Highlights
-
•
Diaphragm contraction in neonates evoked a sequence of three event-related potentials.
-
•
Hiccups can be encoded by the brain as early as ten weeks prior to average time of birth.
-
•
Hiccups – frequent in neonates – provide afferent input to the developing brain.
Keywords: Somatosensory, Proprioceptive, Afferent, Diaphragm, Hiccup, Evoked potential
Abstract
Objective
Involuntary isolated body movements are prominent in pre-term and full-term infants. Proprioceptive and tactile afferent feedback following limb muscle contractions is associated with somatotopic EEG responses. Involuntary contractions of respiratory muscles, primarily the diaphragm – hiccups – are also frequent throughout the human perinatal period during active behavioural states. Here we tested whether diaphragm contraction provides afferent input to the developing brain, as following limb muscle contraction.
Methods
In 13 infants on the neonatal ward (30–42 weeks corrected gestational age), we analysed EEG activity (18-electrode recordings in six subjects; 17-electrode recordings in five subjects; 16-electrode recordings in two subjects), time-locked to diaphragm contractions (n = 1316) recorded with a movement transducer affixed to the trunk.
Results
All bouts of hiccups occurred during wakefulness or active sleep. Each diaphragm contraction evoked two initial event-related potentials with negativity predominantly across the central region, and a third event-related potential with positivity maximal across the central region.
Conclusions
Involuntary contraction of the diaphragm can be encoded by the brain from as early as ten weeks prior to the average time of birth.
Significance
Hiccups – frequently observed in neonates – can provide afferent input to developing sensory cortices in pre-term and full-term infants.
1. Introduction
Involuntary isolated limb movements are prominent in pre-term and full-term infants (Fukumoto et al., 1981, Hadders-Algra et al., 1993, Grigg-Damberger, 2016). Unilateral hand movements evoke somatotopic electroencephalography (EEG) activity overlying the contralateral fronto-central scalp area (Milh et al., 2007, Whitehead et al., 2018b), while bilateral body movements evoke symmetrical EEG activity (Losito et al. 2017). This coupling indicates that frequent limb muscle contractions in the perinatal period may provide afferent input to the developing cortex, which in neonatal animal models allows the refinement of body surface representations (Khazipov et al., 2004, Tiriac et al., 2012).
Alongside the external body map, the mature somatomotor cortex has dedicated areas representing the internal body environment, including the thoracic cavity (Maskill et al. 1991). These are crucial for monitoring the status of vital functions such as breathing, and thereby allow adaptive motor control of respiratory musculature (McKay et al., 2003, Wheeler-Hegland et al., 2011). As for external body representations, these internal body maps may also require early afferent input for their development.
In parallel with the high frequency of involuntary limb muscle contractions, involuntary contractions of respiratory muscles – hiccups – are also common in the equivalent of the last trimester of gestation: they are a dominant motor activity pattern in pre-term infants, occupying an estimated 1% of the day (Lipton et al., 1964, Swann, 1978, Brouillette et al., 1980, van Woerden et al., 1989, Pillai and James, 1990, de Vries and Fong, 2006). Hiccups typically occur in bouts, which last for approximately 8 minutes, and happen predominantly during active behavioural states in fetuses, and during wakefulness in neonates (Wagner, 1939, Brouillette et al., 1980, Pillai and James, 1990). These events are a form of reflex motor activity. The efferent limb is mainly the phrenic and external intercostal nerves, which trigger contraction of the diaphragm primarily and the intercostal muscles, as well as the vagus nerve which innervates the striated muscles of larynx and pharynx (Video 1) (Kahrilas and Shi, 1997, Kandel et al., 2000). The afferent limb of the hiccup reflex arc is poorly defined but appears to be mediated by multiple tracts including the phrenic and vagus nerves (Kahrilas and Shi, 1997, Ceriani et al., 2010). To investigate whether these respiratory muscle contractions could provide afferent input to the developing cortex from the internal body environment, we analysed EEG time-locked to hiccups in pre-term and full-term neonates.
2. Methods
2.1. Subjects
We identified infants who had hiccups by reviewing 217 research EEG recordings, each from a unique subject with corrected gestational age at study (CGA) 28 + 2–47 + 6 weeks + days, acquired between September 2015 and March 2019 (the CGA and other demographic details of the subset of infants who had hiccups are presented in Table 1). Infants who required mechanical ventilation were unsuitable due to difficulty in accessing EEG electrode placement sites, but all other infants including those who required a low to moderate degree of respiratory support (High flow oxygen or Continuous Positive Airway Pressure) were eligible. Research complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and ethical approval was obtained from the NHS Health Research Authority. Parents gave informed written consent, and separate informed written consent was obtained to publish video of one infant. EEG was recorded for approximately 70–90 minutes, in line with recommended best practice (Shellhaas et al. 2011). The presence of a bout of hiccups was recorded at the cot side, alongside annotations of the infant’s vigilance state which was categorised according to behavioural, respiratory and EEG criteria: wakefulness and active sleep are both characterised by movement, irregular breathing, and largely continuous relatively low voltage EEG (Supplementary Fig. 1); quiet sleep is characterised by the absence of movement, regular breathing, and an EEG pattern which fluctuates in amplitude (Tsuchida et al., 2013, Grigg-Damberger, 2016, Whitehead et al., 2018a).
Table 1.
