Abstract
Ultraviolet (UV) irradiation can be considered as a double-edged sword: not only is it a crucial environmental factor that can cause skin-related disorders but it can also be used for phototherapy of skin diseases. Inducible heme oxygenase-1 (HO-1) in response to a variety of stimuli, including UV exposure, is vital to maintain cell homeostasis. Heme oxygenase-2 (HO-2), another member of the heme oxygenase family, is constitutively expressed. In this review, we discuss how heme oxygenase (HO), a vital rate-limiting enzyme, participates in heme catabolism and cytoprotection. Phylogenetic analysis showed that there may exist a functional differentiation between HO-1 and HO-2 during evolution. Furthermore, depending on functions in immunomodulation and antioxidation, HO-1 participates in disease progression, especially in pathogenesis of skin diseases, such as vitiligo and psoriasis. To further investigate the particular role of HO-1 in diseases, we summarized the profile of the HO enzyme system and its related signaling pathways, such as Nrf2 and endoplasmic reticulum crucial signaling, both known to regulate HO-1 expression. Furthermore, we report on a C-terminal truncation of HO-1, which is generally considered as a signal molecule. Also, a newly identified alternative splice isoform of HO-1 not only provides us a novel perspective on comprehensive HO-1 alternative splicing but also offers us a basis to clarify the relationship between HO-1 transcripts and oxidative diseases. To conclude, the HO system is not only involved in heme catabolism but also involved in biological processes related to the pathogenesis of certain diseases, even though the mechanism of disease progression still remains sketchy. Further understanding the role of the HO system and its relationship to UV is helpful for revealing the HO-related signaling networks and the pathogenesis of many diseases.
1. Introduction
Heme oxygenase (HO) is an important rate-limiting enzyme and widely distributed in mammalian tissues. The HO system can degrade the heme into biliverdin (BV), free ferrous iron (Fe2+), and carbon monoxide (CO) [1]. These metabolic products participate in physiological processes including oxidative stress, inflammation, and apoptosis. The heme oxygenase occurs in two isoforms, HO-1 and HO-2 (gene names HMOX1 and HMOX2). HO-1 is the inducible isoform that can be induced by a variety of environmental stimuli, such as UV radiation, heavy metal, lipopolysaccharide, heat shock, growth factors, hydrogen peroxide, phorbol esters, nitric oxide, inflammatory cytokines, endotoxins, hyperoxia, and hypoxia [2–5]. Hence, it is a general concept that HO-1 not only is an oxidative stress marker but also has some cytoprotective properties.
The study on the HO-1 transcriptional regulatory region shows the presence of regulatory sequences for the binding of various transcription factors such as AP-1, AP-2, NF-κB, ATF4, Nrf2, Jun B, and HIF-1, which illustrates that HO-1 could also maintain cellular homeostasis [6, 7]. In contrast to HMOX1, only a few regulatory elements have been identified in the promoter region of HMOX2, such as a glucocorticoid response element (GRE). Indeed, corticosterone or dexamethasone treatment can increase the expression of HMOX2 [8].
Human HO-2 is constitutively expressed and plays a role in the production of CO in neuronal populations. In cerebral tissue, HO-2 is induced in response to cellular oxidative damage and NO sources whereas hypoxia could reduce its expression [9]. HO-2 is also a potential oxygen sensor through BKCa channel activity and hypoxic response in mammalian cells [10].
Despite the well-known role in heme catabolism, HO-1 participates in some disease progressions with properties of immunomodulatory and antioxidation, especially some skin diseases such as vitiligo and psoriasis.
UV as a common environmental factor for skin regulates HO-1 through a complicated signaling network. In this review, we explore the relationship between UVA and HO-1 and focus on Nrf2/Keap1-HO-1 and Eif2α-HO-1 signaling pathways, which are significant pathways in cellular antioxidation [11–13]. We elucidate the function of HO by introducing the transcripts of HO-1 and HO-2. Depending on different cell types, tissues, organs, and species, the HO system will generate various transcripts that may achieve distinctive functions. Different from HO-1, HO-2 renders several transcripts [14, 15]. A truncated form of HO-1 as a signal transducer localized to the nucleus was already introduced [16]. In previous studies, Bian et al. identified a novel isoform 14 kDa HO-1 form that might be related to tumor growth [17]. HO-1 is highly inducible following UVA irradiation in skin fibroblasts, with much lower levels in keratinocytes [18, 19]. Furthermore, we found that silencing of HO-2 in keratinocytes increases HO-1, which also further increases UVA-mediated HO-1 expression in HaCaT cells [20]. Although the vital function of the HO system in heme catabolism and maintenance of cell homeostasis has been well elucidated, recent new findings about the multifunctional role of the HO system in many skin diseases and UV irradiation are worthy to be reviewed in detail. Further understanding of the role of the HO system is helpful for revealing the pathogenesis of many diseases.
2. Heme Oxygenase System
2.1. The General Role of the Heme Oxygenase System in Heme Catabolism and Oxidative Stress
Heme oxygenase (HO) is the vital rate-limiting enzyme in heme catabolism and widely exists in mammalian tissues [21]. HO isoenzymes are located in the endoplasmic reticulum (ER) [22]. The enzyme can degrade the heme into biliverdin (BV), free ferrous iron (Fe2+), and carbon monoxide (CO) [1]. With the function of biliverdin reductase (BVR), biliverdin is converted to bilirubin (BR) and all the metabolic products of HO activity can participate in the physiological process including oxidative stress, inflammation, and apoptosis [23, 24]. The bile pigments biliverdin and bilirubin can scavenge ROS and nitrogen reactive species (NRS) through the recycling mechanism [25, 26]. It was noted that bilirubin can suppress the inflammatory response and decrease the cellular toxicity [27].
As a product of the HO enzymatic activity, CO can modulate the mitogen-activated protein kinase (MAPK) and p38β pathways to induce antiapoptotic, antiproliferative, and anti-inflammatory properties [28]. CO stabilizes the hypoxia-inducible factor 1α (HIF-1α), which plays a role in cytoprotection in macrophages. CO can inhibit cytochromes of the respiratory chain and NADPH oxidase (NOX), thus attributed to the reduction of ROS [29, 30]. Fe2+ is a product of the HO system and can be rapidly removed by ferritin to avoid the prooxidant capacity. With intracellular thiols, Fe2+ can form an iron-sulfur complex [3, 9, 31, 32]. The extreme hydrophobicity of heme can generate reactive oxygen species (ROS) and easily bind to the lipids leading to membrane lipid peroxidation. This can disrupt the membranes of several cellular organelles such as the endoplasmic reticulum (ER), nuclei, and cell membrane [33].
The HO system has the ability to keep the heme protein in balanced levels and protects cells from intracellular free heme damage [34]. Therefore, the cytoprotective role of the HO system is important in the biological process [35–37].
2.2. Homologous Alignment and Phylogenetic Analysis of HO System
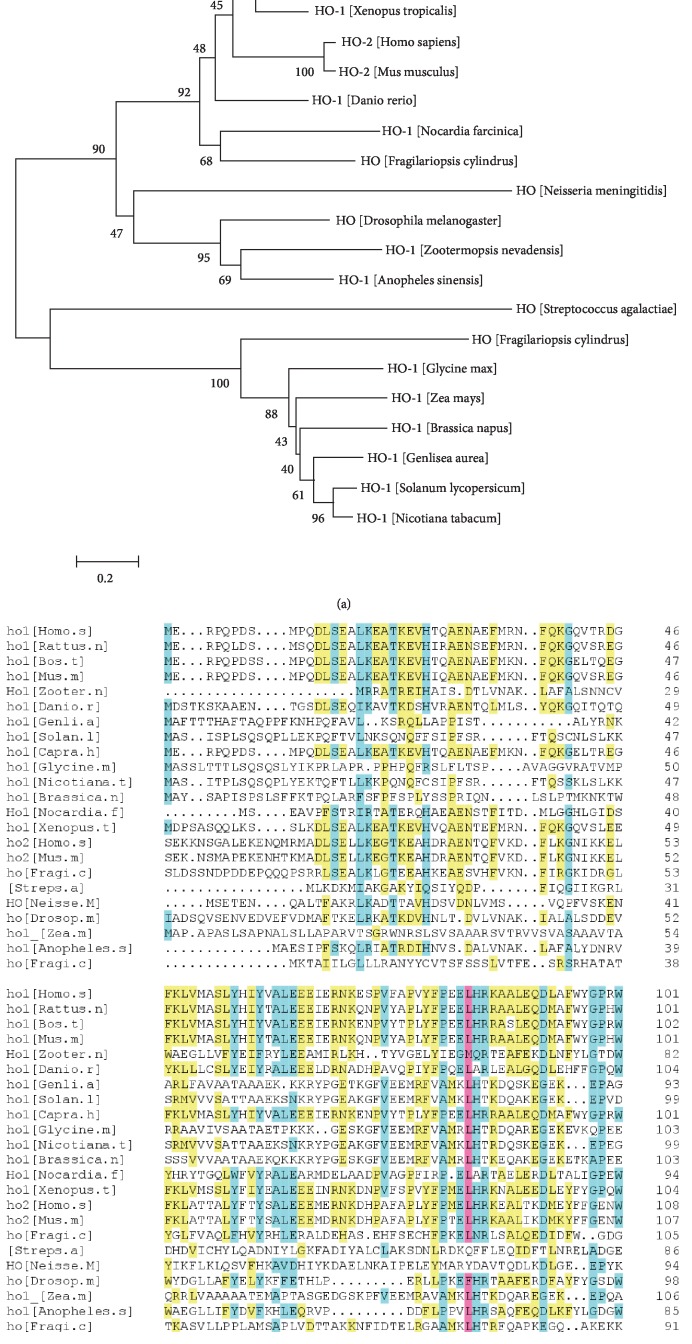
Homologous alignment revealed that the HMOX1 gene encodes 288 amino acids and HMOX2 encodes 313 amino acids [38]. Figure 1 shows that HMOX1 presents with 21.71% identity to HMOX2 and HMOX1 produced significant alignments with those from Bos taurus (41.37%), Mus musculus (40.51%), Xenopus tropicalis (29.57%), Danio rerio (21.71%), Drosophila melanogaster (9.23%), Zootermopsis nevadensis (7.18%), Nicotiana tabacum (4.44%), and Zea mays (4.10%). To analyze the evolutionary relationship of HO with the HO-like protein of other species, the neighbor-joining method was used to construct an HO phylogenetic tree. The results demonstrated that Hmox1 might have a closer relationship with Bos taurus and Capra hircus, while HMOX2 has a closer relationship with Mus musculus than other species (Figure 1).
