Abstract
Human (gamma delta) γδ T cells are unconventional innate-like lymphocytes displaying a broad array of anti-tumor activities with promising perspectives in cancer immunotherapy. In this context, Vδ2pos T cells represent the preferential target of several immunotherapy protocols against solid tumors. However, the impact of both aging and chemotherapy (CHT) on Vδ2pos T cells is still unknown. The present study evaluates with multi-parametric flow cytometry the frequencies, terminal differentiation, senescence and effector-functions of peripheral blood and tumor infiltrating Vδ2pos T cells purified from liver metastases (CLM) of patients affected by colorectal cancer (CRC) compared to those of sex- and age-matched healthy donors. The peripheral blood of CLM patients underwent CHT is characterized by decreased amounts of Vδ2pos T cells showing a relative increase of terminally-differentiated CD27neg/CD45RApos (TEMRA) cells. The enrichment of this latter subset is associated with an increased expression of the senescent marker CD57. The acquisition of CD57 on TEMRA Vδ2pos T cells is also coupled with impairments in cytotoxicity and production of TNF-α and IFN-γ. These features resemble the acquisition of an immune-senescent profile by Vδ2pos T cells from CLM patients that received CHT, a phenomenon that is also associated with the loss of the co-stimulatory marker CD28 and with the induced expression of CD16. The group of CLM patients underwent CHT and older than 60 years old showed higher frequencies of CD57pos and TEMRA Vδ2pos T cells. Similar results were found for tumor infiltrating Vδ2pos T cell subset purified from CLM specimens of patients treated with CHT. The toxicity of CHT regimens also affects the homeostasis of Vδ2pos T cells by inducing higher frequencies of circulating CD57pos TEMRA subset in CLM underwent CHT and younger than 60 years old. Taken together, our data demonstrate that the enrichment of senescent Vδ2pos T cells in CLM patients is not only induced by patients’ aging but also by the toxicity of CHT that further accelerates the accumulation of CD57pos TEMRA cells highly dysfunctional in their anti-tumor activities. These results are important to both predict the clinical outcome of CLM and to optimize those protocols of cell cancer immunotherapy employing unconventional Vδ2pos T cells.
Keywords: γδ T cells, Immune-senescence/Aging, Cancer, Chemotherapy
Introduction
Human γδ T lymphocytes are divided in the two main Vδ1pos and Vδ2pos subsets on the basis of their TCRδ-chain repertoire. While Vδ1pos cells preferentially localize in mucosal tissues and skin, Vδ2pos T cells are mainly enriched in peripheral blood (PB) where they represent about 5% of all circulating T cells. The activation of Vδ2pos T cells relies on the recognition of non-peptidic compounds (i.e. microbial or stress- or tumor-induced “phosphoantigens”) in association with butyrophilin 3A1 (BTN3A1 also known as CD277). Besides the TCR interactions with phosphoantigens/BTN3A1 complexes, several Natural Killer Receptors (NKRs) are involved in triggering the anti-tumor functions of Vδ2pos T cells, with the C-lectin type NKG2D playing a major role [1, 2]. The differential expressions of CD27 and CD45RA surface markers identify different Vδ2pos T cell subsets: CD27pos/CD45RApos naïve cells (TNaïve), CD27pos/CD45RAneg central memory (TCM), CD27neg/CD45RAneg effector-memory (TEM) and the terminally-differentiated (TEMRA) CD27neg/CD45RApos cells. These Vδ2pos T cell subsets diverge not only for their maturation/differentiation status, but also for proliferative capacities, effector functions and resistance to cell death in response to antigens and/or cytokine stimulations [3].
Growing evidences highlighted the high impact of Vδ2pos T cells in cancer immune-surveillance with promising perspectives in cancer immunotherapy [4, 5]. In this context, two main clinical approaches have been employed to boost anti-tumor activities of Vδ2pos T cells. The first one activates them through the in vivo administration of either IL-2 or synthetic nitrogen-containing bisphosphonates (NBPs) drugs that, in turn, induce intracellular accumulation of phosphoantigens. A second strategy relies on adoptive transfers of Vδ2pos T cells expanded in vitro with several methodologies such as the activation with zoledronate [5, 6]. However, these procedures showed both experimental and clinical limits and many efforts are currently being implemented to further improve the effector-functions and persistence in vivo of Vδ2pos T cells. In this context, cellular senescence is certainly one of the main issues to solve considering that age-related changes of T cells greatly impair their capacity to expand and proliferate, thus leading to dysfunctional immune responses against tumors and pathogens [7]. The shift to senescence and accumulation of mature T cells physiologically occur after 60 years old when both αβ and γδ T lymphocytes lose their co-stimulatory molecules (i.e. CD27 and CD28), acquire terminally-differentiated TEM and TEMRA phenotypic profiles, express high constitutive levels of the senescence marker CD57 and shorten their telomerase lengths [8–11]. However, it is still controversial whether CD57 can be used as a single marker to identify senescent Vδ2pos T cells regardless of differential expression of CD27 and CD45 [3, 11, 12].
