Abstract
OBJECTIVES:
Access to subspecialty care may be difficult for patients with liver disease, but it is unknown whether access influences outcomes among this population. Our objectives were to determine rates and predictors of access to ambulatory gastrointestinal (GI) subspecialty care for patients with liver disease and to determine whether access to subspecialty GI care is associated with better survival.
METHODS:
We studied 28,861 patients within the Veterans Administration VISN 11 Liver Disease cohort who had an ICD-9-CM diagnosis code for liver disease from 1 January 2000 through 30 May 2011. Access was defined as a completed outpatient clinic visit with a gastroenterologist or hepatologist at any time after diagnosis. Multivariable logistic regression was used to determine predictors of access to a GI subspecialist. Survival curves were compared between those who did and those who did not see a specialist, with propensity score adjustment to account for other covariates that may affect access.
RESULTS:
Overall, 10,710 patients (37%) had a completed GI visit. On multivariable regression, older patients (odds ratio (OR) 0.98, P<0.001), those with more comorbidities (OR 0.98, P=0.01), and those living farther from a tertiary-care center (OR 0.998/mi, P<0.001) were less likely to be seen in clinic. Patients who were more likely to be seen included those who had hepatitis C (OR 1.5, P<0.001) or cirrhosis (OR 3.5, P<0.001) diagnoses prior to their initial visit. Patients with an ambulatory GI visit at any time after diagnosis were less likely to die at 5 years when compared with propensity-score-matched controls (hazard ratio 0.81, P<0.001).
CONCLUSIONS:
Access to ambulatory GI care was associated with improved 5-year survival for patients with liver disease. Innovative care coordination techniques may prove beneficial in extending access to care to liver disease patients.
INTRODUCTION
Liver disease is a growing medical problem both in the Department of Veterans Affairs (VA) Health Systems, as well as in the United States and worldwide. The prevalence of hepatitis C is estimated at 5% in the VA (1). Approximately 30% of Americans likely have non-alcoholic fatty liver disease (2). Worldwide, cirrhosis is thought to be the eighth leading cause of death (3). Because liver disease may require complex care, referral to subspecialty care in gastroenterology/hepatology (GI) is often warranted. However, which factors determine whether patients ultimately receive care from GI subspecialists and whether this impacts overall survival is not fully known.
Patients with liver disease may experience problems with access to subspecialty care, due to many factors such as geographic clustering of specialists in tertiary-care centers. Although access to healthcare has been sparsely studied in liver disease, it has been recognized anecdotally as a problem in some practice areas. For example, despite the existence of multiple grade 1/class 1 recommendations, adherence to guideline-based care for patients with some forms of chronic liver disease remains low (4, 5, 6, 7, 8). Only 38% of patients with cirrhosis nationwide undergo screening for hepatocellular carcinoma, and only 30% of those with spontaneous bacterial peritonitis receive antibiotics for secondary prophylaxis (7, 9, 10, 11, 12, 13).
One possible cause for such poor guideline adherence may be a lack of access to subspecialty care, which has been shown to influence treatment decisions in a variety of gastroenterological conditions (7, 9, 10, 12, 13, 14, 15, 16). As such, innovative coordination and communication interventions have been designed to increase access to specialty care and knowledge (11, 17). The Department of Veterans Affairs (VA) recently implemented the Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) program, a knowledge network linking primary care physicians to tertiary-care subspecialists. In 2011, SCAN-ECHO began for liver disease within Veterans Integrated Service Network (VISN) 11. The goal of this study was to examine access to subspecialty care and its impact on survival prior to implementation of SCAN-ECHO, in order to establish a baseline for future comparison and develop a better understanding of the access problem. Our aims were (i) to determine rates and predictors of access to ambulatory GI subspecialty care for patients with liver disease and (ii) to determine whether access to ambulatory GI subspecialty care was associated with better survival.