Figure 1.
Homologous alignment and phylogenetic analysis of heme oxygenase and HO-like proteins. (a) Phylogenetic analysis of HO from different species. The amino acid sequences were downloaded from the NCBI website. Amino acid position is presented by a 0.2 bar. (b) Alignment of deduced HO proteins with other species.
2.3. Heme Oxygenase-1
The 32 kDa HO-1 protein belongs to a family of stress proteins as inducible isoform of HO, which is highly expressed in the liver, spleen, and bone marrow [39]. HO-1 can be induced by a variety of environmental stimuli, including UV radiation, heavy metals, lipopolysaccharides, heat shock, growth factors, hydrogen peroxide, phorbol esters, nitric oxide, inflammatory cytokines, endotoxins, hyperoxia, and hypoxia [2–5]. Due to its expression at low levels under basal conditions, but quickly upregulated, HO-1 has been recognized as a biomarker of oxidative stress. The human HO-1 gene is located on chromosome 22q12 and it contains 4 introns and 5 exons [40, 41]. It is generally cytoprotective, antiapoptotic, anti-inflammatory, and antioxidant [23].
We have explored the relationship between HO-1 and UVA. As an environmental factor, UVA irradiation releases heme from microsomes and generates reactive oxygen species (ROS), which could regulate HO-1 expression [42, 43].
Being a multifunctional molecule, HO-1 also participated in some skin diseases [44]. A recent report noted that HO-1 is a powerful immunomodulator, and elevated levels of HO-1 can eliminate inflammatory atopic dermatitis-like lesions in mice [45]. As a multifunctional protein, HO-1 can suppress dendritic cell maturation, T cell activation, and B cell infiltration [46]. In experimental models of ischemia/reperfusion, HO-1 has the ability to protect against cell death, thus making HO-1 a promising target in diverse disease phenotypes, such as myocardial infarction, sepsis, and stoke [47]. In endothelial cells (EC), HO-1 expression could protect EC from undergoing programmed cell death and the antiapoptotic property of HO-1 is mediated heme catabolism to the carbon monoxide (CO) [48]. The major molecular mechanism is when HO-1 inhibits the extrinsic and intrinsic apoptotic pathway, including elevated CO production wherein CO could inhibit P53 expression, decrease prooxidant levels, and increase bilirubin [49]. HO-1 could stimulate various types of cell proliferation and growth, and high levels of HO-1 expression occur in some tumors because of its antiapoptosis and antioxidation [50–52]. Depending on HO-1 which is related to the tumor growth, we are also provided a view that the HO-1 inhibitor could become a novel antitumor chemotherapy.
The function of HO-1 showed extreme similarities among the pathogenesis of vitiligo and psoriasis [53, 54]. In vitiligo, T cells mediated immune responses against melanocytes and against keratinocytes in psoriasis [55]. A previous study demonstrated that vitiligo melanocytes are equipped with the dysfunctional Nrf2-HO-1 antioxidant signaling pathway, as well as the aberrant expression of miRNAs [56–58]. Oxidative stress is considered as a contributing factor in T cell-mediated attack against melanocytes and therefore depigmentation of vitiligo skin; the dysfunctional Nrf2-HO-1 may contribute to pathogenesis of vitiligo. Furthermore, HO-1 expression has been associated with immunosuppressive effects, such as immunoregulatory function of Tregs [59]; the attenuated function of Tregs affecting progressive vitiligo has been confirmed [55, 60, 61]. In melanoma, HO-1 gene promoter mutations have been reported [57, 62]. Different from HO-1, HO-2 is constitutively expressed and has hardly been induced, but HO-2 still plays a vital role in heme homeostasis and antioxidation.
2.4. Heme Oxygenase-2
HO-2 is a 36 kDa protein that is encoded on human chromosome 16q12 [40]. HO-2 is mainly expressed in the brain, testis, spleen, neurons, and endothelial and glial cells [63]. In the brain, HO-2 is expressed in an abundant form, since HO-2 is constitutively expressed in neurons and is involved in antiapoptosis in the cortical and hippocampal [64, 65]. HO-2 acts in the production of CO in neuronal populations, and due to its high expression, in cerebral tissue, HO-2 can respond to cellular damage [9, 66]. Unlike HO-1, HO-2 is hardly inducible and can only be induced by NO donors, which is reduced by hypoxia [67, 68]. Owing to the deficient cysteine motifs in HO-1, HO-2 is a potential oxygen sensor through the BKCa channel activity and hypoxic response in mammalian cells [10]. In contrast to HO-1, HO-2 is mainly constitutively expressed and a few regulatory elements have been identified in the promoter region of HO-2 [9], such as a glucocorticoid response element (GRE) [8]. The expression of HO-2 can be induced under a few conditions. It is upregulated by adrenal glucocorticoids; in endothelial cells, estrogen also upregulates HO-2 [8]. A previous study noted that adrenal glucocorticoids can also modulate the HO-2 expression [8]. In cerebral and smooth muscle cells, HO-2 is also activated by glutamate and increased CO production. As an enzyme, HO-2 activity can be affected by posttranslational modifications; it can also be regulated by the presence of NO and ROS [9, 69]. Basal levels of HO-2 have the ability to maintain heme homeostasis; meanwhile, it can protect against cellular oxidative stress as well [70]. In contrast to HO-2, there are still some publications that reported that HO-1 is a multifunctional protein involved in some vital biological processes and further investigating its transcriptional regulation has become a matter of significance.
3. Ultraviolet Radiation and HO System
Ultraviolet (UV) light is electromagnetic radiation with wavelengths in the range of 200-400 nm. Based on the wavelength of UV light, it can be divided into three parts, UVA (320-400 nm), UVB (280-320 nm), and UVC (lower than 280 nm) [71, 72]. In general, the solar radiation is an environmental factor, which can trigger some skin diseases, such as polymorphic solar eruption (PMLE), photoaging, and skin cancer. Melanoma, squamous cell carcinoma (SCC), and basal cell carcinoma (BCC) are the three main types of skin cancer, and UV radiation (UVR) is the major risk factor for the occurrence of skin cancers [73]. Melanin, produced in melanocytes, plays a critical role in protecting against UV-mediated mutagenesis. However, a recent study observed a decrease in the risk of melanoma and nonmelanoma skin cancer in vitiligo subject with the absence of melanin in vitiligo skin, which may be explained by the inverse relationship between the risk of vitiligo and skin cancers in the RALY-EIF252-ASIPAHCY-ITCH, IRF4, TYR, and MC1R genes [74–77]. UVR could disrupt skin keratinocytes, which cause inflammatory disorders. However, UV radiation exhibited both beneficial and detrimental effects. Ultraviolet radiation, including narrowband UVB (311-313 nm), broadband UVB (290-320 nm), and UVA-1 (340-400 nm), was employed as phototherapy for several chronic inflammatory skin diseases, including atopic dermatitis, vitiligo, pruritus, cutaneous mastocytosis, and psoriasis [78–80].
Long-time exposure to UVA radiation can accumulate reactive oxygen species (ROS), which leads to cellular oxidative stress and activates antioxidation pathways [81]. High doses of UVA (>300 J) can cause DNA damage in either direct or indirect ways related to pathogenesis [82]. Our lab has shown that different wavelengths of UV can activate specific signal pathways [13, 83–85]. As the long wavelength UVA radiation mainly exists in the living environment, it has attracted our attention to UVA radiation research.
HO-1, which belongs to the heme oxygenase family, can be upregulated by low and medium doses of UVA irradiation; the induction of HO-1 contributes to cellular redox homeostasis. We have explored the relationship between HO-1 and UVA. As an environmental factor, UVA irradiation releases heme from microsomal and generates reactive oxygen species (ROS), which could regulate HO-1 expression [42, 43]. Both UVA and UVB can induce HO-1 expression, though much higher levels of induction were found for UVA irradiation. When UVA induction of Nrf2 and HO-1 is abolished in skin cells, they are more sensitive to oxidative stress, such as UVA and H2O2, indicating that the Nrf2/HO-1 system has a protective role in skin cells [86].
4. UV-Related Signal Pathways and Transcription Involved in Regulation of HO-1
4.1. Transcription Regulation of HO-1
In humans, HMOX1 transcription is involved in a variety of signal transduction pathways that activate different transcription factors. HO-1 can be upregulated by various inducers, and the transcriptional regulation is essential to explore the relationship between UVA and HO-1. It is well known that UVA is an oxidative agent, so we mainly focused on the molecular mechanism of UVA which actives the antioxidant signal pathways which affect the transactivation of HMOX1 and other antioxidant genes [87–89]. Previously, transcription factor binding sites have been identified in the HO-1 promoter region, such as AP-1, AP-2, NF-κB, ATF4, Nrf-2, Jun B, and HIF-1, which are associated with the immediate response to tissue injury, inflammatory, and oxidation stress [6, 90].
AP-1 binding sites have been identified, which suggest that a contribution of Jun/Fos transcription factor family induces HO-1 gene transcription by multiple agents [91]. AP-2 and NF-κB binding sites may be implicated as HO-1 in response to tissue injury, oxidation stress, cell growth control, and differentiation processes. As for the NF-κB transcription factor, it is involved in many cell type challenges and pathogenic stimuli, including virus, bacterial, stress, and inflammatory cytokines. HIF-1 is a factor that is related to hypoxia, and ATF4 is an activating transcription factor that can upregulate some genes [12, 92, 93].