Clinical data of infants who had hiccups.
| Subject/Sex | CGA | GA+PNA | Neurology | Respiratory support | No. epochs analysed |
|---|---|---|---|---|---|
| #1/F | 30 + 3 | 27 + 6 + 18 | Normal | High flow oxygen | 118 |
| #2/M | 30 + 4a | 27 + 3 + 22 | Normal | High flow oxygen | 49 |
| #3/M | 32 + 6 | 26 + 4 + 44 | Normal | High flow oxygen | 80 |
| #4/M | 33 + 6 | 32 + 6 + 7 | Mild ventriculomegaly (L > R) | Nil | 94 |
| #5/M | 34 + 5 | 33 + 6 + 6 | Normal | Nil | 82 |
| #6/F | 34 + 5a | 34 + 0 + 5 | Normal | Nil | 138 |
| #7/F | 34 + 6 | 23 + 5 + 78 | GM-IVH (grade III R > L) | High flow oxygen | 234 |
| #8/F | 35 + 5 | 35 + 3 + 2 | Normal | Nil | 7 |
| #9/M | 35 + 6 | 30 + 0 + 41 | GM-IVH (IPL R; grade I L)b | Nil | 42 |
| #10/F | 36 + 5a | 35 + 6 + 6 | Normal | Nil | 83 |
| #11/F | 37 + 1 | 35 + 3 + 12 | Normal | Nil | 22 |
| #12/M | 37 + 5 | 37 + 2 + 3 | Normal | Nil | 132 |
| #13/F | 42 + 0 | 41 + 3 + 4 | Normal | Nil | 235 |
| Median | 34 + 6 | 33 + 6 + 7 |
CGA = corrected gestational age at study (weeks + days); GA = gestational age at birth (weeks + days); PNA = postnatal age (days). CGA is defined as gestational age at birth plus postnatal age. For example, an infant born at 35 weeks + 2 days, who is 3 days old, is CGA 35 weeks + 5 days. Term is defined as ≥ 37 weeks.
GM-IVH = germinal matrix-intraventricular haemorrhage; IPL = intraparenchymal lesion secondary to GM-IVH. R = right; L = left.
These infants were in active sleep at onset of hiccups. All other infants were awake.
Magnetic resonance imaging (MRI) delimited the intraparenchymal lesion to the right basal ganglia, thalami and posterior limb of the internal capsule.
2.2. EEG recordings
Eighteen recording electrodes (disposable Ag/AgCl cup electrodes) were positioned individually by a clinical neurophysiologist (KW) according to the international 10/20 electrode placement system (F7, F8, F3, F4, Cz, C3, C4, T7, T8, P7, P8, O1, O2), with additional central-parietal and temporal coverage (CPz, CP3, CP4, TP9, TP10). In 2/13 infants, 2/18 electrodes were sacrificed because the infant became slightly unsettled during set-up (F7/F8 or TP9/TP10). The EEG reference electrode was placed at Fz. Target impedance was < 10 kΩ (André et al. 2010).
2.3. Polygraphy recordings
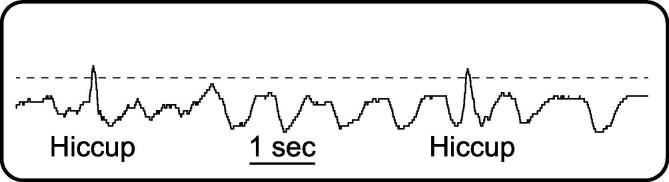
A movement transducer was applied to the lower trunk and a single lead I ECG was recorded from the upper trunk, both time-locked to the EEG recordings (Video 1). After a bout of hiccups was annotated at the cotside, one of these recordings was utilised offline as a hiccups registration trace (lower trunk 10/13 infants, upper trunk 3/13 infants). Each individual contraction was identified by thresholding this signal, on which a deflection occurred with each event (Fig. 1, Supplementary Fig. 1).
Fig. 1.
Representative 10-second long hiccups registration trace (movement recording from lower trunk) in which two hiccups occur, from subject #3. The event onset (0 ms) is identified by thresholding (dashed horizontal line) this signal.
2.4. Pre-processing
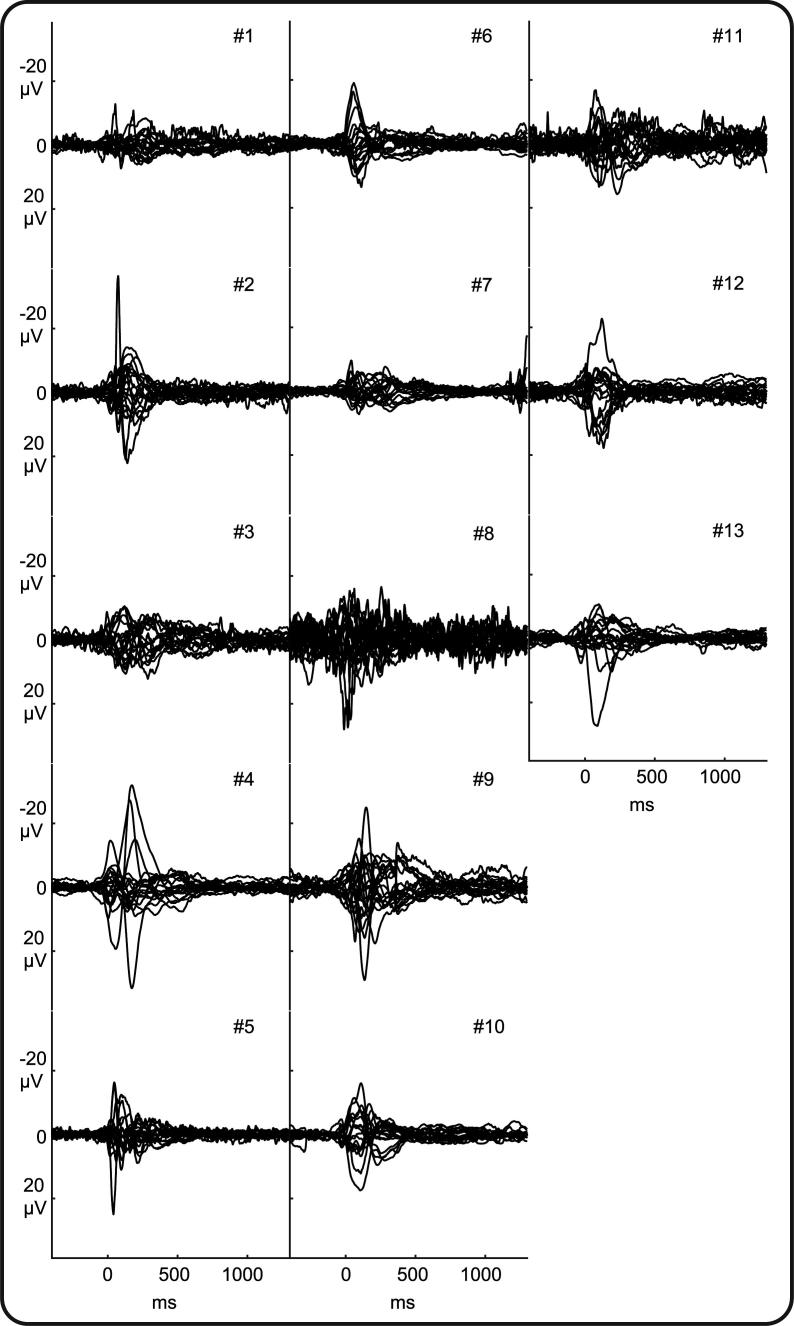