METHODS
Population
The cohort included all adult patients over the age of 18 years within the VISN 11 with an International Classification of Diseases, 9th Edition (ICD-9) code for liver disease entered into the electronic medical record between 1 January 2000 and 31 May 2011 (n=38,709). VISN 11 is one of the 21 integrated service networks within the VA healthcare system and provides inpatient and outpatient care for veterans within a 90,100 mi2 geographic area including Michigan, central Indiana, and northwest Ohio. Liver disease was broadly defined as the presence of any one of the several ICD-9 codes for liver diseases including hepatitis C and cirrhosis (see Supplementary Appendix A online). We specifically chose ICD-9 codes that were the most common reasons for referral to GI subspecialty care and that, for the most frequent diagnoses (hepatitis C and cirrhosis), have been previously validated within the VA system (18). Cirrhosis patients were defined as patients with at least two inpatient or outpatient ICD-9 codes for cirrhosis (571.2, 571.5, 571.6) or one ICD-9 code for cirrhosis plus either a code for a cirrhosis complication (456.0, 456.1, 456.21, 572.2, 572.3, 572.4, 572.8, 789.5), or an aspartate aminotransferase/platelet ratio index >2 (7). To account for late referral bias, i.e., very sick patients who may have died before being able to complete an ambulatory GI clinic visit, we excluded all patients who died within 30 days of their index diagnosis (n=622). Patients can be enrolled in VA care for medication benefits only, which requires only a single yearly visit with a PCP with the remainder of their care provided in non-VA facilities. In order to control for misclassification bias, we further excluded all patients with 10 or fewer visits who also had no GI clinic visit and no inpatient stay over the 10-year study period (n=1,161), which resulted in a final cohort size of n=28,861 (see Supplementary Figure S1 online).
This analysis was conducted as part of an evaluation of the Chronic Liver Disease SCAN-ECHO program at VA Ann Arbor Healthcare System (VAAAHS). It was deemed non-research under VHA Handbook 1058.05 and conducted under the approval of clinical leadership at VAAAHS.
Outcome and predictors
To evaluate access, we defined the presence of a GI clinic visit at any time point within the study period after diagnosis, as indicated by the presence of codes in the electronic record indicating a completed GI or Liver clinic visit. Within VISN 11 during the time period studied, subspecialty care by either a gastroenterologist or a hepatologist was within these two clinic designations. For the purposes of this study, “diagnosis” reflects the first time a liver disease code appears for a given patient during the study period. Five-year survival was determined based on the VA’s Beneficiary Identification Records Locator Subsystem (BIRLS) Death File. Deaths were recorded from the beginning of the study period until 31 May 2011.
Predictors of access were defined as follows: demographic predictors were age at diagnosis, gender, and race/ethnicity. Urban vs. rural patient residence was determined based on VA Planning Systems Support Group geocoding (19). GI referral rates were also calculated and were defined as the presence of a request in the electronic medical record for consultation with a GI physician. Referrals that were non-liver specific (e.g., for colonoscopy only) were excluded. Given that most GI subspecialists are clustered at tertiary-care sites, the driving distance from the patient’s home address to the nearest tertiary-care site was measured in miles. Tertiary-care sites within VISN 11 are Ann Arbor, MI, Indianapolis, IN, and Detroit, MI. The alanine aminotransferase value used was the highest alanine aminotransferase level within 365 days, but prior to diagnosis, to account for the triggers for consultation. The bilirubin level used was the value closest to diagnosis date and within 180 days prior to diagnosis to account for severity of liver disease. Elixhauser comorbidity scores were calculated using diagnosis codes present 365 days prior to the first liver disease diagnosis and excluded liver disease and alcoholism (20). Cirrhosis comorbidities including ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, variceal bleed, hepatocellular carcinoma, and hepatic encephalopathy were determined by ICD-9-CM codes (see Supplementary Appendix A). A supplemental analysis was performed that divided the cohort into two groups, low-grade and high-grade fibrosis, based on the FIB-4 score, a serologic scoring system for fibrosis that utilizes common lab values (age, platelet count, aspartate aminotransferase, and alanine aminotransferase) to create a score that is predictive of high-grade fibrosis (21).
Statistical analysis
The GI referral rate was calculated with the number of patients in the cohort with at least one GI consult request in the electronic health record after diagnosis as the numerator and total cohort population as the denominator. GI visit rate was calculated as the number of patients with a completed GI visit code at any time after diagnosis as the numerator with total number of patients in the cohort as the denominator. Pearson’s [chi]2-test, Wilcoxon rank-sum, and Student’s t-test were used as appropriate for bivariate analyses of categorical and continuous predictors of access, respectively. Multivariable logistic regression was performed to determine independent predictors of a GI visit. All relevant variables were included in the final model, regardless of their statistical significance in bivariate testing.