4.2. Nrf2/Keap1-HO-1 Signaling
Nrf2 (nuclear factor erythroid-derived 2 related factor 2) belongs to the basic leucine zipper family of transcription factors and is responsible for the regulation of cellular redox balance and antioxidation [94]. The antioxidant response element (ARE) is attributed to a consensus binding sequence, identified in HMOX1, thioredoxin reductase 1 (Txnrd1), and series of antioxidative genes [95]. Antioxidation genes could be induced in response to environmental stimuli, such as UV. The procedural activation of cascade affects the status of the cells and provides protection against cellular oxidative stress [96]. Apart from Nrf2, some factors like Nrf1 and Nrf3 as well as transcriptional repressors Bach1 and Bach2 are also members of the bZIP family of transcription factors [97]. Keap1 is a cysteine-rich protein, serving as an adaptor protein for the Cul3-dependent E3 ubiquitin ligase complex [98–100].
Under oxidative stress, including UV irradiation, Nrf2 is separated from Keap1 and translocates to the cell nucleus [95]. Nrf2 combines with small Maf proteins (sMaf) and CBP (CREB-binding protein) and then binds to the antioxidant responsive elements (ARE) in the promoters of target genes [101]. However, Nrf2 can be degraded in the nucleus via the β-TrCP-GSK3β axis or it may translocate back to the cytoplasm and is degraded by Keap1 [102]. Under normal conditions, Keap1 promotes ubiquitination and degradation of Nrf2 and Nrf2 exhibiting a short nearly 20 min half-life, which keeps the low level of Nrf2 to maintain cellular homoeostasis [103]. Keap1, as a thiol-rich protein, contains cysteine residues; the Cys273 and Cys288 are important for Keap1 to regulate Nrf2 under oxidation stress conditions and Cys151 is vital to active Keap1 under cellular stress conditions [99, 104, 105]. It was found that silencing of Keap1 increases the expression of HO-1 by several fold [103].
Therefore, the Nrf2/Keap1-HO-1 pathway is an indispensable route to minimize oxidative stress. Nrf2 is an essential factor through binding to the Maf recognition element (MARE) thereby activating the antioxidant responsive element (ARE), which participates in oxidative stress response [106]. We conclude that Keap1 acts as a sensor in response to oxidative stress and leads to translocation of activated Nrf2 which in turn regulates transcription of a series of antioxidant genes, including HO-1, so that the Nrf2/Keap1-HO-1 signaling pathway is sensitive to oxidative stress.
4.3. Bach1/HO-1 Signaling
Both Bach1 and Bach2 consist of the BTB and CNC homology family, as a transcription factor that belongs to the basic region-leucine zipper factor family (bZIP) [97, 107]. In general, Bach1 and Bach2 form heterodimers with sMaf proteins and bind to the MARE to become transcription repressors [108, 109]. The BTB domain is required for protein-protein interactions and the bZIP domain possesses the nuclear localization signal [107, 110]. Bach2 has the ability to bind a TPA (12 O-tetra decanoylphorbol-13-acetate) response element (TRE) in its promoter region (5′UTR). Except for TRE, Bach2 can also bind to MARE (MAF response element) and ARE (antioxidant response element) in complex with the MAF protein, which results in repressed transcription. Depending on the same consensus sequence (TGAG/CTCA), TRE, MARE, and ARE elements can be bound by Maf family proteins [111]. Bach1 as a competitive binder to the ARE motifs leads to exclusion of Nrf2. As a repressive transcription factor, Bach1 regulates gene induction by release from enhancer elements. Otherwise, Bach1 plays a vital role in the Nrf2/Keap1-HO-1 pathway. Silencing of Bach1 increases HO-1 mRNA and protein dramatically, and the strong suppression of HO-1 activation is primarily mediated by Bach1 in HaCaT cells [43]. The phenomenon also illustrates that competitive binding of Bach1 to ARE motifs ensures that ARE motifs are not overstimulated by oxidation so that this mechanism probably maintains the heme balance by tightly regulating HO-1 expression.
4.4. PERK-HO-1 Signaling
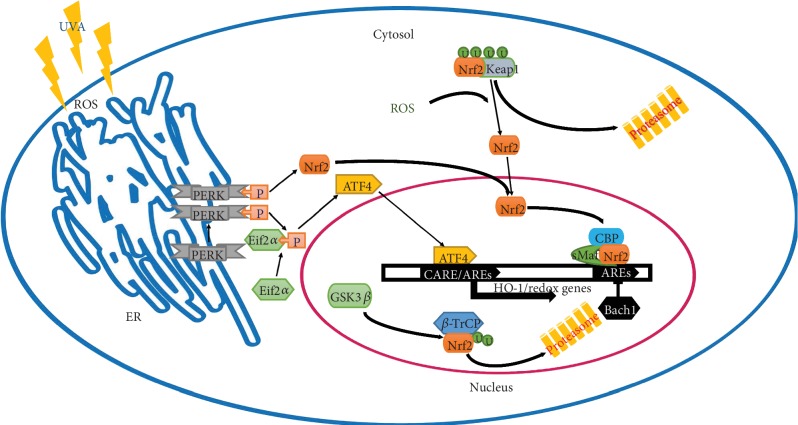
In addition to the Nrf2-HO-1 pathway, there is another important signal pathway that can regulate the expression of HO-1. The PERK-ATF4-HO-1 pathway, which belongs to the cellular homeostatic pathways, can be activated by integrated stress response (ISR) [12, 112]. UVA irradiation leads to oxidative stress and generation of ROS, and all these stressors may trigger disruptions of endoplasmic reticulum (ER) homeostasis, thereby causing ER stress [113, 114]. PERK belongs to the transmembrane ER receptor with a serine/threonine cytoplasmic domain; activated protein kinase RNA-like endoplasmic reticulum kinase (PERK) makes the eukaryotic initiation factor 2 alpha (eIF2α) phosphorylation, especially for Ser51 phosphorylation of eIF2α, and affects the repression of global protein synthesis and preferential translation of selected genes [114, 115]. In the mouse epidermal cell, Xue et al. found that UVA irradiation could activate eIF2α phosphorylation and Nrf2-HO-1 signaling and that modulated eIF2α phosphorylation status could change the Nrf2-HO-1 pathway [116].
Increasing eIF2α phosphorylation enhanced expression of activating transcription factor 4 (ATF4); ATF4 is a bZIP transcription factor that can be upregulated by multiple effectors that determine cell fate [117, 118]. Since ATF4 is downstream of PERK, it could participate in the metastatic cascade and is also critical for the regulation of autophagy [12]. ATF4 transcriptionally regulates several antioxidant genes in response to oxidative stress, including HO-1 and superoxide dismutase 2 (SOD2) [119, 120]. In general, ATF4 regulates the expression of genes by mainly binding to C/EBP-ATF regulatory elements (CARE) in the gene promoter region; however, latest studies of the HO-1 promoter have shown that ATF4 binds to unique ARE sites in the HO-1 promoter and interacts with Nrf2 to upregulate expression following matrix detachment [12, 121]. However, PERK directly phosphorylates Nrf2 to activate a cascade of antioxidant signaling. Nrf2 is also widely regarded as the primary transcriptional inducer of HO-1, which implies a cooperative activity of ATF4 and Nrf2 that may regulate the transcription of HO-1 [11, 12]. It also reveals that the intersection node between PERK-eIF2α and PERK-Nrf2 signaling toward regulating the transcription of HO-1 suggests that PERK could potentially be a therapeutic target for disease [122–124]. For further studies, various pathways can be activated in association with distinct wavelengths of UV, especially PERK-eIF2α signaling. It is known that ER stress signaling in response to unfolded protein stress (UPR) and based on diverse degrees of UPR could determine cell fate through the ER stress pathway. However, PERK can phosphorylate not only eIF2α but also Nrf2 [11]. Increasing eIF2α phosphorylation enhanced ATF4 expression and ATF4 could also regulate HO-1 expression, as shown in Figure 2. Zong et al. found that 60Coγ radiation induces ATF4 mRNA and protein expression in a dose- and time-dependent manner in AHH1 lymphoblast cells. Following 60Coγ radiation, ATF4 expression was increased in murine spleen cells, endothelial cells, and liver LO2 cells [125]. ATF4 is sensitive to ionizing radiation, which further confirms that HO-1 in response to diverse radiation modes may be related to ATF4 as an inducer. Therefore, we hypothesized that there may be a cross-talk relation between ATF4 and Nrf2 signaling through phosphorylation cascades (Figure 2). The phosphorylation status probably demonstrates the dose equivalent of radiation and also provides a new way to explore the principles of biological processes in response to different wavelength radiation modes.
Figure 2.
The mechanism of UVA-modulated HO-1 regulation through the PERK and Nrf2 signal pathway. U: ubiquitination; P: phosphorylation; ROS: reactive oxygen species; ARE: antioxidant response elements; CARE: C/EBP-ATF regulatory element; HO-1: heme oxygenase-1; ER: endoplasmic reticulum; Maf: small Maf protein; Bach1: transcription repressor; Nrf2: nuclear factor 2-related erythroid factor-2; CBP: CREB-binding protein.
4.5. Bioinformatics Analyzation of the HO-1 5′UTR Region
In order to further illustrate the mechanism of HO-1 regulatory relation, MatInspector was performed to predict the 5′-flanking region and some cis-regulatory elements (CREs) that were detected in the HO-1 5′-untranslated region. Although there are still some diverse mutations that exist on the different types of the human HO-1 promoter region, it is sufficient information to illustrate the potential regulatory relationship. Table 1 shows the AARE binding factors that were found. An ATF4 binding site means that either ATF4 or a heterodimer of CEBP epsilon and ATF4 could regulate HO-1 expression. Activator protein 1 could be induced in response to stimuli that have been reported. Estrogen-related receptor alpha binding site showed that estrogen could affect HO-1 and HO-1 expression suggesting that HO-1 levels may be different in male vs. female organisms. In addition, a binding site for the leucine zipper protein NF-E2 was predicted. A binding site for the C/EBP homologous protein (CHOP) could mean that HO-1 may be involved in the apoptosis process. Heat shock factor 1 showed that heat temperature difference may affect HO-1 expression. The hypoxia-inducible factor, bHLH/PAS protein family, is related to oxygen deficit. Nuclear factor kappa B (p50, p65) may be involved in inflammatory response. Signal transducer and activator of transcriptions 1, 3, 5, and 6 were likely related to signal transmission and proliferation. Besides, there are still some attractive binding sites that were found by predication, such as autoimmune regulatory element binding factor, nuclear factor Y binding factor, calcium-response factor, tumor suppressor p53, and tumor protein p63, which require further investigations to demonstrate that these factors are relevant for HO-1 regulation.