Aging is certainly a major burden for social health systems in the industrialized countries as the populations are longer exposed to several pro-tumorigenic risk factors. This leads to a significant higher incidence of cancer onsets in the 6th, 7th and 8th decades of life [13]. Hence, there are rising numbers of elderly patients undergoing anti-cancer conventional chemotherapies (CHT), whose high toxicities greatly hamper both duration and quality of life. In this regard, several lines of clinical and experimental evidence pointed out that these anti-neoplastic treatments further accelerate immune-cell senescence, thus representing negative prognostic factors in aging and worsening the overall clinical outcomes of cancer patients [14, 15].
Since the use of Vδ2pos T cells is currently considered one of the most promising tools in cancer immunotherapy [4, 5], understanding the exact impact of CHT on their immune-senescence is key to better predict the clinical outcomes of cancer in elderly and to optimize those therapeutic protocols targeting these highly cytotoxic unconventional T cell effectors. Colorectal cancer (CRC) represents the 3rd most frequent solid cancer and more than 50% of CRC patients undergo hepatic dissemination of the primary tumor. The gold-standard therapeutic approach of CRC patients with liver metastasis (CLM) is the surgical removal of hepatic secondary lesions after neoadjuvant combination CHT with or without biological therapy (BT) (Table 1) [16, 17]. Moreover, a higher infiltration of competent immune cells in tumor mass greatly improves the prognosis of CLM patients and increases their overall survival (OS) [18, 19]. Here, we analyze the impact of conventional CHT regimens on the homeostasis and effector-functions of Vδ2pos T cells in a cohort of CLM elderly patients.
Table 1.
Neoadjuvant combination chemotherapy (CHT) with or without biological therapy (BT) of enrolled CLM patients
| Patients (number) |
Patients (%) |
CHT cycles (mean number ± SD) |
|
|---|---|---|---|
| CHT/BT Regimensa | 58 | 82 | 8.7 ± 5.3 |
| Combination Therapy with Biologicals | |||
| FOLFOX + VEGF-A mAb | 12 | 21.5 | 7.7 ± 1.4 |
| FOLFIRI + EGFR mAb | 11 | 19.0 | 11.7 ± 4.3 |
| FOLFIRI + VEGF-A mAb | 10 | 17.2 | 7.5 ± 3.3 |
| FOLFIRI + FOLFOX + VEGF-A mAb | 7 | 12.0 | 13.0 ± 3.2 |
| FOLFOX + EGFR mAb | 6 | 10.3 | 11.0 ± 2.3 |
| XELOX + VEGF-A mAb | 4 | 6.9 | 8.5 ± 3.4 |
| Combination Therapy without Biologicals | |||
| FOLFOX | 4 | 6.9 | 5.0 ± 1.6 |
| XELOX | 2 | 3.4 | 4.6 ± 1.2 |
| FOLFIRI | 2 | 3.4 | 7.0 ± 6.0 |
| Naïve for CHT | 13 | 18 | 0.0 |
| Total Patients | 71 | ||
FOLFOX: 5-fluorouracil/oxaliplatin; XELOX: capecitabine/oxaliplatin; FOLFIRI: 5-fluorouracil/irinotecan
EGFR mAb Epidermal Growth Factor Receptor inhibitor monoclonal antibody
VEGF-A mAb Vascular Endothelial Growth Factor A monoclonal antibody
aNote:
a) All CLM patients completed their last CHT cycle at least 6 weeks before the blood draws used for our experiments and before surgical procedures
b) The table refers all therapies received by CLM patients before surgery
c) More than 91% of all CLM patients received one line therapy and all other patients received two lines (1st and 2nd) combination therapy: 3 patients received 1st FOLFOX and 2nd FOLFIRI + VEGF-A; 1 patient received 1st FOLFIRI + VEGF-A and 2nd FOLFOX + VEGF-A, and 1 patient received 1st FOLFIRI + VEGF-A and 2nd FOLFOX
Methods
Patients and specimen collections
Biological specimens from CLM patients underwent CHT (n = 58), or from CHT naïve patients (n = 13) and aged- and sex-matched healthy donors (n = 40) (Table 1). Patients’ recruitment was performed according to the Declaration of Helsinki and the protocol had been approved by the Institutional Review Board (IRB) of Humanitas Research Hospital (HRH) (Approval N.168/18). All enrolled patients signed the related consent forms. Liver specimens and peripheral blood mononuclear cells (PBMCs) were isolated and stored as we previously described [19, 20].