Data pre-processing was carried out using Curry v.7, EEGLAB v.14, and custom-written MATLAB code. EEG data were bandpass filtered at 1.5–40 Hz (2nd order Butterworth filter) with a 50 Hz notch filter (4th order Butterworth filter) and then epoched from −400 until +1300 ms around event onset. One epoch containing movement artefact was discarded from three datasets, and two datasets were de-noised using independent component analysis (component representing ECG breakthrough was removed) (Onton and Makeig 2006). The number of epochs analysed per infant was: 235, 234, 138, 132, 118, 94, 83, 82, 80, 49, 42, 22, and 7 (resulting in 1316 epochs analysed) (Table 1). The median time interval between events was 3.1 seconds (inter-quartile range: 2.1 seconds, minimum interval: 1.2 seconds). The number of epochs analysed per infant was not associated with the median interval between their hiccups (Pearson correlation p = .520, IBM SPSS version 25). Missing and discarded electrode recordings were estimated with spherical interpolation as implemented in EEGLAB v.14 (1/18 electrode recordings were discarded due to artefact in five infants). All EEG epochs were re-referenced to common average (retrieving the reference channel Fz) and baseline corrected by subtracting the mean baseline signal (-400 to 0 ms). Individual responses were estimated by averaging epochs within subject. The signal to noise ratio of each subject’s response was not associated with the number of epochs analysed (Supplementary Data; Fig. 2 visually demonstrates the good signal to noise ratio for each infant independently of number of epochs analysed).
Fig. 2.
Individual EEG responses following hiccups. Butterfly plots of each recording electrode for each of 13 infants (Table 1). Negative amplitudes are plotted upwards as per convention.
2.5. Analysis of hiccup event-related potential
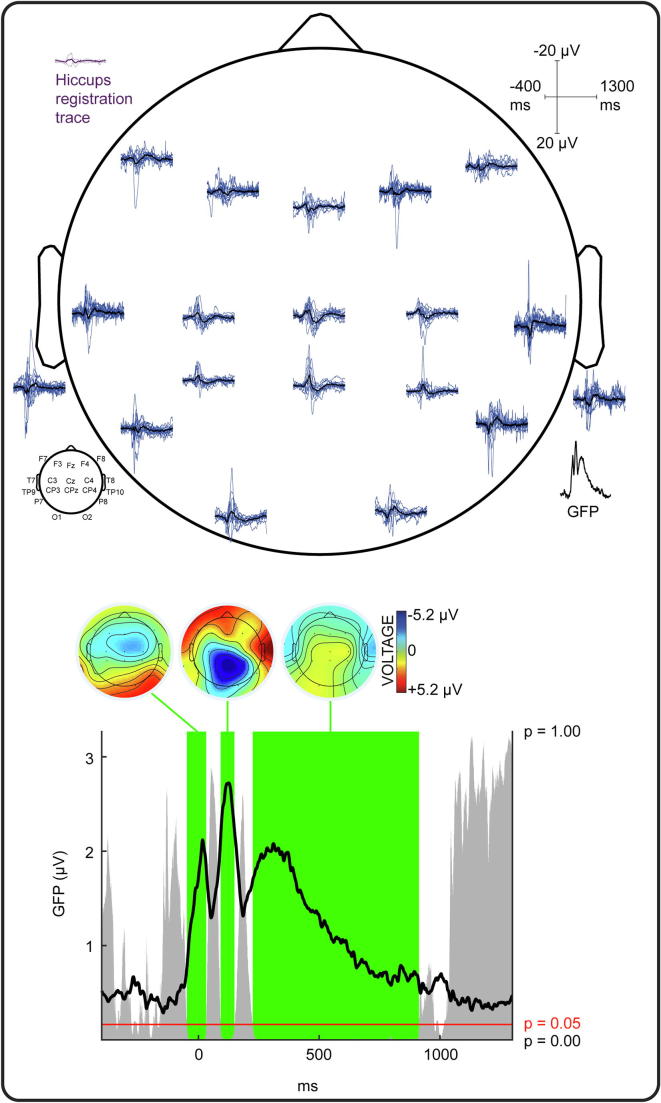
The presence of an event-related potential (ERP) was established using the Topographic Consistency Test, which examines if and at what latencies a stimulus consistently elicits the same scalp field distribution across subjects using Global Field Power (GFP) measurements (standard deviation of the recordings across electrodes at each time point) analysed with non-parametric permutation statistics timepoint by timepoint (n = 1000 randomization runs among channels) (Koenig and Melie-García 2010). Data analysis was implemented in Ragu (Koenig et al. 2011). Statistical significance threshold was set to 0.05 for all tests. An ERP was considered significant if the time period in which the test resulted in p < .05 exceeded 30 ms. Unlike methods to control for multiple comparisons such as false discovery rate, this considers the probability that consecutive samples pass the significance threshold (Guthrie and Buchwald 1991). To provide a visual representation of the topography of each ERP, we generated grand average and individual subject scalp field maps. To facilitate the comparison of topographies across individual subjects, the scalp field map of each ERP for each individual subject was symmetrically scaled to its own peak value.