Table 1.
The predication of HO-1 promoter region.
| Gene | Detail | Matrix name | Start | End | Ration | Strand | Sequence |
|---|---|---|---|---|---|---|---|
| AARE binding factors | ATF4 binding site | V$AARE.01 | 361 | 369 | 0.953 | (−) | gTTTCacca |
| AARE binding factors | ATF4 binding site | V$AARE.01 | 2098 | 2106 | 0.953 | (+) | gTTTCacca |
| Autoimmune regulatory element binding factor | Autoimmune regulator | V$AIRE.01 | 1834 | 1848 | 0.785 | (+) | ccTTATttatggggt |
| AP1, activating protein 1 | Basic leucine zipper transcription factor, ATF-like | V$BATF.01 | 807 | 819 | 0.977 | (−) | cattgaCTCAagg |
| AP1, activating protein 1 | Activator protein 1 | V$AP1.02 | 1208 | 1220 | 0.892 | (+) | atctGAGTgagcc |
| AP1, activating protein 1 | Transcription factor Jun B | V$JUNB.01 | 807 | 819 | 0.977 | (+) | ccttgaGTCAatg |
| MAF- and AP1-related factors | Heterodimers with small Maf proteins | V$MARE.03 | 669 | 693 | 0.92 | (+) | gctctgGCTGaatcatgaaagggga |
| MAF- and AP1-related factors | Bach2 bound TRE | V$BACH2.01 | 802 | 826 | 0.951 | (+) | catcccctTGAGtcaatgctgttat |
| MAF- and AP1-related factors | Leucine zipper protein NF-E2 (nuclear factor, erythroid-derived) | V$NFE2.02 | 2530 | 2554 | 0.805 | (+) | ggggctggcgaGTCActgacccgcc |
| MAF- and AP1-related factors | NF-E2 p45 | V$NFE2.01 | 2707 | 2731 | 1 | (+) | gattttgCTGAgtcaccagtgcctc |
| Activator protein 2 | Transcription factor AP-2, beta | V$TCFAP2B.01 | 1173 | 1187 | 0.891 | (−) | ggaGCCCctgggccc |
| Heterodimers between bZIP family members | Heterodimer of CEBP epsilon and ATF4 | V$CEBPE_ATF4.02 | 1678 | 1690 | 0.89 | (−) | aattatGCAAgct |
| Heterodimers between bZIP family members | Heterodimer of CEBP epsilon and ATF4 | V$CEBPE_ATF4.02 | 1960 | 1972 | 0.869 | (+) | agtggtGCAAtct |
| Heterodimers between bZIP family members | Heterodimer of CEBP epsilon and ATF4 | V$CEBPE_ATF4.02 | 2907 | 2919 | 0.883 | (+) | aatgttGCAAtcc |
| CCAAT binding factors | Nuclear factor Y (Y-box binding factor) | V$NFY.04 | 2192 | 2206 | 0.922 | (−) | tgggCCAAttgtggt |
| CCAAT binding factors | Nuclear factor Y (Y-box binding factor) | V$NFY.01 | 3692 | 3706 | 0.91 | (+) | ctttCCAAtgggggg |
| CCAAT binding factors | Nuclear factor Y (Y-box binding factor) | V$NFY.04 | 1663 | 1677 | 0.948 | (+) | tgatCCAAttagact |
| Calcium-response elements | Calcium-response factor | V$CARF.01 | 752 | 762 | 0.936 | (−) | acagtGAGGct |
| Calcium-response elements | Calcium-response factor | V$CARF.01 | 2437 | 2447 | 0.911 | (−) | agagtGAGGag |
| Cell cycle regulators: cell cycle-dependent element | Cell cycle-dependent element, CDF-1 binding site | V$CDE.01 | 2609 | 2621 | 0.937 | (+) | gagtCGCGatttc |
| C/EBP homologous protein (CHOP) | Heterodimers of CHOP and C/EBPalpha | V$CHOP.02 | 1978 | 1990 | 0.974 | (+) | cacTGCAacctcc |
| Cell cycle regulators: cell cycle homology element | CDE/CHR tandem elements regulate cell cycle-dependent repression | V$CHR.01 | 3922 | 3934 | 0.97 | (+) | tagtTTGAatcct |
| C/EBP homologous protein (CHOP) | Heterodimers of CHOP and C/EBPalpha | V$CHOP.01 | 2909 | 2921 | 0.923 | (+) | tgttGCAAtccac |
| cAMP-responsive element binding proteins | c-Jun/ATF2 heterodimers | V$CJUN_ATF2.01 | 1362 | 1382 | 0.998 | (+) | ccttgcTGACttcaaggagct |
| cAMP-responsive element binding proteins | Activating transcription factor 1 | V$ATF1.02 | 2643 | 2663 | 0.918 | (−) | caaacaTGACgcagcagaaat |
| cAMP-responsive element binding proteins | X-box-binding protein 1 | V$XBP1.01 | 6597 | 6617 | 0.93 | (−) | ggcgggtcACGTgggccaggc |
| CTCF and BORIS gene family | Insulator protein CTCF (CCCTC-binding factor) | V$CTCF.05 | 3782 | 3808 | 0.834 | (−) | attcagcgaccctAGAGggtaaaactc |
| E2F-myc activator/cell cycle regulator | E2F transcription factor 4, p107/p130-binding protein | V$E2F4.01 | 2542 | 2558 | 0.969 | (−) | gggggGCGGgtcagtga |
| Estrogen-related receptors | Estrogen-related receptor alpha | V$ESRRA.01 | 4197 | 4219 | 0.9 | (−) | aatagatgcttcAAGGtctcttt |
| Estrogen-related receptors | Estrogen-related receptor alpha, homodimer DR5 binding site | V$ESRRA.05 | 1331 | 1353 | 0.829 | (+) | taagggcataagAAGGtgaatct |
| Fork head domain factors | Alternative splicing variant of FOXP1, activated in ESCs | V$FOXP1_ES.01 | 1466 | 1482 | 1 | (−) | gctgtaaAACAgattct |
| Fork head domain factors | Hepatic nuclear factor 3 beta (FOXA2) | V$HNF3B.03 | 4490 | 4506 | 0.886 | (−) | cttttgTAAAgaagtgt |
| Glucocorticoid responsive and related elements | Progesterone receptor binding site, IR3 sites | V$pre.01 | 3545 | 3563 | 0.892 | (−) | caggccagagtTGTTctgg |
| Glucocorticoid responsive and related elements | Androgene receptor binding site, IR3 sites | V$are.02 | 5282 | 5300 | 0.903 | (+) | ggacagcgtcttGTTCtgt |
| Human acute myelogenous leukemia factors | Runt-related transcription factor 2/CBFA1 | V$AML3.01 | 2567 | 2581 | 0.91 | (−) | agaaGTGGtttgggc |
| Heat shock factors | Heat shock factor 2 | V$HSF2.02 | 195 | 219 | 0.961 | (−) | tttcaaatgtccAGAAaagtcgcaa |
| Heat shock factors | Heat shock factor 1 | V$HSF1.02 | 481 | 505 | 0.753 | (+) | tgcaatgagcCGAGatagtgccatt |
| Hypoxia-inducible factor, bHLH/PAS protein family | Hypoxia-inducible factor, bHLH/PAS protein family | V$HIF1.02 | 6599 | 6615 | 0.964 | (+) | ctggcccaCGTGacccg |
| Hypoxia-inducible factor, bHLH/PAS protein family | Aryl hydrocarbon receptor nuclear translocator-like, homodimer | V$ARNTL.01 | 6600 | 6616 | 1 | (−) | gcgggtcaCGTGggcca |
| Myc-associated zinc fingers | Myc-associated zinc finger protein (MAZ) | V$MAZ.01 | 2661 | 2673 | 0.951 | (+) | ttggGAGGgggga |
| Myc-associated zinc fingers | MYC-associated zinc finger transcription factor | V$MAZR.01 | 2663 | 2675 | 0.885 | (+) | gggaggGGGGact |
| Cellular and viral myb-like transcriptional regulators | v-Myb, AMV v-myb | V$VMYB.04 | 3596 | 3616 | 0.89 | (+) | gggctgttctAACGaagtctg |
| NGFI-B response elements | Nuclear hormone receptor NUR77 (NR4A1) | V$NUR77.01 | 5088 | 5102 | 0.93 | (−) | gacaaaaGTCAggtt |
| NeuroD, beta2, HLH domain | Neuronal differentiation 1 | V$NEUROD1.02 | 5648 | 5662 | 0.929 | (+) | gaatCATCtggtcca |
| Nuclear factor kappa B/c-rel | NF-kappaB (p50) | V$NFKAPPAB50.01 | 1629 | 1643 | 0.882 | (−) | gagGGGAttaccaag |
| Nuclear factor kappa B/c-rel | NF-kappaB | V$NFKAPPAB.01 | 2396 | 2410 | 0.944 | (−) | agGGGAcgtccccag |
| Nuclear factor kappa B/c-rel | NF-kappaB (p65) | V$NFKAPPAB65.01 | 4464 | 4478 | 0.992 | (+) | tctggaatTTCCtta |
| “Negative” glucocorticoid response elements | Repressive binding sites for glucocorticoid receptor (IR2) | V$IR2_NGRE.01 | 1182 | 1196 | 0.803 | (+) | ggCTCCtagggcagc |
| Nuclear receptor subfamily 2 factors | DR1 binding sites for TR2 homodimers or TR2/TR4 heterodimers | V$TR2_TR4.01 | 612 | 636 | 0.841 | (−) | tgcagtagggcgggGGTCactaaca |
| Nuclear receptor subfamily 2 factors | TR4 homodimer, DR1 site | V$TR4.02 | 2740 | 2764 | 0.782 | (+) | ctctttAGGTgggaggtgaaagggc |
| OVO homolog-like transcription factors | Zinc finger transcription factor OVO homolog-like 1 | V$OVOL1.01 | 3600 | 3614 | 0.836 | (−) | gacttcGTTAgaaca |
| p53 tumor suppressor | Tumor suppressor p53 | V$P53.08 | 399 | 423 | 0.889 | (+) | ctgggcatggtggtgCATGcctgta |
| p53 tumor suppressor | Tumor protein p63 | V$TP63.02 | 5402 | 5426 | 0.933 | (+) | acaggcatgtgccacCATGcccagc |
| Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma, DR1 sites | V$PPARG.03 | 540 | 562 | 0.852 | (+) | tctaaaaaaaaaAAAGgtaaata |
| SOX/SRY-sex/testis | SRY-box containing gene 3 | V$SOX3.01 | 1765 | 1787 | 0.979 | (−) | aacagaCAAAagatgaacattcc |