Flow cytometry
Absolute γδ T cell counts were performed on 100 μl of fresh PB stained with following anti-human monoclonal antibodies (mAbs): CD3 (SK7; BV605) and CD45 (H130; AF700) (BioLegend) and Vδ2 (IMMU-389; FITC) (Beckman Coulter). We then used CountBright™ Absolute Counting Beads (Invitrogen) according to the manufacturer’s instructions.
For both regular and intracellular staining, γδ T cells were first screened for viability with Zombie Aqua™ Fixable Viability kit (BioLegend) and then processed as previously described [20]. The following mAbs were used: CD28 (CD28.2; PE-Cy7) (BioLegend); Vδ2 (B6; BUV395), CD3 (UCHT1; BUV661), CD45RA (H100; BUV737), CD16 (348; BUV496) (BD); CD57 (REA769; PE-Vio615) (Miltenyi); CD27 (0322; APCeFluor780) (eBioscience). The intracellular amounts of TNF-α (Mab11; PE) and IFN-γ (B27; Bv711) (BD) as well as the frequency of cytotoxic CD107apos cells (H4A3, PE) (BD Biosciences) was evaluated after stimulating γδ T cells with Phorbol myristate acetate (PMA; 0.5 μg/mL) and Ionomycin (0.1 μg/mL) (Sigma Aldrich).
Flow cytometry experiments were performed on FACS Symphony™ (BD). All data and t-SNE algorithm were analyzed with FlowJo Software (version 9.6) (FlowJo LLC) using single stained controls BD CompBeads™ (BD).
Statistical analyses
The data were assessed by non-parametric Mann-Whitney U (unpaired) or Wilcoxon (matched-paired) tests by using GraphPad Prism version 7. For all correlation analysis Pearson’s coefficient was applied. Statistically significant p values were represented with GraphPad (GP) style and summarized with following number of asterisks (*): *P ≤0.05; **P ≤0.01; ***P ≤0.001; ****P ≤0.0001.
Results
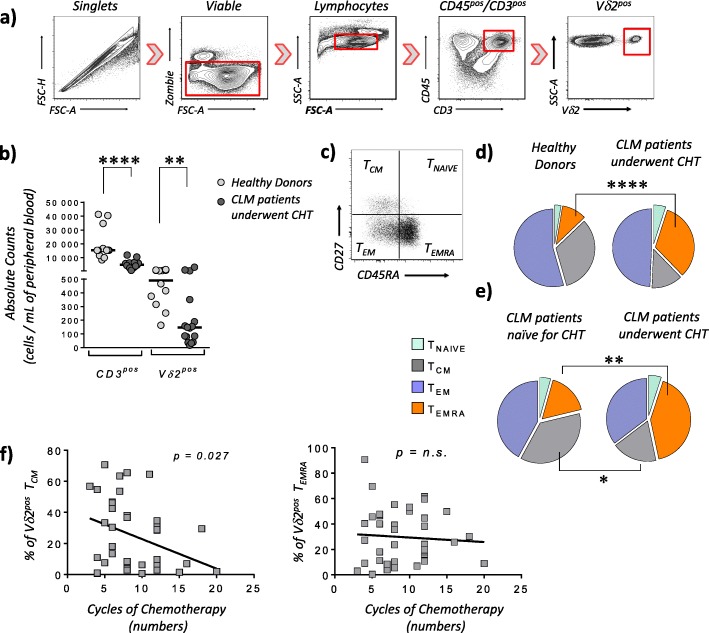
Vδ2pos T cells were gated within viable CD3pos/CD45pos lymphocytes and their absolute counts are significantly lower in the PB of CLM patients underwent CHT compared to those of healthy donors (Fig. 1a-b). We then analyzed the surface expression of CD27 and CD45RA to track the differentiation and distribution of Vδ2pos T cell subsets. Our data showed a significant increase of Vδ2pos TEMRA in CLM patients underwent CHT (28.9 ± 20.6%) compared to healthy controls (9.4 ± 6.4%). This phenomenon is associated with the previous administration of CHT, as the frequency of circulating Vδ2pos TEMRA in those CLM patients naïve for CHT (16.7% ±12.6) is similar to that of healthy donors and significantly lower to that of CLM patients underwent CHT (41.6% ±19.6). The increased amounts of Vδ2pos TEMRA in CLM patients treated with CHT is counterbalanced by a significant decrease of Vδ2pos TCM in the same patients compared to their counterparts naïve for CHT (Fig. 1c-d-e). The great impact of neoadjuvant CHT in shaping the distribution of Vδ2pos T cell subsets in CLM patients is also confirmed by our findings showing that the number of CHT cycles (8.7 ± 2.7) inversely correlates with the percentages of PB Vδ2pos TCM, while not affecting at all the overall frequencies of PB Vδ2pos TEMRA (Fig. 1f). This latter dichotomy reflects the different homeostatic status of Vδ2pos TCM compared to that of Vδ2pos TEMRA, as the first subset is composed of proliferating lymphocytes high susceptible to the toxicity of those chemotherapy compounds that kills all dividing cells without any specificity against tumor blasts. Instead, TEMRA Vδ2pos cells are terminally differentiated and not proliferating effectors resistant to CHT, thus explaining their high frequency even after several cycles of neoadjuvant anti-tumor chemotherapies.