3. Results
Six percent of infants (13/217, Table 1) had a bout of hiccups during their EEG study, with median duration of 7 consecutive minutes (range 1–16). In line with previous reports (Wagner 1939), a bout of hiccups was more likely to occur in infants who were awake during EEG monitoring (chi-squared test (n = 217): p = .005, Phi 0.193; 10 infants were awake at the onset of hiccups, and 3 infants were in active sleep). On the other hand, the incidence of a bout of hiccups was not associated with CGA (binary logistic regression using the Enter Method (n = 217): p = .144).
Cot side observation indicated that hiccups were not associated with changes in respiratory rate (e.g. Fig. 1) or oxygen saturation level (available in 10/13 infants), and statistical analysis demonstrated that the heart-rate of the infants was also unaffected (mean 150 beats per minute (standard deviation (SD): 16) immediately prior to the hiccup bout and 149 beats per minute (SD: 15) immediately after the hiccup bout, LabChart HRV software: paired t-test p = .925 (n = 11 because of poor ECG quality in two infants)). Taken together these data indicate that hiccups were well-tolerated by this cohort, which is in line with previous reports of hiccups in non-mechanically ventilated infants (Brouillette et al., 1980, Niemarkt and Andriessen, 2012).
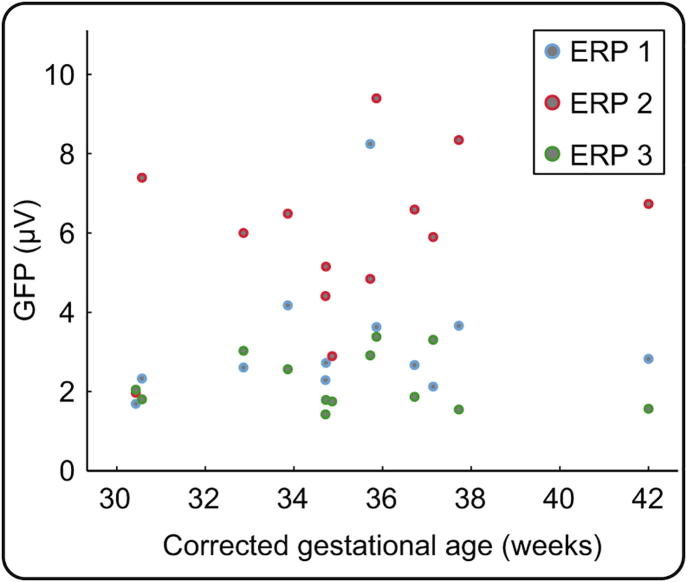
Diaphragm contraction evoked a change in EEG activity compared to baseline for every infant (illustrated in Fig. 2). (This included the two infants with germinal matrix-intraventricular haemorrhage (Table 1), in line with reports that sensory responses can be evoked in infants with this injury (Slater et al., 2010, Nevalainen et al., 2015)). Even if there was inter-subject variability, diaphragm contraction-related EEG activity had statistically consistent topography across infants, i.e. ERPs, between −49 to 35 ms (GFP peak latency: 16 ms), 91 to 150 ms (GFP peak latency: 125 ms), and 223 to 913 ms (GFP peak latency: 310 ms) (Fig. 3, Fig. 4). The first ERP comprised fronto-central-temporal negativity, with positivity most prominent across the posterior region. The second ERP comprised central and posterior negativity, with positivity most prominent across the anterior and bi-temporal regions. The third ERP comprised central and posterior positivity, with negativity most prominent bi-temporally (except in the very youngest subject #1 (Fig. 4)). The strength of this event-related EEG activity was not associated with the CGA of the subjects (mean Global Field Power across the latencies the stimulus elicited topographically consistent EEG activity, Pearson correlations: first ERP: p = .561, second ERP: p = .216, third ERP: p = .774; Supplementary Fig. 2).
Fig. 3.