| Sterol regulatory element binding proteins | Sterol regulatory element binding protein | V$SREBP.03 | 3083 | 3097 | 0.948 | (−) | tgaTCACctgaggtc |
| Signal transducer and activator of transcription | Signal transducer and activator of transcription 3 | V$STAT3.02 | 4259 | 4277 | 0.966 | (−) | ccagTTCCtggaatagtgc |
| Signal transducer and activator of transcription | STAT5: signal transducer and activator of transcription 5 | V$STAT5.01 | 5105 | 5123 | 0.927 | (−) | aaggTTCCccgaaaaccaa |
| Signal transducer and activator of transcription | STAT6: signal transducer and activator of transcription 6 | V$STAT6.01 | 5117 | 5135 | 0.92 | (−) | cttcTTCCtctgaaggttc |
| Signal transducer and activator of transcription | Signal transducer and activator of transcription 1 | V$STAT1.01 | 5810 | 5828 | 0.793 | (+) | aatgtgcctGGAAgagtgt |
| TGF-beta induced apoptosis proteins | Cysteine-serine-rich nuclear protein 1 | V$CSRNP1.01 | 2165 | 2171 | 1 | (+) | AGAGtgc |
| TCF11 transcription factor | TCF11/LCR-F1/Nrf1 homodimers | V$TCF11.01 | 3967 | 3973 | 1 | (−) | GTCAttt |
| Activator/repressor binding to transcription initiation site | Transcription factor yin yang 2 | V$YY2.01 | 3156 | 3178 | 0.981 | (+) | catcctCCATcttcaaagctagc |
| Activator/repressor binding to transcription initiation site | Transcription factor yin yang 2 | V$YY2.02 | 3794 | 3816 | 0.853 | (−) | gtctgtCCATtcagcgaccctag |
| Members of ZIC family, zinc finger protein of the cerebellum | Zinc finger protein of the cerebellum (Zic3) | V$ZIC3.03 | 2978 | 2992 | 0.918 | (+) | gcctcCAGCaggaga |
5. The Effects of HO Transcripts and Truncated HO-1
5.1. Transcripts of HO
As opposed to HO-1, HO-2 possesses some transcripts [15, 126]. Depending on different cell types, tissues, organs, and species, it generates various transcripts that have distinct functions. Different sizes of HO-2 transcripts have been identified; most of them are associated with tissue- and development-specific regulation [14]. However, in other species, HO-1 and HO-2 exert a similar mechanism. Various HO-2 transcripts can be generated by alternative splicing, alternative usage of polyadenylation sites, stage-specific exon utilization, or transcriptional site initiation [127]. The promoter region is important for the transcript formation [128, 129]. There also exists evidence that genetic variations of HMOX1 impact on the physiological function [14], especially for the single nucleotide polymorphism (SNP) and a microsatellite GT-dinucleotide repeat in the promoter region that is related to incidence and progression of disease [6, 130]. These polymorphisms may be a potential component of the pathogenesis through HMOX-1 transcription or translation regulation. The length of the polymorphism is also associated with susceptibility to many diseases such as cardiovascular disease, peripheral artery diseases, lung adenocarcinoma, and Parkinson's disease [131–136]. Moreover, common polymorphisms usually can affect alternative splicing [137]. A recent report by Bian et al. identified an alternative splice isoform of 14 kDa HO-1, which may be involved in tumor growth and telomere modulation [17]. The alternative splice isoform of HO-1 has been found, which helps to clarify its potential function in diseases and provides some meaningful data.
5.2. Truncated HO-1
In mouse 3T3 cells, a 28 kDa HO-1 band was induced under hypoxic exposure; the 28 kDa HO-1 was primarily localized to the nucleus and known as nuclear proteins. This isoform of HO-1 missing 52 amino acids from the C terminus was found to be enzymatically inactive [16]. Hori et al. showed that an enzymatically inactive form of HO-1 was also able to protect against oxidation damage; it can bind to heme but cannot degrade it into biliverdin [138]. In addition, Kassovska-Bratinova et al. used mass spectroscopy to identify a 27 kDa nuclear form of HO-1 that lacks the C terminus [139].
The truncation of the C terminus of human HO-1 by 23 amino acids maintains enzyme activity, but further truncation by 56-68 amino acids reduces HO activity [140]. The C-terminal truncation of HO-1 does not alter the heme catalytic pocket [16]. Meanwhile, the truncated HO-1 modulates stabilization and nuclear accumulation of Nrf2, so the truncated HO-1 protein may play a role in cellular signaling through migration to the nucleus or affect nuclear transcription [141].
There are several examples of cytoplasmic enzymes serving functions in the nucleus; we are also interested in the procession of HO-1 translocation to the nucleus. In general, nuclear localization sequences (NLS) are essential for the majority of proteins that migrate to the nucleus. So far, no NLS has been identified in HO-1; however, HO-1 has a nuclear export sequence (NES). The oxidative modification can modify the function of an NES [142]. In most instances, CRM1 binds with RanGTP to form a complex to allow the nuclear pore through to the cytoplasm [143].
HO-1 may bind to the CRM1 complex for nuclear import rather than for nuclear export [144]. This suggests that CRM1 may shuttle across the nuclear pore and that truncated HO-1 may participate in intercellular signaling [16, 20]. According to the HO transcripts and truncated HO-1, we found that the HO system may play a vital role in cellular homeostasis, which transforms into different transcriptional profiles and performs diverse functions.
6. Conclusion
HO proteins are vital rate-limiting enzymes, which participate in heme catabolism and protect against cellular oxidative stress. HO-1 can be induced by UV irradiation [145], and UV regulates several pathways involving phosphorylation of eIF2α, phosphatidylinositol- (PI-) 3 kinase, mitogen-activated protein kinases (MAPKs), ATM, and ATR [13, 146–148]. A complex signaling network between HO-1 and UV irradiation has recently been revealed, including Nrf2-HO-1 signaling, eIF2α-ATF4-HO-1 signaling and Bach1/HO-1. The phosphorylation of eIF2α could induce HO-1 expression. Moreover, Nrf2/Keap1-HO-1 signaling is another crucial antioxidative signaling pathway that is activated in response to UV exposure. PERK not only phosphorylated Nrf2 but also phosphorylated eIF2α, suggesting that there may exist a relation between Nrf2 and ER stress signaling. Furthermore, We also introduced a new form of truncated HO-1 which is revealed to be related to tumor growth and telomere modulation. Associated with immunomodulation and antioxidation, HO-1 plays a crucial role in pathology. Taken together, this review describes the character of the HO enzyme system, and its relationship to UV is helpful for revealing the HO-related signaling networks and the pathogenesis of many diseases, which also might provide new insights into potential therapeutic applications, i.e., by manipulating potential genetic targets.
Acknowledgments
The study was funded by the Fundamental Research Funds for the Central Universities (2019CDQYGD038), Chongqing Science and Technology Commission (grant no. Cstc2017jcyjbx0044), Chongqing Basic Research and Frontier Exploration Project (cstc2018jcyjAX0830), and Special Project for Performance Incentive and Guidance of Chongqing Scientific Research Institute (cstc2018jxjl130085). We appreciate the useful discussions of Dr. Mingxing Lei, Prof. Rex Tyrrell, Prof. C Pourzand, and Prof. Jorg W Bartsch.
Contributor Information
Mao Lin, Email: lmsleeper@aliyun.com.
Julia Li Zhong, Email: jlzhong@cqu.edu.cn.
Conflicts of Interest
The authors declared that there are no potential conflicts of interest.
