburton DE, Eng JJ, Krassioukov A, Sproule S. Cardiovascular health and exercise rehabilitation in spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;13(1):98–122. doi: 10.1310/sci1301-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson KF, Wilt TJ, Taylor BC et al. Effect of exercise on disorders of carbohydrate and lipid metabolism in adults with traumatic spinal cord injury: Systematic review of the evidence. J Spinal Cord Med. 2009;32(4):361–378. doi: 10.1080/10790268.2009.11754465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akkurt H, Karapolat HU, Kirazli Y, Kose T. The effects of upper extremity aerobic exercise in patients with spinal cord injury: A randomized controlled study. Eur J Phys Rehabil Med. 2017;53(2):219–227. doi: 10.23736/S1973-9087.16.03804-1. [DOI] [PubMed] [Google Scholar]
- 27.Sheel AW, Reid WD, Townson AF, Ayas NT, Konnyu KJ. Effects of exercise training and inspiratory muscle training in spinal cord injury: A systematic review. J Spinal Cord Med. 2008;31(5):500–508. doi: 10.1080/10790268.2008.11753645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alm M, Saraste H, Norrbrink C. Shoulder pain in persons with thoracic spinal cord injury: Prevalence and characteristics. J Rehabil Med. 2008;40(4):277–283. doi: 10.2340/16501977-0173. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs PL, Nash MS. Modes, benefits, and risks of voluntary an delectrically induced exercise in persons with spinal cord injury. J Spinal Cord Med. 2001;24(1):10–18. doi: 10.1080/10790268.2001.11753549. [DOI] [PubMed] [Google Scholar]
- 30.Olive JL, Slade JM, Dudley GA, McCully KK. Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol (Bethesda, Md: 1985) 2003;94(2):701–708. doi: 10.1152/japplphysiol.00736.2002. [DOI] [PubMed] [Google Scholar]
- 31.Ashley EA, Laskin JJ, Olenik LM et al. Evidence of autonomic dysreflexia during functional electrical stimulation in individuals with spinal cord injuries. Paraplegia. 1993;31(9):593–605. doi: 10.1038/sc.1993.95. [DOI] [PubMed] [Google Scholar]
- 32.Wouda MF, Wejden L, Lundgaard E, Strom V. Energetic and cardiovascular responses to treadmill walking and stationary cycling in subjects with incomplete spinal cord injury. Spinal Cord. 2016;54(1):51–56. doi: 10.1038/sc.2015.120. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho DC, de Cassia Zanchetta M, Sereni JM, Cliquet A. Metabolic and cardiorespiratory responses of tetraplegic subjects during treadmill walking using neuromuscular electrical stimulation and partial body weight support. Spinal Cord. 2005;43(7):400–405. doi: 10.1038/sj.sc.3101730. [DOI] [PubMed] [Google Scholar]
- 34.Ditor DS, Kamath MV, MacDonald MJ, Bugaresti J, McCartney N, Hicks AL. Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J Appl Physiol (Bethesda, Md: 1985) 2005;98(4):1519–1525. doi: 10.1152/japplphysiol.01004.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ditor DS, Macdonald MJ, Kamath MV et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord. 2005;43(11):664–673. doi: 10.1038/sj.sc.3101785. [DOI] [PubMed] [Google Scholar]
- 36.Soyupek F, Savas S, Ozturk O, Ilgun E, Bircan A, Akkaya A. Effects of body weight supported treadmill training on cardiac and pulmonary functions in the patients with incomplete spinal cord injury. J Back Musculoskeletal Rehabil. 2009;22(4):213–218. doi: 10.3233/BMR-2009-0237. [DOI] [PubMed] [Google Scholar]
- 37.Hoekstra F, van Nunen MP, Gerrits KH, Stolwijk-Swuste JM, Crins MH, Janssen TW. Effect of robotic gait training on cardiorespiratory system in incomplete spinal cord injury. J Rehabil Res Dev. 2013;50(10):1411–1422. doi: 10.1682/JRRD.2012.10.0186. [DOI] [PubMed] [Google Scholar]