Grand average of the EEG responses following hiccups. Upper panel: Hiccups registration trace (purple solid line) and standard deviation (dashed lines); EEG recordings at each electrode from individual infants (blue lines) and grand average (black lines); Global Field Power (GFP) of the grand average EEG recordings. Negative amplitudes are plotted upwards as per convention. Lower panel: GFP of the grand average EEG recordings showing timing and duration of consistent EEG activity across subjects, i.e. event-related potentials (green shading) and their topographies (averaged across their duration as defined by the Topographic Consistency Test). The height of the grey area indicates the p-value of the Topographic Consistency Test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
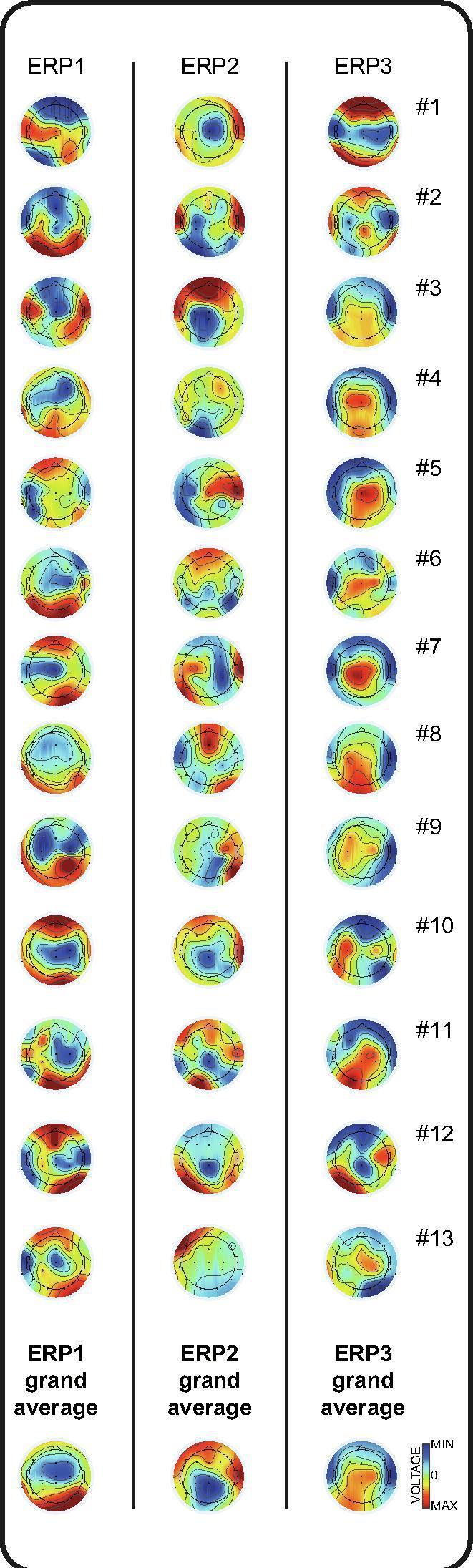
Individual EEG topographies following hiccups. Topographies of each event-related potential (averaged across their duration as defined by the Topographic Consistency Test, and individually symmetrically scaled to their own peak value) for each of 13 infants (Table 1) and the grand average. (MIN = Minimum voltage (-µV); MAX = Maximum voltage (+µV)).
4. Discussion
Diaphragm contraction can evoke a clear cortical response in neonates between 30–42 weeks CGA. This shows that hiccups provide afferent input to the cortex over the equivalent of the last trimester of gestation.
The first two potentials recorded have comparable topography to potentials recorded in neonates up to 185 ms following bilateral myoclonus (Losito et al. 2017) and mechanical somatosensory stimulation of limbs and face, which are somatotopically distributed in pre- and early-term infants (Desmedt and Manil, 1970, Hrbek et al., 1973, Laget et al., 1976, Karniski et al., 1992, Taylor et al., 1996, Pike et al., 1997, Pihko et al., 2004, Tombini et al., 2009, Fabrizi et al., 2011, Nevalainen et al., 2015, Donadio et al., 2018, Whitehead et al., 2019). In older children and adults, perception of respiratory muscle contraction, and other signals from the thoracic cavity, is associated with a sequence of scalp-recorded ERPs across the fronto‐central region lasting until 600 ms post stimulus (Macefield and Gandevia, 1992, Davenport et al., 2000, Frøkjær et al., 2011, Gentsch et al., 2019). In non-human primates, comparable short-latency potentials are recorded from the cortical surface of trunk representation of primary somatosensory cortex, as well as motor and posterior parietal cortex (Amassian 1951). The initial potentials following diaphragm contraction may therefore reflect encoding of afferent input associated with respiratory muscle contraction within the developing somatosensory cortex.
The final potential, positive across the central region, has a similar topography to that recorded in neonates between approximately 200–315 ms following bilateral myoclonus (Losito et al. 2017) and somatosensory stimulation of the body, but is much longer lasting (Karniski et al., 1992, Nevalainen et al., 2015, Whitehead et al., 2019). Consequently, the later part of the cortical response associated with hiccups may differ, at least in part, from that following simple somatosensory feedback. Hiccups are sometimes associated with auditory input produced by abrupt closure of the glottis (Video 1; Supplementary Fig. 1) (Wagner, 1938, Lewis, 1985). Simple auditory stimuli evoke an ERP in neonates with latency and scalp topography very similar to the final potential observed here (Fifer et al., 2010, Chipaux et al., 2013, Kaminska et al., 2018). Therefore, this final potential following diaphragm contraction could encode a multi-sensory stimulus. Further, the stimulus here is most often processed while the infant is awake. To our knowledge this is the first study of neonatal cortical responses, of any modality, largely acquired during wakefulness, because newborns spend so little time awake (Curzi-Dascalova et al. 1993). During wakefulness - i.e. a state of heightened attention - sensory information may be encoded differently. For example, in adults long-latency ERPs are only recorded if the stimulus has entered awareness (Libet et al., 1967, Kitazawa, 2002). Although animal models indicate that, until postnatal day 11, afferent input following body movements during wakefulness is relatively unlikely to evoke cortical activity (Tiriac et al., 2014, Dooley and Blumberg, 2018), here we show in human infants 30–42 weeks CGA that brief contractions of a discrete set of respiratory muscles during wakefulness can evoke pronounced cortical responses of similar strength across the age range.