References
- 1.Yoshida T., Migita C. T. Mechanism of heme degradation by heme oxygenase. Journal of Inorganic Biochemistry. 2000;82(1-4):33–41. doi: 10.1016/s0162-0134(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 2.Dennery P. A. Current Topics in Cellular Regulation. Vol. 36. Elsevier; 2001. Regulation and role of heme oxygenase in oxidative injury; pp. 181–199. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y. M., Choi B. M., Kim Y. S., et al. Protective effect of p53 in vascular smooth muscle cells against nitric oxide-induced apoptosis is mediated by up-regulation of heme oxygenase-2. BMB Reports. 2008;41(2):164–169. doi: 10.5483/bmbrep.2008.41.2.164. [DOI] [PubMed] [Google Scholar]
- 4.Schipper H. M. Heme oxygenase-1: role in brain aging and neurodegeneration. Experimental Gerontology. 2000;35(6-7):821–830. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Furuyama K., Kaneko K., et al. Hypoxia reduces the expression of heme oxygenase-2 in various types of human cell lines. FEBS Journal. 2006;273(14):3136–3147. doi: 10.1111/j.1742-4658.2006.05319.x. [DOI] [PubMed] [Google Scholar]
- 6.Lavrovsky Y., Schwartzman M. L., Levere R. D., Kappas A., Abraham N. G. Identification of binding-sites for transcription factors Nf-kappa-B and Ap-2 in the promoter region of the human heme oxygenase-1 gene. Proceedings of the National Academy of Sciences. 1994;91(13):5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizzardini M., Chiesa R., Angeretti N., et al. Prion protein fragment 106-126 differentially induces heme oxygenase-1 mRNA in cultured neurons and astroglial cells. Journal of Neurochemistry. 1997;68(2):715–720. doi: 10.1046/j.1471-4159.1997.68020715.x. [DOI] [PubMed] [Google Scholar]
- 8.Raju V. S., McCoubrey W. K., Jr., Maines M. D. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1997;1351(1-2):89–104. doi: 10.1016/S0167-4781(96)00183-2. [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Sanchez J., Chanez-Cardenas M. E. A review on hemeoxygenase-2: focus on cellular protection and oxygen response. Oxidative Medicine and Cellular Longevity. 2014;2014:16. doi: 10.1155/2014/604981.604981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams S. E. J., Wootton P., Mason H. S., et al. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306(5704):2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 11.Cullinan S. B., Zhang D., Hannink M., Arvisais E., Kaufman R. J., Diehl J. A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and Cellular Biology. 2003;23(20):7198–7209. doi: 10.1128/mcb.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey S., Sayers C. M., Verginadis I. I., et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. Journal of Clinical Investigation. 2015;125(7):2592–2608. doi: 10.1172/JCI78031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong J. L., Yang L., Lü F., et al. UVA, UVB and UVC induce differential response signaling pathways converged on the eIF2α phosphorylation. Photochemistry and Photobiology. 2011;87(5):1092–1104. doi: 10.1111/j.1751-1097.2011.00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Kramer M., Sponholz C., Slaba M., et al. Alternative 5′ untranslated regions are involved in expression regulation of human heme oxygenase-1. Plos One. 2013;8(10, article e77224) doi: 10.1371/journal.pone.0077224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T., Takahashi S., Kikuchi G. Partial Purification and reconstitution of the heme oxygenase system from pig spleen microsomes. Journal of Biochemistry. 1974;75(5):1187–1191. doi: 10.1093/oxfordjournals.jbchem.a130494. [DOI] [PubMed] [Google Scholar]
- 16.Lin Q., Weis S., Yang G., et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. Journal of Biological Chemistry. 2007;282(28):20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 17.Bian C., Zhong M., Nisar M. F., et al. A novel heme oxygenase-1 splice variant, 14kDa HO-1, promotes cell proliferation and increases relative telomere length. Biochemical and Biophysical Research Communications. 2018;500(2):429–434. doi: 10.1016/j.bbrc.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 18.Ding Y., Zhang Y. Z., Furuyama K., Ogawa K., Igarashi K., Shibahara S. Down-regulation of heme oxygenase-2 is associated with the increased expression of heme oxygenase-1 in human cell lines. FEBS Journal. 2006;273(23):5333–5346. doi: 10.1111/j.1742-4658.2006.05526.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu R. Q., Liu W. Q., Zhang J., et al. Light induced heme oxygenase 1 is suppressed by Bach1 in human skin keratinocytes. Optics in Health Care and Biomedical Optics IV; November 2010; Beijing, China. [Google Scholar]
- 20.Raval C. M., Zhong J. L., Mitchell S. A., Tyrrell R. M. The role of Bach1 in ultraviolet A-mediated human heme oxygenase 1 regulation in human skin fibroblasts. Free Radical Biology and Medicine. 2012;52(1):227–236. doi: 10.1016/j.freeradbiomed.2011.10.494. [DOI] [PubMed] [Google Scholar]
- 21.Maines M. D., Trakshel G. M., Kutty R. K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. only one molecular species of the enzyme is inducible. Journal of Biological Chemistry. 1986;261(1):411–419. [PubMed] [Google Scholar]
- 22.Tyrrell R. M. Solar ultraviolet a radiation: an oxidizing skin carcinogen that activates heme oxygenase-1. Antioxidants & Redox Signaling. 2004;6(5):835–840. doi: 10.1089/ars.2004.6.835. [DOI] [PubMed] [Google Scholar]
- 23.Alam J., Igarashi K., Immenschuh S., Shibahara S., Tyrrell R. M. Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the international conference (Uppsala, 2003) on heme oxygenase. Antioxidants & Redox Signaling. 2004;6(5):924–933. doi: 10.1089/ars.2004.6.924. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi G., Yoshida T., Noguchi M. Heme oxygenase and heme degradation. Biochemical and Biophysical Research Communications. 2005;338(1):558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Baranano D. E., Rao M., Ferris C. D., Snyder S. H. Biliverdin reductase: a major physiologic cytoprotectant. Proceedings of the National Academy of Sciences. 2002;99(25):16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancuso C., Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Current Drug Metabolism. 2009;10(6):579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicology Letters. 2005;157(3):175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Morita T., Mitsialis S. A., Koike H., Liu Y., Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. Journal of Biological Chemistry. 1997;272(52):32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 29.Kim H. P., Ryter S. W., Choi A. M. K. CO as a cellular signaling molecule. Annual Review of Pharmacology and Toxicology. 2006;46(1):411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 30.Morse D., Sethi J., Choi A. M. K. Carbon monoxide-dependent signaling. Critical Care Medicine. 2002;30(Supplement 1):S12–S17. doi: 10.1097/00003246-200201001-00002. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y. M., Chung H. T., Simmons R. L., Billiar T. R. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. Journal of Biological Chemistry. 2000;275(15):10954–10961. doi: 10.1074/jbc.275.15.10954. [DOI] [PubMed] [Google Scholar]
- 32.Ryter S. W., Tyrrell R. M. The heme synthesis and degradation pathways: role in oxidant sensitivity: heme oxygenase has both pro- and antioxidant properties. Free Radical Biology and Medicine. 2000;28(2):289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 33.Haeger M., Unander M., Andersson B., Tarkowski A., Arnestad J. P., Bengtsson A. Increased release of tumor necrosis factor-alpha and interleukin-6 in women with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Acta Obstetricia et Gynecologica Scandinavica. 1996;75(8):695–701. doi: 10.3109/00016349609065729. [DOI] [PubMed] [Google Scholar]
- 34.Tracz M. J., Alam J., Nath K. A. Physiology and pathophysiology of heme: implications for kidney disease. Journal of the American Society of Nephrology. 2007;18(2):414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 35.Arruda M. A., Rossi A. G., de Freitas M. S., Barja-Fidalgo C., Graca-Souza A. V. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-κB. Journal of Immunology. 2004;173(3):2023–2030. doi: 10.4049/jimmunol.173.3.2023. [DOI] [PubMed] [Google Scholar]
- 36.Belcher J. D., Beckman J. D., Balla G., Balla J., Vercellotti G. Heme degradation and vascular injury. Antioxidants & Redox Signaling. 2010;12(2):233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halliwell B., Gutteridge J. M. C. Oxygen-toxicity, oxygen radicals, transition-metals and disease. Biochemical Journal. 1984;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoubrey W. K., Maines M. D. The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene. 1994;139(2):155–161. doi: 10.1016/0378-1119(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 39.Ryter S. W., Alam J., Choi A. M. K. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiological Reviews. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kutty R. K., Kutty G., Rodriguez I. R., Chader G. J., Wiggert B. Chromosomal Localization of the Human Heme Oxygenase Genes: Heme Oxygenase-1 (HMOX1) Maps to Chromosome 22q12 and Heme Oxygenase-2 (HMOX2) Maps to Chromosome 16p13.3. Genomics. 1994;20(3):513–516. doi: 10.1006/geno.1994.1213. [DOI] [PubMed] [Google Scholar]
- 41.Muller R. M., Taguchi H., Shibahara S. Nucleotide-sequence and organization of the rat heme oxygenase gene. Journal of Biological Chemistry. 1987;262(14):6795–6802. [PubMed] [Google Scholar]
- 42.Ossola J. O., Tomaro M. L. Heme oxygenase induction by UVA radiation. A response to oxidative stress in rat liver. International Journal of Biochemistry & Cell Biology. 1998;30(2):285–292. doi: 10.1016/s1357-2725(97)00109-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhong J. L., Raval C., Edwards G. P., Tyrrell R. M. A role for Bach1 and HO-2 in suppression of basal and UVA-induced HO-1 expression in human keratinocytes. Free Radical Biology and Medicine. 2010;48(2):196–206. doi: 10.1016/j.freeradbiomed.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Liu N., Wang X., McCoubrey W. K., Maines M. D. Developmentally regulated expression of two transcripts for heme oxygenase-2 with a first exon unique to rat testis: control by corticosterone of the oxygenase protein expression. Gene. 2000;241(1):175–183. doi: 10.1016/s0378-1119(99)00439-4. [DOI] [PubMed] [Google Scholar]
- 45.Kirino M., Kirino Y., Takeno M., et al. Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: Implications for human disease. Journal of Allergy and Clinical Immunology. 2008;122(2):290–297.e8. doi: 10.1016/j.jaci.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Noordeloos A. M., Cheng C., van Deal E., et al. Heme oxygenase 1 expression in dendritic calls drives Cd4+T cell activation in transplantation atherosclerosis. Circulation. 2008;118:S863–SS63. [Google Scholar]
- 47.Kotsch K., Martins P. N. A., Klemz R., et al. heme oxygenase-1 ameliorates ischemia/reperfusion injury by targeting dendritic cell maturation and migration. Antioxidants & Redox Signaling. 2007;9(12):2049–2064. doi: 10.1089/ars.2007.1801. [DOI] [PubMed] [Google Scholar]