Fig. 1.
Frequency and distributions of peripheral blood Vδ2pos T cell subsets in patients affected by liver metastasis of colorectal cancer and underwent chemotherapy. a Representative dot plot flow cytometric graphs showing the gating strategy of viable CD45pos/CD3pos/Vδ2pos T lymphocytes. b Statistical dot plot graph showing the absolute number of CD3pos (left) and Vδ2pos (right) T cells per 1 mL of blood in healthy donors (n = 12; mean age: 49.3 ± 9.5) and CLM patients underwent CHT regimens (n = 16; mean age: 51.5 ± 8.1). c-e Representative dot plot graph flow cytometric graph (c) and pie charts (d and e) showing respectively the distribution and the percentages of CD27pos/CD45RApos TNaive (upper right in dot plot graph and light green in pie charts), CD27pos/CD45RAneg central memory (TCM) (upper left in dot plot graph and gray in pie charts), CD27neg/CD45RAneg effector-memory (TEM) (lower left in dot plot graph and purple in pie charts) and terminally-differentiated CD27neg/CD45RApos (TEMRA) (lower right in dot plot graph and orange in pie charts) Vδ2pos T cell subsets. Pie charts compare the frequencies of Vδ2pos T cell subsets between healthy donors (n = 34; mean age: 51.7 ± 10.8) with age-matched CLM patient underwent CHT (n = 33; mean age: 51.5 ± 8.1) d as well as between CLM patients naïve for CHT (n = 13; mean age: 69.5 ± 8.1) and age-matched CLM patients underwent CHT (n = 41; mean age: 70.1 ± 6.5) (e). f Statistical analysis showing the Pearson correlations between the frequency (%) of either TCM (left) or TEMRA (right) Vδ2pos T cells with the number of CHT cycles (mean number: 8.7 ± 6.5) administered to patients affected by CLM (n = 40)
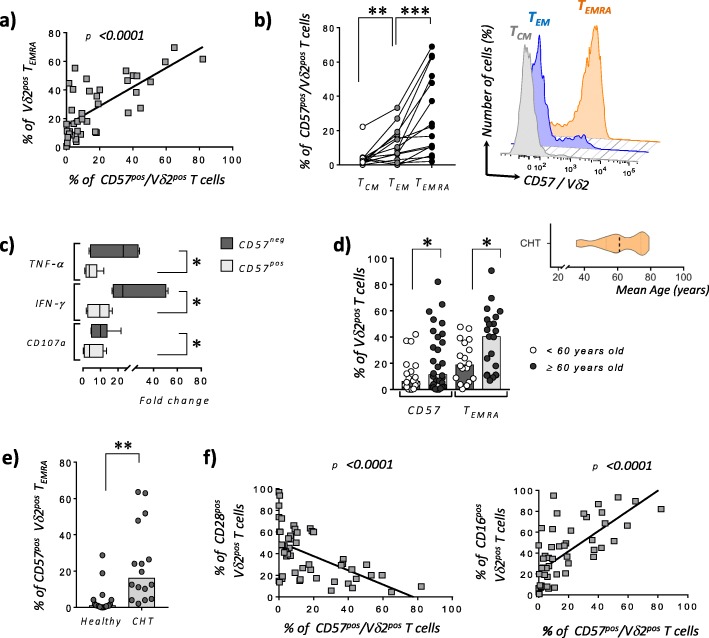
The relative increased frequency of PB TEMRA Vδ2pos in CLM patients underwent CHT correlates with their higher expression of CD57. Notably, the expression of this latter marker of immune senescence follows the terminal differentiation of Vδ2pos T cells. Indeed, the frequency of PB CD57pos TEMRA Vδ2pos T cells resulted significantly higher compared to that of CD57pos TEM Vδ2pos T cells that, in turn, showed significantly higher amounts of CD57 when compared to TCM Vδ2pos T cells (Fig. 2a-b). The acquisition of CD57 by terminal-differentiated Vδ2pos T cells is also associated with significantly impaired effector-functions in term of anti-tumor cytokines production (i.e. IFN-γ and TNF-α) and ability to degranulate (i.e. decreased amounts of cytotoxic CD107apos cells) when compared to CD57neg/Vδ2pos T cells (Fig. 2c). Taken together, these data indicate that the PB of CLM patients underwent CHT is highly enriched of senescent CD57pos/ TEMRA Vδ2pos T cells dysfunctional in their anti-tumor effector functions.