The cortical response characterised in this study is clearly not explained by hiccup-related movement artefact (i.e. electrodes displacement) because a) it is topographically organised, especially across the central region, b) the central electrodes are less likely to be affected by movement as they are placed at the crown of the head and therefore do not brush against bedding or the caregiver (Scher 2006), c) the morphology of the full response recorded does not resemble the movement recorded by the hiccups registration trace and lasts for much longer (Fig. 3).
Fetal ultrasound imaging demonstrates that hiccups are present from just nine weeks gestational age, at which time they are particularly frequent, and then plateau across the third trimester (Pillai and James, 1990, de Vries and Fong, 2006). Therefore repetitive contractions of the diaphragm are one of the earliest established motor activity patterns within the rudimentary functional systems of the fetus. We show here that the sensory feedback from these contractions can be encoded by the brain from as early as 30 weeks CGA, ten weeks prior to the average time of birth. The establishment of early sensory circuits is a crucial developmental milestone for newborn infants (Fabrizi et al. 2011). Our study demonstrates that contractions of respiratory muscles provide sensory input from the internal body environment to the developing brain and may provide the necessary information for the formation of interoceptive representations. This would explain the marked prevalence of hiccups in neonates compared to adults (Brouillette et al. 1980).
Acknowledgments
Acknowledgements
This work was supported by the Medical Research Council UK (MR/L019248/1, MR/M006468/1, and MR/S003207/1), which had no role in the collection, analysis and interpretation of data, or the writing of the manuscript. We acknowledge the support of the UCL/UCLH Biomedical Research Centre. We thank the families who participated in our neonatal research program.
Declaration of Competing Interest
None of the authors have potential conflicts of interest to be disclosed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinph.2019.09.008.
Contributor Information
Kimberley Whitehead, Email: k.whitehead@ucl.ac.uk.
Laura Jones, Email: laura.a.jones@ucl.ac.uk.
Maria Pureza Laudiano-Dray, Email: m.laudiano-dray@ucl.ac.uk.
Judith Meek, Email: judith.meek@nhs.net.
Lorenzo Fabrizi, Email: l.fabrizi@ucl.ac.uk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
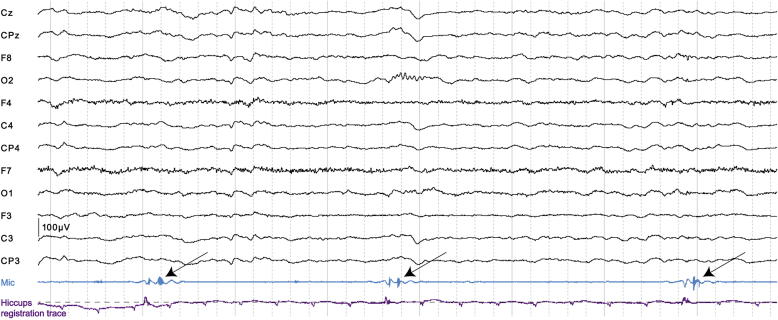
Supplementary figure 1.
Examples of the stereotyped sound time-locked to each hiccup event. EEG and audio recordings (Mic: signal proportional to sound intensity as recorded with a microphone affixed to the collar of subject #6’s clothing (Unimed electrode supplies)) in which three hiccups occur. Note the stereotyped sound associated with abrupt closure of the glottis (arrows) following the hiccup event onset identified by thresholding (dashed horizontal line) the signal of the hiccups registration trace (in this infant, ECG recording from upper trunk). EEG is displayed referred to Fz (acquisition montage) and with a bandpass filter of 1.5-70 Hz. Scale bar bottom left. Solid grey vertical lines mark each second and dashed grey vertical lines mark each 200 milliseconds.
Supplementary figure 2.
The strength of the EEG responses following hiccups was not associated with corrected gestational age. Dots represent the Global Field Power (GFP) of each individual infant’s EEG recordings during three event-related potentials (ERP 1-3).
References
- Amassian V.E. Cortical representation of visceral afferents. J Neurophysiol. 1951;14:433–444. doi: 10.1152/jn.1951.14.6.433. [DOI] [PubMed] [Google Scholar]
- André M., Lamblin M.-D., d’Allest A.M., Curzi-Dascalova L., Moussalli-Salefranque F., Nguyen The Tich S. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Brouillette R.T., Thach B.T., Abu-Osba Y.K., Wilson S.L. Hiccups in infants: characteristics and effects on ventilation. J Pediatr. 1980;96:219–225. doi: 10.1016/s0022-3476(80)80806-7. [DOI] [PubMed] [Google Scholar]
- Ceriani F, Fogliani R, Kustermann A. Hiccups, Yawning and Gasping. In: Development of Normal Fetal Movements. Springer, Milano; 2010 [cited 2018 Mar 7]. p. 29–38. https://link.springer.com/chapter/10.1007/978-88-470-1402-2_4
- Chipaux M., Colonnese M.T., Mauguen A., Fellous L., Mokhtari M., Lezcano O. Auditory stimuli mimicking ambient sounds drive temporal “delta-brushes” in premature infants. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzi-Dascalova L., Figueroa J.M., Eiselt M., Christova E., Virassamy A., D’allest A.M. Sleep state organization in premature infants of less than 35 weeks’ gestational age. Pediatr Res. 1993;34:624–628. doi: 10.1203/00006450-199311000-00013. [DOI] [PubMed] [Google Scholar]
- Davenport P.W., Cruz M., Stecenko A.A., Kifle Y. Respiratory-related Evoked Potentials in Children with Life-threatening Asthma. Am J Respir Crit Care Med. 2000;161:1830–1835. doi: 10.1164/ajrccm.161.6.9903077. [DOI] [PubMed] [Google Scholar]
- Desmedt J.E., Manil J. Somatosensory evoked potentials of the normal human neonate in REM sleep, in slow wave sleep and in waking. Electroencephalogr Clin Neurophysiol. 1970;29:113–126. doi: 10.1016/0013-4694(70)90114-8. [DOI] [PubMed] [Google Scholar]