In order to determine whether subspecialty access was independently associated with mortality, Cox proportional hazard ratios were calculated with multivariate models identical to those used in prior regression for Aim i. To adjust for differences between the patients who received a visit and those who did not, a propensity score was created by fitting a logistic regression model for receipt of a GI visit based on 23 different predictors including demographics, geographic location, disease state and severity, and receipt of selected imaging and procedures (predictors used were identical to those used in the multivariable regression in Table 2). A ratio of 3:1 nearest neighbor matching with replacement was used with a caliper of 0.2 (22). Supplemental analyses including multivariate regression and propensity-adjusted analysis were performed on the low-grade fibrosis and high-grade fibrosis cohorts, respectively, using the same methodology as described above. Statistical analyses were performed using STATA version 12.1 (College Station, TX).
Table 2.
Variables associated with receipt of GI subspecialty care among patients with liver disease on unadjusted and multivariable adjusted analysis
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Odds ratio | P value | Odds ratio | P value | |
| Age | 0.98 | <0.001 | 0.98 | <0.001 |
| Male gender | 0.9 | 0.34 | 0.96 | 0.49 |
| Race/ethnicity | ||||
| 1=White, ethnicity unknown, or not Hispanic | 1.1 | 0.02 | 1.1 | 0.027 |
| 2=White, Hispanic ethnicity | 1.5 | 0.004 | 1.2 | 0.25 |
| 3=Black, ethnicity unknown, or not Hispanic | 1.8 | <0.001 | 1.4 | <0.001 |
| 4=Black, Hispanic ethnicity | 1.3 | 0.57 | 0.99 | 0.98 |
| 5=All other races, ethnicity unknown, or not Hispanic | 1.3 | 0.14 | 1.2 | 0.34 |
| 6=All other races. Hispanic ethnicity | 0.8 | 0.7 | 0.8 | 0.7 |
| Urban location | 1.5 | <0.001 | 1.1 | <0.001 |
| Elixhauser comorbidity score | 0.9 | <0.001 | 0.98 | 0.013 |
| Hepatitis C | 1.8 | <0.001 | 15 | <0.001 |
| Cirrhosis | 2.8 | <0.001 | 3.5 | <0.001 |
| ALT (IU/dI) | 1.0a | <0.001 | 1.0a | 0.028 |
| Hepatitis B | 0.99 | 0.87 | 0.8 | 0.001 |
| SBP | 0.9 | 0.7 | 0.7 | 0.26 |
| Variceal bleed | 1.6 | 0.001 | 0.9 | 0.4 |
| HRS | 0.5 | 0.028 | 0.3 | <0.001 |
| HCC | 0.4 | <0.001 | 0.4 | <0.001 |
| Ascites | 1.9 | <0.001 | 0.7 | 0.001 |
| Abdominal ultrasound | 1.4 | <0.001 | 1.4 | <0.001 |
| Abdominal CT | 0.9 | 0.005 | 0.9 | 0.016 |
| Abdominal MRI | 0.9 | 0.29 | 0.9 | 0.23 |
| Liver biopsy | 2.2 | <0.001 | 1.9 | <0.001 |
| Paracentesis | 1.3 | 0.25 | 0.8 | 0.27 |
| EGD | 1.2 | <0.001 | 1.3 | <0.001 |
| Driving distance in miles | 0.997 | <0.001 | 0.998 | <0.001 |
| Bilirubin (mg/dI) | 1.1 | <0.001 | 1.0 | <0.001 |
ALT, alanine aminotransferase; CT, computed tomography: EGD, upper endoscopy: Gl. gastrointestinal: HCC, hepatocellular carcinoma: HRS. hepatorenal syndrome; MRI, magnetic resonance imaging; SBP, spontaneous bacterial peritonitis.
ALT values were significant at odds ratios of 1.0002 for both bivariate and multivariate analyses.