Fig. 2.
Senescence of peripheral blood Vδ2pos T cell in patients affected by liver metastasis of colorectal cancer and underwent chemotherapy. a Statistical analysis showing the correlations between the frequencies (%) of Vδ2pos TEMRA and CD57pos/Vδ2pos T and in CLM patients underwent CHT (n = 40). b Statistical dot plot (left) and representative histogram (right) graphs showing the expressions (%) of CD57 on matching TCM, TEM and TEMRA Vδ2pos T cell subsets in CLM patients underwent CHT (n = 15). c Statistical bar graphs showing the fold change increases of CD107a expression as well as of intracellular amounts of IFN-γ and TNF-α by CD57neg and CD57pos Vδ2 T cell effector subsets (i.e. TEMRA and TEM) from CLM patients underwent CHT and following in vitro stimulation with PMA and Ionomycin (n = 6). d Statistical dot plot analysis showing the expressions (%) of CD57 and the frequencies (%) of TEMRA within Vδ2pos T cell compartments in CLM patients underwent CHT and divided in two groups of respectively < (white circles; n = 18) and ≥ (black circles; n = 21) of 60 years old. The mean age of the entire cohort of CLM patients underwent CHT is of 61 ± 10.7 years old as shown in statistical graph on right upper side. e Statistical dot plot analysis showing the expressions (%) of CD57 on Vδ2pos TEMRA cells from CLM patients underwent CHT and under 60 years old (n = 16) compared to age-matched healthy donors (n = 16). f Statistical analysis showing the correlations between the surface levels (%) of CD57 and CD28 (n = 51) (left graph) or CD16 (n = 51) (right graph) on Vδ2pos T cells in CLM patients underwent CHT
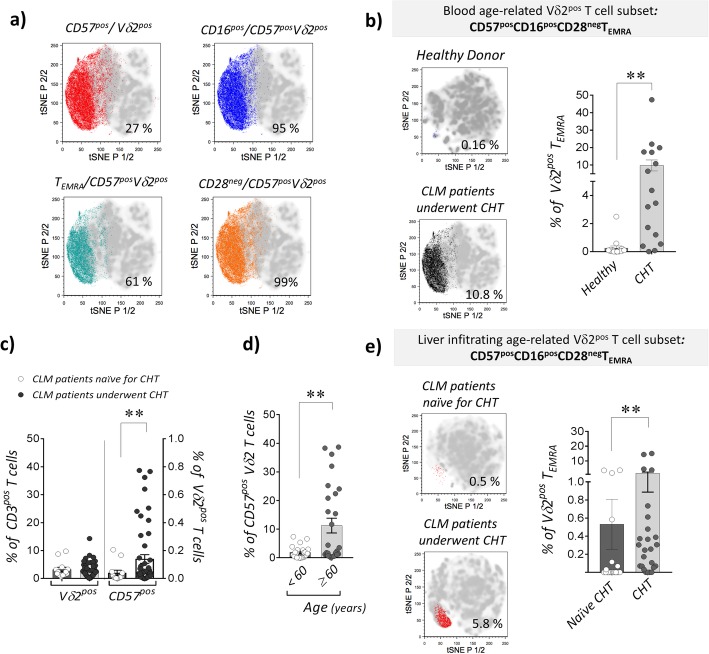
To assess the impact of patients’ aging in the higher frequencies of CD57pos and TEMRA Vδ2pos T cells in CLM patients underwent CHT, we divided this cohort in subjects younger or older than 60 years old. Our data confirmed that the age-induced immune-senescence significantly increases the percentages of both CD57pos and TEMRA Vδ2pos T cells in those patients > 60 years old (Fig. 2d). We also showed that CHT alone induces immune-senescence regardless of patients’ age. Indeed, the percentage of CD57pos TEMRA Vδ2pos cells resulted significantly higher in those CLM underwent CHT and younger than 60 years old compared to that of age-matched healthy donors (Fig. 2e). These data clearly indicate that both CHT and aging play synergic roles in the regulation of Vδ2pos T cell homeostasis in CLM patients with the final result of greatly accelerating their terminal differentiation towards a senescent CD57pos/TEMRA subset highly impaired in its anti-tumor effector-functions. We also demonstrate here that the acquisition of CD57 inversely correlates with the surface expression of CD28 while being associated with increased surface amounts of CD16 (Fig. 2f), the FcγRIII receptor known to define highly differentiated human Vδ2pos TEMRA cells [21]. The clustering of CD57pos/Vδ2pos TEMRA co-expressing CD16 and lacking CD28 in CLM patients underwent CHT is confirmed and better visualized by the t-Distributed Stochastic Neighbor Embedding (t-SNE) analysis (Fig. 3a). This analytic approach also allowed us to compare the impact of CHT in inducing high frequencies of PB CD57pos/CD16pos/CD28neg/Vδ2pos TEMRA cells in CLM patients compared to those of age-matched healthy donors (Fig. 3b).