- Donadio A., Whitehead K., Gonzalez F., Wilhelm E., Formica D., Meek J. A novel sensor design for accurate measurement of facial somatosensation in pre-term infants. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J.C., Blumberg M.S. Developmental “awakening” of primary motor cortex to the sensory consequences of movement. eLife. 2018;7:e41841. doi: 10.7554/eLife.41841. Martin JH, Ivry RB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi L., Slater R., Worley A., Meek J., Boyd S., Olhede S. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr Biol. 2011;21:1552–1558. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifer W.P., Byrd D.L., Kaku M., Eigsti I.-M., Isler J.R., Grose-Fifer J. Newborn infants learn during sleep. Proc Natl Acad Sci. 2010;107:10320–10323. doi: 10.1073/pnas.1005061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær J.B., Olesen S.S., Graversen C., Andresen T., Lelic D., Drewes A.M. Neuroimaging of the human visceral pain system–A methodological review. Scand J Pain. 2011;2:95–104. doi: 10.1016/j.sjpain.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Fukumoto M., Mochizuki N., Takeishi M., Nomura Y., Segawa M. Studies of body movements during night sleep in infancy. Brain Dev. 1981;3:37–43. doi: 10.1016/s0387-7604(81)80004-6. [DOI] [PubMed] [Google Scholar]
- Gentsch A., Sel A., Marshall A.C., Schütz-Bosbach S. Affective interoceptive inference: Evidence from heart-beat evoked brain potentials. Hum Brain Mapp. 2019;40:20–33. doi: 10.1002/hbm.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg-Damberger M.M. The Visual Scoring of Sleep in Infants 0 to 2 Months of Age. J Clin Sleep Med. 2016;12:429–445. doi: 10.5664/jcsm.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie D., Buchwald J.S. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M., Nakae Y., Van Eykern L.A., Klip-Van den Nieuwendijk A.W.J., Prechtl H.F.R. The effect of behavioural state on general movements in healthy full-term newborns. A polymyographic study. Early Hum Dev. 1993;35:63–79. doi: 10.1016/0378-3782(93)90140-p. [DOI] [PubMed] [Google Scholar]
- Hrbek A., Karlberg P., Olsson T. Development of visual and somatosensory evoked responses in pre-term newborn infants. Electroencephalogr Clin Neurophysiol. 1973;34:225–232. doi: 10.1016/0013-4694(73)90249-6. [DOI] [PubMed] [Google Scholar]
- Kahrilas P.J., Shi G. Why do we hiccup? Gut. 1997;41:712–713. doi: 10.1136/gut.41.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska A., Delattre V., Laschet J., Dubois J., Labidurie M., Duval A. Cortical auditory-evoked responses in preterm neonates: revisited by spectral and temporal analyses. Cereb Cortex. 2018;28:3429–3444. doi: 10.1093/cercor/bhx206. [DOI] [PubMed] [Google Scholar]
- Kandel E., Schwartz J., Jessell T. Principles of Neural Science. Fourth. McGraw-Hill; 2000. Brain stem, reflexive behavior, and the cranial nerves. [Google Scholar]
- Karniski W., Wyble L., Lease L., Blair R.C. The late somatosensory evoked potential in premature and term infants. II. Topography and latency development. Electroencephalogr Clin Neurophysiol Potentials Sect. 1992;84:44–54. doi: 10.1016/0168-5597(92)90067-l. [DOI] [PubMed] [Google Scholar]
- Khazipov R., Sirota A., Leinekugel X., Holmes G.L., Ben-Ari Y., Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kitazawa S. Where conscious sensation takes place. Conscious Cogn. 2002;11:475–477. doi: 10.1016/s1053-8100(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Koenig T., Kottlow M., Stein M., Melie-García L. Ragu: A Free Tool for the Analysis of EEG and MEG Event-related Scalp Field Data Using Global Randomization Statistics. Intell Neurosci. 2011;2011(938925):14. doi: 10.1155/2011/938925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig T., Melie-García L. A method to determine the presence of averaged event-related fields using randomization tests. Brain Topogr. 2010;23:233–242. doi: 10.1007/s10548-010-0142-1. [DOI] [PubMed] [Google Scholar]
- Laget P., Salbreux R., Raimbault J., D’Allest A.M., Mariani J. Relationship between changes in somesthetic evoked responses and electroencephalographic findings in the child with hemiplegia. Dev Med Child Neurol. 1976;18:620–631. doi: 10.1111/j.1469-8749.1976.tb04207.x. [DOI] [PubMed] [Google Scholar]
- Lewis J.H.M.D. Hiccups: causes and cures. J Clin Gastroenterol. 1985;7:539–552. doi: 10.1097/00004836-198512000-00021. [DOI] [PubMed] [Google Scholar]
- Libet B., Alberts W.W., Wright J., Feinstein B. Responses of human somatosensory cortex to stimuli below threshold for conscious sensation. Science. 1967;158:1597–1600. doi: 10.1126/science.158.3808.1597. [DOI] [PubMed] [Google Scholar]
- Lipton E.L., Steinschneider A., Richmond J.B. Autonomic function in the neonate: VIII. Cardio-Pulmonary Observat Pediat. 1964;33:212–215. [PubMed] [Google Scholar]
- Losito E., Eisermann M., Vignolo P., Hovhannisyan S., Magny J.F., Kaminska A. Benign neonatal sleep myoclonus evokes somatosensory responses. J Clin Neurophysiol. 2017;34:484. doi: 10.1097/WNP.0000000000000412. [DOI] [PubMed] [Google Scholar]
- Macefield G., Gandevia S.C. Peripheral and central delays in the cortical projections from human truncal muscles. Rapid central transmission of proprioceptive input from the hand but not the trunk. Brain. 1992;115:123–135. doi: 10.1093/brain/115.1.123. [DOI] [PubMed] [Google Scholar]