RESULTS
Characteristics of the cohort are included in Table 1. After application of inclusion and exclusion criteria (see Supplementary Figure S1), 28,861 patients were diagnosed with liver disease over the 10-year study time frame with 10,710 (37%) receiving a GI clinic visit. The average age at first diagnosis was 56 years with those receiving a clinic visit being younger than those who did not (54 years vs. 58 years, P<0.001). Ninety-six percent were male and two-thirds lived in an urban area. As of 31 May 2011, 24% of the cohort had died. Thirty-five percent had a hepatitis C diagnosis and 9% had a diagnosis of cirrhosis. For those with hepatitis C, 44% received a clinic visit. The average travel distance from the patient’s home to the closest tertiary-care site was 79 mi, with patients receiving a clinic visit traveling 69 mi vs. 84 mi for those who did not (P<0.001). Overall, 55% of the entire cohort received a GI referral, whereas 37% received a GI clinic visit. The mean number of GI visits per patient was 4.41 (s.d. 5.82), whereas the median number of GI visits per patient was 2.00 (interquartile range 1.00–5.00).
Table 1.
Characteristics of patients with and without a GI clinic visit
| Full cohort | No visit | Visit | P value | |
|---|---|---|---|---|
| n = 28,861 | n = 18,151 | n = 10,710 | ||
| Age at first diagnosis of OLD (years), mean (s.d.) | 5637 (12.00) | 57.64 (12.85) | 54,23 (10.03) | <0,001 |
| Gender, n (%) | 0.23 | |||
| Female | 1,121 (3.88) | 686 (3.78) | 435 (4.06) | |
| Male | 27,740 (96.12) | 17,465 (96.22) | 10,275 (95.94) | |
| Race/ethnicity, n (%) | <0.001 | |||
| White, ethnicity unknown, or not Hispanic | 19,808 (68.63) | 12,950 (71.35) | 6,858 (64.03) | |
| White, Hispanic ethnicity | 299 (1.04) | 178 (0.98) | 121 (1.13) | |
| Black, elhnicity unknown, or not Hispanic | 6,677 (23.14) | 3,621 (19.95) | 3,056 (28.53) | |
| Black, Hispanic ethnicity | 19 (0.07) | 12 (0.07) | 7 (0.07) | |
| All other races, ethnicity unknown, or not Hispanic | 170 (0.59) | 106 (0.58) | 64 (0.60) | |
| All other races, Hispanic ethnicity | 21 (0.07) | 15 (0.08) | 6 (0.06) | |
| Urban/rural status, n (%) | <0.001 | |||
| Rural | 9,870 (34.20) | 6,795 (37.44) | 3,075 (28.71) | |
| Urban | 18,991 (65.80) | 11.356 (62.56) | 7,635 (71.29) | |
| Elixhauser comorbidity. mean (s.d.) | 2.83 (2.17) | 2.95 (2.23) | 2.62 (2.04) | <0,001 |
| Hepatitis C,n(%) | 10,167 (3523) | 5,472 (30.15) | 4,695 (43.84) | <0,001 |
| Cirrhosis, n(%) | 2,652 (9.19) | 1,107 (6.10) | 1,545 (14.43) | <0,001 |
| Maximum ALT value (IU/I), mean (s.d.) | 97.64 (214.94) | 94.60 (225.82) | 102.79 (195.02) | 0.001 |
| Thrombocytopenia, n (%) | 1,284 (4.45) | 877 (4.83) | 407 (3.80) | <0,001 |
| Hepatitis B, n(%) | 889 (3.08) | 558(3.07) | 331 (3.09) | 0.94 |
| Spontaneous bacterial peritonitis, n(%) | 52 (0.18) | 37 (0.20) | 15 (0.14) | 0.22 |
| Variceal bleed, n (%) | 231 (0.80) | 125 (0.69) | 106 (0.99) | 0.006 |
| Hepatorenal syndrome, n (%) | 74 (0.26) | 61 (0.34) | 13 (0.12) | 0.001 |
| Hepatocellular carcinoma, n (%) | 312 (1.08) | 253 (1.39) | 59 (0.55) | <0,001 |
| Ascites, n (%) | 922 (3.19) | 456 (2.51) | 466 (4.35) | <0.001 |
| Abdominal ultrasound, n (%) | 9,074 (31.44) | 5,164 (28.45) | 3,910 (36.51) | <0.001 |
| Abdominal CT, n (%) | 4,401 (15.25) | 2,832 (15.60) | 1,569 (14.65) | 0.03 |
| Abdominal MRI, n (%) | 489 (1.69) | 315 (1.74) | 174 (1.62) | 0.48 |
| Liver biopsy, n (%) | 191 (0.66) | 87 (0.48) | 104 (0.97) | <0.001 |
| Paracentesis, n (%) | 114 (0.39) | 67 (0.37) | 47 (0.44) | 0.36 |
| EGD, n (%) | 2,786 (9.65) | 1,650 (9.09) | 1,136 (10.61) | <0.001 |
| Driving distance in miles, mean (s.d.)a | 78.56 (77.65) | 84.20 (78.09) | 69.00 (75.94) | <0.001 |
| Bilirubin (mg/dl), mean (s.d.) | 0.97(1.78) | 0.94 (1.75) | 1.03(1.83) | <0.001 |
ALT, alanine aminotransferase; CLD, chronic liver disease; CT, computed tomography; EGD, upper endoscopy; GI, gastrointestinal; MRI, magnetic resonance imaging.