- 38.Millar PJ, Rakobowchuk M, Adams MM, Hicks AL, McCartney N, MacDonald MJ. Effects of short-term training on heart rate dynamics in individuals with spinal cord injury. Autonom Neurosci Basic Clin. 2009;150(1–2):116–121. doi: 10.1016/j.autneu.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Stevens SL, Morgan DW. Heart rate response during underwater treadmill training in adults with incomplete spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21(1):40–48. doi: 10.1310/sci2101-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho DC, Cliquet A., Jr. Response of the arterial blood pressure of quadriplegic patients to treadmill gait training. Brazilian J Med Biol Res. 2005;38(9):1367–1373. doi: 10.1590/s0100-879x2005000900011. [DOI] [PubMed] [Google Scholar]
- 41.Terson de Paleville D, McKay W, Aslan S, Folz R, Sayenko D, Ovechkin A. Locomotor step training with body weight support improves respiratory motor function in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2013;189(3):491–497. doi: 10.1016/j.resp.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiftik T, Gokkaya NK, Malas FU et al. Does locomotor training improve pulmonary function in patients with spinal cord injury? Spinal Cord. 2015;53(6):467–470. doi: 10.1038/sc.2014.251. [DOI] [PubMed] [Google Scholar]
- 43.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Scheer JW, Martin Ginis KA, Ditor DS et al. Effects of exercise on fitness and health of adults with spinal cord injury: A systematic review. Neurology. 2017;89(7):736–745. doi: 10.1212/WNL.0000000000004224. [DOI] [PubMed] [Google Scholar]
- 45.Eng JJ, Teasell R, Miller WC et al. Spinal cord injury rehabilitation evidence: Methods of the SCIRE systematic review. Topics Spinal Cord Inj Rehabil. 2007;13(1):1–10. doi: 10.1310/sci1301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Groot PC, Hjeltnes N, Heijboer AC, Stal W, Birkeland K. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord. 2003;41(12):673–679. doi: 10.1038/sj.sc.3101534. [DOI] [PubMed] [Google Scholar]
- 47.Wirz M, Colombo G, Dietz V. Long term effects of locomotor training in spinal humans. J Neurol Neurosurg Psychiatr. 2001;71(1):93–96. doi: 10.1136/jnnp.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knikou M. Functional reorganization of soleus H-reflex modulation during stepping after robotic-assisted step training in people with complete and incomplete spinal cord injury. Exp Brain Res. 2013;228(3):279–296. doi: 10.1007/s00221-013-3560-y. [DOI] [PubMed] [Google Scholar]
- 49.Pinter MM, Dimitrijevic MR. Gait after spinal cord injury and the central pattern generator for locomotion. Spinal Cord. 1999;37(8):531–537. doi: 10.1038/sj.sc.3100886. [DOI] [PubMed] [Google Scholar]
- 50.Minassian K, Hofstoetter US, Dzeladini F, Guertin PA, Ijspeert A. The human central pattern generator for locomotion: Does it exist and contribute to walking? Neuroscientist. 2017;23(6):649–663. doi: 10.1177/1073858417699790. [DOI] [PubMed] [Google Scholar]
- 51.MacKay-Lyons M. Central pattern generation of locomotion: A review of the evidence. Phys Ther. 2002;82(1):69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- 52.Hicks AL, Ginis KA. Treadmill training after spinal cord injury: It's not just about the walking. J Rehabil Res Dev. 2008;45(2):241–248. doi: 10.1682/jrrd.2007.02.0022. [DOI] [PubMed] [Google Scholar]
- 53.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Human Hypertension. 2010;24(3):175–182. doi: 10.1038/jhh.2009.51. [DOI] [PubMed] [Google Scholar]
- 54.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 55.Haddad S, Silva PR, Barretto AC, Ferraretto I. [The effects of aerobic physical training of short duration using upper limbs in paraplegic persons with mild hypertension] Arquivos brasileiros de cardiologia. 1997;69(3):169–173. [PubMed] [Google Scholar]
- 56.Faghri PD, Glaser RM, Figoni SF. Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch Phys Med Rehabil. 1992;73(11):1085–1093. [PubMed] [Google Scholar]
- 57.Jack LP, Purcell M, Allan DB, Hunt KJ. Comparison of peak cardiopulmonary performance parameters during robotics-assisted treadmill exercise and arm crank ergometry in incomplete spinal cord injury. Technol Health Care. 2010;18(4–5):285–296. doi: 10.3233/THC-2010-0591. [DOI] [PubMed] [Google Scholar]
- 58.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94(4):2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]