Fig. 3.
Clustering of peripheral blood and tissue infiltrating senescent CD57pos/CD28neg/CD16pos TEMRA Vδ2pos T cells in patients affected by liver metastasis of colorectal cancer and underwent chemotherapy. a t-SNE analysis plots in CLM patients underwent CHT (n = 16) showing the cluster of PB CD57pos/ Vδ2pos T cells (red, upper left plot) co-expressing CD16 (blue, upper right plot), CD45RA but not CD27 (TEMRA in green, lower left plot) and negative for CD28 (black, lower right plot). b t-SNE analysis plots (left) and statistical dot plot graph (right) showing the frequency (%) of senescent PB CD57pos/CD28neg/CD16pos TEMRA Vδ2pos T cells in healthy donors (upper plot; n = 12; mean age: 51.7 ± 10.8) and CLM underwent CHT (lower plot; n = 16; mean age: 61 ± 10.7). c Summary dot plot analysis showing the frequencies (%) of liver tumor-associated Vδ2pos T cells within total CD3pos T lymphocytes or CD57pos/Vδ2pos T cells in CLM patients receiving CHT regimen (black circles; n = 58) and naïve for CHT (white circles; n = 13). d Statistical dot plot analysis showing the frequencies (%) of CD57pos cells on liver tumor infiltrating Vδ2pos T cells in CLM patients underwent CHT regimen and sub-divided in two groups of respectively < (white circles; n = 22) and ≥ (black circles; (n = 27) of 60 years old. e t-SNE analysis plots (left plots) and statistical chart (right graph) of the CHT-mediated changes in the frequency (%) of the age-related, liver tumor infiltrating CD57posCD28negCD16posTEMRA Vδ2pos T cell cluster in CHT treated CLM patients (lower plot, n = 25; mean age: 61 ± 10.7) and naïve for CHT patients (upper plot, n = 13; mean age: 69.5 ± 8.1)
Although the overall frequency of tumor infiltrating Vδ2pos T cells purified from CLM specimens is not affected by the administration of CHT, we found a significant increase of CD57 expression on those Vδ2pos T cells from patients underwent CHT compared to naïve ones (Fig. 3c). Similar to their PB counterparts, the frequency of CD57pos/Vδ2pos T cells is significantly higher in elderly CHT patients ≥60 years old (Fig. 3d). Consistently with these data, t-SNE analysis showed also in CLM specimens of patient administered with CHT an increased frequency of age-related tumor infiltrating CD57pos/CD28neg/CD16pos TEMRA Vδ2pos T lymphocytes (Fig. 3e).
Discussion
The present study is aimed to measure the true impact of conventional CHT regimens on unconventional T cell senescence in elderly cancer patients, since the toxicity of conventional anti-tumor therapies greatly impairs their ability to clearance malignant cells [7, 12, 14, 15]. In particular, we focused our investigations on circulating Vδ2pos cells that are endowed with great anti-tumor potentials currently being targeted by several protocols of cancer immunotherapies [4–6]. Our data showed that CLM patients underwent CHT, although showing lower absolute counts of circulating Vδ2pos cells, retain high relative frequencies of terminally differentiated and senescent CD57pos/CD28neg/CD16pos TEMRA Vδ2pos cells greatly impaired in their effector-functions. This latter subset is resistant to the toxicity exerted by repeated CHT cycles administering DNA-damaging drugs that, in contrast, are highly toxic against less differentiated and still proliferating TCM Vδ2pos cells.