Defined as distance in miles to closest tertiary-care site (Ann Arbor, Ml; Indianapolis, IN; Detroit, Ml).
On multivariable logistic regression (see Table 2), older patients (odds ratio (OR) 0.98, P<0.001) and those with more comorbidities (OR 0.98, P=0.01) were less likely to be seen in clinic. Furthermore, patients with certain complications of cirrhosis, such as thrombocytopenia (OR 0.60), hepatorenal syndrome (OR 0.3), hepatocellular carcinoma (OR 0.4), and ascites (OR 0.7), were less likely to be seen in clinic (all P<0.001). Caucasians (OR 1.1, P=0.03) and African-Americans (OR 1.4, P<0.001) were more likely to be seen in clinic, as were patients in urban locations (OR 1.1, P<0.001) and those with HCV and cirrhosis diagnoses prior to their initial GI visit (OR 1.5 and 3.5, respectively; P<0.001). Patients who lived further from a tertiary-care site were less likely to be seen in clinic (OR 0.998/mi, P<0.001).
Table 2Opens a popup window Opens a popup window Opens a popup window
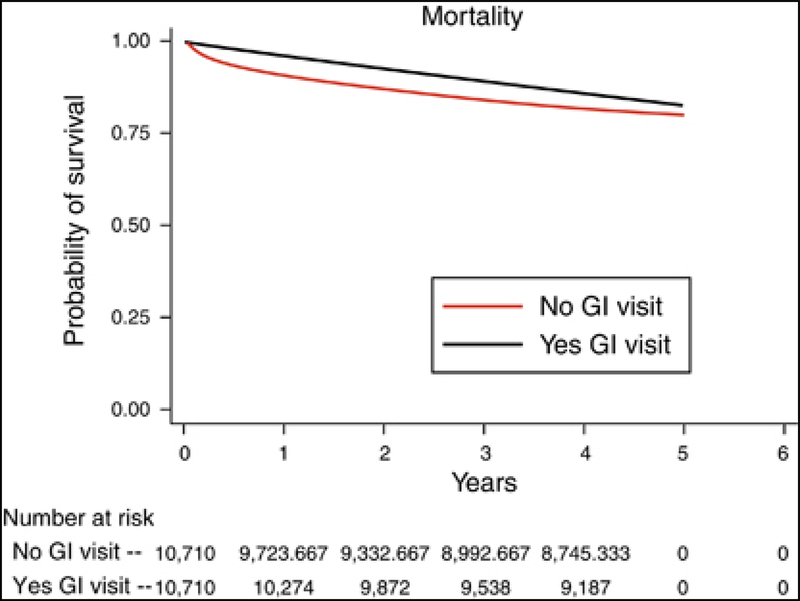
In the overall cohort, patients with an ambulatory GI visit at any time after diagnosis had a lower likelihood of death at 5 years than those without a GI visit when compared with propensity-score- matched controls (hazard ratio 0.81, P<0.001). The propensity-adjusted survival curves are shown in Figure 1. To determine whether there was improved survival with a greater number of GI visits (a “dose-response”), we performed an additional propensity-adjusted survival analysis of patients above or below the median number of GI visits. Patients with 1–2 GI visits had poorer 5-year survival compared with patients who had >2 GI visits (hazard ratio 0.72, P<0.001). To determine whether there was a difference in survival between patients with more severe chronic liver disease, additional propensity analyses of low-grade and high-grade fibrosis cohorts were performed. Patients with both low-grade and high-grade fibrosis had improved survival at 5 years (hazard ratio 0.78 and 0.82, P<0.001 respectively).