- 48.Soares M. P., Usheva A., Brouard S., et al. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxidants & Redox Signaling. 2002;4(2):321–329. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- 49.Fang J., Akaike T., Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004;9(1):27–35. doi: 10.1023/B:APPT.0000012119.83734.4e. [DOI] [PubMed] [Google Scholar]
- 50.Doi K., Akaike T., Fujii S., et al. Induction of haem oxygenase-1 by nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. British Journal of Cancer. 1999;80(12):1945–1954. doi: 10.1038/sj.bjc.6690624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodman A. I., Choudhury M., da Silva J.-L., Schwartzman M. L., Abraham N. G. Overexpression of the heme oxygenase gene in renal cell carcinoma. Experimental Biology and Medicine. 1997;214(1):54–75. doi: 10.3181/00379727-214-44069. [DOI] [PubMed] [Google Scholar]
- 52.Torisu-Itakura H., Furue M., Kuwano M., Ono M. Co-expression of thymidine phosphorylase and heme oxygenase-1 in macrophages in human malignant vertical growth melanomas. Japanese Journal of Cancer Research. 2000;91(9):906–910. doi: 10.1111/j.1349-7006.2000.tb01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asarch A., Barak O., Loo D. S., Gottlieb A. B. Th17 cells: a new paradigm for cutaneous inflammation. The Journal of Dermatological Treatment. 2008;19(5):259–266. doi: 10.1080/09546630802206686. [DOI] [PubMed] [Google Scholar]
- 54.Hanselmann C., Mauch C., Werner S. Haem oxygenase-1: a novel player in cutaneous wound repair and psoriasis? The Biochemical Journal. 2001;353(Part 3):459–466. doi: 10.1042/0264-6021:3530459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elassiuty Y. E., Klarquist J., Speiser J., et al. Heme oxygenase-1 expression protects melanocytes from stress-induced cell death: implications for vitiligo. Experimental Dermatology. 2011;20(6):496–501. doi: 10.1111/j.1600-0625.2010.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Y., Li S., Zhang W., et al. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Scientific Reports. 2017;7(1, article 42394) doi: 10.1038/srep42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jian Z., Li K., Song P., et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. Journal of Investigative Dermatology. 2014;134:2221–2230. doi: 10.1038/jid.2014.152. [DOI] [PubMed] [Google Scholar]
- 58.Shi Q., Zhang W., Guo S., et al. Oxidative stress-induced overexpression of miR-25: the mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death and Differentiation. 2016;23(3):496–508. doi: 10.1038/cdd.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q., Cui T., Chang Y., et al. HO-1 regulates the function of Treg: association with the immune intolerance in vitiligo. Journal of Cellular and Molecular Medicine. 2018;22(9):4335–4343. doi: 10.1111/jcmm.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briganti S., Wlaschek M., Hinrichs C., et al. Small molecular antioxidants effectively protect from PUVA-induced oxidative stress responses underlying fibroblast senescence and photoaging. Free Radical Biology & Medicine. 2008;45(5):636–644. doi: 10.1016/j.freeradbiomed.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Song P., Li K., Liu L., et al. Genetic polymorphism of the Nrf2 promoter region is associated with vitiligo risk in Han Chinese populations. Journal of Cellular and Molecular Medicine. 2016;20(10):1840–1850. doi: 10.1111/jcmm.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Was H., Cichon T., Smolarczyk R., et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. The American Journal of Pathology. 2006;169(6):2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maines M. D. The heme oxygenase system: a regulator of second messenger gases. Annual Review of Pharmacology and Toxicology. 1997;37(1):517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 64.Doré S., Takahashi M., Ferris C. D., Hester L. D., Guastella D., Snyder S. H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proceedings of the National Academy of Sciences. 1999;96(5):2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dore S., Sampei K., Goto S., et al. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Molecular Medicine. 1999;5(10):656–663. doi: 10.1007/BF03401984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dore S., Goto S., Sampei K., et al. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 2000;99(4):587–592. doi: 10.1016/S0306-4522(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 67.Ding Y., McCoubrey W. K., Jr., Maines M. D. Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular ‘sink’ for NO? European Journal of Biochemistry. 1999;264(3):854–861. doi: 10.1046/j.1432-1327.1999.00677.x. [DOI] [PubMed] [Google Scholar]
- 68.He J. Z., Ho J. J. D., Gingerich S., Courtman D. W., Marsden P. A., Ward M. E. Enhanced translation of heme oxygenase-2 preserves human endothelial cell viability during hypoxia. Journal of Biological Chemistry. 2010;285(13):9452–9461. doi: 10.1074/jbc.M109.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. diseases related to heme biosynthesis. Molecular Biology & Medicine. 1990;7(5):405–421. [PubMed] [Google Scholar]
- 70.Maines M. D., Panahian N. The heme oxygenase system and cellular defense mechanisms. Advances in Experimental Medicine and Biology. 2001;502:249–272. doi: 10.1007/978-1-4757-3401-0_17. [DOI] [PubMed] [Google Scholar]
- 71.Sklar L. R., Almutawa F., Lim H. W., Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochemical & Photobiological Sciences. 2013;12(1):54–64. doi: 10.1039/c2pp25152c. [DOI] [PubMed] [Google Scholar]
- 72.Tyrrell R. M. Activation of mammalian gene expression by the UV component of sunlight - from models to reality. BioEssays. 1996;18(2):139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- 73.Natarajan V. T., Ganju P., Ramkumar A., Grover R., Gokhale R. S. Multifaceted pathways protect human skin from UV radiation. Nature Chemical Biology. 2014;10(7):542–551. doi: 10.1038/nchembio.1548. [DOI] [PubMed] [Google Scholar]
- 74.Kaidbey K. H., Agin P. P., Sayre R. M., Kligman A. M. Photoprotection by melanin--a comparison of black and Caucasian skin. Journal of the American Academy of Dermatology. 1979;1(3):249–260. doi: 10.1016/s0190-9622(79)70018-1. [DOI] [PubMed] [Google Scholar]
- 75.Kollias N., Sayre R. M., Zeise L., Chedekel M. R. New trends in photobiology: Photoprotection by melanin. Journal of Photochemistry and Photobiology B-Biology. 1991;9(2):135–160. doi: 10.1016/1011-1344(91)80147-A. [DOI] [PubMed] [Google Scholar]
- 76.Teulings H. E., Overkamp M., Ceylan E., et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. British Journal of Dermatology. 2013;168(1):162–171. doi: 10.1111/bjd.12111. [DOI] [PubMed] [Google Scholar]
- 77.Wu W., Amos C. I., Lee J. E., Wei Q., Sarin K. Y., Han J. Inverse relationship between vitiligo-related genes and skin cancer risk. Journal of Investigative Dermatology. 2018;138(9):2072–2075. doi: 10.1016/j.jid.2018.03.1511. [DOI] [PubMed] [Google Scholar]
- 78.Lim H. W., Silpa-archa N., Amadi U., Menter A., Van Voorhees A. S., Lebwohl M. Phototherapy in dermatology: a call for action. Journal of the American Academy of Dermatology. 2015;72(6):1078–1080. doi: 10.1016/j.jaad.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Meduri N. B., Vandergriff T., Rasmussen H., Jacobe H. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatology Photoimmunology & Photomedicine. 2007;23(4):106–112. doi: 10.1111/j.1600-0781.2007.00291.x. [DOI] [PubMed] [Google Scholar]
- 80.Sidbury R., Davis D. M., Cohen D. E., et al. Guidelines of care for the management of atopic dermatitis. Journal of the American Academy of Dermatology. 2014;71(2):327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisher G. J., Datta S. C., Talwar H. S., et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 82.York N. R., Jacobe H. T. UVA1 phototherapy: a review of mechanism and therapeutic application. International Journal of Dermatology. 2010;49(6):623–630. doi: 10.1111/j.1365-4632.2009.04427.x. [DOI] [PubMed] [Google Scholar]
- 83.de Gruijl F. R., Van der Leun J. C. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion. Health Physics. 1994;67(4):319–325. doi: 10.1097/00004032-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Nisar M. F., Parsons K. S. G., Bian C. X., Zhong J. L. UVA irradiation induced heme oxygenase-1: a novel phototherapy for morphea. Photochemistry and Photobiology. 2015;91(1):210–220. doi: 10.1111/php.12342. [DOI] [PubMed] [Google Scholar]
- 85.Tewari A., Grage M. M. L., Harrison G. I., Sarkany R., Young A. R. UVA1 is skin deep: molecular and clinical implications. Photochemical & Photobiological Sciences. 2013;12(1):95–103. doi: 10.1039/C2PP25323B. [DOI] [PubMed] [Google Scholar]
- 86.Tian F., Zhang F., Lai X., et al. Nrf2-mediated protection against UVA radiation in human skin keratinocytes. Bioscience Trends. 2011;5(1):23–29. doi: 10.5582/bst.2011.v5.1.23. [DOI] [PubMed] [Google Scholar]
- 87.Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proceedings of the National Academy of Sciences. 1989;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nascimento A. L. T. O., Luscher P., Tyrrell R. M. Ultraviolet-a (320-380 nm) radiation causes an alteration in the binding of a specific protein protein complex to a short region of the promoter of the human heme oxygenase-1 gene. Nucleic Acids Research. 1993;21(5):1103–1109. doi: 10.1093/nar/21.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tyrrell R. M. Modulation of gene expression by the oxidative stress generated in human skin cells by UVA radiation and the restoration of redox homeostasis. Photochemical & Photobiological Sciences. 2012;11(1):135–147. doi: 10.1039/c1pp05222e. [DOI] [PubMed] [Google Scholar]
- 90.Rizzardini M., Terao M., Falciani F., Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochemical Journal. 1993;290(2):343–347. doi: 10.1042/bj2900343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alam J., Den Z. N. Distal Ap-1 binding-sites mediate basal level enhancement and Tpa induction of the mouse heme oxygenase-1 gene. Journal of Biological Chemistry. 1992;267(30):21894–21900. [PubMed] [Google Scholar]
- 92.Dawn B., Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? American Journal of Physiology-Heart and Circulatory Physiology. 2005;289(2):H522–H524. doi: 10.1152/ajpheart.00274.2005. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X., Liu X., Li Y., et al. Downregulation of microRNA-155 ameliorates high glucose-induced endothelial injury by inhibiting NF-κB activation and promoting HO-1 and NO production. Biomedicine & Pharmacotherapy. 2017;88:1227–1234. doi: 10.1016/j.biopha.2017.01.122. [DOI] [Google Scholar]
- 94.Kensler T. W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology. 2007;47(1):89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 95.Kansanen E., Kuosmanen S. M., Leinonen H., Levonen A. L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biology. 2013;1(1):45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life Sciences. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sykiotis G. P., Bohmann D. Stress-activated cap‘n’collar transcription factors in aging and human disease. Science Signaling. 2010;3(112, article re3) doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Itoh K., Wakabayashi N., Katoh Y., et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & Development. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang M. I., Kobayashi A., Wakabayashi N., Kim S. G., Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proceedings of the National Academy of Sciences. 2004;101(7):2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang S., Zhou B., Xu W., et al. Nrf2- and Bach1 may play a role in the modulation of ultraviolet A-induced oxidative stress by Acetyl-11-Keto-β-Boswellic acid in skin keratinocytes. Skin Pharmacology and Physiology. 2017;30(1):13–23. doi: 10.1159/000452744. [DOI] [PubMed] [Google Scholar]