The preferential accumulation in PB of senescent CD57pos TEMRA Vδ2pos cells in CLM patients underwent CHT is associated with two major mechanisms. The first one is linked to natural immune-senescence of people aging as the incidence of many cancers is higher in patients ≥ of 60 years old. In this context, liver metastatic CRC is one of the most common causes of cancer deaths worldwide with a higher incidence in elderly [16, 17]. Indeed, our cohort of recruited CLM subjects had a mean age of 61 ± 10.7 years old and both the frequencies of CD57pos and TEMRA Vδ2 T cell subsets resulted higher in that fraction of patients older than 60 years. The second mechanism is associated with a direct toxicity of CHT on immune cells, as also highlighted by several studies both in pediatric and geriatric cancer patients [14, 15, 22]. As a matter of fact, we show here that the expression of CD57 on TEMRA Vδ2pos cells is much higher on those CLM patients underwent CHT and younger than 60 years old compared to age-matched healthy donors. This demonstrates that neoadjuvant CHT induces immune senescence also on unconventional T cells regardless of CLM patients’ age. Notably, high frequencies of impaired CD57pos/TEMRA Vδ2pos cells were able to persist in PB of CLM patients even after 6 weeks from the completion of the last CHT cycle and before surgical removal of liver metastases. Further prospective studies are required to assess how long senescent and functional impaired Vδ2pos T cells survive after CHT and what clinical impact they have on the OS of CLM patients. In this regard, it has been already reported that the enrichment of circulating subsets of CD57pos αβ T cells represents a negative prognostic factor in the clinical outcome of gastrointestinal cancers [23].
Our study also contributes to better characterize immune-senescence of Vδ2pos T cells, since it has been recently reported that expression of CD57 can define alone their senescent status without the need of also evaluating the expression of both CD27 and CD45RA [11]. This represents a key point that is currently being debated both in physiological and pathological conditions. We found that, at least in a human cancer setting, the expression of CD57 on senescent Vδ2pos T cell parallels their terminal differentiation towards TEMRA (CD27neg/CD45RApos), a phenomenon associated with the loss of CD28 and the acquired expression of CD16. These results are in line with a previous study showing that Vδ2pos TEMRA are refractory to phosphoantigen stimulation, but rather respond to activation via FcγRIII [21].
The majority of cancer patients are older than 65 years old in line with population aging [14]. In this context, several clinical trials in the elderly are currently being implemented to optimize the anti-tumor activities of unconventional T cells. These therapeutic protocols are mostly aimed to expand Vδ2pos T cells both in vivo and in vitro [6]. Hence, a better understanding of the mechanisms accelerating immune-senescence in aging is fundamental to boost the effector-functions of these cytotoxic and cytokine-producer T lymphocytes. We show here that, neoadjuvant CHT regimens, although absolutely required to reduce tumor mass in CLM patients before surgery, greatly speed the senescence of Vδ2pos T cells in synergy with aging of cancer patients. This knowledge will allow us to better optimize immune-therapies against cancers in elderly. Indeed, senescence process can be reversed through the inhibition of p38 mitogen-activated protein kinase (MAPK) signaling [24]. This methodology could be then approached to develop new protocols implementing pre-treatment with MAPK inhibitors in elderly patients with CRC [25]. Alternatively, new methodology can be implemented in vitro to select and expand CD57neg/Vδ2pos T cells that better resist to the terminal differentiations and senescence induced by CHT. Further studies are also required to better identify those CHT associated accumulation of impaired and senescent circulating Vδ2pos T cells.
Acknowledgments
The authors thank the patients for their generosity and participation in this study and all the nurses and clinicians of the Department of Hepatobiliary and General Surgery (Humanitas Clinical and Research Center).
Abbreviations
- CHT
Chemotherapy
- CLM
Colorectal liver metastatic cancer
- CRC
Colorectal cancer
- NBPs
Nitrogen-containing bisphosphonates
- TCM
Central memory T
- TEM
Effector memory T
- TEMRA
Terminally differentiated T
- TNaïve
Naïve T
- t-SNE
t-Distributed Stochastic Neighbor Embedding
- γδ T
Gamma delta T
Authors’ contributions
JM and DM developed the study. EB, VC, GS and GL performed the experiments and statistical analyses. MC, MD and GT relucted patients and collected biological specimens. JM, EB, FD and DM contributed to the interpretation of the data and wrote article. All authors read and approved the final manuscript.
Funding
This work was supported by Associazione Italiana per la Ricerca sul Cancro (IG-14687 and IG 21567 to D.M.), Italian Ministry of Health (Bando Ricerca Finalizzata PE-2016-02363915 to D.M.) and Intramural Research Funding of Istituto Clinico Humanitas (to D.M.).
Availability of data and materials
The dataset generated and analyzed in the current study are available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
The collection of human samples for research purposes was ethically approved by the Institutional Review Board (IRB) of Humanitas Research Hospital (HRH) (Approval 168/18).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joanna Mikulak and Domenico Mavilio contributed equally to this work.