Figure 1.
Propensity-adjusted survival difference between patients with and without an ambulatory gastrointestinal clinic visit. GI, gastrointestinal.
DISCUSSION
In this large cohort of VA patients diagnosed with liver disease, receipt of an ambulatory GI clinic visit was associated with improved survival at 5 years in a propensity-score adjusted analysis. Furthermore, receipt of >2 visits over the time period was associated with improved survival compared with those who had fewer visits. Patients who lived farther from tertiary-care sites were less likely to be seen, whereas those who had diagnoses of hepatitis C and cirrhosis were more likely to be seen.
Our study demonstrated an association between access to GI specialty care and improved survival at 5 years, and this association was present for patients with both high- and low-grade fibrosis. Similar associations between access to specialty care and improved survival have been shown in other specialties, particularly in cardiology, where studies in cardiac patients post hospitalization for myocardial infarction have shown that specialist involvement was also associated with improved mortality (4). The reasons for these associations are unclear, however. Within gastroenterology and hepatology, subspecialty involvement has been associated with improved processes and some outcomes in a variety of diseases including GI bleeding, diverticulitis, celiac disease, inflammatory bowel disease, decompensated cirrhosis, ascites, hepatitis C, and hepatocellular carcinoma, although none was able to show a mortality benefit (6, 7, 8, 9, 11, 12, 13). A trend toward improved inpatient survival for admitted patients with end-stage liver disease seen by GI subspecialists in the inpatient setting has been reported, although this trend did not reach statistical significance (14). Furthermore, local access to GI subspecialty care has been shown to improve chances of receiving a liver transplant (15). It must be noted, however, that the association between GI specialty access and improved survival may not be related to GI specialty care exclusively but may represent an overall improvement in mortality, resulting from connection to the healthcare system in general. Patients who receive a diagnosis of liver disease may not have been substantially connected to the VA or other healthcare systems prior to this diagnosis; hence, improvement in mortality may reflect the combined effects of both primary and specialty care of the patients’ liver disease and other comorbidities. In addition, patients with low-grade fibrosis would be expected to have low mortality; hence, the findings of a mortality benefit in this group may be unrelated to the GI clinic visit.
Taken together, these results suggest that access to GI subspecialty care for patients with liver disease may improve outcomes, but more research is required to confirm the presence of this association in other healthcare systems and to determine the causes behind such an association. Precisely how subspecialist access may influence survival is unknown, but improved quality of care, higher rates of screening for hepatocellular carcinoma, greater familiarity with and rapid recognition of complications, and greater likelihood of referral for liver transplant when appropriate may all have a role (15, 23). However, access to care is a multifaceted concept. It has been conceptualized as containing five different domains: availability, accessibility, accommodation, affordability, and acceptability (24). Although the goal of efforts to improve access is to connect patients with the medical care that they need, often culminating in the traditional clinic visit, there are many different barriers to access that patients must overcome before arriving in the clinic. These include financial barriers, insurance status, lack of social support, perceived stigma associated with liver disease, and patient-related beliefs about their disease, as well as poor care coordination to name a few (5, 25). Although our models accounted for many of these factors, there are likely patient, provider, and clinic-level traits that are not captured and that may help confirm and further characterize this association, as well as reasons for it.