- Maskill D., Murphy K., Mier A., Owen M., Guz A. Motor cortical representation of the diaphragm in man. J Physiol. 1991;443:105–121. doi: 10.1113/jphysiol.1991.sp018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L.C., Evans K.C., Frackowiak R.S.J., Corfield D.R. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- Milh M., Kaminska A., Huon C., Lapillonne A., Ben-Ari Y., Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Nevalainen P., Rahkonen P., Pihko E., Lano A., Vanhatalo S., Andersson S. Evaluation of somatosensory cortical processing in extremely preterm infants at term with MEG and EEG. Clin Neurophysiol. 2015;126:275–283. doi: 10.1016/j.clinph.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Niemarkt H., Andriessen P., Halbertsma F.J. Artefacts in the amplitude-integrated EEG background pattern of a full-term asphyxiated neonate caused by diaphragm spasms. Case Rep. 2012;2012 doi: 10.1136/bcr.12.2011.5363. bcr1220115363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onton J., Makeig S. Information-based modeling of event-related brain dynamics. Prog Brain Res. 2006;159:99–120. doi: 10.1016/S0079-6123(06)59007-7. Neuper C, Klimesch W, editors. [DOI] [PubMed] [Google Scholar]
- Pihko E., Lauronen L., Wikström H., Taulu S., Nurminen J., Kivitie-Kallio S. Somatosensory evoked potentials and magnetic fields elicited by tactile stimulation of the hand during active and quiet sleep in newborns. Clin Neurophysiol. 2004;115:448–455. doi: 10.1016/s1388-2457(03)00349-3. [DOI] [PubMed] [Google Scholar]
- Pike A.A., Marlow N., Dawson C. Posterior tibial somatosensory evoked potentials in very preterm infants. Early Hum Dev. 1997;47:71–84. doi: 10.1016/s0378-3782(96)01774-4. [DOI] [PubMed] [Google Scholar]
- Pillai M., James D. Hiccups and breathing in human fetuses. Arch Dis Child. 1990;65:1072–1075. doi: 10.1136/adc.65.10_spec_no.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M.S. Clinical Neurophysiology of Infancy, Childhood, and Adolescence. Butterworth Heinemann Elsevier; Philadelphia: 2006. Electroencephalography of the Newborn: Normal Features. [Google Scholar]
- Shellhaas R.A., Chang T., Tsuchida T., Scher M.S., Riviello J.J., Abend N.S. The American clinical neurophysiology Society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–617. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- Slater R., Fabrizi L., Worley A., Meek J., Boyd S., Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. NeuroImage. 2010;52:583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- Swann I. Intrauterine hiccup. Br Med J. 1978;2:1497–1498. doi: 10.1136/bmj.2.6150.1497-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., Boor R., Ekert P.G. Preterm maturation of the somatosensory evoked potential. Electroencephalogr Clin Neurophysiol Potentials Sect. 1996;100:448–452. [PubMed] [Google Scholar]
- Tiriac A., Del Rio-Bermudez C., Blumberg M.S. Self-generated movements with “unexpected” sensory consequences. Curr Biol. 2014;24:2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A., Uitermarkt B.D., Fanning A.S., Sokoloff G., Blumberg M.S. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombini M., Pasqualetti P., Rizzo C., Zappasodi F., Dinatale A., Seminara M. Extrauterine maturation of somatosensory pathways in preterm infants: A somatosensory evoked potential study. Clin Neurophysiol. 2009;120:783–789. doi: 10.1016/j.clinph.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Tsuchida T.N., Wusthoff C.J., Shellhaas R.A., Abend N.S., Hahn C.D., Sullivan J.E. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the american clinical neurophysiology society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- de Vries J.I.P., Fong B.F. Normal fetal motility: an overview. Ultrasound Obstet Gynecol. 2006;27:701–711. doi: 10.1002/uog.2740. [DOI] [PubMed] [Google Scholar]
- Wagner I.F. A note on the hiccough of the neonate. Pedagog Semin J Genet Psychol Prov Mass Etc. 1938;52:233–234. [Google Scholar]
- Wagner I.F. Curves of sleep depth in newborn infants. Pedagog Semin J Genet Psychol Prov Mass Etc. 1939;55:121–135. [Google Scholar]
- Wheeler-Hegland K., Pitts T., Davenport P.W. Cortical gating of oropharyngeal sensory stimuli. Front Physiol. 2011;1:167. doi: 10.3389/fphys.2010.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K., Laudiano-Dray M.P., Meek J., Fabrizi L. Emergence of mature cortical activity in wakefulness and sleep in healthy preterm and full-term infants. Sleep. 2018;41 doi: 10.1093/sleep/zsy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K., Meek J., Fabrizi L. Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants. Sci Rep. 2018;8:17516. doi: 10.1038/s41598-018-35850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K., Papadelis C., Laudiano-Dray M.P., Meek J., Fabrizi L. The Emergence of Hierarchical Somatosensory Processing in Late Prematurity. Cereb Cortex. 2019;29:2245–2260. doi: 10.1093/cercor/bhz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden E.E., van Geijn H.P., Caron F.J.M., Mantel R., Swartjes J.M., Arts N.F.T. Fetal hiccups; characteristics and relation to fetal heart rate. Eur J Obstet Gynecol Reprod Biol. 1989;30:209–216. doi: 10.1016/0028-2243(89)90003-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.