Contributor Information
Joanna Mikulak, Email: joanna.mikulak@humanitasresearch.it.
Domenico Mavilio, Email: domenico.mavilio@unimi.it.
References
- 1.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willcox BE, Willcox CR. gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. 2019;20(2):121–128. doi: 10.1038/s41590-018-0304-y. [DOI] [PubMed] [Google Scholar]
- 3.Caccamo N, Meraviglia S, Ferlazzo V, Angelini D, Borsellino G, Poccia F, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol. 2005;35(6):1764–1772. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 4.Zou C, Zhao P, Xiao Z, Han X, Fu F, Fu L. gammadelta T cells in cancer immunotherapy. Oncotarget. 2017;8(5):8900–8909. doi: 10.18632/oncotarget.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol. 2015;15(11):683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T cell targets for cancer immunotherapy. Immunity. 2018;48(3):453–473. doi: 10.1016/j.immuni.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19(9):1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larbi A, Fulop T. From “truly naive” to “exhausted senescent” T cells: when markers predict functionality. Cytometry A. 2014;85(1):25–35. doi: 10.1002/cyto.a.22351. [DOI] [PubMed] [Google Scholar]
- 9.Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016;8(1):3–11. doi: 10.18632/aging.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14(11):2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Monaco G, Wong EH, Tan WLW, Kared H, Simoni Y, et al. Mapping of gamma/delta T cells reveals Vdelta2+ T cells resistance to senescence. EBioMedicine. 2019;39:44–58. doi: 10.1016/j.ebiom.2018.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Larbi A. Markers of T cell senescence in humans. Int J Mol Sci. 2017;18(8):1742. doi: 10.3390/ijms18081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smetana K, Jr, Lacina L, Szabo P, Dvorankova B, Broz P, Sedo A. Ageing as an important risk factor for cancer. Anticancer Res. 2016;36(10):5009–5017. doi: 10.21873/anticanres.11069. [DOI] [PubMed] [Google Scholar]
- 14.Hurria A, Jones L, Muss HB. Cancer treatment as an accelerated aging process: assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book. 2016;35:e516–e522. doi: 10.14694/EDBK_156160. [DOI] [PubMed] [Google Scholar]
- 15.Onyema OO, Decoster L, Njemini R, Forti LN, Bautmans I, De Waele M, et al. Chemotherapy-induced changes and immunosenescence of CD8+ T-cells in patients with breast cancer. Anticancer Res. 2015;35(3):1481–1489. [PubMed] [Google Scholar]
- 16.Konopke R, Roth J, Volk A, Pistorius S, Folprecht G, Zophel K, et al. Colorectal liver metastases: an update on palliative treatment options. J Gastrointestin Liver Dis. 2012;21(1):83–91. [PubMed] [Google Scholar]
- 17.Kolligs FT. Diagnostics and epidemiology of colorectal cancer. Visc Med. 2016;32(3):158–164. doi: 10.1159/000446488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 19.Donadon M, Hudspeth K, Cimino M, Di Tommaso L, Preti M, Tentorio P, et al. Increased infiltration of natural killer and T cells in colorectal liver metastases improves patient overall survival. J Gastrointest Surg. 2017;21:1226–1236. doi: 10.1007/s11605-017-3446-6. [DOI] [PubMed] [Google Scholar]
- 20.Roberto A, Di Vito C, Zaghi E, Mazza EMC, Capucetti A, Calvi M, et al. The early expansion of anergic NKG2A(pos)/CD56(dim)/CD16(neg) natural killer represents a therapeutic target in haploidentical hematopoietic stem cell transplantation. Haematologica. 2018;103(8):1390–1402. doi: 10.3324/haematol.2017.186619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, et al. FcgammaRIII discriminates between 2 subsets of Vgamma9Vdelta2 effector cells with different responses and activation pathways. Blood. 2004;104(6):1801–1807. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- 22.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014:e423–30. 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed]
- 23.Akagi J, Baba H. Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer. Int J Clin Oncol. 2008;13(6):528–535. doi: 10.1007/s10147-008-0789-8. [DOI] [PubMed] [Google Scholar]
- 24.Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol. 2014;15(10):965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pancione M, Giordano G, Parcesepe P, Cerulo L, Coppola L, Curatolo AD, et al. Emerging insight into MAPK inhibitors and immunotherapy in colorectal cancer. Curr Med Chem. 2017;24(14):1383–1402. doi: 10.2174/0929867324666170227114356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analyzed in the current study are available from the corresponding authors on reasonable request.