The burden of liver disease is increasing nationwide, and this, coupled with the demand for newly available hepatitis C treatments, means that access to subspecialty care for patients with liver disease will continue to be important (26). To address access issues, innovative communication technologies are being developed and implemented to bridge the gap between patients and subspecialists. Programs such as SCAN-ECHO, e-referral, e-consults, and other telehealth initiatives are emerging as important ways for patients with a variety of chronic diseases including hepatitis C and liver disease to obtain care (11, 17, 27). Within VISN 11, for example, implementation of SCAN-ECHO for patients with liver disease saved patients 187 mi in travel on average (28). Despite this, geographic barriers remain important. Our finding that patients with hepatitis C and cirrhosis were more likely to be referred and to complete visits suggests a place for projects that extend subspecialty knowledge on these diseases from the academic health center to patients’ and providers’ locations. As newer, more tolerable, all-oral regimens with shorter treatment durations and fewer side effects become available for hepatitis C treatment, demand for access to subspecialty care may rise.
There were several limitations to our study. First, it was conducted within the VA healthcare system, which may diminish generalizability of results given the markedly different health-care delivery system within which patients received care. Patients are eligible for VA benefits if they have served in the US military branches and, as such, are more likely to be male, as reflected in our demographic information. As a consequence, our findings may not be generalizable to female patients. Second, although all patients within the cohort received care within the VA health-care system, we were unable to capture care that may have been provided by non-VA providers, as some Veteran patients carry private insurance or Medicare and opt for medical care in the private sector. We controlled for this misclassification bias, however, by excluding patients with 10 or fewer visits over the study time frame who had no GI visit or inpatient visit during that time. This exclusion increases the likelihood that patients in the cohort are primarily cared for within the VA system and minimizes misclassification. Third, the use of ICD-9 codes in administrative databases may be inaccurate in representing true disease. Within the VA system, however, ICD-9 codes for the most common diagnoses in our cohort (HCV and cirrhosis) have been validated and have been shown to have high positive predictive values, representing accurate diagnoses (18). In addition, our finding that patients with >2 GI visits after their liver disease diagnosis had an even greater survival benefit than those with fewer visits provides support for the presence of true liver disease, as patients with inaccurate diagnoses or those without liver disease (for example, HCV antibody-positive patients without positive viral loads) would be less likely to continue to receive ongoing Gl-related care. Fourth, we were unable to capture the presence or absence of inpatient Gl consultation. Patients with chronic liver disease may present with more advanced complications such as ascites, variceal bleeding, or hepatic encephalopathy, which are initially managed in the inpatient setting with inpatient Gl consultation and management. Unfortunately, the VA electronic health record during the bulk of the study time frame did not allow for the reliable identification of access to inpatient Gl consultation; hence, we are unable to characterize the effect of inpatient access to Gl subspecialty care. Fifth, whether or not a patient was referred for a Gl visit is an important factor in determining whether patients receive a Gl visit. However, data on referrals within the VA system are less robust and, early in the cohort time frame, an electronic referral was not necessary for a patient to receive a Gl clinic visit. As a consequence, ~35% of patients who completed a Gl clinic visit did not have a referral. Finally, any care provided at VA installations outside VlSN 11 would not have been captured by our methodology.
There are several strengths to our study. First, our cohort consists of a large number of patients from a diverse geographical background followed over a 10-year time frame, allowing for enough time to observe a survival benefit. Second, all liver diseases were included with subgroups for hepatitis C and cirrhosis, making the results more broadly generalizable. We also included additional sensitivity analyses of low-grade and high-grade fibrosis in order to determine whether findings were robust to differences in severity of liver disease. Third, we used propensity score matching to determine survival between patients with and without a Gl clinic visit. Propensity score matching is a technique that allows for all the observed predictors in a given observational study to be collapsed into a single predictor (20, 29). This method has been used successfully in many observational studies to account for the presence of multiple covariates and produces more reliable results by reducing selection bias found in the observed covariates (4, 22, 30).
ln conclusion, our findings show that, for patients with liver disease, access to Gl specialty care in the form of a face-to-face clinic visit was associated with improved survival. Although the reasons for this association are unclear, more research is necessary to determine whether these findings are replicable in other patient populations, such as those with private insurance or those with Medicare/Medicaid, as well as to determine what factors may influence these associations. Additional research is also necessary to study and implement new and innovative communication and coordination techniques to improve subspecialty care access for this growing population.
Supplementary Material
Box 1:
WHAT IS CURRENT KNOWLEDGE
✓Liver disease is commom and rising.