- 101.Baird L., Dinkova-Kostova A. T. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of Toxicology. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 102.Rada P., Rojo A. I., Chowdhry S., McMahon M., Hayes J. D., Cuadrado A. SCF/β-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Molecular and Cellular Biology. 2011;31(6):1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kobayashi M., Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Advances in Enzyme Regulation. 2006;46(1):113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 104.Hu C. Q., Eggler A. L., Mesecar A. D., van Breemen R. B. Modification of Keap1 cysteine residues by sulforaphane. Chemical Research in Toxicology. 2011;24(4):515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kobayashi A., Kang M. I., Watai Y., et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Molecular and Cellular Biology. 2006;26(1):221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Motohashi H., Yamamoto M. Carcinogenesis and transcriptional regulation through Maf recognition elements. Cancer Science. 2007;98(2):135–139. doi: 10.1111/j.1349-7006.2006.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oyake T., Itoh K., Motohashi H., et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Molecular and Cellular Biology. 1996;16(11):6083–6095. doi: 10.1128/MCB.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kobayashi A., Yamagiwa H., Hoshino H., et al. A combinatorial code for gene expression generated by transcription factor Bach2 and MAZR (MAZ-related factor) through the BTB/POZ domain. Molecular and Cellular Biology. 2000;20(5):1733–1746. doi: 10.1128/MCB.20.5.1733-1746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swaminathan S., Duy C., Müschen M. BACH2-BCL6 balance regulates selection at the pre-B cell receptor checkpoint. Trends in Immunology. 2014;35(3):131–137. doi: 10.1016/j.it.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoshino H., Kobayashi A., Yoshida M., et al. Oxidative stress abolishes leptomycin B-sensitive nuclear export of transcription repressor Bach2 that counteracts activation of Maf recognition element. Journal of Biological Chemistry. 2000;275(20):15370–15376. doi: 10.1074/jbc.275.20.15370. [DOI] [PubMed] [Google Scholar]
- 111.Muto A., Tashiro S., Tsuchiya H., et al. Activation of Maf/AP-1 repressor Bach2 by oxidative stress promotes apoptosis and its interaction with promyelocytic leukemia nuclear bodies. Journal of Biological Chemistry. 2002;277(23):20724–20733. doi: 10.1074/jbc.M112003200. [DOI] [PubMed] [Google Scholar]
- 112.Starck S. R., Tsai J. C., Chen K., et al. Translation from the 5′ untranslated region shapes the integrated stress response. Science. 2016;351(6272, article aad3867) doi: 10.1126/science.aad3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harding H. P., Zhang Y. H., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum- resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 114.Koumenis C., Naczki C., Koritzinsky M., et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Molecular and Cellular Biology. 2002;22(21):7405–7416. doi: 10.1128/mcb.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harding H. P., Zhang Y. H., Zeng H. Q., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 116.Xue F., Chen S., Chunxiang B., et al. eIF2 alpha phosphorylation alleviates UVA-induced HO-1 expression in mouse epidermal cells. Free Radical Research. 2018;52(11-12):1359–1370. doi: 10.1080/10715762.2018.1489127. [DOI] [PubMed] [Google Scholar]
- 117.Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor Atf Cdna clones - an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes & Development. 1989;3(12b):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 118.Vattem K. M., Wek R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proceedings of the National Academy of Sciences. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han J., Back S. H., Hur J., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature Cell Biology. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He C. H., Gong P., Hu B., et al. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein - implication for heme oxygenase-1 gene regulation. Journal of Biological Chemistry. 2001;276(24):20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 121.Kilberg M. S., Shan J., Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends in Endocrinology & Metabolism. 2009;20(9):436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Diehl J. A., Fuchs S. Y., Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141(1):38–41.e2. doi: 10.1053/j.gastro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature Reviews Molecular Cell Biology. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 124.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 125.Zong Y., Feng S., Cheng J., Yu C., Lu G. Up-regulated ATF4 expression increases cell sensitivity to apoptosis in response to radiation. Cellular Physiology and Biochemistry. 2017;41(2):784–794. doi: 10.1159/000458742. [DOI] [PubMed] [Google Scholar]
- 126.McCoubrey W. K., Ewing J. F., Maines M. D. Human heme oxygenase-2: characterization and expression of a full-length cDNA and evidence suggesting that the two HO-2 transcripts may differ by choice of polyadenylation signal. Archives of Biochemistry and Biophysics. 1992;295(1):13–20. doi: 10.1016/0003-9861(92)90481-b. [DOI] [PubMed] [Google Scholar]
- 127.McCoubrey W. K., Eke B., Maines M. D. Multiple transcripts encoding heme oxygenase-2 in rat testis - developmental and cell-specific regulation of transcripts and protein1. Biology of Reproduction. 1995;53(6):1330–1338. doi: 10.1095/biolreprod53.6.1330. [DOI] [PubMed] [Google Scholar]
- 128.Rosenstiel P., Huse K., Franke A., et al. Functional characterization of two novel 5′ untranslated exons reveals a complex regulation of NOD2 protein expression. Bmc Genomics. 2007;8(1):p. 472. doi: 10.1186/1471-2164-8-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang G., Guo X., Floros J. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2005;289:L497–L508. doi: 10.1152/ajplung.00100.2005. [DOI] [PubMed] [Google Scholar]
- 130.Motovali-Bashi M., Hamidy M. Association between GT-repeat polymorphism at heme oxygenase-1 gene promoter and gastric cancer and metastasis. Tumor Biology. 2015;36(6):4757–4762. doi: 10.1007/s13277-015-3125-8. [DOI] [PubMed] [Google Scholar]
- 131.Dick P., Schillinger M., Minar E., et al. Haem oxygenase-1 genotype and cardiovascular adverse events in patients with peripheral artery disease. European Journal of Clinical Investigation. 2005;35(12):731–737. doi: 10.1111/j.1365-2362.2005.01580.x. [DOI] [PubMed] [Google Scholar]
- 132.Funk M., Endler G., Schillinger M., et al. The effect of a promoter polymorphism in the heme oxygenase-1 gene on the risk of ischaemic cerebrovascular events: the influence of other vascular risk factors. Thrombosis Research. 2004;113(3-4):217–223. doi: 10.1016/j.thromres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Wu M. M., Chiou H. Y., Chen C. L., et al. GT-repeat polymorphism in the heme oxygenase-1 gene promoter is associated with cardiovascular mortality risk in an arsenic-exposed population in northeastern Taiwan. Toxicology and Applied Pharmacology. 2010;248(3):226–233. doi: 10.1016/j.taap.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 134.Kikuchi A., Yamaya M., Suzuki S., et al. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Human Genetics. 2005;116(5):354–360. doi: 10.1007/s00439-004-1162-2. [DOI] [PubMed] [Google Scholar]
- 135.Infante J., Garcia-Gorostiaga I., Sanchez-Juan P., et al. Synergistic effect of two oxidative stress-related genes (heme oxygenase-1 and GSK3β) on the risk of Parkinson’s disease. European Journal of Neurology. 2010;17(5):760–762. doi: 10.1111/j.1468-1331.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- 136.Infante J., Rodriguez-Rodriguez E., Mateo I., et al. Gene-gene interaction between heme oxygenase-1 and liver X receptor-β and Alzheimer’s disease risk. Neurobiology of Aging. 2010;31(4):710–714. doi: 10.1016/j.neurobiolaging.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 137.Kwan T., Benovoy D., Dias C., et al. Genome-wide analysis of transcript isoform variation in humans. Nature Genetics. 2008;40(2):225–231. doi: 10.1038/ng.2007.57. [DOI] [PubMed] [Google Scholar]
- 138.Hori R., Kashiba M., Toma T., et al. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. Journal of Biological Chemistry. 2002;277(12):10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 139.Kassovska-Bratinova S., Yang G., Dennery P. Heme oxygenase-1 (HO-1) localizes to the nucleus in hyperoxia. Free Radical Biology and Medicine. 2006;41:S38–S39. [Google Scholar]
- 140.Wilks A., Medzihradszky K. F., Ortiz de Montellano P. R. Heme oxygenase active-site residues identified by heme-protein cross-linking during reduction of CBrCl3†. Biochemistry. 1998;37(9):2889–2896. doi: 10.1021/bi972720x. [DOI] [PubMed] [Google Scholar]
- 141.Biswas C., Shah N., Muthu M., et al. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. Journal of Biological Chemistry. 2014;289(39):26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Connor M. K., Kotchetkov R., Cariou S., et al. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Molecular Biology of the Cell. 2003;14(1):201–213. doi: 10.1091/mbc.e02-06-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yan C., Lee L. H., Davis L. I. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO Journal. 1998;17(24):7416–7429. doi: 10.1093/emboj/17.24.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kose S., Imamoto N., Tachibana T., Shimamoto T., Yoneda Y. Ran-unassisted nuclear migration of a 97-kD component of nuclear pore-targeting complex. Journal of Cell Biology. 1997;139(4):841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reeve V. E., Tyrrell R. M. Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proceedings of the National Academy of Sciences. 1999;96(16):9317–9321. doi: 10.1073/pnas.96.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deng J., Harding H. P., Raught B., et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Current Biology. 2002;12(15):1279–1286. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- 147.Harding H. P., Calfon M., Urano F., Novoa I., Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annual Review of Cell and Developmental Biology. 2002;18(1):575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 148.Wek R. C., Jiang H. Y., Anthony T. G. Coping with stress: eIF2 kinases and translational control. Biochemical Society Transactions. 2006;34(Part 1):7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]