✓Specialty care for patients with liver disease improves some quality measures and outcomes.
✓Access to specialty care can be difficult for patients.
WHAT IS NEW HERE
✓Access to gastrointestinal (GI) specialty care was associated with improved 5-years survival for patients with liver disease.
✓Patients with cirrhosis and hepatitis C were more likely to be seen, whereas older patients with more comorbidities were less likely to be seen.
✓Ever in an integrated healthcare system with a universal electronic health record, specialty clinic visit rates were low.
REFERENCES
- 1.Dominitz JA, Boyko EJ, Koepsell TD et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology 2005;41:88–96. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 3.Asrani SK, Larson JJ, Yawn B et al. Underestimation of liver-related mortality in the United States. Gastroenterology 2013;145:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayanian JZ, Landrum MB, Guadagnoli E et al. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N Engl J Med 2002;347:1678–1686. [DOI] [PubMed] [Google Scholar]
- 5.Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin Gastroenterol Hepatol 2013;11:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005. 112:154–235 [DOI] [PubMed] [Google Scholar]
- 7.Kanwal F, Kramer JR, Buchanan P et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology 2012;143:70–77. [DOI] [PubMed] [Google Scholar]
- 8.Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med 1999;14:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provenzale D, Ofman J, Gralnek I et al. Gastroenterologist specialist care and care provided by generalists-an evaluation of effectiveness and efficiency. Am J Gastroenterol 2003;98:21–28. [DOI] [PubMed] [Google Scholar]
- 10.Davila JA, Kramer JR, Duan Z et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology 2013;57:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora S, Thornton K, Murata G et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011;364:2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanwal F, Hoang T, Chrusciel T et al. Process of care for hepatitis C infection Is linked to treatment outcome and virologic response. Clin Gastroenterol Hepatol 2012;10:1270–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bini EJ, Weinshel EH, Generoso R et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology 2001;34:1089–1095. [DOI] [PubMed] [Google Scholar]
- 14.Ko CW, Kelley K, Meyer KE. Physician specialty and the outcomes and cost of admissions for end-stage liver disease. Am J Gastroenterol 2001;96:3411–3418. [DOI] [PubMed] [Google Scholar]
- 15.Barritt AS, Telloni SA, Potter CW et al. Local access to subspecialty care influences the chance of receiving a liver transplant. Liver Transpl 2013;19:377–382. [DOI] [PubMed] [Google Scholar]
- 16.Kramer JR, Kanwal F, Richardson P et al. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol 2011;106:483–491. [DOI] [PubMed] [Google Scholar]
- 17.Chen AH, Murphy EJ, Yee HF. eReferral-a new model for integrated care. N Engl J Med 2013;368:2450–2453. [DOI] [PubMed] [Google Scholar]
- 18.Kramer JR, Davila JA, Miller ED et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther 2008;27:274–282. [DOI] [PubMed] [Google Scholar]
- 19.West AN, Lee RE, Shambaugh-Miller MD et al. Defining rural for veterans healthcare planningJ Rural Health 2010;26:301–309. [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy V, Zhang P, Ethiraj S et al. Use of analytic morphomics of liver, spleen, and body composition to identify patients at risk for cirrhosis. Clin Gastro Hep 2015;13:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceut Statist 2011;10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talwalker JA. Health services and policy research in hepatology. Curr Opin Gastroenterol 2014;30:1–6. [DOI] [PubMed] [Google Scholar]
- 24.Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care 1981;19:127–140. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn-Sandler V, Sherman C, Aronsohn A et al. Consequences of perceived stigma among patients with cirrhosis. Digest Dis Sci 2014;59:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peery AF, Dellon ES, Lund J et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology 2012;143:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arora S, Geppert CMA, Kalishman S et al. Academic health center management of chronic diseases through knowledge networks: project ECHO. Acad Med 2007;82:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su GL, McCurdy H, Tai WA et al. Implementation of the first Department of Veterans Affairs Specialty Care Access Network-Extension of Community Healthcare Outcomes (SCAN-ECHO) Program for Chronic Liver Disease. Hepatology 2012;56:73A. [Google Scholar]
- 29.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 30.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.